Abstract
Adrenal steroidogenesis is a robust process, involving a series of enzymatic reactions that facilitate conversion of cholesterol into biologically active steroid hormones under the stimulation of angiotensin II, adrenocorticotropic hormone and other regulators. The biosynthesis of mineralocorticoids, glucocorticoids, and adrenal-derived androgens occur in separate adrenocortical zones as a result of the segregated expression of steroidogenic enzymes and cofactors. This mini review provides the principles of adrenal steroidogenesis, including the classic and under-appreciated 11-oxygenated androgen pathways. Several adrenal diseases result from dysregulated adrenal steroid synthesis. Herein, we review growing evidence that adrenal diseases exhibit characteristic modifications from normal adrenal steroid pathways that provide opportunities for the discovery of biomarker steroids that would improve diagnosis and monitoring of adrenal disorders.
Keywords: adrenal, steroids, adrenal disease, androgens, biomarkers
1. Introduction to the human adrenal gland and its steroid pathways
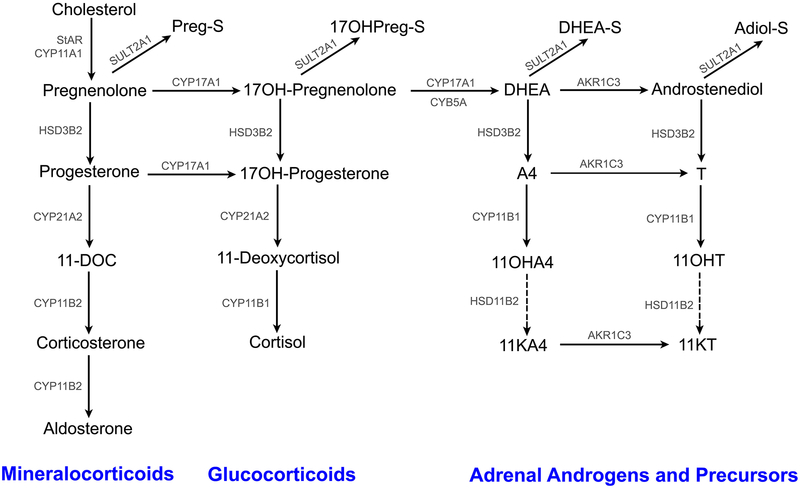
The human adrenal gland is a multifaceted endocrine organ. It consists of an inner medulla and an outer cortex that have different embryological origins - neural crest cells for the medulla and mesoderm for the cortex. The adult adrenal cortex produces three distinct classes of steroid hormones, including mineralocorticoids, glucocorticoids and androgens (Fig. 1). The cytoarchitecture of the adrenal cortex was first described by Arnold in 1866, who classified the three layers as zona glomerulosa (ZG), zona fasciculata (ZF) and zona reticularis (ZR) [1]. The relevant functional characterization of these zones has defined their specific roles in adrenal steroidogenesis: the ZG synthesizes the mineralocorticoid aldosterone, the ZF produces the glucocorticoid cortisol [2, 3], whereas the human ZR produces adrenal-derived androgens and precursors including dehydroepiandrostenone sulfate (DHEA-S) [4–7]. While angiotensin II is the major agonist for the ZG [8], adrenocorticotropic hormone (ACTH) is the primary stimulus for the ZF and ZR [9].
Fig. 1.
Normal Adrenal Steroidogenesis Pathway
The key enzymes involved in adrenal steroid production are either cytochrome P450 enzymes (CYPs) or hydroxysteroid dehydrogenases (HSDs) (Fig. 1). Even though some of the steroidogenic enzymes and cofactors/proteins are present in all the cortical zones [ex: cytochrome P450 cholesterol side-chain cleavage (CYP11A1) and steroidogenic acute regulatory protein (StAR)], the zone-specific production of steroids results from the differential expression of CYP/HSD enzymes and cofactors [7, 10]. The ZG-specific presence of aldosterone synthase (CYP11B2) [11] and absence of 17α-hydroxylase/17,20-lyase (CYP17A1) [12] enables the production of aldosterone. The 17α-hydroxylase activity of CYP17A1 facilitates the synthesis of cortisol in the ZF [12]. The biosynthesis of DHEA-S in the ZR is contingent on the presence of only three steroidogenic enzymes, namely CYP11A1, CYP17A1 and steroid sulfotransferase (SULT2A1) [10]. Furthermore, ZR expression of cytochrome b5 (CYB5A), an allosteric regulator of CYP17A1 that enhances its 17, 20-lyase activity, is required for robust production of DHEA-S [12]. Another distinctive ZR feature that allows its production of DHEA-S is the lack of 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2), which contrasts to high expression in the ZG and ZF where it is needed for both aldosterone and cortisol synthesis [12, 13] (Fig. 1). The relative lack of ZR HSD3B2 expression decreases its enzymatic competition with CYP17A1 for pregnenolone (Preg) and 17α-hydroxypregnenolone (17OHPreg), thus increasing the flux of substrate toward DHEA-S. Historically, there have been reports that the adrenal also produces low but significant amounts of bioactive androgens including testosterone (T) [14–17]. Recently, we demonstrated an intra-adrenal pathway for androstenedione (A4) conversion to T via type 5 17β-hydroxysteroid dehydrogenase (AKR1C3), which has its highest cortex expression in the ZR [6, 10, 12, 18]. In addition, we recently characterized the adrenal synthesis of 11-oxygenated androgens and their precursors [6, 19]. These steroid derivatives of A4 and T include: 11β-hydroxyandrostenedione (11OHA4), 11β-hydroxytestosterone (11OHT), 11-ketoandrostenedione (11KA4) and 11-ketotestosterone (11KT). These steroids are most likely of adrenal origin because 11β-hydroxylase (CYP11B1), an enzyme mainly expressed in adrenocortical cells, readily converts T and 11OHA to 11OHT and 11OHA4, respectively [20, 21] (Fig. 1). 11OHT and 11OHA4 can in turn be oxidized to their respective keto-derivatives, 11KT and 11KA4 in peripheral tissues expressing 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2) [6, 21–23] (Fig. 1). Furthermore, 11KA4 is a much better substrate for AKR1C3 than A4, leading ultimately to efficient production of 11KT [24]. Unlike the conventional adrenal steroids DHEA and DHEA-S, 11OHT and 11KT effectively activate the androgen receptor [6, 25, 26].
2. Diseases that Disrupt Adrenal Steroidogenic Pathways
2.1. Disorders causing Adrenal Androgen Excess
2.1.1. Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is a term used collectively for a group of autosomal recessive genetic defects in cortisol biosynthesis [27]. 21-hydroxylase (CYP21A2) deficiency (21OHD) is the most common form of CAH, accounting for more than 90% of all CAH cases [28]. Based on the presence or absence of cortisol insufficiency, diagnosis of 21OHD is routinely classified into classic and nonclassic forms, respectively [29]. Worldwide, the incidence of classic 21OHD is 1:16,000 live births [30, 31]; whereas nonclassic 21OHD is much more frequent, having an occurrence of up to 1:200 in Caucasians and Ashkenazi Jews [32]. The combined prevalence for classic and nonclassic 21OHD makes it one of the more common genetic diseases. The classic forms of 21OHD are divided into two groups: “salt wasting” and “simple virilizing”, depending on whether or not mineralocorticoid synthesis is sufficiently defective to cause hypotensive crises in newborns. Although subclassification of 21OHD is generally helpful, the spectrum of the disease severity actually depends on the underlying CYP21A2 mutations, the resulting residual activity of the enzyme, and poorly understood factors such as modifier genes.
CYP21A2 is a steroid-metabolizing enzyme required for the synthesis of both aldosterone and cortisol (Fig. 1). The decrease in circulating cortisol biosynthesis in 21OHD releases negative feedback on the hypothalamus and the pituitary gland. This leads to a steep augmentation in the secretion of the corticotropin-releasing hormone (CRH) and ACTH, which in turn causes hyperplasia of the adrenal cortex. An enzyme block at the CYP21A2 step in the steroidogenic pathway results in a build-up of the upstream substrates, particularly 17α-hydroxyprogesterone (17OHP4). Elevated serum 17OHP4 has been traditionally used as a biomarker of 21OHD and for diagnosis and treatment monitoring [31, 33] (Table 1). Serum 17OHP4 testing has several drawbacks that include: 1) false-positive results are commonplace in premature and sick newborns [34, 35]; 2) false-negative results have been reported in newborn screening [36] and are more prevalent in girls [37], particularly when mothers were prenatally exposed to glucocorticoids [38]; 3) dynamic confirmatory testing under cosyntropin stimulation is mandatory for most cases [39]; 4) even after cosyntropin stimulation, some 17OHP4 values cannot distinguish between nonclassic 21OHD and the carrier state. In order to overcome the pitfalls of 17OHP4 testing 21OHD, researchers began searching for additional steroid biomarkers. Milewicz et al first proposed the utilization of 21-deoxycortisol (21dF) concurrently with 17OHP4 after ACTH stimulation for improved diagnosis of 21OHD [40]. They hypothesized that since 21dF is an 11β‐hydroxylated product of 17OHP4 and is produced solely by the adrenal cortex, its serum levels would be elevated in tandem with and more consistently than 17OHP4 in a 21OHD patient. Later studies by Costa-Barbosa et al suggested the analysis of (21dF+17OHP4)/cortisol ratio to improve the positive predictive value of newborn screening and diagnosis of the carrier state [41, 42]. Our recent study also indicated 21dF as a valuable discriminatory steroid between treated 21OHD patients and age- and sex-matched controls [43]. In addition to 17OHP4 and 21dF, we also demonstrated a significant increase in two other upstream steroids: 16α-hydroxyprogesterone (16OHP4) and 11β-hydroxyprogesterone (11OHP4) [43] (Table 1).
Table 1.
Potential Serum Steroid Biomarkers in Human Adrenal Disorders
| Disease/Disorder | Discriminating Steroids | References |
|---|---|---|
| 21-hydroxylase Deficiency | ↑ 17OH-Progesterone, ↑ 16OH-Progesterone, ↑ 11OH-Progesterone, ↑ 21-Deoxycortisol, ↑ A4, ↑ 11OHA4, ↑ 11KA4, ↑ 11OHT, ↑ 11KT, ↑ Preg-S ↓ DHEA, ↓ DHEA-S |
[19, 39–41, 48, 49] |
| Premature Adrenarche | ↑ DHEA, ↑ A4, ↑ 11OHA4, ↑ 11KA4, ↑ 11OHT, ↑ 11KT, ↑ Preg-S, ↑ 17OHPreg-S, ↑ DHEA-S, ↑ Adiol-S | [26, 64–67, 70–72, 75] |
| Polycystic Ovary Syndrome | ↑ DHEA, ↑ DHEA-S, ↑ A4, ↑ T, ↑ 11OHA4, ↑ 11KA4, ↑ 11OHT, ↑ 11KT | [80–82, 88–90, 93–95, 138] |
| Adrenocortical Cancer | ↑ 11-Deoxycortisol, ↑ Preg-S, ↑ 17OHPreg-S | [104, 106–109] |
| Primary Aldosteronism |
↑ 18OH-Cortisol, ↑ 18oxo-Cortisol (KCNJ5) ↑ Aldosterone (ATPase) Other discriminating steroid between mutations in KCNJ5, ATPase and CACNA1D: 11-Deoxycorticosterone, Corticosterone, Cortisol, 21-Deoxycortisol |
[117–121, 129] |
| Cushing Syndrome | ↑ Cortisol, ↑11-Deoxycortisol, ↑ 21-Deoxycortisol, ↑ 11-Deoxycorticosterone, ↑ Corticosterone ↓ DHEA-S, ↓ DHEA, ↓ A4 (ACTH-independent) ↓ Aldosterone (ACTH-dependent, ectopic) |
[134, 136, 137] |
DHEA, Dehydroepiandrosterone; DHEA-S, DHEA sulfate; A4, Androstenedione; T, Testosterone; 11OHA4, 11β-Hydroxyandrostenedione; 11KA4, 11-Ketoandrostenedione; 11OHT, 11β-Hydroxytestosterone; 11KT, 11-Ketotestosterone; Preg-S, Pregnenolone sulfate; 17OHPreg-S, 17α-hydroxypregnenolone sulfate; Adiol-S, Androstenediol sulfate.
Underlined steroids are products of aberrant steroid pathways found in adrenal disease that are only minor products of normal adrenals.
Along with an elevation in serum 17OHP4, another central hallmark of 21OHD is androgen excess, which prompts the diagnosis at birth in females [28, 44]. Females with classic 21OHD are born with ambiguous genitalia [28], while adult women with nonclassic 21OHD might present with hirsutism, irregular menses, and infertility [44]. In both males and females, the excess adrenal androgen production can lead to premature pubarche, rapid somatic growth, advanced bone age, and subfertility [45, 46]. The excess 17OHP4 resulting from CYP21A2 deficiency is diverted through the pathways left accessible, to form potent androgens, such as T (Table 1). CYP17A1 mediates the conversion of 17OHPreg to DHEA (Δ5 pathway) and of 17OHP4 to A4 (Δ4 pathway). The catalytic efficiency of the human 17,20-lyase, however, is ~100 times greater for the Δ5 reaction, as compared with the Δ4 reaction [47], in part explaining the enormous 17OHP4 accumulation in 21OHD. In patients with 21OHD, significant A4 synthesis might still occur via the Δ4 pathway due to very high intra-adrenal 17OHP4. Curiously, the routinely measured androgens, DHEA, DHEA-S, A4 and T do not show a reliable correlation with the clinical evidence of androgen excess in 21OHD patients, particularly women and children [48, 49]. With that in mind, we hypothesized that the 21OHD adrenal must produce other unrecognized androgens to account for this paradox. Indeed, detailed characterization of the androgens and androgen precursors in classic 21OHD demonstrated that the four 11-oxygenated steroids, 11OHA4, 11KA4, 11OHT, and 11KT, along with A4 and T (Table 1) are significantly higher in both male and female patients with classic 21OHD compared to age-matched controls. The increased androgen release may reflect a disrupted adrenal zonation with intermingled expression of both HSD3B2 and CYB5A that was recently observed in a 21OHD adrenal [19, 50]. In women and children with classic 21OHD, 11KT is the dominant bioactive androgen in the circulation, while among men with well controlled disease, testis-derived T is the dominant circulating androgen [19, 50]. Of note, DHEA-S is paradoxically low in patients with 21OHD, including those in poor control despite chronic ACTH stimulation and elevation of other androgens [19, 51] (Table 1). In contrast, our studies indicated that patients with classic 21OHD had increased pregnenolone sulfate (Preg-S) and 17OH-Preg sulfate levels compared to sex- and age-matched controls [19, 50] (Table 1).
In summary, several advances have been made in the identification of novel steroids and steroid panels in classic and non-classic CAH. These include elevated circulating steroid pathway intermediates that may improve diagnosis and monitoring as well as novel bioactive androgens that appear to contribute significantly to the phenotypic properties seen in these diseases. Larger prospective studies are now underway to validate the utility of these markers and to optimize parameters for diagnosis, assessment of disease control, and treatment monitoring.
2.1.2. Premature Adrenarche
The developmental biology of the human adrenal cortex extends well into postnatal life and represents one of the most complex ontogeny schemes of human endocrine physiology. The fetal adrenal gland synthesizes abundant DHEA-S and little cortisol. The fetal zone regresses at birth, and the DHEA-S production in the neonatal adrenal abruptly stops. Adrenarche refers to the onset of adrenal production of DHEA and DHEA-S that reflects the expansion of the ZR that appears to start around 5–6 years (y) of age in both boys and girls [12, 52]. This slow rise in androgenic steroids and their precursors manifests clinically as pre-pubertal initiation of axillary hair, pubic hair, and acquisition of adult body odor. The adrenarche-associated increase in DHEA and DHEA-S has been attributed to the segregated expression of HSD3B2 in the ZF, and of CYB5A and SULT2A1 in the developing ZR (Fig. 1) [53–56]. While DHEA-S is an appropriate marker for the progression of adrenarche, it is not an active androgen, and it requires peripheral conversion to more potent androgens to mediate the phenotypic manifestations of adrenarche. Normal adrenarche is a gradual process that precedes and is independent of puberty. Premature adrenarche (PremA) is defined as an early rise in adrenal androgen production resulting in the appearance of pubic or axillary hair before age 8 y in girls and 9 y in boys. PremA is reported to occur in 1.5% of Caucasian and as high as 9.5% in African-American girls. A number of studies suggest that PremA could be a harbinger of adolescent and adult diseases, most notably increased insulin resistance and cardiovascular risk. Girls with PremA have a higher susceptibility for developing polycystic ovary syndrome (PCOS) later in life, which is present in 5–10% of women of reproductive age. In addition, obesity has been associated with increased risk of PremA, suggesting an association with the early onset in adrenal androgens seen in PremA.
The molecular origins for the vast majority of PremA cases remain unknown; however, rare genetic causes of PremA have been defined, providing insights into the role of certain steroidogenic pathways in adrenal androgen synthesis. One such cause of premature pubarche and/or hyperandrogenemia is apparent cortisone reductase deficiency due to decreased HSD11B1 (11β-hydroxysteroid dehydrogenase type 1) activity [57, 58]. Although genetic studies demonstrated a normal sequence of the HSD11B1 gene in most affected individuals, inactivating mutations have been found in the H6PDH (hexose-6-phosphate dehydrogenase) gene [59, 60]. Inactivating mutations in H6PDH disrupts HSD11B1 oxo-reductase activity resulting in decreased peripheral conversion of cortisone to cortisol. The impact of decreased cortisol regeneration is a mild rise in ACTH, and augmentation in adrenal steroid production that includes androgen androgens. Another rare genetic cause of PremA is mutations in 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase type 2 (PAPSS2) [61]. The sulfonation of steroids by SULT2A1 requires PAPS, a sulfate donor, and synthesis of PAPS requires the enzymes PAPSS1 or PAPSS2 [62, 63]. On examination of an 8-year-old girl with early pubic and axillary hair, Noordam et al concluded that the cause of hyperandrogenism was a set of compound heterozygous mutations in PAPSS2 [61]. As a result of impaired DHEA sulfonation, the unconjugated DHEA pool in this subject was available for conversion into bioactive androgens, such as T.
The age-related patterns in the production of other potent androgens and their precursors have been investigated in PremA with varying results. Historically, children with PremA have been shown to exhibit higher serum concentrations of androgen precursors, such as DHEA, DHEA-S and A4 (Table 1), as well as their urinary metabolites [64–70]. It should be noted that these steroids are poor androgen receptor agonists and hence would not be the direct androgen receptor activators that cause the clinical manifestations of PremA. Until now, T has been the measure of choice as a marker of androgen excess in pre-pubertal children. Unfortunately, the contribution of T to the progression of normal adrenarche and PremA has been controversial. Although many studies have shown that T levels do not rise during early adrenarche [71–73], several other analyses have suggested that T does increase and can therefore contribute to the progression of PremA [64, 67, 68, 72, 74–76]. We recently characterized alterations in the childhood circulating steroid biome, including adrenal-specific 11-oxyandrogens in girls with PremA and an age-matched reference population [26]. Our analysis indicated that PremA is characterized by broad changes in the steroid biome, including early elevations in circulating concentrations of adrenal-derived steroid sulfates, T, and all the 11-oxyandrogens (Table 1). Another highlight of this study was the observation that children have 3-fold higher circulating levels of 11KT than T. Given that 11KT has an androgenic potency approaching that of T and is the dominant circulating androgen in girls during adrenarche and PremA, we suggested that 11KT is an important mediator of the precocious androgenic manifestations of PremA [26].
These recent findings support the need to expand studies of 11-oxyandrogens in normal physiology and diseases of androgen excess. A specific role for this novel adrenal androgen pathway in both normal and PremA seems likely, and measurements of these steroids might help to differentiate precocious puberty from PremA. Whether or not an early elevation in adrenal production of these steroids is an indicator of a higher risk for adult-onset disease remains to be investigated.
2.1.3. Polycystic Ovary Syndrome (PCOS)
PCOS is a common ovarian disorder, affecting 5–10% of women of reproductive age. PCOS is a complex medical condition that manifests as oligomenorrhea, hyperandrogenism, with or without polycystic ovaries. The probable link between the development of PCOS and its phenotypic symptoms is thought to involve genetic and epigenetic mechanisms [77–79]. More importantly, PCOS patients are at an increased risk of developing metabolic anomalies, including obesity, insulin resistance, type 2 diabetes mellitus and cardiovascular disease.
Androgen excess is a hallmark of PCOS, but the exact origin of hyperandrogenemia remains in question [80]. While the ovary has traditionally been considered the primary source of androgens in PCOS women, elevated adrenal androgens, especially DHEA, DHEA-S and A4 (Table 1), have been documented in a subset of patients with PCOS [81–83]. Thus, along with the ovary, the adrenal gland has been acknowledged as a source of hyperandrogenism in PCOS for the past two decades [84]. Indeed, the few studies that examine ovarian and adrenal vein sampling in PCOS women have shown that the adrenal, ovary or both can show elevated T secretion compared to controls [14, 15, 17]. While some PCOS patients do not decrease androgen production after suppression of the pituitary-gonadal axis, a subset of other PCOS patients decrease circulating androgens following glucocorticoid suppression of the pituitary-adrenal axis. This also suggests that both the adrenal and the ovary can contribute to androgen excess in this disorder [85, 86]. Nonetheless, the mechanisms causing both ovarian and adrenal androgen excess in PCOS remain ambiguous. Some researchers have speculated a generalized adrenocortical hyper-reactivity to ACTH in patients with PCOS, after observing an exaggerated response of adrenal androgens and cortisol to cosyntropin stimulation in this population [87, 88].
Researchers have tried to identify biomarkers that could help pinpoint the glandular source of excess androgens in PCOS, and 11OHA4 has been considered a candidate biomarker for PCOS adrenal dysregulation. Studies from the late 1980s and early 1990s demonstrated that 11OHA4 is elevated in some [89–91] but not all [92, 93] patients with PCOS. A 2017 study comprising of 114 women with PCOS and 49 healthy controls quantified the conventional and 11-oxyandrogens in peripheral serum, as well as their metabolites in 24-h urine collections using LC-MS/MS and GC-MS, respectively. [94]. Along with serum 11OHA4, 11KA4, 11OHT, and 11KT (Table 1), the 11-oxyandrogen urine metabolite 11β-hydroxyandrosterone was significantly higher in patients with PCOS than in controls. Interestingly, the authors demonstrated that serum 11OHA4 and 11KA4, but not 11OHT or 11KT, correlated significantly, albeit weakly, with metabolic risk markers such as BMI, insulin, and HOMA-IR. A smaller study from Japan recently confirmed elevations in 11-oxyandrogens in some PCOS patients and suggested that BMI associated with elevated 11-oxyandrogens [95]. A follow-up study by the Arlt group defined an intra-adipose mechanism of androgen precursor activation via AKR1C3 that drives lipid accumulation and insulin resistance in PCOS [96]. Taken together, these studies suggest that there might be dysregulated synthesis of adrenal-derived 11-oxyandrogens and precursors in certain forms of PCOS and might also explain the clinical manifestations associated with androgen excess in women with normal serum T.
2.2. Adrenocortical Carcinoma
Adrenocortical Carcinoma (ACC) is a rare disease with an incidence of 1 per million per year and a dismal prognosis, with a 5-year survival rate of <30% [97]. Early detection of these tumors is imperative, as the primary curative approach is early surgical resection [98]. Once tumor cells extend beyond the adrenal capsule, cure rates drop drastically, and metastatic involvement of distant organs is not curable. Therefore, distinguishing ACC from benign adrenocortical adenomas (ACA) is important to provide appropriate adrenal clinical care. Both ACC and ACA originate from steroidogenic cells and can overproduce steroid hormones, leading to clinical syndromes, such as Cushing syndrome, virilization or primary aldosteronism. Paradoxically, overt hormone excess is found in only 60% of ACC and a small fraction of ACA [97]. Currently, the differential ACC vs ACA diagnosis is primarily based on imaging, which can be subjective and poses some risk due to radiation exposure. Growing evidence suggests that urine and/or serum-based steroid biomarker analysis might provide an additional diagnostic tool that is less expensive and virtually risk-free (Table 1).
Several steroid intermediates have long been a useful tool in the diagnosis of ACC. The first study of urinary steroid metabolites by Touchstone and colleagues (1954) demonstrated the exceptionally high levels of tetrahydro-11-deoxycortisol (THS), the major 11-deoxycortisol (11dF) metabolite, in the urine of a patient with metastatic adrenocortical carcinoma [99]. This observation was subsequently confirmed by other small studies [100–102]. Urinary Δ5 steroid metabolites including 5-pregnenediol (5-PD) and 5-pregnenetriol (5-PT)—degradation products of pregnenolone (Preg) and 17OHPreg are also elevated in ACC [100, 102–104]. One of the initial studies of serum steroids in ACC by Gröndal et al demonstrated elevated levels of Preg-S in all and of 11dF in 72% of the ACC patients (Table 1) [105]. More recently, a comprehensive study from the European Network for the Study of Adrenal Tumors (ENS@T, www.ensat.org) used GC/MS to compared the excretion of 32 urinary steroid metabolites from patients with ACA (n=102) or ACC (n=45) [106]. They observed that ACC patients often had increased urinary THS, and the steroid precursor metabolites, 5-PD and 5-PT. The ENS@T group then developed a sophisticated mathematical algorithm for predicting if an adrenal tumor is an ACC from urine steroid profiling [106]. Kerkhofs et al. and Hines et al later confirmed the results of the ENS@T group, thus proving that urine steroid profiling is a highly specific and sensitive method to diagnose ACC pre-operatively and distinguish ACC from ACA [107, 108].
The comprehensive LC-MS/MS analysis of serum steroids can also be used to identify ACC steroid biomarkers and allow rapid and frequent monitoring of disease progression and response to therapy. We recently evaluated a panel of 15 steroids by LC-MS/MS in random serum samples from patients with ACC and ACA along with age- and sex-matched controls. 11dF and 17OH-Preg sulfate were significantly augmented in patients with ACC as compared to those with ACA and controls (Table 1) [109]. Recent studies by Taylor et al and Schweitzer et al also demonstrated that elevated serum/plasma 11dF is a consistent biomarker for ACC [110, 111]. The serum and urinary steroid findings collectively suggest decreased ACC expression of HSD3B2 and/or CYP11B1 when compared to ACA [112]. The above steroid metabolomics studies, thus, indicate that increased concentrations of steroid synthetic pathway intermediates allow improved discrimination of ACC from ACA. The non-invasive nature of serum biomarker analyses might prove to be useful and practical in clinical care for patients with adrenal lesions.
2.3. Primary Aldosteronism
Primary aldosteronism (PA) comprises two main subtypes: unilateral aldosteronism, mainly caused by aldosterone-producing adenomas (APA) and bilateral adrenal hyperplasia (BHA). Histologic studies have shown that some APAs display a ZG-like histology, with small, compact cells, while other APAs are composed of large, lipid-rich cells, similar to those seen in ZF [113, 114]. The latter group of APAs appear to be the source of 18-hydroxycortisol (18OHF) and 18-oxocortisol (18oxoF), which are structurally related to both aldosterone and cortisol, and often referred to as “hybrid steroids” [115, 116] (Table 1). These two steroids are produced by CYP11B2 using cortisol as a substrate, although some 18OHF can be produced by CYP11B1 [117, 118]. In normal adrenal tissue, expression of CYP11B2 is limited to the ZG, while CYP17A1 and CYP11B1 are selectively expressed in the ZF and ZR, and therefore the production of 18OHF and 18oxoF in normal subjects is very low. Numerous studies have found higher 18OHF and 18oxoF in PA patients compared to patients with essential hypertension. More specifically, within the PA population, levels of these hybrid steroids were much higher in subjects with APAs compared to BHA [119–121]. A more recent study of 234 Japanese PA patients suggested that peripheral plasma 18oxoF and 18OHF can discriminate APAs from BHA with promising sensitivity and specificity (0.83/0.99 for 18oxoF and 0.62/0.96 for 18OHF, respectively) [122]. In contrast, a European study found significant overlap for both 18oxoF and 18OHF between APAs and BHA, with limited utility of these steroids to discriminate between the two subtypes [123]. In this same study, however, a panel incorporating 12 different steroids correctly classified 80% of PA patients as having APA or BHA. The reason for greater steroid overlap in the European study was made clear by comparing the analysis of steroids with the somatic mutations causing tumor aldosterone production.
Somatic mutations in genes encoding ions channels and pumps have been identified in APA, including: KCNJ5 (encoding the Kir3.4 (GIRK4) potassium channel), ATP1A1 (encoding a Na+/K+ ATPase alpha subunit), ATP2B3 (encoding a Ca2+ ATPase), and CACNA1D (encoding a voltage-dependent L-type calcium channel) that all lead to an increase in constitutive aldosterone production [124–129]. A recent European study demonstrated that specific somatic mutations in APAs define distinct steroid profiles in adrenal vein plasma, which might reflect differences in the underlying biology of these tumors [130]. The authors showed increased concentrations of the hybrid steroids 18OHF and 18oxoF in plasma samples from APA carrying a KCNJ5 mutation, owing to a predominantly ZF phenotype (increased expression of CYP11B1 and CYP17A1) as opposed to APAs with ATPase or CACNA1D mutations that display principally a ZG phenotype. Furthermore, a 7-steroid panel, including aldosterone, 18oxoF, 18OHF, 11-deoxycorticosterone, corticosterone, cortisol, and 21dF, correctly classified 92% of APAs according to genotype (Table 1). Follow-up studies, however, showed no significant differences in concentrations of peripheral venous adrenal steroids from PA patients according to histopathologic phenotypes (solitary functional adenoma, hyperplasia, and aldosterone-producing cell clusters) [131]. With the advent of the more sensitive and specific LC-MS/MS methodology for measuring serum steroids, it appears that peripheral and adrenal vein steroid biomarkers might prove to be a major factor in not just differentiating APA from BHA, but also in subtyping the various forms of APAs. Additional prospective outcome-based studies should be able to reveal whether this technique is a better alternative to AVS and can be translated to routine clinical practice.
2.4. Cushing syndrome
Cushing syndrome (CS) ensues from chronic exposure to excess glucocorticoids, either derived from exogenous pharmacological doses of corticosteroids or from an endogenous adrenal overproduction of cortisol [132]. Current guidelines suggest that initial screening for CS should include measurements of 24 h urinary, evening salivary free cortisol or dexamethasone suppression testing (DST) [132, 133]. If results are convincingly positive and corroborated with clinical history and physical exam findings, further dynamic testing and imaging procedures are aimed at differentiating the cause of excess cortisol. CS can be ACTH-independent, as a result of excess cortisol production from an adrenal adenoma or from bilateral micro- or macronodular adrenal hyperplasia. Alternatively, ACTH-dependent CS can result from Cushing disease, meaning an ACTH-secreting pituitary adenoma, from ectopic ACTH secretion, or rarely, from ectopic CRH production [132, 133].
Several studies, using plasma or urinary multisteroid profiles, have suggested the release of precursor steroids that could be useful for the differential diagnosis of CS [101, 134–137] (Table 1). The first report on the application of LC-MS/MS–based multisteroid profiling in subclinical CS, published in 2015, showed that suppressed adrenal androgens had good accuracy in predicting subclinical hypercortisolism [138]. This finding was followed by two reports that were limited to a small series of patients with ACC and CS [108, 110]. The most recent large cohort report implemented a panel of 15 steroids from LC-MS/MS analysis to characterize distinct plasma steroid profiles that might assist the differential diagnosis of pituitary, ectopic, and adrenal subtypes of CS [139]. The largest increases in plasma steroids among patients with CS were observed for 11dF, 21dF, 11-deoxycorticosterone, corticosterone, and cortisol when compared to patients in whom disease was excluded (Table 1). Patients with ectopic ACTH production showed the most prominent increases, but there was considerable variation for other steroids according to subtype. Patients with ACTH-independent CS had the lowest concentrations of adrenal androgens, whereas those with ectopic and pituitary ACTH-dependent CS showed the lowest concentrations of aldosterone (Table 1). Although this study is a strong step towards offering a supplementary single-test alternative for subtyping CS, steroid profiling as a method for characterizing the underlying cause of CS remains still in its infancy. More confirmatory analyses are required for validation of evidence-based steroid panels for diagnosis and subtype classification of CS.
3. Conclusion
Adrenal biosynthesis of mineralocorticoids, glucocorticoids, as well as both weak and potent androgens requires the tight regulation of zone-specific steroidogenic enzyme expression via physiologic trophic hormones. Although steroid biosynthesis has been studied for the past six decades, the significance of minor and alternate pathways continues to emerge. Owing to technical improvements and expanded use of steroid mass spectrometry, our understanding of complex adrenal steroidogenic pathways in physiology and disease has progressed considerably over the last twenty years. It is likely that utilization of steroid ‘metabolomics’ in concert with advances in our understanding of the genetics of adrenal diseases will aid in the diagnostic workup and monitoring of patients that exhibit aberrant production of adrenal steroid hormones.
Highlights.
The adult adrenal cortex produces three distinct classes of steroid hormones, including mineralocorticoids, glucocorticoids and androgens owing to the zone-specific expression of key steroidogenic enzymes.
21-hydroxylase deficiency, premature adrenarche and some cases of polycystic ovary syndrome are associated with excess 11-oxygenated adrenal androgen production.
Several steroid pathway intermediates have been defined in urine and blood that are set to improve the diagnosis and monitoring of patients with Cushing syndrome and adrenocortical cancer.
Hybrid steroids that rely on the aberrant expression of adrenal glomerulosa and fasciculata steroid metabolizing enzymes have the potential of simplifying the current workup of primary aldosteronism and Cushing syndrome patients.
Funding:
This work was supported by grants from the National Institutes of Health (NIH) R01DK069950 and R01DK43140 to W.E.R., R01GM086596 to R.J.A., 1K08DK109116 to A.F.T.
Steroid Abbreviations:
- DHEA
Dehydroepiandrosterone
- DHEA-S
DHEA sulfate
- A4
Androstenedione
- T
Testosterone
- 11OHA4
11β-Hydroxyandrostenedione
- 11KA4
11-Ketoandrostenedione
- 11OHT
11β-Hydroxytestosterone
- 11KT
11-Ketotestosterone
- Preg-S
Pregnenolone sulfate
- 17OHPreg-S
17α-hydroxypregnenolone sulfate
- Adiol-S
Androstenediol sulfate
- 11dF
11-Deoxycortisol
- 21dF
21-Deoxycortisol
- 11-DOC
11-Deoxycorticosterone
- Preg
Pregnenolone
- 17OH-Preg
17α-hydroxypregnenolone
- 17OHP4
17α-Hydroxyprogesterone
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- [1].Arnold J, Ein beitrag zu der feiner struktur und dem chemismus der nebennieren., Virchows Arch., 35 (1866) 64–107. [Google Scholar]
- [2].Vinson GP, Adrenocortical zonation and ACTH, Microsc Res Tech, 61 (2003) 227–239. [DOI] [PubMed] [Google Scholar]
- [3].Vinson GP, Ho MM, Origins of zonation: the adrenocortical model of tissue development and differentiation, Clin Exp Pharmacol Physiol Suppl, 25 (1998) S91–96. [DOI] [PubMed] [Google Scholar]
- [4].Hornsby PJ, Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline, Ann N Y Acad Sci, 774 (1995) 29–46. [DOI] [PubMed] [Google Scholar]
- [5].Parker LN, Odell WD, Control of adrenal androgen secretion, Endocr Rev, 1 (1980) 392–410. [DOI] [PubMed] [Google Scholar]
- [6].Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE, Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation, J Clin Endocrinol Metab, 98 (2013) 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ, Differentially expressed genes in zona reticularis cells of the human adrenal cortex, Mol Cell Endocrinol, 173 (2001) 127–134. [DOI] [PubMed] [Google Scholar]
- [8].Nogueira EF, Bollag WB, Rainey WE, Angiotensin II regulation of adrenocortical gene transcription, Mol Cell Endocrinol, 302 (2009) 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Waterman MR, Simpson ER, Regulation of the biosynthesis of cytochromes P-450 involved in steroid hormone synthesis, Mol Cell Endocrinol, 39 (1985) 81–89. [DOI] [PubMed] [Google Scholar]
- [10].Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE, Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis, J Clin Endocrinol Metab, 99 (2014) E518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kawamoto T, Mitsuuchi Y, Toda K, Yokoyama Y, Miyahara K, Miura S, Ohnishi T, Ichikawa Y, Nakao K, Imura H, et al. , Role of steroid 11 beta-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans, Proc Natl Acad Sci U S A, 89 (1992) 1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE, Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies, Clin Endocrinol (Oxf), 53 (2000) 739–747. [DOI] [PubMed] [Google Scholar]
- [13].Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR, Adrenarche is associated with decreased 3 beta-hydroxysteroid dehydrogenase expression in the adrenal reticularis, Endocr Res, 22 (1996) 723–728. [DOI] [PubMed] [Google Scholar]
- [14].Stahl NL, Teeslink CR, Greenblatt RB, Ovarian, adrenal, and peripheral testosterone levels in the polycystic ovary syndrome, Am J Obstet Gynecol, 117 (1973) 194–200. [DOI] [PubMed] [Google Scholar]
- [15].Stahl NL, Teeslink CR, Beauchamps G, Greenblatt RB, Serum testosterone levels in hirsute women: a comparison of adrenal, ovarian and peripheral vein values, Obstet Gynecol, 41 (1973) 650–654. [PubMed] [Google Scholar]
- [16].Tzingounis VA, Aksu MF, Natrajan PK, Greenblatt RB, The significance of adrenal and ovarian catheterization in patients with polycystic ovary syndrome, Int J Gynaecol Obstet, 17 (1978) 78–82. [DOI] [PubMed] [Google Scholar]
- [17].Parker CR Jr., Bruneteau DW, Greenblatt RB, Mahesh VB, Peripheral, ovarian, and adrenal vein steroids in hirsute women: acute effects of human chorionic gonadotropin and adrenocorticotrophic hormone, Fertil Steril, 26 (1975) 877–888. [PubMed] [Google Scholar]
- [18].Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE, Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis, J Clin Endocrinol Metab, 94 (2009) 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ, Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency, Eur J Endocrinol, 174 (2016) 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, Park HW, Structural insights into aldosterone synthase substrate specificity and targeted inhibition, Mol Endocrinol, 27 (2013) 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Swart AC, Schloms L, Storbeck KH, Bloem LM, Toit T, Quanson JL, Rainey WE, Swart P, 11beta-hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5alpha-reductase yielding 11beta-hydroxy-5alpha-androstanedione, J Steroid Biochem Mol Biol, 138 (2013) 132–142. [DOI] [PubMed] [Google Scholar]
- [22].Swart AC, Storbeck KH, 11beta-Hydroxyandrostenedione: Downstream metabolism by 11betaHSD, 17betaHSD and SRD5A produces novel substrates in familiar pathways, Mol Cell Endocrinol, 408 (2015) 114–123. [DOI] [PubMed] [Google Scholar]
- [23].Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC, 11beta-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer?, Mol Cell Endocrinol, 377 (2013) 135–146. [DOI] [PubMed] [Google Scholar]
- [24].Barnard M, Quanson JL, Mostaghel E, Pretorius E, Snoep JL, Storbeck KH, 11-Oxygenated androgen precursors are the preferred substrates for aldo-keto reductase 1C3 (AKR1C3): Implications for castration resistant prostate cancer, J Steroid Biochem Mol Biol, 183 (2018) 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Campana C, Rege J, Turcu AF, Pezzi V, Gomez-Sanchez CE, Robins DM, Rainey WE, Development of a novel cell based androgen screening model, J Steroid Biochem Mol Biol, 156 (2016) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rege J, Turcu AF, Kasa-Vubu JZ, Lerario AM, Auchus GC, Auchus RJ, Smith JM, White PC, Rainey WE, 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche, J Clin Endocrinol Metab, 103 (2018) 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Turcu AF, Auchus RJ, Adrenal steroidogenesis and congenital adrenal hyperplasia, Endocrinol Metab Clin North Am, 44 (2015) 275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Speiser PW, White PC, Congenital adrenal hyperplasia, N Engl J Med, 349 (2003) 776–788. [DOI] [PubMed] [Google Scholar]
- [29].Auchus RJ, The classic and nonclassic concenital adrenal hyperplasias, Endocr Pract, 21 (2015) 383–389. [DOI] [PubMed] [Google Scholar]
- [30].Therrell BL, Newborn screening for congenital adrenal hyperplasia, Endocrinol Metab Clin North Am, 30 (2001) 15–30. [DOI] [PubMed] [Google Scholar]
- [31].Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI, High frequency of nonclassical steroid 21-hydroxylase deficiency, Am J Hum Genet, 37 (1985) 650–667. [PMC free article] [PubMed] [Google Scholar]
- [32].Hannah-Shmouni F, Morissette R, Sinaii N, Elman M, Prezant TR, Chen W, Pulver A, Merke DP, Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians, Genet Med, 19 (2017) 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Auchus RJ, Arlt W, Approach to the patient: the adult with congenital adrenal hyperplasia, J Clin Endocrinol Metab, 98 (2013) 2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Coulm B, Coste J, Tardy V, Ecosse E, Roussey M, Morel Y, Carel JC, Group DS, Efficiency of neonatal screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency in children born in mainland France between 1996 and 2003, Arch Pediatr Adolesc Med, 166 (2012) 113–120. [DOI] [PubMed] [Google Scholar]
- [35].Cavarzere P, Samara-Boustani D, Flechtner I, Dechaux M, Elie C, Tardy V, Morel Y, Polak M, Transient hyper-17-hydroxyprogesteronemia: a clinical subgroup of patients diagnosed at neonatal screening for congenital adrenal hyperplasia, Eur J Endocrinol, 161 (2009) 285–292. [DOI] [PubMed] [Google Scholar]
- [36].Sarafoglou K, Banks K, Kyllo J, Pittock S, Thomas W, Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota, JAMA, 307 (2012) 2371–2374. [DOI] [PubMed] [Google Scholar]
- [37].Varness TS, Allen DB, Hoffman GL, Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls, J Pediatr, 147 (2005) 493–498. [DOI] [PubMed] [Google Scholar]
- [38].Gatelais F, Berthelot J, Beringue F, Descamps P, Bonneau D, Limal JM, Coutant R, Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: implication for neonatal screening of congenital adrenal hyperplasia, Pediatr Res, 56 (2004) 701–705. [DOI] [PubMed] [Google Scholar]
- [39].Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC, Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline, J Clin Endocrinol Metab, 103 (2018) 4043–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Milewicz A, Vecsei P, Korth-Schutz S, Haack D, Rosler A, Lichtwald K, Lewicka S, von Mittelstaedt G, Development of plasma 21-deoxycortisol radioimmunoassay and application to the diagnosis of patients with 21-hydroxylase deficiency, J Steroid Biochem, 21 (1984) 185–191. [DOI] [PubMed] [Google Scholar]
- [41].Costa-Barbosa FA, Tonetto-Fernandes VF, Carvalho VM, Nakamura OH, Moura V, Bachega TA, Vieira JG, Kater CE, Superior discriminating value of ACTH-stimulated serum 21-deoxycortisol in identifying heterozygote carriers for 21-hydroxylase deficiency, Clin Endocrinol (Oxf), 73 (2010) 700–706. [DOI] [PubMed] [Google Scholar]
- [42].Costa-Barbosa FA, Carvalho VM, Nakamura OH, Bachega TA, Vieira JG, Kater CE, Zona fasciculata 21-hydroxysteroids and precursor-to-product ratios in 21-hydroxylase deficiency: further characterization of classic and non-classic patients and heterozygote carriers, J Endocrinol Invest, 34 (2011) 587–592. [DOI] [PubMed] [Google Scholar]
- [43].Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ, Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency, J Clin Endocrinol Metab, 100 (2015) 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Azziz R, Dewailly D, Owerbach D, Clinical review 56: Nonclassic adrenal hyperplasia: current concepts, J Clin Endocrinol Metab, 78 (1994) 810–815. [DOI] [PubMed] [Google Scholar]
- [45].Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ, Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency, Obstet Gynecol Surv, 58 (2003) 275–284. [DOI] [PubMed] [Google Scholar]
- [46].Cabrera MS, Vogiatzi MG, New MI, Long term outcome in adult males with classic congenital adrenal hyperplasia, J Clin Endocrinol Metab, 86 (2001) 3070–3078. [DOI] [PubMed] [Google Scholar]
- [47].Auchus RJ, Lee TC, Miller WL, Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer, J Biol Chem, 273 (1998) 3158–3165. [DOI] [PubMed] [Google Scholar]
- [48].Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, Lesser M, New MI, White PC, Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency, J Clin Invest, 90 (1992) 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP, Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany, J Clin Endocrinol Metab, 85 (2000) 1059–1065. [DOI] [PubMed] [Google Scholar]
- [50].Turcu AF, Mallappa A, Elman MS, Avila NA, Marko J, Rao H, Tsodikov A, Auchus RJ, Merke DP, 11-Oxygenated Androgens Are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency, J Clin Endocrinol Metab, 102 (2017) 2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rezvani I, Garibaldi LR, Digeorge AM, Artman HG, Disproportionate suppression of dehydroepiandrosterone sulfate (DHEAS) in treated patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency, Pediatr Res, 17 (1983) 131–134. [DOI] [PubMed] [Google Scholar]
- [52].Dhom G, The prepuberal and puberal growth of the adrenal (adrenarche), Beitr Pathol, 150 (1973) 357–377. [DOI] [PubMed] [Google Scholar]
- [53].Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE, Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis, J Clin Endocrinol Metab, 83 (1998) 3695–3701. [DOI] [PubMed] [Google Scholar]
- [54].Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H, Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence, J Endocrinol, 203 (2009) 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rege J, Karashima S, Lerario AM, Smith JM, Auchus RJ, Kasa-Vubu JZ, Sasano H, Nakamura Y, White PC, Rainey WE, Age-dependent Increases in Adrenal Cytochrome b5 and Serum 5-Androstenediol-3-sulfate, J Clin Endocrinol Metab, 101 (2016) 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A, Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche, Clin Endocrinol (Oxf), 82 (2015) 712–718. [DOI] [PubMed] [Google Scholar]
- [57].Jamieson A, Wallace AM, Andrew R, Nunez BS, Walker BR, Fraser R, White PC, Connell JM, Apparent cortisone reductase deficiency: a functional defect in 11beta-hydroxysteroid dehydrogenase type 1, J Clin Endocrinol Metab, 84 (1999) 3570–3574. [DOI] [PubMed] [Google Scholar]
- [58].Malunowicz EM, Romer TE, Urban M, Bossowski A, 11beta-hydroxysteroid dehydrogenase type 1 deficiency (‘apparent cortisone reductase deficiency’) in a 6-year-old boy, Horm Res, 59 (2003) 205–210. [DOI] [PubMed] [Google Scholar]
- [59].Lavery GG, Idkowiak J, Sherlock M, Bujalska I, Ride JP, Saqib K, Hartmann MF, Hughes B, Wudy SA, De Schepper J, Arlt W, Krone N, Shackleton CH, Walker EA, Stewart PM, Novel H6PDH mutations in two girls with premature adrenarche: ‘apparent’ and ‘true’ CRD can be differentiated by urinary steroid profiling, Eur J Endocrinol, 168 (2013) K19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lavery GG, Walker EA, Tiganescu A, Ride JP, Shackleton CH, Tomlinson JW, Connell JM, Ray DW, Biason-Lauber A, Malunowicz EM, Arlt W, Stewart PM, Steroid biomarkers and genetic studies reveal inactivating mutations in hexose-6-phosphate dehydrogenase in patients with cortisone reductase deficiency, J Clin Endocrinol Metab, 93 (2008) 3827–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, Smeitink JA, Smeets R, Sweep FC, Claahsen-van der Grinten HL, Arlt W, Inactivating PAPSS2 mutations in a patient with premature pubarche, N Engl J Med, 360 (2009) 2310–2318. [DOI] [PubMed] [Google Scholar]
- [62].Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB, Sulfation and sulfotransferases 1: Sulfotransferase molecular biology: cDNAs and genes, FASEB J, 11 (1997) 3–14. [PubMed] [Google Scholar]
- [63].Strott CA, Sulfonation and molecular action, Endocr Rev, 23 (2002) 703–732. [DOI] [PubMed] [Google Scholar]
- [64].Doberne Y, Levine LS, New MI, Elevated urinary testosterone and androstanediol in precocious adrenarche, Pediatr Res, 9 (1975) 794–797. [DOI] [PubMed] [Google Scholar]
- [65].Ibanez L, Dimartino-Nardi J, Potau N, Saenger P, Premature adrenarche--normal variant or forerunner of adult disease?, Endocr Rev, 21 (2000) 671–696. [DOI] [PubMed] [Google Scholar]
- [66].Korth-Schutz S, Levine LS, New MI, Dehydroepiandrosterone sulfate (DS) levels, a rapid test for abnormal adrenal androgen secretion, J Clin Endocrinol Metab, 42 (1976) 1005–1013. [DOI] [PubMed] [Google Scholar]
- [67].Korth-Schutz S, Levine LS, New MI, Evidence for the adrenal source of androgens in precocious adrenarche, Acta Endocrinol (Copenh), 82 (1976) 342–352. [DOI] [PubMed] [Google Scholar]
- [68].Likitmaskul S, Cowell CT, Donaghue K, Kreutzmann DJ, Howard NJ, Blades B, Silink M, ‘Exaggerated adrenarche’ in children presenting with premature adrenarche, Clin Endocrinol (Oxf), 42 (1995) 265–272. [DOI] [PubMed] [Google Scholar]
- [69].Rosenfield RL, Lucky AW, Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess, Endocrinol Metab Clin North Am, 22 (1993) 507–532. [PubMed] [Google Scholar]
- [70].Rosenfield RL, Rich BH, Lucky AW, Adrenarche as a cause of benign pseudopuberty in boys, J Pediatr, 101 (1982) 1005–1009. [DOI] [PubMed] [Google Scholar]
- [71].Ducharme JR, Forest MG, De Peretti E, Sempe M, Collu R, Bertrand J, Plasma adrenal and gonadal sex steroids in human pubertal development, J Clin Endocrinol Metab, 42 (1976) 468–476. [DOI] [PubMed] [Google Scholar]
- [72].Korth-Schutz S, Levine LS, New MI, Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche, J Clin Endocrinol Metab, 42 (1976) 117–124. [DOI] [PubMed] [Google Scholar]
- [73].Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM, A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data, J Clin Endocrinol Metab, 95 (2010) 2399–2409. [DOI] [PubMed] [Google Scholar]
- [74].Voutilainen R, Perheentupa J, Apter D, Benign premature adrenarche: clinical features and serum steroid levels, Acta Paediatr Scand, 72 (1983) 707–711. [DOI] [PubMed] [Google Scholar]
- [75].Dorn LD, Rose SR, Rotenstein D, Susman EJ, Huang B, Loucks TL, Berga SL, Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche, J Pediatr Endocrinol Metab, 21 (2008) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Liimatta J, Laakso S, Utriainen P, Voutilainen R, Palvimo JJ, Jaaskelainen T, Jaaskelainen J, Serum androgen bioactivity is low in children with premature adrenarche, Pediatr Res, 75 (2014) 645–650. [DOI] [PubMed] [Google Scholar]
- [77].Dunaif A, Perspectives in Polycystic Ovary Syndrome: From Hair to Eternity, J Clin Endocrinol Metab, 101 (2016) 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF 3rd, Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype, Proc Natl Acad Sci U S A, 111 (2014) E1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pan JX, Tan YJ, Wang FF, Hou NN, Xiang YQ, Zhang JY, Liu Y, Qu F, Meng Q, Xu J, Sheng JZ, Huang HF, Aberrant expression and DNA methylation of lipid metabolism genes in PCOS: a new insight into its pathogenesis, Clin Epigenetics, 10 (2018) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].E.A.-S.P.c.w.g. Rotterdam, Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS), Hum Reprod, 19 (2004) 41–47. [DOI] [PubMed] [Google Scholar]
- [81].Hoffman DI, Klove K, Lobo RA, The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women, Fertil Steril, 42 (1984) 76–81. [DOI] [PubMed] [Google Scholar]
- [82].Azziz R, Black V, Hines GA, Fox LM, Boots LR, Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis, J Clin Endocrinol Metab, 83 (1998) 2317–2323. [DOI] [PubMed] [Google Scholar]
- [83].O’Reilly MW, Taylor AE, Crabtree NJ, Hughes BA, Capper F, Crowley RK, Stewart PM, Tomlinson JW, Arlt W, Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione, J Clin Endocrinol Metab, 99 (2014) 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Turcu AF, Nanba AT, Auchus RJ, The Rise, Fall, and Resurrection of 11-Oxygenated Androgens in Human Physiology and Disease, Horm Res Paediatr, 89 (2018) 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Escobar-Morreale H, Pazos F, Potau N, Garcia-Robles R, Sancho JM, Varela C, Ovarian suppression with triptorelin and adrenal stimulation with adrenocorticotropin in functional hyperadrogenism: role of adrenal and ovarian cytochrome P450c17 alpha, Fertil Steril, 62 (1994) 521–530. [DOI] [PubMed] [Google Scholar]
- [86].Carmina E, Gonzalez F, Chang L, Lobo RA, Reassessment of adrenal androgen secretion in women with polycystic ovary syndrome, Obstet Gynecol, 85 (1995) 971–976. [DOI] [PubMed] [Google Scholar]
- [87].Lucky AW, Rosenfield RL, McGuire J, Rudy S, Helke J, Adrenal androgen hyperresponsiveness to adrenocorticotropin in women with acne and/or hirsutism: adrenal enzyme defects and exaggerated adrenarche, J Clin Endocrinol Metab, 62 (1986) 840–848. [DOI] [PubMed] [Google Scholar]
- [88].Azziz R, Boots LR, Parker CR Jr., Bradley E Jr., Zacur HA, 11 beta-hydroxylase deficiency in hyperandrogenism, Fertil Steril, 55 (1991) 733–741. [PubMed] [Google Scholar]
- [89].Carmina E, Stanczyk FZ, Chang L, Miles RA, Lobo RA, The ratio of androstenedione:11 beta-hydroxyandrostenedione is an important marker of adrenal androgen excess in women, Fertil Steril, 58 (1992) 148–152. [DOI] [PubMed] [Google Scholar]
- [90].Stanczyk FZ, Chang L, Carmina E, Putz Z, Lobo RA, Is 11 beta-hydroxyandrostenedione a better marker of adrenal androgen excess than dehydroepiandrosterone sulfate?, Am J Obstet Gynecol, 165 (1991) 1837–1842. [DOI] [PubMed] [Google Scholar]
- [91].Owen EJ, Holownia P, Conway GS, Jacobs HS, Honour JW, 11 beta-hydroxyandrostenedione in plasma, follicular fluid, and granulosa cells of women with normal and polycystic ovaries, Fertil Steril, 58 (1992) 713–718. [PubMed] [Google Scholar]
- [92].Hudson RW, Lochnan HA, Danby FW, Margesson LJ, Strang BK, Kimmett SM, 11 beta-hydroxyandrostenedione: a marker of adrenal function in hirsutism, Fertil Steril, 54 (1990) 1065–1071. [PubMed] [Google Scholar]
- [93].Polson DW, Reed MJ, Franks S, Scanlon MJ, James VH, Serum 11 beta-hydroxyandrostenedione as an indicator of the source of excess androgen production in women with polycystic ovaries, J Clin Endocrinol Metab, 66 (1988) 946–950. [DOI] [PubMed] [Google Scholar]
- [94].O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W, 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome, J Clin Endocrinol Metab, 102 (2017) 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yoshida T, Matsuzaki T, Miyado M, Saito K, Iwasa T, Matsubara Y, Ogata T, Irahara M, Fukami M, 11-oxygenated C19 steroids as circulating androgens in women with polycystic ovary syndrome, Endocr J, 65 (2018) 979–990. [DOI] [PubMed] [Google Scholar]
- [96].O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W, AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome, J Clin Endocrinol Metab, 102 (2017) 3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD, Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma, J Clin Endocrinol Metab, 99 (2014) 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD, Adrenocortical carcinoma, Endocr Rev, 35 (2014) 282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Touchstone JC, Richardson EM, Bulaschenko H, Landolt I, Dohan FC, Isolation of pregnane-3-alpha, 17-alpha, 21-triol-20-one (tetrahydro compound S) from the urine of a woman with metastatic adrenocortical carcinoma, J Clin Endocrinol Metab, 14 (1954) 676–678. [DOI] [PubMed] [Google Scholar]
- [100].Grondal S, Eriksson B, Hagenas L, Werner S, Curstedt T, Steroid profile in urine: a useful tool in the diagnosis and follow up of adrenocortical carcinoma, Acta Endocrinol (Copenh), 122 (1990) 656–663. [DOI] [PubMed] [Google Scholar]
- [101].Kikuchi E, Yanaihara H, Nakashima J, Homma K, Ohigashi T, Asakura H, Tachibana M, Shibata H, Saruta T, Murai M, Urinary steroid profile in adrenocortical tumors, Biomed Pharmacother, 54 Suppl 1 (2000) 194s–197s. [DOI] [PubMed] [Google Scholar]
- [102].Minowada S, Kinoshita K, Hara M, Isurugi K, Uchikawa T, Niijima T, Measurement of urinary steroid profile in patients with adrenal tumor as a screening method for carcinoma, Endocrinol Jpn, 32 (1985) 29–37. [DOI] [PubMed] [Google Scholar]
- [103].Tiu SC, Chan AO, Taylor NF, Lee CY, Loung PY, Choi CH, Shek CC, Use of urinary steroid profiling for diagnosing and monitoring adrenocortical tumours, Hong Kong Med J, 15 (2009) 463–470. [PubMed] [Google Scholar]
- [104].Malunowicz EM, Ginalska-Malinowska M, Romer TE, Ruszczynska-Wolska A, Dura M, Heterogeneity of urinary steroid profiles in children with adrenocortical tumors, Horm Res, 44 (1995) 182–188. [DOI] [PubMed] [Google Scholar]
- [105].Grondal S, Curstedt T, Steroid profile in serum: increased levels of sulphated pregnenolone and pregn-5-ene-3 beta,20 alpha-diol in patients with adrenocortical carcinoma, Acta Endocrinol (Copenh), 124 (1991) 381–385. [DOI] [PubMed] [Google Scholar]
- [106].Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM, Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors, J Clin Endocrinol Metab, 96 (2011) 3775–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kerkhofs TM, Kerstens MN, Kema IP, Willems TP, Haak HR, Diagnostic Value of Urinary Steroid Profiling in the Evaluation of Adrenal Tumors, Horm Cancer, 6 (2015) 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hines JM, Bancos I, Bancos C, Singh RD, Avula AV, Young WF, Grebe SK, Singh RJ, High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders, Clin Chem, 63 (2017) 1824–1835. [DOI] [PubMed] [Google Scholar]
- [109].Rege J, Wilson T, Kuick R, Rainey W, Else T, Steroid Panels As a Tool for the Differential Diagnosis of Adrenocortical Carcinoma Vs. Benign Adrenal Adenomas, The 99th Meeting of the Endocrine Society Boston, MA., 2017. [Google Scholar]
- [110].Taylor DR, Ghataore L, Couchman L, Vincent RP, Whitelaw B, Lewis D, Diaz-Cano S, Galata G, Schulte KM, Aylwin S, Taylor NF, A 13-Steroid Serum Panel Based on LC-MS/MS: Use in Detection of Adrenocortical Carcinoma, Clin Chem, 63 (2017) 1836–1846. [DOI] [PubMed] [Google Scholar]
- [111].Schweitzer S, Kunz M, Kurlbaum M, Vey J, Kendl S, Deutschbein T, Hahner S, Fassnacht M, Dandekar T, Kroiss M, Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma, Eur J Endocrinol, (2018). [DOI] [PubMed] [Google Scholar]
- [112].Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G, Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling, Clin Cancer Res, 15 (2009) 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA, Mulatero P, Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas, Mol Cell Endocrinol, 411 (2015) 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T, Clinical and Steroidogenic Characteristics of Aldosterone-Producing Adenomas With ATPase or CACNA1D Gene Mutations, J Clin Endocrinol Metab, 101 (2016) 494–503. [DOI] [PubMed] [Google Scholar]
- [115].Ulick S, Blumenfeld JD, Atlas SA, Wang JZ, Vaughan ED Jr., The unique steroidogenesis of the aldosteronoma in the differential diagnosis of primary aldosteronism, J Clin Endocrinol Metab, 76 (1993) 873–878. [DOI] [PubMed] [Google Scholar]
- [116].Lenders JWM, Williams TA, Reincke M, Gomez-Sanchez CE, DIAGNOSIS OF ENDOCRINE DISEASE: 18-Oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids?, Eur J Endocrinol, 178 (2018) R1–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mulatero P, Curnow KM, Aupetit-Faisant B, Foekling M, Gomez-Sanchez C, Veglio F, Jeunemaitre X, Corvol P, Pascoe L, Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol, J Clin Endocrinol Metab, 83 (1998) 3996–4001. [DOI] [PubMed] [Google Scholar]
- [118].Freel EM, Shakerdi LA, Friel EC, Wallace AM, Davies E, Fraser R, Connell JM, Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects, J Clin Endocrinol Metab, 89 (2004) 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, Veglio F, 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes, J Clin Endocrinol Metab, 97 (2012) 881–889. [DOI] [PubMed] [Google Scholar]
- [120].Stowasser M, Bachmann AW, Tunny TJ, Gordon RD, Production of 18-oxo-cortisol in subtypes of primary aldosteronism, Clin Exp Pharmacol Physiol, 23 (1996) 591–593. [DOI] [PubMed] [Google Scholar]
- [121].Mosso L, Gomez-Sanchez CE, Foecking MF, Fardella C, Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives, Hypertension, 38 (2001) 688–691. [DOI] [PubMed] [Google Scholar]
- [122].Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y, Ito S, Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism, Hypertension, 65 (2015) 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, Willenberg HS, Beuschlein F, Deinum J, Lenders JW, Reincke M, Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism, Clin Chem, 62 (2016) 514–524. [DOI] [PubMed] [Google Scholar]
- [124].Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP, K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension, Science, 331 (2011) 768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Okada S, Rokutanda N, Takata D, Koibuchi Y, Horiguchi J, Oyama T, Takeyoshi I, Mori M, Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas, J Clin Endocrinol Metab, 97 (2012) 1311–1319. [DOI] [PubMed] [Google Scholar]
- [126].Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M, Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension, Nat Genet, 45 (2013) 440–444, 444e441–442. [DOI] [PubMed] [Google Scholar]
- [127].Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, Lucatello B, Ronconi V, Fallo F, Bernini G, Maccario M, Giacchetti G, Veglio F, Warth R, Vilsen B, Mulatero P, Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas, Hypertension, 63 (2014) 188–195. [DOI] [PubMed] [Google Scholar]
- [128].Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Kusters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ, Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension, Nat Genet, 45 (2013) 1055–1060. [DOI] [PubMed] [Google Scholar]
- [129].Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP, Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism, Nat Genet, 45 (2013) 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M, Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas, Hypertension, 67 (2016) 139–145. [DOI] [PubMed] [Google Scholar]
- [131].Meyer LS, Wang X, Susnik E, Burrello J, Burrello A, Castellano I, Eisenhofer G, Fallo F, Kline GA, Knosel T, Kocjan T, Lenders JWM, Mulatero P, Naruse M, Nishikawa T, Peitzsch M, Rump LC, Beuschlein F, Hahner S, Gomez-Sanchez CE, Reincke M, Williams TA, Immunohistopathology and Steroid Profiles Associated With Biochemical Outcomes After Adrenalectomy for Unilateral Primary Aldosteronism, Hypertension, 72 (2018) 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lacroix A, Feelders RA, Stratakis CA, Nieman LK, Cushing’s syndrome, Lancet, 386 (2015) 913–927. [DOI] [PubMed] [Google Scholar]
- [133].Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM, The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline, J Clin Endocrinol Metab, 93 (2008) 1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Martin MM, Hamman BL, Patterns of urinary excretion of steroids in Cushing’s syndrome, J Clin Endocrinol Metab, 26 (1966) 257–267. [DOI] [PubMed] [Google Scholar]
- [135].Schoneshofer M, Weber B, Oelkers W, Nahoul K, Mantero F, Urinary excretion rates of 15 free steroids: potential utility in differential diagnosis of Cushing’s syndrome, Clin Chem, 32 (1986) 93–96. [PubMed] [Google Scholar]
- [136].Ueshiba H, Segawa M, Hayashi T, Miyachi Y, Irie M, Serum profiles of steroid hormones in patients with Cushing’s syndrome determined by a new HPLC/RIA method, Clin Chem, 37 (1991) 1329–1333. [PubMed] [Google Scholar]
- [137].Phillipou G, Investigation of urinary steroid profiles as a diagnostic method in Cushing’s syndrome, Clin Endocrinol (Oxf), 16 (1982) 433–439. [DOI] [PubMed] [Google Scholar]
- [138].Di Dalmazi G, Fanelli F, Mezzullo M, Casadio E, Rinaldi E, Garelli S, Giampalma E, Mosconi C, Golfieri R, Vicennati V, Pagotto U, Pasquali R, Steroid Profiling by LC-MS/MS in Nonsecreting and Subclinical Cortisol-Secreting Adrenocortical Adenomas, J Clin Endocrinol Metab, 100 (2015) 3529–3538. [DOI] [PubMed] [Google Scholar]
- [139].Eisenhofer G, Masjkur J, Peitzsch M, Di Dalmazi G, Bidlingmaier M, Gruber M, Fazel J, Osswald A, Beuschlein F, Reincke M, Plasma Steroid Metabolome Profiling for Diagnosis and Subtyping Patients with Cushing Syndrome, Clin Chem, 64 (2018) 586–596. [DOI] [PubMed] [Google Scholar]