Abstract
In addition to being the leading cause of morbidity and mortality in premature infants, germinal matrix hemorrhage (GMH) is also the leading cause of acquired infantile hydrocephalus. The pathophysiology of post-hemorrhagic hydrocephalus development after GMH is complex and vaguely understood, although evidence suggests fibrosis and gliosis in the periventricular and subarachnoid spaces disrupts normal cerebrospinal fluid dynamics. Theories explaining general hydrocephalus etiology have substantially evolved from the original bulk flow theory developed by Dr. Dandy over a century ago. Current clinical and experimental evidence supports a new hydrodynamic theory for hydrocephalus development involving redistribution of vascular pulsations and disruption of Starling forces in the brain microcirculation. In this review, we discuss cerebrospinal fluid flow dynamics, history and development of theoretical hydrocephalus pathophysiology, and GMH epidemiology and etiology as it relates to post-hemorrhagic hydrocephalus development. We highlight known mechanisms and propose new avenues that will further elucidate GMH pathophysiology, specifically related to hydrocephalus.
Keywords: Post-Hemorrhagic Hydrocephalus, Post-Hemorrhagic Ventricular Dilation, Germinal Matrix Hemorrhage, Intraventricular Hemorrhage, Neonatal Brain Hemorrhage, Subarachnoid Hemorrhage, Intracerebral Hemorrhage, Cerebrospinal Fluid, Choroid Plexus, Glymphatic System
Introduction
Germinal matrix hemorrhage (GMH) occurs in approximately 3 live births per 1,000, has a 20–30% mortality rate, and accounts for 1.7% of all neonatal deaths in the United States (Ballabh 2014; Osterman et al. 2015). Premature infants have a much higher rate of occurrence; for infants born before 32 weeks of gestation up to 20%, (about 12,000 infants) develop GMH each year in the US (Kochanek et al. 2012). Fortunately, the premature birthrate and percentage of low birthweight (<2500g) infants have steadily declined between 2006 and 2013, although remaining higher than in the 1980s and 1990s. In 2006, the preterm birthrate was 12.8% and, in 2013, the preterm birthrate declined to 11.39% while the percentage of low birthweight infants was relatively unchanged at 8.02%. The percentage of very low birthweight (<1500g) was 1.41% in 2013 (Osterman et al. 2015). A study investigating premature infants dating back to 1914 determined median postnatal survival increased from 2 to 26 days, and median gestational age decreased from 33 to 27 weeks. Interestingly, GMH incidence was 4.7% before 1960, but it increased to 50.0% between 1975 and 1980, and then decreased to 12.5% after 2005 (Hefti et al. 2015). The introduction of positive pressure ventilation in preterm clinical management after the 1960s increased survival while simultaneously increasing GMH incidence, which may be attributed to cardiorespiratory and hemodynamic instability associated with mechanical ventilation, and the decline in GMH incidence after the 1980s may be attributed to improvements in mechanical ventilation methodology as well as the use of antenatal steroids and surfactant. Despite improving trends in premature birth incidences and outcomes, GMH remains the leading cause of morbidity and mortality in premature and/or very low birthweight infants, and its incidence has remained steady in the past decade.
Premature and very low birthweight infants are prone to hemodynamic and cardiorespiratory instability, leading to abrupt fluctuations in cerebral blood flow (Ballabh 2014). The fetal brain is hypothesized to lack vascular autoregulatory mechanisms to adequately prevent cerebral blood flow fluctuations, although clinical research involving cerebral blood flow monitoring in preterm infants has produced ambiguous results (Alderliesten et al. 2013; Caicedo et al. 2011; du Plessis 2008; Soul et al. 2007; Tsuji et al. 2000; Wong et al. 2008). The germinal matrix layer, which is present in the fetus and matures by term, contains many neuronal and glial precursor cells and is a site of rapid angiogenesis relative to other parts of the brain (Ballabh et al. 2004; Ballabh et al. 2007). The germinal matrix neurovascular unit, consisting of neurons, astrocytes, pericytes, vascular smooth muscle cells, and vascular endothelial cells, is deficient in fibronectin at the endothelial basal lamina, glial fibrillary acidic protein at astrocyte end-feet, and pericyte coverage (Ballabh 2010; Ballabh 2014). Thus, the germinal matrix vasculature is inherently weak and vulnerable to hemorrhage under abnormal conditions, regardless if the premature infant brain has autoregulatory mechanisms to adequately prevent cerebral blood flow fluctuations.
GMH severity is graded on an I-IV scale based on the extent and localization of bleeding. Incidence of higher GMH grades (III-IV) increases as gestational age and/or birthweight decreases (Robinson 2012). Between 50–75% of GMH survivors develop long-term neurocognitive sequelae, including cerebral palsy, learning disabilities, psychiatric disorders, and post-hemorrhagic hydrocephalus (PHH), and higher-grade GMH survivors are most vulnerable to worse long-term outcomes (Ballabh 2010; Ballabh 2014). The mortality rate for severe grade (III-IV) GMH is approximately 44%, with 60% of survivors developing PHH and 25% requiring surgical installation of shunts (Vassilyadi et al. 2009). Another study estimates 10% of GMH patients (any grade) and 20% of severe GMH patients (III-IV) will require surgical insertion of permanent shunts (Robinson 2012). Shunt dependency is not desirable, given the large, costly, detrimental complications that occur due to shunt infection, occlusion, and displacement. Additional approaches to manage or prevent PHH include serial lumbar punctures, ventricular taps, external ventricular drainage, ventricular access device, ventricular-subgaleal shunt, endoscopic third ventriculostomy, and endoscopic coagulation of the choroid plexus (Tully and Dobyns 2014). A non-invasive, therapeutic approach towards ameliorating PHH would significantly improve long-term quality of life for GMH patients.
PHH pathophysiology after GMH remains vague and complex, and minimal advancements have been made in its clinical management. In this review, we discuss CSF flow dynamics, particularly focusing on its importance in GMH. We highlight advancements made in hydrocephalus research after Dr. Dandy first proposed the bulk flow theory over a century ago. We discuss the current hydrodynamic theory for hydrocephalus pathophysiology and how it applies to PHH development after GMH. Special attention is given to CSF dynamics, CSF production at the choroid plexus, and CSF circulation through the glymphatic system. We identify gaps in current research and propose avenues for further exploration.
Cerebrospinal Fluid Flow Dynamics
Cerebrospinal fluid (CSF) is an isotonic solution that primarily acts as a mechanical cushion for the brain, although it serves many other physiologically vital functions as well (Chakravarthi 2012). CSF has a lower specific gravity than brain tissue, creating a buoyant force that reduces the effective mass of the brain. CSF [H+] concentration is detected by central chemoreceptors located at the ventrolateral medullary surface, which help regulate pulmonary ventilation and cerebral blood flow to ensure the brain receives ample oxygen and nutrients. CSF also maintains a stable external environment for growth and development of neurons and glia (Chakravarthi 2012). Importantly, CSF removes brain metabolic waste and transporting neuropeptides, glucose, and lipids (Iliff et al. 2012; Xie et al. 2013).
Production
In adults, between 400–600 mL of CSF is produced per day and the brain renews its CSF between 3–4 times within a 24 hour period (Cutler et al. 1968; Pierce et al. 1962; Sahar 1972; Sato et al. 1975). CSF is primarily produced by the choroid plexus epithelial lining and, to a minimal extent, the cerebral ventricular ependymal lining, which compose the blood-CSF barrier. Choroid plexus epithelial cells are interconnected by tight junctions that are leakier than endothelial cells of the blood-brain barrier. Over two thirds of produced CSF originates from the choroid plexus (Pollay 1975; Segal and Pollay 1977). The choroid plexus lines the lateral ventricles from the inferior horns to the interventricular foramen, where it becomes continuous into the third ventricle and continues into the fourth ventricle. The choroid epithelium protrudes into the ventricles through invaginations of the pia matter containing choroidal capillaries, called tela choroidea, which significantly increase the surface area of the choroidal epithelium (Davson and Segal 1970; Johanson et al. 2011; Keep and Jones 1990; Speake and Brown 2004). The choroid plexus vasculature is also fenestrated to better facilitate CSF production. Non-choroidal ependymal cells, brain interstitial fluid, and capillaries may be other CSF sources as well, which is secreted by transependymal seepage into the brain ventricles or transpinal seepage into the subarachnoid space (Davis and Milhorat 1975; Milhorat et al. 1975; Pollay and Curl 1967; Saunders et al. 1999).
The posterior choroidal, anterior choroidal, inferior cerebellar and superior cerebellar arteries supply the choroid plexus of the lateral ventricles, third ventricle, fourth ventricle, and temporal horns, respectively (Chakravarthi 2012; Milhorat 1978; Sakka et al. 2011). In adults, blood flow to the choroidal epithelium is estimated at 4 – 6 mL / minute / gram tissue, which is significantly greater than blood flow to other brain tissue estimated at 0.9 – 1.8 mL / minute / gram tissue (Maktabi et al. 1991). The choroidal interstitial compartment is the region between choroidal capillaries and choroidal ependymal cells. Choroidal capillaries lack tight junction proteins in their endothelial cells, making them more permeable, and blood plasma filtrate passively crosses into the choroidal interstitial compartment from the choroidal capillaries primarily by Starling forces (Welch 1975; Wright 1972). Starling forces are hydrostatic, and oncotic forces that govern the movement of fluid across capillary membranes. Hydrostatic forces refer to the difference in fluid pressure between the capillary and interstitium, where higher capillary fluid pressure will drive water into the interstitium. Oncotic forces refer to the difference in solute concentration between the capillary and interstitium, where higher interstitial solute and macromolecule concentration will drive fluid from the capillary into the interstitium. Net fluid movement is the net combined hydrostatic and oncotic forces. Thus the main source for produced CSF is technically choroidal capillaries, not the choroid plexus itself (Bulat and Klarica 2011; Oreskovic and Klarica 2010; Oreskovic and Klarica 2011), although this assertion is contentious.
[Na+] and [Cl−] from choroidal interstitium are actively exchanged for [H+] and [HCO3−], generated by cytosolic carbonic anhydrase on choroidal ependymal cells, using carrier proteins in the choroidal ependymal basolateral membrane. Pumps on the choroidal ependymal apical membrane then expel [Na+], [Cl−], [K+], and [HCO3−] into the ventricle lumen, which generates an osmotic pressure (Keep and Jones 1990; Pollay 1975; Spector and Johanson 1989). Water flows down the created osmotic gradient with the help of aquaporin 1 on the choroidal ependymal apical membrane (Reiber 2003). The CSF contains higher concentrations of [Na+], [Mg2+], and [Cl−] than blood plasma but less [Ca2+], [K+], [HCO3−], [PO4+], protein (contains 0.3% plasma proteins), amino acids, and glucose (Felgenhauer 1974). Several holes exist in the assertion that CSF is produced by cerebral capillaries via filtration, which need to be kept in mind. The difference in small solutes between plasma and interstitium is small and the concentration of plasma protein is significantly less in brain tissue interstitium, which significantly diminishes the oncotic pressure gradient. Additionally, flux between cerebral capillaries and the interstitium is not unidirectional in reality, as there is almost as much back flux as forward flux (Hladky and Barrand 2014; Hladky and Barrand 2016).
The choroidal epithelium can alter CSF secretion in response to multiple factors and mechanisms. Most regulatory mechanisms target membrane transporters, carbonic anhydrase, and aquaporins (Faraci et al. 1990; Sakka et al. 2011). The NaK2Cl cotransporter, located on the choroidal ependymal apical membrane, helps regulate CSF composition and secretion by its bidirectional transport ability. Arginine vasopressin, atrial natriuretic peptide, serotonin, melatonin, and dopamine receptors are located on choroidal epithelium. Arginine vasopressin and atrial natriuretic peptide decrease CSF secretion. CSF secretion can also be increased by sympathetic innervation and decreased by cholinergic innervation (Chakravarthi 2012). Pharmaceutical drugs that inhibit carbonic anhydrase or sodium transporters, such as diuretics, reduce CSF production, while drugs that augment cerebral blood flow tend to increase CSF production. Increased intracranial pressure also tends to decrease CSF production, although evidence suggests CSF production tends to remain constant despite large increases in hydrostatic pressure (Sakka et al. 2011).
Circulation
CSF flows from the sites of secretion at the choroidal epithelium to the sites of absorption in the subarachnoid space. The mean CSF volume within the adult brain is 150 mL, with 25 mL in the ventricles and 125 mL in the subarachnoid space (Sakka et al. 2011). Generally, CSF flows from the lateral ventricles, passes through the interventricular foramen of Monro into the third ventricle, and finally passes into the cerebral aqueduct of Sylvius into the fourth ventricle. From the fourth ventricle, CSF enters through three openings, the lateral apertures of Lushka and median aperture of Magendie, into the subarachnoid space where it is absorbed. A portion of the CSF exits the cranium through arachnoid villi and cranial nerves while the remainder enters along the spinal cord and exit through spinal nerve roots (Chakravarthi 2012; Dichiro 1964; Milhorat 1976).
CSF circulates through the brain’s ventricular system and spinal cord in a pulsatile manner. Cerebral arterial pulse waves are the primary drivers of CSF circulation, although jugular venous pressure, respiratory waves, and even physical activity play minor roles as well (Post et al. 1974; Williams 1976). CSF flow, however, is very slow and sometimes occurs bi-directionally through ventricle compartments with each cardiac and/or respiratory cycle, but net CSF flow occurs from the lateral ventricles to the subarachnoid space. Additionally, ventricular ependymal cells have cilia that mix CSF while it is circulating. CSF pressure gradients, which are generated by continuous CSF secretion and arterial pulsations, are also important for maintaining CSF flow. This pressure gradient is particularly important in driving CSF flow through the subarachnoid spaces and venous sinuses. In adults, CSF flow across the subarachnoid epithelium is driven by a 6 cm H2O pressure difference between subarachnoid CSF pressure (approximately 15 cm H2O) and superior sagittal sinus pressure (approximately 9 cm H2O), and the pressure continues to drop into the jugular vein and systemic venous system (Bradley 1970; Milhorat 1975; Sahar et al. 1970; Shulman et al. 1964).
Reabsorption
Conventionally CSF is reabsorbed in the subarachnoid space and enters through the dural venous sinuses, where it returns to the internal jugular system (Chakravarthi 2012). Subarachnoid villi, called pacchonian bodies, were originally thought to be the main reabsorption sites (Brierley and Field 1948; Welch 1975; Welch and Friedman 1960), but evidence suggests other potential CSF outflow and reabsorption routes through either cerebral lymphatic channels or the venous system [(Bradbury et al. 1981; Bulat and Klarica 2011; Oreskovic and Klarica 2010; Oreskovic and Klarica 2011; Zakharov et al. 2003). Evidence for the venous system being the main reabsorption site suggests the vast majority of CSF outflow occurs at the superior sagittal sinus with the remainder occurring at dural sinusoids in dorsal root nerves. CSF outflow is driven by pressure gradients between the subarachnoid space and venous sinuses (Cutler et al. 1968; Pollay 2010; Saunders et al. 1999; Zlokovic et al. 1990). Increased intracranial pressure tends to increase CSF outflow, but very high intracranial pressure that persists for a long period of time tends to actually decrease CSF outflow, mostly because venous pressure tends to increase with intracranial pressure while the overall pressure gradient diminishes. Evidence for cerebral lymphatic channels being the main reabsorption site suggests CSF flows along cranial nerves and spinal nerve roots and is reabsorbed in lymphatic channels (Bradbury et al. 1981; Zakharov et al. 2003). Indeed, CSF outflow in the nasal submucosal lymphatic channels through the cribriform plate, which feed into the cervical lymph nodes, is relatively important (Courtice and Simmonds 1951; Cserr et al. 1992; Erlich et al. 1986; Kida et al. 1993; Mollanji et al. 2002; Silver et al. 1999). Lymphatic vessels have also been recently characterized surrounding the dural sinuses, which are also connected to cervical lymph nodes, further suggesting the lymphatic system plays an important role in CSF outflow (Aspelund et al. 2015; Bradbury et al. 1981; Iliff et al. 2015; Louveau et al. 2015; Zakharov et al. 2003; Zervas et al. 1982). Lymphatic-mediated CSF reabsorption is thought to play a greater role in neonates, since subarachnoid granulations are more sparsely distributed.
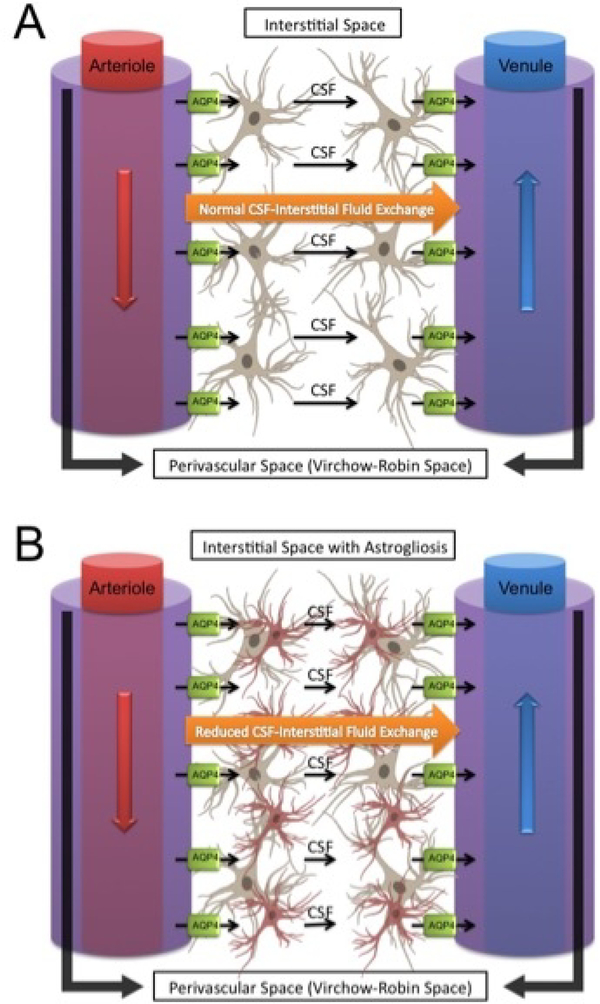
While the central nervous system lacks a conventional lymphatic system, evidence suggests the presence of a functional waste clearance pathway involving exchange between CSF and interstitial fluid, occurring mostly within perivascular Virchow-Robin spaces in the brain parenchyma (Iliff and Nedergaard 2013; Iliff et al. 2012; Jessen et al. 2015). This exchange system is called the glymphatic system for its lymphatic-like function and dependence upon glial cells (Figure 1). Cerebral arteries at the cortical surface extend into pial arteries running through the subarachnoid space and subpial space, which turn into arterioles surrounded by astrocyte end-feet as they run deeper into the brain parenchyma. The Virchow-Robin space is the CSF containing perivascular space between the astrocyte end-feet and arteriole, with both walls lined by a leptomeningeal cell layer (Kulik et al. 2008; Prince and Ahn 2013; Zhang et al. 1990; Zlokovic 2011). Virchow-Robin spaces along veins lack this leptomeningeal cell layer. Arteriole Virchow-Robin spaces become continuous with the basal lamina, which has minimal resistance to CSF flow due to its loosely structured extracellular matrix (ECM). CSF flows along arteriole Virchow-Robin space, through basal lamina surrounding capillaries, and exits through the venous Virchow-Robin space. Arterial pulsation is the main force driving perivascular fluid bulk movement from the subarachnoid space into the Virchow-Robin spaces; although respiration, slow vasomotion, and CSF pressure gradients play minor roles too (Iliff and Nedergaard 2013; Iliff et al. 2012; Jessen et al. 2015). Astrocyte end-feet have high expression of aquaporin 4 and are important for CSF exchange with interstitial fluid, since astrocyte end-feet surround perivascular spaces and facilitates water movement across cell membranes down osmotic pressure gradients from periarterial to perivenous spaces. Interstitial fluid then drains into cervical lymph channels from perivenous spaces (Johnston et al. 2004; Murtha et al. 2014).
Figure 1:
Overview of the glymphatic system. Cerebrospinal fluid enters within para-arterial Virchow-Robin spaces in the brain parenchyma and an astroglia-mediated mechanism exchanges cerebrospinal fluid with interstitial fluid and flushes wastes out within para-venous Virchow-Robin spaces (A). Astrogliosis (B) from brain injury possibly disrupts this astroglia-dependent mechanism.
The glymphatic system is particularly important for removing soluble proteins and metabolites from the brain (Rangroo Thrane et al. 2013). Glymphatic-mediated exchange is greatest during sleep, which is thought to be important for removing metabolic waste during the resting state (Xie et al. 2013). In rodent models of Alzheimer’s disease, glymphatic-mediated exchange was reduced by 65% in aquaporin 4 knockout mice, resulting in increased accumulation of β-amyloid plaques (Iliff et al. 2012). In a mouse repeated traumatic brain injury model, glymphatic exchange was reduced at 24 hours after the last injury and persisted for up to 4 weeks, which was attributed to gliosis (Plog et al. 2015). Furthermore, the glymphatic system was significantly impaired after subarachnoid hemorrhage, due to blood clots occluding perivascular spaces, and during ischemic stroke, due to reduced arterial pulsations (Gaberel et al. 2014). The glymphatic hypothesis, however, has been challenged by a few groups who identified a few shortcomings with the model, which need to be taken into consideration (Abbott et al. 2018; Smith and Verkman 2018). For instance, it is not entirely clear the role aquaporin-4 plays in interstitial fluid flow and no evidence has been provided for its ability to transport solutes. In addition, the brain extracellular matrix significantly hinders fluid movement. The brain extracellular space also allows for the diffusion of small and large molecules naturally (Abbott et al. 2018; Smith and Verkman 2018). Regardless, a system in which CSF enters perivascular arterioles, diffuses with the brain extracellular space, and is cleared along with interstitial fluid and waste products through perivascular venules does have a presence and warrants further investigation. More research is further elucidating the pathophysiological role the glymphatic system plays in multiple neurodegenerative diseases and injuries, and this system may be particularly important in neonatal GMH and consequent PHH pathophysiology due to the role it plays in CSF dynamics.
Hydrocephalus
The International Hydrocephalus Imaging Working Group defines hydrocephalus as “an active distension of the ventricular system resulting from inadequate passage of cerebrospinal fluid from its point of production within the cerebral ventricles to its point of absorption into the systemic circulation” (Rekate 2008). Clinical consequences can include increased intracranial pressure, seizures, mental deterioration, tunnel vision, gait disturbance, headaches, mental impairment, urinary incontinence, dementia, vomiting, and nausea. Most treatments involve surgical implantation of shunts that divert CSF from the brain or surgery, if possible, to repair any malformations that contribute towards hydrocephalus development (Kahle et al. 2015). Dr. Dandy and Dr. Blackfan classified hydrocephalus into communicating and non-communicating hydrocephalus in 1914 after inducing hydrocephalus in dogs by obstructing the foramen of Monro (Dandy 1914). They proposed the bulk flow theory, which states that CSF flows in bulk from the sites of production in the ventricles to the sites of reabsorption in the subarachnoid space. Hydrocephalus, according to bulk flow theory, had to result from an imbalance in CSF production and absorption. Using the same conceptual framework, Dr. Russell proposed a more specific classification of hydrocephalus in 1949 into non-obstructive and obstructive hydrocephalus, which corresponds to communicating and non-communicating hydrocephalus, respectively (Russell 1949). The original terms proposed by Dr. Dandy, however, remain the most pervasively utilized. Advancements involving CSF tracers and imaging technology, however, has produced evidence challenging the bulk flow theory (Symss and Oi 2013). New hydrocephalus classifications have been proposed based on more recent experimental and clinical evidence, which will be discussed.
Non-communicating Hydrocephalus
Hydrocephalus resulting from an obstruction of CSF flow through ventricular and subarachnoid spaces is called non-communicating hydrocephalus, also known as obstructive hydrocephalus (Kahle et al. 2015; McAllister 2012). Non-communicating hydrocephalus is typically caused by congenital cerebral malformations. Arnold-Chiari malformation, which is the displacement of the cerebellar tonsils through the foramen magnum, often obstructs the fourth ventricle, leading to dilation of the lateral ventricles and cerebral aqueduct (Gardner 1965). Dandy-Walker malformations, characterized by the absence of the cerebellar vermis, often obstruct the foramina of Luschka and foramen of Magendie, resulting in prominent dilation of the fourth ventricle (Hirsch et al. 1984). Colloid cysts may obstruct the Foramen of Monro, resulting in lateral ventricular dilation (Camacho et al. 1989). Other lesions may cause abhorrent narrowing of the aqueduct of Sylvius, called aqueductal stenosis, resulting in third and lateral ventricular dilation.
Communicating Hydrocephalus
Communicating hydrocephalus is impaired CSF reabsorption in the absence of any obstruction to CSF flow through the ventricles and subarachnoid spaces (Kahle et al. 2015; McAllister 2012). Communicating hydrocephalus was believed to primarily result from impaired arachnoid granulations, resulting in reduced reabsorption of CSF. Indeed, cerebral malformations resulting in the absence of arachnoid villi has resulted in hydrocephalus development (Gutierrez et al. 1975). Subarachnoid hemorrhage and intraventricular hemorrhage, which induce inflammation and glial scarring in the subarachnoid space, can cause communicating hydrocephalus as well (Korobkin 1975; Vassilouthis and Richardson 1979). Accumulating evidence, however, challenges the presumption that CSF is mostly absorbed by subarachnoid villi (Greitz 2004; Oreskovic and Klarica 2011). Normal pressure hydrocephalus is a form of communicating hydrocephalus that results in ventriculomegaly without increased CSF pressure. CSF pressure readings are within normal range because ventricular dilation compensates for accumulated CSF in the ventricles, thus increased CSF pressure is compensated by increased ventricular volume in this pressure-volume compensatory relationship (Black and Ingraham 2008). In general, the elderly population is most vulnerable to normal pressure hydrocephalus, and causes are either idiopathic or related to other central nervous system diseases and injuries, particularly subarachnoid hemorrhaging. Hydrocephalus ex vacuo is different from normal pressure hydrocephalus because ventricular dilation results from brain tissue atrophy, usually due to a neurodegenerative disorder, and not as a compensatory mechanism for increased CSF pressure (Rekate 2009). Normal pressure hydrocephalus, however, seems to contradict bulk flow theory, because the ventricles should not dilate without increased mean CSF pressure, although differing pressure waveforms may lead to hydrocephalus despite average pressures remaining the same.
Current Hydrodynamic Theory
Although bulk flow theory is congruent with non-communicating hydrocephalus development, when a ventricular obstruction creates back pressure that dilates the ventricles preceding the obstruction, it is incongruent with communicating hydrocephalus, because the apparent obstruction is within the subarachnoid space, which does not dilate or increase in volume (Greitz 2004; Oreskovic and Klarica 2011). In 1914, Dr. Weed injected Prussian blue into the ventricles of dog and cat brains and found the dye accumulated near pacchonian bodies (Weed 1914). Prussian blue, however, was also found in other brain parenchymal areas, and further research concluded Prussian blue cannot cross pacchonian bodies under normal conditions (Symss and Oi 2013). Even Dr. Dandy recognized reduced bulk flow across pacchonian bodies should result in subarachnoid CSF pressure being greater than ventricular CSF pressure and the subarachnoid space should expand before the ventricles, neither of which is observed. Dr. Dandy concluded CSF is primarily reabsorbed in the subarachnoid space and quickly enters the circulatory system, based on intrathecal dye injections that rapidly entered the blood and urine (Dandy 1929). The idea that CSF is mostly reabsorbed at pacchonian bodies, however, remained pervasive. In 1960, Dr. Welch reported pacchionian bodies could act as mechanical valves, although future anatomical studies found no mechanical valve presence (Welch and Friedman 1960). Dr. Di Chiro started experimenting with radionuclide cisternography and, in 1966, suggested CSF was reabsorbed at pacchionian bodies because radionuclide accumulated there after 24 hours (Di Chiro 1966). Future studies, however, challenged this conclusion since other radionuclides enter the circulatory system within minutes and most are reabsorbed in the spinal canal (Greitz 1993; Greitz et al. 1997; Greitz and Hannerz 1996). Furthermore, sites where radionuclides accumulate after a long period of time may indicate sites where CSF reabsorption is actually very limited. A radionuclide cisternography study in patients with venous vasculitis and high intracranial pressure, performed by Dr. Greitz and Dr. Hannerz in 1996, found no tracer in vessel outlets near capillary beds of pacchonnian bodies, providing evidence for an alternative site of CSF reabsorption (Greitz and Hannerz 1996). Another major issue is pacchonian bodies are absent in infants and young children, suggesting CSF must be reabsorbed by a different mechanism (Papaiconomou et al. 2002).
Some scientists investigated if abnormal vascular and CSF pulsations may be the root cause for communicating hydrocephalus. In 1943, after observing normal pressure hydrocephalus patients and noting inconsistencies with the bulk flow theory, Dr. O’Connell proposed communicating hydrocephalus may result from increased ventricular pulse pressure (O’Connell 1943). Dr. Bering provided experimental evidence in 1962 that choroid plexus pulsations deliver the means for ventricular enlargement instead of increased mean CSF pressure (Bering 1962). Dr. Bering used a kaolin-induced hydrocephalic dog model and excised the choroid plexus from one lateral ventricle, which resulted in asymmetric ventricular dilation. Increased mean CSF pressure, therefore, could not account for asymmetric ventricular dilation. Dr. Di Rocci provided additional experimental evidence in 1978 in which extreme ventricular pulsation, caused by inflating and deflating a microballon inserted into the lateral ventricles, can produce hydrocephalic ventricular dilation in sheep (Di Rocco et al. 1978). Concurrently, Dr. Guinane in 1977 produced olfactory ventricular dilation, which lacks a choroid plexus, in rabbits by obstructing surrounding subarachnoid spaces with silicone rubber (Guinane 1977). The silicone rubber obstruction decreased subarachnoid arterial and venous compliance as well as increased capillary pulsations. Increased capillary pulsations, therefore, had to generate the force necessary for the observed ventricular dilation. Using magnetic resonance imaging and radionuclide cisternography, Dr. Greitz reported in the early to mid-1990s arterial pulsation and expansion provides the force necessary for CSF pulsatile circulation in both the brain and spinal cord, and arterial compliance is important for keeping capillary and venous pulsation low (Greitz 1993; Greitz 2004; Greitz et al. 1997; Greitz and Hannerz 1996).
According to bulk flow theory in which CSF malabsorption is a causative factor for communicating hydrocephalus, the subarachnoid CSF-venous pressure gradient would increase, the subarachnoid space would expand, and the ventricles would dilate after subarachnoid space compliance is at maximum. In actuality, the subarachnoid space is smaller, and the subarachnoid CSF-venous pressure gradient is diminished, although both the subarachnoid CSF pressure and venous pressure increase. In 2002, Dr. Egnor developed a mathematical model of communicating hydrocephalus caused by a redistribution of CSF pulsations in the brain (Egnor et al. 2002). Decreased intracranial compliance causes abnormal distribution of vascular pulsations, such that arterial pulsations are weaker while capillary and venous pulsations are stronger, and stronger pulsations reach the ventricles while weaker pulsations reach the subarachnoid space. Thus, this vascular pulsation redistribution causes the ventricles to expand at the expense of the subarachnoid space and decreases the subarachnoid CSF-venous pressure gradient. Dr. Edgor’s model, based on alternating current electric circuitry, accounted for experimentally and clinically observed CSF malabsorption, increased resistive index, ventricular dilation, intracranial pressure waves, reduced cerebral blood flow, and diminished CSF-venous pressure gradient. Dr. Greitz elaborated on this concept in 2004 in his discussion of hydrodynamic theory of chronic hydrocephalus development. Reduced intracranial compliance causes decreased arterial pulsations and increased compensatory capillary pulsations, generating transmantle pulsatile stress responsible for hydrocephalus. CSF malabsorption, therefore, is not a causative factor of communicating hydrocephalus but an effect from vascular pulsatile redistribution (Greitz 2004). Dr. Oreskovic further suggests that disruption of Starling forces in the brain parenchymal microvasculature lead to an imbalance in interstitial fluid and CSF exchange, contributing to hydrocephalus development (Oreskovic and Klarica 2011).
In light of our increased understanding of hydrocephalus pathophysiology, Dr. Oi and Dr. Di Rocco proposed a new classification based on the involved pathway: major pathway hydrocephalus and minor pathway hydrocephalus (Oi and Di Rocco 2006). Major pathway hydrocephalus accounts for CSF circulation disruption from the ventricles to the subarachnoid spaces. Major pathway hydrocephalus encompasses most obstructive / non-communicating hydrocephalus cases. Minor pathway hydrocephalus accounts for disruptions in CSF circulation within the subarachnoid space and brain parenchyma. Evidence suggests this pathway is very important for CSF reabsorption in the embryo, fetus, and infants, making it critical for infantile hydrocephalus development (Papaiconomou et al. 2002). Minor pathway hydrocephalus is disruption of CSF flow and reabsorption in newly elucidated channels in the brain parenchyma, which involve deep vascular structures and lymphatic channels (Figure 2). Dr. Nedergaard further characterized this pathway in rodents using in vivo two photon imaging and coined the term “glymphatic system”, since this functional waste clearance pathway involves astroglia and lymphatic-like paravascular channels (Iliff et al. 2012). CSF enters from the subarachnoid space into paravascular artery channels and exchanges with interstitial fluid, which is cleared through paravascular veins. Additional lymphatic channels lining the dural sinuses and meningeal arteries were characterized by Dr. Louveaue and Dr. Aspelund (Aspelund et al. 2015; Louveau et al. 2015). However, it is unclear if these meningeal lymphatic vessels are anatomically connected with the glymphatic system. As the cerebral glymphatic / lymphatic systems are further characterized, more research is warranted on their potential pathophysiological roles played in hydrocephalus development.
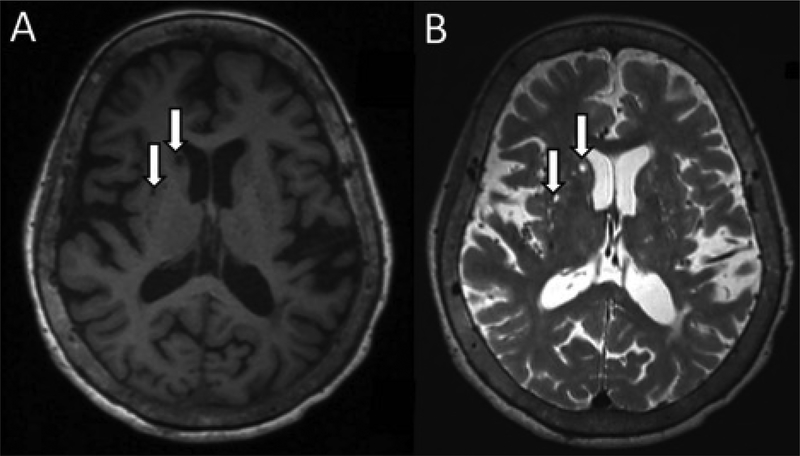
Figure 2:
Axial non-contrasted adult brain MRI demonstrating perivascular spaces (arrows) that appear hypointense to brain tissue and isointense to CSF in T1-weighted (A) and T2-weighted (B) sequences.
Post-hemorrhagic Hydrocephalus Pathophysiology and Potential Mechanisms
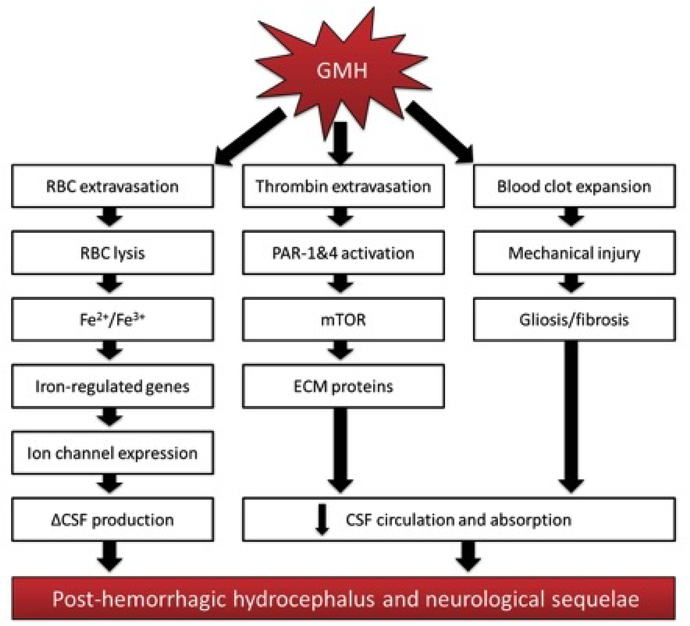
PHH is a common debilitating consequence of severe grade GMH, and the mechanisms contributing to PHH development remain to be elucidated. Cerebroventricular expansion leads to mechanical compression of surrounding brain tissue, causing injury and consequent neurological deficits in patients surviving the initial bleed (Robinson 2012). PHH was commonly theorized to be caused by blood clots obstructing the cerebral aqueduct or foramina of Luschka and Magendie or by microthrombi obstructing small CSF outflow passages in the subarachnoid space. Much evidence suggests a variety of inter-related pathophysiological mechanisms that potentially alter normal CSF dynamics play significant roles in PHH development as well (Strahle et al. 2012; Tang et al. 2016; Whitelaw and Aquilina 2012). Applying concepts in current hydrocephalus theory towards PHH development after GMH may better illuminate potential mechanisms for therapeutic intervention (Figure 3).
Figure 3:
Know pathways and potential mechanisms disrupting cerebrospinal fluid dynamics and contributing to post-hemorrhagic hydrocephalus development after germinal matrix hemorrhage.
Blood Clots, Hemoglobin, and Iron
Non-communicating / obstructive PHH may result from cerebroventricular blood clots and microthrombi directly impairing CSF circulation and absorption by obstructing the cerebral aqueduct, foramina of Luschka and Magendia, and subarachnoid CSF outflow passages. Subsequently, it was hypothesized intraventricular fibrinolytic therapy would remove cerebroventricular blood clots and reduce PHH incidence (Whitelaw and Aquilina 2012). In an adult intraventricular hemorrhage dog model, in which intraventricular blood injection resulted in 80% of dogs developing PHH, intraventricular urokinase injection reduced PHH incidence to 10% (Pang et al. 1986). Clinical investigations of intraventricular streptokinase, urokinase, or tissue plasminogen activator injections after GMH, however, concluded fibrinolytic therapy did not improve long-term dependence on ventriculo-peritoneal shunts (Whitelaw 1993). Thus, cerebroventricular obstruction from thrombi may play only a minor role in long-term PHH development.
Although intraventricular fibrinolytic therapy failed to improve clinical PHH outcomes, evidence suggests hemoglobin and iron may play an important role in PHH development (Strahle et al. 2014). Erythrocyte lysis after hemorrhage, typically from complement activation and consequent membrane attack complex formation, releases hemoglobin and iron into surrounding brain tissue. Experimental adult cerebral hemorrhage models conclude hemoglobin metabolites and iron contributes towards brain edema (Chen et al. 2011). Hemoglobin metabolites were also found in the CSF of rabbit pups with intraventricular hemorrhage, and iron was elevated in the CSF of preterm infants with PHH (Lee et al. 2010; Savman et al. 2001). Intraventricular injection of hemoglobin or iron into neonatal rat pups also resulted in significant acute ventricular dilation (Strahle et al. 2014). Additionally, acute and delayed iron chelation by Deferoxamine reduced long-term PHH development in neonatal rats after GMH (Klebe et al. 2014). Iron, thus, is a quintessential player in PHH formation, although the exact mechanisms remain unclear.
Gene deletion studies determined iron transport and iron-dependent metabolic proteins are highly expressed in the ependymal lining compared to other brain tissue (Keep and Smith 2011). Thus, the ependymal lining may be adversely affected from iron overload due to GMH. Indeed, ependymal cells are theorized to prevent iron diffusion into the brain parenchyma by up-taking it from the CSF (Moos 2002). Additionally, iron overload has been associated with increased expression of aquaporin 4 in adult rats with cerebral hemorrhage, and Deferoxamine treatment reduced aquaporin 4 expression (Qing et al. 2009). Iron, thus, may regulate expression of ependymal ion and water channels, such as aquaporin 4, and contribute towards PHH by altering CSF production dynamics at the ependymal layer. It should be noted, however, that combinatorial furosemide and acetazolamide diuretic treatments targeting choroid plexus epithelial transport were evaluated in clinical trials of preterm GMH patients and determined to have no clinical benefit, although other diuretics have been recommended for further investigation (Whitelaw et al. 2001). More research is needed to further elucidate iron’s pathophysiological role in development of hydrocephalus.
Inflammation, Fibrosis, and Gliosis
Inflammation has been associated with subependymal gliosis, fibrosing arachnoiditis, and meningeal fibrosis after GMH (Cherian et al. 2004b; Oi and Di Rocco 2006). GMH patients also have increased expression levels of inflammatory markers in their CSF, including TNF-α (Savman et al. 2002). Vessel rupture results in blood and serum components entering the brain parenchyma. Resident immune cells, namely microglia, are activated by stimulating toll-like receptors and nod-like receptors with damage-associated molecular patterns, molecules that induce a non-infectious inflammatory response (Klebe et al. 2015). Activated microglia secrete pro-inflammatory cytokines, extracellular proteases, and oxidative species, which damage surrounding tissue and recruit leukocytes that exacerbate inflammation (Chen et al. 2015; Yang et al. 2015). In neonatal rat pups with GMH, microglia proliferation was observed in the perihematoma region, microglia activation was associated with phosphorylated ERK, and modulating microglia activation with minocycline or cannabinoid receptor 2 agonist ameliorate inflammation and improved outcomes (Tang et al. 2015a; Tang et al. 2015b). Additionally, in an IVH adult rat model, IVH caused a TLR4 and NF-κB-dependent inflammatory response in the choroid epithelium, causing an up-to 3-fold increase in CSF production and consequent PHH (Karimy et al. 2017). In the same study, genetic depletion of TLR4 or SPAK as well as pharmacological inhibition of TLR4-NF-κB or SPAK-NKCC1 signaling ameliorated excess CSF production in the choroid epithelium and attenuated PHH. Interestingly, microglia may play an important role in hematoma resolution, since stimulating PPARγ improved short-term hematoma resolution, which was dependent upon CD36 scavenger receptor and was associated with inducing the alternatively activated M2 microglia/macrophage phenotype (Flores et al. 2016).
Fibrosis is the forming of excess connective tissue as a consequence of a reparative process after inflammation (Birbrair et al. 2014). Excess fibrous tissue formation may disrupt the normal functioning of surrounding tissue. Multiple factors trigger fibrosis after GMH. Thrombin, which is significantly active up to 10 days after GMH in neonatal rats, cleaves fibrinogen into fibrin to form fibrin clots, activates the complement pathway to augment inflammation, and stimulates protease-activated receptors (PARs), a family of G protein-coupled receptors (Babu et al. 2012; Lekic et al. 2015; Luo et al. 2007). PAR stimulation has been associated with fibrosis in several tissues, including liver, renal, pulmonary, and cardiac tissues. PAR stimulation upregulates mammalian target of rapamycin (mTOR), which is associated with ECM protein proliferation. Additionally, PAR stimulation exacerbates inflammation by upregulating cyclooxygenase 1 and 2 activity (Kataoka et al. 2003; Luo et al. 2007; Steinhoff et al. 2005). Phosphorylated mTOR and cyclo-oxygenase 2 levels were increased by 72 hours after GMH in rats, which were both reduced by combinatorial PAR-1,4 inhibitor administration (Lekic et al. 2015). ECM proteins are theorized to deposit within the cerebroventricular system, similar to blood clots and microthrombi (Bowen et al. 2013; Strahle et al. 2012; Tang et al. 2016). ECM protein overproduction, therefore, may obstruct normal CSF flow pathways. Indeed, fibronectin and vitronectin expression levels are significantly increased in GMH rats with long-term PHH (Klebe et al. 2014; Manaenko et al. 2014). Inhibiting mTOR with rapamycin and inhibiting cyclo-oxygenase 2 activity ameliorated long-term PHH and neurocognitive deficits in GMH rats, although expression levels of ECM proteins was not determined in this study (Lekic et al. 2015).
TGF-β stimulates mesenchymal stem cells and fibroblasts, which produce ECM matrix proteins and deposit connective tissue (Bowen et al. 2013). TGF-β can be secreted from activated microglia, and TGF-β secretion can be induced by thrombin (Schuliga 2015). ECM production induced by TGF-β stimulation may deposit in the cerebroventricular system, disrupting CSF dynamics (Tada et al. 1994). A rabbit pup GMH model indicated TGF-β, fibronectin, and laminin expression levels were significantly increased in the ependymal and subependyma tissue after GMH (Cherian et al. 2004a). Mice with transgenic TGF-β overexpression developed hydrocephalus with higher expression of ECM proteins in the brain than wild-types (Wyss-Coray et al. 1995). In a clinical study, increased TGF-β1 and ECM protein expression in the CSF were associated with PHH development in preterm infants (Aquilina et al. 2012; Douglas-Escobar and Weiss 2012). The TGF-β1 isoform is most associated with PHH after IVH in neonates and adults (Gomes et al. 2005). Intrathecal TGF-β1 injection in mice resulted in hydrocephalus development, and TGF-β1 expression was significantly increased in brains of neonatal rats with PHVD after intraventricular blood injection (Cherian et al. 2004a; Tada et al. 1994). Indeed, TGF-β1 was elevated in both animal models and premature infants with PHH, although some studies dispute this (Heep et al. 2004). In a rat GMH model, TGF-β1 was elevated within hours after GMH, but normalized by 24 hours post-ictus (Tang et al. 2015a). Additionally, inhibiting TGF-β1 ameliorated long-term PHH and neurocognitive deficits as well as reduced vitronectin and GFAP expression in rats (Manaenko et al. 2014). Although the mechanism of TGF-β signaling after GMH and its association with PHH development has been established, studies are lacking that discern the changes to CSF dynamics as a consequence of TGF-β signaling and fibrosis.
Gliosis results from damage to the central nervous system and is characterized by the nonspecific reactive proliferation of astrocytes, microglia, and oligodendrocytes (Sofroniew 2009). Hydrocephalus development is also associated with neuroinflammation and reactive gliosis (Del Bigio et al. 2003; Deren et al. 2009). Gliosis was observed in cerebral cortical biopsies from hydrocephalic children with shunts (Glees and Hasan 1990). Increased expression of Iba-1 and GFAP, markers for microglia and astrocytes respectively, were also observed in the brains of neonatal rats with hydrocephalus (Deren et al. 2010). Reactive gliosis in the subarachnoid space was associated with hydrocephalus development after subarachnoid hemorrhage in rats. In an IVH rat model, long-term GFAP expression is markedly increased, and injection of umbilical cord blood-derived mesenchymal stem cells reduced GFAP expression as well as long-term PHH development (Ahn et al. 2013). Aquaporin 4 knockout mice more rapidly developed hydrocephalus after kaolin injection, although increased aquaporin 4 expression is observed in hydrocephalus too (Bloch et al. 2006; Mao et al. 2006). Aquaporin 1 is expressed on the choroid plexus apical membrane and aquaporin 1 knockout mice had decreased CSF production (Oshio et al. 2005). Given the important role astrocytes play in the blood-brain barrier function as well as in glymphatic mediated CSF-interstitial fluid exchange, gliosis may have a profound effect on CSF dynamics and PHH development, which warrants further investigation.
Conclusions
Our understanding of hydrocephalus has changed significantly since Dr. Dandy’s first experiments in the early 20th century and the bulk flow theory was proposal. CSF is produced by the choroid plexus epithelial lining and, to a minimal extent, the cerebral ventricular ependymal lining, and, following glymphatic mediated CSF-interstitial fluid exchange, CSF outflow occurs through perivascular channels, meningeal lymph vessels, spinal nerve roots, and the cribriform plate. Our purpose is to reconcile our knowledge of GMH and PHH development with the current hydrodynamic theory of hydrocephalus. PHH after GMH may be obstructive, non-communicating hydrocephalus during the acute phase due to the hematoma, but generally develops as chronic communicating hydrocephalus into adolescence and adulthood. Our proposed mechanisms explore the latter. Indeed, many GMH/IVH studies suggest PHH is a consequence of obstructions within the cerebroventricular system and subarachnoid drainage pathways due to thrombi, gliosis, and fibrosis. In line with current hydrocephalus school of thought, we suggest thrombi, gliosis, and fibrosis after GMH are not merely obstructing CSF passages but are altering barrier dynamics in the microvasculature and ependymal lining, altering CSF dynamics and CSF-interstitial fluid exchange to cause PHH development. Future research should elucidate these potential mechanisms.
Significance Statement.
Germinal matrix hemorrhage (GMH) is a leading cause of morbidity and mortality in preterm and very low birthweight infants. A common long-term consequence of GMH is hydrocephalus. This comprehensive review discusses cerebrospinal fluid dynamics, our current knowledge of hydrocephalus development and GMH pathophysiology, and proposes new mechanisms that warrant further investigation.
Footnotes
Conflict of Interests
The authors have no conflict of interests to disclose
References
- Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. 2018. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135(3):387–407. [DOI] [PubMed] [Google Scholar]
- Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, Oh WI, Park WS. 2013. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke; a journal of cerebral circulation 44(2):497–504. [DOI] [PubMed] [Google Scholar]
- Alderliesten T, Lemmers PM, Smarius JJ, van de Vosse RE, Baerts W, van Bel F. 2013. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop periintraventricular hemorrhage. The Journal of pediatrics 162(4):698–704 e692. [DOI] [PubMed] [Google Scholar]
- Aquilina K, Chakkarapani E, Thoresen M. 2012. Early deterioration of cerebrospinal fluid dynamics in a neonatal piglet model of intraventricular hemorrhage and posthemorrhagic ventricular dilation. Journal of neurosurgery Pediatrics 10(6):529–537. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. 2015. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine 212(7):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu R, Bagley JH, Di C, Friedman AH, Adamson C. 2012. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurgical focus 32(4):E8. [DOI] [PubMed] [Google Scholar]
- Ballabh P 2010. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research 67(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P 2014. Pathogenesis and prevention of intraventricular hemorrhage. Clinics in perinatology 41(1):47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. 2004. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatric research 56(1):117–124. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. 2007. Angiogenic inhibition reduces germinal matrix hemorrhage. Nature medicine 13(4):477–485. [DOI] [PubMed] [Google Scholar]
- Bering EA Jr. 1962. Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. Journal of neurosurgery 19:405–413. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. 2014. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem cell research & therapy 5(6):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P, Ingraham FD. 2008. Normal pressure hydrocephalus. Progress in Clinical Neurosciences 22:289. [Google Scholar]
- Bloch O, Auguste KI, Manley GT, Verkman AS. 2006. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 26(12):1527–1537. [DOI] [PubMed] [Google Scholar]
- Bowen T, Jenkins RH, Fraser DJ. 2013. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. The Journal of pathology 229(2):274–285. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Cserr HF, Westrop RJ. 1981. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. The American journal of physiology 240(4):F329–336. [DOI] [PubMed] [Google Scholar]
- Bradley KC. 1970. Cerebrospinal fluid pressure. Journal of neurology, neurosurgery, and psychiatry 33(3):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley JB, Field EJ. 1948. The connexions of the spinal sub-arachnoid space with the lymphatic system. Journal of anatomy 82(3):153–166. [PubMed] [Google Scholar]
- Bulat M, Klarica M. 2011. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain research reviews 65(2):99–112. [DOI] [PubMed] [Google Scholar]
- Caicedo A, De Smet D, Naulaers G, Ameye L, Vanderhaegen J, Lemmers P, Van Bel F, Van Huffel S. 2011. Cerebral tissue oxygenation and regional oxygen saturation can be used to study cerebral autoregulation in prematurely born infants. Pediatric research 69(6):548–553. [DOI] [PubMed] [Google Scholar]
- Camacho A, Abernathey CD, Kelly PJ, Laws ER Jr. 1989. Colloid cysts: experience with the management of 84 cases since the introduction of computed tomography. Neurosurgery 24(5):693–700. [DOI] [PubMed] [Google Scholar]
- Chakravarthi A 2012. Cerebrospinal Fluid Dynamics. Textbook of Contemporary Neurosurgery (Volumes 1 & 2). [Google Scholar]
- Chen S, Yang Q, Chen G, Zhang JH. 2015. An update on inflammation in the acute phase of intracerebral hemorrhage. Translational stroke research 6(1):4–8. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. 2011. Role of iron in brain injury after intraventricular hemorrhage. Stroke; a journal of cerebral circulation 42(2):465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian S, Thoresen M, Silver IA, Whitelaw A, Love S. 2004a. Transforming growth factorbetas in a rat model of neonatal posthaemorrhagic hydrocephalus. Neuropathology and applied neurobiology 30(6):585–600. [DOI] [PubMed] [Google Scholar]
- Cherian S, Whitelaw A, Thoresen M, Love S. 2004b. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14(3):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtice FC, Simmonds WJ. 1951. The removal of protein from the subarachnoid space. The Australian journal of experimental biology and medical science 29(4):255–263. [DOI] [PubMed] [Google Scholar]
- Cserr HF, DePasquale M, Harling-Berg CJ, Park JT, Knopf PM. 1992. Afferent and efferent arms of the humoral immune response to CSF-administered albumins in a rat model with normal blood-brain barrier permeability. Journal of neuroimmunology 41(2):195–202. [DOI] [PubMed] [Google Scholar]
- Cutler RW, Page L, Galicich J, Watters GV. 1968. Formation and absorption of cerebrospinal fluid in man. Brain : a journal of neurology 91(4):707–720. [DOI] [PubMed] [Google Scholar]
- Dandy WE. 1914. Internal hydrocephalus. An experimental, clinical and pathological study. Am J Dis Child 8:406–482. [Google Scholar]
- Dandy WE. 1929. Where is cerebrospinal fluid absorbed? Journal of the American Medical Association 92(24):2012–2014. [Google Scholar]
- Davis DA, Milhorat TH. 1975. The blood-brain barrier of the rat choroid plexus. The Anatomical record 181(4):779–789. [DOI] [PubMed] [Google Scholar]
- Davson H, Segal MB. 1970. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. The Journal of physiology 209(1):131–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR, Wilson MJ, Enno T. 2003. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Annals of neurology 53(3):337–346. [DOI] [PubMed] [Google Scholar]
- Deren KE, Forsyth J, Abdullah O, Hsu EW, Klinge PM, Silverberg GD, Johanson CE, McAllister JP 2nd. 2009. Low levels of amyloid-beta and its transporters in neonatal rats with and without hydrocephalus. Cerebrospinal fluid research 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deren KE, Packer M, Forsyth J, Milash B, Abdullah OM, Hsu EW, McAllister JP 2nd. 2010. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Experimental neurology 226(1):110–119. [DOI] [PubMed] [Google Scholar]
- Di Chiro G 1966. Observations on the circulation of the cerebrospinal fluid. Acta radiologica: diagnosis 5:988–1002. [DOI] [PubMed] [Google Scholar]
- Di Rocco C, Pettorossi VE, Caldarelli M, Mancinelli R, Velardi F. 1978. Communicating hydrocephalus induced by mechanically increased amplitude of the intraventricular cerebrospinal fluid pressure: experimental studies. Experimental neurology 59(1):40–52. [DOI] [PubMed] [Google Scholar]
- Dichiro G 1964. Movement of the Cerebrospinal Fluid in Human Beings. Nature 204:290–291. [DOI] [PubMed] [Google Scholar]
- Douglas-Escobar M, Weiss MD. 2012. Biomarkers of brain injury in the premature infant. Frontiers in neurology 3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis AJ. 2008. Cerebrovascular injury in premature infants: current understanding and challenges for future prevention. Clinics in perinatology 35(4):609–641, v. [DOI] [PubMed] [Google Scholar]
- Egnor M, Zheng L, Rosiello A, Gutman F, Davis R. 2002. A model of pulsations in communicating hydrocephalus. Pediatric neurosurgery 36(6):281–303. [DOI] [PubMed] [Google Scholar]
- Erlich SS, McComb JG, Hyman S, Weiss MH. 1986. Ultrastructural morphology of the olfactory pathway for cerebrospinal fluid drainage in the rabbit. Journal of neurosurgery 64(3):466473. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Mayhan WG, Heistad DD. 1990. Effect of vasopressin on production of cerebrospinal fluid: possible role of vasopressin (V1)-receptors. The American journal of physiology 258(1 Pt 2):R94–98. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K 1974. Protein size and cerebrospinal fluid composition. Klinische Wochenschrift 52(24):1158–1164. [DOI] [PubMed] [Google Scholar]
- Flores JJ, Klebe D, Rolland WB, Lekic T, Krafft PR, Zhang JH. 2016. PPARgamma-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiology of disease 87:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M. 2014. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke; a journal of cerebral circulation 45(10):3092–3096. [DOI] [PubMed] [Google Scholar]
- Gardner W 1965. Arnold-Chiari malformation and hydrocephalus. J of Neurology, Neurosurgery and Psychiatry 28:247. [Google Scholar]
- Glees P, Hasan M. 1990. Ultrastructure of human cerebral macroglia and microglia: maturing and hydrocephalic frontal cortex. Neurosurgical review 13(3):231–242. [DOI] [PubMed] [Google Scholar]
- Gomes FC, Sousa Vde O, Romao L. 2005. Emerging roles for TGF-beta1 in nervous system development. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 23(5):413–424. [DOI] [PubMed] [Google Scholar]
- Greitz D 1993. Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta radiologica Supplementum 386:1–23. [PubMed] [Google Scholar]
- Greitz D 2004. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurgical review 27(3):145–165; discussion 166–147. [DOI] [PubMed] [Google Scholar]
- Greitz D, Greitz T, Hindmarsh T. 1997. A new view on the CSF-circulation with the potential for pharmacological treatment of childhood hydrocephalus. Acta Paediatr 86(2):125–132. [DOI] [PubMed] [Google Scholar]
- Greitz D, Hannerz J. 1996. A proposed model of cerebrospinal fluid circulation: observations with radionuclide cisternography. AJNR American journal of neuroradiology 17(3):431–438. [PMC free article] [PubMed] [Google Scholar]
- Guinane JE. 1977. Why does hydrocephalus progress? Journal of the neurological sciences 32(1):1–8. [DOI] [PubMed] [Google Scholar]
- Gutierrez Y, Friede RL, Kaliney WJ. 1975. Agenesis of arachnoid granulations and its relationship to communicating hydrocephalus. Journal of neurosurgery 43(5):553–558. [DOI] [PubMed] [Google Scholar]
- Heep A, Stoffel-Wagner B, Bartmann P, Benseler S, Schaller C, Groneck P, Obladen M, Felderhoff-Mueser U. 2004. Vascular endothelial growth factor and transforming growth factor-beta1 are highly expressed in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus. Pediatric research 56(5):768–774. [DOI] [PubMed] [Google Scholar]
- Hefti MM, Trachtenberg FL, Haynes RL, Hassett C, Volpe JJ, Kinney HC. 2015. A Century of Germinal Matrix Intraventricular Hemorrhage in Autopsied Premature Infants: A Historical Account. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. [DOI] [PubMed] [Google Scholar]
- Hirsch J-F, Pierre-Kahn A, Renier D, Sainte-Rose C, Hoppe-Hirsch E. 1984. The Dandy-Walker malformation: a review of 40 cases. Journal of neurosurgery 61(3):515–522. [DOI] [PubMed] [Google Scholar]
- Hladky SB, Barrand MA. 2014. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids and barriers of the CNS 11(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky SB, Barrand MA. 2016. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids and barriers of the CNS 13(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Goldman SA, Nedergaard M. 2015. Implications of the discovery of brain lymphatic pathways. Lancet Neurol 14(10):977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Nedergaard M. 2013. Is there a cerebral lymphatic system? Stroke; a journal of cerebral circulation 44(6 Suppl 1):S93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine 4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. 2015. The Glymphatic System: A Beginner’s Guide. Neurochemical research 40(12):2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Stopa EG, McMillan PN. 2011. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol 686:101–131. [DOI] [PubMed] [Google Scholar]
- Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. 2004. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, nonhuman primates and other mammalian species. Cerebrospinal fluid research 1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Kulkarni AV, Limbrick DD Jr., Warf BC. 2015. Hydrocephalus in children. Lancet. [DOI] [PubMed] [Google Scholar]
- Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, Furey CG, Zhou X, Mansuri MS, Montejo J, Vera A, DiLuna ML, Delpire E, Alper SL, Gunel M, Gerzanich V, Medzhitov R, Simard JM, Kahle KT. 2017. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nature medicine 23(8):997–1003. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR. 2003. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 102(9):3224–3231. [DOI] [PubMed] [Google Scholar]
- Keep RF, Jones HC. 1990. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain research Developmental brain research 56(1):47–53. [DOI] [PubMed] [Google Scholar]
- Keep RF, Smith DE. 2011. Choroid plexus transport: gene deletion studies. Fluids and barriers of the CNS 8(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Pantazis A, Weller RO. 1993. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathology and applied neurobiology 19(6):480–488. [DOI] [PubMed] [Google Scholar]
- Klebe D, Krafft PR, Hoffmann C, Lekic T, Flores JJ, Rolland W, Zhang JH. 2014. Acute and delayed deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke; a journal of cerebral circulation 45(8):2475–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe D, McBride D, Flores JJ, Zhang JH, Tang J. 2015. Modulating the Immune Response Towards a Neuroregenerative Peri-injury Milieu After Cerebral Hemorrhage. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 10(4):576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek KD, Kirmeyer SE, Martin JA, Strobino DM, Guyer B. 2012. Annual summary of vital statistics: 2009. Pediatrics 129(2):338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobkin R 1975. The relationship between head circumference and the development of communicating hydrocephalus in infants following intraventricular hemorrhage. Pediatrics 56(1):74–77. [PubMed] [Google Scholar]
- Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. 2008. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology 55(3):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. 2010. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30(11):1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic T, Klebe D, McBride DW, Manaenko A, Rolland WB, Flores JJ, Altay O, Tang J, Zhang JH. 2015. Protease-activated receptor 1 and 4 signal inhibition reduces preterm neonatal hemorrhagic brain injury. Stroke; a journal of cerebral circulation 46(6):1710–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wang Y, Reiser G. 2007. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain research reviews 56(2):331–345. [DOI] [PubMed] [Google Scholar]
- Maktabi MA, Heistad DD, Faraci FM. 1991. Effects of central and intravascular angiotensin I and II on the choroid plexus. The American journal of physiology 261(5 Pt 2):R1126–1132. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Lekic T, Barnhart M, Hartman R, Zhang JH. 2014. Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke; a journal of cerebral circulation 45(3):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Enno TL, Del Bigio MR. 2006. Aquaporin 4 changes in rat brain with severe hydrocephalus. The European journal of neuroscience 23(11):2929–2936. [DOI] [PubMed] [Google Scholar]
- McAllister JP 2nd. 2012. Pathophysiology of congenital and neonatal hydrocephalus. Seminars in fetal & neonatal medicine 17(5):285–294. [DOI] [PubMed] [Google Scholar]
- Milhorat TH. 1975. The third circulation revisited. Journal of neurosurgery 42(6):628–645. [DOI] [PubMed] [Google Scholar]
- Milhorat TH. 1976. Structure and function of the choroid plexus and other sites of cerebrospinal fluid formation. International review of cytology 47:225–288. [DOI] [PubMed] [Google Scholar]
- Milhorat TH. 1978. Pediatric neurosurgery. Contemporary neurology series 16:1–389. [PubMed] [Google Scholar]
- Milhorat TH, Davis DA, Hammock MK. 1975. Localization of ouabain-sensitive Na-K-ATPase in frog, rabbit and rat choroid plexus. Brain research 99(1):170–174. [DOI] [PubMed] [Google Scholar]
- Mollanji R, Bozanovic-Sosic R, Zakharov A, Makarian L, Johnston MG. 2002. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. American journal of physiology Regulatory, integrative and comparative physiology 282(6):R1593–1599. [DOI] [PubMed] [Google Scholar]
- Moos T 2002. Brain iron homeostasis. Danish medical bulletin 49(4):279–301. [PubMed] [Google Scholar]
- Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, McLeod DD. 2014. Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids and barriers of the CNS 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JE. 1943. The vascular factor in intracranial pressure and the maintenance of the cerebrospinal fluid circulation. Brain : a journal of neurology 66(3):204–228. [Google Scholar]
- Oi S, Di Rocco C. 2006. Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 22(7):662669. [DOI] [PubMed] [Google Scholar]
- Oreskovic D, Klarica M. 2010. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain research reviews 64(2):241–262. [DOI] [PubMed] [Google Scholar]
- Oreskovic D, Klarica M. 2011. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: facts and illusions. Progress in neurobiology 94(3):238–258. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. 2005. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 19(1):76–78. [DOI] [PubMed] [Google Scholar]
- Osterman MJ, Kochanek KD, MacDorman MF, Strobino DM, Guyer B. 2015. Annual summary of vital statistics: 2012–2013. Pediatrics 135(6):1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, Sclabassi RJ, Horton JA. 1986. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery 19(4):553–572. [DOI] [PubMed] [Google Scholar]
- Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M. 2002. Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 283(4):R869–R876. [DOI] [PubMed] [Google Scholar]
- Pierce EC Jr., Lambertsen CJ, Deutsch S, Chase PE, Linde HW, Dripps RD, Price HL. 1962. Cerebral circulation and metabolism during thiopental anesthesia and hyper-ventilation in man. The Journal of clinical investigation 41:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. 2015. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. The Journal of neuroscience : the official journal of the Society for Neuroscience 35(2):518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollay M 1975. Formation of cerebrospinal fluid. Relation of studies of isolated choroid plexus to the standing gradient hypothesis. Journal of neurosurgery 42(6):665–673. [DOI] [PubMed] [Google Scholar]
- Pollay M 2010. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal fluid research 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollay M, Curl F. 1967. Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. The American journal of physiology 213(4):1031–1038. [DOI] [PubMed] [Google Scholar]
- Post RM, Allen FH, Ommaya AK. 1974. Cerebrospinal fluid flow and iodide 131 transport in the spinal subarachnoid space. Life sciences 14(10):1885–1894. [DOI] [PubMed] [Google Scholar]
- Prince EA, Ahn SH. 2013. Basic vascular neuroanatomy of the brain and spine: what the general interventional radiologist needs to know. Seminars in interventional radiology 30(3):234239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing WG, Dong YQ, Ping TQ, Lai LG, Fang LD, Min HW, Xia L, Heng PY. 2009. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. Journal of neurosurgery 110(3):462–468. [DOI] [PubMed] [Google Scholar]
- Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, Nagelhus EA, Nedergaard M. 2013. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Scientific reports 3:2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber H 2003. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and sourcerelated dynamics. Restorative neurology and neuroscience 21(3–4):79–96. [PubMed] [Google Scholar]
- Rekate HL. 2008. The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal fluid research 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekate HL. A contemporary definition and classification of hydrocephalus; 2009. Elsevier; p 9–15. [DOI] [PubMed] [Google Scholar]
- Robinson S 2012. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. Journal of neurosurgery Pediatrics 9(3):242–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DS. 1949. Observations on the pathology of hydrocephalus: HM Stationery Office.
- Sahar A 1972. Choroidal origin of cerebrospinal fluid. Israel journal of medical sciences 81(5):594–596. [PubMed] [Google Scholar]
- Sahar A, Hochwald GM, Ransohoff J. 1970. Cerebrospinal fluid and cranial sinus pressures. Relationship in normal and hydrocephalic cats. Archives of neurology 23(5):413–418. [DOI] [PubMed] [Google Scholar]
- Sakka L, Coll G, Chazal J. 2011. Anatomy and physiology of cerebrospinal fluid. European annals of otorhinolaryngology, head and neck diseases 128(6):309–316. [DOI] [PubMed] [Google Scholar]
- Sato O, Bering EA, Jr., Yagi M, Tsugane R, Hara M, Amano Y, Asai T 1975. Bulk flow in the cerebrospinal fluid system of the dog. Acta neurologica Scandinavica 51(1):1–11. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Habgood MD, Dziegielewska KM. 1999. Barrier mechanisms in the brain, I. Adult brain. Clinical and experimental pharmacology & physiology 26(1):11–19. [DOI] [PubMed] [Google Scholar]
- Savman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A. 2002. Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr 91(12):1357–1363. [DOI] [PubMed] [Google Scholar]
- Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. 2001. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatric research 49(2):208–212. [DOI] [PubMed] [Google Scholar]
- Schuliga M 2015. The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediators of inflammation 2015:437695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal MB, Pollay M. 1977. The secretion of cerebrospinal fluid. Experimental eye research 25 Suppl:127–148. [DOI] [PubMed] [Google Scholar]
- Shulman K, Yarnell P, Ransohoff J. 1964. Dural Sinus Pressure. In Normal and Hydrocephalic Dogs. Archives of neurology 10:575–580. [DOI] [PubMed] [Google Scholar]
- Silver I, Li B, Szalai J, Johnston M. 1999. Relationship between intracranial pressure and cervical lymphatic pressure and flow rates in sheep. The American journal of physiology 277(6 Pt 2):R1712–1717. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Verkman AS. 2018. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32(2):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. 2009. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in neurosciences 32(12):638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. 2007. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatric research 61(4):467–473. [DOI] [PubMed] [Google Scholar]
- Speake T, Brown PD. 2004. Ion channels in epithelial cells of the choroid plexus isolated from the lateral ventricle of rat brain. Brain research 1005(1–2):60–66. [DOI] [PubMed] [Google Scholar]
- Spector R, Johanson CE. 1989. The mammalian choroid plexus. Scientific American 261(5):68–74. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. 2005. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocrine reviews 26(1):1–43. [DOI] [PubMed] [Google Scholar]
- Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. 2012. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Translational stroke research 3(Suppl 1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle JM, Garton T, Bazzi AA, Kilaru H, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. 2014. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery 75(6):696–705; discussion 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symss NP, Oi S. 2013. Theories of cerebrospinal fluid dynamics and hydrocephalus: historical trend. Journal of neurosurgery Pediatrics 11(2):170–177. [DOI] [PubMed] [Google Scholar]
- Tada T, Kanaji M, Kobayashi S. 1994. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. Journal of neuroimmunology 50(2):153–158. [DOI] [PubMed] [Google Scholar]
- Tang J, Chen Q, Guo J, Yang L, Tao Y, Li L, Miao H, Feng H, Chen Z, Zhu G. 2015a. Minocycline Attenuates Neonatal Germinal-Matrix-Hemorrhage-Induced Neuroinflammation and Brain Edema by Activating Cannabinoid Receptor 2. Molecular neurobiology. [DOI] [PubMed] [Google Scholar]
- Tang J, Tao Y, Jiang B, Chen Q, Hua F, Zhang J, Zhu G, Chen Z. 2016. Pharmacological Preventions of Brain Injury Following Experimental Germinal Matrix Hemorrhage: an Up-to-Date Review. Translational stroke research 7(1):20–32. [DOI] [PubMed] [Google Scholar]
- Tang J, Tao Y, Tan L, Yang L, Niu Y, Chen Q, Yang Y, Feng H, Chen Z, Zhu G. 2015b. Cannabinoid receptor 2 attenuates microglial accumulation and brain injury following germinal matrix hemorrhage via ERK dephosphorylation in vivo and in vitro. Neuropharmacology 95:424–433. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe JJ. 2000. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106(4):625–632. [DOI] [PubMed] [Google Scholar]
- Tully HM, Dobyns WB. 2014. Infantile hydrocephalus: a review of epidemiology, classification and causes. European journal of medical genetics 57(8):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilouthis J, Richardson AE. 1979. Ventricular dilatation and communicating hydrocephalus following spontaneous subarachnoid hemorrhage. Journal of neurosurgery 51(3):341351. [DOI] [PubMed] [Google Scholar]
- Vassilyadi M, Tataryn Z, Shamji MF, Ventureyra EC. 2009. Functional outcomes among premature infants with intraventricular hemorrhage. Pediatric neurosurgery 45(4):247255. [DOI] [PubMed] [Google Scholar]
- Weed LH. 1914. Studies on Cerebro-Spinal Fluid. No. III : The pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. The Journal of medical research 31(1):51–91. [PMC free article] [PubMed] [Google Scholar]
- Welch K 1975. The principles of physiology of the cerebrospinal fluid in relation to hydrocephalus including normal pressure hydrocephalus. Advances in neurology 13:247332. [PubMed] [Google Scholar]
- Welch K, Friedman V. 1960. The cerebrospinal fluid valves. Brain : a journal of neurology 83:454–469. [DOI] [PubMed] [Google Scholar]
- Whitelaw A 1993. Endogenous fibrinolysis in neonatal cerebrospinal fluid. European journal of pediatrics 152(11):928–930. [DOI] [PubMed] [Google Scholar]
- Whitelaw A, Brion LP, Kennedy CR, Odd D. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database of Systematic Reviews. 2001(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A, Aquilina K. 2012. Management of posthaemorrhagic ventricular dilatation. Archives of disease in childhood Fetal and neonatal edition 97(3):F229–223. [DOI] [PubMed] [Google Scholar]
- Williams B 1976. Cerebrospinal fluid pressure changes in response to coughing. Brain : a journal of neurology 99(2):331–346. [DOI] [PubMed] [Google Scholar]
- Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, Walker AM. 2008. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121(3):e604–611. [DOI] [PubMed] [Google Scholar]
- Wright EM. 1972. Mechanisms of ion transport across the choroid plexus. The Journal of physiology 226(2):545–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. 1995. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. The American journal of pathology 147(1):53–67. [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. 2013. Sleep drives metabolite clearance from the adult brain. Science 342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. 2015. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. Journal of neuroinflammation 12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov A, Papaiconomou C, Djenic J, Midha R, Johnston M. 2003. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathology and applied neurobiology 29(6):563–573. [DOI] [PubMed] [Google Scholar]
- Zervas NT, Liszczak TM, Mayberg MR, Black PM. 1982. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. Journal of neurosurgery 56(4):475–481. [DOI] [PubMed] [Google Scholar]
- Zhang ET, Inman CB, Weller RO. 1990. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. Journal of anatomy 170:111–123. [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. 2011. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews Neuroscience 12(12):723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Segal MB, Davson H, Lipovac MN, Hyman S, McComb JG. 1990. Circulating neuroactive peptides and the blood-brain and blood-cerebrospinal fluid barriers. Endocrinologia experimentalis 24(1–2):9–17. [PubMed] [Google Scholar]