Abstract
This study was conducted to evaluate the possible clinical and bacteriologic features associated with 30-day mortality from Acinetobacter baumannii (A. baumannii) bloodstream infections (BSIs). We conducted a prospective, multicenter, observational study of 181 entire episodes of A. baumannii BSI from six general hospitals between May 2016 and April 2017 in South Korea. Cox proportional-hazards regression model was used to estimate risks of the primary endpoint, i.e., all-cause mortality within 30 days from the initial blood culture. Most (84.5%) of the A. baumannii blood isolates belonged to the international clonal lineage II (ICLII) and 89.5% of the isolates were either multidrug- or extensively-drug resistant. We identified three risk factors including the old age of patient {hazard ratio, 1.033; [95% Confidential Interval (CI), 1.010–1.056]}, the sequential organ failure assessment score [1.133 (1.041–1.233)], and causative A. baumannii sequence type (ST) 191 belonging to ICLII [1.918 (1.073–3.430)], and three protective factors including causative A. baumannii ST451 belonging to ICLII [0.228 (0.078–0.672)], platelet count [0.996 (0.993–0.999)], and definitive therapy within 72 h [0.255 (0.125–0.519)]. Differing 30-day mortality rate in the dominant ICLII was observed by ST, which was much high in ST191 and low in ST451 and it was likely associated with the molecular traits, rather than the drug resistance.
Keywords: Acinetobacter baumannii, bloodstream infection, international clonal lineage II, ST191, ST451
Introduction
Acinetobacter baumannii is dominant in clinical settings primarily targeting immunocompromised patients in intensive care units (ICUs) and has the potential to cause healthcare-associated infections (1). Infections caused by A. baumannii have repeatedly been reported to have grave clinical outcomes (2, 3). A. baumannii has an epidemic potential of emerging diseases because of its ability to persist in hospital environments by resisting desiccation and disinfectants (1). A propensity toward rapid acquisition of foreign DNA, including antimicrobial resistance determinants and virulence determinants, also contributes to its effective dissemination (4).
Previous studies have demonstrated that A. baumannii infections are accompanied by serious morbidity and mortality associated with a high sequential organ failure assessment (SOFA) score (5), comorbidities (6), immunosuppression (3), and antimicrobial treatment failure, mainly due to the multidrug resistance (MDR) of the causative A. baumannii (5, 7, 8). The rates of resistance to the major anti-Acinetobacter agents are increasing (9). In particular in South Korea, predominance of the multidrug-resistant international clonal lineage II (ICLII) in hospital settings is of great concern (10–12).
This study was performed to evaluate the possible clinical and bacteriologic features associated with 30-day mortality from A. baumannii bloodstream infection (BSI) for the entire 1-year incidence of A. baumannii BSI cases of multiple general hospitals in South Korea.
Methods
Study Design
A prospective, multicenter, observational study was designed to monitor the entire A. baumannii BSI episodes between May 2016 and April 2017 occurring in six general hospitals with 715–1,050 beds participating in a national surveillance study of antimicrobial resistance in South Korea (13). Local ethics committee approvals were expedited or waived at each participating hospital since the study had a purely observational nature. The A. baumannii BSI cases were detected through reviewing daily blood culture results. One authorized investigator at each hospital reviewed patients' medical records, all of the raw data were recorded in a pre-formatted spreadsheet, and the datasheet was submitted via email to an analysis center without monitoring. Prognoses of patients were followed for at least 30 days and the cases of transfer and hospital discharge were recorded as they were. The Charlson comorbidity index (14) and the SOFA score (15) were calculated in the analysis center using the collected raw data for the day of initial blood culture. For the sequential isolation of A. baumannii from one patient, the first isolate was used for analyses and the ensuing isolates were discarded. Among a total of 67,803 patients subjected to blood culture, A. baumannii isolates were recovered from blood specimens of 188 patients, and following the elimination of 7 episodes of death on the day of the initial blood culture, 181 cases were included in the analysis. The microbiological assessment was carried out in the analysis center.
Definitions
Laboratory-confirmed A. baumannii BSI was defined as an A. baumannii-positive blood culture from one or more blood specimens (16). Hospital-originated (HO) infection was specified by ≥2 calendar days of hospitalization at the day of initial blood culture, including the time spent at a previous health care facility before transfer. The 30-day mortality was defined as all-cause mortality within 30 days from the initial blood culture. Non-susceptibility (NS) to a drug class was defined as NS to at least one drug in a class and resistance phenotypes were categorized following Magiorakos et al. (17): MDR (NS to three or more drug classes), extensively drug resistance (XDR, NS to at least one agent in all but one or two drug classes), and pan-drug resistance (NS to at least one agent in all drug classes). An empirical antimicrobial therapy was defined as an initial antimicrobial treatment before the susceptibility testing results for the causative pathogen. A definitive antimicrobial therapy was defined as prescribing a revised antimicrobial regimen based on the antimicrobial susceptibility testing results and, if the revised regimen was administered after 72 h, it was deemed to be a delayed definitive therapy. Adequate antimicrobial therapy must include at least one antimicrobial agent active in vitro and both the dosage and route of administration should meet current medical standards.
Microbiological Assessment
Bacterial species were initially identified by matrix assisted laser desorption/ionization time-of-flight mass spectrometry using a Bruker Biotyper™ system (Billerica, MA, USA) and the detailed species of Acinetobacter spp. were confirmed by PCR and sequencing the rpoB gene. For A. baumannii, multilocus sequence typing was followed through the Oxford scheme (18). Antimicrobial susceptibility was tested following the Clinical and Laboratory Standards Institute (CLSI) guidelines (19). Antimicrobial susceptibilities to 13 anti-Acinetobacter agents belonging to seven drug classes were tested by the disk diffusion method. For carbapenem-resistant isolates, the blaOXA−23 gene and the ISAba1-blaOXA−51−like were identified by PCR and direct sequencing (20). Colistin MICs were determined by the broth microdilution method (21). Twenty-six virulence genes associated with eight virulence characteristics (22) were investigated by real-time PCR using gene-specific primers (23) and a KAPA SYBR Fast quantitative PCR master mix (Kapa biosystems, Inc., Wilmington, MA, USA). The resistance islands AbaR and AbGRI1 were specified by PCR using a set of primers, comMtr3_145-126F (5′-CAAGCGGTTGGCGTAAGACT-3′) and reverse primers tniB_546-525R (5′-CAAGTACATGCTCTGCAAGATG-3′) only for the full tniB gene and tniB_37-16R (5′-CAGACTCAATTCATTGCTGAGG-3′) for both the full and the truncated tniB genes.
Statistical Analysis
Statistical analyses were performed in SPSS statistics (version 23, IBM Corp., Armonk, NY, USA). Data are expressed as the mean ± standard deviation if no special description is indicated. Categorical variables were compared by Pearson's X2 test, and continuous variables were compared by an independent two sample t-test. Associations between 30-day mortality and risk factors were evaluated using univariable and multivariable Cox proportional hazard regression models. The proportional hazards assumption was evaluated by including an interaction term between variables, and variables were natural-log transformed by follow-up time and by log-minus-log survival plots. For multivariable analyses, variables with a P value <0.1 in univariable analyses were considered to include any possible variables. Multicollinearity between variables was diagnosed by calculating the variance inflation factors, and a multivariable analysis was conducted by the backward stepwise method. All tests of significance were two-tailed; P values <0.05 were considered to be significant.
Results
Characteristics of Enrolled Patients
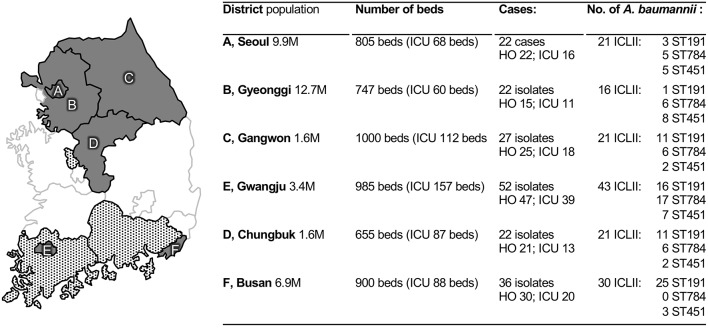
A total of 181 laboratory-confirmed A. baumannii BSI cases were enrolled from the six participating hospitals and the number of cases was ranged from 22 to 52 by hospital (Figure 1). Among patients with A. baumannii BSIs, the sex ratio of male vs. female was 115:66, and their mean age was 67.7 ± 14.3 years (Table 1). The mean Charlson comorbidity index score was 4.1 ± 2.2. Solid tumors (26.5%, 48/181), diabetes mellitus (23.2%, n = 42), cerebrovascular diseases (18.2%, n = 33), kidney diseases (13.8%, n = 25), and chronic pulmonary diseases (6.6%, n = 12) were the five most observed underlying diseases and dementia (n = 9) and chronic liver diseases (n = 7) were followed. Of the total, 88.4% (n = 160) of cases were HO infections, 95.0% (n = 172) were inpatients, and 68.0% of those (117/172) stayed in ICUs. The SOFA score had a mean value of 5.9 ± 3.5. Secondary BSIs were identified in 44.2% (80/181) of cases, and the majority (86.3%, 69/80) were originated from a pulmonary tract infection.
Figure 1.
Participating sentinel hospitals and collected Acinetobacter baumannii blood isolates. Locations of the hospitals (in gray) and covering parts (in gray patterns) are indicated in a map of the South Korean peninsula, respectively, and the associated table represent information regarding blood isolates from each hospital including demographic information from 2016. HO, hospital-origin; ICLII, international clonal lineage II; ST, sequence type.
Table 1.
Thirty day mortality-associated factors for patients with an Acinetobacter baumannii BSI.
| Variables | Total* | Survival | 30-day death† | P‡ | Univariate model | |
|---|---|---|---|---|---|---|
| n = 181 | n = 104 | n = 77 | HR [95%CI]§ | P | ||
| Demographic information | ||||||
| Male | 115 (63.5%) | 67 (64.4%) | 48 (62.3%) | 0.876 | 0.883 [0.557–1.400] | 0.596 |
| Age | 67.7 ± 14.3 | 64.5 ± 15.5 | 72.1 ± 11.1 | <0.001 | 1.033 [1.014–1.054] | <0.001 |
| Inpatients | 172 (95%) | 98 (94.2%) | 74 (96.1%) | 0.735 | 1.355 [0.427–4.298] | 0.606 |
| Hospital origin | 160 (88.4%) | 87 (83.7%) | 73 (94.8%) | 0.033 | 2.808 [1.026–7.685] | 0.044 |
| Intensive care unit | 117 (64.6%) | 63 (60.6%) | 54 (70.1%) | 0.210 | 1.342 [0.824–2.187] | 0.238 |
| Underlying disease¶ | ||||||
| Charlson index | 4.1 ± 2.2 | 3.9 ± 2.2 | 4.4 ± 2.2 | 0.108 | 1.086 [0.981–1.202] | 0.112 |
| Solid tumor | 48 (26.5%) | 27 (26.0%) | 21 (27.3%) | 0.866 | 1.183 [1.109–1.261] | <0.001 |
| Diabetes mellitus | 42 (23.2%) | 28 (26.9%) | 14 (18.2%) | 0.213 | 0.049 [0.000–7177] | 0.620 |
| Cerebrovascular disease | 33 (18.2%) | 23 (22.1%) | 10 (13.0%) | 0.125 | 0.597 [0.307–1.161] | 0.129 |
| Kidney disease | 25 (13.8%) | 15 (14.4%) | 10 (13.0%) | 0.831 | 1.043 [0.631–1.722] | 0.870 |
| Chronic pulmonary diseases | 12 (6.6%) | 7 (6.7%) | 5 (6.5%) | >0.999 | 6.176 [0.840–45.40] | 0.074 |
| Chronic liver diseases | 7 (3.9%) | 2 (1.9%) | 5 (6.5%) | 0.137 | 2.182 [0.878–5.420] | 0.093 |
| Dementia | 9 (5.0%) | 5 (4.8%) | 4 (5.2%) | >0.999 | 1.069 [0.432–2.649] | 0.885 |
| Other# | 5 (2.8%) | 2 (1.9%) | 3 (3.9%) | 0.652 | 1.019 [0.372–2.787] | 0.971 |
| Severity variables | ||||||
| SOFA score | 5.9 ± 3.5 | 4.8 ± 3.2 | 7.4 ± 3.5 | <0.001 | 1.183 [1.109–1.261] | <0.001 |
| Lab data | ||||||
| WBC | 11.5 ± 7.8 | 12.2 ± 6.9 | 10.6 ± 8.8 | 0.185 | 0.967 [0.934–1.000] | 0.052 |
| Hb | 10.4 ± 7.8 | 10.9 ± 10.4 | 9.6 ± 1.6 | 0.262 | 0.910 [0.788–1.051] | 0.201 |
| Platelet | 169.7 ± 132.1 | 206.9 ± 138.3 | 119.5 ± 104.8 | <0.001 | 0.994 [0.991–0.997] | <0.001 |
| Origin of infection¶ | ||||||
| Pulmonary tract | 69 (38.1%) | 36 (34.6%) | 33 (42.9%) | 0.281 | 1.259 [0.801–1.978] | 0.317 |
| Wound | 7 (3.9%) | 5 (4.8%) | 2 (2.6%) | 0.700 | 0.653 [0.160–2.662] | 0.553 |
| Urinary tract | 4 (2.2%) | 4 (3.8%) | 0 (0%) | 0.138 | 0.048 [0.000–18.92] | 0.319 |
| Causative pathogen | ||||||
| Polymicrobial infection | 44 (24.3%) | 30 (28.8%) | 14 (18.2%) | 0.116 | 0.636 [0.356–1.135] | 0.125 |
| ICLII†† | 153 (84.5%) | 83 (79.8%) | 70 (90.9%) | 0.060 | 2.083 [0.958–4.533] | 0.064 |
| ST191 (1-3-3-2-2-94-3) | 73 (40.3%) | 29 (27.9%) | 44 (57.1%) | <0.001 | 2.630 [1.671–4.139] | <0.001 |
| ST784 (1-3-3-2-2-107-3) | 31 (17.1%) | 17 (16.3%) | 14 (18.2%) | 0.842 | 0.983 [0.551–1.755] | 0.954 |
| ST451 (1-3-3-2-2-142-3) | 29 (16.0%) | 24 (23.1%) | 5 (6.5%) | 0.004 | 0.293 [0.118–0.727] | 0.008 |
| Virulence factors | ||||||
| Bacterial lipocalin, Blc | 145 (80.1%) | 79 (76.0%) | 66 (85.7%) | 0.132 | 1.540 [0.813–2.916] | 0.185 |
| Pro-apoptotic porin, PorB | 146 (80.7%) | 79 (76.0%) | 67 (87.0%) | 0.086 | 1.839 [0.946–3.575] | 0.073 |
| Small protein A, SmpA | 177 (97.8%) | 101 (97.1%) | 76 (98.7%) | 0.638 | 1.773 [0.247–12.75] | 0.569 |
| Pili, Csu | 73 (40.3%) | 45 (43.3%) | 28 (36.4%) | 0.363 | 0.781 [0.491–1.243] | 0.297 |
| PNAG, Pga | 173 (95.6%) | 96 (92.3%) | 77 (100%) | 0.022 | 22.02 [0.307–1579] | 0.156 |
| Polysaccharide exporter, EpsA | 9 (5.0%) | 7 (6.7%) | 2 (2.6%) | 0.305 | 0.425 [0.104–1.730] | 0.232 |
| Capsule protein, MviM | 7 (3.9%) | 4 (3.8%) | 3 (3.9%) | >0.999 | 1.047 [0.330–3.322] | 0.938 |
| Lipopolysaccharide, VipA | 16 (8.8%) | 9 (8.7%) | 7 (9.1%) | >0.999 | 1.021 [0.469–2.220] | 0.959 |
| Lipopolysaccharide, Wzx | 48 (26.5%) | 26 (25%) | 22 (28.6%) | 0.613 | 1.144 [0.698–1.875] | 0.595 |
| Glyconokinase, GntK | 179 (98.9%) | 102 (98.1%) | 77 (100%) | 0.508 | 20.54 [0.004–93770] | 0.482 |
| Quorum Sensing, LuxR | 159 (87.8%) | 91 (87.5%) | 68 (88.3%) | >0.999 | 1.000 [0.499–2.004] | 0.999 |
| Motility and secretion, PilA | 18 (9.9%) | 12 (11.5%) | 6 (7.8%) | 0.460 | 0.723 [0.314–1.664] | 0.446 |
| Siderophore, BasD | 179 (98.9%) | 102 (98.1%) | 77 (100%) | 0.508 | 20.54 [0.004–93770] | 0.482 |
| Antimicrobial resistance | ||||||
| Penicillins NS | 157 (86.7%) | 82 (78.8%) | 75 (97.4%) | <0.001 | 7.352 [1.804–29.96] | 0.005 |
| 3-, 4- gen. cephalosporins NS | 162 (89.5%) | 85 (81.7%) | 77 (100%) | <0.001 | 25.33 [1.463–438.6] | 0.026 |
| Carbapenems NS | 160 (88.4%) | 84 (80.8%) | 76 (98.7%) | <0.001 | 13.15 [1.828–94.63] | 0.010 |
| Aminoglycosides NS | 141 (77.9%) | 76 (73.1%) | 65 (84.4%) | 0.073 | 1.515 [0.818–2.805] | 0.187 |
| Fluoroquinolones NS | 162 (89.5%) | 85 (81.7%) | 77 (100%) | <0.001 | 25.33 [1.463–438.6] | 0.026 |
| Tetracyclines NS | 13 (7.2%) | 11 (10.6%) | 2 (2.6%) | 0.045 | 0.284 [0.070–1.156] | 0.079 |
| Drug susceptible | 18 (9.9%) | 18 (17.3%) | 0 (0%) | <0.001 | 0.040 [0.002–0.743] | 0.031 |
| MDR/XDR | 162 (89.5%) | 85 (81.7%) | 77 (100%) | <0.001 | 25.33 [1.463–438.6] | 0.026 |
| Treatment | ||||||
| Adequate empirical therapy | 49 (27.1%) | 30 (28.8%) | 19 (24.7%) | 0.613 | 0.744 [0.443–1.249] | 0.263 |
| Definitive therapy within 72 h | 49 (27.1%) | 39 (37.5%) | 10 (13.0%) | <0.001 | 0.246 [0.124–0.488] | <0.001 |
The percentage was calculated from the total number of AB-BSI.
All-cause mortality within 30 days from the initial blood culture.
P value is either from Pearson's X2 test for categorical data or from the t-test for continuous data. Bonferroni corrected significance level from 32 hypotheses was considered to be P <0.0016, and the significant P values are indicated in bold.
Cox proportional hazards regression model was applied to calculate the hazard ratio (HR) and the 95% confidence interval (CI).
Underlying disease and primary site of infection could be multiple by patient.
Ulcer (n = 2), connective tissue disease (n = 2), congestive cardiac insufficiency (n = 2), and leukemia (n = 2) are included.
Multilocus sequence typing profiles are indicated with the combination of alleles (gltA-gyrB-gdhB-recA-cpn60-gpi-rpoD).
SD, standard deviation; ICLII, the international clonal lineage II.
The BSI-Causative A. baumannii Isolates
Sequence Types
The majority of the A. baumannii blood isolates belonged to ICLII (84.5%, 153/181) and the ICLII mostly comprised sequence type (ST) 191 (allele numbers of gltA-gyrB-gdhB-recA-cpn60-gpi-rpoD, 1-3-3-2-2-94-3; 47.7%, 73/153), ST784 (1-3-3-2-2-107-3; 20.3%, n = 31), and ST451 (1-3-3-2-2-142-3; 19.0%, n = 29). The 30-day mortality rate of patients with A. baumannii ICLII BSIs was 1.83-fold higher than that with A. baumannii BSIs by non-ICLII strains (45.8 vs. 25.0%); and among the ICLII BSIs, the 30-day mortality rate with ST191 BSIs was the highest and, the lowest for ST451 BSIs (60.3 vs. 17.2%).
Antimicrobial Resistance
Majority of the A. baumannii blood isolates (89.5%, 162/181) were either MDR or XDR, and the proportion was markedly greater in A. baumannii ICLII (98.7%, 151/153; 18 MDR and 133 XDR) than in non-ICLII (39.3%, 11/28; 5 MDR, and 6 XDR). All of the tested isolates were susceptible to colistin, and no pan-drug resistant isolate was observed. All but one carbapenem-NS A. baumannii harbored the blaOXA−23 gene (99.4%, 159/160) and the remaining one carried an insertion sequence ISAba1 upstream from the blaOXA−51−like gene. Two thirds and one-quarter of A. baumannii possessed AbGRI1 (63.0%, 114/181) and AbaR (26.5%, 48/181), respectively.
Virulence Factors
Factors for adhesion and membrane integrity, biofilm formation, carbohydrate metabolism, quorum sensing, and siderophore were detected in A. baumannii blood isolates (Table 1). The acinetobactin-associated basD (98.9%, 179/181), the glycokinase gntK (98.9%, n = 179), the adhesion-associated smpA (97.8%, n = 177), and the biofilm formation-associated pga genes (95.6%, n = 173) were harbored by most of the A. baumannii blood isolates, while the capsule protein mviM (3.9%, n = 7), the polysaccharide exporter epsA (5.0%, n = 9), lipopolysaccharide vipA (8.8%, n = 16), and the type IV pili pilA (9.9%, n = 18) genes were occasionally identified. Among the virulence factors tested, the pga gene was significantly associated with 30-day death and the adhesion-associated porB had a tendency of 30-day mortality-associated.
A different frequency of each gene by ST was occasionally identified (Table 2). The psaB gene, formerly the pab2 gene (24), was identified in all but one (96.6%, 28/29) ST451 isolates.
Table 2.
Characteristics of the A. baumannii ICLII blood isolates.
| Characteristics | ICLII | P* | ST191 | P* | ST451 | P* |
|---|---|---|---|---|---|---|
| n = 153 | n = 73 | n = 29 | ||||
| Death within 30 days | 70 (45.8%) | 0.002 | 44 (60.3%) | 0.000 | 5 (17.2%) | 0.013 |
| SOFA score (mean ± SD) | 5.9 ± 3.6 | 0.005 | 6.2 ± 3.9 | 0.031 | 6.4 ± 3.6 | 0.161 |
| Pulmonary tract of primary site of BSI | 61 (39.9%) | 0.000 | 24 (32.9%) | 1.000 | 17 (58.6%) | 0.002 |
| Drug resistance | ||||||
| MDR/XDR | 151 (98.7%) | 0.821 | 72 (98.6%) | 0.003 | 29 (100%) | 0.545 |
| Penicillins NS | 146 (95.4%) | 0.000 | 70 (95.9%) | 0.000 | 27 (93.1%) | 0.004 |
| 3-, 4- gen. cephalosporins NS | 151 (98.7%) | 0.000 | 72 (98.6%) | 0.000 | 29 (100%) | 0.000 |
| Carbapenems NS | 149 (97.4%) | 0.000 | 70 (95.9%) | 0.000 | 29 (100%) | 0.000 |
| Aminoglycosides NS | 136 (88.9%) | 0.000 | 58 (79.5%) | 0.006 | 29 (100%) | 0.000 |
| Fluoroquinolones NS | 151 (98.7%) | 0.000 | 72 (98.6%) | 0.000 | 29 (100%) | 0.000 |
| Tetracyclines NS | 11 (7.2%) | 0.354 | 3 (4.1%) | 0.553 | 6 (20.7%) | 0.003 |
| Polymyxin | 0 | 0 | 0 | |||
| VIRULENCE FACTORS | ||||||
| Drug resistance | ||||||
| AbGRI1 (Tn6022) | 112 (73.2%) | 0.000 | 69 (94.5%) | 0.000 | 5 (17.2%) | 0.000 |
| AbaR (Tn6019) | 38 (24.8%) | 0.164 | 2 (2.7%) | 0.000 | 24 (82.8%) | 0.000 |
| OXA-23 | 148 (96.7%) | 0.000 | 70 (95.9%) | 0.000 | 28 (96.6%) | 0.001 |
| AdeC | 32 (20.9%) | 0.857 | 10 (13.7%) | 0.110 | 6 (20.7%) | 1.000 |
| Adhesion/Membrane Integrity | ||||||
| Blc | 123 (80.4%) | 0.002 | 56 (76.7%) | 0.521 | 22 (75.9%) | 1.000 |
| PorB | 124 (81%) | 0.000 | 64 (87.7%) | 0.001 | 23 (79.3%) | 0.505 |
| SmpA | 149 (97.4%) | 0.000 | 72 (98.6%) | 0.002 | 27 (93.1%) | 0.747 |
| Biofilm formation | ||||||
| Csu | 133 (86.9%) | 0.000 | 62 (84.9%) | 0.000 | 29 (100%) | 0.000 |
| Pga | 153 (100%) | 0.000 | 73 (100%) | 0.000 | 29 (100%) | 0.002 |
| Capsule protein | ||||||
| EpsA | 7 (4.6%) | 0.742 | 1 (1.4%) | 0.107 | 3 (10.3%) | 0.160 |
| MviM | 6 (3.9%) | 0.440 | 0 (0%) | 0.099 | 2 (6.9%) | 0.228 |
| Lipopolysaccharide | ||||||
| PsaB | 36 (23.5%) | 0.000 | 2 (2.7%) | 0.000 | 28 (96.6%) | 0.000 |
| VipA | 12 (7.8%) | 0.448 | 7 (9.6%) | 0.808 | 0 (0%) | 0.084 |
| Wzx | 42 (27.5%) | 0.129 | 17 (23.3%) | 0.869 | 10 (34.5%) | 0.172 |
| Carbohydrate metabolism | ||||||
| GntK | 153 (100%) | 0.000 | 73 (100%) | 0.000 | 29 (100%) | 0.003 |
| Quorum sensing system | ||||||
| LuxR | 138 (90.2%) | 0.000 | 65 (89%) | 0.175 | 27 (93.1%) | 0.183 |
| Motility and secretion | ||||||
| PilA | 14 (9.2%) | 0.808 | 2 (2.7%) | 0.015 | 5 (17.2%) | 0.164 |
| Siderophore | ||||||
| BasD | 153 (100%) | 0.000 | 73 (100%) | 0.000 | 29 (100%) | 0.010 |
| Treatment | ||||||
| Adequate empirical therapy | 31 (20.3%) | 0.000 | 16 (21.9%) | 0.003 | 3 (10.3%) | 0.002 |
| Definitive therapy within 72 h | 46 (30.1%) | 0.013 | 20 (27.4%) | 0.624 | 11 (37.9%) | 0.109 |
P value is either from Pearson's X2 test for categorical data or from the t-test for continuous data. The Bonferroni corrected significance level from 33 hypotheses was considered to be P < 0.0015, and the significant P values are indicated with asterisks.
Antimicrobial Treatment
The majority (95.0%, 172/181) of patients with A. baumannii BSIs received an empirical antimicrobial therapy with anti-Acinetobacter drugs and 28.5% (49/172) of those were adequate (Table 3). The other nine patients received either not-for-Acinetobacter drugs (n = 7) or none due to refusal of treatment (n = 2). Among the 132 patients who received an inadequate empirical therapy, 37.1% (n = 49) of patients were treated with a definitive antimicrobial therapy within 72 h and 20.4% (10/49) of those patients met death within 30 days, much less than that of patients who received a delayed definitive therapy (57.9%, 48/83).
Table 3.
Antimicrobial regimens for the patients with A. baumannii BSIs.
| Regimen | No. of | cases | Proper | Improper |
|---|---|---|---|---|
| Ampicillin | 1 | 0.6% | 1 | 0 |
| Pipercillin/tazobactam | 26 | 14.4% | 5 | 21 |
| comb.w/ciprofloxacin | 12 | 6.6% | 1 | 11 |
| comb.w/tigecycline | 2 | 1.1% | 1 | 1 |
| comb.w/colistin | 2 | 1.1% | 2 | 0 |
| 3-, 4- gen. cephalosporins | 20 | 11.0% | 5 | 15 |
| comb.w/ciprofloxacin | 8 | 4.4% | 0 | 8 |
| comb.w/aminoglycosides | 2 | 1.1% | 0 | 2 |
| Carbapenems | 55 | 30.4% | 5 | 50 |
| comb.w/aminoglycosides | 2 | 1.1% | 0 | 2 |
| comb.w/co-trimoxazole | 2 | 1.1% | 1 | 1 |
| comb.w/ciprofloxacin | 9 | 5.0% | 1 | 8 |
| comb.w/colistin | 10 | 5.5% | 10 | 0 |
| Tigecycline | 6 | 3.3% | 5 | 1 |
| comb.w/co-trimoxazole | 1 | 0.6% | 1 | 0 |
| comb.w/fluoroquinolones | 2 | 1.1% | 2 | 0 |
| Colistin | 4 | 2.2% | 4 | 0 |
| Ciprofloxacin | 6 | 3.3% | 4 | 2 |
| Aminoglycosides | 1 | 0.6% | 0 | 1 |
| Co-trimoxazole | 1 | 0.6% | 1 | 0 |
| Not treated* | 9 | 5.0% | 0 | 9 |
| 181 | 49 | 132 |
Patients received drugs not-for-Acinetobacter (n = 7) or none due to refusal of treatment (n = 2).
Risk Factors for 30-Day Mortality
Risk and protective factors associated with the 30-day mortality in patients with A. baumannii BSIs were searched for among the variables of P <0.1 through a Cox-regression univariable analysis. Most of the variables were omitted by stepwise selection procedure, and each three of risk factors and protective factors associated with the 30-day mortality in patients with A. baumannii BSIs were identified through a Cox-regression multivariable analysis (Table 4): the age of patients of hazard ratio (95% CI), 1.033 (1.010–1.056); the SOFA score of 1.133 (1.041–1.233); and the BSI-causative A. baumannii ST191 of 1.918 (1.073–3.430). Three protective factors were also identified: the platelet count of 0.996 (0.993–0.999), the BSI-causative A. baumannii ST451 of 0.228 (0.078–0.672), and the definitive therapy within 72 h of 0.255 (0.125–0.519).
Table 4.
Risk factors associated with the 30-day mortality in patients with A. baumannii BSI.
| Variables | VIF* | HR [95%CI]† | P |
|---|---|---|---|
| Age | 1.058 | 1.033 [1.010–1.056] | 0.005 |
| SOFA score | 1.335 | 1.133 [1.041–1.233] | 0.004 |
| Platelet | 1.290 | 0.996 [0.993–0.999] | 0.016 |
| ST191 | 1.245 | 1.918 [1.073–3.430] | 0.028 |
| ST451 | 1.255 | 0.228 [0.078–0.672] | 0.007 |
| Definitive therapy within 72 h | 1.094 | 0.255 [0.125–0.519] | <0.001 |
VIF, variance inflation factor. The collinearity was diagnosed for the variables including the multivariate analysis.
A Cox-regression multivariate analysis was conducted by the backward method using the Wald model.
Discussion
During the surveillance study period, the overall incidence of A. baumannii was the third-highest (1.1/10,000 patient-days) BSI-causative Gram-negative bacteria followed by Escherichia coli and Klebsiella pneumoniae. Besides, the incidence was strikingly higher in ICUs (6.6/10,000 patient-days) than in general wards (0.4/10,000 patient-days) (25). The 30-day mortality rate of patients with A. baumannii BSIs is pretty varied by study depending on the subjected patients, and we observed 42.5% of 30-day mortality for entire A. baumannii BSI cases.
Among the variables studied, the old age and the high SOFA score were identified as the patient-associated risk factors. Those were reasonable hazardous factors since both factors pronounced the severity of illness directly or indirectly. A patient-associated protective factor, platelet counts in peripheral blood reflecting the patients' condition also made sense. As well, a treatment-associated protective factor, a proper definitive therapy within 72 h was comprehensible.
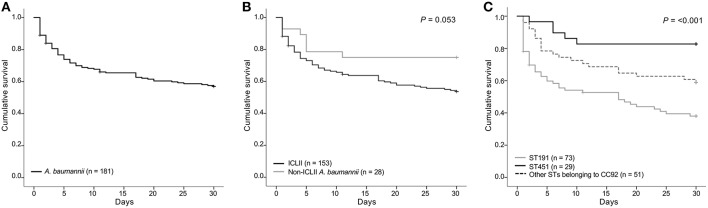
Curiously among the causative-pathogen-associated variables, the A. baumannii ST191 was a risk factor, while the ST451, another ICLII member, was a protective factor. A. baumannii ICLII, is a well-known multidrug resistant clone (26, 27). The treatment options for patients infected by the clone are often limited to the very last resorts, such as carbapenems, tigecycline, minocycline, and polymyxins. For the infections cause by multidrug-resistant A. baumannii, the use of combination antimicrobial therapy is recommended (28) and two thirds (66.3%, 120/181) of the patients were treated empirically by antimicrobial combinations. Carbapenem resistance is already common in ICLII, and the remaining choices are always risky because tigecycline (29) and minocycline (30) have a bacteriostatic activity, which requires a combinational treatment; colistin has a severe dose-dependent neuro- and nephrotoxicity (31); and the colistin resistance is easily developed by prior use of the drug (32). The importance of an adequate antimicrobial therapy (33), including the proper use of colistin (34), has been addressed to diminish the 30-day mortality rate caused by A. baumannii infections. The present results confirmed the notion through a significant protective effect of the definitive therapy including colistin. Among ICLII members, ST191 was the most and both ST784 and ST451 are recently emerging ICLII clones (27, 32). In this study, ST784 and ST451 were the second and third most ICLII clones and the clones were evenly recovered from the six hospitals (Figure 2).
Figure 2.
Mortality of BSI patients by causative pathogen. (A) Mortality of patients with BSIs by A. baumannii, (B) comparison of mortality of BSI patients by ICLII and by non-ICLII A. baumannii, (C) comparison of mortality of BSI patients by ST191, ST451, and other STs belonging to CC91. The Kaplan-Meier plot illustrated survival in patients with AB-BSI infected with the indicated bacterial clone. Differences between groups were compared by the Log-Rank test of the Mantel-Cox model, and the P values are presented. ICLII, international clonal lineage II; ST, sequence type.
In this study, all but two (98.7%) ICLII blood isolates, one ST191 and the other ST357, exhibited MDR/XDR. Since drug resistance is one of the noted hazardous factors obstructing an adequate antimicrobial therapy (7, 8), similar mortality rates were presumed for BSIs caused by any STs belonging to the ICLII. However, a marked difference in 30-day mortality was observed in the three dominant STs: 60.3% in ST191 BSIs, 45.2% in ST784 BSIs, and 17.2% in ST451 BSIs. Curiously, the least proportion of patients with ST451 BSIs (10.3%) were treated by an adequate empirical therapy (Table 2), indicating that the drug resistance and the relevant adequacy of treatment were not related to the 30-day mortality rate at least to BSIs caused by ST191 and ST451. ST451 was more associated with a primary pulmonary tract infection (58.6%) than ST191 (32.9%), however the primary site of infection was insignificantly associated with a 30-day death (Table 1). Similarly, ST451 harbored the Tn6019-associated AbaR (82.8%) more frequently than the Tn6022-associated AbGRI1 (17.2%) differently from ST191 (2.7 vs. 94.5%), however no difference was expected since both may harbor the blaOXA−23 gene (12). The lipooligosaccharide biosynthesis-associated psaB gene was harbored strikingly more by ST451 than by ST191 (96.6 vs. 2.7%). The psaB gene is a part of the oligosaccharide gene clusters generating an ordinary side-branch sugar Gal, differing from the A. baumannii ST191 often carries the gene cluster producing FucNAc sugar composing the oligosaccharide (24). Since the lipooligosaccharides of pathogenic bacteria have long been recognized as a major virulence factor (35), the difference in oligosaccharides between the two clones is possibly associated with the 30-day mortality rate and the detailed impact should be further studied.
This study should be considered with the following caveats in mind. First, the enrolled cases had a lack of external validation due to a distinct epidemiology of A. baumannii clinical isolates in South Korea in terms of high carbapenem resistance, mostly conferred by OXA-23. Second, analyses of bacterial lipooligosaccharides differed by the clones are remained for further study. However, to the best of our knowledge, this is the first notification of the counter clinical prognoses of the patients with a BSI by dominant ICLII clones, drawing an attention to a further follow-up for the clonal exchange and expansion of ICLII clones in clinical settings.
This delicate prognosis assessment combined with an evaluation of the molecular epidemiology for the causative A. baumannii blood isolates confirmed (i) the predominance of ICLII in clinical settings, (ii) the hazardousness of antimicrobial resistance and the importance of an adequate antimicrobial therapy, and finally, (iii) we highlighted the differing 30-day mortality rates in patients with BSIs between causative multidrug-resistant A. baumannii clones ST191 and ST451 belonging ICLII probably due to the differing lipooligosaccharides.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The research, which has no involvement of human subject but the clinical isolates, does meet the exempt category without approval from Ethics Committee on Human Research of the Health Ministry in South Korea and the study design has not been reviewed by the committee.
Author Contributions
SJ conceived the study. E-JY and SJ designed the study. E-JY, HSL, and SJ analyzed the data. E-JY and SJ wrote the manuscript. DK, HL, JongS, YU, KS, YK, YP, JeongS, and SJ collected the clinical data and bacterial isolates.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the Research Programme funded by the Korea Centers for Disease Control and Prevention (2017E4400101#). We would like to thank to Yena Oh for technical support for microbiological experiments.
References
- 1.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. (2007) 5:939–51. 10.1038/nrmicro1789 [DOI] [PubMed] [Google Scholar]
- 2.Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore). (1995) 74:340–9. 10.1097/00005792-199511000-00004 [DOI] [PubMed] [Google Scholar]
- 3.Gu Z, Han Y, Meng T, Zhao S, Zhao X, Gao C, et al. Risk factors and clinical outcomes for patients with Acinetobacter baumannii bacteremia. Medicine. (2016) 95:e2943. 10.1097/MD.0000000000002943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallenet D, Nordmann P, Barbe V, Poirel L, Mangenot S, Bataille E, et al. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS ONE. (2008) 3:e1805. 10.1371/journal.pone.0001805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas ME, Karveli EA, Siempos II, Vardakas KZ. Acinetobacter infections: a growing threat for critically ill patients. Epidemiol Infect. (2008) 136:1009–19. 10.1017/S0950268807009478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra T, Marchaim D, Johnson PC, Awali RA, Doshi H, Chalana I, et al. Risk factors and outcomes for patients with bloodstream infection due to Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother. (2014) 58:4630–5. 10.1128/AAC.02441-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra T, Marchaim D, Awali RA, Krishna A, Johnson P, Tansek R, et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. (2013) 57:6270–5. 10.1128/AAC.01520-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freire MP, de Oliveira Garcia D, Garcia CP, Campagnari Bueno MF, Camargo CH, Kono Magri ASG, et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect. (2016) 22:352–8. 10.1016/j.cmi.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 9.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG, et al. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin Infect Dis. (2006) 42:657–68. 10.1086/499819 [DOI] [PubMed] [Google Scholar]
- 10.Heo ST, Oh WS, Kim SJ, Bae IG, Ko KS, Lee JC. Clinical impacts of a single clone (sequence type 92) of multidrug-resistant Acinetobacter baumannii in intensive care units. Microb Drug Resist. (2011) 17:559–62. 10.1089/mdr.2011.0087 [DOI] [PubMed] [Google Scholar]
- 11.Selasi GN, Nicholas A, Jeon H, Lee YC, Yoo JR, Heo ST, et al. Genetic basis of antimicrobial resistance and clonal dynamics of carbapenem-resistant Acinetobacter baumannii sequence type 191 in a Korean hospital. Infect Genet Evol. (2015) 36:1–7. 10.1016/j.meegid.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 12.Yoon EJ, Kim JO, Yang JW, Kim HS, Lee KJ, Jeong SH, et al. The blaOXA-23-associated transposons in the genome of Acinetobacter spp. represent an epidemiological situation of the species encountering carbapenems. J Antimicrob Chemother. (2017) 72:2708–14. 10.1093/jac/dkx205 [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Yoon EJ, Kim D, Jeong SH, Shin JH, Shin JH, et al. Establishment of the South Korean national antimicrobial resistance surveillance system, Kor-GLASS, in 2016. Euro Surveill. (2018) 23:1700734. 10.2807/1560-7917.ES.2018.23.42.1700734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, de Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 16.CDC Bloodstream Infection Event (2018). Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection. Device-associated Module. [Google Scholar]
- 17.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 18.Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. (2005) 43:4382–90. 10.1128/JCM.43.9.4382-4390.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI Performance Standards for Antimicrobial Susceptibility Testing. 25th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; (2016). [Google Scholar]
- 20.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. (2011) 70:119–23. 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 21.EUCAST Recommendations for MIC Determination of Colistin (Polymyxin E) As Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. (2016). Available online at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf
- 22.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of acinetobacter infections: a century of challenges. Clin Microbiol Rev. (2017) 30:409–47. 10.1128/CMR.00058-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lannan FM, O'conor DK, Broderick JC, Tate JF, Scoggin JT, et al. Evaluation of virulence gene expression patterns in Acinetobacter baumannii using quantitative real-time polymerase chain reaction array. Mil Med. (2016) 181:1108–13. 10.7205/MILMED-D-15-00437 [DOI] [PubMed] [Google Scholar]
- 24.Hu D, Liu B, Dijkshoorn L, Wang L, Reeves PR. Diversity in the major polysaccharide antigen of Acinetobacter baumannii assessed by DNA sequencing, and development of a molecular serotyping scheme. PLoS ONE. (2013) 8:e70329. 10.1371/journal.pone.0070329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. (2018) 23:1800047. 10.2807/1560-7917.ES.2018.23.42.1800047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. (2010) 5:e10034. 10.1371/journal.pone.0010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents. (2013) 41:11–9. 10.1016/j.ijantimicag.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 28.Durante-Mangoni E, Utili R, Zarrilli R. Combination therapy in severe Acinetobacter baumannii infections: an update on the evidence to date. Future Microbiol. (2014) 9:773–89. 10.2217/fmb.14.34 [DOI] [PubMed] [Google Scholar]
- 29.Karageorgopoulos DE, Kelesidis T, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant (including carbapenem-resistant) Acinetobacter infections: a review of the scientific evidence. J Antimicrob Chemother. (2008) 62:45–55. 10.1093/jac/dkn165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie DJ, Garavaglia-Wilson A. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis. (2014) 59(Suppl. 6):S374–380. 10.1093/cid/ciu613 [DOI] [PubMed] [Google Scholar]
- 31.Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. (2006) 10:R27. 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi ZA, Hittle LE, O'hara JA, Rivera JI, Syed A, Shields RK, et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis. (2015) 60:1295–303. 10.1093/cid/civ048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemos EV, de La Hoz FP, Einarson TR, Mcghan WF, Quevedo E, Castaneda C, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii. infection: systematic review and meta-analysis. Clin Microbiol Infect. (2014) 20:416–23. 10.1111/1469-0691.12363 [DOI] [PubMed] [Google Scholar]
- 34.Liu CP, Shih SC, Wang NY, Wu AY, Sun FJ, Chow SF, et al. Risk factors of mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. (2016) 49:934–40. 10.1016/j.jmii.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 35.Moran AP, Prendergast MM, Appelmelk BJ. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. (1996) 16:105–15. 10.1111/j.1574-695X.1996.tb00127.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.