Abstract
Skeletal muscle is a highly plastic tissue that remarkably adapts to diverse stimuli including exercise, injury, disuse, and, as discussed here, aging. Humans achieve peak skeletal muscle mass and strength in mid-life and then experience a progressive decline of up to 50% by the ninth decade. The loss of muscle mass and function with aging is a phenomenon termed sarcopenia. It is evidenced by the loss and atrophy of muscle fibers and the concomitant accretion of fat and fibrous tissue. Sarcopenia has been recognized as a key driver of limitations in physical function and mobility, but is perhaps less appreciated for its role in age-related metabolic dysfunction and loss of organismal resilience. Similar to other tissues, muscle is prone to multiple forms of age-related molecular and cellular damage, including disrupted protein turnover, impaired regenerative capacity, cellular senescence, and mitochondrial dysfunction. The objective of this review is to highlight the clinical consequences of skeletal muscle aging, and provide insights into potential biological mechanisms. In light of population aging, strategies to improve muscle health in older adults promise to have a profound public health impact.
Keywords: Sarcopenia, physical function, strength, autophagy, senescence, mitochondria, exercise
Introduction
In nearly all species from c. elegans to humans, skeletal muscle exhibits marked changes in its form and function with advancing age that profoundly impact health. As a primary driver of movement, age-related changes in muscle compromise the ability of an organism to move efficiently within its environment. Mobility is required for survival and represents one of the most essential and necessary forms of physical function across all species. Skeletal muscle is also a robust metabolic organ, which can store, utilize, and provide vast amounts of energy. These physiological processes commonly falter with age, however, and contribute to the pathogenesis of metabolic disease. Skeletal muscle also appears to bestow resilience to physical challenges, and the loss of muscle with advancing age increases vulnerability to adverse outcomes following medical and surgical interventions. Collectively, these consequences of skeletal muscle aging pose substantial socioeconomic burden. In light of population aging, new strategies to optimize muscle health in older adults are desperately needed.
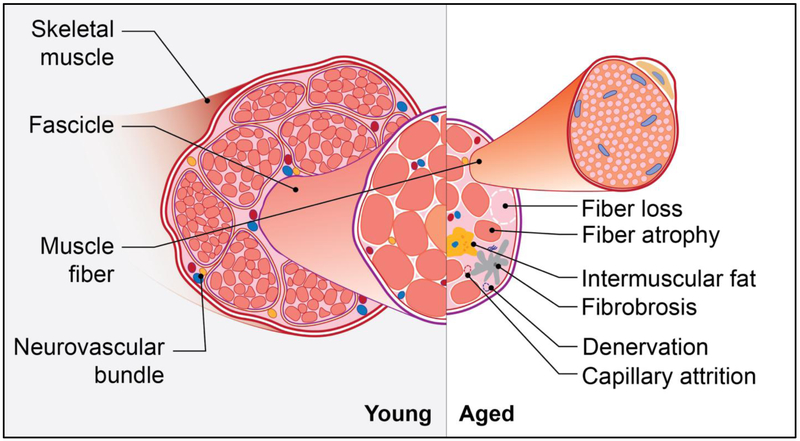
The phenomenon of skeletal muscle aging has been termed sarcopenia, from the Greek words sarx (flesh) and penia (poverty) [1]. The most widely appreciated feature of sarcopenia is the attrition of muscle mass. This has been largely attributed to both the loss and atrophy of postmitotic, multinucleated muscle cells, termed fibers. Lexell and colleagues, for example, observed a 40% reduction in the cross-sectional area of whole vastus lateralis muscle from 20 to 80 years of age, and noted that the number of both type 1 (slow-twitch and more oxidative) and type 2 (fast-twitch and more glycolytic) fibers were reduced, and the size of type 2 fibers was particularly diminished at advanced ages [2](Figure 1). Their analysis further revealed that fat and fibrous tissue replaced lost and shrunken muscle fibers. Though arguably less studied, such alterations in the cellular composition of muscle may equally contribute to the clinical impact of muscle aging as the reduction in muscle mass.
Figure 1. Age-related changes in skeletal muscle.
The considerable loss of muscle mass and volume with advancing age is attributed to both the loss and atrophy of muscle fibers. The progressive decrease in functional contractile tissue is matched with the accretion of fat and fibrotic tissue. These alterations, along with progressive loss of innervating motor neurons and capillaries, contribute to the age-related loss of muscle strength, power, and endurance.
The recognition of sarcopenia as an important clinical syndrome has led to multidisciplinary efforts to identify, understand, prevent, and treat this condition [3-5]. One evidence-based [6] and several consensus-based [7-10] definitions of sarcopenia have been proposed. Despite progress, there is not yet a universally accepted clinical definition; however, a unique International Classification of Diseases, 10th Revision (ICD10), code for sarcopenia was assigned in 2016 [11, 12], The heterogeneity in sarcopenia definitions has made estimates of its prevalence in older adults vary widely, ranging from 0.5 to 13% [13].
Remarkable progress has been made in elucidating the causal molecular pathways of aging. The diverse forms of age-related molecular and cellular damage, commonly referred to as hallmarks [14] or pillars [15] of aging, which include loss of proteostasis, deregulated nutrient sensing, stem cell exhaustion/dysfunction, cellular senescence, genomic instability, mitochondrial dysfunction, and epigenetic alterations, are plausible contributors to sarcopenia. To date, however, there are currently no approved pharmacologic therapies. The burden of sarcopenia on older adults combined with population aging highlight the need to better understand and address age-related muscle loss and dysfunction. To this end, the objective of this review is to discuss the clinical consequences of skeletal muscle aging and highlight recent discoveries relevant to fundamental biological mechanisms.
Clinical consequences of skeletal muscle aging
Physical Function.
Skeletal muscle’s unique capacity to generate force and power is perhaps one of its most important functions. Maximum skeletal muscle force generating capacity, strength, is a product of the cross-sectional area of the muscle and the capacity of the nervous system to fully activate the corresponding motor neurons. Cross sectional analyses reveal a close association between muscle size and maximum strength among older adults. However, longitudinal studies examining changes in muscle mass/size and strength have revealed a more complex relationship. Data from both the Baltimore Longitudinal Study of Aging and the Health Aging and Body Composition (HABC) Study have confirmed age-related changes in muscle size or appendicular lean mass to occur at a rate of approximately 0.5 to 1.0% per year; whereas changes in lower extremity muscle strength occurs at rates between 1.0 to 2.0% per years [16, 17]. The longitudinal divergence observed between muscle mass/size and strength suggest that other biological factors independent of changes in muscle size/mass may play a fundamental role in the well characterized changes in motor performance throughout the lifespan.
The age-related declines in muscle mass and strength occur in conjunction with reductions in mobility and physical functioning [17-23]. Sarcopenia leads to age-related functional limitations, including difficulties in walking, climbing stairs and carrying objects [24, 25]. In turn, the penalties of functional decline include falls [26], disability [27-30], institutionalization [31], and even death [32-35]. Methods to indirectly quantify skeletal muscle mass in humans have relied on imaging techniques such as dual-energy X-ray absorptiometry (DXA), computed-tomography (CT), and magnetic resonance imaging [5]. Somewhat surprisingly, the reported associations between muscle/lean mass estimated from DXA and measures of physical function and other distal outcomes, including falls, fractures, and mortality, have been inconsistent [36-41]. Studies using CT to assess muscle size and quality may be more reliable and assess a direct physical property of muscle composition in comparison to measures of lean mass assessed by DXA. Specifically, muscle attenuation as assessed using CT, a relative measure intramyocellular fat infiltration, independently predicts incident mobility disability (self-reported difficulty walking ¼ mile) in community dwelling older adults [42]. Moreover, changes in muscle cross sectional area over 5 years as measured by CT in HABC Study participants were related to an increased risk of mortality (relative risk 1.21 C.I.: 1.08-1.35) [41]. More recent approaches to measure direct properties of muscle quantity by using the isotopic dilution of deuterium-labelled creatine have revealed stronger associations between skeletal muscle mass (estimated by creatine dilution) and measures of physical functioning (Short Physical Performance Battery (a composite measure of gait speed, repeated chair stand time, and standing balance) and usual walking speed), incident mobility limitation (self-reported difficulty walking 2-3 blocks), and serious fall injury [43]. These findings suggest that tools that more directly assess skeletal muscle mass, size, and/or quality may be more sensitive and thus be more predictive of poor physical function, mobility limitations and more distal clinically relevant outcomes. Future studies need to more extensively interrogate the performance characteristics of new methodologies such as the creatine-dilution technique and other imaging tools in more diverse populations of older adults.
Metabolic Homeostasis.
Aging is strongly associated with T2D, a condition characterized by a resistance to insulin action that accounts for > 90% of cases of diabetes (DC ADA position statement [44]. The incidence of T2D notably increases from 3.1 diagnoses per 1000 persons age 18-44 years to 10.9 diagnoses per 1000 persons age 45 to 65 years [45]. This dramatic mid-life shift in T2D onset, commonly attributed to obesity and physical inactivity, also corresponds to the onset and progression of skeletal muscle aging.
Skeletal muscle is a robust metabolic organ. It is the primary site of insulin-mediated glucose disposal. With the advent of the glycemic clamp technique, the laboratories of DeFronzo and Olefsky demonstrated that insulin sensitivity and, more specifically, post-receptor insulin action, are significantly reduced in healthy, non-obese older women and men [46, 47]. Despite effective binding to its cognate receptor, insulin-mediated activation of Akt2 and the subsequent phosphorylation and inactivation of RabGTPases, which enable glucose transporter type 4-containing vesicles to translocate to the cell membrane to import glucose, are decreased in aged skeletal muscle [48]. Exercise-stimulated muscle glucose transport also diminishes with advancing age. This has been attributed to blunted activation of AMP-activated protein kinase (AMPK), a master regulator of glucose and lipid homeostasis, insulin sensitivity, and mitochondrial biogenesis [49]. Alterations in muscle glucose uptake partly contribute to a marked ~38% reduction in muscle glycogen content in older compared to younger adults [50]. This has notable consequences since skeletal muscle is the largest reservoir of glycogen in the human body and reduced glycogen stores impact endurance, performance, and onset of fatigue [51].
Skeletal muscle further impacts whole-body metabolism as a chief determinant of both resting energy expenditure ((REE), often referred to as basal metabolic rate) and activity-associated energy expenditure (AEE) [52]. REE routinely accounts for 50-70% of total energy expenditure among adults and has been show to decline by 1-2% each decade [53]. This is predominantly a consequence of the loss of lean mass, and skeletal muscle in particular, as nicely summarized by Roberts and Rosenberg [54]. Finally, age-associated changes in AEE are perhaps even more striking and reflect the progressive decrease in both habitual physical activity and purposeful exercise with advancing age. The use of an objective measure of physical activity (accelerometry) in the National Health and Nutrition Examination Survey revealed a 55% decrease in moderate to vigorous activity counts from age 39 to 70+ years, and a 75% decrease in time spent performing moderate to vigorous activity [55]. Indeed, physical activity and exercise have profound effects on skeletal muscle energy demand, including increasing skeletal muscle glucose uptake and fatty acid oxidation, and, in turn, systemic metabolic homeostasis. Determinants of physical activity and participation in structured exercise are undoubtedly multifactorial (e.g., physical, social, environmental, sex, race), and innovative strategies to counteract the age-associated decline in these behaviors are desperately needed as they promise to profoundly impact human health.
Physical Resilience.
The loss of resilience, defined as the ability to resist and/or recover from a stress or challenge, is a critical phenotype of aging that has recently gained increased attention [56-59]. Challenges to physical resilience, such as infections, surgery, medication exposures, and falls, intensify with advancing age. Considerable evidence suggests sarcopenia is a contributor to the age-related loss of resilience. For example, older persons with low muscle mass experience delayed recovery [60] and higher rates of complications and infections following surgery [61], greater drug toxicity [62], and higher disease-specific and all-cause mortality [63-65]. Moreover, performance-based measures of grip strength and gait speed and self-reported measures of endurance and physical activity, are core components of the frailty phenotype and, arguably, indicative of skeletal muscle health [66]. The associations between frailty and adverse outcomes are robust and have been extensively studied and reviewed elsewhere (e.g., [67-69]). Support for a causal relationship between low muscle mass and the loss of resilience stems from preclinical studies in which blockade of factors responsible for muscle wasting, such as activin A, at least in the context of cancer, enhance survival without altering tumor growth or the systemic inflammation [70]. The potential of muscle-based interventions to improve the resilience of older adults to adverse health outcomes following medical and surgical challenges and/or enhancing their subsequent functional recovery offers a unique clinical trial paradigm.
Collectively, the clinical consequences of skeletal muscle aging profoundly impact the health, independence, and quality of life of older adults and pose a significant burden on healthcare resources and health expenditures. Through a better understanding of the biological mechanisms that cause muscle aging, new interventions can be pursued.
Biological mechanisms of skeletal muscle aging
Proteostasis.
Under physiological conditions, a dynamic interplay between protein degradation and synthesis ensures the maintenance of protein homeostasis within skeletal muscle and allows an adequate adaptation to changes in physical activity, nutritional input and metabolic needs [71, 72]. A progressive loss in protein homeostasis is believed to contribute to the pathogenesis of muscle loss and dysfunction during aging, although the precise mechanisms are still far from being fully elucidated.
Among the proteolytic systems, the ubiquitin-proteasome and the autophagy-lysosome pathways play a prominent role in skeletal muscle protein degradation [73]. In the ubiquitin-proteasome system proteins destined for degradation are first covalently attached to a chain of ubiquitin molecules through an enzymatic cascade (involving the ATP-dependent E1 ubiquitin activating enzymes, the E2 ubiquitin conjugating enzymes and the E3 ubiquitin ligases) and are then processed by the 26S proteasome [74]. Hyperactivation of the ubiquitin-proteasome system has been implicated in several muscle wasting conditions [75], but its role in skeletal muscle aging is more controversial. Indeed, studies on aged skeletal muscle have reported both increased and decreased proteasome activity [76, 77]. Moreover, several studies on skeletal muscle aging have reported unchanged and even reduced mRNA levels for atrogin-1/MAFbx and MuRF1 [76-82], which are two important muscle-specific E3 ubiquitin ligases transcriptionally upregulated in many conditions associated to muscle atrophy [73, 83]. Interestingly, experimental studies have shown that MuRF1-null mice display preserved muscle mass at old age, but a decline in muscle force [77, 80], while mice lacking atrogin-1/MAFbx, which did not reach old age because of precocious death, displayed a reduction of muscle mass and force at 15 months of age [80].
Macroautophagy (hereafter referred as autophagy) is an evolutionary conserved homeostatic mechanism that allows the degradation and recycling of cellular components (bulk cytoplasm, long-lived or misfolded proteins, damaged organelles) by their sequestration in a double membrane vesicle called an autophagosome that next fuses with the lysosome for enzymatic digestion of its content [84]. Although long-considered a non-specific degradative pathway, it is becoming increasingly evident that autophagy can also selectively remove protein aggregates or damaged organelles (such as mitochondria via mitophagy) [85]. Autophagy plays an essential role in the maintenance of skeletal muscle mass and its alteration has been linked to the pathogenesis of muscle atrophy during different pathological conditions [80, 86]. Recently, several studies on aging have evaluated the muscular expression of proteins commonly used to monitor autophagy such as beclin-1, microtubule-associated protein 1 light chain 3 isoform I (LC3I) and the lipidated isoform LC3II, and SQSTM1/p62 [87, 88] . Although the patterns of autophagy markers assessed and reported in these studies were slightly different, overall, they suggested impaired autophagic degradation in aged skeletal muscle [89-93]. However, since autophagy is a highly dynamic process and measurement of these markers in static conditions has some limitations [87, 94], recent experiments have been also performed using the autophagy inhibitor colchicine, which blocks the autophagosome-lysosome fusion [95]. Results obtained in whole muscle extracts as well as in mitochondria isolated from the muscle of aged rodents would suggest an increase in basal autophagy and mitophagy flux, although further studies are needed to evaluate whether autophagosome formation is followed by an efficient lysosomal degradation of the cargo during the final step of autophagy [82, 96, 97]. Indeed, an accumulation of lipofuscin has been previously reported in aged skeletal muscle, which would suggest some impairment in lysosomal function [96, 98].
Interestingly, experiments conducted using mice with a muscle-specific deletion of Atg7 (a crucial autophagy gene) suggested that impaired autophagy may contribute to sarcopenia. Indeed, genetic inhibition of autophagy in murine muscle reduced survival and induced muscular alterations resembling those observed during aging such as atrophy and weakness, neuromuscular junction degeneration, mitochondrial dysfunction and enhanced oxidative stress [86, 91]. In addition, in a recent study genetic impairment of autophagy by Atg7 deletion in the satellite cells of young mice induced entry into senescence and regenerative failure [99]. Of note, a decline in basal autophagy was also observed in physiologically aged satellite cells and its reactivation by pharmacological treatment or Atg7 overexpression prevented senescence and restored their regenerative capacity.
In coordination with degradative processes, an important contribution to the maintenance of skeletal muscle mass is made by protein synthesis. The insulin-like growth factor 1 (IGF-1)/ Akt/mechanistic/mammalian target of rapamycin (mTOR) signaling is a well characterized anabolic pathway, which also plays an important role in regulating protein degradation [100]. Indeed, Akt activation, besides stimulating mTOR and thereby protein synthesis, can also inhibit the Forkhead Box O (FoxO) transcription factors, which control the expression of genes involved in the autophagy as well as in the ubiquitin proteasome-pathway [101, 102]. mTOR functions through formation of two different complexes: mTORC1, which stimulates protein synthesis by phosphorylating p70S6 kinase (S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), and mTORC2, which phosphorylates Akt in a feedback loop [71]. Besides regulating protein synthesis, mTORC1 can also suppress autophagy by inhibiting ULK1 (a kinase involved in autophagy induction) and the transcription factor EB (TFEB), which regulates the expression of genes involved in lysosomal biogenesis and autophagy [103]. Importantly, several lines of evidence suggest that mTORC1 activity can be further regulated by diverese inputs such as amino acids and mechanical stimuli, although the molecular mechanisms involved still need to be fully elucidated [103, 104]. The role of the IGF-1/Akt/mTOR pathway in the pathogenesis of skeletal muscle aging is still controversial, but a few studies have suggested that mTORC1 signaling is hyperactivated in the skeletal muscle of aged rodents [80, 93, 105]. Interestingly, different patterns of modulation of the Akt/mTOR pathway have also been shown in the skeletal muscle of female and male aged rodents as well as in different muscles suggesting that the regulation of this pathway may vary with aging by sex and muscle type, at least under the experimental conditions analyzed [106-108]. In humans, reduced, unchanged and increased mTORC1 signaling has been reported [80, 109, 110], underscoring the need for additional research to better understand how modulation of this pathway may contribute to skeletal muscle aging.
Skeletal muscle aging is not associated with a reduction in basal muscle protein synthesis, but rather an impaired protein synthetic response to anabolic stimuli, a phenomenon called anabolic resistance [72]. Data obtained in the skeletal muscle of aged rodents have shown, in spite of high basal mTORC1 signaling, a blunted activation in response to a single bout of muscle contraction compared to younger animals [106]. An impaired activation of mTORC1 signaling has also been shown in the skeletal muscle of elderly subjects in response to acute bout of resistance exercise or essential amino acids ingestion [111, 112]. Despite evidence for aging-associated anabolic resistance, a recent systematic review was less conclusive. Several differences, however, were noted among the included studies in terms of anabolic stimuli utilized as well as experimental methodology and design adopted, which may have contributed to the resultant inconsistencies [113]. Additional research is needed to advance our understanding of anabolic resistance and identify strategies to counteract this consequence of aging.
Stem Cell Exhaustion and Function.
Satellite cells are the essential skeletal muscle stem cell. As their name implies [114], these cells reside juxtaposed to the mature muscle fiber; external to its plasma membrane yet enveloped by the surrounding basal laminae. In addition to their location, satellite cells can be identified by their expression of several molecular markers, including the paired homeodomain transcript factor, Pax7, which is essential for satellite cell specification, expansion and function [115, 116]. Considerable research has been conducted to understand the impact of aging on satellite cells and their role in skeletal muscle health.
Satellite cells are absolutely essential for skeletal muscle regeneration following injury. Indeed, targeted depletion of satellite cells in adult mice harboring a human diphtheria toxin receptor under the control of the Pax7 promoter completely disrupted muscle regeneration following both extreme (intramuscular injection of cardiotoxin (see also [117]) and physiological (downhill running on a treadmill) muscle injury [118]. In the absence of satellite cells, skeletal muscle atrophy, fiber loss, fibrosis, fat accumulation, immune cell infiltration, and inflammation are evident and severe following injury. The extent to which sarcopenia per se is influenced by the health and function of satellite cells is less clear. Targeted elimination of most (~83%) Pax7-expressing cells in mice failed to hasten or exacerbate the age-related loss in muscle fiber cross-sectional area or muscle mass, despite clear defects in muscle regenerative capacity [119]. It is possible that the satellite cells that remained were sufficient to maintain muscle fiber size and mass, as reported in a separate study [120], particularly given the limited demands placed on skeletal muscle by captive laboratory mice. Interestingly, mice deficient in satellite cells did exhibit significant increases in fibrosis, a phenotype of aging muscle that further highlights the dynamic interplay between the diverse cell types residing in skeletal muscle [119], In sum, the findings by Fry and colleagues are provocative, and have stimulated further investigations into the contributions of satellite cells to muscle adaptations to aging and other physiological stimuli.
Muscle regenerative capacity is markedly impaired by aging. The number of muscle stem cells and their capacity to self-renew have been shown to decline with age in both mice [121, 122] and humans [123], particularly in fast-twitch glycolytic muscle fibers. In addition to number, satellite cells exhibit age-associated changes in their molecular phenotype. Purified muscle progenitor cells (highly enriched for PaxT) obtained from aged 24-month-old mice demonstrated markedly impaired (two-thirds reduction) engraftment compared to young 2-month-old donor cells when transplanted into muscle of young immunodeficient recipient mice [120], Further analyses revealed increased activation of p38α/β stress signaling and increased expression of senescence-associated cyclin dependent kinase inhibitors p16Ink4a and p21Cip1 in the aged muscle stem cells, consistent with their diminished proliferative potential [120], Pharmacological inhibition of p38α/β restored aged muscle stem cell proliferative capacity in vitro, and markedly improved engraftment in vivo. A recent analysis of satellite cells derived from geriatric mice (28 to 32 months of age) also revealed elevated expression of p16Ink4a, a cyclin-dependent kinase inhibitor involved in the cell fate of senescence (see below) [124], Following cardiotoxin injury to the muscle of young mice, transplanted satellite cells from geriatric mice were unable to activate and expand. Targeted repression of p16Ink4a by short hairpin RNA, however, restored satellite cell activation in culture, and augmented engraftment and self-renewal. Collectively, these studies provide compelling evidence for intrinsic changes in satellite cells as a consequence of aging that, at a minimum, severely compromise skeletal muscle’s capacity for regeneration.
During muscle regeneration, the transition of satellite cells from quiescence and their subsequent proliferation and differentiation are also regulated by extrinsic factors in the local environment, or niche [125], These cues include cytokines, growth factors, and extracellular matrix proteins and remodelers, which are produced by the mature myofibers and diverse myogenic (i.e., satellite cells) and non-myogenic (e.g., endothelial cells, fibroadipogenic progenitors, immune cells, and invading monocytes, etc.) cell types [126, 127], potentially in the context of senescence as discussed below. Dysregulation of the niche during muscle regeneration results in aging-like phenotypes, including impaired satellite cell proliferation and differentiation and increased fibrosis and fat accumulation.
The experimental model of parabiosis, in which two organisms are joined in a manner so that they share a common circulatory network, has led to the study of progeronic factors from an older organism that adversely affect the health and function of cells in a younger organism, and antigeronic factors from a younger organism that positively affect the health and function of cells in an older organism. Indeed, injury to the skeletal muscle of a younger mouse that shares the circulation of an older mouse leads to compromised tissue regeneration. In contrast, an injured older mouse coupled to a younger mouse experiences restored satellite cell function and ameliorated age-related tissue fibrosis [128], Progeronic factors include Wnt proteins, a large family of secreted glycoproteins, that promote the transition of myogenic satellite cells to a fibrogenic lineage [129], Transforming growth factor-β, which is particularly liberated from aged muscle, also exhibits progeronic activity that involves the phosphorylation of Smad3 and the disruption of Notch signaling in satellite cells [130], Notch signaling is a critical determinant of the satellite cell pool and satellite cell fate [131-133], In older mice, failed activation of Notch in satellite cells leads to “mitotic catastrophe [134].” Antigeronic factors have remained more elusive; however, one example is oxytocin, a hormone produced by the hypothalamus whose abundance declines with age. Systemic administration of oxytocin to aged mice rescued satellite cell proliferation and skeletal muscle regeneration to levels observed in young mice following injury [135], Collectively, these data demonstrate that age-associated alterations in the niche can markedly effect satellite cell function and muscle regeneration. The biological mechanisms that drive alterations in the niche warrant further study.
Cellular Senescence.
Senescent cells and their dynamic secretome—the senescence-associated secretory phenotype (SASP)—have been strongly implicated as drivers of aging [136-139] and aging-related diseases, including vascular dysfunction [140], atherosclerosis [141], lung disease [142], diabetes [143, 144], osteoarthritis [145], osteoporosis [146], and neuropathology [147, 148]. Senescence, a state of stable growth arrest, is principally a fate of proliferating cells. In response to genomic, proteomic, metabolic, or replicative stress, the senescence program is initiated by cell cycle inhibitor proteins, including p16Ink4a and p21Cip1, that antagonize the actions of cyclin dependent kinases to ultimately halt cell proliferation [149, 150]. Senescence is therefore an inherently protective, tumor-suppressive program [151], With advancing age, however, senescent cells accumulate [152, 153], presumably due to their resistance to apoptosis and inefficient removal by the immune system [139, 154]. By depleting the mitotically active progenitor pool and abundantly secreting a mix of cytokines, chemokines, matrix remodeling proteins, and growth factors, senescent cells compromise regeneration and mediate inflammation, deterioration, and fibrosis in tissues of older organisms [154, 155]. Intuitively, senescent cells and the SASP could underlie skeletal muscle aging and sarcopenia, yet this concept has not been methodically tested.
Support for senescent cells and the SASP as drivers of skeletal muscle dysfunction comes from a number of observations. First, transplantation of senescent cells (either syngeneic preadipocytes or autologous ear fibroblasts) into young adult mice compromised measures of grip strength and physical function, including walking speed and hanging endurance [137]. In turn, systemic elimination of p16Ink4a-expressing cells through either activation of a suicide transgene, or the pharmacological targeting of senescent cells (based on their significant upregulation of pro-survival factors (see [139])), modestly improved measures of physical function in older (24-month-old) mice. Whether the detrimental effects of senescent cells on physical function, or the beneficial effects of their removal, resulted from a direct influence on parameters of muscle aging (e.g., atrophy, fibrosis, denervation, or cellular composition) was not studied. Instead, the effects on adipose tissue senescent cell burden and SASP and, correspondingly, systemic inflammation, was demonstrated. This potential mechanism is in line with a prior report showing pharmacological inhibition of the Jak pathway, which was shown to regulate the SASP in senescent preadipocytes and endothelial cells in vitro, suppressed markers of both adipose tissue and systemic inflammation and improved parameters of physical function in aged mice [156]. It is also worth noting that associations between the number of p16Ink4a-expressing cells in thigh adipose tissue and measures of muscle performance and physical function have been observed in older humans [157].
It is unclear whether senescent cell populations within muscle mechanistically contribute to age-related changes in its mass, composition or function. As discussed above, Sousa-Victor and colleagues have demonstrated the significant impact of p16lnk4a-expressing satellite cells on muscle regeneration [124]. However, the longer-term effects of senescent cells or their removal on sarcopenia, fibrosis, fat infiltration and measures of muscle performance have not been carefully investigated. In part, this is a practical challenge as sarcopenia is relatively late phenomenon in mice, compared to its progressive nature in humans. As emphasized, aged muscle is compositionally heterogenous, and senescence of resident cell populations beyond satellite cells, including fibroadipogenic progenitor, endothelial, and immune cells, may also contribute to muscle aging. Consequently, there is a need to identify and comprehensively phenotype the cell populations within aged muscle that are prone to senesce and determine the extent to which they mechanistically contribute to muscle loss and dysfunction.
Mitochondrial function.
Mitochondria play a pivotal role in skeletal muscle function. These organelles are recognized for their role in generating chemical energy in the form of ATP to fuel the metabolic demands within muscle fibers, including contractile function, maintenance of membrane potential, calcium handling, and overall cellular maintenance and homeostasis. In addition to their role as energetic powerhouses, mitochondria are also a primary source of reactive oxygen species (ROS). As reviewed authoritatively elsewhere [158], ROS function as important signaling molecules but can simultaneously initiate cell death through damage to cellular components such as DNA, proteins, and lipids. Given their critical role for cellular homeostasis, mitochondria have been implicated in the etiology of sarcopenia and multiple age-related diseases (e.g., diabetes, cancer, cardiovascular disease) [159-161] and pursued as therapeutic targets [162, 163].
More than 20 years ago, Rooyackers and colleagues first demonstrated that the activities of two key mitochondrial enzymes, citrate synthase and cytochrome c oxidase, decrease with age in skeletal muscle biopsies from men and women across a wide age range [164]. Additional studies supported the premise that aging adversely affects skeletal muscle mitochondria, including decreased mitochondrial ATP production rates [165-167], decreased mitochondrial gene expression [168], decreased mitochondrial DNA copy number [169, 170], reduced mitochondrial volume density [171], and increased oxidative damage [172]. These findings were not universal; however, as a number of carefully conducted studies failed to observe age-related impairments in skeletal muscle mitochondrial function [173-177]. Indeed, several important factors may confound the effects of aging on muscle mitochondrial physiology:
Aging vs. disuse. Plasticity of mitochondria makes them exquisitely responsive to use and disuse (i.e., exercise and sedentariness). To what extent do observed changes in skeletal muscle mitochondria simply reflect changes in muscle use across the lifespan? Multiple studies have shown that age-related changes in muscle oxidative capacity and mitochondrial function are not evident when studies carefully control for habitual physical activity [174, 178, 179]. However, some age-related mitochondrial changes occur independent of physical activity levels. For example, mitochondrial DNA copy number decreases with age in young and older adults who are matched for physical activity levels [180]. Furthermore, the age-associated reduction in mtDNA abundance is also evident even in highly-trained masters level endurance athletes [180].
Age-related mitochondrial changes may be muscle-specific. Fast-glycolytic muscle fibers undergo a more profound age-related atrophy than slow-oxidative muscle fibers [181]. The vastus lateralis muscle, characterized by a mixed fiber type composition, is often studied in humans because its large size, accessibility, and location relative to major blood vessels makes it convenient for repeated sampling by percutaneous biopsy with minimal complications. Samples from vastus lateralis typically reveal age-related declines in mitochondrial function [166, 167, 182, 183], whereas studies in other muscle groups such as tibialis anterior [174], forearm muscles [184], and plantarflexors [185] report similar mitochondrial function in young and old. Indeed, muscle oxidative capacity, measured in vivo by 31P magnetic resonance spectroscopy, was found to be reduced with age in vastus lateralis, but not in tibialis anterior [186, 187]. A recent systematic review on this topic clearly shows that muscle group is a significant moderator of age-related changes in skeletal muscle oxidative capacity [188].
Reductionistic vs. Integrative Studies of Mitochondrial Physiology. Studies commonly draw conclusions based on the expression of a gene or the activity of a single cytochrome chain or TCA cycle enzyme. Even gold-standard measurements of oxygen consumption and ATP production in intact mitochondria isolated from skeletal muscle have been criticized because the organelles are studied ex vivo under non-physiological conditions where cellular circulatory and regulatory systems have been stripped away. Picard and colleagues nicely demonstrated how measurements in isolated mitochondria may overstate the degree to which aging influences muscle mitochondrial biology [176]. In this study they show that salient indices defining mitochondrial function, including respiration, ROS production, and calcium sensitivity, are significantly impaired with aging when examined in isolated muscle mitochondria. However, the effects of age were less apparent when measurements were made in permeabilized yet intact muscle fibers. Phosphorous magnetic resonance spectroscopy (31P-MRS) enables the study of human skeletal muscle bioenergetics in vivo [189], and was first applied to study aging skeletal muscle in the 1980s [177]. Kent and Fitzgerald have authored an authoritative review of studies that have used 31P-MRS to investigate the effects of aging on mitochondrial oxidative capacity [190]. They concluded that much of the ambiguity regarding the effects of aging can be ascribed to differences in muscle group investigated or subject characteristics (e.g., physical activity, age, sex, and presence of age-related comorbidities). An important message from this body of literature is that not all of the mitochondrial changes observed in muscles of older adults are consequences of aging per se, but may be largely driven by environmental and lifestyle factors.
Regardless whether age-related functional changes in muscle mitochondria are consequent to biological aging or simply paraphenomena, the changes experienced by older adults are real. The extent to which age-related changes in muscle mitochondria drive aging phenotypes (e.g., sarcopenia, insulin resistance) is a topic that remains at the leading edge of aging research [191, 192].
Interventions for skeletal muscle aging
Despite the prevalence and impact, there are no currently approved pharmacological interventions for sarcopenia. A number of anabolic interventions to augment protein synthesis have been trialed, such as testosterone and growth hormone, and shown to have minor to modest effects on muscle mass, strength, and/or physical function (e.g., [193-196]), but have also generated significant concerns about safety [197, 198]. There has been great interest in approaches to disrupt myostatin signaling, a catabolic transforming growth factor β (TGFP) superfamily member that is predominantly synthesized and secreted by skeletal muscle that triggers protein degradation and disrupts protein synthesis pathways, as we have recently reviewed [199, 200]. A number of early phase clinical trials in older adults have been completed [201-204], and shown favorable effects on muscle mass or volume, muscle strength, and/or some functional outcomes, including stair climb time, chair rise time, maximal gait speed, and six-minute walk distance. These studies and their outcomes have been nicely summarized in a recent issue of Bone [205]. It is worth noting that other TGFβ family members, including GDF11 and activin A, also compromise skeletal muscle health and function and serve as potential therapeutic targets for sarcopenia [206-211]. Of note, neither myostatin nor GDF11 increase with chronological age in humans [212, 213], and an important study by Latres and colleagues demonstrated critically important species differences in the abundance of myostatin, GDF11, and Activin A [214]. Specifically, circulating concentrations of myostatin are markedly higher in mice than humans, whereas activin A is much higher in humans than in mice, and GDF11 is extremely low in both species. These data potentially help explain why the profound effects of myostatin inhibition on muscle mass in mice do not necessarily translate to humans and may help inform future therapeutic approaches.
Finally, the search for small molecules and biologics to enhance skeletal muscle health and function should not overshadow nor trivialize the remarkably protective effects of exercise against the biology of aging. Indeed, exercise is safe and scalable, and effectively improves measures of muscle mass, strength, power, and physical function, even in those with overt age-associated deficits. The salutary effects of exercise on aging-associated changes in skeletal muscle have recently and very thoughtfully been reviewed [215]. The power of exercise, and the challenge to harness or mimic it in a pill, stems from the array of mechanical, metabolic, hormonal, and neural signals it elicits. Impressively, exercise has been show to counter a range of age-induced forms of damage, including those discussed here; i.e.,proteotoxic stress by inducing autophagy [216], stem cell dysfunction by stimulating muscle satellite cell activation [217], cellular senescence by mitigating senescence-inducing stressors (e.g., DNA damage [218]), and mitochondrial dysfunction by restoring the hermetic response to oxidative stress [219]. As new interventions targeting the biology of aging emerge, there will be value in studying how their effects are augmented by exercise.
Conclusions
Population aging coupled with the clinical consequences of sarcopenia have generated considerable interest to understand and identify strategies to counter the biological mechanisms of skeletal muscle aging. The underlying causes of this process are multifactorial, and in no way is this review all inclusive. We apologize to the authors of the important studies we have not included. An outstanding, incredibly comprehensive review on sarcopenia, led by Dr. Marco Sandri, was very recently published [220].
In sum, strategies to promote muscle health in late life promise to have beneficial effects on physical function, metabolism, and resilience, and subsequently, the independence and quality of life of older adults. While new interventions are on the horizon, physical activity and structured exercise can have an immediate and far-reaching impact. Given physical activity and exercise delay the onset and progression of the majority of age-related diseases and geriatric syndromes, broad reaching public health strategies are warranted to promote adoption and compliance.
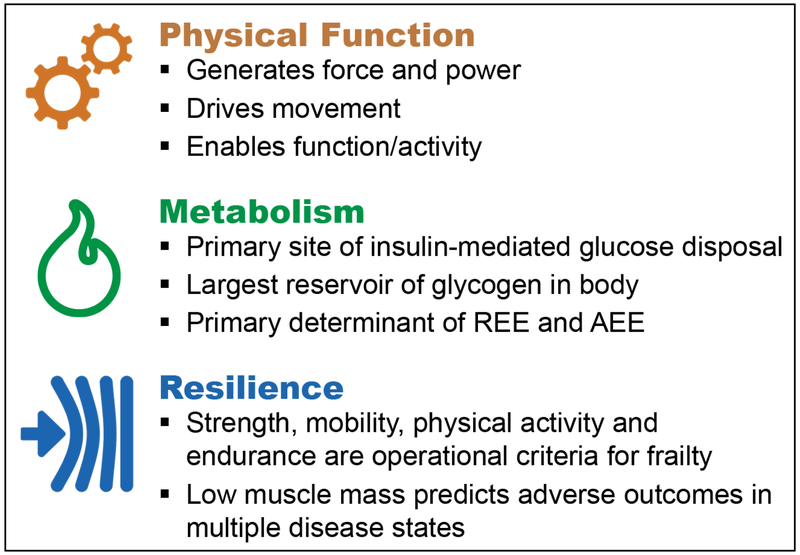
Figure 2. Skeletal muscle in health, aging and disease.
Skeletal muscle uniquely generates force and power for diverse forms of physical function, including mobility. It is also a critical metabolic organ that is responsible for the storage of glucose, the oxidation of fatty acids, and is a rich source of amino acids. Based on its size and metabolic activity during both rest and movement, muscle strongly influences both resting energy expenditure (REE) and activity associated energy expenditure (REE). Measures of muscle performance, physical function, and mass are also determinants of physical resilience, the capacity to resist and recover from diverse challenges.
Highlights.
Muscle mass and cellular composition are altered with advancing age
Muscle aging negatively affects physical function, metabolism, and resilience
Diverse forms of age-related molecular and cellular damage drive muscle aging
New interventions targeting hallmarks of aging may benefit late-life muscle health
Notably, exercise effectively counters muscle aging and its consequences
Acknowledgements
The authors would like to acknowledge the generous support of the National Institutes of Health, National Institute on Aging AG AG054454 (IRL), AG53832 (NKL), and AG55529 (RAF and NKL), and the Pritzker Foundation (ZA, XZ, and NKL).
Footnotes
Declaration of interest
N.K. LeBrasseur has a financial interest related to this work: patents on senolytic drugs are held by Mayo Clinic. This work has been revised by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic conflict of interest policies. No other conflicts of interest, financial or otherwise, are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statement
Zaira Aversa: Data curation; Roles/Writing – original draft
Xu Zhang: Data curation; Roles/Writing – original draft
Roger Fielding: Conceptualization; Roles/Writing- original draft
Ian Lanza: Data curation; Roles/Writing – original draft
Nathan LeBrasseur: Conceptualization; Roles/Writing- original draft; Writing -– review & editing
References
- [1].Rosenberg IH, Sarcopenia: origins and clinical relevance, J Nutr 127(5 Suppl) (1997) 990S–991S. [DOI] [PubMed] [Google Scholar]
- [2].Lexell J, Taylor CC, Sjostrom M, What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men, J Neurol Sci 84(2-3) (1988) 275–94. [DOI] [PubMed] [Google Scholar]
- [3].Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, Reid K, Boonen S, Dere W, Epstein S, Mitlak B, Tsouderos Y, Sayer AA, Rizzoli R, Reginster JY, Kanis JA, Tools in the assessment of sarcopenia, Calcif Tissue Int 93(3) (2013) 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reginster JY, Cooper C, Rizzoli R, Kanis JA, Appelboom G, Bautmans I, Bischoff-Ferrari HA, Boers M, Brandi ML, Bruyere O, Cherubini A, Flamion B, Fielding RA, Gasparik AI, Van Loon L, McCloskey E, Mitlak BH, Pilotto A, Reiter-Niesert S, Rolland Y, Tsouderos Y, Visser M, Cruz-Jentoft AJ, Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia, Aging Clin Exp Res 28(1) (2016) 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, Maggi S, Dennison E, Al-Daghri NM, Allepaerts S, Bauer J, Bautmans I, Brandi ML, Bruyere O, Cederholm T, Cerreta F, Cherubini A, Cooper C, Cruz-Jentoft A, McCloskey E, Dawson-Hughes B, Kaufman JM, Laslop A, Petermans J, Reginster JY, Rizzoli R, Robinson S, Rolland Y, Rueda R, Vellas B, Kanis JA, Pitfalls in the measurement of muscle mass: a need for a reference standard, J Cachexia Sarcopenia Muscle 9(2) (2018) 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT, The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates, J Gerontol A Biol Sci Med Sci 69(5) (2014) 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European P Working Group on Sarcopenia in Older, Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People, Age Ageing 39(4) (2010) 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD, C. Society on Sarcopenia, W. Wasting Disorders Trialist, Sarcopenia with limited mobility: an international consensus, Journal of the American Medical Directors Association 12(6) (2011) 403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M, Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia, Journal of the American Medical Directors Association 12(4) (2011) 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC, Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics", Clinical nutrition 29(2) (2010) 154–9. [DOI] [PubMed] [Google Scholar]
- [11].Anker SD, Morley JE, von Haehling S, Welcome to the ICD-10 code for sarcopenia, J Cachexia Sarcopenia Muscle 7(5) (2016) 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vellas B, Fielding RA, Bens C, Bernabei R, Cawthon PM, Cederholm T, Cruz-Jentoft AJ, Del Signore S, Donahue S, Morley J, Pahor M, Reginster JY, Rodriguez Manas L, Rolland Y, Roubenoff R, Sinclair A, Cesari M, Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force, J Frailty Aging 7(1) (2018) 2–9. [DOI] [PubMed] [Google Scholar]
- [13].Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S, An evidence-based comparison of operational criteria for the presence of sarcopenia, J Gerontol A Biol Sci Med Sci 69(5) (2014) 584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging, Cell 153(6) (2013) 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F, Geroscience: linking aging to chronic disease, Cell 159(4) (2014) 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB, The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study, J Gerontol A Biol Sci Med Sci 61(10) (2006) 1059–64. [DOI] [PubMed] [Google Scholar]
- [17].Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L, Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia, J Appl Physiol (1985) 95(5) (2003) 1851–60. [DOI] [PubMed] [Google Scholar]
- [18].Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA, Lower extremity muscle mass predicts functional performance in mobility-limited elders, J Nutr Health Aging 12(7) (2008) 493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA, Impact of muscle power and force on gait speed in disabled older men and women, J Gerontol A Biol Sci Med Sci 59(11) (2004) 1200–6. [DOI] [PubMed] [Google Scholar]
- [20].Suzuki T, Bean JF, Fielding RA, Muscle power of the ankle flexors predicts functional performance in community-dwelling older women, J Am Geriatr Soc 49(9) (2001) 1161–7. [DOI] [PubMed] [Google Scholar]
- [21].Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA, The relationship between leg power and physical performance in mobility-limited older people, J Am Geriatr Soc 50(3) (2002) 461–7. [DOI] [PubMed] [Google Scholar]
- [22].Guralnik JM, Ferrucci K, Simonnick EM, Salive ME, Wallace RB, Lower extremity function over the age of 70 years as a predictor of subsequent disability., N. Engl. J. Med. 332 (1995) 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sallinen J, Manty M, Leinonen R, Kallinen M, Tormakangas T, Heikkinen E, Rantanen T, Factors associated with maximal walking speed among older community-living adults, Aging Clin Exp Res 23(4) (2011) 273–8. [DOI] [PubMed] [Google Scholar]
- [24].Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M, Basic ADL disability and functional limitation rates among older AMERICANS from 2000-2005: the end of the decline?, J Gerontol A Biol Sci Med Sci 64(12) (2009) 1333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Janssen I, Heymsfield SB, Ross R, Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability, J Am Geriatr Soc 50(5) (2002) 889–96. [DOI] [PubMed] [Google Scholar]
- [26].Tinetti ME, Speechley M, Ginter SF, Risk factors for falls among elderly persons living in the community, N Engl J Med 319(26) (1988) 1701–7. [DOI] [PubMed] [Google Scholar]
- [27].Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB, Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability, N Engl J Med 332(9) (1995) 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guralnik JM, LaCroix AZ, Abbott RD, Berkman LF, Satterfield S, Evans DA, Wallace RB, Maintaining mobility in late life. I. Demographic characteristics and chronic conditions, Am J Epidemiol 137(8) (1993) 845–57. [DOI] [PubMed] [Google Scholar]
- [29].Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, Harris T, Ensrud K, Orwoll E, Lee CG, Chandler JM, Newman AB, Cauley JA, Guralnik JM, Ferrucci L, Studenski SA, Gait Speed Predicts Incident Disability: A Pooled Analysis, J Gerontol A Biol Sci Med Sci 71(1) (2016) 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cummings SR, Studenski S, Ferrucci L, A Diagnosis of Dismobility-Giving Mobility Clinical Visibility: A Mobility Working Group Recommendation, JAMA (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM, Physical performance measures in the clinical setting, J Am Geriatr Soc 51(3) (2003) 314–22. [DOI] [PubMed] [Google Scholar]
- [32].Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA, Improvement in usual gait speed predicts better survival in older adults, J Am Geriatr Soc 55(11) (2007) 1727–34. [DOI] [PubMed] [Google Scholar]
- [33].Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB, Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort, J Gerontol A Biol Sci Med Sci 61(1) (2006) 72–7. [DOI] [PubMed] [Google Scholar]
- [34].Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB, Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability, JAMA 295(17) (2006) 2018–26. [DOI] [PubMed] [Google Scholar]
- [35].Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J, Gait speed and survival in older adults, Jama 305(1) (2011) 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schaap LA, Koster A, Visser M, Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons, Epidemiol Rev 35 (2013) 51–65. [DOI] [PubMed] [Google Scholar]
- [37].Schaap LA, van Schoor NM, Lips P, Visser M, Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam, J Gerontol A Biol Sci Med Sci 73(9) (2018) 1199–1204. [DOI] [PubMed] [Google Scholar]
- [38].Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB, Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women, J Am Geriatr Soc 55(5) (2007) 769–74. [DOI] [PubMed] [Google Scholar]
- [39].Zoico E, Di Francesco V, Mazzali G, Zivelonghi A, Volpato S, Bortolani A, Dioli A, Coin A, Bosello O, Zamboni M, High baseline values of fat mass, independently of appendicular skeletal mass, predict 2-year onset of disability in elderly subjects at the high end of the functional spectrum, Aging Clin Exp Res 19(2) (2007) 154–9. [DOI] [PubMed] [Google Scholar]
- [40].Hars M, Trombetti A, Body composition assessment in the prediction of osteoporotic fractures, Curr Opin Rheumatol 29(4) (2017) 394–401. [DOI] [PubMed] [Google Scholar]
- [41].Santanasto AJ, Goodpaster BH, Kritchevsky SB, Miljkovic I, Satterfield S, Schwartz AV, Cummings SR, Boudreau RM, Harris TB, Newman AB, Body Composition Remodeling and Mortality: The Health Aging and Body Composition Study, J Gerontol A Biol Sci Med Sci 72(4) (2017) 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB, Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons, J Gerontol A Biol Sci Med Sci 60(3) (2005) 324–33. [DOI] [PubMed] [Google Scholar]
- [43].Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, Stefanick ML, Shikany JM, Strotmeyer ES, Glynn NW, Caserotti P, Shankaran M, Hellerstein M, Cummings SR, Evans WJ, Osteoporotic G Fractures in Men Study Research, Strong Relation between Muscle Mass Determined by D3-creatine Dilution, Physical Performance and Incidence of Falls and Mobility Limitations in a Prospective Cohort of Older Men, J Gerontol A Biol Sci Med Sci (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].American Diabetes Association, Diagnosis and classification of diabetes mellitus, Diabetes Care 37 Suppl 1 (2014) S81–90. [DOI] [PubMed] [Google Scholar]
- [45].Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017, Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services, 2017. [Google Scholar]
- [46].DeFronzo RA, Glucose intolerance and aging, Diabetes Care 4(4) (1981) 493–501. [DOI] [PubMed] [Google Scholar]
- [47].Fink RI, Kolterman OG, Griffin J, Olefsky JM, Mechanisms of insulin resistance in aging, J Clin Invest 71(6) (1983) 1523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Petersen KF, Morino K, Alves TC, Kibbey RG, Dufour S, Sono S, Yoo PS, Cline GW, Shulman GI, Effect of aging on muscle mitochondrial substrate utilization in humans, Proc Natl Acad Sci U S A 112(36) (2015) 11330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI, Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis, Cell Metab 5(2) (2007) 151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J, Evans WJ, Peripheral effects of endurance training in young and old subjects, J Appl Physiol (1985) 66(6) (1989) 2844–9. [DOI] [PubMed] [Google Scholar]
- [51].Ortenblad N, Westerblad H, Nielsen J, Muscle glycogen stores and fatigue, J Physiol 591(18) (2013) 4405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zurlo F, Larson K, Bogardus C, Ravussin E, Skeletal muscle metabolism is a major determinant of resting energy expenditure, J Clin Invest 86(5) (1990) 1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Keys A, Taylor HL, Grande F, Basal metabolism and age of adult man, Metabolism 22(4) (1973) 579–87. [DOI] [PubMed] [Google Scholar]
- [54].Roberts SB, Rosenberg I, Nutrition and aging: changes in the regulation of energy metabolism with aging, Physiol Rev 86(2) (2006) 651–67. [DOI] [PubMed] [Google Scholar]
- [55].Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M, Physical activity in the United States measured by accelerometer, Med Sci Sports Exerc 40(1) (2008) 181–8. [DOI] [PubMed] [Google Scholar]
- [56].Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS, Physical Resilience in Older Adults: Systematic Review and Development of an Emerging Construct, J Gerontol A Biol Sci Med Sci 71(4) (2016) 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hadley EC, Kuchel GA, Newman AB, S. Workshop, Participants, Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging, J Gerontol A Biol Sci Med Sci 72(7) (2017) 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kirkland JL, Stout MB, Sierra F, Resilience in Aging Mice, J Gerontol A Biol Sci Med Sci 71(11) (2016) 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].LeBrasseur NK, Physical Resilience: Opportunities and Challenges in Translation, J Gerontol A Biol Sci Med Sci 72(7) (2017) 978–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE, Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery, Br J Cancer 107(6) (2012) 931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN, Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation, Liver Transpl 19(12) (2013) 1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB, Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment, Clin Cancer Res 15(8) (2009) 2920–6. [DOI] [PubMed] [Google Scholar]
- [63].Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, Stewart SB, Thapa P, Tarrell RF, Cheville JC, Tollefson MK, Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality, Cancer 120(18) (2014) 2910–8. [DOI] [PubMed] [Google Scholar]
- [64].Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP, Frailty as a Predictor of Surgical Outcomes in Older Patients, Journal of the American College of Surgeons 210(6) (2010) 901–908. [DOI] [PubMed] [Google Scholar]
- [65].Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP, Phenotype of frailty: characterization in the women's health and aging studies, J Gerontol A Biol Sci Med Sci 61(3) (2006) 262–6. [DOI] [PubMed] [Google Scholar]
- [66].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Frailty in older adults: evidence for a phenotype, J Gerontol A Biol Sci Med Sci 56(3) (2001) M146–56. [DOI] [PubMed] [Google Scholar]
- [67].Lin HS, Watts JN, Peel NM, Hubbard RE, Frailty and post-operative outcomes in older surgical patients: a systematic review, BMC Geriatr 16(1) (2016) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vermeiren S, Vella-Azzopardi R, Beckwee D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I, g. Gerontopole Brussels Study, Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis, J Am Med Dir Assoc 17(12) (2016) 1163 e1–1163 e17. [DOI] [PubMed] [Google Scholar]
- [69].Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, Sibley S, Rockwood K, The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis, Intensive Care Med 43(8) (2017) 1105–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ, Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival, Cell 142(4) (2010) 531–543. [DOI] [PubMed] [Google Scholar]
- [71].Egerman MA, Glass DJ, Signaling pathways controlling skeletal muscle mass, Critical reviews in biochemistry and molecular biology 49(1) (2014) 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ, Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise, Acta Physiol (Oxf) 216(1) (2016) 15–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sandri M, Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome, Int J Biochem Cell Biol 45(10) (2013) 2121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bilodeau PA, Coyne ES, Wing SS, The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation, Am J Physiol Cell Physiol 311(3) (2016) C392–403. [DOI] [PubMed] [Google Scholar]
- [75].Cohen S, Nathan JA, Goldberg AL, Muscle wasting in disease: molecular mechanisms and promising therapies, Nat Rev Drug Discov 14(1) (2015) 58–74. [DOI] [PubMed] [Google Scholar]
- [76].Altun M, Besche HC, Overkleeft HS, Piccirillo R, Edelmann MJ, Kessler BM, Goldberg AL, Ulfhake B, Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway, J Biol Chem 285(51) (2010) 39597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC, Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age, Aging Cell 13(1) (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Edstrom E, Altun M, Hagglund M, Ulfhake B, Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle, J Gerontol A Biol Sci Med Sci 61(7) (2006) 663–74. [DOI] [PubMed] [Google Scholar]
- [79].Gaugler M, Brown A, Merrell E, DiSanto-Rose M, Rathmacher JA, Reynolds T.H.t., PKB signaling and atrogene expression in skeletal muscle of aged mice, J Appl Physiol (1985) 111(1) (2011) 192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Munoz-Canoves P, Musaro A, Pende M, Reggiani C, Rizzuto R, Schiaffino S, Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway, Biogerontology 14(3) (2013) 303–23. [DOI] [PubMed] [Google Scholar]
- [81].Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL, Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison, J Gerontol A Biol Sci Med Sci 69(8) (2014) 1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC, Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis, Aging (Albany NY) 8(1) (2016) 127–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bodine SC, Baehr LM, Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1, Am J Physiol Endocrinol Metab 307(6) (2014) E469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mizushima N, Komatsu M, Autophagy: renovation of cells and tissues, Cell 147(4) (2011) 728–41. [DOI] [PubMed] [Google Scholar]
- [85].Bonaldo P, Sandri M, Cellular and molecular mechanisms of muscle atrophy, Disease models & mechanisms 6(1) (2013) 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M, Autophagy is required to maintain muscle mass, Cell Metab 10(6) (2009) 507–15. [DOI] [PubMed] [Google Scholar]
- [87].Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, et al. , Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition), Autophagy 12(1) (2016) 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Funderburk SF, Wang QJ, Yue Z, The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond, Trends Cell Biol 20(6) (2010) 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C, Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise, Exp Gerontol 45(2) (2010) 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Penna F, Costamagna D, Pin F, Camperi A, Fanzani A, Chiarpotto EM, Cavallini G, Bonelli G, Baccino FM, Costelli P, Autophagic degradation contributes to muscle wasting in cancer cachexia, Am J Pathol 182(4) (2013) 1367–78. [DOI] [PubMed] [Google Scholar]
- [91].Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M, Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging, Cell Rep 8(5) (2014) 1509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sakuma K, Kinoshita M, Ito Y, Aizawa M, Aoi W, Yamaguchi A, p62/SQSTM1 but not LC3 is accumulated in sarcopenic muscle of mice, J Cachexia Sarcopenia Muscle 7(2) (2016) 204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].White Z, White RB, McMahon C, Grounds MD, Shavlakadze T, High mTORC1 signaling is maintained, while protein degradation pathways are perturbed in old murine skeletal muscles in the fasted state, Int J Biochem Cell Biol 78 (2016) 10–21. [DOI] [PubMed] [Google Scholar]
- [94].Yoshii SR, Mizushima N, Monitoring and Measuring Autophagy, International journal of molecular sciences 18(9) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ju JS, Varadhachary AS, Miller SE, Weihl CC, Quantitation of "autophagic flux" in mature skeletal muscle, Autophagy 6(7) (2010) 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Carter HN, Kim Y, Erlich AT, Zarrin-Khat D, Hood DA, Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity, J Physiol 596(16) (2018) 3567–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chen CCW, Erlich AT, Crilly MJ, Hood DA, Parkin is required for exercise-induced mitophagy in muscle: impact of aging, Am J Physiol Endocrinol Metab 315(3) (2018) E404–e415. [DOI] [PubMed] [Google Scholar]
- [98].O'Leary MF, Vainshtein A, Iqbal S, Ostojic O, Hood DA, Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle, Am J Physiol Cell Physiol 304(5) (2013) C422–30. [DOI] [PubMed] [Google Scholar]
- [99].Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Munoz-Canoves P, Autophagy maintains stemness by preventing senescence, Nature 529(7584) (2016) 37–42. [DOI] [PubMed] [Google Scholar]
- [100].Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M, Mechanisms regulating skeletal muscle growth and atrophy, Febs j 280(17) (2013) 4294–314. [DOI] [PubMed] [Google Scholar]
- [101].Schiaffino S, Mammucari C, Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models, Skelet Muscle 1(1) (2011) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M, Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy, Nature communications 6 (2015) 6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Saxton RA, Sabatini DM, mTOR Signaling in Growth, Metabolism, and Disease, Cell 168(6) (2017) 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Goodman CA, Hornberger TA, Robling AG, Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms, Bone 80 (2015) 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Barns M, Gondro C, Tellam RL, Radley-Crabb HG, Grounds MD, Shavlakadze T, Molecular analyses provide insight into mechanisms underlying sarcopenia and myofibre denervation in old skeletal muscles of mice, Int J Biochem Cell Biol 53 (2014) 174–85. [DOI] [PubMed] [Google Scholar]
- [106].Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA, Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle, J Appl Physiol (1985) 97(1) (2004) 243–8. [DOI] [PubMed] [Google Scholar]
- [107].Paturi S, Gutta AK, Katta A, Kakarla SK, Arvapalli RK, Gadde MK, Nalabotu SK, Rice KM, Wu M, Blough E, Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model, Mech Ageing Dev 131(3) (2010) 202–9. [DOI] [PubMed] [Google Scholar]
- [108].Baar EL, Carbajal KA, Ong IM, Lamming DW, Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice, Aging Cell 15(1) (2016) 155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Li M, Verdijk LB, Sakamoto K, Ely B, van Loon LJ, Musi N, Reduced AMPK-ACC and mTOR signaling in muscle from older men, and effect of resistance exercise, Mech Ageing Dev 133(11-12) (2012) 655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Markofski MM, Dickinson JM, Drummond MJ, Fry CS, Fujita S, Gundermann DM, Glynn EL, Jennings K, Paddon-Jones D, Reidy PT, Sheffield-Moore M, Timmerman KL, Rasmussen BB, Volpi E, Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women, Exp Gerontol 65 (2015) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ, Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle, Faseb j 19(3) (2005) 422–4. [DOI] [PubMed] [Google Scholar]
- [112].Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB, Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis, Skelet Muscle 1(1) (2011) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shad BJ, Thompson JL, Breen L, Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review, Am J Physiol Endocrinol Metab 311(5) (2016) E803–e817. [DOI] [PubMed] [Google Scholar]
- [114].Mauro A, Satellite cell of skeletal muscle fibers, J Biophys Biochem Cytol 9 (1961) 493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA, Pax7 is required for the specification of myogenic satellite cells, Cell 102(6) (2000) 777–86. [DOI] [PubMed] [Google Scholar]
- [116].von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA, Pax7 is critical for the normal function of satellite cells in adult skeletal muscle, Proc Natl Acad Sci U S A 110(41) (2013) 16474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lepper C, Partridge TA, Fan CM, An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration, Development 138(17) (2011) 3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A, Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration, Development 138(17) (2011) 3647–56. [DOI] [PubMed] [Google Scholar]
- [119].Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA, Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia, Nat Med 21(1) (2015) 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM, Rejuvenation of the muscle stem cell population restores strength to injured aged muscles, Nature Medicine 20(3) (2014) 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Chakkalakal JV, Jones KM, Basson MA, Brack AS, The aged niche disrupts muscle stem cell quiescence, Nature 490(7420) (2012) 355–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z, Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle, Developmental biology 294(1) (2006) 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJC, Satellite cells in human skeletal muscle; from birth to old age, Age 36(2) (2014) 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P, Geriatric muscle stem cells switch reversible quiescence into senescence, Nature 506(7488) (2014) 316–21. [DOI] [PubMed] [Google Scholar]
- [125].Bentzinger CF, von Maltzahn J, Rudnicki MA, Extrinsic regulation of satellite cell specification, Stem Cell Res Ther 1(3) (2010) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA, Cellular dynamics in the muscle satellite cell niche, EMBO Rep 14(12) (2013) 1062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA, Cellular dynamics in the muscle satellite cell niche, Embo Rep 14(12) (2013) 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, Rejuvenation of aged progenitor cells by exposure to a young systemic environment, Nature 433(7027)(2005)760–4. [DOI] [PubMed] [Google Scholar]
- [129].Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA, Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis, Science 317(5839) (2007) 807–10. [DOI] [PubMed] [Google Scholar]
- [130].Carlson ME, Hsu M, Conboy IM, Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells, Nature 454(7203) (2008) 528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Conboy IM, Conboy MJ, Smythe GM, Rando TA, Notch-mediated restoration of regenerative potential to aged muscle, Science 302(5650) (2003) 1575–1577. [DOI] [PubMed] [Google Scholar]
- [132].Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA, Notch Signaling Is Necessary to Maintain Quiescence in Adult Muscle Stem Cells, Stem Cells 30(2) (2012) 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S, A Critical Requirement for Notch Signaling in Maintenance of the Quiescent Skeletal Muscle Stem Cell State, Stem Cells 30(2) (2012) 243–252. [DOI] [PubMed] [Google Scholar]
- [134].Liu L, Charville GW, Cheung TH, Yoo B, Santos PJ, Schroeder M, Rando TA, Impaired Notch Signaling Leads to a Decrease in p53 Activity and Mitotic Catastrophe in Aged Muscle Stem Cells, Cell Stem Cell 23(4) (2018) 544–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM, Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration, Nature communications 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM, Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan, Nature 530(7589) (2016) 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer A, Weivoda MM, Inman CL, Ogrodnik M, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, Senolytics Enhance Physical Function in Old Age and Extend Post-Treatment Survival, Nat Med In press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM, Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders, Nature 479(7372) (2011) 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, The Achilles' heel of senescent cells: from transcriptome to senolytic drugs, Aging Cell 14(4) (2015) 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD, Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice, Aging Cell 15(5) (2016) 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM, Senescent intimal foam cells are deleterious at all stages of atherosclerosis, Science 354(6311) (2016) 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK, Cellular senescence mediates fibrotic pulmonary disease, Nature communications 8 (2017) 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T, van Deursen JM, Kirkland JL, LeBrasseur NK, Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue, Diabetes 65(6) (2016) 1606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL, Targeting senescent cells enhances adipogenesis and metabolic function in old age, Elife 4 (2015)e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH, Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment, Nat Med (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S, Targeting cellular senescence prevents age-related bone loss in mice, Nat Med 23(9) (2017) 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME, Tau protein aggregation is associated with cellular senescence in the brain, Aging Cell 17(6) (2018)e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ, Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline, Nature 562(7728) (2018) 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC, Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts, Proc Natl Acad Sci U S A 93(24) (1996) 13742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J, Reversal of human cellular senescence: roles of the p53 and p16 pathways, EMBO J 22(16) (2003) 4212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Campisi J, Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors, Cell 120(4) (2005) 513–22. [DOI] [PubMed] [Google Scholar]
- [152].Waaijer ME, Parish WE, Strongitharm BH, van Heemst D, Slagboom PE, de Craen AJ, Sedivy JM, Westendorp RG, Gunn DA, Maier AB, The number of p16INK4a positive cells in human skin reflects biological age, Aging cell 11(4) (2012) 722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM, Cellular senescence in aging primates, Science 311(5765) (2006) 1257. [DOI] [PubMed] [Google Scholar]
- [154].Campisi J, d'Adda di Fagagna F, Cellular senescence: when bad things happen to good cells, Nat Rev Mol Cell Biol 8(9) (2007) 729–40. [DOI] [PubMed] [Google Scholar]
- [155].Munoz-Espin D, Serrano M, Cellular senescence: from physiology to pathology, Nat Rev Mol Cell Biol 15(7) (2014) 482–96. [DOI] [PubMed] [Google Scholar]
- [156].Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL, JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age, Proc Natl Acad Sci U S A 112(46) (2015) E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ, Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women, J Gerontol A Biol Sci Med Sci (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Balaban RS, Nemoto S, Finkel T, Mitochondria, oxidants, and aging, Cell 120(4) (2005) 483–95. [DOI] [PubMed] [Google Scholar]
- [159].Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW 2nd, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, Wallace D, Youle RJ, C.o.C.C. American Heart Association Council on Basic Cardiovascular Sciences, G. Council on Functional, B. Translational, Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association, Circ Res 118(12) (2016) 1960–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wallace DC, Mitochondria and cancer, Nat Rev Cancer 12(10) (2012) 685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Patti ME, Corvera S, The role of mitochondria in the pathogenesis of type 2 diabetes, Endocr Rev 31(3) (2010) 364–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hesselink MK, Schrauwen-Hinderling V, Schrauwen P, Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus, Nature reviews. Endocrinology 12(11) (2016)633–645. [DOI] [PubMed] [Google Scholar]
- [163].D'Souza GG, Wagle MA, Saxena V, Shah A, Approaches for targeting mitochondria in cancer therapy, Biochim Biophys Acta 1807(6) (2011) 689–96. [DOI] [PubMed] [Google Scholar]
- [164].Rooyackers OE, Adey DB, Ades PA, Nair KS, Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle, Proc Natl Acad Sci U S A 93(26) (1996) 15364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS, Decline in skeletal muscle mitochondrial function with aging in humans, Proc Natl Acad Sci U S A 102(15) (2005) 5618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K, Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans, Pflugers Arch 446(2) (2003) 261–9. [DOI] [PubMed] [Google Scholar]
- [167].Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI, Mitochondrial dysfunction in the elderly: possible role in insulin resistance, Science 300(5622) (2003) 1140–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Welle S, Bhatt K, Thornton CA, High-abundance mRNAs in human muscle: comparison between young and old, J Appl Physiol 89(1) (2000) 297–304. [DOI] [PubMed] [Google Scholar]
- [169].Barazzoni R, Short KR, Nair KS, Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart, J Biol Chem 275(5) (2000) 3343–7. [DOI] [PubMed] [Google Scholar]
- [170].Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH, Effects of exercise on mitochondrial content and function in aging human skeletal muscle, J Gerontol A Biol Sci Med Sci 61(6) (2006) 534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Conley KE, Jubrias SA, Esselman PC, Oxidative capacity and ageing in human muscle, J Physiol 526 Pt 1 (2000) 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Melov S, Shoffner JM, Kaufman A, Wallace DC, Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle, Nucleic Acids Res 23(20) (1995) 4122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN, Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria, Exp Gerontol 38(8) (2003) 877–86. [DOI] [PubMed] [Google Scholar]
- [174].Kent-Braun JA, Ng AV, Skeletal muscle oxidative capacity in young and older women and men, J Appl Physiol 89(3) (2000) 1072–8. [DOI] [PubMed] [Google Scholar]
- [175].Lanza IR, Befroy DE, Kent-Braun JA, Age-related changes in ATP-producing pathways in human skeletal muscle in vivo, J Appl Physiol 99(5) (2005) 1736–44. [DOI] [PubMed] [Google Scholar]
- [176].Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T, Hepple RT, Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers, Aging Cell 9(6) (2010) 1032–46. [DOI] [PubMed] [Google Scholar]
- [177].Taylor DJ, Crowe M, Bore PJ, Styles P, Arnold DL, Radda GK, Examination of the energetics of aging skeletal muscle using nuclear magnetic resonance, Gerontology 30(1) (1984) 2–7. [DOI] [PubMed] [Google Scholar]
- [178].Brierley EJ, Johnson MA, James OF, Turnbull DM, Effects of physical activity and age on mitochondrial function, QJM 89(4) (1996) 251–8. [DOI] [PubMed] [Google Scholar]
- [179].Chilibeck PD, McCreary CR, Marsh GD, Paterson DH, Noble EG, Taylor AW, Thompson RT, Evaluation of muscle oxidative potential by 31P-MRS during incremental exercise in old and young humans, Eur J Appl Physiol Occup Physiol 78(5) (1998) 460–5. [DOI] [PubMed] [Google Scholar]
- [180].Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS, Endurance exercise as a countermeasure for aging, Diabetes 57(11) (2008) 2933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lexell J, Human aging, muscle mass, and fiber type composition, J Gerontol A Biol Sci Med Sci 50 Spec No (1995) 11–6. [DOI] [PubMed] [Google Scholar]
- [182].Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS, Decline in skeletal muscle mitochondrial function with aging in humans, Proceedings of the National Academy of Sciences of the United States of America 102(15) (2005) 5618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Lalia AZ, Dasari S, Robinson MM, Abid H, Morse DM, Klaus KA, Lanza IR, Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults, Aging (Albany NY) 9(4) (2017) 1096–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Horska A, Fishbein KW, Fleg JL, Spencer RG, The relationship between creatine kinase kinetics and exercise intensity in human forearm is unchanged by age, Am J Physiol Endocrinol Metab 279(2) (2000) E333–9. [DOI] [PubMed] [Google Scholar]
- [185].Hart CR, Layec G, Trinity JD, Liu X, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS, Evidence of Preserved Oxidative Capacity and Oxygen Delivery in the Plantar Flexor Muscles With Age, J Gerontol A Biol Sci Med Sci 70(9) (2015) 1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA, Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior, Appl Physiol Nutr Metab 37(1) (2012) 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA, In vivo oxidative capacity varies with muscle and training status in young adults, J Appl Physiol 107(3) (2009) 873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Fitzgerald LF, Christie AD, Kent JA, Heterogeneous effects of old age on human muscle oxidative capacity in vivo: a systematic review and meta-analysis, Appl Physiol Nutr Metab 41(11) (2016) 1137–1145. [DOI] [PubMed] [Google Scholar]
- [189].Chance B, Eleff S, Leigh JS Jr., Sokolow D, Sapega A, Mitochondrial regulation of phosphocreatine/inorganic phosphate ratios in exercising human muscle: a gated 31P NMR study, Proc Natl Acad Sci U S A 78(11) (1981) 6714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Kent JA, Fitzgerald LF, In vivo mitochondrial function in aging skeletal muscle: capacity, flux, and patterns of use, Journal of applied physiology 121(4) (2016) 996–1003. [DOI] [PubMed] [Google Scholar]
- [191].Picca A, Calvani R, Bossola M, Allocca E, Menghi A, Pesce V, Lezza AMS, Bernabei R, Landi F, Marzetti E, Update on mitochondria and muscle aging: all wrong roads lead to sarcopenia, Biol Chem 399(5) (2018) 421–436. [DOI] [PubMed] [Google Scholar]
- [192].Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C, Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials, Int J Biochem Cell Biol 45(10) (2013) 2288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT, Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial, Jama 299(1) (2008) 39–52. [DOI] [PubMed] [Google Scholar]
- [194].Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, Wu FC, Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study, J Clin Endocrinol Metab 95(2) (2010) 639–50. [DOI] [PubMed] [Google Scholar]
- [195].Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC, The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial, J Clin Endocrinol Metab 91(2) (2006) 477–84. [DOI] [PubMed] [Google Scholar]
- [196].Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GAW, Schambelan M, Grunfeld C, Growth Hormone Replacement in Healthy Older Men Improves Body Composition but Not Functional Ability, Ann Intern Med 124(8) (1996) 708–716. [DOI] [PubMed] [Google Scholar]
- [197].Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S, Adverse events associated with testosterone administration, N Engl J Med 363(2) (2010) 109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM, Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial, Jama 288(18) (2002) 2282–92. [DOI] [PubMed] [Google Scholar]
- [199].White TA, LeBrasseur NK, Myostatin and sarcopenia: opportunities and challenges - a mini-review, Gerontology 60(4) (2014) 289–93. [DOI] [PubMed] [Google Scholar]
- [200].Jasuja R, LeBrasseur NK, Regenerating skeletal muscle in the face of aging and disease, Am J Phys Med Rehabil 93(11 Suppl 3) (2014) S88–96. [DOI] [PubMed] [Google Scholar]
- [201].Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, Hochberg MC, Ferrari SL, Blain H, Binder EF, Rolland Y, Poiraudeau S, Benson CT, Myers SL, Hu L, Ahmad QI, Pacuch KR, Gomez EV, Benichou O, S. Group, Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial, Lancet Diabetes Endocrinol 3(12) (2015) 948–57. [DOI] [PubMed] [Google Scholar]
- [202].Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR, Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer, J Clin Endocrinol Metab 99(10) (2014) E1967–75. [DOI] [PubMed] [Google Scholar]
- [203].Attie KM, Borgstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE, Kumar R, Willins DA, Seehra JS, Sherman ML, A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers, Muscle Nerve 47(3) (2013) 416–23. [DOI] [PubMed] [Google Scholar]
- [204].Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, Lach-Trifilieff E, Roubenoff R, Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study, J Am Geriatr Soc 65(9) (2017) 1988–1995. [DOI] [PubMed] [Google Scholar]
- [205].Marty E, Liu Y, Samuel A, Or O, Lane J, A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease, Bone 105 (2017) 276–286. [DOI] [PubMed] [Google Scholar]
- [206].Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ, GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration, Cell Metab 22(1) (2015) 164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Rodgers BD, Eldridge JA, Reduced Circulating GDF11 Is Unlikely Responsible for Age-Dependent Changes in Mouse Heart, Muscle, and Brain, Endocrinology 156(11) (2015) 3885–8. [DOI] [PubMed] [Google Scholar]
- [208].Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ, Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells, Aging Cell 15(3) (2016) 582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Glass DJ, Elevated GDF11 Is a Risk Factor for Age-Related Frailty and Disease in Humans, Cell Metab 24(1) (2016) 7–8. [DOI] [PubMed] [Google Scholar]
- [210].Hammers DW, Merscham-Banda M, Hsiao JY, Engst S, Hartman JJ, Sweeney HL, Supraphysiological levels of GDF11 induce striated muscle atrophy, EMBO Mol Med 9(4) (2017) 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zimmers TA, Jiang Y, Wang M, Liang TW, Rupert JE, Au ED, Marino FE, Couch ME, Koniaris LG, Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting, Basic Res Cardiol 112(4) (2017) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Bergen HR 3rd, Farr JN, Vanderboom PM, Atkinson EJ, White TA, Singh RJ, Khosla S, LeBrasseur NK, Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay, Skelet Muscle 5 (2015) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S, Miller JD, Bergen HR 3rd, LeBrasseur NK, Quantification of GDF11 and Myostatin in Human Aging and Cardiovascular Disease, Cell Metab 23(6) (2016) 1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Latres E, Mastaitis J, Fury W, Miloscio L, Trejos J, Pangilinan J, Okamoto H, Cavino K, Na E, Papatheodorou A, Willer T, Bai Y, Hae Kim J, Rafique A, Jaspers S, Stitt T, Murphy AJ, Yancopoulos GD, Gromada J, Activin A more prominently regulates muscle mass in primates than does GDF8, Nature communications 8 (2017) 15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Cartee GD, Hepple RT, Bamman MM, Zierath JR, Exercise Promotes Healthy Aging of Skeletal Muscle, Cell Metab 23(6) (2016) 1034–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].He C, Sumpter R Jr., Levine B, Exercise induces autophagy in peripheral tissues and in the brain, Autophagy 8(10) (2012) 1548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B, Kjaer M, Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise, The Journal of physiology 558(Pt 1) (2004) 333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S, Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle, Pflugers Arch 445(2) (2002) 273–8. [DOI] [PubMed] [Google Scholar]
- [219].Radak Z, Chung HY, Koltai E, Taylor AW, Goto S, Exercise, oxidative stress and hormesis, Ageing research reviews 7(1) (2008) 34–42. [DOI] [PubMed] [Google Scholar]
- [220].Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M, Sarcopenia: Aging-Related Loss of Muscle Mass and Function, Physiol Rev 99(1) (2019) 427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]