Abstract
Purpose
Mouse mutants for proteins expressed in the dystrophinߝglycoprotein complex at the photoreceptor terminal have electroretinogram (ERG) b-waves with a delayed onset and time course. The b-wave is defined by the sum of PII generated by depolarizing bipolar cells and slow PIII generated by Müller glial cells. In this study, we evaluated the hypothesis that the abnormalities observed in one of these mutants, Largevls, are caused by abnormal response properties of slow PIII.
Methods
To isolate slow PIII, we crossed the Largevls mutant to a mouse line (Gpr179nob5) that lacks the ERG b-wave but maintains normal photoreceptor function and in which retinal degeneration does not occur. ERGs were recorded to strobe flash stimuli after overnight dark adaptation.
Results
In comparison with control responses, the a-wave and slow PIII had comparable waveforms but were reduced in amplitude in Largevls mice. The magnitude of this reduction was comparable for these components, and across stimulus luminance. There was no stimulus condition where the amplitude of slow PIII was larger than control.
Conclusions
The data obtained are inconsistent with the idea that the b-wave abnormalities noted in Largevls mutant mice are caused by abnormal response properties of slow PIII.
Keywords: B-wave, Electroretinogram, Müller cell, Slow PIII
Introduction
The electroretinogram (ERG) reflects the algebraic summation of several underlying generators. In response to a strobe flash presented in darkness, the ERG is comprised of two main components, the a-wave and the b-wave. The a-wave reflects the light-induced closure of sodium ion channels along the rod outer segment [1]. The positive polarity b-wave reflects the summation of a positive polarity component (PII [2]) generated by rod depolarizing bipolar cells (DBCs [3]) and a negative polarity component (slow PIII) that reflects Kir4.1 channel activity in Müller glial cells induced as a secondary response to light-evoked photoreceptor activity [3-7]. These underlying generators interact to define the b-wave recorded by electrodes contacting the corneal surface. An altered b-wave could, therefore, reflect changes in PII and/or slow PIII.
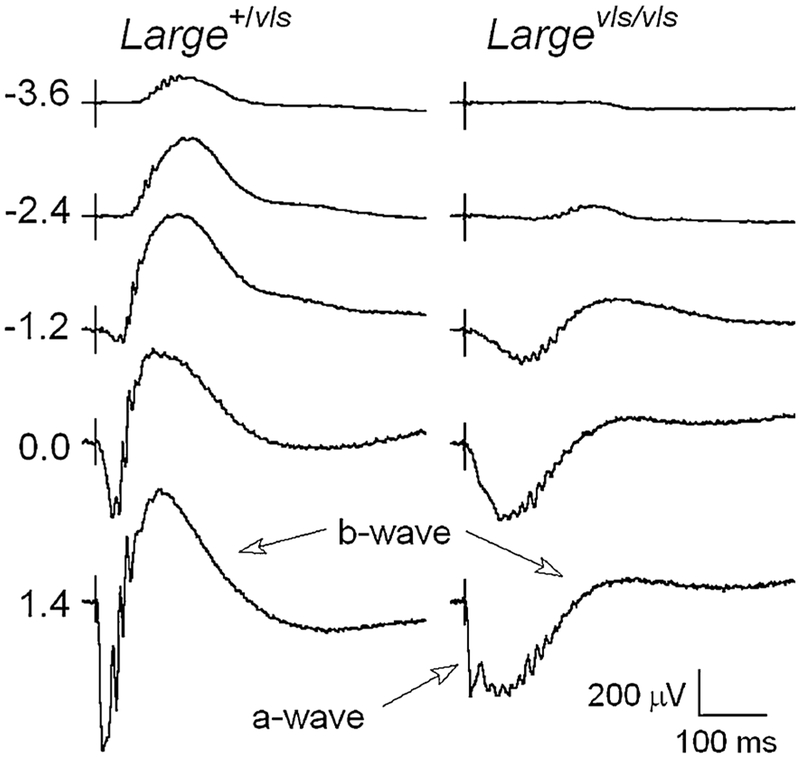
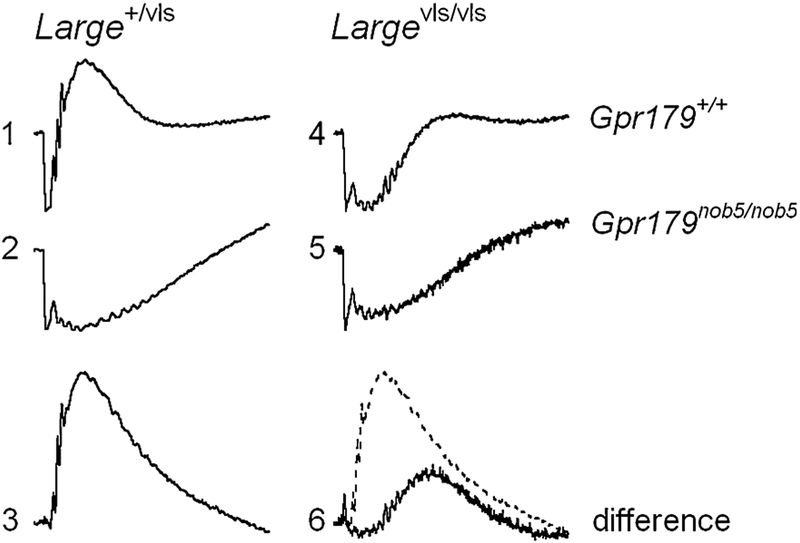
An unusual ERG waveform has been reported in mutant mouse models for proteins expressed in the dystrophin-glycoprotein complex at the photoreceptor terminal, including dystrophin [8], LARGE [9, 10], pikachurin [11], and protein O-mannose N-acetylglucosaminyltransferase 1 (POMGnT1 [12]). The hallmark of these responses is a marked delay in the timing of the b-wave component, as well as amplitude reductions. These abnormalities are exemplified by the ERG waveforms shown in Fig. 1, which were evoked by a series of flashes presented to dark-adapted Large+/vls and Largevls/vls littermates. While responses of Large+/vls heterozygotes are comparable to those of control mice, the responses of Largevls/vls homozygotes have reduced a-waves and b-waves that have reduced amplitude and delayed implicit time. The basis for these response abnormalities has not been established, although they are apparent across a broad range of stimulus luminance. Pattnaik et al. [13] examined a dystrophin mutant, mdxCv3, and suggested that the abnormal b-waves noted in this model could reflect abnormal response properties of slow PIII, which together with PII defines the b-wave waveform. This suggestion was supported by in vitro studies [13] in which recordings were made from mdxCv3 retinas before and after the addition of barium, an ion that blocks Kir channels and eliminates slow PIII [3]. Subtraction of these responses provides a measure of slow PIII, which was larger in mdxCv3 mutant mice than in control animals. Based on these in vitro results, Pattnaik et al. [13] suggested that the abnormal waveform noted in mdxCv3 mice may reflect abnormal response properties of slow PIII.
Fig. 1.
Comparison of dark-adapted ERGs obtained from Large+//vls and Largevls/vls> mice. Values to the left of each row of waveforms indicate flash luminance in log cd s/m2
In the present study, we used a genetic approach to evaluate the hypothesis that changes in the response properties of slow PIII underlie the b-wave abnormalities observed in ERGs obtained from Largevls/vls mice [10]. To do this, we crossed the Largevls allele onto the Gpr179nob5 line [14]. Because Gpr179nob5/nob5 mice lack the ERG b-wave, the response properties of slow PIII can be evaluated in vivo [7, 15].
Methods
Mice
We obtained Large+/vls mice from The Jackson Laboratory (Bar Harbor, ME) and Gpr179nob5 mice from a local colony. A two-generation cross was used to generate Largevls/vls and control (Large+/vls or Large+/+) littermates that were Gpr179nob5/nob5 homozygotes.
Genotyping
DNA was extracted from tail snips. Mice were genotyped for wild-type and mutant alleles of Large and Gpr179 using PCR protocols. TheLargevls mutation arose on a Castaneous background and has been crossed to C57BL/6 J for more than 10 generations [10]. By selecting for the Largevls allele, the only region that remains Castaneous is near that locus. We used two pairs of primers (D8MIT74, D8MIT179) for single-nucleotide polymorphisms that flank the Large locus to distinguish mice that carried Castaneous (Largevls) or C57BL/6 J (Large+) alleles. We confirmed PCR genotyping by two phenotypic features that characterize homozygotes: small stature and an epiretinal vasculature that persists into adulthood [10].
The following primers were used to screen mice for Gpr179 alleles:
For the Gpr179+ allele: sense (5′- TGTGCCTG GGTATCTGTTGA-3′)
antisense (5′- GCTTACACACTTACACACAGA- TAGATG-3′)
For the Gpr179nob5 allele: sense (5′- GCATGTGC- CAAGGGTATCTT-3′)
antisense (5′- GCTTACACACTTACACACAGA- TAGATG-3′)
ERG
After overnight dark adaptation, mice were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg). The cornea was anesthetized (1 % proparacaine HCl) and the pupil was dilated (2.5 % phenylephrine HCl, 1 % tropicamide, and 1 % cyclopentolate HCl). Mice were placed on a temperature-regulated heating pad throughout the recording session. All procedures involving animals were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Because the photoreceptor degeneration of Largevls/vls mice is progressive, all studies were conducted on 1-month-old mice.
Strobe flash ERGs were recorded using a stainless steel electrode in contact with the corneal surface via 1 % methylcellulose. Needle electrodes were placed in the cheek and the tail for reference and ground leads, respectively. Dark-adapted ERGs were evoked by full-field flashes (LKC Technologies, Gaithersburg, MD), with time-integrated flash luminances ranging from −3.6 to 2.1 log cd s/m2. Stimuli were presented in order of increasing luminance and the number of successive responses averaged together decreased from 20 for low-luminance flashes to 2 for the highest luminance stimuli. The duration of the interstimulus interval increased from 4 s for low-luminance flashes to 90 s for the highest luminance stimuli. Responses were differentially amplified (0.3–1,500 Hz), averaged, and stored using a UTAS E-3000 signal averaging system (LKC Technologies, Gaithersburg, MD).
The amplitude of the a-wave was measured at 8 ms after flash presentation from the pre-stimulus baseline. The amplitude of slow PIII was measured from the pre-stimulus baseline to the value of the trough at 150 ms after flash presentation.
Results
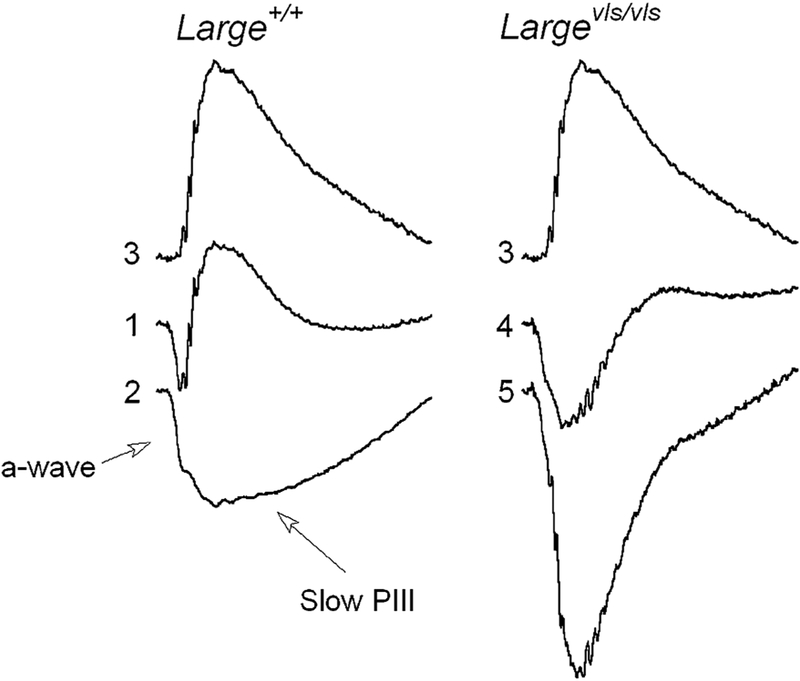
Figure 2 illustrates the expected results if changes in the response properties of slow PIII cause the b-wave abnormalities observed in ERGs obtained from Largevls/vls mice. Response (1) re-plots the Large+/vls response to a 0.0 log cd s/m2 stimulus from Fig. 1. Response (2) is the response of a Gpr179nob5/nob5 mouse to the same stimulus. The absence of the b-wave in the Gpr179nob5/nob5 mouse response allows slow PIII to be measured. Responses (1) and (2) have been normalized at 24 ms. Response (3) is the result of subtracting response (2) from response (1). This difference waveform reflects all of the ERG components that are missing in the responses of Gpr179nob5/nob5 mice and resembles the PII component extracted by others using curve-fitting or pharmacological approaches [16, 17]. Response (4) re-plots from Fig. 1 the Largevls/vls mutant response to a 0.0 log cd s/m2 stimulus. Response (4) was normalized in the same manner as responses (1) and (2). Working from the presumption that the Largevls/vls mutant PII component generated by DBCs has a normal waveform, we subtracted response (3) from response (4) to derive the predicted Largevls/vls mutant PIII component. This is shown as response (5) and is seen to be substantially larger in amplitude than the corresponding component obtained from control Gpr179nob5/nob5 mice [response (2)]. If abnormal slow PIII response properties underlie the abnormal Largevls b-wave, we expect to see a similar slow PIII phenotype in Largevls/vls/Gpr179nob5/nob5 double mutant mice.
Fig. 2.
Predicted result if changes in response properties of slow PIII underlie abnormal Largevls ERG waveform. Responses (1) and (2) were evoked by 0.0 log cd s/m2 stimulus flashes from control (1) and Gpr179nob5/nob5 (2) mice. Response (3) represents the result of subtracting response (2) from response (1). Response (4) was evoked by 0.0 log cd s/m2 stimuli from a Largevls/vls mouse. Response (5) indicates the result of subtracting response (3) from response (4) and represents the waveform expected if Largevls/vls ERGs include a normal waveform P2 component. All responses are normalized to a-wave at 24 ms, corresponding to the trough of response (1)
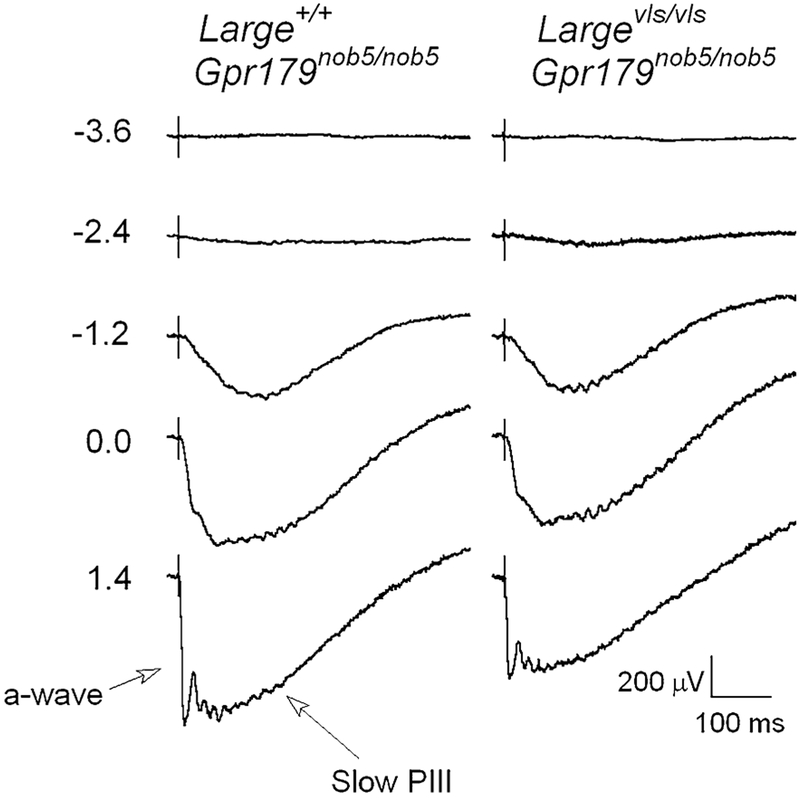
Figure 3 presents a series of dark-adapted ERGs obtained from Large+/+ (left) and Largevls/vls mutant (right) littermates. Because both mice are Gpr179nob5/nob5 homozygotes, the b-wave is eliminated and slow PIII is revealed. At low stimulus luminances, the response is comprised of a small negative polarity deflection. At high stimulus luminances, the a-wave becomes apparent ahead of slow PIII. The response of the Largevls/vls mutant has a waveform that resembles that of the control, but is reduced in amplitude.
Fig. 3.
Comparison of dark-adapted ERGs obtained from Largevls/vls/Gpr179nob5/nob5 and Large+/+/Gpr179nob5/nob5 mice. Values to the left of each row of waveforms indicate flash luminance in log cd s/m2
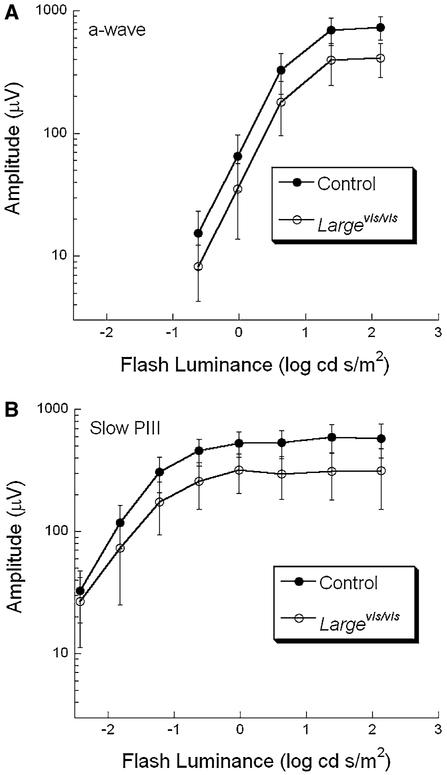
Figure 4 presents luminance-response functions for the a-wave (A) and for slow PIII (B). In comparison with control, both response components are reduced in Largevls/vls mutants. To determine whether the magnitude of reductions in a-wave and slow PIII amplitude are comparable, at each flash luminance the average mutant response was divided by the corresponding control average. Across the stimulus range used here, the Largevls/vls responses are 55 ± 1.5 (a-wave) and 55.7 ± 2.9 % (slow PIII) of control. These values are not significantly different from one another (t < 1), indicating that the a-wave and slow PIII are reduced by equivalent amount in Largevls/vls mice.
Fig. 4.
Response functions for the ERG a-wave (A) and slow PIII (B) measures of Largevls/vls/Gpr179nob5/nob5 and control/Gpr179nob5/nob5 mice. Data points indicate the average (± s.d.) of 7 Largevls/vls/Gpr179nob5/nob5 and 18 control/Gpr179nob5/nob5 mice
The Largevls/vls/Gpr179nob5/nob5 responses do not resemble the waveforms predicted (response (5), Fig. 2) if the PII component of Largevls/vls ERGs had a normal waveform and the abnormal response was due to changes in the response properties of slow PIII. There was no stimulus condition in which the amplitude of slow PIII was larger in Largevls/vls than control mice.
To define the Largevls/vls PII waveform, we subtracted Gpr179nob5/nob5 ERG from Gpr179+/+ responses, obtained to 1.4 log cd s/m2 stimulus flashes. In Fig. 5, response (1) plots a Large+/vls Gpr179+/+ ERG and response (2) is the ERG of a Large+/vls/Gpr179nob5/nob5 mouse. These waveforms were normalized at 8 ms. Response (3) is the result of subtracting response (2) from response (1) and reflects the normal PII component obtained under this stimulus condition. Response (4) plots aLargevls/vls/Gpr179+/+ ERG and response (5) is the ERG of a Largevls/vls/Gpr179nob5/nob5 mouse; these waveforms were also normalized at 8 ms. Response (6) is the result of subtracting response (5) from response (4) and represents the Largevls/vls PII component with response (3) re-plotted as the dashed line. The superimposition of the difference waveforms shows that in comparison with the control, the Largevls/vls PII component is delayed and has a reduced amplitude.
Fig. 5.
Isolation of PII from Large+/vls and Largevls/vls ERGs. ERGs evoked by 1.4 log cd s/m2 stimulus flashes from Large+/vls (1, 2) and Largevls/vls (4, 5) mice. All responses were normalized at 8 ms. Response (3) was obtained by subtracting response (2) from response (1); response (6) was obtained by subtracting response (5) from response (4). The dashed line superimposed on response (6) is response (3)
Discussion
In the present study, we have examined the in vivo response properties of slow PIII to evaluate the possibility that abnormal response properties of this ERG component underlie the b-wave abnormalities observed in Largevls/vls mutant mice. We isolated slow PIII genetically, by crossing the Largevls/vls mutant to a mouse line that does not generate a b-wave due to a Gpr179 null mutation [14]. While the Gpr179nob5/nob5 mutant does not generate a b-wave, the retina appears normal by light and electron microscopy [14]. The main abnormality noted in double mutant Largevls/vls/Gpr179nob5/nob5 mice is an overall reduction in slow PIII amplitude that is proportional to the amplitude reduction noted for the a-wave. Samuels et al. [15] reported a similar analysis of PrphRd2/+/Nyxnob mice and noted that the amplitude of slow PIII was somewhat larger than predicted by the a-wave reduction. Periphe-rin/rds is an outer segment protein [15,18], and PrphRd2/+ mice do not display the b-wave abnormalities noted in Largevls/vls mice [19]. If we take these results as a benchmark, the amplitude reductions noted in slow PIII of the Largevls/vls mutant are somewhat greater than expected based on the a-wave loss and inconsistent with the idea that an abnormally large amplitude slow PIII underlies the b-wave abnormalities noted in this mutant.
These findings do not rule out the possibility that an increase in slow PIII could contribute to the b-wave abnormalities noted in other mutants, including the mdxCv3 mouse. We chose to examine the Largevls mutant based on our prior experience [10]. It would be interesting to address this possibility in vivo by crossing the mdxCv3 mutant to the Gpr179nob5 mutant or any of the other no b-wave mutants that have been described for Nyx [20, 21], Grm6 [22-24], and Trpm1 [25-28].
While the present results indicate that slow PIII abnormalities do not underlie the delayed onset and slow kinetics of the Largevls b-wave (Fig. 1), they do not provide an explanation for these abnormalities. A recent study of the Pikachurin-/- mouse, which has ERG abnormalities comparable to those of Largevls/vls, indicates that DBC invaginations into photoreceptor terminals are abnormal, resulting in a larger gap between the pre-synaptic active zone and the post-synaptic membrane [29]. A larger synaptic gap could delay clearance of glutamate and thus result in a delayed b-wave. It remains to be determined whether the synaptic abnormalities present in Largevls/vls mice [10] include this feature.
Acknowledgments
This research was supported by the US Veterans Administration Medical Research Service, a Foundation Fighting Blindness Center Grant to the Cole Eye Institute, Cleveland Clinic, and an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine.
Footnotes
Conflict of interest None
Contributor Information
Neal S. Peachey, Louis Stokes Cleveland VA Medical Center, 10701 East Boulevard, Cleveland, OH 44106, USA; Cole Eye Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA; Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, 9500 Euclid Avenue, Cleveland, OH 44195, USA
Gwen M. Sturgill-Short, Louis Stokes Cleveland VA Medical Center, 10701 East Boulevard, Cleveland, OH 44106, USA
References
- 1.Penn RD, Hagins WA (1969) Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 223:201–204 [DOI] [PubMed] [Google Scholar]
- 2.Granit R (1933) The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 77:207–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA (2000) Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20:5733–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg RH, Miller S (1973) Aspects of electrolyte transport in frog pigment epithelium. Exp Eye Res 16:365–372 [DOI] [PubMed] [Google Scholar]
- 5.Oakley B II, Green DG (1976) Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol 39: 1117–1133 [DOI] [PubMed] [Google Scholar]
- 6.Witkovsky P, Dudek FE, Ripps H (1975) Slow PIII component of the carp electroretinogram. J Gen Physiol 65:119–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Marmorstein AD, Kofuji P, Peachey NS (2004) Contribution of Kir4.1 to the mouse electroretinogram. Mol Vision 10:650–654 [PMC free article] [PubMed] [Google Scholar]
- 8.Pillers DA (1999) Dystrophin and the retina. Mol Genet Metab 68:304–309 [DOI] [PubMed] [Google Scholar]
- 9.Holzfeind PJ, Grewal PK, Reitsamer HE, Kechvar J, Lass-mann H, Hoeger H, Hewitt JE, Bittner RE (2002) Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Largemyd mouse defines a natural model for glycosylation-deficient muscle-eye-brain disorders. Hum Mol Genet 11: 2673–2687 [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Kameya S, Cox GA, Hsu J, Hicks W, Maddatu TP, Smith RS, Naggert JK, Peachey NS, Nishina PM (2005) Ocular abnormalities in Largemydand Largevls mice, spontaneous models for muscle, eye and brain diseases. Mol Cell Neurosci 30:160–172 [DOI] [PubMed] [Google Scholar]
- 11.Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T (2008) Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci 11:923–931 [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H (2006) A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose beta1,2-N-acetylglucosaminyltransferase (POMGnT1). Mech Dev 123:228–240 [DOI] [PubMed] [Google Scholar]
- 13.Pattnaik BR, Green DG, Pillers D-AM (2009) Aberrant slow wave (Slow PIII) component superimposed on a normal b-wave, accounts for the abnormal electroretinogram in the mdxCv3 mouse. Invest Ophthalmol Vis Sci 50:E-Abstract 3599 [Google Scholar]
- 14.Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, Contreras-Alcantara S, Baba K, Tosini G, Pozdeyev N, Iuvone PM, Bojang P Jr, Pearring JN, Simonsz HJ, van Genderen M, Birch DG, Traboulsi EI, Dorfman A, Lopez I, Ren H, Goldberg AFX, Nishina PM, Lachapelle P, McCall MA, Koenekoop RK, Bergen AAB, Kamermans M, Gregg RG (2012) GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 90:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels IS, Sturgill GM, Grossman GH, Rayborn ME, Hollyfield JG, Peachey NS (2010) Light-evoked responses of the retinal pigment epithelium: changes accompanying photoreceptor loss in the mouse. J Neurophysiol 104: 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood DC, Birch DG (1992) A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci 8:107–126 [DOI] [PubMed] [Google Scholar]
- 17.Robson JG, Frishman LJ (1995) Response linearity and kinetics of the cat electroretinogram: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci 12:837–850 [DOI] [PubMed] [Google Scholar]
- 18.Goldberg AF (2006) Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol 253:131–175 [DOI] [PubMed] [Google Scholar]
- 19.Cheng T, Peachey NS, Li S, Goto Y, Cao Y, Naash MI (1997) The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci 17:8118–8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS (1998) A naturally-occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci 39:2443–2449 [PubMed] [Google Scholar]
- 21.Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA (2007) Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol 98:3023–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, Takada M, Nakamura K, Nakao K, Katsuki M, Nakanishi S (1995) Specific deficit of the ON response in visual transmission by targeted disruption of the mGIuR6 gene. Cell 80:757–765 [DOI] [PubMed] [Google Scholar]
- 23.Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, Bal-annik V, McDearmon EL, Omura C, Lumayag S, Invergo BM, Glawe B, Cantrell DR, Inayat S, Olvera MA, Vessey KA, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS (2007) Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci 24:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddox DM, Vessey KA, Yarbrough GL, Invergo BM, Cantrell DR, Inayat S, Balannik V, Hicks WL, Hawes NL, Byers S, Smith RS, Hurd R, Howell D, Gregg RG, Chang B, Naggert JK, Troy JB, Pinto LH, Nishina PM, McCall MA (2008) Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4 results in differences in retinal ganglion cell visual responses. J Physiol 586: 4409–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL (2009) TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA 106:19174–19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y, Heimel JA, Kammermans M, Peachey NS, Gregg RG, Nawy S (2009) A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci 29:6088–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T (2010) TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA 107:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peachey NS, McCall MA, Nobles RD, Hirschtritt ME, Pearring JN, Bojang P, Shen Y, Nawy SA, Nishina PM, Gregg RG (2011) Trpml point mutation underlies retinal dysfunction in the Mtvr27 mouse model of complete congenital stationary night blindness. Invest Ophthalmol Vis Sci 51:E-Abstract 4124 [Google Scholar]
- 29.Omori Y, Araki F, Chaya T, Kajimura N, Irie S, Terada K, Muranishi Y, Tsujii T, Ueno S, Koyasu T, Tamaki Y, Kondo M, Amano S, Furukawa T (2012) Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells. J Neurosci 32:6126–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]