The fibroblast growth factor (Fgf) signaling pathway has been implicated in many developmental processes, based largely on disruption of Fgf ligand gene functions. However, a mechanistic understanding of how Fgfs regulate each process will require...
Keywords: Fibroblast growth factor signaling, posterior mesoderm, pectoral fin, midbrain-hindbrain boundary, viscerocranium, neurocranium
Abstract
Fibroblast growth factor (Fgf) signaling regulates many processes during development. In most cases, one tissue layer secretes an Fgf ligand that binds and activates an Fgf receptor (Fgfr) expressed by a neighboring tissue. Although studies have identified the roles of specific Fgf ligands during development, less is known about the requirements for the receptors. We have generated null mutations in each of the five fgfr genes in zebrafish. Considering the diverse requirements for Fgf signaling throughout development, and that null mutations in the mouse Fgfr1 and Fgfr2 genes are embryonic lethal, it was surprising that all zebrafish homozygous mutants are viable and fertile, with no discernable embryonic defect. Instead, we find that multiple receptors are involved in coordinating most Fgf-dependent developmental processes. For example, mutations in the ligand fgf8a cause loss of the midbrain-hindbrain boundary, whereas, in the fgfr mutants, this phenotype is seen only in embryos that are triple mutant for fgfr1a;fgfr1b;fgfr2, but not in any single or double mutant combinations. We show that this apparent fgfr redundancy is also seen during the development of several other tissues, including posterior mesoderm, pectoral fins, viscerocranium, and neurocranium. These data are an essential step toward defining the specific Fgfrs that function with particular Fgf ligands to regulate important developmental processes in zebrafish.
THE coordination of cellular events that drive developmental processes requires robust communication between cells. One common communication mechanism is the Fibroblast growth factor (Fgf) signaling pathway, which involves a diffusible Fgf ligand that is secreted into the extracellular space, where it interacts with heparan sulfate and an Fgf receptor (Fgfr) (reviewed in Mohammadi et al. 2005). Fgfrs are single-pass transmembrane proteins composed of an extracellular region containing immunoglobulin (Ig) domains and an intracellular tyrosine kinase domain. Upon ligand binding, receptor dimerization leads to transphosphorylation-dependent activation of the kinase domain (Lemmon and Schlessinger 1994). Fgf signaling can activate several intracellular signal transduction pathways, including the MAPK, PLCγ, and PI3K/Akt cascades (Pawson 1995). While Fgf signaling can result in transcriptional changes, the ultimate cellular response depends on context and ranges from proliferation to migration and differentiation (reviewed in Powers et al. 2000).
Although the Fgf signaling pathway likely arose in eumetazoans (Bertrand et al. 2014), the Fgf ligand and receptor gene repertoire has increased in more complex animals. For example, the mammalian genome contains 22 ligand and four receptor genes, and the zebrafish genome has 31 ligand and five receptor genes (Ornitz and Itoh 2001). These genes are expressed widely throughout both developing and mature tissues, often in overlapping domains (Sleptsova-Friedrich et al. 2001; Thisse et al. 2001, 2008; Tonou-Fujimori et al. 2002; Scholpp et al. 2004; Nechiporuk et al. 2005; Thisse and Thisse 2005; Harvey and Logan 2006; Rohner et al. 2009; Camarata et al. 2010; Ota et al. 2010; Larbuisson et al. 2013; Rohs et al. 2013; Koch et al. 2014; Lovely et al. 2016). Tissue culture-based experiments have indicated that individual Fgf ligands have some degree of preference for the receptors they activate (Ornitz et al. 1996). This preference seems to be conferred by interactions between the glycine box, a ∼10 AA stretch near the C-terminus of an Fgf ligand (Luo et al. 1998), and the third Ig domain (IgIII) of the receptors (Johnson et al. 1991; Yayon et al. 1992). Interestingly, FGFR1, FGFR2, and FGFR3 in mammals, and Fgfr1a and Fgfr2 in zebrafish, are subject to alternative splicing in this IgIII domain, and these alternative isoforms have different affinities for particular ligands (Johnson et al. 1991; Werner et al. 1992; Chellaiah et al. 1994; Yeh et al. 2003).
Previously, studies have investigated the roles of Fgf signaling by disrupting the function of particular ligands. In zebrafish alone, the function of many Fgf ligands during early development has been determined using genetic mutation or morpholino (MO) knockdown. In some cases, disrupting a single Fgf gene leads to a developmental defect. For example, disrupting fgf24, fgf10a, or fgf16 signaling results in the absence of pectoral fins, whereas loss of fgf8a leads to midbrain-hindbrain boundary (MHB) defects (Brand et al. 1996; Reifers et al. 1998; Fischer et al. 2003; Nomura et al. 2006; Manfroid et al. 2007). In other contexts, however, Fgf ligands appear to function redundantly. While both fgf8a and fgf24 single mutants have normal mesoderm development, disrupting both ligands simultaneously leads to loss of posterior mesodermal derivatives and a consequent shortening of the embryonic axis (Draper et al. 2003). Similarly, simultaneous loss of both fgf8a and fgf3 leads to severe defects in pharyngeal pouch development, whereas this tissue develops normally in either single mutant (Crump et al. 2004a; McCarthy et al. 2016). These and similar data suggest that genetic redundancy in the Fgf signaling components creates a robust developmental system (Brand et al. 1996; Reifers et al. 1998; Draper et al. 2003; Fischer et al. 2003).
In contrast to the known requirements of many Fgf ligands during development, comparatively less is known about the requirements for specific Fgfrs. In the mouse, null mutation of Fgfr1 or Fgfr2 is embryonic lethal (Deng et al. 1994; Yamaguchi et al. 1994; Arman et al. 1998), whereas tissue-specific disruption of these genes reveals their roles during later development in limbs and/or brain (Xu et al. 1998, 1999a; Trokovic et al. 2003). By contrast, Fgfr3 and Fgfr4 homozygous mutants are embryonic viable, though Fgfr3 single mutants have skeletal dysplasia, and Fgfr3;Fgfr4 double mutants have defective lung development. These latter results suggest that, like certain Fgf ligands, Fgf receptors also function redundantly in some developmental contexts (Colvin et al. 1996; Deng et al. 1996; Weinstein et al. 1998). The zebrafish genome contains single copies of fgfr2-4 orthologs, and two copies of an fgfr1 ortholog, called fgfr1a and fgfr1b, that appear to have arisen during the teleost-specific whole genome duplication (Rohner et al. 2009). In contrast to several fgf ligand mutants, which have been isolated in phenotype-based forward genetic screens for recessive mutations, only one mutation in an Fgf receptor has been identified in a recessive screen—a result that could be explained if Fgf receptors function redundantly, an idea supported by the extensive spatial overlap of Fgfr gene expression in zebrafish embryos (Reifers et al. 1998; Fischer et al. 2003; Norton et al. 2005). The one exception is fgfr1a, where a point mutation in the kinase domain, proposed to be a strong hypomorph, is embryonic viable, but has defects in scale formation during juvenile development (Rohner et al. 2009).
To determine the precise requirements of each Fgf receptor during embryonic development, we have produced loss-of-function mutations in each of the five zebrafish fgf receptor genes. Because of the known requirements for Fgf signaling during early zebrafish development, we expected that some of the receptor mutants would phenocopy known Fgf ligand mutants. However, we found that all single mutants are viable with no overt embryonic phenotypes; instead, we discovered that only certain double and triple mutant combinations have developmental defects in the posterior mesoderm, brain, pectoral fin, and pharyngeal arch derived cartilages. These findings suggest significant genetic redundancy between various Fgf receptors, and indicate that some ligands are capable of activating signaling through as many as three different receptors.
Materials and Methods
Husbandry
The wild-type strain *AB was used for the generation of fgfr3uc51 and fgfr4uc42. The wild-type strain NHGRI-1 was used for the generation of fgf1a3uc61, fgfr1buc62, and fgfr2uc64. Zebrafish husbandry was performed as previously described (Westerfield 2000; Leerberg et al. 2017).
Generation of alleles
For fgfr3uc51 and fgfr4uc42, sgRNAs were designed using ZiFiT. Two oligonucleotides (see Supplemental Material, Table S1) were annealed and cloned into plasmid pDR274 (Addgene Plasmid #42250). For fgfr1auc61, fgfr1buc62, and fgfr2uc64, sgRNAs were designed using CRISPRscan (Moreno-Mateos et al. 2015) and produced as described (Shah et al. 2016). Briefly, overlap PCR of a T7 RNA promoter containing a gene-specific oligonucleotide and a PAGE-purified scaffold oligonucleotide (see Table S1) was used to generate the DNA template for in vitro transcription. cas9 mRNA was produced using the pT3TS-nls-zCas9-nls containing a codon-optimized Cas9 with two nuclear localization sequences as described in Jao et al. (2013). The plasmid was linearized with XbaI and transcribed using the mMESSAGE mMACHINE T3 Transcription Kit (Cat. No. AM1348; Thermo Fisher). The sgRNA and cas9 mRNA were co-injected into one-cell embryos with phenol red (5% in 2 M KCl) at concentrations of 60 ng/μl and 30 ng/μl, respectively.
CRISPR efficiency was determined by comparing the targeted loci of eight injected embryos and eight control embryos [24 hr postfertilization (hpf)] by High Resolution Melt Analysis (HRMA) as described (Dahlem et al. 2012) (see Table S2 for primers used). Germline mutations were identified by PCR analysis of sperm DNA from injected males (see Table S2 for primers used). Indels were sequenced, and individuals containing frameshift inducing indels were outcrossed to *AB or NHGRI-1 to obtain F1s. To reduce the potential for off-target effects, all lines were outcrossed at least four times prior to analysis.
Genotyping
Genomic DNA was extracted from caudal fin tissue. Genotypes were determined using standard PCR conditions (fgfr1auc61, fgfr1buc62, fgfr2uc64, and fgfr3uc51) or HRMA (fgfr4uc42), and primers listed in Table S2.
RNA in situ hybridization
RNA probes that detect the following genes were used: ta (Schulte-Merker et al. 1992); myod (Begemann and Ingham 2000); pax2a (Krauss et al. 1991); tbx5a (Begemann and Ingham 2000); fgf24 (Draper et al. 2003); etv4 (Münchberg et al. 1999); fgf8a (Reifers et al. 1998). For has2, dlx2a, alcama, fgf10a, and en2a probe synthesis, mRNA was isolated from 24 hpf embryos using TRI reagent (Cat. No. T9424; Sigma-Aldrich) and synthesized into cDNA using the RETROScript Reverse Transcription Kit (Cat. No. AM1710; ThermoFisher). DNA templates for in vitro transcription were PCR amplified with Phusion polymerase (Cat. No. M0530L; New England BioLabs) and the primers listed in Table S3. Reverse primers contained a T7 RNA polymerase promoter (Table S3, underlined portion), and in vitro transcription yielded antisense probes (Roche T7 RNA polymerase, Cat. No. 10881775001). Probes were purified with the RNA Clean and Concentrator kit (Cat. No. R1015; Zymo) and G-50 sephadex columns (Cat. No. 45-001-398; GE Healthcare). Probes were used at a concentration of 0.5–2 ng/μl in hybridization solution.
Samples were fixed in 4% paraformaldehyde (PFA) overnight at 4° or 4 hr at room temperature. Samples were dehydrated with 100% methanol and stored at −20° for at least 16 hr. Embryos >30 hpf were bleached for ∼10 min prior to proteinase K digestion in 3% H2O2, 0.5% KOH. Color in situ hybridizations were performed by a procedure similar to that of Thisse et al. (2008), with the exception that 5% dextran sulfate was included in the hybridization solution.
Bone and cartilage stains
For the scale stain depicted in Figure S2, adults were fixed in 4% PFA for 3 days, followed by 3 × 30 min rinses in deionized water. Samples were bleached with 0.5% H2O2/1% KOH to remove pigment, and scales were stained with 1% alizarin red/1% KOH. Cartilage stains in Figure 6 and Figure 7 were performed as previously described (Walker et al. 2006).
Figure 6.
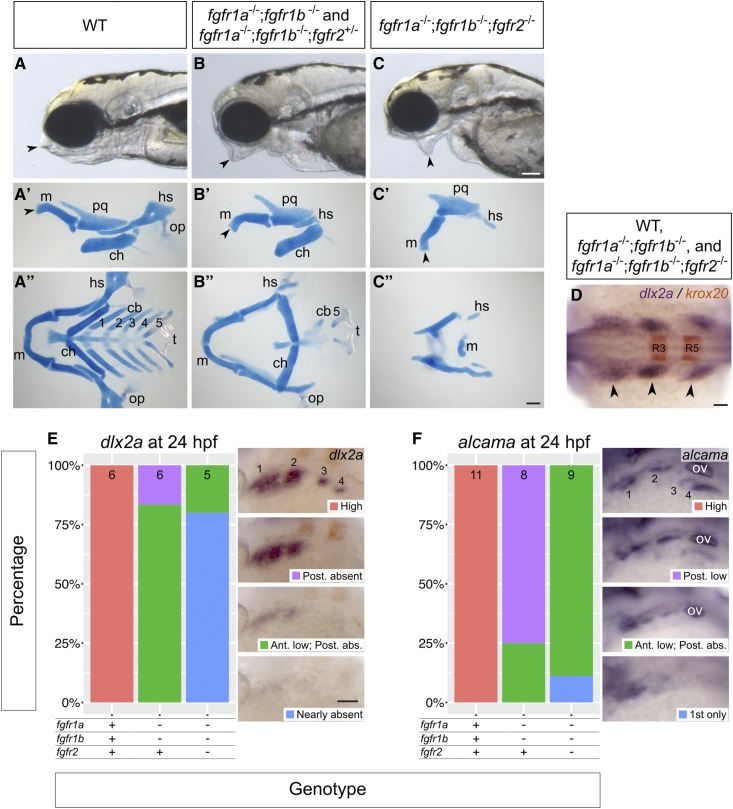
fgfr1a, fgfr1b, and fgfr2 function redundantly to regulate viscerocranial development. (A–C) Lateral views of 5 dpf wild-type (WT, A; n = 12), fgfr1a−/−;fgfr1b−/− (n = 3)/fgfr1a−/−;fgfr1b−/−;fgfr2+/− (B; n = 6), fgfr1a−/−;fgfr1b−/−;fgfr2−/− (C; n = 7) mutant larvae. (A′–C′′) Alcian blue cartilage stains of 5 dpf larvae; arrowheads noting corresponding jaw features between the live larvae in (A–C) and lateral view cartilage mounts in (A′–C ′); m, Meckel’s cartilage; pq, palatoquadrate; hs, hyosymplectic; ch, ceratohyal; op, operculum; cb 1–5, ceratobranchials; t, teeth. (D–F) Pharyngeal arch (D and E) and pouch (F) marker analysis of fgfr double and triple mutant embryos at the 18-somite stage (dlx2a in purple/krox20 in brown labeling rhombomeres 3 (R3) and 5 (R5); D) and 24 hpf (dlx2a, E; alcama; F). Whole mount in situ hybridization was performed, embryos were scored for expression, and genotypes were determined post hoc. All embryos had indistinguishable dlx2a expression at the 18-somite stage (D; n = 7, 26, 6, for WT, fgfr1a−/−;fgfr1b−/−, and fgfr1a−/−;fgfr1b−/−;fgfr2−/−, respectively). In (E and F), the percentage of embryos expressing particular levels of each marker gene is represented in a stacked column chart on the left (sample size for each genotype is listed at the top of each bar), and representative images of those expression levels are shown for each marker to the right (dorsolateral views, rostral to the left and dorsal up; pharyngeal arches (E) and pouches (F) are labeled 1–4. Bars: in (C), 100 μm for (A–C); in (C′′), 100 μm for (A′–C′′); in (D), 50 μm; in (E), 100 μm for (E and F). abs., absent; Ant., Anterior arches/pouches; in (F), ov, otic vesicle; Post., Posterior arches/pouches.
Figure 7.
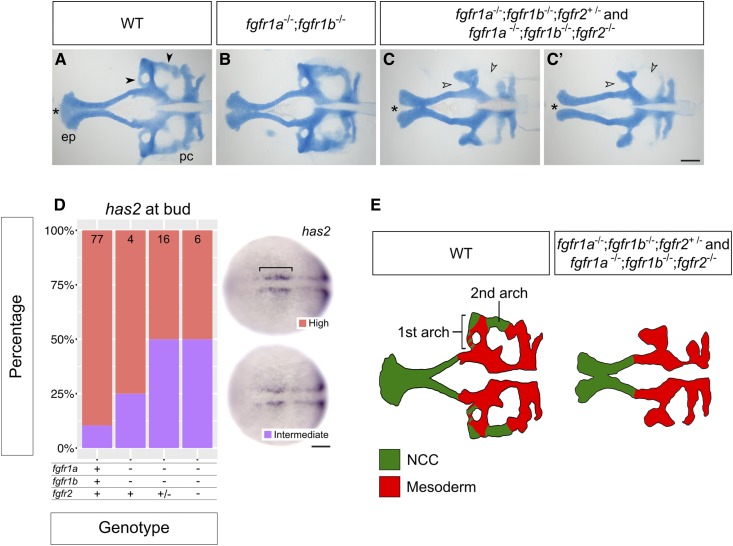
fgfr1a, fgfr1b, and fgfr2 function redundantly to regulate neurocranial development. (A–C′) Alcian blue cartilage staining of 5 dpf wild-type (WT, A; n = 12), fgfr1a−/−;fgfr1b−/− (B; n = 3), fgfr1a−/−;fgfr1b−/−;fgfr2+/− (n = 6)/fgfr1a−/−;fgfr1b−/−;fgfr2−/− (n = 7) (C and C′) mutant larvae. Notice the variable fusion of the trabeculae (*) in fgfr1a−/−;fgfr1b−/−;fgfr2−/− triple mutants (C and C′) compared to WT (A); full fusion in 3/7 animals, partial fusion in 2/7 animals, no fusion in 2/7 animals. Open arrowheads in (C and C′) denote missing regions of the postchordal neurocranium [compare filled arrowheads in (A)]. (D) Cephalic mesoderm marker analysis of fgfr double and triple mutant embryos at the bud stage using has2. Whole mount in situ hybridization was performed, embryos were scored for expression, and genotypes were determined post hoc. The percentage of embryos expressing “High” or “Intermediate” has2 expression is represented in a stacked column chart on the left (sample size for each genotype is listed at the top of each bar), and representative images of those expression levels are shown for each marker to the right (dorsal views, rostral to the left; bracket denotes cells specified for cephalic development. (E) Traces of cartilage mounts in (A and C), filled in with expected lineage contributions, adapted with permission from McCarthy et al. (2016). Bars: in (C), 100 μm for (A–C′); in (D), 50 μm. ep, ethmoid plate; pc, postchordal neurocranium.
Imaging
Embryos were mounted in 4% methylcellulose and imaged on a Leica MZ16 F stereomicroscope. Embryos in Figure 3, E–G were flat-mounted in 70% glycerol and imaged on a Zeiss Axiophot microscope. Images were collected with a Leica DFC500 digital camera.
Figure 3.
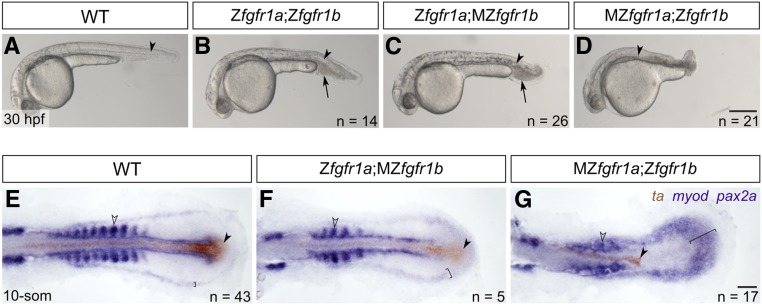
fgfr1a and fgfr1b function redundantly to regulate posterior mesoderm development. (A–D) Lateral view of 30 hpf wild-type (WT; A), and Zfgfr1a;Zfgfr1b (B), Zfgfr1a;MZfgfr1b (C), and MZfgfr1a;Zfgfr1b (D) mutant embryos. Arrowheads denote the notochord; arrows mark pooled blood cells in (B and C). Anterior is to the left, dorsal is up. (E–G) Mesodermal derivative marker analysis of Zfgfr1a;MZfgfr1b and MZfgfr1a;Zfgfr1b double mutant embryos at the 10-somite stage. The notochord (labeled with ta, brown; filled arrowhead) extends down the length of the trunk and tail in wild-type (E) and Zfgfr1a;MZfgfr1b double mutant embryos (F), but is truncated in MZfgfr1a;Zfgfr1b double mutant embryos (G). Defined somites (labeled with myod, purple; open arrowhead) are present in wild-type embryos (E) and Zfgfr1a;MZfgfr1b embryos (F); however, the latter have distinctly fewer somites (5 compared to 10). Although MZfgfr1a;Zfgfr1b mutants retain some myod-positive cells, there are no definitive somites (G). Pronephric precursors (labeled with pax2a, purple; brackets) are restricted to a defined band around the trunk and tail of wild-type embryos (E), a region that is expanded in both Zfgfr1a;MZfgfr1b (F) and MZfgfr1a;Zfgfr1b double mutant embryos (G). Bars: in (D), 200 μm for (A–D); in (G), 50 μm for (E–G).
RT-qPCR
For RT-qPCR experiments, tail biopsies were collected from 24 hpf embryos and placed immediately in genomic DNA lysis buffer (10 mM Tris pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 0.3% Tween-20, 0.3% NP-40). Bodies were collected in 1.6 ml microcentrifuge tubes and stored in liquid nitrogen until tail tissue could be genotyped using the primers listed in Table S2. Total RNA was isolated from the bodies according to (de Jong et al. 2010) with the following exceptions: disposable pestles (Cat. No. 1415–5390; USA Scientific) were used in lieu of metal; Tri Reagent (Cat. No. AM9738; Thermo Fisher Scientific) in lieu of Qiazol; 100 μl of chloroform was added to homogenate instead of 60 μl; vacuum grease was used in lieu of phase lock gel heavy; RNA Clean and Concentrator with on-column DNase treatment (Cat. No. R1014; Zymo Research) in lieu of RNeasy MinElute Cleanup; RNA was eluted with 8.5 μl nuclease-free water instead of 14 μl. RNA was quantified using a NanoDrop 1000 (Thermo Fisher Scientific). cDNA libraries were prepared using 200 ng of total RNA in a 10 μl reaction using the RETROscript Reverse Transcription Kit (Cat. No. AM1710; Thermo Fisher Scientific), and diluted 1:5 after reverse transcription.
qPCR reactions (20 μl) were prepared with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), 1 μl of diluted cDNA, and primers (final concentration: 0.25 μM) listed in Table S4. Only primer sets with a PCR efficiency between 1.9 and 2.1, and an R2 value above 0.98 were used for qPCR experiments. Reactions were performed on a Bio-Rad CFX96 machine with three technical replicates. Samples whose technical replicates had a standard deviation (SD) >0.26 cycles were discarded. Fold change between wild-type and mutant animals was determined using the ddCt method and rpl13a as the reference gene. Unpaired Student’s t-tests were performed to determine whether the expression of individual fgfr genes changed between wild-type and mutant siblings (e.g., expression of fgfr1b in wild type compared to fgfr1a−/− mutants). MANOVA tests were performed to determine whether the combined changes in wild-type fgfr mRNAs were significantly different between wild-type and mutant siblings (e.g., combined expression of fgfr2, fgfr3, and fgfr4 in wild-type compared to fgfr1a−/−;fgfr1b−/− mutants).
Statistics and plotting
Statistics were performed in R and RStudio, using standard packages (R Core Team 2015). Graphing was performed in R, using ggplot2 (Wickham 2009).
Data availability
All fish lines and plasmids are available upon request. Fish lines will be deposited at the Zebrafish International Resource Center. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8162003.
Results and Discussion
Generation of mutant alleles
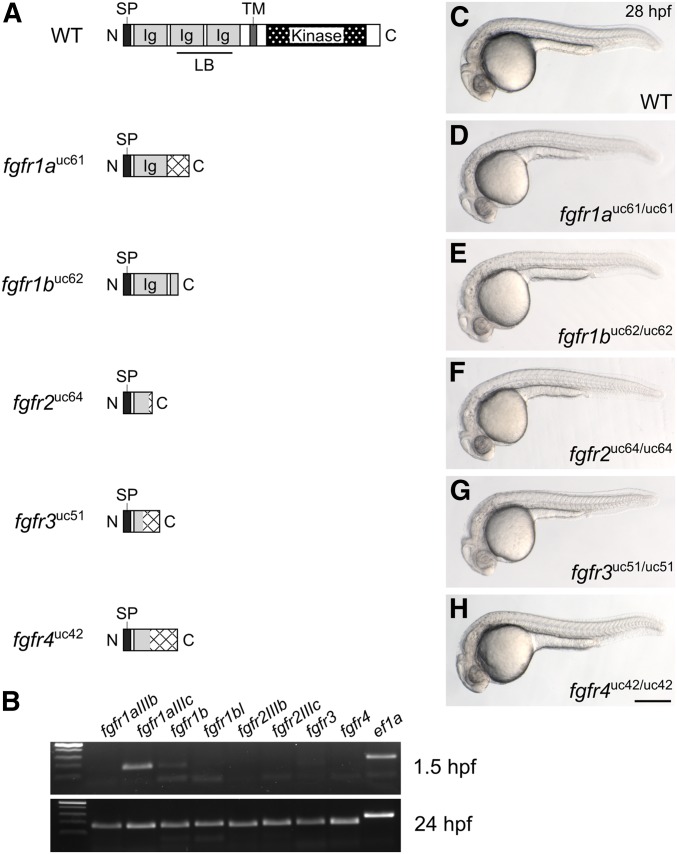
To determine the function of Fgf receptors in zebrafish development, we generated a null allele for each gene using the CRISPR/Cas9 gene editing system. Fgf receptors are composed of an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular kinase domain. We used single-stranded guide RNAs to target Cas9 endonuclease to the 5′ end of each coding sequence to induce frameshift-causing indel mutations, which were confirmed by sequencing genomic DNA and then cDNA to assess the possibility of exon skipping that could result in a truncated, but functional, protein (Mou et al. 2017; Sharpe and Cooper 2017; Figure 1A and Table 1). A 127-bp insertion into fgfr1a exon 5 was the only mutation that resulted in exon skipping. However, because exon 5 is not a multiple of three (173 bp), the mis-spliced transcript goes out of frame at the aberrant splice junction. The predicted peptides that can be potentially translated from each of the five mutant alleles are illustrated in Figure 1A.
Figure 1.
Fgf receptor mutants are embryonic viable. (A) Schematic diagram of a typical full-length Fgfr protein and the predicted truncated peptides resulting from the fgfr1auc61, fgfr1buc62, fgfr2uc64, fgfr3uc51, and fgfr4uc42 alleles. Hatching indicates missense amino acids. (B) RT-PCR of wild-type embryos. While all fgfr isoforms are detected in 24 hr postfertilization (hpf) embryos (postzygotic genome activation, bottom panel), only fgfr1a (isoform IIIc) and fgfr1b are detected in 1.5 hpf embryos (prezygotic genome activation, top panel). ef1a is shown as a positive control. (C–H) Lateral view of ∼28 hpf wild-type (WT, C) or homozygous mutant (D–H) embryos. Anterior is to the left, dorsal is up. Bar in (H), 200 μm for (C–H). Ig, immunoglobulin; LB, ligand-binding domain; SP, signal peptide; TM, transmembrane domain.
Table 1. Nature of CRISPR/Cas9-generated alleles.
| Gene | Allele | Nature of genomic disruption | Exons skipped during splicing | WT peptide length (AA) | Predicted length of resulting peptide (AA) | # of AA in frame |
|---|---|---|---|---|---|---|
| fgfr1a | uc61 | 127 bp inserted into exon 5 | Exon 5 | 810 | 202 | 138 |
| fgfr1b | uc62 | 5 bp deleted from exon 6 | — | 741 | 220 | 220 |
| fgfr2 | uc64 | 47 bp deleted from exon 3 | — | 838 | 76 | 71 |
| fgfr3 | uc51 | 50 bp deleted from exon 3 | — | 821 | 95 | 49 |
| fgfr4 | uc50 | 4 bp deleted from exon 3 | — | 922 | 134 | 64 |
Fgf signaling is involved in many developmental processes, and all five fgfr genes are expressed during zebrafish embryogenesis (Sleptsova-Friedrich et al. 2001; Thisse et al. 2001; Tonou-Fujimori et al. 2002; Scholpp et al. 2004; Nechiporuk et al. 2005; Thisse and Thisse 2005; Harvey and Logan 2006; Thisse et al. 2008; Rohner et al. 2009; Camarata et al. 2010; Ota et al. 2010; Larbuisson et al. 2013; Rohs et al. 2013; Koch et al. 2014; Lovely et al. 2016; Figure 1B). It was therefore surprising that all five fgfr homozygous mutants were embryonic viable and had no apparent defects when compared to wild-type siblings (Figure 1, C–H). Because zygotic defects are often masked by maternally provided gene products (e.g., Waskiewicz et al. 2002; Giraldez et al. 2005; Ciruna et al. 2006; Cheng et al. 2017; Hino et al. 2018), we asked if any of the Fgf receptor mRNAs are maternally provided, and found that, indeed, fgfr1a mRNA (specifically the splice isoform IIIc), and, to a lesser extent, fgfr1b RNA, are maternally provided (Scholpp et al. 2004; Rohner et al. 2009; Ota et al. 2010; Figure 1B). It was therefore possible that this maternal contribution could explain the absence of a phenotype when only the zygotic fgfr expression is lost. To test this, we produced maternal-zygotic (MZ) mutants, which are mutant embryos derived from homozygous mutant mothers, and, therefore, lack both maternal (M) and zygotic (Z) gene products. We found that MZfgfr1a and MZfgfr1b single mutant embryos were phenotypically wild-type (Figure S1). Thus, the absence of phenotypes for any of the receptor single mutants is not likely due to rescue by maternally provided gene products. These data also suggest that the receptor genes act in a redundant or compensatory manner during early development.
Previously, a point mutation in fgfr1a, called fgfr1a(t3R05H), was shown to affect juvenile scale development in zebrafish (Rohner et al. 2009). Animals homozygous for this mutation, which affects a conserved arginine in the intracellular kinase domain and is predicted to be a strong hypomorph, develop with fewer flank scales, and the remaining scales are significantly larger than those of wild-type animals. We therefore asked if a similar scale phenotype in present in animals homozygous for fgfr1a(uc61) null mutations. Indeed, three of five fgfr1a(uc61) mutants analyzed had noticeably larger flank scales compared to wild-type siblings (dotted outlines, Figure S2). However, we did not observe the severe reduction in scale number reported for fgfr1a(t3R05H) mutants (Rohner et al. 2009), suggesting that the severity of this phenotype may be influenced by the genetic background. Alternatively, it is possible that fgfr1a(t3R05H) is a weak antimorph mutation, as it is known that overexpression of an Fgfr lacking a functional intercellular kinase domain can be dominant-negative (Amaya et al. 1991; Griffin et al. 1995).
Genetic redundancy and transcriptional compensation in Fgf receptor mutants
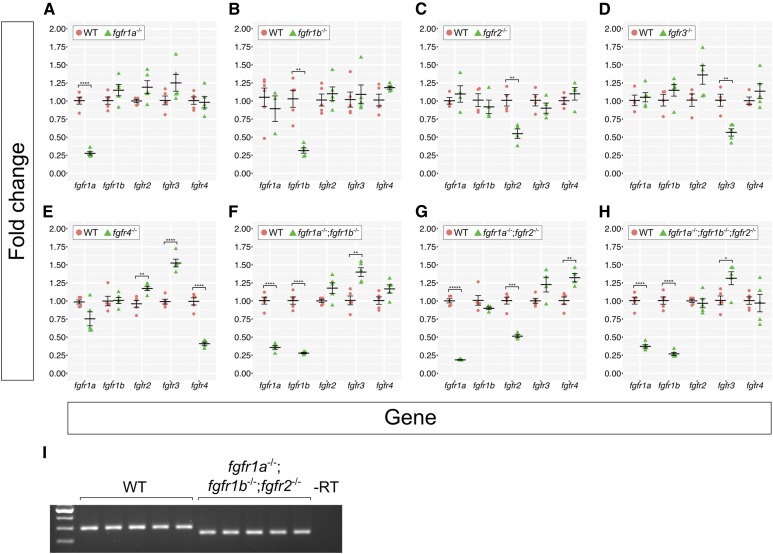
Recently, it has been shown that indel alleles generated by genome editing technologies can result in phenotypes that are weaker than either point mutant alleles or MO oligo knockdown. This is likely due to a mechanism known as genetic compensation, where the transcription of a gene(s) related to the mutated gene is upregulated in mutants and functionally compensates, either partially or completely, for the mutated gene (Rossi et al. 2015; El-Brolosy et al. 2019; Ma et al. 2019). Given that the Fgf receptors share extensive sequence similarity, it was possible that transcriptional compensation could account for the lack of phenotype in our Fgfr mutants. To test this, we used reverse transcription quantitative real-time PCR (RT-qPCR) to examine the expression of all fgfr genes in our fgfr mutants. We compared fgfr gene expression between individual wild-type, single mutant, and select double and triple mutant 24 hpf embryos (Figure 2). Although expression changes are modest, trends emerge from our data. First, with one exception, the mRNA of the mutated gene is detected at significantly lower levels compared to wild type, suggesting that the mutant mRNAs are subject to nonsense-mediated decay (NMD). The exception to this is that, in comparison to wild-type controls, fgfr2 mRNA is significantly decreased in fgfr2−/− single (Figure 2C) and fgfr1a−/−;fgfr2−/− double mutants (Figure 2G), but not in fgfr1a−/−;fgfr1b−/−;fgfr2−/− triple mutant embryos (Figure 2, H and I shows confirmation that these triple mutant embryos are indeed mutant for fgfr2). The reason for this is not known.
Figure 2.
Fgf receptor mRNAs are overexpressed in some Fgf receptor mutants compared to wild-type embryos. (A–H) Fold changes calculated from RT-qPCR experiments comparing Fgf receptor mRNA levels between wild-type (WT, pink circles) and various Fgf receptor mutants (green triangles). Each point represents the mean fold change of an individual embryo, relative to WT. Error bars represent ± the SEM (center bar). Number of asterisks represents P-values calculated using a Student’s t-test: no asterisk between WT and mutant denotes P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, *****P < 0.00001. Results of the Student’s t-test were confirmed using Multivariate ANOVA (MANOVA) tests (see Materials and Methods). (I) Because the fgfr1a;fgfr1b;fgfr2 triple mutants represented in (H) displayed near-WT levels of fgfr2 mRNA, fgfr2 genotypes were confirmed with standard RT-PCR. Note the decreased band size in all fgfr1a;fgfr1b;fgfr2 mutants.
Given that NMD appears to induce transcriptional adaption (El-Brolosy et al. 2019; Ma et al. 2019), we tested whether our mutants have increased expression of the wild-type fgfr mRNAs. With the exception of fgfr4 mutants, which have a significant increase in the expression of wild-type fgfr2 and fgfr3 mRNA (Figure 2E), none of the other single mutants displayed significant increases in the expression of any of the wild-type fgfr RNAs (Figure 2, A–D). By contrast, all double and triple mutant combinations we examined had significantly higher levels of wild-type fgfr3 mRNA (in fgfr1a−/−;fgfr1b−/− and fgfr1a−/−;fgfr1b−/−;fgfr2−/− mutant combinations) or fgfr4 mRNA (in fgfr1a−/−;fgfr2−/− mutants) when compared to wild-type controls (Figure 2, F–H). Whether these increases in wild-type fgfr mRNA expression in the double and triple mutants result in less severe mutant phenotypes remains to be determined. Importantly, because significant upregulation of any of the wild-type fgfr mRNAs in single mutants was only found for fgfr4 mutants, the lack of phenotypes in single fgfr1a, fgfr1b, fgfr2, and fgfr3 mutants is likely due to genetic redundancy, not genetic compensation.
fgfr1a and fgfr1b are required for posterior mesoderm development
In vertebrates, the tail derives from the tailbud—a population of multipotent cells that forms at the caudal end of the embryo at the end of gastrulation. (Kimelman 2016). These cells undergo proliferation and differentiation, resulting in the elongation of the body axis in the posterior direction. Lineage analyses have shown that the tailbud gives rise to the posterior neural tube, notochord, and somites (Kanki and Ho 1997; Davis and Kirschner 2000). Fgf signaling is known to play a role in posterior mesoderm development; both Fgfr1 and Fgf8 mouse mutants lack posterior mesoderm due to failures in mesodermal specification and morphogenesis at the primitive streak (Deng et al. 1994; Yamaguchi et al. 1994; Sun et al. 1999; Ciruna and Rossant 2001). In zebrafish, animals expressing a dominant-negative Fgfr form no posterior mesoderm (Griffin et al. 1995), whereas animals deficient for both fgf8a and fgf24 have a somewhat less severe reduction of posterior mesoderm (Draper et al. 2003). In this latter study, it was shown that fgf8a and fgf24 are together required to maintain, but not initiate, the expression of ta (ntl/brachyury homolog a) and tbx16 (spt), T-box transcription factor genes known to be required for mesodermal specification (Kimmel et al. 1989; Halpern et al. 1993; Conlon et al. 1996; Zhang et al. 1998; Amacher et al. 2002; Warga et al. 2013). These expression defects are visible ∼80% epiboly (Draper et al. 2003)—a time at which only fgfr1a and fgfr1b are expressed highly at the margin of the gastrulating embryo where mesodermal precursors reside (Rohner et al. 2009; Ota et al. 2010). By contrast, during gastrulation, fgfr2 and fgfr3 have minimal expression in mesodermal precursors, and fgfr4 expression appears to be restricted to cells that reside closer to the animal pole (Ota et al. 2010). These expression data therefore identify fgfr1a and fgfr1b as the likely candidate receptors involved in posterior mesoderm development.
Previously, Rohner et al. (2009) showed a variable posterior defect in animals homozygous for fgfr1a(t3R05H) that were also injected with MOs targeting fgfr1b, suggesting that these two receptor genes may play redundant roles in the formation of the posterior mesoderm. We therefore asked whether our fgfr1a;fgfr1b double mutant animals had defects in posterior mesoderm development. Although these double mutants die ∼5 days postfertilization (dpf), we found that, in comparison to wild-type animals (Figure 3A), fgfr1a;fgfr1b double mutant animals have shorter and slightly kinked tails, and an accumulation of blood on the ventral side posterior to the yolk extension (Figure 3B). Because both fgfr1a and fgfr1b mRNAs are maternally provided (Figure 1B), it was possible that the mild defects observed were due to maternal gene product. To test this, we produced various combinations of maternal and/or zygotic loss of fgfr1a and fgfr1b. We found that Zfgfr1a;MZfgfr1b mutants were largely indistinguishable from Zfgfr1a;Zfgfr1b mutants (Figure 3, B and C), arguing that maternally provided fgfr1b was not responsible for the mild phenotype. By contrast, we found that MZfgfr1a;Zfgfr1b embryos had significantly shorter tails than Zfgfr1a;Zfgfr1b mutant embryos (Figure 3, B and D). These data argue that normal posterior mesoderm development requires zygotically expressed fgfr1a and fgfr1b, but also maternally expressed fgfr1a. Because fgfr2 appears to be redundant with fgfr1a and fgfr1b in the other developmental contexts reported here (Figure 4, Figure 5, Figure 6, and Figure 7), we also asked whether the additional removal of functional fgfr2 from Zfgfr1a;Zfgfr1b or MZfgfr1a;Zfgfr1b double mutant embryos enhanced the respective phenotypes. However, these triple mutants underwent similar posterior mesoderm development to their double mutant counterparts, suggesting that fgfr2 does not play a major role in this process (Figure S1, C and D).
Figure 4.
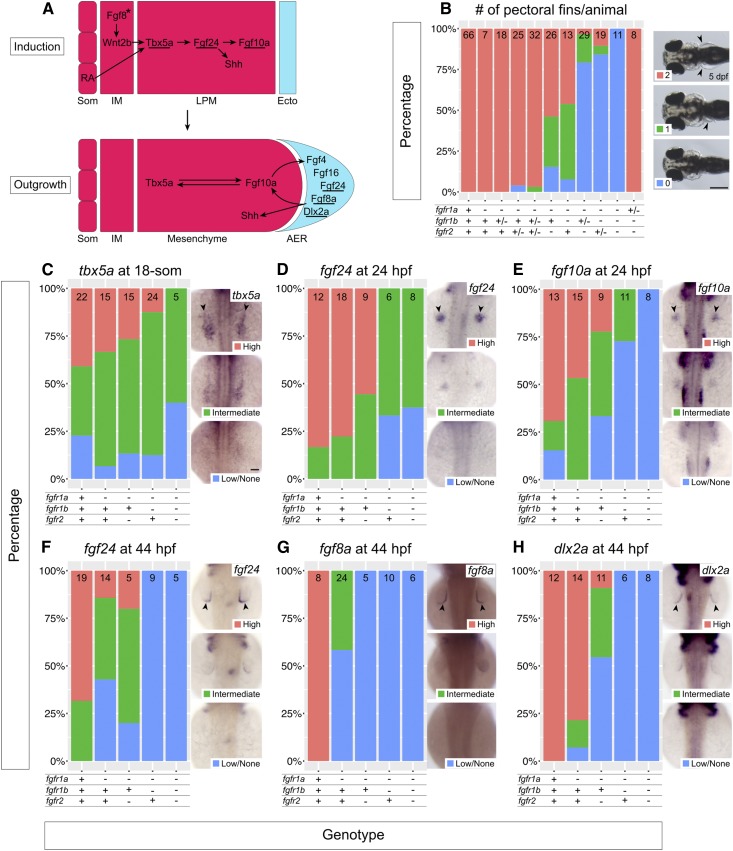
fgfr1a, fgfr1b, and fgfr2 function redundantly to regulate pectoral fin development. (A) Model of pectoral fin bud development during pectoral fin bud Induction (top) and Outgrowth (bottom). Underlines denote genes assayed in (C–H). Arrows denote an epistatic (but not direct) link between molecules. Asterisk signifies that Fgf8 has not yet been shown to play this role in zebrafish, but is hypothesized from forelimb work in chick and mouse. (B) Stacked column chart depicting the average number of pectoral fins per animal at 5 dpf, according to genotype. Sample size for each genotype is listed at the top of each bar. Representative images of larvae with 2, 1, or 0 pectoral fins to the right: dorsal views, anterior to the left, with arrowheads denoting pectoral fins where present. (C–H) Fin bud marker analysis of fgfr double and triple mutant embryos at the 18-somite stage (tbx5a, C), 24 hpf (fgf24, D; fgf10a, E), and 44 hpf (fgf24, F; fgf8a, G; dlx2a, H). Whole mount in situ hybridization was performed, embryos were scored for expression, and genotypes were determined post hoc. In each panel, the percentage of embryos expressing particular levels of each marker gene is represented in a stacked column chart on the left, and representative images of those expression levels are shown for each marker to the right (dorsal views, anterior up; developing fin buds are seen as two spots on either side of the embryo, denoted by arrowheads). Bars: in (B), 200 μm; in (C), 50 μm for (C–H).
Figure 5.
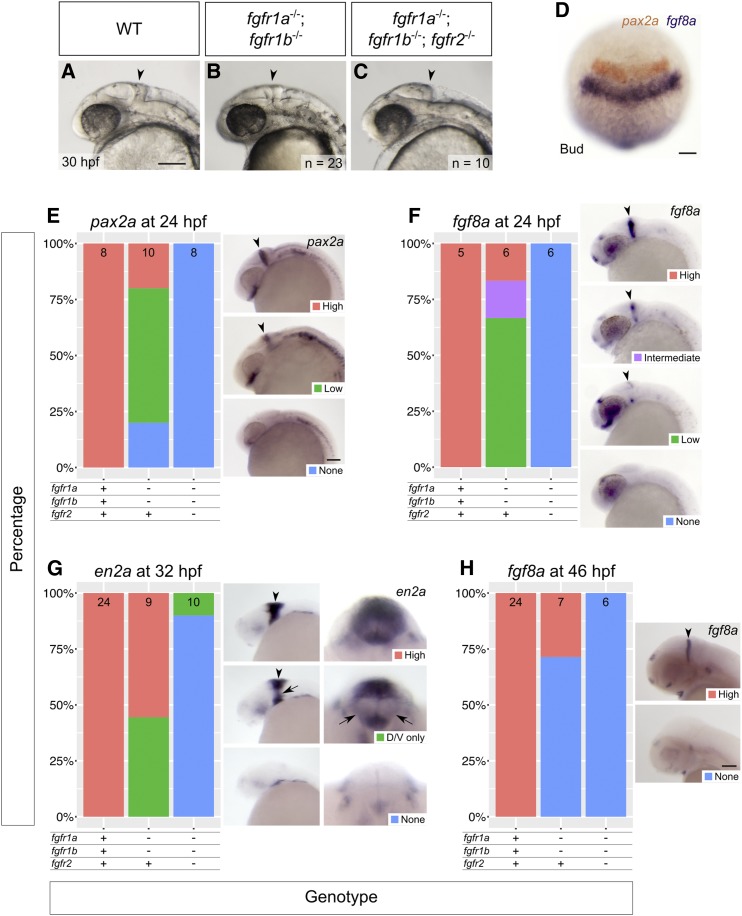
fgfr1a, fgfr1b, and fgfr2 function redundantly to regulate MHB development. (A–C) Lateral view of 30 hpf wild-type (WT, A), and fgfr1a−/−;fgfr1b−/− (B; n = 23), fgfr1a−/−;fgfr1b−/−;fgfr2−/− (C; n = 10) mutant embryos. Arrowheads denote region where the MHB should form. Rostral is to the left, dorsal is up. (D–H) MHB marker analysis of fgfr double and triple mutant embryos at the bud stage (pax2a in brown/fgf8a in purple, D), 24 hpf (pax2a, E; fgf8a, F), 32 hpf (en2a, G), and 46 hpf (fgf8a, H). Whole mount in situ hybridization was performed, embryos were scored for expression, and genotypes were determined post hoc. All embryos had indistinguishable pax2a/fgf8a expression at the bud stage (D; n = 28, 6, 4, for WT, fgfr1a−/−;fgfr1b−/−, and fgfr1a−/−;fgfr1b−/−;fgfr2−/−, respectively). In (E–H), the percentage of embryos expressing particular levels of each marker gene is represented in a stacked column chart on the left (sample size for each genotype is listed at the top of each bar), and representative images of those expression levels are shown for each marker to the right (lateral views, rostral to the left and dorsal up; developing MHBs are denoted by arrowheads). Rightmost images in (G) are magnified frontal views (dorsal up) showing low en2a staining in the left and right regions of the cerebellum (arrows). Bars: in (A), 100 μm for (A–C); in (D), 100 μm; in (E), 100 μm for (E–G); in (H), 100 μm.
To further examine the posterior defects, we compared gene expression between wild-type, Zfgfr1a;MZfgfr1b, and MZfgfr1a;Zfgfr1b 10-somite stage embryos by RNA in situ hybridization to assess the relative amounts of mesodermal derivatives produced. We costained embryos for the axial mesoderm marker ta (formerly ntl), which labels notochord cells (Schulte-Merker et al. 1992), and the paraxial mesodermal marker myod, which labels somitic mesoderm (Weinberg et al. 1996). We found that, while wild-type and Zfgfr1a;MZfgfr1b embryos have a notochord that extends down the entire length of the trunk and tail, MZfgfr1a;Zfgfr1b mutant embryos formed notochord only in the trunk region (filled arrowheads, Figure 3, E–G). Similarly, we found that, at 14 hpf, when wild-type embryos had produced 10 somites, Zfgfr1a;MZfgfr1b embryos have formed only 5 somites (Figure 3, E and F). Finally, in MZfgfr1a; Zfgfr1b mutant embryos, although somitic mesoderm appears to have formed based on myod expression, proper somite morphogenesis appears to have failed (Figure 3G; open arrowhead). These findings are similar to that of fgf8a mutants injected with fgf24 MOs, suggesting that these ligands signal, at least in part, via Fgfr1a and Fgfr1b during posterior mesoderm development. However, MZfgfr1a;Zfgfr1b mutants form more posterior mesoderm than fgf8a mutant; fgf24MO animals (Draper et al. 2003), and significantly more than animals expressing a dominant-negative Fgfr (Griffin et al. 1995). We therefore hypothesize that residual activity of one or more of the remaining three Fgf receptors (Fgfr2, Fgfr3, Fgfr4) must be sufficient to promote partial production of posterior tissue.
pax2a is a marker of pronephric precursors, and, in wild-type embryos, is restricted to a discrete domain of lateral plate mesoderm in the 10-somite stage embryo (Krauss et al. 1991; Draper et al. 2003; bracket, Figure 3E). By contrast, Zfgfr1a;MZfgfr1b, and, to a greater extent, MZfgfr1a;Zfgfr1b mutants have an expanded region of pax2a-expressing cells, suggesting that these mutants produce an increased number of pronephric precursors relative to wild-type embryos (brackets, Figure 3, F and G). Additionally, Zfgfr1a;Zfgfr1b and Zfgfr1a;MZfgfr1b mutants have an accumulation of blood cells just posterior to the yolk extension, similar to dorsalized mutants, such as chordin (Wagner and Mullins 2002; arrows, Figure 3, B and C). Given that the pronephros and blood are derived from lateral plate mesoderm, and somites and notochord arise from more dorsal paraxial and axial mesoderm, respectively, these data are consistent with previous analyses of fgf8a mutants, which concluded that Fgf signaling is important for promoting dorsal mesodermal fates (Furthauer et al. 1997, 2004; Schier and Talbot 2005).
fgfr1a, fgfr1b, and fgfr2 are required for pectoral fin development
Pectoral fins are the equivalent of mammalian forelimbs, and, with few exceptions, their development is regulated by orthologs of genes that regulate mammalian forelimb development (Mercader 2007). Studies using mouse, chick, and zebrafish have identified the important and conserved signaling ligands that participate in this process (Zuniga 2015; Figure 4A). For example, in both forelimb and pectoral fin development, the limb field within the lateral plate mesoderm (LPM) is specified by signals produced by the paraxial (retinoic acid) and intermediate (FGF8 and WNT2B) mesoderm, which initiates expression of the T-box transcription factor gene, TBX5/tbx5a, in the LPM (Cohn et al. 1995; Crossley et al. 1996b; Kawakami et al. 2001; Ng et al. 2002; Gibert et al. 2006). Subsequently, TBX5/Tbx5a activates the expression of an Fgf ligand, Fgf10/fgf10a within the LPM (Min et al. 1998; Ng et al. 2002). In zebrafish, this step is mediated by another Fgf ligand, Fgf24 (Min et al. 1998; Ng et al. 2002; Fischer et al. 2003). FGF10/Fgf10a in turn acts upon the overlying ectoderm to initiate formation of the apical ectodermal ridge (AER)—a structure that reciprocally signals back to the mesoderm via additional Fgf ligands (e.g., FGF2, FGF4, FGF8 in mouse and chick; Fgf4, Fgf8a, Fgf24, and Fgf16 in zebrafish; Niswander and Martin 1992; Fallon et al. 1994; Laufer et al. 1994; Crossley et al. 1996b; Kengaku et al. 1998; Min et al. 1998; Kawakami et al. 2001; Sun et al. 2002; Fischer et al. 2003; Nomura et al. 2006) to maintain the expression of fin development genes within the fin bud mesenchyme. This Fgf-dependent feedback loop is maintained for the duration of limb development, and is required for limb outgrowth and patterning along the proximodistal axis (reviewed in Xu et al. 1999b). Thus, limb development requires a complex signaling network—of which Fgf signaling is a key component—to coordinate its development.
In mouse, null mutations of Fgfr1 and Fgfr2 result in embryonic lethality before the end of gastrulation, precluding their use for determining their role in limb development (Deng et al. 1994; Yamaguchi et al. 1994; Arman et al. 1998). However, the use of hypomorphic alleles has led to the conclusion that FGFR1 is involved in limb patterning, while FGFR2 has a more prominent role in limb bud induction and outgrowth (Xu et al. 1998, 1999a,b; De Moerlooze et al. 2000). Given these findings, and the established roles for Fgf ligands throughout limb development, it was surprising that all five single fgfr mutants have normal pectoral fin development (arrowheads, Figure 4B, and data not shown). However, fgfr1a, fgfr1b, and fgfr2 are all expressed in the developing fin bud (Thisse and Thisse 2005; Harvey and Logan 2006; Thisse et al. 2008; Camarata et al. 2010; Rohs et al. 2013), suggesting that these receptors may play redundant roles in fin development. Consistent with this hypothesis, at 5 dpf we found that 54% of fgfr1a−/−;fgfr1b−/− double mutants, and 46% of fgfr1a−/−;fgfr2−/− double mutants, lack at least one pectoral fin (n = 13 and n = 26, respectively), establishing a role for all three of these receptors in pectoral fin development. Removing the function of an additional fgfr allele in these double homozygous mutants increases the penetrance of the pectoral fin phenotype: 90% of fgfr1a−/−;fgfr1b−/−;fgfr2+/− and 97% of fgfr1a−/−;fgfr1b+/−;fgfr2−/− lack at least one pectoral fin (n = 19 and n = 29, respectively). Finally, 100% of fgfr1a−/−;fgfr1b−/−;fgfr2−/− triple mutants fail to form any pectoral fins (n = 11; Figure 4B), indicating that these three receptors act redundantly to promote zebrafish pectoral fin development. Interestingly, the presence of a single wild-type copy of fgfr1a is sufficient to rescue this phenotype completely (Figure 4B), suggesting that fgfr1a is particularly important for pectoral fin development.
In zebrafish, pectoral fin bud initiation occurs around the 18-somite stage (18 hpf), as evident by the expression of tbx5a, one of two orthologs of mouse and chick Tbx5 (Ahn et al. 2002; Garrity et al. 2002; Ng et al. 2002). Shortly thereafter, Tbx5a promotes the transcription of fgf24 within the fin bud mesoderm, which is then required for mesodermal transcription of fgf10a and sonic hedgehog (shh; involved in anterior-posterior patterning of fins and limbs) (Krauss et al. 1991, 1993; Neumann et al. 1999; Ng et al. 2002; Fischer et al. 2003). Fgf10a maintains tbx5a expression in the fin bud mesoderm, and is likely responsible for signaling to the overlying ectoderm to induce AER formation, which includes the induction of fgf8a, fgf4, fgf24, and fgf16 expression in the ectoderm by ∼30–36 hpf (Reifers et al. 1998; Grandel et al. 2000; Ng et al. 2002; Fischer et al. 2003; Nomura et al. 2006). In chick and mouse, this induction appears to be mediated by WNT signaling (Kengaku et al. 1998; Kawakami et al. 2001); however, this has yet to be established in zebrafish. Similar to chick and mouse, it is likely that the AER Fgfs signal back to the fin bud mesenchyme to stabilize fgf10a expression, thus establishing a positive regulatory feedback loop required for fin bud outgrowth (Camarata et al. 2010; Figure 4A).
Because Fgf signaling is known to mediate many tissue interactions during limb and fin development, we sought to characterize at what level the various mutant combinations affect fin development by assessing the expression of marker genes using RNA in situ hybridization at different stages of fin bud development. Following in situ hybridization, embryos were scored for marker gene expression first and then genotyped. We initially asked if any of the mutant combinations affected fin bud initiation by assaying the expression of tbx5a—the earliest marker of pectoral fin bud induction (Ahn et al. 2002). In wild-type embryos, tbx5a expression in the LPM could be detected in most, but not all, 18-somite stage embryos—a stage that precedes feedback regulation from the LPM expressed Fgfs (Figure 4, A and C). Expression was similarly detected in all other genotypes examined, including fgfr1a−/−;fgfr1b−/−;fgfr2−/− triple mutants, though the triple mutants had, on average, less intense staining (Figure 4C). These results suggest that the first steps of fin bud induction are only mildly affected by loss of Fgfr1a, Fgfr1b, and Fgfr2 functions (Figure 4C).
In zebrafish, Tbx5a induces the expression of fgf24 in the LPM, and Fgf24 signals within the LPM to stimulate fgf10a expression, which, in turn, is thought to form a feedback loop to maintain tbx5a expression (Ng et al. 2002; Fischer et al. 2003; Figure 4A). While the expression of fgf24 and fgf10a is easy to detect by in situ hybridization in most 24 hpf wild-type fin buds, their expression is reduced or not detected in 24 hpf fgfr1a;fgfr1b and fgfr1a;fgfr2 double mutant and fgfr1a;fgfr1b;fgfr2 triple mutant fin buds (Figure 4, D and E). These results suggest that, although the fin bud is induced in these mutants, Fgfr1a, Fgfr1b, and Fgfr2 are redundantly required to maintain gene expression within the fin bud mesenchyme.
During the outgrowth phase of fin development (24 – ∼48 hpf; Grandel and Schulte-Merker 1998), Fgfs from the fin bud mesenchyme signal the ectoderm to form the AER. We therefore asked if AER-specific gene expression was reduced in fgfr1a;fgfr1b and fgfr1a;fgfr2 double mutants and fgfr1a;fgfr1b;fgfr2 triple mutants. Indeed, whereas fgf24, fgf8a, and dlx2a are all expressed in the AER of wild-type animals at 44 hpf, the number of embryos with reduced or no detectable expression by in situ hybridization is greatly increased in the various mutant combinations (Figure 4, F–H). Interestingly, in fgfr1a single-mutant embryos, which have normal pectoral fin development, we also observed a reduction in AER-expressed fgf24 and fgf8a at 44 hpf (Figure 4, F and G). Together, these results suggest that Fgfr1a, Fgfr1b, and Fgfr2 function redundantly to maintain proper gene expression in the fin bud mesenchyme, and, subsequently, in the AER, but that fgfr1a may be particularly important at the mesenchyme/AER interface. This may explain why the presence of a single wild-type copy of fgfr1a is sufficient to rescue the fgfr1a;fgfr1b;fgfr2 triple mutant pectoral fin phenotype (Figure 4B).
fgfr1a, fgfr1b, and fgfr2 are required for brain development
The vertebrate brain develops from the relatively simple neural plate. One of the earliest patterning events of the neural plate is its subdivision into rostral and caudal domains that can be identified by expression of the transcription factors Otx2 and Gbx2, respectively (Broccoli et al. 1999; Millet et al. 1999). A signaling center called the midbrain-hindbrain organizer forms at the boundary of these domains, which acts to pattern the surrounding neural tissues (Marin and Puelles 1994; Martínez et al. 1995). Fgf signaling is the most prominent signaling pathway in the MHB, and, while many Fgf ligands are known to be expressed in the MHB organizer (Fgf8, Fgf17, Fgf18, Fgf4), Fgf8 appears to have the most critical role: in chick, FGF8-soaked beads ectopically induce midbrain development (Crossley et al. 1996a), whereas mutations in mouse Fgf8, or its ortholog fgf8a in zebrafish, result in loss of the MHB and cerebellum (Brand et al. 1996; Meyers et al. 1998; Reifers et al. 1998; Chi et al. 2003).
In mouse, tissue-specific knockout of Fgfr1 in MHB cells, or an Fgfr1 hypomorphic mutation, leads to the loss of certain MHB structures (Trokovic et al. 2003). However, this phenotype is less severe than that of Fgf8 mutants, suggesting that other receptors are involved in FGF8 signal transduction during MHB development (Chi et al. 2003; Trokovic et al. 2003). In zebrafish, fgfr1a is expressed at high levels in the developing MHB region (Tonou-Fujimori et al. 2002; Scholpp et al. 2004; Rohner et al. 2009; Ota et al. 2010; Larbuisson et al. 2013; Rohs et al. 2013; Koch et al. 2014) and MO knockdown of fgfr1a has been reported to phenocopy fgf8a mutants (Scholpp et al. 2004). By contrast to the fgfr1a morphants, fgfr1a(uc61) (Figure 1D) and fgfr1a(t3R05H) (Rohner et al. 2009) mutants have normal MHB development, arguing that, in zebrafish, Fgf receptors in addition to Fgfr1a are able to mediate Fgf8a signaling during MHB development.
Zebrafish fgf8a mutants first display gene expression abnormalities in the hindbrain region during early somitogenesis, with increasing severity by late somitogenesis (18-somite stage) (Reifers et al. 1998). At the 18-somite (18 hpf) and Prim-5 (24 hpf) stages, when the MHB signaling center is active, fgfr1a, fgfr1b, and fgfr2 are expressed in, or immediately adjacent to, the MHB (Thisse et al. 2001, 2008; Tonou-Fujimori et al. 2002; Scholpp et al. 2004; Thisse and Thisse 2005; Rohner et al. 2009; Ota et al. 2010; Larbuisson et al. 2013; Rohs et al. 2013; Koch et al. 2014; Lovely et al. 2016). Considering the apparent role for FGFR1 in maintenance of midbrain and hindbrain tissue in the mouse, we first tested if fgfr1a;fgfr1b double mutants would lead to a brain defect similar to that of the fgf8a mutation. However, the MHBs of these animals are morphologically indistinguishable from wild type (arrowheads, Figure 5, A and B). In contrast, the triple mutant fgfr1a;fgfr1b;fgfr2 appears to phenocopy the acerebellar phenotype of fgf8a mutants (arrowhead, Figure 5C).
To further assess the brain development of these animals, we used RNA in situ hybridization to assay the expression of genes known to play a role in MHB development. At the bud stage of development (10 hpf), pax2a and fgf8a label the prospective MHB (Krauss et al. 1991; Mikkola et al. 1992; Reifers et al. 1998). In all genotypes examined, including fgfr1a;fgfr1b;fgfr2 triple mutants, we found that both genes are expressed in their stereotypic locations, suggesting that the specification of MHB cells is unaffected (Figure 5D). In contrast, at 24 hpf, when both pax2a and fgf8a are still expressed in the MHB of wild-type embryos, their expression is not detected in the MHB of fgfr1a;fgfr1b;fgfr2 triple mutants (Figure 5, E and F). Consistently, the cerebellar marker en2a is absent from most triple mutants by 32 hpf (Millen et al. 1994; Figure 5, G and H).
Although the induction of prospective MHB genes and MHB morphology is normal in fgfr1a;fgfr1b double mutants (Figure 5, B and D), we found that marker gene expression was reduced in many double mutant embryos when compared to wild-type animals at 24 hpf, and, by 46 hpf, fgf8a expression is not detectable in ∼70% of double mutant embryos (Figure 5, E–H). These data suggest that a partial reduction in Fgf signaling is sufficient to affect gene expression in the MHB, but not overt MHB morphology. Though it is possible that brain patterning, and therefore brain function, is compromised in these animals, we have not been able to test this prospect, as they do not survive past 5 dpf.
fgfr1a, fgfr1b, and fgfr2 are required for craniofacial skeletal development
Skeletal structures of the head are largely derived from cranial neural crest cells. During development, neural crest cells (NCCs) migrate ventrolaterally from the dorsal neuroectoderm of the hindbrain into endodermal pockets called pharyngeal pouches. There, intrinsic cues and inductive signaling from the surrounding tissue instruct NCC development into cartilage (reviewed in Kimmel et al. 2001; Mork and Crump 2015). In zebrafish, seven arches form between the endodermally derived pouches, and most arch derivatives compose the viscerocranium: the first arch gives rise to Meckel’s cartilage and the palatoquadrate; the second gives rise to the ceratohyal and hyosemplectic; and arches three to seven give rise to the ceratobranchials (Schilling and Kimmel 1994; Kimmel et al. 2001; Crump et al. 2004b, 2006). However, lineage tracing has revealed that NCCs also give rise to the neurocranium; NCCs from the first two arches contribute to discrete portions of the postchordal neurocranium, whereas the prechordal neurocranium arises from more anteriorly derived NCCs (Wada et al. 2005; Eberhart et al. 2006; Swartz et al. 2011; McCarthy et al. 2016).
Many Fgfs and their receptors are expressed throughout the head during the time of cranial cell specification and differentiation (Scholpp et al. 2004; Nechiporuk et al. 2005; Rohner et al. 2009; Ota et al. 2010; Larbuisson et al. 2013; Rohs et al. 2013), and several of the processes underlying cranial morphogenesis are known to be driven by Fgf signaling. In mouse, Fgf8 is required for proper development of several pharyngeal arch-derived craniofacial structures, and FGFR1 and FGFR2 play an important role in mammalian palatogenesis (Abu-Issa et al. 2002; Rice et al. 2004; Yu et al. 2015). In zebrafish, fgf8a;fgf3 double mutants do not form the posterior viscerocranium, have severely deformed anterior viscerocranium, and do not form the mesodermally derived cartilages of the postchordal neurocranium (Crump et al. 2004a; McCarthy et al. 2016). Furthermore, MO analysis suggests that the Fgf receptors Fgfr1a and Fgfr2 can each regulate late cartilage formation in the viscerocranium, although the effects are attributed to later defects compared to those caused by loss of fgf8a and fgf3 (Larbuisson et al. 2013). However, we found that cranial development in fgfr1a and fgfr2 single mutants, as well as fgfr1a; fgfr2 double mutants were indistinguishable from wild type (data not shown). By contrast, we found that fgfr1a;fgfr1b double mutants have reduced viscerocrania, including a loss of most of the ceratobranchials and hyosymplectic, and also have misshapen palatoquadrate and Meckel’s cartilage (Figure 6, B–B′′). Although fgfr1a;fgfr1b double mutant animals that are also heterozygous for a null allele of fgfr2 (i.e., fgfr1a−/−;fgfr1b−/−;fgfr2+/−) are indistinguishable from fgfr1a−/−;fgfr1b−/−;fgfr2+/+ animals with respect to viscerocrania development (Figure 6, B–B′′), both fgfr1a−/−;fgfr1b−/−;fgfr2+/− and fgfr1a−/−;fgfr1b−/−;fgfr2−/− mutants lack portions of the postchordal neurocranium that are thought to be derived from the first and second pharyngeal arches (Figure 7, C and C′). Finally, of all genotypes analyzed, fgfr1a;fgfr1b;fgfr2 triple mutants have the most severe cranial defects: in addition to an abnormal postchordal neurocranium (Figure 7, C and C′), the viscerocranial ceratobranchials, ceratohyal, and most of the hyosymplectic fail to form, the palatoquadrate is misshapen, and Meckel’s cartilage is displaced downward (Figure 6, C–C′′). Together, these data suggest that Fgfr1a, Fgfr1b, and Fgfr2 function together to promote cranial cartilage formation.
Recent studies have proposed that, during development of the head skeleton, Fgf signaling is required either during pharyngeal pouch/arch formation or maintenance (Crump et al. 2004a), or later during cartilage formation (Larbuisson et al. 2013). We therefore asked at which stage our Fgfr mutations affect cranial development by assessing the expression of known marker genes of pharyngeal endoderm and NCCs—the cells that primarily form the pharyngeal pouches and arches, respectively. In a wild-type 18-somite stage embryo, NCCs expressing dlx2a have migrated into the pouches, where they form three distinct clusters on each side of the embryo, at the anterioposterior level of the midbrain and hindbrain (arrowheads, Figure 6D). The most anterior of these clusters will give rise to the first pharyngeal arch, the middle to the second arch, and the most posterior will later separate to give rise to arches three to seven. We found that embryos of all genotypes, including fgfr1a;fgfr1b;fgfr2 triple mutants, have normal dlx2a expression at the 18-somite stage, indicating that NCCs successfully migrate to, and populate, the pharyngeal pouches (Figure 6D).
In a wild-type 24 hpf embryo (Prim-5 stage), the most posterior cluster of NCCs has begun to separate into two distinct domains (labeled 3 and 4 in Figure 6E), and alcama-labeled pharyngeal endoderm, which separates the arches, also separates into two domains posteriorly (labeled 3 and 4 in Figure 6F). In all 24 hpf fgfr1a;fgfr1b double- and fgfr1a;fgfr1b;fgfr2 triple-mutant embryos, however, dlx2a and alcama expression is either reduced or not detected, with the largest reduction seen in the posterior-most arches and pouches, respectively (Figure 6, E and F). Together, these data suggest that NCC migration into the pharyngeal pouches occurs normally in mutants, but that these cells are not maintained. These data therefore argue for an early role of Fgf signaling in cranial development similar to that previously proposed by Crump et al. (2004a).
Previous fate-mapping experiments in zebrafish indicate that discrete regions of the postchordal neurocranium derive from both mesoderm and NCC (McCarthy et al. 2016; Figure 7E). Fgf8a and Fgf3 function redundantly to establish the mesodermal precursors that will make up this tissue, and embryos deficient for both ligands exhibit severely reduce postchordal neurocranium (McCarthy et al. 2016). By contrast, the portions of cartilage missing from fgfr1a−/−;fgfr1b−/−;fgfr2+/− and fgfr1a−/−;fgfr1b−/−;fgfr2−/− mutant neurocrania appear to correspond solely with the NCC-derived regions of wild-type neurocrania (arrowheads, Figure 7, A, C, and D), while the mesodermally derived regions are retained. To confirm that mesodermal precursors are in fact preserved in fgfr1a−/−;fgfr1b−/−;fgfr2+/− and fgfr1a−/−;fgfr1b−/−;fgfr2−/− mutants, we used in situ hybridization to assess the expression of has2, which, in wild-type bud-stage embryos, is expressed in cephalic mesoderm precursor cells that localize to discrete bilateral domains flanking the anterior midline (Camenisch et al. 2000; McCarthy et al. 2016; bracket in Figure 7D). Unlike embryos deficient for both fgf8a and fgf3, which have reduced or no detectable expression of has2 (McCarthy et al. 2016), we found little to no detectable difference in the expression of has2 between wild type and fgf receptor mutants (Figure 7D). These results suggest that loss of Fgfr1a, Fgfr1b, and Fgfr2 functions does not affect the initial formation of the cephalic mesoderm, but is instead required for NCC maintenance in this tissue, similar to what we found for the NCCs during viscerocranial development (Figure 6, D–F).
Conclusions
The Fgf signaling pathway regulates numerous developmentally important processes. Although the identity of the specific Fgf ligand(s) that regulates these processes is known in many cases, less is known about the identity of the receptors that mediate particular cellular interactions. Here, we have described the generation and initial characterization of mutations in each of the five zebrafish Fgf receptor genes. We showed that all single mutants are viable and fertile as adults, but that double and triple mutant combinations have defects similar to those of known ligand mutants. These mutants have allowed us to genetically identify for the first time, or to confirm previous studies using MO oligo-based analysis, which receptors likely transduce the signaling of select ligands. One surprise is that all phenotypes described here require loss of Fgfr1a function, suggesting that this receptor is of prime importance for Fgf-dependent processes in early development. It is interesting that Fgfr1a is also the predominant maternally supplied receptor, and, as such, is likely uniformly translated in all cells during early development.
In most cases, estimating relevant receptor–ligand selectivity has relied on in vitro mitogenic assays, where ligands are tested for their ability to induce the proliferation of cells expressing a particular receptor or receptor isoform (Ornitz et al. 1996; Zhang et al. 2006). By contrast, probable receptor–ligand interactions investigated by in vivo mutational analysis have been limited, in part due to the lethal effect of introducing null mutations of Fgfr1 or Fgfr2 into the mouse genome (Deng et al. 1994; Yamaguchi et al. 1994; Arman et al. 1998). Our in vivo analysis suggests that there is not a one-to-one relationship, where each ligand stimulates a single receptor type, but that each ligand appears capable of interacting with multiple receptors. It remains to be seen if these ligands bind only to receptor homodimers, or if ligand binding is able to induce heterodimerization between different receptors that are expressed in the same cell.
Genetic redundancy or transcriptional compensation?
We anticipated that we would find genetic redundancy between fgfr1a and fgfr1b, as these ohnologs arose from a more recent genome duplication event than the more ancient event that gave rise to the other receptor orthologs (Rohner et al. 2009). The apparent genetic redundancy between the fgfr genes was especially striking in light of previous reports that MO knockdown of single fgfr genes can result in morphological abnormalities. For example, MOs that block translation or splicing of fgfr1a were shown to cause deformation of the MHB and pharyngeal cartilages (Scholpp et al. 2004; Larbuisson et al. 2013). Likewise, MO knockdown of fgfr2 was reported to cause viscerocranial cartilage and left/right asymmetry defects (Liu et al. 2011; Larbuisson et al. 2013). It is possible that the MO gene knockdowns do not represent the true receptor loss-of-function phenotypes. Alternatively, it is possible that transcriptional adaptation, which can be induced by indel mutations that cause premature termination codons that triggers NMD of mutant mRNAs, is lessening the phenotypic severity of our indel mutations (Rossi et al. 2015; El-Brolosy et al. 2019; Ma et al. 2019). However, with the exception of fgfr4 mutants, we do not detect significant changes in wild-type fgfr mRNA expression in our fgfr single mutants, suggesting that the lack of phenotype in these animals is likely due to redundancy, not genetic compensation (Figure 2, A–H). This notion is supported by previous studies showing that the expression domains of fgfr1a, fgfr1b, and fgfr2 overlap extensively throughout the developing embryo, including in each of the tissues discussed here (Sleptsova-Friedrich et al. 2001; Thisse et al. 2001, 2008; Tonou-Fujimori et al. 2002; Scholpp et al. 2004; Nechiporuk et al. 2005; Thisse and Thisse 2005; Harvey and Logan 2006; Rohner et al. 2009; Camarata et al. 2010; Ota et al. 2010; Larbuisson et al. 2013; Rohs et al. 2013; Koch et al. 2014; Lovely et al. 2016). With regard to our double and triple mutant analysis, we detected a significant increase in wild-type fgfr3 and fgfr4 mRNAs, raising the possibility that the phenotypes we describe for these mutant combinations could be less severe than those caused by alleles that do not induce genetic compensation. Regardless, these mutant alleles have clearly allowed us to identify Fgf receptors that function in specific developmental processes, and to pair these receptors with their probable Fgf ligand(s).
We have shown several developmental processes that appear to use multiple Fgf receptors. This is not unique to zebrafish, as receptor redundancy has also been reported in mammals. For example, during lung development in mice, Fgfr3−/−;Fgfr4−/− double mutants exhibit disrupted alveogenesis, whereas the lungs of single mutants are normal (Weinstein et al. 1998). Additionally, Zhao and colleagues used conditional knockout of Fgfr1 and Fgfr2 in concert with Fgfr3 mutation to show that these three receptors act redundantly during mouse lens development (Zhao et al. 2008). It is possible that this type of redundancy exists elsewhere in the mouse as well, but the early embryonic lethality of Fgfr1 and Fgfr2 mutations makes this a difficult area of study. Going forward, the zebrafish is an attractive model to investigate these questions, in the context of Fgf signaling.
Acknowledgments
We thank Kira Lin and Lan-Uyen Nguyen for assistance identifying and maintaining the alleles used in this report. We are grateful to members of the Draper, C. Erickson, P. Armstrong, L. Jao, S. Burgess, and C. Juliano laboratories (UC Davis) for valuable discussion of this work, and to Carol Erickson, Matt McFaul, and Sydney Wyatt for their critical review of this manuscript. This work was supported by the National Institute of Child Health and Development (1R01 HD081551-01A1) to B.W.D. and by the National Institute of General Medical Science (2 T32 GM007377) to D.M.L. The authors declare no competing interests.
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8162003.
Communicating editor: D. Greenstein
Literature Cited
- Abu-Issa R., Smyth G., Smoak I., Yamamura K., Meyers E. N., 2002. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129: 4613–4625. [DOI] [PubMed] [Google Scholar]
- Ahn D. G., Kourakis M. J., Rohde L. A., Silver L. M., Ho R. K., 2002. T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature 417: 754–758. 10.1038/nature00814 [DOI] [PubMed] [Google Scholar]
- Amacher S. L., Draper B. W., Summers B. R., Kimmel C. B., 2002. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 129: 3311–3323. [DOI] [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W., 1991. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66: 257–270. 10.1016/0092-8674(91)90616-7 [DOI] [PubMed] [Google Scholar]
- Arman E., Haffner-Krausz R., Chen Y., Heath J. K., Lonai P., 1998. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl. Acad. Sci. USA 95: 5082–5087. 10.1073/pnas.95.9.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G., Ingham P. W., 2000. Developmental regulation of Tbx5 in zebrafish embryogenesis. Mech. Dev. 90: 299–304. 10.1016/S0925-4773(99)00246-4 [DOI] [PubMed] [Google Scholar]
- Bertrand S., Iwema T., Escriva H., 2014. FGF signaling emerged concomitantly with the origin of Eumetazoans. Mol. Biol. Evol. 31: 310–318. 10.1093/molbev/mst222 [DOI] [PubMed] [Google Scholar]
- Brand M., Heisenberg C. P., Jiang Y. J., Beuchle D., Lun K., et al. , 1996. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development 123: 179–190. [DOI] [PubMed] [Google Scholar]
- Broccoli V., Boncinelli E., Wurst W., 1999. The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401: 164–168. 10.1038/43670 [DOI] [PubMed] [Google Scholar]
- Camarata T., Krcmery J., Snyder D., Park S., Topczewski J., et al. , 2010. Pdlim7 (LMP4) regulation of Tbx5 specifies zebrafish heart atrio-ventricular boundary and valve formation. Dev. Biol. 337: 233–245. 10.1016/j.ydbio.2009.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch T. D., Spicer A. P., Brehm-Gibson T., Biesterfeldt J., Augustine M. L., et al. , 2000. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106: 349–360. 10.1172/JCI10272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah A. T., McEwen D. G., Werner S., Xu J., Ornitz D. M., 1994. Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J. Biol. Chem. 269: 11620–11627. [PubMed] [Google Scholar]
- Cheng X. N., Shao M., Li J. T., Wang Y. F., Qi J., et al. , 2017. Leucine repeat adaptor protein 1 interacts with Dishevelled to regulate gastrulation cell movements in zebrafish. Nat. Commun. 8: 1353 10.1038/s41467-017-01552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C. L., Martinez S., Wurst W., Martin G. R., 2003. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 130: 2633–2644. 10.1242/dev.00487 [DOI] [PubMed] [Google Scholar]
- Ciruna B., Rossant J., 2001. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1: 37–49. 10.1016/S1534-5807(01)00017-X [DOI] [PubMed] [Google Scholar]
- Ciruna B., Jenny A., Lee D., Mlodzik M., Schier A. F., 2006. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439: 220–224. 10.1038/nature04375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. J., Izpisua-Belmonte J. C., Abud H., Heath J. K., Tickle C., 1995. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80: 739–746. 10.1016/0092-8674(95)90352-6 [DOI] [PubMed] [Google Scholar]
- Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G., Ornitz D. M., 1996. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12: 390–397. 10.1038/ng0496-390 [DOI] [PubMed] [Google Scholar]
- Conlon F. L., Sedgwick S. G., Weston K. M., Smith J. C., 1996. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122: 2427–2435. [DOI] [PubMed] [Google Scholar]
- Crossley P. H., Martinez S., Martin G. R., 1996a Midbrain development induced by FGF8 in the chick embryo. Nature 380: 66–68. 10.1038/380066a0 [DOI] [PubMed] [Google Scholar]
- Crossley P. H., Minowada G., MacArthur C. A., Martin G. R., 1996b Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell 84: 127–136. 10.1016/S0092-8674(00)80999-X [DOI] [PubMed] [Google Scholar]
- Crump J. G., Maves L., Lawson N. D., Weinstein B. M., Kimmel C. B., 2004a An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development 131: 5703–5716. 10.1242/dev.01444 [DOI] [PubMed] [Google Scholar]
- Crump J. G., Swartz M. E., Kimmel C. B., 2004b An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2: E244 10.1371/journal.pbio.0020244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump J. G., Swartz M. E., Eberhart J. K., Kimmel C. B., 2006. Moz-dependent Hox expression controls segment-specific fate maps of skeletal precursors in the face. Development 133: 2661–2669. 10.1242/dev.02435 [DOI] [PubMed] [Google Scholar]
- Dahlem T. J., Hoshijima K., Jurynec M. J., Gunther D., Starker C. G., et al. , 2012. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8: e1002861 10.1371/journal.pgen.1002861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Kirschner M. W., 2000. The fate of cells in the tailbud of Xenopus laevis. Development 127: 255–267. [DOI] [PubMed] [Google Scholar]
- de Jong M., Rauwerda H., Bruning O., Verkooijen J., Spaink H. P., et al. , 2010. RNA isolation method for single embryo transcriptome analysis in zebrafish. BMC Res. Notes 3: 73 10.1186/1756-0500-3-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L., Spencer-Dene B., Revest J. M., Hajihosseini M., Rosewell I., et al. , 2000. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127: 483–492. [DOI] [PubMed] [Google Scholar]
- Deng C., Wynshaw-Boris A., Zhou F., Kuo A., Leder P., 1996. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84: 911–921. 10.1016/S0092-8674(00)81069-7 [DOI] [PubMed] [Google Scholar]
- Deng C. X., Wynshaw-Boris A., Shen M. M., Daugherty C., Ornitz D. M., et al. , 1994. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 8: 3045–3057. 10.1101/gad.8.24.3045 [DOI] [PubMed] [Google Scholar]
- Draper B. W., Stock D. W., Kimmel C. B., 2003. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development 130: 4639–4654. 10.1242/dev.00671 [DOI] [PubMed] [Google Scholar]
- Eberhart J. K., Swartz M. E., Crump J. G., Kimmel C. B., 2006. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development 133: 1069–1077. 10.1242/dev.02281 [DOI] [PubMed] [Google Scholar]
- El-Brolosy M. A., Kontarakis Z., Rossi A., Kuenne C., Gunther S., et al. , 2019. Genetic compensation triggered by mutant mRNA degradation. Nature 568: 193–197. 10.1038/s41586-019-1064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon J. F., Lopez A., Ros M. A., Savage M. P., Olwin B. B., et al. , 1994. FGF-2: apical ectodermal ridge growth signal for chick limb development. Science 264: 104–107. 10.1126/science.7908145 [DOI] [PubMed] [Google Scholar]
- Fischer S., Draper B. W., Neumann C. J., 2003. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development 130: 3515–3524. 10.1242/dev.00537 [DOI] [PubMed] [Google Scholar]
- Furthauer M., Thisse C., Thisse B., 1997. A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development 124: 4253–4264. [DOI] [PubMed] [Google Scholar]
- Furthauer M., Van Celst J., Thisse C., Thisse B., 2004. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development 131: 2853–2864. 10.1242/dev.01156 [DOI] [PubMed] [Google Scholar]
- Garrity D. M., Childs S., Fishman M. C., 2002. The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development 129: 4635–4645. [DOI] [PubMed] [Google Scholar]
- Gibert Y., Gajewski A., Meyer A., Begemann G., 2006. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development 133: 2649–2659. 10.1242/dev.02438 [DOI] [PubMed] [Google Scholar]
- Giraldez A. J., Cinalli R. M., Glasner M. E., Enright A. J., Thomson J. M., et al. , 2005. MicroRNAs regulate brain morphogenesis in zebrafish. Science 308: 833–838. 10.1126/science.1109020 [DOI] [PubMed] [Google Scholar]
- Grandel H., Schulte-Merker S., 1998. The development of the paired fins in the zebrafish (Danio rerio). Mech. Dev. 79: 99–120. 10.1016/S0925-4773(98)00176-2 [DOI] [PubMed] [Google Scholar]
- Grandel H., Draper B. W., Schulte-Merker S., 2000. Dackel acts in the ectoderm of the zebrafish pectoral fin bud to maintain AER signaling. Development 127: 4169–4178. [DOI] [PubMed] [Google Scholar]
- Griffin K., Patient R., Holder N., 1995. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development 121: 2983–2994. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Ho R. K., Walker C., Kimmel C. B., 1993. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75: 99–111. 10.1016/S0092-8674(05)80087-X [DOI] [PubMed] [Google Scholar]
- Harvey S. A., Logan M. P., 2006. sall4 acts downstream of tbx5 and is required for pectoral fin outgrowth. Development 133: 1165–1173. 10.1242/dev.02259 [DOI] [PubMed] [Google Scholar]
- Hino H., Nakanishi A., Seki R., Aoki T., Yamaha E., et al. , 2018. Roles of maternal wnt8a transcripts in axis formation in zebrafish. Dev. Biol. 434: 96–107. 10.1016/j.ydbio.2017.11.016 [DOI] [PubMed] [Google Scholar]
- Jao L. E., Wente S. R., Chen W., 2013. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 110: 13904–13909. 10.1073/pnas.1308335110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Lu J., Chen H., Werner S., Williams L. T., 1991. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol. Cell. Biol. 11: 4627–4634. 10.1128/MCB.11.9.4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki J. P., Ho R. K., 1997. The development of the posterior body in zebrafish. Development 124: 881–893. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Capdevila J., Buscher D., Itoh T., Rodriguez Esteban C., et al. , 2001. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 104: 891–900. 10.1016/S0092-8674(01)00285-9 [DOI] [PubMed] [Google Scholar]
- Kengaku M., Capdevila J., Rodriguez-Esteban C., De La Pena J., Johnson R. L., et al. , 1998. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science 280: 1274–1277. 10.1126/science.280.5367.1274 [DOI] [PubMed] [Google Scholar]
- Kimelman D., 2016. Tales of tails (and trunks): forming the posterior body in vertebrate embryos. Curr. Top. Dev. Biol. 116: 517–536. 10.1016/bs.ctdb.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Kane D. A., Walker C., Warga R. M., Rothman M. B., 1989. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature 337: 358–362. 10.1038/337358a0 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Miller C. T., Moens C. B., 2001. Specification and morphogenesis of the zebrafish larval head skeleton. Dev. Biol. 233: 239–257. 10.1006/dbio.2001.0201 [DOI] [PubMed] [Google Scholar]
- Koch P., Lohr H. B., Driever W., 2014. A mutation in cnot8, component of the Ccr4-not complex regulating transcript stability, affects expression levels of developmental regulators and reveals a role of Fgf3 in development of caudal hypothalamic dopaminergic neurons. PLoS One 9: e113829 10.1371/journal.pone.0113829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Fjose A., 1991. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development 113: 1193–1206. [DOI] [PubMed] [Google Scholar]
- Krauss S., Concordet J. P., Ingham P. W., 1993. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75: 1431–1444. 10.1016/0092-8674(93)90628-4 [DOI] [PubMed] [Google Scholar]
- Larbuisson A., Dalcq J., Martial J. A., Muller M., 2013. Fgf receptors Fgfr1a and Fgfr2 control the function of pharyngeal endoderm in late cranial cartilage development. Differentiation 86: 192–206. 10.1016/j.diff.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Laufer E., Nelson C. E., Johnson R. L., Morgan B. A., Tabin C., 1994. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell 79: 993–1003. 10.1016/0092-8674(94)90030-2 [DOI] [PubMed] [Google Scholar]
- Leerberg D. M., Sano K., Draper B. W., 2017. Fibroblast growth factor signaling is required for early somatic gonad development in zebrafish. PLoS Genet. 13: e1006993 10.1371/journal.pgen.1006993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J., 1994. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem. Sci. 19: 459–463. 10.1016/0968-0004(94)90130-9 [DOI] [PubMed] [Google Scholar]
- Liu D. W., Hsu C. H., Tsai S. M., Hsiao C. D., Wang W. P., 2011. A variant of fibroblast growth factor receptor 2 (Fgfr2) regulates left-right asymmetry in zebrafish. PLoS One 6: e21793 10.1371/journal.pone.0021793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely C. B., Swartz M. E., McCarthy N., Norrie J. L., Eberhart J. K., 2016. Bmp signaling mediates endoderm pouch morphogenesis by regulating Fgf signaling in zebrafish. Development 143: 2000–2011. 10.1242/dev.129379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Lu W., Mohamedali K. A., Jang J. H., Jones R. B., et al. , 1998. The glycine box: a determinant of specificity for fibroblast growth factor. Biochemistry 37: 16506–16515. 10.1021/bi9816599 [DOI] [PubMed] [Google Scholar]
- Ma Z., Zhu P., Shi H., Guo L., Zhang Q., et al. , 2019. PTC-bearing mRNA elicits a genetic compensation response via Upf3a and COMPASS components. Nature 568: 259–263. 10.1038/s41586-019-1057-y [DOI] [PubMed] [Google Scholar]
- Manfroid I., Delporte F., Baudhuin A., Motte P., Neumann C. J., et al. , 2007. Reciprocal endoderm-mesoderm interactions mediated by fgf24 and fgf10 govern pancreas development. Development 134: 4011–4021. 10.1242/dev.007823 [DOI] [PubMed] [Google Scholar]
- Marin F., Puelles L., 1994. Patterning of the embryonic avian midbrain after experimental inversions: a polarizing activity from the isthmus. Dev. Biol. 163: 19–37. 10.1006/dbio.1994.1120 [DOI] [PubMed] [Google Scholar]
- Martínez S., Marin F., Nieto M. A., Puelles L., 1995. Induction of ectopic engrailed expression and fate change in avian rhombomeres: intersegmental boundaries as barriers. Mech. Dev. 51: 289–303. 10.1016/0925-4773(95)00376-2 [DOI] [PubMed] [Google Scholar]
- McCarthy N., Sidik A., Bertrand J. Y., Eberhart J. K., 2016. An Fgf-Shh signaling hierarchy regulates early specification of the zebrafish skull. Dev. Biol. 415: 261–277. 10.1016/j.ydbio.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader N., 2007. Early steps of paired fin development in zebrafish compared with tetrapod limb development. Dev. Growth Differ. 49: 421–437. 10.1111/j.1440-169X.2007.00942.x [DOI] [PubMed] [Google Scholar]
- Meyers E. N., Lewandoski M., Martin G. R., 1998. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18: 136–141. 10.1038/ng0298-136 [DOI] [PubMed] [Google Scholar]
- Mikkola I., Fjose A., Kuwada J. Y., Wilson S., Guddal P. H., et al. , 1992. The paired domain-containing nuclear factor pax[b] is expressed in specific commissural interneurons in zebrafish embryos. J. Neurobiol. 23: 933–946. 10.1002/neu.480230802 [DOI] [PubMed] [Google Scholar]
- Millen K. J., Wurst W., Herrup K., Joyner A. L., 1994. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development 120: 695–706. [DOI] [PubMed] [Google Scholar]
- Millet S., Campbell K., Epstein D. J., Losos K., Harris E., et al. , 1999. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature 401: 161–164. 10.1038/43664 [DOI] [PubMed] [Google Scholar]
- Min H., Danilenko D. M., Scully S. A., Bolon B., Ring B. D., et al. , 1998. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12: 3156–3161. 10.1101/gad.12.20.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., Olsen S. K., Ibrahimi O. A., 2005. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 16: 107–137. 10.1016/j.cytogfr.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Moreno-Mateos M. A., Vejnar C. E., Beaudoin J. D., Fernandez J. P., Mis E. K., et al. , 2015. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 12: 982–988. 10.1038/nmeth.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mork L., Crump G., 2015. Zebrafish craniofacial development: a window into early patterning. Curr. Top. Dev. Biol. 115: 235–269. 10.1016/bs.ctdb.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H., Smith J. L., Peng L., Yin H., Moore J., et al. , 2017. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol. 18: 108 10.1186/s13059-017-1237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münchberg S. R., Ober E. A., Steinbeisser H., 1999. Expression of the Ets transcription factors erm and pea3 in early zebrafish development. Mech. Dev. 88: 233–236. 10.1016/S0925-4773(99)00179-3 [DOI] [PubMed] [Google Scholar]
- Nechiporuk A., Linbo T., Raible D. W., 2005. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development 132: 3717–3730. 10.1242/dev.01876 [DOI] [PubMed] [Google Scholar]
- Neumann C. J., Grandel H., Gaffield W., Schulte-Merker S., Nusslein-Volhard C., 1999. Transient establishment of anteroposterior polarity in the zebrafish pectoral fin bud in the absence of sonic hedgehog activity. Development 126: 4817–4826. [DOI] [PubMed] [Google Scholar]
- Ng J. K., Kawakami Y., Buscher D., Raya A., Itoh T., et al. , 2002. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development 129: 5161–5170. [DOI] [PubMed] [Google Scholar]
- Niswander L., Martin G. R., 1992. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 114: 755–768. [DOI] [PubMed] [Google Scholar]
- Nomura R., Kamei E., Hotta Y., Konishi M., Miyake A., et al. , 2006. Fgf16 is essential for pectoral fin bud formation in zebrafish. Biochem. Biophys. Res. Commun. 347: 340–346. 10.1016/j.bbrc.2006.06.108 [DOI] [PubMed] [Google Scholar]
- Norton W. H., Ledin J., Grandel H., Neumann C. J., 2005. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development 132: 4963–4973. 10.1242/dev.02084 [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Itoh N., 2001. Fibroblast growth factors. Genome Biol. 2: REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Xu J., Colvin J. S., McEwen D. G., MacArthur C. A., et al. , 1996. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271: 15292–15297. 10.1074/jbc.271.25.15292 [DOI] [PubMed] [Google Scholar]
- Ota S., Tonou-Fujimori N., Tonou-Fujimori N., Nakayama Y., Ito Y., et al. , 2010. FGF receptor gene expression and its regulation by FGF signaling during early zebrafish development. Genesis 48: 707–716. 10.1002/dvg.20682 [DOI] [PubMed] [Google Scholar]
- Pawson T., 1995. Protein modules and signalling networks. Nature 373: 573–580. 10.1038/373573a0 [DOI] [PubMed] [Google Scholar]
- Powers C. J., McLeskey S. W., Wellstein A., 2000. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7: 165–197. 10.1677/erc.0.0070165 [DOI] [PubMed] [Google Scholar]
- R Core Team , 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Reifers F., Bohli H., Walsh E. C., Crossley P. H., Stainier D. Y., et al. , 1998. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 125: 2381–2395. [DOI] [PubMed] [Google Scholar]
- Rice R., Spencer-Dene B., Connor E. C., Gritli-Linde A., McMahon A. P., et al. , 2004. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 113: 1692–1700. 10.1172/JCI20384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner N., Bercsenyi M., Orban L., Kolanczyk M. E., Linke D., et al. , 2009. Duplication of fgfr1 permits Fgf signaling to serve as a target for selection during domestication. Curr. Biol. 19: 1642–1647. 10.1016/j.cub.2009.07.065 [DOI] [PubMed] [Google Scholar]
- Rohs P., Ebert A. M., Zuba A., McFarlane S., 2013. Neuronal expression of fibroblast growth factor receptors in zebrafish. Gene Expr. Patterns 13: 354–361. 10.1016/j.gep.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Rossi A., Kontarakis Z., Gerri C., Nolte H., Holper S., et al. , 2015. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524: 230–233. 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- Schier A. F., Talbot W. S., 2005. Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39: 561–613. 10.1146/annurev.genet.37.110801.143752 [DOI] [PubMed] [Google Scholar]
- Schilling T. F., Kimmel C. B., 1994. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120: 483–494. [DOI] [PubMed] [Google Scholar]
- Scholpp S., Groth C., Lohs C., Lardelli M., Brand M., 2004. Zebrafish fgfr1 is a member of the fgf8 synexpression group and is required for fgf8 signalling at the midbrain-hindbrain boundary. Dev. Genes Evol. 214: 285–295. 10.1007/s00427-004-0409-1 [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Ho R. K., Herrmann B. G., Nusslein-Volhard C., 1992. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116: 1021–1032. [DOI] [PubMed] [Google Scholar]
- Shah A. N., Moens C. B., Miller A. C., 2016. Targeted candidate gene screens using CRISPR/Cas9 technology. Methods Cell Biol. 135: 89–106. 10.1016/bs.mcb.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Sharpe J. J., Cooper T. A., 2017. Unexpected consequences: exon skipping caused by CRISPR-generated mutations. Genome Biol. 18: 109 10.1186/s13059-017-1240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleptsova-Friedrich I., Li Y., Emelyanov A., Ekker M., Korzh V., et al. , 2001. fgfr3 and regionalization of anterior neural tube in zebrafish. Mech. Dev. 102: 213–217. 10.1016/S0925-4773(01)00280-5 [DOI] [PubMed] [Google Scholar]
- Sun X., Meyers E. N., Lewandoski M., Martin G. R., 1999. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13: 1834–1846. 10.1101/gad.13.14.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Mariani F. V., Martin G. R., 2002. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418: 501–508. 10.1038/nature00902 [DOI] [PubMed] [Google Scholar]
- Swartz M. E., Sheehan-Rooney K., Dixon M. J., Eberhart J. K., 2011. Examination of a palatogenic gene program in zebrafish. Dev. Dyn. 240: 2204–2220. 10.1002/dvdy.22713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B., Pflumio S., Fürthauer M., Loppin B., Heyer V., et al. , 2001. Expression of the Zebrafish Genome During Embryogenesis. ZFIN Direct Data Submission, Eugene, OR. [Google Scholar]
- Thisse B., Wright G. J., Thisse C., 2008. Embryonic and Larval Expression Patterns from a Large Scale Screening for Novel Low Affinity Extracellular Protein Interactions. ZFIN Direct Data Submission, Eugene, OR. [Google Scholar]
- Thisse C., Thisse B., 2005. High Throughput Expression Analysis of ZF-Models Consortium Clones. ZFIN Direct Data Submission, Eugene, OR. [Google Scholar]
- Tonou-Fujimori N., Takahashi M., Onodera H., Kikuta H., Koshida S., et al. , 2002. Expression of the FGF receptor 2 gene (fgfr2) during embryogenesis in the zebrafish Danio rerio. Mech. Dev. 119: S173–S178. 10.1016/S0925-4773(03)00112-6 [DOI] [PubMed] [Google Scholar]
- Trokovic R., Trokovic N., Hernesniemi S., Pirvola U., Vogt Weisenhorn D. M., et al. , 2003. FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 22: 1811–1823. 10.1093/emboj/cdg169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N., Javidan Y., Nelson S., Carney T. J., Kelsh R. N., et al. , 2005. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 132: 3977–3988. 10.1242/dev.01943 [DOI] [PubMed] [Google Scholar]
- Wagner D. S., Mullins M. C., 2002. Modulation of BMP activity in dorsal-ventral pattern formation by the chordin and ogon antagonists. Dev. Biol. 245: 109–123. 10.1006/dbio.2002.0614 [DOI] [PubMed] [Google Scholar]
- Walker M. B., Miller C. T., Coffin Talbot J., Stock D. W., Kimmel C. B., 2006. Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev. Biol. 295: 194–205. 10.1016/j.ydbio.2006.03.028 [DOI] [PubMed] [Google Scholar]
- Warga R. M., Mueller R. L., Ho R. K., Kane D. A., 2013. Zebrafish Tbx16 regulates intermediate mesoderm cell fate by attenuating Fgf activity. Dev. Biol. 383: 75–89. 10.1016/j.ydbio.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz A. J., Rikhof H. A., Moens C. B., 2002. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev. Cell 3: 723–733. 10.1016/S1534-5807(02)00319-2 [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Allende M. L., Kelly C. S., Abdelhamid A., Murakami T., et al. , 1996. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122: 271–280. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Xu X., Ohyama K., Deng C. X., 1998. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125: 3615–3623. [DOI] [PubMed] [Google Scholar]
- Werner S., Duan D. S., de Vries C., Peters K. G., Johnson D. E., et al. , 1992. Differential splicing in the extracellular region of fibroblast growth factor receptor 1 generates receptor variants with different ligand-binding specificities. Mol. Cell. Biol. 12: 82–88. 10.1128/MCB.12.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M., 2000. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). University of Oregon Press, Eugene, OR. [Google Scholar]
- Wickham H., 2009. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. [Google Scholar]
- Xu X., Weinstein M., Li C., Naski M., Cohen R. I., et al. , 1998. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125: 753–765. [DOI] [PubMed] [Google Scholar]
- Xu X., Li C., Takahashi K., Slavkin H. C., Shum L., et al. , 1999a Murine fibroblast growth factor receptor 1alpha isoforms mediate node regression and are essential for posterior mesoderm development. Dev. Biol. 208: 293–306. 10.1006/dbio.1999.9227 [DOI] [PubMed] [Google Scholar]
- Xu X., Weinstein M., Li C., Deng C., 1999b Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 296: 33–43. 10.1007/s004410051264 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. P., Harpal K., Henkemeyer M., Rossant J., 1994. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 8: 3032–3044. 10.1101/gad.8.24.3032 [DOI] [PubMed] [Google Scholar]
- Yayon A., Zimmer Y., Shen G. H., Avivi A., Yarden Y., et al. , 1992. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 11: 1885–1890. 10.1002/j.1460-2075.1992.tb05240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh B. K., Igarashi M., Eliseenkova A. V., Plotnikov A. N., Sher I., et al. , 2003. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc. Natl. Acad. Sci. USA 100: 2266–2271. 10.1073/pnas.0436500100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Karuppaiah K., Ornitz D. M., 2015. Mesenchymal fibroblast growth factor receptor signaling regulates palatal shelf elevation during secondary palate formation. Dev. Dyn. 244: 1427–1438. 10.1002/dvdy.24319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Houston D. W., King M. L., Payne C., Wylie C., et al. , 1998. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94: 515–524. 10.1016/S0092-8674(00)81592-5 [DOI] [PubMed] [Google Scholar]
- Zhang X., Ibrahimi O. A., Olsen S. K., Umemori H., Mohammadi M., et al. , 2006. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281: 15694–15700. 10.1074/jbc.M601252200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., et al. , 2008. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318: 276–288. 10.1016/j.ydbio.2008.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga A., 2015. Next generation limb development and evolution: old questions, new perspectives. Development 142: 3810–3820. 10.1242/dev.125757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All fish lines and plasmids are available upon request. Fish lines will be deposited at the Zebrafish International Resource Center. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8162003.