The ability of bacterial cells to control motility during early steps in biofilm formation is critical for the transition to a nonmotile, biofilm lifestyle. Recent studies have clearly demonstrated the ability of c-di-GMP to control motility via a number of mechanisms, including through controlling transcription of motility-related genes and modulating motor function. Here, we provide evidence that motor components can in turn impact c-di-GMP levels. We propose that communication between motor components and the c-di-GMP synthesis machinery allows the cell to have a robust and sensitive switching mechanism to control motility during early events in biofilm formation.
KEYWORDS: Pseudomonas aeruginosa, biofilm, c-di-GMP, flagella, motility, stator
ABSTRACT
Flagellar motility is critical for surface attachment and biofilm formation in many bacteria. A key regulator of flagellar motility in Pseudomonas aeruginosa and other microbes is cyclic diguanylate (c-di-GMP). High levels of this second messenger repress motility and stimulate biofilm formation. c-di-GMP levels regulate motility in P. aeruginosa in part by influencing the localization of its two flagellar stator sets, MotAB and MotCD. Here, we show that while c-di-GMP can influence stator localization, stators can in turn impact c-di-GMP levels. We demonstrate that the swarming motility-driving stator MotC physically interacts with the transmembrane region of the diguanylate cyclase SadC. Furthermore, we demonstrate that this interaction is capable of stimulating SadC activity. We propose a model by which the MotCD stator set interacts with SadC to stimulate c-di-GMP production under conditions not permissive to motility. This regulation implies a positive-feedback loop in which c-di-GMP signaling events cause MotCD stators to disengage from the motor; then disengaged stators stimulate c-di-GMP production to reinforce a biofilm mode of growth. Our studies help to define the bidirectional interactions between c-di-GMP and the flagellar machinery.
IMPORTANCE The ability of bacterial cells to control motility during early steps in biofilm formation is critical for the transition to a nonmotile, biofilm lifestyle. Recent studies have clearly demonstrated the ability of c-di-GMP to control motility via a number of mechanisms, including through controlling transcription of motility-related genes and modulating motor function. Here, we provide evidence that motor components can in turn impact c-di-GMP levels. We propose that communication between motor components and the c-di-GMP synthesis machinery allows the cell to have a robust and sensitive switching mechanism to control motility during early events in biofilm formation.
INTRODUCTION
Two key features in biofilm formation, motility repression and exopolysaccharide (EPS) production, are controlled by the second-messenger cyclic diguanylate (c-di-GMP). Intracellular levels of c-di-GMP are governed by diguanylate cyclases (DGCs) that synthesize c-di-GMP from GTP and phosphodiesterases (PDEs) that hydrolyze c-di-GMP to an inactive form (1, 2). Two DGCs in Pseudomonas aeruginosa PA14 are critical for controlling biofilm formation: SadC and RoeA. SadC controls flagellar motility (3), and RoeA controls production of the EPS known as Pel (4). Like many bacterial species, P. aeruginosa has multiple DGCs and PDEs, and it is not well understood how the activities of these enzymes are coordinated to achieve signaling specificity.
Flagellar motility in P. aeruginosa is ultimately controlled by the flagellar motor, which generates torque from two proton-powered stator sets, MotAB and MotCD (5, 6). Only one of these stator sets, MotCD, can support swarming motility. In P. aeruginosa, swarming motility allows movement across a semisolid agar surface and requires a functional flagellum and production of rhamnolipid surfactants (7). Previous studies have shown that c-di-GMP impacts stator localization and, thus, flagellar motor function by decreasing the polar localization (and likely motor engagement) of MotCD (8). Furthermore, we showed that the c-di-GMP effector protein FlgZ physically interacts with MotC in a c-di-GMP-dependent manner. We proposed that FlgZ-MotC interactions resulted in displacement of the MotC stator from the motor, thereby reducing swarming motility (9).
Here, we describe a role for the flagellar stators in controlling the c-di-GMP level. We demonstrate an interaction between the DGC SadC and the stator protein MotC via SadC’s transmembrane (TM) domain. We provide evidence that this interaction stimulates SadC activity, resulting in the increased production of c-di-GMP. We propose that removal of MotC from the motor by the FlgZ:c-di-GMP effector allows enhanced opportunity for MotC and SadC to interact, resulting in a further enhancement of c-di-GMP production and ultimately resulting in a positive-feedback loop of second-messenger production that we predict would effectively and rapidly reduce swarming motility.
RESULTS
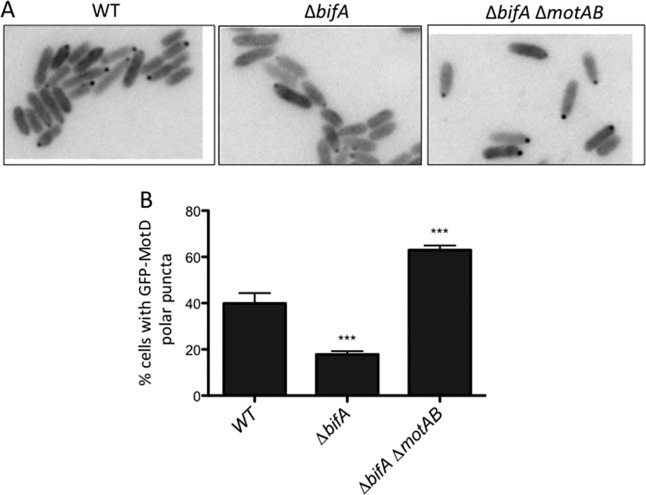
Loss of MotAB stators increases polar localization of GFP-MotD.
We have previously reported that mutating the phosphodiesterase BifA leads to an increase in intracellular c-di-GMP and swarming motility repression in P. aeruginosa (10). We also demonstrated that loss of the MotAB stator restores swarming motility to the ΔbifA mutant, indicating that MotAB participates in c-di-GMP-mediated swarming repression (8). We hypothesized that both stator sets of P. aeruginosa, MotAB and MotCD, compete for engagement with the flagellar motor and that loss of MotAB allows for increased incorporation of MotCD at the motor. Since MotCD is necessary for swarming motility, increased motor engagement by MotCD would lead to increased swarming.
To test the hypothesis that the localization of MotCD is altered upon loss of MotAB in the ΔbifA ΔmotAB mutant, we replaced the motD gene at its native chromosomal locus with gfp-motD. We previously reported that this green fluorescent protein (GFP) fusion does not interfere with MotD’s function in swarming motility (8). Consistent with previous findings (8), we found that a significantly lower percentage of ΔbifA mutant cells than wild-type (WT) cells have GFP-MotD polar puncta (Fig. 1). In the ΔbifA ΔmotAB mutant, we observed that GFP-MotD polar localization increased significantly compared to that in the ΔbifA mutant (Fig. 1). This finding supports our hypothesis that loss of the MotAB stator leads to increased polar localization of the MotCD stator set. Interestingly, the percentage of ΔbifA ΔmotAB cells with a polar localization of GFP-MotD is significantly higher than even that of WT cells. This may indicate that MotAB and MotCD do indeed compete for motor occupancy.
FIG 1.
Localization of GFP-MotD is impacted by MotAB. (A) Representative fluorescence microscopy images of the indicated strains expressing GFP-MotD from the chromosome. Grayscale images were inverted using ImageJ. (B) Percentages of cells with GFP-MotD polar puncta for the indicated strains expressing GFP-MotD. These data are from two independent experiments, with at least 200 total cells counted per strain per experiment. Values are reported as means ± standard errors of the means (SEM). Significance was determined by analysis of variance and Dunnett’s posttest for comparison for differences relative to the WT. ***, P < 0.001.
Loss of stators impacts intracellular c-di-GMP levels.
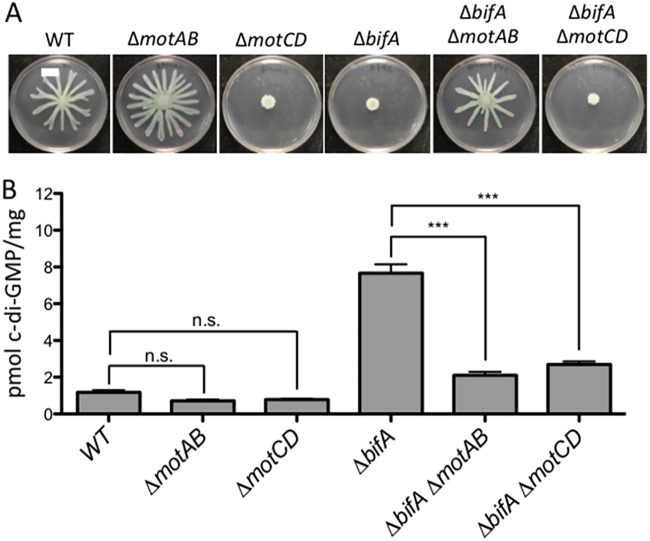
It is possible that increased polar localization of MotCD, rather than low c-di-GMP levels, explains the ability of the ΔbifA ΔmotAB mutant to swarm (8) (Fig. 2A). Thus, we hypothesized that c-di-GMP levels remain elevated in the ΔbifA ΔmotAB mutant despite this strain’s increased motility. To test this hypothesis, we used mass spectrometry to quantify levels of intracellular c-di-GMP in stator mutants. Unexpectedly, we found that c-di-GMP levels significantly decrease in the ΔbifA ΔmotAB mutant relative to those in the ΔbifA mutant (Fig. 2B). We also observe that the ΔmotAB mutant shows lower c-di-GMP levels than the WT, but this change does not appear significant. This lack of significance is likely due to the fact that the level is already quite low in the WT, and statistical tests have difficulty comparing values close to zero. Together, these findings indicate that the loss of MotAB is associated with decreased c-di-GMP production, particularly when levels of this dinucleotide are high.
FIG 2.
Loss of stators impacts cellular c-di-GMP levels. (A) Representative swarm plates of the strains indicated. (B) Quantification of cellular c-di-GMP levels by LC-MS for the indicated strains grown on swarm plates. Data are expressed as picomoles of c-di-GMP per milligram (dry weight) of cells from which nucleotides were extracted. The data represent results from three independent experiments, each with three biological replicates. Values are reported as means ± SEM. Significance was determined by analysis of variance and Tukey’s post hoc test comparison for differences between strains indicated. n.s., not significant; ***, P < 0.001. As previously reported, the ΔbifA mutant is significantly different from the WT (P < 0.001).
We also asked whether the “swarming-powering stator set,” MotCD, affects c-di-GMP levels. We found that the ΔbifA ΔmotCD mutant also has a significantly smaller pool of c-di-GMP than the ΔbifA mutant (Fig. 2B). Notably, the ΔbifA ΔmotCD mutant lacks the powering stator and therefore is nonmotile despite having lower levels of c-di-GMP (Fig. 2). The ΔmotCD mutant also has a nonsignificant decrease in c-di-GMP relative to the level in the WT.
Together, these results suggest that the localization and/or activities of stator proteins in the motor can impact the production of c-di-GMP. This unexpected finding links the two stator sets of P. aeruginosa to c-di-GMP levels of the cell.
MotC interacts with diguanylate cyclase SadC.
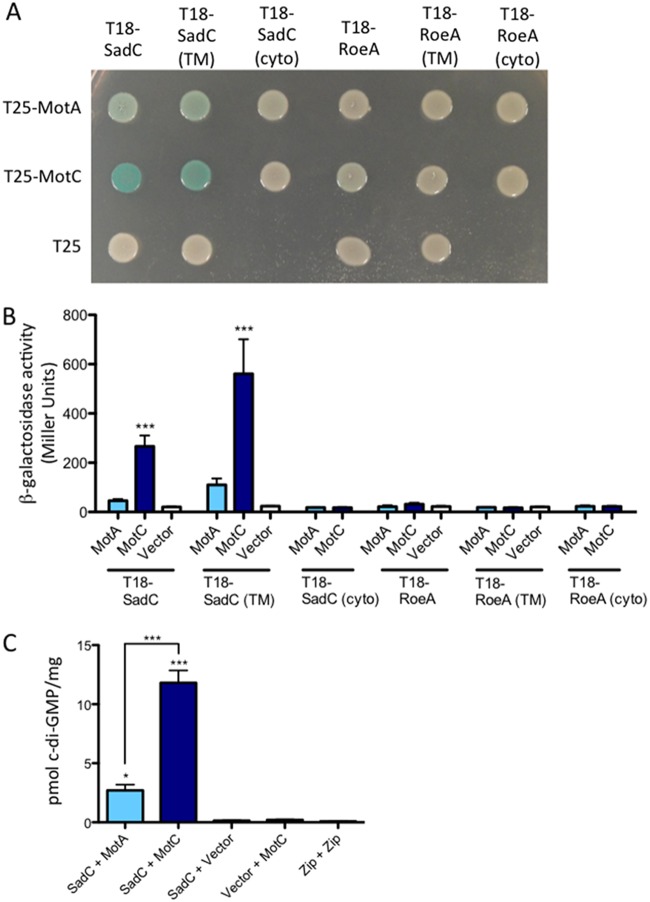
The effects of stator mutations on c-di-GMP levels led us to hypothesize that one or both stator sets may interact directly with the c-di-GMP enzymatic machinery. We used a bacterial adenylate cyclase two-hybrid (B2H) assay of Escherichia coli to probe for interactions between stator proteins (MotA, MotC) and two diguanylate cyclases important for swarming motility regulation, SadC and RoeA (3, 4). Full-length proteins were fused to either the T18 or the T25 subunit of adenylate cyclase and then coexpressed in E. coli BTH101 to test for interaction. An interaction between hybrid proteins was detected as blue colonies on medium containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside); deeper blue indicates a stronger interaction. As shown in Fig. 3A, we observed an interaction between MotC and SadC and a weaker interaction between MotA and SadC. Neither MotA nor MotC interacted with RoeA, indicating that the MotC-SadC interaction is specific. The strength of these interactions was quantified using β-galactosidase assays, and only strains coexpressing SadC and MotC fusion proteins showed significantly higher β-galactosidase than the negative control (Fig. 3B).
FIG 3.
Detection of a physical interaction between MotC and SadC by bacterial two-hybrid analysis. (A) The sadC and roeA genes (and truncated versions) were cloned into the vector pUT18C. “TM” indicates the predicted transmembrane domain, and “cyto” indicates the predicted cytoplasmic region. The motA and motC genes were cloned into the vector pKT25. Plasmids were cotransformed into E. coli BTH101 cells. The transformants were spotted (2 μl) onto LB agar containing Cb, Kan, IPTG, and X-Gal. Plates were incubated at 30°C for 40 h. Cleavage of X-Gal (blue) indicates a positive protein-protein interaction. (B) Bacterial two-hybrid interactions were quantified by measuring β-galactosidase activity of transformants grown at 30°C overnight in LB broth supplemented with Cb and Kan. “Vector” indicates an empty pKT25 vector. The data represent results from 2 experiments, each with 2 to 5 replicates. Values are means ± SEM. Significance was determined by analysis of variance and Dunnett’s posttest comparison for differences from the negative control (T18-SadC’s Vector bar). ***, P < 0.001. (C) Quantification of cellular c-di-GMP levels by LC-MS from B2H assays. The x axis displays the two fusion proteins (listed, in order, as pUT18C and pKT25) cotransformed into BTH101 cells. After being cotransformed with 2 fusion plasmids, cells were spotted onto LB agar containing Cb, Kan, and IPTG (without X-Gal). Plates were incubated at 30°C for 40 h, and then cells were scraped off plates and nucleotides were extracted. Data are expressed as picomoles of c-di-GMP per milligram (dry weight) of cells from which nucleotides were extracted. The data represent results from 2 experiments, each performed in triplicate. Values are means ± SEM. Significance was determined by analysis of variance and Tukey’s post hoc test comparison for differences from the negative control (T18-SadC + Vector) and differences between the indicated samples. Zip + Zip, positive control for the B2H assay. *, P < 0.05; ***, P < 0.001.
Both SadC and RoeA have an N-terminal transmembrane region and a C-terminal cytoplasmic domain. To identify which region of SadC may interact with the stators, we tested for interaction between both stators and either the transmembrane region of SadC or the cytoplasmic domain of SadC. Our results showed that MotC interacts with the transmembrane portion of SadC but apparently does not interact with the cytoplasmic domain of SadC. An important caveat of the lack of interaction between MotC and the cytoplasmic domain of SadC is that we have not shown that this cytoplasmic domain is stable; thus, it is formally possible that both the transmembrane and cytoplasmic domains of SadC contribute to the interaction with MotC. As expected, neither MotA nor MotC interacts with either the transmembrane or cytoplasmic regions of RoeA (Fig. 3A and B).
The interaction between MotC and SadC stimulates SadC’s diguanylate cyclase activity.
We hypothesized that association between MotC and SadC may occur when MotCD complexes are disengaged from the motor; the SadC-MotC interaction would then serve as a means to stimulate SadC activity. To test this hypothesis, we quantified c-di-GMP levels in E. coli BTH101 cells expressing two-hybrid constructs; we used this heterologous system because the background level of c-di-GMP produced is low and we can isolate the c-di-GMP produced by SadC from the ∼30 other candidate DGCs in P. aeruginosa. We found that coexpression of SadC and MotC resulted in a significant increase in c-di-GMP level compared to that in cells coexpressing MotA and SadC (Fig. 3C). These data suggest that MotC’s interaction with SadC stimulates SadC activity.
Importantly, negative controls for the B2H assay (SadC plus a vector, a vector plus MotC) produce relatively low levels of c-di-GMP (Fig. 3C). The positive control for the B2H assay, which shows robust interaction (not shown), also produces low levels of c-di-GMP, indicating again that positive bacterial two-hybrid interactions do not generally stimulate c-di-GMP production (Fig. 3C).
Residues of the N-terminal transmembrane domain contribute to the SadC-MotC interaction and c-di-GMP stimulation.
We next used the bacterial two-hybrid assay to screen for mutants of SadC (TM domain) with decreased interaction with MotC in order to learn more about the interaction interface between these two proteins. We used mutagenic PCR to generate mutant libraries of the T18-SadC(TM) B2H vector. We then cotransformed these libraries with a wild-type version of MotC and screened for light-blue or white colonies, indicating a deficiency in interaction. These mutant T18-SadC(TM) plasmids were isolated, and their mutations were mapped by sequencing. A list of mutations found in this screen is presented in Table S1 in the supplemental material.
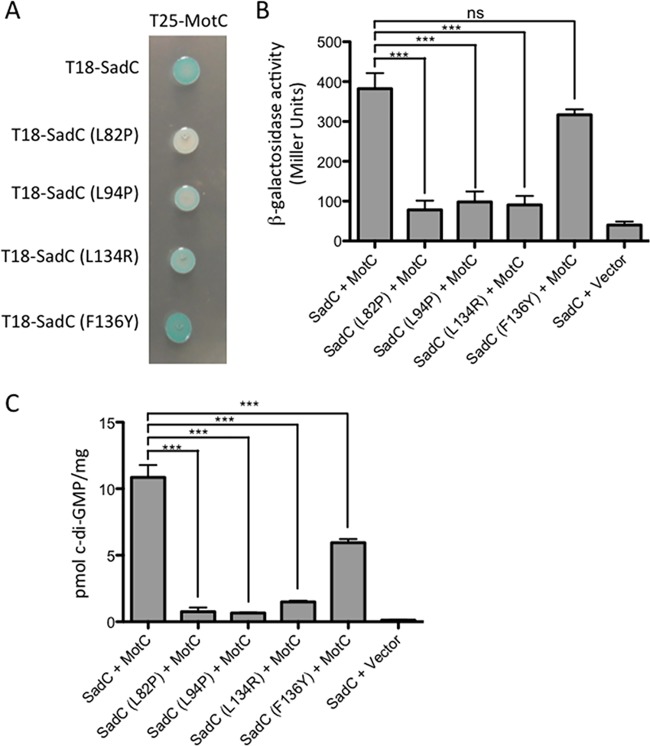
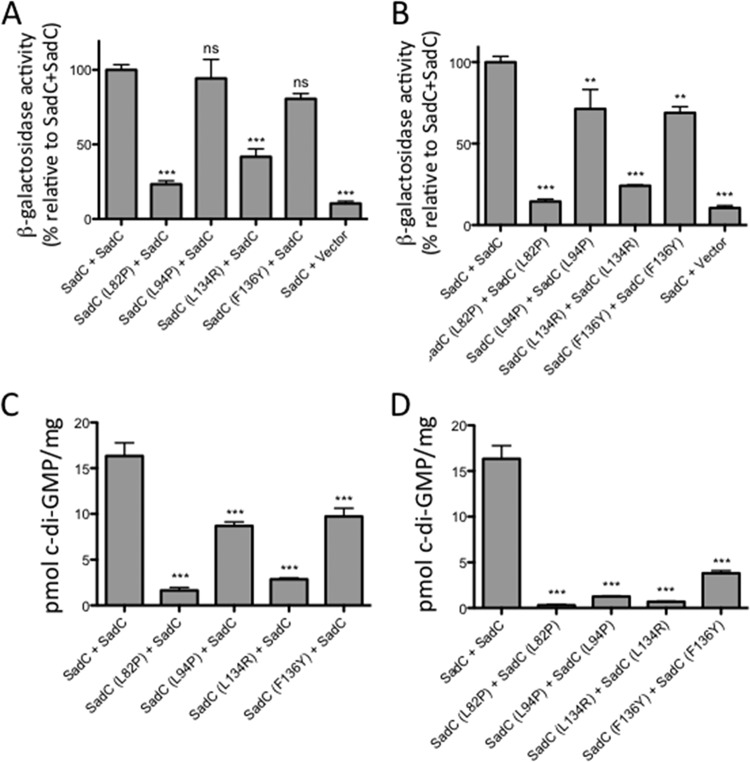
Mutations were found throughout SadC’s six predicted transmembrane domains. It is likely that some of these mutations disrupt the overall structure or function of SadC along with interacting with MotC. We chose four mutations (L82P, L94P, L134R, and F136Y) to introduce into the full-length SadC construct and reexamined their ability to interact with MotC. The L82P, L94P, and L134R mutants are all deficient in their ability to interact with MotC (Fig. 4A and B). On the other hand, the F136Y mutant shows interaction with MotC that is comparable to that of wild-type SadC. It is possible that the F136Y mutation had a large effect on the interaction in the SadC(TM) construct; alternatively, it may be a false positive from our initial screen. We also selected these mutations because, as shown in Table S1, other interaction mutants that we isolated also had mutations near the four residues that we selected, indicating that these residues were likely important for interaction of the proteins.
FIG 4.
Point mutations in the transmembrane domain of SadC disrupt both its interaction with MotC and c-di-GMP production. (A) The wild-type sadC gene and point mutant variants were cloned into the vector pUT18C. The motC gene was cloned into the vector pKT25. Plasmids were cotransformed into E. coli BTH101 cells. The transformants were spotted (2 μl) onto LB agar containing Cb, Kan, IPTG, and X-Gal. Plates were incubated at 30°C for 30 h. Cleavage of X-Gal (blue) indicates a positive protein-protein interaction. (B) Bacterial two-hybrid interactions were quantified by measuring the β-galactosidase activity of transformants grown at 30°C overnight in LB broth supplemented with Cb and Kan. “Vector” indicates an empty pKT25 vector. The data represent results from two experiments, each performed in triplicate. Values are means ± SEM. Significance was determined by analysis of variance and Dunnett’s posttest comparison for difference from the wild-type interaction (T18-SadC plus T25-MotC). ***, P < 0.001; ns, not significant. (C) Quantification of cellular c-di-GMP levels by LC-MS from B2H assays. The x axis displays the two fusion proteins (listed, in order, as pUT18C and pKT25) cotransformed into BTH101 cells. After being cotransformed with 2 fusion plasmids, cells were spotted onto LB agar containing Cb, Kan, and IPTG (with no X-Gal). Plates were incubated at 30°C for 40 h, and then cells were scraped off plates and nucleotides were extracted. Data are expressed as picomoles of c-di-GMP per milligram (dry weight) of cells from which nucleotides were extracted. Data represent results from three experiments, each performed in triplicate. Values are means ± SEM. Significance was determined by analysis of variance and Tukey’s post hoc test comparison for differences between the strains indicated. ***, P < 0.001.
We next quantified c-di-GMP levels in BTH101 cells coexpressing the selected SadC point mutants (L82P, L94P, L134R, or F136Y mutant) and MotC. We found that coexpression of MotC with each of the four SadC mutants resulted in a significant decrease in c-di-GMP production compared to that of coexpression of MotC with wild-type SadC (Fig. 4C), although the magnitude of decrease for the F136Y mutant was markedly less than for the other three mutants, indicating that the F136 residue likely does not contribute robustly to the SadC-MotC interaction. We also observed that the relative strength of the SadC-MotC interaction determined in the B2H assay (Fig. 4B) correlates with c-di-GMP production (Fig. 4C). These results suggest that mutations that weaken SadC’s interaction with MotC lead to decreased c-di-GMP production by SadC.
Mutations that disrupt the SadC-MotC interaction also disrupt SadC dimerization.
We next asked whether any of these mutations in SadC impacted the ability of the protein to dimerize, given that DGCs like SadC function as dimers. We found that both the L82P and L134R mutants showed reduced interaction with wild-type SadC (Fig. 5A) and were unable to homodimerize (Fig. 5B). The L82P and L134R mutants were also unable to interact with MotC (Fig. 4A). In contrast, both the L94P and F136Y mutants were able to interact with wild-type SadC (Fig. 5A) and showed only a modest change in homodimerization (Fig. 5B). It is interesting that these mutants have similar abilities to dimerize, given that they differ in their abilities to interact with MotC: F136Y interacts with MotC, while L94P does not (Fig. 4A).
FIG 5.
TM domain mutations in SadC impact dimerization and c-di-GMP production. (A, B) The wild-type sadC gene was cloned into the vectors pUT18C and pKT25, and mutant sadC plasmids were generated using in vitro site-directed mutagenesis. Plasmids were cotransformed into E. coli BTH101 cells. The transformants were spotted (2 μl) onto LB agar containing Cb, Kan, and IPTG. Plates were incubated at 30°C for 30 h. Bacterial two-hybrid interactions were quantified by measuring β-galactosidase activity in transformants grown at 30°C overnight in LB broth supplemented with Cb and Kan. “Vector” indicates an empty pKT25 vector. Calculated Miller units are represented as percentages of the SadC-SadC interaction (set at 100%). Data are from 2 experiments, each performed in triplicate. Values are means ± SEM. Significance was determined by analysis of variance and Dunnett’s posttest comparison for differences from the SadC-SadC interaction. **, P < 0.01; ***, P < 0.001; ns, not significant. (C, D) Quantification of cellular c-di-GMP levels by LC-MS from B2H assays. The x axis displays the two fusion proteins (listed, in order, as pUT18C and pKT25) cotransformed into BTH101 cells. After being cotransformed with 2 fusion plasmids, cells were spotted onto LB agar containing Cb, Kan, and IPTG (with no X-Gal). Plates were incubated at 30°C for 40 h, and then cells were scraped off plates and nucleotides were extracted. Data are expressed as picomoles of c-di-GMP per milligram (dry weight) of cells from which nucleotides were extracted. Data in panels C and D are from three experiments, each performed in triplicate. Values are means ± SEM. Significance was determined by analysis of variance and Dunnett’s posttest comparison for differences from the wild-type interaction (T18-SadC plus T25-SadC). ***, P < 0.001.
We next asked whether these SadC mutants could produce c-di-GMP as part of a SadC dimer. We found that coexpression of wild-type SadC with itself produces high levels of c-di-GMP (Fig. 5C). Coexpression of wild-type SadC with any mutant SadC yielded significantly less c-di-GMP (Fig. 5C). However, coexpression of SadC and SadC (L94P) or SadC (F136Y) still produced modest levels of c-di-GMP (Fig. 5C). This result suggests that the L94P and F136Y mutations have less of a negative impact on SadC activity than the L82P and L134R mutations. Overall, the L94P mutation appears to have a more specific effect than the other mutations on disrupting SadC’s ability to interact with MotC and to stimulate c-di-GMP production, while having only a moderate effect on SadC dimerization.
L82P and L134R homodimers produced little c-di-GMP (Fig. 5D), which was expected because the physical interaction between these dimers was weak. Interestingly, although both the L94P and F136Y mutants were able to homodimerize similarly to wild-type SadC (Fig. 5B and D), these mutant homodimers produced very little c-di-GMP (Fig. 5D). These data indicate that physical interaction demonstrated in the B2H assay is not sufficient for c-di-GMP production; SadC may need to interact with a partner such as MotC for full activity.
We noticed an unusual phenomenon in the context of the B2H assays here and in Fig. 3 and 4. That is, the T18-SadC fusion in Fig. 3B and 4C apparently could not form homodimers to generate c-di-GMP, whereas in Fig. 5, when SadC was fused to both the T18 and T25 fragments, it was able to form homodimers and show diguanylate cyclase activity. In Fig. 3B and 4C, we have a situation where SadC is expressed from one B2H plasmid (T18-SadC) and another protein (for example, MotA or MotC) is present on the other B2H plasmid (and fused to the T25 fragment). We presume that when expressed in this context, SadC readily dimerizes and becomes active only in the context of its partner protein (i.e., MotC). In contrast, in Fig. 5, SadC is fused to both the T18 and T25 fragments in the same strain, and perhaps in this context the protein more readily dimerizes.
Mutations that disrupt the SadC-MotC interaction alter swarming motility and reduce c-di-GMP production in P. aeruginosa.
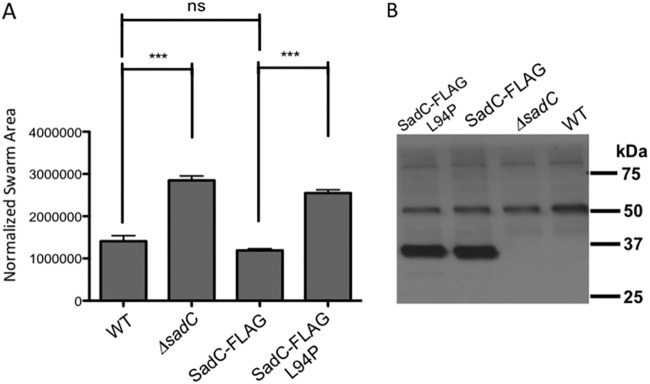
We selected two of the mutants described above (L94P and L82P mutants) to analyze in P. aeruginosa to determine whether defects in the SadC-MotC interaction in the E. coli B2H system resulted in altered function in the native context of P. aeruginosa. These mutant variants of SadC were introduced onto the chromosome in the context of a C-terminally FLAG-tagged variant of SadC. Our initial analysis revealed that the SadC-L82P mutant variant protein was unstable, and thus, we did not work further with this mutant.
We first demonstrated that the SadC-FLAG construct conferred the same swarming phenotype as the wild-type SadC protein (Fig. 6A), indicating that the C-terminal FLAG epitope did not disrupt SadC function. Next, we analyzed the impact of the L94P mutation on SadC for its ability to support swarming motility; this mutant (SadC-L94P) was moderately less stable than the WT protein (the ratio of FLAG-tagged SadC to SadC-L94P was 0.79 ± 0.20, n = 4, Student's t test, P = 0.03) (Fig. 6B) but resulted in a hyper-swarming phenotype very similar to that of the ΔsadC mutant (Fig. 6A). Thus, the SadC-L94P mutant protein, which does not interact with MotC, behaves as if the cell has lost all SadC function. These data are consistent with the model that the SadC-MotC interaction is critical for normal swarm motility regulation.
FIG 6.
Analysis of SadC point mutations in P. aeruginosa. (A) Swarming motility as assessed for the indicated strains. Significance was determined by analysis of variance and Tukey’s post hoc test comparison for differences from the WT or the SadC-FLAG expression strain swarm zone, as indicated. ***, P < 0.0001; ns, not significant. (B) Western blot analysis of the WT and mutant FLAG-tagged SadC. The positions of the molecular weight size standards are shown on the right. The bands at ∼50 and ∼80 kDa are nonspecific, cross-reacting bands and serve as additional loading controls. Each lane contains 250 μg of crude cell extract of the P. aeruginosa carrying the indicated allele of the SadC or SadC-FLAG gene.
Role of MotA in the control of c-di-GMP production.
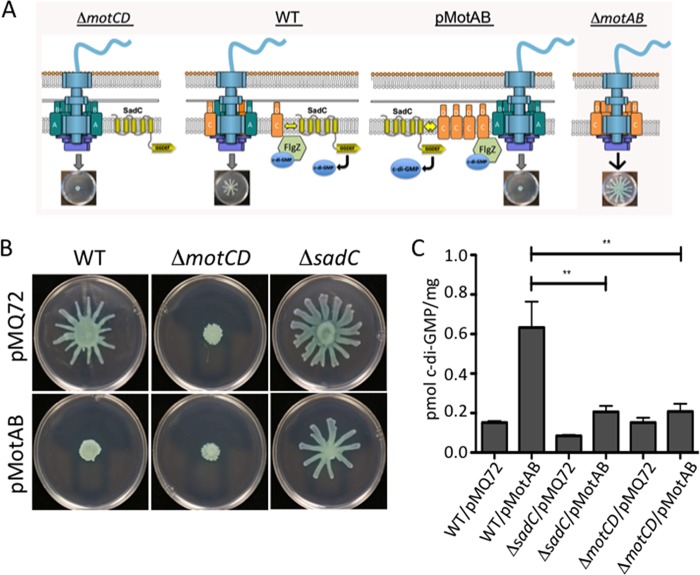
We next sought to better understand the role of the MotAB stator set in the control of surface motility. We showed above that introducing a ΔmotAB mutation into the ΔbifA mutant background reduces c-di-GMP levels similarly to the introduction of a ΔmotCD mutation into the same background (Fig. 1). This result was surprising in that MotAB is not known to interact with SadC (Fig. 3) or the c-di-GMP receptor FlgZ (9). Based on our previous studies (9) and the work above suggesting the possibility that MotAB and MotCD compete for motor occupancy, we considered the possibility that the loss of MotAB would result in more MotCD in the motor and therefore less MotCD to interact with SadC to stimulate biofilm formation.
The model proposed above suggests two strong predictions. First, if MotAB and MotCD indeed compete for motor occupancy, and given that MotCD function is required for swarming, we predict that overexpression of MotAB displaces MotCD and, thus, inhibits swarming (Fig. 7A, third panel). This is indeed what we observed (Fig. 7B, bottom row). Furthermore, the data above suggest that it is the stimulation of SadC activity by MotC interaction that drives the increased c-di-GMP levels that contribute to reduced swarming. Again, our data are consistent with this model in that MotAB overexpression does not repress swarming motility in the ΔsadC mutant.
FIG 7.
MotAB-MotCD dynamics impact c-di-GMP levels. (A) Shown is a model of the flagellar motor with various configurations of the stators, including a ΔmotCD mutant (left), the wild type (center, left), a strain expressing MotAB from a multicopy plasmid (center, right), and the ΔmotAB mutant (left). Also shown is the c-di-GMP effector FlgZ, which can interact with MotC when FlgZ is in the c-di-GMP-bound state. A, B, C, and D refer to MotA, MotB, MotC and MotD, respectively. (B) Swarming motility as assessed for the indicated strains. (C) c-di-GMP quantification assays. Data are expressed as picomoles of c-di-GMP per milligram (dry weight) of cells from which nucleotides were extracted. Values are means ± SEM. Significance was determined by analysis of variance and Dunnett’s posttest comparison to the WT carrying the vector plasmid (pMQ72). **, P < 0.01.
Second, if MotAB indeed displaces MotCD from the stator, we predict that there would be more MotCD available to interact with SadC and, thus, an increase in c-di-GMP level. We further predict that the overexpression of MotAB would require both MotCD and SadC to increase levels of c-di-GMP. As shown in Fig. 7C, overexpression of MotAB from a plasmid in the wild-type P. aeruginosa results in an ∼5-fold increase in c-di-GMP compared to the level produced with the vector control. Furthermore, this stimulation in c-di-GMP levels is dependent on the presence of SadC and the MotCD stator. Thus, it appears that MotAB can indirectly impact c-di-GMP levels via impacting the availability of MotCD to interact with SadC and, thus, modulate c-di-GMP production.
DISCUSSION
Regulation of flagellar motility via c-di-GMP is well documented and occurs at multiple levels: transcriptionally, posttranscriptionally, and posttranslationally (2, 11, 12). Here, we present a new kind of regulation; we show that flagellar motor components can influence c-di-GMP production. It is established that stators are dynamic and engage and disengage from the motor in response to environmental factors, such as load on the flagellum and ion availability (13). Our results indicate that the status of stators in the motor may be communicated directly to the rest of the cell through c-di-GMP signaling.
In previous work, we showed that the bifA mutant was not able to swarm and, furthermore, that mutating the MotAB stator set rescued this loss of swarming motility (8), but until this report, we did not have an adequate explanation for this finding. The data presented here help us explain this observation. We show here that deleting motAB in the ΔbifA mutant results in increased incorporation of MotCD at the motor as well as decreased c-di-GMP production. Furthermore, we found that MotC interacts with SadC in a bacterial two-hybrid assay and that this interaction stimulates c-di-GMP production. These results may explain our finding that the ΔbifA ΔmotCD mutant produces low levels of c-di-GMP; that is, the loss of MotC means that this stator component cannot stimulate SadC activity.
We found that just the transmembrane domain of SadC was able to interact with MotC in the B2H assay. Recent work with P. aeruginosa PAO1 demonstrated that while overexpression of full-length SadC inhibits swimming motility, a truncated version of SadC lacking its transmembrane domains cannot suppress motility (even though it can still synthesize c-di-GMP) (14). Our results support the findings that membrane association is important for SadC activity and perhaps for the ability of SadC to interact with the stator components. These data are also consistent with a model in which protein-protein interactions can drive signaling specificity, as we and others have suggested (15–19).
Using a B2H screen, we identified point mutations in the transmembrane domain of SadC that disrupt its interaction with MotC. We retested a small subset of these mutations and found that three mutations (L82P, L94P, and L134R) decrease SadC’s ability to interact with MotC and to stimulate c-di-GMP production when coexpressed with MotC in E. coli BTH101 cells. We further tested these SadC mutants for their ability to dimerize and the ability of those dimers to produce c-di-GMP in the context of the B2H assay. Two of these mutations (L82P and L134R) resulted in proteins that had a decreased ability to form homodimers and “heterodimers” with wild-type SadC. For these mutants, decreased dimerization led to decreased production of c-di-GMP in the B2H system. Thus, interpreting these mutants is somewhat complicated by their apparent impact on SadC dimerization. Nevertheless, the L94P mutant could still form a dimer producing ∼50% of the c-di-GMP detected for WT SadC, and when introduced into the chromosome of P. aeruginosa, despite the fact that the SadC-L94P mutant protein was almost as stable as the WT, the L94P mutation behaved like a sadC-null allele. Thus, we argue that this mutation’s inability to effectively interact with MotC is, at least in part, responsible for its loss-of-function phenotype.
In previous work, we proposed that MotCD disengages from the motor through its interaction with FlgZ:c-di-GMP as intracellular c-di-GMP levels increase (9). Based on results presented here, we propose that as MotCD becomes disengaged from the motor it is able to bind SadC (see Fig. 7A for a model). This MotC-SadC interaction stimulates SadC activity and, thus, serves as a positive-feedback mechanism to enhance c-di-GMP production when the motility-promoting stator is not in the motor. This mechanism allows cells to directly reduce flagellar motility through stator rearrangement while further promoting sessile growth by stimulating other c-di-GMP-dependent biofilm functions (e.g., exopolysaccharide production). It is important to note two additional points here. First, this positive-feedback model may be relevant only for surface-associated cells, since the role of SadC appears to predominantly impact swarming motility rather than swimming motility (3). Ongoing studies are specifically targeted at understanding why SadC might largely impact surface-associated swarming rather than planktonic swimming. Second, there presumably must be a way to overcome or reverse SadC-mediated swarming inhibition and biofilm promotion, but we have not yet explored that mechanism. BifA is known to turn over the c-di-GMP produced by SadC (4); thus, perhaps the activity of BifA is stimulated to reduce c-di-GMP levels and ultimately restore swarming.
Furthermore, we observed that loss of the MotAB stator also resulted in reduced c-di-GMP levels. We propose that this is an indirect effect resulting from increased MotCD associating with the motor (and thus, not with SadC) in the absence of MotAB, resulting in lower levels of SadC activity. Together, these data indicate a dynamic interaction among the MotAB and MotCD stators (and perhaps a competition for motor occupancy), with the SadC DGC, which allows the status of motor occupancy by the stators to be communicated to the c-di-GMP network of the cell.
MATERIALS AND METHODS
Strains and media.
Bacterial strains used in this study are listed in Table S2 in the supplemental material. P. aeruginosa PA14 and E. coli S17-1 λpir and BTH101 cells were routinely cultured in lysogeny broth (LB) or on 1.5% agar LB plates. When antibiotic selection was required, gentamicin (Gm) was used at 30 μg/ml for P. aeruginosa. For E. coli, Gm was used at 10 μg/ml, carbenicillin (Cb) at 50 μg/ml, kanamycin (Kan) at 50 μg/ml, and nalidixic acid at 20 μg/ml.
Saccharomyces cerevisiae strain InSc1 (Invitrogen) was used for plasmid construction using in vivo homologous recombination (20). InSc1 was grown in yeast extract-peptone-dextrose (1% Bacto yeast extract, 2% Bacto peptone, and 2% dextrose). Synthetic defined medium lacking uracil was used when selecting for plasmid-harboring yeast.
Construction of mutant strains and plasmids.
Plasmids used in this study are listed in Table S3, and primers used in plasmid and mutant construction are listed in Table S4. In-frame deletion mutants were generated using allelic exchange as previously described (20). Genes were cloned into the bacterial two-hybrid vectors pKT25 and pUT18C by ligation.
To build the SadC-FLAG construct, the amino acid sequence of the SadC protein was based on the annotation of the PA14_56280 open reading frame (ORF) in the P. aeruginosa PA14 genome according to https://pseudomonas.com. The PA14_56280 ORF is predicted to be translated as a 375-amino-acid protein. While there is a 111-amino-acid N-terminal sequence upstream of this protein sequence, there is a stop codon at position 86 of the sequence, so this 111-amino-acid sequence was not included in our constructs. Therefore, we used the 375-amino-acid sequence for the full-length SadC protein (GenBank entry ABJ13602), and the numbering of all mutations in this paper is based on this sequence.
Point mutations made in the sadC-containing plasmids were constructed using a modified protocol for in vitro site-directed mutagenesis. Forward and reverse oligonucleotide primers were designed to contain mismatches for generating the point mutation of interest. These primers were used separately to amplify the parental plasmid using Phusion polymerase (NEB). After four amplification cycles, products of these reactions were combined and amplified for an additional 18 cycles. The parental plasmid was digested using DpnI prior to transforming the PCR products into E. coli. Plasmids containing the desired mutation were identified by DNA sequencing.
Swarming motility assays.
Swarming assays were performed as previously described (21). Swarm medium contained M8 medium supplemented with glucose (0.2%), Casamino Acids (0.5%), MgSO4 (1 mM), and 0.53% agar. Arabinose was used at 0.2% where indicated to induce expression of genes under the control of the PBAD promoter. Swarm plates were incubated for 16 to 18 h at 37°C.
c-di-GMP measurements.
P. aeruginosa cells were collected from swarm plates after incubation at 37°C for 18 h. For bacterial two-hybrid assay, BTH101 cells were collected from LB agar plates after 40 h at 30°C. Nucleotide extraction was performed as previously described (22, 23). c-di-GMP quantification was performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) at the Mass Spectrometry Facility at Michigan State University.
Fluorescence microscopy and data processing.
P. aeruginosa cells for fluorescence images were picked from the edges of swarm plate colonies as described in references 21 and 8 and resuspended in motility buffer (M8 medium with 0.2% glucose, 0.5% Casamino Acids, 1 mM MgSO4), and 1.5 μl of the resuspension was placed on a microscope slide layered with a pad of 1.5% agarose in motility buffer. Fluorescence microscopy was performed as previously described (24), with minor modifications: a DeltaVision Spectris optical sectioning microscope (Applied Precision) equipped with a UPlanApo 100× 1.35-numerical-aperture oil objective (Olympus), resulting in a pixel size of 64.8 nm. A CoolSNAP_HQ2 camera (Photometrics) was used to take differential interference contrast (DIC) and fluorescence photomicrographs. For fluorophore visualization, a GFP filter set was used. DIC frames were taken with 0.01-s and fluorescence frames with 1.0-s exposure times. For each image, an initial z-stack containing 20 frames with a spacing of 150 nm was acquired. DIC frames corresponding to the center of the bacterium were selected, and 20 subsequent GFP fluorescence images at a z position corresponding to the center of the bacterium were taken. To select for stable fluorescent foci while reducing the unspecific background fluorescence present in the first two frames, frames 3 to 16 were averaged and background corrected with the ImageJ software (25). The number of foci in 493 to 718 bacteria per strain was determined by eye by two observers blind to their identifications.
Bacterial two-hybrid assays.
Protein-protein interactions were assessed by the bacterial adenylate cyclase two-hybrid (B2H) system obtained from Euromedex (Souffelweyersheim, France) as previously described (26, 27). Proteins of interest were fused to the T18 or T25 fragment of Bordetella pertussis adenylate cyclase. T18 and T25 fusion proteins were then coexpressed in E. coli BTH101 to test for interaction. Interaction between the two hybrid proteins reconstitutes the catalytic domain of adenylate cyclase, leading to cyclic AMP (cAMP) synthesis and transcription of the lac operon.
BTH101 transformants were 10-fold serially diluted and spotted (2 μl) on LB agar containing Cb, Kan, X-Gal (40 μg/ml), and IPTG (isopropyl-β-d-thiogalactopyranoside, 0.5 mM) and incubated at 30°C for 24 to 40 h. The efficiencies of these interactions were quantified using β-galactosidase activity assays, as previously described (28).
Western blot analysis.
The indicated strains (WT, ΔsadC, and chromosomal FLAG-tagged SadC) were grown overnight in LB at 37°C and diluted 1:100 in liquid swarm medium [M8 medium supplemented with glucose (0.2%), Casamino Acids (0.5%), and MgSO4 (1 mM)], and subcultured for ∼3 h to an OD600 of ∼0.6. Cells were normalized to an OD600 of 2 and lysed with sample buffer. Samples were separated on a 12% Tris-HCl gel and blotted onto a nitrocellulose membrane. The membrane was incubated in blocking buffer (5% milk powder) for 1 h at room temperature. Proteins were detected with a 1:10,000 dilution of monoclonal anti-FLAG M2 antibody (Sigma).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant R37 AI83256-06 to G.A.O.
We thank Anna Townley from the University of Oxford for assistance with analysis of fluorescence microscopy experiments and Lijun Chen at the Michigan State University Mass Spectrometry Facility for quantitative analysis of c-di-GMP.
Footnotes
For a commentary on this article, see https://doi.org/10.1128/JB.00186-19.
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00741-18.
REFERENCES
- 1.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 2.Romling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merritt JH, Brothers KM, Kuchma SL, O'Toole GA. 2007. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J Bacteriol 189:8154–8164. doi: 10.1128/JB.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merritt JH, Ha DG, Cowles KN, Lu W, Morales DK, Rabinowitz J, Gitai Z, O'Toole GA. 2010. Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases. mBio 1:e00183-10. doi: 10.1128/mBio.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle TB, Hawkins AC, McCarter LL. 2004. The complex flagellar torque generator of Pseudomonas aeruginosa. J Bacteriol 186:6341–6350. doi: 10.1128/JB.186.19.6341-6350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toutain CM, Zegans ME, O'Toole GA. 2005. Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J Bacteriol 187:771–777. doi: 10.1128/JB.187.2.771-777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caiazza NC, Shanks RM, O'Toole GA. 2005. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol 187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchma SL, Delalez NJ, Filkins LM, Snavely EA, Armitage JP, O'Toole GA. 2015. Cyclic di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the MotAB stator. J Bacteriol 197:420–430. doi: 10.1128/JB.02130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker AE, Diepold A, Kuchma SL, Scott JE, Ha DG, Orazi G, Armitage JP, O'Toole GA. 2016. PilZ domain protein FlgZ mediates cyclic di-GMP-dependent swarming motility control in Pseudomonas aeruginosa. J Bacteriol 198:1837–1846. doi: 10.1128/JB.00196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchma SL, Brothers KM, Merritt JH, Liberati NT, Ausubel FM, O'Toole GA. 2007. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 12.Hengge R, Grundling A, Jenal U, Ryan R, Yildiz F. 2016. Bacterial signal transduction by cyclic di-GMP and other nucleotide second messengers. J Bacteriol 198:15–26. doi: 10.1128/JB.00331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker AE, O'Toole GA. 2017. Bacteria, rev your engines: stator dynamics regulate flagellar motility. J Bacteriol 199:e00088-17. doi: 10.1128/JB.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B, Liu C, Liu S, Cong H, Chen Y, Gu L, Ma LZ. 2016. Membrane association of SadC enhances its diguanylate cyclase activity to control exopolysaccharides [sic] synthesis and biofilm formation in Pseudomonas aeruginosa. Environ Microbiol 18:3440–3452. doi: 10.1111/1462-2920.13263. [DOI] [PubMed] [Google Scholar]
- 15.Dahlstrom KM, Collins AJ, Doing G, Taroni JN, Gauvin TJ, Greene CS, Hogan DA, O'Toole GA. 2018. A multimodal strategy used by a large c-di-GMP network. J Bacteriol 200:e00703-17. doi: 10.1128/JB.00703-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlstrom KM, Giglio KM, Collins AJ, Sondermann H, O'Toole GA. 2015. Contribution of physical interactions to signaling specificity between a diguanylate cyclase and its effector. mBio 6:e01978-15. doi: 10.1128/mBio.01978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlstrom KM, Giglio KM, Sondermann H, O'Toole GA. 2016. The inhibitory site of a diguanylate cyclase is a necessary element for interaction and signaling with an effector protein. J Bacteriol 198:1595–1603. doi: 10.1128/JB.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarenko O, Klauck G, Wilke FM, Pfiffer V, Richter AM, Herbst S, Kaever V, Hengge R. 2017. More than enzymes that make or break cyclic di-GMP-local signaling in the interactome of GGDEF/EAL domain proteins of Escherichia coli. mBio 8:e01639-17. doi: 10.1128/mBio.01639-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengge R. 2016. Trigger phosphodiesterases as a novel class of c-di-GMP effector proteins. Philos Trans R Soc Lond B Biol Sci 371:20150498. doi: 10.1098/rstb.2015.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha DG, Kuchma SL, O'Toole GA. 2014. Plate-based assay for swarming motility in Pseudomonas aeruginosa. Methods Mol Biol 1149:67–72. doi: 10.1007/978-1-4939-0473-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuchma SL, Griffin EF, O'Toole GA. 2012. Minor pilins of the type IV pilus system participate in the negative regulation of swarming motility. J Bacteriol 194:5388–5403. doi: 10.1128/JB.00899-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newell PD, Yoshioka S, Hvorecny KL, Monds RD, O'Toole GA. 2011. Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1. J Bacteriol 193:4685–4698. doi: 10.1128/JB.05483-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diepold A, Kudryashev M, Delalez NJ, Berry RM, Armitage JP. 2015. Composition, formation, and regulation of the cytosolic c-ring, a dynamic component of the type III secretion injectisome. PLoS Biol 13:e1002039. doi: 10.1371/journal.pbio.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battesti A, Bouveret E. 2012. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods 58:325–334. doi: 10.1016/j.ymeth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A 95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.