Abstract
Background:
Mothers must consume a nutrient-rich diet to ensure healthy neural tube development during early pregnancy. Neural tube defect risk can be reduced through folic acid fortification of grain products and taking folic acid supplements. Fortification of grain products with folic acid is not required in Bangladesh, and rates of maternal supplement use are low, in line with other countries globally. This study evaluates maternal dietary intake during pregnancy to identify possible nutritional interventions.
Methods:
A food frequency questionnaire (FFQ) assessed maternal diet during pregnancy. The primary aim compared dietary intake (calories, fat, carbohydrate, protein, fiber, vitamins, and minerals) between mothers of infants with myelomeningocele (cases) and mothers of controls. Secondary aims included (i) comparing foods consumed and (ii) evaluating if increased rice exposure correlated with increased arsenic exposure. Statistical tests used included paired t-tests, Wilcoxon signed rank tests, McNemar’s chi-square test, and linear regression.
Results:
This study included 110 matched mother-infant pairs (55 cases/55 controls). Mothers of cases and mothers of controls had similar caloric intake [median 2406 kcal/day vs. 2196 kcal/day (p=0.071)]. Mothers of cases and mothers of controls consumed less than half the daily recommended 600 µg of folate. Diets were potentially deficient in vitamins A, D, E, potassium, sodium, and iron. Steamed rice was the primary food consumed for both groups, with eggs, lentils, and milk other common dietary components. Neither group frequently consumed certain foods high in folate, such as spinach, okra, and cauliflower. Increased steamed rice intake was not associated with increased toenail arsenic concentrations.
Conclusions:
Dietary interventions should increase folate, vitamins A, D, E, potassium, sodium, and iron intake in Bangladeshi mothers. Folic acid fortification of grain products may be the only viable strategy to achieve adequate folate intake for mothers. Given the central role of rice to the Bangladeshi diet, folic acid fortification of rice may be a viable option.
Keywords: Nutrition, maternal nutrition, myelomeningocele, neural tube defects, spina bifida, epidemiology, prenatal, Bangladesh, arsenic
INTRODUCTION
Neural tube defects are debilitating birth defects characterized by high rates of mortality and lifelong disabilities in surviving children. Over the past 30 years, researchers have identified the importance of having adequate folate intake during pregnancy to reduce neural tube defect risk (Hewitt et al., 1992; Castilla et al., 2003; Yi et al., 2011; Williams et al., 2015). Currently, pregnant women are recommended to consume 600 µg/day of dietary folate equivalents, which is equal to 1 µg of food folate, 0.6 µg of folic acid from food or folic acid supplements, or 0.5 µg of folic acid on an empty stomach (Institute of Medicine, 1998; Office of Dietary Supplements, no date). Foods rich in folate include meats, beans, lentils, spinach, eggs, and lettuce (USDA, 2018).
Landmark studies include two large-scale randomized clinical trials showing the protective effect of folic acid supplementation in early pregnancy on reoccurrence and first occurrence of neural tube defects, respectively (Medical Research Council Vitamin Study Research Group, 1991; Czeizel and Dudas, 1992). In the U.S., a reduction in neural tube defect prevalence was reported between the pre- (1995–1996) and post-folic acid food fortification (1999–2011) period from 4.2 to 2.9 for anencephaly and from 6.5 to 4.0 for spina bifida per 10,000 live births (Williams et al., 2015). Food fortification in Canada reduced the incidence of more rostral, and therefore more severe, spina bifida presentations (De Wals et al., 2008). These reductions have occurred in several other countries that adopted food fortification (Castillo-Lancellotti et al., 2012), and higher rates of spina bifida occur in countries without folic acid fortification (Atta et al., 2016).
The combination of folic acid food fortification and folic acid supplement use for pregnant women is intended to ensure women have sufficient blood folate levels. In the United States, food fortification increased serum and red blood cell folate levels in post-fortification time periods for all tested groups (Yetley and Johnson, 2011). If women avoid eating fortified grains, then these potential benefits from increased folic acid intake will not be realized (Desrosiers et al., 2018). Similarly, not all pregnant women report taking folic acid supplements. Globally summaries identified supplement usage rates varying between 1% and 50% (Ray et al., 2004) and 0% and 78% (Toivonen et al., 2018). Regional differences occur in folic acid supplement use rates. For example, in North America rates varied between 32 to 51% and in Asia rates varied between 21 and 46% (Toivonen et al., 2018).
The reported rates of supplement use in Bangladesh are similar to the rates reported in countries around the world. In rural, northern Bangladesh, women who recently delivered reported taking iron and folic acid supplements at rates of 46% and 55% (Nguyen et al., 2017a). A separate, national survey in Bangladesh found 12% of women reported taking iron tablets during pregnancy for 4 or more months, and 11% reported taking tablets for 3 months (Bangladesh Bureau of Statistics, 2013). Assuming these iron tablets may have contained folic acid, these data indicate consistent, long-term supplement use rates are low. Recent analysis suggests 36 weeks of folic acid supplement use is needed to reach new, higher steady-state red blood cell folate concentrations (Crider et al, 2019).
Many nutrients other than folate are suspected of being essential to healthy neural tube development. Lower intakes of plant proteins, iron, magnesium, and niacin were all associated with increases in spina bifida risk of 2- to 5-fold in a Dutch population (Groenen et al., 2004b). In this same population, spina bifida risk was 3.5-fold higher in mothers with serum vitamin B12 values less than or equal to 185 pmol/L (Groenen et al., 2004a). In a Mexican-American cohort, higher choline and betaine intake tended to reduce neural tube defect risk. However, Lavery et al. (2014) noted the risk estimates included the null effect value of 1.0. Across the United States, findings from the National Birth Defects Prevention Study indicated higher overall diet quality scores were associated with 0.72 (0.54–0.95 95% confidence interval) lower odds of spina bifida when compared to lower diet quality scores. Diet quality scores were calculated by giving positive and negative scores to dietary components, including positive scores for vegetables, grains, folate, fruits, calcium, and iron, and negative scores for increasing percentages of fat- and sweet-derived calories. As overall diet quality increased, odds for other birth defects, such as orofacial clefts, decreased as well (Carmichael et al., 2012). Additionally, food insecurity, a broader measure of dietary intake that includes several socioeconomic factors, can be considered when evaluating birth defect risk (Carmichael et al., 2007).
Eating fortified foods or an overall high-quality diet does not eliminate all neural tube defect risk. Studies estimate that less than half of neural tube defects are explained by known factors, such as obesity, inadequate dietary folate intake, female infant sex, and not using folic acid supplements (Agopian et al., 2013). Combinations of genetic and environmental factors, along with folate’s role as a carbon donor in one-carbon metabolism, appear to be a key set of interactions that require further investigation (Copp et al., 2013). The MTHFR gene, specifically 677 (C→T) and 1298 (A→C) variants, has received attention due to its role in one-carbon metabolism as a possible explanatory variable for neural tube defects because these variants modify homocysteine (Nguyen et al., 2017b) and serum folate (Nishio et al., 2008) concentrations. However, complete absence of MTHFR in knockout mice did not directly lead to neural tube defects (Nguyen et al., 2017b).
Arsenic exposure is one environmental factor that interacts with folate and may enhance neural tube defect risk. Populations in Bangladesh may be exposed to high arsenic concentrations due to contaminated groundwater (British Geological Survey and Department of Public Health Engineering [Bangladesh], 2001). Diet could be a source of arsenic exposure as well. Rice is a primary food consumed in Bangladesh (Waid et al., 2018), and rice is known to bioaccumulate environmental arsenic (Halder et al., 2014). Multiple experimental animal model systems have documented arsenic’s ability to induce congenital malformations, including neural tube defects (Ferm and Carpenter, 1968; Hood and Bishop, 1972; Leonard and Lauwerys, 1980; Gilani and Alibhai, 1990; Machado et al., 1999; Carter et al., 2003).
Ingested arsenic compounds are methylated to less toxic forms via a one-carbon metabolism pathway that is dependent on folate for methyl group recruitment (Gamble et al., 2005a). The strongest evidence supporting the role of folate in arsenic metabolism comes from a double-blind, placebo-controlled, folic acid supplementation trial in Bangladesh. Compared to those who did not receive supplements, subjects who received folic acid-supplements had a greater proportion of urinary arsenic excreted as dimethylarsenic acid (DMA). Having a greater proportion of arsenic present as DMA, as opposed to inorganic arsenic, suggested that a greater amount of arsenic methylation occurred in the folic acid supplementation group (Gamble et al., 2006). Recent work continues to support this finding that folic acid supplementation can increase arsenic methylation (Bozack et al., 2018).
Developing effective supplement use and food fortification strategies for Bangladesh requires learning more about the types of foods currently consumed. The primary aim of our study compared the estimated dietary intake of several nutrients (calories, fat, carbohydrate, protein, fiber, vitamins [including folate], and minerals) between mothers of cases and mothers of controls using responses to food frequency questionnaires. Secondary aims included: (i) comparing food intake between mothers of cases and mothers of controls and (ii) analyzing if increased rice exposure correlated with increased arsenic exposure. The findings from this study provide implications for addressing neural tube defects in low- and middle-income countries and those countries with environmental arsenic exposure.
METHODS
Study Sample and Case Ascertainment
We used the same case-control study sample as Mazumdar et al. (2015a, 2015b) and Kancherla et al. (2017). We established a program between April and November 2013 for identifying cases of myelomeningocele in rural communities served by Dhaka Community Hospital, primarily in the Munshiganj and Pabna Districts of Bangladesh. Dhaka Community Hospital provides health care services to the communities throughout Bangladesh, many of which are high priorities for arsenic remediation by the Bangladeshi government and nongovernmental organizations. The primary aim of the original study was to investigate the association between environmental arsenic exposure during pregnancy and risk of neural tube defects (Mazumdar et al., 2015a).
As there is no active surveillance for birth defects in Bangladesh, our case ascertainment strategy relied on those who presented for clinical care or who were identified otherwise by midwives or other health officials. Eligible cases were infants who were 1 year old or younger with neural tube defects. Infants with any type of neural tube defect including anterior neural tube defects (e.g., anencephaly) were eligible for enrollment. However, our study did not capture any cases of anencephaly. Most deliveries in our study area occur at home. Infants with anencephaly are likely to die immediately after the delivery. Mothers with pregnancies affected by anencephaly were less likely to be identified and enrolled because the infant would not survive long enough to present at a clinic. A trained pediatrician with expertise in classifying neural tube defects confirmed all cases by performing a physical examination and medical record review. This examination included identifying the vertebral position of the defect and the presence of other congenital anomalies. The pediatrician collected information regarding chromosomal abnormalities when it was available.
Controls (live-born infants with no neural tube defects or other congenital anomalies) and their mothers were randomly enrolled from the pregnancy registries of Dhaka Community Hospital-affiliated clinics. Cases and controls were matched (1:1) by infant sex and age (age differences of ± 2 months). Matching by infant age was intended to provide a control for temporal aspects of the study. Assuming mothers were exposed to arsenic, matching by infant age sought to sample mothers who had exposures at similar times in the past. For example, as we assessed toenail arsenic, we sought to ensure the time since birth, and by extension the time since exposure to arsenic that could have affected the developing fetus, remained as similar as possible for each case and control matched pair. Informed consent was obtained from parents of infants. The study was approved by the Human Research Committees at Boston Children’s Hospital and Dhaka Community Hospital.
Maternal Nutrient Intake During Pregnancy
Trained interviewers administered a 39-item semi-quantitative food frequency questionnaire (FFQ) to measure a broad array of nutrient intake of mothers throughout pregnancy. Mothers were asked to retrospectively estimate their dietary intake during pregnancy. The FFQ was conducted when infants were enrolled in the study. We used an instrument that was previously developed for use in rural Bangladeshi populations and validated by comparison to 7-day food diaries (Chen et al., 2004). This instrument has been used in epidemiological studies in Bangladesh in settings similar to that of our population (Chen et al., 2006; Chen et al., 2007; Argos et al., 2010; Pierce et al., 2011; Chen et al., 2013). When participants completed the FFQ, the amount of food consumed was expressed in either cups (i.e. eggs, rice, tomato) or pieces (i.e. fish, meat, bread). Participants indicated the amount they ate per meal, the number of times per day they ate this amount, and the number of times per year they ate this meal. Another variable collected by the FFQ was the number of times per week the food was consumed. As the FFQ included data on the number of times per day and number of times per year a food was consumed, the collected week variable was not used in the presented analysis.
Environmental Arsenic Exposure
Environmental arsenic exposure was assessed using water samples from the mother’s primary drinking source during pregnancy and mother’s toenail samples (Mazumdar et al., 2015a; Mazumdar et al., 2015b; Tauheed et al., 2017). Trained field staff collected a water sample from each tubewell identified as a mother’s primary source of drinking water at the time when she became aware of her pregnancy. The mother identified the tubewell retrospectively when completing a study questionnaire. Arsenic levels from the water samples were quantified by inductively coupled plasma mass-spectrometry (ICP-MS) following the US EPA method 200.8 (Spectrum Analytical, Inc., Agawam, Massachusetts, USA) (Creed et al., 1994). The limit of detection for the water samples was 0.15 μg/L. Toenail samples were collected from the mother when the infant was presented for care. All toenails were clipped using stainless steel scissors. Collected samples were stored at room temperature until shipped to the Harvard T.H. Chan School of Public Health for analysis. Samples were processed using microwave-assisted acid digestion and analyzed for arsenic concentrations using ICP-MS following the methods of Chen et al. (1999). The average detection limit for the samples was 0.14 µg/g.
Maternal Plasma Folate
Whole blood samples were collected using ethylenediaminetetraacetic acid (EDTA) tubes and analyzed for maternal plasma folate concentrations. Blood samples were collected from the mother when the infant was enrolled in the study. After centrifuging the samples at 2,000 rpm for 12 minutes, plasma samples were aliquoted into 5 ml cryovials and stored at −20 oC. Samples were later shipped to the Vitamin Metabolism Laboratory at the Jean Mayer United States Department of Agriculture (USDA) Human Nutrition Research Center at Tufts University for analysis. The total folate of the plasma samples was assessed by microbial assay with the use of Lactobacillus casei (Kalmbach et al., 2011). Detailed methods for the assessment of the maternal plasma folate concentrations have been previously described (Mazumdar et al., 2015a; Mazumdar et al., 2015b; Tauheed et al., 2017).
Maternal Health Status
Trained interviewers asked mothers about their demographic and medical histories, including questions about family medical history, reproductive history, and medication use during pregnancy. The medication use form included questions about maternal betel nut (the fruit of the betel or areca palm tree) use during pregnancy and periconceptional folic acid use. Periconceptional folic acid use was defined as taking any folic acid supplements (with or without other nutrients) within the two months prior to awareness of the pregnancy. Betel nut is an addictive psychoactive substance that is commonly consumed among Bangladeshi people by chewing the wrapping products inside betel leaves or with tobacco (Gupta et al., 2004). Previous findings indicated betel nut chewing is associated with lower plasma folate concentrations because betel nut use may affect folate levels through oxidative degradation by generating reactive oxygen species (Gamble et al., 2005b; Nair et al., 2004; Pilsner et al., 2009). Genetic covariates related to folate metabolism, including MTHFR677 and MTHFR1298 variants, were compiled from existing single nucleotide polymorphisms analysis conducted on the study population (Mazumdar et al., 2015b). The proportions of MTHFR677 and MTHFR1298 variants were reported by group to evaluate the distribution between mothers of cases and mothers of controls.
Statistical Analysis
The study used a matched case-control design. All analyses were performed with R software, version 3.5.1 (http://www.r-project.org/), including the moments package (Komsta and Novomestky, 2015). McNemar’s Chi-squared test was used for comparing categorical variables. The responses for each count variable were summarized by case and control pairs and the McNemar Chi-squared test was calculated based on these summary tables. For continuous variables, either a paired t-test or a Wilcoxon Signed rank test was used. Ninety-five percent confidence intervals were generated for both tests. The following steps were used to determine when to use the parametric or non-parametric test. We generated 100,000 random samples of 110 observations each from a normal distribution. After calculating the skewness and kurtosis values for these 100,000 random samples, we plotted their skewness and kurtosis values (Supplemental Figure 1). For all continuous variables in Tables 1, 2, and 3, we calculated the skewness and kurtosis values and added these points to Supplemental Figure 1. The skewness and kurtosis values were calculated based on all observations for each variable, not split into case and control group. We used a decision criteria of skewness values greater than 2 as the cut-off for evaluating a sample using a two-sample t-test (skewness < 2) or Wilcoxon Signed Rank test using a continuity correction (skewness > 2). For interested readers, mean, medians, paired t-test p-values, and Wilcoxon Signed Rank test p-values are provided for all variables for which a Wilcoxon Signed Rank test was reported in the paper (Supplemental Table 2). Reported p-values do not account for the multiple statistical tests that occurred across the nutrient or the food intake analyses. For Tables 2 and 3 in the paper, p-values meeting the criteria of q= 0.05, 0.10, and 0.15 from false discovery rate tests (Benjamini and Hochberg, 1995). are indicated. This statistical approach was applied to the following four aspects of the project.
Table 1.
Comparison of sample characteristics between matched pair cases and controls.
| Characteristics | Cases (n=55) | Controls (n=55) | p-value† |
|---|---|---|---|
| Parental Characteristics | |||
| Mother’s Age at Delivery (Years) | 24.5 | 22.3 | 0.031 |
| Father’s Age at Delivery (Years) | 32.4 | 31.5 | 0.432 |
| Periconceptional Folic Acid Use (# Yes) | 27 | 39 | 0.038¶ |
| Periconceptional Vitamin Use (# Yes) | 21 | 27 | 0.327¶ |
| Mother’s Betel Nut Use during Pregnancy (# Yes) | 6 | 1 | 0.131¶ |
| Any Medication Use during Pregnancy (# Yes) | 26 | 32 | 0.361¶ |
| Place of Birth (# Yes) | 0.161¶ | ||
| Home | 27 | 21 | |
| Birth center | 15 | 11 | |
| Hospital | 13 | 23 | |
| Reported Ultrasound During Pregnancy (# Yes) | 46 | 50 | 0.387¶ |
| Plasma Folate (ng/mL) | 4.7 | 4.7 | 0.918†† |
| Maternal MTHFR677 / MTHFR1298 variants‡ | § | ||
| CC / AA | 17 | 15 | |
| CT / AA | 6 | 5 | |
| TT / AA | 0 | 1 | |
| CC / AC | 11 | 23 | |
| CT / AC | 4 | 6 | |
| CC/ CC | 15 | 5 | |
| Water Arsenic Concentrations (μg/L) | 20.5 | 49.4 | 0.047†† |
| Toenail Arsenic Concentrations (μg/g) | 1.0 | 2.1 | 0.002†† |
| Infant Characteristics | |||
| Birth weight (kg) | 2.6 | 2.8 | 0.053 |
| Age at Enrollment (days) | 175 | 240 | 0.426‡‡ |
| Female (# Yes) | 23 | 23 | 0.999¶ |
| First Born (# Yes) | 23 | 25 | 0.823¶ |
| Toenail Arsenic Concentrations (μg/g) | 0.58 | 0.75 | 0.321 |
Reported p-value for comparison between cases and controls using a paired t-test unless indicated differently.
MTHFR variants only available for 53 of 55 cases.
Insufficient counts in multiple cells to conduct the McNemar test. Further reducing the number of categories to 4 [(1)677CC 1298AA, (2) 677CC 1298AC + 677CC 1298CC, (3) 677CT 1298 AA + 677TT + 1298 AA, (4) 677CT 1298AC] did not result in sufficient counts in cells for the McNemar test.
McNemar’s Chi-squared test used for comparison.
Wilcoxon Signed Rank test used for comparison.
Indicated p-value is for a paired t-test against the alternative of 60 days difference to test if the ± 2 month age at enrollment criteria was followed in the study.
Table 2.
Estimated mean and standard deviation for daily intake of total kilocalories, macronutrients, vitamins, and elements, from dietary sources only for mothers of cases and mothers of controls using the Food Frequency Questionnaire.
| Nutrients | Cases (n=55) | Controls (n=55) | DRI† | p-value¶ |
|---|---|---|---|---|
| Total Energy (Kcal) §§c | 2545 ± 1180 | 2218 ± 530 | ND | 0.071†† |
| Macronutrients | ||||
| Carbohydrates (g) §§a | 377 ± 267 | 292 ± 77 | 175 | 0.002†† |
| Protein (g) | 148 ± 57 | 159 ± 55 | 71 | 0.272 |
| Fat (g) | 41 ± 18 | 40 ± 16 | ND | 0.691 |
| Dietary Fiber (g) §§a | 34 ± 15 | 26 ± 8 | 28† | <0.001†† |
| Percent Carbohydrate (%) | 59 | 53 | ||
| Percent Protein (%) | 23 | 29 | ||
| Percent Fat (%) | 15 | 16 | ||
| Vitamins | ||||
| Vitamin A (µg)‡ §§b | 471 ± 211 | 406 ± 151 | 770 | 0.049 |
| Vitamin A1 (µg) §§c | 343 ± 183 | 293 ± 122 | ND | 0.065 |
| Thiamin (Vitamin B1) (mg) §§a | 1.5 ± 0.4 | 1.3 ± 0.3 | 1.4 | 0.008 |
| Riboflavin (Vitamin B2) (mg) | 1.8 ± 0.6 | 1.8 ± 0.6 | 1.4 | 0.615 |
| Niacin (Vitamin B3) (mg) §§c | 25 ± 12 | 21 ± 9 | 18 | 0.087†† |
| Vitamin B6 (mg) | 2.1 ± 0.7 | 1.9 ± 0.6 | 1.9 | 0.131 |
| Dietary Folate (Vitamin B9) (µg) §§a | 297 ± 125 | 242 ± 81 | 600 | 0.005†† |
| Vitamin D (µg) §§b | 4.8 ± 2.5 | 3.9 ± 2.0 | 15 | 0.023 |
| Vitamin C (mg) §§a | 205 ± 217 | 144 ± 110 | 85 | 0.013†† |
| Vitamin E (mg) §§b | 4.8 ± 2.0 | 4.1 ± 1.9 | 15 | 0.018†† |
| Elements | ||||
| Calcium (mg) §§a | 1017 ± 558 | 1377 ± 680 | 1000 | 0.001 |
| Copper (mg) §§a | 3.3 ± 1.1 | 2.9 ± 0.7 | 1 | 0.010†† |
| Iron (mg) §§b | 18 ± 6 | 20 ± 8 | 27 | 0.048 |
| Magnesium (mg) §§c | 488 ± 185 | 433 ± 123 | 350 | 0.100†† |
| Phosphorus (mg) | 1757 ± 557 | 1716 ± 505 | 700 | 0.687 |
| Potassium (mg) | 3135 ± 941 | 2946 ± 1128 | 4700† | 0.317†† |
| Sodium (mg) | 570 ± 186 | 530 ± 165 | 1500† | 0.247 |
| Zinc (mg) §§b | 17 ± 7 | 14 ± 5 | 11 | 0.036†† |
Dietary Reference Intakes for pregnant women aged 19–30 (Office of Dietary Supplements, no date). Values marked with † are Adequate Intakes (AI). All other values are the Recommended Dietary Allowance (RDA) and data not available is indicated as No Data (ND).
Reported as Retinol Activity Equivalent.
Percent total energy intake for carbohydrates, proteins, and fat estimated by 4 kcal/g for carbohydrates and proteins and 9 kcal/g for fat.
Reported p-value for comparison between cases and controls using a paired t-test unless indicated differently.
Reported p-value from a Wilcoxon Signed Rank test.
Meets criteria for False Discovery Rate test at q=0.05, q=0.10, and q=0.15.
Meets criteria for False Discovery Rate test at q=0.10 and q=0.15.
Meets criteria for False Discovery Rate test at q=0.15 and above.
Table 3.
Mean food intake in grams with standard deviations for mothers of cases and mothers of controls with the estimated folate intake per food item sorted in descending order for cases intake.
| Food Frequency Questionnaire Item | Cases (n=55) | Controls (n=55) | p-value† | Cases Meeting Folate Requirement (n=4) | Estimated Folate mcg in 100 g food‡ |
|---|---|---|---|---|---|
| Steam Rice | 1082 ± 1090 | 828 ± 236 | 0.107§ | 965 ± 98 | 3 |
| Eggs¶††b | 117 ± 75 | 91 ± 48 | 0.020 | 201 ± 117 | 45 |
| Lentil | 113 ± 52 | 105 ± 49 | 0.349 | 95 ± 52 | 9 |
| Milk | 113 ± 83 | 134 ± 66 | 0.171 | 194 ± 128 | 9 |
| Water Rice††a | 80 ± 110 | 26 ± 47 | 0.004§ | 150 ± 129 | 3 |
| Poultry | 76 ± 63 | 73 ± 70 | 0.811 | 86 ± 60 | 7 |
| Banana††b | 75 ± 73 | 68 ± 198 | 0.017§ | 177 ± 182 | 20 |
| Red Meat | 71 ± 83 | 69 ± 74 | 0.975§ | 98 ± 136 | 9 |
| Potato | 61 ± 29 | 60 ± 15 | 0.667§ | 56 ± 16 | 11 |
| Mango¶ | 59 ± 82 | 44 ± 64 | 0.209§ | 304 ± 107 | 71 |
| Jackfruit††a | 44 ± 91 | 8 ± 14 | 0.002§ | 149 ± 209 | 24 |
| Guava¶ | 32 ± 67 | 18 ± 25 | 0.127§ | 137 ± 198 | 49 |
| Big Fish | 31 ± 33 | 29 ± 28 | 0.744 | 23 ± 22 | 0 |
| Watermelon | 30 ± 55 | 16 ± 24 | 0.116§ | 133 ± 139 | 3 |
| Green Papaya¶ | 29 ± 32 | 22 ± 22 | 0.222 | 70 ± 56 | 58 |
| Salted Fish | 27 ± 31 | 23 ± 23 | 0.432§ | 32 ± 32 | 14 |
| Tomato††b | 24 ± 27 | 14 ± 12 | 0.021§ | 39 ± 32 | 15 |
| Small Fish††a | 21 ± 28 | 46 ± 37 | <0.001 | 1 ± 1 | 30 |
| Yam | 19 ± 24 | 22 ± 19 | 0.411 | 8 ± 9 | 23 |
| Dried Fish††a | 19 ± 43 | 33 ± 32 | 0.001§ | 1 ± 1 | 0 |
| Bean¶ | 14 ± 17 | 9 ± 6 | 0.133§ | 19 ± 16 | 96 |
| Brinjal††b | 13 ± 13 | 8 ± 8 | 0.033§ | 24 ± 9 | 34 |
| Wheat Bread | 12 ± 16 | 14 ± 14 | 0.580 | <1 ± <1 | 29 |
| Bottle Gourd | 12 ± 11 | 14 ± 17 | 0.608§ | 17 ± 11 | 6 |
| Cauliflower¶ | 12 ± 7 | 10 ± 7 | 0.162 | 14 ± 8 | 57 |
| Bitter Gourd¶ | 11 ± 15 | 6 ± 6 | 0.079§ | 40 ± 32 | 45 |
| Cabbage | 9 ± 6 | 8 ± 5 | 0.197 | 11 ± 5 | 43 |
| Pumpkin | 8 ± 8 | 8 ± 6 | 0.964 | 17 ± 16 | 16 |
| Okra¶ | 7 ± 8 | 7 ± 11 | 0.928§ | 21 ± 16 | 60 |
| Puff Rice | 6 ± 5 | 7 ± 5 | 0.241 | 2 ± 3 | 12 |
| Parwar | 5 ± 5 | 6 ± 12 | 0.506§ | 13 ± 10 | 16 |
| Snake Gourd††b | 4 ± 4 | 2 ± 4 | 0.028 | 8 ± 7 | 16 |
| Ridge Gourd | 4 ± 4 | 4 ± 6 | 0.520§ | 8 ± 7 | 7 |
| Radish | 4 ± 5 | 4 ± 6 | 0.899§ | 2 ± 2 | 25 |
| Ghosala | 4 ± 5 | 3 ± 3 | 0.818§ | 2 ± 4 | 7 |
| Sweet Potato | 3 ± 8 | 4 ± 9 | 0.188§ | 1 ± 2 | 7 |
| Spinach Stalk¶ | 1 ± 1 | 1 ± 1 | 0.838 | 2 ± 1 | 194 |
| Spinach¶ | 1 ± 1 | 1 ± 1 | 0.684§ | 1 ± 1 | 194 |
| Tea | 1 ± 1 | 1 ± 2 | 0.661§ | 2 ± 1 | 1 |
Reported p-value comparing cases and controls using a paired t-test unless indicated.
From Shaheen et al., 2013.
Reported p-value from a Wilcoxon Signed Rank test.
Food has a folate concentration in the top quartile of foods listed (>44 µg per 100 g).
Meets criteria for False Discovery Rate test at q=0.05, q=0.10, and q=0.15.
Meets criteria for False Discovery Rate test at q=0.15. No variables were in the range of meeting criteria for both q=0.10 and q=0.15 only as occurred in Table 2.
First, summary statistics were generated to compare mothers of cases and mothers of controls across several variables including age of parents at delivery, maternal periconceptional supplement and medication use, birth location, maternal genetic status of MTHFR677 and MTHFR1298 variants, arsenic exposure measures, and selected child characteristics.
Second, mean daily nutrient intake was compared between cases and controls using the FFQ across several categories including total calories (Kcal), carbohydrates (g), protein (g), fat (g), vitamins (µg), and minerals (mg). The method of analysis followed Chen et al. (2004) and utilized an updated source for nutrient data specific for Bangladesh (Shaheen et al., 2013). This nutrient table was used previously in research about Bangladesh diets (Nguyen et al., 2017a). The amount of food consumed per year was calculated by multiplying the total amount consumed per meal, the number of times per day the food was consumed, and the number of times per year the food was consumed. This value was divided by 365 to express the total food consumption on a daily basis. The amount of food expressed as either cups or pieces was converted to grams using data available from the USDA Food Composition Database (USDA, 2018). Nutrient intake was estimated by multiplying the grams of food consumed by the nutrient values from Shaheen et al. (2013). The nutrient values from Shaheen et al. (2013) were reported based on 100 g of food consumed. The mean daily intake in grams per food was divided by 100 prior to multiplying by the values in Shaheen et al. (2013). Supplemental Table 1 provides an overview for the sources of information used to convert the FFQ data. The estimated nutrient intake for cases and controls was compared to dietary reference intake values for pregnant women aged 19–30 (Office of Dietary Supplements, no date).
Third, mean daily intake in grams for all 39 foods included in the FFQ was compared between cases and controls. The values for the daily intake in grams was taken from the previous analytical steps used to convert the FFQ results into estimated nutrient intake.
Finally, the estimated daily dietary intake of rice was plotted against the maternal toenail arsenic concentration to evaluate if a trend in increasing rice consumption was associated with increased toenail arsenic. This trend was evaluated using linear regression comparing these two variables.
RESULTS
Enrollment summary
A total of 112 mother-infant pairs were enrolled, including 57 cases of myelomeningocele and 55 controls and their respective mothers, with participation rates of 98% among potential cases and 83% among potential controls (Mazumdar et al., 2015a; Mazumdar et al., 2015b). Recruitment was stopped after 55 controls due to political unrest in Bangladesh at the time that made travel in rural areas unsafe. For these analyses, the 55 mother-infant controls were matched to 55 mother-infant case pairs based on the matching criteria described above (n=110).
Descriptive Characteristics
Table 1 summarizes the characteristics of our study participants. Case mothers were 2.2 ± 2.0 (95% confidence interval) years older than controls when they delivered (p=0.031). Control mothers were more likely to use folic acid supplements than case mothers (p=0.038). Control mothers reported higher arsenic exposure through toenail samples with an estimated median toenail arsenic 0.7 µg/g higher than cases (95% confidence interval 0.3 to 1.3 µg/g, p=0.002). Control mothers had greater water arsenic exposure with an estimated median of 16 µg/L more arsenic than cases (95% confidence interval 0.1 to 37 µg/L, p=0.047).
The enrollment criteria for matching case and control infants by sex (p=0.999) and by ±2 months of age (p=0.426) were met. The results suggested control infants were 0.20 ± 0.20 kg heavier than case infants as birth (p=0.053). Plasma folate concentrations were 4.7 ng/mL for both cases and control mothers (p=0.918). These mean plasma folate concentrations are greater than the 3.0 ng/mL threshold that are commonly used to define low plasma folate (Yetley and Johnson, 2011).
Estimated Nutrient Intake For Cases and Controls
Both case and control mothers had difficulty meeting recommended dietary intakes (DRI) (Table 2). The nutrient intake status between cases and controls was similar in relation to the DRI. No case or control mother met all the DRI values through their diet. One case mother met all the DRI values except for vitamin D, vitamin E, sodium, and calcium requirements. No control mothers met the dietary folate requirement of 600 µg/day, but four case mothers met this requirement with estimated daily food folate intakes ranging from 601 µg/day to 709 µg/day.
Across the estimated daily nutrient intakes, paired t-tests or Wilcoxon signed rank tests indicated there were statistically significant (p<0.05, unadjusted for multiple comparisons) differences between cases and controls for carbohydrates, dietary fiber, vitamin A, thiamin, folate, vitamin C, vitamin E, calcium, copper, iron, and zinc (Table 2). Several additional variables were above a p-value of 0.05, but met False Discovery Rate tests at q=0.15, including total energy, Vitamin A1, niacin, and magnesium (Table 2).
However, it can be more useful to compare the dietary intakes between mothers of cases and mothers of controls in relation to the listed DRI because this comparison is useful for contextualizing the differences between cases and controls. Three outcomes are possible: (i) both case and control mothers have group means/medians at or above the DRI, (ii) below the DRI, or (iii) one group is above and one is below. For example, mothers of cases were estimated to consume a median of 46 µg/day more folate from their diets than mothers of controls (95% confidence interval 12.8 to 79.6 µg/day, p=0.005. Neither mothers of cases nor mothers of controls consumed close to the recommended 600 µg/day folate. Testing separate one-sample Wilcoxon signed rank tests against the alternative of 600 µg/day showed cases (p=2.0*10−10) and controls (p=1.1*10−10) were both below this recommended level.
Mothers of cases and mothers of controls consumed, on average, sufficient carbohydrates, protein, riboflavin, niacin, vitamin B6, vitamin C, calcium, copper, magnesium, phosphorus, and zinc intakes at levels at or above the DRI. We did not analyze these nutrients further because the FFQ estimated that, on average, mothers of cases and mothers of controls had a diet that already provided these nutrients in sufficient quantities. The nutrients for which cases and controls were both below the DRI when analyzed using either one sample t-tests or one sample Wilcoxon signed rank tests were folate (discussed above), vitamin A, vitamin D, vitamin E, iron, potassium, and sodium. All p-values for cases or controls relative to the DRI for these nutrients were at least as small as or smaller than p = 3.5*10-8. Mothers of cases and mothers of controls were estimated to have diets deficient in folate, vitamin A, vitamin D, vitamin E, iron, potassium, and sodium based on the FFQ results.
The final two nutrients in Table 2 with DRI values were fiber and thiamin. Case mothers’ consumption of fiber and thiamin was on average at or above the DRI. Control mothers’ consumption was below the DRI for fiber using a one sample Wilcoxon signed rank test (95% confidence interval 24.1 to 27.1 g, p=0.005). Control mothers were also below the DRI for thiamin using a one sample t-test (95% confidence interval 1.22 to 1.38 mg, p=0.016).
Estimated Food Intake For Cases and Controls
Rice was the primary food consumed for case and control mothers (Table 3). In addition to rice, both diets for case and control mothers included eggs, lentils, and milk as commonly consumed foods. Table 3 provides the mean daily food intakes sorted by case mother results. Across the 39 foods asked about on the FFQ, 9 foods showed a statistical trend towards cases or controls with p-values of 0.05 or less, including eggs, water rice, banana, jackfruit, tomato, small fish, dried fish, brinjal (eggplant), and snake gourd. Six of these trends were based on Wilcoxon Signed Rank tests, demonstrating the variability in food group consumption seen in this study.
Case mothers consumed higher quantities of water rice, eggs, bananas, jackfruit, tomatoes, brinjal, and snake gourds. Case mothers consumed an estimated median of 62 grams of water rice more than controls (95% confidence interval 19 to 121 grams, p=0.004). Case mothers consumed 26 ± 21 grams of eggs more than controls (p=0.020). Banana consumption by case mothers was a median of 28 grams more than control mothers (95% confidence interval 5 to 49 grams, p=0.017). Jackfruit consumption by case mothers was a median of 16 grams more than control mothers (95% confidence interval 5 to 46 grams, p=0.002). Case mothers consumed a median of 8 grams more tomatoes than control mothers (95% confidence interval 1 to 15 grams, p=0.021). Case mothers consumed a median of 4 grams more brinjal (eggplant) than control mothers (95% confidence interval 0.4 to 8 grams, p=0.033). Case mothers consumed 2 ± 1 (95% confidence interval) more grams of snake gourds than control mothers (p=0.028).
Control mothers consumed higher quantities of small fish (p<0.001) and dried fish (p=0.001). Given the 39 pairwise comparisons occurring in Table 3, a p-value of 0.001 could be used as a decision criteria, rather than 0.05, to account for these multiple comparisons (0.05/39). In this case, the only true differences between case and control mothers were related to fish consumption. Control mothers consumed 26 ± 10 (95% confidence interval) grams per day of smaller fish more than cases (p<0.001). With dried fish, control mothers consumed a median of 19 more grams than cases (95% confidence interval 9 to 28 grams, p=0.001). The pairwise comparisons were also evaluated using False Discovery Rate criteria. Small fish, dried fish, jackfruit, and water rice consumption met the False Discovery Rate criteria of q=0.05, and eggs, banana, tomato, brinjal, and snake gourd met criteria for q=0.15.
Table 3 also provides the estimated folate (µg in 100 g food) from Shaheen et al. (2013). Most of the foods consumed by case and control mothers in the highest quantities, such as rice, are not foods that have higher folate concentrations. Beans and spinach are two foods with higher folate contents, but these were not frequently consumed by either case or control mothers.
For comparison, Table 3 includes the estimated daily food intakes for the four case mothers who were estimated to consume sufficient folate through their diets. These four case mothers consumed high folate foods, such as eggs, mango, guava, green papaya, beans, bitter gourd, and okra. However, the standard deviation associated with each of these high folate foods indicates that these four mothers did not follow the same type of diet to achieve an estimated daily folate intake greater than 600 µg.
Nutritional Associations with Arsenic Exposure
As rice was the primary food consumed for both mothers of cases and mothers of controls, arsenic exposure could be occurring due to high rice consumption relative to the other foods in the FFQ (Table 3). Though mothers of cases and mothers of controls consumed similar amounts of steam rice (Table 3), mothers of controls were estimated to have higher toenail arsenic concentrations (Table 1). A main effect of increasing toenail arsenic due to increasing steamed rice consumption was not evident across mothers in the study (Figure 1). A linear regression comparing the amount of steam rice consumed per day as a predictor for toenail arsenic concentration was not significant (p=0.642). Responses tended to bunch at 7 cups per day of steamed rice. Two respondents were estimated to consume more than 10 cups of rice per day and were not included in the plot to allow for a better visual analysis of the majority of respondents. All respondents were included in the linear regression line shown in Figure 1.
Figure 1.
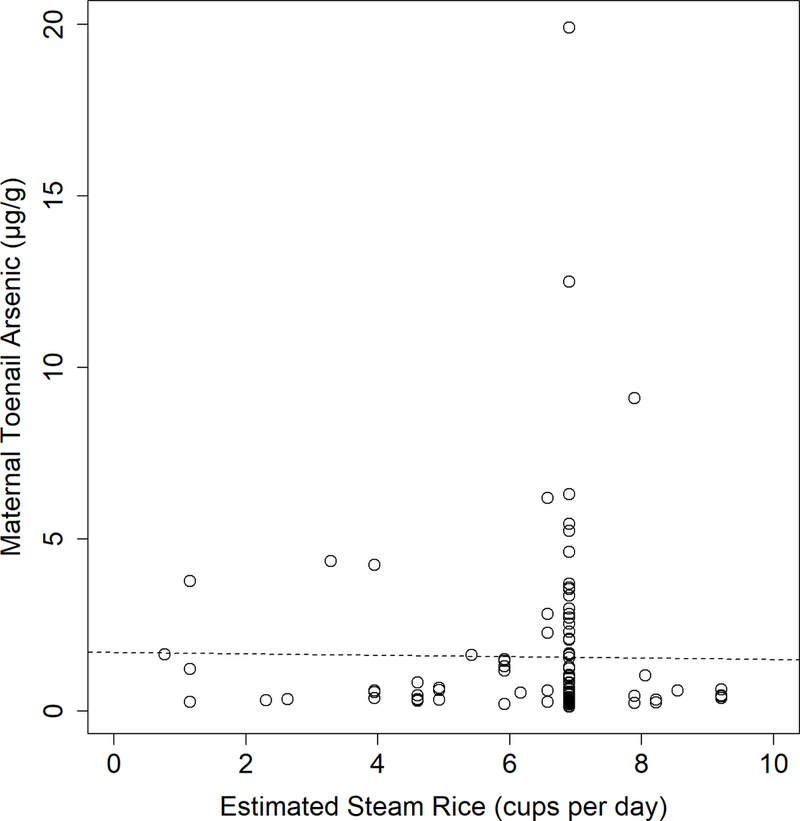
Comparison between estimated steam rice consumption from the Food Frequency Questionnaire and maternal toenail arsenic concentration with the regression line plotted (p=0.643). Horizontal axis plotting range truncated to remove two outlying values of an estimated >20 cups of steam rice per day.
DISCUSSION
The primary finding in our study is that nutritional intake of folate was less than half the recommended intake of 600 µg/day for both mothers of cases and mothers of controls, underscoring the need to expand folic acid supplementation programs and folic acid fortification of food products in Bangladesh to prevent neural tube defects. Foods high in folate were not frequently consumed, including spinach, okra, bitter gourd, cauliflower, and beans. However, the lower dietary folate intake was not directly associated with neural tube defects as mothers of cases were estimated to consume a median of 46 µg/day more folate than mothers of controls (p=0.005). Seventy-one percent of control mothers reported consuming periconceptional folic acid supplements compared to 49 percent of case mothers (p=0.038). Even with these differences in dietary folate intake and supplement use, both case and control mothers had similar plasma folate levels of 4.7 ng/mL when measured post-pregnancy (p=0.918). For the four mothers of cases estimated to consume sufficient folate from their diets (>600 µg/day), their postpartum plasma folate measurements ranged from 1.1 to 8.9 ng/mL with a mean of 5.1 and median of 5.2 ng/mL. More research is needed to match current dietary choices and plasma folate levels in these mothers.
Along with this finding, a secondary finding is that several other dietary components were not consumed in sufficient quantities for both cases and controls, including vitamin A, vitamin D, vitamin E, iron, potassium, and sodium. Sodium values may be lower than actual because the FFQ did not directly ask about salt added to meals or use of canned foods. Given that no mother in either case or control groups met all DRI recommendations, there may be underlying limitations in the types of foods available to pregnant women in Bangladesh to meet their nutritional requirements. Foods high in vitamins A (i.e. sweet potato, spinach), vitamin D (fish), vitamin E (nuts and oils), iron (beans, lentils, spinach, fish), and potassium (apricots, lentils, prunes, squash) were not consumed frequently or were not directly asked about on the FFQ (Office of Dietary Supplements 2018a, 2018b, 2018c, 2018d, 2018e). Increasing folate, iron, and vegetable consumption would likely increase the women’s dietary quality index scores as these factors were all positively scored in a previous index approach (Carmichael et al., 2012). An index score approach may be useful for future research in Bangladeshi populations to better understand the overall dietary composition of pregnant mothers.
These dietary results are similar to previously estimated nutrient intakes collected in Bangladesh (Nguyen et al., 2017a). Nguyen et al. (2017a) surveyed 600 pregnant women, split them into 2 treatment groups of 300 each, and measured their dietary intakes before and after a planned dietary intervention. Their reported baseline conditions provide comparative data for this current study. Pregnant women were estimated to consume approximately half of the recommended 600 µg/day of folate, with reported means of 309 and 287 µg/day. Other vitamins that below recommended intakes included calcium, iron, zinc, riboflavin, vitamin B-12, and vitamin A. Nguyen et al. (2017a) did not estimate consumption for vitamins D and E. The pregnant mothers consumed approximately 2,300 kcal/day in both treatment groups during the baseline sampling. This daily caloric intake is similar to the estimated intakes of 2,556 and 2,218 kcal/day estimated from this current study with one sample Wilcoxon Signed Rank test p-values of 0.095 for cases and 0.173 for controls against an alternative value of 2,300 kcal/day.
Sufficient intakes of several nutrients are required for healthy neural tube development. Bangladesh is known to have high prevalence of chronic malnutrition (Shannon et al., 2008; Goudet et al., 2011), and believed to have a high prevalence of neural tube defects (Christianson et al., 2006). Previous studies have shown that lower intake of protein, vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B12, choline, betaine, vitamin C, vitamin E, iron, and magnesium are associated with increased risk of neural tube defects (Groenen et al., 2004a; Groenen et al., 2004b; Carmichael et al, 2010; Lavery et al., 2014). Our study showed low dietary intake of vitamins A, D, E, iron, potassium, and sodium across cases and controls, suggesting that there may be additional opportunities for nutritional interventions to prevent neural tube defects. Of note, findings of these studies and this current study may not be directly comparable due to the heterogeneity of study sample characteristics (e.g., obese vs. malnourished populations).
The combination of a dietary intervention with increased folic acid food fortification, such as rice, could be considered for ensuring adequate nutrient intakes, particularly due to the central role that rice plays in the Bangladeshi diet (Table 3). Increasing bean and spinach intakes, along with other foods higher in folate than rice, would be helpful, but this might not be achievable in all Bangladeshi households. Providing folic acid fortification of rice may be a suitable intervention given the high intakes of rice reported for cases and controls. Increasing dietary intake of rice, particularly if the rice is grown in arsenic polluted water, could increase arsenic exposure. However, increasing estimated steamed rice consumption did not lead to increased toenail arsenic concentrations for cases and controls (Figure 1).
Previous dietary interventions increased the likelihood for having adequate nutrient intake in a study involving pregnant women in Bangladesh (Nguyen et al. 2017a). Following a nutrition-focused Maternal, Neonatal, and Child Health (MNCH) intervention, the likelihood for achieving adequate intake of several necessary vitamins and minerals increased, including for folate, calcium, iron, vitamin C, and vitamin A. Consumption of pulses (+89 g/day), dairy (+93 g/day), meat (+27 g/day), eggs (+20 g/day), and dark-green leafy vegetables (+200 g/day) all increased following the intervention. For many of these foods, the effect size was almost a doubling of intake from previously consumption estimates (Nguyen et al., 2017a).
Nutritional interventions could be paired with encouraging supplement use to increase the likelihood for women in Bangladesh to meet nutritional requirements. However, previous studies in Bangladesh have noted efforts to increase supplement use have not always been successful. Rural, married women in Bangladesh were found to have low iron levels, with over one-third of sampled women anemic (Khambalia et al., 2009b). Providing pregnant women with iron and folic acid supplements (IFA) did not reduce anemia or increase iron status when measured at 15 weeks gestation (Khambalia et al., 2009a). The supplements did have the intended effect of reducing anemia and increasing iron stores in non-pregnant women (Khambalia et al., 2009a). Khambalia et al. (2009) noted that pregnant women’s low adherence to routinely taking the supplements could have reduced the beneficial effect of folic acid supplements.
Multiple barriers exist for ensuring adherence to new diets or to routine supplement use, including mothers’ perceptions of risks due to low nutrient intakes, awareness and access to anenatal care, and sufficient supplement supplies (Siekmans et al., 2018). Two factors that can help overcome these barriers include community-based supplement delivery methods and including family-members in the nutritional and supplement programs (Goudet al., 2018). Nutritional interventions that involved husbands helped improve nutritional outcomes for pregnant wives (Nguyen et al., 2018).
Ensuring mothers in Bangladesh are getting adequate folate levels could have multiple benefits. Theoretically, a mother with higher arsenic exposure in Bangladesh may require greater folate intake to address two potential health risks. First, the mother may need additional folate to methylate arsenic to prevent negative health effects from the arsenic exposure. Second, the mother will need sufficient folate to reduce neural tube defect risks. In agreement with existing recommendations for folic acid supplementation, our previous work in Bangladesh suggested prenatal folic acid supplement use was protective for myelomeningocele, with an adjusted odds ratio of 0.43 (0.18–1.02 95% confidence interval) (Kancherla et al., 2017). This finding is consistent with many other studies of neural tube defects.(Hewitt et al., 1992; Castilla et al., 2003; Yi et al., 2011; Williams et al., 2015). Though we did not find a direct main effect of arsenic exposure on myelomeningocele risk, our findings suggested that arsenic may increase the risk of myelomeningocele by reducing the effectiveness of folic acid supplementation (Mazumdar et al., 2015a; Mazumdar et al., 2015b). Therefore, increasing folic acid supplement use and folic acid fortification of food products may be needed to provide a protective effect against higher arsenic concentrations.
Our study suggests that folic acid supplementation and fortification is needed in this population to ensure sufficient folate intakes in pregnant mothers. These efforts should be paired with dietary intervention strategies to increase intakes of vitamins A, D, and E, along with iron, potassium, and sodium. Of the foods listed on the FFQ, the ones with the highest concentration of vitamin A were sweet potatoes, spinach, pumpkin, and egg. Vitamin D is found most often in fish, eggs, and meat, and vitamin E is found in spinach, eggs, and pumpkins. Based on the foods shown in Table 3, mothers in Bangladesh could be encouraged to shift their consumption away from cereals such as rice in exchange for an increase in fish, eggs, meat, and vegetable intake. There are multiple socioeconomic factors that affect food access, and these factors would need to be explored in future studies.
Study Strengths and Limitations
This study has multiple strengths. First, this study successfully collected dietary recall data from mothers of infants diagnosed with myelomeningocele. If there are future efforts aimed at dietary interventions to help reduce neural tube defects in Bangladesh, this study provides data that may help inform best methods for achieving adequate folate intake. Second, this study collected arsenic exposure data through toenail and water samples in combination with the dietary recall data. These findings will be of interest to future research projects evaluating potential arsenic exposure sources through diet. By identifying the foods most frequently consumed by mothers, researchers could use a targeted approach to analyze these primary foods for arsenic content. Finally, another strength of this study is that it continues to provide data on birth defects in Bangladesh, a country that does not have a nationwide birth defects registry. Even with a modest sample size of 55 infants with myelomeningocele, this study contributes to the limited information about birth defects in Bangladesh.
Our study has some limitations. Due to the lack of birth defect surveillance in Bangladesh, our study was based on livebirths only and our cases, therefore, represent a less severe phenotype. It is possible that environmental and nutritional exposures have different associations with risk in more severely affected cases, and these potential associations are not captured in our study design. Future research projects on neural tube defects in Bangladesh should consider expanded recruitment procedures, such as large cohort studies that capture all pregnancies to identify cases to reduce potential sampling bias.
Another important limitation is recall bias. The FFQ specifically queried dietary intake during pregnancy, but the questionnaire was administered after the infant was born. The average age for case infants was 175 days and control infants was 240 days (Table 1). Mothers were asked to retrospectively estimate their dietary intake during pregnancy, which occurred generally between 6 months and 12 months prior to participating in the study. Pairing the FFQ with additional dietary sampling and analysis from pregnant women could help overcome some of the challenges with relying on retrospective FFQs alone (Hackett 2011; Kristal et al. 2005). The FFQ asked about 39 food items, but there could have been other items that were consumed by women but not reported because they were not included on the FFQ. Future studies could also better differentiate between foods consumed at the household level and the individual level. For example, an individual might not consume nearly 1 kg of rice per day (Table 3), but this amount might be prepared for an entire household. In addition, mother’s blood samples were collected during the participant’s enrollment which was after childbirth. Accordingly, plasma folate levels may not directly reflect the status of food intake and prenatal folic acid supplement use during pregnancy.
CONCLUSIONS
We used a validated food frequency questionnaire in a rural Bangladeshi population to assess dietary risk factors for neural tube defects among mothers. Though we did not find a particular pattern of dietary intake that was associated with increased risk of neural tube defects in our study sample, our population had very low dietary intake of folate and other micronutrients known to be associated with increased risk of neural tube defects. Our study supports current recommendations for folic acid supplementation and folic acid fortification of food products. As rates of supplement use in Bangladesh are low, one important consider could be rice fortification with folic acid because rice is a central part of the Bangladeshi diet. In addition, increasing dietary intake of vegetables and proteins could help address vitamins A, D, and E, iron, sodium, and potassium levels as well. Case and control mothers were estimated to consume less than half of the recommended daily folate intake of 600 µg, demonstrating that nutritional intervention is needed to improve maternal health in Bangladesh and reduce rates of neural tube defects.
Supplementary Material
Acknowledgments:
This work was supported by the National Institutes of Environmental Health Sciences (NIEHS), National Institutes of Health (R01 ES 026317), the Child Neurology Foundation, the Harvard T.H. Chan School of Public Health NIEHS Center (P30 ES 000002), and the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center (P30 HD 18655).
Footnotes
Disclosures: The authors declare they have no actual or potential competing financial interests.
Contributor Information
John F. Obrycki, Email: john.obrycki@childrens.harvard.edu.
Jane J. Lee, Email: ntrjane@gmail.com.
Kush Kapur, Email: kush.kapur.79@gmail.com.
Ligi Paul, Email: Ligi.Paul_Pottenplackel@tufts.edu.
Md Omar Sharif Ibne Hasan, Email: dr.sharif0909@gmail.com.
Quazi Quamruzzaman, Email: dcht87@gmail.com.
David C. Christiani, Email: dchris@hsph.harvard.edu.
REFERENCES
- Agopian AJ, Tinker SC, Lupo PJ, Canfield MA, Mitchell LE, National Birth Defects Prevention Study. 2013. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res A Clin Mol Teratol 97:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Rathouz PJ, Pierce BL, Kalra T, Parvez F, Slavkovich V, Ahmed A, Chen Y, Ahsan H. 2010. Dietary B vitamin intakes and urinary total arsenic concentration in the Health Effects of Arsenic Longitudinal Study (HEALS) cohort, Bangladesh. Eur J Nutr 49:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta CAM, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, Rajapakse T, Kaplam GG, Metcalfe A. 2016. Global birth prevalence of spina bifida by folic acid fortification status: A systematic review and meta-analysis. Am J Public Health 106(159):e24–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangladesh Bureau of Statistics. 2013. Child and mother nutrition survey 2012. Statistics and Informatics Division http://www.bbs.gov.bd/site/page/56067433-bdd2-4f62-b2df-7ac875659d64/- [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statis Soc B 57(1):289–300. [Google Scholar]
- Bozack AK, Hall MN, Liu X, Ilievski V, Lomax-Luu AM, Parvez F, Siddique AB, Shahriar H, Uddin MN, Islam T, Graziano JH, Gamble MV. 2018. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. Am J Nutr doi: 10.1093/ajcn/nqy148. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Geological Survey and Department of Public Health Engineering (Bangladesh). 2001. Arsenic contamination of groundwater in Bangladesh. Kinniburgh DG and Smedley PL (Editors). British Geological Survey Technical Report WC/00/19 British Geological Survey: Keyworth: https://www.bgs.ac.uk/research/groundwater/health/arsenic/Bangladesh/home.html [Google Scholar]
- Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, Pearson K, Devine O, Mulinare J, National Birth Defects Preventin Network. 2005. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth Defects Res A Clin Mol Teratol 73:679–689. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Feldkamp ML, Munger RG, Siega-Riz AM, Botto LD, Shaw G, National Birth Defects Prevention Study. 2012. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Arch Pediatr Adolesc Med 166(2):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Herring A, Abrams B, Shaw GM. 2007. Maternal food insecurity is associated with increased risk of certain birth defects. J Nutr 137:2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Shaw GM. 2010. Periconceptional nutrient intakes and risks of neural tube defects in California. Birth Defects Res A Clin Mol Teratol 88:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DE, Aposhian HV, Gandolfi AJ. 2003. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol 193:309–334. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Orioli IM, Lopez‐Camelo JS, Dutra Mda G, Nazer‐Herrera J, Latin American Collaborative Study of Congenital Malformations (ECLAMC). 2003. Preliminary data on changes in neural tube defect prevalence rates after folic acid fortification in South America. Am J Med Genet A 123:123–128. [DOI] [PubMed] [Google Scholar]
- Castillo-Lancellotti C, Tur JA, Uauy R. 2012. Impact of folic acid fortification of flour on neural tube defects: A systematic review. Public Health Nutr 16(5):901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2010. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep 59:980–984. [PubMed] [Google Scholar]
- Chen KL, Amarasiriwardena CJ, Christiani DC. 1999. Determination of total arsenic concentrations in nails by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 67:109–125. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ahsan H, Parvez F, Howe GR. 2004. Validity of a food-frequency questionnaire for a large prospective cohort study in Bangladesh. Br J Nutr 92:851–859. [DOI] [PubMed] [Google Scholar]
- Chen Y, Factor-Litvak P, Howe GR, Parvez F, Ahsan H. 2006. Nutritional influence on risk of high blood pressure in Bangladesh: a population-based cross-sectional study. Am J Clin Nutr 84:1224–1232. [DOI] [PubMed] [Google Scholar]
- Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, van Geen A, Ahsan H. 2007. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol 165:541–552. [DOI] [PubMed] [Google Scholar]
- Chen Y, McClintock TR, Segers S, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Ahsan H. 2013. Prospective investigation of major dietary patterns and risk of cardiovascular mortality in Bangladesh. Int J Cardiol 167:1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson A, Howson CP, Modell B. 2006. March of Dimes global report on birth defects: the hidden toll of dying and disabled children White Plains, NY: March of Dimes Birth Defects Foundation. [Google Scholar]
- Crider KS, Devine O, Qi YP, Yeung LF, Sekkarie A, Zaganjor I, Wong E, Rose CE, Berry RJ. 2019. Systematic review and Bayesian meta-analysis of the dose-response relationship between folic acid intake and changes in blood folate concentrations. Nutrients 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Stanier P, Green NDE. 2013. Neural tube defects - recent advances, unsolved questions and controversies. Lancet Neurol 12(8):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed JT, Brockhoff CA, Martin TD. 1994. Method 200.8, Revision 5.4: Determination of trace elements in waters and wastes by inductively coupled plasma-mass spectrometry Cincinnati, OH, USA: U.S. Environmental Protection Agency. [Google Scholar]
- Czeizel AE, Dudas I. 1992. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 327:1832–1835. [DOI] [PubMed] [Google Scholar]
- Desrosiers TA, Siega-Riz AM, Mosley BS, Meyer RE, National Birth Defects Prevention Study. 2018. Low carbohydrate diets may increase risk of neural tube defects. Birth Defects Research 110(11):901–909. [DOI] [PubMed] [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, Lowry RB, Evans JA, Van den Hof MC, Crowley M, Uh S- H, Zimmer P, Sibbald B, Fernandez B, Lee NS, Niyonsenga T. 2008. Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A 82:622–626. [DOI] [PubMed] [Google Scholar]
- Ferm VH, Carpenter SJ. 1968. Malformations induced by sodium arsenate. J Reprod Fertil 17:199–201. [DOI] [PubMed] [Google Scholar]
- Gamble MV, Ahsan H, Liu X, Factor-Litvak P, Ilievski V, Slavkovich V, Parvez F, Graziano JH. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. 2005b. Am J Clin Nutr 81:1372–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, Parvez F, Chen Y, Levy D, Factor-Litvak P. 2006. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. Am J Clin Nutr 84:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. 2005a. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 113:1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani SH, Alibhai Y. 1990. Teratogenicity of metals to chick embryos. J Toxicol Environ Health 30:23–31. [DOI] [PubMed] [Google Scholar]
- Goudet SM, Faiz S, Bogin BA, Griffiths PL. 2011. Pregnant women’s and community health workers’ perceptions of root causes of malnutrition among infants and young children in the slums of Dhaka, Bangladesh. Am J Public Health 101:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet S, Murira Z, Torlesse H, Hatchard J, Busch-Hallen J. 2018. Effectiveness of programme approaches to imporve the coverage of maternal nutrition interventions in South Asia. Matern Child Nutr 14(S4):e12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen PM, van Rooij IA, Peer PG, Gooskens RH, Zielhuis GA, Steegers-Theunissen RP. 2004a. Marginal maternal vitamin B12 status increases the risk of offspring with spina bifida. Am J Obstet Gynecol 191:11–17. [DOI] [PubMed] [Google Scholar]
- Groenen PM, van Rooij IA, Peer PG, Ocké MC, Zielhuis GA, Steegers-Theunissen RP. 2004b. Low maternal dietary intakes of iron, magnesium, and niacin are associated with spina bifida in the offspring. J Nutr 134:1516–1522. [DOI] [PubMed] [Google Scholar]
- Gupta P, Ray C. Epidemiology of betel quid usage. 2004. Ann Acad Med Singapore 33:31–36. [PubMed] [Google Scholar]
- Hackett A 2011. Food frequency questionnaires: simple and cheap, but are they valid? Matern Child Nutr 7:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder D, Biswas A, Šlejkovec Z, Chatterjee D, Nraigu J, Jacks G, Bhattacharya P. 2014. Arsenic species in raw and cooked rice: Implications for human health in rural Bengal. Sci Total Environ 497–498:200–208. [DOI] [PubMed] [Google Scholar]
- Hewitt SM, Crowe CMW, Navin AW, Miller ME. 1992. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects Atlanta, GA, USA: Centers for Disease Control and Prevention; 41:980–984. [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. 2001. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 285:2981–2986. [DOI] [PubMed] [Google Scholar]
- Hood RD, Bishop SL. 1972. Teratogenic effects of sodium arsenate in mice. Arch Environ Health 24:62–65. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. 1998. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, panthothenic acid, biotin, and choline Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Kancherla V, Ibne Hasan MOS, Hamid R, Paul L, Selhub J, Oakley G, Quamruzzaman Q, Mazumdar M. 2017. Prenatal folic acid use associated with decreased risk of myelomeningocele: A case-control study offers further support for folic acid fortification in Bangladesh. PloS One 12:e0188726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach R, Paul L, Selhub J. 2011. Determination of unmetabolized folic acid in human plasma using affinity HPLC. Am J Clin Nutr 94:343S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambalia AZ, O’Connor DL, Macarthur C, Dupuis A, Zlotkin SH. 2009. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. Am J Clin Nutr 90:1295–1302. [DOI] [PubMed] [Google Scholar]
- Khambalia A, O’Connor DL, Zlotkin S. 2009b. Periconceptional iron and folate status is inadequate among married, nulliparous women in rural Bangladesh. J Nutr 139:1179–1184. [DOI] [PubMed] [Google Scholar]
- Komsta L, Novomestky F. 2015. moments: Moments, cumulants, skewness, kurtosis and related tests. R package version 0.14 https://CRAN.R-project.org/package=moments [Google Scholar]
- Kristal AR, Peters U, Potter JD. 2005. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev 14(12):2826–2828. [DOI] [PubMed] [Google Scholar]
- Lavery AM, Brender JD, Zhao H, Sweeney A, Felkner M, Suarez L, Canfield MA. 2014. Dietary intake of choline and neural tube defects in Mexican Americans. Birth Defects Res A Clin Mol Teratol 100:463–471. [DOI] [PubMed] [Google Scholar]
- Leonard A, Lauwerys RR. 1980. Carcinogenicity, teratogenicity and mutagenicity of arsenic. Mutat Res 75:49–62. [DOI] [PubMed] [Google Scholar]
- Machado AF, Hovland DN, Pilafas S, Collins MD. 1999. Teratogenic response to arsenite during neurulation: relative sensitivities of C57BL/6J and SWV/Fnn mice and impact of the splotch allele. Toxicol Sci 51:98–107. [DOI] [PubMed] [Google Scholar]
- Mazumdar M, Ibne Hasan MOS, Hamid R, Valeri L, Paul L, Selhub J, Rodrigues EG, Silva F, Mia S, Mostofa MG. 2015a. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: a case control study in Bangladesh. Environ Health 14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M, Valeri L, Rodrigues EG, Ibne Hasan MO, Sharif MO, Hamid R, Paul L, Selhub J, Silva F, Mostofa MG. 2015b. Polymorphisms in maternal folate pathway genes interact with arsenic in drinking water to influence risk of myelomeningocele. Birth Defects Res A Clin Molec Teratol 103:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, Scott JM, Mills JL. 2009. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Vitamin Study Research Group. 1991. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338:131–137. [PubMed] [Google Scholar]
- Nair U, Bartsch H, Nair J. 2004. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis 19:251–262. [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Frongillo EA, Sanghvi T, Wable G, Mahmud Z, Tran LM, Aktar B, Afsana K, Alayon S, Ruel MT, Menon P. 2018. Engagement of husbands in a maternal nutrition program substantially contributed to greater intake of micronutrient supplements and dietary diversity during pregnancy: Results of a cluster-randomized program evaluation in Bangladesh. J Nutr 148:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PH, Kim SS, Sanghvi T, Mahmud Z, Tran LM, Shabnam S, Aktar B, Haque R, Afsana K, Frongillo EA, Ruel MT, Menon P. 2017a. Integrating nutrition interventions into an existing maternal, neonatal, and child health program increased maternal dietary diversity, micronutrient intake, and exclusive breastfeeding practices in Bangladesh: Results of a cluster-randomized program evaluation. J Nutr 147:2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MP, Lupo PJ, Northup H, Morrison AC, Cirino PT, Au KS. 2017b. Maternal gene-micronutrient interactions related to one-carbon metabolism and the risk of myelomeningocele among offspring. Birth Defects Res 109:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio K, Goto Y, Kondo T, Ito S, Ishida Y, Kawai S, Naito M, Wakai K, Hamajima N. 2008. Serum folate and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism adjusted for folate intake. J Epidemiol 18(3):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Dietary Supplements. No date. Nutrient recommendations: dietary reference intakes (DRI) National Institutes of Health. https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx [Google Scholar]
- Office of Dietary Supplements. 2018a. Iron Fact Sheet for Health Professionals National Institutes of Health. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ [Google Scholar]
- Office of Dietary Supplements. 2018b. Potassium Fact Sheet for Health Professionals National Institutes of Health. https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/ [Google Scholar]
- Office of Dietary Supplements. 2018c. Vitamin A Fact Sheet for Health Professionals National Institutes of Health. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ [Google Scholar]
- Office of Dietary Supplements. 2018d. Vitamin D Fact Sheet for Health Professionals National Institutes of Health. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ [Google Scholar]
- Office of Dietary Supplements. 2018e. Vitamin E Fact Sheet for Health Professionals National Institutes of Health. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ [Google Scholar]
- Pierce BL, Argos M, Chen Y, Melkonian S, Parvez F, Islam T, Ahmed A, Hasan R, Rathouz PJ, Ahsan H. 2011. Arsenic exposure, dietary patterns, and skin lesion risk in Bangladesh: a prospective study. Am J Epidemiol 173:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Graziano JH, Gamble MV. 2009. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect 117:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JG, Singh G, Burrows RF. 2004. Evidence for suboptimal use of periconceptional folic acid supplements globally. Br J Obstet Gynaecol 111:399–408. [DOI] [PubMed] [Google Scholar]
- Shaheen N, Rahim ATMA, Mohiduzzaman M, Banu CP, Bari ML, Tukun AB, Mannan MA, Bhattacharjee L, Stadlmayr B. 2013. Food Composition Table for Bangladesh First edition. Dhaka, Bangladesh: Intergraphic Limited. [Google Scholar]
- Shannon K, Mahmud Z, Asfia A, Ali M. 2008. The social and environmental factors underlying maternal malnutrition in rural Bangladesh: implications for reproductive health and nutrition programs. Health Care Women Int 29:826–840. [DOI] [PubMed] [Google Scholar]
- Siekmans K, Roche M. Kung’u JK, Desrochers RE, De-Regil LM. 2018. Barriers and enables for iron folic acid (IFA) supplementation in pregnant women. Matern Child Nutr 14(S5):e12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauheed J, Sanchez-Guerra M, Lee JJ, Paul L, Ibne Hasan MOS, Quamruzzaman Q, Selhub J, Wright RO, Christiani DC, Coull BA, Baccarelli AA, Mazumdar M. 2017. Associations between post translational histone modifications, myelomeningocele risk, environmental arsenic exposure, and folate deficiency among participants in a case control study in Bangladesh. Epigenetics 12:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen KI, Lacroix E, Flynn M, Ronksley PE, Oinonen KA, Metcalfe A, Campbell TS. 2018. Folic acid supplementation during the preconception period: A systematic review and meta-analysis. Prev Med 114:1–17. [DOI] [PubMed] [Google Scholar]
- USDA. 2018. USDA Food Compostion Databases National Agricultural Library. https://ndb.nal.usda.gov/ndb/ [Google Scholar]
- Waid JL, Ali M, Thilsted SH, Gabrysch S. 2018. Dietary change in Bangladesh from 1985 to 2010. Global Food Security 17:221–232. [Google Scholar]
- Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS. 2015. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification-United States, 1995–2011. MMWR Morb Mortal Wkly Rep 64:1–5. [PMC free article] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, Sever LE, Miller LA, Meaney FJ, Levitt M. 2002. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology 66:33–39. [DOI] [PubMed] [Google Scholar]
- Yetley EA, Johnson CL. 2011. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr 94(suppl):1S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Lindemann M, Colligs A, Snowball C. 2011. Economic burden of neural tube defects and impact of prevention with folic acid: a literature review. Eur J Pediatr 170:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


