Abstract
Molecular clocks are present in almost every cell to anticipate daily recurring and predictable changes, such as rhythmic nutrient availability, and to adapt cellular functions accordingly. At the same time, nutrient-sensing pathways can respond to acute nutrient imbalance and modulate and orient metabolism so cells can adapt optimally to a declining or increasing availability of nutrients. Organismal circadian rhythms are coordinated by behavioral rhythms such as activity–rest and feeding–fasting cycles to temporally orchestrate a sequence of physiological processes to optimize metabolism. Basic research in circadian rhythms has largely focused on the functioning of the self-sustaining molecular circadian oscillator, while research in nutrition science has yielded insights into physiological responses to caloric deprivation or to specific macronutrients. Integration of these two fields into actionable new concepts in the timing of food intake has led to the emerging practice of time-restricted eating. In this paradigm, daily caloric intake is restricted to a consistent window of 8–12 h. This paradigm has pervasive benefits on multiple organ systems.
Keywords: time-restricted feeding, time-restricted eating, metabolic disease, circadian rhythms
INTRODUCTION
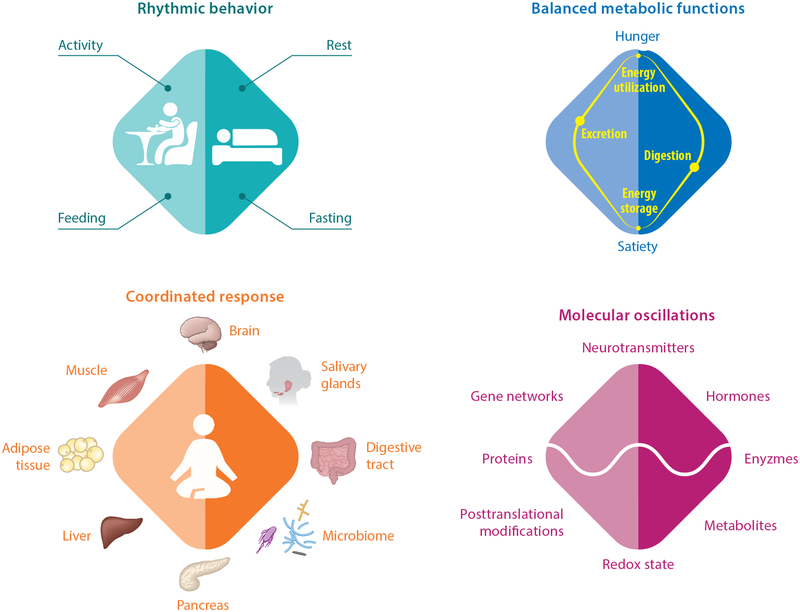
The quality and quantity of nutrition are well-accepted determinants of health. However, recent progress in the field of circadian rhythms has led to the idea that the time of day when food is ingested affects body weight, body composition, glucose regulation, lipid homeostasis, the gut microbiome, cardiac function, inflammation, sleep, and overall health (90). Daily fluctuation in nutrient absorption, assimilation, substrate interconversion, and utilization are lending increasing support to this concept (Figure 1).
Figure 1.
The extensive roles of the circadian clock in regulating nutritional and energetic balance, from behavior to molecules. The master clock controls daily rhythms in activity–rest and associated feeding–fasting behaviors. Accordingly, metabolic functions oscillate between nutrient digestion and energy storage during satiety and between nutrient excretion and energy mobilization during hunger. This nutritional and energetic equilibrium engages multiple organs to ensure balanced digestion and excretion (for example, the salivary glands, pancreas, digestive tract, microbiome, liver) and balanced energy storage and utilization (for example, the liver, muscle, adipose tissue). The secretion of digestive enzymes and hormones, as well as gut peristalsis also vary during the day. At the molecular level, metabolic rhythms are associated with daily oscillations in the activity of gene networks, protein expression, posttranslational modifications, the level of metabolites, and redox state. The master clock and peripheral clocks play critical roles in the daily temporal coordination of these processes.
Circadian rhythms are ~24-h rhythms in biological processes that are produced by endogenous circadian clocks. The circadian clock in animals is based on a cell-autonomous transcription–translation feedback circuit composed of nearly a dozen transcription factors and more than 50 accessory proteins (90). The circadian clock regulates nutrient utilization at the cellular level through clock transcription factors that modulate the expression of many downstream genes involved in nutrient utilization, and at the behavioral level, it also regulates ingestion, through circadian rhythms in activity–rest cycles and the dependent rhythm in feeding–fasting (9). Feeding and fasting are also known to acutely activate nutrient-sensing pathways that act at both the transcriptional and posttranscriptional levels to maintain cellular and organismal nutrient homeostasis (24).
Circadian clock components interact with nutrient-sensing pathways, and this interaction serves three major functions: (a) When feeding occurs at an anticipated time, the integrated anticipatory response, driven by the circadian clock, and an acute response, initiated by nutrient-sensing pathways, act synergistically to maintain nutrient homeostasis; (b) when feeding occurs at an unanticipated time, the nutrient-sensing pathways act on the circadian clocks to adjust the phase of the clocks, so that on the subsequent days, food is anticipated at the new feeding time; (c) circadian regulation ensures that the appropriate pathways that help assimilate nutrients begin to rise in anticipation of feeding, so that the organism can better handle a rush of nutrients. However, this circadian clock–driven rise in gene expression that optimizes nutrient utilization declines after a few hours. During this decline or at its nadir, feeding can activate nutrient-sensing pathways separate from the circadian system, but the suboptimal expression of circadian-driven genes compromises how the food is processed. In sum, this interaction defines a limited time window for optimal nutrient metabolism. Accordingly, time-restricted feeding (TRF) or time-restricted eating (TRE, when referring to humans), in which food is consumed within a consistent 8–12-h interval appears to sustain optimal nutrient utilization and promote health (90). The concept of TRF and its molecular correlates are being extensively studied in animal models, and human studies of TRE are beginning to emerge.
THE MOLECULAR CIRCADIAN CLOCK
Circadian timekeeping mechanisms are present in almost every brain region and peripheral organ implicated in nutrient metabolism, including hunger, digestion, absorption, substrate interconversion, utilization and storage, detoxification, and excretion (Figure 1).
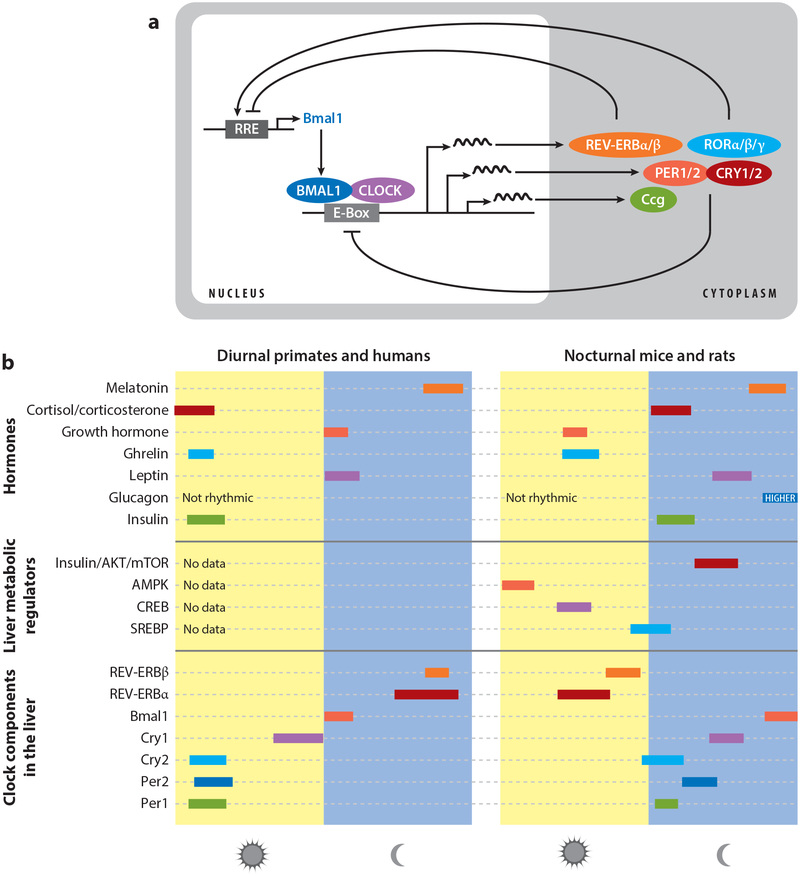
At the molecular level, circadian rhythms arise from a cell-autonomous feedback circuit driven by the bHLH (basic helix-loop-helix)-PAS transcription factors BMAL1 (also known as ARNTL or MOP3) and CLOCK, or the CLOCK homolog NPAS2 (for simplicity, this review utilizes CLOCK/BMAL1, when referring to all heterodimer forms). In this circuit, CLOCK/BMAL1 heterodimers bind to target E-box site (CACGTG) elements present in the promoter regions of the period (PER1 and PER2) and cryptochrome (CRY1 and CRY2) genes to activate their transcription. In turn, the PER and CRY proteins heterodimerize to inhibit CLOCK/BMAL1 activity, thus producing ~24-h rhythms in PER and CRY transcription. In addition, CLOCK/BMAL1 and PER/CRY generate rhythmic transcription of the ROR and REV-ERB classes of nuclear hormone receptors, whose opposing actions on the BMAL1 promoter result in an ~24-h rhythm in BMAL1 transcription (reviewed in 97, 117). This self-sustained oscillation persists in the absence of any external timing cues, such as food or light, thus offering the organism an intrinsic timing system (Figure 2).
Figure 2.
From molecular circadian oscillations to daily rhythms in metabolic regulators. (a) Representation of the core cell-autonomous circadian transcriptional–translational feedback loop. Activators are depicted in blues and repressors in red. (b) Schematic of the daily peak of clock components (messenger RNA expression), liver metabolic regulators (pathway activity), and hormones (serum level) both in diurnal primates and humans and nocturnal mice and rats. Abbreviations: AKT, protein kinase B; AMPK, adenosine monophosphate–activated protein kinase; CREB, cyclic adenosine monophosphate response element binding protein; Ccg: clock-controlled genes; mTOR, mammalian target of rapamycin; SREBP, sterol regulatory element binding protein.
While circadian rhythm research began with the search for the autonomous 24-h timekeeping mechanism that is self-sustaining in the absence of periodic cues, such as light–dark or feeding–fasting events, in the natural world, daily rhythms are a function of the circadian clock and inter-locked environmental and behavioral rhythms. Therefore, it is equally important to understand how light–dark and feeding–fasting cycles interact with the circadian clocks that ultimately dictate the daily rhythms in behavior, physiology, and metabolism.
The suprachiasmatic nucleus (SCN) of the hypothalamus acts as the master circadian clock. Developmental or acute surgical ablation of the SCN completely abolishes activity–rest rhythm and almost all physiological rhythms (127). The SCN uses both synaptic and diffusible factors to synchronize circadian clocks in other brain regions and peripheral organs. The SCN is monosynaptically innervated with intrinsically photosensitive and melanopsin-expressing retinal ganglion cells (50). The intrinsically photosensitive retinal ganglion cells constitute the principal conduit for light entrainment of the master circadian clock in the SCN (45, 47). Melanopsin photopigment is most sensitive to blue light, and the circadian activity–rest rhythm as well as light suppression of the sleep-promoting hormone melatonin are also most sensitive to blue light (48). Accordingly, chronic exposure to white light or blue-spectrum-enriched light or frequent changes in light exposure pattern from one day to another—as occurs during shift work or travel across time zones—can disrupt the normal functioning of the master circadian oscillator and have systemic circadian disruptive effects. While the effects of light on circadian rhythms are well studied, there is emerging evidence that the timing of food ingestion also has a profound impact on circadian clocks in peripheral organs and in some brain regions.
NUTRIENT-SENSING PATHWAYS
In mammals, feeding behavior is cyclic with periods of fasting separating feeding bouts. Accordingly, nutrient metabolism, from individual cells to the whole organism, has adapted mechanisms to switch between nutrient storage during periods of availability and the use of stored nutrients during periods of fasting. Elaborate regulatory pathways (neuroendocrine, cellular signaling, substrate-mediated enzymatic control) have evolved to sense nutrients or energy status and to cycle between anabolic and catabolic states to maintain homeostasis. Such cycles apply to carbohydrate, fat, and protein metabolism. For example, glycogen synthesis or glycogenolysis (and gluconeogenesis), fatty acid synthesis or β-oxidation, and protein synthesis and amino acid degradation exhibit daily rhythms (9, 10). The metabolism of cholesterol and nucleotides are interlocked within the metabolism of carbohydrate, fat, and protein.
Several key metabolic sensors, regulators, and effectors have been identified. Genetic inactivation of these components disrupts one or multiple steps in the anabolic or catabolic arm of the normal metabolic cycle and eventually leads to metabolic diseases. These molecules range from (a) endocrine regulators, hormones such as insulin or glucagon, to (b) cell-autonomous signaling modules, such as the insulin receptor and adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling pathways, to (c) transcriptional regulators, such as peroxisome proliferator–activated receptor-gamma (PPARγ), PPARγ coactivator 1-alpha (PGC1α), liver X receptor (LXR) and retinoid X receptor (RXR) to (d) enzymes, such as phosphoenolpyruvate carboxykinase (PCK1), glucokinase (GCK), fatty acid synthase (FAS), and cytochrome P450 family 7 subfamily A member 1 (CYP7A1). Unlike the self-sustained circadian oscillator, the expression or activity of these metabolic regulators is dampened or arrhythmic under prolonged fasting in whole animals or in tissue culture cells (52, 125). However, these regulators respond to feeding or fasting in a manner comparable to that of a switch or an hourglass (reviewed in 13). For example, in response to feeding and consequent insulin signaling, protein kinase B (AKT) phosphorylation aligns with the period of feeding, while glucagon signaling during fasting drives cAMP response element binding (CREB) protein phosphorylation and targets gene transcription (125) (Figure 2).
CROSS TALK BETWEEN THE CIRCADIAN OSCILLATOR AND NUTRIENT-SENSING PATHWAYS
Nutrient-sensing pathways can impact the circadian clock, so the clock can be entrained to changes in the timing of nutrient availability (27, 114). Conversely, the circadian system also modulates nutrient-sensing and-response mechanisms, such that responses to feeding or fasting at predictable times are amplified.
The circadian system and nutrient-sensing pathways interact at multiple levels, from the whole organism to individual molecules. At the molecular level, clock and metabolic regulators interact at multiple nodes. For instance, both CRY and BMAL1 are involved in the coupling between nutrient pathway and the clock. The clock protein CRYs also function as repressors of glucocorticoid receptor–mediated transcription (62). Mice lacking Cry exhibit overexpression of glucocorticoid receptor target genes, including Pck1, and consequently display impaired glucose tolerance. CRY proteins also exhibit extranuclear roles in repressing the function of the metabolic regulator CREB (134). Consequently, acute knockdown of Cry in hepatocytes and Cry knockout hepatocytes show elevated expression of the gluconeogenic CREB target glucose 6-phosphatase (134). Acute knockdown of Cry in the liver elevates fasting plasma glucose levels, while acute overexpression suppresses gluconeogenesis (134). Bmal1 is induced and required for adipocyte differentiation in vitro (111). The BMAL1 protein is found at the promoter of several genes involved in lipid and sterol biogenesis pathways, and its phase of expression coincides with the increased expression of genes including sterol regulatory element binding transcription factor 1 (Srebf1), Fasn, cluster of differentiation 36 (Cd36), acetoacetyl-coenzyme A (CoA) synthetase (Aacs), and 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr) (60, 100). Additionally, the cellular energy sensor AMPK phosphorylates and tunes the degradation of CRY proteins, which in turn modulates the repression of CLOCK/BMAL1-mediated transcription of the target genes (63). CLOCK/BMAL1 modulates the transcription of key components of the AMP salvage pathway and imposes rhythms on cellular levels of nicotinamide adenine dinucleotide (NAD) (84, 96). NAD levels modulate activities of the NAD-dependent poly(ADP-ribose) polymerase enzymes and sirtuins that impact the circadian oscillator (8, 83). Feeding itself causes changes in core body temperature and activates heat shock factor 1, which upregulates Per2 transcription (98). Additional cross talk through the mammalian target of rapamycin (mTOR), glycogen synthase kinase, casein kinase, many nuclear hormone receptors, and their coactivators has also been demonstrated (reviewed in 14). This extensive cross talk imposes three principal temporal regulations: (a) The circadian clock gates the cellular responses to feeding and fasting; (b) fasting duration and feeding bouts impact the oscillator and change its robustness; and (c) the interaction between the temporal feeding pattern and the clock determines the global transcriptional adaptation to energy availability.
THE INTERACTION BETWEEN CIRCADIAN RHYTHMS AND FEEDING–FASTING CYCLES DRIVES ROBUST OSCILLATIONS
Although the cell-autonomous circadian oscillator can sustain transcriptional oscillations under constant environmental and nutrient conditions, robust genome-wide transcriptional rhythms in intact animals arise from interactions between the circadian clock and feeding–fasting cycles. For example, in the liver of fasted mice, a smaller number of transcripts, including the clock components, and their direct output targets oscillate with 24-h rhythms. When mice are fed a normal chow ad libitum, they consume the majority of their calories at night and a smaller fraction during the day, and their liver shows rhythmic expression of a relatively large number of transcripts. Consolidating feeding to ~8 h without altering caloric intake further increases the number of rhythmic transcripts to a few thousand protein-coding genes. In the absence of a circadian clock, as in Cry1−/−;Cry2−/− mice, the feeding rhythm is abolished, and the liver transcriptome does not show any significant rhythm. However, consolidating feeding to ~8 h drives rhythmic transcription of a few hundred transcripts in the liver, including downstream targets of CREB, activating transcription factor 6 (ATF6), mTOR, forkhead box protein O (FoxO), and PGC1α. Furthermore, consistent feeding during the daytime phase shifts peak expression levels of most liver rhythmic transcripts by 12 h relative to those in mice fed mostly at night, suggesting that the hepatic circadian transcriptome is largely driven by the time of feeding (125). Similarly, daily rhythms in levels of protein and metabolites are also found in circadian mutant mice subject to a defined feeding–fasting cycle (2, 11, 86).
The relevance of the effect of food on the circadian system has also been examined in models resembling human shift work. In a rodent model of shift work in which mice are forced to be active during the day (when they would normally sleep) on weekdays and allowed to sleep and eat when they like on weekends (105), an increase in daytime food consumption is observed during weekdays. This paradigm dampens the global gene expression rhythm in the liver (12), which roughly resembles the dampened gene expression rhythm found in mice fed a high-fat diet ad libitum (59). Together, these results indicate that (a) feeding consolidation reinforces diurnal rhythms in thousands of hepatic transcripts; (b) the temporal spreading of caloric intake and an irregular feeding schedule, as found in shift work, dampens diurnal rhythms; (c) the feeding–fasting cycle can drive the rhythmic expression of hundreds of hepatic transcripts even in the absence of a circadian oscillator; and (d) consistent daytime feeding in mice can drive robust transcriptional oscillations with phases that are ~12 h away from those of animals fed during the night. These observations suggest that consistent feeding during the day in nocturnal animals may still sustain robust diurnal oscillations. Such eating patterns in animals have shown attenuation of metabolic diseases commonly found among shift workers (109).
CIRCADIAN RHYTHM DISRUPTION: CAUSE, FACILITATOR, OR EFFECT OF METABOLIC DISEASE?
The intimate interaction among circadian clock, light–dark and feeding–fasting cycles implies that an inconsistent duration of light exposure or a change in eating time from one day to another can disrupt daily rhythms. Additionally, in humans, reduced sleep can also indirectly affect circadian rhythms, as sleep deprivation increases the probability of consuming an energy-dense diet (73) and prolongs exposure to lighting, both of which disrupt circadian rhythms. Therefore, circadian rhythm disruption (CRD) can be defined as a misalignment in the timing of and/or change in the duration of ambient illumination, sleep, and period of eating from a reference range. Because the impact of food and light on circadian rhythms in humans has only recently been documented, it is too early to come to a consensus around the reference timing and duration for light exposure, sleep, and nutrition that sustain robust circadian rhythms. Nevertheless, with sleep duration as a relatively better agreed-upon reference value (51, 89), an ideal circadian day for an average adult would involve (a) 8 h for sleep, (b) waiting for at least 1 h after waking up before the first caloric intake of the day, (c) at least 1 h of exposure to bright light (1,000–10,000 lux) within the first half of the waking hour to entrain the hypothalamic clock to ambient light and to suppress melatonin (131), (d) exposure to dim or blue-depleted light for 2–3 h prior to bedtime to build sleep pressure, and (e) no caloric ingestion for 2–3 h prior to bedtime. As the wake-up time in humans is closely linked to light exposure, an abrupt change in the habitual wake-up time or bedtime by ≥2 h can disrupt normal circadian rhythms. Subjectively, most individuals also experience the discomfort of 2 h of jet lag after traveling across two time zones within a day.
Shift workers, who account for ~20% of the workforce worldwide, experience chronic circadian rhythm disruption. Even those who do not work shifts tend to change their weekday and weekend sleep and eating patterns, and some studies have estimated that nearly 80% of the general population experiences social jet lag (129). Actual shift work and chronic CRD under experimental conditions that mimic shift work or social jet lag can increase risks for noninfectious chronic diseases, including glucose intolerance, weight gain, adiposity, liver diseases, various forms of cancer, depression, and cardiovascular diseases, among others (3, 30, 57, 76, 81, 108, 124). Chronic CRD also compromises the immune system so that experimental animals become more susceptible to elevated inflammation and septic shock (29). As these chronic diseases now account for more than 85% of healthcare cost in the United States (19), experimentally testing the effect of circadian rhythm optimization by improving patterns of light exposure, sleep, and caloric intake to prevent and better manage these diseases will lead to significant impacts on public health. Among these three factors, the daily timing of nutrition is an attractive target as it can complement the rich scientific knowledge and public health infrastructure that are based on the impact of nutrition quality and quantity on health and disease.
TIME-RESTRICTED FEEDING AND TIME-RESTRICTED EATING
The concepts of TRF in animals and TRE in humans arose from studies examining the effects of food timing on the circadian system. The idea was to restrict feeding to a certain time of the day or night and test how this restriction affected daily rhythms in activity–rest cycles and clock components in metabolic organs (primarily the liver). The initial TRF experiments were done in rodents by restricting the timing of food access to a few hours (typically 4–8 h) during the day, when rodents usually rest and eat less, and then testing whether the daily activity–rest cycle would follow the new eating routine (36, 78). Restricting the time of food access to less than 8 h often reduces caloric intake. These experiments showed that rodents would wake up a few hours prior to the arrival of food and start ambulatory activity as if they were anticipating food. Such food anticipatory activity also occurred when calories were reduced and presented at night, and the magnitude of activity increased with the reduction in calories (79). Therefore, caloric restriction (CR) did not compromise physical activity, rather it increased the opportunistic food-seeking behavior that is important for survival (22, 25). In parallel, these TRF experiments also revealed that daytime access to food changed the phase of the circadian clock components in the liver of mice, such that clock components whose expression typically rises at night, instead rose during the day and vice versa (27, 114). Therefore, it was concluded that the liver clock is largely driven by the timing of food intake and not by the SCN clock, whose phase is tied to the light–dark cycle.
Conversely, in rodent models of diet-induced obesity, in which mice are given ad libitum access to a high-fat and high-sucrose diet, the daily feeding rhythm dampens and mice distribute their caloric intake throughout the 24-h day. This eating pattern also dampens the liver’s circadian clock, and it systemically affects almost all of the circadian transcriptome, both in metabolic organs and the brain (59). To test the relative contribution of an obesogenic diet and the disrupted eating pattern to obesity and metabolic diseases in these mice, we and others have subjected mice to isocaloric food consumption from a high-fat and high-sucrose diet at 8–12 h intervals. The rodents that eat the same calories from the same food source as the ad libitum cohort are largely protected from high-fat diet-induced obesity and related metabolic illnesses (21, 23, 49, 109, 133).
The initial rodent experiments were done with 8 h access to food and, hence, a popular diet called the 8-h diet or 8:16 diet arose. In popular media, it is also grouped under the umbrella of intermittent fasting, which describes all types of fasting, lasting from a few hours to a few days, without any explicit reference to circadian rhythms. Subsequently, TRF of 8–12 h without reducing caloric intake in rodents was shown to prevent obesity and diseases in a dose (fasting-duration)-dependent manner, with 8-h TRF having maximum benefits and 12-h TRF having less benefit (23). TRF in daytime also shows some benefit for weight loss relative to ad libitum controls (109). There is some debate about whether daytime TRF and nighttime TRF offer the same benefits in mice because daytime TRF has shown slightly reduced benefits in some experiments(7). Daytime TRF in rodents disturbs their habitual sleep and also temporally decouples their normal physical activity, which is aligned with feeding time (95). Therefore, it is not clear whether it is the direct effect of daytime TRF or an indirect effect of sleep loss and lack of physical activity after consuming a meal that contributes to the slightly attenuated benefits of daytime TRF in mice.
Many CR studies in rodents are done in a manner that also imposes time restriction in feeding. Traditionally, in CR studies control mice receive food ad libitum while the CR mice are given a meal at a fixed time each day that contains 20–30% fewer calories. The mice typically consume the reduced calories within 2–3 h, leaving a prolonged 20–22 h fasting interval (1, 80). Other rodent CR experiments have been done in which the CR cohort received the food in the morning, afternoon, or evening. However, irrespective of the timing of the calorie-restricted diet, all of these experiments extended the life span (85). In short-term studies, however, CR mice receiving food in the morning (when they are supposed to be sleeping) had less weight loss than mice that received the same calories at night (1). In a recently concluded controlled experiment designed to test the effect of time of meal in CR experiments, when the control mice were also given the daily ration at a fixed time, they finished the food within 10–12 h. These mice also lived longer than the control ad libitum–fed mice but shorter than the CR mice (80). This raises the idea that some benefits of CR also arise from time restriction (31).
Human studies on the timing of diet and health outcomes with respect to circadian physiology have a relatively long history. For more than 30 years it has been well known that in response to a standard test meal, postprandial glucose remains relatively higher in the evening or late night than in the morning (115, 123). This also fits with the action of circadian rhythms in insulin release and the suppressive effect of the sleep hormone melatonin on insulin release (92, 110, 122). Together these effects suggest that consuming a bigger portion of daily caloric intake during the first half of wakeful hours may be preferred for better blood glucose regulation and weight control. Accordingly, it is likely that the reports of breakfast skipping having detrimental health effects also ignored that those skipping breakfast might have had late-night eating events (20), so the causal link between breakfast skipping and health outcomes remains inconclusive (113). Relatively recent studies of caloric reduction interventions in humans have retrospectively found greater weight loss if lunch is consumed earlier rather than later (41). Epidemiological studies on the timing of food intake or length of overnight fasting on health outcomes are also emerging. These studies are revealing that a prolonged overnight fast of 13 h or longer, or eating an earlier dinner, correlates with a reduced risk for cancer (58, 72).
A big difference between laboratory rodents and humans is the production of melatonin and its effect on glucose homeostasis. Most laboratory mouse strains, specifically the widely used C57BL/6J strain, do not produce melatonin. In humans, melatonin levels in the plasma or saliva begins to rise 2–3 h before habitual bedtime (99). A receptor for melatonin, melatonin receptor 1B (MTNR1B), is expressed in insulin-producing pancreatic islets. The binding of melatonin with its receptor in these cells leads to the attenuation of glucose-stimulated insulin release, and this inhibition is further accentuated in humans carrying a mutant allele of MTNR1B (93). The functional consequence of this interaction is the reduced production of insulin in response to a meal late at night, which is further worsened in individuals carrying the mutant allele (68). As a result, carriers of this mutant allele have increased risks for hyperglycemia and type 2 diabetes (16, 71, 102). In summary, this interaction between melatonin and insulin release suggests it may be beneficial to avoid meals for 2–3 h before going to bed and for up to an hour after waking up, after which melatonin levels decline. Altogether, multiple lines of observation support the notion that confining all caloric intake to within a defined time interval that is a few hours separated from the daily sleep interval has multiple health benefits.
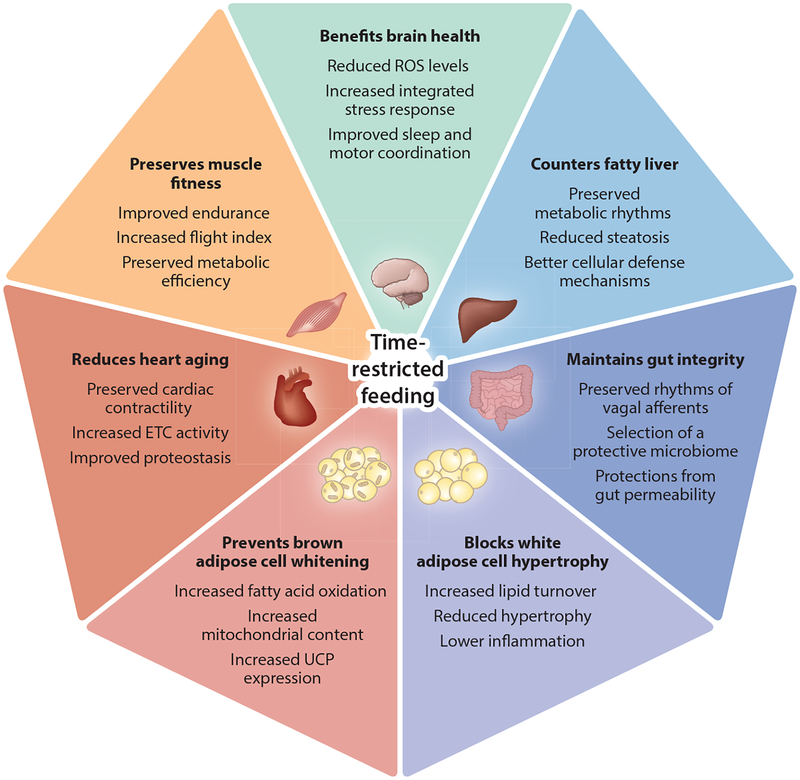
The underlying biochemical or gene expression basis for the health benefits of TRF is better studied in animal models. In the following sections we discuss mechanisms, with a specific emphasis on metabolic organs (Figure 3).
Figure 3.
Pervasive benefits of time-restricted feeding. Chronic circadian rhythm disruption is a risk factor for metabolic diseases. Studies in animal models (flies, mice, rats) and emerging studies in humans show that time-restricted feeding protects metabolic tissues from metabolic disturbances. Time-restricted feeding may also benefit brain health and could delay the development of neurodegenerative diseases. Abbreviations: ETC, electron transport chain; ROS, reactive oxygen species; UCP, uncoupling protein.
TIME-RESTRICTED FEEDING OR EATING AND LIVER FUNCTION
Human metabolic health and the diagnosis of disease are often linked to the metabolism of glucose, fat, and cholesterol. The liver plays an important role in the metabolism of all three factors. Hence, we discuss the molecular changes during TRF and TRE that affect these metabolic pathways in the liver.
Time-Restricted Feeding/Eating and Control of Blood Glucose Levels
Both the hormonal regulation of glucose (glucagon, insulin, and incretins) and cell-autonomous enzymatic regulation of glucose metabolism are modulated by the circadian clock and nutrient-sensing mechanisms (90). Time series analyses of gene expression, protein abundance, posttranslational modifications, and levels of metabolites in rodents that undergo TRF have shed light on the potential mechanisms by which TRE maintains glucose homeostasis. Most of the rodent studies have been done in mice fed a high-fat and high-sucrose diet, as this diet accelerates insulin resistance and the effect of any pharmacological or lifestyle intervention can be observed within a short period of time.
It is known that ad libitum access to a high-fat diet dampens the feeding rhythm and also dampens the rhythmic expression of many clock components, primarily by reducing peak levels(59). Conversely, TRF increases the peak expression levels both of activators and repressors of core clock components, including that of the repressors Cry1, Rev-erbα, and Per2, and of the activator Bmal1 (49). Core clock components affect metabolism through direct transcriptional regulation or through modulating signaling or transcription of interacting factors (14). Accordingly, increased expression of clock components for a few hours every 24 h modulates the expression or function of several regulators of glucose homeostasis.
Blood glucose is tightly regulated and is maintained within a narrow range. The liver plays an important role in glucose homeostasis. During the fasting state, the pancreatic hormone glucagon increases phosphorylation of the transcription factor CREB, which activates downstream target genes to promote gluconeogenesis to maintain blood glucose (5, 75). In mice fed a high-fat diet ad libitum, CREB remains phosphorylated during both the fasted and fed states. CREB phosphorylation during the fed state fails to suppress gluconeogenesis when it is not needed, and this excess gluconeogenesis likely contributes to the elevated blood glucose level. TRF restores the daily rhythm in CREB phosphorylation, with elevated levels of phosphorylated CREB (pCREB) during the fasted phase (49). Such rhythmic abundance of pCREB, with reduced pCREB levels during the fed state, also parallels a reduction in the expression of CREB target genes, which are implicated in gluconeogenesis. Parallel liver metabolomic analyses have also indicated that TRF reduces gluconeogenesis, promotes the tricarboxylic acid cycle (TCA), and shuttles glucose toward the pentose phosphate pathway (49).
The pentose sugars generated by the pentose phosphate pathway and some intermediates of the TCA cycle are substrates both for the de novo and the salvage pathways of nucleotide biosynthesis. Accordingly, increased levels of several nucleotides in mice exposed to TRF are also observed (49). During the fed state, glucose is also utilized for glycogen synthesis. Electron microscopy images of TRF mouse liver show increased levels of glycogen (49). Overall, these coordinated changes in gene expression and metabolites imply that TRF reprograms glucose metabolism away from gluconeogenesis and toward anabolic pathways.
TRF reduces insulin resistance by an unknown mechanism. Both fasting and postprandial levels of serum insulin levels are relatively high in mice fed a high-fat diet ad libitum, while TRF reverses this trend (23, 49). Insulin activates mTOR, which in turn phosphorylates S6 (24, 34). Levels of phosphorylated S6 (pS6) are typically higher in the fed state or at night. However, mice on ad libitum feeding of a high-fat diet show an inverted pS6 rhythm, with higher levels during the day. TRF corrects this temporal activation of pS6 and aligns the higher levels with the fed state (49).
Recent human studies have also shown that TRE can improve cardiometabolic health. TRE with a 6-h daily interval (from 8:00 a.m. to 2:00 p.m.) for 5 weeks was able to increase β-cell function and insulin sensitivity and to decrease postprandial insulin, oxidative stress, blood pressure, and appetite (116). A crossover study in men at risk for type 2 diabetes found that early TRE (from 8:00 a.m. to 5:00 p.m.) and delayed TRE (from 12:00 p.m. to 9:00 p.m.) improved glycemic response to a test meal, but only the early TRE led to a decrease in fasting glucose (53).
Time-Restricted Feeding and Lipid Metabolism in the Liver
Both nutrient-sensing pathways and circadian clock components are implicated in hepatic lipid homeostasis (9, 86). The prolonged fasting in TRF mice mildly elevates hepatic AMP levels, which in turn activate AMP kinase, one of the key energy sensors of the cell. One of the known targets of AMPK is acetyl-CoA carboxylase (ACC). ACC catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the first and rate-limiting step in fatty acid synthesis. Phosphorylated ACC (pACC) is enzymatically inactive and, hence, cannot mediate the first step in lipid synthesis. Increased AMP levels in the liver of TRF mice activate AMPK, which, in turn, phosphorylates ACC, and this may reduce de novo lipogenesis. TRF also increases the peak expression level of the circadian clock repressor component Rev-erb. Rev-erb acts as a repressor of several genes implicated in lipid synthesis (26). Mice carrying the loss-of-function allele of Rev-erb show excessive fat accumulation in the liver and fatty liver disease (4, 18, 37). Conversely, increased expression of Rev-erb in the liver of TRF mice parallels reduced expression of its direct target and a key lipogenic gene, Fasn. Reduced Fasn messenger RNA levels and increased relative pACC levels act synergistically to reduce fatty acid synthesis, leading to reduced levels of several free fatty acids.
Reduced fatty acid synthesis also promotes fatty acid oxidation. Malonyl-CoA, a product of ACC activity in the first step of fatty acid synthesis, allosterically inhibits mitochondrial carnitine palmitoyltransferase (CPT). CPT is essential for the transit of long-chain fatty acids and acylcarnitine esters into the mitochondria for β-oxidation. In the liver of ad libitum–fed mice, elevated levels of malonylcarnitine imply increased inhibition of CPT and reduced β-oxidation caused by impaired entry of fatty acids into the mitochondria (49). Conversely, in the liver of TRF mice, decreased ACC activity reduces malonylcarnitine levels. Although definitive proof of more fatty acid oxidation in the TRF liver is lacking, the reduced volume of intracellular lipid droplets in hepatocytes and increased levels of β-hydroxybutyrate, one of the end products of β-oxidation, in liver from TRF mice relative to liver from ad libitum–fed mice indicate that TRF enhances lipolysis and β-oxidation, further contributing to a reduction in liver free fatty acids.
A beneficial by-product of reduced fatty acid synthesis and increased β-oxidation is the overall reduction in the free fatty acid pool in the TRF liver and reduced inflammation. The increased pool of free fatty acids in the liver of ad libitum–fed mice becomes a substrate for the increased production of the proinflammatory long-chain n-6 fatty acids dihomo-linoleate (20:2n-6) and arachidonate (20:4n-6). Liver from ad libitum–fed mice is prone to more oxidative stress as it contains relatively less reduced glutathione, a major cellular antioxidant. Oxidation of arachi-donate and linoleic acid in the liver of the ad libitum–fed mice further increased the levels of the proinflammatory eicosanoids 15-hydroxyeicosatetraenoic acid (HETE), 5-HETE, and 13-hydroxyoctadecadienoic acid. In contrast, reduced hepatic lipid levels along with the glutathione-enriched cellular environment in the liver of TRF mice attenuated the levels of proinflammatory lipids and reduced the levels of plasma alanine transaminase, a biomarker of fatty liver disease (49). Histological examination of liver from TRF mice also supports a healthier cellular content and reduced pathological phenotype. The liver of TRF mice had significantly less hepatic steatosis compared with the liver from ad libitum–fed mice (49, 109).
Time-Restricted Feeding and Cholesterol Metabolism in the Liver
The mammalian liver synthesizes up to 20% of the whole body cholesterol pool and also regulates cholesterol break down for the production of bile acids and other sterols (74). Both the diurnal rhythms in food intake and the clock component Rev-erb are known to affect cholesterol biosynthesis and break down to bile acids through the transcriptional regulation of several genes, including Cyp7 (42, 64, 65). The messenger RNA expression of two enzymes at the committing step of the classical and acidic pathways of bile acid synthesis, Cyp7a1 and Cyp7b1, respectively, are indicators of cholesterol catabolic activity. Relative to liver from ad libitum–fed mice, the peak expression of Cyp7a1 was elevated in TRF groups. Additionally, the expression of Cyp7b1 was elevated throughout 24 h in the liver of TRF mice (23, 49). Gene expression of cholesterol anabolic and catabolic enzymes is also regulated by the transcription factor sterol regulatory element binding protein (SREBP). Hepatic SREBP was elevated in TRF mice compared with ad libitum–fed mice. Specifically, the active cleaved form of SREBP was rhythmic, with higher expression during the dark–feeding phase in the liver of TRF mice (23). The total active form is reduced in the liver of ad libitum-fed mice. Together, these changes in Cyp7a1 and Cyp7b1 correlated with a net increase in the break down of cholesterol, leading to a concomitant reduction in cholesterol and increase in various bile acids in the liver of TRF mice (23, 49).
TIME-RESTRICTED FEEDING/EATING AND ADIPOSE TISSUE FUNCTION
Rodents on TRF always exhibit a reduction in adipose tissue mass, while lean mass is preserved (21, 23, 49, 109). This is also observed among some humans undergoing TRE (82). The difference in body weight between ad libitum–fed and TRF groups fed an obesogenic diet for 18 weeks can be as high as 28%, and most of the difference in body weight is due to a difference in adiposity (21, 23, 49, 109). Gene expression and biochemical studies are largely focused on the liver, and little is known about molecular changes in adipose tissues. The reduction in adipose tissue mass in obese mice subjected to TRF suggests that an overall increase in fatty acid oxidation under TRF may contribute to reduced adiposity in TRF mice.
Both white adipose tissue and brown adipose tissue in mice fed an obesogenic diet ad libitum show hypertrophy and whitening of the brown fat. Conversely, white adipose tissue in TRF mice has smaller adipocytes, and the brown adipose tissue has more browning, as observed under hematoxylin and eosin staining of tissue sections (21, 23, 49).
Activation of tissue-resident macrophages is known to increase tissue inflammation and contribute to obesity-related comorbidities. White adipose tissue in TRF mice shows reduced levels of crown-like structures, which are largely infiltrating macrophages, and a parallel reduction in many proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6 and chemokine (C-X-C motif) ligand 2 (49).
TIME-RESTRICTED FEEDING AND HEART HEALTH
One of the major adverse outcomes of chronic circadian disruption, as occurs in shift workers, is an increased risk for cardiovascular diseases (70, 94). In fact, among active duty firefighters, it is the major cause of death and disability while at work (32, 112). Even among the general population, cardiovascular diseases remain the number one cause of death worldwide (69). Accordingly, there is increasing interest in the circadian regulation of heart health. Time series gene expression studies of the cardiovascular system—including the atrium, ventricle, aorta, and endothelial cells—have found hundreds to thousands of genes showing circadian rhythms (17, 60, 104). The functional significance of these rhythms in heart health is clearly demonstrated in various genetic perturbation models (reviewed in 91). For example, Bmal1 knockout mice show increased susceptibility to atherosclerosis, which may partly arise from systemic metabolic disruption in these mice (103).
The effect of TRF on age-related cardiac decline has been extensively studied in Drosophila. The Drosophila model has been well established for exploring the genetic basis of the deterioration of cardiac function that arises due to aging or an energy-dense diet (15, 77, 88, 130). The primary function of the fruit fly’s heart is to pump hemolymph through the rhythmic diametric expansion (diastole) and contraction (systole) of the heart tube. By combining semi-intact in vivo preparation with high-speed imaging, cardiac rhythms can be imaged for a sufficient period of time to quantitatively assess various cardiac function parameters. TRF of young Drosophila from 2 weeks of age for 12 h every day did not reduce their daily caloric intake, yet the flies did not gain weight between 3 and 7 weeks of age, a substantial portion of the lifespan for Drosophila. Ad libitum–fed flies showed characteristic features of cardiac aging, marked by increases in the diastolic interval, systolic interval, heart period, and arrhythmia and by reductions in systolic diameter, diastolic diameter, and in radial contractility (43). Both male and female TRF flies exhibited attenuation of this age-dependent deterioration of cardiac function independent of strain background (43).
Gene expression profiling of head, body, and heart tissues from Drosophila revealed that the gene expression signature of TRF is distinct from that of CR (38, 43). In the Drosophila heart, TRF led to downregulation of a cluster of genes encoding for mitochondrial electron transport chains, which may indirectly reduce reactive oxygen species and protect the heart. TRF also led to a coordinated increase in the expression of various subunits of an adenosine triphosphate-dependent chaperonin complex (chaperonin containing T-complex protein 1 subunits, or CCT complex) (33, 132). This chaperonin complex is known to facilitate the proper folding of cytoskeletal proteins.
While CCT complex expression increased in the heart, the expression of genes encoding various cytoskeletal monomers or subunits actually decreased by as much as 20%. Increased chaperonin expression coupled with reduced expression of cytoskeleton genes likely reduced the overall fraction of unfolded or misfolded cytoskeletal proteins and improved cardiac function. Genetic perturbation of CCT subunits or of mitochondrial electron transport chain components supported the hypothesis that these changes in gene expression contributed to improved cardiac function in fruit flies (43). While these experiments are yet to be verified in vertebrates, genome-wide association studies have shown that a point mutation in CCT7 is associated with ventricular arrhythmia(35). Overall, the Drosophila TRF studies have revealed that changes in mitochondrial function and improvements in proteostasis may underlie some of the benefits of TRF.
Another outcome from the Drosophila TRF experiments was the observation that the TRF fruit flies showed improved sleep relative to the ad libitum–fed flies flies. Sleep improvement was seen in flies in group-housed condition, while no difference in sleep in older ad libitum–fed or TRF flies was found when flies were individually housed (43). Together these observations suggest that sleep improvement under group-housed condition is not due to changes in overall sleep pressure (which remained similar in individually housed flies) but rather to increases in the arousal threshold. Self-reported sleep improvement is also found in human volunteers undergoing TRE (44). As sleep improvement also benefits cardiac health, TRE might contribute to improved heart health both through direct tissue-autonomous mechanisms and indirect system-wide effects on improving sleep.
TIME-RESTRICTED FEEDING/EATING AND GUT HEALTH
Hunger and satiety operate at a higher level for ingestive behavior and, consequently, for optimal gut health. Although it may seem reasonable to assume that the daily rhythm of activity–rest cycles drive the daily rhythm of hunger, experiments both in animals and humans have shown that there is a circadian drive to hunger, which is likely a product of activity–rest cycles. In a constant-routine paradigm in humans, hunger rises in the afternoon and peaks in the evening (at about 8:00 p.m.) (106). Rodents on light–dark cycles or in constant darkness exhibit a feeding rhythm that aligns with the onset of activity in the evening (or subjective evening). However, mice carrying a point mutation in the clock gene Per1, exhibit an altered feeding rhythm that is uncoupled from their activity rhythm (67). These mice begin to eat a few hours before the habitual feeding time of wild-type mice, but they show no change in their daily activity–rest cycles. Ad libitum access to food in these Per1 mutant mice renders them obese. However, TRF can prevent excessive obesity and metabolic disease phenotypes in Per1 mutant mice (67). Such daytime eating behavior in Per1 mutant mice resembles night-eating syndrome in humans, in which individuals wake up in the middle of their sleep with extreme hunger and consume a substantial meal (107). Although this syndrome has not been subject to rigorous genetic analyses, the Per1 mutant mice support the idea that some eating disorders in humans may have an underlying circadian genetic basis. Nevertheless, the observation that the metabolic diseases in Per1 mutant mice can be prevented by TRF offers hope that some of the genetic predisposition to metabolic diseases in humans can be prevented by a behavioral intervention such as TRE.
Humans exhibit a daily rhythm in the production of saliva, gastric acids, digestive enzymes, and bile salts, the production of all of which declines late at night. Aligned with the production of these digestive agents, intestinal peristalsis also shows a daily rhythm, with reduced contractions at night. Finally, colonic movement increases in the early morning, driving a daily rhythm in excretion (39,61). With these rhythms in ingestion, secretion, digestion, absorption, and excretion, there is a daily rhythm in the gut chemical environment. Accordingly, the composition and function of the gut microbiome also vary throughout the day (118–120). The nightly rise in growth hormone from the pituitary works in concert with the nightly rise in mucus secretion and cellular repair and replication in the gut lining to maintain gut integrity (39, 61). Maintaining intestinal lining integrity is critical in to support the intestinal barrier function that is essential to prevent potential food allergens and bacterial lipopolysaccharides from breaching the gut and causing systemic inflammation. Although it is too early to measure how TRE affects these gut functions, these daily rhythms in gut physiology offer a framework and rationale for adopting TRE to sustain gut health.
One of the important effects of gut–brain communication is to assess meal size and provide information to the brain to modulate hunger and satiety. Mechanosensitive pathways that sense gut distension independent of nutrient composition and chemical pathways that sense meal composition participate in this gut–brain communication. Mechanosensitive gastric vagal afferents (GVAs) exhibit diurnal rhythmicity in their response to food-related stimuli, allowing for specific time-of-day satiety signaling (55). When an obesogenic diet is given ad libitum to rodents, this diurnal rhythmicity is ablated (56), which may enable animals to consume food at a non-preferred time. Therefore, a loss of diurnal rhythm in the GVA axis can further accentuate hyperphagia and obesity. TRF of the same obesogenic diet has been reported to restore the daily rhythm of GVA responsiveness to meal size (54). This result from studies in rats might explain the reduced hunger at bedtime seen among human volunteers practicing TRE (44, 116).
The gut microbiome is presumed to play an important role in host metabolism and physiology. Although it is generally accepted that having a diverse group of species in the gut microflora is protective against obesity and metabolic diseases, there was no significant difference in overall diversity or species richness between mice fed an obesogenic diet ad libitum or mice on TRF (133). However, it cannot be ruled out that the gene expression pattern of these species is affected by an ad libitum or TRF pattern, which might affect microbiome–host interaction, leading to changes in luminal metabolite content.
Metabolomic analyses of fecal matter from ad libitum–fed and TRF mice showed significant differences that may explain some of the improvements seen in the TRF mice. Hemicellulose in the diet is typically broken down to xylose and galactose by the gut microbes, and some of this is absorbed by the host. Relative to ad libitum–fed mice, TRF mice excreted significantly more xylose and galactose in their stool, which implies that TRF reduced host absorption of these simple sugars. The stool of TRF mice was also rich both in primary and secondary bile acids (133). Usually, a significant portion of bile acids is reabsorbed from the gut. Elevated levels of bile acids in the stool of TRF mice indicate that some of the reduction in hepatic and serum cholesterol in TRF mice may be due to the net elimination of bile acids in stool.
TIME-RESTRICTED FEEDING AND BRAIN HEALTH
Most studies in rodents and humans have focused on body weight, body composition, and metabolic health. Some preliminary results from animal experiments indicate that TRF may also benefit brain health, specifically by protecting against or delaying the onset or development of neurodegenerative diseases. Gene expression and phenotypic assessments of TRF and ad libitum–fed flies and mice have shown several molecular signatures that support this notion: (a) TRF reduces mitochondrial electron transport chain activity, thereby potentially reducing the levels of reactive oxygen species in Drosophila heart (43); (b) TRF improves the structural integrity of the endoplasmic reticulum and mitochondria in Drosophila heart and mouse liver (43, 49); (c) TRF enhances the integrative stress response by slightly reducing mTOR activation and increasing the unfolded protein response in mouse liver (21); (d) in rodents, TRF improves motor coordination (21, 23,49); and (e) Drosophila on TRF show sleep improvement (43) (Figure 3). If some of these results from non neuronal tissues extend to the nervous system, we should anticipate the alleviation of disease severity in animal models of neurodegenerative diseases under TRF. Two recent studies have demonstrated that mouse models of Huntington’s disease subject to TRF show improvement in motor coordination, sleep, and autonomic nervous system function (126, 128). Also, gene expression markers of the disease in the striatum were normalized in TRF mice (126). These early indications in animal models raise the hope that TRE in humans may delay the onset of neurode-generative diseases or reduce the severity of these diseases.
PERSPECTIVE ON THE FUTURE OF TIME-RESTRICTED EATING
The demonstrated success of TRF in combating obesity and metabolic diseases in animals is raising substantial hope for its long-term potential in improving public health and reducing healthcare costs. Although nutrition management is considered to be central to combat obesity and metabolic diseases, the emphasis has so far been on caloric reduction and nutritional quality amelioration. The effectiveness of these approaches for health improvement may be substantially increased if they are combined with optimal timing for meals. As rodent studies have indicated, if ingested at the wrong circadian time, even reduced-calorie diets may not achieve their full potential in reducing body weight (1). Similarly, human volunteers going through nutrition-based weight-loss programs fail to achieve optimal weight loss if calories are consumed later in the circadian day (41). Recently, the ketogenic diet has been considered to be effective in managing metabolic diseases, including diabetes. However, as seen in a recent rodent study, a ketogenic diet given ad libitum led to excessive weight gain, while a ketogenic diet combined with TRF improved several markers of health and increased the healthy life span (87, 101). Altogether, these observations suggest that traditional and emerging approaches for weight and disease management can benefit from being combined with TRE.
Rigorous TRE without caloric reduction, as rodents were subject to, is difficult to implement in large human cohorts. To our knowledge, only a few studies have been published that used TRE as a health intervention (6, 40, 44, 53, 66, 82, 116, 121) (Table 1), yet many are ongoing. Nevertheless, a controlled study on a small cohort has shown that TRE of 6 hours (versus 12 h) for 5 weeks did not lead to weight loss, but it did increase β-cell function and insulin sensitivity and decreased post-prandial insulin, oxidative stress, blood pressure, and appetite (116). This preliminary human study lends support to the idea that TRE can improve human health even without a reduction in weight.
Table 1.
Studies of time-restricted eating (TRE) interventions in humans
| Study | Duration of eating window | Time of eating window | Duration of TRE | Participants | Study design | Nutrition during TRE | Result of TRE |
|---|---|---|---|---|---|---|---|
| Hutchison et al. (53) | 9 h | Early: 8:00 a.m. to 5:00 p.m. Delayed: 12:00 p.m. to 9:00 p.m. |
7 days in each condition | N = 15 males; overweight; age 55 ± 3 years | Crossover | No data | ↓ Body weight No change in body fat ↓ Glucose incremental area under the curve for a mixed-nutrient meal for both early and delayed TRE ↓ Mean fasting glucose in early TRE using a continuous glucose monitor ↓ Fasting triglycerides for both early and delayed TRE |
| Gabel et al. (40) | 8 h | 10:00 a.m. to 6:00 p.m. | 12 weeks | TRE group: n = 23 (20 females and 3 males); BMI 35 ± 1; age 50 ± 2 years Control group: n = 23 (21 females and 2 males); BMI 34 ± 1; age 48 ± 2 years |
Matched historical controls | No data | ↓ Body weight No change in body fat ↓ Energy intake ↓ Blood pressure |
| Antoni et al. (6) | Varied: mean 517 min (8 h 37 min), SEM = 22 min | Breakfast delayed and dinner advanced by 1.5 h each | 10 weeks | TRE group: n = 7 (6 female, 1 male) Control group: n = 6 (females) Baseline BMI for both groups: 20–39 Baseline age for both groups: 29–57 years |
Randomized controlled trial | No data | No change in body weight ↓ Body fat ↓ Energy intake |
| Sutton et al. (116) | 6 h or 12 h | 8:00 a.m. to 2:00 p.m. or 8:00 a.m. to 8:00 p.m. | 5 weeks in each condition | N = 8 (males); overweight and prediabetic; age 56 ± 9 years | Crossover | 3 meals/day provided with matched nutrients | No change in body weight Body fat not measured ↑ Insulin sensitivity, (3-cell function, and fasting triglycerides ↓ Blood pressure, oxidative stress, postprandial insulin, evening appetite |
| Moro et al. (82) | 8 h | TRE group: 1:00 p.m.to 9:00 p.m. or Control group: 8:00 a.m.to 9:00 p.m. |
8 weeks | TRE group: n = 17 (males); RT; BMI = 29.94 ± 4.07 Control group: n = 17 (males); RT; BMI = 28.47 ± 3.48 |
Randomized controlled trial | 3 meals/day with nutrients matched to food journals | No change in body weight ↓ Body fat ↓ Testosterone and insulin-like growth factor 1 Maintained muscle mass |
| Tinsley et al. (121) | 4 h/4 days per week and then unrestricted | 4 h between 4:00 p.m. and 12:00 a.m. on non-workout days | 8 weeks | TRE and RT: n = 10 (males); age 22.9 it 4.1 years Unrestricted eating and RT: n = 8 (males); age 22.0 ± 2.4 years |
Randomized controlled trial | No data | No change in body weight or body fat ↑ Upper and lower body strength and lower body muscular endurance ↓ Energy intake No adverse effects on lean mass retention or muscular improvements with short-term RT |
| Gill & Panda (44) | 10–12 h | Decided by participant | 16 weeks and 1 year follow-up | N = 8 (5 males, 3 females); BMI >25; and >14 h eating window at baseline; age 24–30 years | Within-participant | No data | ↓ Body weight Body fat not measured ↓ Hunger at night ↑ Morning and overall energy levels and sleep satisfaction |
| LeCheminant et al. (66) | 13 h | 6:00 a.m. to 7:00 p.m. | 2 weeks | N = 29 (males); healthy; age 20.9 ± 2.5 years | Crossover | No data | ↓ Body weight Body fat not measured ↓ Energy intake |
Abbreviations: BMI, body mass index; RT, resistance training.
A few pilot feasibility studies have shown that time restriction is often accompanied by caloric reduction (40, 44). In some of the pilot studies, CR was as much as 20% (44). Aiming for caloric reduction without making an overt attempt at calorie counting is remarkable because the most extensive and long-term caloric reduction study (CALERIE-2) achieved only 12% caloric reduction against a targeted reduction of 25% (28). In lieu of the practical outcome of TRE in reducing caloric intake in many individuals, it is highly likely that long-term efficacy studies of TRE on health outcomes will emerge from the contributions of CR, TRE, and circadian optimization.
The obvious first step in implementing TRE as a public health intervention will be to assess the size of the populations that habitually spread caloric intake over a longer period of time or changes sleep and eating patterns on the weekend. These populations will likely benefit from TRE. The next step will be to assess whether long-term TRE is a sustainable modifiable behavior. Although conventional thinking is that people usually eat three meals within a 12-h window every day (116), studies using the objective measures of temporal patterns of caloric intake among those who do not do shift work have found that only 10% of the adult population in a relatively small US cohort consumed all caloric intake within 12 h or less, and 50% consumed calories during a period of 15 h or longer (44). This eating pattern was also found among those in India who do not do shift work(46)—that is, in a country experiencing a rapid increase in metabolic diseases. Research into sleep habits among the general population in developed countries has also indicated that more than 75% of the adult population delays their wake-up time on the weekend, and they are also likely to shift their breakfast time on the weekend. Altogether, it is safe to assume that a large fraction of the population in both developing and developed countries would benefit from consistent TRE lasting for a period of 10–12 h.
There are few studies on the efficacy and mechanisms of TRE in humans (Table 1). TRE lasting for a period of 10 h was sustained for up to a year in a small cohort of eight adults. Although the sample size is small, the feasibility of sustaining TRE for such a long period and the accompanying weight loss of ~3.85% among overweight (not obese) adults is encouraging (44). Preliminary results from an obese cohort of 23 participants who lost a modest 2.6% of body weight and achieved a 7-mm reduction in systolic blood pressure are also encouraging (40). Although the study in the obese cohort targeted TRE to 8 h, participants were not closely monitored for adherence, whereas the study in overweight adults targeting TRE lasting for 10 h (in which participants achieved greater weight loss) was monitored through daily self-reporting using the myCircadianClock app (https://mycircadianclock.org/). Therefore, it is important to monitor time restriction both through self-reports and via sensors, such as continuous glucose monitors, and this practice should be followed in future studies to objectively assess adherence to TRE and health outcomes.
In summary, TRE has opened new avenues to assess the effects of the timing of eating on metabolism, physiology, and behavior. Although animal experiments have produced great results in preventing or reversing chronic metabolic diseases, the underlying mechanisms remain to be explored. More rigorous human studies are also needed to assess the mechanisms and efficacy of TRE in a wide range of diseases.
ACKNOWLEDGMENTS
S.P. received funding from the US National Institutes of Health (grants EY016807, DK118278, DK115214), Department of Defense (grant W81XWH1810645), and Department of Home-land Security (grant EMW-2016-FP-00788), as well as the Robert Wood Johnson Foundation (grant 76014), the Paul F. Glenn Center for the Biology of Aging, the Leona M. and Harry B. Helmsley Charitable Trust (grant2012-PG-ME002), and the Brown Foundation. E.N.C.M. is supported by a fellowship from the Larry L. Hillblom Foundation. A.C. received an American Heart Association Career Development Award (grant 18CDA34110292) and has received support from the Philippe Foundation; A.C. has also received a Women in Science Award from the Salk Institute.
DISCLOSURE STATEMENT
S.P. is the author of The Circadian Code: Lose Weight, Supercharge Your Energy and Transform Your Health from Morning to Midnight, for which he receives royalties. The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. 2017. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26:267–77.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, et al. 2014. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 19:319–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerstedt T, Knutsson A, Alfredsson L, Theorell T. 1984. Shift work and cardiovascular disease. Scand.J. Work Environ. Health 10:409–14 [DOI] [PubMed] [Google Scholar]
- 4.Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, et al. 2008. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456:997–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altarejos JY, Montminy M. 2011. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12:141–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoni R, Robertson TM, Robertson MD, Johnston JD. 2018. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci 7:e22 [Google Scholar]
- 7.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. 2009. Circadian timing of food intake contributes to weight gain. Obesity 17:2100–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, et al. 2008. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–28 [DOI] [PubMed] [Google Scholar]
- 9.Asher G, Sassone-Corsi P. 2015. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161:84–92 [DOI] [PubMed] [Google Scholar]
- 10.Asher G, Schibler U. 2011. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 13:125–37 [DOI] [PubMed] [Google Scholar]
- 11.Atger F, Gobet C, Marquis J, Martin E, Wang J, et al. 2015. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. PNAS 112:E6579–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, et al. 2012. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLOS ONE 7:e37150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bass J 2012. Circadian topology of metabolism. Nature 491:348–56 [DOI] [PubMed] [Google Scholar]
- 14.Bass J, Takahashi JS. 2010. Circadian integration of metabolism and energetics. Science 330:1349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, et al. 2010. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab 12:533–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, et al. 2009. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet 41:89–94 [DOI] [PubMed] [Google Scholar]
- 17.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, et al. 2008. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am. J. Physiol. Heart Circ. Physiol 294:H1036–47 [DOI] [PubMed] [Google Scholar]
- 18.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, et al. 2012. Rev-erbαand Rev-erbβcoordinately protect the circadian clock and normal metabolic function. Genes Dev 26:657–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buttorff C, Ruder T, Bauman M. 2017. Multiple Chronic Conditions in the United States. Santa Monica, CA.: Rand Corp. [Google Scholar]
- 20.Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, et al. 2013. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 128:337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaix A, Lin T, Le HD, Chang MW, Panda S. 2019. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29:303–19.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaix A, Panda S. 2016. Ketone bodies signal opportunistic food-seeking activity. Trends Endocrinol. Metab 27:350–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaix A, Zarrinpar A, Miu P, Panda S. 2014. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20:991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chantranupong L, Wolfson RL, Sabatini DM. 2015. Nutrient-sensing mechanisms across evolution. Cell 161:67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavan R, Feillet C, Costa SS, Delorme JE, Okabe T, et al. 2016. Liver-derived ketone bodies are necessary for food anticipation. Nat. Commun 7:10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, et al. 2012. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485:123–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das SK, Roberts SB, Bhapkar MV, Villareal DT, Fontana L, et al. 2017. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am. J. Clin. Nutr 105:913–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. 2006. Chronic jet-lag increases mortality in aged mice. Curr. Biol 16:R914–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis S, Mirick DK, Stevens RG. 2001. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst 93:1557–62 [DOI] [PubMed] [Google Scholar]
- 31.Di Francesco A, Di Germanio C, Bernier M, de Cabo R. 2018. A time to fast. Science 362:770–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donovan R, Nelson T, Peel J, Lipsey T, Voyles W, Israel RG. 2009. Cardiorespiratory fitness and the metabolic syndrome in firefighters. Occup. Med 59:487–92 [DOI] [PubMed] [Google Scholar]
- 33.Dunn AY, Melville MW, Frydman J. 2001. Cellular substrates of the eukaryotic chaperonin TRiC/CCT.J. Struct. Biol 135:176–84 [DOI] [PubMed] [Google Scholar]
- 34.Efeyan A, Comb WC, Sabatini DM. 2015. Nutrient-sensing mechanisms and pathways. Nature 517:302–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, et al. 2013. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 504:432–36 [DOI] [PubMed] [Google Scholar]
- 36.Escobar C, Díaz-Muñoz M, Encinas F, Aguilar-Roblero R. 1998. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am. J. Physiol 274:R1309–16 [DOI] [PubMed] [Google Scholar]
- 37.Everett LJ, Lazar MA. 2014. Nuclear receptor Rev-erbα: up, down, and all around. Trends Endocrinol. Metab 25:586–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farhadian SF, Suarez-Farinas M, Cho CE, Pellegrino M, Vosshall LB. 2012. Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiol. Behav 105:544–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster JA, McVey Neufeld KA. 2013. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–12 [DOI] [PubMed] [Google Scholar]
- 40.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, et al. 2018. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging 4:345–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. 2013. Timing of food intake predicts weight loss effectiveness. Int. J. Obes 37:604–11. Erratum. 2013. Int. J. Obes. 37:624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatfield D, Le Martelot G, Vejnar CE, Gerlach D, Schaad O, et al. 2009. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev 23:1313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill S, Le HD, Melkani GC, Panda S. 2015. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347:1265–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill S, Panda S. 2015. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 22:789–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, et al. 2008. Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature 453:102–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta NJ, Kumar V, Panda S. 2017. A camera-phone based study reveals erratic eating pattern and disrupted daily eating–fasting cycle among adults in India. PLOS ONE 12:e0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, et al. 2008. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLOS ONE 3:e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatori M, Panda S. 2010. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol. Med 16:435–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, et al. 2012. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15:848–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295:1065–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, et al. 2015. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health 1:233–43 [DOI] [PubMed] [Google Scholar]
- 52.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, et al. 2009. Harmonics of circadian gene transcription in mammals. PLOS Genet 5:e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutchison AT, Regmi P, Manoogian EN, Fleischer JG, Wittert GA, et al. 2013. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity. 27:724–32 [DOI] [PubMed] [Google Scholar]
- 54.Kentish SJ, Hatzinikolas G, Li H, Frisby CA, Wittert GA, Page AJ. 2018. Time restricted feeding prevents ablation of diurnal rhythms in gastric vagal afferent mechanosensitivity observed in high-fat diet-induced obese mice. J. Neurosci 38:5088–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kentish SJ, Page AJ. 2014. Plasticity of gastro-intestinal vagal afferent endings. Physiol. Behav 136:170–78 [DOI] [PubMed] [Google Scholar]
- 56.Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. 2016. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J. Neurosci 36:3199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knutsson A 2003. Health disorders of shift workers. Occup. Med 53:103–8 [DOI] [PubMed] [Google Scholar]
- 58.Kogevinas M, Espinosa A, Castello A, Gomez-Acebo I, Guevara M, et al. 2018. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study). Int. J. Cancer 143:2380–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, et al. 2007. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–21 [DOI] [PubMed] [Google Scholar]
- 60.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, et al. 2012. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338:349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konturek PC, Brzozowski T, Konturek SJ. 2011. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol 62:139–50 [PubMed] [Google Scholar]
- 62.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, et al. 2011. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480:552–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, et al. 2009. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326:437–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavery DJ, Schibler U. 1993. Circadian transcription of the cholesterol 7αhydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev 7:1871–84 [DOI] [PubMed] [Google Scholar]
- 65.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, et al. 2009. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLOS Biol 7:e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LeCheminant JD, Christenson E, Bailey BW, Tucker LA. 2013. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br. J. Nutr 110:2108–13 [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, Huang M, Wu X, Shi G, Xing L, et al. 2014. PER1 phosphorylation specifies feeding rhythm in mice. Cell Rep 7:1509–20 [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Minguez J, Saxena R, Bandín C, Scheer FA, Garaulet M. 2017. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin. Nutr 37:1133–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, et al. 2017. Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ 607–608:1073–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, et al. 2009. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet 41:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, et al. 2015. Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009–2010). Cancer Epidemiol. Biomark. Prev 24:783–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, et al. 2013. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. PNAS 110:5695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maxfield FR, Tabas I. 2005. Role of cholesterol and lipid organization in disease. Nature 438:612–21 [DOI] [PubMed] [Google Scholar]
- 75.Mayr B, Montminy M. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol 2:599–609 [DOI] [PubMed] [Google Scholar]
- 76.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. 2005. Night work and breast cancer risk: a systematic review and meta-analysis. Eur. J. Cancer 41:2023–32 [DOI] [PubMed] [Google Scholar]
- 77.Melkani GC, Trujillo AS, Ramos R, Bodmer R, Bernstein SI, Ocorr K. 2013. Huntington’s disease induced cardiac amyloidosis is reversed by modulating protein folding and oxidative stress pathways in the Drosophila heart. PLOS Genet 9:e1004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mistlberger RE. 1994. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev 18:171–95 [DOI] [PubMed] [Google Scholar]
- 79.Mitchell SE, Delville C, Konstantopedos P, Derous D, Green CL, et al. 2016. The effects of graded levels of calorie restriction: V. Impact of short term calorie and protein restriction on physical activity in the C57BL/6 mouse. Oncotarget 7:19147–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, et al. 2019. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 29:221–28.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohren DC, Jansen NW, Kant IJ, Galama J, van den Brandt PA, Swaen GM. 2002. Prevalence of common infections among employees in different work schedules. J. Occup. Environ. Med 44:1003–11 [DOI] [PubMed] [Google Scholar]
- 82.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, et al. 2016. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med 14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, et al. 2008. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134:329–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. 2009. Circadian control of the NAD+ salvage pathway by CLOCK–SIRT1. Science 324:654–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson W, Halberg F. 1986. Meal-timing, circadian rhythms and life span of mice. J. Nutr 116:2244–53 [DOI] [PubMed] [Google Scholar]
- 86.Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, et al. 2016. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. PNAS 113:E1673–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, et al. 2017. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 26:547–57.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ocorr K, Akasaka T, Bodmer R. 2007. Age-related cardiac disease model of Drosophila. Mech. Ageing Dev 128:112–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, et al. 2017. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health 3:6–19 [DOI] [PubMed] [Google Scholar]
- 90.Panda S 2016. Circadian physiology of metabolism. Science 354:1008–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paschos GK, FitzGerald GA. 2010. Circadian clocks and vascular function. Circ. Res 106:833–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, et al. 2015. Pancreatic βcell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350:aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Persaud SJ, Jones PM. 2016. A wake-up call for type 2 diabetes? N. Engl. J. Med 375:1090–92 [DOI] [PubMed] [Google Scholar]
- 94.Puttonen S, Harma M, Hublin C. 2010. Shift work and cardiovascular disease—pathways from circadian stress to morbidity. Scand. J. Work Environ. Health 36:96–108 [DOI] [PubMed] [Google Scholar]
- 95.Ramirez-Plascencia OD, Saderi N, Escobar C, Salgado-Delgado RC. 2017. Feeding during the rest phase promotes circadian conflict in nuclei that control energy homeostasis and sleep–wake cycle in rats. Eur. J. Neurosci 45:1325–32 [DOI] [PubMed] [Google Scholar]
- 96.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, et al. 2009. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324:651–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddy AB, O’Neill JS. 2010. Healthy clocks, healthy body, healthy mind. Trends Cell Biol 20:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reinke H, Saini C, Fleury-Olela F, Dibner C, Benjamin IJ, Schibler U. 2008. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev 22:331–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reiter RJ. 1991. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev 12:151–80 [DOI] [PubMed] [Google Scholar]
- 100.Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. 2011. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLOS Biol 9:e1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, et al. 2017. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 26:539–46.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ronn T, Wen J, Yang Z, Lu B, Du Y, et al. 2009. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 52:830–33 [DOI] [PubMed] [Google Scholar]
- 103.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, et al. 2004. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLOS Biol 2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, et al. 2005. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112:2716–24 [DOI] [PubMed] [Google Scholar]
- 105.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. 2010. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 151:1019–29 [DOI] [PubMed] [Google Scholar]
- 106.Scheer FA, Morris CJ, Shea SA. 2013. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 21:421–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schenck CH, Mahowald MW. 1994. Review of nocturnal sleep-related eating disorders. Int. J. Eat. Disord 15:343–56 [DOI] [PubMed] [Google Scholar]
- 108.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, et al. 2003. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl. Cancer Inst 95:825–28 [DOI] [PubMed] [Google Scholar]
- 109.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. 2012. Timed high-fat diet resets circa-dian metabolism and prevents obesity. FASEB J 26:3493–502 [DOI] [PubMed] [Google Scholar]
- 110.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. 2013. Circadian disruption leads to insulin resistance and obesity. Curr. Biol 23:372–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, et al. 2005. Brain and muscle Arnt-like protein-1(BMAL1), a component of the molecular clock, regulates adipogenesis. PNAS 102:12071–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soteriades ES, Smith DL, Tsismenakis AJ, Baur DM, Kales SN. 2011. Cardiovascular disease in US firefighters: a systematic review. Cardiol. Rev 19:202–15 [DOI] [PubMed] [Google Scholar]
- 113.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, et al. 2017. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 135:e96–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. 2001. Entrainment of the circadian clock in the liver by feeding. Science 291:490–93 [DOI] [PubMed] [Google Scholar]
- 115.Sturis J, Scheen AJ, Leproult R, Polonsky KS, van Cauter E. 1995. 24-hour glucose profiles during continuous or oscillatory insulin infusion: demonstration of the functional significance of ultradian insulin oscillations. J. Clin. Investig 95:1464–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. 2018. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27:1212–21.e3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi JS. 2017. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet 18:164–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, et al. 2016. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167:1495–510.e12 [DOI] [PubMed] [Google Scholar]
- 119.Thaiss CA, Zeevi D, Levy M, Segal E, Elinav E. 2015. A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes 6:137–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, et al. 2014. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159:514–29 [DOI] [PubMed] [Google Scholar]
- 121.Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, et al. 2017. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci 17:200–7 [DOI] [PubMed] [Google Scholar]
- 122.Tuomi T, Nagorny CL, Singh P, Bennet H, Yu Q, et al. 2016. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab 23:1067–77 [DOI] [PubMed] [Google Scholar]
- 123.Van Cauter E, Desir D, Decoster C, Fery F, Balasse EO. 1989. Nocturnal decrease in glucose tolerance during constant glucose infusion. J. Clin. Endocrinol. Metab 69:604–11 [DOI] [PubMed] [Google Scholar]
- 124.Viswanathan AN, Hankinson SE, Schernhammer ES. 2007. Night shift work and the risk of endometrial cancer. Cancer Res 67:10618–22 [DOI] [PubMed] [Google Scholar]
- 125.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. 2009. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. PNAS 106:21453–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang HB, Loh DH, Whittaker DS, Cutler T, Howland D, Colwell CS. 2018. Time-restricted feeding improves circadian dysfunction as well as motor symptoms in the Q175 mouse model of Huntington’s disease. eNeuro 5:0431–17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties.Annu. Rev. Physiol 72:551–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whittaker DS, Loh DH, Wang HB, Tahara Y, Kuljis D, et al. 2018. Circadian-based treatment strategy effective in the BACHD mouse model of Huntington’s disease. J. Biol. Rhythms 33:535–54 [DOI] [PubMed] [Google Scholar]
- 129.Wittmann M, Dinich J, Merrow M, Roenneberg T. 2006. Social jetlag: misalignment of biological and social time. Chronobiol. Int 23:497–509 [DOI] [PubMed] [Google Scholar]
- 130.Wolf MJ, Rockman HA. 2008. Drosophila melanogaster as a model system for genetics of postnatal cardiac function. Drug Discov. Today Dis. Models 5:117–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wood B, Rea MS, Plitnick B, Figueiro MG. 2013. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl. Ergon 44:237–40 [DOI] [PubMed] [Google Scholar]
- 132.Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. 1992. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature 358:245–48 [DOI] [PubMed] [Google Scholar]
- 133.Zarrinpar A, Chaix A, Yooseph S, Panda S. 2014. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 20:1006–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, et al. 2010. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med 16:1152–56 [DOI] [PMC free article] [PubMed] [Google Scholar]