Abstract
Objective
To assess the efficacy and safety of green-light laser photoselective vaporisation of the prostate (PVP) compared with transurethral resection of the prostate (TURP) for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH).
Design
Systematic review and meta-analysis, conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.
Data sources
PubMed, EMBASE, the Cochrane Library until October 2018.
Eligibility criteria
Randomised controlled trials and prospective studies comparing the safety and efficacy of PVP versus TURP for LUTS manifesting through BPH.
Data extraction and synthesis
Perioperative parameters, complications rates and functional outcomes including treatment-related adverse events such as International Prostate Symptom Score (IPSS), maximum flow rate (Qmax), postvoid residual (PVR), quality of life (QoL) and International Index of Erectile Function (IIEF).
Results
22 publications consisting of 2665 patients were analysed. Pooled analysis revealed PVP is associated with reduced blood loss, transfusion, clot retention, TUR syndrome, capsular perforation, catheterisation time and hospitalisation, but also with a higher reintervention rate and longer intervention duration (all p<0.05). No significant difference in IPSS, Qmax, QoL, PVR or IIEF at 3, 24, 36 or 60 months was identified. There was a significant difference in QoL at 6 months (MD=−0.08; 95% CI −0.13 to −0.02; p=0.007), and IPSS (MD = −0.10; 95% CI −0.15 to −0.05; p<0.0001) and Qmax (MD=0.62; 95% CI 0.06 to 1.19; p=0.03) at 12 months, although these differences were not clinically relevant.
Conclusion
PVP is an effective alternative, holding additional safety benefits. PVP has equivalent long-term IPSS, Qmax, QoL, PVR, IIEF efficacy and fewer complications. The main drawbacks are dysuria and reintervention, although both can be managed with non-invasive techniques. The additional shortcoming is that PVP does not acquire histological tissue examination which removes an opportunity to identify prostate cancer.
Keywords: benign prostatic hyperplasia (BPH), lower urinary tract symptoms (LUTS), meta-analysis, photoselective vaporisation of the prostate (PVP), transurethral resection of the prostate (TURP)
Strengths and limitations of this study.
This updated meta-analysis included a larger number of studies involving more participants which adds precision to previous findings.
This study analysed both safety and efficacy, focusing on sexual functioning and quality of life measures because lower urinary tract symptoms treatment related adverse events have a hugely detrimental impact on ones’ psychological well-being.
Quality assessment methods used did not highlight substantial differences between studies because blinding is not possible given the characteristics of the two interventions under investigation.
Due to the limited number of studies in this field, we were unable to conduct subgroup analysis around laser power (ie, 80W, 120W, 180W and so on) which is necessary to identify the most effective/efficient standard.
Surgical experience with laser technology, drop outs and withdrawals as well as other important factors were seldom reported in any detail which inhibits further analysis.
Introduction
Lower urinary tract symptoms (LUTS) commonly occur in the ageing male population, affecting >1 in 4 of those above 50 years of age. LUTS manifest through benign prostatic hyperplasia (BPH) and often have a hugely negative impact on quality of life (QoL).1 Treatments for BPH range from medicinal interventions to surgery, where transurethral resection of the prostate (TURP) remains the surgical gold standard. Surgical therapy is recommended for patients whom have not benefitted from medical interventions such as, 5-alpha-reductase inhibitors and alpha-blockers.1 2 TURP has been found to have a high success rate and low reintervention rate at long-term follow-up,3 however; increasingly evidence indicates this invasive procedure is also associated with serious complications such as bleeding, urethral strictures, urinary incontinence and transurethral resection (TUR) syndrome.4–6 Consequently, there is an urgent need to develop minimally invasive therapies which do not have such a negative impact on patients’ lives.
Laser therapies offer a new direction in BPH therapies and photoselective vaporisation of the prostate (PVP) is increasingly being studied as a potential new first line treatment.7–11 This technique is generally performed with a 532 nm green laser generated using potassium-titanyl-phosphate (KTP) or lithium triborate crystals.12 Unlike other types of laser, the green laser is easily absorbed by soft tissue haemoglobin, while hardly at all by other fluid mediums, which leads to improved coagulation and lowers the risk of deeper tissue injuries during vaporisation.13 14 Numerous studies provide supporting evidence of increased benefit, demonstrating that PVP has superior mid-term clinical efficacy compared with TURP across functional outcomes including International Prostate Symptom Score (IPSS), maximum flow rate (Qmax), postvoid residual volume (PVR), International Index of Erectile Function (IIEF) and QoL.15 16
In a previous meta-analysis published in 2013, Teng et al 17 found that PVP and TURP have similar treatment efficacies, although due to the minimally invasive nature, PVP offers several potential benefits. While this early research provided some optimism, studies have yet to compare sexual function outcomes or efficacy results at 24 months, and across all available randomised controlled trials (RCTs) and prospective studies. Consequently, we sought to conduct an updated systematic review and meta-analysis of high quality studies to support clinical decision-makers treating BPH.
Materials and methods
Patient and public involvement
Neither patients nor the public were involved in the design and planning of the study.
Literature search and article selection
A comprehensive literature search was performed using biomedical databases including PubMed, EMBASE and the Cochrane Library up until October 2018. The following MeSH terms and free text words were used: benign prostatic hyperplasia, BPH, transurethral resection of the prostate, TURP, green-light laser, vaporization, photoselective vaporization of the prostate and PVP. These terms were used singly and in combination (for further details please see online supplement file 1). Additionally, manual searches were commenced for references and citations included within pertinent reviews. Language was restricted to English and the search and selection strategy was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.18 Randomised controlled trials and prospective studies meeting the following criteria were included: (1) studies comparing the safety and efficacy of PVP versus TURP for surgical treatment of LUTS secondary to manifesting BPH, (2) endpoints such as treatment-related adverse events and functional outcomes such as IPSS, Qmax, PVR, QoL and IIEF when available, and (3) providing the full text of the study could be accessed.
bmjopen-2018-028855supp001.pdf (49.2KB, pdf)
Literature searching, selection and data extraction was undertaken independently by two reviewers (SL and PP) which was then cross-checked. Any discrepancies were resolved through discussion. A flowchart representing the search and selection process is presented in figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart.
Assessment of study quality
Study quality was assessed in accordance with criteria recommended by the Oxford Centre for Evidence Based Medicine.19 Methodological reporting quality of RCTs was assessed using Jadad20 and the Newcastle–Ottawa scale21 was used to evaluate the quality of the prospective cohort studies included.
Data extraction and statistical analysis
Preoperative parameters were extracted together with intraoperative data including operation times, changes in haemoglobin and transfusion rates. Postoperative data including length of hospitalisation, duration of catheterisation and treatment-related complications were also analysed. Functional results including IPSS, Qmax, PVR, QoL and IIEF were assessed at 3, 6, 12, 24, 36 and 60 months after surgery.
Mean difference (MD) was used to assess continuous parameters. Authors were contacted when data were expressed as medians with corresponding range values. Otherwise, the statistical formula elaborated by Hozo et al 22 was implemented to back-calculate means and SD in accordance with the recommended methods described in the Cochrane Handbook for Systematic Reviews.23
Results were expressed as risk ratios (RR) with corresponding 95% CI for dichotomous variables. I2 was utilised to assess heterogeneity across studies. An I2<50% is generally considered an acceptable level of heterogeneity therefore a fixed effect model was applied. In instances where the I2>50% a random-effects model was applied as is the standard procedure for higher levels of heterogeneity. Pooled effects were synthesised using Z test and a p value <0.05 was set at the threshold for statistical significance.
Sensitivity analysis
Sensitivity analysis was conducted to assess the reliability of the findings of this study. As such, Qmax at 24 months, PVR at 3 months, operation times and period of hospitalisation were further analysed by removing non-RCTs. All data analyses were conducted with Review Manager V.5.3 software.
Results
The predetermined search and selection criteria yielded 22 publications,2 7–11 24–39 reporting 19 separate clinical studies. Three studies (ie, Bachman et al,10 29 Thomas et al 30) refer to an identical study, and two studies24 31 were from the same trials over different periods of time. In total, there were 2665 patients involved, 1455 of whom had been treated with PVP and 1210 with TURP. Patient characteristics and study characteristics are summarised in table 1. Overall, RCTs included in this meta-analysis can be considered of reasonably high quality with eight studies achieving a score of 3, while seven slightly lower quality achieved Jadad scores of 2. All prospective studies included can be considered high quality having been awarded nine using the Newcastle-Ottowa Scale.
Table 1.
Baseline characteristics of comparative studies
| Authors and year | Design | Group | Laser power (W) | No of patients | Age (years) | Prostate size (mL) | IPSS | Qmax (mL/s) | PVR (mL) | QoL | IIEF | Follow-up (months) | LE | Study quality |
| Kumar et al, 201324 | RCT | PVP | 120 | 58 | 64.58±6.64 | 52.79±16.13 | 20.05±2.75 | 6.68±2.00 | 143.35±52.67 | 3.60±1.01 | 16.65±2.80 | 12 | 2a | 3* |
| TURP | 60 | 63.68±6.57 | 52.20±15.93 | 20.71±2.68 | 7.00±1.97 | 139.25±54.28 | 3.73±0.97 | 16.95±2.86 | ||||||
| Lukacs et al 2012 2 | RCT | PVP | 120 | 68 | 66.9±7.8 | 50.54±16.53 | 22 (17–26)% | 7.79±2.75 | 89.5 (30, 158)% | 70 (68, 80)% | 12 | 2a | 3* | |
| TURP | 68 | 67.6±7.6 | 50.11±14.73 | 20 (15–23)% | 7.76±2.64 | 75 (28,126)% | 75 (65, 85)% | |||||||
| Pereira-Correia et al 201225 | RCT | PVP | 120 | 10 | 66.4 (52, 76)$ | 43.4 (30, 58)$ | 22 (9, 33)$ | 10 (3, 18)$ | 150 (25, 250)$ | 23 (22, 24)$ | 24 | 2a | 2* | |
| TURP | 10 | 63.5 (56, 78)$ | 47 (30, 60)$ | 25 (15, 31)$ | 6.4 (4,11)$ | 177 (50, 300)$ | 23 (22, 25)$ | |||||||
| Capitan et al 20117 | RCT | PVP | 120 | 50 | 69.8±8.44 | 51.29±14.72 | 23.74±5.24 | 8.03±3.14 | 4.52±0.27 | 12 | 2a | 3* | ||
| TURP | 50 | 67.7±6.7 | 53.10±13.75 | 23.52±4.38 | 3.88±2.71 | 4.14±1.06 | ||||||||
| Al-Ansari et al 201026 | RCT | PVP | 120 | 60 | 66.3±9.4 | 61.8±22 | 27.2±2.3 | 6.9±2.2 | 53.2±25 | 36 | 2a | 3* | ||
| TURP | 60 | 67.1±8 | 60.3±20 | 27.9±2.7 | 6.4±2 | 57±21 | ||||||||
| Xue et al 201327 | RCT | PVP | 120 | 100 | 72.1±11.3 | 65.8±23.6 | 23.0±5.1 | 8.0±3.6 | 148.3±101.6 | 4.2±0.9 | 36 | 2a | 2* | |
| TURP | 100 | 71.0±10.8 | 67.3±24.7 | 23.2±5.0 | 8.2±3.8 | 151.1±105.2 | 4.3±0.8 | |||||||
| Horasanli et al 200838 | RCT | PVP | 80 | 39 | 69.2±7.1 | 86.1±8.8 | 18.9±5.1 | 8.6±5.2 | 183±50.1 | 19.9±5.1 | 6 | 2a | 2* | |
| TURP | 37 | 68.3±6.7 | 88±9.2 | 20.2±6.8 | 9.2±5.6 | 176.9±45.3 | 20.1±5.5 | |||||||
| Mohanty et al 201228 | RCT | PVP | 80 | 60 | 66.68±8.62 | 44.77±14.09 | 19.98±3.27 | 7.41±2.07 | 145.8±70.33 | 3.97±0.82 | 17.98±3.55 | 12 | 2a | 3* |
| TURP | 57 | 65.74±9.09 | 49.02±15.93 | 20.88±3.87 | 6.75±1.63 | 143.23±65.96 | 3.91±0.78 | 17.40±4.76 | ||||||
| Bouchier-Hayes et al 200911 | RCT | PVP | 80 | 60 | >50 | 25.28±5.93 | 8.81±2.55 | 129.2±155.7 111.3±113.7 | 4.74±1.23 | 12 | 2a | 3* | ||
| TURP | 59 | 25.41±5.72 | 8.86±2.99 | 5.08±0.94 | ||||||||||
| Bachmann et al 201410 | RCT | PVP | 180 | 136 | 65.9±6.8 | 48.6±19.2 | 21.2±5.9 | 9.5±3.0 | 110.1±88.5 | 4.6±1.1 | 13.2±7.6 | 6 | 2a | 3* |
| TURP | 133 | 65.4±6.6 | 46.2±19.1 | 21.7±6.4 | 9.9±3.5 | 109.8±103.9 | 4.5±1.4 | 13.7±7.5 | ||||||
| Bachmann et al 201529 | RCT | PVP | 180 | 136 | 65.9±6.8 | 48.6±19.2 | 21.2±5.9 | 9.5±3.0 | 110.1±88.5 | 4.6±1.1 | 13.2±7.6 | 12 | 2a | 2* |
| TURP | 133 | 65.4±6.6 | 46.2±19.1 | 21.7±6.4 | 9.9±3.5 | 109.8±103.9 | 4.5±1.4 | 13.7±7.5 | ||||||
| Thomas et al 201630 | RCT | PVP | 180 | 136 | 65.9±6.8 | 48.6±19.2 | 21.2±5.9 | 9.5±3.0 | 110.1±88.5 | 4.6±1.1 | 13.2±7.6 | 24 | 2a | 3* |
| TURP | 133 | 65.4±6.6 | 46.2±19.1 | 21.7±6.4 | 9.9±3.5 | 109.8±103.9 | 4.5±1.4 | 13.7±7.5 | ||||||
| Telli et al 201532 | RCT | PVP | 120 | 39 | 67 (51, 87)$ | 60 (41, 75)$ | 20 (12, 30)$ | 10.6 (5, 17)$ | 60 (20, 220)$ | 24 | 2a | 2* | ||
| TURP | 62 | 69 (56, 87)$ | 55 (40, 72)$ | 19 (10, 31)$ | 12.5 (3, 21)$ | 65 (10, 220)$ | ||||||||
| Kumar et al 201631 | RCT | PVP | 120 | 58 | 64.58±6.64 | 52.79±16.13 | 20.05±2.75 | 6.68±2.00 | 143.35±52.67 | 3.60±1.01 | 16.65±2.80 | 36 | 2a | 2* |
| TURP | 60 | 63.68±6.57 | 52.20±15.93 | 20.71±2.68 | 7.00±1.97 | 139.25±54.28 | 3.73±0.97 | 16.95±2.86 | ||||||
| Mordasini et al 201839 | RCT | PVP | 80 | 112 | 68.4±8.7 | 36.1±11.5 | 20.3±7.0 | 8.9±4.1 | 91.1±88.3 | 4.2±1.1 | 60 | 2a | 2* | |
| TURP | 126 | 67.6±8.4 | 37.9±14.3 | 20.4±7.5 | 8.5±4.6 | 114.5±136.4 | 4.3±14 | |||||||
| Chen et al 2011 | PCS | PVP | 160 | 57 | 69.5±7.4 | 60.2±27.8 | 19.7±6.0 | 6.9±4.0 | 93.7±79.7 | 6 | 2b | 9† | ||
| TURP | 51 | 67.1±6.9 | 58.3±26.2 | 21.8±7.3 | 6.8±2.3 | 102.2±70.1 | ||||||||
| Bachmann et al 200537 | PCS | PVP | 37 | 71.0±9.3 | 65.1±36.9 | 18.1±5.9 | 6.9±2.2 | 146.1±106.9 120.7±49.0 | 3.3±1.7 | 6 | 2b | 9† | ||
| TURP | 64 | 68.7±7.9 | 48.9±21.2 | 17.3±6.3 | 6.9±2.2 | 3.4±1.6 | ||||||||
| Ruszat et al 20088 | PCS | PVP | 80 | 113 | 62.3±5.0 | 56.3±27.4 | 20±6.4 | 8.5±4.1 | 203±226 | 24 | 2b | 9† | ||
| TURP | 75 | 61.7±5.5 | 45.3±21.0 | 19±6.9 | 9.8±5.0 | 104±108 | ||||||||
| PVP | 91 | 75.0±2.8 | 64.8±26.8 | 18.6±5.8 | 7.3±2.7 | 215±247 | ||||||||
| TURP | 40 | 74.0±2.6 | 54.2±21.2 | 16.0±7.1 | 9.2±5.4 | 124±141 | ||||||||
| PVP | 65 | 84.3±3.1 | 69.3±32.7 | 14.1±7.4 | 7.1±4.2 | 200±219 | ||||||||
| TURP | 12 | 82.4±2.8 | 44.9±22.1 | 15.5±6.7 | 7.6±3.9 | 231±350 | ||||||||
| Tasci et al 200833 | PCS | PVP | 40 | 71.8±5.9 | 108.4±15.8 | 22.3±5.6 | 6.2±2.2 | 116.5±60.5 | 3.6±0.7 | 24 | 2b | 9† | ||
| TURP | 41 | 70.1±5.4 | 104.2±12.5 | 22.6±3.9 | 6.5±1.8 | 110.7±59.8 | 3.5±0.6 | |||||||
| Tugcu et al 200833 | PCS | PVP | 112 | 67.5±7.4 | 49.1±11.9 | 17.9±4.9 | 6.9±1.9 | 107.9±63.0 100.3±57.1 | 3.4±0.6 | 24 | 2b | 9† | ||
| TURP | 98 | 66.3±7.9 | 47.7±8.4 | 17.7±3.5 | 7.2±1.7 | 3.4±0.5 | ||||||||
| Nomura et al 200935 | PCS | PVP | 80 | 78 | 72.0 (67.0, 78.0)$ | 50.5 (38.6, 70.3)$ | 23 (17, 27)$ | 6.8 (5.2, 9.5)$ | 69 (31, 139)$ | 5 (5, 6)$ | 12 | 2b | 9† | |
| TURP | 51 | 70.5 (66.5, 76.0)$ | 42.8 (34.6, 54.0)$ | 22 (16, 27)$ | 7.3 (5.3, 10.2)$ | 60 (31, 140)$ | 5 (4, 5)$ | |||||||
| Guo et al 201536 | PCS | PVP | 80 | 257 | 69.7±8.9 66.4±8.4 | 52.3±19.3 44.2±19.1 | 19.4±6.3 18.4±6.3 | 8.3±6.0 | 119.5±83.8 95.6±98.4 | 3.7±1.7 3.7±1.3 | 60 | 2b | 9† | |
| TURP | 104 | 10.0±5.2 |
Continuous variables were expressed as (mean±SD), mean (range)$ or median (IQR)%.
Bachmann et al, 201410 Bachmann et al, 2015 and30 Thomas et al, 2016 are from the same trials in different period.24 Kumar et al, 2013 and Kumar et al, 2016 are from the same trials in different period.
*Using Jadad scale (score from 0 to 5).
†Using Newcastle-Ottawa Scale (score from 0 to 9).
IIEF, international index of erectile function; IPSS, International Prostate Symptom Score;LE, level of evidence; PCS, prospective cohort study; PVP, photoselective vaporisation of the prostate; PVR, postvoid residual volume; Qmax, maximum flow rate; QoL, quality of life;RCT, randomised controlled trial; TURP, transurethral resection of the prostate.
Meta-analysis of functional outcomes
Baseline data including IPSS, Qmax, PVR, QoL and IIEF for all participants in both the PVP and TURP groups were similar (table 2).
Table 2.
Meta-analytical outputs summarising baseline parameters of PVP compared with TURP
| Parameter | No of studies | Sample size | Heterogeneity (Total) | Mean difference (95% CI) | Test for overall effect | |||||
| PVP | TURP | χ2 | df | I2 (%) | P value | |||||
| IPSS | ||||||||||
| Baseline | 14 | 1179 | 989 | 11.32 | 13 | 0 | 0.58 | −0.29 (–0.68 to 0.10) | Z=1.47 | P=0.14 |
| Qmax | ||||||||||
| Baseline | 14 | 1179 | 989 | 70.23 | 13 | 81 | <0.01 | 0.05 (–0.51 to 0.61) | Z=0.17 | P=0.87 |
| PVR | ||||||||||
| Baseline | 12 | 1016 | 864 | 9.24 | 11 | 0 | 0.6 | 2.19 (–3.22 to 7.6) | Z=0.79 | P=0.43 |
| QoL | ||||||||||
| Baseline | 10 | 910 | 766 | 11.15 | 9 | 19 | 0.27 | 0.01 (–0.07 to 0.10) | Z=0.33 | P=0.74 |
| IIEF | ||||||||||
| Baseline | 5 | 351 | 297 | 1.58 | 4 | 0 | 0.81 | −0.13 (–0.86 to 0.60) | Z=0.34 | P=0.73 |
IIEF, International Index of Erectile Function; IPSS, International Prostate Symptom Score; PVP, photoselective vaporisation of the prostate; PVR, postvoid residual volume; Qmax, maximum flow rate; QoL, quality of life; RR, risk ratio; TURP, transurethral resection of the prostate.
IPSS at 3-month, 6-month, 12-month and 24-month follow-up
Pooled analysis suggests there is no significant difference in IPSS at the 3-month, 6-month or 24-month follow-up points. At 3 months the MD=0.01 (p=0.85) please see figure 2 a1. At 6 months the MD=0.30 (p=0.15), see figure 2 a2. At the 12-month follow-up stage, there was a statistically significant difference with a MD=−0.10 (p<0.01), see figure 2 a3, however; at 24 months, there was no significant difference (MD=0.02, p=0.92), see figure 2 a4.
Figure 2.
Forest plot of IPSS at 3 months (a1), 6 months (a2), 12 months (a3) and 24 months (a4); forest plot of Qmax at 3 months (b1), 6 months (b2), 12 months (b3) and 24 months (b4); forest plot of Pvr at 3 months (c1), 6 months (c2), 12 months (c3) and 24 months (c4); forest plot of QoL at 3 months (d1), 6 months (d2), 12 months (d3) and 24 months (d4); forest plot of IIEF at 3 months (e1), 6 months (e2), 12 months (e3) and 24 months (e4). IIEF, International Index of Erectile Function; IPSS, International Prostate Symptom Score; PVR, postvoid residual volume; Qmax, maximum flow rate; QoL, quality of life
Qmax at 3-month, 6-month, 12-month and 24-month follow-up
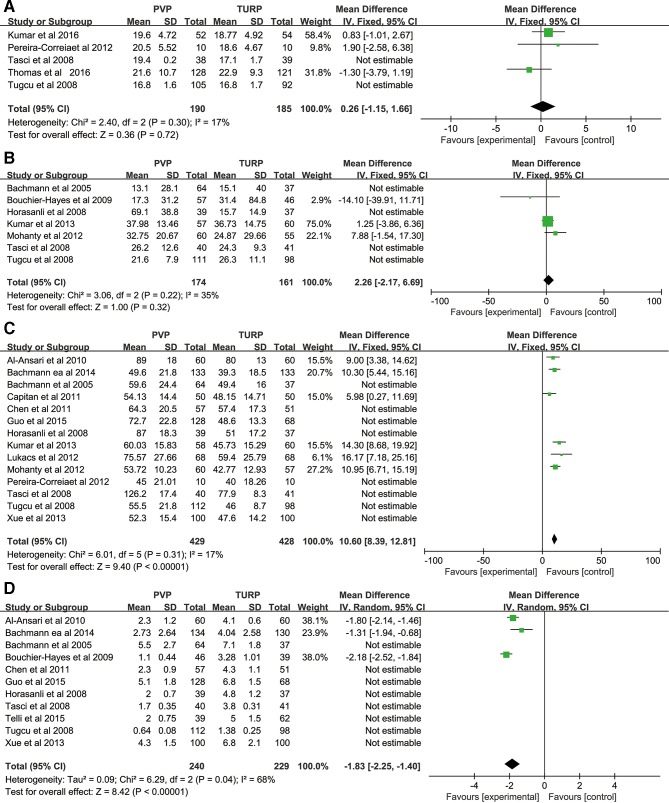
Pooled analysis suggests there is no significant difference between PVP and TURP regarding Qmax at the 3-month follow-up stage with an MD=−0.07 (p=0.91), see figure 2 b1. At the 6-month follow-up the MD=−0.17 (p=0.67), see figure 2 b2. At 12 months, Qmax measures were slightly higher in the PVP group (MD=0.62), which may be considered a statistically significant difference (p=0.03), although only borderline when considering confidence intervals (95% CI 0.06 to 1.19), see figure 2 b3 for details. At 24 months, the MD=0.74 although was again non significant (p=0.34), see figure 2 b4 for details. However, an extreme level of heterogeneity were observed (I2=91%) hence sensitivity analysis was conducted at the 24-month follow-up point which yielded an MD=0.26, although this was not a significant finding (p=0.72), see figure 3a.
Figure 3.
Sensitivity analysis of the Qmax at 24-month follow-up (A); PVR at 3-month follow-up (B); operation times (C); and period of hospitalisation (D). PVR, postvoid residual volume; Qmax, maximum flow rate.
PVR at 3-month, 6-month, 12-month and 24-month follow-up
PVR between the two groups, yielded no significant difference at 3 months (MD=6.65, p=0.16), see figure 2 c1, at 6 months (MD=2.07, p=0.35), see figure 2 c2, at 12 months (MD=0.85, p=0.11), see figure 2 c3, or at the 24-month follow-up point (MD=1.58, p=0.23), see figure 2 c4. Again, a high level of heterogeneity (I2=93%) was observed and so sensitivity analysis was conducted at the 3-month follow-up juncture. This did not highlight a significant difference between groups (p=0.38), see figure 3b for details.
QoL at 3-month, 6-month, 12-month and 24-month follow-up
There was no clinically relevant difference in QoL across the time points analysed. At the 3-month point, there was an MD=0.02, (p=0.59) see figure 2 d1. However; there was one statistically significant difference at 6 months (MD=−0.08), although this is not clinically relevant and can only be considered of borderline significance (95% CI –0.13 to –0.02), despite the low p value (p=0.007), see figure 2 d2. At 12 months (MD=0.01, p=0.75), see figure 2 d3 and at 24 months (MD=−0.07, p=0.10), see figure 2 d4, there was no significant difference.
IIEF at 6-month, 12-month and 24-month follow-up
An analysis of sexual functioning was performed using IIEF. There was no significant difference between the two groups in terms of the IIEF at the 3-month point (MD=−0.06, p=0.76) see figure 2 e1, at the 6-month (MD=−0.07, p=0.78) see figure 2 e2 or at the 12-month point (MD=−0.06, p=0.82) see figure 2 e3. Pooled analysis does highlight a lower IIEF at the 24-month follow-up in the PVP group compared with the TURP group with a MD=−0.68, which is statistically significant but again must be interpreted with caution because to the upper CI is so close to zero (95% CI −1.20 to −0.15, p=0.01), see figure 2 e4.
Meta-analysis of perioperative parameters
Operation time
Fourteen studies comparing PVP against TURP reported operation times. Overall, TURP takes less time than PVP with a MD=15.24 min, and this was a significant finding (p<0.01) see table 3. However, there was extreme heterogeneity across this sample (I2=94%). As such, sensitivity analysis was conducted by removing low-quality trials (figure 3c) which lowered the level of heterogeneity (I2=17%) and lowered the MD to 10.60 min (95% CI 8.39 to 12.81, p<0.01), see table 3.
Table 3.
Meta-analytical outputs for the safety of PVP compared with TURP
| Outcomes | No of studies | Sample size | Heterogeneity (total) | MD or RR (95% CI) | Test for overall effect | |||||
| PVP | TURP | χ2 | df | I2 (%) | P value | |||||
| Z | P value | |||||||||
| Operation time | 14 | 979 | 870 | 216.27 | 13 | 94 | <0.01 | 15.24 (8.91 to 21.54) | 4.72 | <0.01 |
| 6* | 429* | 428* | 6.01* | 5* | 17* | 0.31* | 10.60 (8.39 to 12.81)* | 9.40* | <0.01* | |
| Hospitalisation time | 11 | 819 | 723 | 600.62 | 10 | 98 | <0.01 | −1.98 (−2.56 to −1.39) | 6.59 | <0.01 |
| 3* | 240* | 229* | 6.29* | 2* | 68* | <0.01* | −1.83 (−2.25 to −1.40)* | 8.42* | <0.01* | |
| Catheterisation time | 14 | 861 | 794 | 964.75 | 13 | 99 | <0.01 | −1.25 (−1.58 to −0.92) | 7.48 | <0.01 |
| Blood loss | 6 | 389 | 335 | 46.05 | 5 | 89 | <0.01 | −1.33 (−2.05 to −0.61) | 3.62 | <0.01 |
| Transfusion | 14 | 1110 | 946 | 10.87 | 13 | 0 | 0.62 | 0.14 (0.08 to 0.26) | 6.10 | <0.01 |
| TUR syndrome | 7 | 590 | 435 | 0.73 | 6 | 0 | 0.99 | 0.19 (0.06 to 0.61) | 2.82 | <0.01 |
| Capsular perforation | 7 | 641 | 451 | 1.84 | 6 | 0 | 0.93 | 0.09 (0.03 to 0.26) | 4.51 | <0.01 |
| Clot retention | 8 | 699 | 504 | 1.72 | 7 | 0 | 0.97 | 0.14 (0.07 to 0.29) | 5.32 | <0.01 |
| Urinary tract infection | 13 | 1049 | 860 | 8.79 | 12 | 0 | 0.72 | 1.15 (0.85 to 1.55) | 0.89 | 0.38 |
| Acute urinary retention | 10 | 694 | 653 | 5.55 | 9 | 0 | 0.78 | 1.19 (0.80 to 1.75) | 0.86 | 0.39 |
| Urinary incontinence | 4 | 296 | 263 | 4.28 | 3 | 30 | 0.23 | 1.45 (0.74 to 2.86) | 1.08 | 0.28 |
| Bladder neck contracture | 8 | 523 | 520 | 4.32 | 7 | 0 | 0.74 | 1.05 (0.57 to 1.94) | 0.16 | 0.87 |
| Urethral stricture | 15 | 1172 | 980 | 9.37 | 14 | 0 | 0.81 | 0.81 (0.57 to 1.16) | 1.14 | 0.25 |
| Retrograde ejaculation | 4 | 320 | 314 | 15.06 | 3 | 80 | <0.01 | 0.72 (0.49 to 1.07) | 1.62 | 0.11 |
| Dysuria | 12 | 1079 | 854 | 24.80 | 11 | 58 | 0.01 | 1.76 (1.17 to 2.65) | 2.71 | <0.01 |
| Reintervention | 12 | 980 | 809 | 14.58 | 11 | 25 | 0.20 | 1.81 (1.28 to 2.56) | 3.35 | <0.01 |
*Using sensitivity analysis.
MD, mean difference; PVP, photoselective vaporisation of the prostate; RR, risk ratio; TR, syndrome=transurethral resection syndrome; TURP, transurethral resection of the prostate.
Operative blood loss
Six studies involving 724 participants (PVP n=389, TURP n=335) provided blood loss estimates during operations. The pooled statistic suggested that the drop in haemoglobin levels in the PVP group was significantly lower than in the TURP group with a MD of –1.33 g/dL (p<0.01), see table 3 for details.
Periods of hospitalisation
Eleven studies involving 1542 participants met our inclusion criteria for the analysis of periods of hospitalisation. Pooled statistics highlighted a significant reduction in hospitalisation times with a MD=−1.98 days (p<0.01) for PVP compared with TURP. However, again the level of heterogeneity across this sample was extreme (I2=98%) therefore sensitivity analysis (figure 3d) was again performed although this had a negligible impact on the results (MD=−1.83 days, 95% CI −2.25 to −1.40, p<0.01). See table 3 for further details.
Catheterisation time
Fourteen available studies including 1655 participants (861 in the PVP group and 794 in the TURP group) were involved in this meta-analysis. Pooled data revealed that the PVP group had a significantly shorter catheterisation time with an MD=−1.25 days, (p<0.01) see table 3.
Meta-analysis of complications
Perioperative complications
The overall effect of perioperative complications including bleeding-related transfusion, TUR syndrome, capsular perforation, clot retention, urinary tract infection and acute urinary retention are summarised in table 3. According to this meta-analysis, PVP was found to have significantly lower incidence of transfusion with an RR=0.14 (p<0.01), and clot retention (RR=0.14, p<0.01). There was also a substantial and significant difference in the occurrence of TUR syndrome (RR=0.19, p<0.01) and capsular perforations (RR=0.09, p<0.01). Furthermore, PVP appears to have a higher risk of mild to moderate dysuria (RR=1.76, 95% CI 1.17 to 2.65, p<0.01), although there was no substantial or significant difference regarding urinary tract infection (RR=1.15, 95% CI 0.85 to 1.55, p=0.38) or acute urinary retention rate (RR=1.19, 95% CI 0.80 to 1.75, p=0.39).
Long-term complications
Analysis of long-term complications such as bladder neck contracture, retrograde ejaculation and urethral stricture, suggests there is no significant difference between PVP and TURP. Bladder neck contracture (RR=1.05, p=0.87), retrograde ejaculation (RR=0.72, p=0.11) and urethral stricture (RR=0.81, p=0.25), see table 3 for further details. However, PVP was found to have a significantly higher risk of re-intervention (RR=1.81, p<0.01) see table 3 for details.
Discussion
Over the last two decades, TURP has remained the gold standard surgical intervention for symptomatic BPH despite having high rates of treatment-related morbidities and complications which have a hugely negative impact on ~20% of those receiving this intervention.3 6 11 Urologists continue to search for safer techniques without diminishing clinical efficacy compared with TURP.
Endoscopic technologies are being developed, and PVP emerged as a promising intervention which attracted our attention because this is a minimally invasive surgical procedure. The first generation PVP laser system utilised high-powered KTP lasers (60W) at 532 nm and was initially introduced in 1998.40 More advanced generations including the KTP laser (80W), the Green-light high-performance system laser (120W), the Green-light lithium triboride laser (160W) and the Green-light X-ray photoelectron spectroscopy (XPS) laser (180W) systems were then sequentially introduced up until 2018, raising hopes of treating symptomatic BPH, effectively and safely.
Previous research comparing PVP and TURP has demonstrated that there is no significant difference in medium-term efficacy or safety when treating BPH, however; the long-term efficacy between these two techniques remains controversial. In this updated systematic review and meta-analysis, we reviewed all available RCTs and prospective studies (n=22) up until October 2018 which involved a total of 2665 participants. Pooled analyses and sensitivity analysis suggests both PVP and TURP have similar long-term function outcomes, which were analysed using both subjective (IPSS, QoL) and objective (Qmax, PVR) measures. IPSS at 12-month follow-up, Qmax at 6 months and QoL at 12 months highlighted a statistically significant difference, although the differences were only small.
This study adds to the current evidence base in terms of understanding sexual functioning post-intervention. Previous clinical studies have evaluated retrograde ejaculation rates, although conclusions could not be provided with any authority because findings were generally inconsistent and gathered over relatively short periods of time.7 10 25 27 38 The longest running RCT which compared PVP with TURP had a 60-month follow-up, and suggested there is similar improvement in IPSS, Qmax, PVR, QoL and IIEF.36 39
Previously conducted meta-analyses have not had the opportunity to evaluate IIEF due to the relatively small number of studies collecting and reporting this particular outcome. Fortunately, IIEF is increasingly being used to analyse sexual functioning which enabled us to design and perform this meta-analysis given the increased availability of evidence in this area. Pooled analysis, however, suggests there is no significant difference in the retrograde ejaculation rate nor is there a significant difference in IIEF outcomes between PVP and TURP.
This meta-analysis did highlight substantial differences in perioperative factors analysed across this sample of studies. Pooled analyses and sensitivity analyses show that operation times are significantly longer for PVP, whereas the duration of hospitalisation and catheterisation are significantly shorter. Prolonged operative duration involved in PVP interventions appears to be associated with laser power and individual surgeon’s experience and related skills. Laser power is determined for each individual device, and evidence from previous studies suggest that overall operation times are prolonged by ~23 min for PVP with an 80W laser, ~9 min with 120W and 7 min with 120W and 160W lasers. Furthermore, literature shows a surgeon’s overall technical skills and confidence place him/her at a point on a learning curve for new technologies which is likely to be an important factor in the length of operations.
Safety is another key issue because the most serious TURP complications, such as bleeding and TUR syndrome are known to correlate with prostate size and longer operative times.6 41 This analysis highlighted additional benefits, in that the incidence of perioperative complications including bleeding, blood transfusion, clot retention, capsule perforation and TUR syndrome are significantly lower for those receiving the PVP intervention. Although this can be explained by the characteristics of the green light laser, where the 532 nm wavelength is easily absorbed by haemoglobin in prostatic tissues but not by water.13 Likewise in vaporisation, high-power laser energy is instantly absorbed by the blood, ensuring quicker vaporisation into the tissue which creates a prostate cavity with minimal blood loss.42
Other bleeding-related complications occur less frequently for those receiving PVP. However, another possible explanation could be that KTP laser energy penetrates only 1–2 mm of tissue. Therefore, high-power laser energy might be concentrated into the surface coat of prostatic tissue, which then ensures rapid vaporisation, leaving a 0.2 cm rim of residual coagulated tissue.13 It may also be the case that the fluid medium used for PVP procedures is saline solution rather than glycine, therefore TUR syndrome does not occur in PVP. However, further research is necessary to understand this treatment-related complication.
Additional postoperative complications such as acute urinary retention, UTIs, bladder neck contracture and urethral stricture were analysed although no significant differences between TURP and PVP interventions were identified. However, PVP had two distinct disadvantages when compared with TURP. PVP appears to be associated with a higher risk of developing dysuria and for reintervention. Dysuria rates after PVP have been reported to be between 6% and 30%.33 43 There may be several reasons for this, although most likely postoperative dysuria is caused by thermal damage and oedema in urethral tissue. Also, shorter catheterisation times could be another cause of this irritable symptom. That said, research suggests this symptom is generally classified as mild to moderate across all patients, and therefore can be effectively managed, if not resolved altogether within 2 months of follow-up.27 33 As such, transient dysuria is not a serious PVP complication, the more serious complication is reintervention.
There may be a number of reasons post-PVP patients are at a higher risk of re-intervention. There may be inadequate energy delivery, leading to incomplete tissue removal which might play an important role regarding the outcome of the procedure.38 44 According to our analysis, those who received an 80W PVP intervention were at significantly higher risk of reintervention compared with TURP. However, researchers have found the differences between other higher power PVP laser groups (ie, 120W, 160W and 180W) and TURP cohort are not statistically significant. Although the GOLIATH study suggests that the 180W XPS laser system is superior to TURP when considering this particular parameter. Logically, this type of adverse event would markedly decrease with the advent of higher power laser systems.
As well as having a higher risk of dysuria and reintervention, PVP is administered in the absence of histological tissue examination, which might limit opportunities to incidentally identify prostate cancer. In order to address this, clinicians might want to consider when there is a rapidly increasing, or higher levels of prostate-specific antigen (PSA), it might be more beneficial to use TURP rather than laser evaporation techniques. In addition, an extensive examination including PSA measures, digital rectal examinations and ultrasonography could be used to guide prostate biopsies administration, if cancer is suspected.12 45 Prostate cancer is often diagnosed in the late stages which is nearly always too late and therefore opportunities to diagnose this insidious disease must not be disregarded.
LUTS manifest secondarily through BPH and is a chronic health condition. The management of these symptoms create additional economic burden for patients and healthcare systems, generally.2 46 It is vital to evaluate the cost effectiveness of the two surgical therapies in clinical practice. Based on a cost-effectiveness analysis, Armstrong et al suggest that the PVP procedure is unlikely to be cost effective because of the relatively expensive consumables.47 However, Patel argues that there is an absence of high-quality and long-term data, in fact only two RCTs with short-term follow-ups were available at the time.48 This meta-analysis suggests that any initial investment in equipment and surgeon’s training may be at least partially offset by shorter lengths of hospitalisation and lower incidence of postoperative complications for PVP compared with TURP. Considering high number of cases each year, PVP may actually lower the demand for medical resources in this field, although this also requires further research.
This meta-analysis was undertaken using all currently available comparative clinical studies, however; there are some limitations. First of all, despite designing a systematic search strategy, our inclusion criteria meant that non-English documents were omitted, therefore there must be some language bias. Second, there are very few RCTs with long-term follow-up endpoints in this field of interest which must be addressed. To overcome this, we designed this study to incorporate five prospective cohort studies which added a layer of sophistication to this analysis.
None of the RCTs included described blinding methods which is considered a distinct quality deficit but this is to be expected given the nature of the interventions explored. Actually, this perhaps highlights the need to use the Consolidated Standards of Reporting Trials (CONSORT) quality appraisal method or the Delphi method in further studies. While studies have demonstrate high levels of agreement49 between these quality assessment tools and the methods implemented in this meta-analysis, the CONSORT and Delphi methods contain an increased number of variables and are therefore more likely to differentiate. A more substantial concern, however, is that several studies did not report withdrawal or drop outs. This appears to have been is significant factor in our quality assessment and must be addressed in further research. Third, there was consistently, substantial to extreme heterogeneity across this study sample. Sensitivity analysis only partially accounted for such high levels of heterogeneity. Increased sample sizes, or multicentre trials involving larger numbers of participants as well as reporting age stratification may elaborate on our present understanding. Despite these limitations, this study provides the most up-to-date information concerning the comparison of PVP and TURP in surgical management of BPH.
Conclusion
These findings confirm previous studies which suggested that PVP is superior in long-term efficacy to TURP. PVP appears to have only slightly increased IPSS, Qmax, QoL, PVR and IIEF benefit, but is associated with fewer complications. As such, we recommend PVP is offered as the first-line treatment for LUTS secondary to BPH rather than the traditional TURP method. The only addendum is that PVP cannot acquire histological tissue examination which removes an opportunity to identify prostate cancer. Withdrawals and drop outs are not always reported in full and there is a need to use a more comprehensive quality assessment tool to appraise studies in this field. Further research is of course necessary, and should be conducted with larger samples, over longer periods.
Supplementary Material
Footnotes
SL and PP contributed equally.
ML and JW contributed equally.
Contributors: SL and PP designed and conducted the systematic search to identify all relevant studies. SL and PP then assessed eligibility and the quality of each study, before extracting data and conducting statistical analysis. TD and HH coordinated the study and performed data acquisition. XW, WZ and YZ participated in data interpretation and drafting this article. SS, ML and JW reviewed and revised this report for critical content and scientific rigour. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1. Oelke M, Bachmann A, Descazeaud A, et al. . EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64:118–40. 10.1016/j.eururo.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 2. Lukacs B, Loeffler J, Bruyère F, et al. . Photoselective vaporization of the prostate with GreenLight 120-W laser compared with monopolar transurethral resection of the prostate: a multicenter randomized controlled trial. Eur Urol 2012;61:1165–73. 10.1016/j.eururo.2012.01.052 [DOI] [PubMed] [Google Scholar]
- 3. Bachmann A, Descazeaud A, Drake M, et al. . Guidelines on the management of non-neurogenic male lower urinary tract symptoms (LUTS), INCL. benign prostatic obstruction (BHO. Madrid, Spain: Presented at 30th Annual EAU Congress, 2015. [Google Scholar]
- 4. Reich O, Gratzke C, Bachmann A, et al. . Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol 2008;180:246–9. 10.1016/j.juro.2008.03.058 [DOI] [PubMed] [Google Scholar]
- 5. Rassweiler J, Teber D, Kuntz R, et al. . Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol 2006;50:969–80. 10.1016/j.eururo.2005.12.042 [DOI] [PubMed] [Google Scholar]
- 6. Mebust WK, Holtgrewe HL, Cockett AT, et al. . Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol 1989;141:243–7. 10.1016/S0022-5347(17)40731-2 [DOI] [PubMed] [Google Scholar]
- 7. Capitán C, Blázquez C, Martin MD, et al. . GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: a randomized clinical trial with 2-year follow-up. Eur Urol 2011;60:734–9. 10.1016/j.eururo.2011.05.043 [DOI] [PubMed] [Google Scholar]
- 8. Ruszat R, Wyler SF, Seitz M, et al. . Comparison of potassium-titanyl-phosphate laser vaporization of the prostate and transurethral resection of the prostate: update of a prospective non-randomized two-centre study. BJU Int 2008;102:1432–8. 10.1111/j.1464-410X.2008.07905.x [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Wang M, Wang S, et al. . 160-Watt lithium triboride laser vaporization versus transurethral resection of prostate: a prospective nonrandomized two-center trial. Urology 2012;79:650–4. 10.1016/j.urology.2011.11.039 [DOI] [PubMed] [Google Scholar]
- 10. Bachmann A, Tubaro A, Barber N, et al. . 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial--the GOLIATH study. Eur Urol 2014;65:931–42. 10.1016/j.eururo.2013.10.040 [DOI] [PubMed] [Google Scholar]
- 11. Bouchier-Hayes DM, Van Appledorn S, Bugeja P, et al. . A randomized trial of photoselective vaporization of the prostate using the 80-W potassium-titanyl-phosphate laser vs transurethral prostatectomy, with a 1-year follow-up. BJU Int 2010;105:964–9. 10.1111/j.1464-410X.2009.08961.x [DOI] [PubMed] [Google Scholar]
- 12. Malek RS, Kuntzman RS, Barrett DM. High power potassium-titanyl-phosphate laser vaporization prostatectomy. J Urol 2000;163:1730–3. 10.1016/S0022-5347(05)67530-1 [DOI] [PubMed] [Google Scholar]
- 13. Kuntzman RS, Malek RS, Barrett DM, et al. . Potassium-titanyl-phosphate laser vaporization of the prostate: a comparative functional and pathologic study in canines. Urology 1996;48:575–83. 10.1016/S0090-4295(96)00247-6 [DOI] [PubMed] [Google Scholar]
- 14. Kuntzman RS, Malek RS, Barrett DM, et al. . High-Power (60-watt) potassium-titanyl-phosphate laser vaporization prostatectomy in living canines and in human and canine cadavers. Urology 1997;49:703–8. 10.1016/S0090-4295(97)00232-X [DOI] [PubMed] [Google Scholar]
- 15. Thangasamy IA, Chalasani V, Bachmann A, et al. . Photoselective vaporisation of the prostate using 80-W and 120-W laser versus transurethral resection of the prostate for benign prostatic hyperplasia: a systematic review with meta-analysis from 2002 to 2012. Eur Urol 2012;62:315–23. 10.1016/j.eururo.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 16. Ding H, Du W, Lu Z-P, et al. . Photoselective green-light laser vaporisation vs. TURP for BPH: meta-analysis. Asian J Androl 2012;14:720–5. 10.1038/aja.2012.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teng J, Zhang D, Li Y, et al. . Photoselective vaporization with the green light laser vs transurethral resection of the prostate for treating benign prostate hyperplasia: a systematic review and meta-analysis. BJU Int 2013;111:312–23. 10.1111/j.1464-410X.2012.11395.x [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 19. Phillips B, Ball C, Sackett D, et al. . Oxford centre for evidence-based medicine – lev ELS of evidence. 2009, 2009. Available: http://www.cebm.net/oxford-centre-evidence-based-medicine levels-evidencemarch-2009/ [Accessed May 2018].
- 20. Clark HD, Wells GA, Huët C, et al. . Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials 1999;20:448–52. [DOI] [PubMed] [Google Scholar]
- 21. Wells GA, Shea B, O’Connell, et al. . The Newcastle Ottawa scale (NOS) for assessing the Qual ity of nonrandomized studies in Metaanaly Ses. Ottawa Hospital research Institute, 2018. Available: http://www.ohri.ca/programs/clinical_epi demiology/oxford.asp [Accessed May 2018].
- 22. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Green S. Cochrane Handbook for systematic reviews of interventions, version 5.1.0. 2011 Cochrane Collaboration, 2011. [Google Scholar]
- 24. Kumar A, Vasudeva P, Kumar N, et al. . A prospective randomized comparative study of monopolar and bipolar transurethral resection of the prostate and photoselective vaporization of the prostate in patients who present with benign prostatic obstruction: a single center experience. J Endourol 2013;27:1245–53. 10.1089/end.2013.0216 [DOI] [PubMed] [Google Scholar]
- 25. Pereira-Correia JA, de Moraes Sousa KD, Santos JBP, et al. . GreenLight HPS™ 120-W laser vaporization vs transurethral resection of the prostate (<60 mL): a 2-year randomized double-blind prospective urodynamic investigation. BJU Int 2012;110:1184–9. 10.1111/j.1464-410X.2011.10878.x [DOI] [PubMed] [Google Scholar]
- 26. Al-Ansari A, Younes N, Sampige VP, et al. . GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia: a randomized clinical trial with midterm follow-up. Eur Urol 2010;58:349–55. 10.1016/j.eururo.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 27. Xue B, Zang Y, Zhang Y, et al. . GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia: a prospective randomized trial. J Xray Sci Technol 2013;21:125–32. 10.3233/XST-130359 [DOI] [PubMed] [Google Scholar]
- 28. Mohanty NK, Vasudeva P, Kumar A, et al. . Photoselective vaporization of prostate vs. transurethral resection of prostate: a prospective, randomized study with one year follow-up. Indian J Urol 2012;28:307–12. 10.4103/0970-1591.102708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bachmann A, Tubaro A, Barber N, et al. . A European multicenter randomized noninferiority trial comparing 180 W GreenLight XPS laser vaporization and transurethral resection of the prostate for the treatment of benign prostatic obstruction: 12-month results of the Goliath study. J Urol 2015;193:570–8. 10.1016/j.juro.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 30. Thomas JA, Tubaro A, Barber N, et al. . A multicenter randomized Noninferiority trial comparing GreenLight-XPS laser vaporization of the prostate and transurethral resection of the prostate for the treatment of benign prostatic obstruction: Two-yr outcomes of the Goliath study. Eur Urol 2016;69:94–102. 10.1016/j.eururo.2015.07.054 [DOI] [PubMed] [Google Scholar]
- 31. Kumar N, Vasudeva P, Kumar A, et al. . Prospective randomized comparison of monopolar TURP, bipolar TURP and Photoselective vaporization of the prostate in patients with benign prostatic obstruction: 36 months outcome. Low Urin Tract Symptoms 2018;10:17–20. 10.1111/luts.12135 [DOI] [PubMed] [Google Scholar]
- 32. Telli O, Okutucu TM, Suer E, et al. . A prospective, randomized comparative study of monopolar transurethral resection of the prostate versus photoselective vaporization of the prostate with GreenLight 120-W laser, in prostates less than 80 cc. Ther Adv Urol 2015;7:3–8. 10.1177/1756287214556643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tasci AI, Tugcu V, Sahin S, et al. . Rapid communication: photoselective vaporization of the prostate versus transurethral resection of the prostate for the large prostate: a prospective nonrandomized bicenter trial with 2-year follow-up. J Endourol 2008;22:347–54. 10.1089/end.2007.0137 [DOI] [PubMed] [Google Scholar]
- 34. Tugcu V, Tasci AI, Sahin S, et al. . Comparison of photoselective vaporization of the prostate and transurethral resection of the prostate: a prospective nonrandomized bicenter trial with 2-year follow-up. J Endourol 2008;22:1519–26. 10.1089/end.2007.0321 [DOI] [PubMed] [Google Scholar]
- 35. Nomura H, Seki N, Yamaguchi A, et al. . Comparison of photoselective vaporization and standard transurethral resection of the prostate on urodynamics in patients with benign prostatic hyperplasia. Int J Urol 2009;16:657–62. 10.1111/j.1442-2042.2009.02333.x [DOI] [PubMed] [Google Scholar]
- 36. Guo S, Müller G, Lehmann K, et al. . The 80-W KTP GreenLight laser vaporization of the prostate versus transurethral resection of the prostate (TURP): adjusted analysis of 5-year results of a prospective non-randomized bi-center study. Lasers Med Sci 2015;30:1147–51. 10.1007/s10103-015-1721-x [DOI] [PubMed] [Google Scholar]
- 37. Bachmann A, Schürch L, Ruszat R, et al. . Photoselective vaporization (PVP) versus transurethral resection of the prostate (TURP): a prospective bi-centre study of perioperative morbidity and early functional outcome. Eur Urol 2005;48:965–72. discussion 972 10.1016/j.eururo.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 38. Horasanli K, Silay MS, Altay B, et al. . Photoselective potassium titanyl phosphate (KTP) laser vaporization versus transurethral resection of the prostate for prostates larger than 70 mL: a short-term prospective randomized trial. Urology 2008;71:247–51. 10.1016/j.urology.2007.09.017 [DOI] [PubMed] [Google Scholar]
- 39. Mordasini L, Di Bona C, Klein J, et al. . 80-W GreenLight laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic obstruction: 5-year outcomes of a single-center prospective randomized trial. Urology 2018;116:144–9. 10.1016/j.urology.2018.01.037 [DOI] [PubMed] [Google Scholar]
- 40. Malek RS, Barrett DM, Kuntzman RS. High-Power potassium-titanyl-phosphate (KTP/532) laser vaporization prostatectomy: 24 hours later. Urology 1998;51:254–6. 10.1016/S0090-4295(97)00613-4 [DOI] [PubMed] [Google Scholar]
- 41. Olsson J, Nilsson A, Hahn RG. Symptoms of the transurethral resection syndrome using glycine as the irrigant. J Urol 1995;154:123–8. 10.1016/S0022-5347(01)67246-X [DOI] [PubMed] [Google Scholar]
- 42. Stafinski T, Menon D, Harris K, et al. . Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia. Can Urol Assoc J 2008;2:124–34. [PMC free article] [PubMed] [Google Scholar]
- 43. Kuntz RM. Current role of lasers in the treatment of benign prostatic hyperplasia (BPH). Eur Urol 2006;49:961–9. 10.1016/j.eururo.2006.03.028 [DOI] [PubMed] [Google Scholar]
- 44. Sandhu JS, Ng C, Vanderbrink BA, et al. . High-Power potassium-titanyl-phosphate photoselective laser vaporization of prostate for treatment of benign prostatic hyperplasia in men with large prostates. Urology 2004;64:1155–9. 10.1016/j.urology.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 45. Malek RS, Kuntzman RS, Barrett DM. Photoselective potassium-titanyl-phosphate laser vaporization of the benign obstructive prostate: observations on long-term outcomes. J Urol 2005;174:1344–8. 10.1097/01.ju.0000173913.41401.67 [DOI] [PubMed] [Google Scholar]
- 46. Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2005;173:1256–61. 10.1097/01.ju.0000155709.37840.fe [DOI] [PubMed] [Google Scholar]
- 47. Armstrong N, Vale L, Deverill M, et al. . Surgical treatments for men with benign prostatic enlargement: cost effectiveness study. BMJ 2009;338:b1288 10.1136/bmj.b1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel A. Words of wisdom. re: surgical treatments for men with benign prostatic enlargement: cost effectiveness study. Eur Urol 2009;56:741–2. 10.1016/j.eururo.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 49. Dreier M. Quality Assessment in Meta-analysis : Doi S, Williams G, Methods of clinical epidemiology. Springer series on epidemiology and public health. Berlin: Springer, 2013: 213–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028855supp001.pdf (49.2KB, pdf)