Abstract
Objective
Esophageal and gastric cardia adenocarcinoma (EA/GCA) are among the most rapidly increasing cancers in Western countries. Elevated body mass index (BMI, kg/m2) in adulthood is a known risk factor, but associations in early life are unclear.
Methods
We assessed weight change between childhood and early adulthood in relation to EA/GCA. Measured weights and heights during childhood (7–13 years) and early adulthood (17–26 years) were available for 64,695 young men from the Copenhagen School Health Records Register and Danish Conscription Database. Individuals were categorized as normal or overweight. Linkage with the Danish Cancer Registry identified 275 EA/GCA cases. Hazard ratios (HRs) and 95% confidence intervals (CI) were estimated using Cox proportional hazards regression.
Results
Men first classified as overweight at age 7 were at a 2.5-times increased EA/GCA risk (HR=2.49, 95%CI: 1.50–4.14), compared with men never classified as overweight. Men with persistent overweight at ages 7, 13, and early adulthood had 3.2-times increased EA/GCA risk (HR=3.18, 95%CI: 1.57–6.44). However, there was little evidence of increased EA/GCA risk for men with overweight during childhood and subsequent remittance by early adulthood.
Conclusion
Persistent overweight in early life was associated with increased EA/GCA risk, which declines if body weight is reduced.
Keywords: adenocarcinoma, body mass index, obesity
INTRODUCTION
Incidence of esophageal and gastric cardia adenocarcinoma (EA/GCA) has been rapidly increasing over the last two decades in Denmark and other Western countries (1, 2, 3, 4) and has generally paralleled the increasing prevalence of obesity (5, 6). EA and GCA are glandular epithelial cancers originating in or near the gastroesophageal junction with 5-year survival rates of approximately 26% and an overlapping etiopathogenesis (7).
Elevated body mass index (BMI, kg/m2) in adulthood is consistently associated with increasing incidence of EA and GCA (8). Additionally, our recent study of two U.S. cohort studies reported that overweight in early adulthood and weight gain in later life were associated with increased EA and GCA risks (9). While the underlying mechanisms of the BMI–EA/GCA association are unclear, leading hypotheses include mediation by gastroesophageal reflux (10) or metabolic dysfunction (11, 12, 13).
A prior report, utilizing the Copenhagen School Health Records Register (CSHRR), examined early life adiposity in relation to EA and reported higher childhood BMI was associated with an increased EA risk (14). The associations between childhood BMI and EA/GCA may be due to 1) direct effects of childhood obesity on gastroesophageal reflux disease (15) and metabolic disorders (16), which may directly affect future risks of EA and GCA(10, 11, 12) or 2) “tracking” of adiposity from childhood through early and mid-adulthood (17). These possibilities lead to question the effects of adiposity changes between childhood and early adulthood in relation to EA or GCA.
The importance of these associations is underscored by the fact that the prevalence of obesity (BMI≥30 kg/m2) has been increasing over the past four decades. Although data suggests a recent plateau, the excess adiposity remains widespread (18) with 23% of European adults classified as having obesity (19) and 10–30% of European children classified as having overweight or obesity (20, 21).
Therefore, elucidating the etiologically relevant timing of weight gain in association with EA and GCA could provide insight into possible mechanisms as well as suggestion of the time point for the most efficacious interventions. In the current study, we examined the timing of overweight and weight changes from childhood to early adulthood in relation to EA and GCA, utilizing a large population-based cohort of men.
METHODS
Study Population
We used height and weight data from the CSHRR (22) and the Danish Conscription Database (DCD) (23). For childhood measures, we used the CSHRR, which includes 188,360 male children who attended public or private schools in Copenhagen, Denmark and were born between 1930 and 1989. Annual school health assessments were performed by school doctors or health nurses and included anthropometric measurements as part of a comprehensive examination. With the child wearing no shoes and minimal clothing, medical personnel measured and recorded weight (to the nearest 100 g) and height (to the nearest ½ cm) on the child’s personal health card.
For early adulthood measures, we utilized the DCD, which includes 728,159 men who entered compulsory conscription and were born between 1939 and 1959. Mandatory health examinations were performed by conscripted board physicians and included anthropometric measurement, physical exam, educational level ascertainment, and a cognitive ability assessment (23). With the men wearing underwear and no shoes, medical personnel measured and recorded weight (to the nearest 1 kg) and height (to the nearest 1 cm). Information on self-reported educational ascertainment was categorized as short: 7–10 years primary school with or without finals; medium: skilled training in industry, trade and craft; and long: 9–12 years middle and secondary school, secondary school final, medium length or higher education (24).
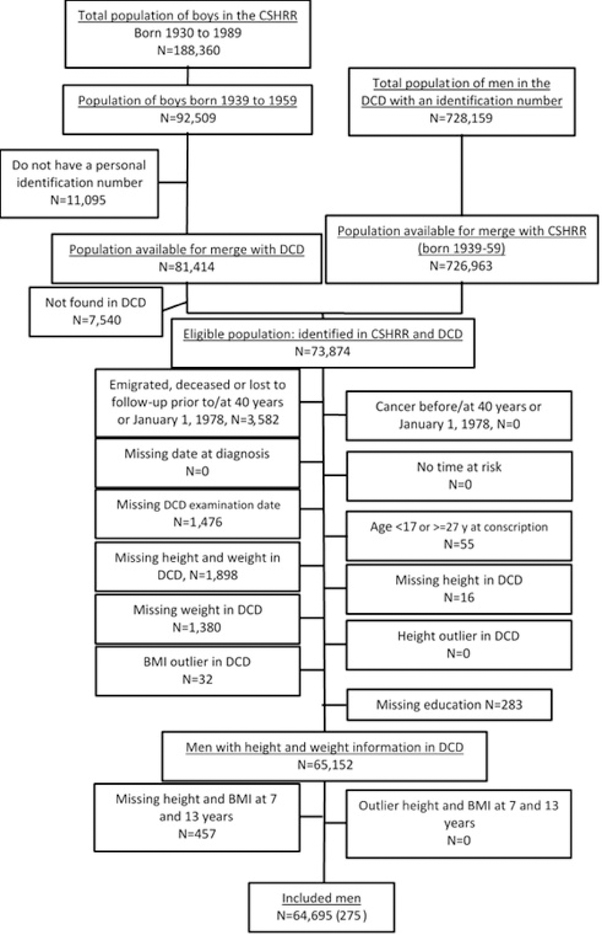
The eligible study population included 73,874 men with a national personal identification (ID) number, who were born between 1939 and 1959 (Figure 1) and were included in both the CSHRR and DCD. Men were excluded due to emigration, death, or loss to follow-up prior to 40 years of age or January 1, 1978 (n=3,582). Further exclusions included missing examination date (n=1,476), missing height and weight in childhood or early adulthood (n=3,751), BMI outlier (z-score <−4.5 or >4.5, n=32), age <17 or ≥27 years at conscription (n=55), and missing educational attainment report (n=283). Thus, our final analytic population included 64,695 men.
Figure 1.
Flow chart of eligible and included individuals in the study. CSHRR: Copenhagen School Health Records Register, DCD: Danish Conscription Database, BMI: body mass index (kg/m2).
Exposure
Weight and height were used to calculate BMI, as weight in kilograms (kg) divided by height in meters (m) squared (kg/m2). As there were suggestions of non-linearity of the association between BMI z-score and EA/GCA, men were categorized as having a normal weight or overweight at 7 and 13 years and in early adulthood (i.e., conscription). The association between BMI z-score at specific ages and EA/GCA is presented as a supplemental analysis but should be interpreted with caution. For boys and young men <18 years of age, we utilized the age- and sex specific cut points defined by the International Obesity Task Force to classify men with overweight: ≥17.88 kg/m2 for boys at age 7 years, ≥21.89 kg/m2 for boys at age 13 years, and ≥24.46–24.96 kg/m2 for young men between 17 and 18 years (utilizing month-specific cut-points) (25). For men ≥18 years, overweight was defined as ≥25 kg/m2 according to World Health Organization (26).
Record linkages
The CSHRR and DCD were linked to the Danish Cancer Registry and Danish Civil Registration System (vital statistics) using the ID number. Beginning in April 1968, Danish citizens of all ages were assigned ID numbers (27). Children attending school in or after 1968 had the ID number recorded on their health card (27). For indiviudals with no ID number recorded on their health card, ID numbers were obtained from the Danish Civil Registration System based on the person’s forename(s), surname, sex, and date of birth. Utilizing this approach, ID numbers were identified for 88% of the study population. For indiviudals that died or emigrated prior to 1968, an ID number was never issued (22).
Outcomes
Incident esophageal cancers (International Classification of Diseases [ICD]- 10 topography: C15.0–C15.9) and gastric cardia cancer (ICD-10: C16.0), were ascertained by linkage with the Danish Cancer Registry (28). Cases were then classified as adenocarcinoma (ICD for Oncology, 3rd edition morphology codes: 8140–8575). Follow-up began on January 1, 1978 or 40 years of age (as there were few recorded cases of these malignancies at earlier ages) and continued until an EA/GCA diagnosis, death, emigration, loss to follow-up or December 31, 2014, whichever came first. This resulted in 146 EA cases and 129 GCA cases.
Statistical Analysis
To assess the associations between anthropometric factors and risk of EA and GCA, we utilized Cox proportional hazards regression analysis to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), with age as the underlying time metric and baseline hazard stratified by birth cohort (5-year intervals). All models were adjusted for educational attainment and age at conscription. We examined weight status at ages 7 and 13 and early adulthood (i.e., conscription) and weight patterns: (1) between age 7 and early adulthood, (2) between age 13 and early adulthood, and (3) at ages 7, 13, and early adulthood. We also examined age when individuals were first classified with overweight (29). Tests for deviations from linearity were performed using linear splines with three knot points. The proportional hazards assumption was tested by likelihood ratio test, comparing two nested models one with and one without an interaction term between BMI z-score and a categorical variable of age at diagnosis. The proportional hazards assumption was not observed to be violated (p≥0.17).
All statistical analyses were performed using Stata (version 14, StataCorp LP, College Station, TX, USA). The study was approved by the Danish Data Protection Agency. According to Danish legislation, no approval from an ethics committee or informed consent from patients is required for register-based studies in Denmark (30), and this analysis was determined to be excluded from IRB review per 45 CFR 46.102 by the NIH Office of Human Subjects Research Protections.
RESULTS
Of the 64,695 men included in the study, a majority also had a BMI measurement at age 7 years (n=62,590, 96.7%) or at age 13 years (n=63,322, 97.9%; Table 1). The average BMI was 15.5 kg/m2 at 7 years, 18.1 kg/m2 at 13 years, and 21.6 kg/m2 at conscription. The majority of the cohort had medium (36.0%) or long (40.5%) educational attainment. Follow-up (from January 1, 1978 or 40 years of age) averaged 24 years (standard deviation=7.3), and the average age of diagnosis with EA/GCA was 60.3 years (standard deviation=7.4).
Table 1.
Body Mass Index (BMI) characteristics of the included men by birth cohort.
| BMI (kg/m2) | ||||
|---|---|---|---|---|
| Birth Cohort | N (%)* | Mean | SD | |
| Age | 1939– 49 | 37,652 (58.2) | 15.49 | 1.12 |
| 7 years | 1939– 49 | 37,652 (58.2) | 15.49 | 1.12 |
| 1950–59 | 24,938 (38.5) | 15.40 | 1.22 | |
| 13 years | 1939– 49 | 38,131 (58.9) | 18.16 | 2.00 |
| 1950–59 | 25,191 (38.9) | 18.00 | 2.23 | |
| Early adulthood | 1939– 49 | 39,056 (60.4) | 21.66 | 2.34 |
| 1950–59 | 25,639 (39.6) | 21.51 | 2.74 | |
| Education† | – | 15,207 (23.5) | 21.77 | 2.75 |
| Short | – | 15,207 (23.5) | 21.77 | 2.75 |
| Medium | – | 23,294 (36.0) | 21.64 | 2.41 |
| Long | – | 26,194 (40.5) | 21.47 | 2.43 |
Percentage of total study population (n=64,695).
Education defined as short: 7–10 years primary school with or without finals; medium: skilled training in industry, trade and craft; and long: 9–12 years middle and secondary school, secondary school final, medium length or higher education.
The prevalence of overweight increased with increasing age: 2.9% at 7 years, 5.2% at 13 years, and 8.2% in early adulthood (Supplemental Table S1). Of boys with overweight at age 7 or 13 years, 49.3% and 57.5% also had overweight in early adulthood, respectively.
Overweight in childhood was associated with a 2-times increased risk of EA/GCA at both ages 7 (HR=2.36, 95% CI: 1.42–3.92) and 13 years (HR=1.77, 95% CI: 1.15–2.71; Table 2). In early adulthood, overweight was associated with a 2-times increased risk of EA (HR=2.11, 95% CI: 1.33–3.33) but not GCA (HR=0.91, 95% CI: 0.46–1.79), resulting in a 50% increased risk of EA/GCA combined (HR=1.52, 95% CI: 1.05–2.22). Further, when BMI was categorized into quintiles rather than as dichotomous values, there was a general pattern of elevated risks for men in the highest quintile than the median quintile, although these were statistically significant only at ages 13 and early adulthood (Supplemental Table S2). These patterns were less evident for GCA. Similar results were observed when we examined the associations between a one-unit increase in BMI z-score and EA/GCA (Supplemental Table S3).
Table 2.
Adjusted hazard ratios (HR)* and 95% confidence intervals (CI) for associations between weight status and esophageal and gastric cardia adenocarcinoma incidence.
| Esophageal Adenocarcinoma | Gastric Cardia Adenocarcinoma | Combined Cases | |||||
|---|---|---|---|---|---|---|---|
| Total (n) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | |
| Age, 7 years | |||||||
| Normal weight | 60,773 | 130 | Referent | 120 | Referent | 250 | Referent |
| Overweight | 1,817 | 11 | 3.11 (1.68, 5.76) | 5 | 1.55 (0.63, 3.79) | 16 | 2.36 (1.42, 3.92) |
| Age, 13 years | |||||||
| Normal weight | 60,013 | 131 | Referent | 117 | Referent | 248 | Referent |
| Overweight | 3,309 | 13 | 1.88 (1.06, 3.33) | 10 | 1.64 (0.86, 3.13) | 23 | 1.77 (1.15, 2.71) |
| Early Adulthood | |||||||
| Normal weight | 59,395 | 124 | Referent | 120 | Referent | 244 | Referent |
| Overweight | 5,300 | 22 | 2.11 (1.33, 3.33) | 9 | 0.91 (0.46, 1.79) | 31 | 1.52 (1.05, 2.22) |
Adjusted for education level and age at conscription.
Men who were classified with overweight at both age 7 and conscription had 3-times the increased risk of EA/GCA (HR=3.35, 95% CI: 1.83–6.15; Table 3). Results were also increased when examining men who were classified with overweight at both age 13 and conscription (HR=1.94, 95% CI: 1.13–3.34). There was little evidence for an increased risk of EA/GCA for men with overweight during childhood and subsequent remittance by early adulthood. For example, men with overweight at age 7 and a normal weight in early adulthood had a non-significant 48% increased risk of EA/GCA (HR=1.48, 95% CI: 0.61–3.59). Men with overweight in early adulthood but not at age 7 were at an increased risk of EA (HR=1.83, 95% CI: 1.06–3.14) but not GCA (HR=0.63, 95% CI: 0.26–1.54). Examining the cross-classification (Table 4), individuals with persistent overweight at ages 7 and 13 years and in early adulthood were at three-fold higher risk of EA/GCA (HR=3.18, 95% CI: 1.57–6.44), compared with men never classified as overweight. Individuals with overweight only in early adulthood were not at a significantly increased risk (HR=1.25, 95% CI: 0.73–2.15).
Table 3.
Adjusted hazard ratios (HR)* and 95% confidence intervals (CI) for associations between weight patterns and esophageal and gastric cardia adenocarcinoma incidence.
| Esophageal Adenocarcinoma | Gastric Cardia Adenocarcinoma | Combined Cases | |||||
|---|---|---|---|---|---|---|---|
| Total (n) | Cases (n)** | HR (95% CI) | Cases (n)** | HR (95% CI) | Cases (n) | HR (95% CI) | |
| Age 7 and Early Adulthood | |||||||
| Normal weight | 56,541 | 115 | Referent | 115 | Referent | 230 | Referent |
| Overweight at age 7 only | 919 | – | – | – | – | 5 | 1.48 (0.61, 3.59) |
| Overweight at early adulthood only | 4,232 | 15 | 1.83 (1.06, 3.14) | 5 | 0.63 (0.26, 1.54) | 20 | 1.24 (0.78, 1.96) |
| Overweight at age 7 and early adulthood | 898 | 7 | 4.29 (1.99, 9.21) | – | – | 11 | 3.35 (1.83, 6.15) |
| Age 13 and Early Adulthood | |||||||
| Normal weight | 56,727 | 118 | Referent | 113 | Referent | 231 | Referent |
| Overweight at age 13 only | 1,406 | – | – | 5 | 1.83 (0.75, 4.49) | 9 | 1.62 (0.83, 3.15) |
| Overweight at early adulthood only | 3,286 | 13 | 2.01 (1.13, 3.58) | – | – | 17 | 1.37 (0.83, 2.24) |
| Overweight at age 13 and early adulthood | 1,903 | 9 | 2.42 (1.22, 4.78) | 5 | 1.44 (0.59, 3.53) | 14 | 1.94 (1.13, 3.34) |
Adjusted for education level and age at conscription.
Cells with less than 5 cases are not presented.
Table 4.
Adjusted hazard ratios (HR)* and 95% confidence intervals (CI) for associations between weight patterns and combined esophageal and gastric cardia adenocarcinoma incidence.
| Combined Cases |
|||
|---|---|---|---|
| Total (n) | Cases (n)** | HR (95% CI) | |
| Weight Pattern | |||
| Never overweight | 54289 | 218 | Referent |
| Overweight at age 7 only | 531 | – | – |
| Overweight at age 13 only | 1008 | 8 | 1.97 (0.97, 3.99) |
| Overweight in early adulthood only | 2996 | 14 | 1.25 (0.73, 2.15) |
| Overweight at age 7 and 13 | 370 | – | – |
| Overweight at age 7 and early adulthood | 176 | – | – |
| Overweight at age 13 and early adulthood | 1142 | 6 | 1.38 (0.61, 3.11) |
| Persistently overweight | 705 | 8 | 3.18 (1.57, 6.44) |
Adjusted for education level and age at conscription.
Cells with less than 5 cases are not presented.
The group of individuals with first overweight at age 7 had more than two-fold increased risk of EA/GCA (HR=2.49, 95% CI: 1.50–4.14), irrespective of the subsequent weight pattern when compared with individuals with normal weight from childhood to early adulthood (Table 5). Associations were weaker and non-significant among individuals with first overweight at age 13 (HR=1.66, 95% CI: 0.97–2.86) or early adulthood (HR=1.25, 95% CI: 0.73–2.16).
Table 5.
Adjusted hazard ratios (HR)* and 95% confidence intervals (CI) for associations between first registration with overweight and esophageal and gastric cardia adenocarcinoma incidence.
| Esophageal Adenocarcinoma | Gastric Cardia Adenocarcinoma | Combined Cases | |||||
|---|---|---|---|---|---|---|---|
| Total (n) | Cases (n) | HR (95% CI) | Cases (n)** | HR (95% CI) | Cases (n) | HR (95% CI) | |
| Time First Overweight | |||||||
| Never | 57,767 | 116 | Referent | 115 | Referent | 231 | Referent |
| Age 7 | 1782 | 11 | 3.41 (1.83, 6.34) | 5 | 1.57 (0.64, 3.85) | 16 | 2.49 (1.50, 4.14) |
| Age 13 | 2150 | 8 | 1.90 (0.93, 3.89) | 6 | 1.43 (0.63, 3.25) | 14 | 1.66 (0.97, 2.86) |
| Early adulthood | 2996 | 11 | 1.95 (1.04, 3.62) | – | – | 14 | 1.25 (0.73, 2.16) |
Adjusted for education level and age at conscription.
Cells with less than 5 cases are not presented.
DISCUSSION
This population-based cohort study indicates that persistent overweight across the early life course, particularly when exposure begins in early childhood, is associated with increased risks of EA and GCA. The strongest associations between adiposity and EA/GCA were observed for men who were classified with persistently overweight at ages 7 and 13 years and in early adulthood. Among this group of individuals, we found a 3-times or more increased risk of EA/GCA, compared with men who were classified as normal weight at all three time points. Similarly, men with first overweight at age 7 were at a 2.5-times increased risk of EA/GCA irrespectively of later weight development. However, men with overweight at age 7 that remitted by early adulthood did not have a significantly increased risk of EA/GCA, although the confidence intervals were wide.
Prior studies have reported that excess early adulthood weight status or adult weight gain have each, independently, been associated with increased risk of EA and GCA (9, 14, 31, 32, 33, 34, 35). Our findings extend these analyses by examining weight changes between childhood (ages 7 and 13) and early adulthood. These results support our prior findings, which showed that childhood BMI was associated with an increased risk of EA (14). Additionally, the current report also expands the age of BMI assessment to include early adulthood, which is conceivably more related to later adulthood weight. For EA, we report that overweight only in early adulthood was associated with a 2-times increased risk, similar to prior reports (8). However, utilizing both childhood and early adulthood BMI enabled us to identify critical periods for overweight and weight gain, specifically early childhood overweight and persistent overweight. Our prior study, utilizing data from NIH-AARP Diet and Health Study and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, examined weight in early adulthood and weight gain during adulthood and reported that individuals with persistent overweight in adulthood were at a 3-times or greater increased risk of EA and GCA (9). However, this was based on self-report, and there was no information about weight status in childhood. Another previous study, utilizing data from the Nurses’ Health Study and Health Professionals Follow-Up Study, examined self-reported, recalled somatotypes of body shape beginning at age 5 through age 60 and reported a 2-times increased risk of EA associated with a body shape trajectory of heavy-stable/increasing (36). Despite the limitations of recalled body shape and the small number of EA outcomes assessed (n=98), the results of this study are supportive of our findings presented herein, namely that cumulative effects of overweight as important.
The current study highlights the adverse health effects of children with persistent overweight into early adulthood and provides support that there is a possibility to reduce risk by remitting from childhood overweight by early adulthood. The increased risk of EA/GCA that is noted for individuals with first overweight at age 7 or 13 may be indicative of individuals with persistent overweight, as 49% and 58% of boys at ages 7 and 13 years, respectively, who are classified as overweight remain as such in early adulthood. Similarly, a recent study (37) reported that adolescents with obesity had the greatest acceleration in BMI between ages 2 and 6 years. Additionally, 53% of adolescents with obesity were with overweight or obesity from age 5 years through 18 years (37). However, BMI tracking is reported to be weaker from childhood to late adulthood than to early adulthood (17). In the current report, the nonsignificant reduced risks of EA/GCA among men that are classified as overweight in childhood, but normal weight by early adulthood shows that there is intervention potential in childhood to reduce risks of adverse health outcomes.
While the adiposity-EA/GCA association is well-established, the exact underlying mechanisms remains to be elucidated. One hypothesis is that central adiposity leads to increased intra-abdominal pressure which, in turn, can promote gastroesophageal reflux disease, a risk factor for the precursor lesion of EA (38) – Barrett’s esophagus (39). Another hypothesis is that dysfunctional adipose tissue may lead to metabolic sequelae, such as glucose intolerance, hypertension, metabolic syndrome, or chronic, low-grade inflammation. These metabolic disturbances may have carcinogenic effects and could potentially increase risks of EA or GCA (11, 12, 13).
A prior study in the CSHRR reported that higher childhood BMI was associated with an increased risk of EA (14). One hypothesis for this association is that adiposity could “track” from childhood through early and mid-adulthood (17). However, only 49% of men with overweight in childhood were also with overweight at conscription. The current study supports that childhood obesity may have direct effects on future EA/GCA risks, as it begins a cascade of individuals with persistent overweight who are at the highest risk of EA/GCA. Thus, there may be direct effects of childhood obesity on gastroesophageal reflux disease (15) and metabolic disorders (16), which may directly affect future risks of EA and GCA (10, 11, 12). However, individuals that remit with overweight between childhood and early adulthood, or are only overweight in early adulthood, had a non-significantly lower risk of EA/GCA, compared with those with persistent overweight. Thus, the cumulative effect of overweight from childhood through early adulthood appears to be the most important adiposity influence on EA/GCA development.
Overall, the associations were stronger between excess adiposity and EA, which reinforces the fact that EA is the primary driver of the observed associations (40). Associations with GCA were similar but weaker. However, in early adulthood, overweight was associated with a 2-times increased risk of EA but not GCA. This could be due in part to the limited sample size but is also consistent with the hypothesis that GCA represents a heterogeneous set of tumors with a less clear origin than EA (41). Genomic studies of EA and GCA have revealed that 99% of EA tumors are characterized by chromosomal instability (CIN) while 80% of GCA tumor subtypes are classified as CIN with the remainder of GCA tumors classified as either Epstein-Barr virus (EBV) infection-positive or microsatellite instability (MSI)positive subtypes (42). If we had the capability to limit our examination to only CIN-positive tumors, we may have observed greater homogeneity in results between EA and GCA.
We only had data at three time points—ages 7 and 13 years, and age at conscription (i.e., early adulthood). Thus, we were only able to examine weight status and weight changes in the early life course until entry to adulthood and not at time points closer to cancer diagnosis. However, we know from prior studies that adult weight status is associated with an increased risk of EA/GCA (8, 9). Thus, this suggests that adiposity might be involved at all stages of cancer – initiation, promotion, and progression. Categorizing men into normal weight and overweight may result in some non-differential misclassification, which would usually bias results toward the null. To classify men with overweight, we utilized contemporary definitions. However, men with overweight in the current report are likely leaner than a contemporary cohort of men with overweight (43). Thus, children with overweight today could be at even further increased risk.
We were able to adjust for education status at conscription, but this may not be the final educational attainment for these men. However, there was little difference between adjusted and unadjusted models (data not shown). This study also did not have information on some other important risk factors for EA and GCA. Specifically, gastroesophageal reflux disease was not assessed. However, gastroesophageal reflux disease may be an important stratification, whereby some individuals’ risks of EA/GCA due to excess adiposity may be mediated through gastroesophageal reflux versus metabolic sequalae. Despite the large sample size, we had a limited number of cases in some strata due to the rare outcomes of EA and GCA. Finally, our study population was a homogeneous population from Denmark and may not be generalizable to other high-risk populations (44).
This study utilized the CSHRR linked to the DCD, which is a unique resource in that it has measured height and weight recorded for childhood and early adulthood, whereas prior studies have relied on participant recall of weight or somatotype (9, 36). This allowed for prospective evaluation of the association between weight change in early life and EA/GCA risk. However, even with a large sample size, we were limited in some analyses, specifically examining the cross-classification of weight status at ages 7 and 13 years, and early adulthood. Due to reliance on conscription information for the early adulthood BMI, we were unable to examine weight changes in relation to EA/GCA risk for women. However, our prior study on childhood BMI suggested that associations were similar, albeit slightly stronger for women than men.
In summary, this analysis of a large, prospective Danish cohort provides evidence that persistent early life overweight is a strong risk factor for EA and GCA, but that remitting from overweight by early adulthood may decrease risk of EA and GCA, suggesting a causal role of childhood adiposity. These results indicate that weight control programs could be targeted in childhood or adolescence for reducing the incidence of these highly lethal cancers and improving overall health.
Supplementary Material
STUDY IMPORTANCE.
Excess adiposity and weight gain in adulthood is a known risk factor for esophageal and gastric cardia adenocarcinoma.
However, associations of excess adiposity and weight gain in childhood and early adulthood are unclear.
In this study of men, persistent overweight in early life was associated with a 3-times increased risk of esophageal and gastric cardia adenocarcinoma, highlighting the adverse health effects of boys with persistent overweight into early adulthood.
However, this study also provides support that there is a possibility to reduce risk by remitting from childhood overweight by early adulthood in men.
ACKNOWLEDGEMENTS
The authors thank M Osler, K Christensen, D Molbo, EL Mortensen and TIA Sørensen who established the Danish Conscription Database. The Danish Conscription Database is housed at the Department of Public Health, University of Copenhagen.
Funding: NIH Intramural Research Program and Dr. Sofus Carl Emil Friis and Wife Olga Dorus Friis’ Legacy Fund.
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer 2015;51: 1164–1187. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol 2017;112: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 3.Xie SH, Rabbani S, Petrick JL, Cook MB, Lagergren J. Racial and Ethnic Disparities in the Incidence of Esophageal Cancer in the United States, 1992–2013. Am J Epidemiol 2017;186: 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer 2009;101: 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M, Colquhoun A, Cook MB, Ferlay J, Forman D, Soerjomataram I. Obesity and the Incidence of Upper Gastrointestinal Cancers: An Ecological Approach to Examine Differences across Age and Sex. Cancer Epidemiol Biomarkers Prev 2016;25: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015;33: 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijnhoven BP, Siersema PD, Hop WC, van Dekken H, Tilanus HW. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg 1999;86: 529–535. [DOI] [PubMed] [Google Scholar]
- 8.Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol 2012;41: 1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrick JL, Kelly SP, Liao LM, Freedman ND, Graubard BI, Cook MB. Body weight trajectories and risk of oesophageal and gastric cardia adenocarcinomas: a pooled analysis of NIH-AARP and PLCO Studies. Br J Cancer 2017;116: 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook MB, Corley DA, Murray LJ, Liao LM, Kamangar F, Ye W, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One 2014;9: e103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer 2010;10: 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan AM, Duong M, Healy L, Ryan SA, Parekh N, Reynolds JV, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: epidemiology, etiology and new targets. Cancer Epidemiol 2011;35: 309–319. [DOI] [PubMed] [Google Scholar]
- 13.Cook MB, Barnett MJ, Bock CH, Cross AJ, Goodman PJ, Goodman GE, et al. Prediagnostic circulating markers of inflammation and risk of oesophageal adenocarcinoma: a study within the National Cancer Institute Cohort Consortium. Gut 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook MB, Freedman ND, Gamborg M, Sorensen TI, Baker JL. Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br J Cancer 2015;112: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koebnick C, Getahun D, Smith N, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Extreme childhood obesity is associated with increased risk for gastroesophageal reflux disease in a large population-based study. Int J Pediatr Obes 2011;6: e257–263. [DOI] [PubMed] [Google Scholar]
- 16.Owens S, Galloway R. Childhood obesity and the metabolic syndrome. Curr Atheroscler Rep 2014;16: 436. [DOI] [PubMed] [Google Scholar]
- 17.Aarestrup J, Bjerregaard LG, Gamborg M, Angquist L, Tjonneland A, Overvad K, et al. Tracking of body mass index from 7 to 69 years of age. Int J Obes (Lond) 2016;40: 1376–1383. [DOI] [PubMed] [Google Scholar]
- 18.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390: 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Health Observatory. Prevalence of obesity among adults, BMI ≥ 30, age-standardized Estimates by WHO region Geneva: World Health Organization; 2016. [updated September 22, 2017; cited 2018 August 23]. Available from: http://apps.who.int/gho/data/view.main.REGION2480A?lang=en. [Google Scholar]
- 20.Currie C, Zanotti C, Morgan A, Currie D, de Looze M, Roberts C, et al. Social determinants of health and well-being among young people Health Behaviour in School-aged Children (HBSC) study: international report from the 2009/2010 survey. WHO Regional Office for Europe: Copenhagen, 2012. [Google Scholar]
- 21.van Stralen MM, te Velde SJ, van Nassau F, Brug J, Grammatikaki E, Maes L, et al. Weight status of European preschool children and associations with family demographics and energy balancerelated behaviours: a pooled analysis of six European studies. Obes Rev 2012;13 Suppl 1: 29–41. [DOI] [PubMed] [Google Scholar]
- 22.Baker JL, Olsen LW, Andersen I, Pearson S, Hansen B, Sorensen T. Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol 2009;38: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen GT, Molbo D, Angquist LH, Mortensen EL, Christensen K, Sorensen TI, et al. Cohort Profile: The Danish Conscription Database(DCD): A cohort of 728,160 men born from 1939 through 1959. Int J Epidemiol 2015;44: 432–440. [DOI] [PubMed] [Google Scholar]
- 24.Teasdale TW, Owen DR. The Influence of Paternal Social-Class on Intelligence and Educational-Level in Male Adoptees and Non-Adoptees. Brit J Educ Psychol 1986;56: 3–12. [Google Scholar]
- 25.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012;7: 284–294. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894: i–xii, 1–253. [PubMed] [Google Scholar]
- 27.Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39: 22–25. [DOI] [PubMed] [Google Scholar]
- 28.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011;39: 42–45. [DOI] [PubMed] [Google Scholar]
- 29.Adams KF, Leitzmann MF, Ballard-Barbash R, Albanes D, Harris TB, Hollenbeck A, et al. Body mass and weight change in adults in relation to mortality risk. American journal of epidemiology 2014;179: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Haberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 2015;7: 491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng KK, Sharp L, McKinney PA, Logan RF, Chilvers CE, Cook-Mozaffari P, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer 2000;83: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1998;90: 150–155. [DOI] [PubMed] [Google Scholar]
- 33.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130: 883–890. [DOI] [PubMed] [Google Scholar]
- 34.Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 2007;56: 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control 2001;12: 721–732. [DOI] [PubMed] [Google Scholar]
- 36.Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer 2015;138: 2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med 2018;379: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, Spechler S, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127: 310–330. [DOI] [PubMed] [Google Scholar]
- 39.Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention, 3rd edn. Oxford University Press: Oxford; New York, 2006. [Google Scholar]
- 40.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375: 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gertler R, Stein HJ, Loos M, Langer R, Friess H, Feith M. How to classify adenocarcinomas of the esophagogastric junction: as esophageal or gastric cancer? Am J Surg Pathol 2011;35: 1512–1522. [DOI] [PubMed] [Google Scholar]
- 42.Cancer Genome Atlas Research N, Analysis Working Group: san U, Agency BCC, Brigham Women’s H, Broad I, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bua J, Olsen LW, Sorensen TI. Secular trends in childhood obesity in Denmark during 50 years in relation to economic growth. Obesity (Silver Spring) 2007;15: 977–985. [DOI] [PubMed] [Google Scholar]
- 44.Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am 2009;18: 469485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.