Abstract
Over 70% of older adults report chronic or acute pain, and pain threatens affective wellbeing. The strategies older adults use to maintain affective wellbeing following acute pain remain unknown. Specific strategies that can be used to manage pain include recalling, recognizing, and responding to positive stimuli and prioritizing close over knowledgeable social partners. The study tested whether older adults used positivity-enhancing strategies and maintained affective wellbeing following acute pain better than younger adults. Fifty older (ages 65-85) and 50 younger (ages 18-30) pain-free adults experienced a control and a pain condition and were given the chance to employ positivity-enhancing strategies. Older and younger adults similarly used positivity-enhancing strategies following pain. Younger adults demonstrated reduced preference for knowledgeable social partners after experiencing pain. Pain-related affective changes were similar between age groups. Older and younger adults may cope with acute pain similarly, highlighting future directions for exploring age differences in pain coping.
Keywords: aging, cold-pressor, executive function, heart rate variability, socioemotional selectivity
Introduction
Positive affect is associated with psychological wellbeing and health in older adults (Diener & Chan, 2011; Pressman & Cohen, 2005). Unfortunately, pain can decrease positive affect and increase negative affect (e.g., Zautra et al., 2001). This is particularly relevant for older adults, as over 70% report experiencing chronic or acute pain within the previous month (Thomas et al., 2004).
Older adults may be adept at maintaining affective wellbeing (maximizing positive affect and minimizing negative affect) in the face of pain. Older pain patients report better quality of life, marital and social satisfaction, mood, and less pain interference than younger pain patients (Boggero et al., 2016; Cook, & Chastain, 2001; Rustøen et al., 2004). Changes in pain appear unrelated to changes in wellbeing in older adults (Phelan & Heidrich, 2007), and older adults with “extreme” pain have similar life satisfaction than those with “no” or “moderate” pain (Lohmann et al., 1998). Yet, little is known about how older adults maintain affective wellbeing following acute pain, whether they do so better than younger adults, or what makes some older and younger adults better able to do so than others. The current study aimed to answer these questions.
One explanation for how older adults maintain affective wellbeing comes from socioemotional selectivity theory (SST). SST posits that as people age, they become increasingly motivated to maximize positive affect (Carstensen et al., 1999). Older adults achieve this goal via a positivity bias in attention and memory that enhances positive emotions (Mather & Carstensen, 2005). They recall, recognize, and respond to positive more than negative stimuli in their environments, and prioritize close over novel but knowledgeable social contacts, providing affective over informational benefits (Fung et al., 2001; Murphy & Isaacowitz, 2008). However, no research has examined whether older adults use such positivity-enhancing strategies in acute pain control. Additionally, no study has tested whether older adults use positivity-enhancing strategies differently than younger adults do to maintain affective wellbeing following acute pain. The current study tests whether younger and older adults differ in their use of positivity-enhancing strategies to maintain affective wellbeing following acute pain.
Additionally, executive functioning (EF) and vagal tone may be important resources for managing pain. EFs are interrelated cognitive abilities that allow people to plan and modify their actions and have been previously linked to adaptive pain coping, as they may allow people to shift attention away from pain and toward activities that promote positive affect (e.g., Berryman et al., 2014; Boggero et al., 2016; Solberg Nes et al., 2009). Similarly, vagal tone, as indexed by heart rate variability, is an index of parasympathetic nervous system activity and may reflect physiological inhibitory ability. Greater vagal tone has been linked to lower depression and pain, highlighting its importance as a resource for pain management (e.g. Carlson et al., 2000; Koenig et al., 2014). Thus, the study also tests whether EF or vagal tone serve as resources for maintaining affective wellbeing following acute pain independently of age.
It was hypothesized that:
Hypothesis 1: Relative to younger adults, older adults would recall, recognize, and respond more quickly to positive than negative pictures following acute pain, and would demonstrate stronger preference for close versus knowledgeable social partners following acute pain..
Hypothesis 2: Older adults would maintain positive affect and minimize increases in negative affect following acute pain better than younger adults.
Hypothesis 3: Older and younger adults with better EF and vagal tone would use more positivity-enhancing strategies following acute pain.
If confirmed, these hypotheses would suggest that older adults use different strategies and resources to manage acute pain than younger adults and do so more effectively. They would help explain one potential mechanism for why older adults demonstrate greater affective wellbeing than younger adults despite experiencing pain more frequently and intensely and can highlight strategies for future interventions aimed at maintaining affective wellbeing following acute pain. Further, positive affect buffers against pain-related declines in physical functioning and promotes positive pain outcomes above and beyond negative affect (Finnan & Garland, 2015), making it a particularly important target for investigations into successful acute pain management.
Methods
Participants
With an alpha level set at .05 and an estimated effect size of d = .48 (Connelly et al., 2007), an a-priori power analysis indicated that a total sample size of 48 per group would yield power of .80 to detect the predicted effects using a repeated measure design. Fifty older adults between ages 65 and 85 were recruited from a research database maintained by the University of Kentucky’s Sanders-Brown Center on Aging. Fifty sex- and race-matched younger adults aged 18-30 were recruited from introductory psychology students at the University of Kentucky. Inclusion criteria included: a) a score of 0-3 to the question of “On a scale from 0-10, with 0 being no pain and 10 being the worse pain imaginable, what is your current level of pain?”; b) absence of disorders that would make pain testing unsafe (e.g., seizure disorders, severe cardiovascular disorder); c) absence of medications that would significantly alter pain processing or vagal tone, such as psychiatric medications or pain medication; d) fluency in English, and e) normal or corrected-to-normal vision. Data were collected between September 2014 and December 2015.
Design and Procedures
Due to the effects of exercise, caffeine, and alcohol on vagal tone and pain, participants were asked at the time of scheduling to abstain from these activities for 4 hours prior to participation in the study. Adherence to these criteria were confirmed upon arriving at the lab. Participants then provided informed consent.
Baseline ECG data were collected for seven minutes using the Type II electrode configuration and a sampling rate of 1000 samples/second. During this baseline period, participants provided demographic information, completed questionnaires assessing affect, and were introduced to the social preference task (described below). Blood pressure was taken three times using a blood pressure cuff on the nondominant arm (Omron Healthcare Inc, Bannockburn, IL).
Following the blood pressure measurement, participants underwent a control condition in which they submerged their hand in a 71.6°F (room temperature) bath of circulating water for one minute. Every 20 seconds during and immediately after hand submersion, participants rated pain intensity. Participants were then shown a series of 8 positive, 16 neutral, and 8 negative International Affective Picture System (IAPS) pictures in random order, at 2-sec intervals. Positive and negative pictures were matched on salience. Participants were instructed to attend to these pictures “as if they were watching television” and were not told they would have to memorize the pictures. Next, participants performed a social preference task (described below) which has been used for assessing social preferences in older adults (Segerstrom et al., 2016). They were then told to rest for ten minutes while they read magazines or used their phones so that the experimenter could monitor ECG; in reality, the rest period was implemented as a delay period for the memory tasks which were to follow. The magazines available to them were specifically selected not to be arousing, and due to the nature of the lab space, few participants were able to get reception on their phones. Most participants did not attempt to look at their phones or magazines and instead chose to sit quietly for the rest period. After the rest period, the experimenter returned to the room and instructed the participants to provide a brief written description of all the pictures they were able to remember (recall). They were then shown a set of pictures containing half novel and half previously-seen pictures. Participants were instructed to press the “a” key on the computer keyboard if they thought the picture was novel, or the “l” key if they thought it was previously seen (recognition and response time). The methodology for the recognition task (including the pictures) was based on that used by Charles et al. (2003). After the recognition task, participants provided ratings of positive and negative affect.
Following the control condition tasks, participants repeated the same procedures in the pain condition. In the pain condition, participants were asked to place their hand in a 39.2°F bath of circulating ice water for one minute – a temperature which has previously been shown to elicit pain in older adults (Edwards et al., 2003). Participants were then again shown a different set of positive, neutral, and negative pictures following the pain induction task. These pictures were administered in counterbalanced order such that half of the participants were randomly assigned to see one set of pictures first (i.e., following the control condition), and the other half were randomly assigned to see that same set of pictures second (i.e., following the pain condition). Following the picture presentation, participants again underwent the social preference task, a rest period, and a picture recall and recognition task as described above to test the positivity-enhancing strategies they used following pain. After the recognition task, participants again provided ratings of positive and negative affect. The order of the pain condition was not counterbalanced between participants (i.e., participants completed the control condition first, and the pain condition second) so that carryover effects from the pain condition would not influence control ratings.
Following these tasks, participants completed neuropsychological tests of working memory, inhibition, and task-switching, in counterbalanced order, as detailed below. These tests were administered last to prevent possible frustration with the tests from influencing affect ratings. After EF testing, participants were detached from the ECG equipment, debriefed, and thanked for their participation. A flowchart of procedures is presented in Figure 1. All procedures were approved by the Institutional Review Board at the university where the study was conducted (IRB# 14-0398-P4S).
Figure 1.
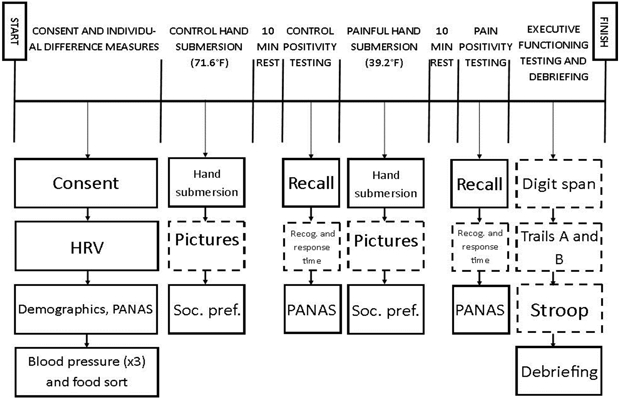
Graphical Representation of Study Procedures.
Note: Participants proceeded through the study in order, moving from left to right across the solid black line. Specific tasks completed at each stage are depicted by the boxes under the corresponding section. During the 60 sec hand submersion tasks, participants provided pain intensity ratings every 20 sec that their hand was in the water. Dashed boxes signify that tasks were counterbalanced between participants. Abbreviations: HRV = heart rate variability; PANAS = positive and negative affect schedule; F = Fahrenheit; Soc. pref. = social preference; Recog. = recognition.
Materials
Demographics.
Participants self-reported their age, sex, education level, relationship status, height, weight, and ethnicity.
Affect.
The Positive and Negative Affect Scale was used to assess affect (Watson & Clark, 1999). Affect was measured at three points in the study: during the baseline ECG, after the control condition, and after the pain condition (see Figure 1). The means, standard deviations, and internal consistencies at these three time points were M = 3.4, SD = 0.74, α = .89; M =3.3, SD = 0.86, α = .93; and M = 3.1, SD = 0.95, α = .94 for positive affect. For negative affect, the values at baseline, after the control condition, and after the pain condition were M = 1.18, SD = 0.23, α = .74; M = 1.14, SD = 0.19, α = .81; and M =1.14, SD = 0.22, α = .86. To assess pain-related changes in positive and negative affect, the differences between the post-control condition and post-pain condition values were taken. Variables were coded so that negative scores reflected decreases in positive affect but increases in negative affect. This scale has been previously shown to be sensitive to pain-related changes in affect (e.g., Zautra et al., 2001).
Pain Intensity.
Participants provided verbal pain intensity ratings every 20 seconds while their hand was submerged in the water using a scale of “No Pain” (0) to “The Worst Pain You Can Imagine” (10). If participants withdrew their hand from the water prior to 20 seconds, they were asked to provide a pain intensity rating at withdrawal so that pain intensity data was available for all participants. The average of all available ratings (up to 3) was computed so that data from those who withdrew before the minute was up (and therefore had fewer ratings) could still be used. Reliability of the ratings for the pain intensity scale in the control condition was α = .94 and in the pain condition, α = .95.
Positivity-Enhancing Strategy: Recall, Recognition, and Response Time.
Recall refers to the ability of remembering previously-experienced stimuli; it requires self-directed processing and is influenced by current goals and motivation (Charles et al., 2003). It was operationalized as the number of correctly identified pictures for each of the three valence categories (positive, neutral, and negative) participants wrote down following the rest period. Recognition refers to the ability to accurately identify if a stimulus was previously experienced after being presented with it again; it requires proper encoding of the stimulus when it was originally encountered. Recognition was operationalized by calculating a C score for each of the three picture valence categories. C is a signal-detection-theory-based estimate of response bias, where values of C above zero indicate a conservative bias (less willing to judge an item as having been previously seen) and values of C below zero indicate a liberal bias (more willing to judge as having been previously seen; Macmillan, & Creelman, 1991). Response time is an index of processing and/or psychomotor speed, and was operationalized as the average time in milliseconds participants took to respond to each of the previously-seen pictures within each valence category.
Positivity-Enhancing Strategy: Social Preference.
Social preferences were assessed using the social preference task from Lang and Carstensen (2002). Participants were first oriented to the task by sorting cards by preference on which 8 foods were printed. In the control and pain conditions, participants were given a stack of 18 cards each containing a potential social partner in different categories ranging from close family and friends (“a member of your immediate family”) to knowledgeable partners (“an author of a book that you have read”). Participants sorted the cards into piles representing how much they would like to spend half an hour with that person, with leftmost piles containing the people with whom they would most like to spend time and rightmost piles containing the people with whom they would least like to spend time. Based on previous cluster analysis conducted on a similar sample, two knowledgeable social partners (“author” and “poet”) and three close social partners (“close friend,” “member of your immediate family,” and “sibling”) were used for analysis; these two categories were specifically selected because they demonstrated consistent clustering across older and younger adults across different experimental conditions in previous data (Segerstrom et al., 2016). Social preference for each partner was operationalized as pile rank, standardized from 0 (most preferred pile) to 1 (least preferred pile) using the following equation: (pile number – 1)/(number of piles – 1). For example, the values assigned if there were four piles were 0 (most preferred), 1/3, 2/3, and 1 (least preferred).
EF.
To assess task-switching, participants completed the Trail Making Test Parts A and B (Reitan, 1958). The difference in completion time between Parts A and B was used to assess task-switching ability. To assess working memory, Digit Span Forward, Backward, and Sequencing from the Wechsler Adult Intelligence Scale (4th Edition) task was used. A total score was computed by summing the correct number of responses across Forward, Backward, and Sequencing. To assess inhibition, the Stroop task was used (West & Alain, 2000). Response times of correct responses were measured in milliseconds using Inquisit. Inhibition was operationalized as (response time incongruent – response time congruent)/ response time control * (−1) so that higher scores reflected better inhibition ability. An error variable summing the number of errors for congruent, incongruent, and control trials was also computed.
Vagal Tone and Blood Pressure.
The ECG was collected using a Biopac Systems (Goleta, CA) EKG100B amplifier. The ECG was sampled continuously and recorded using Biopac Acqknowledge software. Mindware (Gahanna, OH) HRV software was used to visually inspect and edit data in accordance with the guidelines described by the Task Force of the European Society of Cardiology (1996). Mindware HRV software provides spectral analysis of the data, yielding log HF HRV (.15-.4 Hz) as a measure of vagally mediated HRV. The mean HF HRV across the last 5 of the 7-minute baseline period comprised baseline HRV for each individual (data from the first two minutes were discarded to allow acclimatization to the testing environment). Following the baseline, three separate blood pressure measurements using a blood pressure cuff on participant’s non-dominant arm were taken to calculate average diastolic blood pressure, systolic blood pressure, and heart rate.
Data Analysis Plan
Prior to analysis, all variables were checked for missing data, outliers, and normality. Missing values were treated using pairwise deletion. Outliers were identified using a criterion of +/− 4 SD from the mean. Next, descriptive statistics and bivariate correlations were computed among all variables. Student’s t-tests were used to compare demographic differences between age groups and to conduct manipulation checks.
Hypothesis 1.
To test for recall, recognition, and response time, three separate age group (younger vs. older, between-person) X condition (control vs. pain; within-person) X valence (positive vs. neutral vs. negative; within-person) mixed model ANOVAs were conducted.
To test for social preferences, multilevel models with social partners at Level 1 and participants at Level 2 were conducted in SAS version 9.4 using PROC MIXED with standardized pile rank as the dependent variable. The Kenward-Rogers correction was used to calculate degrees of freedom due to the small sample size of the current study. An unstructured covariance structure was used for all models because it is particularly good at handling balanced data, and because results from likelihood ratio tests comparing it to compound symmetry and autoregressive structures revealed that it was a significantly better fit for the data.
Main effects of age group, pain condition, valence, and their interactions were tested for all models. Main effects models only included the main effect term without any other variables in the model. Interaction models included all main effect and interaction terms entered simultaneously. To control for multiple comparisons, the Holm-Bonferroni method was used (Aickin, & Gensler, 1996).
Hypothesis 2.
To test for pain-related changes in positive affect, an age group (younger vs. older, between-person) X condition (control vs. pain; within-person) mixed-factor ANOVA was conducted. These same analyses were repeated for negative affect.
Hypothesis 3.
Main effects models with EF or HRV entered as the unitary predictor of each of the four positivity-enhancing strategy were computed using linear regression. Then, an EF/HRV (between-person) X pain condition (control vs. pain; within-person) X valence (positive vs. neutral vs. negative; within-person) interaction was independently tested for each EF/HRV variable with all lower-order main effect and interaction terms included in the model. All models were run with and without picture-set order, blood pressure, and heart rate entered as a covariate. Inclusion of these variables did not substantively change results; thus, only models without these covariates are reported.
Results
Missing Data, Outliers, and Normality
There were no missing cases of positive or negative affect, 1 missing case for pain intensity (scale misuse), 2 missing cases for recognition in the control condition (administration error), 12 missing cases for response times in the control condition (administration error [n = 6] or > 4 SD from the mean [n = 6]), 1 missing case for social preference in the control condition (administration error), 1 missing case for Trails (administration error), 4 missing cases for Digit Span (participant refusal), 8 missing cases for the Stroop task (administration error), and 4 missing cases for HRV (equipment malfunction). Missing values were assumed to be missing at random, and due to the small amount of missingness, it is unlikely that they influenced results in any substantive way.
Nine people in the sample did not have siblings and as such could not rate them on the social preference task. In those cases, preference for close social partners was computed by averaging across the remaining close social partners.
The Trails difference variable was significantly negatively skewed. A square root transformation was conducted to normalize the distribution; as such, models below use the squared Trails difference. HRV was positively skewed. To correct for this, a log transformation of the log HF power was used. Subsequent analyses of HRV therefore use the log of the logged HF power. All other variables were normally distributed. No outliers were identified on any variable.
Manipulation Check
The average water temperature was 72.9°F (SD = 4.43) in the control condition and 42.2°F (SD = 3.54) in the pain condition. Forty-eight of 50 younger adults kept their hand submerged for the entire minute in the pain condition. Average length of hand submersion for the other two younger adults was 24.95 seconds. Thirty-nine of 50 older adults kept their hand submerged for the entire minute in the pain condition. The average length of hand submersion for the other eleven was 28.46 seconds.
Both younger and older adults reported the cold water as more painful than the room temperature water (p <.001 for both younger and older adults). Older adults rated the cold water as more painful than the younger adults (older: M = 5.62, SD = 2.52; younger: M = 4.46, SD = 2.26; t(97) = 2.41, p = .018).
Descriptive Statistics
Demographic characteristics of the sample by age group are presented in Table 1. The sample was entirely Caucasian and 62% female. Older adults demonstrated significantly lower EF and HRV than younger adults.
Table 1.
Demographic Characteristics of the Sample by Age Group
| Younger Adults (n = 50) |
Older Adults (n = 50) |
t(df) or χ2 | p | |
|---|---|---|---|---|
| Mean Age (SD) | 19.06 (1.81) | 73.44 (4.73) | ||
| Observed Age Range | 18-28 | 65-84 | ||
| Relationship Status | 81.19 | <.001 | ||
| Single | 96.0% | 6.0% | ||
| Married/Cohabitating | 4.0% | 62.0% | ||
| Divorced | 0% | 12.0% | ||
| Widowed | 0% | 20.0% | ||
| Mean BMI (SD) | 23.59 (4.08) | 18.36 (5.11) | −2.23 (97) | .028 |
| Mean Years of Education (SD) | 12.51 (0.77) | 16.06 (2.57) | −9.26 (95) | <.001 |
| Trails A (SD) | 24.33 (7.77) | 34.08 (11.59) | −4.93 (97) | <.001 |
| Trails B (SD) | 53.14 (15.95 | 93.13 (46.22) | −5.77(97) | <.001 |
| Trails A – B (SD) | −28.81 (15.63) | −60.11 (40.29) | 5.11 (96) | <.001 |
| Digit Span Total (SD) | 26.64 (4.51) | 24.00 (4.68) | 2.81 (94) | .006 |
| Stroop Inhibition (SD) | −0.22 (0.18) | −0.14 (0.23) | −1.86 (89) | .067 |
| Stroop Error (SD) | 2.79 (2.40) | 2.54 (2.37) | 0.49 (86) | .62 |
| Log HRV (SD) | 2.80 (0.36) | 2.03 (0.59) | 7.82 (94) | <.001 |
Note: Pearson’s Chi Square test was used to compare relationship status between age groups. All other variables were compared using a t-test.
Hypothesis 1: Positivity-Enhancing Strategies Following Pain
Recall.
A main effect of condition revealed that participants recalled more pictures following the control condition (M = 6.42, SD = 2.65) than the pain condition (M = 4.71, SD = 2.65), F(1, 97) = 36.23, p < .001. There was a significant two-way valence by age group interaction such that older adults remembered significantly fewer negative (but not positive or neutral) pictures than did younger adults (Mold = 1.54, Myoung = 2.14, t(98) = 3.18, p = .002, see Figure 2). Contrary to Hypothesis 1, there was not a significant three-way pain condition by age group by valence interaction in predicting recall, F(2, 194) = 0.55, p = .58. Results from the full model are presented in Table 2 (Model 1).
Figure 2.
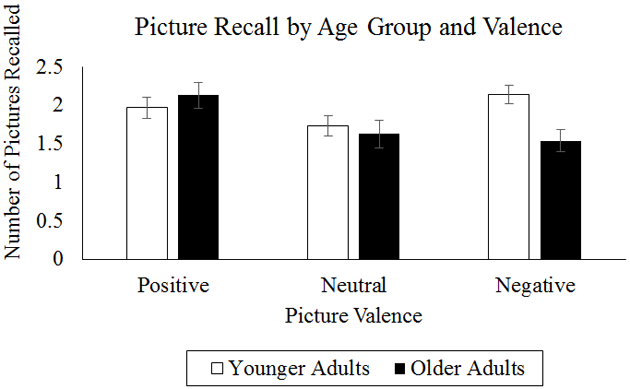
Graphical Representation of Age Group by Valence Interaction on Picture Recall.
Table 2.
Effects of Age Group, Pain Condition, and Picture Valence on Positivity-Enhancing Mechanism Usage.
| Model 1: Recall | Model 2: Recognition | Model 3: Response Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F(df) | p | ɳ2 | F(df) | p | ɳ2 | F(df) | p | ɳ2 | |
| Age Group | 1.53 (1, 97) | .22 | .02 | 13.21 (1, 96) | < .001 | .12 | 50.81 (1, 91) | < .001 | .36 |
| Condition | 36.23 (1, 97) | <.001 | .27 | 1.56 (1, 96) | .22 | .02 | 17.36 (1, 91) | <.001 | .16 |
| Valence | 3.93 (2, 194) | .021 | .04 | 6.56 (2, 192) | .002 | .06 | 2.13 (2, 182) | .12 | .02 |
| Age Group × Condition | 0.17 (1, 97) | .68 | .002 | 0.35 (1, 96) | .56 | .004 | 1.73 (1, 91) | .19 | .02 |
| Age Group × Valence | 4.60 (2, 194) | .011 | .04 | 1.27 (2, 192) | .28 | .01 | 3.88 (2, 182) | .022 | .04 |
| Condition × Valence | 0.62 (2, 194) | .54 | .01 | 3.16 (2, 192) | .032 | .03 | 0.62 (2, 182) | .94 | .001 |
| Age Group × Condition × Valence | 0.55 (2, 194) | .58 | .01 | 2.53 (2, 192) | .083 | .03 | 0.02 (2, 182) | .98 | .000 |
Note: Age group: 0 = young, 1 = older; Condition: 0 = control, 1 = pain.
Symbols: ɳ2 = partial eta squared.
Recognition.
A main effect of age group revealed that younger adults were significantly less willing to judge pictures as previously seen than older adults (C scores: Myoung = 0.22, Mold = 0.03, F(1, 96) = 13.21, p <.001). A two-way pain condition by valence interaction (F(2, 192) = 3.16, p = .032) indicated that after the pain condition only, both older and younger adults were marginally more willing to judge negative pictures as having been previously seen (t(97) = 1.79, p = .077), whereas no condition effects were found for the neutral or positive pictures (p’s > .05; see Table 2, Model 2). There was not a predicted three-way pain condition by age group by valence interaction in predicting recognition, F(2, 192) = 2.53, p = .083.
Response Time.
A main effect of age group revealed that both older and younger adults were quicker to recognize pictures following the pain condition than the control condition (M = 1146.51 vs 1231.33, respectively), F(1, 91) = 17.36, p <.001 (see Table 2, Model 3). Contrary to the hypothesis, there was not a predicted three-way pain condition by age group by valence interaction in predicting response time, F(2, 182) = 0.02, p = .98.
Social Preferences.
Participants preferred close versus knowledgeable social partners (γ = −0.33, t(134) = −8.88, p <.001; see Table 3). There was a significant 3-way interaction between social partner, pain condition, and age group (γ = −0.15, t(125) = −2.45, p = .016). In contrast to the control condition, after the pain condition, younger adults demonstrated lesser preference for knowledgeable social partners than older adults (see Figure 3).
Table 3.
Three-Way Interaction of Partner Type, Age Group, and Pain Condition on Social Preference.
| Variable | Effect | Estimate | SE | Df | p |
|---|---|---|---|---|---|
| Intercept | 0.58 | 0.05 | 141 | <.001 | |
| Partner Type | Close (ref = knowledge) | −0.54 | 0.05 | 127 | <.001 |
| Age Group | Older (ref = younger) | −0.36 | 0.07 | 141 | <.001 |
| Condition | Control (ref = pain) | −0.14 | 0.04 | 113 | .001 |
| Partner Type*Age Group | Close, Older (ref = knowledge, younger) | 0.36 | 0.08 | 129 | <.001 |
| Partner Type*Condition | Close, Control (ref = knowledge, pain) | 0.14 | 0.04 | 125 | .003 |
| Age Group*Condition | Older, Control (ref = younger, pain) | 0.15 | 0.06 | 113 | .012 |
| Partner Type*Age Group*Condition | Close, Older, Control (ref = knowledge, younger, pain) | −0.15 | 0.06 | 125 | .016 |
Figure 3.
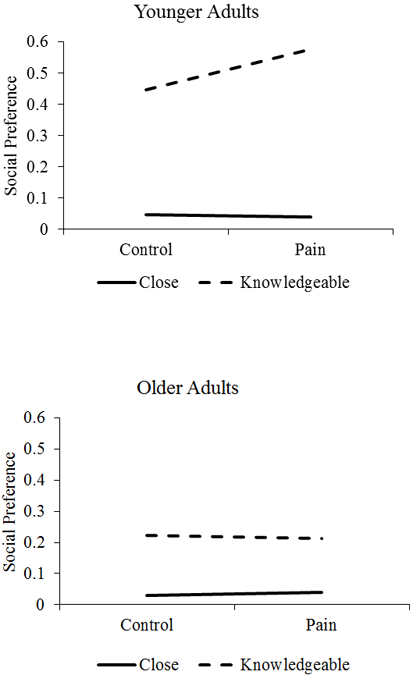
Graphical Representation of 3-Way Interaction of Age Group, Partner Type, and Pain Condition in predicting Social Preference (higher = less preferred).
Hypothesis 2: Changes in Affect Following Pain
Positive Affect.
Older adults reported more positive affect (M = 3.56, SD = 0.90) than younger adults (M = 2.84, SD = 0.72, t(98) = 4.47, p <.001). Positive affect was significantly lower after the pain condition (M = 3.11, SD = 0.95) than after the control condition (M = 3.28, SD = 0.86), F(1, 99) = 17.77, p <.001. However, there was not a predicted age group by pain condition interaction (F(1, 98) = 0.79, p = .38), suggesting that positive affect change after experiencing pain did not significantly differ between age groups.
Negative Affect.
Older adults (M = 1.12, SD = 0.16) and younger adults (M = 1.16, SD = 0.21) did not differ in levels of negative affect, t(98) = 1.20, p = .23. There was no main effect of pain condition on negative affect (F(1, 95) = 0.06, p = .81), nor was the predicted age group by pain condition interaction statistically significant (F(1, 95) = 0.14, p = .71).
Hypothesis 3: The Influence of EF and Vagal Tone
There were no significant hypothesized three-way interactions between either EF or vagal tone, pain condition, and valence on recall, recognition, or response times. Tables with complete results of these analyses are available by request to the first author.
However, there was a three-way interaction of task-switching performance by pain condition by partner type on social preference. After the control condition, better task-switching performance was associated with greater preference for knowledgeable social partners, whereas after the pain condition, better task-switching performance was associated with reduced preference for knowledgeable social partners (γ = 0.00001, t(120) = 2.07, p = .041, see Figure 4).
Figure 4.
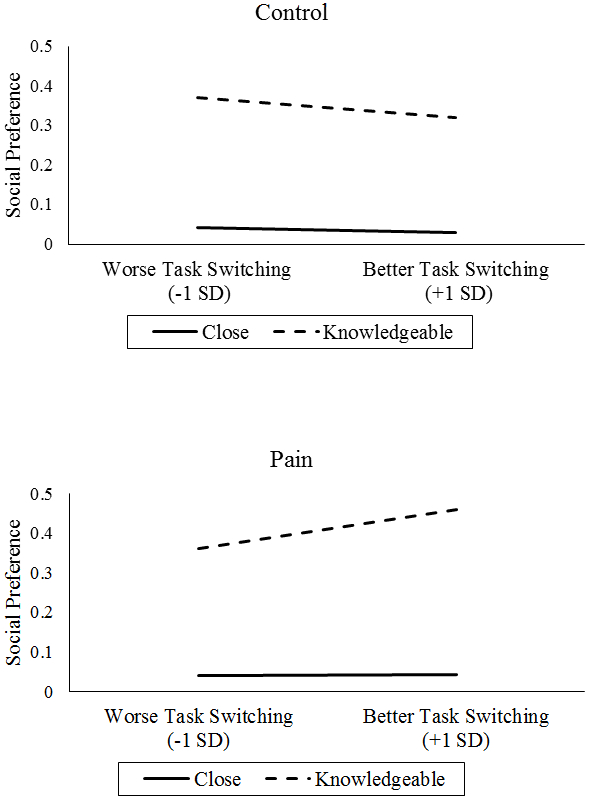
Interaction of Task-Switching, Pain Condition, and Partner Type on Social Preference (higher = less preferred).
Similarly, there was a three-way interaction of vagal tone by pain condition by partner type on social preference, γ = 0.12, t(119) = 2.33, p = .022. Specifically, after the pain condition, vagal tone more strongly predicted reduced preference for knowledgeable social partners compared with the control condition (see Figure 5).
Figure 5.
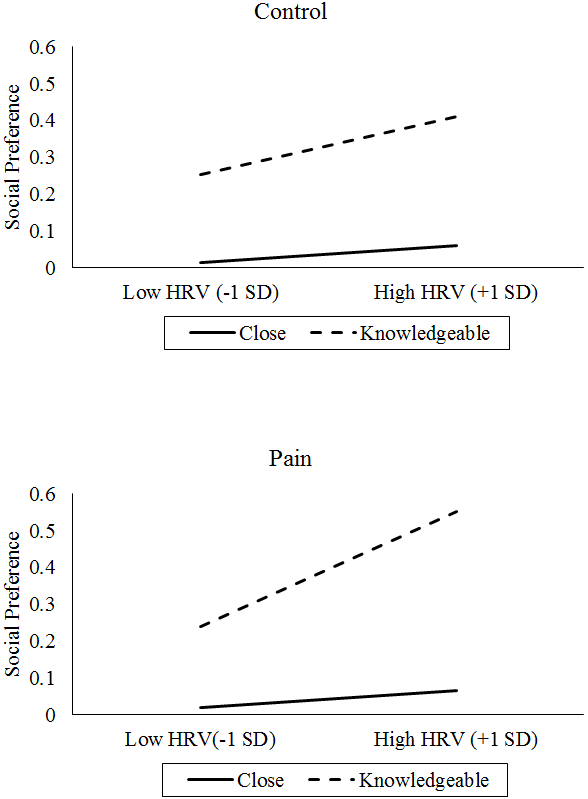
Interaction of Vagal Tone (assessed by HRV), Pain Condition, and Partner Type on Social Preference (higher = less preferred).
Discussion
On one hand, older adults more frequently experience and report pain than younger adults (Thomas et al., 2004). On the other hand, they report higher positive affect than younger adults, possibly guided by increased motivation for affective wellbeing as proposed by SST (Carstensen et al., 1999). Do older adults maintain affective wellbeing following acute pain better than younger adults, and if so, how?
The first aim of the study was to examine use of four positivity-enhancing strategies between older and younger adults following acute pain. It was hypothesized that older adults would recall, recognize, and respond more quickly to positive than negative pictures following acute pain and prefer closer versus knowledgeable social partners; in addition, older adults would do it to a greater extent than younger adults. The first strategy was recall for positive pictures – a strategy that older adults are known to use for emotion regulation purposes (e.g., Sims et al., 2015). Contrary to the hypothesis, people recalled fewer pictures of all valences following pain. This is consistent with literature showing that pain demands attention and disrupts executive functioning (Bushnell et al., 2013). Because participants were shown the pictures immediately following the pain task, it is also possible that performance on the recall task reflected problems with encoding rather than recall.
In partial support for the hypothesis, positive pictures were better recalled than neutral or negative pictures, but this was not specific to older adults. Older adults did, however, recall fewer negative pictures than younger adults (Figure 2), consistent with previous work showing that negative pictures are more easily forgotten by older than younger adults (Charles et al., 2003). The fact that there were no age differences in changes in negative affect following pain contribute to a wide yet ambiguous body of literature examining age differences in emotional reactivity. Some studies find that older adults are more emotionally reactive than younger adults (e.g., Röcke et al., 2009), whereas other studies find that they are less emotionally reactive (Kliegel et al., 2007; Kunzmann and Grühn, 2005; Smith et al., 2005) or equally as emotionally reactive as younger adults (Charles et al., 2009; Labouvie-Vief et al., 2003; Stawski et al., 2008). Our results are more in line with the latter set of findings, although emotional reactivity in older adults may be context-specific and may depend more on individual differences in perceptions of stress than on objective situational factors (Stawski et al., 2008). Given that participants always had the choice to withdraw their hand from the water, it is possible that the pain task was not as stressful as naturally-occuring pain and may not have elicited significant negative emotional reactivity from younger or older adults. Whether the lack of increase in negative affect following pain was due to differences in emotional reactivity or to perceptions of stress represents an important area for future inquiry.
A second positivity-enhancing strategy that older adults were expected to use more than younger adults following pain was recognition of positive stimuli. Instead, we found that both older and younger adults were marginally more willing to judge negative pictures as previously seen following acute pain. It may be that pain signals threat and primes people to attend to negative or threatening stimuli. Pain patients selectively attend toward painful faces relative to neutral ones in the dot-probe task (Khatibi et al., 2009). Chronic musculoskeletal pain and headache populations, among others, show similar attentional bias toward negative stimuli (Baum et al., 2013; Fashler & Katz, 2016; Schoth & Liossi, 2010). The attentional bias was corroborated by the fact that older and younger adults were quicker to respond to negative pictures following pain. This is the first study that demonstrates negativity bias following acute pain in healthy older and younger adults. These results suggest that pain instead of pain chronicity causes attentional shift, but this interpretation is speculative and should be tested in future research.
Despite these main effects and two-way interactions, the hypothesized three-way interactions between age group, pain condition, and picture valence were not found for either recall, recognition, or response times, suggesting that older and younger adults may be using similar strategies to maintain affective wellbeing following pain.
The second aim of the study was to investigate changes in affect following acute pain in older and younger adults. It was hypothesized that older adults would maintain positive affect and minimize increases in negative affect following acute pain better than younger adults. Older adults reported higher levels of positive affect and similar levels of negative affect than younger adults, consistent with previous findings and SST (Sims et al., 2015). There were no significant pain condition by age group interactions on pain-related changes in affect. As expected, positive affect was higher after the control than after the pain condition, but unexpectedly, there were no differences in negative affect change between the control and the pain conditions. Because affect was measured after the rest period and positivity tasks, it could be that the lack of pain-related changes in affect were due to affect having already returned to baseline by the time it was measured. Because the study population was relatively pain-free, the strategies employed by this population may be different than those employed by older adults with chronic pain. Results from this study suggest that one difference is that pain-free older adults tend to cope with pain by attempting to avoid it altogether, as 39/50 older adults kept their hand submerged for the entire minute in the pain condition compared to 48/50 of the younger adults. Avoiding pain is not always a viable strategy, especially in chronic pain, but to the extent that it is possible, it may be that older adults attempt to prevent pain rather than cope with its aftereffect. This interpretation is consistent with hedonic theories of wellbeing where the goal is to maximize pleasure and avoid pain (for a review, see Ryan & Deci, 2001), and with theories of aging that propose that older adults will avoid negative affect because of the costs associated with returning to homeostasis following sustained emotional arousal (Charles, 2010). Nevertheless, a direct test of these theories within the context of older adults and pain coping remains to be confirmed with future research.
Even though older and younger adults did not differ in their abilities to maintain affective wellbeing following pain, older adults reported more intense pain and worse mean levels of EF than younger adults. SST would posit that motivational differences account for these effects: older adults may have been more motivated to maintain positive affect, thereby obtaining the same outcome as younger adults despite having fewer cognitive resources. Alternatively, it may be that older adults are motivated to minimize increases in negative affect instead of increases in positive affect- a possibility that fits nicely with extant literature showing negative stimuli are more easily forgotten in older than younger adults (Charles et al., 2003). Another possibility is that older adults may have used additional unmeasured resources or different cognitive strategies like distraction, reappraisal, and reframing to cope with pain. A fourth possibility is that older adults have more expertise than younger adults in emotion regulation, and that this experience extends to having more effective strategies for managing pain. These possibilities pave the way for future research into how older adults manage pain-related affect.
With regard to the third aim that older and younger adults with better EF and vagal tone would use more positivity-enhancing strategies following acute pain, there was mixed support for EF or vagal tone serving as resources for maintaining affective wellbeing following acute pain. Despite not influencing recall, recognition, or response time as predicted, there were significant EF and vagal tone effects for social preference. Although younger and older adults both demonstrated strong preferences for close social partners across conditions, younger adults preferred knowledgeable social partners less following pain, whereas older adults’ preferences toward knowledgeable social partners did not change. It is unclear if maintaining interest in knowledgeable social partners following pain is adaptive or maladaptive. On one hand, older adults may be motivated to establish, learn from, and maintain social connections, and acute pain may not dissuade them from these goals. On the other, it may be a sign of resource misallocation. Under conditions of pain, spending time with a knowledgeable social partner probably does not provide as many affective benefits as spending time with a close social partner. Those older and younger adults with better task-switching performance and vagal tone demonstrated reduced preference for knowledgeable social partners following pain, suggesting they may have been prioritizing positive affect or emotional support over obtaining information or other types of social connections. One possibility is that task switching or vagal tone serve as resources that allow people to implement strategies that will help cope with pain more generally- a possibility that remains to be confirmed with future research. Another possibility is that better EF and HRV are the results of older adults successfully implementing positivity-enhancing strategies over extended periods of time, and that the relationship between EF/HRV and positive pain outcomes are bidirectional and reinforce one another. Whether these patterns extend to actual patterns of social network utilization represent another important extension to these findings.
The current study informs the pain and aging literature in several ways. Older adults have been found to maintain affective wellbeing following chronic pain (Boggero et al., 2016; Cook, & Chastain, 2001; Lohmann et al., 1998; Phelan & Heidrich, 2007; Rustøen et al., 2004), and the results from this study add to that literature by showing that they also successfully maintain affective wellbeing following acute pain; moreover, the strategies and resources (HRV, EF) that allow for successfully coping with pain appear consistent across the lifespan. One exception is that results from this study suggest older adults may be more prone than younger adults to avoid acute pain when they can – a finding that fits nicely with recent literature showing that older adults may be more sensitive to certain kinds of pain than their younger counterparts (El Tumi et al., 2017). Furthermore, literature in older adults with chronic pain is finding discrete subgroups based on psychological functioning and pain intensity (Larsson et al., 2017). Similarly, psychological variables may also differentiate subgroups of how older adults maintain affective wellbeing following acute pain, and this possibility remains to be explored in future research.
The study is not without limitations. First, a laboratory pain task was used to induce pain. Pain in the lab is different than pain in the real world. In the real world, the duration, threat value, and uncontrollability of pain may be greater, making it harder to manage (Crombez et al., 1998; Salomons, Johnstone et al., 2004). Another limitation is that participants always underwent the control condition prior to the pain condition. This was done to ensure that the pain condition did not contaminate the control measurements, but it eliminated the possibility of making conclusive statements about order effects. Affect in the current study was measured with the PANAS, which assesses high-arousal positive and negative affect in the present moment. However, an emerging body of literature is finding that maintaining low-arousal positive affect (i.e., calm, pleased) over the course of days to weeks is particularly important to health outcomes (see Steptoe et al., 2009 for a review). Future work should examine how trait levels and variability in low-arousal affect influence pain coping. Another major limitation is that previous experience with pain was not assessed, and as such, well-established relationships between pain self-efficacy and outcomes could not be examined (Skidmore et al., 2016). Finally, the sample was not ethnically diverse. Given racial differences in pain outcomes, results cannot generalize to other populations (Edwards et al., 2001). Results also cannot generalize to chronic pain populations.
Despite these limitations, the study also has considerable strengths. The sex- and race-matching of older and younger adults eliminated the possibility of potential gender or race effects in the age group comparisons. The inclusion of multiple strategies, EFs, and affective outcomes allowed for a thorough examination of psychological responses to acute pain in healthy older and younger adults. The repeated-measures design allowed for participants to serve as their own controls. Finally, stringent inclusion criteria and a laboratory setting provided high internal validity for the study. Maintaining affective wellbeing is an important health goal for many older adults, and learning how older adults do it effectively following pain has the potential to elucidate strategies that promote healthy aging.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers F31AG048692, K02-033629) and the American Psychological Association (Dissertation Award).
The authors would like to thank Charles Carlson for feedback on important aspects of study design and Lawrence Gottlob for assistance with analyzing and interpreting results from the cognitive tasks.
Footnotes
The authors declare no conflicts of interests.
References
- Aickin M, & Gensler H (1996). Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. American Journal of Public Health, 86(5), 726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C, Schneider R, Keogh E, & Lautenbacher S (2013). Different stages in attentional processing of facial expressions of pain: a dot-probe task modification. The Journal of Pain, 14(3), 223–232. [DOI] [PubMed] [Google Scholar]
- Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, & Moseley GL (2014). Do people with chronic pain have impaired executive function? A meta-analytical review. Clinical Psychology Review, 34(7), 563–579. [DOI] [PubMed] [Google Scholar]
- Boggero IA, Eisenlohr-Moul TA, & Segerstrom SC (2016). Task-switching ability protects against the adverse effects of pain on health: A longitudinal study of older adults. British Journal of Health Psychology, 21(2), 434–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggero IA, Geiger P, Segerstrom SC, & Carlson CR (2015). Pain intensity moderates the relationship between age and interference in chronic orofacial pain patients. Experimental Aging Research, 41(4), 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Čeko M, & Low LA (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews Neuroscience, 14(7), 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, & Charles ST (1999). Taking time seriously: A theory of socioemotional selectivity. American Psychologist, 54(3), 165–181. [DOI] [PubMed] [Google Scholar]
- Charles ST (2010). Strength and vulnerability integration: A model of emotional well-being across adulthood. Psychological Bulletin, 136(6), 1068–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, & Carstensen LL (2003). Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General, 132(2), 310–324. [DOI] [PubMed] [Google Scholar]
- Charles ST, Piazza JR, Luong G, & Almeida DM (2009). Now you see it, now you don’t: Age differences in affective reactivity to social tensions. Psychology and Aging, 24(3), 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, & Waters S (2007). Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain, 131(1), 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AJ, & Chastain DC (2001). The classification of patients with chronic pain: Age and sex differences. Pain Research & Management, 6(3), 142–151. [DOI] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Baeyens F, & Eelen P (1998). Attentional disruption is enhanced by the threat of pain. Behaviour Research and Therapy, 36(2), 195–204. [DOI] [PubMed] [Google Scholar]
- Diener E, & Chan MY (2011). Happy people live longer: Subjective well‐being contributes to health and longevity. Applied Psychology: Health and Well‐Being, 3(1), 1–43. [Google Scholar]
- Edwards CL, Fillingim RB, & Keefe F (2001). Race, ethnicity and pain. Pain, 94(2), 133–137. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, & Ness TJ (2003). Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain, 101(1), 155–165. [DOI] [PubMed] [Google Scholar]
- El Tumi H, Johnson MI, Dantas PBF, Maynard MJ, & Tashani OA (2017). Age‐related changes in pain sensitivity in healthy humans: A systematic review with meta‐analysis. European Journal of Pain, 21(6), 955–964. [DOI] [PubMed] [Google Scholar]
- Fashler SR, & Katz J (2016). Keeping an eye on pain: investigating visual attention biases in individuals with chronic pain using eye-tracking methodology. Journal of Pain Research, 9, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, & Garland EL (2015). The role of positive affect in pain and its treatment. The Clinical Journal of Pain, 31(2), 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung HH, Carstensen LL, & Lang FR (2001). Age-related patterns in social networks among European Americans and African Americans: Implications for socioemotional selectivity across the life span. International Journal of Aging and Human Development, 52(3), 185–206. [DOI] [PubMed] [Google Scholar]
- Khatibi A, Dehghani M, Sharpe L, Asmundson GJ, & Pouretemad H (2009). Selective attention towards painful faces among chronic pain patients: evidence from a modified version of the dot-probe. Pain, 142(1), 42–47. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, & Phillips LH (2007). Emotional development across adulthood: differential age-related emotional reactivity and emotion regulation in a negative mood induction procedure. The International Journal of Aging and Human Development, 64(3), 217–244. [DOI] [PubMed] [Google Scholar]
- Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, & Thayer JF (2014). Heart rate variability and experimentally induced pain in healthy adults: A systematic review. European Journal of Pain, 18(3), 301–314. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, & Grühn D (2005). Age differences in emotional reactivity: the sample case of sadness. Psychology and Aging, 20(1), 47–59. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Lumley MA, Jain E, & Heinze H (2003). Age and gender differences in cardiac reactivity and subjective emotion responses to emotional autobiographical memories. Emotion, 3(2), 115–126. [DOI] [PubMed] [Google Scholar]
- Lang FR, & Carstensen LL (2002). Time counts: Future time perspective, goals, and social relationships. Psychology and Aging, 17(1), 125–139. [DOI] [PubMed] [Google Scholar]
- Larsson B, Gerdle B, Bernfort L, Levin LÅ, & Dragioti E (2017). Distinctive subgroups derived by cluster analysis based on pain and psychological symptoms in Swedish older adults with chronic pain–a population study (PainS65+). BMC Geriatrics, 17(1), 200–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann R, Heuft G, Schneider G, & Kruse A (1998). Pain, coping and psychological well-being in late life. European Journal of Pain, 2(1), 43–52. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, & Creelman CD (1991). Detection theory: A user’s guide. Cambridge Univ. Press, Cambridge. [Google Scholar]
- Mather M, & Carstensen LL (2005). Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences, 9(10), 496–502. [DOI] [PubMed] [Google Scholar]
- Murphy NA, & Isaacowitz DM (2008). Preferences for emotional information in older and younger adults: A meta-analysis of memory and attention tasks. Psychology and Aging, 23(2), 263–286. [DOI] [PubMed] [Google Scholar]
- Phelan CH, & Heidrich SM (2007). Patterns of pain and well-being in older women: A 10-year longitudinal study. Journal of Women & Aging, 19(3-4), 21–35. [DOI] [PubMed] [Google Scholar]
- Pressman SD, & Cohen S (2005). Does positive affect influence health? Psychological Bulletin, 131(6), 925–971. [DOI] [PubMed] [Google Scholar]
- Reitan RM (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8(3), 271–276. [Google Scholar]
- Röcke C, Li SC, & Smith J (2009). Intraindividual variability in positive and negative affect over 45 days: Do older adults fluctuate less than young adults? Psychology and Aging, 24(4), 863–878. [DOI] [PubMed] [Google Scholar]
- Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, & Miaskowski C (2004). Prevalence and characteristics of chronic pain in the general Norwegian population. European Journal of Pain, 8(6), 555–565. [DOI] [PubMed] [Google Scholar]
- Ryan RM, & Deci EL (2001). On happiness and human potentials: A review of research on hedonic and eudaimonic well-being. Annual Review of Psychology, 52(1), 141–166. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, & Davidson RJ (2004). Perceived controllability modulates the neural response to pain. Journal of Neuroscience, 24(32), 7199–7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoth DE, & Liossi C (2010). Attentional bias toward pictorial representations of pain in individuals with chronic headache. The Clinical journal of Pain, 26(3), 244–250. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Geiger PJ, Combs HL, Boggero IA (2016). Time perspective and social preference in older and younger adults: Effects of self-regulatory fatigue. Psychology and Aging, 31, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims T, Hogan CL, & Carstensen LL (2015). Selectivity as an emotion regulation strategy: lessons from older adults. Current Opinion in Psychology, 3, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore JR, Koenig AL, Dyson SJ, Kupper AE, Garner MJ, & Keller CJ (2015). Pain self-efficacy mediates the relationship between depressive symptoms and pain severity. The Clinical Journal of Pain, 31(2), 137–144. [DOI] [PubMed] [Google Scholar]
- Smith DP, Hillman CH, & Duley AR (2005). Influences of age on emotional reactivity during picture processing. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Solberg-Nes L, Roach AR, & Segerstrom SC (2009). Executive functions, self-regulation, and chronic pain: A review. Annals of Behavioral Medicine, 37(2), 173–183. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Sliwinski MJ, Almeida DM, & Smyth JM (2008). Reported exposure and emotional reactivity to daily stressors: the roles of adult age and global perceived stress. Psychology and Aging, 23(1), 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Dockray S, & Wardle J (2009). Positive affect and psychobiological processes relevant to health. Journal of Personality, 77(6), 1747–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology. (1996). Heart rate variability standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17, 354–381. [PubMed] [Google Scholar]
- Thomas E, Peat G, Harris L, Wilkie R, & Croft PR (2004). The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain, 110(1), 361–368. [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1999). The PANAS-X: Manual for the positive and negative affect schedule-expanded form. [Google Scholar]
- West R, & Alain C (2000). Effects of task context and fluctuations of attention on neural activity supporting performance of the Stroop task. Brain Research, 873(1), 102–111. [DOI] [PubMed] [Google Scholar]
- Zautra A, Smith B, Affleck G, & Tennen H (2001). Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect. Journal of Consulting and Clinical Psychology, 69(5), 786–795. [DOI] [PubMed] [Google Scholar]


