Significance
Models of the CO2 concentrating mechanism (CCM) of green algae and diatoms postulate that chloroplast CO2 is generated from HCO3− brought into the acidic thylakoid lumen and converted to CO2 by specific thylakoid carbonic anhydrases. However, the identity of the transporter required for thylakoid HCO3− uptake has remained elusive. In this work, 3 bestrophin-like proteins, BST1–3, located on the thylakoid membrane have been found to be essential to the CCM of Chlamydomonas. Reduction in expression of BST1–3 markedly reduced the inorganic carbon affinity of the alga. These proteins are prime candidates to be thylakoid HCO3− transporters, a critical currently missing step of the CCM required for future engineering efforts of the Chlamydomonas CCM into plants to improve photosynthesis.
Keywords: Chlamydomonas, CO2 concentrating mechanism, bicarbonate transport, photosynthesis, chloroplast thylakoid
Abstract
The green alga Chlamydomonas reinhardtii possesses a CO2 concentrating mechanism (CCM) that helps in successful acclimation to low CO2 conditions. Current models of the CCM postulate that a series of ion transporters bring HCO3− from outside the cell to the thylakoid lumen, where the carbonic anhydrase 3 (CAH3) dehydrates accumulated HCO3− to CO2, raising the CO2 concentration for Ribulose bisphosphate carboxylase/oxygenase (Rubisco). Previously, HCO3− transporters have been identified at both the plasma membrane and the chloroplast envelope, but the transporter thought to be on the thylakoid membrane has not been identified. Three paralogous genes (BST1, BST2, and BST3) belonging to the bestrophin family have been found to be up-regulated in low CO2 conditions, and their expression is controlled by CIA5, a transcription factor that controls many CCM genes. YFP fusions demonstrate that all 3 proteins are located on the thylakoid membrane, and interactome studies indicate that they might associate with chloroplast CCM components. A single mutant defective in BST3 has near-normal growth on low CO2, indicating that the 3 bestrophin-like proteins may have redundant functions. Therefore, an RNA interference (RNAi) approach was adopted to reduce the expression of all 3 genes at once. RNAi mutants with reduced expression of BST1–3 were unable to grow at low CO2 concentrations, exhibited a reduced affinity to inorganic carbon (Ci) compared with the wild-type cells, and showed reduced Ci uptake. We propose that these bestrophin-like proteins are essential components of the CCM that deliver HCO3− accumulated in the chloroplast stroma to CAH3 inside the thylakoid lumen.
Aquatic photosynthetic organisms, which account for close to 50% of the world’s carbon fixation (1), face several challenges in carrying out efficient photosynthesis. Limitations include the slow diffusive rate of gases in water, fluctuations in pH, and the slow interconversion of inorganic carbon (Ci) forms. Thus, most aquatic autotrophs have developed an adaptation called the CO2 concentrating mechanism (CCM) that increases the concentration of CO2 around Ribulose bisphosphate carboxylase/oxygenase (Rubisco) to increase its carboxylase activity. Aside from Rubisco’s slow rate of catalysis, O2 can compete with CO2 for the active site of the enzyme, resulting in the wasteful process of photorespiration (2). Since CO2 and O2 are competitive substrates, the CCM reduces photorespiration and increases photosynthetic efficiency.
The CCM of the unicellular green alga Chlamydomonas reinhardtii (hereafter referred to as Chlamydomonas) has a number of bicarbonate (HCO3−) transporters that help increase the HCO3− concentration in the chloroplast stroma relative to the external HCO3− concentration. These transporters are located on the plasma membrane (LCI1 and HLA3) as well as the chloroplast envelope (NAR1.2/LCIA). Loss of any one of these transporters reduces the ability of the cell to accumulate HCO3− at high external pH (3, 4). In addition, Rubisco is tightly packaged in a microcompartment of the chloroplast called the pyrenoid (5–7). Finally, carbonic anhydrase 3 (CAH3), located in the lumen of pyrenoid-traversing thylakoids, converts the accumulated HCO3− to CO2 near the site of Rubisco (8, 9), increasing photosynthetic and growth rates at otherwise growth-limiting CO2 levels.
Carbonic anhydrases play an essential role in the Chlamydomonas CCM (10). The loss of CAH3 results in cells that cannot grow on air levels of CO2, even though these mutants tend to overaccumulate HCO3− (11). Chlamydomonas CCM models propose that mutants missing CAH3 accumulate the HCO3− brought into the chloroplast by the transport proteins but cannot convert that HCO3− to CO2, the actual substrate of Rubisco (12, 13). These CCM models postulate that the pH gradient across the thylakoid membrane in the light helps drive the conversion of HCO3− to CO2. The apparent acid dissociation constant (pKa) of the interconversion of HCO3− and CO2 is about 6.4, with the chloroplast stoma having a pH close to 8 in the light and the thylakoid lumen having a pH close to 5.7 under low CO2 concentrations (14). Therefore, as HCO3− is brought from the stroma to the thylakoid lumen, it goes from an environment favoring HCO3− to one favoring CO2. Therefore, the acidification of the thylakoid lumen is important to the functioning of the CCM.
The CCM models also proposed the presence of a thylakoid HCO3− transporter that brings in HCO3− from the stroma to the lumen for dehydration by CAH3 (12, 13). In a recent interactome study, the CCM complex LCIB/LCIC is shown to interact with the bestrophin-like proteins encoded by Cre16.g662600 and Cre16.g663450 (15). These proteins were also shown to interact with each other and another bestrophin-like protein encoded by Cre16.g663400 (15). All 3 genes were found to be up-regulated in low CO2 conditions in a transcriptomic study showing they belonged to a cluster of genes that had increased expression in low CO2 and were controlled by CIA5 (16). Bestrophins are typically chloride channels, including the Arabidopsis bestrophin-like protein AtVCCN1 (17). However, they have also been shown to transport a range of anions, with some showing high HCO3− permeability (18). The interactome study also putatively localizes these bestrophin-like proteins to the thylakoid membrane, which makes them promising candidates to be the thylakoid HCO3− transporter in the CCM of Chlamydomonas.
In the present study, we investigate the role of these 3 proteins using an RNA interference (RNAi) approach to knock down the expression of all 3 genes. This approach was feasible as the 3 genes are extremely similar at the DNA sequence level. Knockdown mutants with low expression of all 3 genes grow poorly in limiting CO2 conditions, exhibit a poor affinity for external Ci, and have a severely reduced ability to accumulate HCO3−. This study sheds light on the intracellular location and function of these bestrophin-like proteins in the CCM of Chlamydomonas.
Results
Chlamydomonas Has 3 Very Similar Bestrophin-Like Proteins on the Thylakoid Membrane.
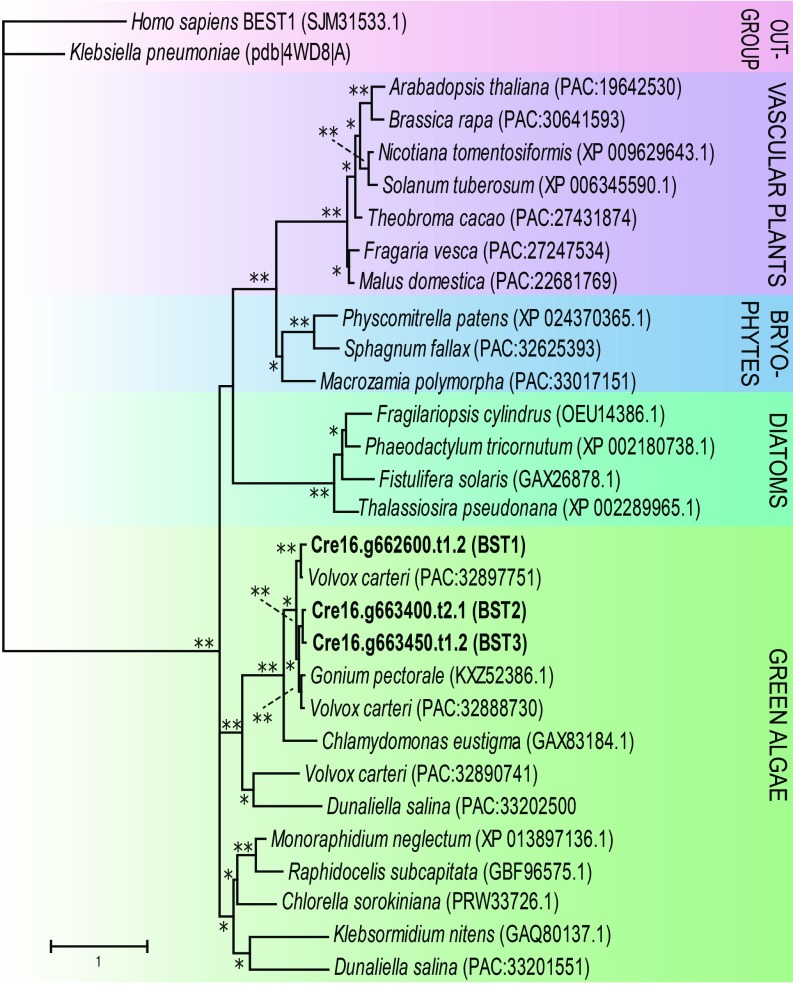
BST1 (Cre16.g662600), BST2 (Cre16.g663400), and BST3 (Cre16.g663450) (collectively BST1–3) are paralogous bestrophin-like genes located within a 130-kilobase pair (kbp) region on the 16th chromosome of Chlamydomonas. Phylogenetic analyses revealed that bestrophin-like proteins are found in a diverse variety of photosynthetic organisms (Fig. 1), including vascular plants, nonvascular plants, and diatoms, with the homologs with the highest sequence identity to BST1–3 found in algae. The amino acid sequences encoded by these genes were analyzed in TMHMM, which predicted that BST1–3 are membrane proteins having 4 predicted transmembrane domains each. Further analysis using PredAlgo predicted that each BST protein had a chloroplast transit peptide and was likely to be a chloroplast membrane protein. BST1 was annotated as a bestrophin-like protein in Phytozome (version 12.1), and BST2 and BST3 were previously reported as LCI11 by Fang et al. (16). An alignment between the 3 Chlamydomonas bestrophin-like proteins showed that the proteins are >80% identical to one another (SI Appendix, Fig. S1). There are 7 more genes annotated as encoding bestrophin-like proteins in the Chlamydomonas genome, but they share less than 50% identity to BST1–3. Sequence alignment of BST1–3 with human Bestrophin 1 (BEST1) showed low sequence identity between BEST1 and BST1–3 (21 to 23%; SI Appendix, Fig. S1). The most similar protein in terrestrial plants, the thylakoid localized AtVCCN1 protein of Arabidopsis (17), has approximately a 30% sequence identity with BST1–3. To further explore the potential structure and function of BST1–3, we did homology modeling using SWISS-MODEL (19). Structural studies show that human and Klebsiella pneumoniae bestrophins are pentameric, and modeling of BST1 in a pentameric assembly is of high confidence (SI Appendix, Fig. S2A). The highest ranking template identified by SWISS-MODEL for BST1–3 was K. pneumoniae bestrophin. BST1–3 contain nonpolar residues along their selective pore that are conserved in proteins of the bestrophin family and are involved in anion transport (20) (SI Appendix, Fig. S2B). The entry pocket of BST1 has a predominantly neutral/negative electrostatic potential, and the selective pore is positively charged, supporting the hypothesis that BST1–3 transport negatively charged ions (19, 21) (SI Appendix, Fig. S2 C and D), as does AtVCCN1 in Arabidopsis (17).
Fig. 1.
Phylogenetic analysis of Chlamydomonas bestrophin-like proteins BST1–3. The evolutionary history of Chlamydomonas bestrophin-like proteins BST1–3 was inferred by using the maximum likelihood method based on the Le and Gascuel (37) model with discrete Gamma distribution (5 categories) and 500 bootstrap replicates. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. *Bootstrap value ≥ 50, **Bootstrap value ≥ 90.
BST1–3 Are Up-Regulated under Low CO2 Growth Conditions and Localized to the Thylakoid.
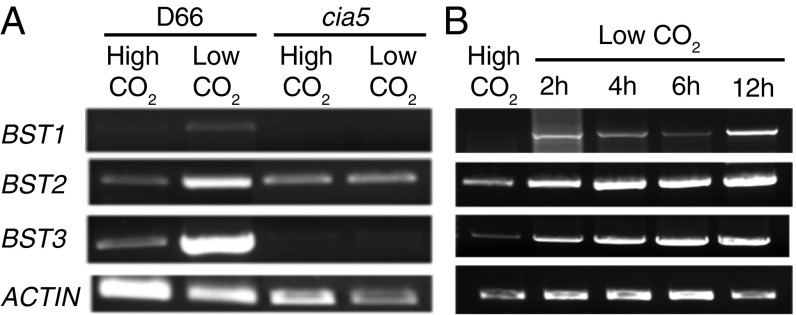
Semiquantitative RT-PCR (Fig. 2A) was performed using complementary DNA isolated from strains D66 and cia5 grown under high CO2 or ambient CO2 conditions. For this work, we have used 5% CO2 (vol/vol) in air as high CO2, 0.04% as ambient CO2, and <0.02% as low CO2. D66 is the wild-type strain for these studies, and cia5 is missing the CCM1 protein, which is required for the induction of the CCM in Chlamydomonas (22). This work demonstrated that all 3 BST genes were up-regulated under ambient CO2 growth conditions in D66 and that this up-regulation was not observed in cia5 (SI Appendix, Fig. S3A). In addition, the cia5 mutant exhibited severely reduced expression of BST1 and BST3 under both CO2 conditions, a transcriptional pattern observed with other CCM genes. BST2 transcript levels in cia5 cells showed reduced induction in ambient CO2 when compared with D66 cells, where BST2 transcript levels increase in ambient CO2 conditions. A time course study of the expression of these 3 genes during induction of the CCM was done by transferring high CO2-grown cells to ambient CO2 levels for 2 to 12 h (Fig. 2B and SI Appendix, Fig. S3B). All 3 genes had increased transcript levels within 2 h after the switch to low CO2, and these elevated levels of expression continued until at least 12 h after induction. BST1 had a lower level of expression than BST2 or BST3 (Fig. 2).
Fig. 2.
Transcript analysis of BST1–3. (A) Semiquantitative RT-PCR showing BST1–3 accumulation in ambient CO2 (0.04% CO2) vs. high CO2 (5% [vol/vol] CO2 in air) in D66 and cia5 cells. (B) Semiquantitative RT-PCR time course showing the expression of BST1–3 in complementary DNA obtained from high CO2 (5% CO2 [vol/vol] in air) and in cells switched to ambient CO2 (0.04% CO2) for the indicated times. Actin has been used as a loading control.
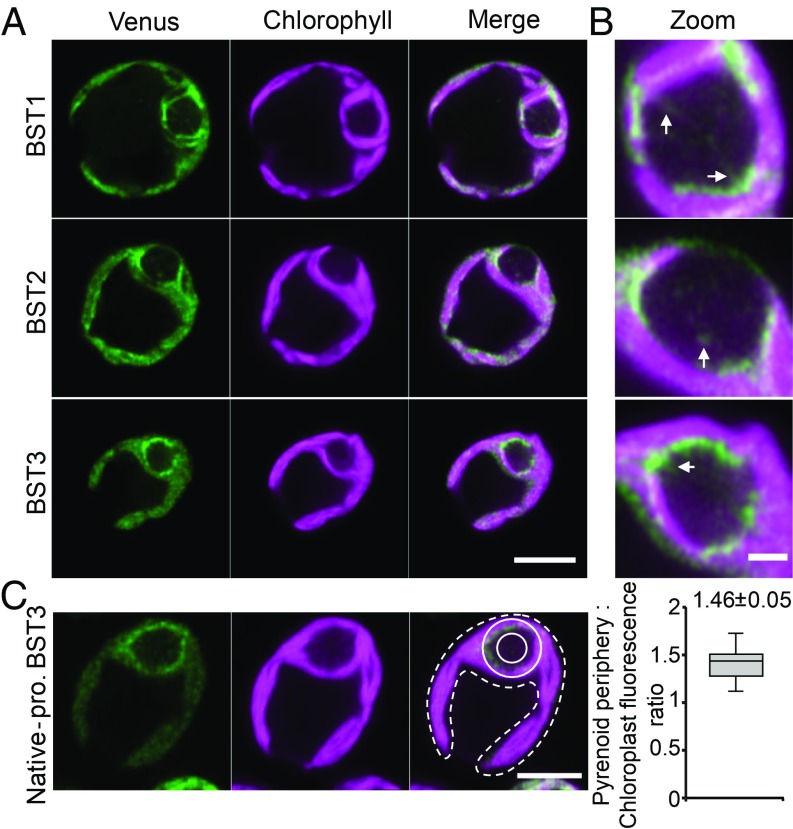
To determine the localization of these 3 BST-like proteins in Chlamydomonas, fluorescent protein fusions were constructed linking Venus to the C terminus of each BST protein. All 3 BST-like proteins localized to the thylakoid membranes of the chloroplast (Fig. 3A), and this localization visibly extended into the thylakoid tubules of the pyrenoid (Fig. 3B). The localization studies visually showed that BST1, BST2, and BST3 were preferentially concentrated near the pyrenoid (Fig. 3B). To confirm that expression using the constitutive PSAD promoter was not affecting localization or pyrenoid periphery enrichment, we constructed a BST3-Venus line with the BST3 gene under its native promoter. This line showed the same localization pattern as BST3 under the constitutive PSAD promoter (Fig. 3C), and quantification of enrichment showed a 1.46-fold enrichment (P < 0.01, Student’s paired t test) around the pyrenoid relative to the rest of the chloroplast. Thus, BST1, BST2, and BST3 are thylakoid localized anion transporters enriched at the pyrenoid periphery that are expressed coordinately with the expression of other Chlamydomonas CCM proteins.
Fig. 3.
Localization of BST1–3. (A) Confocal microscopy of BST1–3 proteins fused with Venus (green) and driven by the constitutive PSAD promoter. Chlorophyll autofluorescence is shown in magenta. (Scale bar, 5 μm.) (B) Zoomed-in images of BST1–3 pyrenoids shown in A. Arrows highlight where Venus fluorescence is seen overlapping with chlorophyll fluorescence in the pyrenoid matrix. (Scale bar, 1 μm.) (C) Localization and quantification of BST3 distribution under its native promoter. The ratio of fluorescence intensity at the pyrenoid periphery (solid line region) and chloroplast (dotted line region) was quantified. The value above the plot denotes the mean ± SE (n = 23). (Scale bar, 4 μm.)
Reduction of BST1–3 Expression Results in Cells that Grow Slowly under Low CO2 Conditions.
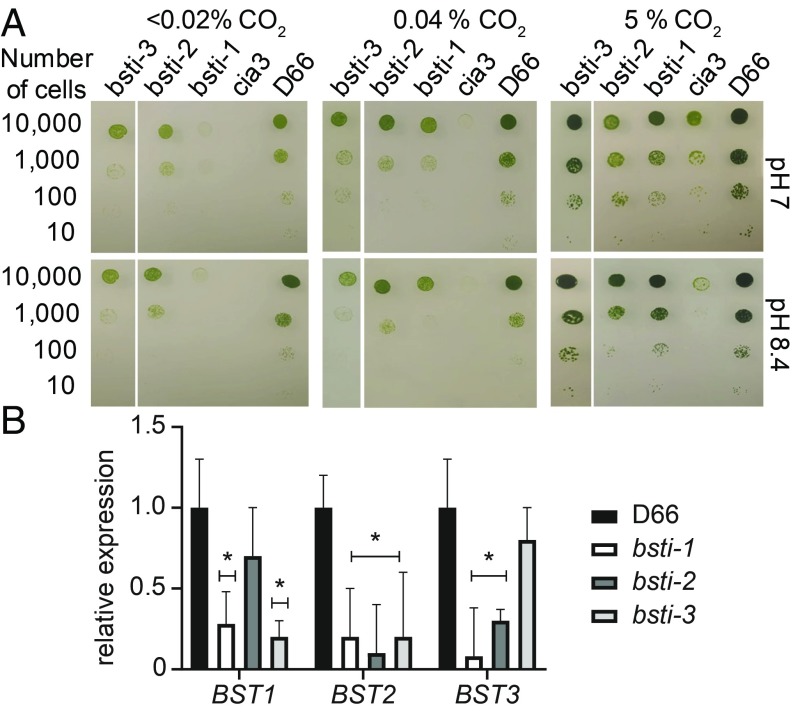
A BST3 knockout (bst3) was obtained from the Chlamydomonas Library Project (CLiP) mutant collection (23) with a paromomycin insert in the last exon of the bst3 gene (SI Appendix, Fig. S4A). The BST3 transcript was not detected in bst3 (SI Appendix, Fig. S4B), and the BST3 protein was absent (SI Appendix, Fig. S4C). We observed a weak growth difference for this strain as compared with wild-type cells under ambient CO2 (SI Appendix, Fig. S5 A and B), but no clear phenotype on plates at pH 7 or pH 8.4 at 100 μmol of photons per m−2⋅s−1 at low CO2 (SI Appendix, Fig. S5C). However, there was no significant difference in Ci affinity between wild type and bst3 grown at ambient CO2 (SI Appendix, Fig. S5D), and Ci uptake by bst3 was only slightly lower than wild type (SI Appendix, Fig. S5 E and F). This led us to think that BST1 or BST2 function might be redundant with BST3 and that the expression of all 3 genes must be reduced to determine their physiological role(s). Therefore, to elucidate the function of BST1–3, RNAi constructs complementary to regions of identity among BST1–3 were designed (SI Appendix, Table S1). The D66 strain was transformed with these constructs, and colonies were kept at high CO2. Colonies were then screened for growth on high CO2 versus low CO2, and BST1–3 expression was quantified using RT-qPCR. Three independent colonies from 2 different transformations were chosen for further study and designated as bsti-1, bsti-2, and bsti-3 (BST RNAi triple-knockdown lines 1, 2, and 3).
The growth of bsti-1, bsti-2, and bsti-3 on high and low CO2 was compared with D66 and the CAH3 knockout mutant, cia3 (Fig. 4A). In low CO2, bsti-1 showed severely reduced growth that was further exacerbated at high pH, resembling the growth of cia3 (Fig. 4A). The bsti-2 and bsti-3 also grew more slowly than wild-type cells, but better than bsti-1. However, at high CO2, the growth of all 3 strains was comparable to wild type. RT-qPCR showed that bsti-1 had significantly reduced expression of BST1, BST2, and BST3 compared with D66 (Fig. 4B), and bsti-2 and bsti-3 had a more moderate knockdown of expression of the 3 genes. To see if reduced transcript levels resulted in decreased protein abundance, we checked BST3 protein levels in the knockdown lines. All showed reduced levels relative to D66, although this was only significant for bsti-2 and bsti-3 (P < 0.05, Student’s t test; SI Appendix, Fig. S6A). Thus, the BSTs are required for wild-type–like growth of Chlamydomonas under low CO2 conditions.
Fig. 4.
Growth of bsti1–3 triple-knockdown RNAi lines and relative expression of BST1–3 in the triple-knockdown lines. (A) Spot tests showing growth of D66, cia3, and bsti1–3. Cells were diluted to 6.6 × 105 cells per milliliter, followed by 1:10 serial dilution 3 times to compare growth in low CO2 (<0.02% CO2), ambient CO2 (0.04% CO2), and high CO2 (5% CO2 [vol/vol] in air) at pH 7 and pH 8.4. Cells were grown for 6 d. The CAH3 mutant, cia3, was included as a CCM-deficient control. (B) RT-qPCR shows that the expression of all 3 BST genes in the triple-knockdown lines is reduced when compared with their expression levels seen in D66. D66 and bsti-1, bsti-2, and bsti-3 were acclimated to air levels of CO2 for 12 h before harvesting the RNA. *P < 0.05 by Student t test.
Reduction of BST1–3 Expression Also Results in Cells that Have a Reduced Capacity to Accumulate Inorganic Carbon.
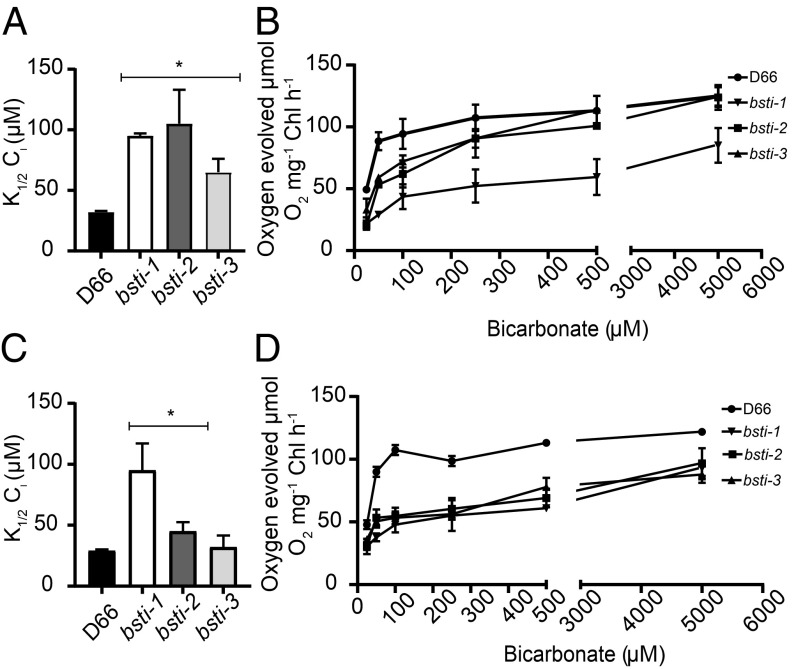
Two characteristics of algal cells with a CCM are a very high affinity for Ci and the ability to accumulate Ci to levels higher than can be obtained by diffusion. The bsti-1, bsti-2, and bsti-3 acclimated to ambient CO2 exhibited a lower affinity for Ci as judged by their measured Ci concentration needed for half-maximum oxygen evolution [K1/2(Ci)] (Fig. 5). When grown at high CO2, bsti1–3 and D66 exhibited similar Ci affinities (SI Appendix, Fig. S6B). These results indicate that the expression of BST1–3 is required for optimal Ci affinity when cells are grown on ambient levels of CO2. At pH 8.4, the K1/2(Ci) values for bsti1–3 are elevated in sharp contrast to a low K1/2(Ci) for D66 (Fig. 5 A and B). At the higher pH of 8.4, the predominant Ci species in the medium would be HCO3−. Thus, the increased affinity of the cells for Ci reflects their ability to actively take up and utilize HCO3−. For bsti-1, where the expression of all 3 BST genes is between 60 and 90% reduced, there is a reduced Ci affinity at both pH 8.4 (Fig. 5 A and B) and pH 7.8 (Fig. 5 C and D). In contrast, bst3, the mutant missing only BST3, the difference in Ci affinity with wild type (SI Appendix, Fig. S2B) is much smaller. Thus, we can conclude that BST1–3 are necessary components of the CCM of Chlamydomonas.
Fig. 5.
Photosynthetic oxygen evolution activity of bst1–3 RNAi lines and D66. Ci affinity was estimated for bsti1–3 and D66 acclimated to ambient CO2 for 12 h at pH 8.4 (A and B) and for bsti1–3 and D66 at pH 7.8 (C and D). Oxygen evolving activity was measured at the indicated pH, and the K1/2(Ci) values were calculated from the O2 evolution versus Ci curves. Triplicate runs were made at each Ci concentration. The differences in K1/2(Ci) were significant (*P < 0.05 by Student’s t-test). At pH 7.8, the maximum velocity (Vmax) of D66 is 121 μmol of O2 per milligram of chlorophyll (Chl) per hour (O2 mg−1 Chl h−1), the Vmax of bsti-1 is 105 μmol of O2 mg−1 Chl h−1, the Vmax of bsti-2 is 87 μmol of O2 mg−1 Chl h−1, and the Vmax of bsti-3 is 90 μmol of O2 mg−1 Chl h−1. At pH 8.4, the Vmax of D66 is 124 μmol of O2 mg−1 Chl h−1, the Vmax of bsti-1 is 85.5 μmol of O2 mg−1 Chl h−1, the Vmax of bsti-2 is 124 μmol of O2 mg−1 Chl h−1, and the Vmax of bsti-3 is 123 μmol of O2 mg−1 Chl h−1. Error bars indicate SD.
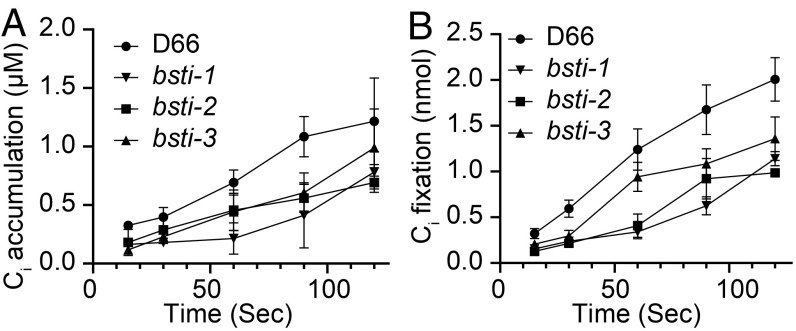
Ci uptake activity was measured in D66, bsti-1, bsti-2, and bsti-3 to evaluate the importance of BST1–3 in accumulation and fixation of Ci. Ambient CO2-acclimated bsti-1 had a notably lower accumulation and fixation of 14Ci compared with D66 at pH 8.4 (Fig. 6), and bsti-2 and bsti-3 also had inhibited 14C uptake and fixation, although not as reduced as bsti-1 (Fig. 6). The most severely affected mutant, bsti-1, accumulated 14Ci to only 30 to 40% of the levels observed in D66 cells. These results indicate that BST1–3 play an important role in Ci uptake and fixation in low CO2 conditions in Chlamydomonas.
Fig. 6.
Ci uptake of D66 and bsti1-3. Ci uptake and Ci accumulation were measured in D66 and bsti1-3 using the silicone oil uptake method (Materials and Methods). Cells were grown in high CO2 and then acclimated to ambient CO2 for 12 h prior to the assays. Cells were harvested and depleted of endogenous Ci before running the assays. A time course of intracellular Ci accumulation (A) and CO2 fixation (B) is shown for pH 8.4. Triplicate samples were run for each time point. The added H14CO3− concentration was 50 μM.
A bestrophin-like protein recently discovered in Arabidopsis, AtVCCN1, is a Cl− channel that helps regulate the proton motive force (pmf) in the Arabidopsis thylakoid (17). Elimination of AtVCCN1 results in plants that have an increased pmf, altering how the plant regulates nonphotochemical quenching and the ΔpH across the thylakoid membrane. It is possible that the reduction of these BST proteins in Chlamydomonas could render cells less able to regulate the membrane potential (Δψ) and ΔpH components of the pmf, leading to photodamage or to an adenosine 5′-triphosphate (ATP)/NADPH imbalance. To investigate if BST1–3, in addition to being critical for Ci affinity and accumulation, have a role in regulating pmf similar to AtVCCN1, we measured electrochromic shift to estimate the pmf in the knockdown lines under HCO3−-depleted conditions (SI Appendix, Fig. S7). We found a small reduction of the pmf in the bsti mutants (SI Appendix, Fig. S7 A and B), which is opposite to what is seen in Arabidopsis. In addition, the pmf decayed slightly faster in the bsti-1 and bsti-3 mutants than in wild type (SI Appendix, Fig. S7C). We also measured the yield of variable chlorophyll a fluorescence to estimate photosystem II function in the mutants and found that Fv/Fm was the same in mutant and wild-type cells (SI Appendix, Fig. S7D). The fact that the bsti1–3 mutants grew normally at relatively high light levels (Fig. 4A) indicates that reducing BST1–3 does not cause severe photodamage.
In conclusion, the localization, the Ci affinity, and the Ci accumulation phenotypes of the bsti triple-knockdown mutants support an essential role for BST1–3 in the CCM.
Discussion
We present evidence here that BST1–3 are chloroplast thylakoid localized anion transporters that are important components of the Chlamydomonas CCM. Cells that have reduced BST1–3 transcript levels fail to grow on low CO2 (Fig. 4), have a lower affinity for Ci (Fig. 5), and have a reduced ability to accumulate added 14Ci (Fig. 6). A key aspect of current Chlamydomonas CCM models is that accumulated HCO3− is converted to CO2 by CAH3, a carbonic anhydrase located in the thylakoid lumen (11–13). This feature of algal CCMs may extend to other algal types, notably diatoms, where Kikutani et al. (24) recently discovered a θ-type carbonic anhydrase within the thylakoid of Phaeodactylum tricornutum that was required for CCM function. These CCM models predict that a thylakoid HCO3− transporter is required to deliver HCO3− from the chloroplast stroma to the thylakoid lumen. We propose that BST1–3 are the transporters that bring HCO3− to CAH3 inside the thylakoid.
Members of the human bestrophin family transport both HCO3− and Cl− ions (18). The homology modeling presented here supports the function of BST1–3 as anion transporters, with BST1–3 having predicted structural and conserved transport residue similarities to chicken and bacterial bestrophins (SI Appendix, Fig. S2).
The expression of all of the CCM transporters discovered previously is induced by ambient or lower CO2 conditions, and their expression is controlled by the transcription factor CIA5/CCM1 (22, 25). We have observed that all 3 BST genes are induced when Chlamydomonas is grown under ambient CO2 conditions (Fig. 2) and that this induction is absent in the cia5 mutant (Fig. 2A). In addition, LCIB and LCIC, possible θ-carbonic anhydrases (24, 26) essential to the CCM (4) that interact with BST1–3 (15), have the same expression pattern (16). Thus, the expression of the BST1–3 genes is consistent with these proteins playing a role in the uptake and accumulation of Ci when Chlamydomonas is exposed to low CO2 conditions.
An alternative hypothesis is that the 3 BST proteins have a function similar to AtVCCN1 (20) and are involved in Cl− transport to regulate the pmf across the thylakoid. The presence of AtVCCN1 decreases pmf in Arabidopsis, but the presence of the 3 BST proteins increases pmf in Chlamydomonas. This result, in combination with our genetic and physiology data, suggests that the function of the BST proteins in Chlamydomonas is not the same as VCCN1 in Arabidopsis. A further understanding of this interconnection and the balancing/regulation of pmf within the context of the CCM is critical.
In Chlamydomonas, there seems to be a built-in redundancy of Ci transporter functions. For example, both LCI1 and HLA3 are present on the plasma membrane, and loss of only one of the proteins fails to cause an extreme growth phenotype at low CO2 (3). However, when more than 1 transporter is knocked down, a significant change in Ci uptake and growth is observed (4, 5). BST1–3 also appear to have redundant or overlapping functions. This is demonstrated in this study, as knocking out BST3 by itself did not cause a drastic change in growth or reduction in Ci affinity at ambient levels of CO2 (SI Appendix, Figs. S4 and S5). However, when the expression of all 3 genes is decreased in bsti1–3, cells could not grow at low CO2 and Ci uptake was severely compromised. In addition, BST1–3 transcript levels in the RNAi strains correlated to Ci affinity and Ci uptake, supporting their Ci transport role and functional redundancy. This redundancy likely explains why BST1–3 were not identified in earlier mutant screens, as these screens typically knock out only 1 gene at a time. The 3 BST proteins do, however, have sequence differences, particularly at their C termini. Therefore, they might have specific (or slightly different) physiological roles that cannot be differentiated under the conditions employed in this present study.
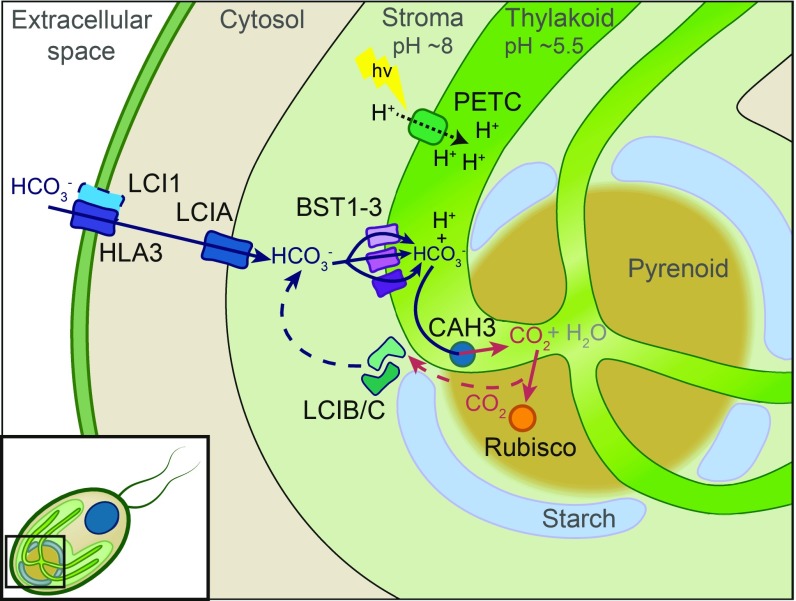
Fig. 7 shows a refined model for the Chlamydomonas CCM, which now includes our proposed function for BST1–3. In this model, HLA3 and LCI1 transport HCO3− across the plasma membrane, bringing HCO3− into the cell (12, 13). At the chloroplast envelope, NAR1.2 (LCIA) transports HCO3− into the chloroplast stroma. Then, BST1–3 on the thylakoid bring HCO3− into the thylakoid lumen, where CAH3 in the pyrenoidal thylakoid tubules converts HCO3− to CO2 to be fixed by Rubisco. Since BST1–3 are found throughout the thylakoid, a potential futile cycle is possible. However, for the futile cycle to take place, CAH3 needs to be present and active in the thylakoid membranes away from the pyrenoid. There is published work that CAH3 is preferentially located and activated in the pyrenoid tubules under low (<0.02%) CO2 conditions (8, 9). The location of BST1–3 is also likely to be important in the recapture of CO2 that is generated by the CCM (Fig. 7). Any CO2 in the pyrenoid not fixed by Rubisco has the potential to simply diffuse out of the cell (4, 27–29). The LCIB/C complex is thought to help recapture this CO2 (27) by directionally driving CO2 to HCO3− or by acting as a tightly regulated carbonic anhydrase (30) at the pyrenoid periphery (28). This is interesting because there are data supporting the interaction of LCIB/C with BST1 and BST3 (15). Our model adds BST1–3 to this hypothesized recapture system (Fig. 7). Having BST1–3 throughout the thylakoid (Fig. 3) would increase the surface area for the reuptake of HCO3− in the stroma. As such, we propose that the recapture of Ci is a 2-step process, with leaked CO2 from the pyrenoid converted to HCO3− by LCIB/C and BST1–3 transporting the HCO3− back into the thylakoid, creating an overall cyclic recapture mechanism. Loss of either BST1–3 or LCIB/C results in cells that cannot accumulate Ci to normal levels, which agrees with experimental observations.
Fig. 7.
Tentative model showing the proposed physiological role of BST1–3 in the CCM of Chlamydomonas. Known transporters (LCI1, HLA3, and LCIA) are indicated on the plasma membrane and chloroplast, respectively. Solid line arrows indicate the movement of HCO3− into the thylakoid by BST1–3. Dashed lines indicate the proposed leakage-reducing pathway that involves recycling CO2 by LCIB/C back to HCO3−. The dotted black line represents the light-driven establishment of a proton gradient across the thylakoid membrane by PSII and the cytochrome b6f complex of the photosynthetic electron transport chain (PETC).
The discovery of CCM components on the thylakoid (BST1–3) and inside the thylakoid lumen (CAH3) also indicates how light energy may be used to energize the CCM. The apparent pKa of the interconversion of HCO3− to CO2 is about 6.4. The pH of the chloroplast stroma, thought to be near 8.0, is well above the pKa, while the pH of the thylakoid lumen is thought to be close to 5.7 (14), below the pKa. When HCO3− moves from the chloroplast stroma to the thylakoid lumen, it moves from an environment that favors HCO3− to one that favors CO2. This effectively allows the algal cells to increase the CO2 concentration to levels higher than could be obtained by the action of carbonic anhydrase alone. Thus, a transthylakoid pH gradient is necessary for this proposed “CO2 pump,” and this pH gradient is set up by the photosystems and requires light. To date, all experimental data available indicate that light and the activity of the photosystems are required for the Chlamydomonas CCM to function. In fact, some of the earliest work in the field indicated that electron transport inhibitors and mutations that disrupt electron transport also inhibited the Chlamydomonas CCM (31, 32). One potential problem with this CO2 pump model is that it would partially reduce the pmf across the thylakoid membrane, thus reducing ATP biosynthesis. However, it should be pointed out that only a single H+ would be consumed per CO2 generated, which is the equivalent of less than one-third of an ATP per CO2 generated. This cost is far less than the 2 additional ATPs required for C4 photosynthesis, and C4 photosynthesis has been shown to be energetically competitive with C3 photosynthesis once the costs of photorespiration are considered (33). In conclusion, BST1–3 are bestrophin-like, thylakoid localized membrane proteins that are synthesized in coordination with other CCM components, and their predicted structures fit well with functionally characterized bestrophins. As such, they are excellent candidates to be the HCO3− transporters that not only bring HCO3− into the thylakoid lumen for CO2 generation but may also play a role in Ci recapture as well.
Materials and Methods
Cell Cultures, Growth, and Photosynthetic Assays.
C. reinhardtii culture conditions were set according to the conditions used previously (34). The D66 strain (nit2−, cw15, mt+) was obtained from Rogene Schnell (University of Arkansas, Fayetteville, AR), and CMJ030 (CC-4533; cw15, mt−) and bst3 (BST3 knockout LMJ.RY0402.089365) were obtained from the CLiP collection at the Chlamydomonas culture collection (23, 35). For acclimation experiments, Tris-acetate-phosphate–grown cells were switched to minimal media and bubbled with high CO2 (5% [vol/vol] CO2 in air) to reach an optical density at 730 nm between 0.2 and 0.3 (∼2 to 3 × 106 cells per milliliter). This was followed by CCM induction when the cells were transferred to ambient CO2 (0.04% CO2) bubbling. For photosynthetic assays, cells acclimated to 5% or 0.04% CO2 were resuspended in Ci-depleted buffer at pH 7.8 or pH 8.4, and O2 evolution was measured at different Ci concentrations. K1/2(Ci) was calculated as the Ci concentration needed for the half-maximal rate of oxygen evolution.
Fluorescence Protein Tagging and Confocal Microscopy.
The BST1–3 genes driven by the constitutive PSAD promoter were cloned as reported by Mackinder et al. (15). Briefly, the open reading frames of BST1–3 genes were PCR-amplified from genomic DNA and cloned into pLM005 with C-terminal Venus-3xFLAG and a PSAD promoter through Gibson assembly. BST3 driven by its native promoter was cloned using recombineering based on methods reported by Sarov et al. (36). Transformation of these genes into Chlamydomonas and selection of colonies are described in SI Appendix, SI Materials and Methods. Images were captured with a laser-scanning microscope (LSM880; Zeiss) equipped with an Airyscan module using a 63× objective with a 1.4 numerical aperture. Argon lasers at 514 nm and 561 nm were used for excitation of Venus and chlorophyll, respectively. Filters were set at 525 to 550 nm for the Venus emission and at 620 to 670 nm for chlorophyll emission.
Additional details of materials and methods are provided in SI Appendix, SI Materials and Methods
Supplementary Material
Acknowledgments
We thank the University of York Biosciences Technology Facility for confocal microscopy support. The project was funded by Biotechnology and Biological Sciences Research Council Grant BB/R001014/1 (to L.C.M.M.); Leverhulme Trust Grant RPG-2017-402 (to L.C.M.M. and C.E.W.); a University of York Biology Pump Priming award (to L.C.M.M.); and a subaward from the University of Illinois as part of the Realizing Increased Photosynthetic Efficiency project (to J.V.M.) funded by the Bill & Melinda Gates Foundation, Foundation for Food and Agriculture Research, and United Kingdom Aid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909706116/-/DCSupplemental.
References
- 1.Falkowski P., et al. , The global carbon cycle: A test of our knowledge of earth as a system. Science 290, 291–296 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Bauwe H., Hagemann M., Fernie A. R., Photorespiration: Players, partners and origin. Trends Plant Sci. 15, 330–336 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Yamano T., Sato E., Iguchi H., Fukuda Y., Fukuzawa H., Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 112, 7315–7320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Spalding M. H., Acclimation to very low CO2: Contribution of limiting CO2 inducible proteins, LCIB and LCIA, to inorganic carbon uptake in Chlamydomonas reinhardtii. Plant Physiol. 166, 2040–2050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkhsenious O. N., Mason C. B., Moroney J. V., The intracellular localization of ribulose-1,5-bisphosphate carboxylase/oxygenase in chlamydomonas reinhardtii. Plant Physiol. 116, 1585–1591 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackinder L. C., et al. , A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proc. Natl. Acad. Sci. U.S.A. 113, 5958–5963 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawat M., Henk M. C., Lavigne L. L., Moroney J. V., Chlamydomonas reinhardtii mutants without ribulose-1,5-bisphosphate carboxylase-oxygenase lack a detectable pyrenoid. Planta 198, 263–270 (1996). [Google Scholar]
- 8.Blanco-Rivero A., Shutova T., Román M. J., Villarejo A., Martinez F., Phosphorylation controls the localization and activation of the lumenal carbonic anhydrase in Chlamydomonas reinhardtii. PLoS One 7, e49063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra M., et al. , The carbonic anhydrase gene families of Chlamydomonas reinhardtii. Can. J. Bot. 83, 780–795 (2005). [Google Scholar]
- 10.Moroney J. V., et al. , The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosynth. Res. 109, 133–149 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Karlsson J., et al. , A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 17, 1208–1216 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moroney J. V., Ynalvez R. A., Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot. Cell 6, 1251–1259 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spalding M. H., Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J. Exp. Bot. 59, 1463–1473 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Takizawa K., Cruz J. A., Kanazawa A., Kramer D. M., The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim. Biophys. Acta 1767, 1233–1244 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Mackinder L. C. M., et al. , A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 171, 133–147 e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang W., et al. , Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. Plant Cell 24, 1876–1893 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herdean A., et al. , A voltage-dependent chloride channel fine-tunes photosynthesis in plants. Nat. Commun. 7, 11654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu Z., Hartzell H. C., Bestrophin Cl− channels are highly permeable to HCO3−. Am. J. Physiol. Cell Physiol. 294, C1371–C1377 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse A., et al. , SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu Z., Chien L. T., Cui Y., Hartzell H. C., The anion-selective pore of the bestrophins, a family of chloride channels associated with retinal degeneration. J. Neurosci. 26, 5411–5419 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane Dickson V., Pedi L., Long S. B., Structure and insights into the function of a Ca2+-activated Cl− channel. Nature 516, 213–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuzawa H., et al. , Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc. Natl. Acad. Sci. U.S.A. 98, 5347–5352 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., et al. , An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell 28, 367–387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikutani S., et al. , Thylakoid luminal θ-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. U.S.A. 113, 9828–9833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroney J. V., et al. , Isolation and characterization of a mutant of Chlamydomonas reinhardtii deficient in the CO2 concentrating mechanism. Plant Physiol. 89, 897–903 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin S., et al. , Structural insights into the LCIB protein family reveals a new group of β-carbonic anhydrases. Proc. Natl. Acad. Sci. U.S.A. 113, 14716–14721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duanmu D., Wang Y., Spalding M. H., Thylakoid lumen carbonic anhydrase (CAH3) mutation suppresses air-Dier phenotype of LCIB mutant in Chlamydomonas reinhardtii. Plant Physiol. 149, 929–937 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamano T., et al. , Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 51, 1453–1468 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Kaplan A., Reinhold L., CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 539–570 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Mackinder L. C. M., The Chlamydomonas CO2 -concentrating mechanism and its potential for engineering photosynthesis in plants. New Phytol. 217, 54–61 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Badger M. R., Kaplan A., Berry J. A., Internal inorganic carbon pool of Chlamydomonas reinhardtii: Evidence for a carbon dioxide-concentrating mechanism. Plant Physiol. 66, 407–413 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spalding M. H., Spreitzer R. J., Ogren W. L., Carbonic anhydrase-deficient mutant of Chlamydomonas reinhardii requires elevated carbon dioxide concentration for photoautotrophic growth. Plant Physiol. 73, 268–272 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moroney J. V., Jungnick N., Dimario R. J., Longstreth D. J., Photorespiration and carbon concentrating mechanisms: Two adaptations to high O2, low CO2 conditions. Photosynth. Res. 117, 121–131 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Ma Y., Pollock S. V., Xiao Y., Cunnusamy K., Moroney J. V., Identification of a novel gene, CIA6, required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol. 156, 884–896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R., et al. , High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell 26, 1398–1409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarov M., et al. , A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat. Methods 3, 839–844 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Le S. Q., Gascuel O., An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.