Abstract
The Hedgehog (Hh) signaling pathway is crucial for the development of vertebrate and invertebrate animals alike. Hh ligand binds its receptor Patched (Ptc), allowing the activation of the obligate signal transducer Smoothened (Smo). The levels and localizations of both Ptc and Smo are regulated by ubiquitination, and Smo is under additional regulation by phosphorylation and SUMOylation. Downstream of Smo, the Ci/Gli family of transcription factors regulates the transcriptional responses to Hh. Phosphorylation, ubiquitination and SUMOylation are important for the stability and localization of Ci/Gli proteins and Hh signaling output. Finally, Suppressor of Fused directly regulates Ci/Gli proteins and itself is under proteolytic regulation that is critical for normal Hh signaling.
Keywords: Hh, Shh, Ptc, Ptch1, Smo, Ci, Gli1, Gli2, Gli3, Sufu, ubiquitin, SUMO, phosphorylation, PKA, GSK3, CK1, Grk2, Gprk2, Smurf, Slimb, Cul1, Cul3, Cul4, Usp8, Uchl5, proteasome, lysosome
1. Introduction
The Hedgehog (Hh) family of signaling proteins plays crucial roles in the development of all vertebrate and many invertebrate animals, and aberrant activation of the signaling pathway downstream of Hh proteins account for many malignancies in humans [1]. More than three decades of research into this pathway has culminated in the FDA approval of Vismodegib, a specific inhibitor of the Hh pathway, for the treatment of basal cell carcinoma, the most common form of skin cancer in the western world, whereas more potential drugs targeting various components of the pathway are being tested for many other types of cancers [2]. Given the great scientific and clinical importance of this signaling pathway, it is critical to understand how it is regulated.
1.1. The Hh signaling cascade
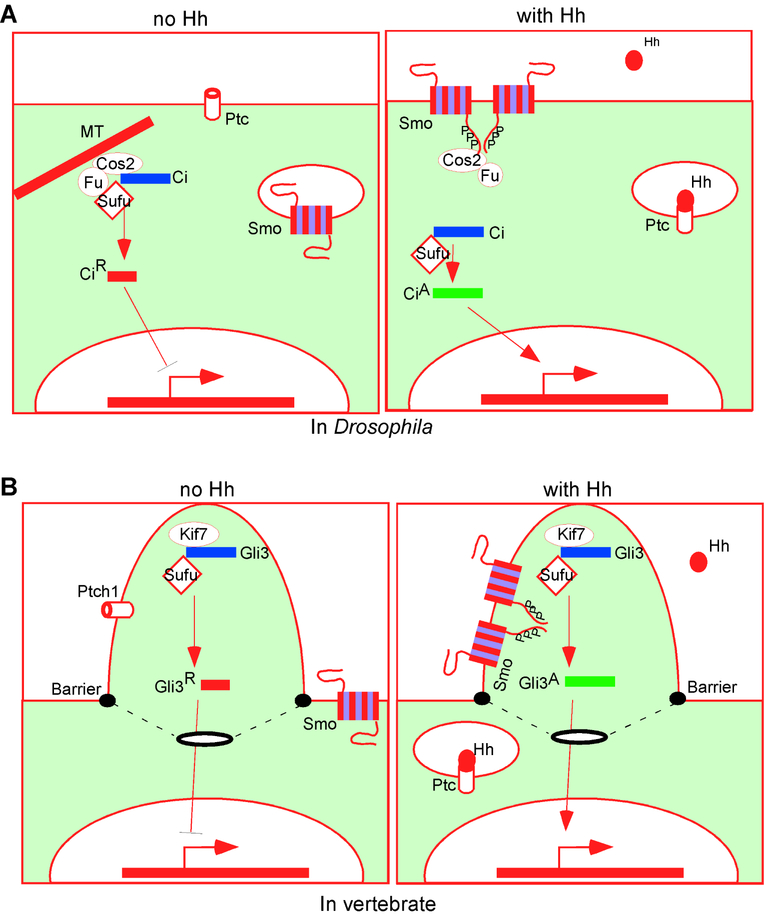
Hh was named after the spiny appearance of the Drosophila mutant cuticle due to the formation of denticles throughout each body segment in the absence of Hh [3]. Secreted Hh proteins bind their signal-transducing receptor Patched (Ptc), a twelve-span transmembrane protein similar to the bacterial proton-driven RND family of transporters [4–7] (Fig. 1A). Ptc inhibits the function of Smoothened (Smo), a seven-pass transmembrane protein similar to G-protein coupled receptors [8, 9]. Hh binding to Ptc relieves Smo from the Ptc inhibition, allowing it to activate the downstream transcription factor Cubitus interruptus (Ci) [10, 11]. In the absence of Hh, Ci is sequestered in a cytoplasmic complex comprising Costal2 (Cos2)/Fused (Fu)/Suppressor of Fused (Sufu), and is proteolytically processed into a shorter transcriptional repressor [10, 12–15]. Smo activation inhibits Ci processing and allows the full-length Ci to enter the nucleus and activate transcription of Ptc, Engrailed(En) and many tissue-specific targets [16–19].
Figure 1.
The outlines of Hh signaling
(A) In Drosophila, Ptc keeps Smo inactive, partly by promoting its internalization and degradation. Full-length Ci is sequestered in the cytoplasm in a complex with Cos2, Fu and Sufu, whereas Ci repressor (CiR) inhibits target gene expression in the nucleus. Hh blocks Ptc function, allowing the phosphorylation and dimerization/oligomerization of Smo, leading to Ci activation (CiA) and target gene expression. MT: microtubule;
(B) In vertebrates, Ptch1 in the primary cilia prevents Smo ciliary localization and activation. Gli3 enters the cilia with Sufu and Kif7 and is processed into Gli3R. Shh binds Ptch1, leading to Smo ciliary translocation, phosphorylation, dimerization/oligomerization and Gli3 activation (Gli3A). A membrane barrier near the base of the cilia prevents free diffusion of Smo.
In vertebrates, the Hh pathway is more complex due to gene duplication and the involvement of the primary cilia. For example, the mammalian Hh family has three members, Sonic hedgehog (Shh), Indian hedgehog (Ihh) and Desert hedgehog (Dhh), which have partially overlapping functions in development and diseases [20–25]. These Hh family members bind two Ptc homolog proteins (Ptch1 and Ptch2) on the target cells, relieving their inhibition on Smo [26–29] (Fig. 1B). There are three Ci homologs in mammals, the Glioma-associated oncogene family that includes Gli1, Gli2 and Gli3 [30]. Similar to Ci, Gli2 and Gli3 can serve as both transcriptional repressors and activators, whereas Gli 1 can only serve as a transcriptional activator [31, 32].
Interestingly, the requirements for Sufu in Hh signaling diverge between fruit flies and mammals. In flies, loss of Sufu suppresses the loss of Hh signaling phenotype of Fu mutants; however, Sufu mutant flies exhibit very subtle phenotype by itself [33]. In striking contrast, Sufu mutant mice exhibit aberrant activation of Hh signaling in the entire embryo, suggesting a possibility that mammals may lack the redundant pathway compensating for the loss of Sufu in fruit flies [34, 35].
The primary cilia are microtubule-based cell surface organelles present in almost all mammalian cells [36]. Genetic analyses revealed a surprising connection between mammalian Hh signaling and the cilia [37–39]. Subsequent cell biology studies indicated that most Hh pathway components, including Ptch1/2, Smo, Gli1/2/3 and Sufu, are localized to the primary cilia [40–44]. The importance of such localization has been demonstrated by removing some of these proteins from the cilia and examining the consequential disruption of Hh signaling [42, 45, 46].
1.2. The critical roles of Hh signaling in animal development
Hh signaling plays essential roles in numerous developmental processes in vertebrate and invertebrate animals [1]. For the better understanding of this review, I will briefly introduce a few systems in which Hh signaling has been extensively studied. In fruit flies, a well-studied organ is the wing imaginal disc (Fig. 2A). The fly wing disc is divided into the anterior (A) and posterior (P) compartments [47]. Hh is expressed at a high level in the P compartment, but P cells are not responsive to Hh due to the lack of Ci expression. In the A compartment, Hh secreted from the P compartment forms a gradient such that the cells by the A/P border receive the highest concentration of Hh, and respond by expressing En [48, 49], Cells a few cell diameters from the border receive intermediate levels of Hh exposure and express Ptc. Cells situated further away from the A/P boundary receive lower levels of Hh and express Decapendaplegic (Dpp) [10, 50]. Ectopic expression of Hh leads to induction of its target genes ectopically and duplication of the wing structure [51].
Figure 2.
Most popular systems for the studies of Hh signaling
(A) The wing imaginal disc in Drosophila. Hh is transcribed only in the posterior compartment, and form a gradient in the anterior compartment. The highest level of Hh signaling induces En expression immediately adjacent to the compartment boundary. An intermediate level of Hh signaling induces Ptc expression, and a lower level of Hh signaling induces Dpp expression, in cells further anterior in the A compartment. A: anterior; P: posterior; D: dorsal; V: ventral.
(B) The vertebrate neural tube. Shh is produced in the notochord (nc) and floor plate (FP), and forms a ventral-to-dorsal gradient. Decreasing concentrations of Shh are required to determine the fates of the V3 interneurons, motor neurons (MN), V2 and V1 interneurons.
(C) The vertebrate limbs. Shh is expressed in the posterior mesenchyme known as the zone of polarizing activity (ZPA), and regulates digit formation by antagonizing Gli3 repressor activity. Wild type (wt) mouse limbs have five digits. Shh mutant limbs have only one digit. Gli3 mutant limbs have 8–9 identical digits. A: anterior; P: posterior; P: proximal; D: distal.
In mammals, Shh is the most prevalent Hh family member that regulates, among many others, the dorsal/ventral (D/V) pattern of the neural tube (Fig. 2B) and the A/P pattern of the limbs (Fig. 2C) [22, 52]. In the neural tube, Shh is produced in the ventral most structure, the floor plate, and notochord, a mesodermal rod ventral to the neural tube [20, 21]. Following a ventral to dorsal order, cells of the floor plate, the V3 interneurons, motor neurons, V2 and V1 interneurons are all dependent on Shh, but require different amount of exposure [52]. In the limb buds, Shh is expressed in a small posterior-distal domain of mesoderm known as “the zone of polarizing activity” [22]. Both gain-of-function and loss-of-function studies in the chicken and mice showed that Shh is important in setting up the A/P polarity of the limbs and promoting the formation of digits [22, 53–55]. Interestingly, numerous digits form in mouse limbs with simultaneous loss of Gli3 and Shh, suggesting that Shh regulates digit formation by limiting the Gli3 repressor activity to the anterior regions of the limb buds [56].
The best-studied developmental role of Ihh is the regulation of skeletal development. Ihh is required for the proliferation and hypertrophy of chondrocytes [23, 57]. Meanwhile, it is also required for the differentiation of osteoblasts that replace chondrocytes in bones [58]. In addition, Ihh is also involved in vascular development and the development of endodermal organs [59, 60]. Dhh plays a crucial role in gonad development and is involved in axon myelination in peripheral nervous system in mice [24, 61].
1.3. The importance of proteostasis in Hh signaling
The levels, localizations and various post-translational modifications (PTMs) of the Hh pathway components are critical for the proper activation of the pathway and the developmental processes it regulates. At the cell surface, Hh activates downstream signaling partly by stabilizing Smo and promoting the internalization and degradation of Ptc. Inside the target cells, partial degradation of Ci, Gli2 and Gli3 through a proteasome-based mechanism leads to the production of their repressor forms. Additional proteasome and lysosome-based mechanisms regulate the stability of all Gli family members. The proteolytic control of Sufu also started to be revealed that could have important implications in Hh signaling regulation in mammals. Finally, Smo and Gli family members are also modified with Small Ubiquitin-like Modifier (SUMO), which generally stabilizes and activates the target proteins. Carefully designed in vivo studies have revealed the physiological significance of such regulations, and have suggested surprising complexities that were not expected from in vitro observation. In the following sections, I will summarize what we know about these processes and some remaining questions.
2. Proteostasis of Smo
2.1. The activation of Smo involves phosphorylation of its carboxyl-terminal tail and change in localization
Smo consists of an extracellular amino-terminal Cysteine-Rich Domain (CRD), seven transmembrane domains, and a cytoplasmic carboxyl-terminal tail (C-tail) (Fig. 3A) [8, 9]. Extensive studies on the biochemistry of Smo indicated that phosphorylation of the C-tail of Smo is an essential step of Smo activation [49, 62–66]. In the absence of Hh, clusters of Arginine residues in the Smo C-tail interact with acidic residues on the same molecule, leading to a closed, inactive conformation [64]. cAMP-dependent protein kinase (PKA) and Casein Kinase 1 (CK1) phosphorylate multiple Serines and Threonines in the Smo C-tail, abrogating the intramolecular electrostatic interaction and changing Smo into an open, active conformation [64]. On the other hand, protein phosphatase 1 (PP1), PP2A and PP4 inhibits Smo activity by removing PKA and CK1-mediated phosphorylation [67, 68]. Increasing Hh concentration leads to progressive phosphorylation of the Smo C-tail by activating the kinases and blocking the phosphatases, resulting in graded downstream responses.
Figure 3.
Proteostatic regulation of Smo and Ptc.
(A) In the absence of Hh, Ptc promotes the ubiquitination of Smo mediated by Smurf, internalization and degradation of Smo. Krz promotes Smo internalization in parallel to Smo ubiquitination. (B) In the presence of Hh, Smo is phosphorylated and SUMOylated, forming dimers/oligomers, dissociating from Smurf, and translocates to cell surface. Smurf associates with and ubiquitinates Ptc, leading to its internalization and degradation.
G protein coupled receptor kinase 2 (Gprk2) is also involved in the activation of Smo, partly by directly phosphorylating the C-tail of the Smo [69]. Interestingly, Gprk2 promotes Smo oligomerization independent of its kinase activity. In addition, Gprk2 enriches phosphatidylinositol 4-phosphate, which promotes Smo activation [70].
Hh and Ptc also regulate the dynamic localization of Smo. Ptc promotes the internalization of Smo, whereas Hh promotes Smo surface localization by inhibiting Ptc [62, 71]. Forced Smo localization to the plasma membrane activates Hh signaling, while trapping activated Smo in the endoplasmic reticulum blocks Hh signaling, indicating that surface localization is critical for its activation. Interestingly, blocking lysosomal degradation of Smo activates Smo without significantly increasing its cell surface localization, suggesting Smo activation does not result directly from its localization per se [72].
In Drosophila, β-arrestin Kurtz (Krz) promotes Smo internalization by recruiting Clathrin to Smo [73]. Gprk2 facilitates this process as β-arrestin specifically binds Smo phosphorylated by Gprk2.
CK1-mediated phosphorylation of the C-tail also underlies the activation of mammalian Smo [74]. In mammals, however, Smo needs to be in the primary cilia to be active [42]. In the absence of Hh, Smo is kept out of the cilia by Ptch1, which is localized to the cilia (Fig. 3B) [40]. The presence of a ligand, such as Shh, leads to the internalization of Ptch1, hence the ciliary translocation and activation of Smo [40]. Smo enters the cilia by lateral diffusion, and β-arrestin 1/2 mediate Smo interaction with a microtubule motor Kif3a [75, 76]. A Septin 2-based diffusion barrier at the base of the cilia keeps Smo from leaving the cilia and is required for cilia-dependent activation of Hh signaling [77].
Despite its structural similarity to the G-protein coupled receptor family, a conventional ligand for Smo has yet to be found. The prevailing view in the field is that the endogenous direct regulator of Smo is likely a small hydrophobic molecule similar to cholesterol. Many synthetic small molecule antagonists and agonists regulate Smo activity by targeting a site in the transmembrane domain [78–81]. In contrast, cholesterol and its derivatives bind the CRD of Smo, which is essential for Hh-induced Smo activation [79, 82–87]. In line with cholesterol or its derivatives being the endogenous Smo regulator, recent structural analyses suggested that Ptc could function as a steroid pump [88–90]. Ptch1 also pumps 7-dehydrocholesterol, a cholesterol precursor, out of the cell, and 7-dehydrochosterol and its derivative vitamin D3 bind and inhibit Smo activity [91].
2.2. The ubiquitination of Smo
In Drosophila, Ptc promotes, and Hh inhibits, the internalization and degradation of Smo [62]. Ubiquitination plays a key role in Smo internalization and degradation as targeting E1 ubiquitin activating enzyme Uba1 increased Smo at the cell surface [92]. Smo is both multi-monoubiquitinated and polyubiquitinated, and is degraded in both the lysosome and proteasome [92, 93]. Hh treatment, PKA and CK1-mediated Smo phosphorylation inhibit its ubiquitination, internalization and degradation, whereas Ptc promotes Smo ubiquitination. The deubiquitinating enzymes UBPY/Usp8 and Uchl5 interact with the C-tail of Smo and inhibits its ubiquitination [92–94]. Interestingly, Hh enchances the interaction between Uchl5 and Smo [94]. Finally, Krz acts in parallel to ubiquitination to promote Smo internalization and degradation [72]. The mammalian Smo is similarly multi-ubiquitinated and Shh treatment reduces its ubiquitination.
Endosomal Sorting Complexes Required for Transport (ESCRT) was known for its roles in mediating endosomal sorting of ubiquitinated membrane proteins [95]. RNAi knockdown of multiple ESCRT components, including Vps25, Vps28, Vps32 and Vps36, in S2 cells and wing imaginal disc, showed that ESCRT is involved in Smo internalization and degradation [72, 96]. Furthermore, the ESCRT complexes interact with ubiquitinated Smo C-tail via Vps36. As expected, Hh, phosphorylation of the Smo C-tail and deubiquitinating enzyme Usp8 inhibit the Smo/Vps36 interaction.
Recent studies through RNAi screen have identified two sets of E3 ligases for Smo-the Smurf family of E3 ubiquitin ligases and the Cul4-DDB1-Gß complex-that act in parallel to regulate Smo ubiquitination and cell surface expression [97, 98]. Gprk2 phosphorylates and activates HECT domain-containing E3 ubiquitin ligase Smurf, which catalyzes the ubiquitination of Smo [98]. Interestingly, in the presence of Hh, Smurf dissociates from Gprk2 and Smo, and associates with Ptc and promotes Ptc internalization. Both PKA and Gprk2 phosphorylate Smo C-tail, leading to the dissociation of Smurf from Smo. These data suggest that Gprk2 plays complex roles in Hh signaling.
A recent study implicated a Cul4-based ubiquitin ligase complex Cul4-DDB1-Gß in the ubiquitination of Smo and Gprk2 [97]. Again, Hh induces the dissociation of Cul4-DDB1 from Smo-Gß, allowing stabilization and surface localization of Smo.
In mammalian cells, Grk2 promotes Smo ciliary localization and Shh signaling, but Smo localization is not affected in Grk2 mutant cells, likely due to functional redundancy with other GRK family members or CK1 [74, 99, 100]. Grk2 acts genetically downstream of Smo but upstream of Gαs and Sufu [99]. Although the molecular mechanisms of its function remain incompletely understood, Grk2 may act, at least in part, by phosphorylating Smo [74].
2.3. The SUMOylation of Smo
Smo is also modified by the small ubiquitin-like modifier (SUMO) proteins. Hh promotes the SUMOylation of Smo, which leads to its stabilization [101, 102]. SUMOylation of Smo is not dependent on, and acts in parallel to, its phosphorylation in the activation of Smo. Furthermore, SUMOylation of Smo recruits the deubiquitination enzyme Usp8. This mechanism appears to be conserved as SUMOylation of mouse Smo leads to its ciliary localization [103].
Excess Krz recruits ubiquitin like protease 1 to prevent the SUMOylation of Smo, adding another mechanism by which it promotes the internalization and degradation of Smo [102].
3. Proteostasis of Gli transcription factors
3.1. Cul1 and βTRCP-based proteolytic processing of Gli family members
3.1.1. Slimb converts phosphorylated Ci into a transcriptional repressor
The roles of Ci in Hh signaling is complex as both loss of Ci and overexpression of Ci results in ectopic activation of Hh signaling in the wing disc [10]. The answer to this complex puzzle lies in the fact that Ci is proteolytically processed into a shorter, repressor form, and Hh inhibits this process (Fig. 4) [104]. As a result, Ci exists as a transcriptional activator in cells exposed to high levels of Hh, such as those near the A/P boundary of the wing disc, converting the Hh signal into a transcriptional response (Fig. 2A) [105]. On the other hand, Ci is efficiently processed into a repressor in cells not being exposed to Hh, such as those in the A compartment of the wing disc far away from the boundary, leading to the silencing of the Hh target genes. Reflecting this molecular complexity, the loss of Ci in Drosophila leads to upregulation of genes that are normally repressed by Ci repressor, and simultaneous downregulation of genes that are normally dependent on Ci activator [106].
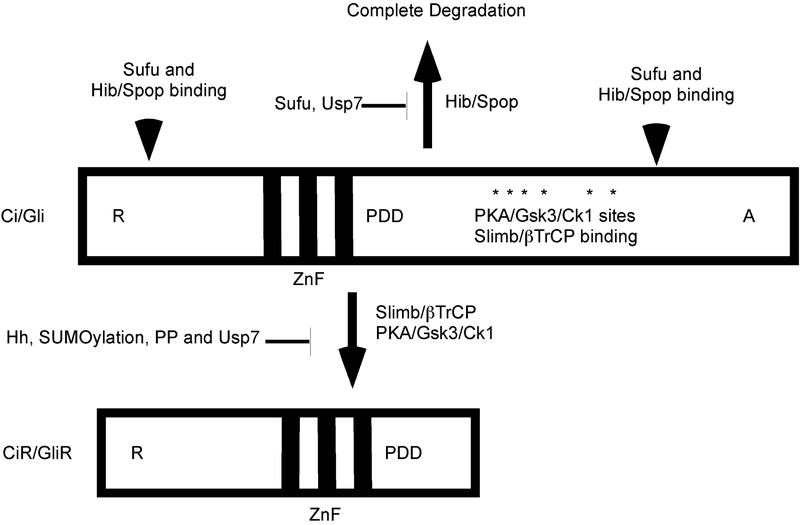
Figure 4.
Proteostatic regulation of Ci/Gli proteins
All Ci/Gli proteins, except mammalian Gli1, have an N-terminal repressor (R), a central zinc-figure (ZnF) and a C-terminal activator (A) domain. PKA/Gsk3/Ck1 phosphorylate a series of Serines/Threonines (asterisks) and promote ubiquitination of Ci/Gli proteins by Slimb/pTrCP and proteolytic processing of Ci/Gli proteins into repressors (CiR/GliR). SUMOylation, protein phosphatases (PP) and deubiquitinating enzymes (Usp8) inhibit Ci/Gli processing. A processing determinant domain (PDD) in Gli3 is required for efficient processing. Hib/Spop catalyzes the complete degradation of Ci/Gli proteins, and Sufu protects Ci/Gli proteins from this degradation. Sufu and Spop bind both the N- and C-termini of the Ci/Gli proteins.
Although PKA phosphorylation of Smo C-tail plays a critical role in Smo activation (see above), genetic analyses in Drosophila wing discs showed that PKA inhibited the expression of Hh target genes, suggesting an additional negative role of PKA downstream of Smo [107–110]. Later it was shown that PKA phosphorylates Ci and promotes its proteolytic processing into repressors [111–113]. Mutating 4 or 5 Serines targeted by PKA renders Ci resistant to proteolytic processing. Furthermore, PKA phosphorylation of Ci primes it for subsequent phosphorylation by Gsk3 and Ck1 at adjacent Serines or Threonines, which is also essential for Ci processing [114, 115].
Slimb, part of the SCF (Skp1/Cullin1/F-box) E3 ubiquitin ligase complex, is required for the proteolytic processing of Ci [116]. The phosphorylation of Ci by PKA, CK1 and GSK3 leads to Slimb binding and subsequent proteolytic processing of Ci [117–119]. Cos2 serves as a scaffold protein to bring the above kinases to Ci, leading to Ci phosphorylation and subsequent degradation [120].
A unique feature of Slimb-mediated Ci processing is that only the C-terminal region of the protein is degraded, whereas the N-terminal and DNA binding regions are spared. The DNA binding Zinc finger domain and a Lysine residue at position 750 together form a protection signal to limit proteasomal degradation of Ci to the C-terminal region [121]. Mutations to this signal prevent the formation of Ci repressor through partial degradation.
3.1.2. β TRCP mediates efficient processing of Gli3 and degradation of Gli2
In mammals, gene duplication adds another layer of complexity to the complex regulation of the Gli protein activities. Genetic studies indicated a primarily positive role of Gli2 and a negative role of Gli3 in Hh signaling, implying a potential difference in their proteolytic processing [122–124]. Indeed, when mammalian Gli proteins are expressed in the fly wing, Gli3 is efficiently processed into a repressor, whereas Gli2 processing is inefficient and Gli 1 is not processed into a repressor at all [125]. More direct evidence for the differential processing of Gli2 and Gli3 came from the examination of these proteins in vivo with antibodies against their N-termini. Gli3 is efficiently processed into a repressor form in the anterior half of the limb buds, whereas Shh limits both the overall expression level and proteolytic processing of Gli3 in the posterior half of the limb buds [31]. In contrast, Gli2 repressor is barely detectable in vivo, and Shh treatment primarily stabilizes full-length Gli2 rather than inhibiting the formation of a repressor [32].
PKA, CK1 and GSK3β phosphorylate Gli2 and Gli3 at multiple sites, and phosphorylation at these sites are critical for Gli3 processing and Gli2 degradation [31, 32]. The phosphorylation of Gli2 and Gli3 allows them to interact with βTrCP, the mammalian homolog of Slimb, which promotes their ubiquitination [32, 126].
The fact that Gli3 is proteolytically processed into a repressor whereas Gli2 is degraded in the proteasome results from their structural difference. A processing determinant domain (PDD) in the central region of Gli3 (residues 648–844) accounts for the much higher proteolytic processing efficiency in Gli3 than Gli2 [127]. Replacing the PDD of Gli3 with the corresponding region of Gli2 (residues 585–780) blocks Gli3 processing, whereas introduction of PDD to Gli2 leads to efficient processing.
The importance of PKA-mediated phosphorylation and subsequent degradation of Gli2 and Gli3 has been investigated genetically by gene replacement in mouse genome. Replacing the four Serines critical for Gli3 processing with Alanines in mouse genome results in the disruption of Gli3 processing and severe polydactyly similar to that of Gli3 null [128]. Replacing the four PKA sites in Gli2 in vivo (in Gli2P1−4 mice) leads to stabilization of the protein [129]. Unfortunately, The Gli2P1−4 heterozygotes in which the mutant version of Gli2 is presumably expressed at the wild type level die prematurely, preventing further characterization. The analysis of a different allele, GliP1−4neo, which transcribes the mutant Gli2 at a much lower level, showed an increase in Hh pathway activity, suggesting that PKA phosphorylation of Gli2 is a critical regulatory mechanism of Hh signaling in development.
Slimb-mediated ubiquitination of Ci may also lead to Ci degradation through the lysosomal pathway in the presence of Debra [130]. This lysosomal pathway of Ci degradation appears to be essential for maintaining gut homeostasis in flies [131]. Similar mechanism has not been reported in vertebrates.
3.1.3. Gli3 processing in vertebrates is dependent on the primary cilia
Gli3 repressor was greatly reduced while full-length Gli3 accumulated in mouse mutants with complete loss of the primary cilia, suggesting that Gli3 processing requires the cilia [37–39, 132]. Consistent with the decrease in Gli3 repressor, these mutants exhibit polydactyly. The localization of Gli3 to the tips of the cilia raises the possibility that Gli3 processing may occur in or near the cilia [41]. Interestingly, increasing cAMP levels by treating cells with Forskolin and/or IBMX blocks the ciliary entry of Gli3, raising the possibility that PKA phosphorylation of Gli3 may inhibit its ciliary entry or promote its ciliary exit [133, 134]. However, using a phospho-Gli3 specific antibody, it was recently found that phosphorylated Gli3 is localized to the ciliary tip and is reduced by Shh treatment [135]. Furthermore, Forskolin and IBMX treatment blocks Gli3 ciliary localization in PKA mutant cells, suggesting that Gli3 localization to the cilia is regulated by a PKA-independent mechanism [136]. Surprisingly, a recent report showed that blocking Gli3 ciliary entry did not affect Gli3 processing, raising the possibility that the roles of the cilium in Gli3 processing may be indirect [46].
3.2. Cul3 based degradation of Gli transcription factors
3.2.1. Hib targets Ci for degradation
In addition to the Slimb-mediated proteolytic processing, Ci is also under the regulation of a Cul3-based E3 ubiquitin ligase. Hh-induced MATH and BTB domain containing protein (Hib, also known as roadkill/rdx), the substrate-recognition subunit of a Cul3-based E3 ligase, is expressed in response to high levels of Hh signaling, and targets Ci for ubiquitination and degradation [137, 138]. The MATH domain of Hib interacts with both the N and C termini of Ci (Fig. 4) [137]. Unlike Slimb, which partially degrades Ci to produce a transcriptional repressor, Hib-mediated ubiquitination leads to complete degradation of Ci. Consistent with this role in Ci degradation, loss of Hib leads to ectopic activation of Hh signaling, making it a negative regulator of the pathway.
Multiple Serine and Threonine-rich motifs in Ci mediate its interaction with Hib [139]. Both Ci and Hib form dimers/oligomers, and the dimerization/oligomerization appears to be critical for efficient ubiquitination and degradation of Ci.
Although loss of Sufu does not cause drastic activation of Hh pathway, it does enhance Hh target gene expression on sensitized background, suggesting that Sufu is a negative regulator of the pathway [140]. Paradoxically, the full-length Ci was greatly reduced in the absence of Sufu, suggesting that Sufu protects Ci from degradation. Sufu prevents the association between Hib and Ci, implying that Hib may target activated Ci not protected by Sufu [137]. Downstream of Sufu, CK1 phosphorylation of multiple Serines on Ci prevents Hib-mediated Ci degradation, suggesting a surprising positive role for CK1 in Ci activation [141].
Another study found that Hib downregulates Ci in cells with moderate Hh pathway activation, but not in those with maximal Hh pathway activation [142]. Instead, Hib sequesters Ci in the cytoplasm in the presence of Hh. This appears to be inconsistent with the model that Hib specifically targets activated Ci for degradation, and the observation that Hib expression is enriched in cells with highest Hh signaling activity. Further investigation is thus needed to resolve this controversy.
3.2.2. Spop targets Gli2 and Gli3 for degradation in cultured cells
In striking contrast to Drosophila Sufu mutants that exhibit very subtle activation of the Hh pathway, the mouse Sufu mutants exhibit widespread ectopic activation of the Hh pathway [33–35]. Interestingly, the levels of Gli2 and Gli3 were drastically reduced in Sufu mutants [43, 44, 143]. Treating cultured cells with Shh similarly downregulates the Gli3 protein level [144, 145]. Knocking down a mouse Hib homologue, Speckle-type POZ Protein (Spop), in Sufu mutant fibroblasts, restored the levels of Gli2 and Gli3, suggesting that Sufu protects these Gli proteins from Spop-mediated degradation [69, 143]. Spop knockdown leads to a more significant increase in the levels of full-length Gli2 and Gli3 in the presence of Shh, suggesting that Spop preferentially degrades activated full-length Gli2 and Gli3 [145].
Serine and Threonine-rich Spop binding sites are present in all three Gli proteins, although in vitro studies indicated that only Gli2 and Gli3 are ubiquitinated and degraded by Spop [139]. It is worth mentioning that Spop appears to have a higher affinity to Gli3 than to Gli2.
Spop-like (Spopl) shares 81% sequence identity with Spop, but the function of Spopl is poorly understood [146]. Different from Spop, Spopl carries an extra 18-residue insertion in the BACK domain that prevents it from forming higher order assembly (i.e. multimers) and impairs its catalytic activity [147]. By forming heterodimers with Spop, Spopl also shifts Spop into smaller complexes (i.e. dimers), hence impairing the E3 ubiquitin ligase activity of Spop.
3.2.3. In vivo studies reveal a more specific function of Spop in Gli3 ubiquitination and degradation
For the in vivo requirement of Spop in mammalian development, two apparent null alleles of Spop in mice have been characterized [148, 149]. In contrast to Drosophila where Hib expression was under the regulation by Hh, Spop expression is more widespread in mouse embryos [43, 149]. Notably, it is expressed at higher levels in the cartilage and bones. Consistent with this expression pattern, Spop mutants exhibit defects in chondrocyte and osteoblast differentiation [149]. Contrary to the in vitro results suggesting that both Gli2 and Gli3 are Spop substrates, the level of Gli2 was not significantly affected in Spop mutants [43, 143, 145, 149]. On the other hand, both the full-length and the repressor form of Gli3 were significantly increased in the absence of Spop. Significantly, the skeletal defects of Spop mutants were rescued by reducing Gli3 dosage, suggesting that increased Gli3 repressor underlies these defects. Therefore, these in vivo studies reveal an important physiological role for Spop in the regulation of Gli3 repressor.
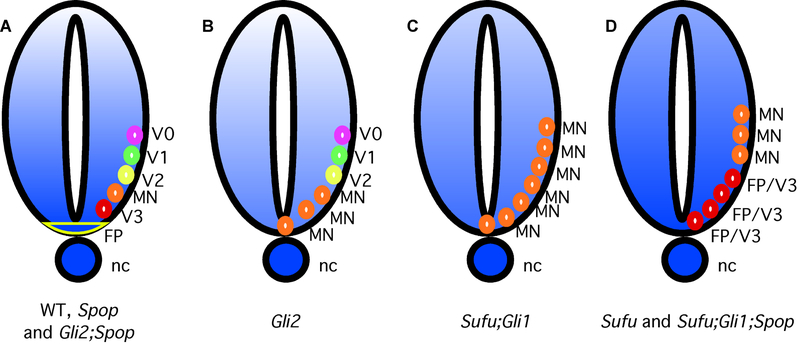
Despite the important roles of Hh signaling in neural tube patterning, the neural tube appears normal in Spop mutants, consistent with a minor role of Gli3 in this process (Fig. 5A) [148]. However, loss of Spop suppresses the partial loss of Hh signaling defects of Gli2 mutants (Fig. 5A and B). As Gli3 is the primary Hh pathway effector in the absence of Gli2, these data indicate that stabilization of Gli3 in the absence of Spop does increase Hh pathway activation.
Figure 5.
The importance of Sufu/Spop-mediated proteostatic regulation of Gli3 in neural tube patterning
(A) Gli2A and Gli3A are sufficient for patterning ventral NT properly in the presence (wt) and absence (Spop) of Spop. Increased Gli3A is sufficient for patterning ventral NT in the absence of Gli2 and Spop (Gli2;Spop). (B) Gli3A is insufficient for FP and V3 formation in Gli2 mutants. (C) In Sufu;Gli1 double mutants, greatly reduced Gli2A and Gli3A are not sufficient for FP and V3. (D) Increased Gli2A and Gli3A activities in Sufu;Gli1;Spop triple mutants support FP and V3 interneurons, similar to Sufu single mutants.
Although Glil is dispensable for neural tube patterning in the presence of Sufu, the floor plate and V3 interneurons that require high levels of Hh signaling activities fail to form in Sufu;Gli1 double mutants, revealing a surprising positive role of Sufu in Hh signaling (Fig. 5C) [150]. Interestingly, the formation of these structures are restored in Sufu;Gli1;Spop triple mutants, suggesting that Spop plays a negative role in Hh signaling in this context (Fig. 5D) [148]. Surprisingly, the levels of Gli2 and Gli3 were not significantly increased in Sufu;Spop double mutants compared to Sufu mutants, suggesting that Spop may inhibit their activities in addition to targeting them for degradation. It is possible that an alternative mechanism, such as altered subcellular localization as suggested earlier in Drosophila [142], may contribute to the difference. Indeed, overexpression of Spop appears to make Gli3 more cytoplasmic (Cai and Liu, unpublished data)[43].
3.3. Deubiquitination of Ci/Gli proteins
The deubiquitination enzyme Usp7 catalyzes the removal of ubiquitin from Ci, antagonizing both Cul1/Slimb-mediated processing and Cul3/Hib-mediated degradation [151]. Hh induces the interaction between Ci and Usp7. Similar function of Usp7 was also observed in zebrafish and mammals, suggesting that it is a conserved regulator of Ci/Gli stability and Hh signaling.
3.4. SUMOylation of the Gli proteins
The E3 SUMO ligase Pias1 mediates mammalian Gli SUMOylation [152, 153]. The roles of Gli phosphorylation in their SUMOylation have been controversial. One report found that phosphorylation by PKA inhibits the SUMOylation of Gli2 and Gli3, but not Gli1, and SUMOylation only happens to the full-length, but not the repressor form, of Gli3 [152]. In contrast, another report suggested that PKA promotes, and Hh inhibits, Gli2 SUMOylation [153]. More importantly, whether SUMOylation promotes or inhibits Gli activity is also under debate. Overexpression of Pias1 in the chicken neural tube induces ectopic Nkx2.2 expression, which can be inhibited by a dominant/negative form of Gli3, suggesting a potential positive role of SUMOylation in Gli activation [152]. On the other hand, Han et al (2012) found that replacing two Lysines critical for Gli2 SUMOylation with Arginines leads to elevated Hh signaling in the mouse neural tube, although the difference appears subtle [153]. The differences may result from different experimental paradigms (chicken vs mouse, overexpression of an enzyme that targets multiple substrates vs mutating one individual substrate), and additional rigorous investigation is needed to resolve this controversy.
SUMOylation also plays a role in regulating Ci activity in Drosophila [154]. RNAi knockdown of E2 conjugase Lwr and E3 SUMO ligase Su(Var) leads to reduced Ptc expression and disrupted testis development. It was found that Lwr interacts with Ci, and mutating all SUMOylation sites on Ci abrogates its activity, supporting a positive role of SUMOylation in Ci activation. It should be noted that mutating the SUMOylation sites on Ci reduces its activity in S2 cells but does not drastically change Hh pathway activity in wing discs [103].
4. Proteostasis of other pathway components
4.1. Proteostasis of Patched
Being an important regulator of Smo, the localization and level of Ptc itself is also under regulation. Upon Hh treatment, Ptc is internalized and degraded despite an increase in its transcription [62]. As reviewed above, Smurf promotes Smo ubiquitination and degradation in the absence of Hh; however, mutating Smurf in Drosophila or zebrafish leads to decreased Hh signaling, suggesting it must target additional components of the pathway [155]. Indeed, Smurf promotes Ptc ubiquitination and internalization by directly interacting with the C-tail of Ptc, and reducing Ptc dosage rescues the Hh signaling defects in Smurf1/2 morphant fish (Fig. 3). Interestingly, Smo promotes Smurf-mediated Ptc ubiquitination and degradation.
Combining the results in Smo and Ptc, a model was proposed to explain the complex roles of Smurf in Hh signaling (Fig. 3) [156]. In the absence of Hh, both Smurf and Gprk associate with the Smo C-tail, leading to Smo ubiquitination and degradation (Fig. 3A). In the presence of Hh, the phosphorylation and SUMOylation of the Smo C-tail reduces binding of Smurf to Smo (Fig. 3B). Instead, Smurf binds and ubiquitinates Ptc.
Itch, another E3 ubiquitin ligase, plays a role in regulating the ubiquitination, internalization and degradation of mammalian Ptchl, but not Ptch2 [157]. Reducing Itch or mutating the Itch target (K1413) in Ptchl stabilizes Ptchl. Itch appears to regulate Ptchl only in cells not receiving Hh signal, thus is not involved in Hh mediated downregulation of Ptchl.
4.2. Proteostasis of Sufu
Sufu directly interacts with the Gli proteins and inhibits their transcriptional activities by sequestering them in the cytoplasm [14, 158–163]. Hh signaling alleviates this inhibition, but how it achieves this remains a hotly debated issue. Some believe that Hh separates Sufu from Gli proteins, allowing Gli to enter the nucleus and activate downstream genes [134, 144]. Others believe Sufu enters the nucleus with the Gli proteins and exhibits an inhibitory role inside the nucleus [14, 164–167]. Among these, some believe that Sufu dissociates from the Gli proteins in the nucleus upon Hh signaling [166]. Others posits that Sufu and Gli proteins remain associated upon Hh pathway activation, and Sufu is turned into a positive regulator of Gli in this context [167]. A clear answer to this question awaits more studies.
The dependence on direct association for its inhibitory function on the Gli proteins suggests that a higher level of Sufu is needed to prevent improper activation of the pathway, which has been confirmed by a study showing Sufu is in great excess compared to Ci in Drosophila [168]. Recent studies have started to reveal mechanisms by which Hh signaling regulates the levels of Sufu in vertebrates and insects. In mammals, Shh promotes the ubiquitination of Sufu at K257 and its subsequent degradation [169]. Decreased Sufu stability appears to be associated with cancer formation. Interestingly, GSK3β and PKA phosphorylate Sufu at Ser342 and Ser346, respectively, and stabilize Sufu [170]. Sufu phosphorylation promotes its ciliary localization and colocalization with Gli3. The levels of total and phosphorylated Sufu are elevated in the absence of Smo, suggesting that Hh signaling may inhibit Sufu phosphorylation.
Nek2A phosphorylates Sufu at T225 and S352 and inhibits Sufu ubiquitination and degradation, hence negatively regulates Hh signaling [171, 172]. Interestingly, Hh signaling induces the transcription of Nek2A, making Nek2A a negative feedback regulator of the Hh pathway.
Hh also regulates the level of Sufu in Drosophila, but through an indirect mechanism [173]. Hh upregulates Hib, which sequesters a spliceosome factor Crn in the nucleus, and interferes with the translation of the Sufu mRNA. However, the direct target of Hib in this context has not been identified.
5. Conclusion
Like all other developmental signaling pathways, Hh signaling is under strict regulation in development and adulthood to avoid developmental errors and malignancies. Proteostasis plays a particularly important role in the regulation of this pathway. Proteasome-based processing of the Ci/Gli proteins and Hh regulation of this process, directly result in the transcriptional output of the pathway. The deubiquitination and cell surface/ciliary translocation of Smo is an integral part of its activation. Although the exact mechanisms by which Hh inhibits the activities of Ptc and Sufu are still actively pursued, the downregulation of these proteins certainly contributes to Hh pathway activation.
New components of the Hh pathway and regulatory mechanisms, including proteostasis, have been revealed at a striking pace. In addition to above proteins, other components of the Hh signaling pathway are also subject to PTMs that change their level, localization and/or activities in the cells. For example, Cos2 is SUMOylated and this modification antagonizes its activity in Hh signaling [102]. Cos2/Kif7 is ubiquitinated by Ubr3, which destabilizes the protein [174].
With nearly three decades of extensive investigation, great progress has been made in understanding the proteostasis in the Hh signaling pathway. However, many outstanding questions remain. As discussed above, several issues remain unsettled, including the roles and regulation of Gli SUMOylation [152, 153], the exact roles of the cilia in Gli3 processing [39, 46, 136, 144] and the exact relationship between Sufu and Gli proteins upon Hh pathway activation [134, 144, 166, 175]. Furthermore, although Smurf family members regulate Ptch1 endocytosis [176], it is not clear whether Smurf-mediated ubiquitination also regulates Smo localization in mammals. It was long known that Hh signaling downregulates Cos2 [19], and a recent study suggested that the E3 ligase Ubr3 regulates the degradation of Cos2 and Kif7 to modulate Hh signaling [174]. However, loss of Ubr3 does not block Hh-induced Cos2 degradation in vivo, suggesting that Hh may promote Cos2 degradation through additional mechanism(s). Finally, although the significance of proteostasis in Hh signaling has been extensively addressed in vivo in Drosophila, more in vivo genetics studies are still needed in mammals.
Acknowledgement
The research in the Liu lab has been supported by the US NSF (IOS-0949877 and IOS-1257540), US NIH (HD083625) and a Penn State University new faculty start-up fund. Due to the limited scope of this review, it is not possible to discuss all important aspects of the Hh pathway regulation. The author apologizes to colleagues whose results are not included.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Briscoe J and Therond PP, The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol, 2013. 14(7): p. 416–29. [DOI] [PubMed] [Google Scholar]
- 2.Guha M, Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nat Rev Drug Discov, 2012. 11(4): p. 257–8. [DOI] [PubMed] [Google Scholar]
- 3.Nusslein-Volhard C and Wieschaus E, Mutations affecting segment number and polarity in Drosophila. Nature, 1980. 287(5785): p. 795–801. [DOI] [PubMed] [Google Scholar]
- 4.Hooper JE and Scott MP, The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell, 1989. 59(4): p. 751–65. [DOI] [PubMed] [Google Scholar]
- 5.Nakano Y, et al. , A protein with several possible membrane-spanning domains encoded by the Drosophila segment polarity gene patched. Nature, 1989. 341(6242): p. 508–13. [DOI] [PubMed] [Google Scholar]
- 6.Marigo V, et al. , Biochemical evidence that patched is the Hedgehog receptor. Nature, 1996. 384(6605): p. 176–9. [DOI] [PubMed] [Google Scholar]
- 7.Stone DM, et al. , The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature, 1996. 384(6605): p. 129–34. [DOI] [PubMed] [Google Scholar]
- 8.Alcedo J, et al. , The Drosophila smoothenedgene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell, 1996. 86(2): p. 221–32. [DOI] [PubMed] [Google Scholar]
- 9.van den Heuvel M and Ingham PW, smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature, 1996. 382(6591): p. 547–51. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez M, et al. , Sending and receiving the hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science, 1996. 272(5268): p. 1621–5. [DOI] [PubMed] [Google Scholar]
- 11.Orenic TV, et al. , Cloning and characterization of the segment polarity gene cubitus interruptus Dominant of Drosophila. Genes Dev, 1990. 4(6): p. 1053–67. [DOI] [PubMed] [Google Scholar]
- 12.Robbins DJ, et al. , Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell, 1997. 90(2): p. 225–34. [DOI] [PubMed] [Google Scholar]
- 13.Sisson JC, et al. , Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell, 1997. 90(2): p. 235–45. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, et al. , Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev, 2000. 14(22): p. 2893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefers MA, Wang QT, and Holmgren RA, Genetic dissection of the Drosophila Cubitus interruptus signaling complex. Dev Biol, 2001. 236(2): p. 411–20. [DOI] [PubMed] [Google Scholar]
- 16.Jia J, Tong C, and Jiang J, Smoothened transduces Hedgehog signal by physically interacting with Costal2/Fused complex through its C-terminal tail. Genes Dev, 2003. 17(21): p. 2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lum L, et al. , Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell, 2003. 12(5): p. 1261–74. [DOI] [PubMed] [Google Scholar]
- 18.Ogden SK, et al. , Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr Biol, 2003. 13(22): p. 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruel L, et al. , Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat Cell Biol, 2003. 5(10): p. 907–13. [DOI] [PubMed] [Google Scholar]
- 20.Echelard Y, et al. , Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNSpolarity. Cell, 1993. 75(7): p. 1417–30. [DOI] [PubMed] [Google Scholar]
- 21.Krauss S, Concordet JP, and Ingham PW, A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell, 1993. 75(7): p. 1431–44. [DOI] [PubMed] [Google Scholar]
- 22.Riddle RD, et al. , Sonic hedgehog mediates the polarizing activity of the ZPA. Cell, 1993. 75(7): p. 1401–16. [DOI] [PubMed] [Google Scholar]
- 23.St-Jacques B, Hammerschmidt M, and McMahon AP, Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev, 1999. 13(16): p. 2072–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitgood MJ, Shen L, and McMahon AP, Sertoli cell signaling by Desert hedgehog regulates the malegermline. Curr Biol, 1996. 6(3): p. 298–304. [DOI] [PubMed] [Google Scholar]
- 25.Chiang C, et al. , Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature, 1996. 383(6599): p. 407–13. [DOI] [PubMed] [Google Scholar]
- 26.Goodrich LV, et al. , Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev, 1996. 10(3): p. 301–12. [DOI] [PubMed] [Google Scholar]
- 27.Motoyama J, et al. , Overlapping and non-overlapping Ptch2 expression with Shh during mouse embryogenesis. Mech Dev, 1998. 78(1–2): p. 81–4. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter D, et al. , Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci U S A, 1998. 95(23): p. 13630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motoyama J, et al. , Ptch2, a second mouse Patched gene is co-expressed with Sonic hedgehog. Nat Genet, 1998. 18(2): p. 104–6. [DOI] [PubMed] [Google Scholar]
- 30.Matise MP and Joyner AL, Gli genes in development and cancer. Oncogene, 18(55): p. 7852–9. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Fallon JF, and Beachy PA, Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell, 2000. 100(4): p. 423–34. [DOI] [PubMed] [Google Scholar]
- 32.Pan Y, et al. , Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol, 2006. 26(9): p. 3365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preat T, Characterization of Suppressor of fused, a complete suppressor of the fused segment polarity gene of Drosophila melanogaster. Genetics, 1992. 132(3): p. 725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper AF, et al. , Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development, 2005. 132(19): p. 4407–17. [DOI] [PubMed] [Google Scholar]
- 35.Svard J, et al. , Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell, 2006. 10(2): p. 187–97. [DOI] [PubMed] [Google Scholar]
- 36.Bangs FK, et al. , Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol, 2015. 17(2): p. 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huangfu D, et al. , Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature, 2003. 426(6962): p. 83–7. [DOI] [PubMed] [Google Scholar]
- 38.Huangfu D and Anderson KV, Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A, 2005. 102(32): p. 11325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu A, Wang B, and Niswander LA, Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development, 2005. 132(13): p. 3103–11. [DOI] [PubMed] [Google Scholar]
- 40.Rohatgi R, Milenkovic L, and Scott MP, Patched1 regulates hedgehog signaling at the primary cilium. Science, 2007. 317(5836): p. 372–6. [DOI] [PubMed] [Google Scholar]
- 41.Haycraft CJ, et al. , Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet, 2005. 1(4): p. e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbit KC, et al. , Vertebrate Smoothened functions at the primary cilium. Nature, 2005. 437(7061): p. 1018–21. [DOI] [PubMed] [Google Scholar]
- 43.Chen MH, et al. , Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev, 2009. 23(16): p. 1910–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia J, et al. , Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol, 2009. 330(2): p. 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zeng H, and Liu A, The loss of Hh responsiveness by a non-ciliary Gli2 variant. Development, 2015. 142(9): p. 1651–60. [DOI] [PubMed] [Google Scholar]
- 46.Han Y, et al. , Regulation of Gli ciliary localization and Hedgehog signaling by the PY-NLS/karyopherin-beta2 nuclear import system. PLoS Biol, 2017. 15(8): p. e2002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabata T and Kornberg TB, Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell, 1994. 76(1): p. 89–102. [DOI] [PubMed] [Google Scholar]
- 48.Ho KS, et al. , Differential regulation of Hedgehog target gene transcription by Costal2 and Suppressor of Fused. Development, 2005. 132(6): p. 1401–12. [DOI] [PubMed] [Google Scholar]
- 49.Jia J, et al. , Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature, 2004. 432(7020): p. 1045–50. [DOI] [PubMed] [Google Scholar]
- 50.Basler K and Struhl G, Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature, 1994. 368(6468): p. 208–14. [DOI] [PubMed] [Google Scholar]
- 51.Kojima T, et al. , Induction of a mirror-image duplication of anterior wing structures by localized hedgehog expression in the anterior compartment of Drosophila melanogaster wing imaginal discs. Gene, 1994. 148(2): p. 211–7. [DOI] [PubMed] [Google Scholar]
- 52.Dessaud E, McMahon AP, and Briscoe J, Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development, 2008. 135(15): p. 2489–503. [DOI] [PubMed] [Google Scholar]
- 53.Liu A, Joyner AL, and Turnbull DH, Alteration of limb and brain patterning in early mouse embryos by ultrasound-guided injection of Shh-expressing cells. Mech Dev, 1998. 75(1–2): p. 107–15. [DOI] [PubMed] [Google Scholar]
- 54.Chiang C, et al. , Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol, 2001. 236(2): p. 421–35. [DOI] [PubMed] [Google Scholar]
- 55.Kraus P, Fraidenraich D, and Loomis CA, Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev, 2001. 100(1): p. 45–58. [DOI] [PubMed] [Google Scholar]
- 56.Litingtung Y, et al. , Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature, 2002. 418(6901): p. 979–83. [DOI] [PubMed] [Google Scholar]
- 57.Karp SJ, et al. , Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and - independent pathways. Development, 2000. 127(3): p. 543–8. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi A, Komori T, and Suda T, Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev, 2000. 21(4): p. 393–411. [DOI] [PubMed] [Google Scholar]
- 59.Byrd N, et al. , Hedgehog is required for murine yolk sac angiogenesis. Development, 2002. 129(2): p. 361–72. [DOI] [PubMed] [Google Scholar]
- 60.Ramalho-Santos M, Melton DA, and McMahon AP, Hedgehog signals regulate multiple aspects of gastrointestinal development. Development, 2000. 127(12): p. 2763–72. [DOI] [PubMed] [Google Scholar]
- 61.Salzer JL, Creating barriers: a new role for Schwann cells and Desert hedgehog. Neuron, 1999. 23(4): p. 627–9. [DOI] [PubMed] [Google Scholar]
- 62.Denef N, et al. , Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell, 2000. 102(4): p. 521–31. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C, et al. , Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc Natl Acad Sci U S A, 2004. 101(52): p. 17900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y, Tong C, and Jiang J, Hedgehog regulates smoothened activity by inducing a conformational switch. Nature, 2007. 450(7167): p. 252–8. [DOI] [PubMed] [Google Scholar]
- 65.Apionishev S, et al. , Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nat Cell Biol, 2005. 7(1): p. 86–92. [DOI] [PubMed] [Google Scholar]
- 66.Li S, et al. , Regulation of Smoothened Phosphorylation and High-Level Hedgehog Signaling Activity by a Plasma Membrane Associated Kinase. PLoS Biol, 2016. 14(6): p. e1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia H, et al. , PP4 and PP2A regulate Hedgehog signaling by controlling Smo and Ci phosphorylation. Development, 2009. 136(2): p. 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su Y, et al. , Sequential phosphorylation of smoothened transduces graded hedgehog signaling. Sci Signal, 2011. 4(180): p. ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, et al. , G protein-coupled receptor kinase 2 promotes high-level Hedgehog signaling by regulating the active state of Smo through kinase-dependent and kinase-independent mechanisms in Drosophila. Genes Dev, 2010. 24(18): p. 2054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang K, et al. , PI(4)P Promotes Phosphorylation and Conformational Change of Smoothened through Interaction with Its C-terminal Tail. PLoS Biol, 2016. 14(2): p. e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu AJ, et al. , Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev, 2003. 17(10): p. 1240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang K, et al. , An intracellular activation of Smoothened that is independent of Hedgehog stimulation in Drosophila. J Cell Sci, 2018. 131(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molnar C, et al. , Role of the Drosophila non-visual ss-arrestin kurtz in hedgehog signalling. PLoS Genet, 2011. 7(3): p. e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, et al. , Sonic Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol, 2011. 9(6): p. e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovacs JJ, et al. , Beta-arrestin-mediated localization of smoothened to the primary cilium. Science, 2008. 320(5884): p. 1777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milenkovic L, Scott MP, and Rohatgi R, Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol, 2009. 187(3): p. 365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu Q, et al. , A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science, 2010. 329(5990): p. 436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang C, et al. , Structure of the human smoothened receptor bound to an antitumour agent. Nature, 2013. 497(7449): p. 338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byrne EFX, et al. , Structural basis of Smoothened regulation by its extracellular domains. Nature, 2016. 535(7613): p. 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen JK, et al. , Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev, 2002. 16(21): p. 2743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen JK, et al. , Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A, 2002. 99(22): p. 14071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao X, et al. , Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling. Mol Cell, 2017. 66(1): p. 154–162 e10. [DOI] [PubMed] [Google Scholar]
- 83.Corcoran RB and Scott MP, Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A, 2006. 103(22): p. 8408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myers BR, et al. , Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell, 2013. 26(4): p. 346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luchetti G, et al. , Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nedelcu D, et al. , Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol, 2013. 9(9): p. 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang P, et al. , Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell, 2016. 166(5): p. 1176–1187 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gong X, et al. , Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science, 2018. 361(6402). [DOI] [PubMed] [Google Scholar]
- 89.Qi X, et al. , Two Patched molecules engage distinct sites on Hedgehog yielding a signaling-competent complex. Science, 2018. 362(6410). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qi X, et al. , Structures of human Patched and its complex with native palmitoylated sonic hedgehog. Nature, 2018. 560(7716): p. 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bijlsma MF, et al. , Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol, 2006. 4(8): p. e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S, et al. , Hedgehog-regulated ubiquitination controls smoothened trafficking and cell surface expression in Drosophila. PLoS Biol, 2012. 10(1): p. e1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia R, et al. , USP8 promotes smoothened signaling by preventing its ubiquitination and changing its subcellular localization. PLoS Biol, 2012. 10(1): p. e1001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Z, et al. , The deubiquitinase UCHL5/UCH37 positively regulates Hedgehog signaling by deubiquitinating Smoothened. J Mol Cell Biol, 2018. 10(3): p. 243–257. [DOI] [PubMed] [Google Scholar]
- 95.Hurley JH, The ESCRTcomplexes. Crit Rev Biochem Mol Biol, 2010. 45(6): p. 463–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X, et al. , Drosophila Vps36 regulates Smo trafficking in Hedgehog signaling. J Cell Sci, 2013. 126(Pt 18): p. 4230–8. [DOI] [PubMed] [Google Scholar]
- 97.Li S, et al. , Regulation of Smoothened ubiquitylation and cell surface expression through a Cul4-DDB1-Gbeta E3 ubiquitin ligase complex. J Cell Sci, 2018. 131(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S, et al. , Hedgehog reciprocally controls trafficking of Smo and Ptc through the Smurf family of E3 ubiquitin ligases. Sci Signal, 2018. 11(516). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pusapati GV, et al. , G protein-coupled receptors control the sensitivity of cells to the morphogen Sonic Hedgehog. Sci Signal, 2018. 11(516). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meloni AR, et al. , Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol, 2006. 26(20): p. 7550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma G, et al. , Regulation of Smoothened Trafficking and Hedgehog Signaling by the SUMO Pathway. Dev Cell, 2016. 39(4): p. 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, et al. , SUMO regulates the activity of Smoothened and Costal-2 in Drosophila Hedgehog signaling. Sci Rep, 2017. 7: p. 42749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma G, et al. , Regulation of Smoothened Trafficking and Hedgehog Signaling by the SUMO Pathway. Dev Cell, 2016. 39(4): p. 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aza-Blanc P, et al. , Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell, 1997. 89(7): p. 1043–53. [DOI] [PubMed] [Google Scholar]
- 105.Methot N and Basler K, Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell, 1999. 96(6): p. 819–31. [DOI] [PubMed] [Google Scholar]
- 106.Methot N and Basler K, An absolute requirement for Cubitus interruptus in Hedgehog signaling. Development, 2001. 128(5): p. 733–42. [DOI] [PubMed] [Google Scholar]
- 107.Jiang J and Struhl G, Protein kinase A and hedgehog signaling in Drosophila limb development. Cell, 1995. 80(4): p. 563–72. [DOI] [PubMed] [Google Scholar]
- 108.Li W, et al. , Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell, 1995. 80(4): p. 553–62. [DOI] [PubMed] [Google Scholar]
- 109.Pan D and Rubin GM, cAMP-dependent protein kinase and hedgehog act antagonistically in regulating decapentaplegic transcription in Drosophila imaginal discs. Cell, 1995. 80(4): p. 543–52. [DOI] [PubMed] [Google Scholar]
- 110.Lepage T, et al. , Signal transduction by cAMP-dependent protein kinase A in Drosophila limb patterning. Nature, 1995. 373(6516): p. 711–5. [DOI] [PubMed] [Google Scholar]
- 111.Chen Y, et al. , Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. Proc Natl Acad Sci U S A, 1998. 95(5): p. 2349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Price MA and Kalderon D, Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development, 1999. 126(19): p. 4331–9. [DOI] [PubMed] [Google Scholar]
- 113.Wang G, Wang B, and Jiang J, Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes Dev, 1999. 13(21): p. 2828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Price MA and Kalderon D, Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell, 2002. 108(6): p. 823–35. [DOI] [PubMed] [Google Scholar]
- 115.Jia J, et al. , Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature, 2002. 416(6880): p. 548–52. [DOI] [PubMed] [Google Scholar]
- 116.Jiang J and Struhl G, Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeatprotein Slimb. Nature, 1998. 391(6666): p. 493–6. [DOI] [PubMed] [Google Scholar]
- 117.Smelkinson MG and Kalderon D, Processing of the Drosophila hedgehog signaling effector Ci-155 to the repressor Ci-75 is mediated by direct binding to the SCF component Slimb. Curr Biol, 2006. 16(1): p. 110–6. [DOI] [PubMed] [Google Scholar]
- 118.Jia J, et al. , Phosphorylation by double-time/CKIepsilon and CKIalpha targets cubitus interruptus for Slimb/beta-TRCP-mediated proteolytic processing. Dev Cell, 2005. 9(6): p. 819–30. [DOI] [PubMed] [Google Scholar]
- 119.Smelkinson MG, Zhou Q, and Kalderon D, Regulation of Ci-SCFSlimb binding, Ci proteolysis, and hedgehog pathway activity by Ci phosphorylation. Dev Cell, 2007. 13(4): p. 481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang W, et al. , Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell, 2005. 8(2): p. 267–78. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y and Price MA, A unique protection signal in Cubitus interruptus prevents its complete proteasomal degradation. Mol Cell Biol, 2008. 28(18): p. 5555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hui CC and Joyner AL, A mouse model of greig cephalopolysyndactyly syndrome: the extra-toes] mutation contains an intragenic deletion of the Gli3 gene.PG −241–6. Nat Genet, 1993. 3(3). [DOI] [PubMed] [Google Scholar]
- 123.Ding Q, et al. , Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development, 1998. 125(14): p. 2533–43. [DOI] [PubMed] [Google Scholar]
- 124.Matise MP, et al. , Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system.PG - 2759–70. Development, 1998. 125(15). [DOI] [PubMed] [Google Scholar]
- 125.Aza-Blanc P, et al. , Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development, 2000. 127(19): p. 4293–301. [DOI] [PubMed] [Google Scholar]
- 126.Wang B and Li Y, Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci U S A, 2006. 103(1): p. 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan Y and Wang B, A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. J Biol Chem, 2007. 282(15): p. 10846–52. [DOI] [PubMed] [Google Scholar]
- 128.Wang C, Ruther U, and Wang B, TheShh-independentactivator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol, 2007. 305(2): p. 460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pan Y, Wang C, and Wang B, Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol, 2009. 326(1): p. 177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai P, Akimaru H, and Ishii S, A hedgehog-responsive region in the Drosophila wing disc is defined by debra-mediated ubiquitination and lysosomal degradation of Ci. Dev Cell, 2003. 4(6): p. 917–28. [DOI] [PubMed] [Google Scholar]
- 131.Li Z, et al. , Debra-mediated Ci degradation controls tissue homeostasis in Drosophila adult midgut. Stem Cell Reports, 2014. 2(2): p. 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.May SR, et al. , Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol, 2005. 287(2): p. 378–89. [DOI] [PubMed] [Google Scholar]
- 133.Zeng H, Jia J, and Liu A, Coordinated translocation of mammalian Gli proteins and suppressor of fused to the primary cilium. PLoS One, 2010. 5(12): p. e15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tukachinsky H, Lopez LV, and Salic A, A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol, 2010. 191(2): p. 415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li J, et al. , PKA-mediated Gli2 and Gli3 phosphorylation is inhibited by Hedgehog signaling in cilia and reduced in Talpid3 mutant. Dev Biol, 2017. 429(1): p. 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tuson M, He M, and Anderson KV, Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development, 2011. 138(22): p. 4921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Q, et al. , A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev Cell, 2006. 10(6): p. 719–29. [DOI] [PubMed] [Google Scholar]
- 138.Kent D, Bush EW, and Hooper JE, Roadkill attenuates Hedgehog responses through degradation of Cubitus interruptus. Development, 2006. 133(10): p. 2001–10. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Q, et al. , Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci U S A, 2009. 106(50): p. 21191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohlmeyer JT and Kalderon D, Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature, 1998. 396(6713): p. 749–53. [DOI] [PubMed] [Google Scholar]
- 141.Shi Q, et al. , Hedgehog-induced phosphorylation by CK1 sustains the activity of Ci/Gli activator. Proc Natl Acad Sci U S A, 2014. 111(52): p. E5651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seong KH, et al. , Inhibition of the nuclear import of cubitus interruptus by roadkill in the presence of strong hedgehog signal. PLoS One, 2010. 5(12): p. e15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang C, Pan Y, and Wang B, Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development, 2010. 137(12): p. 2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Humke EW, et al. , The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev, 2010. 24(7): p. 670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wen X, et al. , Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol, 2010. 30(8): p. 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choo KB, et al. , Evolutionary expansion of SPOP and associated TD/POZgene family: impact of evolutionary route on gene expression pattern. Gene, 2010. 460(1–2): p. 39–47. [DOI] [PubMed] [Google Scholar]
- 147.Errington WJ, et al. , Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure, 2012. 20(7): p. 1141–53. [DOI] [PubMed] [Google Scholar]
- 148.Cai H and Liu A, Spop regulates Gli3 activity and Shh signaling in dorsoventral patterning of the mouse spinal cord. Dev Biol, 2017. 432(1): p. 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cai H and Liu A, Spop promotes skeletal development and homeostasis by positively regulating Ihh signaling. Proc Natl Acad Sci U S A, 2016. 113(51): p. 14751–14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu J, et al. , Dual function of suppressor of fused in Hh pathway activation and mouse spinal cord patterning. Dev Biol, 2012. 362(2): p. 141–53. [DOI] [PubMed] [Google Scholar]
- 151.Zhou Z, et al. , Deubiquitination of Ci/Gli by Usp7/HAUSP Regulates Hedgehog Signaling. Dev Cell, 2015. 34(1): p. 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cox B, Briscoe J, and Ulloa F, SUMOylation by Pias1 regulates the activity of the Hedgehog dependent Gli transcription factors. PLoS One, 2010. 5(8): p. e11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han L, Pan Y, and Wang B, Small ubiquitin-like Modifier (SUMO) modification inhibits GLI2 protein transcriptional activity in vitro and in vivo. J Biol Chem, 2012. 287(24): p. 20483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lv X, et al. , SUMO regulates somatic cyst stem cell maintenance and directly targets the Hedgehog pathway in adult Drosophila testis. Development, 2016. 143(10): p. 1655–62. [DOI] [PubMed] [Google Scholar]
- 155.Huang S, et al. , Activation of Smurf E3 ligase promoted by smoothened regulates hedgehog signaling through targeting patched turnover. PLoS Biol, 2013. 11(11): p. e1001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li S, Wang B, and Jiang J, Hedgehog reciprocally controls trafficking of Smo and Ptc through the Smurf family of E3 ubiquitin ligases. Sci Signal, 2018. 11(516). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen XL, et al. , Patched-1 proapoptotic activity is downregulated by modification of K1413 by the E3 ubiquitin-protein ligase Itchy homolog. Mol Cell Biol, 2014. 34(20): p. 3855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ding Q, et al. , Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr Biol, 1999. 9(19): p. 1119–22. [DOI] [PubMed] [Google Scholar]
- 159.Pearse RV, 2nd, et al. , Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol, 1999. 212(2): p. 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stone DM, et al. , Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci, 1999. 112 (Pt23): p. 4437–48. [DOI] [PubMed] [Google Scholar]
- 161.Methot N and Basler K, Suppressor of fused opposes hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development, 2000. 127(18): p. 4001–10. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y, et al. , Structural insight into the mutual recognition and regulation between Suppressor of Fused and Gli/Ci. Nat Commun, 2013. 4: p. 2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Han Y, Shi Q, and Jiang J, Multisite interaction with Sufu regulates Ci/Gli activity through distinct mechanisms in Hh signal transduction. Proc Natl Acad Sci U S A, 2015. 112(20): p. 6383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sisson BE, Ziegenhorn SL, and Holmgren RA, Regulation of Ci and Sufu) nuclear import in Drosophila. Dev Biol, 2006. 294(1): p. 258–70. [DOI] [PubMed] [Google Scholar]
- 165.Cheng SY and Bishop JM, Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc Natl Acad Sci U S A, 2002. 99(8): p. 5442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin C, et al. , Regulation of Sufu activity by p66beta and Mycbp provides new insight into vertebrate Hedgehog signaling. Genes Dev, 2014. 28(22): p. 2547–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Z, et al. , Suppressor of Fused chaperones Gli proteins to generate transcriptional responses to Sonic Hedgehog signaling. Mol Cell Biol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farzan SF, et al. , A quantification of pathway components supports a novel model of Hedgehog signal transduction. J Biol Chem, 2009. 284(42): p. 28874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yue S, Chen Y, and Cheng SY, Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin-proteasome pathway. Oncogene, 2009. 28(4): p. 492–9. [DOI] [PubMed] [Google Scholar]
- 170.Chen Y, et al. , Dual Phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium. J Biol Chem, 2011. 286(15): p. 13502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou F, et al. , Nek2A/SuFu feedback loop regulates Gli-mediated Hedgehog signaling pathway. Int J Oncol, 2017. 50(2): p. 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Y, et al. , Nek2A phosphorylates and stabilizes SuFu: A new strategy of Gli2/Hedgehog signaling regulatory mechanism. Cell Signal, 2016. 28(9): p. 1304–13. [DOI] [PubMed] [Google Scholar]
- 173.Liu C, et al. , Hedgehog signaling downregulates suppressor of fused through the HIB/SPOP-Crn axis in Drosophila. Cell Res, 2014. 24(5): p. 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li T, et al. , Ubr3, a Novel Modulator of Hh Signaling Affects the Degradation of Costal-2 and Kif7 through Poly-ubiquitination. PLoS Genet, 2016. 12(5): p. e1006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Z, et al. , Suppressor of Fused Chaperones Gli Proteins To Generate Transcriptional Responses to Sonic Hedgehog Signaling. Mol Cell Biol, 2017. 37(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yue S, et al. , Requirement of Smurf-mediated endocytosis of Patched1 in sonic hedgehog signal reception. Elife, 2014. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]