ABSTRACT
The current paradigm that a single long-term hematopoietic stem cell can regenerate all components of the mammalian immune system has been challenged by recent findings in mice. These findings show that adult tissue-resident macrophages and innate-like lymphocytes develop early in fetal hematopoiesis from progenitors that emerge prior to, and apparently independently of, conventional long-term hematopoietic stem cells. Here, we discuss these recent findings, which show that an early and distinct wave of hematopoiesis occurs for all major hematopoietic lineages. These data provide evidence that fetal hematopoietic progenitors not derived from the bona fide long-term hematopoietic stem cells give rise to tissue-resident immune cells that persist throughout adulthood. We also discuss recent insights into B lymphocyte development and attempt to synthesize seemingly contradictory recent findings on the origins of innate-like B-1a lymphocytes during fetal hematopoiesis.
KEY WORDS: B-1a, Fetal hematopoiesis, HSC independent, Innate-like B lymphocytes, Tissue-resident cells
Summary: This Review summarizes the development of fetal immune cells that occurs before and after the appearance of the first bona fide hematopoietic stem cell in the embryo, and the possibility that certain tissue-resident immune cells in adults develop through an alternative pathway.
Introduction
It is generally thought that a single bona fide hematopoietic stem cell (HSC) is capable of sustained regeneration of all components of the immune system, including the erythroid, myeloid, lymphoid and granuloid lineages (Yamamoto et al., 2013; Osawa et al., 1996; Carrelha et al., 2018). Since the purification and characterization of the mouse HSC in the 1980s (Spangrude et al., 1988), much effort has been put into elucidating the early cellular and molecular events that lead to the development of the first bona fide HSC in the fetus. Collectively, these studies on the early events of fetal hematopoiesis (reviewed by Dzierzak and Bigas, 2018; Palis, 2016) have now provided enough evidence to suggest that a distinct wave of hematopoietic progenitors emerge before, and apparently independently of, the bona fide HSC. Most importantly, recent analyses indicate that these fetal-derived HSC-independent progenitors generate functionally distinct immune lineages that persist throughout adulthood as tissue-resident immune cells (Gentek et al., 2018a,b; Hashimoto et al., 2013; Ghosn and Yang, 2015).
In this Review, we will first summarize the early hematopoietic events in the fetus that lead to the development of the first bona fide HSC. We will then focus on the earlier waves of fetal hematopoiesis and discuss the recent findings suggesting that certain lineages of (tissue-resident) immune cells in adults develop from specialized progenitors that emerge in the fetus through a developmental pathway that is separate from the conventional bona fide HSC. These recent findings come from elegant studies using purified (single-) HSC/progenitor transplantation assays, in vivo lineage-tracing assays and transgenic mouse models, which combined have provided strong evidence to support a fetal-derived and HSC-independent origin for certain tissue-resident immune cells in adults.
Finally, we will focus on the current views of murine B lymphocyte development – particularly in light of seemingly contradictory recent data on the origins [HSC dependent (Kristiansen et al., 2016) versus HSC independent (Ghosn et al., 2016; Kobayashi et al., 2014)] of innate-like B-1a cells (see Glossary, Box 1) during fetal hematopoiesis. We attempt to reconcile these findings and accommodate them into a proposed model of B-1a development in the fetus.
Box 1. Glossary.
Aorta-gonad-mesonephros (AGM). A region in the embryo proper that develops from the P-Sp and includes the dorsal aorta, genital ridge (gonads) and mesonephros in the mouse embryo around E10-E11. In the mouse, the P-Sp region is referred to as AGM at or after E10.
B-1a cells. A subtype of B lymphocytes that develops primarily during fetal life and persists throughout adulthood in body cavities and mucosa. Unlike other types of B cells, B-1a cells respond very quickly to antigenic stimuli and do not require T-cell help to differentiate into effector antibody-producing cells; hence, they are commonly referred to as innate-like B cells.
Common lymphoid progenitor (CLP). A hematopoietic progenitor cell in the adult bone marrow that is committed to the lymphoid lineages, including B and T lymphocytes, but lacks long-term self-renewal potential. CLPs develop directly from the MPPs, which in turn develop from LT-HSCs in the bone marrow.
Erythromyeloid progenitor (EMP). A hematopoietic progenitor cell that produces both erythroid and myeloid cells in the embryo. EMPs appear first in the extra-embryonic yolk sac starting at ∼E8.5.
Hemangioblast. A mesodermal-derived cell that can produce both endothelial cells and blood/hematopoietic lineages in the embryo. The existence of a hemangioblast was first proposed based on studies on chicken embryos and on the observation that single-gene knockouts in mouse embryos can result in loss of both endothelial and blood cells. However, the existence of hemangioblasts in vivo remains controversial.
Hemogenic endothelial cell (HEC). An endothelial cell that can produce blood and/or hematopoietic lineages, also known as hemogenic endothelium. HECs are present in the embryo around E9-E12 in both yolk sac and P-Sp/AGM regions. The transcription factor Runx1 is indispensable for hematopoiesis in HECs.
(L)MPP. When a LMPP cannot be distinguished from a MPP, the term (L)MPP is used to indicate that the progenitor referred to could be either a LMPP or a MPP, or both.
Long-term hematopoietic stem cell (LT-HSC). The most-potent hematopoietic precursor cell that harbors the ability to regenerate all types of blood cells (multilineage potential) and to maintain hematopoiesis in vivo throughout life by means of self-renewal. Currently, a bona fide LT-HSC activity can be identified experimentally by the ability of a single cell to engraft, self-renew and regenerate all blood lineages in transplanted recipients for more than 6 months (and sustain long-term blood regeneration in secondary recipients). In the mouse, bona fide LT-HSC activity, at the single cell level, has been identified in cells displaying the phenotype KSL (Kithi, Sca-1hi, Lineage−), CD150+ and CD48−.
Lymphoid-primed MPP (LMPP). A multipotent progenitor (MPP) cell that is biased towards producing the lymphoid lineages, including B and T lymphocytes. LMPPs have been identified in both intra- and extra-embryonic tissues as early as ∼E9, prior to and independently of the LT-HSCs. In adult bone marrow, LMPPs develop from LT-HSCs.
Multipotent progenitor (MPP). A hematopoietic progenitor cell that produces several hematopoietic lineages in the embryo, including the erythroid, myeloid and lymphoid lineages, but which lacks long-term self-renewal capacity and can regenerate blood lineages only for a short period of time. In embryonic life, MPPs can be identified at around E9. They arise from HECs that are present in both extra- and intra-embryonic tissues for a limited period of time (∼E8.5-E11.5). In the adult, MPPs develop from LT-HSCs.
Para-aortic splanchnopleura (P-Sp). A region in the embryo proper that develops from the axial mesoderm during embryogenesis and is located alongside the dorsal aorta of the mouse embryo around E8.5-E9.5.
Pre-hematopoietic stem cells (pre-HSCs). The precursors to LT-HSCs. Pre-HSCs emerge at around E10 in the intra-embryonic AGM region. Pre-HSCs can be separated into type I (CD45−) and type II (CD45+). Type I pre-HSCs represent the most immature stage of LT-HSC development and as such they do not harbor bona fide LT-HSC activity in transplantation assays. Recent studies suggest that pre-HSCs arise from precursors that can be detected in the P-Sp region as early as E9.5; these precursors are referred to as pro-HSCs.
Most of our current understanding on fetal-derived and HSC-independent immune lineages has come from studies in mice – and this is the primary focus of our Review. However, studies using human embryos have now shown that fetal hematopoiesis is largely similar between mice and humans (Ivanovs et al., 2011). Human hematopoiesis has been reviewed in detail elsewhere (Ivanovs et al., 2017), but we briefly describe recent studies on fetal hematopoiesis in human, which suggest that, similar to mice, humans develop separate lineages of immune cells in the fetus that are functionally distinct from the bone marrow HSC-dependent immune cells (Rechavi et al., 2015; Roy et al., 2017; Schroeder and Wang, 1990; Schroeder et al., 1987). We discuss the clinical implications of these findings vis-à-vis current efforts to improve purified HSC transplantation in medical settings and the origins of B-cell-derived childhood and adult leukemia (Boiers et al., 2018; Montecino-Rodriguez et al., 2014; Barrett et al., 2016; Hayakawa et al., 2016a,b; DiLillo et al., 2013).
Taken together, the accumulating evidence supporting an HSC-independent origin for tissue-resident immune lineages challenges the current notion that adult bone marrow HSCs can fully regenerate the immune system. They provide fundamental insights into the developmental landscape of the mammalian immune system and shed new light on the evolutionary mechanisms that led to the development of two separate hematopoietic lineages (i.e. HSC independent and HSC dependent) that can respond to the endogenous and environmental changes that occur from embryonic life to adulthood.
Overview of hematopoietic development in the mouse embryo
In both mice and humans, the first blood cells to develop appear in the extra-embryonic yolk sac mesoderm, in an anatomic region known as the blood islands (Moore and Metcalf, 1970; Palis and Yoder, 2001). In mice, these cells consist primarily of primitive erythroid progenitors (fetal erythrocytes) and primitive macrophages, which emerge at embryonic day 7 (E7) (Palis et al., 1999), surrounded by endothelial cells (ECs) but before the onset of blood circulation (Fig. 1). Both primitive blood and ECs in the blood islands are thought to originate from a common precursor known as the hemangioblast (see Glossary, Box 1) (Nishikawa, 2012). However, lineage-tracing studies using multicolor chimeric mice show that, at ∼E7-E8.5, each blood island contains multiple clones of either endothelial or blood cells, but usually not both lineages from a single clonogenic progenitor (Ueno and Weissman, 2006), challenging the assumption that a single hemangioblast in the blood island gives rise to both blood and endothelial cells (Nishikawa, 2012).
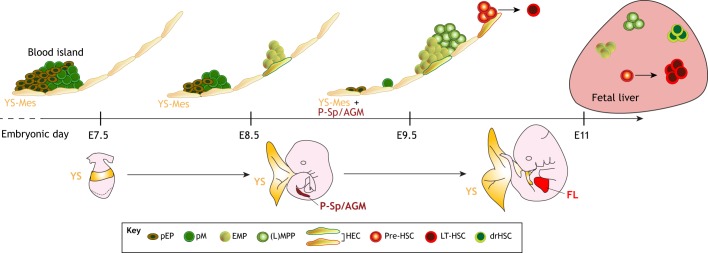
Fig. 1.
A timeline of lineage emergence during mouse embryonic development. The first blood cells to emerge are primitive erythroid progenitors (pEPs) and primitive macrophages (pMs) in the blood island region of the yolk sac (YS) at ∼E7.5. Presumably, pEP and pM develop directly from the YS mesoderm (YS-Mes). At ∼E8.5-E10, erythro-myeloid progenitors (EMPs) and lymphoid (L)-primed multipotent progenitors (MPP) emerge in the YS and para-aortic splanchnopleura (P-Sp) and aorta-gonad-mesonephros (AGM), presumably from hemogenic endothelial cells (HECs). It is not known whether EMPs and (L)MPPs emerge from the same type of HEC, or whether different types of HECs give rise to different types of precursor cells. At ∼E10.5, the first long-term hematopoietic stem cell (LT-HSC) emerges from pre-HSCs, which develop from a specialized HEC (red outline) in the AGM. The cellular origin of the recently described developmentally restricted (dr)HSC is unknown. Green shades represent HSC-independent hematopoiesis; red shades represent HSC-dependent hematopoiesis. FL, fetal liver.
The second proposed wave of fetal hematopoiesis is characterized by the development of erythro-myeloid progenitors (EMPs; see Glossary, Box 1) (Fig. 1). These progenitor cells emerge in the yolk sac at about E8.5 and – unlike the earlier wave that produces primitive (fetal-type) cells – give rise to definitive (adult-type) erythrocytes and myeloid cells (Palis et al., 1999; Lux et al., 2008). The third proposed wave initiates around E9 and is marked by a surge of multipotent progenitors (MPPs; see Glossary, Box 1) (Inlay et al., 2014; Kawamoto et al., 1998; Godin et al., 1995), some of which appear biased towards a lymphoid fate, i.e. lymphoid-primed multipotent progenitors (LMPPs; see Glossary, Box 1) (Boiers et al., 2013; Yoshimoto et al., 2011, 2012; Nishikawa et al., 1998; Yokota et al., 2006; Cumano et al., 1996; Lu and Auerbach, 1998, 1991; Lu et al., 1996). These progenitors emerge in both the extra-embryonic yolk sac and intra-embryonic tissues, including the para-aortic splanchnopleura (P-Sp; see Glossary, Box 1) and aorta-gonad-mesonephros (AGM; see Glossary, Box 1) (Fig. 1).
Unlike the first wave of primitive hematopoiesis, the second and third waves [which generate EMPs and (L)MPPs (see Glossary, Box 1)] develop from specialized ECs, called hemogenic endothelial cells (see Glossary, Box 1), which are present in both yolk sac and P-Sp/AGM, and show hematopoietic activities only within a limited period of time (E8.5-11.5) (Fig. 1) (Tober et al., 2013). At the end of the third wave (or perhaps concomitantly), the precursors to the long-term (LT) adult-repopulating HSCs, the so-called pre-HSCs (see Glossary, Box 1), can be detected in the intra-embryonic AGM at around E10 (Rybtsov et al., 2011). The relationship between (L)MPPs and pre-HSCs is not yet clear, but these cell populations share similar surface markers.
Finally, the LT-HSC (see Glossary, Box 1) – defined by its ability to self-renew and long-term repopulate all hematopoietic lineages in transplantation assays (see Box 2) – emerges at the latest by around E11, possibly from hemogenic EC-derived pre-HSCs in the AGM region and later in the yolk sac and placenta (Kumaravelu et al., 2002; Gekas et al., 2005; Chen et al., 2011; Medvinsky and Dzierzak, 1996; Weissman et al., 1978) (Fig. 1). LT-HSC development can be viewed as the last wave of fetal hematopoiesis, which begins in fetal life (mid-gestation) and continues to give rise to all hematopoietic lineages during adulthood (Sawai et al., 2016; Sawen et al., 2018). Hematopoietic progenitors from all waves of fetal hematopoiesis circulate via the blood vessels and seed the fetal liver to support homeostasis until LT-HSC-derived hematopoiesis is established in the bone marrow at about E18 (Christensen et al., 2004). Bone marrow-resident LT-HSCs regenerate the blood and immune system throughout life (Sawai et al., 2016; Sawen et al., 2018).
Box 2. Transplantation assays.
Experimental approach used to assess the potential of a cell (or population of cells) to regenerate the various types of blood/hematopoietic lineages in vivo after transplantation. In general, donor-derived hematopoietic cells are injected intravenously into irradiated congenic recipient mice or sub-lethally irradiated immunodeficient mice, and after a period of time (usually up to 6 months after injection) the peripheral blood of recipient mice is analyzed, by flow cytometry, for the presence of donor-derived hematopoietic lineages (i.e. erythrocytes, myeloid cells, lymphocytes, etc.). Transplantation assays can be performed with any type of cell to determine its behavior/function in vivo, and represent the current gold standard approach to assess the presence of bona fide Long-term hematopoietic stem cells (LT-HSCs) in a particular cell population by revealing their multi-lineage regeneration and self-renewal abilities. Transplantation assay can also be performed at the single-cell level to determine whether the transplanted cell harbors a bona fide LT-HSC activity.
However, transplantation assays have their limitations: they can reveal information only about the ‘potential’ of a particular cell type when transplanted in vivo, but may not reveal the actual behavior of the cell when located in its native unperturbed environment. This limitation is particularly evident when assessing the LT-HSC activity of explanted embryonic cells, which might require signals from their native microenvironment to express their native behavior. For example, embryonic yolk sac cells engraft better when transplanted into neonatal mice or directly into embryos in utero. In addition, embryonic tissues require enzymatic digestion to obtain single cell suspensions, which can affect the viability and behavior of the cells used in transplantation assays, and could account for some of the discrepancies in the results published by different laboratories. Hence, even though transplantation assays still represent the gold standard approach to evaluate the long-term self-renewal and multi-lineage regeneration ‘potential’ of a cell, the behavior of such a cell might change once excised from its native tissue and transplanted into recipient mice (Rodriguez-Fraticelli et al., 2018).
Although it is generally accepted that the AGM region at ∼E11 represents the first site of de novo LT-HSC development in the mouse embryo (Muller et al., 1994; Medvinsky and Dzierzak, 1996; Medvinsky et al., 2011; Dzierzak and Medvinsky, 2008), the contribution of the extra-embryonic yolk sac to LT-HSC development remains controversial – even though its contribution to the development of EMPs and (L)MPPs is well described and accepted in the field (Lux et al., 2008; Boiers et al., 2013; Yoshimoto et al., 2011). However, as discussed in greater depth below, various studies (Inlay et al., 2014; Lee et al., 2016; Yoder et al., 1997; Weissman et al., 1978; Tanaka et al., 2012, 2014; Samokhvalov et al., 2007) provide evidence supporting the notion that the yolk sac also contributes to the development of LT-HSCs.
Fetal LT-HSC origin
The idea that the first LT-HSC arises in the intra-embryonic AGM region is strongly supported by a collection of studies using in vitro and ex vivo tissue excision and cell transplantation assays, performed before or after the onset of blood circulation (North et al., 2002; de Bruijn et al., 2002; Medvinsky and Dzierzak, 1996; Muller et al., 1994; Kumaravelu et al., 2002; Gekas et al., 2005; Cumano et al., 1996; Cumano and Godin, 2001; Boisset et al., 2010; Ganuza et al., 2018). Collectively, these studies demonstrate that LT-HSC activity can be identified as early as E10.5 from transplants of intra-embryonic P-Sp/AGM, but not yolk sac, tissues.
Lineage-tracing mouse models that specifically mark ECs in vivo reveal that all blood cells, including LT-HSCs in the fetal liver and adult bone marrow, are derived from ECs (Zovein et al., 2008). Moreover, EC-specific deletion of the Runx1 (Runt-related transcription factor 1) gene, which is necessary for the development of all hematopoietic progenitors – from the early EMPs to the late LT-HSCs, results in embryonic lethality due to lack of proper hematopoiesis (Okuda et al., 1996). In this view, LT-HSCs are considered to be derived from hemogenic ECs present in the AGM region.
In support of de novo hematopoietic activity in the AGM, the Medvinsky group (Rybtsov et al., 2014, 2016, 2011) and others (Hadland et al., 2015; Zhou et al., 2016; Baron et al., 2018) have identified and characterized the precursors to the HSC (i.e. pro- and pre-HSCs) in the hemogenic ECs of the P-Sp/AGM region from about E9.5. Unlike bona fide LT-HSCs, pre-HSCs still co-express endothelial markers and do not yet have the potential to engraft lethally irradiated hosts in transplantation assays. However, pre-HSCs from the E10 AGM, but not yolk sac, can acquire bona fide HSC phenotype and are engraftable after in vitro aggregation culture with either stromal cells (OP9/OP9-DL1) or AGM-derived Akt-expressing ECs, further supporting the notion that the AGM represents the main site of de novo LT-HSC development (Zhou et al., 2016; Rybtsov et al., 2011; Hadland et al., 2015). This differentiation process from the pre-HSC to HSC has now been investigated at the single-cell level, providing greater insights into the mechanisms that regulate the early events of HSC development (Baron et al., 2018; Zhou et al., 2016).
On the other hand, the contribution of the extra-embryonic yolk sac to LT-HSC development remains somewhat controversial, perhaps because several studies failed to detect the potential of yolk sac cells to repopulate hematopoietic lineages in transplantation assays (Cumano et al., 1996; Cumano et al., 2001; Medvinsky and Dzierzak, 1996; Muller et al., 1994). In fact, yolk sac cells show engraftment potential only when transplanted either directly into an embryonic environment (in utero) (Weissman et al., 1978) or into preconditioned neonatal mice (Kumano et al., 2003; Yoder et al., 1997), or after co-culture with stromal cells (Matsuoka et al., 2001) – suggesting that their potential may be more influenced by environment than AGM cells (Arora et al., 2014).
To overcome the limitations of transplantation assays (see Box 2) and investigate the origins of the LT-HSC in situ, several in vivo lineage-tracing mouse models have been generated (Samokhvalov et al., 2007; Tanaka et al., 2014, 2012; Lee et al., 2016). In these models, cells expressing the transcription factor Runx1, which is necessary for the development of fetal LT-HSCs, could be labeled by tamoxifen injection. These experiments showed that Runx1+ cells, which were found exclusively in the yolk sac at E7.5, contributed to fetal liver and adult bone marrow LT-HSCs (Samokhvalov et al., 2007). Deletion of Runx1 results in embryonic lethality around E13 due to lack of proper hematopoiesis (Okuda et al., 1996). Using a mouse model in which Runx1 expression could be rescued by tamoxifen injection in Runx1−/− embryos (Tanaka et al., 2012), it was found that embryos could be rescued only when tamoxifen was injected at E7.5, but not thereafter, suggesting that Runx1 expression at E7.5 is indispensable for the development of LT-HSCs that occurs at later stages (Tanaka et al., 2012).
To explain how Runx1+ yolk sac cells at E7.5 contribute to the development of LT-HSCs in the embryo at around E11, Nishikawa's group used time-lapse imaging to track the Runx1+ cells in vivo (Tanaka et al., 2014). Their results suggest that Runx1+ cells move from the yolk sac to embryo proper at around E7.5-8.0, independently of blood circulation, and appear to contribute to a fraction of the ECs of the dorsal aorta (P-Sp/AGM) at later stages (Tanaka et al., 2014). Collectively, these findings suggest that the Runx1+ cells in the yolk sac at E7.5 migrate to the intraembryonic AGM region, where they contribute to a fraction of bona fide LT-HSCs that appear later at around E11. However, further studies are needed to elucidate the role of early Runx1 expression in yolk sac cells and its relationship to the development of the first bona fide LT-HSC that occurs at later stages.
Another relevant lineage-tracing study suggests that a fraction of fetal and adult LT-HSCs is derived from yolk sac cells expressing the membrane receptor Lyve1 (Lee et al., 2016). Lyve1 is a hyaluronan receptor and usually a marker of lymphatic vessels, but it is ubiquitously expressed in the yolk sac ECs at E9.5 to 10.5. The authors suggest that about 40% of total fetal and adult definitive LT-HSCs in the mouse are derived from the yolk sac cells expressing Lyve1 at E9.5-E10.5 (Lee et al., 2016). Nonetheless, it was not clear whether these Lyve1+ cells have given rise to LT-HSCs in situ or instead have migrated from the yolk sac to the AGM region. In fact, Lyve1 expression can also be detected in a small subset of ECs in the AGM region (4% in the AGM, compared with 75% in the yolk sac). As a single HSC can expand vigorously from E10.5 onwards, it is possible that Lyve1+ cells in the AGM may have contributed to the total LT-HSC pool observed in these mice. Indeed, in a separate study, Lyve1 expression has been identified in the intra-embryonic P-Sp region prior to blood circulation (∼E8-E8.5) (Ganuza et al., 2018). In this study, intra-embryonic Lyve1+ cells were capable of multi-lineage reconstitution in recipient mice after in vitro culture, providing further evidence that Lyve1+ cells in the P-Sp/AGM region contribute to the total fetal and adult LT-HSC pool (Ganuza et al., 2018).
Taken together, these studies still suggest that, in addition to the AGM, the yolk sac might function as an autonomous site of de novo LT-HSC development and contribute to a fraction of the total LT-HSC pool that migrates to fetal liver and adult bone marrow. However, the current lack of a known gene that could selectively mark and separate the AGM and yolk sac cells during LT-HSC development makes it challenging to estimate the overall contribution of each hematopoietic tissue to the fetal and adult LT-HSC pool in vivo. If indeed both AGM and yolk sac could contribute to the overall LT-HSC pool in fetal liver and adult bone marrow, future studies should determine whether LT-HSCs represent a heterogeneous cell population that independently emerge at different sites in response to unique signaling from local niches and whether their resulting migratory potential would determine which type of HSC comes to dominate in place and time.
Regardless of their origin(s), LT-HSCs migrate to the mouse fetal liver (and placenta) at around E11.5-E12 (Kumaravelu et al., 2002), where they expand and initiate hierarchical HSC-dependent hematopoiesis. This produces most of the adult-type immune cells (Sawai et al., 2016), including circulating monocytes, granulocytes, T lymphocytes and follicular B lymphocytes. Thus, the fetal liver contains a mixture of yolk sac- and P-Sp/AGM-derived progenitors, including EMPs and (L)MPPs, which develop from both HSC-independent and HSC-dependent precursors (Fig. 1). In the next section, we will discuss the types of immune cells that have been reported to develop in the early embryo prior to, and independently of, the definitive LT-HSC.
Are there HSC-independent immune cells?
It is now accepted that, in both mice and humans, the very first erythroid progenitors to appear in the yolk sac develop prior to, and independently of, the LT-HSC (Moore and Metcalf, 1970; Palis and Yoder, 2001; Palis et al., 1999; Ivanovs et al., 2017). However, it remains unclear whether subsequent myeloid and lymphoid progenitors also develop independently of HSCs or whether they emerge from a subtype of early HSCs that are not detectable by standard transplantation assays and are biased towards producing certain cell types when active in a particular niche. Various lineage-tracing studies and transgenic/knockout mouse models have tried to address these issues.
An elegant study by Nancy Speck's group characterized the role of core-binding factor β (CBFβ) in two separate subsets of hemogenic ECs. CBFb is a non-DNA-binding subunit of the CBF transcription factor family and regulates Runx1 function. CBFb−/− embryos show a similar phenotype to Runx1−/− embryos, in that they fail to generate definitive LT-HSCs and do not survive beyond E12.5. Using a mouse model in which CBFb can be selectively expressed in different subsets of ECs present in both the yolk sac and P-Sp/AGM regions, they show a separate cellular origin for LT-HSCs and EMPs (Chen et al., 2011). Thus, EMPs develop exclusively from a subset of ECs expressing the Tek gene (encoding the angiopoetin receptor Tie2) at around E8.5, distinct from the ECs expressing the gene Ly6a (encoding the surface protein Sca-1), which in turn give rise to LT-HSCs at around E11.5 (Chen et al., 2011). It is important to note, however, that the expression of Tie2 and Sca-1 is spatially and temporally controlled, and not limited to single cell types (Chen et al., 2011). Hence, although the CBFb rescue mouse model showed that Tie2+ ECs fail to generate LT-HSCs at E11.5, there is a possibility that other Tie2+ cells at a different location and time point during fetal development could also give rise to LT-HSCs (Pei et al., 2017; Gomez Perdiguero et al., 2015; Busch et al., 2015; Inlay et al., 2014).
The development of EMPs (and perhaps LMPPs) from specialized precursors that are distinct from LT-HSCs raises the possibility that these progenitors might give rise to immune cells that are functionally distinct from those derived from LT-HSCs. Indeed, recent lineage-tracing studies have demonstrated that EMPs (Gomez Perdiguero et al., 2015; Schulz et al., 2012; Hoeffel et al., 2015) and yolk sac-derived primitive macrophages (Ginhoux et al., 2010), but not LT-HSCs, are the precursors of most adult tissue-resident macrophages, including microglia, Langerhans cells and Kupffer cells (Fig. 2). These tissue-resident macrophages emerge in the mouse embryo around E7.5 from precursors present in the yolk sac (Gomez Perdiguero et al., 2015; Ginhoux et al., 2010; Schulz et al., 2012), and then migrate to their respective tissues (i.e. brain, skin, and liver) before birth, where they are maintained throughout adulthood by in situ self-renewal (Hashimoto et al., 2013).
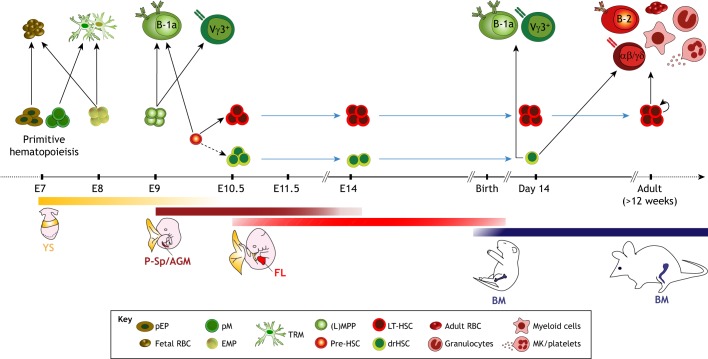
Fig. 2.
HSC-independent and HSC-dependent hematopoietic lineages. This schematic shows the timeline and location for the emergence of hematopoietic lineages during mouse development. Three major waves of hematopoiesis can be identified. The first wave is hematopoietic stem cell (HSC) independent and generates primitive erythroid progenitors (pEPs) and primitive macrophage progenitors (pMs) from the yolk sac (YS) blood islands at around E7.5. These give rise to fetal red blood cells (RBCs) and tissue-resident macrophages (TRMs), including brain microglia. The second wave, also HSC independent, generates erythro-myeloid progenitors at ∼E8.5 that give rise to both fetal and adult-type RBCs and TRMs. Shortly after, at ∼E9.5, the hemogenic endothelial cells from the yolk sac, and perhaps from the para-aortic splanchnopleura (P-Sp) and aorta-gonad-mesonephros (AGM) regions, generate lymphoid (L)-primed multipotent progenitors (LMPPs), which later give rise to innate-like B and T lymphocytes, including B-1a and Vγ3+ T cells. The third wave, in which LT-HSCs emerge, initiates around E9.5 in the AGM region. The precursors to the LT-HSCs, so-called pre-HSCs, can also give rise to innate-like B-1a lymphocytes. The recently identified developmentally restricted (dr) HSCs are detected in the yolk sac, AGM and fetal liver between E10.5 and E14.5. These cells can still be detected in newborns but are not found in adult bone marrow (BM). LT-HSCs migrate from the fetal liver to the bone marrow, where they continually regenerate the LT-HSC-dependent immune lineages, including the FO B-2 lymphocytes, αβ and γδ T lymphocytes, myeloid cells, granulocytes, adult-type RBCs and megakaryocytes (MKs)/platelets (see red cells, upper right) throughout the life of the animal. Blue arrows indicate persistence/survival after birth and/or throughout adulthood. Dotted arrow indicates an unknown, but likely, developmental pathway that has not yet been tested experimentally. Green shades represent HSC-independent hematopoiesis; red shades represent HSC-dependent hematopoiesis. FL, fetal liver.
Similarly, recent lineage-tracing studies in zebrafish show that the first wave of embryonic T-cell development occurs prior to, and independently of, the LT-HSC (Tian et al., 2017). In fact, it has long been appreciated that murine fetal T-cell development also occurs in sequential waves, starting with progenitors that are biased towards the generation of T cells expressing the gamma delta (γδ) T-cell receptor (TCR) (Fig. 2) (Ikuta et al., 1992a, 1990; Havran and Allison, 1988). The first wave of T-cell progenitors seeds the fetal thymus around E11 (Masuda et al., 2005) and is biased towards the Vγ3+ TCR. Vγ3+ T cells are produced only in the early fetal thymus, and become dendritic epidermal T cells (DETCs), which play key roles in skin homeostasis and wound healing (Boismenu et al., 1996; Chen et al., 2002). Subsequently, the Vγ3+ T cells are followed by the development of thymic Vγ4+, Vγ2+ and finally Vγ5+ cells (Ikuta et al., 1992b; Heilig and Tonegawa, 1986).
Given the scarcity of LT-HSCs at around E11, when the first T-cell progenitors seed the fetal thymus, it seems reasonable to consider that the first wave of T-cell development might occur prior to and independent of the LT-HSC. In fact, recent lineage-tracing studies have shown that the γδ DETCs originate from the yolk sac through a distinct LT-HSC-independent developmental pathway (Gentek et al., 2018b). Consistent with these recent findings, T-cell potential has been identified in embryonic yolk sac and P-Sp cells as early as E8 (Liu and Auerbach, 1991; Nishikawa et al., 1998; Cumano et al., 1996; Yoshimoto et al., 2012). Yoshimoto et al. showed that yolk sac and P-Sp cells from embryos before the onset of blood circulation [or from Ncx1−/− (Slc8a1−/−) embryos, which do not develop blood circulation] have the potential to differentiate into mature T cells in vitro, including both γδ (Vγ3, Vγ4, and Vγ5) and αβ T-cell subsets (Yoshimoto et al., 2012). Importantly, transplantation of these freshly isolated yolk sac cells into neonatal mice resulted in a fraction of recipient mice that develop only T cells, supporting the notion that some types of T cells might develop independently of LT-HSCs (Yoshimoto et al., 2012).
Recently, much attention has been given to another subset of γδ T cells that appear only during mouse embryonic development, namely interleukin (IL)-17-producing γδ T cells (γδT17) (Haas et al., 2012). These are innate-like tissue-resident T cells that are located primarily in the dermis, lung and liver, where they contribute to host defense in the epithelial barrier of these tissues. Importantly, in transplantation assays, neither adult bone marrow nor fetal liver cells can regenerate γδT17 cells in adult recipient mice. Although it is possible that the transplantation of fetal liver cells directly into the fetal environment (via an in utero transplantation assay) could promote γδT17 cell differentiation in the fetal thymus, these initial studies suggest that γδT17 cells might represent a separate lineage of fetal thymus-derived T cells that emerge independently of the bone marrow- and liver-derived LT-HSCs (Haas et al., 2012).
Taken together, these data reveal a temporally and spatially controlled landscape for the immune system during mammalian development, in which several waves of hematopoiesis take place in a continuous progression before the development of LT-HSCs (Fig. 2). The immune cells (tissue-resident macrophages and innate-like lymphocytes) that emerge prior to the development of LT-HSCs appear to be functionally distinct from the cells that emerge at later stages from the bona fide LT-HSC (e.g. circulating monocytes and follicular B lymphocytes).
Even though there is enough evidence to demonstrate that separate immune lineages undergo sequential development (progressing from HSC-independent to HSC-dependent), it is still possible that these lineages might also develop simultaneously. Hence, despite recent advances in elucidating HSC-independent hematopoiesis (Sawai et al., 2016; Kobayashi et al., 2014; Gomez Perdiguero et al., 2015; Gentek et al., 2018b; McGrath et al., 2015), current limitations in terms of the availability of biomarkers and in vivo models hinder our ability to distinguish the HSC-independent from the HSC-dependent lineages that emerge during fetal development. These limitations have directly affected the recent attempts to elucidate the origins of murine fetal B lymphocytes, which remain somewhat controversial. In the next section, we will review and synthesize these studies on the early development of fetal, innate-like, B lymphocytes and provide a framework to reconcile these seemingly contradictory findings.
HSC-independent B lymphopoiesis
Recent findings (Sawen et al., 2018, 2016; Ghosn et al., 2016; Kristiansen et al., 2016; Beaudin et al., 2016) on the origins of an innate-like B lymphocyte lineage, known as B-1a cells, raise the issue of whether B-1a cell development is HSC independent or HSC dependent. Here, we discuss the origins of these tissue-resident innate-like B-1a lymphocytes and synthesize the various findings published by our group and others (Fig. 3).
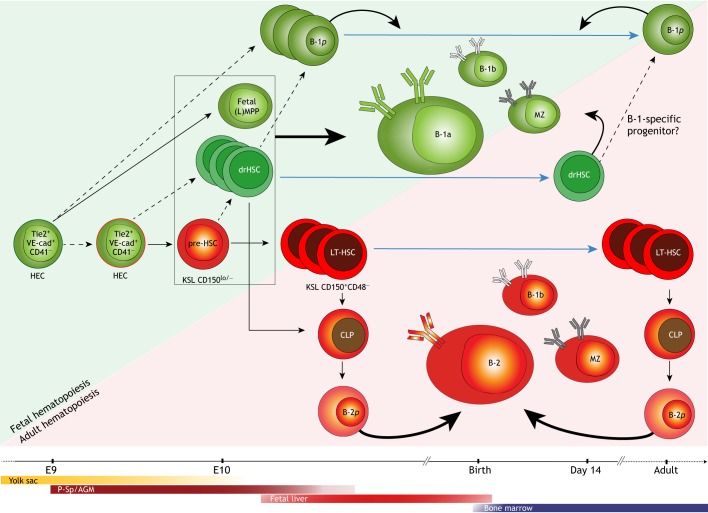
Fig. 3.
Development of B lymphocytes during fetal and adult life. Innate-like B-1a lymphocytes develop early in fetal life from hematopoietic stem cell (HSC)-independent precursors around E8.5-E9.5 in the yolk sac (YS) and para-aortic splanchnopleura (P-Sp). These precursors have been identified as hemogenic endothelial cells (HECs), which can give rise to B-1a, but not follicular (FO) B-2, cells. It is not known whether B-1a precursors emerge from a specialized HEC (green outline), or whether they emerge from the same type of HEC (red outline) that later gives rise to the precursors of LT-HSCs (pre-HSCs), as suggested by recent polylox lineage-tracing assays. Pre-HSCs, which can be identified in the para-aortic splanchnopleura (P-Sp) and aorta-gonad-mesonephros (AGM) around E9.5, have also been shown to give rise to B-1a cells. B-1-specific progenitors (B-1p) can be identified in the fetal liver and later in adult spleen and bone marrow, but their precursors are unknown (dotted arrows indicate potential candidates). Developmentally restricted (dr) HSCs are detected in the yolk sac, aorta-gonad-mesonephros (AGM) and fetal liver between E10.5 and E14.5, but their precursors remain unknown. Fetal (lymphoid-primed) multipotent progenitors [(L)MPPs], drHSCs and pre-HSCs (enclosed in black box) all share similar phenotypes (KSL CD150lo/−) and have been shown to give rise to B-1a lymphocytes in transplantation assays. In adults, the LT-HSC-dependent precursors in the bone marrow continually regenerate FO B-2, B-1b and marginal zone B cells through B-2-specific progenitors (B-2p). Blue arrows indicate persistence/survival after birth and/or throughout adulthood. Green shades represent HSC-independent hematopoiesis; red shades represent HSC-dependent hematopoiesis. CD41, integrin alpha IIb; CLP, common lymphoid progenitors; KSL, cells expressing the phenotype Kit+, Sca-1+ and Lineage−; MZ, marginal zone B cells; Tie2, angiopoietin 1 receptor; VE-cad, vascular endothelial cadherin.
Innate-like B-1 cells
B lymphocytes are a key component of both cellular and humoral immunity, serving both as antigen-presenting cells and antibody-producing cells. Murine B cells are commonly divided into (at least) five functionally distinct subsets: follicular (FO) B-2; marginal zone (MZ); transitional; B-1b; and B-1a B cells. These subsets differ not only by the type of antigens they recognize but also in the mechanisms by which they elicit immune effector functions. B-2 FO cells produce the well-described germinal center, T-cell-dependent, immune responses against protein antigens (McHeyzer-Williams and McHeyzer-Williams, 2005; Nutt et al., 2015). In contrast, innate-like B-1a cells are a subset of tissue-resident lymphocytes that reside primarily in body cavities and mucosa (i.e. in peritoneal and pleural cavities and in the lamina propria of intestines and lung), and produce a rapid, T-cell independent, antibody response primarily to lipids and polysaccharides (Martin et al., 2001).
B-1a cells also secrete most of the circulating natural antibodies (IgM, IgG and IgA) that arise spontaneously in the absence of exogenous antigens, including in germ-free mice (Coutinho et al., 1995; Bos et al., 1989; Baumgarth et al., 2005; Yang et al., 2015). Natural antibodies often recognize endogenous antigens that are detectable on apoptotic cells and on oxidized lipids, making them important for housekeeping activities that clear cellular debris and metabolic waste. However, natural antibodies are also necessary for the immune response against infectious diseases, such as influenza (Choi and Baumgarth, 2008) and pneumonia (Weber et al., 2014; Haas et al., 2005). B-1a cells can also mount rapid antigen-specific antibody responses that are necessary (and sometimes sufficient) to provide long-term protection against certain infectious diseases, particularly tularemia (Yang et al., 2012a,b).
In addition to producing natural and antigen-specific antibodies, innate-like B-1a cells are the main source of B-cell-derived IL-10 (Kurnellas et al., 2015; Aziz et al., 2017; O'Garra et al., 1992). Both IL-10 and natural antibodies produced by B-1a cells are required to maintain physiological and immune homeostasis (Grönwall et al., 2012; Kaveri et al., 2012). As such, their dysregulation is associated with various metabolic and inflammatory disorders, including atherogenesis (Shaw et al., 2000), type 1 diabetes and insulin resistance (Bodogai et al., 2018; Wu et al., 2014; New et al., 2016), colitis (Bouaziz et al., 2008; Shimomura et al., 2008), as well as autoimmunity (Murakami et al., 1995; Duan and Morel, 2006; Nguyen et al., 2015), allergy (Patel et al., 2016), sepsis (Aziz et al., 2017; Rauch et al., 2012) and susceptibility to infectious diseases (Weber et al., 2014; Haas et al., 2005). The functional differences between innate-like B-1a and other B-cell subsets is further revealed by their pre-immune B-cell receptor (IgH) repertoire (Yang et al., 2015).
Even though much is known about the origins of FO B-2 (Hardy et al., 2007) cells and the functional differences between each B-cell subset, the origins of innate-like B-1a cells remain controversial (Graf et al., 2019; Ghosn et al., 2019; Herzenberg et al., 2000; Berland and Wortis, 2002; Ghosn and Yang, 2015). Although it is well-established that B-1a cells emerge early in fetal development, the stem and/or progenitor cell that gives rise to these lymphocytes in the fetus has not been defined. Given the over-arching paradigm that all blood cells come from LT-HSCs, it has generally been assumed that B-1a cells, similar to other B lymphocytes, arise from these stem cells. However, recent studies have now challenged this assumption and suggest instead that B-1a cells emerge prior to, and independent of, the LT-HSC.
An HSC-independent origin for B-1a cells
It has long been known that transfers of total (unsorted) fetal liver cells regenerate B-1a cells in irradiated recipients more efficiently than transfers of total adult bone marrow cells (Kantor et al., 1992; Hayakawa et al., 1985; Osmond, 1986). Subsequent independent studies using adult bone marrow transfers, including purified common lymphoid progenitors (CLPs; see Glossary, Box 1) and/or cells enriched for LT-HSCs and other early hematopoietic progenitors (i.e. cells expressing the phenotype Kit+, Sca-1+ and Lineage−, also known as KSL cells) (Montecino-Rodriguez et al., 2006; Barber et al., 2011; Smith-Berdan et al., 2007; Kikuchi and Kondo, 2006), confirmed the earlier findings and collectively show that adult bone marrow cells have a limited potential to regenerate B-1a cells in irradiated recipients. One of the interpretations for this discrepancy in B-1a potential between the fetal and adult transplantation studies had been that fetal LT-HSCs gradually lose the potential to generate B-1a cells in adult bone marrow (Kantor and Herzenberg, 1993), likely through epigenetic modifications in the aging LT-HSCs (Zhou et al., 2015; Yuan et al., 2012).
To address this issue and directly test the regenerative potential of LT-HSCs in vivo, single cell transplantation assay studies have been carried out (Ghosn et al., 2012). These studies showed that a single LT-HSC (purified as KSL CD150+CD48− cells) harvested from adult bone marrow and transplanted into lethally irradiated recipients fully regenerates FO B-2, MZ B, B-1b and peripheral blood B cells, but selectively fails to regenerate tissue-resident B-1a cells (Ghosn et al., 2012). The ability of fetal LT-HSCs (purified from E15 liver) to regenerate B-1a cells was also tested and, surprisingly, similar to adult LT-HSCs, fetal liver-derived LT-HSCs selectively fail to regenerate B-1a cells while effectively regenerating all other components of the immune system (Ghosn et al., 2016).
It is possible that the adult environment in the recipient mice might not be conducive to B-1a development. However, it has long been known that B-1a cells are readily reconstituted in adult recipients by transfers of total unsorted fetal liver cells (Kantor and Herzenberg, 1993), indicating that the adult environment can support B-1a development. To determine which cell population in mouse fetal liver give rise to B-1a cells in these early transplantation assay studies, studies (Ghosn et al., 2016; Beaudin et al., 2016) have shown that B-1a cells arise from progenitors expressing the marker phenotype KSL CD150−/lo, which is not shared by the conventional LT-HSC characterized in the fetal liver (Kim et al., 2006) and adult bone marrow (Chen et al., 2016; Yamamoto et al., 2013; Carrelha et al., 2018). Of note, the KSL CD150−/lo progenitors reconstitute B-1a cells to the same extent as unsorted fetal liver, suggesting that most B-1a progenitors are contained within the CD150−/lo fraction (Ghosn et al., 2016). Hence, the failure of LT-HSCs to reconstitute B-1a in transplantation assays is not due to a lack of support for B-1a development in the adult recipient environment.
Another limitation of transplantation assay studies is that LT-HSCs might require unique signals from the fetal environment (perhaps from fetal liver) that are not provided by the adult recipient environment in order to successfully regenerate B-1a cells. This limitation has been addressed by two different experimental approaches: transplantation of purified LT-HSCs into neonatal (day 0) recipients (i.e. before the development of host B-1a cells) (Ghosn et al., 2016) and co-transplantation of purified LT-HSCs along with congenic unsorted fetal liver cells (Ghosn et al., 2016). In both cases, the donor LT-HSCs failed to regenerate B-1a cells in the recipient, suggesting that the selective inability of LT-HSCs to reconstitute B-1a cells may reflect an intrinsic genetic program rather than a response that is dictated by the environment (Ghosn et al., 2016).
Taken together, the transplantation assay studies demonstrate that, in an environment conducive to B-1a development, fetal and adult LT-HSC transplantations give rise to FO B-2, MZ B and B-1b cells, but do not give rise to B-1a cells (Fig. 3). However, although the transplantation studies can inform about the hematopoietic potential of the transplanted LT-HSCs, they cannot provide definitive insight regarding the hematopoietic potential of the in situ and undisturbed LT-HSC in homeostatic conditions.
To this end, three recent studies have addressed the origin of B-1a cells using other methods, including in vivo lineage-tracing assays in adults. Using an inducible genetic labeling of adult undisturbed LT-HSCs in vivo, two independent groups (Sawen et al., 2018; Sawai et al. 2016) have shown that LT-HSCs, under homeostatic conditions, fully regenerate all components of the immune system, except for B-1a cells and microglia. Consistent with these findings, Kobayashi et al. (Kobayashi et al., 2014) showed that B-1a progenitors can be detected in HSC-deficient transgenic embryos (Cbfb−/−:Tek-gfp/Cbfb) that fail to develop conventional LT-HSCs (Kobayashi et al., 2014), demonstrating that at least some B-1a cells can develop independently of LT-HSCs. Similar studies using embryonic tissues have confirmed that B-1a progenitors can be detected in the embryo prior to, and independently of, LT-HSCs. These studies demonstrate that early hemogenic endothelial cells from the E9 mouse yolk sac (Yoshimoto et al., 2011), P-Sp (at the 10-18 somite stage) and AGM region (Yoshimoto et al., 2011; de Andres et al., 2002; Godin et al., 1993; Marcos et al., 1997) preferentially reconstitute innate-like B-1a and MZ B cells, but not FO B-2 cells. Collectively, these studies of early B-cell development support an LT-HSC-independent origin for innate-like B-1a lymphocytes.
Could there be HSC-dependent B-1a cells?
Despite the accumulating evidence for HSC-independent B-1a cell genesis, a recent elegant study by Kristiansen et al. (2016) has provided compelling evidence supporting a fetal HSC-dependent origin for these cells. In this study, the authors used individual cell-barcoding technology to define the developmental changes in the immune lineage potential of LT-HSCs at a clonal level. Thus, they transduced fetal liver (E14.5) KSL cells (i.e. KithiSca-1hiLineage−) ex vivo with a lentiviral barcoding library (LV-GFP-lib) and transplanted these cells into lethally irradiated recipient mice.
Characterization of the resulting peritoneal B-cell subsets in the recipient mice showed that cells of distinct subsets shared common barcoding sequences. In essence, the authors demonstrated that a fraction of B-1a, FO B-2 and granulocytes share a common multilineage precursor expressing the same barcode sequence – implying a potential clonal relationship (Kristiansen et al., 2016). They interpreted these findings as suggesting that individual fetal LT-HSCs give rise to B-1a, B-2 and splenic granulocytes, and hence that these cell types are clonally related to one another. As such clones represent more than one half of the peritoneal B-1a cells recovered from the recipient mice, the authors concluded that LT-HSCs are the main source of B-1a cells in the peritoneal cavity.
Reconciling seemingly contradictory findings on B-1a cell origin
The seemingly contradictory findings on the origins of B-1a cells between the cell-barcoding studies showed by Kristiansen et al. (2016) and other recent studies using purified LT-HSC transplantation (Ghosn et al., 2016), in vivo LT-HSC lineage-tracing (Sawen et al., 2018; Sawai et al., 2016) and HSC-deficient mice (Kobayashi et al., 2014) could partially be explained by the significant differences in the experimental design of each of these studies, particularly with respect to the age and cell type of the donor fetal liver. LT-HSC transplantation assay studies (Ghosn et al., 2016) used highly purified, stringently gated, ∼E15 fetal liver KSL CD150+CD48− cells, a phenotype currently described as containing most (if not all) the bona fide LT-HSCs in fetal liver (Kim et al., 2006) and adult bone marrow (Yamamoto et al., 2013; Carrelha et al., 2018). By contrast, Kristiansen et al. transduced the lentiviral library into total E14.5 fetal liver KSL population, of which only ∼1% are LT-HSCs (Ghosn et al., 2016), and cultured this population overnight in cytokine-enriched media (Kristiansen et al., 2016). Thus, and also considering the low lentiviral transduction efficiency of 15-30%, it is possible that the cells that gave rise to the B-1a and B-2 cells and to granulocytes in Kristiansen et al.’s study were different from the fetal liver LT-HSCs used in Ghosn et al.’s study (Ghosn et al., 2016). In fact, the CD150−/lo progenitors are the dominant population in the fetal liver KSL compartment; these were shown to give rise to B-1a cells in transplantation studies (Ghosn et al., 2016). This raises the possibility that the CD150−/lo cells enriched in the cultured KSL population may have been the main cells transduced in the barcoding studies to give rise to B-1a, B-2 and granulocytes.
Kristiansen and colleagues also performed in vivo single-LT-HSC transplantation assays to confirm their findings (Kristiansen et al., 2016), relying on a similar LT-HSC marker phenotype that we used in our previous studies (Ghosn et al., 2016). Consistent with our findings (Ghosn et al., 2016), they show that the recipient mice only poorly regenerated B-1a cells (Kristiansen et al., 2016). Among the total donor-derived peritoneal B cells, only about 2%-10% were identified as true B-1a cells (Kristiansen et al., 2016). It is important to note that B-1a cells represent about 60-80% of total peritoneal B cells in unmanipulated wild-type mice (Ghosn et al., 2008). This selective inefficiency to regenerate B-1a cells (but to fully regenerate FO B-2) does not support the notion that B-1a cells are regenerated by bona fide LT-HSCs (which should regenerate all lineages at appropriate proportions).
Finally, the intriguing result that nearly half of all the ∼200 unique cell barcodes retrieved in these experiments were detected only in B-1a cells (Kristiansen et al., 2016) provides further support for the notion that at least some B-1a cells develop from a committed progenitor, independently of fetal liver LT-HSCs. On the other hand, the single LT-HSC transplant experiments do suggest that some B-1a cells can be derived from these progenitors, implying that the B-1a compartment might contain both HSC-dependent and HSC-independent progeny. Future studies are needed to determine the extent to which these two potential sources contribute to the total peritoneal B-1a cell pool in vivo, in a non-transplantation setting.
In any event, the elegant studies by Kristiansen and colleagues have added a new layer to our understanding of the complex landscape of fetal B-cell development. They demonstrate that the progenitors that give rise to innate-like B-1a cells in fetal liver can also give rise to FO B-2 cells (Kristiansen et al., 2016). This is the first study to report, at the single cell level, that a fraction of B-1a, FO B-2 and granulocytes share a common clonal precursor, raising the possibility that a multilineage progenitor, or perhaps a functional HSC distinct from the phenotypically defined LT-HSC, might exist in the fetal liver.
Along these lines, a recent fate-mapping study (Beaudin et al., 2016) has compared the regenerative potential, and developmental relationship, between fetal and adult LT-HSCs in vivo. The authors suggest that the fetal liver does indeed contain a hitherto unidentified, functionally distinct subset of HSCs that are biased towards regenerating B-1a cells. In essence, these studies indicate that B-1a cells are derived from a developmentally restricted fetal HSC (termed drHSC) that emerges separate from the conventional LT-HSCs (Beaudin et al., 2016). Importantly, the drHSCs express the KSL CD150lo phenotype and are biased towards innate-like, tissue-resident B and T lymphocytes (i.e. B-1a, marginal zone B, and γ3+-γδ T cells) (Beaudin et al., 2016) (Fig. 3).
The drHSC was identified using a Tomato→GFP lineage-tracing mouse model, in which the expression of the tyrosine kinase receptor fetal liver kinase 2 (Flk2) can be traced by an irreversible switch from red (Tomato+ Flk2-off) to green (GFP+ Flk2-on). In the adult bone marrow, virtually all adult-repopulating LT-HSCs are red (Tomato+) as they lack Flk2 expression. Flk2+ KSL cells (i.e. GFP+, Tomato−) in the adult bone marrow fail to engraft in transplantation settings, confirming that LT-HSC activity in the adult bone marrow is confined within the Tomato+/Flk2− phenotype.
By contrast, in the fetal liver, the results were strikingly different. The drHSCs expressed the GFP+ KSL phenotype and displayed HSC activity in a transplantation setting, efficiently regenerating innate-like γδ T cells, B-1a and marginal zone B cells (as well as producing the long-term engraftment of αβ T and B-2 cells) (Beaudin et al., 2016). However, drHSCs do not seem to express all the conventional characteristics used to define a bona fide LT-HSC, in that they lack long-term self-renewal potential and disappear from the bone marrow by postnatal day 14 in unmanipulated mice (Beaudin et al., 2016). Future single cell studies should determine whether drHSCs represent true functional HSCs, in that a single cell from this population should be capable of serial multilineage reconstitution (including B-1a), even though they lack the long-term survival that is characteristic of bona fide LT-HSCs.
Fetal KSL CD150−/lo population: the missing link?
As indicated above, the expression level of CD150 in fetal liver cells may have been a confounding factor that led to the discrepancies among studies aimed at identifying the B-1a precursor, especially when comparing results from E14 versus E15 mouse fetal liver. In the adult bone marrow, the CD150−/lo KSL population contains short-term (ST)-HSCs, MPPs and LMPPs, which are all known to be derived from conventional LT-HSCs (i.e. KSL CD150+CD48−) (Yamamoto et al., 2013). In addition, the bone marrow-derived CD150lo KSL population shows a lymphoid-biased potential when transplanted into irradiated hosts (Beerman et al., 2010; Morita et al., 2010). This population becomes gradually less represented in aging mice, when the CD150hi KSL phenotype – which is myeloid biased – predominates (Beerman et al., 2010; Morita et al., 2010).
In the fetus, however, CD150 is not expressed on LT-HSCs in the E11.5 AGM region and E12.5 placenta, but can be detected on fetal liver LT-HSCs from E14.5 (Kim et al., 2006; McKinney-Freeman et al., 2009; Papathanasiou et al., 2009), when CD150 expression levels gradually increase. After E15, the expression level of CD150 is sufficiently high and distinguishable from CD150−, allowing CD150+ (enriched for LT-HSCs) and CD150− (mostly progenitors) within the fetal KSL population to be separated from each other for functional studies (Papathanasiou et al., 2009; Kim et al., 2006; Ghosn et al., 2016). These data together suggest a change in potential as LT-HSCs acquire the CD150+ phenotype-such that the conventional LT-HSC in E15 liver lacks the ability to regenerate B-1a cells. However, it is still possible that a hitherto unidentified precursor cell with HSC activity at the single cell level, perhaps expressing the CD150−/lo phenotype, might give rise to both B-1a and other haematopoietic cell types.
Embryonic pre-HSCs have the potential to give rise to B-1a cells
Based on the current literature, the emergence of intra-embryonic HSCs in the AGM at ∼E10.5 (Medvinsky and Dzierzak, 1996; Kumaravelu et al., 2002; Chen et al., 2011) overlaps, in time, with the development of B-1a progenitors from hemogenic ECs in the yolk sac and P-Sp (Yoshimoto et al., 2011; de Andres et al., 2002; Godin et al., 1993; Marcos et al., 1997). This raises the issue of whether the HSCs that emerge in the AGM region also have the potential to generate B-1a cell progenitors that significantly contribute to the peritoneal B-1a cell pool.
Hadland et al. (Hadland et al., 2017) have recently addressed this by assessing the potential of embryonic mouse pre-HSCs to give rise to B-1a and B-2 cells following in vitro culture with Akt-overexpressing ECs derived from the AGM region (AGM-ECs). This group have previously reported that AGM-ECs promote pre-HSC maturation and expansion, which enables the transplantation of cells into lethally irradiated recipient mice (Hadland et al., 2015).
Using the AGM-EC co-culture system (Hadland et al., 2017), the authors performed a clonal assay experiment in which single pre-HSCs were sorted from either E9.5 P-Sp or E11.5 AGM and co-cultured with AGM-ECs for 5 days. The clonal progeny containing matured HSCs (identified by a ZsGreen reporter gene) was transplanted into congenic lethally irradiated mice. Both B-1a and B-2 cells were found in the peritoneal cavity of recipient mice, leading to the conclusion that pre-HSCs give rise to both B-1a and B-2 cells (Hadland et al., 2017).
Intriguingly, the potential to generate B-1a cells differs between E9.5 and E11.5 pre-HSCs. The pre-HSCs derived from the E9.5 P-Sp have a higher B-1a cell potential than do pre-HSCs derived from the E11.5 AGM (Hadland et al., 2017). The authors speculate that E9.5 pre-HSCs might develop into the previously identified ‘drHSCs’ that are found in the fetal liver, whereas E11.5 pre-HSCs might develop into conventional LT-HSCs in the fetal liver, with limited B-1a cell potential. If this is the case, further studies should identify when (and how) E11.5 pre-HSCs lose their potential to generate B-1a cells after their maturation into LT-HSCs.
These studies describe the potential of pre-HSCs to give rise to B-1a and B-2 cells under a specific culture condition. If the findings hold true under in vivo homeostasis, they would suggest that pre-HSCs represent the last common precursor of B-1a progenitors and conventional LT-HSCs. (Fig. 3). Support for this idea has come from a recent in vivo lineage tracing study (Pei et al., 2017), which points to the existence of a common precursor for B-1a cells and LT-HSCs that emerges as early as E9.5. The authors employed a polylox barcoding system that can be activated at different developmental stages during embryogenesis. When the polylox was activated in cells expressing the angiopoietin receptor Tie2 at E9.5, more than 95% of adult bone marrow HSCs and their progeny, including B-1a and B-2 cells, show recombined barcodes.
However, the hierarchical clustering analysis place the B-1a lineage separate from the LT-HSC-derived T and B-2 lineages (Pei et al., 2017), suggesting that a lineage split might occur early during embryogenesis (i.e. after E9.5). Thus, although B-1a and LT-HSCs might initially originate from a common Tie2+ endothelial precursor (Fig. 3), the separation of these lineages becomes evident by the low frequency of a common precursor for both B-1a and B-2 cells (Pei et al., 2017). Further investigation of pre-HSC heterogeneity in vivo and in situ, and transplantation assay studies of freshly isolated AGM-derived single HSCs, at different development ages, would reveal the fate of AGM-derived (pre)-HSCs.
Intra- and extra-embryonic sources of B-1a progenitors
When studying precursor potential to (re)generate B-1a cells in vivo, it is important to consider the efficiency with which peritoneal B-1a cells are reconstituted. In all studies that suggest that B-1a cells develop from fetal liver HSCs, the number (and percentage) of B-1a cells that are reconstituted in the recipient peritoneal cavity is well below the numbers observed in unmanipulated wild-type adult mice. For example, regeneration of B-1a cells from fetal liver (even from total unsorted liver) is, at best, ∼20% of the total B-1a numbers found in unmanipulated wild-type mice (Ghosn et al., 2016; Beaudin et al., 2016; Kristiansen et al., 2016; Yenson and Baumgarth, 2014). In striking contrast, all other immune cell lineages, including FO B-2, MZ B, B-1b and transitional B cells, are fully regenerated at appropriate numbers that are comparable with unmanipulated mice, as expected from bona fide multilineage LT-HSC activity. This raises the possibility that some B-1a cells originate from sources other than fetal liver.
Consistent with this idea, intra-embryonic tissues other than liver have shown lymphopoietic potential prior to LT-HSC development. Engraftment of E9.5 P-Sp under the kidney capsule of recipient mice readily regenerates innate-like B-1a and serum IgM in recipient mice (Godin et al., 1993). Similarly, some B-1a cells might also emerge extra-embryonically in the yolk sac at ∼E9 (Yoshimoto et al., 2011). The mechanisms by which B-1a precursor(s) migrate from the yolk sac, P-Sp/AGM and fetal liver to form the B-1a cell pool in the postnatal body cavities and mucosa remain unknown. Collectively, these studies suggest that B-1a cells develop in multiple locations, within and outside the embryo proper, from progenitors (perhaps, multilineage progenitors) that are developmentally separate from the conventional LT-HSC defined as KSL CD150+CD48−.
HSC-independent lineages in humans: clinical implications
These conclusions on HSC-independent hematopoiesis, including B-1a development, have come mainly from studies performed in mice and whether or not they reflect the fetal hematopoiesis in humans needs to be investigated. Although the human hematopoiesis is largely similar to the mouse, especially during fetal development (Ivanovs et al., 2017), it remains unclear whether the human HSC-independent lineages (including B-1a) can also persist throughout adulthood to participate in immune responses alongside HSC-dependent lineages. Here, we briefly summarize the recent relevant literature on human fetal hematopoiesis and discuss the relevance and impact of these findings to the human health.
Similar to mice, the first wave of hematopoiesis in humans appears in the yolk sac, before the development of the LT-HSC. As early as 16-18.5 days post-conception (dpc), before the onset of blood circulation, the human yolk sac gives rise to large primitive nucleated erythrocytes, followed by primitive macrophages and megakaryocytes (Luckett, 1978; Ivanovs et al., 2017). Subsequently, these and other hematopoietic cells appear in the circulation at or after 22 dpc (Carnegie stage, CS >10), still before the development of LT-HSCs (Tavian et al., 1999).
Recent studies have compared the hematopoietic potential of human AGM, yolk sac, liver, umbilical cord and placenta with their mouse counterparts (Ivanovs et al., 2011, 2017; Tavian et al., 2001). These initial studies revealed that the first human HSCs emerge in the intra-embryonic AGM at around 30-32 dpc (CS 14). In the yolk sac, HSCs were first detected 5 days later, at around 35-38 dpc (CS 17). These HSCs later migrate to the fetal liver around >40 dpc, where they expand and give rise to all major hematopoietic lineages. Finally, before birth, HSCs migrate and take residence in the bone marrow, where they are maintained throughout life (Ivanovs et al., 2017).
Red and white blood cells (i.e. erythrocytes and immune cells) can be detected in the fetal circulation at least 10 days before the detection of cells exhibiting LT-HSC activity, which occurs in the AGM around 32 dpc (Ivanovs et al., 2011). These findings suggest that early circulating immune cells, including myeloid cells akin to murine EMP-derived macrophages, are likely the human equivalent of HSC-independent hematopoiesis. Similar to mouse, these early human HSC-independent lineages first appear in the extra-embryonic yolk sac (16-37 dpc), and perhaps later from the intra-aortic hematopoietic clusters in the AGM region (27 dpc), still before the development of the LT-HSC (Ivanovs et al., 2017, 2011).
Studies on human fetal lymphopoiesis also show striking similarities to mouse (Roy et al., 2017; Rechavi et al., 2015; Schroeder and Wang, 1990; Schroeder et al., 1987), particularly to mouse B-1a lymphopoiesis. The human fetal B-cell receptor (BCR) repertoire is tightly regulated and less diverse (i.e. it is biased and not stochastic) than the repertoire of the adult bone marrow HSC-dependent B lymphocytes (Roy et al., 2017; Rechavi et al., 2015; Schroeder and Wang, 1990; Schroeder et al., 1987). In mouse, fetal-derived B lymphocytes represent the innate-like B-1a lineage, which expresses a limited and predictable BCR repertoire (Yang et al., 2015) relevant to housekeeping activities (Shaw et al., 2000), and are likely derived from HSC-independent precursors.
Human fetal B cells can already be identified in the liver around 42 dpc (Thilaganathan et al., 1993; Rechavi et al., 2015). However, the exact location and time for the emergence of their first precursors is not yet known. Because mature fetal B cells (i.e. expressing surface immunoglobulin) have been reported in the human embryo about 1 week after the development of the first LT-HSC, it is not clear whether the first fetal B cells in humans also develop independently of the LT-HSC. In any event, the functional properties of the human fetal B cells, including a biased and limited BCR repertoire, is similar to the murine HSC-independent innate-like B-1a cells.
Understanding the molecular mechanisms that regulate the early HSC-independent hematopoiesis in the human fetus is not only of fundamental biological relevance but can also shed light on the mechanisms that lead to human disease, both in infants and adults. Of note, recent studies of childhood and adult leukemia strongly support the idea that prenatal early fetal B lymphocytes are the likely cell target(s) of childhood B-cell acute lymphoblastic leukemia (B-ALL) (Boiers et al., 2018; Montecino-Rodriguez et al., 2014; Barrett et al., 2016), adult chronic lymphocytic leukemia (CLL) (Hayakawa et al., 2016a,b; DiLillo et al., 2013) and mantle cell lymphoma (Hayakawa et al., 2018). Strikingly, murine fetal-derived B-1a lymphocytes, but not adult bone marrow-derived FO B-2 cells, were shown to be more susceptible to differentiate into leukemic cells when genetically transformed by the mutations that cause B-ALL [MLL-AF4 (Barrett et al., 2016), ETV6-RUNX1 (Boiers et al., 2018) and BCR-ABL (Montecino-Rodriguez et al., 2014)] and CLL [chromosomal loss of 13q14 (Hayakawa et al., 2016b) and TCL1 oncogene overexpression (Hayakawa et al., 2016a)].
Further studies are also needed to determine the types (phenotype and function) of human immune cells that develop early during fetal hematopoiesis prior to, and independently of, the LT-HSC and to assess whether these human fetal HSC-independent lineages persist throughout adulthood, as occurs in mice. Such studies can also inform current efforts to transplant highly purified (Czechowicz and Weissman, 2011) or iPSC-derived (Ivanovs et al., 2017) HSCs, as these HSCs might lack the potential to fully regenerate all components of the immune system. HSC transplantation is widely used to treat individuals with hematological malignancies (leukemias, lymphomas and myelomas), and immune-deficiency and hematopoietic disorders, among other diseases (Liang and Zúñiga-Pflücker, 2015; D'Souza and Fretham, 2018). As such, it is vitally important to determine whether humans, as in mice, generate immune cells independently of the LT-HSC in both the bone marrow and cord blood. If, for example, innate-like B-1a lymphocytes cannot be reconstituted, this might pose a serious impediment to the natural immunity of the host. Hence, the addition of B-1a precursors (and perhaps precursors to other HSC-independent lineages) to highly purified adult HSC transplants in therapeutic settings might prove to be clinically valuable.
Conclusions and future directions
Recent advances in our understanding of fetal hematopoiesis in mice have stimulated a paradigm shift in how we think about the development of the mammalian immune system. These important advances suggest that HSC-independent precursors emerge early in ontogeny to give rise to a myriad of immune lineages that fulfil unique and evolutionarily conserved functions. These lineages are distinct from the immune lineages that develop later on in development from HSC-dependent precursors. For example, in the mouse, some innate-like B-1a cells carry a unique genetic program that ensures the development of germline-encoded B-cell receptor (BCR) repertoires that might be important for maintaining homeostasis in the immune system (Herzenberg et al., 2000). These germline-encoded repertoires, including the VH11 gene family, are strictly regulated. They show little to no mutation in their BCR sequences and are expressed only by innate-like B-1a cells (Kantor et al., 1995; Yang et al., 2015; Ghosn et al., 2008; Hardy et al., 2004).
The HSC-independent developmental strategy for generating innate-like B-1a cells during fetal hematopoiesis appears to apply to all hematopoietic lineages (Figs 1 and 2). Primitive erythrocytes, which express fetal hemoglobin, appear early in ontogeny (Palis et al., 1999), and are followed by definitive erythrocytes, which appear before HSCs and initiate the production of adult erythrocytes (Lux et al., 2008). A similar strategy is seen for the development of the myeloid lineage (Lux et al., 2008). In this lineage, early HSC-independent ‘primitive’ (mesoderm-derived) macrophages and EMPs give rise to brain microglia, skin Langerhans cells and other tissue-resident macrophages (Schulz et al., 2012; Hoeffel et al., 2015; Gomez Perdiguero et al., 2015; Ginhoux et al., 2010; De et al., 2018) (Fig. 2). By contrast, LT-HSCs give rise to circulating monocytes, which represent the precursors to most adult-type macrophages and certain dendritic cells (Sawai et al., 2016). Lymphocyte development follows a similar pattern. The first wave of T-cell development occurs prior to LT-HSC emergence (Tian et al., 2017; Liu and Auerbach, 1991; Nishikawa et al., 1998; Yoshimoto et al., 2012). It principally generates γδ T cells (Havran and Allison, 1988; Ikuta et al., 1990; Masuda et al., 2005) and other T cells of fetal origin (Yoshimoto et al., 2012; Tian et al., 2017; Nishikawa et al., 1998). The subsequent LT-HSC-dependent wave(s) of lymphopoiesis generates most of the αβ T cells that ultimately predominate in adult life (Havran and Allison, 1988) (Fig. 2).
Future lineage-tracing studies in vivo will define the developmental relationships and full reconstitution potential of HSCs and other HSC-independent hematopoietic precursors [hemogenic endothelium, EMP, (L)MPP, drHSC, etc.] that emerge at different stages of development, and at different locations (yolk sac, P-Sp, AGM, placenta, etc.) (Fig. 1). The recent demonstration that the nuclear protein Hoxb5 is expressed in virtually all LT-HSCs in young adult mice (Chen et al., 2016) could provide a potential lineage-tracing approach for investigating the origin(s) of fetal LT-HSCs and their relationship to HSC-independent lineages that emerge at both intra- and extra-embryonic sites.
Importantly from a functional standpoint, the fetal-derived HSC-independent lineages persist throughout adulthood as tissue-resident immune cells by means of in situ self-renewal (Gentek et al., 2018a,b; Hashimoto et al., 2013; Ghosn and Yang, 2015; Kreslavsky et al., 2017), and might participate in tissue homeostasis and possibly in immunopathologies in adults. Future studies are urgently needed to determine the functional role(s) of the HSC-independent lineages from embryonic life to adulthood and to determine whether their precursors also persist throughout adulthood or slowly wane during neonatal life.
The limitations in the experimental methods available and ethical considerations in designing human studies have motivated the development of alternative approaches to study human hematopoiesis, including the direct differentiation of embryonic stem cells of induced pluripotent stem cells (iPSCs) in vitro (Ditadi et al., 2017; Wahlster and Daley, 2016). These efforts represent great advances in the field and have the potential to not only provide a fundamental understanding of the biology of the human immune system but also improve mainstream medical care. Although it has proved challenging to generate and maintain the HSC phenotype in culture, efforts to generate human HSCs in vitro that are suitable for HSC transplantation in clinical settings are under way (Ditadi et al., 2017; Wahlster and Daley, 2016; Ivanovs et al., 2017). So far, these studies using human pluripotent stem cell cultures suggest that human yolk sac hematopoiesis can be recapitulated in vitro (Kennedy et al., 2007; Davis et al., 2008; Vodyanik et al., 2006, 2010). Given that human fetal hematopoiesis initiates in the yolk sac prior to, and independently of, the LT-HSC, future studies should determine whether these in vitro generated yolk sac-like cells represent the human HSC-independent hematopoiesis.
Finally, where and how these HSC-independent (and fetal-derived) immune lineages are maintained in the adult tissues and, most importantly, what their functional role(s) are in both infant and adult immune responses, needs to be determined. Results from such studies can be expected to provide fundamental insights into how evolution has crafted the development and function of the mammalian immune system to respond to the endogenous and environmental changes that occur from embryonic life to adulthood.
Acknowledgement
We thank Dr Koshika Yadava for technical support with graphic design.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors' research is supported by the Lowance Center for Human Immunology; the Pediatric Research Alliance of Atlanta, Center for Transplantation and Immune-mediated Disorders; and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002378). Deposited in PMC for release after 12 months.
References
- Arora N., Wenzel P. L., Mckinney-Freeman S. L., Ross S. J., Kim P. G., Chou S. S., Yoshimoto M., Yoder M. C. and Daley G. Q. (2014). Effect of developmental stage of HSC and recipient on transplant outcomes. Dev. Cell 29, 621-628. 10.1016/j.devcel.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Holodick N. E., Rothstein T. L. and Wang P. (2017). B-1a cells protect mice from sepsis: critical role of CREB. J. Immunol. 199, 750-760. 10.4049/jimmunol.1602056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C. L., Montecino-Rodriguez E. and Dorshkind K. (2011). Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc. Natl. Acad. Sci. USA 108, 13700-13704. 10.1073/pnas.1107172108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C. S., Kester L., Klaus A., Boisset J. C., Thambyrajah R., Yvernogeau L., Kouskoff V., Lacaud G., Van Oudenaarden A. and Robin C. (2018). Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat. Commun. 9, 2517 10.1038/s41467-018-04893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N. A., Malouf C., Kapeni C., Bacon W. A., Giotopoulos G., Jacobsen S. E. W., Huntly B. J. and Ottersbach K. (2016). Mll-AF4 confers enhanced self-renewal and lymphoid potential during a restricted window in development. Cell Rep. 16, 1039-1054. 10.1016/j.celrep.2016.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Tung J. W. and Herzenberg L. A. (2005). Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26, 347-362. 10.1007/s00281-004-0182-2 [DOI] [PubMed] [Google Scholar]
- Beaudin A. E., Boyer S. W., Perez-Cunningham J., Hernandez G. E., Derderian S. C., Jujjavarapu C., Aaserude E., Mackenzie T. and Forsberg E. C. (2016). A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell 19, 768-783. 10.1016/j.stem.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I. L., Bryder D. and Rossi D. J. (2010). Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA 107, 5465-5470. 10.1073/pnas.1000834107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland R. and Wortis H. H. (2002). Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20, 253-300. 10.1146/annurev.immunol.20.100301.064833 [DOI] [PubMed] [Google Scholar]
- Bodogai M., O'connell J., Kim K., Kim Y., Moritoh K., Chen C., Gusev F., Vaughan K., Shulzhenko N., Mattison J. A. et al. (2018). Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 10, eaat4271 10.1126/scitranslmed.aat4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiers C., Carrelha J., Lutteropp M., Luc S., Green J. C., Azzoni E., Woll P. S., Mead A. J., Hultquist A., Swiers G. et al. (2013). Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 13, 535-548. 10.1016/j.stem.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Boiers C., Richardson S. E., Laycock E., Zriwil A., Turati V. A., Brown J., Wray J. P., Wang D., James C., Herrero J. et al. (2018). A human IPS model implicates embryonic B-myeloid fate restriction as developmental susceptibility to b acute lymphoblastic leukemia-associated ETV6-RUNX1. Dev. Cell 44, 362-377.e7. 10.1016/j.devcel.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R., Feng L., Xia Y. Y., Chang J. C. and Havran W. L. (1996). Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J. Immunol. 157, 985-992. [PubMed] [Google Scholar]
- Boisset J. C., Van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E. and Robin C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116-120. 10.1038/nature08764 [DOI] [PubMed] [Google Scholar]
- Bos N. A., Kimura H., Meeuwsen C. G., De Visser H., Hazenberg M. P., Wostmann B. S., Pleasants J. R., Benner R. and Marcus D. M. (1989). Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur. J. Immunol. 19, 2335-2339. 10.1002/eji.1830191223 [DOI] [PubMed] [Google Scholar]
- Bouaziz J. D., Yanaba K. and Tedder T. F. (2008). Regulatory B cells as inhibitors of immune responses and inflammation. Immunol. Rev. 224, 201-214. 10.1111/j.1600-065X.2008.00661.x [DOI] [PubMed] [Google Scholar]
- Busch K., Klapproth K., Barile M., Flossdorf M., Holland-Letz T., Schlenner S. M., Reth M., Hofer T. and Rodewald H. R. (2015). Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518, 542-546. 10.1038/nature14242 [DOI] [PubMed] [Google Scholar]
- Carrelha J., Meng Y., Kettyle L. M., Luis T. C., Norfo R., Alcolea V., Boukarabila H., Grasso F., Gambardella A., Grover A. et al. (2018). Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature 554, 106-111. 10.1038/nature25455 [DOI] [PubMed] [Google Scholar]
- Chen Y., Chou K., Fuchs E., Havran W. L. and Boismenu R. (2002). Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA 99, 14338-14343. 10.1073/pnas.212290499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Li Y., De Obaldia M. E., Yang Q., Yzaguirre A. D., Yamada-Inagawa T., Vink C. S., Bhandoola A., Dzierzak E. and Speck N. A. (2011). Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9, 541-552. 10.1016/j.stem.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y., Miyanishi M., Wang S. K., Yamazaki S., Sinha R., Kao K. S., Seita J., Sahoo D., Nakauchi H. and Weissman I. L. (2016). Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223-227. 10.1038/nature16943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S. and Baumgarth N. (2008). Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 205, 3053-3064. 10.1084/jem.20080979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. L., Wright D. E., Wagers A. J. and Weissman I. L. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2, E75 10.1371/journal.pbio.0020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Kazatchkine M. D. and Avrameas S. (1995). Natural autoantibodies. Curr. Opin. Immunol. 7, 812-818. 10.1016/0952-7915(95)80053-0 [DOI] [PubMed] [Google Scholar]
- Cumano A. and Godin I. (2001). Pluripotent hematopoietic stem cell development during embryogenesis. Curr. Opin. Immunol. 13, 166-171. 10.1016/S0952-7915(00)00200-4 [DOI] [PubMed] [Google Scholar]
- Cumano A., Ferraz J. C., Klaine M., Di Santo J. P. and Godin I. (2001). Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15, 477-485. 10.1016/S1074-7613(01)00190-X [DOI] [PubMed] [Google Scholar]
- Cumano A., Dieterlen-Lievre F. and Godin I. (1996). Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86, 907-916. 10.1016/S0092-8674(00)80166-X [DOI] [PubMed] [Google Scholar]
- Czechowicz A. and Weissman I. L. (2011). Purified hematopoietic stem cell transplantation: the next generation of blood and immune replacement. Hematol. Oncol. Clin. North Am. 25, 75-87. 10.1016/j.hoc.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. P., Ng E. S., Costa M., Mossman A. K., Sourris K., Elefanty A. G. and Stanley E. G. (2008). Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 111, 1876-1884. 10.1182/blood-2007-06-093609 [DOI] [PubMed] [Google Scholar]
- de Andres B., Gonzalo P., Minguet S., Martinez-Marin J. A., Soro P. G., Marcos M. A. and Gaspar M. L. (2002). The first 3 days of B-cell development in the mouse embryo. Blood 100, 4074-4081. 10.1182/blood-2002-03-0809 [DOI] [PubMed] [Google Scholar]
- de Bruijn M. F., Ma X., Robin C., Ottersbach K., Sanchez M. J. and Dzierzak E. (2002). Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16, 673-683. 10.1016/S1074-7613(02)00313-8 [DOI] [PubMed] [Google Scholar]
- De S., Van Deren D., Peden E., Hockin M., Boulet A., Titen S. and Capecchi M. R. (2018). Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development 145, dev152306 10.1242/dev.152306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilillo D. J., Weinberg J. B., Yoshizaki A., Horikawa M., Bryant J. M., Iwata Y., Matsushita T., Matta K. M., Chen Y., Venturi G. M. et al. (2013). Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 27, 170-182. 10.1038/leu.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditadi A., Sturgeon C. M. and Keller G. (2017). A view of human haematopoietic development from the Petri dish. Nat. Rev. Mol. Cell Biol. 18, 56-67. 10.1038/nrm.2016.127 [DOI] [PubMed] [Google Scholar]
- D'Souza A. and Fretham C. (2018). Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2018. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx#RequestSummarySlides
- Duan B. and Morel L. (2006). Role of B-1a cells in autoimmunity. Autoimmun Rev. 5, 403-408. 10.1016/j.autrev.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Dzierzak E. and Bigas A. (2018). Blood development: hematopoietic stem cell dependence and independence. Cell Stem Cell 22, 639-651. 10.1016/j.stem.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Dzierzak E. and Medvinsky A. (2008). The discovery of a source of adult hematopoietic cells in the embryo. Development 135, 2343-2346. 10.1242/dev.021279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganuza M., Chabot A., Tang X., Bi W., Natarajan S., Carter R., Gawad C., Kang G., Cheng Y. and Mckinney-Freeman S. (2018). Murine hematopoietic stem cell activity is derived from pre-circulation embryos but not yolk sacs. Nat. Commun. 9, 5405 10.1038/s41467-018-07769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C., Dieterlen-Lièvre F., Orkin S. H. and Mikkola H. K. (2005). The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365-375. 10.1016/j.devcel.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Gentek R., Ghigo C., Hoeffel G., Bulle M. J., Msallam R., Gautier G., Launay P., Chen J., Ginhoux F. and Bajenoff M. (2018a). Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity 48, 1160-1171.e5. 10.1016/j.immuni.2018.04.025 [DOI] [PubMed] [Google Scholar]
- Gentek R., Ghigo C., Hoeffel G., Jorquera A., Msallam R., Wienert S., Klauschen F., Ginhoux F. and Bajenoff M. (2018b). Epidermal gammadelta T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J. Exp. Med. 215, 2994-3005. 10.1084/jem.2018120611142018c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E. E. and Yang Y. (2015). Hematopoietic stem cell-independent B-1a lineage. Ann. N. Y. Acad. Sci. 1362, 23-38. 10.1111/nyas.12881 [DOI] [PubMed] [Google Scholar]
- Ghosn E. E., Yang Y., Tung J., Herzenberg L. A. and Herzenberg L. A. (2008). CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proc. Natl. Acad. Sci. USA 105, 5195-5200. 10.1073/pnas.0712350105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E. E. B., Yamamoto R., Hamanaka S., Yang Y., Herzenberg L. A., Nakauchi H. and Herzenberg L. A. (2012). Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 109, 5394-5398. 10.1073/pnas.1121632109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E. E. B., Waters J., Phillips M., Yamamoto R., Long B. R., Yang Y., Gerstein R., Stoddart C. A., Nakauchi H. and Herzenberg L. A. (2016). Fetal hematopoietic stem cell transplantation fails to fully regenerate the B-Lymphocyte compartment. Stem Cell Reports 6, 137-149. 10.1016/j.stemcr.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E., Dorshkind K., Herzenberg L. A., Holodick N., Kantor A., Montecino-Rodriguez E., Rothstein T. L., Silverman G. J., Yang Y., Yoder M. C. et al. (2019). B1 B cell progenitors. Science 364, 248 10.1126/science.aax6784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M. F., Conway S. J., Ng L. G., Stanley E. R. et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841-845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin I. E., Garcia-Porrero J. A., Coutinho A., Dieterlen-Lièvre F. and Marcos M. A. R. (1993). Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 364, 67-70. 10.1038/364067a0 [DOI] [PubMed] [Google Scholar]
- Godin I., Dieterlen-Lievre F. and Cumano A. (1995). Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc. Natl. Acad. Sci. USA 92, 773-777. 10.1073/pnas.92.3.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M. F., Geissmann F. and et al. (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547-551. 10.1038/nature13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf R., Seagal J., Otipoby K. L., Lam K. P., Ayoub S., Zhang B., Sander S., Chu V. T. and Rajewsky K. (2019). BCR-dependent lineage plasticity in mature B cells. Science 363, 748-753. 10.1126/science.aau8475 [DOI] [PubMed] [Google Scholar]
- Grönwall C., Vas J. and Silverman G. J. (2012). Protective roles of natural IgM antibodies. Front. Immunol. 3, 66 10.3389/fimmu.2012.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K. M., Poe J. C., Steeber D. A. and Tedder T. F. (2005). B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23, 7-18. 10.1016/j.immuni.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Haas J. D., Ravens S., Düber S., Sandrock I., Oberdörfer L., Kashani E., Chennupati V., Fohse L., Naumann R., Weiss S. et al. (2012). Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity 37, 48-59. 10.1016/j.immuni.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Hadland B. K., Varnum-Finney B., Poulos M. G., Moon R. T., Butler J. M., Rafii S. and Bernstein I. D. (2015). Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J. Clin. Invest. 125, 2032-2045. 10.1172/JCI80137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland B. K., Varnum-Finney B., Mandal P. K., Rossi D. J., Poulos M. G., Butler J. M., Rafii S., Yoder M. C., Yoshimoto M. and Bernstein I. D. (2017). A common origin for B-1a and B-2 lymphocytes in clonal pre- hematopoietic stem cells. Stem Cell Reports 8, 1563-1572. 10.1016/j.stemcr.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Wei C.-J. and Hayakawa K. (2004). Selection during development of VH11+ B cells: a model for natural autoantibody-producing CD5+ B cells. Immunol. Rev. 197, 60-74. 10.1111/j.0105-2896.2004.0100.x [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Kincade P. W. and Dorshkind K. (2007). The protean nature of cells in the B lymphocyte lineage. Immunity 26, 703-714. 10.1016/j.immuni.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D. et al. (2013). Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792-804. 10.1016/j.immuni.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L. and Allison J. P. (1988). Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 335, 443-445. 10.1038/335443a0 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. and Herzenberg L. A. (1985). Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161, 1554-1568. 10.1084/jem.161.6.1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Formica A. M., Brill-Dashoff J., Shinton S. A., Ichikawa D., Zhou Y., Morse H. C. III and Hardy R. R. (2016a). Early generated B1 B cells with restricted BCRs become chronic lymphocytic leukemia with continued c-Myc and low Bmf expression. J. Exp. Med. 213, 3007-3024. 10.1084/jem.20160712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Formica A. M., Colombo M. J., Shinton S. A., Brill-Dashoff J., Morse H. C. III, Li Y.-S. and Hardy R. R. (2016b). Loss of a chromosomal region with synteny to human 13q14 occurs in mouse chronic lymphocytic leukemia that originates from early-generated B-1 B cells. Leukemia 30, 1510-1519. 10.1038/leu.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Formica A. M., Nakao Y., Ichikawa D., Shinton S. A., Brill-Dashoff J., Smith M. R., Morse H. C. III and Hardy R. R. (2018). Early generated B-1-derived B cells have the capacity to progress to become mantle cell lymphoma-like neoplasia in aged mice. J. Immunol. 201, 804-813. 10.4049/jimmunol.1800400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig J. S. and Tonegawa S. (1986). Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322, 836-840. 10.1038/322836a0 [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Baumgarth N. and Wilshire J. A. (2000). B-1 cell origins and VH repertoire determination. Curr. Top. Microbiol. Immunol. 252, 3-13. 10.1007/978-3-642-57284-5_1 [DOI] [PubMed] [Google Scholar]
- Hoeffel G., Chen J., Lavin Y., Low D., Almeida F. F., See P., Beaudin A. E., Lum J., Low I., Forsberg E. C. et al. (2015). C-myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665-678. 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y.-H. and Weissman I. L. (1990). A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 62, 863-874. 10.1016/0092-8674(90)90262-D [DOI] [PubMed] [Google Scholar]
- Ikuta K., Kina T., Macneil I., Uchida N., Peault B., Chien Y. H. and Weissman I. L. (1992a). Development of gamma delta T-cell subsets from fetal hematopoietic stem cells. Ann. N. Y. Acad. Sci. 651, 21-32. 10.1111/j.1749-6632.1992.tb24590.x [DOI] [PubMed] [Google Scholar]
- Ikuta K., Uchida N., Friedman J. and Weissman I. L. (1992b). Lymphocyte development from stem cells. Annu. Rev. Immunol. 10, 759-783. 10.1146/annurev.iy.10.040192.003551 [DOI] [PubMed] [Google Scholar]
- Inlay M. A., Serwold T., Mosley A., Fathman J. W., Dimov I. K., Seita J. and Weissman I. L. (2014). Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem Cell Reports 2, 457-472. 10.1016/j.stemcr.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovs A., Rybtsov S., Welch L., Anderson R. A., Turner M. L. and Medvinsky A. (2011). Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 208, 2417-2427. 10.1084/jem.20111688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovs A., Rybtsov S., Ng E. S., Stanley E. G., Elefanty A. G. and Medvinsky A. (2017). Human haematopoietic stem cell development: from the embryo to the dish. Development 144, 2323-2337. 10.1242/dev.134866 [DOI] [PubMed] [Google Scholar]
- Kantor A. B. and Herzenberg L. A. (1993). Origin of murine B cell lineages. Annu. Rev. Immunol. 11, 501-538. 10.1146/annurev.iy.11.040193.002441 [DOI] [PubMed] [Google Scholar]
- Kantor A. B., Stall A. M., Adams S. and Herzenberg L. A. (1992). Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. USA 89, 3320-3324. 10.1073/pnas.89.8.3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A. B., Merrill C. E., Mackenzie J. D., Herzenberg L. A. and Hillson J. L. (1995). Development of the antibody repertoire as revealed by single-cell PCR of FACS-sorted B-cell subsets. Ann. N. Y. Acad. Sci. 764, 224-227. 10.1111/j.1749-6632.1995.tb55831.x [DOI] [PubMed] [Google Scholar]
- Kaveri S. V., Silverman G. J. and Bayry J. (2012). Natural IgM in immune equilibrium and harnessing their therapeutic potential. J. Immunol. 188, 939-945. 10.4049/jimmunol.1102107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto H., Ohmura K. and Katsura Y. (1998). Presence of progenitors restricted to T, B, or myeloid lineage, but absence of multipotent stem cells, in the murine fetal thymus. J. Immunol. 161, 3799-3802. [PubMed] [Google Scholar]
- Kennedy M., D'souza S. L., Lynch-Kattman M., Schwantz S. and Keller G. (2007). Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109, 2679-2687. 10.1182/blood-2006-09-047704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K. and Kondo M. (2006). Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc. Natl. Acad. Sci. USA 103, 17852-17857. 10.1073/pnas.0603368103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., He S., Yilmaz O. H., Kiel M. J. and Morrison S. J. (2006). Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood 108, 737-744. 10.1182/blood-2005-10-4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Shelley W. C., Seo W., Vemula S., Lin Y., Liu Y., Kapur R., Taniuchi I. and Yoshimoto M. (2014). Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proc. Natl. Acad. Sci. USA 111, 12151-12156. 10.1073/pnas.1407370111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T., Vilagos B., Tagoh H., Poliakova D. K., Schwickert T. A., Wohner M., Jaritz M., Weiss S., Taneja R., Rossner M. J. et al. (2017). Essential role for the transcription factor Bhlhe41 in regulating the development, self-renewal and BCR repertoire of B-1a cells. Nat. Immunol. 18, 442-455. 10.1038/ni.3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen T. A., Jaensson Gyllenbäck E., Zriwil A., Björklund T., Daniel J. A., Sitnicka E., Soneji S., Bryder D. and Yuan J. (2016). Cellular barcoding links B-1a B cell potential to a fetal hematopoietic stem cell state at the single-cell level. Immunity 45, 346-357. 10.1016/j.immuni.2016.07.014 [DOI] [PubMed] [Google Scholar]
- Kumano K., Chiba S., Kunisato A., Sata M., Saito T., Nakagami-Yamaguchi E., Yamaguchi T., Masuda S., Shimizu K., Takahashi T. et al. (2003). Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18, 699-711. 10.1016/S1074-7613(03)00117-1 [DOI] [PubMed] [Google Scholar]
- Kumaravelu P., Hook L., Morrison A. M., Ure J., Zhao S., Zuyev S., Ansell J. and Medvinsky A. (2002). Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 129, 4891-4899. [DOI] [PubMed] [Google Scholar]
- Kurnellas M. P., Ghosn E. E., Schartner J. M., Baker J., Rothbard J. J., Negrin R. S., Herzenberg L. A., Fathman C. G., Steinman L. and Rothbard J. B. (2015). Amyloid fibrils activate B-1a lymphocytes to ameliorate inflammatory brain disease. Proc. Natl. Acad. Sci. USA 112, 15016-15023. 10.1073/pnas.1521206112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. K., Ghorbanian Y., Wang W., Wang Y., Kim Y. J., Weissman I. L., Inlay M. A. and Mikkola H. K. A. (2016). LYVE1 Marks the divergence of yolk sac definitive hemogenic endothelium from the primitive erythroid lineage. Cell Rep. 17, 2286-2298. 10.1016/j.celrep.2016.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H.-C. and Zúñiga-Pflücker J. C. (2015). Hematopoiesis: from start to immune reconstitution potential. Stem Cell Res. Ther. 6, 52 10.1186/s13287-015-0051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. P. and Auerbach R. (1991). In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development 113, 1315-1323. [DOI] [PubMed] [Google Scholar]
- Lu L.-S. and Auerbach R. (1998). Characterization and differentiation of an early murine yolk sac-derived IL-7-independent pre-pro-B cell line. J. Immunol. 161, 1284-1291. [PubMed] [Google Scholar]
- Lu L.-S., Wang S.-J. and Auerbach R. (1996). In vitro and in vivo differentiation into B cells, T cells, and myeloid cells of primitive yolk sac hematopoietic precursor cells expanded >100-fold by coculture with a clonal yolk sac endothelial cell line. Proc. Natl. Acad. Sci. USA 93, 14782-14787. 10.1073/pnas.93.25.14782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckett W. P. (1978). Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am. J. Anat. 152, 59-97. 10.1002/aja.1001520106 [DOI] [PubMed] [Google Scholar]
- Lux C. T., Yoshimoto M., Mcgrath K., Conway S. J., Palis J. and Yoder M. C. (2008). All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 111, 3435-3438. 10.1182/blood-2007-08-107086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos M. A., Morales-Alcelay S., Godin I. E., Dieterlen-Lievre F., Copin S. G. and Gaspar M. L. (1997). Antigenic phenotype and gene expression pattern of lymphohemopoietic progenitors during early mouse ontogeny. J. Immunol. 158, 2627-2637. [PubMed] [Google Scholar]
- Martin F., Oliver A. M. and Kearney J. F. (2001). Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14, 617-629. 10.1016/S1074-7613(01)00129-7 [DOI] [PubMed] [Google Scholar]
- Masuda K., Itoi M., Amagai T., Minato N., Katsura Y. and Kawamoto H. (2005). Thymic anlage is colonized by progenitors restricted to T, NK, and dendritic cell lineages. J. Immunol. 174, 2525-2532. 10.4049/jimmunol.174.5.2525 [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Tsuji K., Hisakawa H., Xu M., Ebihara Y., Ishii T., Sugiyama D., Manabe A., Tanaka R., Ikeda Y. et al. (2001). Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood 98, 6-12. 10.1182/blood.V98.1.6 [DOI] [PubMed] [Google Scholar]
- McGrath K. E., Frame J. M., Fegan K. H., Bowen J. R., Conway S. J., Catherman S. C., Kingsley P. D., Koniski A. D. and Palis J. (2015). Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 11, 1892-1904. 10.1016/j.celrep.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams L. J. and McHeyzer-Williams M. G. (2005). Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487-513. 10.1146/annurev.immunol.23.021704.115732 [DOI] [PubMed] [Google Scholar]
- McKinney-Freeman S. L., Naveiras O., Yates F., Loewer S., Philitas M., Curran M., Park P. J. and Daley G. Q. (2009). Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood 114, 268-278. 10.1182/blood-2008-12-193888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A. and Dzierzak E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897-906. 10.1016/S0092-8674(00)80165-8 [DOI] [PubMed] [Google Scholar]
- Medvinsky A., Rybtsov S. and Taoudi S. (2011). Embryonic origin of the adult hematopoietic system: advances and questions. Development 138, 1017-1031. 10.1242/dev.040998 [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Leathers H. and Dorshkind K. (2006). Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293-301. 10.1038/ni1301 [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Li K., Fice M. and Dorshkind K. (2014). Murine B-1 B cell progenitors initiate B-acute lymphoblastic leukemia with features of high-risk disease. J. Immunol. 192, 5171-5178. 10.4049/jimmunol.1303170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A. S. and Metcalf D. (1970). Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br. J. Haematol. 18, 279-296. 10.1111/j.1365-2141.1970.tb01443.x [DOI] [PubMed] [Google Scholar]
- Morita Y., Ema H. and Nakauchi H. (2010). Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 207, 1173-1182. 10.1084/jem.20091318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A. M., Medvinsky A., Strouboulis J., Grosveld F. and Dzierzak E. (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291-301. 10.1016/1074-7613(94)90081-7 [DOI] [PubMed] [Google Scholar]
- Murakami M., Yoshioka H., Shirai T., Tsubata T. and Honjo T. (1995). Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int. Immunol. 7, 877-882. 10.1093/intimm/7.5.877 [DOI] [PubMed] [Google Scholar]
- New J. S., King R. G. and Kearney J. F. (2016). Manipulation of the glycan-specific natural antibody repertoire for immunotherapy. Immunol. Rev. 270, 32-50. 10.1111/imr.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Elsner R. A. and Baumgarth N. (2015). Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J. Immunol. 194, 1489-1502. 10.4049/jimmunol.1401880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S. (2012). Hemangioblast: an in vitro phantom. Wiley Interdiscip Rev. Dev. Biol. 1, 603-608. 10.1002/wdev.38 [DOI] [PubMed] [Google Scholar]
- Nishikawa S.-I., Nishikawa S., Kawamoto H., Yoshida H., Kizumoto M., Kataoka H. and Katsura Y. (1998). In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8, 761-769. 10.1016/S1074-7613(00)80581-6 [DOI] [PubMed] [Google Scholar]
- North T. E., de Bruijn M. F., Stacy T., Talebian L., Lind E., Robin C., Binder M., Dzierzak E. and Speck N. A. (2002). Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16, 661-672. 10.1016/S1074-7613(02)00296-0 [DOI] [PubMed] [Google Scholar]
- Nutt S. L., Hodgkin P. D., Tarlinton D. M. and Corcoran L. M. (2015). The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160-171. 10.1038/nri3795 [DOI] [PubMed] [Google Scholar]
- O'garra A., Chang R., Go N., Hastings R., Haughton G. and Howard M. (1992). Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 22, 711-717. 10.1002/eji.1830220314 [DOI] [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S. W., Grosveld G. and Downing J. R. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321-330. 10.1016/S0092-8674(00)80986-1 [DOI] [PubMed] [Google Scholar]
- Osawa M., Hanada K., Hamada H. and Nakauchi H. (1996). Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242-245. 10.1126/science.273.5272.242 [DOI] [PubMed] [Google Scholar]
- Osmond D. G. (1986). Population dynamics of bone marrow B lymphocytes. Immunol. Rev. 93, 103-124. 10.1111/j.1600-065X.1986.tb01504.x [DOI] [PubMed] [Google Scholar]
- Palis J. (2016). Hematopoietic stem cell-independent hematopoiesis: emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo. FEBS Lett. 590, 3965-3974. 10.1002/1873-3468.12459 [DOI] [PubMed] [Google Scholar]
- Palis J. and Yoder M. C. (2001). Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 29, 927-936. 10.1016/S0301-472X(01)00669-5 [DOI] [PubMed] [Google Scholar]
- Palis J., Robertson S., Kennedy M., Wall C. and Keller G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073-5084. [DOI] [PubMed] [Google Scholar]
- Papathanasiou P., Attema J. L., Karsunky H., Xu J., Smale S. T. and Weissman I. L. (2009). Evaluation of the long-term reconstituting subset of hematopoietic stem cells with CD150. Stem Cells 27, 2498-2508. 10.1002/stem.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. S., King R. G. and Kearney J. F. (2016). Pulmonary alpha-1,3-glucan-specific IgA-secreting B cells suppress the development of cockroach allergy. J. Immunol. 197, 3175-3187. 10.4049/jimmunol.1601039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W., Feyerabend T. B., Rössler J., Wang X., Postrach D., Busch K., Rode I., Klapproth K., Dietlein N., Quedenau C. et al. (2017). Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 548, 456-460. 10.1038/nature23653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch P. J., Chudnovskiy A., Robbins C. S., Weber G. F., Etzrodt M., Hilgendorf I., Tiglao E., Figueiredo J.-L., Iwamoto Y., Theurl I. et al. (2012). Innate response activator B cells protect against microbial sepsis. Science 335, 597-601. 10.1126/science.1215173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi E., Lev A., Lee Y. N., Simon A. J., Yinon Y., Lipitz S., Amariglio N., Weisz B., Notarangelo L. D. and Somech R. (2015). Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci. Transl. Med. 7, 276ra25 10.1126/scitranslmed.aaa0072 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli A. E., Wolock S. L., Weinreb C. S., Panero R., Patel S. H., Jankovic M., Sun J., Calogero R. A., Klein A. M. and Camargo F. D. (2018). Clonal analysis of lineage fate in native haematopoiesis. Nature 553, 212-216. 10.1038/nature25168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Bystry V., Bohn G., Goudevenou K., Reigl T., Papaioannou M., Krejci A., O'byrne S., Chaidos A., Grioni A. et al. (2017). High resolution IgH repertoire analysis reveals fetal liver as the likely origin of life-long, innate B lymphopoiesis in humans. Clin. Immunol. 183, 8-16. 10.1016/j.clim.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Sobiesiak M., Taoudi S., Souilhol C., Senserrich J., Liakhovitskaia A., Ivanovs A., Frampton J., Zhao S. and Medvinsky A. (2011). Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med. 208, 1305-1315. 10.1084/jem.20102419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Batsivari A., Bilotkach K., Paruzina D., Senserrich J., Nerushev O. and Medvinsky A. (2014). Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Reports 3, 489-501. 10.1016/j.stemcr.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Ivanovs A., Zhao S. and Medvinsky A. (2016). Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 143, 1284-1289. 10.1242/dev.131193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov I. M., Samokhvalova N. I. and Nishikawa S. (2007). Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446, 1056-1061. 10.1038/nature05725 [DOI] [PubMed] [Google Scholar]
- Sawai C. M., Babovic S., Upadhaya S., Knapp D., Lavin Y., Lau C. M., Goloborodko A., Feng J., Fujisaki J., Ding L. et al. (2016). Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity 45, 597-609. 10.1016/j.immuni.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawen P., Eldeeb M., Erlandsson E., Kristiansen T. A., Laterza C., Kokaia Z., Karlsson G., Yuan J., Soneji S., Mandal P. K. et al. (2018). Murine HSCs contribute actively to native hematopoiesis but with reduced differentiation capacity upon aging. Elife 7, e41258 10.7554/eLife.41258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W. Jr and Wang J. Y. (1990). Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc. Natl. Acad. Sci. USA 87, 6146-6150. 10.1073/pnas.87.16.6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W. Jr, Hillson J. L. and Perlmutter R. M. (1987). Early restriction of the human antibody repertoire. Science 238, 791-793. 10.1126/science.3118465 [DOI] [PubMed] [Google Scholar]
- Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S. E., Pollard J. W. et al. (2012). A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86-90. 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- Shaw P. X., Hörkkö S., Chang M. K., Curtiss L. K., Palinski W., Silverman G. J. and Witztum J. L. (2000). Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731-1740. 10.1172/JCI8472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y., Mizoguchi E., Sugimoto K., Kibe R., Benno Y., Mizoguchi A. and Bhan A. K. (2008). Regulatory role of B-1 B cells in chronic colitis. Int. Immunol. 20, 729-737. 10.1093/intimm/dxn031 [DOI] [PubMed] [Google Scholar]
- Smith-Berdan S., Gille D., Weissman I. L. and Christensen J. L. (2007). Reversal of autoimmune disease in lupus-prone New Zealand black/New Zealand white mice by nonmyeloablative transplantation of purified allogeneic hematopoietic stem cells. Blood 110, 1370-1378. 10.1182/blood-2007-03-081497 [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S. and Weissman I. L. (1988). Purification and characterization of mouse hematopoietic stem cells. Science 241, 58-62. 10.1126/science.2898810 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Hayashi M., Kubota Y., Nagai H., Sheng G., Nishikawa S. and Samokhvalov I. M. (2012). Early ontogenic origin of the hematopoietic stem cell lineage. Proc. Natl. Acad. Sci. USA 109, 4515-4520. 10.1073/pnas.1115828109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Sanchez V., Takata N., Yokomizo T., Yamanaka Y., Kataoka H., Hoppe P. S., Schroeder T. and Nishikawa S. (2014). Circulation-independent differentiation pathway from extraembryonic mesoderm toward hematopoietic stem cells via hemogenic angioblasts. Cell Rep. 8, 31-39. 10.1016/j.celrep.2014.05.055 [DOI] [PubMed] [Google Scholar]
- Tavian M., Hallais M. F. and Peault B. (1999). Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development 126, 793-803. [DOI] [PubMed] [Google Scholar]
- Tavian M., Robin C., Coulombel L. and Peault B. (2001). The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity 15, 487-495. 10.1016/S1074-7613(01)00193-5 [DOI] [PubMed] [Google Scholar]
- Thilaganathan B., Nicolaides K. H., Mansur C. A., Levinsky R. J. and Morgan G. (1993). Fetal B lymphocyte subpopulations in normal pregnancies. Fetal Diagn. Ther. 8, 15-21. 10.1159/000263742 [DOI] [PubMed] [Google Scholar]
- Tian Y., Xu J., Feng S., He S., Zhao S., Zhu L., Jin W., Dai Y., Luo L., Qu J. Y. et al. (2017). The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J. Exp. Med. 214, 3347-3360. 10.1084/jem.20170488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober J., Yzaguirre A. D., Piwarzyk E. and Speck N. A. (2013). Distinct temporal requirements for Runx1 in hematopoietic progenitors and stem cells. Development 140, 3765-3776. 10.1242/dev.094961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H. and Weissman I. L. (2006). Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev. Cell 11, 519-533. 10.1016/j.devcel.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Vodyanik M. A., Thomson J. A. and Slukvin I. I. (2006). Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 108, 2095-2105. 10.1182/blood-2006-02-003327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik M. A., Yu J., Zhang X., Tian S., Stewart R., Thomson J. A. and Slukvin I. I. (2010). A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7, 718-729. 10.1016/j.stem.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlster L. and Daley G. Q. (2016). Progress towards generation of human haematopoietic stem cells. Nat. Cell Biol. 18, 1111-1117. 10.1038/ncb3419 [DOI] [PubMed] [Google Scholar]
- Weber G. F., Chousterman B. G., Hilgendorf I., Robbins C. S., Theurl I., Gerhardt L. M., Iwamoto Y., Quach T. D., Ali M., Chen J. W. et al. (2014). Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med. 211, 1243-1256. 10.1084/jem.20131471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I., Papaioannou V. and Gardner R. (1978). Fetal hematopoietic origin of the adult hematolymphoid system. In Differentiation of Normal and Neoplastic Hematopoietic Cells (ed. Clarkson B., Marks P. A. and Till J.E.), pp. 33-43. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Wu L., Parekh V. V., Hsiao J., Kitamura D. and Van Kaer L. (2014). Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance. Proc. Natl. Acad. Sci. USA 111, E4638-E4647. 10.1073/pnas.1324052111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R., Morita Y., Ooehara J., Hamanaka S., Onodera M., Rudolph K. L., Ema H. and Nakauchi H. (2013). Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112-1126. 10.1016/j.cell.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Yang Y., Ghosn E. E. B., Cole L. E., Obukhanych T. V., Sadate-Ngatchou P., Vogel S. N., Herzenberg L. A. and Herzenberg L. A. (2012a). Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proc. Natl. Acad. Sci. USA 109, 5382-5387. 10.1073/pnas.1121631109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ghosn E. E. B., Cole L. E., Obukhanych T. V., Sadate-Ngatchou P., Vogel S. N., Herzenberg L. A. and Herzenberg L. A. (2012b). Antigen-specific memory in B-1a and its relationship to natural immunity. Proc. Natl. Acad. Sci. USA 109, 5388-5393. 10.1073/pnas.1121627109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang C., Yang Q., Kantor A. B., Chu H., Ghosn E. E., Qin G., Mazmanian S. K., Han J. and Herzenberg L. A. (2015). Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife 4, e09083 10.7554/eLife.09083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenson V. and Baumgarth N. (2014). Purification and immune phenotyping of B-1 cells from body cavities of mice. Methods Mol. Biol. 1190, 17-34. 10.1007/978-1-4939-1161-5_2 [DOI] [PubMed] [Google Scholar]
- Yoder M. C., Hiatt K., Dutt P., Mukherjee P., Bodine D. M. and Orlic D. (1997). Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity 7, 335-344. 10.1016/S1074-7613(00)80355-6 [DOI] [PubMed] [Google Scholar]
- Yokota T., Huang J., Tavian M., Nagai Y., Hirose J., Zuniga-Pflucker J. C., Peault B. and Kincade P. W. (2006). Tracing the first waves of lymphopoiesis in mice. Development 133, 2041-2051. 10.1242/dev.02349 [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., Montecino-Rodriguez E., Ferkowicz M. J., Porayette P., Shelley W. C., Conway S. J., Dorshkind K. and Yoder M. C. (2011). Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc. Natl. Acad. Sci. USA 108, 1468-1473. 10.1073/pnas.1015841108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M., Porayette P., Glosson N. L., Conway S. J., Carlesso N., Cardoso A. A., Kaplan M. H. and Yoder M. C. (2012). Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 119, 5706-5714. 10.1182/blood-2011-12-397489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Nguyen C. K., Liu X., Kanellopoulou C. and Muljo S. A. (2012). Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like Lymphopoiesis. Science 335, 1195-1200. 10.1126/science.1216557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li Y.-S., Bandi S. R., Tang L., Shinton S. A., Hayakawa K. and Hardy R. R. (2015). Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J. Exp. Med. 212, 569-580. 10.1084/jem.20141510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Li X., Wang W., Zhu P., Zhou J., He W., Ding M., Xiong F., Zheng X., Li Z. et al. (2016). Tracing haematopoietic stem cell formation at single-cell resolution. Nature 533, 487-492. 10.1038/nature17997 [DOI] [PubMed] [Google Scholar]
- Zovein A. C., Hofmann J. J., Lynch M., French W. J., Turlo K. A., Yang Y., Becker M. S., Zanetta L., Dejana E., Gasson J. C. et al. (2008). Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625-636. 10.1016/j.stem.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]