Abstract
Experience with sexual behavior causes cross-sensitization of amphetamine reward, an effect dependent on a period of sexual reward abstinence. We previously showed that ΔFosB in the nucleus accumbens (NAc) is a key mediator of this cross-sensitization, potentially via dopamine receptor activation. However, the role of mesolimbic dopamine for sexual behavior or cross-sensitization between natural and drug reward is unknown. This was tested using inhibitory designer receptors exclusively activated by designer drugs in ventral tegmental area (VTA) dopamine cells. rAAV5/hSvn-DIO-hm4D-mCherry was injected into the VTA of TH::Cre adult male rats. Males received clozapine N-oxide (CNO) or vehicle injections before each of 5 consecutive days of mating or handling. Following an abstinence period of 7 d, males were tested for amphetamine conditioned place preference (CPP). Next, males were injected with CNO or vehicle before mating or handling for analysis of mating-induced cFos, sex experience-induced ΔFosB, and reduction of VTA dopamine soma size. Results showed that CNO did not affect mating behavior. Instead, CNO prevented sexual experience-induced cross-sensitization of amphetamine CPP, ΔFosB in the NAc and medial prefrontal cortex, and decreases in VTA dopamine soma size. Expression of hm4D-mCherry was specific to VTA dopamine cells and CNO blocked excitation and mating-induced cFos expression in VTA dopamine cells. These findings provide direct evidence that VTA dopamine activation is not required for initiation or performance of sexual behavior. Instead, VTA dopamine directly contributes to increased vulnerability for drug use following loss of natural reward by causing neuroplasticity in the mesolimbic pathway during the natural reward experience.
SIGNIFICANCE STATEMENT Drugs of abuse act on the neural pathways that mediate natural reward learning and memory. Exposure to natural reward behaviors can alter subsequent drug-related reward. Specifically, experience with sexual behavior, followed by a period of abstinence from sexual behavior, causes increased reward for amphetamine in male rats. This study demonstrates that activation of ventral tegmental area dopamine neurons during sexual experience regulates cross-sensitization of amphetamine reward. Finally, ventral tegmental area dopamine cell activation is essential for experience-induced neural adaptations in the nucleus accumbens, prefrontal cortex, and ventral tegmental area. These findings demonstrate a role of mesolimbic dopamine in the interaction between natural and drug rewards, and identify mesolimbic dopamine as a key mediator of changes in vulnerability for drug use after loss of natural reward.
Keywords: FosB, mesolimbic, nucleus accumbens, prefrontal cortex, psychostimulant, reward
Introduction
Drugs of abuse act on the mesolimbic circuit, which mediates natural reward behavior and memory (Frohmader et al., 2010; Olsen, 2011; Pitchers et al., 2013, 2014). There is increasing recognition that experience with natural and social reward behaviors influence drug-related behavior and development of addiction (Heilig et al., 2016). For example, stress and social isolation (Lu et al., 2003; Nader et al., 2012; Koob et al., 2014) and exposure to fat (Kenny, 2011) increase vulnerability to drug-seeking behavior in rodent preclinical studies. In contrast, exposure to an enriched environment (Thiel et al., 2009; Solinas et al., 2010; Gipson et al., 2011) and pair bonding in voles (Aragona et al., 2007; Liu et al., 2011) have protective effects against susceptibility to drugs of abuse. In recent years, the neurobiological basis for the interaction between natural and drug reward has been demonstrated by our laboratory for sexual behavior and the psychostimulant d-amphetamine, in male rats. During sexual behavior, μ opioid receptors (MORs) in the ventral tegmental area (VTA) are activated, resulting in activation of dopamine neurons (Balfour et al., 2004) and dopamine release in the nucleus accumbens (NAc; Pfaus et al., 1990; Damsma et al., 1992; Di Chiara, 1999; Fiorino and Phillips, 1999). Antagonism of MORs in the VTA during the acquisition of sexual experience disrupts the long-term expression of sexual reward memory, without affecting sexual motivation, performance, or reward (Pitchers et al., 2014). Moreover, activation of VTA MORs and dopamine cells occurs upon exposure to contextual conditioned cues predictive of sexual reward (Balfour et al., 2004; Pitchers et al., 2014) and, in turn, mediates cue-induced neural activity in VTA projection areas (Pitchers et al., 2014). Such findings are in agreement with the general role of dopamine in reward-related learning (Wise, 2002; Everitt and Robbins, 2005; Kalivas and Volkow, 2005; Fields et al., 2007; Brom et al., 2014; Stauffer et al., 2016) and encoding of the predictive salience of reward-associated cues (Schultz, 2013; Steinberg et al., 2013; Stauffer et al., 2016). However, the role of VTA dopamine activation in sexual behavior remains unclear. Antagonism of D1 dopamine receptors in the NAc did not affect sexual behavior, but the study was limited to a single dose (Pitchers et al., 2013). Moreover, VTA dopamine may influence sex behavior by acting in other mesolimbic areas.
Sexual experience causes cross-sensitization of amphetamine-induced locomotor activity and conditioned place preference (CPP; Pitchers et al., 2010a, 2013, 2016; Beloate et al., 2016). Importantly, the cross-sensitizing effects of sexual experience on amphetamine reward are dependent on a period of sexual abstinence (Pitchers et al., 2010a, 2013), and a key mediator of this amphetamine cross-sensitization is mating-induced ΔFosB expression in the NAc (Pitchers et al., 2010b, 2013). In turn, ΔFosB is regulated by NAc D1 (Pitchers et al., 2013) and NMDA receptor activation (Beloate et al., 2016), suggesting roles for dopamine and/or glutamate in the cross-sensitizing effects of loss of sexual reward. Sexual experience also causes a decrease in dopamine soma size in the VTA, via mating-induced activation of MOR (Pitchers et al., 2014). However it is unknown whether sex experience-induced plasticity is dependent on dopamine neuron activity during mating behavior.
Together, these results suggest that dopamine cell activation in the VTA is not essential for the initiation or expression of sexual behavior, but regulates sex experience-induced neural plasticity in the mesolimbic pathway and amphetamine reward cross-sensitization. The current study directly tested this hypothesis using designer receptors exclusively activated by designer drugs (DREADDs; Lee et al., 2014) and male rats that express Cre recombinase under the control of the tyrosine hydroxylase promotor (TH::Cre; Witten et al., 2011) for selective manipulation of VTA dopamine cell activation during the acquisition of sexual experience.
Materials and Methods
Animals
All rats were housed in same-sex pairs in standard Plexiglas cages. Food and water were provided ad libitum, and animals were maintained in temperature-controlled and humidity-controlled rooms on a 12 h dark/light cycle. All experiments were performed in accordance with the U.S. National Institutes of Health guidelines involving vertebrate animals in research and in agreement with Dutch laws (Wet op Dierproeven 1996) and European regulations (Guideline 86/609/EEC), and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center or the Animal Ethics Committee of Utrecht University.
TH::Cre males.
Heterozygous transgenic male rats expressing Cre recombinase under the control of the tyrosine hydroxylase (TH) promotor (TH::Cre) were bred from TH::Cre males (National Institutes of Health Rat Resource and Research Center, Columbia, MO; Long–Evans background; RRID:RGD_10401201; Witten et al., 2011; Pandit et al., 2016) and wild-type Long–Evans females (Charles River). Offspring genotyping was done via PCR using DNA isolated from tail biopsies with REDExtract-N-Amp PCR Ready mix (Sigma-Aldrich; catalog #R4775; 10 μl) in 4 μl of double-distilled water (ddH2O) supplemented with 10 μm forward and reverse primers (1 μl of each primer): 5′-CGT TCA CCG GCA TCA ACG TTT-3′; 5′-GCG GCA TGG TGC AAG TTG AAT-3′ (Integrated DNA Technologies). PCR reaction conditions consisted of 1 cycle of 94°C for 3 min; 35 cycles of 94°C for 1 min, 60.8°C for 1 min, and 72°C for 1 min; and 1 cycle of 72°C for 10 min. PCR products were separated by electrophoresis on a 1% agarose gel in 1× TAE buffer (Bio-Rad Laboratories; catalog #161-0743) to visualize the 232 bp product indicative of Cre recombinase.
Females.
Female Sprague Dawley rats (Charles River; 201–225 g; RRID:RGD_734476) used for mating behavior tests were bilaterally ovariectomized and implanted with subcutaneous capsules (Dow Corning tubing; 1.98 mm internal diameter) containing 5% 17-β-estradiol-benzoate (in cholesterol; 1 cm filled area). Females received 500 μg of progesterone in 0.1 ml of sesame oil (Sigma-Aldrich; subcutaneous) 3–6 h before each mating session to induce sexual receptivity.
Behavioral studies
Viral-mediated transfection of DREADD receptors.
A chemogenetic approach was used to specifically inhibit VTA dopamine neurons. TH::Cre males received bilateral VTA microinjections of a recombinant adeno-associated virus (rAAV) encoding a Cre-driven Gi-coupled DREADD (rAAV5/hSyn-DIO-hm4D-mCherry; University of North Carolina Vector Core, Chapel Hill, NC). Animals were deeply anesthetized with ketamine (87 mg/ml/kg)/xylazine (13 mg/ml/kg, i.p.) and placed into a stereotaxic apparatus (Kopf Instruments). Skulls were exposed, and burr holes were drilled to target the VTA. rAAV vectors were injected at a volume of 1 μl/injection using a 10 μl Hamilton syringe (Harvard Apparatus) attached to a microdrive (Kopf Instruments, model 5000). For the behavioral studies, TH::Cre rats were 8–16 weeks old at time of injection and all animals received two injections per hemisphere at two rostrocaudal locations as follows: anteroposterior (AP) −5.0 mm and AP −5.8 mm, mediolateral (ML) ±0.7 mm from bregma, and dorsoventral (DV) −8.4 mm from skull. For ex vivo electrophysiology experiments TH::Cre rats were injected with the virus at 5–7 weeks old, using one injection of 1 μl/hemisphere at AP −4.8 mm, ML ±1.0 mm from bregma, and DV −7.1 mm from skull (5° angle). Each infusion was slowly delivered over 2 min, and the needle was left in place for an additional 5 min. Animals received 5 mg/ml/kg carprofen (subcutaneous) during surgery and 24 h later for analgesia, and were allowed a 3 week recovery before onset of experiments.
Chemogenetic inhibition of VTA dopamine cells during mating.
TH::Cre transgenic males expressing Cre-driven Gi DREADDs received either mating sessions (sexually experienced) or control handling (sexually naive) over 5 consecutive days. Forty minutes before each session, the animals were administered clozapine N-oxide (CNO; 1 mg/ml/kg dissolved in saline, s.c.; Tocris Bioscience) or the vehicle control saline (1 ml/kg, s.c.). The dose of CNO was chosen based on previous behavioral studies using hM4Di manipulations (Robinson et al., 2014; Jennings et al., 2015).
For sexual behavior testing, males were placed in a mating arena (60 × 45 × 50 cm) with clean bedding for 5 min, after which they mated with a receptive female until one ejaculation. If males did not reach ejaculation, tests were terminated 1 h after introduction of the female. Males assigned to the sexually naive group were placed in identical test cages with clean bedding for 30 min, but did not receive sexually receptive females. Naive males were housed and handled in the same rooms as the sexually experienced males and thus subjected to identical environmental conditions. All testing took place 3–6 h after lights off, under dim red lighting, and were videotaped.
The following mating parameters were calculated for sexually experienced animals: latencies to first anogenital investigation and numbers of anogenital investigations during the first 2 min, first mount (time from introduction of female to first mount), intromission (time from introduction of female to first intromission), and ejaculation (time from first intromission to ejaculation); copulation efficiency [number of intromissions/(number of mounts+intromissions)]; and percentages of animals displaying mounts, intromissions, and ejaculations. Parameters were compared between drug-treatment groups and between mating sessions with a repeated-measures two-way ANOVA using 95% confidence levels. Animals were included in analyses only for sexual parameters that they displayed for each of the sessions. Percentages of animals displaying each behavior were compared between groups for each mating session using χ2 analysis.
Amphetamine CPP testing.
One week after the last mating or handling session, all animals were subjected to CPP for amphetamine, as previously described (Pitchers et al., 2010a; Frohmader et al., 2011; Beloate et al., 2016). The CPP apparatus consists of two main chambers separated by a smaller middle chamber, each distinguishable by visual and tactile cues (Med Associates). Testing occurred over 4 d. On day 1, a 15 min pretest was conducted, in which males were allowed to freely roam the CPP apparatus. On days 2 and 3, animals were administered d-amphetamine hemisulfate salt (Sigma-Aldrich; product #A5880; 0.5 mg/ml/kg calculated on the basis of the free base, s.c.) and confined to one chamber of the apparatus for 30 min, or were administered vehicle (saline) and confined to the other chamber for 30 min. The order of treatments was counterbalanced, and the design was unbiased (half of the animals had amphetamine paired with the initially preferred chamber and half with the initially nonpreferred chamber). At this dose, sexually experienced rats develop a CPP while naive rats do not (Pitchers et al., 2010a, 2013; Beloate et al., 2016). On day 4 the change in preference was determined during a post-test procedurally identical to the pretest.
The CPP score (difference in time spent in the amphetamine-paired chamber during the post-test minus the pretest) was calculated for each animal. Animals were excluded from analysis if they displayed an initial preference of ≥120 s for one of the three chambers (n = 1 in each group) or a CPP score >3 SDs from the average of the group (n = 1 in each group). CPP scores were compared between groups and analyzed using a one-way ANOVA on ranks followed by Dunn's post hoc analysis. Saline-pretreated and CNO-pretreated sexually naive groups were not statistically different and were combined for this analysis. For all tests, 95% confidence levels were used.
Tissue processing
Tissue collection.
Five days following CPP testing, the effects of inhibiting VTA dopamine neurons on mating-induced cFos expression were examined. CNO (1 mg/ml/kg dissolved in saline, s.c.) or saline (1 ml/kg, s.c.) was administered 30 min before mating to one ejaculation or handling, using the same group designation as during the initial stage of the experiment (sexually experienced, mating; sexually naïve, no mating). All animals received an overdose of sodium pentobarbital (Vortech Pharmaceutical; 390 mg/ml/kg, i.p.) 1 h after the initiation of mating and were transcardially perfused with 10 ml saline [0.9% NaCl (Sigma-Aldrich) in ddH2O] and 500 ml of 4% paraformaldehyde [Electron Microscopy Sciences; in 0.1 m phosphate buffer (PB)]. Brains were removed and postfixed for 1 h in the same fixative at room temperature and stored in a sucrose solution [Thermo Fisher Scientific; 20% in 0.1 m PB containing 0.01% sodium azide (Sigma-Aldrich)] at 4°C.
Immunohistochemistry.
Using a freezing stage microtome (SM 2000R, Leica Biosystems), brains were sectioned coronally (35 μm) into four parallel series and stored in −20°C in cryoprotectant solution [30% sucrose in 0.1 m PB containing 30% ethylene glycol (Thermo Fisher Scientific) and 0.01% sodium azide] until processing. For all staining procedures detailed below, free-floating sections were incubated and washed at room temperature under gentle agitation, thoroughly washed in PBS, pH 7.4, between all incubations, and exposed to 1% H2O2 (10 min; in PBS) and antibody incubation solution [1 h; PBS containing 0.1% bovine serum albumin (Thermo Fisher Scientific) and 0.4% Triton X-100 (Sigma-Aldrich)]. Sections were mounted onto Superfrost plus glass slides (Fisher Laboratories) and coverslipped with gelvatol containing antifading agent 1,4-diazabicyclo(2,2)octane (Sigma-Aldrich; 50 mg/ml; for all immunofluorescence detection) or dehydrated and coverslipped with dibutyl phthalate xylene (Electron Microscopy Sciences; for chromogen detection). For each of the staining procedures, sections of all animals were processed simultaneously.
mCherry and TH.
One series of sections per animal containing VTA was incubated in mouse anti-TH (EMD Millipore, catalog #MAB5280; RRID:AB_2201526; 1:1000; 17 h), Dylight 488-conjugated goat anti-mouse (Thermo Fisher Scientific, catalog #SA-35503; RRID:AB_1965946; 1:100 in PBS; 30 min), rabbit anti-mCherry (Abcam, catalog #ab167453; RRID:AB_2571870; 1:40,000; 17 h), and Alexa 555-conjugated goat anti-rabbit (Invitrogen, catalog #A21428; RRID:AB_141784; 1:100 in PBS; 30 min). The antibody for TH has been previously used and validated (Balfour et al., 2004; Baltazar et al., 2013; Pitchers et al., 2014), and mCherry immunostaining was validated to be 100% colocalized with the endogenous mCherry expressed by the viral construct in a separate series of sections.
cFos and TH.
One series of sections containing VTA was incubated with rabbit anti-cFos (Santa Cruz Biotechnology, catalog sc-52; RRID:AB_2106783; 17 h), biotinylated goat anti-rabbit (Vector Laboratories, catalog #BA-1000; RRID:AB_2313606; 1:500; 1 h), avidin-biotin-horseradish peroxidase (ABC Elite; Vector Laboratories, catalog #PK-6100; RRID:AB_2336819; 1:1000 in PBS; 1 h), and 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich) with 0.02% nickel sulfate (Sigma-Aldrich) and 0.015% H2O2 in 0.1 m PB (10 min). Next, sections were incubated in a mouse anti-TH (Millipore, catalog #MAB5280; 1:800,000; 17 h), biotinylated goat anti-mouse (Vector Laboratories, catalog #BA-9200; RRID:AB_2336171; 1:500; 1 h), ABC Elite (Vector; 1:1000 in PBS; 1 h), and 0.02% DAB (Sigma-Aldrich) with 0.015% H2O2 in 0.1 m PB (10 min). The cFos antibody has been previously validated to specifically recognize cFos (Pitchers et al., 2010b; Beloate et al., 2016).
cFos.
One series of sections containing VTA projection areas were incubated in rabbit anti-cFos (Santa Cruz Biotechnology, catalog #sc-52; 17 h), biotinylated goat anti-rabbit (Vector Laboratories, catalog #BA-1000; 1:500; 1 h), avidin-biotin-horseradish peroxidase (ABC Elite; Vector Laboratories, catalog #PK-6100; 1:1000 in PBS; 1 h), and 0.02% DAB (Sigma-Aldrich) with 0.02% nickel sulfate (Sigma-Aldrich) and 0.015% H2O2 in 0.1 m PB (10 min).
ΔFosB.
One series of sections containing VTA projection areas were incubated in a rabbit polyclonal antibody raised against an internal region shared by FosB and ΔFosB (pan-FosB; Santa Cruz Biotechnology, catalog #sc-48; RRID:AB_631515; 1:5000; 17 h), biotinylated goat anti-rabbit IgG (Vector Laboratories; 1:500; 1 h), ABC Elite (Vector Laboratories; 1:1000 in PBS; 1 h), and 0.02% DAB (Sigma-Aldrich) with 0.02% nickel sulfate (Sigma-Aldrich) and 0.015% H2O2 in 0.1 m PB (10 min). The FosB antibody has been previously validated to specifically visualize ΔFosB under these conditions (Perrotti et al., 2004, 2008; Pitchers et al., 2010a,b, 2013).
Tissue analyses
DREADD expression.
Sections fluorescently stained for TH and mCherry were examined and the location of mCherry expression was mapped throughout the rostral-to-caudal and medial-to-lateral extent of the VTA. All animals had sufficient spread throughout the VTA and none were excluded from the analyses. For a representative subpopulation of animals (n = 10), images of rostral-caudal levels of VTA were taken at 10× magnification, and numbers of TH+, mCherry+, and TH+/mCherry± cells were quantified in the medial (400 × 300 μm) and lateral (500 × 600 μm) subregions of the VTA using Neurolucida (MBF Bioscience; RRID:SCR_004314).
Mating-induced cFos expression in the VTA.
Numbers of TH+, cFos+, and cFos+/TH+ cells were quantified in medial and lateral VTA subregions at three rostrocaudal levels (−4.56 to −6.00 mm from bregma) using a bright-field microscope (Leica DMR; Leica Microsystems) with a camera lucida phototube attachment at 10× magnification. The VTA subregions were based on the findings of Fu et al. (2012) and the rostrocaudal levels were based on the findings of Balfour et al. (2004). The medial subregions analyzed included the rostral linear nucleus (RLi), interfascicular nucleus (IF), and caudal linear nucleus of raphe (CLi; all 200 × 600 μm area of analysis), and the lateral subregions included the rostral, middle, and caudal parabrachial pigmented nucleus (PBP; 1000 × 800 μm area of analysis). All counts were averaged per animal, per area of analysis, and compared between groups using a two-way ANOVA (sexually experienced vs naïve; CNO vs vehicle) and Holm–Sidak pairwise comparisons, all with p < 0.05 for significance.
Mating-induced cFos and experience-induced ΔFosB in VTA projection areas.
cFos-immunoreactive or FosB-immunoreactive cells were quantified using a bright-field microscope with drawing tube attachment (Leica DMR; Leica Microsystems). The areas analyzed included the NAc shell and core (400 × 600 μm per subregion); the anterior cingulate area (ACA); the infralimbic (IL) and prelimbic (PL) subareas (600 × 800 μm per subregion) of the medial prefrontal cortex (mPFC); and the basolateral amygdala (BLA; 400 × 300 μm). Two-to-three sections per animal were counted and the counts were averaged per animal, per area of analysis. All measures were compared between groups using a two-way ANOVA (sexually experienced vs naïve; CNO vs vehicle) and Holm–Sidak pairwise comparisons, all with p < 0.05 for significance.
Morphological analysis of VTA dopamine cells.
Images of TH-immunoreactive neurons in the lateral PBP subregion at the middle level (−5.28 mm to −5.64 mm from bregma) VTA were taken at 40× magnification from tissue processed for TH and cFos. The soma size was analyzed using ImageJ (National Institutes of Health). Mean area and perimeter were measured as described in Pitchers et al. (2014). An average of ≥20 cells per animal was analyzed and only cells with a clearly visible nucleus were included. For each animal, the mean area and perimeter were calculated. Measures were compared between groups using a two-way ANOVA (sexually experienced vs naïve; CNO vs vehicle) and Holm–Sidak pairwise comparisons, all with p < 0.05 for significance.
Electrophysiological studies
Horizontal slices of the midbrain (300 μm) were prepared from TH::Cre rats injected with rAAV5/hSyn-DIO-hm4D-mCherry (University of North Carolina Vector Core, Chapel Hill, NC) as described above and by Pandit et al. (2016), using a vibratome (Leica VT1200S, Leica Microsystems) in ice-cold modified artificial CSF (ACSF) containing the following (in mm): 92 N-methyl-D-glucamine, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 napyruvate, 0.5 CaCl2·4H2O, and 10 MgSO4·7H2O, bubbled with 95% O2 and 5% CO2, pH 7.3–7.4. Slices were initially recovered in carbogenated modified ACSF for 15 min at 34°C and then transferred into a holding chamber with standard ACSF [containing as follows (in mm): 126 NaCl, 3 KCl, 2 MgSO4, 2 CaCl2, 10 glucose, 1.25 NaH2PO4, and 26 NaHCO3) bubbled with 95% O2 and 5% CO2, pH 7.3, at room temperature for ≥1 h. The slices were transferred one at a time to the recording chamber perfused with standard ACSF continuously bubbled with 95% O2 and 5% CO2 at 30–32°C. Whole-cell patch-clamp recordings were made from VTA dopamine neurons visualized with an Olympus BX61W1 microscope (equipped with an mCherry filter) using infrared video microscopy and differential interference contrast optics. VTA dopaminergic neurons were identified by mCherry fluorescence. Patch electrodes were pulled from borosilicate glass capillaries; they had a resistance of 3–5 MΩ when filled with intracellular solutions. Internal solution contained the following (in mm): 140 K-gluconate, 1 KCl, 10 HEPES, 0.5 EGTA, 4 MgATP, 0.4 Na2GTP, 4 phosphocreatine, pH 7.3 with KOH). Signals were amplified, filtered at 3 kHz, and digitized at 10 kHz using an EPC-10 patch-clamp amplifier and PatchMaster v2x73 software. Series resistance was constantly monitored, and the cells were rejected from analysis if the resistance changed by >20%. No series resistance compensation was used. Resting membrane potential was measured in bridge mode (I = 0) immediately after obtaining whole-cell access. The basic electrophysiological properties of the cells were determined from the voltage responses to a series of hyperpolarizing and depolarizing square current pulses. Input resistance was determined by the slope of the linear regression line through the voltage–current curve. To determine the direct effect of CNO, synaptic inputs onto these neurons were blocked using inhibitors of synaptic transmission [10 μm CNQX (Tocris Bioscience), 50 μm APV (Tocris Bioscience), and 20 μm bicuculline (Tocris Bioscience)]. CNO (kindly provided by Bryan Roth and the National Institute of Mental Health) was dissolved in ACSF at a concentration of 10 μm and was applied to the bath through perfusion. This dose of CNO was chosen based on previous studies (Krashes et al., 2011; Robinson et al., 2014). After 10 min baseline recording, CNO was applied for 5–7 min and recordings were continued during and 10–20 min after the CNO washout period. Passive and active membrane properties were analyzed with Clampfit 10 (Axon Instrument) and Mini Analysis (Synaptosoft; RRID:SCR_014441) software.
Results
DREADD expression was limited to VTA dopamine neurons
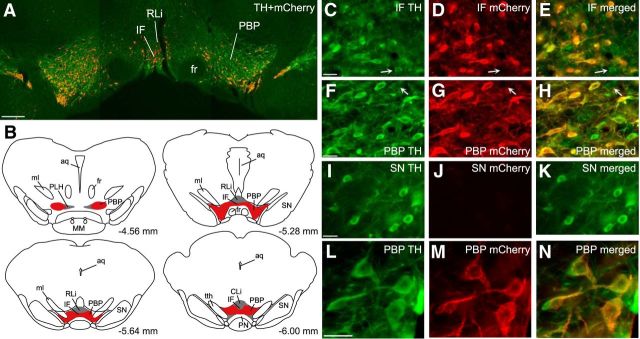
Analysis of hM4D/mCherry following the completion of behavioral studies demonstrated that hM4D/mCherry expression was present throughout the VTA (Fig. 1A–H), but not observed in the substantia nigra (Fig. 1I–K). There was limited variability in the spread of hM4D/mCherry among animals and all animals had mCherry immunoreactivity throughout the rostral-caudal extent of the VTA (from −4.56 to −6.00 mm from bregma; Fig. 1N). In addition, mCherry expression extended rostrally in 36% of animals (all groups) to −4.36 mm from bregma and thus encompassed a portion of the posterior hypothalamus and extended caudally in 43% of animals (all groups) to −6.72 mm from bregma. DREADD expression was also seen throughout the medial-lateral extent of the VTA (Fig. 1B). In the medial subregions of the VTA, hM4D/mCherry expression was observed in 90–100% of animals in the ventral IF (Fig. 1B–E), dorsal RLi, and CLi. In the lateral subdivisions of the VTA, hM4D/mCherry was expressed in 89–100% of animals in the rostral, middle, and caudal PBP and the ventral paranigral nucleus (Fig. 1B, F–H). Six animals (distributed among the groups) had primarily unilateral spread of the DREADD, but these animals did not differ significantly from their respective groups in any outcome measures, and were included in analyses.
Figure 1.
DREADD expression in VTA dopamine cells. A, Representative image of TH (green) and mCherry (red) expression in the VTA. Scale bar, 200 μm. B, Four line drawings of coronal sections indicating the representative spread of mCherry expression through the rostral (−4.56 mm from bregma), middle (−5.28 and −5.64 mm from bregma) and caudal (−6.00 mm from bregma) subregions of the VTA in all (red) and the majority (gray) of animals. C–K, Representative images of TH (C, F, I), mCherry (D, G, J), and merged TH+mCherry (E, H, K) immunoreactivity in the IF (C–E) and PBP (F–H) subregions of the VTA and in substantia nigra (I–K). Arrows illustrate an example of a VTA TH cell without detectable mCherry expression, while all other TH cells coexpress mCherry. Scale bar, 25 μm. L–N, Higher magnification of representative TH (L), mCherry (M), and merged TH+mCherry (N) immunoreactivity in the PBP subregion of the VTA. Scale bar, 25 μm. SN, Substantia nigra; aq, cerebral aqueduct; ml, medial lemniscus; PLH, peduncular part of lateral hypothalamus; fr, fasciculus retroflexus; MM, mammillary nuclei; tth, trigeminothalamic tract.
Within the VTA, hM4D/mCherry was expressed in ∼92% of TH-immunoreactive cells with similar coexpression in medial (91 ± 1.1%) and lateral (93 ± 1.3%) subregions of the VTA. hM4D/mCherry expression was specific to TH cells and was not observed in non-TH cells in VTA or other brain areas. Thus, hM4D/mCherry was restricted to TH cells in the VTA and expressed in a large majority of VTA cells.
Inhibition of VTA dopamine neurons did not affect sexual behavior
VTA dopamine neuron inhibition did not affect any parameter of sexual behavior during the 5 consecutive days of mating, as CNO-treated groups did not differ significantly from saline-treated groups in the initiation or expression of mating behavior (Fig. 2; Tables 1, 2). Moreover, both CNO-injected and saline-injected groups displayed experience-induced facilitation of sexual behavior (Fig. 2A,B). A significant effect of sexual experience (i.e., mating session), but not of CNO, was observed for numbers of anogenital investigations (F(1,29) = 7.1, p = 0.021), mount latencies (F(6,108) = 10.9, p < 0.001), intromission latencies (F(6,108) = 8.5, p < 0.001), and ejaculation latencies (F(6,98) = 6.0, p < 0.001). Post hoc analyses showed decreased latencies to ejaculation on days 3, 4, and 5, mount on days 4 and 5, and intromission on day 5, compared with the first day of mating. This decrease in latencies is indicative of experience-induced facilitation of mating in both groups (all p's < 0.001). There were no effects of CNO, except for a main effect of CNO on intromission latencies (F(1,108) = 4.7, p = 0.043), but no group differences were detected in any post hoc comparisons. The approach behavior to the female was also unaffected by CNO, and both groups showed a lower percentage of males displaying anogenital investigations and decreased numbers of anogenital investigations with sexual experience on day 5 compared with day 1 (p = 0.020). Finally, mating behavior in saline-injected males appeared similar to that observed in males without viral vectors in previous studies (Balfour et al., 2004; Pitchers et al., 2010b, 2013, 2014; Beloate et al., 2016), suggesting that neither expression of DREADD in VTA dopamine neurons or the use of transgenic TH::Cre rats had an impact on the initiation and expression of sexual behavior or experience-induced facilitation. Together, these results indicate that activation of VTA dopamine neurons is not essential for the initiation or expression of male sexual behavior, or for the facilitation of sexual behavior following acquisition of mating experience.
Figure 2.
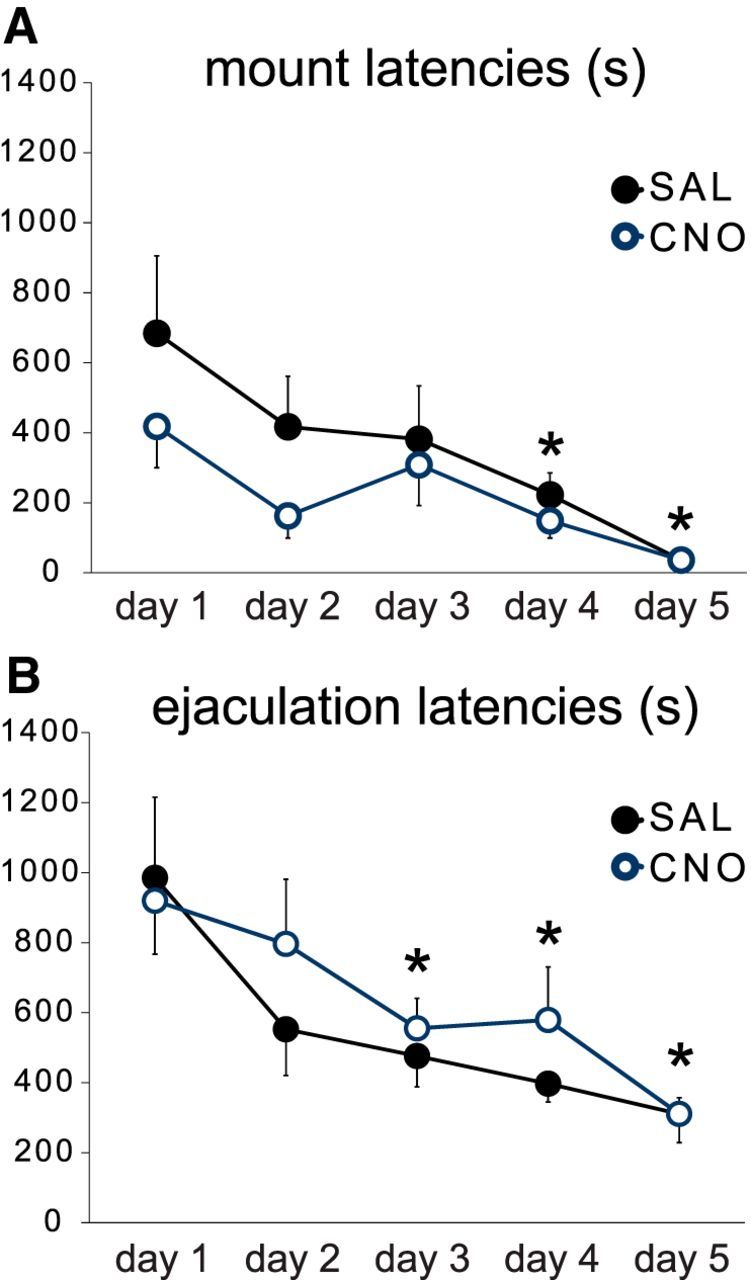
CNO did not affect sexual behavior during acquisition of sexual experience. A, B, Quantitative analysis of latencies (in seconds) to mount (A) and ejaculation (B) during 5 consecutive days of sexual behavior in saline-pretreated (SAL; black line, closed markers; n = 10) and CNO-pretreated (blue line, open markers; n = 11) TH::Cre transgenic rats expressing Gi DREADD in VTA TH cells. Data represent mean ± SEM *, Significant difference compared with day 1 of both groups combined.
Table 1.
Sexual behavior parameters during 5 consecutive days of matinga
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Intromission latency | |||||
| Saline | 716 ± 236 s | 440 ± 161 s | 842 ± 310 s | 365 ± 162 s | 42 ± 12 sb |
| CNO | 434 ± 117 s | 175 ± 64 s | 315 ± 115 s | 154 ± 49 s | 42 ± 13 sb |
| Copulation efficiency [intromissions/( mounts + intromissions)] | |||||
| Saline | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.0 | 0.7 ± 0.0 |
| CNO | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.0 | 0.7 ± 0.1 |
| Mounts (% of males) | |||||
| Saline | 70 | 80 | 90 | 90 | 91 |
| CNO | 91 | 91 | 73 | 100 | 91 |
| Intromissions (% of males) | |||||
| Saline | 90 | 80 | 100 | 90 | 100 |
| CNO | 91 | 100 | 73 | 100 | 100 |
| Ejaculations (% of males) | |||||
| Saline | 70 | 80 | 90 | 90 | 100 |
| CNO | 64 | 91 | 73 | 100 | 100 |
aCNO did not affect sexual behavior during acquisition of sexual experience. Quantitative analysis of intromission latencies (in seconds), copulation efficiency, percentages of males that display mounts, intromissions, and ejaculations, during 5 consecutive days of sexual behavior in saline-pretreated (n = 10) and CNO-pretreated (n = 11) TH::Cre transgenic rats expressing Gi DREADD in VTA TH cells. Data represent mean ± SEM.
bSignificant difference compared to day 1.
Table 2.
Effects of CNO on anogenital investigationa
| Group | Day 1 | Day 5 |
|---|---|---|
| Numbers | ||
| Saline | 7.33 ± 1.26 | 3.17 ± 1.85b |
| CNO | 6.00 ± 0.55 | 4.22 ± 1.18b |
| Latencies | ||
| Saline | 14.33 ± 3.70 s | 17.25 ± 8.72 s |
| CNO | 23.50 ± 1.26 s | 12.71 ± 3.88 s |
| % Males | ||
| Saline | 100.00 | 50.00 |
| CNO | 100.00 | 77.78 |
aCNO did not affect anogenital investigations during acquisition of sexual experience. Quantitative analysis of numbers of anogenital investigations, latencies (seconds) to first anogenital investigation, and percentages of males that display anogenital investigations, during the first 2 min of day 1 and day 5 of 5 consecutive days of sexual behavior in saline-treated (n = 6) and CNO-treated (n = 9) TH::Cre transgenic rats expressing Gi DREADD in VTA TH cells. Data represent mean ± SEM.
bSignificant difference compared to day 1. No significant differences between saline and CNO groups were detected.
Validation of inhibition of VTA dopamine neurons during mating
Next, using cFos as the neural activation marker, it was verified that CNO administration did indeed block VTA dopamine cell activation during sexual behavior (Fig. 3). There were statistically significant main effects of mating behavior on numbers of cFos/TH dual-labeled cells in the IF (F(1,28) = 13.6, p < 0.001), the CLi (F(1,28) = 10.9, p = 0.003), and the middle PBP (F(1,28) = 9.0, p < 0.001), but not in the RLi, the rostral PBP, or the caudal PBP. In addition, there were interaction effects of mating and CNO on numbers of cFos/TH dual-labeled cells in the IF (F(1,28) = 5.2, p = 0.031) and the middle PBP (F(1,28) = 9.0, p < 0.001), but not in the CLi, and an effect of CNO in the middle PBP (F(1,28) = 9.0, p = 0.007). Similar effects were also observed on the percentages of TH cells that coexpressed cFos: main effects of mating in the IF (F(1,28) = 12.2, p = 0.002), CLi (F(1,28) = 6.9, p = 0.014), and caudal PBP (F(1,28) = 4.4, p = 0.044); and a trend in the middle PBP (F(1,28) = 3.7, p = 0.064). Interactions between mating and CNO were found for the IF (F(1,28) = 5.2, p = 0.031) and middle PBP (F(1,28) = 11.6, p = 0.002), with a main effect of CNO in the middle PBP (F(1,28) = 9.4, p = 0.005). Thus, the strongest effects of mating and CNO were noted in the IF and the middle PBP. Overall, the finding that mating activated fewer cells in the lateral and caudal VTA subregions agrees with our previous finding (Balfour et al., 2004). It is important to note that there were no differences in total numbers of TH-immunoreactive cells between groups in any of the VTA subregions, suggesting that repeated CNO administration and dopamine cell inactivation did not cause major damage or changes to TH cells (Table 3).
Figure 3.
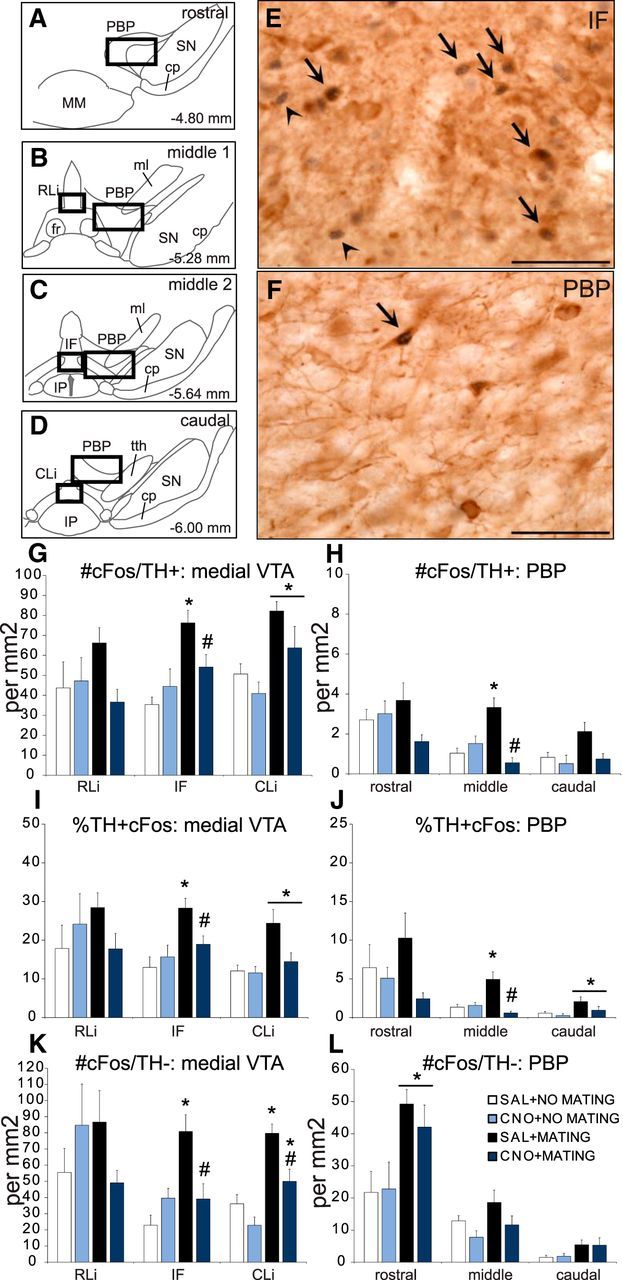
CNO blocked mating-induced cFos in VTA dopamine cells. A–D, Four representative rostral-caudal coronal VTA sections (−4.80, −5.28, −5.64, −6.00 mm from bregma), with boxes indicating the areas of analysis (IF, 200 × 600 μm; PBP, 1000 × 800 μm). E, F, Representative images of cells immunopositive for cFos and TH (cFos/TH+; arrows) or cFos only (cFos/TH−; arrowhead) cells in the IF (E) and PBP (F) subregions of the VTA. Scale bar, 50 μm. MM, Mammillary nuclei; SN, Substantia nigra; cp, cerebral peduncle; fr, fasciculus retroflexus; ml, medial lemniscus; IP, interpeduncular nucleus; tth, trigeminothalamic tract. G–L, Quantitative analysis of the number of cFos/TH+ cells (per mm2; G, H), percentage of TH cells that are also cFos-positive (I, J), and number of cFos/TH− cells (per mm2) in rostral-middle-caudal levels (K, L) through the medial (G, I, K) and lateral (H, J, L) VTA subregions. Groups: saline + no mating (SAL+NO MATING), white bars, n = 6; CNO + no mating (CNO+NO MATING), light blue bars, n = 6; saline + mating (SAL+MATING), black bars, n = 10; CNO + mating (CNO+MATING), dark blue bars, n = 10. Data represent mean ± SEM. * with line, Significant difference, compared with no-mating control groups. *, Significant difference compared with the saline + no-mating control group. #, Significant difference compared with saline + mating group.
Table 3.
TH immunoreactivity in the VTAa
| Group | RLi | IF | CLi | PBP |
||
|---|---|---|---|---|---|---|
| Rostral | Middle | Caudal | ||||
| Saline + no mating | 34 ± 7 | 38 ± 6 | 53 ± 7 | 55 ± 12 | 67 ± 9 | 122 ± 13 |
| CNO + no mating | 27 ± 2 | 35 ± 2 | 43 ± 3 | 53 ± 7 | 76 ± 10 | 123 ± 18 |
| Saline + mating | 30 ± 3 | 34 ± 2 | 46 ± 4 | 52 ± 10 | 67 ± 13 | 109 ± 15 |
| CNO + mating | 29 ± 4 | 35 ± 3 | 52 ± 3 | 62 ± 9 | 68 ± 10 | 101 ± 9 |
aCNO did not affect TH immunoreactivity in the VTA. Quantitative analysis of numbers of TH-positive cells in the RLi, IF, CLi, and rostral, middle, and caudal VTA subregions. Data represent mean ± SEM.
Post hoc analyses in the IF and middle PBP showed that mating increased cFos expression in TH cells in saline-treated mating males compared with nonmating males (all p's < 0.001). In contrast, mating did not increase cFos in TH cells in CNO-treated mated males and numbers and percentages of dual-labeled cFos/TH cells in mated CNO-treated males were significantly lower compared with saline-treated mated animals [number of cFos/TH+ cells: IF (p = 0.014), middle PBP (p < 0.001); percentage of TH/cFos cells: IF (p = 0.008), middle PBP (p < 0.001)], and did not differ from CNO-treated nonmating control males (Fig. 3G–J). In the CLi, CNO-mated males did not show significantly increased cFos in TH cells compared with CNO-treated nonmating controls (Fig. 3G,I). CNO itself had no effect on cFos in nonmating control animals, and cFos in TH cells did not differ from saline-treated nonmating controls (Fig. 3G–J).
Finally, there were also significant effects of mating, CNO, and interactions in the IF, CLi, and rostral PBP on cFos expression in TH-negative neurons (mating: IF, F(1,29) = 6.5, p = 0.017; CLi, F(1,28) = 27.0, p < 0.001; rostral PBP, F(1,28) = 12.1, p = 0.002; CNO: CLi, F(1,28) = 1.4, p = 0.004; interaction between mating and CNO: IF, F(1,28) = 9.0, p = 0.006; Fig. 3K,L). Post hoc analyses showed that mating increased cFos in TH-negative cells in saline-treated males in the IF (p < 0.001), CLi (p < 0.001), and rostral PBP (p = 0.002) compared with saline-treated no-mating controls. CNO significantly reduced mating-induced cFos in the IF (p = 0.002) and CLi (p = 0.004) compared with saline-treated mated controls. In the CLi, this was a partial reduction and mating-induced cFos was significantly higher in CNO-treated mated males compared with nonmated males (p < 0.001). Since DREADD was not found in any non-TH cells in the VTA, the blockade of cFos activation is unlikely a direct effect of CNO, and rather an indirect result of dopamine cell inhibition on neighboring VTA neurons.
VTA dopamine cell inhibition did not affect mating-induced cFos in VTA projection areas
In contrast to the effects of VTA dopamine cell inactivation on mating-induced cFos in the VTA, mating-induced cFos in VTA projection areas remained unaffected by CNO administration. There was a significant main effect of mating behavior on cFos expression in the NAc (core, F(1,29) = 13.9, p < 0.001; shell, F(1,29) = 15.3, p < 0.001), mPFC (ACA, F(1,28) = 12.2, p = 0.002), PL (F(1,28) = 4.4, p < 0.001), IL (F(1,28) = 9.1, p < 0.001), and BLA (F(1,29) = 29.7, p < 0.001), but no effects of CNO treatment or interactions were noted (Fig. 4). Mating increased cFos immunoreactivity in NAc core (p < 0.001), shell (p < 0.001), BLA (p < 0.001), ACA (p = 0.002), PL (p = 0.046), and IL (p = 0.005) compared with the nonmating groups.
Figure 4.
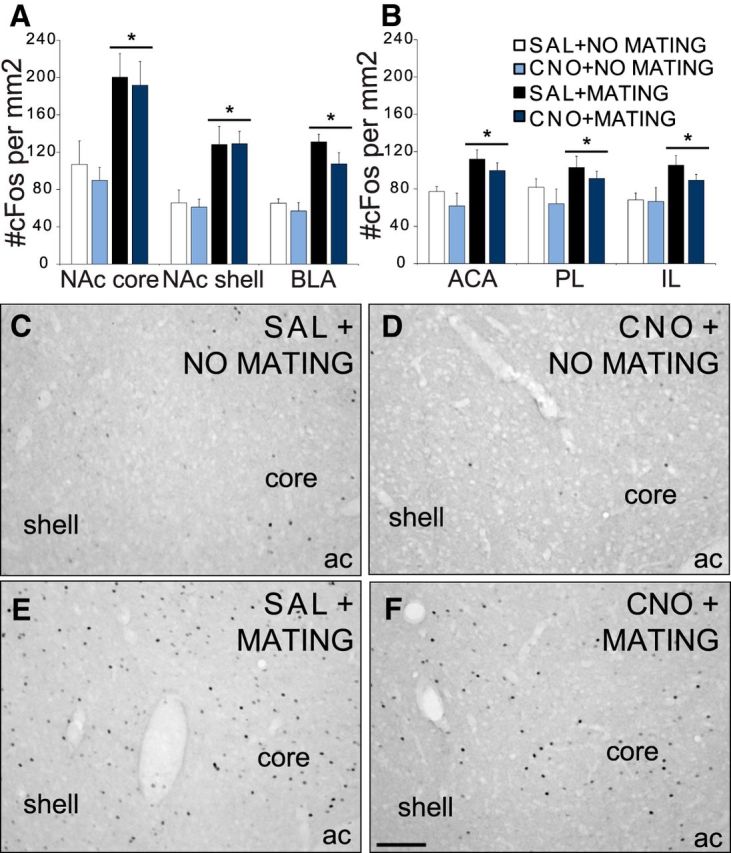
CNO did not affect mating-induced cFos in VTA projection areas. A, B, Quantitative analysis of cFos immunoreactivity (per mm2) in NAc core and shell, BLA (A), and ACA, PL, and IL subregions of mPFC (B) in saline + no mating (SAL+NO MATING; white bars; n = 6) or CNO + no mating (CNO+NO MATING; light blue bars; n = 6) and saline + mating (SAL+MATING; black bars; n = 10) or CNO + mating (CNO+MATING; dark blue bars; n = 11) groups. Data represent mean ± SEM. C–F, Representative images of cFos expression in NAc of SAL+NO MATING (C), CNO+NO MATING (D), SAL+NO MATING (E) and CNO+MATING (F) groups. Scale bar,100 μm. ac, Anterior commissure. *, Significant differences to no-mating controls.
In vitro validation of chemogenetic inhibition of VTA dopamine neurons
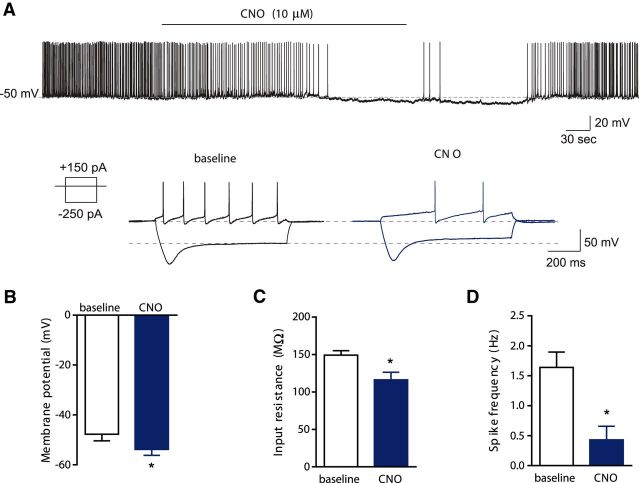
To confirm inhibition of dopamine neurons upon CNO application, targeted whole-cell current-clamp recordings were made from hM4D/mCherry-expressing dopamine neurons in VTA slices prepared from TH::Cre rats with hM4D expression in a separate set of male rats. Similar to the distribution in the behavioral studies, mCherry-expressing neurons were located in both lateral and medial regions of the anterior through posterior VTA. To determine the direct effect of CNO, synaptic inputs onto these neurons were blocked using inhibitors of synaptic transmission (10 μm CNQX, 50 μm APV, and 20 μm bicuculline). Bath application of CNO (10 μm) hyperpolarized the membrane potential by 6.2 ± 0.5 mV (p < 0.0001) and reduced the input resistance (p = 0.001) of hM4D-expressing VTA dopamine neurons compared with the baseline condition (Fig. 5A–C). Furthermore, CNO decreased the firing rate of spontaneous action potentials from 1.64 ± 0.3 Hz to 0.4 ± 0.2 Hz (p = 0.007; Fig. 5D) and the number of evoked action potentials in hM4D-expressing neurons (Fig. 5A, sample trace). Effects of CNO on dopamine neurons were reversible following CNO washout. CNO did not affect the membrane potential of non-hM4D/mCherry-expressing neurons in the VTA. Together, these results show that CNO-activation of hM4D reduced the excitability of VTA dopamine neurons.
Figure 5.
Inhibition of hM4D-expressing VTA dopamine neurons following CNO application. A, Sample traces of spontaneous (top) and evoked action potentials (bottom) in hM4D-expressing dopamine neurons before and after CNO (10 μm) application. B–D, Group data for the effect of CNO on membrane potential (B), input resistance (C), and firing rate of the spontaneous action potentials (D; n = 7) in hM4D-expressing VTA dopamine neurons. Data represent mean ± SEM. *, Significant difference compared with the baseline.
VTA dopamine cell inhibition during sex experience prevented cross-sensitization of amphetamine CPP
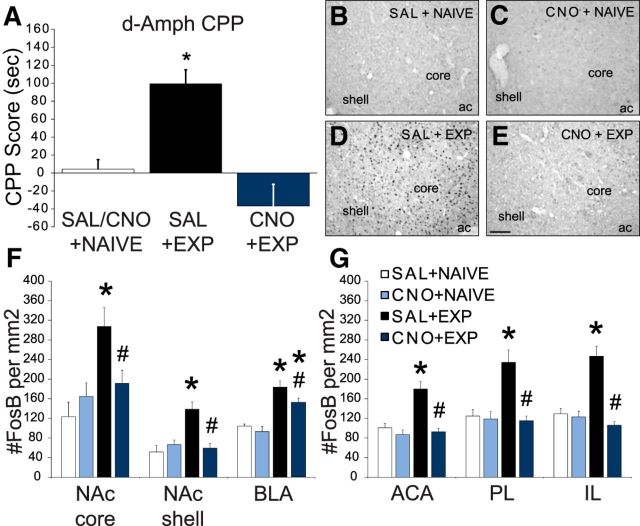
Results thus far have demonstrated that VTA dopamine cell activation is not essential for the initiation, expression, and facilitation of sexual behavior. Next, we tested the hypothesis that VTA dopamine cell activation during the acquisition of sexual experience is essential for the subsequent development of cross-sensitization of amphetamine reward-seeking behavior. First, sexually naive animals did not form an amphetamine CPP, and CNO-pretreated naive males did not differ from vehicle-treated naive males; hence vehicle-pretreated and CNO-pretreated naive groups were combined for analyses. However, CNO administration during sexual experience did affect cross-sensitization of amphetamine CPP (Fig. 6A). Sexual experience in vehicle-pretreated controls significantly increased amphetamine CPP compared with sexually naive controls (H = 9.041, p = 0.011), thereby replicating previously published observations in wild-type Sprague Dawley males (Pitchers et al., 2010a,b, 2013, 2014; Beloate et al., 2016). Moreover, in support of our hypothesis, CNO pretreatment prevented the effects of sexual experience on amphetamine CPP, as the CNO sexually experienced males were significantly lower than vehicle-pretreated sexually experienced males and did not significantly differ from naive controls (p < 0.05).
Figure 6.
CNO blocked effects of sexual experience on amphetamine CPP and ΔFosB expression. A, CPP scores (time spent in amphetamine-paired chamber, post-test–pretest; in seconds) of sexually naive (white bars; saline-treated and CNO-treated groups were combined into one saline/CNO + naive (SAL/CNO+NAIVE) group; n = 8 (including saline, n = 3 and CNO, n = 5), saline + experienced (SAL+EXP; black bars; n = 8), CNO + experienced (CNO+EXP; dark blue bars; n = 9). *, Significant difference, compared with SAL/CNO+NAIVE. B–E, Representative images of ΔFosB immunoreactivity in NAc of SAL+NAIVE (B), CNO+NAIVE (C), SAL+EXP (D), and CNO+EXP (E) groups. Scale bar, 100 μm. ac, Anterior commissure. F, G, Quantitative analysis of numbers of ΔFosB-positive cells (per mm2) in VTA projection areas in SAL+NAIVE (white bars; n = 6), CNO+NAIVE (light blue bars; n = 6), SAL+EXP (black bars; n = 10), and CNO+EXP (dark blue bars; n = 9) groups. Data represent mean ± SEM. *, Significant difference from naive group within same treatment: compared with SAL+NAIVE or CNO+NAIVE. #, Significant difference from SAL+EXP.
VTA dopamine cell inhibition during sex experience prevented ΔFosB expression in VTA projection areas
VTA dopamine cell inactivation during sexual experience also blocked induction of ΔFosB in areas that receive dopaminergic input from the VTA (Fig. 6B–G). There were significant main effects of both sexual experience and CNO, and significant interactions between sexual experience and CNO treatment in the NAc, mPFC, and BLA (NAc: sex experience: core, F(1,27) = 9.4, p = 0.005; shell, F(1,27) = 9.3, p = 0.005; CNO: shell, F(1,27) = 6.1, p = 0.020; interaction: core, F(1,27) = 5.2, p = 0.030; shell, F(1,27) = 12.9, p = 0.001; subregions of the mPFC: sex experience: ACA, F(1,25) = 15.4, p < 0.001; PL, F(1,25) = 10.4, p = 0.003; IL, F(1,25) = 14.2, p < 0.001; CNO: ACA, F(1,25) = 12.6, p < 0.001; PL, F(1,25) = 14.4, p < 0.001; IL, F(1,25) = 30.4, p < 0.001; interaction: ACA, F(1,25) = 11.3, p = 0.003; PL, F(1,25) = 11.8, p = 0.002; IL, F(1,25) = 25.6, p < 0.001; BLA: sex experience, F(1,25) = 47.7, p < 0.001; CNO, F(1,25) = 5.1, p = 0.033). In these areas, sexual experience induced ΔFosB in vehicle-pretreated males, replicating previous findings in Sprague Dawley males (Pitchers et al., 2010b, 2014; Beloate et al., 2016) as saline-injected sexually experienced rats had increased ΔFosB expression in the NAc core and shell (p < 0.001); in the ACA, PL, and IL subregions (all p's < 0.001) of the mPFC; and in the BLA (p = 0.015), compared with saline-injected sexually naive controls (Fig. 6F,G). However, CNO significantly blocked the effect of sexual experience in all areas (NAc and mPFC, p < 0.001; BLA, p = 0.015) compared with saline-treated sexually experienced males. In the BLA, this reduction was partial, and CNO-treated sexually experienced males had higher ΔFosB expression (p < 0.001) compared with CNO-treated sexually naive controls. Finally, CNO pretreatment during handling did not affect ΔFosB expression in these brain areas of sexually naive males.
VTA dopamine cell activation during sex experience is essential for dopamine neuron morphological changes
Along with prevention of sex experience-induced amphetamine reward cross-sensitization and ΔFosB expression, CNO administration during sexual experience partially attenuated experience-induced morphological changes in VTA dopamine soma size (Fig. 7). First, the effects of sex experience on reduction of soma area and perimeter were confirmed as previously described for Sprague Dawley rats (Pitchers et al., 2014), and there was a significant interaction effect of sexual experience and CNO treatment on soma perimeter (sex experience, F(1,25) = 28.7, p < 0.001; interaction, F(1,25) = 10.5, p = 0.004) and area (sex experience, F(1,25) = 29.0, p < 0.001; interaction, F(1,25) = 5.0, p = 0.036). Post hoc analyses revealed that compared with the saline-injected naive control group, the saline-injected sexually experienced group had a significantly decreased perimeter (92% of baseline; p < 0.001) and area (87%; p < 0.037) of VTA TH cells. Moreover, CNO administration attenuated the effects of sex experience on soma size, as cell perimeter in CNO-treated sexually experienced males did not differ from naive controls, and both cell perimeter and area were significantly larger than saline-treated sex experienced controls (perimeter, p < 0.001; area, p = 0.015). Finally, CNO treatment had no effect on TH soma size in naive controls.
Figure 7.
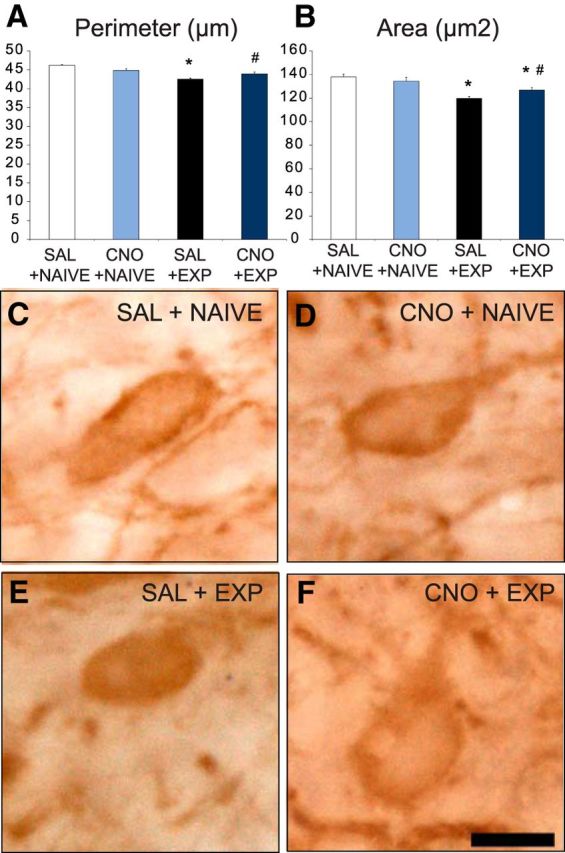
CNO blocked effects of sexual experience on morphology of VTA dopamine cells. A, B, Quantitative analyses of VTA dopamine soma perimeter (A) and area (B) in saline + naive (SAL+NAIVE; white bars; n = 6), CNO + naive (CNO+NAIVE; light blue bars; n = 6; 97% perimeter, 97% area compared with SAL+NAIVE), saline + experienced (SAL+EXP; black bars; n = 9; 92% perimeter, 87% area compared with SAL+NAIVE), and CNO + experienced (CNO+EXP; dark blue bars; n = 10; 95% perimeter, 92% area compared with SAL+NAIVE) groups. Data represent mean ± SEM. *, Significant difference from SAL+NAIVE or CNO+NAIVE. #, Significant difference from SAL+EXP. C–F, Representative images of TH-immunoreactive cells in the PBP of SAL+NAIVE (C), CNO+NAIVE (D), SAL+EXP (E), and CNO+EXP (F) groups. Scale bar, 10 μm.
Discussion
Findings from these studies showed that inactivation of VTA dopamine neurons during mating did not disrupt the initiation, performance, or short-term facilitation of sexual behavior. In contrast, chemogenetic inhibition of VTA dopamine neurons during mating prevented effects of sexual experience on neuronal plasticity in the mesolimbic pathway and on cross-sensitization of amphetamine CPP following sex abstinence. These results support the role for VTA dopamine activity in long-term reward-related learning. Moreover, these findings demonstrate that dopamine activation during natural reward behavior contributes to neuroplasticity mimicked by exposure to drugs of abuse, as VTA dopamine activation during mating was critical for sex experience-induced ΔFosB expression in the NAc, mPFC, and BLA, and the reduction of VTA dopamine soma size. In conclusion, these studies demonstrate that mesolimbic dopamine activation during natural reward exposure can influence vulnerability for drug-seeking behavior following the loss of the natural reward via neural plasticity in the NAc, PFC, BLA, and VTA.
Technological considerations
The current study used chemogenetic inhibition of VTA dopamine neurons, and hM4D/mCherry expression was confirmed to be specific to TH-immunoreactive neurons. Expression of hM4D/mCherry was not observed in non-TH neurons in the VTA or other areas, ruling out nonspecific expression due to retrograde transport of the AAV (Burger et al., 2004; Aschauer et al., 2013) or Cre-recombinase expression in non-TH neurons as reported in mice (Lammel et al., 2008) but not rats (Liu et al., 2016). The TH::Cre rats have previously been validated for optogenetic manipulations of VTA dopamine cells in reward-related learning processes (Steinberg et al., 2013; Gunaydin et al., 2014; Chang et al., 2016). In agreement, here it was confirmed that CNO administration blocked both mating-induced cFos and excitability in VTA dopamine cells. In addition, CNO also decreased cFos expression in non-TH cells in the medial subregions IF and CLi, but not in the lateral subregions of the VTA. Since hM4D/mCherry was not expressed in non-TH cells in these regions, this may reflect reduced inputs from neighboring VTA TH cells, suggesting that dopamine neurons synapse locally on nondopamine neurons in the medial VTA.
VTA dopamine does not regulate initiation, expression, or short-term facilitation of male sexual behavior
VTA dopamine neuron inhibition did not affect approach toward the female, initiation, or performance of mating behavior, suggesting that VTA dopamine activation is not required for the control of sexual behavior per se. This further extends our previous demonstration of the lack of effects of VTA MOR blockade (Pitchers et al., 2014) and NAc D1 receptor blockade (Pitchers et al., 2013). Some effects of dopamine receptor blockade on sexual behavior have been shown using systemic administration (Pfaus and Phillips, 1991; Balthazart et al., 1997), suggesting actions in brain areas outside the mesolimbic pathway, including the medial preoptic area (Will et al., 2014). However, the lack of effects of dopamine cell inhibition on approach to the female and short-term facilitation of approach behavior are not consistent with a general role for dopamine in reward learning and incentive salience (Baik, 2013; Robinson et al., 2016; Hamid et al., 2016). Instead, these findings parallel our previous demonstration using MOR blockade in the VTA, demonstrating that the mesolimbic dopamine system is involved in the long-term memory for sexual reward, but not the initial short-term facilitation of the reward behavior (Pitchers et al., 2014). Finally, VTA dopamine neuron inhibition did not affect mating-induced cFos in the NAc, mPFC, and BLA, demonstrating that this neural activity is independent from dopamine inputs. Instead, NMDA receptor antagonism in the NAc blocks mating-induced cFos (Beloate et al., 2016), suggesting that glutamate, rather than dopamine, is the essential neurotransmitter in this brain region to induce cFos during sexual behavior.
VTA dopamine activity mediates amphetamine cross-sensitization and expression of ΔFosB in the NAc
In line with our hypothesis, chemogenetic inhibition of VTA dopamine activity during mating blocked sex experience-induced increases in amphetamine CPP, even though this cross-sensitization is dependent on abstinence from sexual behavior. These findings suggests that activation of dopamine neurons during sexual behavior results in long-lasting neural alterations that, in turn, influence responses to drugs of abuse. A key mediator for the role of dopamine in long-term cross-sensitization to psychostimulants is likely the transcription factor ΔFosB. Previously, we showed that sex experience-induced ΔFosB expression in the NAc is prevented by NAc infusions of D1 receptor antagonist before mating (Pitchers et al., 2013). ΔFosB expression can be induced in D1-expressing and D2-expressing neurons in the NAc, depending on the type of stimulus, and is induced by optogenetic stimulation of the VTA (Lobo et al., 2013). However, NAc ΔFosB is also prevented by local infusions of NMDA receptor antagonists during mating (Beloate et al., 2016) or can be induced by chronic stimulation of areas that provide glutamatergic inputs to the NAc, including the PFC and the amygdala (Lobo et al., 2013). Therefore, interactions between glutamate and dopamine receptor activation may contribute to ΔFosB induction. A small subpopulation of VTA dopamine neurons coexpress vGluT2 (Yamaguchi et al., 2015; Zhang et al., 2015) and potentially corelease dopamine and glutamate in the NAc (Tecuapetla et al., 2010; Zhang et al., 2015) during sexual behavior. Another question that remains is whether there are specific dopamine cell subpopulations based on afferent or efferent connections that contribute to the cross-sensitization of mating and drug reward, as has been shown for the VTA–NAc pathway in both sucrose self-administration (Boender et al., 2014) and reinforcing intracranial self-stimulation (Beier et al., 2015).
VTA dopamine activity mediates natural reward-induced expression of ΔFosB in the mPFC
In the current study, chemogenetic inactivation of VTA dopamine activity during mating also blocked ΔFosB expression in the IL, PL, and ACA subregions of the mPFC, demonstrating an essential role of dopamine receptor activation for ΔFosB induction in these areas. Such a role of dopamine for ΔFosB in areas outside the NAc has not been established, although D1R has been shown to play a role in Δ9-tetrahydrocannabinol-induced ΔFosB expression in mouse mPFC (Lazenka et al., 2014, 2015). In contrast to the extensive investigation of ΔFosB in the NAc (Nestler, 2015), less is understood regarding the functional role of ΔFosB in the mPFC or BLA. In mice mPFC, ΔFosB is induced by stress partly via cholecystokinin receptor-B activation and mediates increased stress susceptibility (Lehmann and Herkenham, 2011; Vialou et al., 2014). Sexual behavior reduces stress and anxiety-related behaviors, including decreased anxiety-like behavior (Fernández-Guasti et al., 1989; Waldherr and Neumann, 2007) and decreased fear responses (Bai et al., 2009) shortly following mating. However, the effects of a prolonged period of sexual reward abstinence on stress coping and susceptibility and potential roles for dopamine-induced ΔFosB in these behaviors remain to be tested.
VTA dopamine activity mediates natural reward-induced morphological changes
In addition to dopamine-dependent neural alterations in areas downstream from the VTA, sexual experience also causes decreased soma size of VTA dopamine neurons, an effect dependent on MOR activation (Pitchers et al., 2014). Here, it is demonstrated that this effect is also partially regulated by activity of the dopamine neuron itself. Similar alterations in soma size have been reported following chronic exposure to opiates (Mazei-Robison et al., 2011; Mazei-Robison and Nestler, 2012) and are associated with decreased dopamine trafficking and release (Mazei-Robison et al., 2011), and hyperexcitability of VTA dopamine neurons (Mazei-Robison et al., 2011), and are mediated via reduced BDNF signaling (Sklair-Tavron et al., 1996; Russo et al., 2007; Koo et al., 2012; Collo et al., 2014). Opiate-induced soma size reduction is associated with opiate reward tolerance following opiate exposure (Russo et al., 2007) and sexual experience (Pitchers et al., 2014). Even though involvement of BDNF in the effects of sex experience have not been tested, BDNF is expressed by dopamine neurons throughout the brain (Seroogy et al., 1994; Baquet et al., 2005; Graham et al., 2007) and thus potentially regulated by dopamine neuron activity. Hence, VTA dopamine inhibition during mating may partially block soma size reduction by reducing BDNF signaling. However, the dopamine soma size reduction is unlikely causally related to the cross-sensitization of amphetamine reward, as psychostimulants cause no change in soma size and increase BDNF expression (Russo et al., 2009; Fanous et al., 2011; Mazei-Robison et al., 2014). Moreover, overexpression of BDNF in the VTA increases cross-sensitization to amphetamines (Wang et al., 2013). One possibility is that psychostimulants, opiates, and sexual behavior activate BDNF-TrKB signaling via different signaling pathways, thereby differentially regulating dopamine neuron excitability.
In conclusion, the current findings demonstrate that VTA dopamine neuron activity during sexual behavior does not prevent the motivation, expression, or short-term facilitation of this natural reward behavior. Instead, VTA dopamine regulates the cross-sensitizing effects of abstinence from sexual reward on amphetamine reward, via neural alterations, including ΔFosB expression in VTA target areas, and changes in VTA dopamine cell soma size.
These findings demonstrate that mesolimbic dopamine has a role in the interaction between natural and drug rewards and is a key mediator of changes in vulnerability for drug use after loss of natural reward.
Footnotes
The authors declare no competing financial interests.
References
- Aragona BJ, Detwiler JM, Wang Z. Amphetamine reward in the monogamous prairie vole. Neurosci Lett. 2007;418:190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai HY, Cao J, Liu N, Xu L, Luo JH. Sexual behavior modulates contextual fear memory through dopamine D1/D5 receptors. Hippocampus. 2009;19:289–298. doi: 10.1002/hipo.20505. [DOI] [PubMed] [Google Scholar]
- Baik JH. Dopamine signaling in reward-related behaviors. Front Neural Circuits. 2013;7:152. doi: 10.3389/fncir.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Baltazar RM, Coolen LM, Webb IC. Diurnal rhythms in neural activation in the mesolimbic reward system: critical role of the medial prefrontal cortex. Eur J Neurosci. 2013;38:2319–2327. doi: 10.1111/ejn.12224. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/S0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM. Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats. Neuropharmacology. 2016;101:154–164. doi: 10.1016/j.neuropharm.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MC, van der Plasse G, Adan RA. Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS One. 2014;9:e95392. doi: 10.1371/journal.pone.0095392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom M, Both S, Laan E, Everaerd W, Spinhoven P. The role of conditioning, learning and dopamine in sexual behavior: a narrative review of animal and human studies. Neurosci Biobehav Rev. 2014;38:38–59. doi: 10.1016/j.neubiorev.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Chang CY, Esber GR, Marrero-Garcia Y, Yau HJ, Bonci A, Schoenbaum G. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat Neurosci. 2016;19:111–116. doi: 10.1038/nn.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Cavalleri L, Spano P. Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: critical role of BDNF and dopamine. Front Pharmacol. 2014;5:259. doi: 10.3389/fphar.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037/0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/S0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fanous S, Lacagnina MJ, Nikulina EM, Hammer RP., Jr Sensitized activation of Fos and brain-derived neurotrophic factor in the medial prefrontal cortex and ventral tegmental area accompanies behavioral sensitization to amphetamine. Neuropharmacology. 2011;61:558–564. doi: 10.1016/j.neuropharm.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guasti A, Roldán-Roldán G, Saldívar A. Reduction in anxiety after ejaculation in the rat. Behav Brain Res. 1989;32:23–29. doi: 10.1016/S0166-4328(89)80068-3. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after d-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohmader KS, Pitchers KK, Balfour ME, Coolen LM. Mixing pleasures: review of the effects of drugs on sex behavior in humans and animal models. Horm Behav. 2010;58:149–162. doi: 10.1016/j.yhbeh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, Lehman MN, Laviolette SR, Coolen LM. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31:16473–16482. doi: 10.1523/JNEUROSCI.4013-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yuan Y, Halliday G, Rusznák Z, Watson C, Paxinos G. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct Funct. 2012;217:591–612. doi: 10.1007/s00429-011-0349-2. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19:117–126. doi: 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016 doi: 10.1038/nrn.2016.67. Advance online publication. Retrieved Aug. 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S, Stuber GD. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lazenka MF, Selley DE, Sim-Selley LJ. DeltaFosB induction correlates inversely with CB(1) receptor desensitization in a brain region-dependent manner following repeated Delta(9)-THC administration. Neuropharmacology. 2014;77:224–233. doi: 10.1016/j.neuropharm.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Tomarchio AJ, Lichtman AH, Greengard P, Flajolet M, Selley DE, Sim-Selley LJ. Role of dopamine type 1 receptors and dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa in Delta9-tetrahydrocannabinol-mediated induction of DeltaFosB in the mouse forebrain. J Pharmacol Exp Ther. 2015;354:316–327. doi: 10.1124/jpet.115.224428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Giguere PM, Roth BL. DREADDs: novel tools for drug discovery and development. Drug Discov Today. 2014;19:469–473. doi: 10.1016/j.drudis.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J Neurosci. 2011;31:7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Brown A, Fisher D, Wu Y, Warren J, Cui X. Tissue specific expression of Cre in rat tyrosine hydroxylase and dopamine active transporter-positive neurons. PLoS One. 2016;11:e0149379. doi: 10.1371/journal.pone.0149379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, et al. ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/S0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med. 2012;2:a012070. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M, Krishnan V, Kim S, Siuta MA, Galli A, Niswender KD, Appasani R, Horvath MC, Neve RL, Worley PF, Snyder SH, Hurd YL, Cheer JF, Han MH, Russo SJ, et al. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron. 2011;72:977–990. doi: 10.1016/j.neuron.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Appasani R, Edwards S, Wee S, Taylor SR, Picciotto MR, Koob GF, Nestler EJ. Self-administration of ethanol, cocaine, or nicotine does not decrease the soma size of ventral tegmental area dopamine neurons. PLoS One. 2014;9:e95962. doi: 10.1371/journal.pone.0095962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Chauvet C, Claudia C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology. 2012;37:1579–1587. doi: 10.1038/npp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. FosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol. 2015;753:66–72. doi: 10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, Omrani A, Luijendijk MC, de Vrind VA, Van Rozen AJ, Ophuis RJ, Garner K, Kallo I, Ghanem A, Liposits Z, Conzelmann KK, Vanderschuren LJ, la Fleur SE, Adan RA. Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology. 2016;41:2241–2251. doi: 10.1038/npp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of ΔFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037/0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol Psychiatry. 2010a;67:872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. DeltaFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010b;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Coppens CM, Beloate LN, Fuller J, Van S, Frohmader KS, Laviolette SR, Lehman MN, Coolen LM. Endogenous opioid-induced neuroplasticity of dopaminergic neurons in the ventral tegmental area influences natural and opiate reward. J Neurosci. 2014;34:8825–8836. doi: 10.1523/JNEUROSCI.0133-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Di Sebastiano AR, Coolen LM. mGluR5 activation in the nucleus accumbens is not essential for sexual behaviour or cross-sensitization of amphetamine responses by sexual experience. Neuropharmacology. 2016;107:122–130. doi: 10.1016/j.neuropharm.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Fischer AM, Ahuja A, Lesser EN, Maniates H. Role of “wanting” and “liking” in motivating behavior: gambling, food, and drug addictions. Curr Top Behav Neurosci. 2016;27:105–136. doi: 10.1007/7854_2015_387. [DOI] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci. 2014;34:10982–10988. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-4 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Kobayashi S, Schultz W. Components and characteristics of the dopamine reward utility signal. J Comp Neurol. 2016;524:1699–1711. doi: 10.1002/cne.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fanous S, Terwilliger EF, Bass CE, Hammer RP, Jr, Nikulina EM. BDNF overexpression in the ventral tegmental area prolongs social defeat stress-induced cross-sensitization to amphetamine and increases DeltaFosB expression in mesocorticolimbic regions of rats. Neuropsychopharmacology. 2013;38:2286–2296. doi: 10.1038/npp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will RG, Hull EM, Dominguez JM. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacol Biochem Behav. 2014;121:115–123. doi: 10.1016/j.pbb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/S0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci. 2015;41:760–772. doi: 10.1111/ejn.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]