Abstract
Though human prostate cancer (PCa) heterogeneity can best be studied using multiple cell types isolated from clinical specimens, the difficulty of establishing cell lines from clinical tumors has hampered this approach. In this proof-of-concept study we established a human PCa cell line from a prostatectomy surgical specimen without the need for retroviral transduction. In a previous report, we characterized the stromal cells derived from PCa specimens. Here, we characterized the epithelial cells isolated from the same tumors. Compared to the ease of establishing prostate stromal cell lines, prostatic epithelial cell lines are challenging. From three matched pairs of normal and tumor tissues, we established one new PCa cell line, HPE-15. We confirmed the origin of HPE-15 cells by short tandem repeat (STR) microsatellite polymorphism analysis. HPE-15 cells are androgen-insensitive and express marginal androgen receptor (AR), prostate specific antigen (PSA), and prostate specific membrane antigen (PSMA) proteins. HPE-15 expresses luminal epithelial markers of E-cadherin and cytokeratin 18, basal cell markers of cytokeratin 5 and p63, and neuroendocrine marker of chromogranin A (CgA). Interestingly, HPE-15 cells showed no tumorigenicity in different strains of immune-deficient mcie, but can become tumorigenic through interaction with aggressive cancer cell types. HPE-15 cells can thus serve as an experimental model for the study of PCa progression, metastasis, and tumor cell dormancy.
Keywords: Prostate cancer, cell line, transit amplifying, cell-cell interaction
Introduction
One of the major hurdles in prostate cancer (PCa) research is the lack of representative cell lines from primary lesions to simulate clinical progression under experimental conditions 1. Currently all PCa cell lines derived from primary tumor tissues require genetic manipulation. All the available cell lines are either derived from metastatic tumors 2, 3 or from normal or malignant prostate tissues through gene transduction using retroviral vector encoding human telomerase reverse transcriptase (hTERT) 4, simian virus SV40 or human papillomavirus 18 (HPV-18) 5. With exogenous viral oncogenes supporting proliferation and survival, these cell lines are probably not suitable for investigating the natural history of PCa development and progression.
We conducted ex vivo tumor culture to establish new PCa cell lines of both mesenchymal origin and epithelial cell lineage. In a previous report on the mesenchymal compartment, we established and characterized three pairs of mesenchymal stromal cell lines from tumor and the matched “normal” zone 6. Using a direct co-culture system to simulate in vivo cancer-stromal interaction, we determined that the main effect of cancer-associated stromal cells was to preserve epithelial cancer cell vitality. We propose that in the absence of stromal cells, the survival of cancer cells is severely compromised by the loss of outside-in signaling 6. We further used cancer-stromal co-culture to demonstrate that cancer-associated stromal cells could promote PCa cell heterogeneity 7. Characterizing newly established cell lines from clinical specimens provides a unique opportunity to uncover new behavioral traits of prostate tumors, as well as their interactions with cells in the tumor microenvironment.
This report characterizes a newly established epithelial cell line from the same clinical PCa specimens used to establish the HPS-15 prostate stromal cell line 6. This new cell line, HPE-15, is unique because it can be propagated indefinitely but is non-tumorigenic. Though these cells do not form xenograft tumor in athymic mice, HPE-15 cells can be used as a tool to study the mechanism of cellular interaction and reprogramming that promotes phenotypic and behavioral transition of PCa cells from indolent to aggressive states.
Materials and Methods
Human PCa cell lines.
The LNCaP (RRID:CVCL_0395) cell line established from lymph node metastasis 8, 9 was a kind gift from the late Dr. Gary Miller (University of Colorado, Denver, CO) 10. The PC-3 (RRID:CVCL_0035) cell line, derived from a bone aspirate sample, was obtained from American Type Cell Culture (ATCC, Manassas, VA). Passage 4 of the PC-3 cells from the time of purchase were used in this study. These cells were cultured in RPMI 1640 medium (Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), penicillin (100 units/ml) and streptomycin (100 μg/ml) at 37˚C, in humidified atmospheric air supplemented with 5% CO2. Primary culture of human prostate epithelial PrEC cells were purchased from Lonza (Rockland, ME) and cultured in the recommended PrEGM medium (Lonza) for 4 passages before being used for whole cell lysate preparation. IH10 and IIG5 cells represent selected tumorigenic ARCaP (RRID:CVCL_4830) subclones established originally from the ascites of a PCa patient with widely deseminated PCa to bone and soft tissues11, 12. IH10 and IIG5 cells were cultured and maintained in T-medium 10 (Formula LS0020056DJ, Life Technologies) supplemented with 10% FBS, penicillin (100 units/ml) and streptomycin (100 μg/ml). We have reported the isolation and characterization of HPS-14 and HPS-15, matched pair of patient-derived prostate stromal cell lines 6. All human cell lines used in the study have been authenticated using STR profiling.
Culturing prostate epithelial cells from clinical PCa specimens.
Three matched pairs of clinical prostate specimens from three patients were used in this study, as reported previously 6. To establish epithelial cell lines, a similar protocol to the one for establishing stromal cell lines was used 6. Briefly, diced prostate tissue specimens were digested with Dispase II (Roche Diagnostics, Indianapolis, IN) and cultured in a thin layer of T-medium 10 for outgrowth. Cells with epithelial morphology were isolated based on weaker attachment to the plastic surface than the stromal cells, and were stored as passage 1 (p1) in vapor phase liquid nitrogen. Continued passaging was initiated by replating p1 cells at a 1 : 3 ratio. Cell detachment was achieved by treatment with trypsin (Life technologies), accutase (Sigma-Aldrich, St. Louis, MO), or citric saline (135 mM potassium chloride and 15 mM sodium citrate treatment). The method of limiting dilution was used for cloning cells of interest.
Analyses for androgen responsiveness.
Changes in cell proliferation, androgen receptor (AR) level and prostate specific antigen (PSA) production were assayed as parameters of androgen response, following our previously reported protocols 13, 14. Briefly, cells under androgen-starvation conditions for 48 hours were treated for 24 hours with the synthetic androgen analog methyltrienolone (R1881, Sigma-Aldrich). The cells were then subjected to crystal violet staining for cell proliferation 6. PSA production was determined from the culture medium by enzyme-linked immunosorbent assay (ELISA) as we reported 6.
Western blotting.
The protocol for western blotting was previously reported 14. Antibodies to human AR (sc-7305), p63 (sc-8431), chromogranin A (CgA, sc-13090), cytokeratin 5 (CK5, sc-32721), cytokeratin 18 (CK18, sc-6259), and β-actin (sc-69879) were obtained from Santa Cruz Biotechnology (sc-7305, Santa Cruz, CA). Antibodies to E-cadherin (E-cad, #5296) and FOXA2 (#8186) were from Cell Signaling Technology (Danvers, MA). The J591 monoclonal antibody to prostate specific membrane antigen (PSMA) was generously provided by Dr. Neil Bander of Cornell University.
Xenograft tumor formation assay.
Tumorigenicity of the human PCa cells was assessed by xenograft tumor formation in immune compromised mice. Male mice between the age of 4 to 8 weeks underwent bilateral subcutaneous (s.c.) or intra-tibial (i.t.) tumor cell inoculation following our reported protocols 12, 14. After recovery from anesthesia, the mice were kept for a 12-month observation period for in vivo tumor formation and metastasis assay. Mouse strains used in this study included nude mice (NCrnu/nu, NCI, Frederick, MD), SCID mice (NOD.SCID/NCr, NCI, Frederick, MD), and NSG mice (NOD scid gamma, Jackson Laboratory, Bar Harbor, ME). Matrigel has been shown to promote xenograft tumor formation 15. In some experiments, to facilitate tumor formation cells were pre-mixed with an equal volume of Matrigel (BD Biosciences, Bedford, MA) before inoculation. Protocols for the tumorigenicity and metastasis assay and bioluminescence imaging (BLI) have been reported 14, 16.
Genotyping analysis.
Authentication of isolated cell lines was based on microsatellite polymorphism through short tandem repeat (STR) analysis. For each analysis, 1 × 106 cells in 100 μl phosphate buffered saline (PBS) were spotted onto a sample collection card and submitted for STR profiling analysis by Cell Line Authentication Service in DDC Medical (Fairfield, OH) or ATCC.
Karyotype analysis.
The protocol used for chromosome preparations and staining was reported previously 16, 17. Cells at 70% confluency in fresh media were exposed to Colcemid (20 ng/ml, Sigma-Aldrich) at 37°C for 2 hours, and then to hypotonic solution (75 mM KCl) for 20 minutes at room temperature temperature and fixed with cold fixative solution of methanol and glacial acetic acid (3:1). Optimally aged slides were G-banded using trypsin solution and stained in Giemsa. Images were captured using a Nikon 80i microscope equipped with karyotyping software from Applied Spectral Imaging Inc. (ASI, Vista, CA).
Fluorescence protein and luciferase tagging.
Red fluorescence protein (RFP) tagging was performed by transfecting plasmid DNA (1 μg) of the expression vector pCDH-CMV-MSC-EF1-RFP-puro (CD516B-2, System Biosciences) following our reported protocol 6, 7, 14. HPE-15RFP, a representative clone of RFP-tagged HPE-15 cells after 2 weeks of puromycin (2 μg/ml, Life Technologies) selection and limiting dilution, was used in the study. To track xenograft tumor formation in NSG mice, HPE-15RFP cells were further tagged with luciferase protein (luc) by transfection with the MSCV Luciferase PGK-hygro plasmid (Addgene, Cambridge, MA). HPE-15RFP cells expressing luciferase, HPE-15RFP/luc were selected with 200 μg/ml hygromycin (Gemini Bio-products, West Sacramento, CA).
Co-culture with inductive PCa cells.
Our previously reported cell co-culture protocol 6, 7, 14 was used to test if HPE-15 could be induced to gain tumorigenic and metastatic potential. To determine if the cell phenotype can be switched after co-culture, we determined the optimal in vitro conditions to be 3-dimensional (3-D) spheroid co-culture. The indolent HPE-15RFP/luc cells (1 × 106) were mixed with 5 × 105 aggressive IH10 or IIG5 cells in a 10-cm Ultra-low attachment culture dish (Corning Inc., Corning, NY) in RPMI 1640 medium containing 10% FBS, penicillin (100 units/ml) and streptomycin (100 μg/ml) for 4 days. HPE-15RFP/luc cells were then isolated from the co-culture by fluorescence-acitvated cell sorting (FACS) to collect top 10% counts with the highest red fluorescnce. To amplify recovered HPE-15REF/luc cells, sorted cells (1 × 105) were plated onto a 15-cm culture dish in 25 ml culture medium containing 4 μg/ml puromycin for 14 days to purge any IH10 or IIG5 contamination at the same time. Purified HPE-15RFP/luc without visible IH10 or IIG5 contamination were used for further analyses.
Phase contrast and fluorescence microscopy.
Phase contrast images of cultured cells were documented with an Eclipse Ti inverted microscope (Nikon Instruments Inc., Melville, NY). Fluorescence imaging was obtained with an EVOSfl inverted microscope and the Eclipse Ti microscope with 488 nm laser excitation. To facilitate comparison, an identical exposure time was set for all fluorescence imaging with both green and red fluorescence 7.
Immunohistochemical staining (IHC).
Xenograft tumors were harvested and fixed in 10% neutral-buffered formalin for tissue processing and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) for histology. Our previously reported protocol was used for IHC staining 14. Primary antibodies to RFP (ABIN129578, Antibodies-online), pan-cytokeratin (pan-CK, Santa Cruz), and EpCAM (Novus Biolgicals, Littleton, CO) were used. Images were acquired by Digital Sight DS-SM camera (Nikon, Melville, NY).
Results
We used a specially formulated T-medium 10 to culture clinical prostate specimens, with the expectation of obtaining matched pairs of prostate stromal and epithelial cell lines for the study of cancer-stromal interaction 18. As previously reported, we established matched pairs of prostate stromal cell lines from three patients with confirmed localized PCa 6. In the current study we focused on the establishment and characterization of prostate epithelial cells from the same surgical specimens.
1. General features of ex vivo prostate tissue culture.
With our ex vivo culture protocol, all three matched pairs of prostate specimens produced similar types of morphologically distinctive outgrowth from diced tissues. An epithelial cell population crept out four days into the culture (Figure 1A), forming a monolayer of fast-growing, mostly cobblestone-like cells reminiscent of cell lines with known epithelial cell properties 12, 19. As the outgrowth proceeded, the monolayer became interspersed with long fiber-like cells (Figure 1B) with a neuroendocrine cell morphology 20 as defined in the culture of PrEC cells, which were considered as normal primary human prostate epithelial cells. Cells with stromal cell morphology started to appear later, around two weeks of culture 6. Taking advantage of the differences in outgrowth time and attachment strength, we obtained large numbers (≈ 4 × 107) of epithelial cells from each of the six prostate tissue specimens, three each from normal and tumor zones, for the generation of prostate stromal and prostate epithelial cells. We concluded that primary prostate epithelial cells could be expanded readily from clinical specimens.
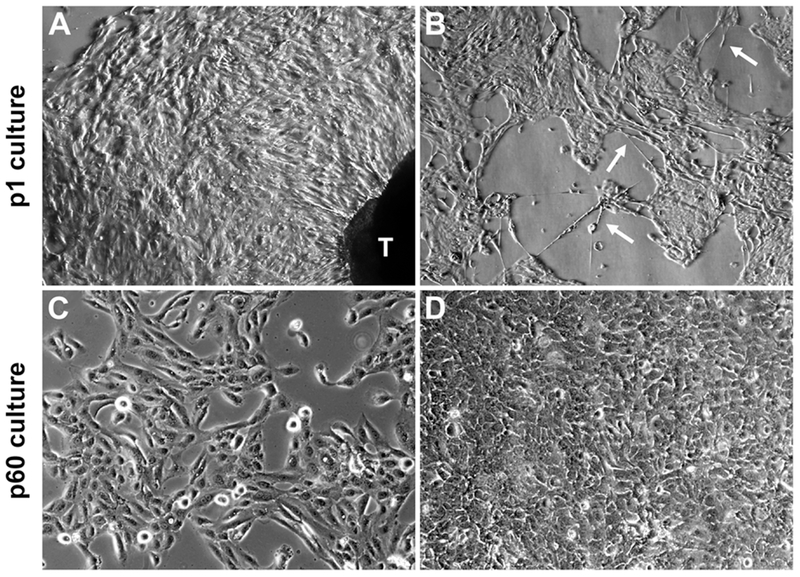
Figure 1. Epithelial cells from ex vivo tissue culture of clinical prostate tumor specimens.
Representative results illustrate the common features from ex vivo culture of three matched pairs of patient specimens. A, in primary culture (p1) outgrow of cells with epithelial morphology from prostate tissue dice (labeled as T) became obvious after 7 days of ex vivo culture (40×). B, the p1 epithelial cell culture contained at least two different cell types, cobblestone-like cells interspersed with long neuroendocrine-like cells (arrowheads, 40×). C, a few HPE-15 cells from primary culture survived continuous passaging as a cell line. Low density HPE-15 cells at passage 60 (p60) (100×). D, high density HPE-15 culture at p60 (100×).
Though it was quite easy to obtain epithelial cells from primary prostate tissue culture, continued culture of these cells was difficult, since these cells appeared unable to survive replating. Most cells that survived replating showed markedly retarded growth and ceased proliferation completely within 15 passages (Supplementary Data, Table S1). Notably, after testing many commercially formulated cell culture media including RPMI 1640, αMEM, DMEM, and K-SFM, even a combination of popular growth factors for primary cell culture in the case of PrEGM medium 21, we found no culture medium or culture conditions we have tested could reverse the tendency of growth arrest and cell death in cultured PCa epithelial cells. Our observation is in agreement with previous ex vivo culture results which concluded that prostate epithelial cells could undergo only limited numbers of cell division 22, 23. The inability to survive ex vivo seems an inherent trait of primary prostate epithelial cells.
Despite the low survival rate, certain epithelial cells from one tumor specimen did survive repeated plating in continuous culture. Derived from the same tumor specimen as the HPS-15 cancer-associated prostate stromal cells 6, this surviving epithelial cell-like population was named HPE-15. Within the primary (p1) HPE-15 cell population, while most cells would die upon replating, a few colonies always arose in secondary culture, in the middle of many surviving but senescent cells. Based on the number of colonies in secondary culture, we estimated that there could be between 6 and 15 colony-forming units among 1 × 106 cells of the primary culture. The colony-forming cells probably reflected the presence of a small fraction of cells with extraordinary growth and survival potential in the original tumor specimen.
2. HPE-15 as a newly established prostate epithelial cell line.
The colonies of HPE-15 cells in secondary culture could be propagated through replating in T-medium 10. We conducted three separate experiments to confirm that HPE-15 cells could be cultured continuously for more than 60 passages without showing any signs of senescence (Figures 1C and 1D). These cells grew slowly in the first 10 passages, and acquired a moderately accelerated rate of growth afterwards, with a doubling time of about 31 hours. In contrast, the long neuroendocrine-like cells kept their slow growth rate, and disappeared after the first 20 passages. The remaining HPE-15 cells were quite tolerant to different culture media, and after 20 passages the cells grew equally well in either RPMI 1640 or T-medium with 5% FBS. In all three experiments, HPE-15 cells grew continuously beyond 60 passages, demonstrating immortality. Though out of three pairs of prostate tissues specimens we were successful in establishing only a single epithelial cell line, HPE-15 is the first PCa cell line established spontaneously from the outgrowth of a primary PCa tissue.
3. Androgen insensitivity of the HPE-15 cell line.
Androgen-responsive PSA production is considered as a marker of prostate epithelial cells. When examining culture media for PSA production, however, we found that tumor specimens would rapidly lose PSA production in continued culture (Figure 2A). In all six ex vivo cultures, PSA concentration was dropped beyond passage 5 and became undetectable at the 10th passaging. These observations were in accord with previous findings in which prostate tissue in ex vivo culture rapidly lost PSA expression 23, 24, even under 3-dimensional microgravity culture conditions that more closely simulated in situ tumor growth 25.
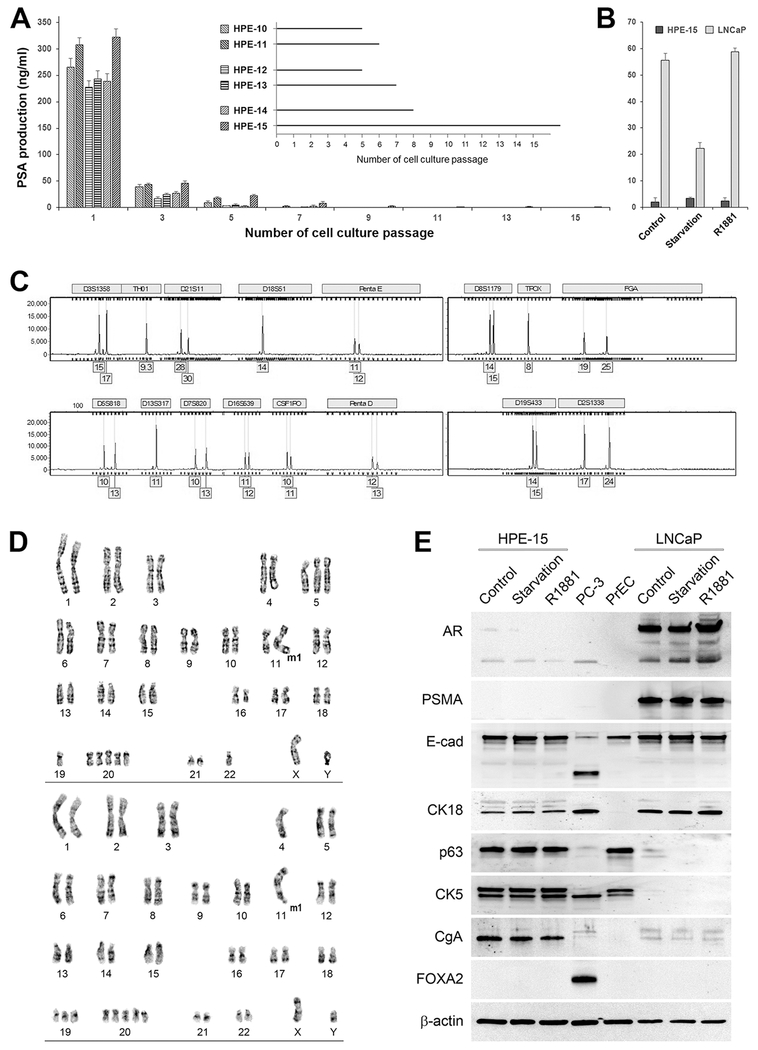
Figure 2. Characteristics of the HPE-15 cell line.
General features of HPE-15 are: A, loss of PSA production in ex vivo culture. ELISA was used to detect PSA levels in cell culture medium. Quadruplet assays were conducted for each sample. Fresh medium was used to culture each prostate tissue for 48 hours, followed by ELISA for PSA detection. Inset: passages from each surviving epithelial culture are shown. B, non-responsiveness to androgen treatment. Cells were first treated in androgen starvation medium for 48 hours, then replenished with regular medium (Control), kept in androgen-deprivation medium (Starvation), or stimulated with 5 nM R1881 in androgen-deprivation medium (R1881). After another 24 hours, culture medium from each treatment was subjected to PSA detection. C, unique STR genotype profile. An electropherogram of HPE-15 STR analysis is shown. The STR genotype was determined to be identical to pair-matched HPS-15 stromal cells (not shown), but different from any known established cell lines of human origin. Some genotype data are not shown to protect the identity of the donor. D, aneuploidy of HPE-15 cells. HPE-15 cells at passage 64 were used for analysis. Representative results from two metaphase spreads are shown. E, unique marker protein expression. HPE-15 cells at passage 34 under regular culture (Control), androgen starvation (Starvation), and androgen stimulation (R1881, 5 nM) for 24 hours were subjected to western blotting analyses. PC-3 cells were used as a positive control for FOXA2 expression. PrEC cells at passage 5 were used as a “normal” prostate epithelial cell control.
Similarly, growth and survival of HPE-15 cells were not affected by androgen. In a comparative study of HPE-15 versus LNCaP cells, we determined that while addition of androgen R1881 would generate a biphasic growth response, i.e., stimulatory at lower R1881 concentration but suppressive at 5 nM, as documented previously 9, 26, HPE-15 cells were not sensitive to androgen. After treatment under androgen-deprivation conditions, the addition of R1881 did lead to increased PSA production by LNCaP cells, but this was not observed in HPE-15 cells (Figure 2B).
In two limiting dilution studies with HPE-15 cells at the 25th and 34th passages, no colonies were formed from 384 cells in 1,152 wells or from 576 cells in 1152 wells respectively. In parallel experiments, addition of androgen (R1881, 5 nM) did not help HPE-15 cells survive limiting dilution. In the third limiting dilution study, in which 576 HPE-15 cells mixed with equal numbers of red fluorescent LNCaP RL1 cells 6 were plated to 2,304 wells, 37 red fluorescent colonies were counted (≈ 6.4%) while still no HPE-15 colonies were observed. These results indicated that HPE-15 had only a little intrinsic colony formation capability or response to extrinsic influences, a phenotypic feature not seen in other prototypical human PCa cell lines.
4. Unique features of the HPE-15 cell line.
Cell line authentication.
To exclude any possibility that HPE-15 is an artifact of cross contamination, we submitted HPE-15 cells for genotyping. STR analysis revealed that HPE-15 cells have a unique STR profile differing from any known cells of human origin (Figure 2C). Furthermore, STR analysis showed that HPE-15 shared an identical STR genotype with its matched stromal HPS-15 pair 6, confirming that these cell lines were isolated properly from the same tumor specimen.
Karyotypic features.
The potential of indefinite cell growth is often accompanied by gross chromosomal abnormalities. We conducted karyotype analysis to investigate whether HPE-15 cells harbored visible abnormalities. At passage 64, all 20 HPE-15 cells examined were hyperdiploid aneuploid, with chromosomal numbers ranging from 45 to 54 and a modal number of 48 (Figure 2D). Polysomy 20 was frequent (≈ 75%). X chromosome was present in all the cells, while Y chromosome was present in 17 of 20 cells. A marker chromosome (M1) was found in 19 of the 20 cells. Relative to the complex chromosomal rearrangements seen in commonly used PCa cell lines of LNCaP 9, DU145 27, PC-3 28, ARCaP 11 and CW22Rv1 29, HPE-15 cells seem to contain the simplest chromosomal abnormalities (Table 1), much like virally transformed RWPE-1 prostate epithelial cells 30.
Table 1.
Chromosomal numbers in HPE-15 and other common PCa cell lines.
Expression of differentiation markers.
Prostate epithelial cells are known to express different marker proteins during the process of differentiation 31. We used western blotting to determine marker protein expression. This series of analyses revealed that HPE-15 cells had complicated patterns of marker protein expression. Among the four marker proteins of prostate luminal epithelial cells, HPE-15 cells did not express detectable AR or PSMA proteins, but expressed CK18 and E-cad (Figure 2E). Meanwhile, the same cells expressed basal cell marker proteins of p63 and CK5, together with expression of the neuroendocrine marker protein CgA. Importantly, none of the marker proteins expressed in HPE-15 cells were affected by androgen, because similar levels of marker protein expression were observed under regular culture conditions, during androgen starvation, and upon androgen stimulation (Figure 2E). The results from these analyses suggest that the HPE-15 cell line is a unique cell type which, under normal conditions, is probably present in a transitional stage from basal- to luminal-epithelial cell differentiation 32.
5. Lack of xenograft tumorigenicity in the HPE-15 cell line.
As an epithelial cell line isolated from a clinical prostate tumor and with genomic abnormality, HPE-15 was expected to harbor tumorigenic potential. Surprisingly, though HPE-15 was derived from a clinical specimen of primary PCa, no tumorigenicity was detected through repeated xenograft tumor formation assays (Table 2). Three strains of immunocompromised mice were used to investigate the incidence of tumor formation, though immune compromised, NCrnu/nu and SCID mice might have remnant immune capacity 33–35. The NCrnu/nu mouse, for instance, reserves a certain capacity for antibody production and even T cell function. The fact that HPE-15 cells could not form tumors in any of the tested mouse strains suggests that null tumor formation was an intrinsic feature of this cell line, most likely independent of the host immunity.
Table 2.
Null tumorigenicity in HPE-15 cellsa as determined by xenograft tumor formation.
| Subject mouse strains | Group size | Route of inoculation | Cell numbers per site | Total number of sites | Matrigel co-injectionb | Number of tumors formedc |
|---|---|---|---|---|---|---|
| NCrnu/nu | 5 | s.c. | 2 × 106 | 10 | 0 | |
| 5 | s.c. | 5 × 106 | 10 | 0 | ||
| 6 | i.f | 2 × 106 | 12 | 0 | ||
| SCID | 5 | s.c. | 2 × 106 | 10 | Yes | 0 |
| 5 | s.c. | 5 × 106 | 10 | Yes | 0 | |
| 5 | s.c. | 2 × 107 | 10 | Yes | 0 | |
| NSG | 5 | s.c. | 2 × 106 | 10 | Yes | 0 |
| 5 | s.c. | 5 × 106 | 10 | Yes | 0 |
Cells between passages 35 and 45 were used in the assay.
Cells pre-mixed with 50% Matrigel were used in the inoculation.
Xenograft tumor formation was determined 12 months after the inoculation.
6. HPE-15 cell xenograft tumor formation following interaction with PCa cells.
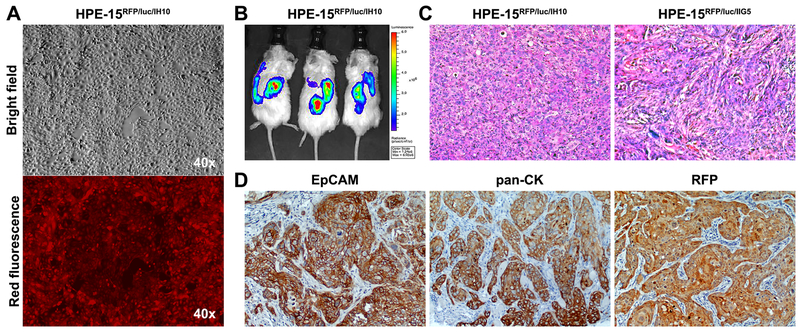
Though HPE-15 cells were non-tumorigenic, we found that these cells were highly susceptible to external influences via interaction with other cancer cells under 3-D spheroid co-culture conditions. For instance, a 4-day co-culture with aggressive IH10 and IIG5 cells, sublines of the ARCaP PCa cells rendered HPE-15 cells tumorigenic in mice. Using our reported xenograft tumor formation assay protocol 11, 12, we determined that both sublines were highly tumorigenic by themselves, each forming palpable s.c tumors within 2 weeks of inoculation, yielding 100% tumor formation in 8 weeks (n = 9). To track the growth of xenograft tumors, dual RFP- and luc-tagged HPE-15 cells of the HPE-15RFP/luc clone were co-cultured with either IH10 or IIG5 PCa cells (Table 3 and Figure 3). After 4 days in 3-D co-culture with IH10 or IIG5 cells, HPE-15RFP/luc cells were isolated by FACS, and were purified and amplified by 2-weeks of high-dose puromycin selection (Figure 3A). HPE-15RFP/luc cells after the co-culture became highly clonogenic in colony formation assays with rate of colony formation of 43.2% and 30.7%, respectively. Importantly, HPE-15RFP/luc cells from the co-culture became capable of forming s.c tumors in NSG mice (Figure 3B). The tumors became palpable at 2 weeks after inoculation, and reached 1 cm in the largest dimension in 2 months, when the assay was terminated. In these experiments, purity of HPE-15RFP/luc cells from IH10 or IIG5 contamination was validated by STR analyses (Figure 2C and Table 4). Though an extra D8S1179 allele was detected in the HPE-15RFP/luc cells after 3-D co-culture with IH10 cells, it could be a result of the call-cell interaction rather than a contamination, because the same alleles was absent from IH10 cells. The xenograft tumors displayed glandular structure (Figure 3C) with expression of pan-cytokeratin (CK) and EpCAM, together with RFP indicative of the HPE-15 origin of the tumor cells (Figure 3D). Intriguingly, HPE-15RFP/luc cells recovered from xenograft tumors through ex vivo culture were found to have a karyotype similar to the parental HPE-15 cells as shown in Figure 2D, probably indicating that the malignant transition was mainly an epigenetic event. Though the underlying molecular and cellular mechanism has yet to be elucidated, these findings support our previous finding that a cell can acquire tumorigenicity from interactions with other cells in the tumor microenvironment 36.
Table 3.
Acquired tumorigenicity of RHPE-15 derivative cells from 3-D co-culture.
| Cells | Source of the HPE-15 cells | Rate of xenograft tumor formation* |
|---|---|---|
| HPE-15RFP/luc/con | 3-D mono-culture of HPE-15RFP/luc cells | 0/10 (0%) |
| HPE-15RFP/luc/IH10 | 3-D co-culture of HPE-15RFP/luc with IH10 cells | 10/10 (100%) |
| HPE-15RFP/lucIIG5 | 3-D co-culture of HPE-15RFP/luc with IIG5 cells | 8/8 (100%) |
Results were obtained 6 months after s.c inoculation in NSG mice.
Figure 3. Acquisition of tumorigenicity through interaction with highly tumorigenic PCa cells.
HPE-15RFP/luc cells, recovered from 3-D co-culture with two aggressive PCa cells, IH10 or IIG5, were found to have acquired tumorigenic potential by s.c xenograft tumor formation. A, photograph of HPE-15RFP/luc cells isolated from 3-D co-culture with IH10 cells and amplified under puromycin selection conditions (HPE-15RFP/luc/IH10). No contaminating IH10 cells were seen since all the cells were red fluorescent. B, representative BLI results of NSG mice bearing s.c HPE-15RFP/luc/IH10 tumors. C, representative H&E staining of s.c xenograft tumors of HPE-15RFP/luc/IH10 and HPE-15RFP/luc/IIG5 cells isolated from 3-D co-culture with metastatic IH10 or IIG5 cells in mice (200×). D, representative IHC images of HPE-15RFP/luc/IH10 tumors, which expressed pan-CK and EpCAM epithelial markers. Strong RFP staining confirmed the origin of the tumor formation to be from HPE-15RFP/luc/IH10 cells (100×).
Table 4.
STR profiles of major cell preparations used in the study.
| HPE-15 related stromal cells |
HPE-15 derivative clones |
ARCaP derivative clones |
|||||
|---|---|---|---|---|---|---|---|
| STR locusa | HPS-14 | HPS-15 | HPE-15b | HPE-15RFP/luc/IH10 inoculatedc | HPE-15RFP/luc/IH10 recoveredd | IH10 | IIG5 |
| D5S818 | 10, 13 | 10, 13 | 10, 13 | 10, 13 | 10, 13 | 10, 11 | 10, 11 |
| D13S317 | 11 | 11 | 11 | 11 | 11 | 11 | 11 |
| D7S820 | 10, 13 | 10, 13 | 10, 13 | 10, 13 | 10, 13 | 10, 11 | 10, 11 |
| D16S539 | 11, 12 | 11, 12 | 11, 12 | 11, 12 | 11, 12 | 11 | 11 |
| vWA | 14, 17 | 14, 17 | 14, 17 | 14, 17 | 14, 17 | 18, 19 | 18, 19 |
| THO1 | 9.3 | 9.3 | 9.3 | 9.3 | 9.3 | 8, 9.3 | 8, 9.3 |
| TPOX | 8 | 8 | 8 | 8 | 8 | 8, 10 | 8, 10 |
| CSF1PO | 10, 11 | 10, 11 | 10, 11 | 10, 11 | 10, 11 | 10, 13 | 10, 13 |
| Amelogenin | X, Y | X, Y | X | X | X | X, Y | X, Y |
Not all the results from 18 loci were shown to protect patient information. Omission of two alleles does not affect the study conclusion.
A representative STR microsatellite electrophoretic profile of HPE-15 cells is shown in Figure 2C.
HPE-15RFP/luc cells isolated from 3-D co-culture with cells of the ARCaP IH10 clone and amplified to be inoculated for xenograft tumor formation (Figure 3A).
HPE-15RFP/luc cells recovered from ex vivo culture of the s.c mouse xenograft tumor.
Discussion
After isolation and characterization of a panel of human prostate mesenchymal stromal cell lines 6, we established matched pairs of PCa epithelial cell lines from the same tumor specimens. These specimens were from patients whose PCa tumors were confirmed with indications for radical prostatectomy 6. This study demonstrates that, compared to mesenchymal stromal cells, PCa epithelial cell lines are much more difficult to establish (Supplementary Data, Table S1), since most of these cells become senescent in continuous passaging and die following prolonged growth arrest. These observations agree with previous failures in establishing PCa cell lines, as virtually no cell line has been established directly from freshly harvested PCa tumor tissues without the use of viral gene transduction. Though the biological function of prostate epithelial cells is maintained by androgen stimulation, addition of androgen in ex vivo tumor culture has not facilitated isolation and establishment of PCa cell lines. The cause of this failure remains unresolved.
The difficulty with prostate epithelial cells ex vivo could be attributed to several reasons. In our prostate tissue culture, we observed drastic death of primary epithelial cells at the first passage. A majority (≥ 85%) of primary epithelial cells, from a full monolayer with high vitality, would undergo apoptotic cell death immediately after the first passaging (data not shown). We tested several mild detachment agents, such as diluted trypsin (0.0025%), dispase II and accutase (1 : 10) and citric saline, and found that none could help the cells avoid widespread post-passaging death. These observations indicate that death is not caused by harsh treatment of cells during detachment, but rather that the primary epithelial cells per se are sensitive to any standard forms of monolayer disintegration.
Detachment-induced apoptosis, or anoikis, is a biological mechanism for maintaining structural integrity 37, 38. Both cell-cell adhesion and cell-extracellular matrix attachment are supported by surface protein-mediated intercellular signaling. The dimeric E-cad protein, for example, binds to another E-cad dimer on an apposed cell in a Ca++-dependent and homophilic manner, while heterodimeric integrins bind to specific extracellular matrix epitopes. Importantly, the subcellular region of the surface adhesion proteins is linked to mechanisms of cell growth, survival and differentiation 39. It is probable that abrupt detachment during replating nullifies outside-in survival signaling. Elucidation of the adhesion-dependent survival mechanism may help establish PCa cell lines from prostate specimens, and may also help better understand PCa development and progression.
It is not clear why only one of the tumor specimens yielded the established HPE-15 cell line, although the same ex vivo culture protocol was applied to all the prostate specimens. HPE-15 was derived from the tumor of a late life Caucasian with a 4 + 4 Gleason score at diagnosis, without detectable extraprostatic metastasis. We speculate that the success culturing HPE-15 cells was particular to the intrinsic properties of the tumor specimen in which HPS-15 was shown to be one of the most effective stromal fibroblasts tested for sustaining the survival of PCa cells in an experimental co-culture model 6. Unlike the other five specimens, this specimen could also have contained certain cells with stronger survival potential and robust proliferation capability suitable for ex vivo culture conditions. This postulation is supported by the fact that only a small fraction of cells could survive initial replating to form colonies in secondary culture.
Though the established HPE-15 cells share morphologic features with PrEC prostate epithelial cells (Figure 1), HPE-15 has unlimited growth potential while PrEC cells have only limited proliferation. HPE-15 shares with PrEC cells a minimal PSA level and androgen insensitivity when cultured in vitro. In fact, we identified a rapid loss of PSA expression in all prostate epithelial specimens during ex vivo culture (Figure 2). It seems that with the exception of LNCaP cells, prostate epithelial cells tend to lose PSA expression and androgen responsiveness in ex vivo culture. With the availability of a homotypic HPS-15 stromal cell line, it would prove to be of interest to investigate if prostate stromal cells can activate AR and PSA in vitro and in vivo if recombined or co-cultured with HPE-15 cells.
In the initial characterization of this newly established cell line, HPE-15 was determined to share a unique STR profile with its matched stromal cell line (Figure 2C), confirming that HPE-15 represents a new prostate epithelial cell line. Though these cells are aneuploid with visible chromosomal re-arrangement (Figure 2D) and indefinite proliferation potential, HPE-15 is non-tumorigenic as evaluated in experimental mice with different degrees of immune compromise (Table 2). In future studies, orthotopically inoculating HPE-15 cells directly to the prostate gland of athymic mice could determine whether tumorigenicity in this cell line depends on the prostatic stromal microenvironment of the host. It is, however, not uncommon that a tumor-derived cell line is non-tumorigenic in experimental mice. LNCaP cells, for example, are non-tumorigenic, though this cell line was derived from PCa lymph node metastasis 8, 9.
To assess the pathological relevance of the HPE-15 cell line, we compared its marker protein expression to PrEC, LNCaP, and PC-3 cells (Figure 2E). HPE-15 cells may represent a unique cell type in the transit amplifying stage from basal cell to luminal epithelial cell differentiation 40, 41. Though controversial, epithelial cells at this stage could contribute to PCa oncogenesis and development 42. With this possibility in mind, we investigated especially whether HPE-15 cells could be induced to become tumorigenic cells through cellular interaction instead of using genomic manipulation with exogenous genes. We demonstrated that HPE-15 cells acquired aggressive phenotype and tumorigenicity simply through in vitro 3-D co-culture with aggressive tumorigenic PCa cells (Table 3 and Figure 3). In this regard, HPE-15 cells were found to be particularly susceptible to induction by other cancer cells through direct cell-cell contact. Because initial HPE-15 lacks any tumorigenicity, this cell line should be a promising model for studying the role of cellular interaction and intercellular programming in the co-evolution observed in PCa tumors 43, 44. Further studies are ongoing in our laboratory focusing on the mechanisms of induction and phenotypic transition of PCa cells from indolent to aggressive behaviors.
In conclusion, we isolated and established a new PCa cell line, HPE-15, directly from clinical prostate tumor specimens. Both genomic and gene expression results show that this cell line uniquely represents a new cell type of the prostate epithelia. As these cells are highly susceptible to modulation by exogenous inductive cues, HPE-15 will be an excellent experimental model for PCa oncogenesis and anti-tumor treatment. This cell line may also prove valuable in defining the underlying molecular mechanisms accounting for the transition of prostate basal cells to luminal epithelial cells during prostate carcinogenesis.
Supplementary Material
Novelty and Impact:
This article describes the establishment and characterization of a epithelium-like cell line from a prostatectomy specimen without exogenous gene transfer. Not in itself tumorigenic, this new cell line can become highly tumorigenic via cell-cell interaction with aggressive prostate cancer cells. This newly established cell line is thus an ideal model for investigating the mechanism of prostate cancer progression and metastasis.
Acknowledgements
We thank Dr. Zhihui Xie, who contributed to the initial culture of HPE-15. We thank Dr. Neil Bander of Cornell University for providing the J591 monoclonal antibody to PSMA for this study. This work was supported in part by NIH-P01-CA098912 (LWKC), the Cedars-Sinai Endowed Cancer Research Chair (LWKC), NIH-R21-CA112330 (RXW), and DoD-PC040578 (RXW) and DoD PC170916 (CYC).
Abbreviations:
- AR
androgen receptor
- CgA
chromogranin A
- CK
cytokeratin
- CO2
carbon dioxide
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin stain
- IHC
immunohistochemical staining
- luc
luciferase
- PBS
phosphate buffered saline
- PCa
prostate cancer
- PSA
prostate specific antigen
- PSMA
prostate specifie membrane antigen
- RFP
red fluorescence protein
- STR
short tandem repeat
References
- 1.Rhim JS. Research into molecular and genetic mechanisms underlying prostate carcinogenesis would be greatly advanced by in vitro prostate cell models. Drugs Today (Barc) 2003;39: 837–47. [DOI] [PubMed] [Google Scholar]
- 2.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. J Urol 2005;173: 342–59. [DOI] [PubMed] [Google Scholar]
- 3.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 2. J Urol 2005;173: 360–72. [DOI] [PubMed] [Google Scholar]
- 4.Kogan I, Goldfinger N, Milyavsky M, Cohen M, Shats I, Dobler G, Klocker H, Wasylyk B, Voller M, Aalders T, Schalken JA, Oren M, et al. hTERT-immortalized prostate epithelial and stromal-derived cells: an authentic in vitro model for differentiation and carcinogenesis. Cancer Res 2006;66: 3531–40. [DOI] [PubMed] [Google Scholar]
- 5.Webber MM, Bello D, Quader S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications. Part I. Cell markers and immortalized nontumorigenic cell lines. Prostate 1996;29: 386–94. [DOI] [PubMed] [Google Scholar]
- 6.Sun X, He H, Xie Z, Qian W, Zhau HE, Chung LW, Marshall FF, Wang R. Matched pairs of human prostate stromal cells display differential tropic effects on LNCaP prostate cancer cells. In Vitro Cell Dev Biol Anim 2010;46: 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Sun X, Wang CY, Hu P, Chu CY, Liu S, Zhau HE, Chung LW. Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression. PLoS One 2012;7: e42653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 1980;37: 115–32. [PubMed] [Google Scholar]
- 9.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res 1983;43: 1809–18. [PubMed] [Google Scholar]
- 10.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res 1991;51: 3753–61. [PubMed] [Google Scholar]
- 11.Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang H, Kao C, Sang QA, Pathak SJ, Chung LW. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A 1996;93: 15152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, Chung LW, Zhau HE. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate 2006;66: 1664–73. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Xu J, Saramaki O, Visakorpi T, Sutherland WM, Zhou J, Sen B, Lim SD, Mabjeesh N, Amin M, Dong JT, Petros JA, et al. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer Res 2004;64: 1589–94. [DOI] [PubMed] [Google Scholar]
- 14.Chu GC, Zhau HE, Wang R, Rogatko A, Feng X, Zayzafoon M, Liu Y, Farach-Carson MC, You S, Kim J, Freeman MR, Chung LW. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr Relat Cancer 2014;21: 311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweeney TM, Kibbey MC, Zain M, Fridman R, Kleinman HK. Basement membrane and the SIKVAV laminin-derived peptide promote tumor growth and metastases. Cancer Metastasis Rev 1991;10: 245–54. [DOI] [PubMed] [Google Scholar]
- 16.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res 1994;54: 2577–81. [PubMed] [Google Scholar]
- 17.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer 1994;57: 406–12. [DOI] [PubMed] [Google Scholar]
- 18.Chung LW, Huang WC, Sung SY, Wu D, Odero-Marah V, Nomura T, Shigemura K, Miyagi T, Seo S, Shi C, Molitierno J, Elmore J, et al. Stromal-epithelial interaction in prostate cancer progression. Clin Genitourin Cancer 2006;5: 162–70. [DOI] [PubMed] [Google Scholar]
- 19.He H, Yang X, Davidson AJ, Wu D, Marshall FF, Chung LW, Zhau HE, Wang R. Progressive epithelial to mesenchymal transitions in ARCaP E prostate cancer cells during xenograft tumor formation and metastasis. Prostate 2010;70: 518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res 2006;66: 8598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freshney RI. Culture of animal cells - A mannual of basic technique and specialized applications, sixth edition ed. Hoboken, NJ: John Wiley & Sons, Inc., 2011. [Google Scholar]
- 22.Chaproniere DM, McKeehan WL. Serial culture of single adult human prostatic epithelial cells in serum-free medium containing low calcium and a new growth factor from bovine brain. Cancer Res 1986;46: 819–24. [PubMed] [Google Scholar]
- 23.Kozlowski JM, McEvan R, Keer H, Sensibar J, Sherwood ER, Lee C, Grayhack JT, Ambini A, and Martin GR Prostate cancer and the invasive phenotype: application of new in vivo and in vitro approaches. ed. New York, NY: Alan R. Liss, Inc., 1988. [Google Scholar]
- 24.Sherwood ER, Berg LA, McEwan RN, Pasciak RM, Kozlowski JM, Lee C. Two-dimensional protein profiles of cultured stromal and epithelial cells from hyperplastic human prostate. J Cell Biochem 1989;40: 201–14. [DOI] [PubMed] [Google Scholar]
- 25.Margolis L, Hatfill S, Chuaqui R, Vocke C, Emmert-Buck M, Linehan WM, Duray PH. Long term organ culture of human prostate tissue in a NASA-designed rotating wall bioreactor. J Urol 1999;161: 290–7. [PubMed] [Google Scholar]
- 26.Song W, Khera M. Physiological normal levels of androgen inhibit proliferation of prostate cancer cells in vitro. Asian J Androl 2014;16: 864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 1978;21: 274–81. [DOI] [PubMed] [Google Scholar]
- 28.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979;17: 16–23. [PubMed] [Google Scholar]
- 29.van Bokhoven A, Caires A, Maria MD, Schulte AP, Lucia MS, Nordeen SK, Miller GJ, Varella-Garcia M. Spectral karyotype (SKY) analysis of human prostate carcinoma cell lines. Prostate 2003;57: 226–44. [DOI] [PubMed] [Google Scholar]
- 30.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 1997;18: 1215–23. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation 2001;68: 270–9. [DOI] [PubMed] [Google Scholar]
- 32.Buhler P, Wolf P, Katzenwadel A, Schultze-Seemann W, Wetterauer U, Freudenberg N, Elsasser-Beile U. Primary prostate cancer cultures are models for androgen-independent transit amplifying cells. Oncol Rep 2010;23: 465–70. [PubMed] [Google Scholar]
- 33.Radzikowski C, Rygaard J, Budzynski W, Stenvang JP, Schou M, Vangsted A, Zeuthen J. Strain- and age-dependent natural and activated in vitro cytotoxicity in athymic nude mice. APMIS 1994;102: 481–8. [PubMed] [Google Scholar]
- 34.Silobrcic V, Zietman AL, Ramsay JR, Suit HD, Sedlacek RS. Residual immunity of athymic NCr/Sed nude mice and the xenotransplantation of human tumors. Int J Cancer 1990;45: 325–33. [DOI] [PubMed] [Google Scholar]
- 35.Taghian A, Budach W, Zietman A, Freeman J, Gioioso D, Ruka W, Suit HD. Quantitative comparison between the transplantability of human and murine tumors into the subcutaneous tissue of NCr/Sed-nu/nu nude and severe combined immunodeficient mice. Cancer Res 1993;53: 5012–7. [PubMed] [Google Scholar]
- 36.Chung LW, Gleave ME, Hsieh JT, Hong SJ, Zhau HE. Reciprocal mesenchymal-epithelial interaction affecting prostate tumour growth and hormonal responsiveness. Cancer Surv 1991;11: 91–121. [PubMed] [Google Scholar]
- 37.Chiarugi P From anchorage dependent proliferation to survival: lessons from redox signalling. IUBMB Life 2008;60: 301–7. [DOI] [PubMed] [Google Scholar]
- 38.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol 2008;76: 1352–64. [DOI] [PubMed] [Google Scholar]
- 39.Horbinski C, Mojesky C, Kyprianou N. Live free or die: tales of homeless (cells) in cancer. Am J Pathol 2010;177: 1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudson DL, O’Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest 2000;80: 1243–50. [DOI] [PubMed] [Google Scholar]
- 41.Hudson DL. Epithelial stem cells in human prostate growth and disease. Prostate Cancer Prostatic Dis 2004;7: 188–94. [DOI] [PubMed] [Google Scholar]
- 42.Xin L Cells of origin for cancer: an updated view from prostate cancer. Oncogene 2013;32: 3655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, et al. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res 2008;68: 9996–10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang R, Xu J, Juliette L, Castilleja A, Love J, Sung SY, Zhau HE, Goodwin TJ, Chung LW. Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Semin Cancer Biol 2005;15: 353–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.