Abstract
Glucocorticoids influence a wide array of metabolic, anti-inflammatory, immunosuppressive, and cognitive signaling processes, playing an important role in homeostasis and preservation of normal organ function. Synthesis is regulated by the hypothalamic-pituitary-adrenal (HPA) axis of which cortisol is the primary glucocorticoid in humans. Synthetic glucocorticoids are important pharmacological agents that augment the anti-inflammatory and immunosuppressive properties of endogenous cortisol and are widely used for the treatment of asthma, Crohn’s disease, and rheumatoid arthritis, amongst other chronic conditions. The homeostatic activity of cortisol is disrupted by the administration of synthetic glucocorticoids and so there is interest in developing treatment options that minimize HPA axis disturbance while maintaining the pharmacological effects. Studies suggest that optimizing drug administration time can achieve this goal. The present review provides an overview of endogenous glucocorticoid activity and recent advances in treatment options that have further improved patient safety and efficacy with an emphasis on chronopharmacology.
Keywords: Cortisol, synthetic glucocorticoids, chronopharmacology, chronopharmacokinetics, HPA axis disruption, circadian rhythms
1. Introduction
Glucocorticoids are a group of cholesterol-derived hormones secreted from the adrenal glands. They are involved in a wide array of metabolic, anti-inflammatory, immunosuppressive, and cognitive signaling processes with the discovery of such properties by Hench and Kendal in 1948 [1–3]. Glucocorticoids are thought to be the most important mediators of systemic inflammation and play an essential role in restoring homeostasis [4]. Glucocorticoids help maintain the proper balance between proinflammatory and anti-inflammatory mediators, which can be upset by serious infections and diseases [5, 6]. Cortisol, the primary glucocorticoid in humans, and its precursors vary in a predictable time-dependent manner in humans with both circadian and ultraradian patterns of secretion and elimination [4, 7]. The oscillatory behavior is driven by signals received from the central pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN) and external cues, such as stress [8, 9]. Cortisol is a key entraining signal for synchronization of peripheral circadian clock genes that coordinate various biological processes across their residing tissues to ensure homeostasis [10–13]. Glucocorticoids impact the rhythmic expression of circadian genes in almost all tissues [14]. The maintenance of proper circadian rhythmicity is critical in humans with peak cortisol concentrations in the early morning and nadirs at night [7, 15]. The timing of the peak concentration is highly regulated and in conjunction with other signaling hormones enables the separation of physiological processes throughout the day for optimal biological functioning and survival [11, 13, 16]. The natural rhythms of plasma cortisol are sensitive to internal physiological and external environmental cues which can lead to altered or disrupted oscillations throughout the day. Phase shifts, atypical absolute concentrations, and dampened or heightened amplitudes are all indicators of a disrupted diurnal rhythm [12]. For example, clinically significant increases in glucose and insulin occur with misalignment of the cortisol rhythm compared to normal cortisol activity [14].
Synthetic glucocorticoids are an important class of pharmacological agents to augment or substitute, in the case of adrenal insufficiency, the anti-inflammatory and immunosuppressive properties and physiological actions of endogenous glucocorticoids [17–19]. Despite the abundance of clinical benefits and years since its first clinical use in the 1950s, glucocorticoid therapies are associated with serious adverse effects, especially during high-dose administration [20–24]. Patients are at a risk of a host of clinical manifestations, including psychiatric disorders like depression, drug-induced hyperglycemia, long-term diabetes mellitus, glaucoma, osteoporosis, obesity, gastritis and cardiovascular disease [6, 14, 22, 25–31]. The systemic effects arise from the pleiotropic influence of glucocorticoids on liver, skeletal muscle, adipose tissue, and pancreatic functioning, amongst other tissues [32]. Glucocorticoids influence carbohydrate metabolism, inhibit gluconeogenesis, modulate lipid disposition and storage, inhibit processes such as proinflammatory cytokine production in response to injury and pathogens, and regulate proinflammatory mediators under normal physiological conditions [33, 34]. Furthermore, glucocorticoids regulate blood pressure, bone resorption, the cell cycle, and energy homeostasis [35]. High glucocorticoid levels during the day facilitate energy allocation to the brain, muscles, and immunosurveillance while low levels during the night facilitate activation of the immune system [16, 33].
Although widely accepted as the leading treatment option for a host of health conditions over the last century, challenges remain before glucocorticoid therapies are no longer accompanied by serious adverse effects. A major downfall of long-term term use of synthetic glucocorticoids is the modification of endogenous cortisol secretary patterns which is thought to lead to misalignment of peripheral clock genes and improper physiological functioning [10, 20, 36–38]. This unmet medical need is particularly true for patients receiving treatment for chronic conditions requiring lifetime administration, often at high doses. While significant progress has been made to understand the genomic and non-genomic actions of synthetic glucocorticoids, the mechanisms have not yet been elucidated, given that dissecting drug effect from inherent inflammatory processes is rather challenging [30, 31, 39]. Today, glucocorticoid research continues to strive for long-term treatment options that balance safety and efficacy to minimize complications as shown in Figure 1. There is interest in minimizing the disruption of the homeostatic activity of cortisol while retaining the pharmacological actions of synthetic glucocorticoids by leveraging the interplay between the HPA axis and the immune system [20, 40]. Evidence suggests that this goal can be achieved by optimizing drug administration time [41, 42]. In general, chronopharmacokinetics are most beneficial for drugs with narrow therapeutic ranges, for drugs with high variability, and to treat diseases with circadian-dependent symptomology [43]. All of which may be true for glucocorticoids, supporting the potential to improve the effectiveness and safety of treatment options through chronopharmacological intervention. This review provides an overview of the role of glucocorticoids in health and disease, as well as recent advances in pharmacology that have improved patient safety and effectiveness with an emphasis on chronopharmacokinetics and chronopharmacodynamics.
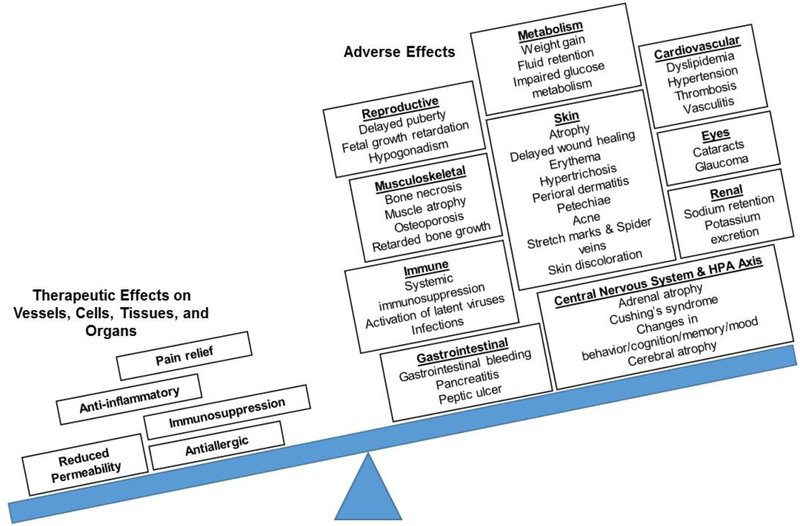
Figure 1:
Therapeutic and adverse effects associated with glucocorticoid therapies. Glucocorticoid treatment is accompanied by several adverse effects across peripheral tissues and many biological processes. For safe and effective treatment, the therapeutic benefits of synthetic glucocorticoid administration must outweigh the risk of these adverse effects. Figure adapted from Liu et al. [6] and Buttgereit et al. [30].
2. Overview of glucocorticoid activity: the key aspects of systemic and tissue-level regulation
2.1. Synthesis of Glucocorticoids
Glucocorticoid synthesis is regulated by the hypothalamic-pituitary-adrenal (HPA) axis in which cortisol, the final HPA axis product, is the primary glucocorticoid in humans [3, 20]. The HPA axis is the principal stress response mechanism, both physical and physiological, along with the autonomic nervous system, constituting a carefully regulated signaling network [44, 45]. The HPA axis consists of stimulatory signals and negative feedback loops which are important for maintaining both resting and stress-related homeostasis, and for preserving the normal physiology of organ systems and almost all physiologic, cellular and molecular networks [46, 47]. When an individual is exposed to stress, the HPA axis becomes activated, leading to an increase in cortisol secretion, which along with other mediators, facilitates the modification of underlying physiological properties in the body and brain for maintenance of homeostasis, a process referred to as allostasis [48, 49]. A schematic of the HPA axis is given in Figure 2. The release of corticotrophin-releasing hormone (CRH) in the hypothalamus triggers the release of adrenocorticotrophic hormone (ACTH) in the pituitary gland and finally cortisol from the adrenal glands. Cortisol, in addition to mediating downstream physiological effects, also provides negative feedback to the HPA axis by inhibiting the release of CRH and ACTH and subsequently its own secretion [50]. This negative feedback loop is important for maintaining normal physiological levels of circulating cortisol, enabling the host to preserve homeostasis in response to stress or disease activity.
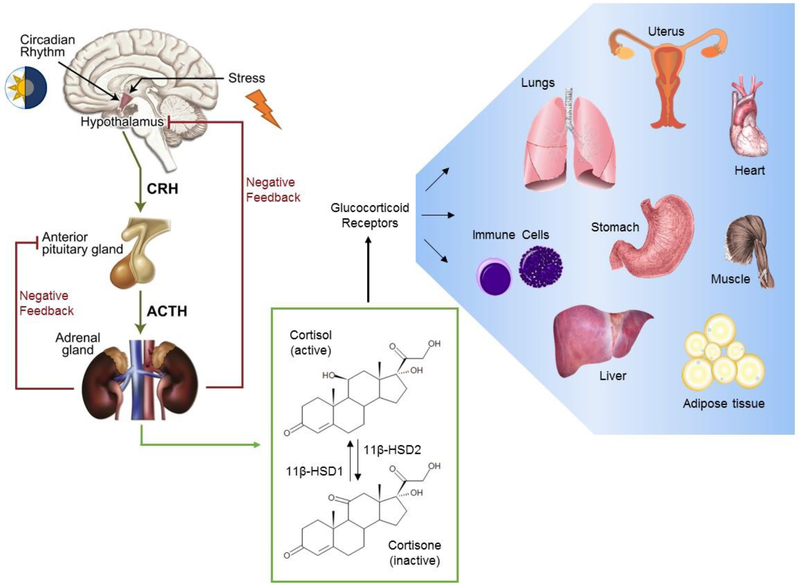
Figure 2:
Systemic and tissue-level regulation of glucocorticoids. Glucocorticoid synthesis is regulated by the hypothalamic-pituitary-adrenal (HPA) axis, comprised of stimulatory and negative feedback signals which control the secretion of corticotrophin-releasing hormone (CRH) from the hypothalamus, adrenocorticotrophic hormone (ACTH) from the pituitary gland and finally cortisol from the adrenal glands. The 11β-hydroxysteroid dehydrogenase (11β-HSD) enzyme system is responsible for the activation and deactivation of cortisol, which in part is responsible for local regulation of cortisol. Cortisol induces its pleiotropic influences on peripheral tissues through glucocorticoid receptor-mediated actions, supporting the normal physiology and functioning of these organ systems. Figure adapted from Cruz-Tropete et al. [34, 51] and Oakley et al. [52].
2.2. Transport of Plasma Cortisol
Once secreted from the adrenal glands, cortisol is circulated throughout the host to peripheral tissues. Endogenous cortisol is highly bound (90–95%) to plasma proteins, corticoid binding globulin (CBG or transcortin) and albumin, which influence the half-life, storage and volume of distribution of circulating cortisol [53–55]. Cortisol has a typical half-life of 70 to 120 minutes [3]. Protein-bound cortisol is circulated throughout the host to various tissues and is subsequently liberated from CBG by elastase. Upon its release, cortisol can freely cross cell membranes due to its lipophilic nature and bind to cytosolic glucocorticoid receptors [29, 56]. Only free, unbound cortisol is considered to be biologically active [45, 55]. Furthermore, CBG exhibits its own circadian rhythmicity that is in antiphase with the circadian patterns of cortisol secretion [7]. Unbound cortisol concentrations outside the physiological range may in part be due to disturbances in the time course of CBG [54, 55].
2.3. Glucocorticoid Receptors
Unbounded cortisol freely crosses the cell membranes of immune and other cell types, where many of cortisol’s physiological actions are regulated by glucocorticoid receptors. Upon binding in the cytoplasm, the bound glucocorticoid-receptor complex undergoes a transformational change and translocates to the nucleus where subsequent regulation of gene transcription occurs through glucocorticoid response elements [7, 31]. The glucocorticoid receptor (Type II) primarily regulates the anti-inflammatory effects of circulating cortisol through transrepression, or inhibition, of genes involved in the production of proinflammatory cytokines and other mediators involved in immune response [20, 29, 30, 57]. A second kind of glucocorticoid receptor (Type I) has mineralocorticoid activity and is important for maintaining fluids and electrolyte homeostasis [57, 58].
While the glucocorticoid receptor is expressed in almost all tissues, mineralocorticoid receptors are localized to the kidney, colon, salivary and sweat glands, and the hippocampus [59]. Mineralocorticoid receptors have a high affinity, but low capacity for glucocorticoids, whereas glucocorticoid receptors have low affinity, but high capacity. As such, the mineralocorticoid receptors respond to low glucocorticoid concentrations and glucocorticoid receptors respond to both basal and stress concentrations [60]. The mineralocorticoid receptor is almost always in an activated state in the nucleus and the glucocorticoid receptors remains latent in the cytoplasm until activation [7]. The balance between these receptors is believed to have important behavioral and cognitive influence in response to stress [57, 58]. Additionally, rapid non-genomic effects of glucocorticoids are explained by the activity of membrane-associated receptors [3, 7, 34]. Together, the various receptor types are responsible for maintaining the fast and slow signaling cascades controlled by glucocorticoids corresponding with the non-genomic and genomic mechanisms of action, respectively [57]. While non-genomic influences of glucocorticoids occur within minutes to hours, genomic actions mediated by glucocorticoid receptors, such as altered transcription of genes, have much longer time scales.
2.4. Enzymatic Regulation of Cortisol
Cortisol undergoes interconversion between its active and inactive forms by 11β-hydroxysteroid dehydrogenase (11β-HSD) types 1 and 2, respectively, for regulation of cortisol concentrations in peripheral tissues [45, 55]. This interconversion protects the mineralocorticoid receptor from excess cortisol-induced activity and plays an important role in modulating systemic inflammatory response [61]. Cortisol is inactivated to cortisone by 11β-HSD2 in the kidneys while 11β-HSD1 activates cortisol from cortisone in the liver, adipose and other bodily tissues [62]. Readers are directed to the review by Chapman et al. for a detailed description of the influences of 11β-HSD enzymes on glucocorticoid actions [56]. Cortisol is also metabolized by CYP3A4, an important cytochrome p450 enzyme in the liver [62]. Both cortisol and cortisone derivatives are further metabolized by reductase enzymes or conjugated to glucuronic acid, and rapidly excreted in urine [3].
3. Glucocorticoids in disease
Clinical manifestations associated with cortisol outside the normal physiological range arise from disruption of the HPA axis, which affects adrenal secretion and circulating glucocorticoids, as well as from modifications in local glucocorticoid activity that upsets normal functioning of peripheral tissues. Irregular HPA axis activity is associated with changes in the rest-activity cycle as a result of shift work or jet lag, hypersecretion due to various physiological factors (aging, sleep disorders, depression, and adrenal morbidities, etc.), the onset and pathogenesis of several inflammatory or immune conditions, and pharmacological intervention [14, 63, 64]. Adrenal insufficiency is associated with many immune and inflammatory conditions such as sepsis, severe pneumonia, adult respiratory stress syndrome, and HIV infection, as a result of both decreased cortisol secretion and impaired signal transduction, although these mechanisms are not fully understood [65]. The extent of HPA axis disruption varies from normal to complete adrenal insufficiency depending on the severity of HIV infection [66]. Similarly, chronic allergic inflammatory disorders are accompanied by reduced HPA axis activity leading to flattened diurnal rhythms and low peak concentrations of cortisol due to interactions between the stress and immune systems involved in allergic conditions [67]. Conversely, surgery and fevers due to infection are strong activators of the HPA axis, which lead to the stimulation of ACTH and cortisol secretion [64]. Several other conditions associated with prolonged disruption of the HPA axis are shown in Table 1 (adapted from [47]).
Table 1:
Conditions associated with altered activity of the hypothalamic-pituitary-adrenal (HPA) axis
| Increased HPA axis activity | Decreased HPA axis activity |
|---|---|
| Chronic stress | Adrenal insufficiency |
| Melancholic depression | Atypical/seasonal depression |
| Anorexia nervosa | Chronic fatigue syndrome |
| Malnutrition | Fibromyalgia |
| Obsessive-compulsive and panic disorders | Hypothyroidism |
| Excessive exercise | Nicotine withdrawal |
| Chronic active alcoholism | Post discontinuation of glucocorticoid therapy |
| Alcohol and narcotic withdrawal | Cured Cushing’s syndrome |
| Diabetes mellitus | Premenstrual tension syndrome |
| Truncal obesity | Postpartum period |
| Childhood sexual abuse | Following chronic stress |
| Psychosocial short stature | Rheumatoid arthritis |
| Attachment disorder of infancy | Perimenopause |
| “Functional” gastrointestinal disease | |
| Hyperthyroidism | |
| Cushing’s syndrome | |
| Pregnancy (last trimester) |
Note: Table is adapted from Charmandari et al. [47].
Abnormal glucocorticoid activity in peripheral tissues can arise due to local dysregulation of glucocorticoids. This dysregulation interrupts the normal signaling cascades of cortisol necessary for homeostasis. Glucocorticoid activity in peripheral tissues influences the strength of negative feedback to the HPA axis, which then induces a compensatory increase or decrease in cortisol secretion to account for differences at the tissue-level [47]. Primary generalized glucocorticoid resistance (PGGR) or Chrousos syndrome is a rare condition in which all tissues have decreased sensitivity or resistance to glucocorticoids [68]. PGGR is accompanied by hypersecretion of cortisol and its precursors due to impaired feedback to the HPA axis [69]. Conversely, primary generalized glucocorticoid hypersensitivity (PGGH) leads to increased sensitivity to glucocorticoids in all peripheral tissues and compensatory suppression of the HPA axis [70]. While PGGR and PGGH are rare, various levels of resistance and hypersensitivities are observed in chronic inflammatory conditions [71]. Rheumatoid arthritis, lupus, asthma and AIDS have shown regular activity of the HPA axis but altered glucocorticoid activity in various tissues [72]. These irregularities are attributed to hypersensitivity or resistance to glucocorticoids in peripheral tissues due to interference by disease on glucocorticoid receptors and signal transduction [73–75]. Changes in receptor-mediated activity are a result of modified affinity, nuclear translocation, glucocorticoid response element binding and communication with transcription factors or cofactors [76]. Furthermore, patients suffering from rheumatoid arthritis or inflammatory bowel disease (IBD) have also shown increased levels of 11β-HSD1 and decreased levels of 11β-HSD2 which would lead to locally high cortisol concentrations [77]. Readers are directed to the review by Quax for further discussion of glucocorticoid sensitivity in health and disease [77] and to the reviews by Charmandari and Chrousos [47, 78] for a deeper look at the role that glucocorticoids play in maintaining homeostasis and stress management.
4. Pharmacological intervention with synthetic glucocorticoids
Synthetic glucocorticoids are widely used for the treatment of a variety of chronic conditions such as asthma, skin infections, Crohn’s disease, and rheumatoid arthritis, as well as the management of immune response following organ transplantation [6, 30]. Acute exposure is associated with reduced inflammation, whereas chronic exposure has immunosuppressive action [34]. An almost immediate decline in blood lymphocytes and basophils, and an increase in neutrophils are observed after administration, while the induction of interleukins and other immunosuppression mediators, such as IκBα (inhibitor of transcription factor NFκB), are observed after several hours [79]. Importantly, the adverse phenotypes that appear following synthetic glucocorticoid administration are fundamentally the same as those associated with atypical endogenous cortisol levels [80]. Many of the adverse effects of glucocorticoid treatment are due to local excesses or deficiencies in peripheral tissues, as shown in Table 2, resulting from atypical cortisol secretion and/or abnormal signal transduction [47, 81–84]. Generally, low levels of glucocorticoids are associated with more severe inflammatory responses due to rising levels of proinflammatory mediators, whereas high levels of glucocorticoids, particularly in the absence of inflammation, leads to increased risk of infections and issues with immunity due to the immunosuppressive properties of glucocorticoids [23].
Table 2:
Clinical manifestations associated with tissue-specific glucocorticoid excess or deficiency
| Affected Tissue | Excess Glucocorticoid Activity | Deficient Glucocorticoid Activity |
|---|---|---|
| Central nervous system | Insomnia, anxiety, depression, defective cognition | Fatigue, somnolence, malaise, defective cognition |
| Liver | Increased gluconeogenesis, increased lipogenesis | Hypoglycemia, resistance to diabetes mellitus |
| Fat | Visceral fat accumulation | Loss body weight, resistance to weight gain |
| Blood Vessels | Hypertension | Hypotension |
| Bone | Stunted growth, osteoporosis | |
| Inflammation/immunity | Immunomodulation, anti-inflammation, susceptibility to infections and tumors | Increased Inflammation, autoimmunity, and allergy |
The chemical structures of glucocorticoids are similar to endogenous compounds but with modifications to enhance therapeutic properties while minimizing adverse effects [29, 31, 85]. Given that the primary action of glucocorticoids is through receptors, there is particular interest in the development of selective glucocorticoid receptor agonists that have minimal mineralocorticoid receptor activity. Mineralocorticoid activity is thought to be associated with many of the transactivating properties that lead to adverse effects, while glucocorticoid receptor activity is thought to be associated with many of the transrepression properties that suppress proinflammatory genes [20, 30, 86]. Unfortunately, the therapeutic effects mediated by glucocorticoid receptors are accompanied by HPA axis suppression. Early glucocorticoids, such as hydrocortisone, have both glucocorticoid and mineralocorticoid activity while more recently developed drugs have less mineralocorticoid activity like prednisolone and almost no mineralocorticoid activity like dexamethasone [29]. A brief summary of pharmacodynamics for select glucocorticoids are provided in Table 3 (adapted from [75]). In general, the clinical effects of synthetic glucocorticoids are dependent on drug solubility, absorption rate, receptor affinity, metabolic rate, and dose, which vary by drug [87]. Therefore, differences in the pharmacodynamics across this class of drugs must be accompanied by an understanding of pharmacokinetics to optimize the dose-exposure-response relationship as discussed in Sections 4.1 and 4.2.
Table 3:
Pharmacodynamics of select synthetic glucocorticoids
| Synthetic Glucocorticoids | Dose (mg) | Anti-inflammatory Activity (relative to hydrocortisone) | Mineralocorticoid Activity (relative to hydrocortisone) | Biological Half-life (hours) |
|---|---|---|---|---|
| Hydrocortisone | 20 | 1 | 1 | 8–12 |
| Prednisone/Prednisolone | 5 | 4 | 0.3 | 12–36 |
| Methylprednisolone | 4 | 5 | 0.5 | 12–36 |
| Dexamethasone | 0.75 | 30 | 0 | 36–72 |
Note: Table contains data from R.M. Paragliola, G. Papi, A. Pontecorvi, S.M. Corsello, Treatment with Synthetic Glucocorticoids and the Hypothalamus-Pituitary-Adrenal Axis, Int J Mol Sci, 18 (2017).
4.1. Differences in use and administration routes
When glucocorticoids are administered for health emergencies, high doses are usually given intravenously whereas chronic diseases are managed with low oral doses [85]. Any of the glucocorticoids may be administered intravenously or orally depending on the indication and severity of the condition. Similar immunosuppressive and anti-inflammatory properties can be maintained using dose equivalency, enabling clinicians to switch between compounds as needed [88]. It is known that the duration and extent of suppressive effects on the HPA axis are dependent on the length of time systemic concentrations remain above the IC50, which is inherently a function of the dose, clearance, and potency of the drug administered [19]. For example, greater suppression of the HPA axis is usually observed with higher doses due to increased negative feedback signals [53, 89]. Consequently, the choice of one synthetic glucocorticoid over another stems from the nature of the condition being treated, frequency of dosing, and the severity of the condition. Generally, hydrocortisone is considered a short-acting glucocorticoid. Prednisolone and methylprednisolone are recognized as intermediate-acting, while dexamethasone has extended suppressive effects on the HPA axis even after administration of a low single dose [88].
Hydrocortisone is most often used for hormone replacement due to adrenal insufficiency [90, 91]. Considering its structural equivalency to endogenous cortisol, hydrocortisone is the preferred drug due to its dual glucocorticoid and mineralocorticoid activity that matches endogenous cortisol action [89]. Prednisolone and methylprednisolone are commonly used to treat a variety of inflammatory and immune disorders, including rheumatoid arthritis and asthma [92, 93]. When administered for hormone replacement, prednisolone and methylprednisolone must be administered in conjunction with a mineralocorticoid drug to compensate for the reduced mineralocorticoid activity [92]. Prednisolone is amenable to chronic treatment due to its short half-life, and is often administered orally as the prodrug, prednisone [92, 94, 95]. Dexamethasone is used pharmacologically for the treatment of inflammation, allergic and autoimmune disorders, leukemia, nausea, and vomiting, amongst other indications, as well as in diagnostics to probe HPA axis functionality for disease identification, such as Cushing’s syndrome or depression [25, 96, 97]. Dexamethasone is also commonly used to suppress the HPA axis to mimic disease states for analysis of other synthetic glucocorticoids [90, 98, 99]. This drug is rarely used for hormone replacement because it lacks mineralocorticoid activity [97]. Given its association with severe HPA axis suppression, dexamethasone is typically used for short-term treatment in severe, acute conditions [6].
4.2. Absorption and disposition of synthetic glucocorticoids
Synthetic glucocorticoids are lipophilic drugs with the bioavailability of oral dosage forms ranging from 60 to 100% [85]. Glucocorticoids are rapidly absorbed when administered orally, with the maximum concentration typically seen within 1 to 3 hours after administration for immediate release formulations [85]. At higher doses, hydrocortisone is associated with reduced dissolution rates and may be classified as class II of the Biopharmaceutical Classification System (BCS) [100]. Prednisolone is considered a BCS class I drug with both high permeability and solubility [101], whereas the permeability and solubility of prednisone are on the borderline of being considered class I [102]. While highly soluble, methylprednisolone and dexamethasone are associated with high or low permeability depending on the study and are classified as BCS classI or III accordingly [103, 104]. Dexamethasone undergoes hepatic recirculation [85], which may contribute to variability in systemic exposure for this particular drug.
Both hydrocortisone and prednisolone have nonlinear, dose-dependent pharmacokinetics in part due to plasma protein binding, whereas methylprednisolone and dexamethasone typically have linear pharmacokinetics with pharmacological doses [19, 21, 85, 105]. Hydrocortisone and prednisolone bind to both corticosteroid binding globulin and albumin, while methylprednisolone and dexamethasone bind only to albumin [21, 45, 85, 89, 95]. Hydrocortisone and prednisolone will compete with endogenous cortisol for binding sites on corticosteroid binding globulin, affecting the circulating concentrations of unbound endogenous and synthetic glucocorticoids. Within the therapeutic range of prednisolone doses, CBG can become saturated, causing the bound fraction of endogenous cortisol to drop nonlinearly from 95% to 60–70% [95]. Synthetic glucocorticoids are also substrates for 11β-hydroxysteroid dehydrogenase types 1 and 2 [21, 85, 95], which will influence the relative amounts of active and inactive glucocorticoid forms. Similar to the interconversion between cortisol and cortisone, prednisone and prednisolone will undergo interconversion by the 11β-HSD enzyme system. Therefore, compounds like prednisone are considered both a prodrug and a metabolite [21, 95]. The possible activity of 11β-HSD2 in the colon may influence absorption of formulations released late in the gastrointestinal tract [98].
All drugs are eliminated by hepatic metabolism and renal excretion of inactive metabolites as well as some un-metabolized drug [85]. Hydrocortisone and prednisolone have short half-lives, typically less than 3.5 hours with no drug accumulation between doses, even when patients are administered multiple daily doses, making these compounds preferable for chronic treatment with short dosing intervals [89, 94]. Prednisolone clearance is dose-dependent due to plasma protein binding with greater clearance and apparent volume of distribution at higher doses given a higher fraction of unbound drug [101]. The half-life of methylprednisolone is comparable to hydrocortisone and prednisolone with reported values up to 2.5 hours [85]. In contrast, dexamethasone has a much longer half-life of 36 to 72 hours and is typically administered at lower doses because of its potent glucocorticoid receptor activity and longer duration of action [91].
Studies exploring the pharmacokinetics of synthetic glucocorticoids have shown that the number of compartments necessary to describe distribution and elimination vary by drug type, dose, and administration route. This observation highlights the potential complexities of pharmacokinetics for this drug class and the underlying influences that circulating cortisol has on drug exposure. Prednisolone is often described by a 2-compartment model following intra-venous administration while oral delivery requires a 1 or 2 compartment model depending on dose [85, 106]. Variability in prednisolone pharmacokinetics are likely attributed to saturable processes, such as plasma protein binding or interconversion by the 11β-HSD enzymes, that influence drug distribution to tissues and eventually its elimination from the body, which is at least partially dependent on circulating cortisol levels. Methylprednisolone requires a 2-compartment model following injections of high doses whereas oral or low intra-venous doses are described by 1-compartment. Dexamethasone is typically described by 2-compartment models [88].
5. Case Study: Modeling of synthetic glucocorticoids for dose equivalency evaluation
As discussed in Section 4, several differences exist in the pharmacokinetics and pharmacodynamics of synthetic glucocorticoids, which ultimately influence the magnitude and duration of the biological effects of each drug. Physicians often leverage these differences to optimize patient treatment, requiring an understanding of the equivalent doses needed to produce similar therapeutic effects. This case study demonstrates how models are used to improve our understanding of synthetic glucocorticoid for successful pharmacological intervention. The pharmacokinetics and pharmacodynamics (PKPD) of prednisolone (PNL), methylprednisolone (MPL), dexamethasone (DEX), and hydrocortisone (HC) were described in healthy male subjects using compartment models to describe drug exposure and indirect response models to describe the effects on cortisol secretion [88]. The compartments models for the time-dependent drug concentration are given in Equations 1–6, where ka is the first-order absorption rate constant, kel is the first-order elimination constant, k12 and k21 are the rate constants for transfer of drug between tissue and plasma, and kout is the first-order elimination constant of hydrocortisone which is indistinguishable from that of endogenous cortisol. The model for prednisolone considers the free drug due to nonlinear plasma protein binding.
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
| Eq. 4 |
| Eq. 5 |
| Eq. 6 |
Synthetic glucocorticoids have a suppressive effect on endogenous cortisol secretion (Equations 7–8), characterized by the inhibitory function, IC(t), where IC50 is the drug concentration that achieves 50% inhibition of cortisol secretion and CGC is the time-dependent concentration of the synthetic glucocorticoids determined by the compartment models [88]. Cortisol secretion in this model is described by a periodic, time-dependent production function, kin(t), and cortisol elimination is described by the first-order elimination rate constant, kout. The production function is described by a two-harmonic Fourier series (Equation 9).
| Eq. 7 |
| Eq. 8 |
| Eq. 9 |
The pharmacokinetic and pharmacodynamic parameters were determined by fitting the model to clinical data and are shown in Table 4. Hydrocortisone and cortisol are indistinguishable and thus a joint PKPD model was used to estimate parameter values. For the other drugs, pharmacokinetic parameters were first estimated and then the pharmacodynamic parameters were determined [88]. The concentration-time profiles for the synthetic glucocorticoids and cortisol before and after a single dose are shown in Figure 3.
Table 4:
Estimated Pharmacokinetic and Pharmacodynamic Parameters
| Model | Parameter | Hydrocortisone | Free Prednisolone | Methylprednisolone | Dexamethasone |
|---|---|---|---|---|---|
| Pharmacokinetics | Dose (mg) | 149 | 37 | 29 | 5.7 |
| k12 (h−1) | NA | NA | NA | 1.11 (39) | |
| k21 (h−1) | NA | NA | NA | 0.919 (13) | |
| ka (h−1) | NA | 1.25 (21) | NA | NA | |
| kel (h−1) | 0.405 (9.4) | 0.272 (4.8) | 0.27 (4.2) | 0.416 (20) | |
| V/F (L)a | 119 (15) | 168 (9.9) | 83.4 (8.1) | 42.8 (22) | |
| CL/F (L/h)a | 48.2 | 45.7 | 22.5 | 17.8 | |
| Pharmacodynamics | IC50 (ng/mL) | 8.01 (70) | 1.25 (14) | 0.52 (37) | 0.172 (30) |
| kout (h−1) | 0.405 (9.4) | 1.54 (26) | 0.405b | 0.405b | |
| AUEC (ng∙h/mL)c | 1070 (230) | 1510 (452) | 1150 (394) | 1430 (237) | |
| Duration of Suppression (hr) | ~15 | ~15 | ~28 | ~32 |
Data reported as estimate and percent coefficient of variance unless noted. NA = Not applicable; k12, k21 = first-order rate constants of drug transport between central and peripheral compartments; ka = first-order absorption rate constant; kel = first-order elimination rate constant; V/F = volume of distribution corrected for bioavailability; CL/F = total system clearance corrected for bioavailability; IC50 = drug concentration leading to 50% suppression of cortisol secretion; kout = first order rate constant of cortisol elimination; AUEC =area under effect curve from 0 to 20 hours (|AUECbaseline − AUECtreatment|). Table contains data from D.E. Mager, S.X. Lin, R.A. Blum, C.D. Lates, W.J. Jusko, Dose equivalency evaluation of major corticosteroids: Pharmacokinetics and cell trafficking and cortisol dynamics, Journal of Clinical Pharmacology, 43 (2003) 1216–1227.
F=1 for intravenous hydrocortisone, methylprednisolone, and dexamethasone
Fixed values.
Data in parentheses corresponds to standard deviation.
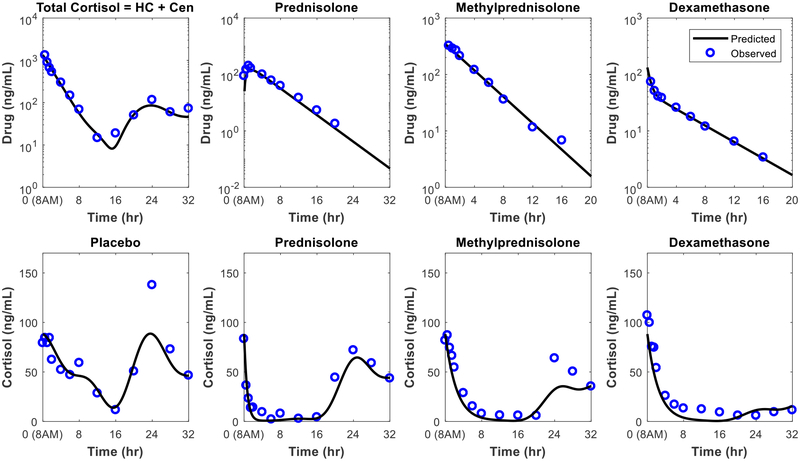
Figure 3:
Concentration-time profiles for synthetic glucocorticoids and endogenous cortisol. The plasma concentration profiles after administration of a single dose at 8 AM are shown for a hydrocortisone injection, prednisolone tablet, methylprednisolone injection, and dexamethasone injection. The cortisol profiles for the baseline (placebo) and after treatment with prednisolone, methylprednisolone, or dexamethasone are given. Because endogenous cortisol and hydrocortisone are indistinguishable in the biochemical assay used for this study, the total cortisol level following dosing with hydrocortisone is shown. Figure drawn using data from D.E. Mager, S.X. Lin, R.A. Blum, C.D. Lates, W.J. Jusko, Dose equivalency evaluation of major corticosteroids: Pharmacokinetics and cell trafficking and cortisol dynamics, Journal of Clinical Pharmacology, 43 (2003) 1216–1227.
The model showed that similar drug response can be achieved by adjusting the dose of each synthetic glucocorticoid considering differences in absorption and drug disposition, as well as the strength and duration of the inhibitory effects [88]. Similar models were applied to study the influence of sex and ethnicity on prednisone PKPD [105], sex differences in methylprednisolone PKPD [107], and the effects of administration time on methylprednisolone PKPD [19]. In these examples, indirect response models were used to describe the suppressive effects of synthetic glucocorticoids on endogenous cortisol secretion.
6. Importance of chronopharmacology for glucocorticoid treatment
Several advances have been made to optimize drug structure for enhanced specificity of glucocorticoid receptor-mediated actions and reduced mineralocorticoid activity, leading to fewer adverse effects [11, 29, 58]. Despite these improvements, HPA axis disruption remains a major downfall of long-term term treatment. However, chronopharmacology has proven to be a successful approach to maintaining the balance between homeostatic cortisol activity and the therapeutic effects of synthetic glucocorticoids. Drawing from a strong understanding of the natural rhythms of interest, chronopharmacological intervention supports the return to normal activity in the case of altered or absent rhythms, and additionally can lead to reduced adverse effects associated with disrupted rhythmicity [108]. The success of chronopharmacology relies on balancing the circadian rhythmicity of disease manifestation and adverse effects with the desired therapeutic effects [61]. Diurnal oscillations in physiological mechanisms lead to variability in drug efficacy, pharmacokinetics and drug toxicity with regard to administration time [109]. These concepts have proven to be highly beneficial for other drug classes [43, 110–119] and shows great promise in the advancement of long-term glucocorticoid therapies for patients with inflammatory and immune disorders, and those receiving hormone replacement therapy. Several studies have investigated chronopharmacological therapies that minimize the disruption of endogenous cortisol secretion or mimic the circadian rhythmicity in patients with adrenal insufficiency through careful timing of peak drug exposure with the circadian pattern of various compounds involved in disease activity [19, 21, 98, 120–122].
Importantly, negative feedback to the HPA axis exhibits circadian rhythmicity in normal diurnally active patients [121]. Negative feedback signals are strengthened by drug administration just before the nocturnal rise in ACTH production, resulting in maximum suppression of cortisol secretion, while morning administration has limited influence on HPA axis activity [21, 95]. Additionally, glucocorticoid receptors show diurnal variations in peripheral tissues which could potentiate or negate the local effects of synthetic glucocorticoid dosing [123]. As such, understanding the signal cascades of glucocorticoids in peripheral tissues throughout the day will ensure the desired local activity of synthetic glucocorticoids is achieved. Interestingly, the intrinsic sensitivity of receptors to cortisol is constant throughout the day [19] and so diurnal variations in glucocorticoid activity are attributed to other factors, such as the expression patterns of glucocorticoid receptors or variability in gene transcription susceptible to transrepression or transactivation by glucocorticoids.
Historically, glucocorticoids have been given in the morning for timing with disease symptomology to maximize treatment of allergic, inflammatory, and autoimmune conditions such as asthma, lupus, rheumatoid arthritis or Crohn’s disease [21]. The best example of effective chronopharmacological dosing of synthetic glucocorticoids is associated with the treatment of rheumatoid arthritis. Rheumatoid arthritis is a chronic inflammatory condition in which symptoms are closely linked to the circadian rhythmicity of proinflammatory and anti-inflammatory mediators, which peak during the evening and day, respectively [24, 121]. Morning administration has been shown to alleviate symptoms by timing drug release with the release of proinflammatory cytokines while also reducing the risk of HPA suppression [121, 124]. Thus, the secretion patterns of cortisol from the adrenal glands are not influenced and cortisol peak concentrations in the morning are still observed as shown in Figure 4 [121]. Although the endogenous cortisol circadian rhythm of rheumatoid arthritis with low or moderate activity remains normal, the natural rhythms can be disturbed when patients have high disease activity [121]. Consequently, any shifts in the endogenous cortisol rhythm due to disease must also factor into selection of an administration time to maintain the lowered risk of HPA suppression.
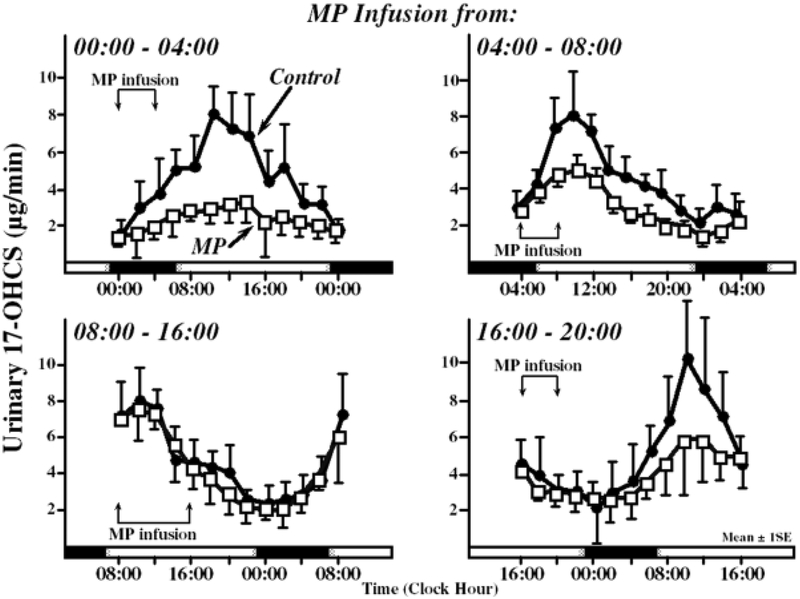
Figure 4:
Time-dependent differences in adrenal suppression following methylprednisolone infusions. The time-concentration profiles of 17-OHCS, a metabolite of cortisol excreted in urine, are shown following 4-hour methylprednisolone infusions for the following time windows: 00:00 to 04:00, 04:00 to 08:00, 08:00 to 16:00, and 16:00 to 20:00. Solid circles represent the control (no drug infusion) and open squares represent the drug-modified cortisol rhythm. Figure drawn using data from Angeli A. Circadian ACTH-adrenal rhythm in man. Chronobiologia. 1974 Sep;1 Suppl 1:253–68. Reprinted from: Haus E, Sackett-Lundeen L, Smoilensky MH. Rheumatoid arthritis: circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications. Bull Hosp Jt Dis. 2012;70(Suppl 1):S3–10. With permission.
Rhythmicity in symptom severity is also observed in other conditions. For example, nasal congestion and sneezing associated with allergies tends to be greater in the morning for a majority of patients [43], whereas the risk of an asthma attack is greatest at night [125, 126]. Glucocorticoid receptors mediate diurnal variations in pulmonary inflammatory response which is important during asthma attacks, chronic obstructive pulmonary disease (COPD) exacerbations, and respiratory bacterial infections [127]. Similar to the treatment of rheumatoid arthritis, careful timing of synthetic glucocorticoid administration with the rhythmicity of mediators involved in immune and inflammatory response for these pulmonary indications help to improve symptom relief [128–130]. Patients benefited from prednisone administration at 3:00 PM compared to early morning or late night dosing with improved expiratory volumes [126]. The success of glucocorticoid therapy for the treatment of asthma is dependent on the ability of glucocorticoids to alter transcription of various proinflammatory mediators (interleukins, tumor necrosis factor-α, and chemokines) and anti-inflammatory mediators (inhibitory protein IκB or interleukins Il-1, IL-10, and IL-12) involved in pulmonary immune response [59]. While asthma is considered both an acute and chronic inflammatory disease, timing glucocorticoid administration to maximally suppress proinflammatory mediators which peak at night may help to manage chronic airway inflammation associated with this disease.
Another successful application of chronopharmacology for glucocorticoids is in the management of Addison’s disease through asymmetrical dosing to reduce fatigue and enable realignment of circadian clocks using high doses in the morning and low doses in the afternoon [131]. Patients with autoimmune disorders also benefited from timing drug administration based on diurnal rhythms of the immune system such that evening dosing of prednisolone led to improved treatment [10]. Multiple sclerosis, which is a chronic inflammatory disease affecting the central nervous system, exhibits both seasonal and diurnal variation. Nighttime dosing of intravenous methylprednisolone led to improved symptom reprieve, in terms of rapid onset and greater overall relief, as well as reduced adverse effects, such as restlessness, palpitations, and hot flashes [132]. Further improvement in treatment options for multiple sclerosis may consider seasonal variation in immunity, owing to differences in the basal HPA axis activity throughout the year [133]. While not the emphasis of the current review, this study suggests that patients may further benefit from considering the impact of biological rhythms with periods longer than 24 hours. A similar argument could be made for the influence of the menstrual cycle for women receiving glucocorticoid therapy due to changes in drug disposition and the role of circulating sex hormones in glucocorticoid activity [105].
6.1. Circadian influence on systemic synthetic glucocorticoid exposure
Another key aspect for successful implementation of chronotherapy is an understanding of how drug exposure varies with administration times. Chronopharmacokinetics considers the natural oscillations of biological processes and functions that influence absorption and disposition, and ultimately the amount of pharmacologically active drug in systemic circulation [134–136]. For orally administered drugs, circadian rhythms in gastric pH, motility and gastric emptying can influence both the extent and rate of absorption throughout the day [43, 137–139]. In general, morning administration of tablets has been associated with faster gastrointestinal transit times in comparison to evening dosing [140]. Moreover, drug distribution to bodily tissues is affected by circadian rhythms in biologically processes, such as blood flow and protein binding [43, 139]. Metabolism and elimination are also altered by biological rhythms due to changes in hepatic and renal perfusion, hepatic Phase I and Phase II enzyme expression, glomerular filtration rate, urine excretion rate, urine pH, and electrolyte balances, amongst other factors [43, 137–139].
While the importance of chronopharmacodynamics has been recognized for glucocorticoids, the influence of circadian variation in absorption and drug disposition properties for synthetic glucocorticoids is less clear. There are conflicting findings for circadian variation in prednisolone pharmacokinetics, such as the volume of distribution, half-life, clearance, and unbound fraction, when given at low doses [19]. Some studies suggested that the rate and extent of absorption of prednisolone or prednisone from the gastrointestinal tract were not influencedby administration time [21, 141]. Other studies indicated that morning administration (8 AM) of methylprednisolone and prednisolone led to higher drug exposure due to reduced clearance [19]. Furthermore, the timing of dexamethasone administration was shown to have a weak effect on pharmacokinetics with a slight increase in the absorption rate when administered at 11 PM compared to administration at 8 AM [142]. General knowledge suggests that circadian influences are expected to be more significant for lipophilic drugs [43]; however, further studies are needed to confirm whether this holds true for synthetic glucocorticoids.
Given that only unbound glucocorticoids have pharmacological influence, variations in plasma protein binding throughout the day may have a significant role in the observed dose-exposure-response relationship. This influence arises from the competition between endogenous cortisol and synthetic glucocorticoids for plasma protein binding sites. Thus, the amounts of unbound and bound circulating glucocorticoids depend on the balance between these species as well as the rhythmicity of corticoid binding globulin expression [143]. This consideration is particularly true for compounds like prednisolone and hydrocortisone which compete with endogenous cortisol for plasma protein binding sites. Maximum binding of prednisolone has been reported at midnight and minimum at 8 AM [144]. Interestingly, the extent of prednisolone binding is in antiphase with the circadian rhythm of cortisol.
6.2. Controlled delivery of synthetic glucocorticoids for chronopharmacological intervention
Synchronization of drug concentrations to rhythms in disease or morbidity activity is aided by the use of specialized formulations or drug delivery systems with controlled release profiles [41, 145]. The need for such advancements stems from the fact that optimal drug release may not coincide with optimal administration time for patient compliance [146]. While single doses in the early morning have been the standard of care due to reduced adverse effects and alignment with peak cortisol concentrations, studies have shown that further relief in inflammatory conditions is achieved when drug exposure peaks before the rise of proinflammatory activity and during the rise of endogenous cortisol, which both occur overnight [24]. Given that glucocorticoids typically achieve peak concentrations within a few hours after oral administration, optimal timing of drug exposure with proinflammatory and endogenous cortisol cycles would lead to sleep disruption and lack of compliance due to administration in the middle of the night. As such, a modified release formulation of prednisone was developed for administration at bedtime with drug release delayed by 4 hours after administration (~2 AM), resulting in less disruption of the HPA axis, while still maintaining anti-inflammatory benefits [20, 24]. There were no signs of adrenal insufficiency even after months of treatment using this formulation strategy, greatly improving the safety of chronic administration [147]. The plasma concentration profiles for immediate release and modified-release formulations are given in Figure 5.
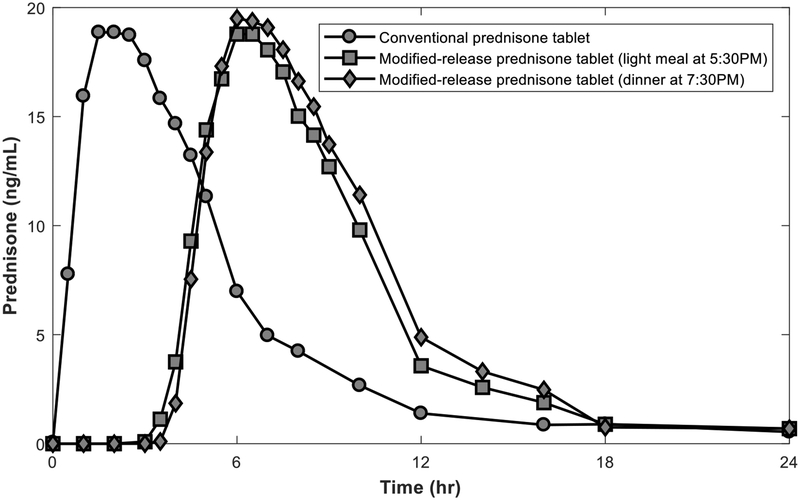
Figure 5:
Plasma concentration profiles for conventional prednisone and modified-release prednisone tablets. The time-concentration profiles of a conventional immediate release prednisone tablet (administered at 2 AM) and modified-release prednisone tablets (administered at 8 PM) after a light meal at 5:30 PM or dinner at 7:30 PM are provided. Figure drawn using data from S.P. Beltrametti, A. Ianniello, C. Ricci, Chronotherapy with low-dose modified-release prednisone for the management of rheumatoid arthritis: a review, Ther Clin Risk Manag, 12 (2016) 1763–1776.
Similarly, modified release prednisone improved treatment of nocturnal asthma when administered at bedtime, reducing the likelihood of nighttime awakenings and symptoms. Timing drug release with the nocturnal activation of inflammatory cells involved in nighttime airflow limitation enhanced the safety and efficacy of the treatment for chronic asthma compared to morning administration at 8 AM [128–130]. Another benefit to chronopharmacological dosing of inhaled corticosteroids was reduced disruption of peripheral clocks in lung tissue. Optimizing the frequency and timing of inhaled dexamethasone minimized phase shifts of clock functions in the lungs compared to once-daily or twice-daily inhaled corticosteroids. While emphasis is often placed on suppression of cortisol levels, maintaining the timing of peak cortisol concentration is also important to host functioning. An animal study showed that a phase advance or phase delay was observed when glucocorticoids were administered at lights on and 6 hours after lights off, respectively [148]. In addition to its pharmacological use, dexamethasone is often used as a probe to diagnose conditions related to the HPA axis and to suppress endogenous cortisol activity while studying other glucocorticoids. In these scenarios, dexamethasone is purposely administered at a time when the HPA axis is most susceptible to disruption for complete knockout of cortisol secretion. As such, dexamethasone is usually taken at 11 PM in the form of immediately release tablets as part of the dexamethasone suppression test [25]. This timing optimizes the suppressive effects of dexamethasone on the HPA axis, inhibiting the nocturnal rise in cortisol.
Adrenal insufficiency often requires lifetime treatment for hormone replacements [91]. As such, it is especially important to develop replacement regimens that successfully replicate diurnal secretory patterns and physiological concentrations [90], while simultaneously minimizing adverse effects associated with chronic use. Patients receiving hormone replacement therapy benefit from using the smallest dose possible to minimize the likelihood of adrenal crisis while maintaining efforts to replicate physiological rhythms [149]. To manage adrenal hyperplasia, glucocorticoids may be administered in the evening or early night hours to induce production of endogenous cortisol [121]. The normal nocturnal rise in concentration has been successfully replicated using modified release formulations when administered at bedtime and released overnight [98]. Previously, patients with adrenal insufficiency were treated using immediate release hydrocortisone given 2 to 3 times daily at a fixed dose or body weight/surface area adjusted dose [98]. Dose splitting throughout the day was associated with an increased risk of suppression [150]. Replicating the complete diurnal rhythm of endogenous cortisol is not feasible with immediate release formulations [11], even with multiple daily doses, and so hormone replacement therapies have turned towards tailored formulation strategies.
The patterns of cortisol secretion have been better matched using continuous infusions which effectively mimicked the circadian rhythmicity of cortisol secretion but were not practical for patient use [11, 14, 151, 152]. Therefore, modified release, including delayed and sustained, oral formulations have been particularly useful for hormone replacement therapy to control the release of drug into systemic circulation to better match the endogenous cortisol secretion rate. Cortisol profiles following conventional and modified-release oral replacement therapy as well as circadian infusions of hydrocortisone are given in Figure 6.
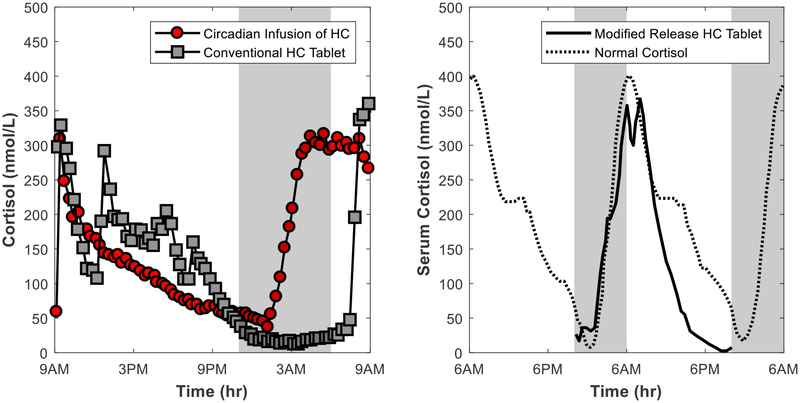
Figure 6:
Concentration-time profiles for cortisol replacement studies. The left panel shows cortisol profiles following a circadian infusion of hydrocortisone (HC) via a pump and administration of a conventional hydrocortisone tablet in patients with Addison’s disease and congenital adrenal hyperplasia. The right panel shows the cortisol profiles following administration of a modified release hydrocortisone tablet at 10PM in healthy individuals with suppression of endogenous cortisol by dexamethasone. Figure drawn using data from M. Debono, R.J. Ross, J. Newell-Price, Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy, Eur J Endocrinol, 160 (2009) 719–729.
Furthermore, once-daily delayed release hydrocortisone given in the morning upon wakening significantly reduced adverse effects on the cardiovascular system, glucose metabolism, and quality of life. Weight gain, impaired metabolism, and the frequency of infections were also reversed by switching to the modified release formulation [153]. The improvement in safety was attributed to the absence of the afternoon peak typically associated with other treatment options. Additionally, a modified release glucocorticoid with pH-dependent dissolution along the gastrointestinal tract was shown to have less adverse effects and comparable effectiveness to prednisolone for the treatment of Crohn’s disease [154].
6.3. Monitoring Glucocorticoid Activity
Irregularities in cortisol levels and diurnal rhythms outside the normal physiological range following treatment with synthetic glucocorticoids can be detected by direct measurement of plasma cortisol. Cushing’s syndrome, which is characterized by supraphysiological cortisol levels, is typically diagnosed by measuring the 24-hour free cortisol and late-night salivary cortisol levels (after 10 PM) [155]. Conversely, first morning cortisol measurements (prior to 8 AM) may be used to assess patient risk of adrenal suppression to determine whether the nocturnal rise in cortisol levels is observed following treatment [6]. Serum cortisol, urinary-free cortisol levels or salivary cortisol measurements have been used to determine whether therapy is successful in patients receiving hormone replacement [152]. While salivary cortisol is a measure of unbound biologically active cortisol, serum cortisol is a measure of the total (bound and unbound) circulating cortisol [96]. Saturation of CBG can skew results for the urinary-free cortisol test, whereas the downside of salivary measurements is the wide inter-individual variability and lack of a correlation to plasma measurements in patients with adrenal insufficiency [152]. Peak cortisol concentration, the time of the peak, and 24-hour cortisol exposure (area-under-the-curve of the cortisol profile) serve as metrics to assess whether the circadian features of the cortisol rhythm have been successfully replicated. Together, these parameters evaluate maximum and cumulative cortisol exposure, along with circadian timing, to ensure the effectiveness and safety of replacement therapy [156].
Furthermore, there are tests to probe the integrity of the HPA axis including the ACTH stimulation test and dexamethasone suppression test. To confirm adrenal insufficiency, the low-dose ACTH stimulation test may be used to detect abnormal cortisol secretion which involves measuring the adrenal response following a bolus injecting of corticotropin [6]. Similarly, the dexamethasone suppression test is used to evaluate the integrity of the HPA axis and degree of suppression following administration of dexamethasone [96]. Atypical results for these diagnostic tests may be indicative of physiological conditions associated with disease states or as a result of long-term use of synthetic glucocorticoids which alters glucocorticoid activity both locally and systemically.
7. Case Study: Modeling chronopharmacological administration of synthetic glucocorticoids using a semi-mechanistic PKPD model
Controlled delivery of synthetic glucocorticoids through chronopharmacological intervention were shown to successfully minimize disruption of endogenous cortisol activity (Section 6), demonstrating how administration time, formulation, and route of administration can be leveraged to achieve the desired biological effects. While many of the aforementioned examples discuss cortisol suppression, fewer clinical results emphasize chronic use and the counterintuitive influences of treatment on the HPA axis activity that can lead to increased cortisol levels. Interestingly, one study found that modified-release prednisone administered at bedtime in rheumatoid arthritis patients resulted in a rise in cortisol levels after 2 weeks of treatment [120]. To further explore the adaptability and responsiveness of the HPA axis following long term synthetic glucocorticoid dosing, a semi-mechanistic model was developed that simulates the diurnal rhythm of endogenous cortisol in the absence of inflammation [157]. This model considers the time-varying feedforward (stimulatory) and feedback (suppressive) processes of the HPA axis. The model describes the secretion of cortisol (CORT) and its precursors ACTH and CRH using nonlinear differential equations with a Goodwin oscillator modified to consider Michaelis-Menten degradation kinetics (Equations 10–12). The model accounts for receptor binding of both synthetic glucocorticoids (GC) and endogenous cortisol (GCbound, Equation 13), and the subsequent pharmacodynamics of the bound receptor complex translocated to the nucleus (GCbound(N), Equation 14), such as negative feedback to the hypothalamus and pituitary gland. Select components of the model are given in Equations 10 to 14 with the complete model provided in Rao et al. [157], where kp1 is the zero-order CRH synthesis rate constant; kp2 and kp3 are the first-order rate constants for ACTH and CORT synthesis; Kp1 and Kp2 are the Michaelis-Menten constants for glucocorticoid-induced CRH and ACTH inhibition; Vd1, Vd2 and Vd3 are the zero-order rate constants for CRH, ACTH and CORT degradation; Kd1, Kd2, and Kd3 are the Michaelis-Menten constants for CRH, ACTH and CORT degradation; kon is the second-order rate constant for glucocorticoid-receptor binding; kt is the translocation rate constant of the glucocorticoid-receptor complex from cytoplasm to nucleus; rf is the recycle fraction of the glucocorticoid-receptor complex from nucleus to cytoplasm; and kre is the recycle rate constant of the glucocorticoid-receptor complex from nucleus to cytoplasm.
| Eq. 10 |
| Eq. 11 |
| Eq. 12 |
| Eq. 13 |
| Eq. 14 |
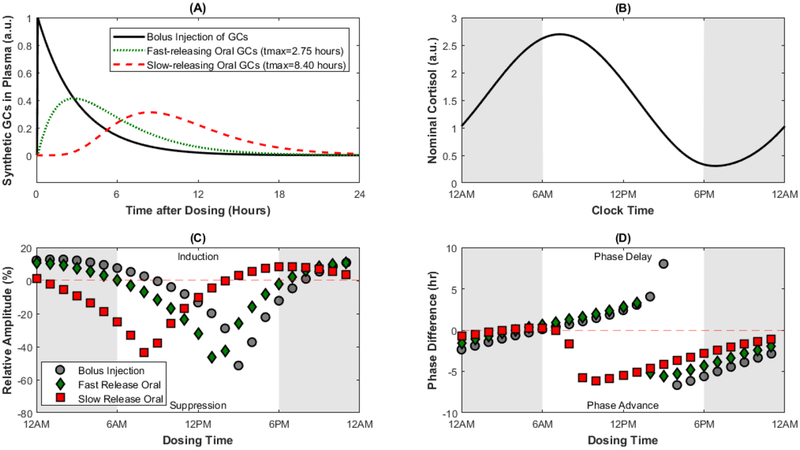
The semi-mechanistic HPA axis model was integrated with compartment models to describe the pharmacokinetics of a once-daily dose of a synthetic glucocorticoid administered by bolus injection and orally as an immediate release or extended release formulation [157]. The concentration-time profiles for drug exposure and the nominal cortisol rhythm are shown in Figure 7A and B, respectively. The change in amplitude and phase of the modified cortisol rhythm are shown in Figure 7C and D following a single daily dose. These simulations reveal alteration of the rhythmic characteristics of endogenous cortisol are highly dependent on the administration time of the once-daily dose, yet normal activity can be preserved by carefully timing drug administration based on the formulation characteristics [157]. The study suggests that formulation properties can be systematically manipulated to optimize dosing time and the extent of cortisol suppression or induction to achieve the desired pharmacodynamic effect. Furthermore, the model reveals the nontrivial influences of synthetic glucocorticoids on HPA axis activity (i.e. induction) that are otherwise not captured by simpler models. Finally, the studies supported by this model-based approach emphasized the need to use multiple metrics (amplitude, phase, 24-hour AUC) to comprehensively study alterations in the diurnal rhythms in response to chronopharmacological intervention [157].
Figure 7:
Modeling chronopharmacological dosing of synthetic glucocorticoids using a physiologically-based HPA axis model. The concentration-time profiles for a bolus injection, fast-releasing oral dose, and a slow-releasing oral dose are given in Panel A. The nominal cortisol profile predicted by the model is given in Panel B. The relative amplitude change (%difference in amplitude before and after once-daily dosing of synthetic glucocorticoids) are given for several dosing times in Panel C, where a positive value indicates induction of the cortisol rhythm and a negative value indicates suppression of the cortisol rhythm. The difference in phase of the cortisol rhythm before and after treatment with synthetic glucocorticoids for several dosing times are given in Panel D, where a positive value indicates delayed timing of the cortisol peak and a negative value indicates an advance in the timing of the cortisol peak. Figure drawn using data from R.T. Rao, M.L. Scherholz, I.P. Androulakis, Modeling the influence of chronopharmacological administration of synthetic glucocorticoids on the hypothalamic-pituitary-adrenal axis, Chronobiol Int, (2018) 1–18.
8. Reducing patient risk through chronopharmacological dosing
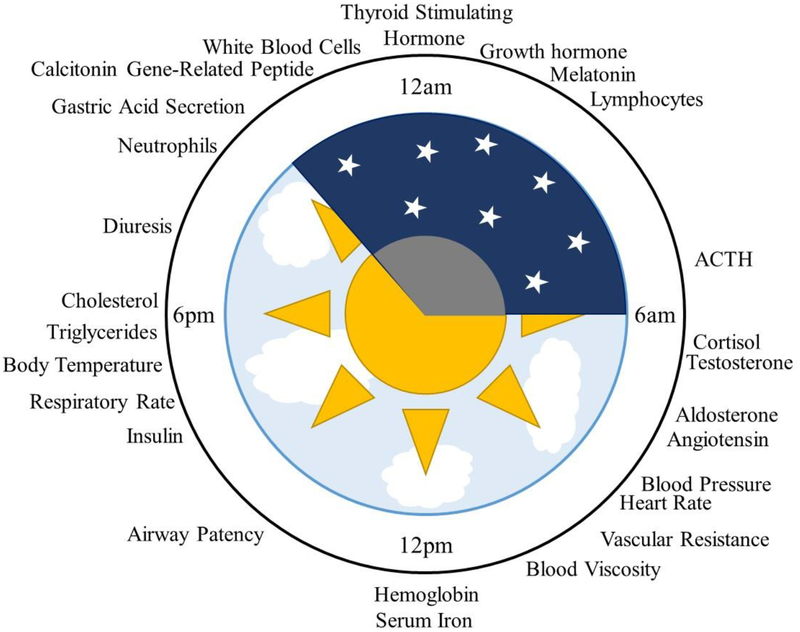
Considering that glucocorticoids have pleiotropic effects, leveraging knowledge of the pathways involved in both therapeutic and adverse effects of systemic glucocorticoids will further the development of effective and safe treatments. The acrophase (time of peak concentration) for various physiological functions relative to the rest-activity cycle in humans is given in Figure 8 [41]. While this list is by no means comprehensive, the schematic highlights how expression of various physiological compounds are distributed throughout the 24-hour day. The benefits of chronopharmacological dosing to minimize HPA axis disruption is generally accepted, but the link between chronopharmacology and other adverse effects is still under study. An example of progress towards this goal is related to the influence of glucocorticoid treatment on glucose levels and insulin secretion. Under normal conditions, endogenous glucocorticoids are responsible for maintaining glucose homeostasis. Elevated glucose levels are observed following both morning and evening doses of hydrocortisone, but reduced hyperglycemic effects were observed when administered in the morning [14, 42]. Similar findings were observed for patients receiving prednisolone to treat COPD [158]. These observations provide evidence that the risk of serious adverse effects, such as treatment-induced diabetes, may be minimized by proper timing of drug administration [6]. Moreover, a common adverse effect of chronic glucocorticoid treatment involves the disruption of normal blood pressure patterns. Glucocorticoids exhibit their influences on the cardiovascular system through angiotensin II receptor signaling which are important modulators of blood pressure [23]. Hypertension and hypotension are associated with supra-physiological and sub-physiological exposure to glucocorticoids, respectively [159]. While the exact mechanisms responsible for glucocorticoid-induced changes in blood pressure are not fully understood, there is evidence that an imbalance between vasoconstriction and vasodilation activity may be important [32]. Thus, reduced cardiovascular effects may be achieved by minimizing the disruption of this balance. Furthermore, glucocorticoid excess leads to drug-induced osteoporosis by transrepression of the genes involved in bone formation. The impact of glucocorticoids on bone and mineral metabolism are both dose and time dependent [160]. Typically, glucocorticoid-induced osteoporosis is treated after its occurrence [160], but chronopharmacological dosing represents a potential approach to prevent adverse effects on the skeletal system by timing dosing with the circadian variation of osteoblast and osteoclast differentiation and turnover [161].
Figure 8:
Circadian rhythmicity of physiological functions in humans. The peak time or acrophase of several biological processes in humans are shown relative to the sleep-wake cycle, indicating distribution of biological processes across the 24-hour day under normal physiology. Figure adapted from Smolensky et al. [41].
Recently, an ancillary study of the DREAM clinical trial formalized the link between adverse effects and altered gene expression in peripheral tissues [37]. The study showed that adverse effects on the cardiovascular system, glucose metabolism, and quality of life were alleviated when disruption of peripheral clock genes was minimized. The improvement in clinical manifestations was attributed to the resynchronization of endogenous and exogenous zeitgebers, that is, the realignment of peak exposure to exogenous hydrocortisone with peripheral tissue functions.
9. Patient factors affecting chronopharmacokinetics
Due to the inherent variability across patients in pharmacokinetics and crosstalk between the neuroendocrine and immune systems, efforts to establish the relationship between dose and HPA axis suppression has been difficult [147]. Physiological factors, such as chronotype, sex, age, race or disease state, contribute to substantial variability in pharmacokinetics as well as variation in the underlying regulatory mechanisms and responsiveness of the HPA axis [14, 162–167]. This behavior is observed in clinical studies evaluating the duration and extent of cortisol suppression, which is shown to be highly variable across patient subgroups as a result of disease, ethnicity or sex [19, 107, 168]. While cortisol levels tend to reflect long-term individual traits, the responsiveness of the HPA axis to synthetic glucocorticoids appears to be more closely tied to clinical state [168]. Patients suffering from post-traumatic stress disorder (PTSD) at the time of the study exhibited greater cortisol suppression following administration of dexamethasone compared to patients without PTSD regardless of whether these patients were previously exposed to trauma [168]. Furthermore, glucocorticoid activity in peripheral tissues can contribute to inter-individual differences due to variability in the negative feedback on ACTH secretion, 11β-HSD activity, CBG levels, and active transporters, such as P-glycoprotein, which regulate local concentrations of cortisol [77]. Polymorphisms in genes involved in glucocorticoid transport and metabolism contribute significantly to inter-individual variability in the efficacy and toxicity of patients suffering from Crohn’s disease and ulcerative colitis [71]. Body weight and body surface area have been recognized as important determinants of clearance and are routinely accounted for in glucocorticoid replacement therapy [151]. Yet, significant variability in patient exposure and response is still observed. Some patients are susceptible to all adverse effects with low doses of glucocorticoids, while others show minimal side effects at high doses.
Sexual dimorphism in susceptibility to autoimmune and chronic inflammatory disorders has, in part, been attributed to differences in the underlying regulatory mechanisms of the HPA axis across sexes, such as greater adrenal sensitivity and weaker negative feedback in females [163]. Given differential regulatory mechanisms in basal activity of the HPA axis, one would expect differences in the response and activity of the HPA to drug administration. One study reported a greater potency of methylprednisolone in female subjects. However, the increased sensitivity to glucocorticoids was balanced by higher methylprednisolone clearance in females such that same overall therapeutic effect was observed across sexes [85]. Another study reported lower clearance and volume of distribution of free prednisolone in women compared to men, when corrected for body weight, following a single oral dose. Interesting, differences in the pharmacokinetic parameters resulted in similar half-life in women and men [105]. Sex differences in pharmacokinetics of synthetic glucocorticoids vary by drug due to differences in the glucocorticoid and mineralocorticoid receptor activity as well as the displacement of cortisol from plasma protein binding sites when the drug is a substrate of corticoid binding globulin. While sex differences were observed for pharmacokinetics but not the pharmacodynamics of prednisolone, the opposite was true when race was treated as the covariate. The sensitivity of various biomarkers was dependent on race, whereas pharmacokinetic parameters showed no differences. This suggest that different patient subgroups may be more susceptible to adverse effects due to differences in drug exposure and sensitivity [105].
Another key consideration is the influence that age has on the basal activity of the HPA axis. Diurnal cortisol rhythms change with age, manifesting as phase advances, lower nadirs, and dampened amplitude in elderly compared to young individuals [156]. Inter-individual differences in clock phase may also be clinically relevant and the success of chronopharmacological dosing relies on proper timing of drug exposure with specific physiological functions [169]. Likewise, differences in glucocorticoid receptor sensitivity may contribute significantly to variability in the dose-response relationship, given the critical role that glucocorticoid receptors play in physiological mechanisms of glucocorticoids [152]. Genetic variation in glucocorticoid pathway regulation and activity is a significant factor in inter-individual differences in glucocorticoid treatment [170]. For example, polymorphisms in the glucocorticoid receptor gene are sometimes accompanied by cardiovascular risks such as irregular blood pressure, low glucose, or low total cholesterol, which may partially explain why some patients are at a greater risk for such adverse effects while receiving treatment [35]. Furthermore, the effectiveness of treatment varies with glucocorticoid activity. For example, some patients with inflammatory bowel disease (IBD) require chronic therapy to stay in remission whereas others require treatment during flare-ups [81]. While intrinsic glucocorticoid resistance leads to drug resistance at pharmacological doses [74], glucocorticoids hypersensitivity can actually aid treatment. For example, lower disease activity of IBD, marked by less gut inflammation and improved mucosal healing, was observed in patients with higher glucocorticoid sensitivity and drug-induced adrenal insufficiency, although other side effects, such as osteoporosis and infections, persisted [171]. Successful treatment is likely due to the favorable increase in glucocorticoid activity in the gut, with epithelium integrity, cell-cell adhesion, and mucus hypersecretion modulated by local receptor activity [172]. Interestingly, the same individual can show significant variability in the magnitude and specificity of action across tissues and even across phases of the cell cycle, requiring a deeper understanding of the signal transduction pathways involved in both therapeutic and adverse effects [173]. Variability inherent to the activity of 11β-HSD enzyme system may contribute to an individual’s risk of adverse effects associated with chronic over-exposure at the tissue-level. Accounting for such differences could provide another opportunity to tailor replacement therapies for protection against detrimental effects on the cardiovascular system, central nervous system or bone [174].
10. Conclusions
Cortisol rhythm irregularity due to pharmacological intervention or adrenal insufficiency is a major health concern given the critical role of glucocorticoids in an array of metabolic, anti-inflammatory, immunosuppressive, and cognitive processes. Over the last several decades, significant progress has been made towards elucidating the genomic and non-genomic actions of systemic glucocorticoids which have led to the development of drugs with reduced mineralocorticoid activity and improved safety. Yet, HPA axis disruption remains a central concern with chronic high dose administration. Disruption of glucocorticoid-mediated functions can be minimized and potentially eliminated through chronopharmacological dosing regimens that maintain the potent therapeutic effects for treatment of immune and inflammatory conditions as well as for hormone replacement therapy. To date, several studies have emphasized the potential for chronopharmacological dosing of glucocorticoids to limit HPA axis disturbance or to successfully replicate the diurnal rhythm of cortisol secretion. Relying on the interplay between the HPA axis and the immune system, specialized formulations and drug delivery systems have enabled peak drug exposure to be synchronized to the nocturnal rise in proinflammatory mediators and cortisol. Manipulation of the pharmacological exposure profile to minimize disruption of endogenous cortisol or to support the development of hormone replacement therapies has yet to be fully explored. This potential was demonstrated theoretically using an exploratory model developed by Rao et al., which showed how administration time, dosing strength, and shape of the drug exposure profile could be leveraged to minimize disruption of the endogenous cortisol profile or to supplement endogenous cortisol levels by inducing its production at select administration times [157]. Furthermore, suppression of the HPA axis is dependent on slow and fast signaling cascades proportional to the rate of change in concentration and the absolute concentration of cortisol, respectively [64]. Understanding diurnal variations in these negative feedback cascades represents another understudied aspect of pharmacological intervention.
Therapy is further complicated by CBG and 11β-HSD activity [155]. Plasma protein binding and enzymatic interconversion can influence drug exposure, echoed by differences in the time to maximum concentration (tmax) and terminal half-life [53]. While the inhibitory effects of synthetic glucocorticoids on secretion of cortisol are widely accepted, the influence of synthetic glucocorticoids on endogenous cortisol elimination are less clear. Originally, elimination of cortisol was thought to be independent of drug clearance [19]. However, displacement of endogenous cortisol by exogenous cortisol at the binding sites of plasma proteins has potential to influence the elimination of endogenous cortisol. Due to this competitive protein binding, endogenous cortisol elimination may not be entirely independent of drug clearance and vice versa. Therefore, dosing strength and frequency need to be adjusted based on absorption of oral glucocorticoids from the gastrointestinal tract and half-life of cortisol in systemic circulation around the time of dosing [53].
Chronopharmacology benefits from a strengthened understanding of glucocorticoid action to balance beneficial immunosuppressive and anti-inflammatory properties with the risk of systemic disruption of various biological functions, such as blood pressure regulation or glucose homeostasis. Physiological factors, such as chronotype, sex, age, race or disease state, contribute to substantial variability in pharmacokinetics as well as variation in the underlying regulatory mechanisms and responsiveness of the HPA axis, inherently complicating the cross talk between the neuroendocrine and immune systems. Incorporating such patient factors show great promise towards maximizing the therapeutic potential of glucocorticoid therapy with lower doses and overall safer treatment options. The case studies presented in this review highlighted the utility of modeling in glucocorticoid research to support such goals, revealing how counterintuitive and nontrivial behavior of the HPA axis were unveiled using physiologically and semi-mechanistic representations of the HPA axis. Similarly, the use of physiologically-based pharmacokinetic models, rather than simpler compartment models to describe drug exposure, would facilitate the integration of circadian dynamics and physiological factors that contribute to the significant time-of-day and inter-individual differences in clinical response.
Funding
The authors acknowledge support from NIH GM024211.
Abbreviations List
- HPA axis
hypothalamic-pituitary-adrenal axis
- CRH
Corticotrophin-releasing hormone
- ACTH
Adrenocorticotrophic hormone
- CBG
Corticoid binding globulin
- 11β-HSD1
11β-hydroxysteroid dehydrogenase type 1
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- BCS
Biopharmaceutical Classification System
- PGGR
Primary generalized glucocorticoid resistance
- PGGH
Primary generalized glucocorticoid hypersensitivity
- COPD
Chronic obstructive pulmonary disease
- PTSD
Post-traumatic stress disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest Statement
The authors have no conflicts of interest to declare.
11 References
- [1].Hench PS, Kendall EC, Slocumb CH, Polley HF, The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocortical hormone in arthritis: preliminary report, Ann Rheum Dis, 8 (1949) 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fietta P, Delsante G, Central nervous system effects of natural and synthetic glucocorticoids, Psychiatry Clin Neurosci, 63 (2009) 613–622. [DOI] [PubMed] [Google Scholar]
- [3].Arlt W, Stewart PM, Adrenal corticosteroid biosynthesis, metabolism, and action, Endocrinol Metab Clin North Am, 34 (2005) 293–313, viii. [DOI] [PubMed] [Google Scholar]
- [4].Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE, Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy, Chest, 136 (2009) 1631–1643. [DOI] [PubMed] [Google Scholar]
- [5].Elenkov IJ, Chrousos GP, Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity, Ann N Y Acad Sci, 966 (2002) 290–303. [DOI] [PubMed] [Google Scholar]
- [6].Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H, A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy, Allergy Asthma Clin Immunol, 9 (2013) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spiga F, Walker JJ, Terry JR, Lightman SL, HPA Axis-Rhythms, in: Terjung R (Ed.) Comprehensive Physiology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2014, pp. 1273–1298. [DOI] [PubMed] [Google Scholar]
- [8].Partch CL, Green CB, Takahashi JS, Molecular architecture of the mammalian circadian clock, Trends Cell Biol, 24 (2014) 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ, Role of the CLOCK Protein in the Mammalian Circadian Mechanism, Science, 280 (1998) 1564–1569. [DOI] [PubMed] [Google Scholar]
- [10].Nicolaides NC, Charmandari E, Kino T, Chrousos GP, Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health, Front Endocrinol (Lausanne), 8 (2017) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chan S, Debono M, Replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy, Ther Adv Endocrinol Metab, 1 (2010) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spencer RL, Chun LE, Hartsock MJ, Woodruff ER, Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems, Front Neuroendocrinol, 49 (2018) 52–71. [DOI] [PubMed] [Google Scholar]
- [13].Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A, The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system, J Endocrinol, 177 (2003) 17–26. [DOI] [PubMed] [Google Scholar]
- [14].Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E, The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids, Endocr Rev, 38 (2017) 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smith SM, Vale WW, The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress, Dialogues Clin Neurosci, 8 (2006) 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sukumaran S, Almon RR, DuBois DC, Jusko WJ, Circadian rhythms in gene expression: Relationship to physiology, disease, drug disposition and drug action, Adv Drug Deliv Rev, 62 (2010) 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Edwards C, Sixty years after Hench--corticosteroids and chronic inflammatory disease, J Clin Endocrinol Metab, 97 (2012) 1443–1451. [DOI] [PubMed] [Google Scholar]
- [18].Kirwan JR, Clarke L, Hunt LP, Perry MG, Straub RH, Jessop DS, Effect of novel therapeutic glucocorticoids on circadian rhythms of hormones and cytokines in rheumatoid arthritis, Ann N Y Acad Sci, 1193 (2010) 127–133. [DOI] [PubMed] [Google Scholar]
- [19].Fisher LE, Ludwig EA, Wald JA, Sloan RR, Middleton E Jr., Jusko WJ, Pharmacokinetics and pharmacodynamics of methylprednisolone when administered at 8 am versus 4 pm, Clin Pharmacol Ther, 51 (1992) 677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beltrametti SP, Ianniello A, Ricci C, Chronotherapy with low-dose modified-release prednisone for the management of rheumatoid arthritis: a review, Ther Clin Risk Manag, 12 (2016) 1763–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu J, Winkler J, Sabarinath SN, Derendorf H, Assessment of the impact of dosing time on the pharmacokinetics/pharmacodynamics of prednisolone, AAPS J, 10 (2008) 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, Kovac SH, Spettell CM, Saag KG, Population-based assessment of adverse events associated with long-term glucocorticoid use, Arthritis Rheum, 55 (2006) 420–426. [DOI] [PubMed] [Google Scholar]
- [23].Rhen T, Cidlowski JA, Antiinflammatory action of glucocorticoids--new mechanisms for old drugs, N Engl J Med, 353 (2005) 1711–1723. [DOI] [PubMed] [Google Scholar]
- [24].Alten R, Doring G, Cutolo M, Gromnica-Ihle E, Witte S, Straub R, Buttgereit F, Hypothalamus-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone, J Rheumatol, 37 (2010) 2025–2031. [DOI] [PubMed] [Google Scholar]
- [25].Queckenberg C, Wachall B, Erlinghagen V, Di Gion P, Tomalik-Scharte D, Tawab M, Gerbeth K, Fuhr U, Pharmacokinetics, pharmacodynamics, and comparative bioavailability of single, oral 2-mg doses of dexamethasone liquid and tablet formulations: a randomized, controlled, crossover study in healthy adult volunteers, Clin Ther, 33 (2011) 1831–1841. [DOI] [PubMed] [Google Scholar]
- [26].Fardet L, Petersen I, Nazareth I, Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care, Am J Psychiatry, 169 (2012) 491–497. [DOI] [PubMed] [Google Scholar]
- [27].Hwang JL, Weiss RE, Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment, Diabetes Metab Res Rev, 30 (2014) 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moghadam‐Kia S, Werth VP, Prevention and treatment of systemic glucocorticoid side effects, International journal of dermatology, 49 (2010) 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alangari AA, Genomic and non-genomic actions of glucocorticoids in asthma, Ann Thorac Med, 5 (2010) 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buttgereit F, Burmester GR, Lipworth BJ, Optimised glucocorticoid therapy: the sharpening of an old spear, Lancet, 365 (2005) 801–803. [DOI] [PubMed] [Google Scholar]
- [31].McMaster A, Ray DW, Modelling the glucocorticoid receptor and producing therapeutic agents with anti-inflammatory effects but reduced side-effects, Exp Physiol, 92 (2007) 299–309. [DOI] [PubMed] [Google Scholar]
- [32].Fardet L, Feve B, Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events, Drugs, 74 (2014) 1731–1745. [DOI] [PubMed] [Google Scholar]
- [33].Straub RH, Cutolo M, Buttgereit F, Pongratz G, Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases, Journal of Internal Medicine, 267 (2010) 543–560. [DOI] [PubMed] [Google Scholar]
- [34].Cruz-Topete D, Cidlowski JA, One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids, Neuroimmunomodulation, 22 (2015) 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yan YX, Dong J, Wu LJ, Shao S, Zhang J, Zhang L, Wang W, He Y, Liu YQ, Associations between polymorphisms in the glucocorticoid-receptor gene and cardiovascular risk factors in a Chinese population, J Epidemiol, 23 (2013) 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dibner C, Schibler U, Albrecht U, The mammalian circadian timing system: organization and coordination of central and peripheral clocks, Annu Rev Physiol, 72 (2010) 517–549. [DOI] [PubMed] [Google Scholar]
- [37].Venneri MA, Hasenmajer V, Fiore D, Sbardella E, Pofi R, Graziadio C, Gianfrilli D, Pivonello C, Negri M, Naro F, Grossman AB, Lenzi A, Pivonello R, Isidori AM, Circadian Rhythm of Glucocorticoid Administration Entrains Clock Genes in Immune Cells: A DREAM Trial Ancillary Study, J Clin Endocrinol Metab, 103 (2018) 2998–3009. [DOI] [PubMed] [Google Scholar]
- [38].Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ, Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures, Endocr Rev, 37 (2016) 584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ohdo S, Koyanagi S, Matsunaga N, Chronopharmacological strategies: Intra- and inter-individual variability of molecular clock, Adv Drug Deliv Rev, 62 (2010) 885–897. [DOI] [PubMed] [Google Scholar]
- [40].Cutolo M, Glucocorticoids and chronotherapy in rheumatoid arthritis, RMD Open, 2 (2016) e000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smolensky MH, Peppas NA, Chronobiology, drug delivery, and chronotherapeutics, Adv Drug Deliv Rev, 59 (2007) 828–851. [DOI] [PubMed] [Google Scholar]
- [42].Ballesta A, Innominato PF, Dallmann R, Rand DA, Levi FA, Systems Chronotherapeutics, Pharmacol Rev, 69 (2017) 161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baraldo M, The influence of circadian rhythms on the kinetics of drugs in humans, Expert Opin Drug Metab Toxicol, 4 (2008) 175–192. [DOI] [PubMed] [Google Scholar]
- [44].Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E, Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis, Mol Cell Endocrinol, 349 (2012) 20–29. [DOI] [PubMed] [Google Scholar]
- [45].Alderson AL, Novack TA, Neurophysiological and clinical aspects of glucocorticoids and memory: a review, J Clin Exp Neuropsychol, 24 (2002) 335–355. [DOI] [PubMed] [Google Scholar]
- [46].Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E, Stress, the stress system and the role of glucocorticoids, Neuroimmunomodulation, 22 (2015) 6–19. [DOI] [PubMed] [Google Scholar]
- [47].Charmandari E, Kino T, Chrousos GP, Glucocorticoids and their actions: an introduction, Ann N Y Acad Sci, 1024 (2004) 1–8. [DOI] [PubMed] [Google Scholar]
- [48].McEwen BS, Kalia M, The role of corticosteroids and stress in chronic pain conditions, Metabolism, 59 Suppl 1 (2010) S9–15. [DOI] [PubMed] [Google Scholar]
- [49].Juster RP, McEwen BS, Lupien SJ, Allostatic load biomarkers of chronic stress and impact on health and cognition, Neurosci Biobehav Rev, 35 (2010) 2–16. [DOI] [PubMed] [Google Scholar]
- [50].Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM, SCN outputs and the hypothalamic balance of life, Journal of biological rhythms, 21 (2006) 458–469. [DOI] [PubMed] [Google Scholar]
- [51].Cruz-Topete D, Cildlowski JA, Glucocorticoids: Molecular Mechanisms of Action, in: Riccardi C, Levi-Schaffer F, Tiligada E (Eds.) Immunopharmacology and Inflammation, Springer International Publishing, Switzerland, 2018. [Google Scholar]
- [52].Oakley RH, Cidlowski JA, The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease, J Allergy Clin Immunol, 132 (2013) 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hindmarsh PC, Charmandari E, Variation in absorption and half-life of hydrocortisone influence plasma cortisol concentrations, Clin Endocrinol (Oxf), 82 (2015) 557–561. [DOI] [PubMed] [Google Scholar]
- [54].Beishuizen A, Thijs LG, Vermes I, Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma, Intensive Care Med, 27 (2001) 1584–1591. [DOI] [PubMed] [Google Scholar]
- [55].Prigent H, Maxime V, Annane D, Science review: mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids, Crit Care, 8 (2004) 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chapman K, Holmes M, Seckl J, 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action, Physiol Rev, 93 (2013) 1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Joels M, Karst H, DeRijk R, de Kloet ER, The coming out of the brain mineralocorticoid receptor, Trends Neurosci, 31 (2008) 1–7. [DOI] [PubMed] [Google Scholar]
- [58].Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR, Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour, Psychoneuroendocrinology, 38 (2013) 648–658. [DOI] [PubMed] [Google Scholar]
- [59].Umland SP, Schleimer RP, Johnston SL, Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma, Pulm Pharmacol Ther, 15 (2002) 35–50. [DOI] [PubMed] [Google Scholar]
- [60].Charmandari E, Tsigos C, Chrousos G, Endocrinology of the stress response, Annu Rev Physiol, 67 (2005) 259–284. [DOI] [PubMed] [Google Scholar]
- [61].Spies CM, Straub RH, Cutolo M, Buttgereit F, Circadian rhythms in rheumatology--a glucocorticoid perspective, Arthritis Res Ther, 16 Suppl 2 (2014) S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thomson AH, Devers MC, Wallace AM, Grant D, Campbell K, Freel M, Connell JM, Variability in hydrocortisone plasma and saliva pharmacokinetics following intravenous and oral administration to patients with adrenal insufficiency, Clin Endocrinol (Oxf), 66 (2007) 789–796. [DOI] [PubMed] [Google Scholar]
- [63].Haus E, Chronobiology in the endocrine system, Adv Drug Deliv Rev, 59 (2007) 985–1014. [DOI] [PubMed] [Google Scholar]
- [64].Kassi EN, Chrousos GP, The central CLOCK system and the stress axis in health and disease, Hormones (Athens), 12 (2013) 172–191. [DOI] [PubMed] [Google Scholar]
- [65].Charmandari E, Nicolaides NC, Chrousos GP, Adrenal insufficiency, Lancet, 383 (2014) 2152–2167. [DOI] [PubMed] [Google Scholar]
- [66].Chrousos GP, Zapanti ED, Hypothalamic-pituitary-adrenal axis in HIV infection and disease, Endocrinol Metab Clin North Am, 43 (2014) 791–806. [DOI] [PubMed] [Google Scholar]
- [67].Priftis KN, Papadimitriou A, Nicolaidou P, Chrousos GP, The hypothalamic-pituitary-adrenal axis in asthmatic children, Trends Endocrinol Metab, 19 (2008) 32–38. [DOI] [PubMed] [Google Scholar]
- [68].Chrousos G, Q&A: primary generalized glucocorticoid resistance, BMC Med, 9 (2011) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nicolaides NC, Charmandari E, Chrousos syndrome: from molecular pathogenesis to therapeutic management, Eur J Clin Invest, 45 (2015) 504–514. [DOI] [PubMed] [Google Scholar]
- [70].Charmandari E, Primary generalized glucocorticoid resistance and hypersensitivity, Horm Res Paediatr, 76 (2011) 145–155. [DOI] [PubMed] [Google Scholar]
- [71].De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G, Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease, World J Gastroenterol, 17 (2011) 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kino T, Chrousos GP, Glucocorticoid and mineralocorticoid resistance/hypersensitivity syndromes, J Endocrinol, 169 (2001) 437–445. [DOI] [PubMed] [Google Scholar]
- [73].Chrousos GP, Charmandari E, Kino T, Glucocorticoid action networks--an introduction to systems biology, J Clin Endocrinol Metab, 89 (2004) 563–564. [DOI] [PubMed] [Google Scholar]
- [74].Kino T, Charmandari E, Chrousos GP, Glucocorticoid receptor: implications for rheumatic diseases, Clin Exp Rheumatol, 29 (2011) S32–41. [PMC free article] [PubMed] [Google Scholar]
- [75].Paragliola RM, Papi G, Pontecorvi A, Corsello SM, Treatment with Synthetic Glucocorticoids and the Hypothalamus-Pituitary-Adrenal Axis, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pujols L, Mullol J, Picado C, Importance of glucocorticoid receptors in upper and lower airways, Front Biosci (Landmark Ed), 15 (2010) 789–800. [DOI] [PubMed] [Google Scholar]
- [77].Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SW, van Rossum EF, Feelders RA, Glucocorticoid sensitivity in health and disease, Nat Rev Endocrinol, 9 (2013) 670–686. [DOI] [PubMed] [Google Scholar]
- [78].Chrousos GP, Stress and disorders of the stress system, Nat Rev Endocrinol, 5 (2009) 374–381. [DOI] [PubMed] [Google Scholar]
- [79].Mager DE, Moledina N, Jusko WJ, Relative immunosuppressive potency of therapeutic corticosteroids measured by whole blood lymphocyte proliferation, J Pharm Sci, 92 (2003) 1521–1525. [DOI] [PubMed] [Google Scholar]
- [80].Pereira AM, Meijer OC, Glucocorticoid Regulation of Neurocognitive and Neuropsychiatric Function, in: Greer EB (Ed.) The Hypothalamic-Pituitary-Adrenal Axis in Health and Disease: Cushing’s Syndrome and Beyond, Springer International Publishing, Switzerland, 2017. [Google Scholar]
- [81].Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G, Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference, Neuroimmunomodulation, 10 (2002) 247–260. [DOI] [PubMed] [Google Scholar]
- [82].Chrousos GP, Kino T, Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders, Ann N Y Acad Sci, 1179 (2009) 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lee MJ, Pramyothin P, Karastergiou K, Fried SK, Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity, Biochim Biophys Acta, 1842 (2014) 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang ZJ, Zhang XQ, Cui XY, Cui SY, Yu B, Sheng ZF, Li SJ, Cao Q, Huang YL, Xu YP, Zhang YH, Glucocorticoid receptors in the locus coeruleus mediate sleep disorders caused by repeated corticosterone treatment, Sci Rep, 5 (2015) 9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Czock D, Keller F, Rasche FM, Haussler U, Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids, Clin Pharmacokinet, 44 (2005) 61–98. [DOI] [PubMed] [Google Scholar]
- [86].Schacke H, Schottelius A, Docke WD, Strehlke P, Jaroch S, Schmees N, Rehwinkel H, Hennekes H, Asadullah K, Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects, Proc Natl Acad Sci U S A, 101 (2004) 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Strehl C, Buttgereit F, Optimized glucocorticoid therapy: teaching old drugs new tricks, Mol Cell Endocrinol, 380 (2013) 32–40. [DOI] [PubMed] [Google Scholar]
- [88].Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ, Dose equivalency evaluation of major corticosteroids: Pharmacokinetics and cell trafficking and cortisol dynamics, Journal of Clinical Pharmacology, 43 (2003) 1216–1227. [DOI] [PubMed] [Google Scholar]
- [89].Hydrocortisone, in: Anderson LA (Ed.), 2017. [Google Scholar]
- [90].Melin J, Parra-Guillen ZP, Hartung N, Huisinga W, Ross RJ, Whitaker MJ, Kloft C, Predicting Cortisol Exposure from Paediatric Hydrocortisone Formulation Using a Semi-Mechanistic Pharmacokinetic Model Established in Healthy Adults, Clin Pharmacokinet, 57 (2018) 515–527. [DOI] [PubMed] [Google Scholar]
- [91].Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, Darzy K, Merke DP, Arlt W, Ross RJ, Modified-release hydrocortisone to provide circadian cortisol profiles, J Clin Endocrinol Metab, 94 (2009) 1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Prednisone, in: Anderson LA (Ed.). [Google Scholar]
- [93].Methylprednisolone, in: Anderson LA (Ed.). [Google Scholar]
- [94].Chow FS, Sharma A, Jusko WJ, Modeling interactions between adrenal suppression and T-helper lymphocyte trafficking during multiple dosing of methylprednisolone, J Pharmacokinet Biopharm, 27 (1999) 559–575. [DOI] [PubMed] [Google Scholar]
- [95].Xu J, Winkler J, Derendorf H, A pharmacokinetic/pharmacodynamic approach to predict total prednisolone concentrations in human plasma, J Pharmacokinet Pharmacodyn, 34 (2007) 355–372. [DOI] [PubMed] [Google Scholar]
- [96].de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EGWM, Westenberg HGM, Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder, Psychoneuroendocrinology, 32 (2007) 215–226. [DOI] [PubMed] [Google Scholar]
- [97].Dexamethasone, in: Anderson LA (Ed.). [Google Scholar]
- [98].Newell-Price J, Whiteman M, Rostami-Hodjegan A, Darzy K, Shalet S, Tucker GT, Ross RJ, Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers, Clin Endocrinol (Oxf), 68 (2008) 130–135. [DOI] [PubMed] [Google Scholar]
- [99].Findling JW, Raff H, Aron DC, The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing’s syndrome, J Clin Endocrinol Metab, 89 (2004) 1222–1226. [DOI] [PubMed] [Google Scholar]
- [100].Plenadren Annex I: Summary of Product Characteristics, European Medicines Agency. [Google Scholar]
- [101].Vogt M, Derendorf H, Kramer J, Junginger HE, Midha KK, Shah VP, Stavchansky S, Dressman JB, Barends DM, Biowaiver monographs for immediate release solid oral dosage forms: prednisolone, J Pharm Sci, 96 (2007) 27–37. [DOI] [PubMed] [Google Scholar]
- [102].Vogt M, Derendorf H, Kramer J, Junginger HE, Midha KK, Shah VP, Stavchansky S, Dressman JB, Barends DM, Biowaiver monographs for immediate release solid oral dosage forms: prednisone, J Pharm Sci, 96 (2007) 1480–1489. [DOI] [PubMed] [Google Scholar]
- [103].Lindenberg M, Kopp S, Dressman JB, Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system, Eur J Pharm Biopharm, 58 (2004) 265–278. [DOI] [PubMed] [Google Scholar]
- [104].Ploger GF, Hofsass MA, Dressman JB, Solubility Determination of Active Pharmaceutical Ingredients Which Have Been Recently Added to the List of Essential Medicines in the Context of the Biopharmaceutics Classification System-Biowaiver, J Pharm Sci, 107 (2018) 1478–1488. [DOI] [PubMed] [Google Scholar]
- [105].Magee MH, Blum RA, Lates CD, Jusko WJ, Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race, J Clin Pharmacol, 41 (2001) 1180–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Meno-Tetang GM, Blum RA, Schwartz KE, Jusko WJ, Effects of oral prasterone (dehydroepiandrosterone) on single-dose pharmacokinetics of oral prednisone and cortisol suppression in normal women, J Clin Pharmacol, 41 (2001) 1195–1205. [DOI] [PubMed] [Google Scholar]
- [107].Lew KH, Ludwig EA, Milad MA, Donovan K, Middleton E Jr., Ferry JJ, Jusko WJ, Gender-based effects on methylprednisolone pharmacokinetics and pharmacodynamics, Clin Pharmacol Ther, 54 (1993) 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ohdo S, Koyanagi S, Matsunaga N, Hamdan A, Molecular basis of chronopharmaceutics, J Pharm Sci, 100 (2011) 3560–3576. [DOI] [PubMed] [Google Scholar]
- [109].Griffett K, Burris TP, The mammalian clock and chronopharmacology, Bioorg Med Chem Lett, 23 (2013) 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Binkhorst L, Kloth JS, de Wit AS, de Bruijn P, Lam MH, Chaves I, Burger H, van Alphen RJ, Hamberg P, van Schaik RH, Jager A, Koch BC, Wiemer EA, van Gelder T, van der Horst GT, Mathijssen RH, Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients, Breast Cancer Res Treat, 152 (2015) 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dallmann R, Okyar A, Levi F, Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy, Trends Mol Med, 22 (2016) 430–445. [DOI] [PubMed] [Google Scholar]
- [112].Rao RT, DuBois DC, Almon RR, Jusko WJ, Androulakis IP, Mathematical Modeling of the Circadian Dynamics of the Neuroendocrine-Immune Network in Experimentally Induced Arthritis, Am J Physiol Endocrinol Metab, (2016) ajpendo 00006 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ovacik MA, Sukumaran S, Almon RR, DuBois DC, Jusko WJ, Androulakis IP, Circadian signatures in rat liver: from gene expression to pathways, BMC Bioinformatics, 11 (2010) 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Almon RR, Yang E, Lai W, Androulakis IP, Ghimbovschi S, Hoffman EP, Jusko WJ, Dubois DC, Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle, Am J Physiol Regul Integr Comp Physiol, 295 (2008) R1031–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Almon RR, Yang E, Lai W, Androulakis IP, DuBois DC, Jusko WJ, Circadian variations in rat liver gene expression: relationships to drug actions, J Pharmacol Exp Ther, 326 (2008) 700–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Mavroudis PD, Corbett SA, Calvano SE, Androulakis IP, Circadian characteristics of permissive and suppressive effects of cortisol and their role in homeostasis and the acute inflammatory response, Math Biosci, 260 (2015) 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mavroudis PD, Scheff JD, Calvano SE, Androulakis IP, Systems biology of circadian-immune interactions, J Innate Immun, 5 (2013) 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP, Entrainment of peripheral clock genes by cortisol, Physiol Genomics, 44 (2012) 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mavroudis PD, Corbett SA, Calvano SE, Androulakis IP, Mathematical modeling of light-mediated HPA axis activity and downstream implications on the entrainment of peripheral clock genes, Physiological Genomics, 46 (2014) 766–778. [DOI] [PubMed] [Google Scholar]
- [120].Kirwan JR, Targeting the time of day for glucocorticoid delivery in rheumatoid arthritis, Int. J. Clin. Rheumatol, 6 (2011) 273–279. [Google Scholar]
- [121].Haus E, Sackett-Lundeen L, Smolensky MH, Rheumatoid arthritis: circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications, Bull NYU Hosp Jt Dis, 70 Suppl 1 (2012) 3–10. [PubMed] [Google Scholar]
- [122].Cutolo M, Straub RH, Circadian rhythms in arthritis: hormonal effects on the immune/inflammatory reaction, Autoimmun Rev, 7 (2008) 223–228. [DOI] [PubMed] [Google Scholar]
- [123].Kino T, Circadian rhythms of glucocorticoid hormone actions in target tissues: potential clinical implications, Sci Signal, 5 (2012) pt4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ravindran V, Choy E, Modified-release prednisone in rheumatoid arthritis: Rationale for chronotherapy, mechanistic considerations, and clinical implications, Indian Journal of Rheumatology, 11 (2016) 216. [Google Scholar]
- [125].Ohdo S, Changes in toxicity and effectiveness with timing of drug administration: implications for drug safety, Drug Saf, 26 (2003) 999–1010. [DOI] [PubMed] [Google Scholar]
- [126].Burioka N, Fukuoka Y, Koyanagi S, Miyata M, Takata M, Chikumi H, Takane H, Watanabe M, Endo M, Sako T, Suyama H, Ohdo S, Shimizu E, Asthma: Chronopharmacotherapy and the molecular clock, Adv Drug Deliv Rev, 62 (2010) 946–955. [DOI] [PubMed] [Google Scholar]
- [127].Sundar IK, Yao H, Sellix MT, Rahman I, Circadian molecular clock in lung pathophysiology, Am J Physiol Lung Cell Mol Physiol, 309 (2015) L1056–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Alavoine L, Taillé C, Ball J, Knauer C, Witte S, Kent J, Aubier M, Nocturnal Asthma: Proof-of-Concept Open-Label Study with Delayed-Release Prednisone, Pulmonary Therapy, 1 (2015) 43–52. [Google Scholar]
- [129].Durrington H, Farrow S, Ray D, Recent advances in chronotherapy for the management of asthma, ChronoPhysiology and Therapy, (2014) 125. [Google Scholar]
- [130].Truong KK, Lam MT, Grandner MA, Sassoon CS, Malhotra A, Timing Matters: Circadian Rhythm in Sepsis, Obstructive Lung Disease, Obstructive Sleep Apnea, and Cancer, Ann Am Thorac Soc, 13 (2016) 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Smolensky MH, Haus E, Circadian rhythms and clinical medicine with applications to hypertension, Am J Hypertens, 14 (2001) 280S–290S. [DOI] [PubMed] [Google Scholar]
- [132].Glass-Marmor L, Paperna T, Ben-Yosef Y, Miller A, Chronotherapy using corticosteroids for multiple sclerosis relapses, J Neurol Neurosurg Psychiatry, 78 (2007) 886–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Pierre K, Schlesinger N, Androulakis IP, The role of the hypothalamic-pituitary-adrenal axis in modulating seasonal changes in immunity, Physiol Genomics, 48 (2016) 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sajan J, Cinu T, Chacko A, LItty J, Jaseeda T, Chronotherapeutics and chronotherapeutic drug delivery systems, Tropical Journal of Pharmaceutical Research, 8 (2009) 467–475. [Google Scholar]
- [135].Lemmer B, Chronopharmacokinetics: Implications for Drug Treatment, Journal of Pharmacy and Pharmacology, 51 (1999) 887–890. [DOI] [PubMed] [Google Scholar]
- [136].Levi F, Schibler U, Circadian rhythms: mechanisms and therapeutic implications, Annu Rev Pharmacol Toxicol, 47 (2007) 593–628. [DOI] [PubMed] [Google Scholar]
- [137].Lemmer B, The importance of biological rhythms in drug treatment of hypertension and sex-dependent modifications, ChronoPhysiology and Therapy, (2012) 9. [Google Scholar]
- [138].Erkekoglu P, Baydar T, Chronopharmacokinetics of drugs in toxicological aspects: A short review for pharmacy practitioners, J Res Pharm Pract, 1 (2012) 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sukumaran S, Xue B, Jusko WJ, Dubois DC, Almon RR, Circadian variations in gene expression in rat abdominal adipose tissue and relationship to physiology, Physiol Genomics, 42A (2010) 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, Jamei M, Lloyd R, Pepin X, Rostami-Hodjegan A, Sjogren E, Tannergren C, Turner DB, Wagner C, Weitschies W, Dressman J, PBPK models for the prediction of in vivo performance of oral dosage forms, Eur J Pharm Sci, 57 (2014) 300–321. [DOI] [PubMed] [Google Scholar]
- [141].McAllister WA, Mitchell DM, Collins JV, Prednisolone pharmacokinetics compared between night and day in asthmatic and normal subjects, Br J Clin Pharmacol, 11 (1981) 303–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lamiable D, Vistelle R, Fay R, Millart H, Caron J, Choisy H, [Chronopharmacokinetics of dexamethasone in young subjects], Therapie, 46 (1991) 405–407. [PubMed] [Google Scholar]
- [143].Musiek ES, Fitzgerald GA, Molecular clocks in pharmacology, Handb Exp Pharmacol, (2013) 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gupta R, Gupta BM, Khajuria V, Bhat NK, Gupta A, Chronopharmacology: An Overview, J Rational pharmacother Res, 1 (2013) 6–9. [Google Scholar]
- [145].Adil MS, HM A, M I, K MA, Haadi A, K MA, Nematullah M, Chronoterapuetics: targeting the disease at its ideal time, The Pharma Innovation Journal, 2 (2014). [Google Scholar]
- [146].Chapman SC, Llahana S, Carroll P, Horne R, Glucocorticoid therapy for adrenal insufficiency: nonadherence, concerns and dissatisfaction with information, Clin Endocrinol (Oxf), 84 (2016) 664–671. [DOI] [PubMed] [Google Scholar]
- [147].Alten R, Wiebe E, Hypothalamic-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with different glucocorticoid approaches, Neuroimmunomodulation, 22 (2015) 83–88. [DOI] [PubMed] [Google Scholar]
- [148].Hayasaka N, Yaita T, Kuwaki T, Honma S, Honma K, Kudo T, Shibata S, Optimization of dosing schedule of daily inhalant dexamethasone to minimize phase shifting of clock gene expression rhythm in the lungs of the asthma mouse model, Endocrinology, 148 (2007) 3316–3326. [DOI] [PubMed] [Google Scholar]
- [149].Behan LA, Rogers B, Hannon MJ, O’Kelly P, Tormey W, Smith D, Thompson CJ, Agha A, Optimizing glucocorticoid replacement therapy in severely adrenocorticotropin-deficient hypopituitary male patients, Clin Endocrinol (Oxf), 75 (2011) 505–513. [DOI] [PubMed] [Google Scholar]
- [150].Naafs MA, Glucocorticoid chronotherapy: a mini-review, Endocrinology & Metabolism International Journal, 6 (2018) 118–122. [Google Scholar]
- [151].Debono M, Price JN, Ross RJ, Novel strategies for hydrocortisone replacement, Best Pract Res Clin Endocrinol Metab, 23 (2009) 221–232. [DOI] [PubMed] [Google Scholar]
- [152].Debono M, Ross RJ, Newell-Price J, Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy, Eur J Endocrinol, 160 (2009) 719–729. [DOI] [PubMed] [Google Scholar]
- [153].Isidori AM, Venneri MA, Graziadio C, Simeoli C, Fiore D, Hasenmajer V, Sbardella E, Gianfrilli D, Pozza C, Pasqualetti P, Morrone S, Santoni A, Naro F, Colao A, Pivonello R, Lenzi A, Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial, Lancet Diabetes Endocrinol, 6 (2018) 173–185. [DOI] [PubMed] [Google Scholar]
- [154].Cutolo M, Sulli A, Pincus T, Circadian use of glucocorticoids in rheumatoid arthritis, Neuroimmunomodulation, 22 (2015) 33–39. [DOI] [PubMed] [Google Scholar]
- [155].Chung TT, Gunganah K, Monson JP, Drake WM, Circadian variation in serum cortisol during hydrocortisone replacement is not attributable to changes in cortisol-binding globulin concentrations, Clin Endocrinol (Oxf), 84 (2016) 496–500. [DOI] [PubMed] [Google Scholar]
- [156].Debono M, Ross RJ, What is the best approach to tailoring hydrocortisone dose to meet patient needs in 2012?, Clin Endocrinol (Oxf), 78 (2013) 659–664. [DOI] [PubMed] [Google Scholar]
- [157].Rao RT, Scherholz ML, Androulakis IP, Modeling the influence of chronopharmacological administration of synthetic glucocorticoids on the hypothalamic-pituitary-adrenal axis, Chronobiol Int, (2018) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN, Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD, J Clin Endocrinol Metab, 96 (2011) 1789–1796. [DOI] [PubMed] [Google Scholar]
- [159].Prigent H, Maxime V, Annane D, Clinical review: corticotherapy in sepsis, Crit Care, 8 (2004) 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Seibel MJ, Cooper MS, Zhou H, Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives, Lancet Diabetes Endocrinol, 1 (2013) 59–70. [DOI] [PubMed] [Google Scholar]
- [161].Rogers TS, Harrison S, Swanson C, Cauley JA, Barrett-Connor E, Orwoll E, Stone KL, Lane NE, Osteoporotic G Fractures in Men Study Research, Rest-activity circadian rhythms and bone mineral density in elderly men, Bone Rep, 7 (2017) 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hartmanshenn C, Scherholz M, Androulakis IP, Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine, J Pharmacokinet Pharmacodyn, 43 (2016) 481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Rao RT, Androulakis IP, Modeling the Sex Differences and Interindividual Variability in the Activity of the Hypothalamic-Pituitary-Adrenal Axis, Endocrinology, 158 (2017) 4017–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ebner K, Singewald N, Individual differences in stress susceptibility and stress inhibitory mechanisms, Current Opinion in Behavioral Sciences, 14 (2017) 54–64. [Google Scholar]
- [165].Cutolo M, Villaggio B, Otsa K, Aakre O, Sulli A, Seriolo B, Altered circadian rhythms in rheumatoid arthritis patients play a role in the disease’s symptoms, Autoimmunity Reviews, 4 (2005) 497–502. [DOI] [PubMed] [Google Scholar]
- [166].Kouri V-P, Olkkonen J, Kaivosoja E, Ainola M, Juhila J, Hovatta I, Konttinen YT, Mandelin J, Circadian Timekeeping Is Disturbed in Rheumatoid Arthritis at Molecular Level, PLoS ONE, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chrousos GP, Stress and sex versus immunity and inflammation, Sci Signal, 3 (2010) pe36. [DOI] [PubMed] [Google Scholar]
- [168].Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C, The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder, Biol Psychiatry, 52 (2002) 393–403. [DOI] [PubMed] [Google Scholar]
- [169].Dallmann R, Brown SA, Gachon F, Chronopharmacology: new insights and therapeutic implications, Annu Rev Pharmacol Toxicol, 54 (2014) 339–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Song QQ, Xie WY, Tang YJ, Zhang J, Liu J, Genetic variation in the glucocorticoid pathway involved in interindividual differences in the glucocorticoid treatment, Pharmacogenomics, 18 (2017) 293–316. [DOI] [PubMed] [Google Scholar]
- [171].Ibrahim A, Dahlqvist P, Olsson T, Lundgren D, Werner M, Suhr OB, Karling P, The clinical course after glucocorticoid treatment in patients with inflammatory bowel disease is linked to suppression of the hypothalamic-pituitary-adrenal axis: a retrospective observational study, Therap Adv Gastroenterol, 10 (2017) 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Pujolsa L, Mullol J, Picado C, Glucocorticoid receptor in human respiratory epithelial cells, Neuroimmunomodulation, 16 (2009) 290–299. [DOI] [PubMed] [Google Scholar]
- [173].Oakley RH, Cidlowski JA, Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids, J Biol Chem, 286 (2011) 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Crown A, Lightman S, Why is the management of glucocorticoid deficiency still controversial: a review of the literature, Clin Endocrinol (Oxf), 63 (2005) 483–492. [DOI] [PubMed] [Google Scholar]