Abstract
We aimed to quantify the contribution of excess mortality in HIV-exposed uninfected (HEU) infants to total mortality in HIV-uninfected infants in Botswana and South Africa in 2013. Population attributable fractions (PAFs) and excess infant deaths associated with HIV exposure in HIV-uninfected infants were estimated. Additionally, the Thembisa South African demographic model estimated the proportion of all infant mortality associated with excess mortality in HEU infants from 1990 to 2013. The PAF (lower bound; upper bound) of mortality associated with HIV exposure in HIV-uninfected infants was 16.8% (2.5; 31.2) in Botswana and 15.1% (2.2; 28.2) in South Africa. Excess infant deaths (lower bound; upper bound) associated with HIV exposure in 2013 were estimated to be 5.6 (0.5; 16.6)/1000 and 4.9 (0.6; 11.2)/1000 HIV-uninfected infants in Botswana and South Africa, respectively. In South Africa, the proportion of all infant (HIV-infected and HIV-uninfected) mortality associated with excess HEU infant mortality increased from 0.4% in 1990 to 13.8% in 2013.
Keywords: HIV-exposed uninfected, HEU, infant mortality, South Africa, Botswana
INTRODUCTION
Globally, infant acquisition of HIV in utero, intrapartum or during breastfeeding has declined substantially, while the number of pregnant and breastfeeding women living with HIV (WLHIV) has remained relatively unchanged. As a result, 1.4 million infants are born to WLHIV annually, 1.25 million being HIV-uninfected, i.e. HIV-exposed uninfected (HEU) [1]. In Botswana and South Africa, the prevalence of HIV in pregnant women is close to 25% and with remarkable reductions in perinatal and postnatal HIV transmission to infants >20% of all infants are HEU [2, 3]. HEU infants have been observed to be at greater risk for mortality than infants born to women living without HIV, i.e. HIV-unexposed uninfected (HUU) infants [4–6]. The reasons for increased mortality in HEU children are complex and multifactorial, likely resulting from a combination of universal infant risk factors, including adverse birth outcomes, suboptimal breastfeeding, maternal mortality or ill health and compromised social circumstances; and exposures unique to HEU infants, including in utero exposure to HIV and antiretroviral drugs [7–10]. Irrespective of the aetiology of increased HEU child mortality, their risk for morbidity and mortality continues to exceed that of HUU infants, despite uptake of safer breast feeding and improving maternal health and survival as WLHIV increasingly access antiretroviral therapy (ART) in high HIV-burden settings [4, 5, 11, 12].
Countries in East and Southern Africa, where more than two-thirds of HIV-infected pregnant women live, are simultaneously grappling with high maternal HIV prevalence and high infant and under-5 mortality [13, 14]. While interventions to reduce infant HIV acquisition and to rapidly identify and treat infants living with HIV have undeniably contributed to recent declines in infant mortality in these regions, there are still gains to be made through intensified efforts in this area [14–18]. There are indications that the rate of decline in overall infant mortality has begun to taper in some countries, and neonatal mortality has remained stagnant for the past decade [19]. Considering the evidence of increased risk for mortality in HEU infants and the large size of this population, attention is needed to understand how this excess mortality, through all potential causes, contributes to infant mortality at a population level. We aimed to quantify the population-level mortality associated with excess HEU infant mortality in high HIV-burden countries, using Botswana and South Africa as case studies.
METHODS
We conducted an epidemiologic exercise informed by existing published estimates to quantify the population-level impact associated with increased mortality in HEU compared with HUU infants in Botswana and South Africa. Owing to the lack of published evidence comparing HEU and HUU infant mortality in the universal maternal ART era, the evidence used to inform the calculations comes from studies conducted before the current era of universal lifelong maternal ART for all pregnant and breastfeeding WLHIV [2, 4–6, 20]. As such, the Year 2013 was chosen as the reference year for this analysis, being the most recent year before wide-scale shift in Botswana’s and South Africa’s national HIV guidelines following WHO recommendations to provide universal lifelong ART to all pregnant and breastfeeding WLHIV [21, 22]. This analysis therefore quantifies the population-level effect of excess HEU infant mortality before expansion of universal maternal ART.
We calculated the attributable fraction (AF) and population attributable fraction (PAF) for mortality in the first year of life associated with HIV exposure in HIV-uninfected infants. We estimated the excess number of infant deaths associated with HIV exposure per 1000 HIV-uninfected infants in 2013 for Botswana and South Africa. Furthermore, we modelled the change from 1990 to 2013 in the proportion of South African infant deaths associated with excess mortality in HEU infants using the Thembisa model [23]. The following calculations and assumptions were used:
Calculations
AFs and PAFs were calculated individually for Botswana and South Africa using the following formulae where RR is risk ratio for mortality in HEU infants compared with HUU infants, and PHEU is the proportion of infants who are HEU:
| [1] |
| [2] |
The excess infant mortality in HEU infants per 1000 HIV-uninfected infants (both HEU and HUU) was calculated using the following formula, where IMR is infant mortality rate, and PHIV Mort is the proportion of infant mortality that is attributable to infant HIV infection:
| [3] |
Similarly, the fraction of all infant mortality attributable to the excess mortality risk in HEU infants was calculated as:
| [4] |
Assumptions
The prevalence of infant HIV exposure was assumed to be 26% in Botswana and 23% in South Africa, based on observed antenatal HIV prevalence in Botswana and modelled antenatal HIV prevalence in South Africa, and the proportion of HIV-exposed infants that become HIV-infected by 12 months of age was assumed to be 3% in Botswana and 3.9% in South Africa [2, 24, 25]. Thus, the prevalence of HEU infants in the population was estimated to be 25.2% in Botswana and 22.1% in South Africa. Of three meta-analyses providing estimates of RR for mortality in HEU compared with HUU children, the most conservative infant-specific RR was used [1.8 (95% confidence interval, CI, 1.1; 2.8)] [4–6]. To understand the potential range of estimates of AF and PAF, these were calculated using the point estimate as well as the lower and upper bounds of the 95% CI of the RR for mortality. It is recognized that the 95% CI for the RR estimate from one of the meta-analyses did include 1 (RR 1.9; 95% CI 0.9–3.8); however, the point estimate was in keeping with the point estimates from the other two meta-analyses [6]. The most recent UNICEF estimates of the IMR and their lower and upper bounds for the year 2013 were used [13]. The proportion of infant mortality occurring in infants living with HIV was assumed to be 8% in both countries informed by a large cohort study in Botswana and the South African National Burden of Disease Study [2, 20].
To assess time trends from 1990 to 2013 in the proportion of total South African infant mortality associated with the excess mortality of HEU infants in South Africa, we used the Thembisa model, a combined South African demographic and HIV model. The HIV model is calibrated to HIV prevalence data from national antenatal clinic and household prevalence surveys [3, 26], and assumptions about perinatal and postnatal HIV transmission validated using routine data from infant HIV screening programmes [27]. Estimates of infant mortality are derived from South Africa’s vital registration system [28], and the proportion of infant deaths that are HIV-related is estimated based on assumed rates of HIV survival in HIV-infected infants and estimated rates of ART initiation [24, 29]. We assumed a constant RR between 1990 and 2013 for mortality in HEU compared with HUU infants.
As this analysis uses only published summary estimates and no individual patient data, approval from a health research ethics committee was not sought.
RESULTS
The AF (fraction of mortality in HEU infants because of their excess mortality risk associated with being born to a WLHIV) was estimated to be 44.4% (range 9.1–64.3%) (Table 1). The PAF estimates indicate that 16.8% (range 1.5–31.2%) and 15.0% (range 2.2–28.5%) of mortality in the population of all HIV-uninfected infants, including both HEU and HUU, in Botswana and South Africa, respectively, were associated with excess mortality in HEU infants.
Table 1.
Attributable and population attributable mortality fractions associated with HIV exposure in HIV-uninfected infants in Botswana and South Africa
| Botswana | South Africa | |
|---|---|---|
| AF (%): | ||
| RR = 1.1 (lower 95% CI) | 9.1 | 9.1 |
| RR = 1.8 (point estimate) | 44.4 | 44.4 |
| RR = 2.8 (upper 95% CI) | 64.3 | 64.3 |
| PAF (%): | ||
| RR = 1.1 (lower 95% CI) | 2.5 | 2.2 |
| RR = 1.8 (point estimate) | 16.8 | 15.0 |
| RR = 2.8 (upper 95% CI) | 31.2 | 28.5 |
AF, Attributable fraction; CI, confidence interval; PAF, Population attributable fraction; RR, relative risk.
Estimated excess deaths associated with HIV exposure per 1000 HIV-uninfected infants (including both HEU and HUU) were 5.6 deaths/1000 in Botswana and 4.9 deaths/1000 in South Africa (Table 2). These estimates vary, depending on the bounds of the IMR estimates and the bounds of the 95% CI for the RR, from 0.5 to 16.6 excess deaths/1000 HIV-uninfected infants in Botswana and 0.6 to 11.2 excess deaths/1000 HIV-uninfected infants in South Africa. Using the Thembisa model for South Africa, which estimates a lower IMR in 2013 than the UNICEF estimate, excess mortality associated with HIV exposure was 3.8/1000 HIV-uninfected infants.
Table 2.
Excess mortality associated with HIV exposure per 1000 HIV-uninfected infants in Botswana and South Africa in 2013
| IMR | RR = 1.1 (lower 95% CI) | RR = 1.8 (point estimate) | RR = 2.8 (upper 95% CI) |
|---|---|---|---|
| Botswana | |||
| 22.2/1000 (lower bound) | 0.5 | 3.4 | 6.4 |
| 36.4/1000 (median) | 0.8 | 5.6 | 10.5 |
| 57.7/1000 (upper bound) | 1.3 | 8.9 | 16.6 |
| South Africa | |||
| 29.1/1000 (lower bound) | 0.6 | 4.0 | 7.6 |
| 35.3/1000 (median) | 0.7 | 4.9 | 9.2 |
| 42.7/1000 (upper bound) | 0.9 | 5.9 | 11.2 |
CI, confidence interval; IMR, Infant Mortality Rate; RR, relative risk.
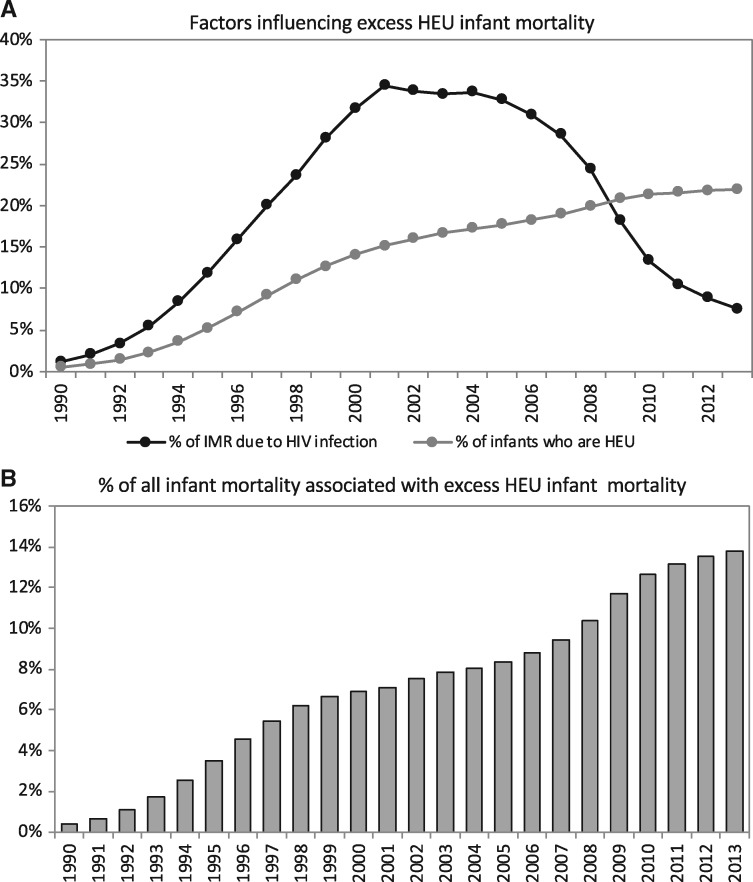
According to the Thembisa model, the fraction of infants born HEU in South Africa has increased from 0.5% in 1990 to 22.0% in 2013 (Fig. 1A—grey line). Over the same time, the proportion of infant deaths because of HIV increased from 1.2% in 1990 to a peak of 33.8% in 2002, with a decline thereafter, dropping to 7.6% in 2013 (Fig. 1A—black line). The estimated proportion of mortality in all South African infants (both HIV-infected and HIV-uninfected) associated with excess mortality in HEU infants increased from 0.4% in 1990 to 6.9% of infant mortality in 2000, as HIV prevalence in pregnant women increased (Fig. 1B). The proportion grew more slowly from 2000 to 2005 as HIV prevalence in pregnant women plateaued, reaching 8.3% in 2005. A second steep increase occurred in the late 2000s, because of sharp declines in overall infant mortality, with the proportion of infant mortality attributable to excess mortality in HEU infants reaching 13.8% by 2013 (Fig. 1B). From the year 2011, the contribution to all infant mortality of excess HEU infant mortality overtook the contribution of infant HIV infection.
Fig. 1.
Factors influencing population-level trends in excess HEU infant mortality (A) and the contribution of excess HEU infant mortality to all infant mortality (B) in South Africa, 1990–2013: estimates from the Thembisa Demographic Model.
DISCUSSION
In 2013, before expansion of universal maternal ART, at a population level, the excess mortality in HEU compared with HUU infants accounted for approximately 15% of all infant mortality in HIV-uninfected children and increased the IMR by 5 excess deaths/1000 HIV-uninfected infants. Following a dramatic decline in the contribution of infant HIV-infection to overall infant mortality, excess mortality in HEU infants in Botswana and South Africa accounted for a greater proportion of infant mortality than that because of infant HIV infection in 2013 [2, 20]. From our modelled estimates in South Africa, the contribution of excess HEU mortality to overall infant mortality has not been static. On the contrary, it has been increasing over time, and in 2013, it reached the magnitude of other leading causes of child mortality such as pneumonia and diarrhoeal disease, exceeding the contribution of infant HIV infection [28, 30].
A large individual patient data meta-analysis evaluating factors associated with mortality in >19 000 HEU children, 75% of whom were born in sub-Saharan Africa and 70% before 2005, confirmed that before universal maternal ART, ART initiated for the health of a WLHIV because of advanced HIV disease, and maternal survival and breastfeeding were of substantial survival benefit to HEU children [31]. It is hoped that universal ART, including its benefits for maternal survival, and safer breastfeeding will be sufficient to eliminate most of the excess mortality risk associated with being an HEU child. However, direct evidence at an individual and population level in the universal ART era is urgently needed to confirm this.
HEU children are not currently monitored within global child health or HIV surveillance systems. Particularly in East and Southern Africa, where 10–20% of children are HEU, a partnership between national health programmes, HEU researchers and global agencies is needed to establish ongoing systematic national, regional and global surveillance of mortality trends to ascertain changes in HEU child survival following expansion of universal ART and safer breastfeeding. Disaggregation of infant and child mortality data by HIV exposure status will allow national health programmes in high-burden HIV settings to quantify the extent to which HEU child survival is improving and the point at which it is equivalent to HUU child survival. As electronic medical records and national health monitoring systems are implemented and expand in sub-Saharan Africa under the Sustainable Development Goal framework, there is an opportunity to strengthen vital registration and mortality surveillance systems for more accurate monitoring and understanding of all child mortality irrespective of HIV exposure [32]. Furthermore, these developing platforms could be leveraged to monitor longitudinal child outcomes for all children with the added benefit of providing critical data in high HIV-burden countries by HIV exposure status. Our findings demonstrate the pressing need for disaggregation by HIV-exposure status in high HIV-burden countries, at a minimum in national monitoring of neonatal and under-5 mortality.
Our findings relate most directly to countries in Southern Africa. Owing to lower maternal HIV prevalence and higher IMRs in sub-Saharan African countries outside of Southern Africa, we would expect the excess mortality in HEU infants to account for a smaller proportion of total infant mortality in these settings. Our analysis is limited by the imprecision of the published estimates of the RR for mortality in HEU and HUU infants and the modelled estimates of IMR in Botswana and South Africa. This results in a wide range in the estimate for the PAF and excess IMRs. To overcome the limitations inherent in modelling approaches, we motivate for empirical population-based estimates and particularly investment in childhood vital registration and mortality surveillance systems.
Infant mortality, however, is only the tip of the iceberg. It is well established that children in settings with high childhood mortality experience substantial infectious, nutritional and developmental morbidity [33, 34]. The results of this analysis do not inform what the population-level consequences of infant HIV exposure could be on other morbidities. However, as we learn more about early life adversities and their impact on adolescent and adult capacity later in life, it is conceivable that the morbidity experienced by the large population of HEU infants could have social and economic implications for HIV high-burden countries like Botswana and South Africa [35].
CONCLUSION
According to current evidence, excess mortality in HEU infants contributed substantially to infant mortality in Botswana and South Africa in 2013 despite tremendous evidence of success in preventing HIV acquisition in this population. Investment in systematic data collection and monitoring systems represents a critical starting point to understanding whether universal maternal ART will be sufficient to reduce inequality in outcomes for HEU compared with HUU children in the future.
FUNDING
This work was supported by an early career development award from the National Institutes of Health (grant number 1K43TW010683 to A.L.S.).
REFERENCES
- 1. Mahy M. What Is Needed for National and Global Monitoring of HEU Child Mortality. In International AIDS Society Conference Paris, France, 2017. http://programme.ias2017.org/Programme/Session/173/ (28 May 2018, date last accessed).
- 2. Zash R, Souda S, Leidner J, et al. HIV-exposed children account for more than half of 24-month mortality in Botswana. BMC Pediatr 2016; 16: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.South African National Department of Health. 2015 National Antenatal Sentinel HIV & Syphilis Survey Report. Pretoria, South Africa, 2017. www.doh.gov.za (12 July 2018, date last accessed).
- 4. Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared to HIV-unexposed uninfected infants and children. AIDS 2016;30:2351–60. [DOI] [PubMed] [Google Scholar]
- 5. Le Roux SM, Abrams EJ, Nguyen K, et al. Clinical outcomes of HIV-exposed, HIV-uninfected children in sub-Saharan Africa. Trop Med Int Heal 2016;21:829–45. [DOI] [PubMed] [Google Scholar]
- 6. Arikawa S, Rollins N, Newell ML, et al. Mortality risk and associated factors in HIV-exposed, uninfected children. Trop Med Int Heal 2016;21:720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans C, Jones CE, Prendergast AJ.. Review HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis 2016;16:e92–107. [DOI] [PubMed] [Google Scholar]
- 8. Desmonde S, Goetghebuer T, Thorne C.. Health and survival of HIV perinatally exposed but uninfected children born to HIV-infected mothers. Curr Opin HIV AIDS 2016;11:465–76. [DOI] [PubMed] [Google Scholar]
- 9. Sherr L, Cluver LD, Betancourt TS, et al. Evidence of impact: health, psychological and social effects of adult HIV on children. AIDS 2014;28:S251–9. [DOI] [PubMed] [Google Scholar]
- 10. Slogrove AL, Goetghebuer T, Cotton MF, et al. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Front Immunol 2016;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verani JR, Groome MJ, Zar HJ, et al. Risk factors for presumed bacterial pneumonia among HIV-uninfected children hospitalized in Soweto, South Africa. Pediatr Infect Dis J 2016;35:1169–74. [DOI] [PubMed] [Google Scholar]
- 12. Slogrove AL, Esser MM, Cotton MF, et al. A Prospective cohort study of common childhood and HIV-unexposed Infants. Pediatr Infect Dis J 2017;36:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNICEF. UNICEF Data: Monitoring the Situation of Children and Women - Child Survival, 2016. https://data.unicef.org/resources/ (12 July 2018, date last accessed).
- 14.UNAIDS. UNAIDS 2017 Estimates, 2017. http://aidsinfo.unaids.org (28 May 2018, date last accessed).
- 15.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach, 2nd edn., 2016http://www.who.int/hiv/pub/arv/arv-2016/en/ (8 June 2018, date last accessed). [PubMed]
- 16.South African National Department of Health. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults, 2015. http://www.sahivsoc.org/Files/ARTGuidelines15052015.pdf (8 June 2018, date last accessed).
- 17. Kerber KJ, Lawn JE, Johnson LF, et al. South African child deaths 1990–2011: have HIV services reversed the trend enough to meet Millenium Development Goal 4? AIDS 2013;27:2637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adetunji J. Trends in under-5 mortality rates and the HIV/AIDS epidemic. Bull World Health Organ 2008;78:1200–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Dorrington R, Bradshaw D, Laubscher R, et al. Rapid Mortality Surveillance Report, 2016. http://www.mrc.ac.za/sites/default/files/files/2018-02-22/RapidMortalitySu rveillanceReport2016.pdf (28 May 2018, date last accessed).
- 20. Pillay-van Wyk V, Msemburi W, Laubscher R, et al. Mortality trends and differentials in South Africa from 1997 to 2012: second national burden of disease study. Lancet Glob Heal 2016;4:642–53. [DOI] [PubMed] [Google Scholar]
- 21.South African National Department of Health. The South African Antiretroviral Treatment Guidelines 2013, 2013. http://www.sahivsoc.org/Files/2013 ART Treatment Guidelines Final 25 March 2013 corrected.pdf (12 July 2018, date last accessed).
- 22.World Health Organisation. Consolidated Guidelines on The Use of Antiretroviral Drugs for Treating and Preventing HIV Infection, 2013, 1–251. http://www.who.int/hiv/pub/guidelines/arv2013/en/ (12 July 2018, date last accessed).
- 23. Johnson LF, Chiu C, Myer L, et al. Prospects for HIV control in South Africa: a model-based analysis. Glob Health Action 2016;9:30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson LF. Thembisa version 2.4: A model for evaluating the impact of HIV/AIDS in South Africa. Cape Town, South Africa: Centre for Infectious Disease Epidemiology and Research, University of Cape Town, 2016. [Google Scholar]
- 25. Goga AE, Dinh T, Jackson DJ, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Commun Health 2015;69:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shisana O, Rehle TM, Simbayi LC, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town, 2014. http://www.hsrc.ac.za/en/research-data/view/6871 (12 July 2018, date last accessed).
- 27. Sherman GG, Lilian RR, Bhardwaj S, et al. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. South. African Med J 2014;104:235–8. [DOI] [PubMed] [Google Scholar]
- 28. Dorrington R, Bradshaw D, Laubscher R, et al. Rapid Mortality Surveillance Report 2015. Cape Town, South Africa, 2016. http://www.mrc.ac.za/sites/default/files/files/2017-02-06/RapidMortalitySurveillanceReport2015.pdf (12 July 2018, date last accessed).
- 29. Johnson LF, Davies MA, Moultrie H, et al. The effect of early initiation of antiretroviral treatment in infants on paediatric AIDS mortality in South Africa: a model-based analysis. Pediatr Infect Dis J 2012;31:474–80. [DOI] [PubMed] [Google Scholar]
- 30. Stephen C, Child PIP, Group. MRC unit for maternal and infant health care strategies. Saving children 2012-2013 An Eighth Survey of Child Healthcare in South Africa. Pretoria, South Africa: Tshepesa Press; 2016. [Google Scholar]
- 31. Arikawa S, Rollins N, Jourdain G, et al. Contribution of maternal ART and breastfeeding to 24-month survival in HIV-exposed uninfected children: an individual pooled analysis of African and Asian studies. Clin Infect Dis 2018;66:1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development, 2015. https://sustainabledevelopment.un.org/post2015/transformingourwor ld (3 September 2018, date last accessed).
- 33. Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384: 957–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mokdad AH, Forouzanfar MH, Daoud F, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:2383–401. [DOI] [PubMed] [Google Scholar]
- 35. Richter LM, Daelmans B, Lombardi J, et al. Investing in the foundation of sustainable development: pathways to scale up for early childhood development. Lancet 2016;389:103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]