Abstract
Purpose
To investigate the natural history in patients with LRAT-associated retinal degenerations (RDs), in the advent of clinical trials testing treatment options.
Methods
A retrospective cohort of 13 patients with LRAT-RDs.
Results
Twelve patients from a genetic isolate carried a homozygous c.12del mutation. One unrelated patient carried a homozygous c.326G>T mutation. The mean follow-up time was 25.3 years (SD 15.2; range 4.8–53.5). The first symptom was nyctalopia (n = 11), central vision loss (n = 1), or light-gazing (n = 1), and was noticed in the first decade of life. Seven patients (54%) reached low vision (visual acuity < 20/67), four of whom reaching blindness (visual acuity < 20/400), respectively, at mean ages of 49.9 (SE 5.4) and 59.9 (SE 3.1) years. The fundus appearance was variable. Retinal white dots were seen in six patients (46%). Full-field electroretinograms (n = 11) were nondetectable (n = 2; ages 31–60), reduced in a nonspecified pattern (n = 2; ages 11–54), or showed rod–cone (n = 6; ages 38–48) or cone–rod (n = 1; age 29) dysfunction. Optical coherence tomography (n = 4) showed retinal thinning but relative preservation of the (para-)foveal outer retinal layers in the second (n = 1) and sixth decade of life (n = 2), and profound chorioretinal degeneration from the eighth decade of life (n = 1).
Conclusions
LRAT-associated phenotypes in this cohort were variable and unusual, but generally milder than those seen in RPE65-associated disease, and may be particularly amenable to treatment. The window of therapeutic opportunity can be extended in patients with a mild phenotype.
Translational Relevance
Knowledge of the natural history of LRAT-RDs is essential in determining the window of opportunity in ongoing and future clinical trials for novel therapeutic options.
Keywords: retinitis pigmentosa, cone-rod degeneration, disease progression, genetic diseases
Introduction
Inherited retinal degenerations (RD) comprise a collection of heterogeneous diseases characterized by variable progressive dysfunction of rods and/or cones. Leber congenital amaurosis (LCA) is the most severe RD, characterized by severe visual impairment, nondetectable rod and cone function in the first year of life, and often nystagmus. Retinitis pigmentosa (RP) or rod–cone degeneration, the most common RD, is characterized by progressive nyctalopia, (mid)peripheral visual field constriction, and finally, loss of central vision.1 Pathogenic variants in several genes that are typically associated with LCA, can also cause RP.2
Genes that are mutated in RDs encode proteins that function through multiple mechanisms and pathways involving structural and functional retinal integrity, such as the retinoid cycle.3 This cycle regenerates the visual pigments that are used after light activation. Key enzymes in the retinoid cycle are retinal pigment epithelium (RPE)-specific protein 65 kDa (RPE65) and lecithin:retinol acetyltransferase (LRAT), which are encoded by the RPE65 and LRAT gene, respectively. Photoactivation induces the configurational change of the visual chromophore 11-cis-retinal into all-trans-retinal in the photoreceptor, and the subsequent reduction to all-trans- retinol. The released all-trans-retinol is shuttled to the RPE cells, and esterified to all-trans-retinyl esters by the LRAT enzyme, providing the substrate for the RPE65 enzyme. Ultimately, 11-cis-retinal is resupplied to the photoreceptors. LRAT and RPE65 are thus essential enzymes in the regeneration of functional visual pigment, and a deficiency of either enzyme leads to an impairment in the visual cycle.4,5 Mutations in RPE65 have been associated with several severe RDs in the autosomal recessive form, causing 6% to 8% of LCA cases, and 5% of cases of childhood-onset RP.6–15 Rarely, cases of autosomal-dominant RD have been described in association with mutations in RPE65.16–18 Although reports on the phenotype associated with LRAT-mutations are scarce, case reports and small case series have shown an association with LCA and childhood-onset RP.19–23 LRAT mutations have been predicted to cause less than 1% of these cases.6
While no established and effective treatment is available for most RDs, the advent of subretinal gene therapy in the treatment of LCA and RP associated with RPE65 mutations and the favorable results have recently led to the first approval of subretinal gene therapy by the US Food and Drug Administration, and provide a promising perspective for related disorders.24–26 A trial investigating the safety and efficacy of oral 9-cis-retinal supplementation has shown promising results in the treatment of RDs associated with mutations in RPE65 and LRAT, combining these two patient groups.27,24 However, due to the rarity of RDs associated with LRAT mutations, little is known about the associated phenotypic spectrum, and the degree of clinical resemblance to phenotypes associated with RPE65 mutations.
The purpose of this study was to provide a description of the initial and longitudinal clinical characteristics of patients with RDs associated with LRAT mutations, and to compare these findings with previously reported clinical characteristics of RDs associated with RPE65 mutations.
Methods
Patient Population and Genetic Analysis
Patients with bi-allelic molecularly confirmed pathogenic variants in LRAT were collected from the database for hereditary eye diseases (Delleman archive) at the Academic Medical Center (AMC) in Amsterdam, the Netherlands, resulting in a cohort of 12 patients from a Dutch genetic isolate. One Turkish–Belgian patient (Turkish descent) was included from Ghent University Hospital, Belgium. Informed consent was obtained from Dutch patients in this study, and the study adhered to the tenets of the Declaration of Helsinki. For the Belgian patient, informed consent for this retrospective study was waivered by the Ethics Committee of Ghent University Hospital.
Mutational analyses for Dutch patients were performed at the AMC, and at the Ghent University Hospital for the Belgian patient. In the Dutch patients, no pathogenic variants were found with the autosomal recessive RP chip (version 2011/2012; Asper Biotech, Tartu, Estonia), which included RPE65, but sequence analysis of LRAT revealed a previously described homozygous frameshift mutation,28 which led to a premature stop codon (c.12del; p.[Met5Cysfs*53]; NM_004744.4), and segregated with the disease. Because the Dutch patients originated from the same genetic isolate as the previously described CRB1-RP cohorts,29,30 the presence of bi-allelic CRB1 mutations was excluded in all patients. The Turkish–Belgian patient carried a homozygous missense mutation in LRAT (c.326G>T; p.[Arg109Leu]), found through identity by descent–guided Sanger sequencing,31 and a heterozygous rare variant in CRB1 (c.4060G>A; p.[Ala1354Thr]), found with LCA chip analysis. Sanger sequencing of the coding regions and intron–exon borders revealed no second CRB1 mutation.
Clinical Assessment and Data Collection
Through a standardized medical records review, data on initial symptoms, best-corrected visual acuity (BCVA), refractive error, slit-lamp biomicroscopy of the anterior segment, and dilated fundus examination were obtained for all patients. International Society for Clinical Electrophysiology of Vision standard full-field electroretinography (ERG) results and data on color vision testing were available for 11 patients (Ishihara in all patients; Farnsworth D-15 dichotomous color blindness test, Roth 28-hue desaturated test, and Hardy-Rand-Rittler plates in a subset).32 Goldmann visual fields (GVFs) were performed in six patients. Fundus photographs were available for 11 patients, and spectral-domain optical coherence tomography (SD-OCT) with Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) was available for four patients. Thirty-degree fundus autofluorescence images (FAF; Heidelberg Engineering) were available for three patients.
Statistical Analysis
Data were analyzed using SPSS version 23.0 (IBM Corp, Armonk, NY). Kaplan Meier's methodology was used to analyze the time-to-event for low-vision (BCVA < 20/67) and blindness (BCVA < 20/400), based on the World Health Organization criteria, using the better-seeing eye. GVF areas of the V4e target were digitized and converted to seeing retinal areas in millimeters squared using a method described by Dagnelie.33 P < 0.05 were considered statistically significant.
Results
Of 13 subjects, 7 were male. The mean age at first examination was 24.4 years (SD 15; range 1.6–53.6 years). The mean follow-up time was 25.3 years (SD 15.2; range 4.8–53.5 years), with a mean number of 9.5 visits per patient (SD 6.4; range 5–26). Symptoms started as early as the first decade of life in all patients (Table 1), but the 12 patients from the genetic isolate reported a slow progression. Nine patients (69%) first started using visual aids other than glasses between the ages of 29 and 43. A comparison of the phenotypes associated with mutations in LRAT and RPE65 is provided in Table 2.
Table 1.
Clinical Characteristics of Patients With LRAT-Associated Retinal Degeneration
| ID |
Sex, Age, (y) |
Symptoms (DOL at Onset)* |
BCVA |
Fundus |
ERG (Age)† |
|||
| Nyctalopia |
VF Loss |
VA Loss |
Macula |
P |
||||
| I | M, 75‡ | + (1st) | + (3rd) | + (2nd) | CF | Profound chorioretinal and RPE atrophy, sharply demarcated. Pigment clumping. | Bone spicule pigmentation, RPE mottling and depigmentation spots. No white–yellow dots. | ND (52) |
| II | M, 49‡ | + (infancy) | + (1st) | + (1st) | CF | Sheen, RPE alterations | Extensive bone spicule pigmentation, RPE atrophy. Extensive white–yellow dots. | CRD (28) |
| III | F, 52 | + (infancy) | + (3rd) | + (3rd) | 20/200 | Retinal and RPE atrophy. | Bone spicule pigmentations, extensive clumping in atrophic supratemporal region, coarse salt and pepper. No white dots, but hypopigmented dots. Paving stone–like degeneration. | ND (30) |
| IV | M, 49 | + (infancy) | + (3rd) | + (2nd) | CF | RPE alterations, choroidal atrophy. | Bone spicule pigmentations, extensive pigment clumping. Salt and pepper. Paving stone–like degeneration in far periphery. RPE mottling. No white–yellow dots. | MR (31) |
| V | F, 49 | + (infancy) | + (2nd) | + (2nd/3rd) | CF | Sheen, RPE atrophic alterations | Extensive bone spicule and coarse pigmentation. Salt and pepper. RPE mottling. Paving stone–like atrophic depigmentation. | NA |
| VI | M, 42 | + (infancy) | + (3rd) | + (2nd) | 20/200 | Chorioretinal and RPE atrophy spanning posterior pole and beyond vascular arcade. | Bone spicule and coarse pigmentation. RPE mottling. Choroid atrophy. No white dots or paving stone degeneration. | NA |
| VII | F, 64 | + (infancy) | + (3rd) | + (<4th) | 20/100 | M: difficult to assess due to synchysis scintillans. Small pigmentations. | Bone spicule pigmentation. RPE mottling. Paving stone–like degenerative depigmentation. White dots, which were no longer observed at the age of 59 onward, after they had been repeatedly observed between the ages of 34–52. | RCD (37) |
| VIII | M 55 | + (1st) | + (<5th) | + (1st) | 20/25 | Sheen, RPE atrophy around spared fovea. | Few bone spicule pigmentations. RPE mottling, lobular atrophy. White–yellow dots in far periphery. | RCD (49) |
| IX | M, 20 | + (1st) | + (1st) | - | 20/20 | Sheen, normal pigmentation, white dots. | Salt and pepper. RPE mottling. Drusen. White–yellow dots. | RCD (16) |
| X | F, 44 | + (1st) | + (2nd) | + (2nd) | 20/20 | No sheen, subtle white dots on a pale retina, subtle granular RPE changes. | Bone spicule pigmentations. Patches of paving stone–like retinal and RPE atrophy, RPE mottling. White yellow dots. Retina generally hypopigmented. | RCD (40) |
| XI | F, 68 | + (infancy) | + (5th) | - | 20/40 | Atrophic RPE alterations. | Choroideremia-like atrophic zones with relatively little bone spicule pigmentation. Paving stone–like degeneration. | RCD (42) |
| XII | F, 58 | + (infancy) | - | - | 20/16 | RPE alterations. | Few clustered bone-spicule and nummular pigmentations, mainly inferiorly. Midperipheral chorioretinal atrophy. | RR (53) |
| XIII | M, 17 | + (infancy) | +* | + (1st) | 20/66 | Central macula with normal appearance, white dots in the superior posterior pole. | Outer retinal atrophy, no intraretinal hyperpigmentation, white–grayish dots | RCD (1) |
CRD, cone–rod degeneration pattern; DOL, decade of life; ERG, electroretinography; ND, nondetectable; MR, minimal response; NA, data not available; ND, no detectable amplitudes; RCD, rod–cone degeneration pattern; RR, reduced response, pattern not specified; P, (mid)periphery; VA, visual acuity; VF, visual field.
The decade of life during which the patient started noticing the symptom is shown between parentheses. Mild adult-onset nystagmus was documented in patients I, II, and X, starting from the fifth decade of life. In patient XIII, light-gazing and nystagmus were noticed by the parents during the first months of life. Subjective visual field complaints were not documented, but concentric constriction on Goldmann kinetic perimetry in the second decade of life. Photopsias were reported in 7/13 patients (54%).
Full-field ERGs were not performed at the last clinical visit, but the results of the initial ERG are shown, and the age at the time of ERG examination is shown in parentheses. In patients II and VIII, later ERGs showed nondetectable dark- and light-adapted, at the ages of 49 and 55 years, respectively. In the Turkish–Belgian patient, patient XIII, dark-adapted responses were nondetectable, with severely diminished light-adapted responses (<5 μV), leading to a diagnosis of early-onset panretinal degeneration.
These patients are siblings.
Table 2.
Overview of the Current Literature of RPE65- and LRAT-Associated Phenotypes
| Phenotype |
Additional Features |
RPE65 |
LRAT |
| IRD* | Biswas et al., 201734 | ||
| LCA | Katagiri et al., 201610 Srilekha et al., 201511 Jakobsson et al., 201412 Chen et al., 201313 Roman et al., 201314 Xu et al., 201235 McKibbin et al., 201015 Pasadhika et al., 201036 Walia et al., 201037 Li et al., 200938 Simonelli et al., 20079 Jacobson et al., 200539; 200840 Galvin et al., 200541 Booij et al., 20052 Silva et al., 200442 Hanein et al., 20047 Sitorus et al., 200343 Hamel et al., 200144 Dharmaraj et al., 200045 | Den Hollander et al., 200720 Sénéchal et al., 200622 | |
| Autosomal-recessive RP | Chebil et al., 2016 (French)46 Walia et al 201037 Booij et al., 20052 Hamel et al., 200144 Morimura et al., 199847 | Preising et al., 2007 (German)23 This study | |
| Autosomal-dominant IRD | Extensive chorioretinal atrophy | Jauregui et al., 201818 Hull et al., 201616* Bowne et al., 201117 (RP) | - |
| AVMD/foveal vitelliform lestions | Hull et al., 201616 | - | |
| EORD | Hull et al., 201616 Kabir et al., 201348 El Matri et al., 200649 Thompson et al., 200050 | Coppieters et al., 201431Dev Borman et al., 201221 | |
| EOSRD/SECORD | Better visual functions than typically seen in LCA | Mo et al., 201451 Weleber et al., 201152 Lorenz et al., 200853 Preising et al., 2007 (German)23 Paunescu et al., 200554 Lorenz et al., 200455 Yzer et al., 200356 Felius et al., 200257 | Thompson et al., 200119 |
| RPA | White–yellow dots | Littink et al., 201228 This study | |
| FA | White–yellow dots | Katagiri et al., 201858 (flecks) Yang et al., 201759 Schatz et al., 201160 | |
| Early and prominent severe cone involvement | Jakobsson et al., 201412 (severe visual impairment in childhood) | ||
| Cone–rod dystrophy | This study (good initial visual function; low vision in third decade of life) |
AdRP, autosomal-recessive retinitis pigmentosa; ArRP, autosomal-recessive retinitis pigmentosa; AVMD, adult-onset vitelliform macular dystrophy; EORD, early-onset retinal dystrophy; EOSRD, early-onset severe retinal dystrophy; FA, fundus albipunctatus; IRD, inherited retinal dystrophy; RPA, retinitis punctata albescens; SECORD, severe early childhood–onset retinal dystrophy.
Retinal dystrophy, not otherwise specified.
Ophthalmic and Fundoscopic Features
The mean spherical equivalent of the refractive error (SER) was +2.2 diopters (D; SD 2.1; range −2.4D to +4.7D), and all but two patients (85%) were mildly (+1D ≤ SER < +3D; n = 6) or moderately (+3D ≤ SER < +6D; n = 5) hyperopic. Hyperopic patients were aged 17 to 59 years, and nonhyperopic patients were aged 11 and 13 years at the time of initial examination of the SER.
Nuclear (n = 2) or posterior subcapsular (n = 2) cataract was observed in four patients. Two patients underwent uncomplicated cataract surgery in their seventh decade of life. Abnormalities in the vitreous were present in 10 of 13 patients (77%), all from the genetic isolate, and consisted of cells or dust-like particles (n = 6), veils (n = 6), or synchysis scintillans (n = 2).
All patients from the genetic isolate had optic disc pallor and vascular attenuation, and 11 of 13 patients (85%) had peripapillary chorioretinal atrophy, varying from a small to moderately sized (n = 2) or large (n = 1) but distinct atrophic border, to a large atrophic zone encompassing both the peripapillary region and parts of the posterior pole (n = 8). Bone spicule–like pigment migration in the (mid)periphery was first seen at the mean age of 44.6 years (SD 8.8; range 31.5–55.2) in all but two patients aged 17.0 and 20.9 years at the last examination, but intraretinal pigmentation remained sparse in three patients (23%). Eight patients (62%) from the genetic isolate had sharply demarcated areas of atrophic depigmentation in the periphery (Table 1). White dots were seen in the (mid)periphery in six patients (46%) aged 17 to 64 years, including the Turkish–Belgian patient. Considerable intrafamilial variability was observed (Fig. 2). Macular RPE involvement ranged from mild RPE alterations (n = 7), first described at a mean age of 39.8 years (SD 17.0; range 11.7–59.4), to profound atrophy of the posterior pole and the central macula (n = 4), first described at a mean age of 42.6 (SD 20.5; range 22.1–68.3). The macular RPE had a healthy aspect in two patients aged 17.0 and 55.2 years.
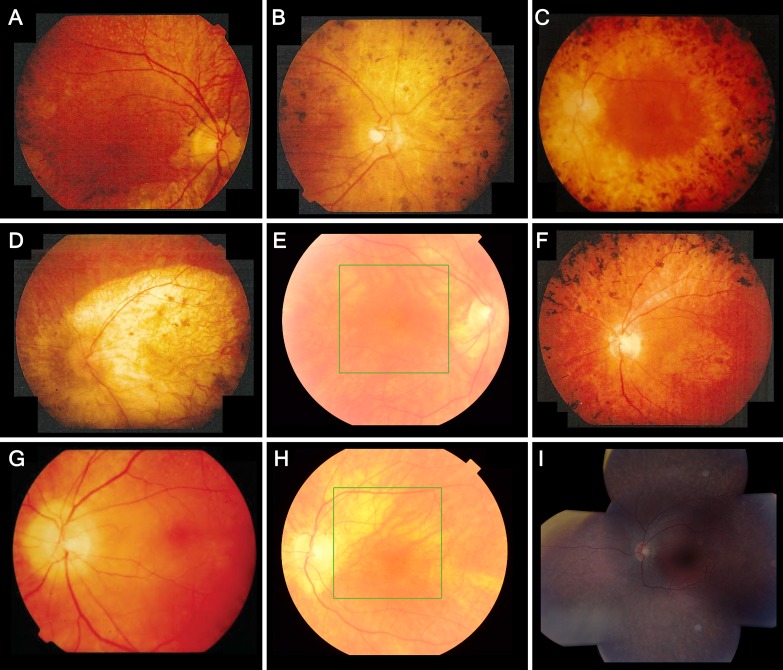
Figure 2.
Fundus photographs showing retinal features and intrafamilial variability in patients with LRAT-associated retinal degeneration. Reported ages are at the time of fundus photography. (A) Patient II, aged 39, showing pigmentary changes in the posterior pole, a macular sheen, and yellow-white dots, associated with retinitis punctata albescens, in the midperiphery. The periphery, not shown here, showed extensive bone-spicule hyperpigmentation. (B) Patient IV, aged 49, showing a pale optic disc, peripapillary atrophy, vascular attenuation, mottled retinal pigmentary epithelium (RPE) changes and clumping in the posterior pole, and atrophy in the nasal midperiphery. In the inferior midperiphery, not shown here, paving stone–like atrophic zones were seen with fundoscopy. (C) Patient VI, aged 42, showing retinal atrophy, and widespread bone spicule–like and coarse hyperpigmentation extending into the posterior pole. The central macula is relatively spared, with mild RPE alterations. (D) Patient I, aged 50, showed profound chorioretinal atrophy of the posterior pole and along the vascular arcade. The periphery, not shown here, showed mild RPE mottling, several paving stone–like degenerations, and little bone spicule–like hyperpigmentation. (E) Patient XII, aged 56, showing peripapillary atrophy and areas of retinal thinning along the superior vascular arcade, with no RPE changes in the central retina. In the inferonasal midperiphery, not shown here, a few round and bone spicule–like pigmentations were clustered. (F) Patient V, aged 47, showed atrophic RPE alterations and a sheen in the central macula, atrophy around the optic disc and along the vascular arcade, and extensive bone spicule–like pigmentation in the midperipheral retina. (G, H) Patient XI, showing the disease progression between the ages of 43 (G) and 67 (H). At the age of 43, this patient showed peripapillary atrophy, yellow–white dots below the superior vascular arcade, and no RPE changes of the central macula. At the age of 67, the white dots were no longer visible, and the posterior pole showed zones of atrophic RPE. (I) Composite fundus photography of the left eye of patient XIII at the age of 15, showing peripapillary atrophy, a normal macula, limited intraretinal white dots in the superior macula that appear less brightly yellow and less sharply circumscribed than the white–yellow dots seen in patients from the genetic isolate, and fleck-like outer retinal atrophy in the (mid-)periphery.
Central Visual Function
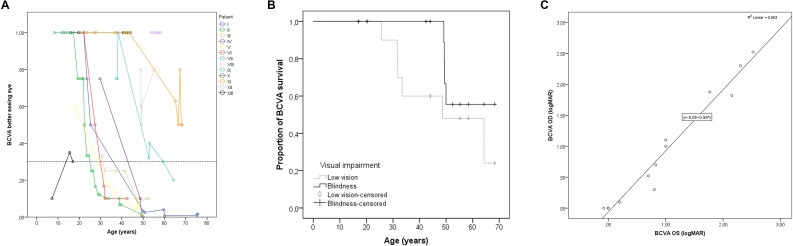
The median initial BCVA was 20/20 (IQR in decimals 0.33; range 20/100–20/20), at a median age of 20.6 years (IQR 20.3; range 7.2–53.6 years). Figure 1 illustrates a better preservation of the BCVA in patients from the genetic isolate than in patient XIII, the youngest patient (aged 17 at the final visit), who was Turkish–Belgian. Survival analysis revealed no BCVA-based visual impairment until the third decade of life (Figs. 1A, 1B). The mean ages for reaching low vision and blindness were 49.9 (SE 5.4; 95%CI 39.3–60.5) and 59.9 years (SE 3.1; 95%CI 53.8–66.0), respectively, with seven patients (54%) reaching low vision in the better seeing eye during the follow-up period, four of whom (31%) reaching blindness. Five patients (38%) maintained a BCVA of 20/40 or more into the third to seventh decades of life (BCVA > 20/25 in 4 patients), while patient XIII had a BCVA of 20/66 at the final visit. Spearman's rank correlation coefficients showed a high degree of intraindividual between-eye symmetry in BCVA at the first (0.78; P = 0.002) and last (0.98; P < 0.00001) visit.
Figure 1.
Visual acuities in patients with LRAT-associated retinal degeneration. (A) The course of decline of the best-corrected visual acuity (BCVA) in individual patients. For illustrative purposes, a BCVA of 20/20 was considered the ceiling, also in patients with a BCVA > 20/20. The dashed line indicates the cutoff value for low vision (20/67), based on the World Health Organization criteria. The individual line for patient XII, the only patient not from the genetic isolate, is shown in black. (B) Kaplan-Meier plots showing the proportion of patients with a BCVA above low vision (BCVA ≥ 20/67) and above blindness (BCVA ≥ 20/400). Censored observations (i.e., patients who had not reached low vision or blindness at the last follow-up moment, are depicted as vertical bars [blindness] or rhombi [low vision]). The median age for reaching low vision was 48.6 years (standard error 14.1). The median age for reaching blindness could not be calculated, as fewer than 50% of subjects became blind during follow-up. (C) The relation of the BCVA in logarithm of the minimum angle of resolution (logMAR) between the right and left eye at the last clinical visit, showing a linear relationship.
Color vision testing in 11 patients (10 from the genetic isolate) showed a severe or near-complete deficiency in all axes in eight patients aged 15.5 to 59.4 (patients I, III, V–IX, XIII), or multiple errors in different axes in three patients aged 12.6 to 43.1 (patient II: protan; patient X and XI: tetartan and tritan; patient XI also deutan), despite a nonimpaired concurrent BCVA in seven of 11 cases.
Visual Fields and Electroretinography
Visual fields were recorded in 10 patients (Supplementary Table S1), using different modalities (GVF in 6 subjects). Visual fields predominantly showed peripheral constriction or midperipheral scotomas with relative preservation of central vision in seven of 10 patients (70%), and a central scotoma in three of 10 patients (30%) including two brothers (Supplementary Fig. S1). Initial seeing retinal areas for the V4e target were large (>250 mm2) in all patients, aged 15.5 to 48.9 years at the time of examination, with a median of 714.3 mm2 (IQR 123.9; range 482.1–774.2). During follow-up, visual field–based low vision (central diameter <20°) or blindness (central diameter <10°) was observed in two patients from the genetic isolate at the ages of 23.6 (patient I) and 54.1 years (patient XII), despite a normal concurrent BCVA (Table 2). Patient I reached visual field–based blindness 26 years before reaching BCVA-based blindness. The degree of between-eye symmetry of seeing retinal areas was high (Spearman's rank correlation coefficient 0.89; P = 0.019).
Initial ERG examination showed a rod–cone pattern (n = 5; ages 37.6–49.0 in the genetic isolate; second year of life in patient XIII), cone–rod pattern (n = 1; age 28.9), severely reduced responses in a nonspecified pattern (n = 3; ages 11.9–53.6), or a nondetectable response (n = 2; ages 30.8–52.1 years). Later ERGs showed progression to nondetectable responses in two more patients in their fifth to sixth decades of life.
Findings on Retinal Imaging
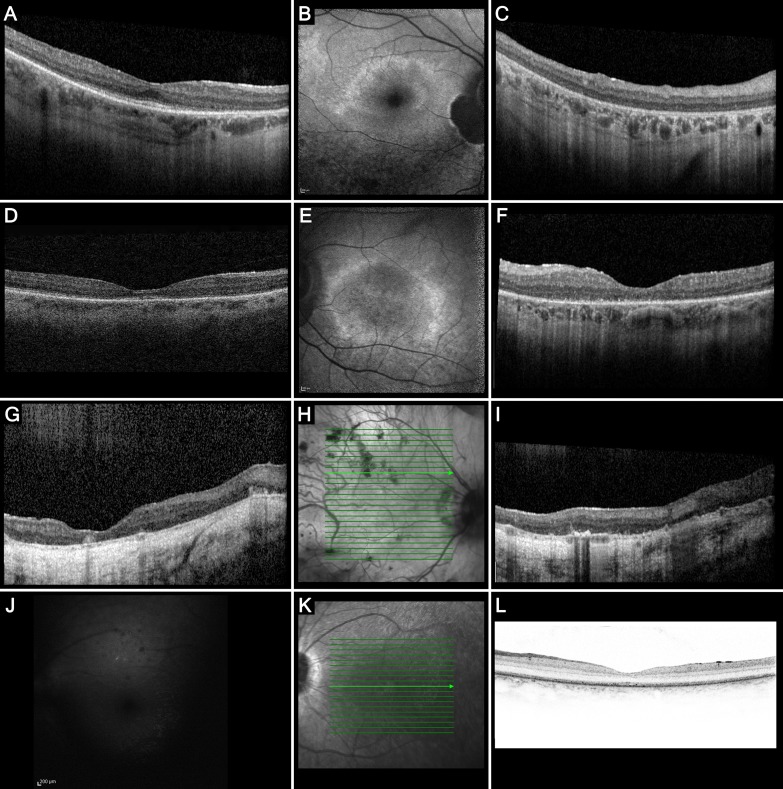
SD-OCT scans of four patients (I, XI, XII, and XIII) showed outer retinal attenuation in the peripheral macula and a relatively well-preserved foveal and parafoveal outer retina in patient XIII during his second decade of life, and in patient XII until her sixth to seventh decades of life. Patient XI showed progressive thinning of the outer nuclear layer and hyperreflective outer retinal bands in her seventh decade of life (Figs. 3D, 3F). In the mildly hyperopic patient I, an SD-OCT scan at the age of 76.5 showed a markedly thinned choroid, and generalized outer retinal atrophy (Figs. 3G–I). FAF images in three patients showed granular or mottled hypoautofluorescence and a hyperautofluorescent ring in two patients from the genetic isolate (Figs. 3B, 3E).
Figure 3.
Imaging findings in patients with LRAT-associated retinal degeneration. (A–C) Imaging in patient XII, aged 57 at the time of examination. Repeated SD-OCT scans between the ages of 54 and 58 showed relative foveal, parafoveal, and temporal perifoveal preservation of the outer nuclear layer and the external limiting membrane (ELM) and ellipsoid zone (EZ), although these hyperreflective outer retinal bands showed a few interruptions, with profound nasal thinning and near-complete disappearance of these layers in areas corresponding with atrophy on the fundus photograph. Follow-up SD-OCT scans over the course of 4 years showed mildly increased outer retinal thinning, mainly in the nasal peri- and parafoveal region. The transition zone between relatively spared outer retina and markedly thinned outer retina colocalized with the hyperautofluorescent ring on FAF (B), which also showed a juxtapapillary patch of absent AF, sharply demarcated by a hyper-AF border. Along the inferior vascular arcade, a zone of dense granular hypo-AF was visible. On SD-OCT of the peripheral macula (C), small hyperreflective foci with no shadowing were visible in the inner plexiform layer and the inner nuclear layer. To a milder degree, these hyperreflective foci are also visible in (A). (D–F) SD-OCT scan of the right eye of patient XI at the age of 65 years (D), showing a mild epiretinal membrane, and generalized outer retinal thinning and attenuation of the outer retinal hyperreflective bands that was more pronounced at the parafovea, with relative preservation more temporally and at the fovea. FAF imaging of the left eye at the age of 68 (E) showed peripapillary atrophy, and a perimacular hyper-AF ring with mottled hypo-AF inside and outside this hyper-AF ring. The corresponding SD-OCT scan of the left eye (F) showed more pronounced outer retinal atrophy, and severe granulation of the EZ band. (G–I) Patient I, aged 75, showing generalized severe atrophy of the outer nuclear layer, ELM, and EZ, a markedly thin choroid and a scleral tunnel. Large hyperreflective outer retinal accumulations above the RPE level were visible. No concurrent fundus photograph was made in order to attempt to colocalize these accumulations to a funduscopic structure, but earlier fundus photographs taken at the age of 52 (Fig. 2D) showed profound atrophy of the retina inside and around the posterior pole. (J–L) FAF imaging (J) of patient XIII at the of 15 years, carrying a homozygous c.326G>T mutation, showing reduced image quality due to the nystagmus, and a generalized reduced AF with small hypo-AF spots in the posterior pole. No corresponding SD-OCT scan was available at that age, but an SD-OCT scan at the age of 17 years (K–L) showed no evident outer retinal thinning, but a granular appearance of the EZ and ELM, with relative preservation of the foveal structure.
Discussion
In this retrospective cohort study of 13 patients with RDs associated with LRAT-mutations, we describe the long-term natural history of an unusually mild, albeit variable, phenotype, adding to the phenotypic spectrum of RDs associated with LRAT-mutations, and providing further insight regarding the window of opportunity for novel therapeutic strategies.27,61 We describe, to the best of our knowledge, the largest cohort to date of patients with RDs associated with LRAT mutations.
While the presentation of the first symptom, usually nyctalopia, was within the first years of life, the retinal phenotypes and the natural disease course reflect a phenotypic variability in RDs associated with LRAT mutations, even within the genetic isolate. Although little is known on the phenotype associated with LRAT mutations, due to its rarity, it has been associated with Leber congenital amaurosis and other types of severe early-onset retinal degeneration.19,20,22 In contrast to earlier reports of visual impairment in childhood or adolescence,20,27 the mean ages for reaching low vision and blindness in the current cohort were 49.9 and 59.9 years, respectively, with five patients maintaining good BCVA until the final examination, in their third to seventh decade of life. In the Turkish–Belgian patient, symptomatology and visual function were evidently worse, with a BCVA less than 20/40 from the first decade of life, although he had not yet reached low vision at the final visit at the age of 17 years. An earlier study of retinitis punctata albescens included four Dutch patients with the same homozygous frameshift mutation in LRAT found in the genetic isolate in this cohort, who had relatively well-preserved BCVA and visual fields compared with patients with retinitis punctata albescens associated with other genes involved in the retinoid cycle, although the patients with LRAT mutations described in the previous study were younger (ages 7–19 years) than the patients in the current cohort.28 In this study, color vision testing was markedly abnormal before BCVA started to decrease. Peripheral visual fields showed more variability, but were relatively well-preserved until the sixth decade of life, with most patients showing relative or absolute concentric constriction with a relatively large central residue, with or without (para)central scotomas. In contrast to these findings, previous reports on LRAT-RDs have lacked quantitative visual field analysis, but where reported, visual field sizes were usually small (<25 mm2) or intermediate (25–250 mm2) in the second to third decades of life, with few exceptions,27 or were reported as severely reduced,19,20 with variably sized peripheral crescents of vision.21
In gene- or cell-based therapeutic trials that are designed to compare the visual and retinal function between the treated and nontreated control eye, between-eye symmetry is an important assumption. Our findings in this cohort show a high degree of symmetry between affected eyes of the same individual in BCVA and seeing retinal area.
Marked intrafamilial variability was observed in the genetic isolate, with phenotypes varying from cone–rod to rod–cone patterns. This variability was most notable upon ophthalmoscopy, with some patients showing central and midperipheral profound chorioretinal atrophy and coarse hyperpigmentation (Figs. 2C, 2D), while others showed midperipheral patchy paving stone–like degeneration, and some patients maintaining a relatively spared posterior pole with only mild RPE alterations (Figs. 2E, 2G, 2H). This considerable phenotypic variability in patients originating from the same genetic isolate and carrying the same homozygous mutation points to the involvement of genetic and/or environmental modifiers. Certain Rpe65 variants have been shown to modify the disease course in mouse models of RP and albinism,62,63 although no such variants were found in our study. Bone spicule–like pigmentation was absent in two patients (15%), and limited or sparsely scattered in three patients (23%; Fig. 2). Eight patients (62%) had distinct areas of depigmentation. This finding is in line with previous observations of no or minimal hyperpigmentation in phenotypes associated with LRAT and RPE65 mutations,21,31,49 and areas of hypo- or depigmentation.20
Retinal imaging, in line with visual function, showed relatively well-preserved outer retinas in two patients in their second and sixth decade of life, and marked retinal or chorioretinal degeneration in two patients in their seventh and eighth decades of life. Preservation of central retinal structure, including outer photoreceptor layers on SD-OCT has been reported before in a younger patient (27 years) with different LRAT mutations.21 The long-term preservation of the outer retina in these patients, at least at the level of the fovea, is a favorable finding, as it has been demonstrated that a relatively preserved photoreceptor layer predicts a positive treatment response in patients with RDs associated with mutations in RPE65 or LRAT receiving oral synthetic 9-cis-retinoid in a clinical trial.61,64
As a systemic therapeutic trial targeting the retinoid cycle in human patients has grouped patients with LRAT- and RPE65-associated RP and LCA,27 and the same systemic or novel cell–based therapeutic strategies have grouped Lrat(−/−) and Rpe65(−/−) mouse models together,65,66 understanding the comparability of these phenotypes is important. Comparable LCA phenotypes have been found in Rpe65(−/−) and Lrat(−/−) mice.67,68 Similarly in human patients, parallels between the phenotypes associated with LRAT- and RPE65-mutations have been suggested in previous case studies of LRAT-RDs.21,22 RPE65-mutations typically lead to severe retinal degeneration, presenting with severely reduced vision in the first year of life, nystagmus, and panretinal photoreceptor degeneration, leading to a diagnosis of LCA or early-onset severe retinal degeneration.∗ However, cases of RPE65-RP and early-onset RD with relatively preserved ambulatory vision into adolescence have also been described.47,52,37,50,56 Distinctive fundus features include white dots, just like we observed in six (46%) of the LRAT-RD patients in the current cohort, sometimes fading with time as also observed in one patient in the current study.52,46,35,58,51 In RPE65-associated RD, these white–yellow dots have also been described in fundus albipunctatus.59,60 SD-OCT scans in several patients with RPE65-LCA have shown preserved retinal thickness and foveal contour, and attenuated but detectable outer photoreceptor layers, even in cases of visual impairment.9,55,70 However, lamellar disorganization and/or outer retinal and photoreceptor layer disintegration have also been found in RPE65-LCA, with differing reports on the relationship with age.40,36,53 Our findings in this LRAT-RD population indicate similarities with the retinal phenotype of RPE65-RDs, although the exceptionally extended period of retinal structural and functional preservation in the genetic isolate is distinct. Moreover, the findings in our study are variable, complicating the ability to make a clear comparison between phenotypes associated with mutations in LRAT and those associated with mutations in RPE65.
The drawbacks of this study include its retrospective design, and the limited historic availability of imaging and all modalities of functional testing, as most tests were available only for a subset of patients. Although we have found a symptomatically and visually more severe phenotype in the patient with the homozygous c.326G>T mutation than in the patients from the genetic isolate with a homozygous c.12del mutation, a larger and more genetically heterogeneous cohort would have allowed for the exploration of genotype–phenotype correlations, subgroup analyses, and for a more comprehensive description of the phenotypic spectrum. As the current literature on phenotypes associated with bi-allelic mutations in LRAT is limited, the generalizability of our findings in this Dutch and Belgian cohort to the general population of patients with LRAT-RDs is uncertain.
In conclusion, our findings in patients with RDs associated with LRAT-mutations due to the c.12del mutation or c.326G>T mutation indicate that LRAT-RDs may be particularly amenable to treatment. Not all pathogenic variants in LRAT lead to early severe visual dysfunction, and the window of therapeutic opportunity may be extended to later decades of life in some patients. While fundus phenotypes were heterogeneous within this genetic isolate, similarities can be found with the heterogeneous phenotypes associated with RPE65 mutations.
Supplementary Material
Acknowledgments
This study was performed as part of a collaboration within the European Reference Network for Rare Eye Diseases (ERN-EYE). ERN-EYE is co-funded by the Health Program of the European Union under the Framework Partnership Agreement #739543 – ‘ERN-EYE' and co-funded by the Hôpitaux Universitaires de Strasbourg.
Supported by grants from Stichting Blindenhulp, Janivo Stichting (CJFB).
Disclosure: M. Talib, None; M.J. van Schooneveld, None; R.J.G. van Duuren, None; C. Van Cauwenbergh, None; J.B. ten Brink, None; E. De Baere, None; R.J. Florijn, None; N.E. Schalij-Delfos, None; B.P. Leroy, None; A.A. Bergen, None; C.J.F. Boon, None
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Booij JC, Florijn RJ, ten Brink JB, et al. Identification of mutations in the AIPL1, CRB1, GUCY2D, RPE65, and RPGRIP1 genes in patients with juvenile retinitis pigmentosa. J Med Genet. 2005;42:e67. doi: 10.1136/jmg.2005.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Soest S, Westerveld A, de Jong PT, et al. Retinitis pigmentosa: defined from a molecular point of view. Surv Ophthalmol. 1999;43:321–334. doi: 10.1016/s0039-6257(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 4.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 5.Batten ML, Imanishi Y, Maeda T, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Hollander AI, Roepman R, Koenekoop RK, Cremers FPM. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Hanein S, Perrault I, Gerber S, et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum Mutat. 2004;23:306–317. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- 8.Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–472. doi: 10.1146/annurev-neuro-060909-153227. [DOI] [PubMed] [Google Scholar]
- 9.Simonelli F, Ziviello C, Testa F, et al. Clinical and molecular genetics of Leber's congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri S, Hayashi T, Kondo M, et al. RPE65 mutations in two Japanese families with Leber congenital amaurosis. Ophthalmic Genet. 2016;37:161–169. doi: 10.3109/13816810.2014.991931. [DOI] [PubMed] [Google Scholar]
- 11.Srilekha S, Arokiasamy T, Srikrupa NN, et al. Homozygosity mapping in Leber Congenital Amaurosis and autosomal recessive retinitis pigmentosa in South Indian families. PLoS One. 2015;10:e0131679. doi: 10.1371/journal.pone.0131679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsson C, Othman IS, Munier FL, et al. Cone-rod dystrophy caused by a novel homozygous RPE65 mutation in Leber congenital amaurosis. Klin Monbl Augenheilkd. 2014;231:405–410. doi: 10.1055/s-0034-1368221. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zhang Q, Shen T, et al. Comprehensive mutation analysis by whole-exome sequencing in 41 Chinese families with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2013;54:4351–4357. doi: 10.1167/iovs.13-11606. [DOI] [PubMed] [Google Scholar]
- 14.Roman AJ, Cideciyan AV, Schwartz SB, et al. Intervisit variability of visual parameters in Leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2013;54:1378–1383. doi: 10.1167/iovs.12-11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKibbin M, Ali M, Mohamed MD, et al. Genotype-phenotype correlation for Leber congenital amaurosis in Northern Pakistan. Arch Ophthalmol. 2010;128:107–113. doi: 10.1001/archophthalmol.2010.309. [DOI] [PubMed] [Google Scholar]
- 16.Hull S, Mukherjee R, Holder GE, et al. The clinical features of retinal disease due to a dominant mutation in RPE65. Mol Vis. 2016;22:626–635. [PMC free article] [PubMed] [Google Scholar]
- 17.Bowne SJ, Humphries MM, Sullivan LS, et al. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur J Hum Genet. 2011;19:1074–1081. doi: 10.1038/ejhg.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauregui R, Park KS, Tsang SH. Two-year progression analysis of RPE65 autosomal dominant retinitis pigmentosa. Ophthalmic Genet. 2018;39:544–549. doi: 10.1080/13816810.2018.1484929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson DA, Li Y, McHenry CL, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001;28:123–124. doi: 10.1038/88828. [DOI] [PubMed] [Google Scholar]
- 20.den Hollander AI, Lopez I, Yzer S, et al. Identification of novel mutations in patients with Leber congenital amaurosis and juvenile RP by genome-wide homozygosity mapping with SNP microarrays. Invest Ophthalmol Vis Sci. 2007;48:5690–5698. doi: 10.1167/iovs.07-0610. [DOI] [PubMed] [Google Scholar]
- 21.Dev Borman A, Ocaka LA, Mackay DS, et al. Early onset retinal dystrophy due to mutations in LRAT: molecular analysis and detailed phenotypic study. Invest Ophthalmol Vis Sci. 2012;53:3927–3938. doi: 10.1167/iovs.12-9548. [DOI] [PubMed] [Google Scholar]
- 22.Sénéchal A, Humbert G, Surget MO, et al. Screening genes of the retinoid metabolism: novel LRAT mutation in Leber congenital amaurosis. Am J Ophthalmol. 2006;142:702–704. doi: 10.1016/j.ajo.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 23.Preising MN, Paunescu K, Friedburg C, Lorenz B. Genetic and clinical heterogeneity in LCA patients. The end of uniformity [in German] Ophthalmologe. 2007;104:490–498. doi: 10.1007/s00347-007-1533-x. [DOI] [PubMed] [Google Scholar]
- 24.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. U.S. Food and Drug Administration, 2017. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm589467.htm Accessed April 2, 2019.
- 27.Koenekoop RK, Sui R, Sallum J, et al. Oral 9-cis retinoid for childhood blindness due to Leber congenital amaurosis caused by RPE65 or LRAT mutations: an open-label phase 1b trial. Lancet. 2014;384:1513–1520. doi: 10.1016/S0140-6736(14)60153-7. [DOI] [PubMed] [Google Scholar]
- 28.Littink KW, van Genderen MM, van Schooneveld MJ, et al. A homozygous frameshift mutation in LRAT causes retinitis punctata albescens. Ophthalmology. 2012;119:1899–1906. doi: 10.1016/j.ophtha.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Mathijssen IB, Florijn RJ, van den Born LI, et al. Long-term follow-up of patients with retinitis pigmentosa type 12 caused by CRB1 mutations: a severe phenotype with considerable interindividual variability. Retina. 2017;37:161–172. doi: 10.1097/IAE.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 30.Talib M, van Schooneveld MJ, van Genderen MM, et al. Genotypic and phenotypic characteristics of CRB1-associated retinal dystrophies: a long-term follow-up study. Ophthalmology. 2017;124:884–895. doi: 10.1016/j.ophtha.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Coppieters F, Van Schil K, Bauwens M, et al. Identity-by-descent-guided mutation analysis and exome sequencing in consanguineous families reveals unusual clinical and molecular findings in retinal dystrophy. Genet Med. 2014;16:671–680. doi: 10.1038/gim.2014.24. [DOI] [PubMed] [Google Scholar]
- 32.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 33.Dagnelie G. Conversion of planimetric visual field data into solid angles and retinal areas. Clin Vis Sci. 1990;5:95–100. [Google Scholar]
- 34.Biswas P, Duncan JL, Maranhao B, et al. Genetic analysis of 10 pedigrees with inherited retinal degeneration by exome sequencing and phenotype-genotype association. Physiol Genomics. 2017;49:216–229. doi: 10.1152/physiolgenomics.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu F, Dong Q, Liu L, et al. Novel RPE65 mutations associated with Leber congenital amaurosis in Chinese patients. Mol Vis. 2012;18:744–750. [PMC free article] [PubMed] [Google Scholar]
- 36.Pasadhika S, Fishman GA, Stone EM, et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51:2608–2614. doi: 10.1167/iovs.09-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walia S, Fishman GA, Jacobson SG, et al. Visual acuity in patients with Leber's congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117:1190–1198. doi: 10.1016/j.ophtha.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Wang H, Peng J, et al. Mutation survey of known genes and loci in the Saudi Arabian population. Invest Ophthalmol Vis Sci. 2009;50:1336–1343. doi: 10.1167/iovs.08-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson SG, Cideciyan AV, Aleman TS, et al. Photoreceptor layer topography in children with Leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2008;49:4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galvin JA, Fishman GA, Stone EM, Koenekoop RK. Evaluation of genotype-phenotype associations in Leber Congenital Amaurosis. Retina. 2005;25:919–929. doi: 10.1097/00006982-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Silva E, Dharmaraj S, Li YY, et al. A missense mutation in GUCY2D acts as a genetic modifier in RPE65-related Leber congenital amaurosis. Ophthalmic Genet. 2004;25:205–217. doi: 10.1080/13816810490513451. [DOI] [PubMed] [Google Scholar]
- 43.Sitorus RS, Lorenz B, Preising MN. Analysis of three genes in Leber congenital amaurosis in Indonesian patients. Vision Res. 2003;43:3087–3093. doi: 10.1016/j.visres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Hamel CP, Griffoin JM, Lasquellec L, et al. Retinal dystrophies caused by mutations in RPE65: assessment of visual functions. Br J Ophthalmol. 2001;85:424–427. doi: 10.1136/bjo.85.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dharmaraj SR, Silva ER, Pina AL, et al. Mutational analysis and clinical correlation in Leber congenital amaurosis. Ophthalmic Genet. 2000;21:135–150. [PubMed] [Google Scholar]
- 46.Chebil A, Falfoul Y, Habibi I, et al. Genotype-phenotype correlation in ten Tunisian families with non-syndromic retinitis pigmentosa [in French] J Fr Ophtalmol. 2016;39:277–286. doi: 10.1016/j.jfo.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Morimura H, Fishman GA, Grover SA, et al. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabir F, Naz S, Riazuddin SA, et al. Novel mutations in RPE65 identified in consanguineous Pakistani families with retinal dystrophy. Mol Vis. 2013;19:1554–1564. [PMC free article] [PubMed] [Google Scholar]
- 49.El Matri L, Ambresin A, Schorderet DF, et al. Phenotype of three consanguineous Tunisian families with early-onset retinal degeneration caused by an R91W homozygous mutation in the RPE65 gene. Graefes Arch Clin Exp Ophthalmol. 2006;244:1104–1112. doi: 10.1007/s00417-005-0096-2. [DOI] [PubMed] [Google Scholar]
- 50.Thompson DA, Gyurus P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41:4293–4299. [PubMed] [Google Scholar]
- 51.Mo G, Ding Q, Chen Z, et al. A novel mutation in the RPE65 gene causing Leber congenital amaurosis and its transcriptional expression in vitro. PLoS One. 2014;9:e112400. doi: 10.1371/journal.pone.0112400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weleber RG, Michaelides M, Trzupek KM, et al. The phenotype of Severe Early Childhood Onset Retinal Dystrophy (SECORD) from mutation of RPE65 and differentiation from Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2011;52:292–302. doi: 10.1167/iovs.10-6106. [DOI] [PubMed] [Google Scholar]
- 53.Lorenz B, Poliakov E, Schambeck M, et al. A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation. Invest Ophthalmol Vis Sci. 2008;49:5235–5242. doi: 10.1167/iovs.07-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paunescu K, Wabbels B, Preising MN, Lorenz B. Longitudinal and cross-sectional study of patients with early-onset severe retinal dystrophy associated with RPE65 mutations. Graefes Arch Clin Exp Ophthalmol. 2005;243:417–426. doi: 10.1007/s00417-004-1020-x. [DOI] [PubMed] [Google Scholar]
- 55.Lorenz B, Wabbels B, Wegscheider E, et al. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004;111:1585–1594. doi: 10.1016/j.ophtha.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 56.Yzer S, van den Born LI, Schuil J, et al. A Tyr368His RPE65 founder mutation is associated with variable expression and progression of early onset retinal dystrophy in 10 families of a genetically isolated population. J Med Genet. 2003;40:709–713. doi: 10.1136/jmg.40.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felius J, Thompson DA, Khan NW, et al. Clinical course and visual function in a family with mutations in the RPE65 gene. Arch Ophthalmol. 2002;120:55–61. doi: 10.1001/archopht.120.1.55. [DOI] [PubMed] [Google Scholar]
- 58.Katagiri S, Hosono K, Hayashi T, et al. Early onset flecked retinal dystrophy associated with new compound heterozygous RPE65 variants. Mol Vis. 2018;24:286–296. [PMC free article] [PubMed] [Google Scholar]
- 59.Yang G, Liu Z, Xie S, et al. Genetic and phenotypic characteristics of four Chinese families with fundus albipunctatus. Sci Rep. 2017;7:46285. doi: 10.1038/srep46285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schatz P, Preising M, Lorenz B, et al. Fundus albipunctatus associated with compound heterozygous mutations in RPE65. Ophthalmology. 2011;118:888–894. doi: 10.1016/j.ophtha.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Scholl HP, Moore AT, Koenekoop RK, et al. Safety and proof-of-concept study of oral QLT091001 in retinitis pigmentosa due to inherited deficiencies of retinal pigment epithelial 65 protein (RPE65) or lecithin:retinol acyltransferase (LRAT) PLoS One. 2015;10:e0143846. doi: 10.1371/journal.pone.0143846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samardzija M, Wenzel A, Naash M, et al. Rpe65 as a modifier gene for inherited retinal degeneration. Eur J Neurosci. 2006;23:1028–1034. doi: 10.1111/j.1460-9568.2006.04639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Danciger M, Matthes MT, Yasamura D, et al. A QTL on distal chromosome 3 that influences the severity of light-induced damage to mouse photoreceptors. Mamm Genome. 2000;11:422–427. doi: 10.1007/s003350010081. [DOI] [PubMed] [Google Scholar]
- 64.Wen Y, Birch DG. Outer segment thickness predicts visual field response to QLT091001 in patients with RPE65 or LRAT mutations. Transl Vis Sci Technol. 2015;4(5):8. doi: 10.1167/tvst.4.5.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maeda T, Lee MJ, Palczewska G, et al. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J Biol Chem. 2013;288:34484–34493. doi: 10.1074/jbc.M113.518571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeda T, Dong Z, Jin H, et al. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Invest Ophthalmol Vis Sci. 2013;54:455–466. doi: 10.1167/iovs.12-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan J, Rohrer B, Frederick JM, et al. Rpe65-/- and Lrat-/- mice: comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008;49:2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Fan J, Li S, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 70.Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci U S A. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.