Introduction
Approximately one out of three people in the United States suffers from chronic pain.[35] A family of conditions characterized in part by pain and varied constitutional symptoms, such as Fibromyalgia (FM), Temporomandibular Disorder (TMD), and Chronic Pelvic Pain, play a significant role in the societal burden of chronic pain as they are both prevalent and difficult to treat. These and other pain conditions are referred to as Chronic Overlapping Pain Conditions (COPCs) because they so often co-occur, in both individuals and families.[58] In COPCs, peripheral pathology corresponds poorly to the location and severity of pain[9; 59]. Many researchers favor a primary (but not exclusive) role for central nervous system (CNS) mechanisms in the etiology and maintenance of COPCs.[65] Functional, chemical, and structural neuroimaging studies reveal abnormalities in the brains of patients with COPCs,[20; 25; 27; 29; 34; 36; 37; 39; 45; 46; 53; 56] and the drugs that are effective in these conditions are thought to work primarily in the CNS.[7; 28] However, defining and describing the CNS pain phenotype is a challenge. As a result, health care providers often assume new onset pain or other symptoms to be a peripheral problem rather than yet another manifestation of an ongoing CNS issue.
Currently the term ‘centralized pain’ is used to encompass the theoretical and empirical basis for the CNS contribution to chronic pain states, but the term may also be used to describe the symptomology characteristic of COPCs with the greatest evidence of pain centralization.[8] Hallmark symptoms of centralized pain include widespread pain, fatigue, negative affect, unrefreshing sleep, and cognitive dysfunction. While these symptoms are assessed by promising instruments like the 2011 survey criteria for FM,[64] COPCs seem to involve additional symptom domains.[43] Namely, many patients with COPCs report sensitivity to non-painful environmental stimuli, including lights and sounds, and increased awareness of non-painful somatic sensations, suggesting aberrant sensory processing outside of the traditional nociceptive pathways.[18; 22; 26; 30; 31; 42; 48; 61] Understanding how these varied symptoms relate to one another will help us build simple methods for characterizing individuals who suffer from these conditions.
Urologic Chronic Pelvic Pain Syndrome (UCPPS) is a COPC characterized by pain in the pelvic region accompanied by urologic symptoms.[47] One of the goals of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network, a multi-site project funded by the National Institute of Diabetes and Digestive and Kidney Diseases,[11; 40] is to define symptom patterns that UCPPS has in common with other COPCs. In its first phase, the MAPP Research Network measured several symptom domains relevant to centralized pain in cohorts with UCPPS, other COPCs, and controls free of COPCs. Our primary objective here was to determine if primary symptoms of centralized pain can be explained by a smaller number of latent factors or symptom clusters. Using factor analysis and structural equation modelling, we identified two factors, representing sensory sensitivity and symptom severity, and then examined their relationship to COPCs and other clinical outcomes.
Methods
Participants
The MAPP Research Network recruited three participant cohorts across six US discovery sites: UCPPS (n= 424), healthy controls without COPCs from the community (n = 415) and a mixed pain cohort comprised of individuals with other COPCs (i.e., FM, Irritable Bowel Syndrome [IBS], TMD, Chronic Fatigue Syndrome [CFS], Migraine; n = 200). The scientific aims of the network, recruitment strategy, and inclusion and exclusion criteria have been described in detail in previous publications.[11; 40]. The MAPP study is registered at Clinicaltrials.gov: “Chronic Pelvic Pain Study of Individuals with Diagnoses or Symptoms of Interstitial Cystitis and/or Chronic Prostatitis (MAPP-EP)”. All procedures were approved by Institutional Review Boards at the participating institutions and all subjects provided informed consent.
In brief, primary inclusion criteria for UCPPS was bladder and/or pelvic pain, pressure or discomfort present the majority of the time over the last three months. The mixed pain cohort met criteria for either IBS, CFS, or FM, though participants from both the UCPPS and mixed pain cohorts could and did meet criteria for more than one condition. The presence of UCPPS symptoms (i.e., pelvic pain) was not exclusionary for the mixed pain cohort though mean levels were well below those of the UCPPS cohort (Table 1). The healthy controls had no UCPPS symptoms and no chronic pain conditions as assessed by the modules of the Complex Medical Symptoms Inventory (described below), but may have endorsed some pain on the body map as would be expected from a community sample. The UCPPS cohort was seen for in-clinic visits at baseline, 6 months, and at one year. The healthy controls and mixed pain cohorts were seen once at baseline.
Table 1.
Type and number of COPCs in the UCPPS and mixed pain cohorts.
| UCPPS (n = 424) |
Mixed Pain (n = 200) |
|
|---|---|---|
| Genitourinary Pain (SYM-Q # 1) | 5.07 (2.20) | 1.40 (2.20)* |
| Non-Genitourinary Pain (SYM-Q # 6) | 3.25 (2.70) | 4.64 (2.83)* |
| frequency (%) | ||
| IBS | 127 (30) | 146 (73)* |
| TMD | 101 (24) | 55 (28) |
| CFS | 49 (12) | 75 (38)* |
| FM | 38 (9) | 84 (42)* |
| MI | 99 (23) | 69 (35)* |
| Number of comorbid COPCs | ||
| None | 201 (47) | 80 (40) |
| 1 | 112 (26) | 54 (27) |
| 2 | 58 (14) | 33 (17) |
| 3 or more | 53 (12) | 33 (17) |
COPCs = Chronic Overlapping Pain Conditions; UCPPS = Urological Chronic Pelvic Pain Syndrome; IBS = Irritable Bowel Syndrome; TMD = Temporomandibular Disorder; CFS = Chronic Fatigue Syndrome; FM = Fibromyalgia; MI=Migraine.
p < 0.01
Measures
Pain Severity
In the UCPPS and the mixed pain cohorts, pain severity was measured by the Symptom and health care utilization Questionnaire (SYM-Q), which was designed specifically for the MAPP Research Network.[40] The pain severity measure ranges from 0–10, and contains a question for genitourinary pain severity (SYM-Q #1), and an analogous question for non-genitourinary pain severity (SYM-Q #6). We chose this measure rather than that used in previous MAPP publications[24] so that a similar pain severity measure could be used in both the UCPPS and mixed pain cohorts.
Urinary Symptom Severity
Urinary symptom severity was assessed using a composite measure derived from the Genitourinary Pain Index (GUPI)[10] and Interstitial Cystitis Symptom Index (ICSI),[49] based on psychometric analyses performed on MAPP baseline data.[24] Individual items assess urinary urgency and frequency, nocturia, and bladder emptying. This results in a urinary severity score (range 0–25), with higher scores indicating greater symptom severity.
Sleep and Fatigue
Sleep disturbances and fatigue were each assessed with the Patient Reported Outcomes Measurement Information System (PROMIS) questionnaires.[5]
Cognitive Dysfunction
Self-reported cognitive difficulties were assessed with the Multiple Ability Self-Report Questionnaire (MASQ). The 38-items of the MASQ cover 5 domains: language ability, attention, visuo-spatial, verbal memory, and visual memory. These domains have been validated against neuropsychological tasks.[55] The sum of the five domains represents the cumulative burden of self-reported cognitive dysfunction.[38]
Depression
Depression was measured using the Hospital Anxiety and Depression Scale (HADS).[66]
Spatial Extent of Pain
The extent or spatial distribution of pain was assessed using a 45-site body map [40] which has been used in previous MAPP studies and is associated with disease burden, immunological, and neuroanatomical findings.[39; 52; 54]
Disability and Quality of Life
Perceived physical and mental well-being were measured using the SF-12 physical components score (PCS; a composite of all physical health subscales), and mental health components score (MCS).[60]
Complex Medical Symptom Inventory (CMSI)
The CMSI provided an overall index of the symptom burden, as well as an assessment of the presence of COPCs.[62] The CMSI is composed of 41 items asking about the presence of functional symptoms for 3 months out of the last year.[62] Ten of the 41 questions act as “trigger” items, which, if answered affirmatively, lead to additional diagnostic modules being administered. For example, checking, “abdominal pain or discomfort,” automatically triggers the IBS module. For this version of the CMSI, possible diagnostic modules included FM,[64] CFS,[21] IBS,[14] TMD,[16] and MI[50]. Additional information about the diagnostic modules is given below. An additional nine items directly reference pain/tenderness or symptoms central to the definition of COPCs investigated in this study (e.g., items about impaired memory, attention, or urinary dysfunction). The remaining 22 items cover non-specific somatic or functional symptoms (18 items) or sensory sensitivity to non-painful environmental stimuli (e.g., to bright lights or odors; 4 items). All CMSI items are shown in Supplemental Table 1.
The 18 functional or somatic symptom items in the CMSI are highly similar to items used to measure a construct variously termed “somatic awareness,” “somatosensory amplification,” “anxious arousal” or “somatic arousal” which refers to heightened awareness of and attention to internal sensations and symptoms. Similarly, the four items representing sensory sensitivity in the CMSI are comparable to items used to measure sensitivity to external physical stimuli. These 22 CMSI items are compared to similar items used in validated measures of analogous constructs in Supplemental Table 2.
For the present analysis, we explored the use of the summed score of the 18 items as a Somatic Awareness subscale (range 0 −18), and the sum of the four sensory items (range 0–4) as a Sensory Sensitivity subscale. The adequacy of these scales was subsequently tested using confirmatory factor analysis (Methods and Results for this analysis are shown in Supplemental Figure 1). We also calculated Chronbach’s α for both Somatic Awareness and Sensory Sensitivity subscales for each cohort.
CMSI Diagnostic Modules
FM was assessed by an adaptation of the 2011 survey criteria for FM.[64] These criteria use a 19-site body index of pain distribution (referred to as the Widespread Pain Index, WPI) and several questions about symptoms of fatigue, cognitive issues, unrefreshing sleep, headache, depression, and gastrointestinal complaints. For each patient to receive a classification of FM, both multisite pain and non-specific symptoms must be present, the symptoms must have been present at similar levels three months or longer.
CFS was assessed by an adaptation of the 1994 Fukuda criteria.[21] These criteria assess fatigue that has been ongoing for at least six months in addition to domains for cognitive dysfunction, unrefreshing sleep, post-exertion malaise, interference with activities, and symptoms like headache, sore throat, and muscle pain. Fatigue relieved by rest or due to strenuous activity does not contribute to case status.
IBS was assessed by an adaptation of the ROME III criteria.[14] These criteria assess pain or discomfort in the abdomen in conjunction with change in the frequency of bowel movements, and change in the appearance or consistency of bowel movements. Pain during menses does not contribute to case status. The pain must be ongoing at least six months.
TMD was assessed by an adaptation of the Research Diagnostic Criteria for TMD.[16] These criteria assess pain in the face, jaw, temple, ear, or in front of the ear, and measure severity and interference with activities during the past six months.
Migraine was assessed by an adaptation of the International classification of headache disorders criteria, 2nd edition.[50] These criteria assess frequency, severity, and symptoms accompanying headache, such as pain confined to one side of the head and vomiting.
The CMSI also included a triggered diagnostic module for vulvodynia. This COPC was not considered in this manuscript as it is female specific and we sought to only include COPCs that affect both sexes.
Data Analysis
Overview
We pursued three analytic aims: a) identify the number of latent factors that explain the centralized pain phenotype in UCPPS using exploratory factor analysis (EFA), b) confirm these factors through confirmatory factor analysis (CFA) in the UCPPS, mixed pain, and healthy control cohorts, and c) evaluate the stability of these factors over time in the UCPPS cohort. Because healthy controls were by definition without pain, we initially excluded the pain intensity/severity measures from the analyses of all groups. However, as pain severity is a critical component of the perceptual burden experienced by chronic pain patients,[12] pain measures were subsequently added to the CFA models in both the UCPPS and mixed pain cohorts.
We report model fit indices, including non-centrality fit indices (Comparative Fit Index [CFI; greater than .95 generally represents adequate fit]); Root Mean Square Error of Approximation and 90% confidence interval (RMSEA; <.06 generally represents adequate fit), and absolute measures of fit (Standardized Root Mean Square Residual [SRMR; < .08 generally represents adequate fit]; χ2 test [where non-significant p values are seen as desirable, but in practice are rarely observed when sample size is greater than 200]). These guidelines are adopted from Hu & Bentler.[32]
The scales used for factor analysis were the total number of painful sites on the 45-site body map, the Somatic Awareness and Sensory Sensitivity subscales derived from the CMSI, the PROMIS-fatigue and PROMIS-sleep measures, the MASQ total score, and the HADS-depression scale. Together these reflect the primary elements of the 2011 FM survey criteria[64] and other posited measures of the centralized pain phenotype, with the addition of the somatic awareness and sensory sensitivity measures.
Exploratory Factor Analysis in UCPPS
The EFA model was fit by Maximum Likelihood robust (MLR) estimation. MLR is able to provide reliable estimates of model fit even when the underlying distribution of data does not meet assumptions embedded in the ML framework, such as normality.[19] We used the lower bound RMSEA (lb.RMSEA) criteria to select the optimal number of factors.[51] Lb.RMSEA is the smallest number of factors that produces a lower bound 90% confidence interval for RMSEA below .05. In simulation studies this metric appears to result in the strongest likelihood of verisimilitude, or identifying the “true” underlying number of factors when compared to other model fit metrics like AIC, BIC, or the simple RMSEA estimate.[51] Using MPLUS v. 8.0 with Geomin, an oblique rotation method that allows identified factors to be correlated, was applied. This EFA was conducted both with and without the pain severity measure.
Confirmatory Factor Analysis in All Cohorts
Confirmatory factor analysis on the factor solution derived from the EFA was performed in the UCPPS, mixed pain, and healthy control cohorts using MLR estimation. CFA models were constructed both with and without the pain severity measures, to allow for analogous models to be fit in all three cohorts (i.e., without pain severity), and separately for the UCPPS and mixed pain cohorts (i.e., with pain severity – genitourinary pain severity for UCPPS, non-genitourinary pain severity for PC).
We then fit the same model to a subset UCPPS participants (n = 332) that completed all measures at baseline, six months, and one year to evaluate the stability of these factors over time.
Associations of Identified Factors with Measures of Disability and Urinary Symptom Severity
To determine if the identified factors were associated with severity of non-painful urinary symptoms (e.g., frequency and urgency) and perceived physical (PCS) and mental (MCS) well-being as measured by the SF-12, we constructed structural models with the identified factors as predictors of these measures controlling for age, sex, and BMI.
Supplemental Analyses
a) As a form of method validation, we also explored the association of the identified factors with individual COPC status and with the total number of COPCs in the full UCPPS and mixed pain cohorts. These analyses were intended to determine whether higher levels of these factors are associated with the presence or absence of COPCs
b) It is possible that while a latent factor may fit a particular dataset well, its association with other outcomes may be driven by a given indicator, rather than the latent construct itself. To evaluate this possibility, we compared the proportion of variance in number of COPCs explained (R2) when the latent variable was used as a predictor, to each of its individual indicators alone.
Results
Participants
UCPPS participants were on average 43 years old and 55% of the sample was female. Healthy controls were 41 years old on average and 56% of the sample was female; mixed pain participants were 42 years old on average and 78% of the sample was female. See Table 1 for comparison of COPCs in the UCPPS and mixed pain cohorts.
Exploratory Factor Analysis in UCPPS
The optimal number of factors in the UCPPS cohort was two, in both models with and without pain severity. The rotated factor loadings are shown in Supplemental Table 3.
The two factors identified appeared to be readily interpretable – number of painful sites, somatic awareness, and sensory sensitivity loaded on the first factor; whereas fatigue, sleep, depression, and cognitive dysfunction, and pain severity loaded on the second factor. The first factor represents a broad amplification or awareness of sensory processes, both somatosensory (internal) and external. The second factor represents severity of clinical pain and non-specific CNS symptoms across multiple domains. All factor loadings were above .45 on the primary factor, and less than .2 on the second factor. Overall model fit was adequate (CFI = .998; RMSEA = .020, 90% CI = .000, .062; χ2 = 9.342, df = 8, p = .314).
Confirmatory Factor Analysis in All Cohorts
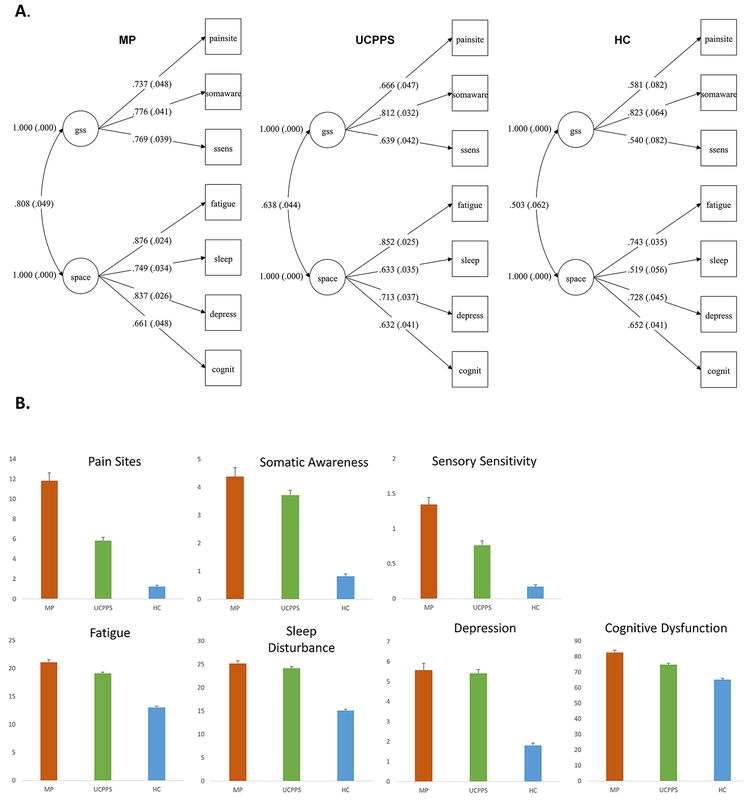
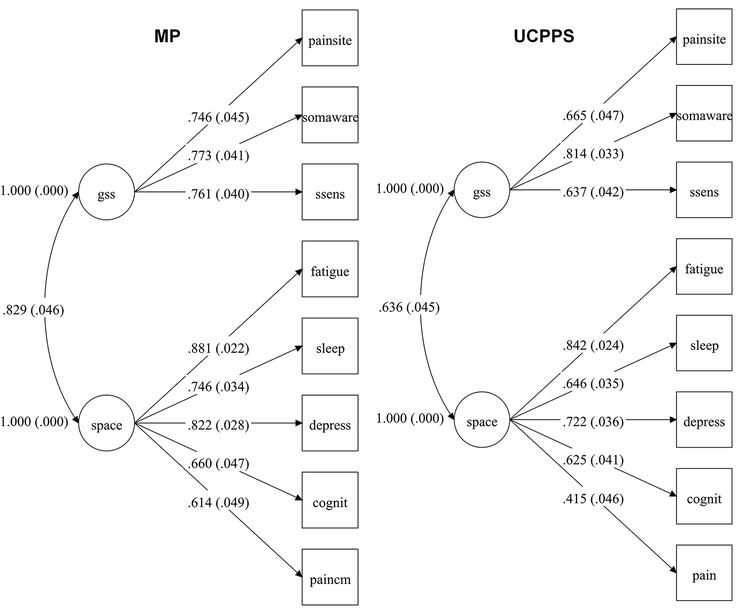
The resulting two-factor models displayed adequate fit in all three cohorts. The model for each cohort is shown in Figure 1A with fit statistics. Mean symptom levels for each cohort are shown in Figure 1B. The models that included pain severity measures for UCPPS and mixed pain cohorts similarly showed adequate fit to the data, with general loadings broadly similar between groups. These are shown in Figure 2 with fit statistics.
Figure 1.
(A) Measurement models for symptoms of centralized pain in UCPPS, mixed pain (MP), and healthy controls (HC). Model fit for UCPPS: Χ2 =20.512, df= 13, p =.0832. RMSEA = .037, 90% CI = .000, .066. CFI = .991. SRMR = .027; Model fit for MP: Χ2 =18.014, df= 13, p =.1570. RMSEA = .044, 90% CI = .000, .088. CFI = .991. SRMR = .027;; Model fit for HC: Χ2 =8.007, df= 13, p =.8431. RMSEA = .000, 90% CI = .000, .028. CFI = 1.000. SRMR = .023. Standardized loadings are shown. (B) Mean symptom levels by group with standard errors.
Figure 2.
Measurement models with pain severity for UCPPS and mixed pain (MP) cohorts. Model fit for UCPPS: Χ2 =41.907, df= 19, p =.0018. RMSEA = .053, 90% CI = .031, .075. CFI = .976. SRMR = .035; Model fit for MP: Χ2 =37.285, df= 19, p =.0073. RMSEA = .069, 90% CI = .035, 1.02. CFI = .972. SRMR = .039. Standardized loadings are shown.
We refer to the first factor as Generalized Sensory Sensitivity or GSS, and the second factor by the acronym SPACE (Sleep, Pain, Affect, Cognition, Energy).
Factor loadings and overall model fit were similar for SPACE, if slightly worse, when the HADS-anxiety subscale was used in place of the HADS-depression subscale (data not shown).
In each cohort, GSS and SPACE were associated with one another (UCPPS standardized φ = .638, 95% CI = .554, .721; Mixed pain standardized φ = .808, 95% CI = .727, .888; Healthy control standardized φ = .503, 95% CI = .395, .611).
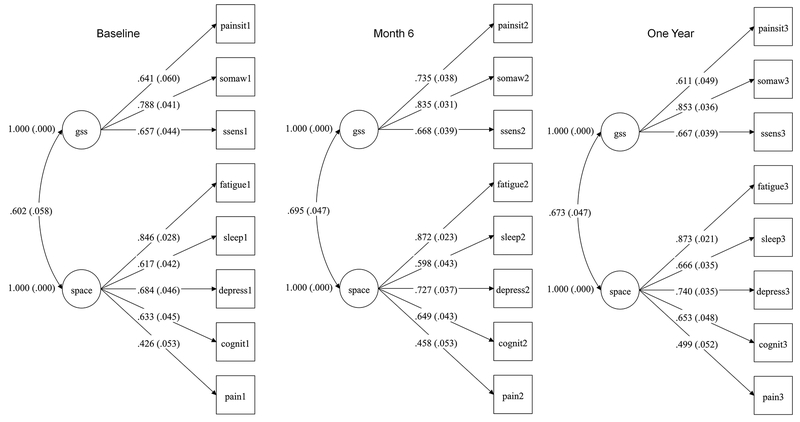
Temporal Stability of GSS and SPACE
In the 332 UCPPS participants that completed symptom assessments at baseline, six months, and one year, the same two-factor model showed adequate fit to the data. These are shown in Figure 3 with fit statistics.
Figure 3.
Measurement model in the UCPPS cohort at baseline, 6 months and one year (n = 332). Model fit for baseline: Χ2 =37.378, df= 19, p =.0071. RMSEA = .054, 90% CI = .027, .079. CFI = .974. SRMR = .038; Model fit for month 6: Χ2 =27.039, df= 19, p =.1038. RMSEA = .036, 90% CI = .000, .064. CFI = .990. SRMR = .029; Model fit for year one: Χ2 =35.551, df= 19, p =.0120. RMSEA = .051, 90% CI = .024, .077. CFI = .981. SRMR = .032.
Relationships between GSS, SPACE, Disability, and Urinary Symptoms
Higher levels of SPACE were significantly associated with worse perceived physical well-being (SF-12 PCS; β = −.175, 95% CI = −.300, - .050, p = .006) and worse urinary symptom severity (β = .181, 95% CI = .223, .491, p < .001). SPACE was not associated with worse perceived mental well-being, and GSS was not associated with any of these outcomes (all p > .05).
Summary of Supplemental Analyses (See Supplemental Material)
a) GSS showed strong relationships with each individual comorbid COPC as well as the number of comorbid COPCs in the UCPPS cohort; these results validated in the mixed pain cohort. GSS particularly showed strong “discrimination” on the probability of a patient having each COPC (See Supplemental Table 4 & Supplemental Figures 2–4).
b) The variance explained in the number of COPCs was substantially greater using the entire GSS construct versus that explained by the indicator variables in isolation. This suggests that these associations cannot be reduced to a patient indicating more painful sites on the body map, or increased somatic awareness or sensory sensitivity (See Supplemental Table 5).
c) Given the strong association of the GSS construct with the prevalence of COPCs, we created a preliminary brief form of the GSS from representative items. This form correlates well with the factor scores derived from entire GSS construct (Supplemental Material; Supplemental Figure 4).
Discussion
Because many chronic pain conditions overlap with one another it has long been assumed that they share common neurobiological mechanisms – these conditions are now collectively termed COPCs. We refer to the neurobiological substrate of COPCs as ‘centralized pain’ because of the overwhelming evidence for central neurobiological dysfunction in these conditions. Changes to the CNS provide the simplest explanation for the co-occurrence of symptoms like sensitivity to many different sensory experiences, widespread pain, and the memory, sleep, and mood issues observed in most COPCs.[8] These CNS changes may proceed from peripheral pathological processes, such as the presence of the Hunner’s ulcers in the wall of the bladder of some UCPPS patients.[57] More often than not however UCPPS patients do not show gross pathological features indicative of local nociceptive input, suggesting that in these patients the CNS changes are independently generated and/or maintained.[65] No matter the role of peripheral nociceptive input in these conditions, there is a clear aggregation of these symptoms into two factors in COPCs, and these primary self-reported symptoms of centralized pain need to be accurately and succinctly assessed in both research and clinical contexts. Here we have begun that work using one of the largest multi-site phenotyping studies of chronic pain patients ever conducted.
Conceptually, we have found that the myriad of COPC symptoms often studied in isolation can be described as part of distinct but closely related constructs. We have coined these Generalized Sensory Sensitivity (GSS) and SPACE (Sleep, Pain, Affect, Cognition, Energy). GSS may be best understood as a tendency to experience, notice, and report increased sensitivity to external stimuli across multiple sensory modalities, increased sensitivity to symptoms or sensations occurring within the body (somatic awareness), and pain or tenderness (hyperalgesia/allodynia) in multiple regions of the body. SPACE is an amalgamation of constitutional symptoms that often become disrupted in tandem. Symptom clusters similar to SPACE have been described in primary care,[12] cancer patients,[6] as well as other chronic diseases.
Exploratory and confirmatory factor analyses support a two-factor model of centralized pain, with strong associations between the two factors. This basic two-factor structure was apparent in each cohort (UCPSS; mixed pain such as FM, IBS, and CFS; and healthy community controls), despite the large differences in average levels of symptoms. Put simply, even when the severity range of each symptom differs enormously, the same symptoms tend to be co-expressed. This supports the idea of a symptom continuum present to some degree in all people, including those described nominally as healthy. This idea was first advanced by Wolfe, using the term ‘fibromyalgianess’ to connote the fact that FM-like symptoms are not confined to FM patients, and that sub-syndromal levels of fibromyalgianess contribute to pain and disability.[63] We previously showed that higher levels of fibromyalgianess are associated with poorer post-surgical outcomes even at levels below criteria for FM.[3; 4; 33] The current analyses considerably expand and refine the concept of centralized pain by identifying additional critical aspects of COPC symptomology. The longitudinal analyses within the UCPPS cohort further demonstrate that the basic two-factor structure can be observed in the same sample over time, suggesting that the general co-expression of these patterns of symptoms is stable.
Recent work in rheumatoid arthritis has demonstrated that higher levels of fibromyalgia symptoms are associated with the same functional connectivity findings seen in centralized pain conditions like FM[46] – specifically, an increase in positive connections between the default mode network (DMN) and insular cortex as fibromyalgianess increases. [2] This agreement, which echoes findings in chronic low back pain[41] and other mixed-pain cohorts [1], suggests that there may be identifiable neurobiological substrates of ‘centralized pain’ across pain conditions. The insula cortex plays an important role in integrating sensory information, monitoring interoceptive processes,[15] and determining their salience. Thus it is possible that the more closely the insula is incorporated into other neural processing streams the more likely it is that sensory information will attain an aversive valence. Recent work using a graph theory framework, which attempts to model patterns of connections across hundreds of brain regions, supports this view. In FM patients with high levels of pain, those brain regions with the greatest importance for relaying and integrating information – hubs – show reorganization that favors connections between the somatosensory cortices and both anterior and posterior insular cortices, compared to both healthy controls and FM patients with low levels of pain [Under Review]. In FM patients, recent work has shown that complex visual stimuli are judged to be more aversive, and that neural activation patterns evoked by these stimuli, particularly within the insula, distinguish these patients from healthy controls with remarkable accuracy.[30] Other studies of the neural response to non-painful sensory stimuli have confirmed augmented insular activation to visual, auditory and tactile stimulation in FM.[42] These findings support a model in which COPCs are characterized by enhanced coupling, or over-integration, of sensory signals with regions and networks that determine salience.
The present work also has implications for applied research in COPCs. COPCs are characterized by an increased prevalence of mood imbalances, cognitive difficulties, and fatigue. However, when we attempted to confirm the association between each factor and the presence of COPCs, we found that the GSS construct has a stronger relationship with the presence of COPCs measured by self-report criteria than SPACE. It may be then, that GSS is a particularly important concept for assessing risk of developing a new COPC – we make this suggestion cautiously, as we do not yet have data suggesting that the relationship extends beyond the cross-sectional. However, the Orofacial Pain Prospective Evaluation and Risk Assessment (OPPERA) study’s findings support this idea. Higher scores on a measure of somatic awareness was the most robust predictor of new-onset TMD over an average observational period of 2.8 years.[17] Similarly, the somatic awareness construct predicted new-onset chronic widespread pain over a 12-month period of observation.[44] Previous research has found that the presence of COPCs is a good predictor of developing a new COPC,[61] but it is not currently simple or easy to comprehensively assess COPCs. A short version of GSS or similar construct, which we have developed a preliminary version of here, may be useful for assessing the broad vulnerability to COPCs. Similarly, the continuous nature of the construct may be helpful in assessing the impact of therapy or adverse events on the underlying vulnerability. For instance, pregabalin has been shown to reduce levels of excitatory neurotransmitter in the posterior insula of FM patients.[28] It is plausible that GSS, if shown to accurately reflect aberrant neurochemistry in sensory processing regions, could be used either to predict treatment response to pregabalin, or as a surrogate marker of the attenuation of multi-modal sensory hypersensitivity in responders.
Limitations
The absence of clinical outcomes over a long follow-up period is a limitation of the current study design. A larger set of items derived from focus groups may be helpful in better defining the sensory sensitivity and somatic awareness constructs. Some caution is warranted in interpreting these findings in the pain-free community sample, as the questions used to define somatic awareness and sensory sensitivity were intended for chronic pain patients – it is reasonable to wonder if the range of difficulty for these items is not broad enough for this group. While UCPPS is an important clinical category, it comprises several conditions (i.e., interstitial cystitis/bladder pain syndrome, chronic prostatitis/chronic pelvic pain syndrome) that may be distinct. Perceived stress and pain catastrophizing, for example, are closely related constructs that have been hypothesized to play critical roles in the development or presence of COPCs,[13; 23] and these should be investigated further in the context of SPACE.
Conclusions
Two constructs encompassing a large number of related symptoms in COPCs have been identified in one of the largest phenotyping studies of chronic pain patients yet undertaken. These constructs appear to be closely linked with one another and the strength of that association, or coupling, may reflect critical pathological processes undergirding the presence and development of COPCs. Generalized Sensory Sensitivity (GSS) and SPACE represent novel expansions of constructs for measuring the various aspects of sensory sensitivity and symptom severity that mark COPCs. Both of these aspects of the centralized pain continuum should be measured in studies of COPCs, and each should be considered in longitudinal studies that evaluate the trajectory of symptoms in COPCs and responses to treatment. Because the GSS construct has not been presented before, we offer a short form that appears to measure the construct well, in the hope that it will be useful to researchers.
Supplementary Material
Acknowledgements
Funding for the MAPP Research Network was obtained under a cooperative agreement from, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes, of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316.) Additional support for author AS came from Grant Number K12 DE023574 from the National Institute of Dental and Craniofacial Research.
Dr. Schrepf reports grants from NIH, during the conduct of the study. Dr. Williams has nothing to disclose. Dr. Gallop has nothing to disclose. Dr. Naliboff has nothing to disclose. Dr. Basu has nothing to disclose. Dr. Kaplan has nothing to disclose. Dr. Harper has nothing to disclose. Dr. Landis has nothing to disclose. Dr. Clemens reports grants from NIDDK, during the conduct of the study. Dr. Strachan has nothing to disclose. Dr. Griffith has nothing to disclose. Dr. Afari reports grants from NIH/NIDDK, during the conduct of the study. Dr. Hassett reports personal fees from AbbVie Pharmaceuticals, outside the submitted work. Dr. Pontari reports grants from NIH, during the conduct of the study; grants and personal fees from Aquinox Pharmaceuticals, outside the submitted work. Dr. Clauw reports personal fees from Abbott Pharmaceutical, grants and personal fees from Aptinyx, personal fees from Astellas Pharmaceutical, grants and personal fees from Cerephex, personal fees from Daiichi Sankyo, grants and personal fees from Pfizer, Inc, personal fees from Pierre Fabre, personal fees from Samumed, personal fees from Theravance, personal fees from Tonix, personal fees from Williams & Connolly LLP, personal fees from Zynerba, outside the submitted work. Dr. Harte reports grants from NIH during the conduct of the study; grants from NIH, VA, Cerephex, Eli Lilly, American Cancer Society, AAOGF; grants and personal fees from Aptinyx; personal fees from SUFU, Longitudinal Capital Management; personal fees and non-financial support from University of North Carolina - Chapel Hill; and an affiliation with Arbor Medical Innovations, outside the submitted work. In addition, Dr. Harte has a patent US 9307906 with royalties paid outside the submitted work.
Footnotes
Registration Number and Registry Name: Clinicaltrials.gov identifier:
Conflicts of Interest
The authors declare no conflicts of interest and no competing financial interests with this work.
References
- [1].Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One 2014;9(9):e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Basu N, Kaplan CM, Ichesco E, Larkin T, Harris RE, Murray A, Waiter G, Clauw DJ. Neurobiological features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis Rheumatol 2018. [DOI] [PubMed] [Google Scholar]
- [3].Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA, Clauw DJ. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology 2013;119(6):1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67(5):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen ML, Tseng HC. Symptom clusters in cancer patients. Support Care Cancer 2006;14(8):825–830. [DOI] [PubMed] [Google Scholar]
- [7].Clauw DJ. Pharmacotherapy for patients with fibromyalgia. J Clin Psychiatry 2008;69 Suppl 2:25–29. [PubMed] [Google Scholar]
- [8].Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311(15):1547–1555. [DOI] [PubMed] [Google Scholar]
- [9].Clauw DJ. What is the meaning of “small fiber neuropathy” in fibromyalgia? Pain 2015;156(11):2115–2116. [DOI] [PubMed] [Google Scholar]
- [10].Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR, Urologic Pelvic Pain Collaborative Research N. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology 2009;74(5):983–987, quiz 987 e981–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ, Group MRNS. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davis LL, Kroenke K, Monahan P, Kean J, Stump TE. The SPADE Symptom Cluster in Primary Care Patients With Chronic Pain. Clin J Pain 2016;32(5):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med 2001;23(3):215–226. [DOI] [PubMed] [Google Scholar]
- [14].Drossman DA. Rome III: the new criteria. Chin J Dig Dis 2006;7(4):181–185. [DOI] [PubMed] [Google Scholar]
- [15].Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience 2005;133(2):533–542. [DOI] [PubMed] [Google Scholar]
- [16].Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain 2002;16(3):207–220. [PubMed] [Google Scholar]
- [17].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 2013;14(12 Suppl):T75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12(11 Suppl):T46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Finney SJ, DiStefano C. Nonnormal and categorical data in structural equation modeling Structural equation modeling: A second course, 2nd ed Charlotte, NC, US: IAP Information Age Publishing, 2013. pp. 439–492. [Google Scholar]
- [20].Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum 2012;64(2):579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994;121(12):953–959. [DOI] [PubMed] [Google Scholar]
- [22].Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008;9(5):417–422. [DOI] [PubMed] [Google Scholar]
- [23].Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004;127(4):835–843. [DOI] [PubMed] [Google Scholar]
- [24].Griffith JW, Stephens-Shields AJ, Hou X, Naliboff B, Pontari M, Edwards TC, Williams DA, Clemens JQ, Afari N, Tu F. Pain and urinary symptoms should not be combined into one score: Psychometric findings from the Multi-disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. The Journal of Urology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. J Pain 2013;14(6):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harper DE, Schrepf A, Clauw DJ. Pain Mechanisms and Centralized Pain in Temporomandibular Disorders. J Dent Res 2016;95(10):1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007;27(37):10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119(6):1453–1464. [DOI] [PubMed] [Google Scholar]
- [29].Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum 2009;60(10):3146–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. Pain 2016;157(9):1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain 2009;141(3):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hu LT, Bentler PM. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria Versus New Alternatives. Structural Equation Modeling-a Multidisciplinary Journal 1999;6(1):1–55. [Google Scholar]
- [33].Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, Brummett CM. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology 2015;122(5):1103–1111. [DOI] [PubMed] [Google Scholar]
- [34].Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 2009;144(1–2):95–100. [DOI] [PubMed] [Google Scholar]
- [35].Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 2010;11(11):1230–1239. [DOI] [PubMed] [Google Scholar]
- [36].Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Patients with Interstitial Cystitis/Painful Bladder Syndrome. Journal of Urology 2015;193(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol 2014;192(3):947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kratz AL, Schilling SG, Goesling J, Williams DA. Development and initial validation of a brief self-report measure of cognitive dysfunction in fibromyalgia. J Pain 2015;16(6):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE, Network MR. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017;158(10):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ, Group MRNS. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain 2013;154(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lopez-Sola M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 2014;66(11):3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, Perez Y, Gatchel RJ. The development and psychometric validation of the central sensitization inventory. Pain Pract 2012;12(4):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: results of a large population-based study. Arthritis Rheum 2001;44(4):940–946. [DOI] [PubMed] [Google Scholar]
- [45].Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 2012;64(7):2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62(8):2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nickel JC, Tripp D, Gordon A, Pontari M, Shoskes D, Peters KM, Doggweiler R, Baranowski AP. Update on urologic pelvic pain syndromes: highlights from the 2010 international chronic pelvic pain symposium and workshop, august 29, 2010, kingston, ontario, Canada. Rev Urol 2011;13(1):39–49. [PMC free article] [PubMed] [Google Scholar]
- [48].O’Brien EM, Atchison JW, Gremillion HA, Waxenberg LB, Robinson ME. Somatic focus/awareness: Relationship to negative affect and pain in chronic pain patients. Eur J Pain 2008;12(1):104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].O’Leary MP, Sant GR, Fowler FJ Jr., Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology 1997;49(5A Suppl):58–63. [DOI] [PubMed] [Google Scholar]
- [50].Olesen J, Steiner TJ. The International classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry 2004;75(6):808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Preacher KJ, Zhang G, Kim C, Mels G. Choosing the Optimal Number of Factors in Exploratory Factor Analysis: A Model Selection Perspective. Multivariate Behav Res 2013;48(1):28–56. [DOI] [PubMed] [Google Scholar]
- [52].Schrepf A, Bradley CS, O’Donnell M, Luo Y, Harte SE, Kreder K, Lutgendorf S, Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research N. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun 2015;49:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta JK, Clauw DJ, Harris RE. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 2016;157(10):2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schrepf A, O’Donnell M, Luo Y, Bradley CS, Kreder K, Lutgendorf S, Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research N. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Pain 2014;155(9):1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol 1994;16(1):93–104. [DOI] [PubMed] [Google Scholar]
- [56].Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 2010;139(1):48–57 e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Simon LJ, Landis JR, Erickson DR, Nyberg LM. The Interstitial Cystitis Data Base Study: concepts and preliminary baseline descriptive statistics. Urology 1997;49:64–75. [DOI] [PubMed] [Google Scholar]
- [58].Veasley C, Clare D, Clauw DJ, Cowley T, Nguyen RHN, Reinecke P, Verron SD, Williams DA. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment: 2015 analysis and policy recommendations. . The Chronic Pain Research Alliance 2015. [Google Scholar]
- [59].Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007;22(1):266–271. [DOI] [PubMed] [Google Scholar]
- [60].Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- [61].Warren JW, Langenberg P, Clauw DJ. The number of existing functional somatic syndromes (FSSs) is an important risk factor for new, different FSSs. J Psychosom Res 2013;74(1):12–17. [DOI] [PubMed] [Google Scholar]
- [62].Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am 2009;35(2):339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wolfe F. Fibromyalgianess. Arthritis Rheum 2009;61(6):715–716. [DOI] [PubMed] [Google Scholar]
- [64].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38(6):1113–1122. [DOI] [PubMed] [Google Scholar]
- [65].Woolf Clifford J Pain amplification—A perspective on the how, why, when, and where of central sensitization. Journal of Applied Biobehavioral Research 2018;0(0):e12124. [Google Scholar]
- [66].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.