Highlights
-
•
Stranded driftwood feedstocks (SD) were steam exploded and hydrolyzed.
-
•
The enzymatic hydrolysis was optimized using a multivariate approach (RSM).
-
•
The conversion of carbohydrates into lipids by S. terricola was high (YL = 25.26%).
-
•
The fatty acid profile achieved was similar to that reported for palm oil.
-
•
SD feedstocks resulted a cheap C-source for biofuels and biochemicals production.
Abbreviations: TAGs, Tryacylglicerols; FA, fatty acid; C/N, carbon/nitrogen; SD, stranded driftwood; SE, steam explosion; WIS, water insoluble substrate; NREL, National Renewable Energy Laboratory; HPLC, high performance liquid chromatography; RI, refractive index; A600, absorbance at 600 nm; ANOVA, analysis of variance; SFA, saturated fatty acid; UFA, unsaturated fatty acid; UI, unsaturation index; PL, total lipid production; DW, dry weight; PL/DW, % of total intracellular lipid on cellbiomass; YL, lipid yied; PL/d, lipid production per day; C8:0, caprylic acid (octanoic acid); C10:0, capric acid (decanoic acid); C12:0, lauric acid (dodecanoic acid); C14:0, myristic acid (tetradecanoic acid); C16:0, palmitic acid (hexadecanoic acid); Δ9C16:1, palmitoleic acid [(9Z)-hexadec-9-enoic acid]; C18:0, stearic acid (octadecanoic acid); Δ9C18:1, oleic acid [(9E9Z)-octadec-9-enoic acid]; Δ9,12C18:2, linoleic acid [(9Z,12Z)-9,12-octadecadienoic acid]; Δ9,12,15C18:3, linolenic acid [(9Z,12Z,15Z)-9,12,15-octadecatrienoic acid]; C20:0, arachic acid (eicosanoic acid); C22:0, behenic acid (docosanoic acid); Δ13C22:1, erucic acid [(13Z)-docos-13-enoic acid]; C24:0, lignoceric acid (tetracosanoic acid); YPD, Yeast Extract Peptone Dextrose; LF, liquid fraction; RSM, response surface methodology; CCD, Central Composite Design; ED, enzyme dosage; SL, solid loading; FPU, filterpaper unit; CBU, cellobiase unit; HLF, hydrolyzed liquid fraction; Rpm, revolutions per minute; g, gravity force; h, hours; min, minutes; v/v, concentration in volume/volume percent; GC, Gas Chromatography; C6, carbohydrates with six carbon atoms; C5, carbohydrates with five carbon atoms; XG, Xilose and Galactose; GC-FID, Gas Chromatography – Flame Ionization Detector; F.A.M.E., fatty acid methyl ester; Yoleic, oleic acid yield; Eq, equation; p, p-value
Keywords: Stranded driftwood feedstocks, Solicoccozyma terricola, Enzymatic hydrolysis, Yeast biochemicals and biofuels, Response surface methodology
Abstract
Stranded driftwood feedstocks may represent, after pretreatment with steam explosion and enzymatic hydrolysis, a cheap C-source for producing biochemicals and biofuels using oleaginous yeasts. The hydrolysis was optimized using a response surface methodology (RSM). The solid loading (SL) and the dosage of enzyme cocktail (ED) were variated following a central composite design (CCD) aimed at optimizing the conversion of carbohydrates into lipids (YL) by the yeast Solicoccozyma terricola DBVPG 5870.
A second-order polynomial equation was computed for describing the effect of ED and SL on YL.
The best combination (ED = 3.10%; SL = 22.07%) for releasing the optimal concentration of carbohydrates which gave the highest predicted YL (27.32%) was then validated by a new hydrolysis. The resulting value of YL (25.26%) was close to the theoretical maximum value.
Interestingly, fatty acid profile achieved under the optimized conditions was similar to that reported for palm oil.
1. Introduction
Lignocellulosic feedstocks, e.g. agricultural residues and woody wastes, represent more than 90% of global plant biomass. Due to their abundance and high content (up to 75%) of polysaccharides they can be considered as a cheap alternative C-rich substrate for developing microbial processes aimed at producing novel molecules including oleochemicals [1,2].
Stranded driftwood feedstocks are accumulated in large amounts after coastal storms along Mediterranean beaches and represent an increasing problem of disposal management for Europe. More than 200,000 tons of woody material are accumulated on Italian shores every year [3,4]. Current Italian laws classify stranded driftwood feedstocks as non-hazardous wastes. So they are managed as ordinary residual biomass and are disposed into landfills or even burned [5]. However, due to their high cellulosic and hemicellulosic content, stranded driftwood feedstocks could be considered suitable C-rich sources for producing chemicals via microbial processes [[6], [7], [8]]. Since some decades oleaginous yeasts are considered potential converters of carbohydrates into lipids due to their ability to accumulate high amounts of intracellular triacylglycerols (TAGs - above 20% of their dry biomass) under appropriate conditions [[9], [10], [11], [12], [13]].
TAGs from oleaginous yeasts may be used as renewable sources of oleochemicals for fuels, food and cosmetic industry, namely biodiesel, soaps, plastics, paints, detergents, textiles, rubbers, surfactants and lubricants, etc. [14]. Interestingly, TAGs sometimes show fatty acid profiles and technological performances comparable with those exhibited by some vegetable oils [13,15]. According to the literature, one of the main fatty acid produced by oleaginous yeasts is represented by oleic acid. Ideally, oils containing a high portion of monounsaturated fatty acids, such as oleic acid, can be considered good candidates to be used in food and cosmetic industry and especially for the biodiesel production in order to reduce the dependence on fossil fuels [11,[16], [17], [18], [19], [20], [21]].
Nevertheless, due to the inability of most microorganisms including oleaginous yeasts, to directly utilize cellulose and hemicellulose, lignocellulosic biomass, including stranded driftwood feedstocks, need to be pretreated and subsequently hydrolyzed to convert recalcitrant polymers into monomeric carbohydrates [22]. Steam explosion (procedure performed to deconstruct the lignocellulosic portion making it accessible to enzymes) coupled with enzymatic hydrolysis of cellulose and hemicellulose represent crucial steps for approaching the release of simple carbohydrates. So, their optimization could determine a real improvement to the whole process [23].
The economic impact of the enzymatic hydrolysis of cellulose of lignocellulosic feedstocks after steam explosion has been studied since early 2000s. Indeed, although the use of high dosages of cellulolytic enzyme cocktails can enhance both the rate and the yield of the hydrolysis, the cost of the process could be significantly increased [24]. In order to overcome this bottleneck, some authors suggested to use high solid loadings (i.e. the amount of suspended solid feedstock during the hydrolysis of cellulose, which should be ≥15% w/w) to obtain a higher efficiency of the release of both glucose and cellobiose and reducing the production costs, energy demand and water requirement [25,26]. Therefore the choice of the best combination of both dosage of cellulolytic enzyme cocktail and solid loading can be considered a crucial step for optimizing the enzymatic hydrolysis of cellulosic fraction obtained from stranded driftwood feedstocks for lipid production by oleaginous yeasts.
Besides the need to reduce the cost of enzymatic hydrolysis, the achievement of high values of lipid yield (YL, defined as the % of fermentable carbohydrates converted into lipids), which quantifies the efficiency of bioconversion from C-rich substrate into lipid, can increase the economic advantage of producing lipids from oleaginous yeasts. Lipid yield is considered a fundamental parameter affecting the cost of the oil accumulation into microbial cells process [[27], [28], [29]].
Computational methods are increasingly applied in microbial processes to orient experimental planning and design of experiments and, consequently modulate enzymatic and microbial metabolic responses [30,31]. Accordingly, in this study a response surface methodology was used to predict the best combination of two independent variables (i.e. the dosage of cellulolytic enzyme cocktail and the solid loading, both related to the enzymatic hydrolysis of cellulose obtained after steam explosion of stranded driftwood feedstocks) in order to optimize the lipid yield achieved by the oleaginous yeast Solicoccozyma terricola DBVPG 5870, which was previously selected as lipid overproducing strain [13,32].
2. Material and methods
2.1. Chemicals
Unless otherwise specified, all chemicals were from Sigma-Aldrich (Saint Louis, Missouri, USA) while all microbiological media were from Oxoid (Basingstoke, Hampshire, UK).
2.2. Yeast strain
The strain Solicoccozyma terricola DBVPG 5870 was used. It was previously selected on the basis of its superior lipogenic aptitude [13,32]. The strain is preserved under frozen conditions (−80 °C) in the Industrial Yeast Collection DBVPG of the Department of Agricultural, Food and Environmental Sciences, University of Perugia, Italy (http://www.dbvpg.unipg.it). Working cultures were grown on YPD agar (g/l): glucose 20, yeast extract 10, peptone 10, agar 20, pH 6.0.
2.3. Biomass feedstock
Stranded driftwood (SD) feedstocks were gathered in 2015 from the Italian coasts of Adriatic Sea. In order to collect a representative sample, different wood sizes were chosen in a 1000 m2 area. The biomass was preliminarily dried at 40 °C for one week and then chipped down (to increase the surface area of chips enhancing the efficiency of steam explosion - approx. diameter =3 cm) by a cutting mill [33,34].
2.4. Biomass pretreatment: steam explosion
The better combination of parameters of steam explosion (SE) has been preliminarily selected for optimizing the recovery of cellulose. The experimental design and the results are reported in Table S1 and Fig. S1 (Supplementary Material). SE of SD was conducted into a 10 l batch reactor (Biochemtex, Tortona, Italy) at 210 °C for 25 min [4,13]. The combined effect of both temperature and time was summarized by the severity factor LogR0 [35] according to Eq. (1):
| R0 = t × e (T−100)/14.75 | (1) |
where:
- t is the time of residence (sec);
- T is the temperature (°C).
The value of the LogR0 used in the present work was 4.64.
Two different fractions were obtained after SE: i) a solid fraction, otherwise namely water insoluble substrate (WIS) containing mainly cellulose and lignin and ii) a liquid fraction (LF) containing mainly hemicelluloses and some inhibitors. After separation of the two fractions by stainless steel filters (cutoff =1 mm), WIS was washed with water at 50 °C for 30 min using a solid/liquid ratio = 10% [36].
2.5. Enzymatic hydrolysis of WIS and design of experiment
The enzymatic hydrolysis of WIS was the step subjected to the optimization procedure. A response surface methodology (RSM) was used in order to predict the best combination of both the dosage of cellulolytic enzyme cocktail and the solid loading, in order to obtain the best concentration of fermentable carbohydrates able to optimize the lipid yield of S. terricola (YL, expressed as the % of fermentable carbohydrates converted into lipids). The experimental design generated by the software Minitab 17 (Minitab Inc., State College, Pennsylvania, USA) was a Central Composite Design (CCD) with two levels (+1 and -1) for two independent variables: i) enzyme dosage [ED = (g of enzyme/g of cellulose loaded into the bioreactor) x 100] and ii) solid loading [SL = (g of dry WIS used during the hydrolysis of cellulose)/(g of WIS + buffered water + cellulolytic enzyme cocktail loaded into the bioreactor) x 100]. The central point was replicated five times (runs 1, 6, 8, 11 and 13) and four axial points (+1.41 and -1.41) were also added for a total of 13 runs (Table 1).
Table 1.
A = experimental design runs (1–13) for enzymatic hydrolysis tests of water insoluble substrates (WIS) of stranded driftwood feedstocks (SD): enzyme dosage [ED = (g of enzyme/g of cellulose loaded into the bioreactor) x 100] and solid loading [SL = (g of dry WIS used during the hydrolysis of cellulose)/(g of WIS + buffered water + cellulolytic enzyme cocktail loaded into the bioreactor) x 100]; B = carbohydrates composition of hydrolyzed liquid fraction (HLF) obtained from runs 1–13; C = quantitative results achieved by S. terricola DBVPG 5870 grown on each HLF (runs 1–13): PL = total lipid production; PL/DW = % of total intracellular lipid on cell biomass; YL = lipid yield; Yoleic = oleic acid yield (calculated using the % of oleic acid reported in Table 2); PL/d = daily productivity. The technological steps for obtaining WIS and HLF from SD are reported in the text.
| A |
B |
C |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Runs | Enzyme Dosage (ED) |
Solid Loading (SL) |
Glucose (g/l) | XG* (g/l) | Cellobiose (g/l) | PL (g/l) | PL/DW (%) | YL (%) | Yoleic (%) | PL/d [g/(l*d)] | ||
| Level | (%) | Level | (%) | |||||||||
| 1 | 0 | 13 | 0 | 15 | 61.22 ± 0.54 | 0.46 ± 0.07 | 2.80 ± 0.11 | 6.58 ± 0.20 | 49.63 ± 0.60 | 11.05 ± 0.10 | 7.52 ± 0.23 | 0.33 ± 0 |
| 2 | −1.41 | 3.10 | 0 | 15 | 24.08 ± 1.23 | 0.18 ± 0.01 | 1.19 ± 0.09 | 3.20 ± 0.10 | 33.37 ± 2.46 | 12.55 ± 0.55 | 6.55 ± 0.18 | 0.25 ± 0.01 |
| 3 | 0 | 13 | +1.41 | 22.07 | 66.42 ± 0.42 | 0.55 ± 0.12 | 4.23 ± 0.15 | 6.75 ± 0.21 | 48.21 ± 1.03 | 9.49 ± 0.30 | 7.0 ± 0.16 | 0.3 ± 0.02 |
| 4 | +1.41 | 22.90 | 0 | 15 | 65.34 ± 1.38 | 0.61 ± 0.09 | 4.20 ± 0.08 | 6.70 ± 0.42 | 48.46 ± 4.80 | 9.56 ± 0.61 | 7.03 ± 0.32 | 0.34 ± 0.02 |
| 5 | −1 | 6 | −1 | 10 | 29.81 ± 0.14 | 0.23 ± 0.03 | 1.45 ± 0.07 | 4.65 ± 0.05 | 38.90 ± 2.50 | 14.76 ± 0.16 | 7.47 ± 0.07 | 0.30 ± 0 |
| 6 | 0 | 13 | 0 | 15 | 55.10 ± 1.27 | 0.43 ± 0.13 | 3.82 ± 0.37 | 6.35 ± 0.15 | 43.25 ± 0.25 | 10.76 ± 0.25 | 7.85 ± 0.17 | 0.35 ± 0.05 |
| 7 | +1 | 20 | −1 | 10 | 43.40 ± 0.29 | 0.40 ± 0.11 | 3.04 ± 0.24 | 6.90 ± 0.10 | 50.25 ± 0.45 | 14.68 ± 0.21 | 10.74 ± 0.17 | 0.50 ± 0 |
| 8 | 0 | 13 | 0 | 15 | 58.68 ± 0.42 | 0.47 ± 0.10 | 4.04 ± 0.52 | 6.70 ± 0.40 | 41.36 ± 0.60 | 10.63 ± 0.10 | 7.55 ± 0.44 | 0.28 ± 0 |
| 9 | 0 | 13 | −1.41 | 7.93 | 30.83 ± 0.17 | 0.26 ± 0.02 | 1.92 ± 0.42 | 7.75 ± 0.05 | 55.55 ± 7.05 | 23.48 ± 0.15 | 12.42 ± 0.06 | 1.10 ± 0 |
| 10 | +1 | 20 | +1 | 20 | 89.18 ± 0.67 | 0.76 ± 0.21 | 5.35 ± 0.37 | i.g.** | i.g.** | i.g.** | i.g.** | i.g.** |
| 11 | 0 | 13 | 0 | 15 | 55.54 ± 1.21 | 0.43 ± 0.12 | 4.11 ± 0.21 | 7.05 ± 0.15 | 44.05 ± 0.15 | 11.75 ± 0.25 | 8.59 ± 0.17 | 0.40 ± 0 |
| 12 | −1 | 6 | +1 | 20 | 30.91 ± 0.20 | 0.22 ± 0.02 | 2.62 ± 0.07 | 7.70 ± 0.20 | 48.88 ± 0.49 | 22.85 ± 0.59 | 11.91 ± 0.33 | 1.10 ± 0.03 |
| 13 | 0 | 13 | 0 | 15 | 51.36 ± 1.27 | 0.40 ± 0.09 | 4.04 ± 0.27 | 6.20 ± 0.10 | 43.00 ± 2.40 | 11.07 ± 0.18 | 8.21 ± 0.12 | 0.30 ± 0 |
XG represent the sum of xylose and galactose.
i.g. = insignificant yeast growth.
The contribution of each variable to the model and the potential interaction between variables were evaluated by regression analysis and ANOVA (confidence level = 95%). The regression model (described by a quadratic polynomial equation) was assessed by RSM. The surfaces generated by regression models were used to indicate the direction in which the original design must be displaced in order to achieve the optimal conditions, namely the most favorable combination of ED and SL to get the best hydrolyzate for obtaining the highest YL by S. terricola [37].
Hydrolyses were carried out in a 5 l Biostat® A-Plus-Sartorius reactor (Sartorius, Goettingen, Germany) equipped with an automatic monitoring system for controlling agitation, pH, aeration, temperature and antifoam. The enzyme cocktail used was NS-22192 (Novozyme, Bagsværd, Denmark) with an activity of 120 FPU/ml and 4500 CBU/ml [13]. Working conditions were T =50 °C, pH = 5 and stirring =300 rpm.
The hydrolyses were stopped after 96 h and the hydrolyzates were heat-treated to quench the residual enzymes activity. After hydrolysis the insoluble residual lignin fraction was separated from the carbohydrate-rich hydrolyzed liquid fraction (HLF) by filtration (filter cutoff 0.45 μm, Filter Lab®) under pressure (73 g/m2). HLF samples were stored at - 20 °C until use.
2.6. Lipid accumulation tests
Batch cultures of S. terricola DBVPG 5870 were conducted at 25 °C on HLF obtained using the different combinations of ED and SL settled by the experimental design (Table 1). A loopful of 48 h yeast cells grown on YPD agar was inoculated in 50 ml orbital shaken flasks (160 rpm) containing 10 ml of pre-culture media (50% of YPD broth and 50% of each different HLF obtained from runs 1–13, according to Table 1). The pH of pre-culture media was adjusted to 5.5 with NaOH 1 M and yeast extract was added to obtain a constant C/N ratio of 40. After 24 h of incubation, 1 ml of the pre-culture (A600 adjusted to 0.1, corresponding to a cell concentration of 1 × 106 cells/ml) was inoculated in 100 ml orbital shaken flasks (160 rpm) containing 20 ml of each different HLF obtained from runs 1–13, according to Table 1. As above, pH was adjusted to 5.5 with 1 M NaOH and yeast extract was added to obtain a C/N ratio of 40. Each sample was incubated until the complete depletion of carbohydrates.
Furthermore, in order to validate the results of design, a batch culture of S. terricola was conducted in triplicate on the HLF obtained using the optimal predicted values of ED and SL and following the above culture conditions.
2.7. Extraction of lipids
The extraction of intracellular lipids was performed using the protocol reported in Filippucci et al. [32]. Briefly, 10 ml of each culture was centrifuged (5000×g for 10 min) and repeatedly washed with distilled water. The cells were then treated with 5 ml of 4 M HCl, incubated at 60 °C for 2 h in a water bath to obtain acid-hydrolyzed cells, mixed with 7.5 ml of a chloroform/methanol 2:1 (v/v) mixture and incubated at room temperature for 2 h in an orbital shaker (160 rpm). After incubation, the samples were centrifuged (3000×g for 10 min) to obtain the separation of the different phases. The organic phase containing the lipids was recovered and put inside glass vials which were fluxed to dryness in the dark by N2 flow. Glasses were then instantly sealed with a rubber septum, weighed to determine the total amount of lipids and stored at −20 °C until the Gas Chromatography (GC) analysis.
2.8. Analytical determinations
The % of cellulose, hemicellulose and lignin of SD before SE was evaluated according to the National Renewable Energy Laboratory (NREL) analytical methods for biomass [38] and are reported in Table S2 (Supplementary Material).
WIS was then analyzed according to NREL analytical methods [38]. Briefly, acid hydrolysis (using H2SO4) was performed in triplicate to obtain C6 and C5 monomers from cellulose and residual hemicellulose. The concentration of both C5 and C6 monomers was detected by Dionex Ultimate 3000 HPLC (Thermo Scientific, Sunnyvale, CA, USA) equipped with a Biorad Aminex HPX-87H column (Biorad, CA, USA) thermo-regulated at 50 °C and a RI detector (Refracto Max 520, Thermo Scientific, Waltham, MA, USA), mobile phase = 0.01 N H2SO4, flow 0.6 ml/min. The concentration of polymeric sugars was calculated using an anhydrous correction of 0.88 and 0.90 for C5 and C6 carbohydrates, respectively. The remaining acid-insoluble residue was used for calculating the acid-insoluble lignin after removing the ash content. The % of cellulose, hemicellulose and lignin of WIS are reported in Table S2 (Supplementary Material).
The concentration of carbohydrates on HLF was determined by HPLC [13] and the total nitrogen content was determined by semi-micro Kjeldahl method [39].
During batch cultures, yeast growth was daily monitored spectrophotometrically (Beckman DU® 640, Brea, CA, USA) by measuring the A600, while the carbohydrates depletion was daily checked by HPLC. The amount (g/l) of yeast biomass produced after batch incubations was determined gravimetrically as cell dry weight (DW). The weight of yeast biomass and the lipids extracted from yeast cells and the content of glucose, xylose/galactose (XG) and cellobiose of the hydrolyzed biomass were used to calculate the following parameters: (i) the total lipid production (PL, g/l); (ii) % of total intracellular lipid on cell biomass (PL/DW); (iii) the lipid yield (YL = ratio between the total lipid production and the amount of carbohydrates used by yeast for growth and metabolism); and (iv) the daily productivity [PL/d, g/(l × day)].
Fatty acid (FA) profiles were determined using the following protocol: 100 mg of each sample of lipids produced by S. terricola, was dissolved in 4 ml of n-hexane (>99%) in a glass vial after that 160 μl of a KOH methanolic solution 2 M were added. This solution was mixed vigorously in vortex shaker for 30 s. After this, 1.6 ml of a NaCl saturated solution was added in order to facilitate the phases separation. The sample was then centrifuged at 1000×g for 5 min and a 1 μl aliquot of the organic phase was analyzed by GC with Flame Ionization Detector (GC-FID) Varian 3300 (Walnut Creek, CA, USA). An TG-WAX MS capillary column (length 30 m, internal diameter 0.25 mm, fill thickness of 0.25 μm) (Thermo Scientific, Sunnyvale, CA, USA) was used for the separation of the different FA. The injection temperature was 250 °C and the oven temperature was programmed as follows: (i) a gradient of 6 °C/min from 140 to 160 °C; (ii) a gradient of 8 °C/min from 160 to 180 °C; (iii) a gradient of 4 °C/min from 180 to 240 °C; (iv) an isotherm of 15 min at 240 °C. Helium and nitrogen were used as mobile phases. FA profiles were identified by comparing their retention times with those of commercial standards of fatty acids methyl esters (F.A.M.E. Mix C8-C24, SUPELCO® - Bellefonte, PA, USA). Peak areas in the total ion chromatograms were used to determine the FA relative amounts. FA profiles were also used to calculate the oleic acid yield (Yoleic = the amounts of carbohydrates converted into oleic acid, expressed as percentage). The % of saturated fatty acids (SFA), the % of unsaturated fatty acids (UFA) and the unsaturation index (UI) [13,40] were also evaluated.
3. Results and discussion
3.1. Composition of SD and WIS
Cellulose was the principal component found in SD (31.4%), while the hemicellulosic fraction (constituted by over 80% of xylose) was about the half of the cellulose. On the contrary, lignin accounted for about 28% of total biomass (Table S2 - Supplementary Material). The WIS composition exhibited an increased content of both cellulose and lignin (44.52% and 48.45%, respectively) (Table S2 - Supplementary Material).
3.2. Responses of experimental design
The carbohydrate composition of HLF obtained from the experimental design realized using different combinations of ED and SL (runs 1–13) is reported in Table 1. Glucose was always over 90% of total carbohydrates. The five central points (runs 1, 6, 8, 11 and 13) exhibited an average glucose concentration = 57.18 ± 3.52 g/l. The highest release of glucose (89.18 ± 0.67 g/l) was observed on run 10 (obtained using 20% of both ED and SL), while the lowest ones (from 24.08 ± 1.23 to 30.91 ± 0.20 g/l) were found on runs 2, 5, 9 and 12 (ED from 3.10 to 13%; SL from 7.93 to 20%, respectively) (Table 1). Table 1 also reports quantitative results on PL; PL/DW; YL; Yoleic; PL/d exhibited by S. terricola DBVPG 5870 after conversion of sugars derived from the HLF obtained from experimental design using different combinations of ED and SL (runs 1–13). Interestingly, the HLF obtained from run 10 (obtained using 20% of both ED and SL and exhibiting the highest release of glucose) did not allow neither significant growth of S. terricola cells, nor lipid accumulation. This effect may be the consequence of the high amount of carbohydrates (95.29 ± 0.99 g/l) released after enzyme hydrolysis and is apparently consistent with a previous study underlining the impact of osmotic stress (due to the high glucose content) on yeast physiology [41]. At the same time, S. terricola grown on HLF from runs 9 and 12 (ED = 13 and 6%; SL = 7.93 and 20%, respectively) exhibited the best results in terms of both YL and PL (23.48 ± 0.15 and 22.85 ± 0.59%; and 7.75 ± 0.05 and 7.70 ± 0.20 g/l, respectively) (Table 1).
The FA profiles of S. terricola grown on the HLF obtained from experimental design using different combinations of ED and SL (runs 1–13, Table 1) are reported in Table 2. Overall, the main FA were palmitic (hexadecanoic acid = C16:0), stearic (octadecanoicacid = C18:0), oleic [(9E9Z)-octadec-9-enoicacid = Δ9C18:1] and linoleic [(9Z,12Z)-9,12-octadecadienoic acid = Δ9,12C18:2] acids. Other minor FA, namely caprylic (octanoic acid = C8:0), capric (decanoic acid = C10:0), lauric (dodecanoic acid = C12:0), myristic (tetradecanoicacid = C14:0), palmitoleic [(9Z)-hexadec-9-enoicacid = Δ9C16:1), linolenic [(9Z,12Z,15Z)-9,12,15-octadecatrienoic acid = Δ9,12,15C18:3], arachic (eicosanoic acid = C20:0), behenic (docosanoic acid = C22:0), erucic [(13Z)-docos-13-enoic acid = Δ13C22:1] and lignoceric (tetracosanoicacid = C24:0) acids were also found (Table 2). In agreement with the current literature [11,13,42], oleic acid was always the dominant FA produced by S. terricola. The Yoleic values obtained on HLF from runs 9 and 12 (ED = 13 and 6%; SL = 7.93 and 20%, respectively) were 12.42 ± 0.06 and 11.91 ± 0.33%, respectively (Table 1). Surprisingly, other HLF exhibited higher contents (i.e. more than 70%) of oleic acid (runs 1, 3, 4, 6, 7, 8, 11 and 13, Table 2), but lower Yoleic values (Table 1). This evidence suggested that the % of oleic acid and Yoleic should be considered as divergent responses.
Table 2.
Fatty acid (FA) profiles, percentages of unsaturated and saturated fatty acids (UFA and SFA), and unsaturation index (UI) of lipids produced by Solicoccozyma terricola DBVPG 5870 grown on hydrolyzed liquid fraction (HLF) (runs 1–13): C6:0 caproic acid (hexanoic acid), C8:0 caprylic acid (octanoic acid), C10:0 capric acid (decanoic acid), C12:0 lauric acid (dodecanoic acid), C14:0 myristic acid (tetradecanoic acid), C16:0 palmitic acid (hexadecanoic acid), Δ9C16:1 palmitoleic acid [(9Z)-hexadec-9-enoic acid), C18:0 stearic acid (octadecanoic acid), Δ9C18:1 oleic acid [(9E9Z)-octadec-9-enoic acid], Δ9,12C18:2 linoleic acid [(9Z,12Z)-9,12-octadecadienoic acid], Δ9,12,15C18:3 α-linolenic acid [(9Z,12Z,15Z)-9,12,15-octadecatrienoic acid], Δ6,9,12C18:3 γ-linolenic acid [(6Z,9Z,12Z)-6,9,12-octadecatrienoic acid], C20:0 arachic acid (eicosanoic acid), Δ11C20:1 gondoic acid [(11Z)-11-eicosenoic acid], C22:0 behenic acid (docosanoic acid), Δ13C22:1 erucic acid [(13Z)-docos-13-enoic acid], C24:0 lignoceric acid (tetracosanoic acid).
| Runs | C8:0 (%) | C10:0 (%) | C12:0 (%) | C14:0 (%) | C16:0 (%) | C16:1 (%) | C18:0 (%) | C18:1 (%) | C18:2 (%) | C18:3 (%) | C20:0 (%) | C22:0 (%) | C22:1 (%) | C24:0 (%) | Saturated fatty acids (%) | Unsaturated fatty acids (%) | UI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.03 ± 0.02 | 0.02 ± 0.02 | 0.04 ± 0.01 | 0.28 ± 0.08 | 15.97 ± 0.72 | 0.13 ± 0.01 | 5.67 ± 0.12 | 73.50 ± 0.68 | 3.14 ± 0.12 | 0.03 ± 0.03 | 0.28 ± 0.03 | 0.15 ± 0.04 | 0.01 ± 0.01 | 0.77 ± 0.05 | 23.21 | 76.79 | 0.80 |
| 2 | 0.02 ± 0.01 | 0 | 0.03 ± 0.01 | 0.41 ± 0.06 | 25.30 ± 1.21 | 0.34 ± 0.04 | 13.41 ± 0.07 | 52.10 ± 0.21 | 6.61 ± 0.67 | 0.08 ± 0.01 | 0.46 ± 0.04 | 0.26 ± 0.01 | 0 | 0.98 ± 0.05 | 40.86 | 59.14 | 0.66 |
| 3 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.26 ± 0.08 | 15.56 ± 0.63 | 0.21 ± 0.12 | 5.76 ± 0.14 | 73.81 ± 0.56 | 3.23 ± 0.13 | 0.02 ± 0.01 | 0.24 ± 0.03 | 0.21 ± 0.02 | 0 | 0.65 ± 0.06 | 22.73 | 77.27 | 0.81 |
| 4 | 0.01 ± 0.01 | 0 | 0.03 ± 0.01 | 0.24 ± 0.07 | 16.12 ± 0.54 | 0.12 ± 0.05 | 5.39 ± 0.13 | 73.65 ± 0.67 | 3.28 ± 0.12 | 0.01 ± 0.02 | 0.25 ± 0.01 | 0.28 ± 0.03 | 0 | 0.62 ± 0.04 | 22.94 | 77.06 | 0.80 |
| 5 | 0 | 0 | 0.02 ± 0.02 | 0.56 ± 0.02 | 26.47 ± 0.94 | 0.42 ± 0.03 | 13.20 ± 0.07 | 50.61 ± 0.20 | 6.87 ± 0.74 | 0.15 ± 0.01 | 0.49 ± 0.06 | 0.25 ± 0.02 | 0 | 0.95 ± 0.07 | 41.94 | 58.06 | 0.67 |
| 6 | 0.01 ± 0 | 0.01 ± 0 | 0.03 ± 0.01 | 0.21 ± 0 | 15.96 ± 0.54 | 0.13 ± 0.02 | 5.73 ± 0.03 | 73.39 ± 0.36 | 3.12 ± 0.05 | 0.07 ± 0.07 | 0.33 ± 0.05 | 0.21 ± 0.04 | 0 | 0.82 ± 0.06 | 23.31 | 76.69 | 0.80 |
| 7 | 0.06 ± 0.03 | 0 | 0.03 ± 0.01 | 0.26 ± 0.02 | 15.23 ± 0.42 | 0.12 ± 0.01 | 7.59 ± 0.46 | 72.90 ± 0.12 | 2.40 ± 0.02 | 0 | 0.69 ± 0.08 | 0.27 ± 0.02 | 0 | 0.45 ± 0.11 | 24.58 | 75.42 | 0.78 |
| 8 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.18 ± 0.02 | 9.74 ± 0.12 | 0.04 ± 0.01 | 14.74 ± 0.02 | 71.25 ± 0.51 | 1.70 ± 0.08 | 0.04 ± 0.01 | 0.71 ± 0.02 | 0.53 ± 0.01 | 0.04 ± 0.01 | 0.97 ± 0.05 | 26.92 | 73.08 | 0.75 |
| 9 | 0.01 ± 0 | 0.01 ± 0 | 0.03 ± 0.01 | 0.39 ± 0 | 26.11 ± 0.28 | 0.46 ± 0.03 | 13.92 ± 0.42 | 52.89 ± 0.22 | 4.49 ± 0.11 | 0.05 ± 0 | 0.54 ± 0.04 | 0.24 ± 0 | 0 | 0.86 ± 0.03 | 42.11 | 57.89 | 0.62 |
| 10 | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* | i.g.* |
| 11 | 0.01 ± 0.01 | 0.06 ± 0.04 | 0.05 ± 0.02 | 0.41 ± 0.22 | 16.56 ± 1.41 | 0.13 ± 0.01 | 5.52 ± 0.02 | 73.20 ± 1.61 | 2.87 ± 0.08 | 0.02 ± 0.02 | 0.24 ± 0.02 | 0.16 ± 0.01 | 0.04 ± 0.04 | 0.74 ± 0.05 | 23.75 | 76.25 | 0.79 |
| 12 | 0.01 ± 0 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.28 ± 0.01 | 26.34 ± 0.43 | 0.32 ± 0.02 | 14.21 ± 0.25 | 52.20 ± 0.67 | 5.22 ± 0.12 | 0.09 ± 0.05 | 0.42 ± 0.03 | 0.18 ± 0.01 | 0 | 0.70 ± 0.04 | 42.17 | 57.83 | 0.63 |
| 13 | 0.07 ± 0.05 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.21 ± 0.02 | 15.38 ± 0.21 | 0.12 ± 0.01 | 5.76 ± 0.32 | 73.90 ± 0.05 | 3.42 ± 0.23 | 0 | 0.28 ± 0.01 | 0.09 ± 0.09 | 0 | 0.73 ± 0.04 | 22.56 | 77.44 | 0.81 |
The technological steps for obtaining HLF from stranded driftwood feedstocks (SD) are reported in the text.
i.g. = insignificant yeast growth.
3.3. Prediction of optimal results
YL obtained from experimental design using different combinations of ED and SL (runs 1–13, Table 1) were used as response to generate a quadratic polynomial model to predict the best combination of ED and SL able to release the optimal concentration of carbohydrates allowing to obtain the highest YL by S. terricola DBVPG 5870. A second-order polynomial equation (Eq. (2)) was computed for describing the effect of independent variables (ED and SL) on dependent one (response = YL).
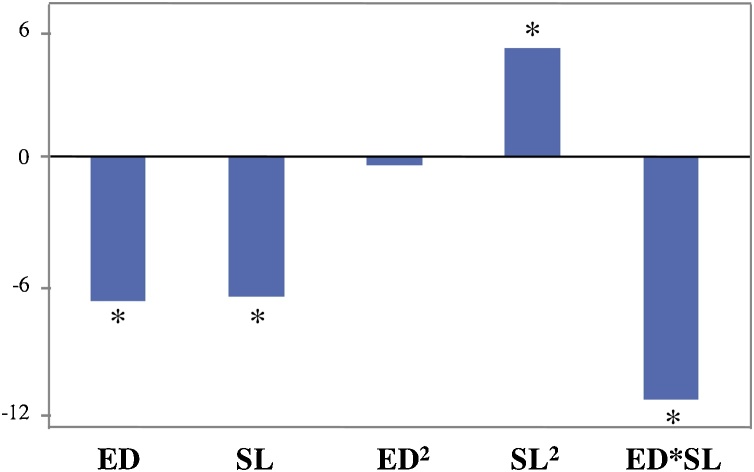
| YL = 17.8 + 2.056 ED – 1.59 SL – 0.0037 ED2 + 0.1017 SL2 – 0.1628 ED*SL | (2) |
Main effects and interactions produced by ED and SL on YL are reported in Fig. 1. On the basis of ANOVA, non significant (p > 0.05) coefficients were removed from the above second-order polynomial equation (Eq. (2); R2 = 0.84). A simplified second-order polynomial equation (Eq. (3) R2 = 0.84) was recomputed accordingly.
| YL = 18.5 + 1.959 ED – 1.62 SL + 0.1026 SL2 – 0.1628 ED*SL | (3) |
Fig. 1.
Second-order polynomial equation (Eq. (3) as reported in the text). Main effects and interactions produced by the independent variables: dosage of enzyme cocktail (ED) and solid loading (SL) computed after the experimental design runs (1–13).
* = significant (p < 0.05) value.
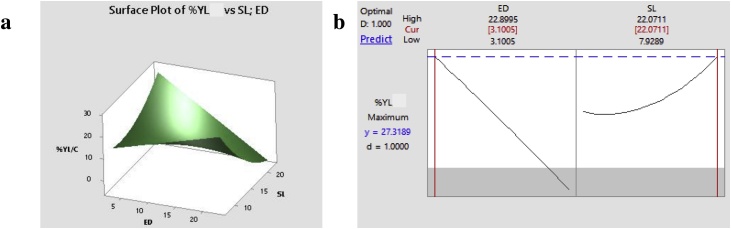
The best combination of ED and SL able to release the optimal concentration of carbohydrates allowing to obtain the highest YL by S. terricola was predicted by the RSM. The highest YL (27.32%), close to the maximum theoretical value (31.6%) described by Ratledge [43], was predicted using the following values: ED = 3.10%; SL = 22.07% (Fig. 2). The joint use of Eq. (3) and Response Surface Plot (Fig. 2) suggested to use low ED and high SL. This combination, which allowed to obtain the highest YL, can be considered convenient also from an economical viewpoint, because the cost of the enzyme cocktail is sometimes considered one of the bottleneck of the process. Likewise, the use of high SL can be positively considered for depleting the costs for energy supply [24,26].
Fig. 2.
Graphical response obtained by the second-order polynomial equation (Eq. (3), as reported in the text). a: Response surface plot of the dependent variable (the lipid yield - YL - of Soliccocozyma terricola) as a function of the two independent variables [dosage of enzyme cocktail (ED) and solid loading (SL)]. b: Optimal values of ED and SL to obtain the maximum YL.
3.4. Validation of the model
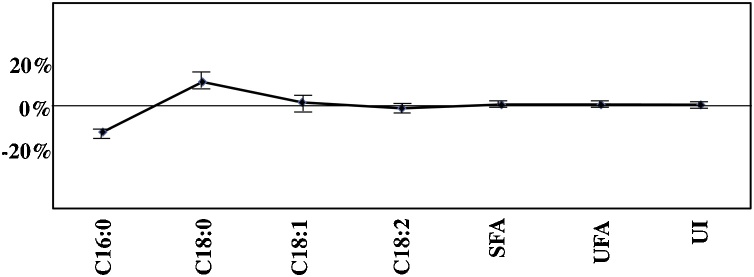
In order to validate the model, a new enzymatic hydrolysis was carried out using the above combination (ED = 3.10%; SL = 22.07%) predicted by Eq. (3) and RSM. After hydrolysis, the carbohydrate composition of HLF was: glucose = 24.90 ± 0.61 g/l, XG = 0.87 ± 0.02 g/l and cellobiose = 3.31 ± 0.32 g/l. After yeast growth, a YL = 25.26 ± 0.79% was found, very close to the value predicted by the model (27.32%), thus confirming the reliability of the above reported simplified second-order polynomial equation (Eq. (3)) in predicting the best combination of ED and SL able to release the optimal concentration of carbohydrates allowing to obtain the highest YL by S. terricola. In addition, under the same conditions the PL was 6.53 ± 0.21 g/l and the main FA were: palmitic, stearic, oleic and linoleic acids (23.94 ± 0.03%, 18.26 ± 1.32%, 47.79 ± 1.13% and 7.42 ± 0.27%, respectively). The Yoleic reaches the value of 12.10 ± 0.46%. Interestingly, the above FA profile was similar to that of palm oil described in literature [42,44], also in terms of SFA, UFA and UI (Fig. 3). The similarity between the oil obtained from S. terricola grown under the above optimal conditions and that from palm represents an alternative for the renewable and sustainable production of biodegradable oils for food, feed, fuels and chemicals [45,46]. Globally, palm oil is the most largely traded oil from oilseed crops due to its versatile use as ingredient of some food and industrial products, especially for the biodiesel production [47,48]. Driven by its raising demand, some concerns associated with palm cultivation (i.e. deforestation, loss of biodiversity, etc.) are going to increase [48]. In this context, oleaginous yeasts including S. terricola could be considered as extra-source of oils exhibiting similar FA profile potentially able to mitigate the problems associated with palm cultivation [13]. Of course, the possible use of yeast oil either as a substitute or a complementary additive for palm oil is a still open question. The possibility to successfully scale up the process from the laboratory to the semi- and to the industrial scale, will also depend to the actual availability of large amounts of stranded driftwood feedstocks for feeding possible future biorefineries.
Fig. 3.
Comparison between the profile of the main fatty acids obtained by Soliccocozyma terricola and those of palm oil. The differences are expressed as % respect to the composition of palm oil (reported as baseline). Main fatty acids were palmitic (hexadecanoic acid = C16:0), stearic (octadecanoic acid = C18:0), oleic [(9E9Z)-octadec-9-enoic acid = Δ9C18:1] and linoleic [(9Z,12Z)-9,12-octadecadienoic acid = Δ9,12C18:2] acids. Saturated Fatty Acids (SFA), Unsaturated Fatty Acids (UFA) and Unsaturation Index (UI) are also reported.
4. Conclusions
Stranded driftwood feedstocks usually represent wastes needing disposal costs, however they can be considered as a C-source to produce lipid by oleaginous yeasts for producing biochemicals and biofuels. The selection of the best combination of ED and SL (ED = 3.10%; SL = 22.07%) able to release the optimal concentration of carbohydrates allowed to obtain a YL by S. terricola DBVPG 5870 close to the theoretical maximum value. The validation of the model confirmed the reliability of the second-order polynomial equation (Eq. (3)) in predicting the best response.
Interestingly, FA profile of oil produced by S. terricola was comparable to that of palm oil; besides its high Yoleic confirmed that such oleaginous yeast can be considered as extra-source of oils for fuels, food and cosmetic industry.
Declaration of Competing Interest
The authors declare that they have no competing interests.
On behalf of all co-authors, I confirm that:
- There is no conflict of interest.
- All authors have approved the manuscript for submission.
- The content of the manuscript is original, it has not been published, or submitted for publication elsewhere.
Acknowledgments
Authors deeply acknowledge the Italian Ministry for Education and Scientific Research (MIUR) for funding the study though the project BIT3G: “Bioraffineria di III Generazione Integrata nel Territorio” (Project No CTN01_00063_49295), 2014–2016.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00367.
Contributor Information
Giorgia Tasselli, Email: tasselligiorgia@gmail.com.
Sara Filippucci, Email: sara.filippucci87@virgilio.it.
Silvia D’Antonio, Email: silviadanto@hotmail.it.
Gianluca Cavalaglio, Email: cavalaglio@crbnet.it.
Benedetta Turchetti, Email: benedetta.turchetti@unipg.it.
Franco Cotana, Email: cotana@crbnet.it.
Pietro Buzzini, Email: pietro.buzzini@unipg.it.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.FitzPatrick M., Champagne P., Cunningham M.F., Whitney R.A. A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010;101(23):8915–8922. doi: 10.1016/j.biortech.2010.06.125. [DOI] [PubMed] [Google Scholar]
- 2.Lee J., Vadlani P.V., Min D. Sustainable production of microbial lipids from lignocellulosic biomass using oleaginous yeast cultures. J. Sustain. Bioenergy Syst. 2017;7(1):36–50. [Google Scholar]
- 3.Johansen S. Origin of driftwood in north Norway and its relevance for transport routes of drift ice and pollution to the Barents Sea. Sci. Total Environ. 1999;231(1-2):201–225. [Google Scholar]
- 4.Cavalaglio G., Gelosia M., D’Antonio S., Nicolini A., Pisello A.L., Barbanera M., Cotana F. Lignocellulosic ethanol production from the recovery of stranded driftwood residues. Energies. 2016;9(8):634. [Google Scholar]
- 5.Bartocci P., Barbanera M., D’Amico M., Laranci P., Cavalaglio G., Gelosia M., Ingles D., Bidini G., Buratti C., Cotana F., Fantozzi F. Thermal degradation of driftwood: determination of the concentration of sodium, calcium, magnesium, chlorine and sulfur containing compounds. Waste Manag. 2017;60:151–157. doi: 10.1016/j.wasman.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Papanikolaou S., Komaitis M., Aggelis G. Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour. Technol. 2004;95(3):287–291. doi: 10.1016/j.biortech.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Chatzifragkou A., Makri A., Belka A., Bellou S., Mavrou M., Mastoridou M., Papanikolaou S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy. 2011;36(2):1097–1108. [Google Scholar]
- 8.Da Silva T.L., Gouveia L., Reis A. Integrated microbial processes for biofuels and high value-added products: the way to improve the cost effectiveness of biofuel production. Appl. Microb. Biotech. 2014;98(3):1043–1053. doi: 10.1007/s00253-013-5389-5. [DOI] [PubMed] [Google Scholar]
- 9.Angerbauer C., Siebenhofer M., Mittelbach M., Guebitz G.M. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour. Technol. 2008;99(8):3051–3056. doi: 10.1016/j.biortech.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Meng X., Yang J., Xu X., Zhang L., Nie Q., Xian M. Biodiesel production from oleaginous microorganisms. Renew. Energy. 2009;34(1):1–5. [Google Scholar]
- 11.Ageitos J.M., Vallejo J.A., Veiga-Crespo P., Villa T.G. Oily yeasts as oleaginous cell factories. Appl. Microb. Biotech. 2011;90(4):1219–1227. doi: 10.1007/s00253-011-3200-z. [DOI] [PubMed] [Google Scholar]
- 12.Tchakouteu S.S., Kalantzi O., Gardeli C., Koutinas A.A., Aggelis G., Papanikolaou S. Lipid production by yeasts growing on biodiesel‐derived crude glycerol: strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microb. 2015;118(4):911–927. doi: 10.1111/jam.12736. [DOI] [PubMed] [Google Scholar]
- 13.Tasselli G., Filippucci S., Borsella E., D’Antonio S., Gelosia M., Cavalaglio G., Turchetti B., Sannino C., Onofri A., Mastrolitti S., De Bari I., Cotana F., Buzzini P. Yeast lipids from cardoon stalks, stranded driftwood and olive tree pruning residues as possible extra sources of oils for producing biofuels and biochemicals. Biotechnol. Biofuels. 2018;11:147. doi: 10.1186/s13068-018-1142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer J.M., Stymne S., Green A.G., Carlsson A.S. High-value oils from plants. Plant J. 2008;54(4):640–655. doi: 10.1111/j.1365-313X.2008.03430.x. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam R., Dufreche S., Zappi M., Bajpai R. Microbial lipids from renewable resources: production and characterization. J. Ind. Microbiol. Biotechnol. 2010;37(12):1271–1287. doi: 10.1007/s10295-010-0884-5. [DOI] [PubMed] [Google Scholar]
- 16.Elkacmi R., Kamil N., Bennajah M., Kitane S. Extraction of oleic acid from Moroccan olive mill wastewater. Biomed Res. Int. 2016:1–9. doi: 10.1155/2016/1397852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal S., Arya D., Khan S. Comparative fatty acid and trace elemental analysis identified the best raw material of jojoba (Simmondsia chinensis) for commercial applications. Ann. Agric. Sci. 2018;63(1):37–45. [Google Scholar]
- 18.Lopez-Huertas E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010;61(3):200–207. doi: 10.1016/j.phrs.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Mennella I., Savarese M., Ferracane R., Sacchi R., Vitaglione P. Oleic acid content of a meal promotes oleoylethanolamide response and reduces subsequent energy intake in humans. Food Funct. 2015;6:204–210. doi: 10.1039/c4fo00697f. [DOI] [PubMed] [Google Scholar]
- 20.Cannilla C., Bonura G., Costa F., Frusteri F. Biofuels production by esterification of oleic acid with ethanol using a membrane assisted reactor in vapour permeation configuration. Appl. Catal. A Gen. 2018;566:121–129. [Google Scholar]
- 21.Alismaeel Z.T., Abbas A.S., Albayati T.M., Doyle A.M. Biodiesel from batch and continuous oleic acid esterification using zeolite catalysts. Fuel. 2018;234:170–176. [Google Scholar]
- 22.Darvekar P., Holtzapple M.T. Assessment of shock pretreatment of corn stover using the carboxylate platform. Appl. Biochem. Biotech. 2016;178(6):1081–1094. doi: 10.1007/s12010-015-1930-6. [DOI] [PubMed] [Google Scholar]
- 23.Alvira P., Tomás-Pejó E., Ballesteros M., Negro M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 2010;101(13):4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 2002;83(1):1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 25.Humbird D., Mohagheghi A., Dowe N., Schell D.J. Economic impact of total solids loading on enzymatic hydrolysis of dilute acid pretreated corn stover. Biotechnol. Prog. 2010;26(5):1245–1251. doi: 10.1002/btpr.441. [DOI] [PubMed] [Google Scholar]
- 26.Modenbach A.A., Nokes E.S. Enzymatic hydrolysis of biomass at high-solids loadings–a review. Biomass Bioener. 2013;56:526–544. [Google Scholar]
- 27.Ykema A., Verbree E.C., Verseveld H.W., Smit H. Mathematical modeling of lipid production by oleaginous yeasts in continuous cultures. A van Leeuwenhoek. 1986;52(6):491–506. doi: 10.1007/BF00423410. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Fang X., Zhu X.L., Li Y., Xu H.P., Zhao B.F., Chen L., Zhang X.D. Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenergy. 2011;35(5):1906–1911. [Google Scholar]
- 29.Gong Z., Shen H., Yang X., Wang Q., Xie H., Zhao Z.K. Lipid production from corn stover by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels. 2014;7(1):158. doi: 10.1186/s13068-014-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braiuca P., Ebert C., Basso A., Linda P., Gardossi L. Computational methods to rationalize experimental strategies in biocatalysis. Trends Biotechnol. 2006;24(9):419–425. doi: 10.1016/j.tibtech.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Bas D., Boyaci I.H. Modeling and optimization I: usability of response surface methodology. J. Food Eng. 2007;78:836–845. [Google Scholar]
- 32.Filippucci S., Tasselli G., Scardua A., Di Mauro S., Cramarossa M.R., Perini D., Turchetti B., Onofri A., Forti L., Buzzini P. Study of Holtermanniella wattica, Leucosporidium creatinivorum, Naganishia adeliensis, Solicoccozyma aeria, and Solicoccozyma terricola for their lipogenic aptitude from different carbon sources. Biotechnol. Biofuels. 2016;28(9):259. doi: 10.1186/s13068-016-0672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballesteros I., Oliva J.M., Negro M.J., Manzanares P., Ballesteros M. Enzymic hydrolysis of steam exploded herbaceous agricultural waste (Brassicacarinata) at different particle sizes. Process Biochem. 2002;38(2):187–192. [Google Scholar]
- 34.Tomas-Pejo E., Oliva J., Ballesteros M. Realistic approach for full-scale bioethanol production from lignocellulose: a review. J. Sci. Ind. 2008;67(11):874–884. [Google Scholar]
- 35.Overend R., Chornet E., Gascoigne J. Fractionation of lignocellulosic by steam-aqueous pretreat. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987;321(1561):523–536. [Google Scholar]
- 36.Sluiter J.B., Ruiz R.O., Scarlata C.J., Sluiter A.D., Templeton D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010;58(16):9043–9053. doi: 10.1021/jf1008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Sluiter J.B., Sluiter A.D. NREL; Colorado: 2010. Summative Mass Closure; pp. 1–10. NREL/TP-510-48087. [Google Scholar]
- 39.16th ed. Garrard Press; Gaithersburg: 1995. Official Methods of Analysis. AOAC International. [Google Scholar]
- 40.Vishniac H.S. Yeast biodiversity in the Antarctic. In: Rosa C.A., Peter G., editors. Biodiversity and Ecophysiology of Yeasts. Springer; Berlin: 2006. pp. 420–440. [Google Scholar]
- 41.Gomar-Alba M., Morcillo-Parra M.Á., Olmo M.L. Response of yeast cells to high glucose involves molecular and physiological differences when compared to other osmostress conditions. FEMS Yeast Res. 2015;15(5) doi: 10.1093/femsyr/fov039. Article fov039. [DOI] [PubMed] [Google Scholar]
- 42.Ramos M.J., Fernández C.M., Casas A., Rodríguez L., Pérez A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009;100(1):261–268. doi: 10.1016/j.biortech.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 43.Ratledge C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie. 2004;86(11):807–815. doi: 10.1016/j.biochi.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Whiffin F., Santomauro F., Chuck C.J. Toward a microbial palm oil substitute: oleaginous yeasts cultured on lignocellulosem. Biofuels Bioprod. Bioref. 2016;10(3):316–334. [Google Scholar]
- 45.Sitepu I.R., Garay L.A., Sestric R., Levin D., Block D.E., Bruce German J., Boundy-Mills K.L. Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol. Adv. 2014;32(7):1336–1360. doi: 10.1016/j.biotechadv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Blomqvist J., Pickova J., Tilami S.K., Sampels S., Mikkelsen N., Brandenburg J., Sandgren M., Passoth V. Oleaginous yeast as a component in fish feed. Sci. Rep. 2018;8(1):15945. doi: 10.1038/s41598-018-34232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ndayishimiye P., Tazerout M. Use of palm oil-based biofuel in the internal combustion engines: performance and emissions characteristics. Energy. 2011;36(3):1790–1796. [Google Scholar]
- 48.Vijay V., Pimm S.L., Jenkins C.N., Smith S.J. The Impacts of oil palm on recent deforestation and biodiversity loss. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159668. Article e0159668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.