Abstract
Stress induces a shift from hippocampus-based “cognitive” toward dorsal striatum-based “habitual” learning and memory. This shift is thought to have important implications for stress-related psychopathologies, including post-traumatic stress disorder (PTSD). However, there is large individual variability in the stress-induced bias toward habit memory, and the factors underlying this variability are completely unknown. Here we hypothesized that a functional deletion variant of the gene encoding the α2b-adrenoceptor (ADRA2B), which has been linked to emotional memory processes and increased PTSD risk, modulates the stress-induced shift from cognitive toward habit memory. In two independent experimental studies, healthy humans were genotyped for the ADRA2B deletion variant. After a stress or control manipulation, participants completed a dual-solution learning task while electroencephalographic (Study I) or fMRI measurements (Study II) were taken. Carriers compared with noncarriers of the ADRA2B deletion variant exhibited a significantly reduced bias toward habit memory after stress. fMRI results indicated that, whereas noncarriers of the ADRA2B deletion variant showed increased functional connectivity between amygdala and putamen after stress, this increase in connectivity was absent in carriers of the deletion variant, who instead showed overall enhanced connectivity between amygdala and entorhinal cortex. Our results indicate that a common genetic variation of the noradrenergic system modulates the impact of stress on the balance between cognitive and habitual memory systems, most likely via altered amygdala orchestration of these systems.
SIGNIFICANCE STATEMENT Stressful events have a powerful effect on human learning and memory. Specifically, accumulating evidence suggests that stress favors more rigid dorsal striatum-dependent habit memory, at the expense of flexible hippocampus-dependent cognitive memory. Although this shift may have important implications for understanding mental disorders, such as post-traumatic stress disorder, little is known about the source of individual differences in the sensitivity for the stress-induced bias toward habit memory. We report here that a common genetic variation of the noradrenergic system, a known risk factor for post-traumatic stress disorder, modulates the stress-induced shift from cognitive to habit memory, most likely through altered crosstalk between the hippocampus and dorsal striatum with the amygdala, a key structure in emotional memory.
Keywords: amygdala, dorsal striatum, hippocampus, memory, noradrenaline, stress
Introduction
Stress has a major impact on health and well-being (McEwen, 1998; de Kloet et al., 2005). These stress effects may be partly due to stress-induced changes in cognition, including altered learning and memory processes (Diamond et al., 2007; Lupien et al., 2009; Schwabe et al., 2010; Roozendaal and McGaugh, 2011; Sandi and Haller, 2015). Stress enhances memory consolidation and impairs memory retrieval. These modulatory effects are critically mediated by noradrenaline, in interaction with glucocorticoids (McGaugh et al., 1996; Roozendaal et al., 2004; de Quervain et al., 2007a; Schwabe et al., 2009; Roozendaal and McGaugh, 2011). Beyond its effects on consolidation and retrieval, stress alters the contribution of multiple, anatomically and functionally distinct memory systems to learning and behavior (Schwabe et al., 2010; Schwabe and Wolf, 2013). Specifically, stress promotes a shift from hippocampus-dependent cognitive toward dorsal striatum-dependent habit memory (Kim et al., 2001; Schwabe and Wolf, 2012, 2013; Schwabe et al., 2007). Neuroimaging data indicate that the amygdala orchestrates this stress-induced shift in the balance between flexible and more rigid memory processes (Packard and Wingard, 2004; Schwabe et al., 2013; Vogel et al., 2016).
However, not all individuals show the bias toward dorsal striatal habit memory after stress (Kim et al., 2001; Schwabe et al., 2007; Schwabe and Wolf, 2012). Given the critical clinical implications associated with the stress-induced modulation of the balance between cognitive and habit memory (Schwabe et al., 2010; Goodman et al., 2012; de Quervain et al., 2017), identifying the source of this variability is crucial. Genetic differences explain part of the variance in human memory (de Quervain and Papassotiropoulos, 2006; de Quervain et al., 2012; Papassotiropoulos et al., 2006, 2013). With respect to the emotional modulation of memory, a deletion variant of the gene encoding the α2b-adrenoceptor (ADRA2B), which is present in ∼30% of Caucasians (Small et al., 2001), is of particular interest. Corroborating studies showing that pharmacological manipulations of α2-adrenoceptors affect emotional memory (O'Carroll et al., 1999; Southwick et al., 2002), healthy ADRA2B deletion carriers exhibited enhanced episodic (i.e., hippocampus-dependent) memory for emotional material (de Quervain et al., 2007b; Rasch et al., 2009). This emotional memory enhancement was also observed in Rwandan civil war survivors carrying the deletion variant (de Quervain et al., 2007b) and directly linked to increased PTSD risk (Liberzon et al., 2014). At the neural level, the ADRA2B deletion variant was associated with increased amygdala activation during encoding of emotional stimuli (Rasch et al., 2009) and following stress (Cousijn et al., 2010) as well as increased connectivity between the amygdala and the insular cortex (Rasch et al., 2009), a region closely linked to the medial temporal lobe network relevant for episodic memory (Miranda and Bermúdez-Rattoni, 2007). Based on this evidence, suggesting that the ADRA2B deletion variant is associated with stronger amygdala activation and enhanced hippocampus-dependent memory for emotional material, we hypothesized that the deletion variant would be linked to enhanced crosstalk between the amygdala and the hippocampus, thereby reducing the stress-induced shift from hippocampal to dorsal striatal memory.
To test the modulatory role of the ADRA2B deletion, we exposed healthy individuals, genotyped for the ADRA2B polymorphism, to a stressor (or control manipulation) before they completed a probabilistic classification learning (PCL) task that can be solved by the hippocampus and the dorsal striatum (Fig. 1) (Knowlton et al., 1996; Poldrack et al., 2001). The engagement of these memory systems can be assessed by the analysis of distinct learning strategies known to rely on the hippocampus and the dorsal striatum, respectively (Shohamy et al., 2004; Schwabe and Wolf, 2012). Additionally, in our first experiment, we used EEG measurements to assess the feedback-related negativity (FRN), which likely reflects striatal processing (Nieuwenhuis et al., 2005; Hauser et al., 2014). In a second experiment, we used the same experimental setup, but fMRI to elucidate the neural correlates of the modulatory effect of the ADRA2B polymorphism on the stress-induced shift toward habit memory.
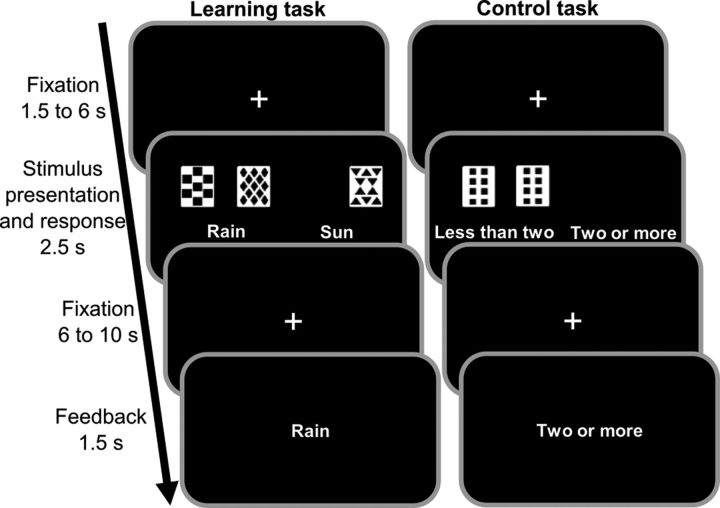
Figure 1.
Probabilistic classification learning task. Participants were presented with 1–3 of 4 possible cues per trial and asked to predict the weather outcome (“rain” or “sun”). Each cue has a certain probability for the 2 outcomes. Feedback about the correct outcome was presented after each trial. The task can be solved by using single-cue strategies based on the hippocampus and multi-cue strategies based on the dorsal striatum. In a visual-motor control task, participants were required to indicate whether ≤2 or >2 cards were presented. The shown task setup refers to Study II. In Study I, there was no visual-motor control task and the timing was slightly different.
Materials and Methods
Study I: role of an ADRA2B deletion variant in the stress-induced modulation of multiple memory systems
Participants and experimental design.
A total of 252 healthy volunteers without medication intake, including adrenergic agonists and antagonists, or any current or previous neurological or psychiatric disorders participated in this experiment (127 women; mean ± SD age, 25.1 ± 3.5 years). To control for factors known to influence the responsiveness of the hypothalamus-pituitary-adrenal axis to stress, smokers as well as women taking hormonal contraceptives were excluded and women were only tested outside their menstrual cycle phase (Kirschbaum et al., 1999; Rohleder and Kirschbaum, 2006). The study was approved by the ethical review board of the German Psychological Society (reference: LS072014). Participants gave written informed consent and received a moderate monetary compensation of 25€ for their participation.
A 2 × 2 between-subjects design with the factors treatment (stress vs control manipulation) and ADRA2B deletion variant (carriers vs noncarriers) was used to examine modulatory effects of the ADRA2B genotype on stress-induced changes in the preferential engagement of multiple memory systems. Participants were randomly assigned to the stress or control condition. To control for the diurnal rhythm of cortisol, all testing took place in the afternoon.
Genotyping.
A common variant (rs29000568) of the ADRA2B gene consists of an inframe deletion of three acidic residues. Specifically, glutamic acid residues 301–303 in the third intracellular loop of the receptor, which are part of a large glutamic acid stretch (glu12; amino acids 297–309), are absent in ∼30% of Caucasians (Small et al., 2001; de Quervain et al., 2007b). This deletion variant is associated with increased extracellular noradrenaline availability (Small et al., 2001). For genetic analysis, DNA was extracted from buccal cells. Automated purification of genomic DNA was conducted by means of the MagNA Pure LC system using a commercial extraction kit (MagNA Pure LC DNA isolation kit; Roche Diagnostics). Genotyping of the polymorphism was performed by real-time PCR using fluorescence melting curve detection analysis by means of the Light Cycler System (Roche Diagnostics) and a LightSNiP Assay (TIB MOLBIOL).
Stress manipulation.
Participants in the stress condition underwent the Trier Social Stress Test (TSST), the gold standard in experimental stress research that reliably induces autonomic nervous system and hypothalamus-pituitary-adrenal axis activation (Kirschbaum et al., 1993). After a short preparation time, each participant was asked to give a 5 min free speech about why he or she is the ideal candidate for a job tailored to his or her interests and subsequently had to solve a difficult mental-arithmetic task for another 5 min (counting backwards from 2043 in steps of 17). Throughout the TSST, participants were videotaped and evaluated by a reserved and nonreinforcing panel. In the control condition, participants were asked to talk about a self-chosen topic and had to count forwards from zero in steps of 15, while being alone in the room and without video recordings.
To assess the effectiveness of the stress induction, subjective and physiological measures were taken at several time points across the experiment. Changes in subjective mood were evaluated using a German mood questionnaire (subscales: depressed vs elevated, restless vs calm, sleepy vs awake; high scores indicate elevated mood, calmness, and wakefulness) (Steyer et al., 1994). In addition, participants rated how difficult, unpleasant, and stressful they had experienced the manipulation on a scale from 0 (not at all) to 100 (very much). Blood pressure was measured using a Dinamap system (Critikon) before (−25 min), during (10 min), and after (20, 60, 80 min) the stress manipulation. Furthermore, saliva samples were collected before (−25 min) and after (20, 30, 40, 80 min) the experimental treatment using Salivette collection devices (Sarstedt). Samples were stored at −18°C; and at the end of the study, the free fraction of cortisol was analyzed from saliva using a commercially available chemiluminescence immunoassay (IBL).
Probabilistic classification learning (PCL) task.
Fifteen minutes after the stress or control manipulation, when peak cortisol levels were expected, participants completed a modified version of the weather prediction task (Fig. 1) (Knowlton et al., 1994; Gluck et al., 2002) while EEG was recorded. In this PCL task, participants had to learn how to classify stimuli into two given categories (“rain” vs “sun”) based on trial-by-trial feedback. Between 1 and 3 (of 4) cards appeared on each trial, yielding 14 different cue patterns. These cue patterns were associated with two possible outcomes (sun and rain) in a probabilistic manner, such that a particular cue was associated with the outcome “sun” with a probability of 75.6%, 57.5%, 42.5%, or 24.4% across 100 trials; these probabilities were the same as in previous studies using this task (Gluck et al., 2002; Lagnado et al., 2006; Schwabe and Wolf, 2012; Schwabe et al., 2013). A response was counted as correct if it matched the outcome with the highest probability for that cue pattern. Participants completed 100 PCL trials (duration ∼25 min). On each trial, they saw 1 of the 14 cue patterns and had 5 s to respond by pressing one of the buttons that corresponded with either “sun” or “rain.” The chosen outcome was highlighted with a red circle for 500 ms. Following a black screen that was shown for another 500 ms, a feedback stimulus in the form of a happy or sad face was presented for 1 s. The intertrial interval varied between 1 and 2.5 s.
Assessment of learning strategies.
The PCL task can be solved by using different learning strategies that rely on distinct brain systems. In particular, patient and neuroimaging studies showed that participants may acquire the task using single-cue strategies supported by a hippocampus-dependent system or by using multi-cue strategies that are based on the dorsal striatum (Knowlton et al., 1996; Shohamy et al., 2004; Foerde et al., 2006; Schwabe et al., 2012). To assess the learning strategy that participants used during the PCL task, participants' actual responses were compared with ideal response patterns for each strategy (Gluck et al., 2002; Lagnado et al., 2006). A least mean squares measure resulted in a fit-value ranging from 0 to 1 (0 indicating a perfect fit). The strategy with the lowest score was defined as the best fit for that participant. If the best fit score was >0.15, participants' strategies were classified as “nonidentifiable” (Gluck et al., 2002); and because the multi-match strategy contains a “random” element (Lagnado et al., 2006), when single-cue and multi-match scores were very close, the single-cue strategy was chosen. In Study I, there were 20 participants for which no strategy could be identified; experimental groups did not differ in the number of participants for which no strategy could be identified (χ(1)2 = 0.220, p = 0.639). For the sake of simplicity and in line with previous studies (Schwabe and Wolf, 2012; Schwabe et al., 2013), we classified the strategies that participants may use to solve the PCL task into hippocampus-dependent single-cue (comprising one cue and singleton) and dorsal striatum-dependent multi-cue strategies.
Behavioral and physiological data analyses.
Subjective and physiological measurements were analyzed using mixed-design ANOVAs with time as within-subject factor and treatment (TSST vs control) as well as ADRA2B deletion variant (carriers vs noncarriers) as between-subjects factors. A mixed-design ANOVA with blocks of 10 trials as within-subject factor was used to assess learning performance on the PCL task. Group differences in learning strategy were analyzed by means of χ2 tests. Statistical analyses were performed using SPSS Statistics 22 (RRID:SCR_002865, IBM). All reported p values are two-tailed. In case of violation of the sphericity assumption, Greenhouse-Geisser corrections were applied. Significant main and interaction effects were followed by the appropriate post hoc tests.
EEG recording and analyses.
During the PCL task, EEG was recorded from 64 active electrodes arranged according to the international 10–20 system. In addition, horizontal electro-oculograms were measured, and the most frontal electrodes served as recording sites for vertical eye movements. A Biosemi Active-Two amplifier system was used with a sampling rate of 2048 Hz (Biosemi). Common mode sense and driven right leg electrodes served as recording reference and ground, respectively.
EEG data were analyzed offline using the Brain Vision Analyzer software (Brain Products) and a costume-written MATLAB script (The MathWorks). After the EEG signal was downsampled to 512 Hz, the data were high-pass filtered at 0.01 Hz. To remove artifacts from electrical lines, a 50 Hz notch filter was applied. EEG data were then visually inspected to discard any extreme artifacts. Additionally, eye-blink and eye-movement artifacts were removed using an independent component-based approach. Bad channels were replaced by means of topographic interpolation, and the data were rereferenced to the average of all electrodes. To analyze ERPs reflecting feedback processing, data were segmented into epochs from −200 to 800 ms with respect to feedback stimulus onset and subsequently baseline corrected relative to the 200 ms preceding the feedback stimulus. Before averaging, trials were rejected if there was a voltage step >50 μV /ms, or a difference of >100 μV as well as a signal <0.1 μV was detected in any of the intervals. Because of technical difficulties or excessive noise, 24 participants were excluded from further EEG data analyses. For the remaining 228 participants (stress group: 68 carriers, 46 noncarriers; control group: 64 carriers, 50 noncarriers), on average 37.5 trials (SD 13.8 trials) in the negative feedback condition were available. The FRN, which likely reflects striatal feedback processing (Nieuwenhuis et al., 2005; Hauser et al., 2014), was calculated as the most negative peak amplitude in the time window between 200 and 350 ms following feedback presentation relative to the preceding positive peak amplitude between 150 ms and the latency of that negative peak (Frank et al., 2005; Eppinger et al., 2008; Rustemeier et al., 2013). A mixed-design ANOVA with electrode site and feedback (positive vs negative) as within-subject factors and treatment as well as genotype as between-subjects factors was used to investigate stress- or genotype-related differences in feedback processing. Frontal electrodes (FC1, Fz, FCz, FC2), where the FRN was most pronounced, were included in the analyses.
Study II: neural underpinnings of the ADRA2B modulation of memory system engagement under stress
Participants and experimental design.
A total of 128 volunteers of the Bonn Gene Brain Behavior Project participated in this experiment (62 women; mean ± SD age, 23.0 ± 3.6 years). Participants were young and healthy nonsmokers without medication intake, including adrenergic agonists and antagonists, or lifetime history of neurological or psychiatric disorders. Furthermore, any contraindications for fMRI measurements served as exclusion criteria. The study was approved by the ethical review board of the German Psychological Society (reference: LS072014) as well as by a local committee at the University of Bonn. Participants gave written informed consent and received a moderate monetary compensation of 35€.
In line with the first experiment, we used a 2 × 2 between-subjects design with the factors treatment (TSST vs control manipulation) and ADRA2B genotype (deletion vs nondeletion carriers). In this experiment, however, participants were prescreened and groups were stratified by the ADRA2B genotype. We aimed for 30 participants in each of the four experimental groups (N = 120). However, because of technical difficulties and excessive head motion in the MRI scanner, 8 participants had to be excluded from the fMRI analyses (stress: 2 deletion, 3 nondeletion; control: 1 deletion, 2 nondeletion) and 8 additional participants were recruited, thus leading to a sample of 120 participants (N = 30/group) for the fMRI analyses. For the behavioral analyses, data from all 128 tested participants were used (stress: 32 deletion, 33 nondeletion; control: 31 deletion, 32 nondeletion).
Experimental procedure.
The experimental procedure, including the stress manipulation, the parameters measured, and the PCL task, was the same as in the first study, with two exceptions. First, fMRI instead of EEG measurements were taken while participants performed the PCL task. Second, the task was slightly modified to accommodate fMRI requirements. More specifically, in addition to 100 PCL trials, participants completed 100 visuo-motor control trials in which they were asked to indicate whether <2 or ≥2 cards appeared on the screen (trial type was randomly alternated; task duration ∼45 min). Moreover, the timing of the events was adjusted to the slow BOLD response. In Study II, there were 31 participants for whom no strategy could be identified; experimental groups did not differ in that number (p > 0.05). The behavioral and statistical analyses were in line with those of the first experiment.
MRI acquisition and analyses.
MRI measurements were acquired using a 3T Trio Scanner (Siemens) equipped with a 32-channel head coil. BOLD T2-weighted echoplanar functional images were acquired parallel to the anterior commissure-posterior commissure plane (37 transversal slices; TR = 2000 ms; TE = 30 ms; ascending acquisition; effective voxel size = 3 × 3 × 3 mm). Additionally, a high-resolution T1-weighted anatomical image was acquired (208 sagittal slices, TR = 1660 ms, TE = 2.54 ms, voxel size = 0.8 × 0.8 × 0.8 mm).
Preprocessing and analyses of the fMRI data using GLMs were performed with the SPM12 MATLAB toolbox (RRID:SCR_007037, Wellcome Trust Centre for Neuroimaging, London). Functional data were slice-time and head-motion corrected as well as coregistered to the structural image using rigid-body transformations. The T1-weighted image was segmented into gray and white matter, cerebrospinal fluid, bone, soft tissue, and air. Forward deformation fields were then used to spatially normalize the functional and structural scans to the MNI standard brain. Finally, normalized functional images were smoothed using an 8 mm FWHM Gaussian kernel.
Correct and incorrect PCL trials as well as visuo-motor control trials were modeled using canonical hemodynamic response functions. Additionally, fixation, button presses and the six movement parameters were included into the model. Data were filtered in the temporal domain using a nonlinear high-pass filter with a 128 s cutoff. Contrast images were generated for PCL minus control trials and for correct minus incorrect PCL trials. These difference contrasts were then entered into a second-level (group) analyses, using a full-factorial model with the factors treatment (control vs stress) and genotype (deletion vs nondeletion carriers). Psycho-Physiological-Interaction (PPI) analyses were performed to assess whether the coupling of the amygdala with the hippocampus and the dorsal striatum (regions of interest [ROIs]) was altered by stress and/or ADRA2B genotype. For this purpose, the first eigenvariate of the time course of each ROI in the contrast PCL correct minus PCL incorrect was extracted from the appropriate brain atlases and used as seed. The PPI was then computed as the element-by-element product of the BOLD signal time course of this seed and a vector coding for successful classification learning. Next, each time course was added separately as a covariate of interest in addition to the first-level regressors. The individual PPI contrasts were then entered in a second-level random-effects analysis. Results of these analyses give insight into brain regions that show a similar and task-dependent pattern of activation. These regions are therefore supposed to be functionally connected during correct classification learning.
Explorative whole-brain analyses as well as ROI analyses were used. A priori ROIs were memory system structures (hippocampus, caudate nucleus, and putamen) that are consistently activated in the PCL task (Poldrack et al., 2001; Foerde et al., 2006; Schwabe and Wolf, 2012; Schwabe et al., 2013) as well as the amygdala because this area has been shown to be affected by the ADRA2B deletion variant (Cousijn et al., 2010) and is known to modulate multiple memory systems (Elliott and Packard, 2008; Schwabe et al., 2013; Vogel et al., 2015). Anatomical masks of the caudate nucleus, the putamen, and the amygdala were taken from the Harvard-Oxford subcortical atlas, whereas masks of the hippocampal subregions were taken from the Anatomy Toolbox for SPM (Institute of Neuroscience and Medicine, Jülich, Germany). For the explorative whole-brain analysis, the significance threshold was set to p < 0.05 at cluster level and corrected for multiple testing (family-wise error [FWE] correction). ROI analyses were performed using small-volume correction with an initial threshold of p < 0.05 uncorrected, followed by FWE correction (p < 0.05). Thresholds at 50% were used to include only voxels with a probability of at least 50% to belong to each subregion. To assess stress- or genotype-dependent alterations in the connectivity between the ROIs, PPI analyses were performed. After voxels of interest of the time courses of the ROIs were extracted from the appropriate brain atlases, they were added separately in the model in addition to the first-level regressors. Correlations between these time series and the activity of the rest of the brain are indicative of similar activation patterns and thus brain regions that are supposedly functionally connected. Again, for ROI analyses, small-volume correction with an initial threshold of p < 0.05 uncorrected, followed by FWE correction (p < 0.05) was performed.
Results
Study I: role of an ADRA2B deletion variant in the stress-induced modulation of multiple memory systems
Genotyping
Our first study included 114 heterozygous and 29 homozygous carriers of the ADRA2B deletion variant. In line with previous studies (de Quervain et al., 2007b; Rasch et al., 2009) and due to the relatively small number of homozygous carriers of the deletion variant, heterozygous and homozygous carriers were statistically treated as one group (N = 143). A total of 109 participants did not carry the deletion variant. Genotype frequencies were in Hardy-Weinberg equilibrium (χ(1)2 = 0.780, p = 0.377) and in line with the genotype and allele frequencies typically observed in Caucasians (Small et al., 2001). Genotype was not significantly associated with sex or age (both p > 0.279) and the distribution of carriers and noncarriers of the ADRA2B deletion variant was comparable in the stress (74 carriers, 52 noncarriers) and control group (69 carriers, 57 noncarriers; χ(1)2 = 0.40, p = 0.525).
Subjective, autonomic, and endocrine stress response
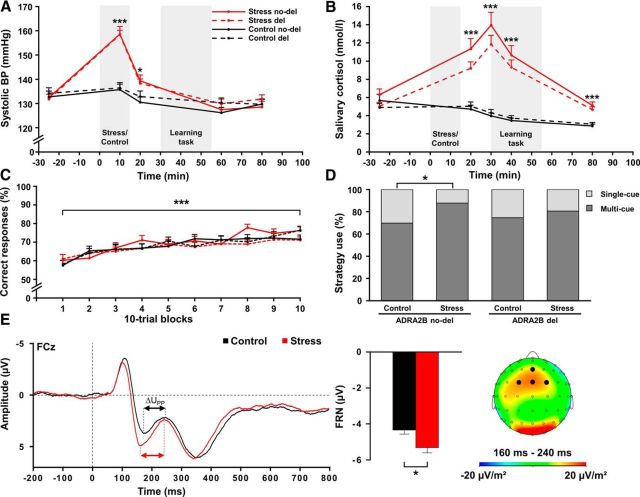
Changes in subjective feeling, blood pressure, and salivary cortisol verified the successful stress induction by the TSST (Table 1). Compared with the control procedure, exposure to the TSST was rated as significantly more difficult, unpleasant, and stressful (all F(1,248) ≥ 223.55, all p < 0.001). Moreover, the TSST, but not the control manipulation, resulted in increases of depressed mood and restlessness (time × treatment: both F(2,2494) ≥ 53.76, both p < 0.001). Additionally, exposure to the TSST led to significant autonomic activation, reflected by increases in systolic (Fig. 2A) and diastolic blood pressure (time × treatment: both F ≥ 62.91, both p < 0.001). Finally, we obtained a significant increase in cortisol concentrations following the stress but not the control manipulation (time × treatment: F(2,493.2) = 71.15, p < 0.001). As shown in Figure 2B, peak cortisol levels were reached ∼15 min following the stressor, when behavioral testing started. Importantly, carriers and noncarriers of the ADRA2B deletion variant did not differ in measures of blood pressure, cortisol, or mood (all p > 0.299).
Table 1.
Subjective, autonomic, and endocrine stress response in Study Ia
| Control |
Stress |
|||
|---|---|---|---|---|
| Deletion | No deletion | Deletion | No deletion | |
| Subjective assessment | ||||
| Stressful | 26.67 ± 2.63 | 29.30 ± 2.90 | 71.76 ± 2.64*** | 68.08 ± 2.03*** |
| Difficult | 25.51 ± 2.58 | 32.63 ± 2.84 | 77.70 ± 2.49*** | 71.73 ± 2.97*** |
| Unpleasant | 27.97 ± 2.78 | 30.53 ± 3.06 | 76.89 ± 2.68*** | 69.42 ± 3.20*** |
| Subjective mood | ||||
| Good versus bad mood | ||||
| Before treatment | 32.58 ± 0.52 | 32.66 ± 0.58 | 33.70 ± 0.51 | 33.56 ± 0.60 |
| 1 min after treatment | 32.30 ± 0.72 | 32.04 ± 0.80 | 27.28 ± 0.69*** | 25.75 ± 0.83*** |
| 65 min after treatment | 31.23 ± 0.68 | 31.00 ± 0.76 | 31.07 ± 0.66 | 29.48 ± 0.79 |
| Calm versus restless | ||||
| Before treatment | 30.58 ± 0.66 | 30.80 ± 0.73 | 31.51 ± 0.63 | 31.15 ± 0.76 |
| 1 min after treatment | 30.06 ± 0.72 | 30.09 ± 0.80 | 23.82 ± 0.70*** | 22.94 ± 0.83*** |
| 65 min after treatment | 31.19 ± 0.71 | 31.02 ± 0.78 | 30.82 ± 0.68 | 29.56 ± 0.81 |
| Tired versus awake | ||||
| Before treatment | 29.06 ± 0.74 | 29.41 ± 0.83 | 30.68 ± 0.72 | 30.14 ± 0.86 |
| 1 min after treatment | 28.33 ± 0.75 | 29.23 ± 0.84 | 30.18 ± 0.73 | 28.65 ± 0.87 |
| 65 min after treatment | 22.70 ± 0.83 | 23.34 ± 0.93 | 23.28 ± 0.81 | 23.00 ± 0.96 |
| Systolic blood pressure (bpm) | ||||
| Before treatment | 134.22 ± 2.20 | 132.75 ± 2.42 | 131.80 ± 2.16 | 132.42 ± 2.54 |
| During treatment | 136.45 ± 2.21 | 135.76 ± 2.43 | 158.22 ± 2.16*** | 158.64 ± 2.54*** |
| 5 min after treatment | 132.91 ± 2.05 | 130.50 ± 2.25 | 138.17 ± 2.00 | 138.35 ± 2.36* |
| 45 min after treatment | 130.34 ± 2.03 | 126.24 ± 2.23 | 130.13 ± 1.98 | 127.49 ± 2.33 |
| 65 min after treatment | 129.70 ± 1.92 | 129.79 ± 2.11 | 132.01 ± 1.88 | 128.56 ± 2.21 |
| Diastolic blood pressure (bpm) | ||||
| Before treatment | 76.90 ± 1.04 | 75.83 ± 1.14 | 76.28 ± 1.02 | 75.85 ± 1.19 |
| During treatment | 81.59 ± 1.32 | 80.25 ± 1.45 | 95.22 ± 1.29*** | 94.10 ± 1.52*** |
| 5 min after treatment | 78.37 ± 1.01 | 76.97 ± 1.11 | 81.98 ± 0.99* | 80.89 ± 1.16 |
| 45 min after treatment | 76.27 ± 0.94 | 74.25 ± 1.04 | 77.52 ± 0.92 | 76.50 ± 1.09 |
| 65 min after treatment | 77.54 ± 0.94 | 75.93 ± 1.04 | 77.88 ± 0.92 | 75.77 ± 1.08 |
| Salivary cortisol (nmol/l) | ||||
| Before treatment | 4.90 ± 0.51 | 5.66 ± 0.56 | 5.04 ± 0.49 | 6.30 ± 0.59 |
| 5 min after treatment | 5.04 ± 0.68 | 4.72 ± 0.75 | 9.21 ± 0.65*** | 11.34 ± 0.78*** |
| 15 min after treatment | 4.31 ± 0.81 | 3.98 ± 0.89 | 11.86 ± 0.78*** | 13.95 ± 0.93*** |
| 25 min after treatment | 3.73 ± 0.64 | 3.46 ± 0.71 | 9.32 ± 0.62*** | 10.65 ± 0.74*** |
| 65 min after treatment | 3.06 ± 0.28 | 2.85 ± 0.31 | 4.66 ± 0.27*** | 5.12 ± 0.32*** |
aData are mean ± SEM.
*p < 0.05;
***p < 0.001.
Figure 2.
Physiological, behavioral, and EEG data of Study I. Compared with a nonstressful control manipulation, exposure to the TSST led to significant increases in (A) systolic blood pressure (BP) and (B) salivary cortisol concentrations, regardless of the allelic variant. C, Classification learning performance increased across trials, without any effects of stress or α2b-adrenoceptor gene (ADRA2B) variant. D, Stress increased the bias toward more multi-cue strategies in noncarriers (ADRA2B no-del) but not in carriers of the deletion variant (ADRA2B del). E, EEG data revealed a significant increase in the FRN at FCz electrode, without differences between carriers and noncarriers of the ADRA2B deletion variant. The FRN was calculated as the most negative peak amplitude in the time window between 200 and 350 ms following feedback presentation relative to the preceding positive peak amplitude between 150 ms and the latency of that negative peak. ΔUPP, Voltage difference between the positive and the negative peak amplitude after negative feedback. *p < 0.05. ***p < 0.001. Error bars indicate SEM.
ADRA2B deletion variant is associated with a reduced stress-induced shift toward multi-cue strategies
In the PCL task, participants' performance gradually improved from 59% to 74% correct responses (F(7.8, 1921.1) = 21.52, p < 0.001; Fig. 2C), thus indicating successful learning. In line with previous studies showing that different memory systems may contribute equally well to learning performance (Schwabe and Wolf, 2009, 2012), stress had no effect on performance (F(3.1, 762.2) = 0.80, p = 0.597). Moreover, there was no difference between carriers and noncarriers of the ADRA2B deletion variant in learning performance (F(3.1, 762.2) = 1.22, p = 0.287).
The engagement of multiple memory systems is reflected in the use of single-cue strategies, which are based on the hippocampus; and multi-cue strategies, which are based on the dorsal striatum. Corroborating earlier results from our laboratory (Schwabe and Wolf, 2012; Schwabe et al., 2013), stressed participants showed a shift toward more multi-cue and fewer single-cue strategies compared with controls (χ(1)2 = 4.25, p = 0.039). Most importantly, however, this stress-induced shift was modulated by ADRA2B genotype (Fig. 2D). In noncarriers of the deletion variant, stress increased the use of multi-cue strategies from 70% to 88% (χ(1)2 = 4.85, p = 0.028). This, however, was not the case in ADRA2B deletion carriers who did not show any changes in the used strategy after stress (χ(1)2 = 0.67, p = 0.412).
Stress increases the amplitude of the feedback-related negativity during probabilistic classification learning
Our EEG data showed a larger FRN, thought to reflect dorsal striatal processing (Nieuwenhuis et al., 2005; Hajcak et al., 2007; Hauser et al., 2014), with a typical frontocentral distribution in stressed participants compared with controls following negative feedback (F(1,224) = 6.62, p = 0.011; Fig. 2E). As the FRN is particularly important for learning from negative feedback (van der Helden et al., 2010), this difference was not observed in response to positive feedback (F(1,223) = 0.33, p = 0.564; feedback × condition: F(1,9.8) = 4.99, p = 0.026). Carriers and noncarriers of the ADRA2B deletion variant, however, neither differed in FRN amplitude, nor was the stress effect on the FRN modulated by ADRA2B genotype (all F < 0.59, all p > 0.321). We did not obtain significant correlations between task performance and FRN amplitude (all p > 0.05).
Study II: neural underpinnings of the ADRA2B modulation of memory system engagement under stress
Subjective, autonomic, and endocrine stress responses
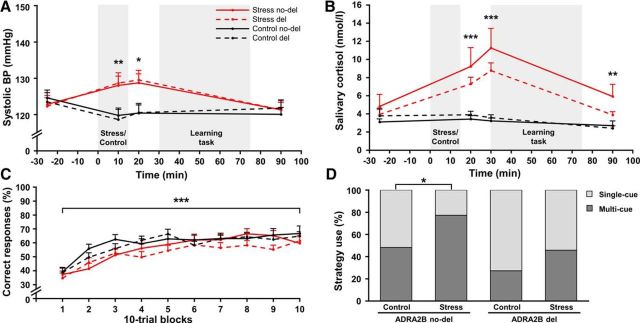
Exposure to the TSST was rated as significantly more difficult, unpleasant, and stressful compared with the control manipulation (all F(1,124) > 58.68, all p < 0.001) and participants' mood decreased only following the TSST (all F(2,246) > 21.48, all p < 0.001; Table 2). Moreover, systolic and diastolic blood pressure (Fig. 3A) as well as salivary cortisol increased following the TSST, but not the control manipulation (all F > 20.28, all p < 0.001). As shown in Figure 3B, cortisol concentrations peaked ∼15 min following the stressor, when the scanning session began. ADRA2B deletion and nondeletion carriers did not differ in their physiological or subjective responses to stress (all p > 0.136).
Table 2.
Subjective, autonomic, and endocrine stress response in Study IIa
| Control |
Stress |
|||
|---|---|---|---|---|
| Deletion | No deletion | Deletion | No deletion | |
| Subjective assessment | ||||
| Stressful | 30.65 ± 3.67 | 28.44 ± 3.61 | 68.13 ± 3.61*** | 63.64 ± 3.55*** |
| Difficult | 26.45 ± 3.85 | 26.25 ± 3.79 | 71.88 ± 3.79*** | 69.70 ± 3.73*** |
| Unpleasant | 34.84 ± 4.41 | 35.94 ± 4.34 | 70.94 ± 4.34*** | 66.36 ± 4.28*** |
| Subjective mood | ||||
| Good versus bad mood | ||||
| Before treatment | 35.10 ± 0.73 | 33.88 ± 0.72 | 34.53 ± 0.72 | 34.59 ± 0.72 |
| 1 min after treatment | 34.87 ± 0.98 | 33.00 ± 0.96 | 28.38 ± 0.97*** | 28.34 ± 0.97*** |
| 75 min after treatment | 33.36 ± 0.93 | 32.06 ± 0.91 | 29.87 ± 0.91 | 32.56 ± 0.91 |
| Calm versus restless | ||||
| Before treatment | 33.03 ± 0.92 | 31.31 ± 0.91 | 31.59 ± 0.91 | 31.84 ± 0.91 |
| 1 min after treatment | 30.58 ± 1.11 | 30.53 ± 1.09 | 24.47 ± 1.09*** | 24.25 ± 1.09*** |
| 75 min after treatment | 32.36 ± 0.91 | 31.34 ± 0.89 | 31.56 ± 0.89 | 32.28 ± 0.89 |
| Tired versus awake | ||||
| Before treatment | 30.87 ± 0.96 | 29.81 ± 0.95 | 30.31 ± 0.95 | 30.81 ± 0.95 |
| 1 min after treatment | 30.45 ± 1.03 | 28.94 ± 1.02 | 29.00 ± 1.02 | 28.44 ± 1.02 |
| 75 min after treatment | 23.61 ± 1.06 | 23.31 ± 1.04 | 22.22 ± 1.04 | 22.34 ± 1.04 |
| Systolic blood pressure (bpm) | ||||
| Before treatment | 123.52 ± 2.40 | 125.19 ± 2.40 | 122.33 ± 2.36 | 122.80 ± 2.32 |
| During treatment | 118.63 ± 2.64 | 119.97 ± 2.64 | 128.64 ± 2.60** | 128.03 ± 2.60** |
| 5 min after treatment | 120.55 ± 2.53 | 120.73 ± 2.53 | 129.55 ± 2.49** | 128.76 ± 2.45** |
| 75 min after treatment | 121.74 ± 2.24 | 120.10 ± 2.24 | 121.31 ± 2.21 | 121.32 ± 2.17 |
| Diastolic blood pressure (bpm) | ||||
| Before treatment | 83.15 ± 1.57 | 82.92 ± 1.57 | 80.83 ± 1.55 | 83.26 ± 1.52 |
| During treatment | 83.37 ± 1.82 | 82.66 ± 1.82 | 90.36 ± 1.79*** | 91.32 ± 1.76*** |
| 5 min after treatment | 84.68 ± 1.63 | 83.13 ± 1.63 | 87.69 ± 1.61** | 89.50 ± 1.58** |
| 75 min after treatment | 83.97 ± 1.59 | 84.10 ± 1.59 | 83.84 ± 1.57 | 83.77 ± 1.54 |
| Salivary cortisol (nm) | ||||
| Before treatment | 3.78 ± 0.88 | 3.10 ± 0.86 | 3.93 ± 0.86 | 4.82 ± 0.85 |
| 5 min after treatment | 3.90 ± 1.19 | 3.43 ± 1.17 | 7.30 ± 1.17*** | 9.22 ± 1.15*** |
| 15 min after treatment | 3.57 ± 1.24 | 3.22 ± 1.22 | 8.78 ± 1.22*** | 11.26 ± 1.20*** |
| 75 min after treatment | 2.41 ± 0.79 | 2.69 ± 0.77 | 3.88 ± 0.77** | 5.92 ± 0.76** |
aData are mean ± SEM.
**p < 0.01;
***p < 0.001.
Figure 3.
Physiological and behavioral data of Study II. Compared with a nonstressful control manipulation, exposure to the TSST led to significant increases in (A) systolic blood pressure (BP) and (B) salivary cortisol concentrations, regardless of the α2b-adrenoceptor gene (ADRA2B) variant. C, Classification learning performance increased across trials, without any effects of stress or ADRA2B variant. D, Stress led to a bias toward more multi-cue strategies only in noncarriers (ADRA2B no-del) but not in carriers of the deletion variant (ADRA2B del). *p < 0.05. **p < 0.01. ***p < 0.001. Error bars indicate SEM.
ADRA2B deletion variant is associated with a reduced stress-induced shift toward multi-cue strategies
Participants gradually learned to correctly classify the card stimuli and correct responses increased from 37% to 63% across trials (F(6.7, 826.5) = 28.64, p < 0.001; Fig. 3C). Stress and the ADRA2B deletion variant had no effect on task performance (both F(6.7, 826.5) < 1.01, both p > 0.421). Analysis of the used learning strategies, however, revealed that carriers of the ADRA2B deletion variant used overall more single-cue strategies compared with noncarriers of the deletion variant (χ(1)2 = 5.49, p = 0.019). Also, replicating the findings of our first study, stressed participants used single-cue strategies significantly less often and multi-cue strategies significantly more often compared with participants in the control group (χ(1)2 = 4.54, p = 0.033). Most importantly, as shown in Figure 3D, this stress effect was again mainly due to noncarriers of the ADRA2B deletion variant who showed a significant stress-induced shift toward multi-cue strategies (χ(1)2 = 4.41, p = 0.036), whereas there was no such effect in carriers of the deletion variant (χ(1)2 = 1.7, p = 0.193).
Neural mechanisms of stress-induced modulation of learning
Corroborating previous studies (Poldrack et al., 2001; Foerde et al., 2006; Schwabe and Wolf, 2012; Schwabe et al., 2013), our fMRI data showed that PCL (vs visuo-motor control trials) was associated with bilateral activation of the caudate nucleus, the putamen, and the hippocampus (all pFWE ≤ 0.031). In addition, we observed activation in other regions known to be involved in learning processes, including the cingulate and paracingulate cortex, the orbitofrontal cortex, the insular cortex, and the precuneus (all p < 0.001; Table 3). There were no significant correlations between activity in those regions and performance (all p > 0.05). In line with the idea that stress biases multiple memory systems toward the dorsal striatum, we obtained significantly increased activity of the caudate nucleus in stressed participants compared with controls (F(1,116) = 12.57, pFWE = 0.028, k = 40; Fig. 4A). This stress-induced increase in dorsal striatal activity, however, was not modulated by the ADRA2B deletion variant. In medial temporal lobe regions, we did not find any differences (all pFWE > 0.05).
Table 3.
Peak voxels and t values of significantly activated clusters during the PCL taska
| PCL > control | Cluster size | MNI coordinates (mm) |
tmax | pcorr | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L supplementary motor area | 866 | 0 | 23 | 47 | 19.51 | <0.001 |
| L insula left; L caudate; R caudate | 3.083 | −30 | 20 | −4 | 18.32 | <0.001 |
| R insula; R inferior frontal gyrus triangular; R precentral gyrus | 900 | 33 | 20 | −4 | 17.11 | <0.001 |
| L inferior parietal sulcus; R angular gyrus; L precuneus | 2.337 | −33 | −58 | 44 | 15.02 | <0.001 |
| R middle occipital gyrus; R cuneus; R fusiform gyrus | 879 | 33 | −88 | −1 | 12.61 | <0.001 |
| L middle occipital gyrus left; inferior temporal gyrus | 512 | −15 | −103 | 2 | 9.03 | <0.001 |
| Middle cingulate cortex | 101 | −3 | −28 | 29 | 8.54 | <0.001 |
| L middle frontal gyrus | 58 | −30 | 5 | 56 | 6.68 | <0.001 |
| R anterior orbitofrontal cortex | 20 | 27 | 38 | −22 | 6.29 | <0.001 |
| Cerebellar crus | 11 | −36 | −61 | −28 | 5.83 | 0.001 |
| Calcarine cortex | 9 | 3 | −88 | −7 | 5.69 | 0.001 |
| R anterior orbitofrontal cortex | 5 | 48 | 47 | −16 | 5.66 | 0.001 |
| R middle frontal gyrus; superior frontal gyrus | 42 | 33 | 53 | 2 | 5.22 | 0.008 |
| L anterior cingulate cortex | 5 | −6 | −1 | 29 | 5.18 | 0.010 |
| L hippocampus CA | 7 | −18 | −37 | 5 | 3.33 | 0.031b |
| R hippocampus CA | 23 | 18 | −34 | 2 | 6.89 | <0.001b |
| L hippocampus DG | 5 | −21 | −37 | 2 | 3.97 | 0.001b |
| L hippocampus DG | 5 | 21 | −34 | 2 | 6.89 | <0.001b |
| L hippocampus | 5 | −21 | −28 | −10 | 3.91 | 0.005b |
| L caudate nucleus | 106 | −9 | 11 | −1 | 15.55 | <0.001b |
| R caudate nucleus | 107 | 9 | 11 | 2 | 14.87 | <0.001b |
| L putamen | 38 | −15 | 8 | −4 | 10.96 | <0.001b |
| R putamen | 36 | 18 | 11 | −1 | 7.32 | <0.001b |
aData indicate local maxima. All labels are taken from the Automatic Anatomical Labeling atlas. The significance threshold was set to p < 0.05 (FWE-corrected).
bSmall-volume corrected (ROI); all other activations are significant at the whole-brain level.
Figure 4.
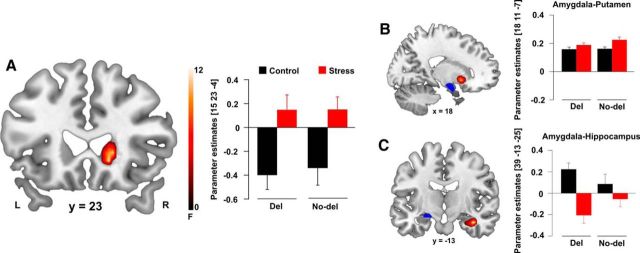
Stress effects on brain activity and connectivity. A, The right caudate nucleus was significantly more activated in the stress compared with the control group (pFWE < 0.05). B, C, Functional connectivity between the left amygdala and the hippocampus was decreased after stress (pFWE < 0.05), whereas functional connectivity between amygdala and putamen tended to increase after stress (pFWE = 0.063). Activations are superimposed on coronal and sagittal axial sections of a T1-weighted template image (red). Blue represents anatomical masks. L, Left side of the brain; R, right side of the brain. Error bars indicate SEM.
ADRA2B deletion variant modulates stress-induced changes in amygdala connectivity with the dorsal striatum
In a next step, we performed functional connectivity analyses to assess how stress altered the crosstalk of multiple memory systems and whether this crosstalk was modulated by the ADRA2B polymorphism. Because the amygdala is known to play a key role in the modulation of memory (Packard and Goodman, 2012; Schwabe, 2013) and previous studies showed altered amygdala coupling with the hippocampus and the dorsal striatum after stress (Schwabe et al., 2013; Vogel et al., 2015), we focused on the connectivity of the amygdala. Overall, stress had opposite effects on amygdala connectivity with the dorsal striatum (F(1,116) = 11.09, pFWE = 0.063, k = 31; Fig. 4B) and the hippocampus (F(1,116) = 17.41, pFWE = 0.005, k = 33; Fig. 4C). More specifically, stress increased amygdala connectivity with the putamen (t(1,118) = 4.15, pFWE = 0.003, k: 38) but decreased amygdala coupling with the hippocampus, in particular with the cornu ammonis subregion (t(1,118) = 4.12, pFWE = 0.003, k = 44).
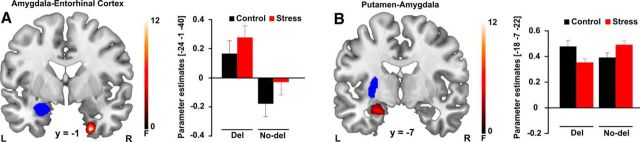
Critically, coupling of the amygdala with the hippocampus and dorsal striatum was significantly affected by ADRA2B genotype (F(1,116) = 13.77, pFWE = 0.017, k = 32). Regardless of stress, carriers compared with noncarriers of the deletion variant showed increased task-related connectivity between the amygdala and the entorhinal cortex (EC; t(1,118) = 3.72, pFWE = 0.008, k = 40; Fig. 5A), the main input structure to the hippocampus that is highly important for hippocampus-dependent declarative memory processes (Squire and Zola, 1996; Eichenbaum, 2000; Hargreaves et al., 2005). Even more interestingly, we obtained a significant stress × ADRA2B interaction for the connectivity between the amygdala and the putamen (F(1,116) = 9.73, pFWE < 0.05, k = 31). As is shown in Figure 5B, noncarriers compared with carriers of the deletion variant showed significantly increased amygdala-putamen connectivity after stress (t(1,58) = 3.72, pFWE = 0.006, k = 53), whereas genotype groups did not differ under control conditions (pFWE > 0.05).
Figure 5.
Alpha2b-adrenoceptor gene (ADRA2B) effects on brain connectivity. A, Regardless of stress, carriers of the ADRA2B deletion variant showed stronger functional connectivity between the left amygdala and the right entorhinal cortex (pFWE < 0.05). B, ADRA2B genotype additionally modulates stress-induced changes in amygdala-putamen connectivity. Under stress, noncarriers of the deletion variant showed significantly increased amygdala-putamen connectivity than carriers of the ADRA2B deletion variant (pFWE = 0.006), whereas genotype groups did not differ under control conditions (pFWE = 0.352). Activations are superimposed on coronal axial sections of a T1-weighted template image (red). Blue represents anatomical masks. L, Left side of the brain; R, right side of the brain. Error bars indicate SEM.
Discussion
Stress biases memory toward rigid dorsal striatum-based processes, and this bias is thought to play a crucial role in stress-related psychopathology (Packard and Goodman, 2012; Schwabe and Wolf, 2013; Schwabe, 2013). We show here, in two independent studies, that a genetic polymorphism on the gene coding the α2b-adrenoceptor modulates the stress-induced bias toward habit memory in healthy individuals. Specifically, the shift toward habit memory was diminished in carriers of an ADRA2B deletion variant compared with noncarriers of this variant. Our neuroimaging data suggest that this modulatory effect of the ADRA2B polymorphism is mediated by altered connectivity of the amygdala with the hippocampus and dorsal striatum.
In line with previous studies (Schwabe and Wolf, 2012; Schwabe et al., 2013), we showed that stress leads to an increase in multi-cue strategies known to rely on the dorsal striatum and a decrease in single-cue strategies supported by the hippocampus (Shohamy et al., 2004; Schwabe and Wolf, 2012). This shift in strategy use may be due to impaired hippocampal or enhanced dorsal striatal memory. Previous studies found evidence for both alternatives (Schwabe and Wolf, 2012; Schwabe et al., 2013; Vogel et al., 2015), which are not mutually exclusive. Our EEG data showed a stress-induced increase in the FRN, which might be taken as evidence for increased striatal processing (Nieuwenhuis et al., 2005; Foti et al., 2011). In line with this interpretation, our fMRI data revealed increased dorsal striatal activation after stress. Moreover, our data support earlier findings suggesting that the amygdala, a region critically involved in emotional memory modulation (McGaugh, 2000; Packard and Wingard, 2004), orchestrates the shift from hippocampal to dorsal striatal memory after stress (Schwabe et al., 2013; Vogel et al., 2016). Specifically, we observed that stress led to increased amygdala-dorsal striatum connectivity but decreased amygdala-hippocampus connectivity.
Most importantly, however, the stress-induced shift in the preferential engagement of hippocampal and striatal memory systems was modulated by the ADRA2B deletion variant. The relative bias toward multi-cue strategies after stress was only observed in noncarriers of the ADRA2B deletion variant. This genotype effect appeared not to be mediated by changes in the activity of the hippocampus or the dorsal striatum because activity of these systems remained unaffected by genotype and the stress-induced increases in FRN amplitude and striatal activity were independent of ADRA2B genotype. Instead, the ADRA2B deletion variant had a significant impact on functional amygdala-EC and amygdala-dorsal striatum connectivity. Regardless of stress, the ADRA2B deletion variant was linked to increased amygdala-EC coupling. This finding suggests that the amygdala-EC crosstalk is particularly strong in carriers of the ADRA2B deletion variant, which may explain their superior episodic memory for emotional stimuli reported earlier (de Quervain et al., 2007b; Rasch et al., 2009). Although the increased amygdala-EC coupling in carriers of the deletion variant remained largely unchanged by stress, we obtained genotype-specific stress effects on the connectivity between amygdala and putamen. Specifically, whereas stress increased amygdala-putamen coupling in noncarriers of the ADRA2B deletion variant, this was not the case in carriers of the deletion variant. Together, these data suggest that the ADRA2B deletion variant, known to affect amygdala processing (Rasch et al., 2009; Cousijn et al., 2010), primarily modulates the crosstalk between amygdala and EC (regardless of stress) as well as between amygdala and putamen (under stress) and that, in line with recent pharmacological data (Schwabe et al., 2013), it is this modulation in connectivity patterns that is critical for the changes in learning strategies under stress in carriers of the ADRA2B deletion variant.
Previous studies on the stress-induced modulation of multiple memory systems suggested a critical role of glucocorticoids in the shift toward habit memory (Vogel et al., 2016). There is, however, also evidence that noradrenaline is critically involved in the shift from cognitive to habit memory. For instance, it has been shown that intra-amygdala injections of an α2-adrenoceptor antagonist, leading to increased noradrenergic stimulation, biased memory toward the dorsal striatum-dependent system in rats (Packard and Wingard, 2004; Wingard and Packard, 2008). At first glance, these findings, suggesting increased habit memory following noradrenergic activation, might seem to be in conflict with the present finding that a deletion variant of the ADRA2B gene, which is also linked to increased noradrenergic activation (Small et al., 2001), reduces the stress-induced shift toward habit memory. However, the α2-adrenoceptor antagonist used in pharmacological studies is not specific for any of the adrenergic receptor subtypes (Hieble and Ruffolo, 1995; Hieble et al., 1996), whereas the effects of the ADRA2B deletion variant are specific for the α2b-adrenoceptor subtype. Although specific adrenergic receptor subtypes may exert different effects on learning under stress, direct evidence supporting this view is still missing. Moreover and perhaps even more importantly, the administration of the α2-adrenoceptor antagonist leads to an acute, transient increase in noradrenergic stimulation, whereas the ADRA2B deletion variant is most likely associated with more constant changes. Specifically, the deletion variant is assumed to be associated with inherent and constant differences in noradrenaline availability (Small et al., 2001), although direct evidence from humans for this claim is still lacking. It is tempting to speculate that the ADRA2B deletion variant is associated with some sort of homeostatic compensations, an important mechanism of neuronal functioning (Marder and Goaillard, 2006). Indeed, our neuroimaging data suggest that carriers of the ADRA2B deletion variant show, compared with noncarriers of this variant, increased amygdala-EC coupling, which may be such a compensatory mechanism. Thus, acute pharmacological manipulations of a neurotransmitter system can hardly be compared with genetic differences in such a system. However, both the available pharmacological data and our behavioral genetics findings argue for an important role of the noradrenergic system in the balance between cognitive and habit memory.
We tested the role of the ADRA2B genotype in the stress-induced modulation of cognitive and habit memory in two experiments. In both experiments, task performance started ∼30 min after stress, when cortisol levels were significantly elevated, and lasted for 25 min (Experiment I) and 45 min (Experiment II), respectively. Thus, stress-induced sympathetic activity should have mainly vanished before behavioral testing and genomic cortisol actions should not have fully developed yet, suggesting that the present effects were mainly the result of nongenomic cortisol actions. Nevertheless, accumulating evidence indicates that there might be substantial variation in stress effects on cognition within relatively short time windows (Bendahan et al., 2016; Vogel and Schwabe, 2016) and the conclusion that the stress effect has been stable across the PCL task in this study might be premature. Such (possible) time-dependent changes in task performance or brain activity due to temporal dynamics of the stress response, however, can hardly be dissociated from changes resulting from learning processes.
The experimental setup used in the two experiments was virtually the same, but different methods were used to measure brain activity (EEG vs fMRI). Because of differences in the temporal resolution of the measured signals, feedback timing varied between the two studies. Feedback timing has considerable implications for the predominance of cognitive versus habit memory. Specifically, the striatum is very important for immediate feedback processing, whereas a delay in feedback presentation leads to increased engagement of the hippocampus (Foerde and Shohamy, 2011). Indeed, in the first experiment, in which feedback followed shortly after the response, larger hippocampal engagement possibly led to overall more multi-cue learning than in the second experiment, in which feedback was delayed by a few seconds and learning has a generally larger striatal contribution. Critically, however, regardless of feedback timing and the general distribution of single-cue versus multi-cue strategies, stress increased multi-cue learning and the ADRA2B deletion variant modulated this effect. This underlines the robustness of the stress-induced bias toward dorsal striatal memory and its modulation by the ADRA2B genotype.
In conclusion, we showed, in two independent experiments, that a deletion variant of the ADRA2B gene encoding the α2b-adrenoceptor reduces the stress-induced shift from hippocampal cognitive toward dorsal striatal habit memory, most likely via overall increased amygdala-EC connectivity and altered amygdala-putamen connectivity under stress. Although the stress-induced bias toward habit memory may hamper memory flexibility (Seehagen et al., 2015; Dandolo and Schwabe, 2016), it is thought to be a generally adaptive mechanism that aids coping with stressful events (Vogel et al., 2016). The reduced ability to shift toward a more suitable memory system under stress, together with aberrant emotional memory formation (de Quervain et al., 2007b; Rasch et al., 2009), may thus be an important factor that renders carriers of the ADRA2B deletion variant particularly vulnerable for developing PTSD (Liberzon et al., 2014).
Footnotes
This work was supported by German Research Foundation Grant SCHW1357/10-1. We thank Alicia Weisener, Angela Schlicker, Parijah Said, Livia Wilheim, Mascha Busch, Maximilian Walther, Paula Hartung, Erik Lang, Felicia Aparicio, and Florian Schwalb for help with data collection.
The authors declare no competing financial interests.
References
- Bendahan S, Goette L, Thoresen J, Loued-Khenissi L, Hollis F, Sandi C (2016) Acute stress alters individual risk taking in a time-dependent manner and leads to anti-social risk. Eur J Neurosci. Advance online publication. Retrieved Sep. 8, 2016. 10.1111/ejn.13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernández G (2010) Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci U S A 107:9867–9872. 10.1073/pnas.1003514107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandolo LC, Schwabe L (2016) Stress-induced cortisol hampers memory generalization. Learn Mem 23:679–683. 10.1101/lm.042929.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Papassotiropoulos A (2006) Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci U S A 103:4270–4274. 10.1073/pnas.0510212103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Roozendaal B (2007a) Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am J Psychiatry 164:967–969. 10.1176/ajp.2007.164.6.967 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A (2007b) A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci 10:1137–1139. 10.1038/nn1945 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ackermann S, Aerni A, Boesiger P, Demougin P, Elbert T, Ertl V, Gschwind L, Hadziselimovic N, Hanser E, Heck A, Hieber P, Huynh KD, Klarhöfer M, Luechinger R, Rasch B, Scheffler K, Spalek K, Stippich C, et al. (2012) PKCα is genetically linked to memory capacity in healthy subjects and to risk for posttraumatic stress disorder in genocide survivors. Proc Natl Acad Sci U S A 109:8746–8751. 10.1073/pnas.1200857109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain D, Schwabe L, Roozendaal B (2017) Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 18:7–19. 10.1038/nrn.2016.155 [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR (2007) The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast 2007:60803. 10.1155/2007/60803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50. 10.1038/35036213 [DOI] [PubMed] [Google Scholar]
- Elliott AE, Packard MG (2008) Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem 90:616–623. 10.1016/j.nlm.2008.06.012 [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mock B, Mecklinger A (2008) Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia 46:521–539. 10.1016/j.neuropsychologia.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Foerde K, Shohamy D (2011) Feedback timing modulates brain systems for learning in humans. J Neurosci 31:13157–13167. 10.1523/JNEUROSCI.2701-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA (2006) Modulation of competing memory systems by distraction. Proc Natl Acad Sci U S A 103:11778–11783. 10.1073/pnas.0602659103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G (2011) Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: response to commentary. Hum Brain Mapp 32:2267–2269. 10.1002/hbm.21357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T (2005) Error-related negativity predicts reinforcement learning and conflict biases. Neuron 47:495–501. 10.1016/j.neuron.2005.06.020 [DOI] [PubMed] [Google Scholar]
- Gluck MA, Shohamy D, Myers C (2002) How do people solve the “weather prediction” task? Individual variability in strategies for probabilistic category learning. Learn Mem 9:408–418. 10.1101/lm.45202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J, Leong KC, Packard MG (2012) Emotional modulation of multiple memory systems: implications for the neurobiology of post-traumatic stress disorder. Rev Neurosci 23:627–643. 10.1515/revneuro-2012-0049 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF (2007) It's worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology 44:905–912. 10.1111/j.1469-8986.2007.00567.x [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ (2005) Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308:1792–1794. 10.1126/science.1110449 [DOI] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Stämpfli P, Drechsler R, Brandeis D, Walitza S, Brem S (2014) The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. Neuroimage 84:159–168. 10.1016/j.neuroimage.2013.08.028 [DOI] [PubMed] [Google Scholar]
- Hieble JP, Ruffolo RR Jr (1996) Subclassification and nomenclature of alpha 1- and alpha 2-adrenoceptors. Prog Drug Res 47:81–130. [PubMed] [Google Scholar]
- Hieble JP, Bondinell WE, Ruffolo RR Jr (1995) Alpha- and beta-adrenoceptors: from the gene to the clinic: I. Molecular biology and adrenoceptor subclassification. J Med Chem 38:3415–3444. 10.1021/jm00018a001 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG (2001) Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 21:5222–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154–162. 10.1097/00006842-199903000-00006 [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA (1994) Probabilistic classification learning in amnesia. Learn Mem 1:106–120. 10.1126/science.273.5280.1399 [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR (1996) A neostriatal habit learning system in humans. Science 273:1399–1402. 10.1101/lm.1.2.106 [DOI] [PubMed] [Google Scholar]
- Lagnado DA, Newell BR, Kahan S, Shanks DR (2006) Insight and strategy in multiple-cue learning. J Exp Psychol Gen 135:162–183. 10.1037/0096-3445.135.2.162 [DOI] [PubMed] [Google Scholar]
- Liberzon I, King AP, Ressler KJ, Almli LM, Zhang P, Ma ST, Cohen GH, Tamburrino MB, Calabrese JR, Galea S (2014) Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry 71:1174–1182. 10.1001/jamapsychiatry.2014.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM (2006) Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7:563–574. 10.1038/nrn1949 [DOI] [PubMed] [Google Scholar]
- McEwen BS. (1998) Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci 840:33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2000) Memory: a century of consolidation. Science 287:248–251. 10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B (1996) Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A 93:13508–13514. 10.1073/pnas.93.24.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, Bermúdez-Rattoni F (2007) Cholinergic activity in the insular cortex is necessary for acquisition and consolidation of contextual memory. Neurobiol Learn Mem 87:343–351. 10.1016/j.nlm.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB (2005) Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci 21:3161–3168. 10.1111/j.1460-9568.2005.04152.x [DOI] [PubMed] [Google Scholar]
- O'Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP (1999) Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med 29:1083–1088. 10.1017/S0033291799008703 [DOI] [PubMed] [Google Scholar]
- Packard MG, Goodman J (2012) Emotional arousal and multiple memory systems in the mammalian brain. Front Behav Neurosci 6:14. 10.3389/fnbeh.2012.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Wingard JC (2004) Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem 82:243–252. 10.1016/j.nlm.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, Huynh KD, Brunner F, Corneveaux J, Osborne D, Wollmer MA, Aerni A, Coluccia D, Hänggi J, Mondadori CR, Buchmann A, Reiman EM, Caselli RJ, Henke K, de Quervain DJ (2006) Common Kibra alleles are associated with human memory performance. Science 314:475–478. 10.1126/science.1129837 [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stefanova E, Vogler C, Gschwind L, Ackermann S, Spalek K, Rasch B, Heck A, Aerni A, Hanser E, Demougin P, Huynh KD, Luechinger R, Klarhöfer M, Novakovic I, Kostic V, Boesiger P, Scheffler K, de Quervain DJ (2013) A genome-wide survey and functional brain imaging study identify CTNNBL1 as a memory-related gene. Mol Psychiatry 18:255–263. 10.1038/mp.2011.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Paré-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA (2001) Interactive memory systems in the human brain. Nature 414:546–550. 10.1038/35107080 [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ (2009) A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A 106:19191–19196. 10.1073/pnas.0907425106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C (2006) The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol 59:236–243. 10.1016/j.ijpsycho.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL (2011) Memory modulation. Behav Neurosci 125:797–824. 10.1037/a0026187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL (2004) Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci 24:8161–8169. 10.1523/JNEUROSCI.2574-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustemeier M, Schwabe L, Bellebaum C (2013) On the relationship between learning strategy and feedback processing in the weather prediction task: evidence from event-related potentials. Neuropsychologia 51:695–703. 10.1016/j.neuropsychologia.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J (2015) Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16:290–304. 10.1038/nrn3918 [DOI] [PubMed] [Google Scholar]
- Schwabe L. (2013) Stress and the engagement of multiple memory systems: integration of animal and human studies. Hippocampus 23:1035–1043. 10.1002/hipo.22175 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT (2009) Stress prompts habit behavior in humans. J Neurosci 29:7191–7198. 10.1523/JNEUROSCI.0979-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT (2012) Stress modulates the engagement of multiple memory systems in classification learning. J Neurosci 32:11042–11049. 10.1523/JNEUROSCI.1484-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT (2013) Stress and multiple memory systems: from ‘thinking’ to ‘doing.’ Trends Cogn Sci 17:60–68. 10.1016/j.tics.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Philippsen C, Richter S, Bohringer A, Wippich W, Schachinger H (2007) Stress modulates the use of spatial versus stimulus-response learning strategies in humans. Learn Mem 14:109–116. 10.1101/lm.435807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Römer S, Richter S, Dockendorf S, Bilak B, Schächinger H (2009) Stress effects on declarative memory retrieval are blocked by a beta-adrenoceptor antagonist in humans. Psychoneuroendocrinology 34:446–454. 10.1016/j.psyneuen.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS (2010) Memory formation under stress: quantity and quality. Neurosci Biobehav Rev 34:584–591. 10.1016/j.neubiorev.2009.11.015 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS (2012) Stress effects on memory: an update and integration. Neurosci Biobehav Rev 36:1740–1749. 10.1016/j.neubiorev.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, Wolf OT (2013) Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol Psychiatry 74:801–808. 10.1016/j.biopsych.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Seehagen S, Schneider S, Rudolph J, Ernst S, Zmyj N (2015) Stress impairs cognitive flexibility in infants. Proc Natl Acad Sci U S A 112:12882–12886. 10.1073/pnas.1508345112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA (2004) Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain 127:851–859. 10.1093/brain/awh100 [DOI] [PubMed] [Google Scholar]
- Small KM, Brown KM, Forbes SL, Liggett SB (2001) Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem 276:4917–4922. 10.1074/jbc.M008118200 [DOI] [PubMed] [Google Scholar]
- Southwick SM, Davis M, Horner B, Cahill L, Morgan CA 3rd, Gold PE, Bremner JD, Charney DC (2002) Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am J Psychiatry 159:1420–1422. 10.1176/appi.ajp.159.8.1420 [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM (1996) Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A 93:13515–13522. 10.1073/pnas.93.24.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M (1994) Theoretical analysis of a multidimensional mood questionnaire (MDBF). Diagnostica 40:9. [Google Scholar]
- van der Helden J, Boksem MA, Blom JH (2010) The importance of failure: feedback-related negativity predicts motor learning efficiency. Cereb Cortex 20:1596–1603. 10.1093/cercor/bhp224 [DOI] [PubMed] [Google Scholar]
- Vogel S, Schwabe L (2016) Stress in the zoo: tracking the impact of stress on memory formation over time. Psychoneuroendocrinology 71:64–72. 10.1016/j.psyneuen.2016.04.027 [DOI] [PubMed] [Google Scholar]
- Vogel S, Klumpers F, Krugers HJ, Fang Z, Oplaat KT, Oitzl MS, Joëls M, Fernández G (2015) Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology 40:947–956. 10.1038/npp.2014.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Fernández G, Joëls M, Schwabe L (2016) Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn Sci 20:192–203. 10.1016/j.tics.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Wingard JC, Packard MG (2008) The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res 193:126–131. 10.1016/j.bbr.2008.05.002 [DOI] [PubMed] [Google Scholar]