Abstract
Individualized treatment rules aim to identify if, when, which, and to whom treatment should be applied. A globally aging population, rising healthcare costs, and increased access to patient-level data have created an urgent need for high-quality estimators of individualized treatment rules that can be applied to observational data. A recent and promising line of research for estimating individualized treatment rules recasts the problem of estimating an optimal treatment rule as a weighted classification problem. We consider a class of estimators for optimal treatment rules that are analogous to convex large-margin classifiers. The proposed class applies to observational data and is doubly-robust in the sense that correct specification of either a propensity or outcome model leads to consistent estimation of the optimal individualized treatment rule. Using techniques from semiparametric efficiency theory, we derive rates of convergence for the proposed estimators and use these rates to characterize the bias-variance trade-off for estimating individualized treatment rules with classification-based methods. Simulation experiments informed by these results demonstrate that it is possible to construct new estimators within the proposed framework that significantly outperform existing ones. We illustrate the proposed methods using data from a labor training program and a study of inflammatory bowel syndrome.
Keywords: Individualized treatment rules, convex surrogate, double-robustness, classification, personalized medicine
1. Introduction
There is a growing consensus that the best possible care results from treatment decisions that are carefully tailored to individual patient characteristics (Sox and Greenfield, 2009). Individualized treatment rules (ITRs) formalize tailored treatment decisions as a function from patient information to a recommended treatment. We define an optimal ITR as maximizing the mean of a pre-specified clinical outcome if applied to recommend treatments in a population of interest (see Linn et al., 2016, for alternative definitions of optimality). With expanding access to patient-level data through electronic health records, adverse event reporting, insurance claims, and billing records, there is increasing interest in estimating optimal ITRs from observational data. An important use of an estimated optimal ITR is hypothesis-generation whereby the estimated optimal rule is used to discover covariate-treatment interactions or identify subgroups of patients with large treatment effects. In such applications, it is useful to directly control the class of ITRs within which the optimal ITR will be estimated. The form of this class can be chosen to ensure interpretability, enforce logistical or cost constraints, or make the tests of certain clinical hypotheses overt.
One approach to estimating an optimal ITR is to model some or all of the conditional distribution of the outcome given treatments and covariates and then to use this estimated distribution to infer the optimal ITR. These approaches are sometimes called indirect methods as they indirectly specify the form of the optimal ITR through postulated models for components of the conditional outcome distribution. Indirect methods have dominated the literature on estimating optimal ITRs; examples of indirect estimation methods include variations of g-estimation in structural nested models (Robins, 1989, 1997; Murphy, 2003; Robins, 2004); Q- and A-learning (Zhao et al., 2009; Qian and Murphy, 2011; Moodie et al., 2012; Chakraborty and Moodie, 2013; Schulte et al., 2014), and regret regression (Henderson et al., 2009). However, a major drawback with these approaches is that the postulated outcome models dictates the class of possible ITRs. A consequence is that to obtain a simple ITR requires specification of simple outcome models, which may not be correctly specified. Moreover, if these outcome models are misspecified, the foregoing methods may not be consistent for the optimal ITR within the class implied by the outcome models. For example, to ensure a linear ITR using Q-learning, it is common to use a linear conditional mean model. It can be shown that if the linear mean model is misspecified then the estimated optimal ITR using Q-learning need not converge to the optimal linear ITR (Qian and Murphy, 2011). Alternatively, flexible outcome models that mitigate the risk of misspecification (e.g., Zhao et al., 2009; Qian and Murphy, 2011; Moodie et al., 2013) can induce a class of ITRs that is difficult or impossible to interpret (see Section 2 for details).
An alternative to indirect estimation is to decouple models for the conditional outcome distribution from the class of ITRs. One way to do this is to form a flexible estimator of the mean outcome as a function of the ITR that is consistent under a large class of potential generative models and then to use the maximizer of this function over a pre-specified class of ITRs as the estimator of the optimal ITR. These approaches are called direct (Laber et al., 2014), policy-search (Sutton and Barto, 1998; Szepesvári, 2010), policy learning (Athey and Wager, 2017) or value-search (Davidian et al., 2014) estimators. An advantage of direct estimators is that they permit flexible, e.g., semi- or non-parametric, models for modeled portions of the outcome distribution yet still control the form of the estimated optimal ITR. Direct estimators include outcome weighted learning (Zhao et al., 2012, 2015a, 2015b), robust value-search estimators (Zhang et al., 2012a, 2012b, 2013); marginal structural mean models (Robins et al., 2008; Orellana et al., 2010); and Q-learning with policy-search (Taylor et al., 2015; Zhang et al., 2015, 2017).
While the foregoing methods represent significant progress in direct estimation, computational and theoretical gaps remain. Outcome weighted learning uses a convex relaxation of an inverse-probability weighted estimator (IPWE) of the mean outcome. This convex relaxation makes their method computationally efficient and scalable to large problems; in addition, convexity simplifies derivations of convergence rates and generalization error bounds. However, the IPWE is known to be unstable under certain generative models (Zhang et al., 2012a, 2012b), and theoretical guarantees for outcome weighted learning were developed only for data from a randomized clinical trial. Robust value-search estimators directly maximize an augmented IPWE (AIPWE). The AIPWE is semi-parametric efficient and is significantly more stable than the IPWE. However, the AIPWE is a discontinuous function of the observed data, which makes direct maximization computationally burdensome even in moderate sized problems and complicates theoretical study of these estimators. We establish the theory for both AIPW and its convex relaxation, which fills the gap in the current literature on direct search methods. Marginal structural mean models are best suited for problems where the ITR depends only on a very small number of covariates. Liu et al. (2016) proposed a robust method for estimating optimal treatment rules in a multi-stage setup. At each stage in a multi-stage setup, they proposed a robust weight to replace the original weight in OWL based on the idea of augmentation. However, they still require consistent estimation of the propensity score at the present stage. In particular, their proposal for the single stage problem still relies on an IPWE, and does not possess the double robustness property.
We propose a class of estimators representable as the maximizer of a convex relaxation of the AIPWE; we term this class of estimators Efficient Augmentation and Relaxation Learning (EARL). EARL is computationally efficient, theoretically tractable, and applies to both observational and experimental data. Furthermore, EARL contains outcome weighted learning (OWL) (Zhao et al., 2012) as a special case. However, EARL is considerably more general than OWL, and this generality leads to new insights about classification-based estimation of ITRs, new algorithms, and new theoretical results. Unlike OWL, EARL makes use of both a propensity score and an outcome regression model. Estimators within the EARL framework are doubly-robust in the sense that they consistently estimate the optimal ITR if either the propensity score model or outcome regression model is correctly specified. Within the EARL framework, we are able to characterize convergence rates across a range of convex relaxations, propensity score models, and outcome regression models. In particular, making use of sample splitting, we are able to remove the dependence in estimating the nuisance functions and in constructing the estimated ITR. We show that under all convex relaxations considered, a fast convergence rate of the estimated optimal ITR can be achieved, and that the estimation of the propensity score and outcome regression models need not affect the upper bound of this rate. Our theoretical results complement existing work on convergence rate for estimating optimal treatment decision rules, which primarily compared the estimated rules to the best-in-class rule (Kitagawa and Tetenov, 2017; Athey and Wager, 2017; ?). The proposed method has been implemented in R and is freely available through the ‘DynTxRegime’ package hosted on the comprehensive R network (cran.org).
In Section 2, we introduce the EARL class of estimators. In Section 3, we investigate the theoretical properties of estimators within this class. In Section 4, we use simulation experiments to investigate the finite sample performance of EARL estimators. In Section 5, we present illustrative case studies using data from a labor training program and an inflammatory bowel disease study. In Section 6, we make concluding remarks and discuss potential extensions.
2. Methods
In this section, we first provide background of the proposed method. We then introduce Efficient Augmentation and Relaxation Learning (EARL) in details.
2.1. Background and preliminaries
The observed data, , comprise n independent, identically distributed copies of (X, A, Y ), where: X ∈ ℝp denotes baseline subject measurements; A ∈ {−1, 1} denotes the assigned treatment; and Y ∈ ℝ denotes the outcome, coded so that higher values are better. In this context, an ITR, d, is a map from ℝp into {−1, 1} so that a patient presenting with X = x is recommended treatment d(x). Let denote a class of ITRs of interest. To define the optimal ITR, denoted d*, we use the framework of potential outcomes (Rubin, 1974; Splawa-Neyman et al., 1990). Let Y (a) denote the potential outcome under treatment a ∈ {−1, 1} and define to be the potential outcome under d. The marginal mean outcome is called the value of the ITR d. The optimal ITR satisfies and V (d*) ≥ V (d) for all . Note that this definition of optimality depends on the class . To express the value in terms of the data generating model, we assume: (i) strong ignorability, {Y(−1), Y(1)} ⫫ A|X (Rubin, 1974; Robins, 1986; Splawa-Neyman et al., 1990); (ii) consistency, Y = Y (A); and (iii) positivity, there exists τ > 0 so that τ < P(A = a|X) for each a ∈ {−1, 1} with probability one. These assumptions are common and well-studied (see Schulte et al., 2014, for a recent review of potential outcomes for treatment rules). Assumption (i) is true in a randomized study but unverifiable in an observational study (Bang and Robins, 2005).
Define , then under the foregoing assumptions, it can be shown that
| (1) |
from which it follows that . Q-learning is a common regression-based indirect approach for estimating d* wherein an estimator of Q(x, a) is constructed and subsequently the estimated optimal rule is . Let denote the postulated class of models for Q(x, a), then the set of possible decision rules obtained using Q-learning is . Thus, there is an inherent trade-off between choosing to be sufficiently rich to reduce the risk of model misspecification and the resultant complexity of the resultant class of ITRs.
Direct estimators specify a class of candidate ITRs independently from postulated models for some or all of the generative model. Let denote a class of ITRS; direct search estimators first construct an estimator of the value function, , and then choose as the estimator of d*. Thus, a complex model space for V (·) need not imply a complex class of rules . However, the class of models for V (·) must be sufficiently rich to avoid implicit, unintended restrictions on . To avoid such restrictions and to avoid model-misspecification, it is common to use a flexible class of semi- or non-parametric models for V (·).
2.2. Augmentation for the value function
Define the propensity score , then
| (2) |
where I{·} denotes the indicator function (e.g., Qian and Murphy, 2011). Unlike (1), the preceding expression does not require an estimator of the Q-function. Given an estimator of the propensity score, , a plug-in estimator for V (d) based on (2) is the inverse probability weighted estimator , where is the empirical distribution. The IPWE has potentially high variance as it only uses outcomes from subjects whose treatment assignments coincide with those recommended by d.
One approach to reduce variability is to augment the IPWE with a term involving both the propensity score and the Q-function that is estimated using data from all of the observed subjects (Robins et al., 1994; Cao et al., 2009). Let denote an estimator of Q(x, a). The augmented inverse probability weighted estimator is
| (3) |
It can be seen that is equal to plus an estimator of zero built using outcomes from all subjects regardless of whether or not their treatment assignment is consistent with d. If then for all d.
Hereafter, we use and to denote generic estimators of the Q-function and propensity score. The following assumption is used to establish double robustness of .
Assumption 1
and converge in probability uniformly to deterministic limits Qm(x, a) and πm(a; x).
This assumption does not require that the estimators , are consistent for the truth, only that they converge to fixed functions. The following result is proved in Web Appendix A.
Lemma 2.1
Let be fixed. If either πm(a; x) = π(a; x) or Qm(x, a) = Q(x, a) for all (x, a) outside of a set of measure zero, then , where
The preceding result shows that is doubly-robust in the sense that if either the propensity model or the modeled Q-function is consistent, but not necessarily both, then is consistent for V (d). Thus, the maximizer of over is termed a doubly-robust estimator of the optimal treatment rule (Zhang et al., 2012a, 2012b, 2013). However, because is not continuous, computing this doubly-robust estimator can be computationally infeasible even in moderate problems (Zhang et al., 2012a). Instead, we form an estimator by maximizing a concave relaxation of . Maximizing this concave relaxation is computationally efficient even in very high-dimensional problems. We show that the maximizer of this relaxed criteria remains doubly-robust. Furthermore, we show that the rates of convergence of the proposed estimators depend on the chosen concave relaxation, the chosen propensity model, and the chosen model for the Q-function. The relationships among these choices provides new knowledge about direct search estimators based on concave surrogates (Zhang et al., 2012; Zhao et al., 2012, 2015a, 2015b).
2.3. Efficient augmentation and relaxation learning (EARL)
Let be the class of measurable functions from ℝp into ℝ. Any decision rule d(x) can be written as d(x) = sgn{f(x)} for some function , where we define sgn(0) = 1. For d(x) = sgn{f(x)}, I{a = d(x)} = I{af(x) ≥ 0}. Define V (f), VIPWE,m(f), and V AIPWE,m(f) by substituting I{Af(X) ≥ 0} for I{A = d(X)} in their respective definitions. Define
The following result shows that maximizing is equivalent to minimizing a sum of weighted misclassification rates; a proof is given in Web Appendix B.
Lemma 2.2
Assume that P{f(X) = 0} = 0. Define , then
where , a = ∈ {−1, 1}.
Lemma 2.2 shows that the estimator, , which maximizes over , can be viewed as minimizing a sum of weighted 0–1 losses. In this view, the class labels are and the misclassification weights are , a = ∈ {−1, 1} (see Zhang et al., 2012b, 2013). Directly minimizing the combined weighted 0–1 loss is a difficult non-convex optimization problem (Laber and Murphy, 2011). One strategy to reduce computational complexity is to replace the indicator function with a convex surrogate and to minimize the resulting relaxed objective function (Freund and Schapire, 1999; Bartlett et al., 2006; Hastie et al., 2009). This strategy has proved successful empirically and theoretically in classification and estimation of optimal treatment rules (Zhao et al., 2012). However, unlike previous applications of convex relaxations to the estimation of optimal treatment rules, we establish rates of convergence as a function of the: (i) choice of convex surrogate; (ii) convergence rate of the postulated propensity score estimator; and (iii) convergence rate the postulated Q-function estimator. We characterize the relationship among these three components in Section 3.
The function f is conceptualized as being a smooth function of x that is more easily constrained to possess certain desired structure, e.g., sparsity, linearity, etc. Thus, we will focus on estimation of f within a class of functions called the approximation space; we assume that is a Hilbert space with norm ‖ · ‖k. Let ϕ: ℝ → ℝ denote a convex function and define EARL estimators as those taking the form
| (4) |
where λn‖f‖2 is included to reduce overfitting and λn ≥ 0 is a (possibly data-dependent) tuning parameter. Throughout, we assume that ϕ(t) is one of the following: hinge loss, ϕ(t) = max(1 – t,0); exponential loss, ϕ(t) = e−t; logistic loss, ϕ(t) = log(1 + e−t); or squared hinge loss, ϕ(t) = {max(1 – t, 0)}2. However, other convex loss functions are possible provided that they are differentiable, monotone, strictly convex, and satisfy (0) = 1 (Bartlett et al., 2006). As noted previously, Zhao et al. (2012) proposed a special case of EARL called outcome weighted learning, which set (t) = max(0, 1 – t), , and assumed that the propensity score was known. Thus, as noted previously, EARL is considerably more general than OWL and, as shown in Section 4, the choice of a non-null model for the Q-function and alternative surrogate loss functions can lead to dramatically improved finite sample performance.
2.4. EARL via sample splitting
To facilitate the analysis of the statistical properties of EARL, we consider the following alternative estimator based on the sample splitting. Let I1,I2, …, IK denote a random partition of the indices {1,2, …, n} with Ij ∩ Ik = ∅ for any j ≠ k and . We assume the size of the partitions is comparable, that is, nk = |Ik| with n1 ≍ n2 ≍ … ≍ nK. In practice, K is taken as a small integer (e.g., 2, or 5) and is assumed fixed. Recall that the EARL estimator based on the full sample is defined in (4). In particular, the same samples are used to estimate the nuisance functions , and construct the estimator in (4). This creates the delicate dependence between the estimators , and the samples used in the empirical risk minimization in (4). To remove this dependence, we now modify the procedure via sample splitting. First, for 1 ≤ k ≤ K, we construct estimators , based on the samples in Ik, i.e., {(Xi, Ai, Yi); i ∈ Ik}. Denote I(−k) = {1, …, n}\Ik. Then, we use the remaining samples I(−k) for the EARL estimator
| (5) |
where , a =∈ {−1, 1} and . We note that independent samples are used for estimating the nuisance functions π, Q and the decision rule f. Thus, the dependence between the estimators , and the samples use in (4) is removed. Finally, to obtain a more stable estimator, we can aggregate the estimators
| (6) |
which is the final estimator based on sample splitting. While the estimator requires more computational cost, it has important advantages over the original EARL estimator in (4). From a theoretical perspective, one can still analyze the EARL estimator based on the empirical process theory. This typically requires the entropy conditions on the function classes of π and Q. In comparison, we show in the following section that the sample splitting estimator does not require this condition. To the best of our knowledge, similar sample splitting technique was first applied by Bickel (1982) in general semiparametric estimation problems; see also Schick (1986). Recently, this approach has received attention in causal inference problems as a means of relaxing technical conditions. We refer to Zheng and van der Laan (2011); Chernozhukov et al. (2016); Robins et al. (2017) for further discussion.
3. Theoretical properties
Let be such that d*(x) = sgn{f*(x)}, and . Define the population risk of function f as
and . We define the risk in this way to be consistent with the convention that higher risk is less desirable; however, inspection shows that the risk equals K – V (f) where K is a constant that does not depend on f. Thus, minimizing risk is equivalent to maximizing value, and . Accordingly, for a convex function ϕ, we define the ϕ-risk
By construction, is convex; we assume that it has a unique minimizer and that . The following result is proved in Web Appendix C.
Proposition 3.1
Assume that either πm(a; x) = π(a; x) or Qm(x, a) = Q(x, a). Define and cm(x) = E{|W1(Y, x, A, πm, Qm)| + |W−1(Y, x, A, πm, Qm)|}. Then:
- and
where ψ(θ) = |θ| for hinge loss, for exponential loss, ψ(θ) = (1 + θ)log(1 + θ)/2 + (1 − θ)log(1 − θ)/2 for logistic loss, and ψ(θ) = θ2 for squared hinge loss.
Part (a) of the preceding proposition states that if either the model for the propensity score or for the Q-function is correctly specified, then the EARL procedure, optimized over the space of measurable functions, is Fisher consistent for the optimal rule. Part (b) bounds the difference between V (f) and V * through the surrogate risk difference . The different forms of ψ(·) are due to the fact that different loss functions induce different distance measures of closeness of f(x) to the true f*(x). We use these risk bounds to derive bounds on the convergence rates of the value of EARL estimators constructd using sample splitting.
Let Π denote the function spaces to which the postulated models for π(a; x) belong; that is, the estimator belongs to Π. Similarly, let denote a postulated class of models for Q(x, a). In this section, we allow the approximation space, , to be arbitrary subject to complexity constraints; our results allow both parametric or non-parametric classes of models. Our primary result is a bound on the rate of convergence of in terms of the ϕ-risk difference .
For any ϵ > 0 and measure P, let denote the covering number of the space , that is, is the minimal number of closed L2(P)-balls of radius ϵ required to cover (Kosorok, 2008). Denote . We make the following assumptions.
Assumption 2
There exists MQ > 0 such that and for all (x, a) ∈ ℝp × {−1, 1} and ; there exists 0 < LΠ < MΠ < 1 such that LΠ ≤ π(a; x) ≤ MΠ for all (x, a) ∈ ℝp × {−1, 1} and π ∈ Π.
Assumption 3
There exist constants 0 < v < 2 and c < ∞ such that for all 0 < ϵ ≤ 1: , where the supremum is taken over all finitely discrete probability measures P.
Assumption 4
For some α, β > 0, and for a = ±1 and 1 ≤ k ≤ K.
Assumption 2 assumes outcomes are bounded, which often holds in practice. Otherwise, we can always use a large constant to bound the outcome. We also assume propensity scores are bounded away from 0 and 1, which is a standard condition for the identification of the treatment effect in causal inference. Assumption 3 controls the complexity of the function spaces for estimating an optimal ITR. For example, if is composed of linear combinations of elements in a fixed base class, , where has finite Vapnik-Chervonenkis (VC) dimension vc, then there exists a constant cvc, depending on vc, so that (Theorem 9.4, Kosorok (2008)). We note that the entropy conditions on and Π are not needed by using the sample splitting technique, due to the independence between estimating π, Q and estimating f.
Assumption 4 specifies the rate of convergence of the estimators and in terms of the ‖ · ‖P,2 norm. It is well known that the L2 rate of convergence is related to the smoothness of the function classes and Π and the dimension of X. For instance, if corresponds to the Holder class with smoothness parameter s on the domain [0, 1]p, then Theorem 7 of Newey (1997) implies , where is the regression spline estimator and K is the number of basis functions.
Define the approximation error incurred by optimizing over as
| (7) |
The following result on the risk bound is the main result in this section and is proved in the Web Appendix D.
Theorem 3.1
Suppose that assumptions 1–4 hold, λn → 0. Define . If Qm(x, a) = Q(x, a) and πm(a; x) = π(a;x), then
In all cases considered, the function ψ is invertible on [0,1], and its inverse is monotone non-decreasing. Thus, for sufficiently large n (making the right-hand-side of the equation sufficiently small) the inequality can be re-arranged to yield a bound on . The form of ψ−1 dictates the tightness of the bound as a function of the ϕ-risk. According to Lemma 3 in Bartlett et al (2006), a flatter loss function leads to better bound on ψ function. In other words, a flatter loss function gives better bounds on V * −V (f) in terms of . In this respect, hinge-loss can be seen to provide the tightest bound; however, the ϕ-risk is not directly comparable across different loss functions as they are not on the same scale.
The right hand side of the bound in Theorem 3.1 consists of three parts: the approximation error due to the size of the approximation space , the error due to the estimation in the function space , and the error incurred from plugging the estimators and . As expected, the approximation error decreases as the complexity of the class increases, whereas the estimation error increases with the complexity of the class and decreases as the sample size increases.
For the error incurred from plugging the estimators and , the component converges to 0 faster than in regular statistical models (i.e., α, β ≤ 1/2). Thus, it suffices to only look at the term . This term can shrink to 0 sufficiently fast as long as one of the estimators and has a fast rate, due to the multiplicative form of the estimation error. For example, if α = β = 1/4, the error from plugging the estimators and is . Hence, the rate of the proposed method is faster compared with the outcome weighted learning method, which is developed based on an IPWE and does not enjoy this multiplicative form of the errors. This phenomenon can be viewed as a nonparametric version of the double robustness property (see Fan et al., 2016; Benkeser et al., 2017, for additional discussion). Compared with the results in Athey and Wager (2017), we allow for the surrogate loss to replace the 0–1 loss in solving for the optimizer. While the orders in the bound of convergence rates are comparable, the differences in the constants in the bounds might be due to the application of the surrogate function.
Remark 1
If α = β and
| (8) |
then
| (9) |
where the upper bound is of the same order as that obtained if the conditional mean Q(x, a) and propensity score π(a; x) are known. We note that the additional constraints on α and v in (8) are necessary to obtain the fast rate of convergence (9). For instance, if the function classes and Π are indexed by finite dimensional parameters, we can obtain α = β = 1/2 under mild conditions. As a result, the first condition in (8) holds and the fast rate of convergence (9) is applied. On the other hand, if is a simple class but and converge at slower rates, the rate for will be driven by .
To estimate the value of the optimal treatment rule V *, one can aggregate the empirical value of the sample splitting estimator in each subsamples I(−k), that is, , where
The following corollary, provides a corresponding bound on the rate for . The proof is given in Web Appendix D.
Corollary 3.1
Suppose that assumptions 1–4 hold, and λn → 0. If Qm(x,a) = Q(x, a) and πm(a; x) = π(a; x), then
where
Remark 2
Athey and Wager (2017) and Kitagawa and Tetenov (2017) investigated the binary-action policy learning problem, and established a risk bound of n−1/2 for both known propensities (Kitagawa and Tetenov, 2017) and unknown propensities (Athey and Wager, 2017). However, they considered a restricted class of decision rules and subsequent risk bound were established with respect to the optimal rule within this restricted class. Hence, there was not consideration of the approximation error. In contrast, we considered the optimal rule within the space consisting of all measurable functions from ℝp (the covariate space) to {−1, 1} (the treatment space). We used a smaller space, for example, a reproducing kernel Hilbert space, to approximate the policy space and to avoid overfitting. This led to a tradeoff between approximation and estimation error, and λn was a tuning parameter to control this bias-variance tradeoff. Consequently, the achieved convergence rates are different.
4. Simulation experiments
We compare EARL estimators with: Q-learning fit using ordinary least squares (QL, Qian and Murphy, 2011); estimating the optimal rule within a restricted class based on an AIPW estimator (AIPWE, Zhang et al., 2012b); and outcome weighted learning (OWL, Zhao et al., 2012). Comparisons are made in terms of the average value of the rule estimated by each method. For Q-learning, we fit a linear model for the Q-function that includes all two-way interactions between predictors and pairwise interactions between these terms and treatment. In the AIPWE method, an AIPW estimator for the value function is constructed and then the optimal linear rule that maximizes the AIPW estimator is identified via a genetic algorithm. Similar to EARL, both a propensity score model and a regression model need to be fitted in AIPWE. We will use the same set of models in EARL and the AIPWE, which are detailed in below. For OWL, we use a linear decision rule; recall that OWL is a special case of EARL with , ϕ(t) = max(0, t), and a known propensity score. All estimation methods under consideration require penalization; we choose the amount of penalization using 10-fold cross-validation of the value. Within the class of EARL estimators, we considered hinge, squared-hinge, logistic, and exponential convex surrogates. An implementation of EARL is available in the R package ‘DynTxRegime;’ this package also includes implementations of AIPWE and OWL and therefore can be used to replicate the simulation studies presented here. We included an example for implementing EARL method using ‘DynTxRegime’ package in Web Appendix H.
We consider generative models of the form: X = (X1, …, Xp) ∼i.i.d. N(0,1) with p = 10; treatments are binary, taking the values in {−1, 1} according to the model p(A = 1|X) = exp{ℓ(X)}/[1 + exp{ℓ(X)}], where ℓ(x) = x1 + x2 + x1x2 in Scenario 1, and ℓ(x) = 0.5x1 – 0.5 in Scenario 2; , where ϵ ~ N(0, 1), and c(x) = x1+x2−0.1. Write X2 to denote . The following modeling choices are considered for the propensity and outcome regression models.
CC. A correctly specified logistic regression model for π(A;X) with predictors X1, X2 and X1X2 in Scenario 1, and with predictor X1 in Scenario 2; and a correctly specified linear regression model for Q(X, A) with predictors X, X2, A, X1A and X2A in both scenarios.
CI. A correctly specified logistic regression model for π(A; X) with predictors X1, X2 and X1X2 in Scenario 1, and with predictor X1 in Scenario 2; and an incorrectly specified linear model for Q(X, A) with predictors X, A, XA in both scenarios.
IC. An incorrectly specified logistic regression model for π(A; X) with predictors X in Scenario 1, and without any predictors in Scenario 2; and a correctly specified linear model for Q(X, A) with predictors X, X2, A, X1A and X2A in both scenarios.
II. An incorrectly specified logistic regression model for π(A; X) with predictors X in Scenario 1, and without any predictors in Scenario 2; and an incorrectly specified linear model for Q(X, A) with predictors X, A, XA in both scenarios.
We use the same model specifications to carry out AIPWE, and denote them as CC-A, CI-A, IC-A, and II-A correspondingly. For the OWL method, we use correct and incorrect propensity models to construct the ITRs, and denote them as C. and I. respectively. Similarly, we use Q-learning to construct the ITRs based on correct and incorrect regression models, and term them as .C and .I respectively.
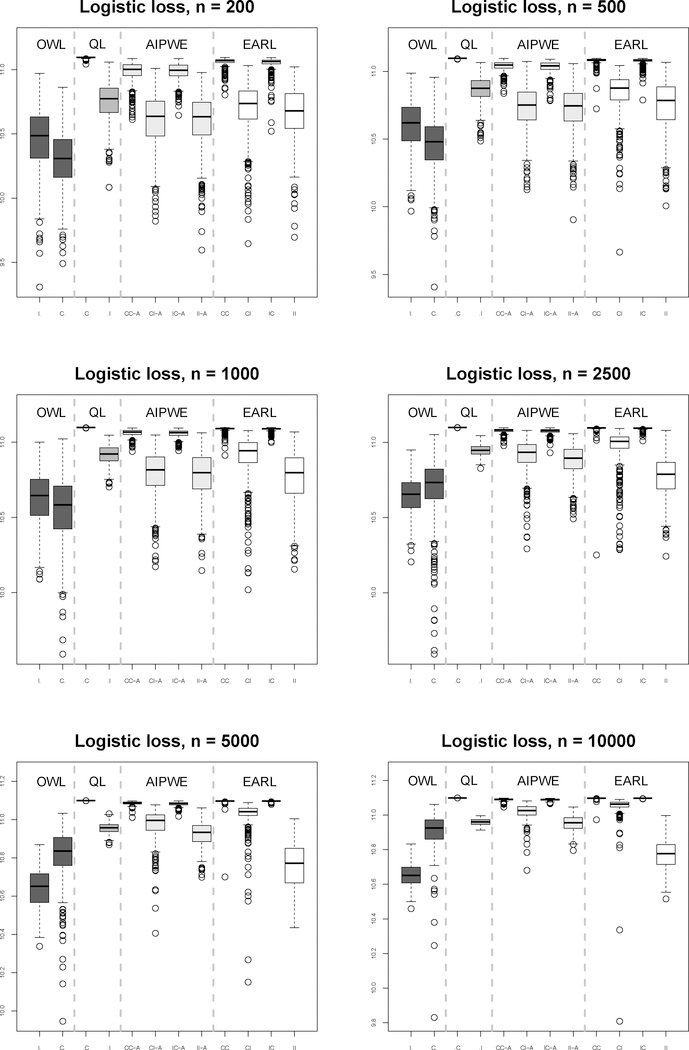
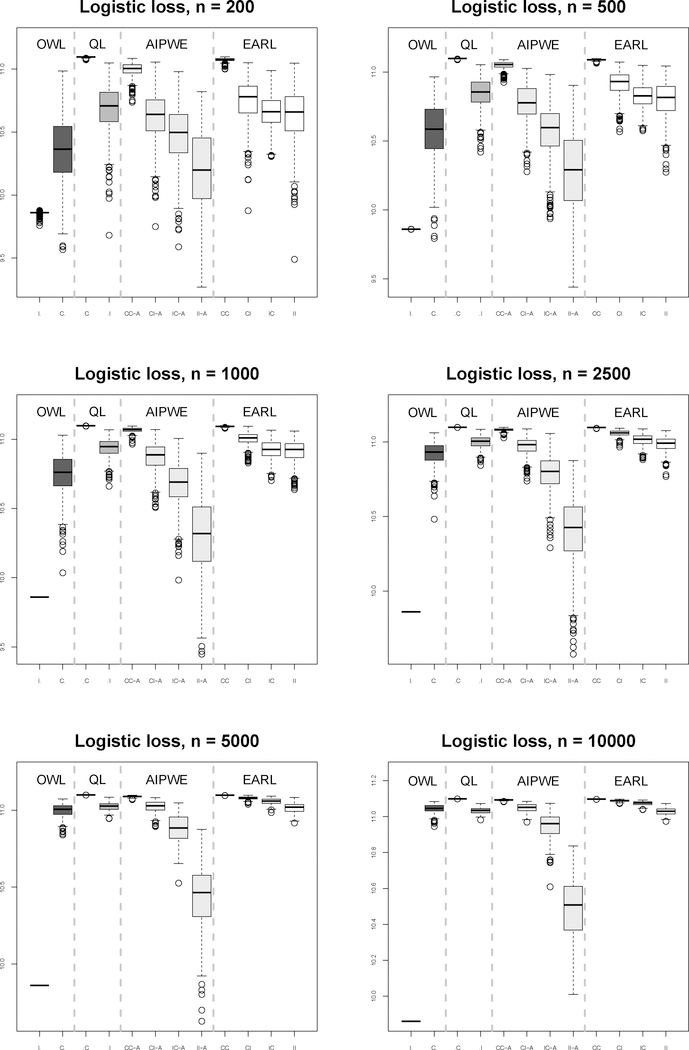
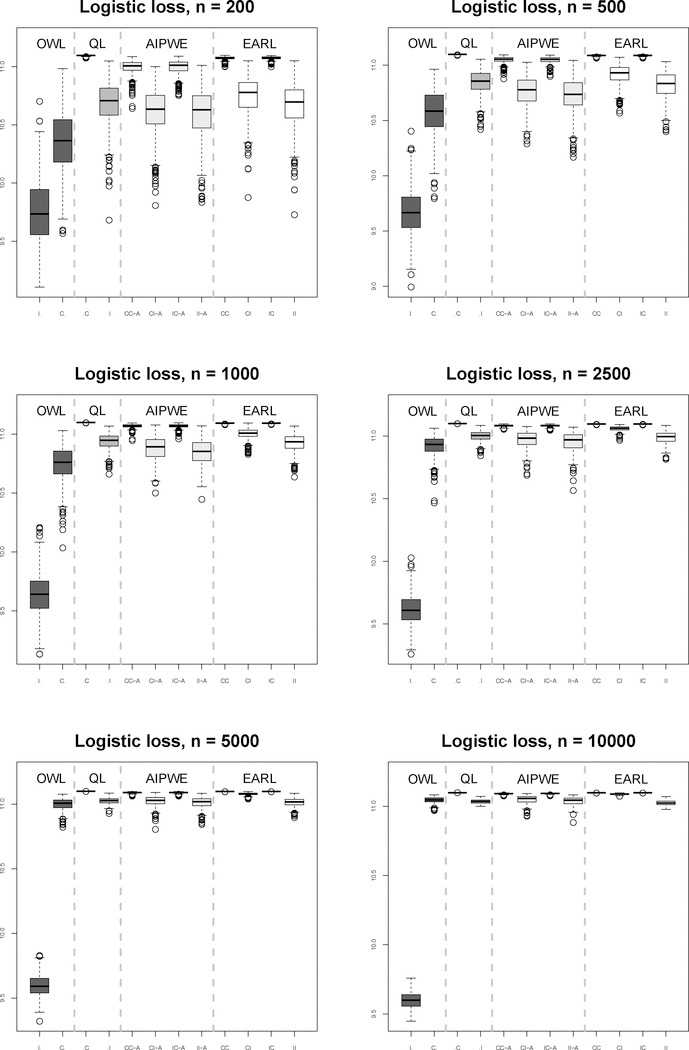
We consider sample sizes 200, 500, 1000, 2500, 5000 and 10000. We generate a large validation data set (size 10000) and 500 training sets under each sample size. The ITRs are constructed based on one training set out of 500 replicates using competing methods. For implementing EARL, we use logistic loss. We observe similar patterns for other surrogate loss functions (see the Web Appendix). We carry out cross-validation to select λn among a pre-specified set of values (2−5,,2−4, …, 25). Then we calculate the mean response had the whole population followed the rule (the value function) by averaging the outcomes over 10000 subjects under the estimated ITRs in the validation data set. Thus, there are 500 values of the estimated rules on the validation set for each sample size. Boxplots of these values are shown in Figures 1 and 3. The performance of OWL was generally worse than that of the EARL estimator or QL. The AIPWE method exhibits a larger bias and a higher variance compared to the proposed method, while running approximately 200 times slower. As expected, the QL method works best when the model is correctly specified but can perform poorly when this model is misspecified.
Figure 1:
Boxplots for Scenario 1 results under QL, AIPWE, and OWL and EARL using logistic loss.
Figure 3:
Boxplots for Scenario 3 results under QL, AIPWE, and OWL and EARL using logistic loss.
It appears that misspecification of the model for the Q-function has a bigger impact than misspecification of the propensity score model on the AIPWE and EARL methods. The relatively poor performance when the propensity is correctly specified but the regression model is not might be attributed in part to inverse weighting by the propensity score, which is problematic when some estimated propensity scores are close to zero, yielding large weights and subsequently induces bias (Kang and Schafer, 2007). This is illustrated by contrasting scenarios 1 and 2. Propensity scores in Scenario 2 are bounded away from zero, which yield a better result compared to Scenario 1. Furthermore, the large variability when the regression model is misspecified may be partly a consequence of the method used to estimate the coefficients in the regression model (see Cao et al., 2009).
Finally, we consider an example to illustrate the impact of a severely misspecified propensity score model. In Scenario 3, the data was generated as in Scenario 2 except that the propensity score was set to 0.025 for all subjects. The ‘CI’ setup outperformed the ‘IC’ setup, especially when the sample size was small. Furthermore, the performance of the AIPWE method was largely affected by this poorly imposed propensity model. The results of ‘CI’ and ‘II’ setups were unsatisfactory even when the sample size was increased to 10000. This example indicates that the performances in the ‘CI’ and ‘IC’ setups depend on the degree of misspecification in the outcome regression model and propensity score model.
We also conducted a set of simulation experiments to investigate the role of parametric and nonparametric models for the propensity score and outcome regression. In addition, we compared the performance across different surrogate loss functions, including logistic loss, exponential loss, squared hinge loss, and hinge loss. These additional simulation results can be found in Web Appendix F. In summary, we found that in the examples considered, using nonparametric working models for propensity scores could improve results over parametric models. Hinge loss has a more robust performance when the regression model is incorrect compared to other smooth losses.
5. Application: Ocean State Crohn’s and Colitis Area Registry (OSCCAR)
OSCCAR is a community—based incident cohort of subjects with inflammatory bowel disease (IBD) residing in the state of Rhode Island that was established in 2008 (Sands et al., 2009). Subjects enrolled in OSCCAR have ulcerative colitis (UC), Crohn’s disease (CD), or indeterminate colitis (IC). Corticosteroids are commonly used to treat active symptoms. Although, corticosteroids often promptly achieve remission, long-term use is complicated by many potential side effects. One treatment strategy for IBD patients is a “step-up” approach in which patients are prescribed medications with increasing potential toxicity based on the severity of their disease. Alternatively, a “top-down” approach uses aggressive therapy early in the disease course to prevent long-term complications. Both approaches have been shown to be clinically effective, however, there is treatment response heterogeneity and it is not clear which treatment is right for each individual patient. Clinical theory dictates that those likely to experience a more aggressive disease progression would benefit more from “top-down” than “step-up”; whereas those likely to experience a less aggressive progression might benefit more from “step-up.”
The primary outcome is the disease activity score measured at the end of the second year, as measured by the Harvey—Bradshaw Index for subjects with CD and the Simple Clinical Colitis Index for subjects with UC. In both measures, higher scores reflect more disease activity. A high-quality treatment rule would reduce disease activity by assigning patients to top-down if it is necessary and step-up otherwise. Among the 274 patients included in the observed data, 32 patients were assigned to the top-down strategy (A = 1) and 242 were assigned to step-up (A = −1). To remain consistent with our paradigm of maximizing mean response we used the negative disease activity score as the response, Y. 11 patient covariates were used, which included age, gender, ethnicity, marital status, race, body mass index, disease type, antibiotics drug usage, antidiarrheal drug usage, indicator for extra-intestinal manifestation and baseline disease activity scores. We used a linear regression model to estimate the Q-function, and a regularized logistic regression model to estimate the propensity score to avoid overfitting. In addition to the EARL estimators we applied QL and OWL to estimate an optimal treatment rule. Because this is an observational study with unknown propensity scores, we evaluated the estimated treatment rules using inverse probability weighting , where is the estimated propensity score. Higher values of , that is, lower disease activity scores, indicate a better overall benefit.
The coefficients of the estimated optimal treatment rules constructed from EARL with logistic loss are presented in Table 1. A permutation test based on 2000 permutation times was conducted to obtain the p-value for each covariate, which showed that body mass index was significant at 0.05 level and gender was significant at 0.1 level. In general, patients with a more severe disease status at baseline are likely to benefit from a top-down therapy. This is consistent with clinical theory as these symptoms are associated with higher disease severity.
Table 1:
Coefficients for the estimated optimal decision rules by EARL with logistic loss (*: significant at 0.05 level).
| Coefficient | p-value | |
|---|---|---|
| Intercept | 2.466 | - |
| Age | −0.001 | 0.905 |
| Gender (Male = 1) | 0.756 | 0.015* |
| Ethnicity (Hispanic = 1) | −1.045 | 0.144 |
| Marital status (Single = 1) | −0.320 | 0.318 |
| Race (White = 1) | −0.233 | 0.478 |
| Body mass index | −0.063 | 0.037* |
| Disease type (UC or IC = 1) | 0.309 | 0.234 |
| Antibiotics drug usage (Yes = 1) | −0.156 | 0.563 |
| Antidiarrheals drug usage (Yes = 1) | −0.580 | 0.167 |
| Extra-intestinal manifestation (Yes = 1) | 0.273 | 0.286 |
| Baseline disease activity scores | 0.050 | 0.427 |
Table 2 describes the agreement between the estimated optimal decision rules constructed using different methods, which shows that the rules estimated using EARL with different loss functions give quite similar treatment recommendations. In this table, we also present the agreement between the estimated decision rules and the observed treatments. Compared to the observed treatment allocations, the estimated rules encourage more patients to receive top-down therapy, where 161 patients are recommended to top-down treatment by EARL methods with all loss functions, 225 patients are recommended by OWL method using logistic loss and 145 patients are recommended by QL method respectively. The estimated disease activity score is 1.75 using logistic loss, compared with 1.80 for the QL estimator, and 1.75 for OWL using logistic loss. Although the achieved benefit of the ITR yielded by OWL and EARL were similar, EARL recommended less patients to the more intensive top-down therapy, which could benefit patients by reducing the side effects. The achieved benefits of the derived ITRs were greater than the benefit that was achieved in the observed dataset, where the average disease activity score was 2.24. Since top-down therapy is relative new in the practice, to be conservative, physicians tend not to provide such therapy to patients. Our analysis encourages the usage of top-down therapy for a greater benefit, which can be tailored according to individual characteristics. By looking into the relationship between the observed treatment and covariates, we found that in current practice, physicians were more likely to follow top-down therapy while giving out antibiotics and antidiarrheals drugs in patients with Crohn’s disease. The ITRs resulted from EARL, on the other hand, were more likely to recommend top-down therapy for ulcerative colitis/indeterminate colitis patients while they are not taking antibiotics and antidiarrheals drugs.
Table 2:
Agreements between the estimated optimal decision rule yield by different methods and the observed treatment. OWL-logit: OWL using logistic loss; EARL: EARL using logistic loss; EARL: EARL using exponential oss; EARL-hinge: EARL using hinge loss; EARL-sqhinge: EARL using squared hinge loss; QL: Q-learning.
| OWL-Logit | EARL-logit | EARL-exp | EARL-hinge | EARL-sqhinge | QL | |
|---|---|---|---|---|---|---|
| OWL-Logit | 1 | 0.642 | 0.821 | 0.639 | 0.639 | 0.577 |
| EARL-logit | 1 | 0.588 | 0.996 | 0.996 | 0.920 | |
| EARL-exp | 1 | 0.591 | 0.591 | 0.529 | ||
| EARL-hinge | 1 | 1 | 0.916 | |||
| EARL-sqhinge | 1 | 0.916 | ||||
| QL | 1 | |||||
| Observed | 0.193 | 0.449 | 0.117 | 0.453 | 0.453 | 0.507 |
We also applied our method to the study of National Supported Work Demonstration, which also showed a superior performance of the proposed method. Results are shown in Web Appendix G.
6. Discussion
We proposed a class of estimators for the optimal treatment rule that we termed EARL. This class of methods is formed by applying a convex relaxation to the AIPWE of the marginal mean outcome. To reduce the risk of misspecification, it is possible to use flexible, e.g., nonparametric, models for the propensity score and the Q-function. However, we showed theoretically and empirically that such flexibility comes at the cost of additional variability and potentially poor small sample performance.
We demonstrated that extreme propensity scores may lead to a large variance in the augmented inverse probability weighted estimator. To alleviate this issue, weight stabilization could potentially help. In particular, we can consider a stabilized weight of the form
Using modified weight leads to consistent estimator of the optimal decision rule if positivity assumption holds, that is, Lemma 2.2 still holds under the modified weight. Alternatively, we may also consider an estimator which achieves the smallest variance among its class of doubly robust estimators when the propensity score model is correctly specified. Such an estimator can be derived following the techniques used in Cao et al. (2009).
There are several important ways this work might be extended. The first is to handle time-to-event outcomes wherein the observed data are subject to right-censoring. In this setting, efficient methods for augmentation to adjust for censoring might be folded into the EARL framework. Another extension is to multi-stage treatment rules, also known as, dynamic treatment regimes (Murphy, 2003; Robins, 2004; Moodie et al., 2007). A challenging component of this extension is that the variability of the AIPWE increases dramatically as the number of treatment stages increases. We believe that the convex relaxation may help in this setting not only in terms of computation but also by reducing variance.
Supplementary Material
Figure 2:
Boxplots for Scenario 2 results under QL, AIPWE, and OWL and EARL using logistic loss.
Acknowledgements
This work was partially supported by R01DK108073, P01CA142538, R01DE024984, P30CA015704, S10 OD020069, DMS-1555141, and grants from the Crohn’s and Colitis Foundation and the Centers for Disease Control and Prevention (DP004785-05).
Footnotes
Supplementary Materials
The Web Appendix referenced in Sections 3, 4 and 5 is available online.
Contributor Information
Ying-Qi Zhao, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA.
Eric B. Laber, Department of Statistics, North Carolina State University, Raleigh, NC, 27695, USA
Yang Ning, Department of Statistical Science, Cornell University, Ithaca, NY, 14853, USA.
Sumona Saha, School of Medicine and Public Health, University of Wisconsin, Madison, WI, 53705, USA.
Bruce E. Sands, Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA
References
- Athey Susan and Wager Stefan. Efficient policy learning. arXiv preprint arXiv:1702.02896, 2017.
- Bang Heejung and Robins James M. Doubly robust estimation in missing data and causal inference models. Biometrics, 61(4):962–973, 2005. [DOI] [PubMed] [Google Scholar]
- Bartlett Peter L, Jordan Michael I, and McAuliffe Jon D. Convexity, classification, and risk bounds. J. of American Statistical Association, 101(473):138–156, 2006. [Google Scholar]
- Benkeser David, Carone Marco, Van Der Laan MJ, and Gilbert PB. Doubly robust nonparametric inference on the average treatment effect. Biometrika, 104(4):863–880, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel Peter J. On adaptive estimation. The Annals of Statistics, pages 647–671, 1982. [Google Scholar]
- Cao Weihua, Tsiatis Anastasios A, and Davidian Marie. Improving efficiency and robustness of the doubly robust estimator for a population mean with incomplete data. Biometrika, 96(3):723–734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty Bibhas and Moodie Erica EM. Statistical Methods for Dynamic Treatment Regimes. Springer, 2013. [Google Scholar]
- Chernozhukov Victor, Chetverikov Denis, Demirer Mert, Duflo Esther, Hansen Christian, and Newey Whitney K. Double machine learning for treatment and causal parameters Technical report, cemmap working paper, Centre for Microdata Methods and Practice, 2016. [Google Scholar]
- Davidian M, Tsiatis AA, and Laber EB Value search estimators In Dynamic Treatment Regimes, pages 1–40. Springer, 2014. [Google Scholar]
- Fan Jianqing, Imai Kosuke, Liu Han, Ning Yang, and Yang Xiaolin. Improving covariate balancing propensity score: A doubly robust and efficient approach. Technical report, 2016.
- Freund Yoav and Schapire Robert E. Large margin classification using the perceptron algorithm. Machine learning, 37(3):277–296, 1999. [Google Scholar]
- Hastie T, Tibshirani R, and Friedman JH The Elements of Statistical Learning. Springer-Verlag New York, Inc., New York, second edition, 2009. [Google Scholar]
- Henderson R, Ansell P, and Alshibani D Regret-Regression for Optimal Dynamic Treatment Regimes. Biometrics, 66(4), 2009. [DOI] [PubMed] [Google Scholar]
- Kang Joseph DY and Schafer Joseph L. Demystifying double robustness: A comparison of alternative strategies for estimating a population mean from incomplete data. Statistical science, pages 523–539, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Toru and Tetenov Aleksey. Who should be treated? empirical welfare maximization methods for treatment choice. 2017. [Google Scholar]
- Kosorok MR Introduction to empirical processes and semiparametric inference. Springer-Verlag, New York, 2008. [Google Scholar]
- Laber Eric B and Murphy Susan A. Adaptive confidence intervals for the test error in classification. Journal of the American Statistical Association, 106(495):904–913, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laber Eric B, Lizotte Daniel J, Qian Min, Pelham William E, Murphy Susan A, et al. Dynamic treatment regimes: Technical challenges and applications. Electronic Journal of Statistics, 8:1225–1272, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn Kristin A, Laber Eric B, and Stefanski Leonard A. Interactive q-learning for quantiles. Journal of the American Statistical Association, (just-accepted):1–37, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Ying, Wang Yuanjia, Kosorok Michael R, Zhao Yingqi, and Zeng Donglin. Robust hybrid learning for estimating personalized dynamic treatment regimens. arXiv preprint arXiv:1611.02314, 2016.
- Moodie Erica E. M., Richardson Thomas S., and Stephens David A. Demystifying optimal dynamic treatment regimes. Biometrics, 63(2):447–455, 2007. [DOI] [PubMed] [Google Scholar]
- Moodie Erica EM, Chakraborty Bibhas, and Kramer Michael S. Q-learning for estimating optimal dynamic treatment rules from observational data. Canadian Journal of Statistics, 40(4):629–645, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie Erica EM, Dean Nema, and Sun Yue Ru. Q-learning: Flexible learning about useful utilities. Statistics in Biosciences, pages 1–21, 2013. [Google Scholar]
- Murphy SA Optimal dynamic treatment regimes. Journal of the Royal Statistical Society, Series B, 65:331–366, 2003. [Google Scholar]
- Newey Whitney K. Convergence rates and asymptotic normality for series estimators. Journal of econometrics, 79(1):147–168, 1997. [Google Scholar]
- Orellana L, Rotnitzky A, and Robins J Dynamic regime marginal structural mean models for estimation of optimal dynamic treatment regimes, part i: Main content. Int. Jrn. of Biostatistics, 6(2):1–19, 2010. [PubMed] [Google Scholar]
- Qian Min and Murphy SA Performance guarantees for individualized treatment rules. The Annals of Statistics, 39:1180–1210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins James. A new approach to causal inference in mortality studies with a sustained exposure periodapplication to control of the healthy worker survivor effect. Mathematical Modelling, 7:1393–1512, 1986. [Google Scholar]
- Robins James. Causal inference from complex longitudinal data. Lect. Notes Statist, 120: 69–117, 1997. [Google Scholar]
- Robins James M. The analysis of randomized and non-randomized aids treatment trials using a new approach to causal inference in longitudinal studies. Health service research methodology: a focus on AIDS, 113:159, 1989. [Google Scholar]
- Robins James M. Optimal structural nested models for optimal sequential decisions. In In Proceedings of the Second Seattle Symposium on Biostatistics, pages 189–326. Springer, 2004. [Google Scholar]
- Robins James M, Rotnitzky Andrea, and Zhao Lue Ping. Estimation of regression coefficients when some regressors are not always observed. Journal of the American Statistical Association, 89(427):846–866, 1994. [Google Scholar]
- Robins James M, Li Lingling, Mukherjee Rajarshi, Tchetgen Eric Tchetgen, Aad van der Vaart, et al. Minimax estimation of a functional on a structured high-dimensional model. The Annals of Statistics, 45(5):1951–1987, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Orellana L, and Rotnitzky A Estimation and extrapolation of optimal treatment and testing strategies. Statistics in Medicine, pages 4678–4721, 2008. [DOI] [PubMed] [Google Scholar]
- Rubin DB Estimating causal effects of treatments in randomized and nonrandomized studies. Journal of Educational Psychology, 66:688–701, 1974. [Google Scholar]
- Sands Bruce E, LeLeiko Neal, Shah Samir A, Bright Renee, and Grabert Stacey. Osccar: ocean state crohn’s and colitis area registry. Medicine and Health Rhode Island, 92(3): 82, 2009. [PubMed] [Google Scholar]
- Schick Anton. On asymptotically efficient estimation in semiparametric models. The Annals of Statistics, pages 1139–1151, 1986. [Google Scholar]
- Schulte Phillip J., Tsiatis Anastasios A., Laber Eric B., and Davidian Marie. Q- and a-learning methods for estimating optimal dynamic treatment regimes. Statistical Science, 29:640–661, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sox Harold C and Greenfield Sheldon. Comparative effectiveness research: a report from the institute of medicine. Annals of Internal Medicine, 151(3):203–205, 2009. [DOI] [PubMed] [Google Scholar]
- Splawa-Neyman J, Dabrowska DM, and Speed TP. On the application of probability theory to agricultural experiments (engl. transl. by d.m. dabrowska and t.p. speed). Statistical Science, 5:465–472, 1990. [Google Scholar]
- Sutton Richard S. and Barto Andrew G. Reinforcement Learning I: Introduction. MIT Press, Cambridge,MA, 1998. [Google Scholar]
- Szepesvári Csaba. Algorithms for reinforcement learning. Synthesis Lectures on Artificial Intelligence and Machine Learning, 4(1):1–103, 2010. [Google Scholar]
- Taylor Jeremy MG, Cheng Wenting, and Foster Jared C. Reader reaction to ga robust method for estimating optimal treatment regimesh by zhang et al.(2012). Biometrics, 71 (1):267–273, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Baqun, Tsiatis Anastasios A, Davidian Marie, Zhang Min, and Laber Eric. Estimating optimal treatment regimes from a classification perspective. Stat, 1(1):103–114, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Baqun, Tsiatis Anastasios A, Laber Eric B, and Davidian Marie. A robust method for estimating optimal treatment regimes. Biometrics, 68(4):1010–1018, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Baqun, Tsiatis Anastasios A, Laber Eric B, and Davidian Marie. Robust estimation of optimal dynamic treatment regimes for sequential treatment decisions. Biometrika, 100:681–695, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yichi, Laber Eric B, Tsiatis Anastasios, and Davidian Marie. Using decision lists to construct interpretable and parsimonious treatment regimes. Biometrics, 71(4):895–904, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yichi, Laber Eric B, Davidian Marie, and Tsiatis Anastasios A. Estimation of optimal treatment regimes using lists. Journal of the American Statistical Association, (just-accepted), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YQ, Zeng Donglin, Rush A. John, and Kosorok Michael R. Estimating individualized treatment rules using outcome weighted learning. Journal of American Statistical Association, 107:1106–1118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Yufan, Kosorok Michael R., and Zeng Donglin. Reinforcement learning design for cancer clinical trials. Statistics in Medicine, 28:3294–3315, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Wenjing and van der Laan Mark J. Cross-validated targeted minimum-loss-based estimation In Targeted Learning, pages 459–474. Springer, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.