Abstract
In smokers, neural responses to smoking cues can be sensitive to acute abstinence, but the degree to which abstinence-related cue reactivity contributes to relapse is not fully understood. This study addressed this question in a sample of 75 smokers who were motivated to quit smoking. Participants underwent blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) during presentation of visual smoking cues and neutral stimuli on two occasions: once during smoking satiety and once following 24-hour abstinence (order counter-balanced). Following the imaging sessions, participants received brief smoking cessation counseling prior to a short-term (7-day) quit attempt. The primary smoking cessation outcome was biochemically confirmed 7-day relapse. The secondary smoking cessation outcome measure was total number of self-reported days of abstinence. During abstinence (vs. satiety), smoking cue reactivity was significantly increased only in the anterior cingulate cortex (ACC); other regions showing a cue (vs. neutral) response did not exhibit an abstinence effect in the stringent whole-brain analysis. Participants that showed greater smoking cue reactivity in the ACC during acute abstinence (compared to smoking satiety) were more likely to relapse (OR=2.10 per standard deviation increase in percent signal change [abstinence minus smoking satiety], 95% CI: 1.05 to 4.20, p=0.036). Greater abstinence-induced change in ACC activation also predicted fewer total days abstinent (β=-0.63, 95% CI=0.43 to 0.66, p<0.0001). This study provides the first evidence that changes in smoking cue reactivity in the ACC during acute abstinence predict smoking relapse, thereby improving our understanding of the neurobiology of smoking cessation.
Clinical trial registry identifier: NCT02837510
Keywords: BOLD fMRI, Short-term Relapse, Smoking Cue Reactivity
INTRODUCTION
Each year, millions of smokers try to quit, but most smokers relapse within a few days (Hughes et al., 2004). One factor that may contribute to the risk of relapse is exposure to smoking-related cues. Frequent pairings between the visual, tactile, and olfactory sensations of smoking with the rewarding effects of nicotine result in a classical conditioning effect, such that even a picture of a cigarette can evoke strong cravings in chronic smokers (Shiffman et al., 2013). Among smokers who are trying to quit, these cue-induced subjective cravings can promote relapse (Conklin et al., 2012; Ferguson and Shiffman, 2009).
Functional magnetic reasoning imaging (fMRI) studies have begun to elucidate neural substrates involved in cue reactivity. A network of limbic and paralimbic regions (e.g. ventral striatum, amygdala, and anterior cingulate cortex [ACC]) has been implicated in cue reactivity across multiple addictive substances (Kuhn and Gallinat, 2011; Wilson et al., 2004). Meta-analyses of studies specifically investigating smoking cue reactivity identified consistent increases in activation in the medial prefrontal cortex (PFC), ACC, and posterior cingulate cortex in response to smoking cues (vs. neutral stimuli) (Engelmann et al., 2012; Wilson et al., 2004). These regions are involved with mesolimbic dopaminergic reward system, which is critical to the reinforcement of addictive drugs (Goldstein and Volkow, 2002). The same regions are also implicated in networks at rest such as the default mode network and salience networks, which are associated with interoceptive processing and attention (Janes et al., 2015; Lerman et al., 2014). Alterations in connectivity of regions involved in interoceptive processing and attention have been associated with smoking cue reactivity (Wilcox et al., 2018) and smoking cessation outcomes (Claus et al., 2013; Wilcox et al., 2017). Exposure to smoking cues may divert attentional resources towards processing cues and trigger behavior resulting in relapse.
Initial evidence supports an association of neural responses to smoking cues and relapse; however, the results are mixed (Courtney et al., 2016; Janes et al., 2017; Janes et al., 2010; Owens et al., 2017; Versace et al., 2014). Among treatment-seeking smokers, those who relapsed showed heightened neural responses during smoking cue reactivity tasks during smoking satiety in a priori regions of interest including the bilateral insula, ACC, posterior cingulate cortex, and amygdala (Janes et al., 2010) as well as the right insula and dorsal striatum in a replication study (Janes et al., 2017). Another study of 55 smokers found that those with heightened brain response in dorsal striatum, medial PFC, and dorsolateral PFC to cigarette-related cues compared to pleasant stimuli during smoking satiety prior to quitting were less likely to be abstinent six months later (Versace et al., 2014). However, another study found the reverse pattern: greater activation in response to smoking cues (vs. neutral stimuli) in the right ventral striatum, left amygdala, and anterior cingulate was associated with longer periods of abstinence following cessation (Owens et al., 2017). The majority of studies of neural cue reactivity conducted to date have examined smokers either in a state of abstinence or of satiety; few have directly examined whether response to cues differs during abstinence, and none of the prior studies utilized a within-subject design to evaluate whether neural responses to smoking cues during abstinence (vs. smoking satiety) predict relapse. In one study that observed greater brain activation in the ACC during smoking cue reactivity following 24-hour abstinence (as compared to smoking satiety), smokers did not complete a quit attempt (McClernon et al., 2009).
To investigate the relevance of abstinence-induced changes during cue reactivity to quit success, we conducted a within-subject investigation of 75 treatment-seeking smokers. We hypothesized that heightened smoking cue reactivity during abstinence (relative to smoking satiety) in the attentional, cognitive control, and reward networks would predict the likelihood of short-term smoking relapse (biochemically confirmed in the first 7 days of a quit attempt).
METHODS
Participants
This paper reports on the effects of abstinence versus satiety on neural cue reactivity as part of a larger ongoing study of neural predictors of smoking relapse. Sample size for the present report was based on an estimated effect size of abstinence on domains involved in smoking behavior (e.g. cue reactivity, stress reactivity); a sample of n=75 provides 80% power to detect an effect size of Cohen’s d=0.33, similar to effect sizes observed in previous studies (Ashare et al., 2016; Loughead et al., 2015; Owens et al., 2017). Participants were 75 treatment-seeking smokers ages 18 to 65 who reported smoking ≥5 cigarettes/day for ≥6 months and were recruited through media advertisements. Exclusion criteria were: exhaled carbon monoxide (CO) breath sample <8ppm at eligibility assessment; current use of nicotine products other than cigarettes (such as chewing tobacco, snuff, e-cigarettes or nicotine replacement therapy); pregnancy, planned pregnancy or breastfeeding; history of DSM-IV Axis I psychiatric or substance disorders within the past two years except nicotine dependence; use of psychotropic medications; history of significant brain injury; left-handedness; fMRI contraindicated material in the body; claustrophobia; low or borderline intelligence (<85 score on Shipley’s Institute of Living Scale; Zachary, 1986); breath alcohol test ≥0.01; and any impairment that would prevent task performance. Fig. 1 shows the CONSORT flow diagram for the study.
Figure 1.

CONSORT participant flow diagram
Screening
All procedures were approved by the University of Pennsylvania Institutional Review Board and carried out in accordance with the Declaration of Helsinki. Initial telephone screen was followed by an in-person eligibility assessment. All participants provided written informed consent, an exhaled CO breath sample to confirm smoking status, a breath alcohol measurement, a urine sample to assess for the use of study-prohibited drugs, and if applicable, participants were provided a self-administered pregnancy screening. Eligible participants completed a smoking history questionnaire (cigarettes per day [CPD]) and the Fagerström Test for Cigarette Dependence (FTCD; Fagerstrom, 2012).
fMRI Sessions
The neuroimaging experiment was a within-subject design with two blood-oxygen-level-dependent (BOLD) fMRI sessions scheduled at least 1 week apart in counterbalanced order: 1) during smoking satiety and 2) following 24-hour abstinence (i.e., abstinence challenge). All sessions were scheduled to begin between 8 a.m.−10 a.m. Participants with a positive urine drug screen, a breath alcohol test ≥0.01, a CO reading ≥8ppm at the abstinent session, or a CO reading <8ppm at the smoking satiety session were excluded. Participants then completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986) and the Questionnaire of Smoking Urges (QSU-Brief; Cox et al., 2001). For the smoking satiety session, participants smoked a single cigarette approximately 1 hour prior to cue exposure (Loughead et al., 2015). Participants completed a short practice session to become familiar with the cue task and response device prior to being escorted to the scanning facility.
fMRI Data Acquisition
BOLD fMRI was acquired with a Siemens Prisma 3T system (Erlangen, Germany) using a whole-brain, single-shot gradient-echo (GE) echoplanar sequence with the following parameters: TR/TE=1000/30ms, FOV=192 mm, matrix=64×64, slice thickness/gap=2.0/0 mm, 78 slices, effective voxel resolution of 2×2×2 mm. RF transmission utilized a quadrature body-coil and reception used a 64-channel head coil. Prior to BOLD fMRI, 5-min magnetization-prepared, rapid acquisition gradient echo T1-weighted image (MPRAGE, TR 2200ms, TE 4.67ms, FOV 240mm, matrix 192×256, effective voxel resolution of 1×1×1mm) was acquired for anatomic overlays of functional data and to aid spatial normalization to standard atlas space.
Cue Reactivity Task
Cue reactivity was assessed during BOLD imaging using a validated event related smoking cue task (Janes et al., 2015). During the task, participants viewed grayscale images of smoking cues and neutral stimuli. Smoking cues (CUE) were images of people smoking, people holding cigarettes, and smoking-related items such as cigarettes. Neutral stimuli were images matched for visual content to a smoking image (e.g. a person with a pen in mouth, neutral items such as pens). To ensure participant engagement, infrequent target stimuli (pictures of animals) were presented and participants were instructed to respond with button press. The cue task consisted of 20 CUE, 20 neutral, and four target stimuli each presented for 4 seconds. Images were presented with a variable ISI (6–24 seconds) during which a fixation point appeared on a grey screen (baseline). Stimuli were pseudo-randomized with no more than two of an image type presented in a row. Before and after the cue reactivity task, participants completed a 2-item questionnaire to assess craving and urge to smoke (Falcone et al., 2016). The total task duration was 10 minutes and 36 seconds.
Smoking Cessation Procedures
Following completion of both imaging sessions, participants had an individual pre-quit counseling session using counseling protocols adapted in previous large placebo-controlled trials (Lerman et al., 2015). During this counseling session, participants discussed strategies for quitting and relapse prevention with a trained smoking cessation counselor, and set a target quit date to occur ~1 week later. Participants completed a brief in-person visit on the target quit date, which included a booster counseling session to reinforce strategies discussed at the pre-quit visit. Following the target quit day, participants received a brief (15 minute) mid-week booster counseling session and verified quit status with a CO reading; quit status was also evaluated at one week following target quit day. At this visit, smoking behavior (cigarettes per day) was assessed for each day following the target quit day using timeline follow-back (Brown et al., 1998). In addition to self-report, quit status was biochemically confirmed using a CO breath sample and NicAlert urine test strips (Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ). NicAlert test strips utilized an immunochromatographic assay to provide a semi-quantitative measure of the concentration of cotinine (the primary metabolite of nicotine) in urine. Results appear as categorical levels of usage. Following manufacturer guidelines, NicAlert results of level two or below (equivalent to a urine cotinine concentration of <100 ng/ml) were required to biochemically confirm abstinence, in addition to a CO reading of ≤5ppm at the two monitoring visits: 3 days and 7 days following target quit date (Perkins et al., 2013).
Image Preprocessing and Time Series Analysis
BOLD time series data were pre-processed using standard image analysis procedures executed with fMRI Expert Analysis Tool (FEAT of FSL [FMRIB’s Software Library, Oxford, UK]). Pre-processing included motion correction (MCFLIRT; Jenkinson and Smith, 2001), slice time correction (interleaved), skull stripping using BET (Smith, 2002), spatial smoothing (6mm), and high pass filtering (100s). The median functional volume was co-registered to the anatomical T1-weighted structural volume and transformed into standard anatomical space (T1 MNI template) with FLIRT (Jenkinson and Smith, 2001). Pre-processed data was analyzed using FILM (FMRIB’s Improved General Linear Model). The model included regressors for CUE, neutral stimuli, and target stimuli convolved with double gamma hemodynamic response function. The temporal derivative and nuisance regressors for standard plus extended motion parameters were also included. The primary contrast was CUE minus neutral. This contrast isolates the additive effects of CUE (vs. neutral) by accounting for the shared cognitive demands of processing visual stimuli. All analyses were completed in subject space and transformation parameters were later applied to statistical maps for group-level analyses.
Image Quality Assessment
Overall signal quality was measured by calculating mean temporal signal to noise ratio (tSNR) and participant motion was assessed with mean relative displacement (Satterthwaite et al., 2014). Participants with low tSNR (>3SD below mean) or mean relative displacement (>3SD from mean) were identified for further evaluation. Using these procedures, two participants were excluded for relative motion greater than 0.5mm. One additional participant was excluded due to incomplete data set, resulting in a final sample of 75 participants.
Whole Brain Image Analysis
Group analyses were conducted using FSL’s local analysis of mixed effects method (FSL FLAME1; Woolrich et al., 2004). First, mean task activation across session was examined to identify regions sensitive to CUE (vs. neutral) stimuli. Next, we tested the resulting contrasts (CUE vs. baseline, neutral vs. baseline, CUE vs. neutral) for between session effects (abstinence vs. smoking satiety) using a whole brain paired t-test. Resulting Z statistic images were corrected for multiple comparisons using voxel-wise correction accounting for the effective resolution (smoothness) of the data (Worsley et al., 1992). Appropriate anatomical assignment for peak activation was determined using the Talairach atlas (Talairach and Tournoux, 1998). Due to the small number of supra-threshold voxels yielded by voxel-wise correction, cluster correction (Z≥2.3, p≤0.01) was used to create an ACC mask for extraction of the mean percent signal change (Worsley, 2001). Percent signal change was used to test the relationship between brain signal and behavioral measures outlined below.
Outcome measures
The primary smoking cessation outcome measure was 7-day relapse; abstinence was biochemically verified with CO and cotinine assessment (see above). The 7-day monitoring period was chosen because the majority of smokers relapse within the first 7 days of a quit attempt (Hughes et al., 2004). This measure is a validated predictor of long-term abstinence (Ashare et al., 2013). The secondary smoking cessation outcome measure was total number of self-reported days quit assessed using timeline follow back for the 7-day monitoring period (Ashare et al., 2013).
Statistical Analysis
Descriptive statistics were obtained for all variables. Paired t-tests (abstinence versus smoking satiety) were used to examine expected abstinence challenge effects on differences in subjective craving and withdrawal. Logistic regressions were used to assess the relationship between percent signal change and short-term quit outcome. For these analyses a standardized difference score (abstinence minus smoking satiety for CUE>neutral percent signal change) was calculated. A logistic regression model (Stata logistic, College Station, TX) was used to predict dichotomized 7-day quit success from the standardized abstinence-induced change in smoking cue reactivity. Abstinence-induced changes in craving and withdrawal, sex, age, baseline cigarettes per day (CPD), and CO at intake were entered as covariates to reduce bias associated with confounding. A second binomial regression model (Stata binreg, College Station, TX) was used for total number of days quit using the same covariates.
RESULTS
Baseline Sample Characteristics and Abstinence Challenge Effects
Seventy-five participants were included in the analysis. Of these, 40 (53.3%) were male, 44 (58.7%) were African-American, and 17 (22.7%) had completed some education beyond high school. The mean age was 43 years (SD 12.7), the mean CPD was 13.9 (SD 5.3), the mean FTCD score was 4.8 (SD 1.7), and mean CO at intake was 15.1 ppm. Exhaled CO was significantly lower at the abstinent session (mean 2.6 ppm, SD 5.3 ppm) than at the smoking satiety session (mean 16.3 ppm, SD 6.6 ppm, p<0.0001), indicating compliance with the abstinence requirement. Subjective craving (QSU) and withdrawal (MNWS) were significantly higher at the abstinence session (craving mean 45.2, SD 14.3; withdrawal mean 15.2, SD 8.5) than at the smoking satiety session (craving mean 29.5, SD 13.6; withdrawal mean 7.84, SD 6.7; ps<0.00001).
Whole Brain Analysis of Cue Reactivity
Whole brain analysis of smoking cue reactivity revealed significantly greater activation to CUE (vs. neutral) in the medial frontal gyrus/ACC, angular gyrus, middle temporal gyrus, posterior cingulate cortex/cingulate gyrus, inferior frontal gyrus, and middle frontal gyrus (Table 1; Fig. 2). This pattern of brain activation is consistent with previous neuroimaging studies and meta-analysis of smoking cue reactivity (Engelmann et al., 2012; Janes et al., 2015; Owens et al., 2017). No voxels survived correction threshold for the neutral vs. CUE contrast.
Table 1.
Brain Reactivity to CUE>Neutral Stimuli.
| RegionA | BAB | HemC | Z-maxD | VoxelsE | XF | Y | Z |
|---|---|---|---|---|---|---|---|
| MFG/ACC | 10 | L | 7.7 | 8518 | −4 | 54 | 16 |
| Angular Gyrus | 39 | L | 9.0 | 6110 | −52 | −62 | 34 |
| MTG | 21 | R | 7.6 | 3638 | 56 | 4 | −34 |
| PCC/Cingulate Gyrus | 9 | L | 8.7 | 3076 | −6 | −52 | 28 |
| IFG | 45 | R | 6.3 | 814 | 52 | 38 | −6 |
| Middle Frontal Gyrus | 11 | L | 6.8 | 216 | −44 | −46 | −22 |
Significant activation p>0.05
BA = Brodmann Area
HEM = Cerebral Hemisphere
Z-MAX values represent peak
Contiguous voxel count
MNI coordinates (mm)
Abbreviations: MFG: Medial Frontal Gyrus; ACC: Anterior Cingulate Cortex; MTG: Middle Temporal Gyrus; PCC: Paracingulate Cortex; IFG: Inferior Frontal Gyrus
Figure 2.
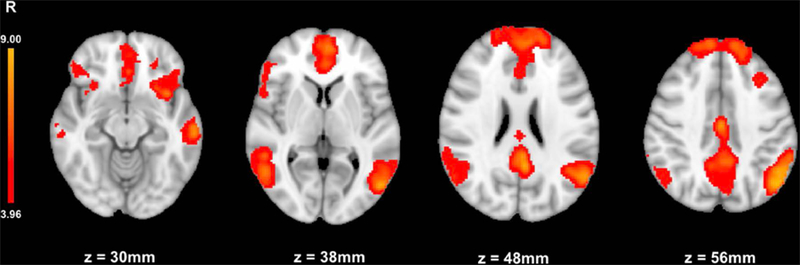
Whole-brain analysis of CUE>neutral stimuli. Mean smoking cue reactivity (CUE>neutral) showing task active brain regions for all sessions (P ≤ 0.05, corrected)
Testing abstinence vs. smoking satiety differences in CUE reactivity (CUE>neutral) revealed significantly greater activation during the abstinence session in the ACC (Fig. 3A). There were no regions with significant activation for smoking satiety>abstinence session. When examining CUE>baseline and neutral>baseline, the ACC showed significant activation during abstinence for the CUE>baseline only (p<0.05 corrected). There were no significant effects of abstinence on neutral>baseline.
Figure 3.

Abstinence-induced change in neural cue reactivity predicts 7-day quit status. A, The whole-brain analysis of the abstinent>smoking satiety session revealed significant activation (red) in the anterior cingulate cortex (P ≤ 0.05, corrected). A mask (yellow) was generated using cluster correction procedures (Z ≥ 2.3, P ≤ 0.01) for percent signal change extraction. B, Participants who showed a greater increase in ACC percent signal change during abstinence (vs satiety) were more likely to relapse (OR = 2.10 per standard deviation increase in percent signal change, 95% CI: 1.05 to 4.20, P = 0.036)
Predicting 7-Day Quit Status
Twenty-three participants (30.7%) were biochemically verified to have remained quit for the 7-day period and 52 (69.3%) had relapsed. Abstinence-induced change in ACC BOLD signal significantly predicted quit outcome; participants who showed a greater increase in BOLD signal during abstinence (compared to satiety) were more likely to relapse (OR=2.10 per standard deviation increase in percent signal change [abstinence minus smoking satiety], 95% CI: 1.05 to 4.20, p=0.036) (Fig. 3B). As a covariate, a greater increase in subjective withdrawal symptoms also significantly predicted increased odds of relapse (OR=1.10, 95% CI=1.02 to 1.19, p=0.016); BOLD signal change in ACC was a significant predictor after controlling for subjective withdrawal and craving.
Predicting Total Number of Days Quit
The mean total number of days quit in the 7-day monitoring period was 4.0 (SD 2.96). Participants who showed a greater increase in BOLD signal during abstinence (vs. smoking satiety) reported fewer days of abstinence following the target quit day (β=−0.63, 95% CI=0.43 to 0.66, p<0.0001).
DISCUSSION
This study evaluated the relationship between brain responses to smoking cues during acute abstinence (vs. satiety) and short-term relapse in 75 treatment-seeking smokers. A whole-brain analysis in this large sample of smokers revealed that of the brain regions sensitive to smoking (vs. neutral) cues, only the ACC is sensitive to abstinence-induced changes in smoking cue reactivity. Regression-based models showed that heightened abstinence-induced BOLD signal change in this region during CUE exposure (vs. neutral stimuli) predicted 7-day quit status and total number of days quit after controlling for changes in subjective withdrawal and craving. These data suggest that the changes that occur in the ACC during an abstinence challenge play a role in relapse during smoking cessation attempts beyond the effects of subjective withdrawal and craving. While previous studies have shown that smoking cue reactivity is associated with relapse (Janes et al., 2017; Versace et al., 2014), this study is the first to examine smoking cue reactivity in acute abstinence vs. satiety.
Increased BOLD signal change in the ACC during exposure to CUE (vs. neutral stimuli) is consistent with prior reports (Brody et al., 2002; McBride et al., 2006; McClernon et al., 2005; Wilson et al., 2005). A meta-analysis using 26 studies (12 studies that required participants to abstain and 14 studies that instructed participants to smoke ad libitum) found that smoking cues were associated with activation of a larger portion of the rostral ACC in nicotine abstinent smokers relative to smokers smoking as usual, underscoring the importance of the ACC for cue reactivity and highlighting the need to measure brain activity in participants during acute abstinence, as well as during satiety (Wilson and Sayette, 2015). The ACC is thought to play a key role in conflict monitoring, and suppression of ACC activity is integral to shifting attention (Botvinick et al., 2004; Bush et al., 2000). Thus, it is plausible that activation in the ACC might be required to manage attention to cues or to cope with disruptive stimuli during acute abstinence (McBride et al., 2006). Indeed, nicotine has been found to improve attention by deactivating regions such as the anterior and posterior cingulate cortex (Hahn et al., 2007). Volitional reduction in ACC activity has also been associated with a reduction in craving, and active resistance to cue-induced craving in bupropion treated smokers is also associated with reduced activation in the bilateral ACC, left ventral striatum, and left medial orbitofrontal cortex (Culbertson et al., 2011; Li et al., 2013). Differential engagement of the ACC may be driven by the differences in the degree to which abstinence vs. satiated smokers experienced the desire to smoke (Wilson and Sayette, 2015); our results suggest that changes in ACC activation during abstinence predict relapse above and beyond the effect of smoking urges. Future studies assessing functional connectivity could further probe the relevance of urge intensity. The ACC is a node of the salience network, and connections between the salience network and neural networks such as the default mode network are disrupted during abstinence (Lerman et al., 2014). Importantly, utilizing the within-subject design allowed us to test the effect of altered cue-elicited activation during abstinence on relapse, an important clinical outcome (Falcone et al., 2016; Loughead et al., 2009). Failure to quit smoking is often attributed to the presence of smoking cues (Ferguson and Shiffman, 2009); the observation that the ACC is both sensitive to abstinence and that changes in activation during abstinence compared to smoking satiety were predictive of smoking cessation indicates an essential role for the ACC in relapse during abstinence.
Several of the task active regions identified by the CUE>neutral contrast did not show an effect of abstinence in the between-session analysis. This is consistent with meta-analysis results suggesting that abstinence does not globally increase activation in all brain regions sensitive to smoking cue reactivity (Engelmann et al., 2012). These findings could indicate that certain cue reactive regions are robustly responsive to cues regardless of acute changes in smoking behavior. It is possible that a high level of salience is already ascribed to cues during satiety and therefore a ceiling effect may prevent substantial increases in activation during abstinence in much of the network (Wilson and Sayette, 2015). However, changes in the degree of connectivity between these regions have been associated with cue reactivity, nicotine dependence, and smoking cessation outcomes. For example, a study by Wilcox et al. found that food cues were associated with greater deactivation of the default mode network compared to smoking cues (Wilcox et al., 2018). This is consistent with previous findings that the default mode network is less suppressed during smoking cue exposure (Janes et al., 2016). A second study showed that changes in connectivity with the left insula in response to smoking vs. food cues correlated with FTCD in areas such as the ACC, pre/post-central gyrus, left caudate, and bilateral thalamus (Claus et al., 2013). Greater coupling of the insula and dorsal ACC at rest is significantly correlated with increased cue reactivity in brain areas associated with attention (Janes et al., 2015). Lastly, enhanced connections between the caudate and dlPFC during rest in participants with high subjective withdrawal significantly predicted worse treatment outcome in a varenicline treatment trial (Wilcox et al., 2017). Together, these findings suggest that functional connectivity within and between regions in the cue reactivity network plays an important role in smoking behavior and treatment outcomes.
The current study is the largest to assess acute abstinence-induced changes in smoking cue reactivity and to link these changes to smoking relapse. Our sample of 75 smokers (23 quit, 52 relapsed) is a strength of our study, and our proportions of quitters and relapsers are representative of quitting in a natural environment (Borland et al., 2012). As part of a larger longitudinal study, 1987 survey respondents had reported a recent quit attempt; 21.5% (95% CI: 19.7–23.3) of respondents reported a quit length of 1–6 days and 29.0% (CI: 27.0–31.0) had reported a quit length of 7–29 days (Borland et al., 2012). However, these results must be interpreted in light of several weaknesses. Due to the task design, the relative contribution of CUE and neutral stimuli to change in subjective craving could not be discerned. Also, the corrected abstinence minus smoking satiety contrast resulted in a relatively small number of contiguous voxels above threshold (32 mm3). However, we utilized a conservative correction to localize the area of peak activation reflecting response of this region as a whole to abstinence. The percent signal change extracted from the larger mask encompassing this region (Z>2.3; volume=19,672 mm3) significantly predicted short-term smoking relapse after correcting for individual differences such as age, sex, and abstinence-induced withdrawal. Future studies should be powered to test the moderating influence of these variables on neural cue reactivity. Additionally, the observed effect in the whole brain abstinence>smoking satiety contrast is consistent with previous findings that ACC activation during smoking cue reactivity is sensitive to early abstinence and relapse (Janes et al., 2010; McClernon et al., 2009; Owens et al., 2017).
The results of this study add to our understanding of the neurobiological effects of early abstinence that may contribute to smoking cessation outcomes (Bough et al., 2013; Loughead et al., 2015). Altered neural activity during early abstinence could provide an early signal of treatment efficacy for medication development, or a directed mechanistic target for novel interventions. In addition, imaging measures can clarify the pathways linking pre-treatment factors (such as cue reactivity) with clinical outcomes (such as treatment response). Measures of brain function may correlate with behavioral phenotypes that contribute to treatment response, or provide insight into the relative contributions of the multiple pathways that may underlie the effects of a treatment. An improved understanding of the mechanisms contributing to relapse could guide research to refine existing treatments; for example, to optimize treatment for certain subpopulations of patients or optimize dosing for individuals.
Acknowledgements
This research was supported by the National Institutes of Health (R35 CA197461 and R01 DA041402 to Dr. Caryn Lerman) and the Pharmacology Graduate Group T-32 Training Grant (T32GM008076 to Dr. Julie Blendy). The funding source had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the article for publication.
References
- Ashare RL, Wileyto EP, Perkins KA, Schnoll RA (2013) The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. J Addict Med 7:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Lerman C, Cao W, Falcone M, Bernardo L, Ruparel K, Hopson R, Gur R, Pruessner JC, Loughead J (2016) Nicotine withdrawal alters neural responses to psychosocial stress. Pyschopharmacology (Berl) 233:2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Partos TR, Yong HH, Cummings KM, Hyland A (2012) How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction 107:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Lerman C, Rose JE, McClernon FJ, Kenny PJ, Tyndale RF, David SP, Stein EA, Uhl GR, Conti DV, Green C, Amur S (2013) Biomarkers for smoking cessation. Clin Pharmacol Ther 93:526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR Jr., Madsen D, Jarvik ME (2002) Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59:1162–1172. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW (1998) Reliability and validity of a smoking timeline followback interview. Pyschology of Addictive Behaviors 12:101–112. [Google Scholar]
- Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE (2013) Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology 38:2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Parzynski CS, Salkeld RP, Perkins KA, Fonte CA (2012) Cue reactivity as a predictor of successful abstinence initiation among adult smokers. Exp Clin Psychopharmacol 20:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA (2016) Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol 21:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3:7–16. [DOI] [PubMed] [Google Scholar]
- Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL (2011) Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry 68:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM (2012) Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage 60:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K (2012) Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res 14:75–78. [DOI] [PubMed] [Google Scholar]
- Falcone M, Cao W, Bernardo L, Tyndale RF, Loughead J, Lerman C (2016) Brain Responses to Smoking Cues Differ Based on Nicotine Metabolism Rate. Biol Psychiatry 80:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S (2009) The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat 36:235–243. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA (2007) Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 27:3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43:289–294. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S (2004) Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 99:29–38. [DOI] [PubMed] [Google Scholar]
- Janes AC, Betts J, Jensen JE, Lukas SE (2016) Dorsal anterior cingulate glutamate is associated with engagement of the default mode network during exposure to smoking cues. Drug Alcohol Depend 167:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, Frederick Bde B, Lukas SE (2015) Insula-Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology 40:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Gilman JM, Radoman M, Pachas G, Fava M, Evins AE (2017) Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings. Drug Alcohol Depend 179:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ (2010) Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2011) Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 33:1318–1326. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA (2014) Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW Jr., Cinciripini P, George TP, Wileyto EP, Swan GE, Benowitz NL, Heitjan DF, Tyndale RF, Group P-PR (2015) Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med 3:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, Lematty T, Brady KT, George MS (2013) Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol 18:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C (2015) Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology 40:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C (2009) Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry 14:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A (2006) Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31:2728–2738. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE (2005) Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology 30:1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE (2009) 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 204:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, MacKillop J, Gray JC, Beach SRH, Stein MD, Niaura RS, Sweet LH (2017) Neural correlates of tobacco cue reactivity predict duration to lapse and continuous abstinence in smoking cessation treatment. Addict Biol [DOI] [PMC free article] [PubMed]
- Perkins KA, Karelitz JL, Jao NC (2013) Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res 15:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, Mentch FD, Sleiman P, Verma R, Davatzikos C, Hakonarson H, Gur RC, Gur RE (2014) Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage 86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S (2013) Smoker reactivity to cues: effects on craving and on smoking behavior. J Abnorm Psychol 122:264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1998) Co-planar stereotaxic atlas of the human brain Thieme, New York. [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-Hage MA, Brown VL, Wetter DW, Cinciripini PM (2014) Prequit fMRI Responses to Pleasant Cues and Cigarette-Related Cues Predict Smoking Cessation Outcome. Nicotine Tob Res 16:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Calhoun VD, Rachakonda S, Claus ED, Littlewood RA, Mickey J, Arenella PB, Hutchison KE (2017) Functional network connectivity predicts treatment outcome during treatment of nicotine use disorder. Psychiatry Res Neuroimaging 265:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Claus ED, Calhoun VD, Rachakonda S, Littlewood RA, Mickey J, Arenella PB, Goodreau N, Hutchison KE (2018) Default mode network deactivation to smoking cue relative to food cue predicts treatment outcome in nicotine use disorder. Addict Biol 23:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA (2015) Neuroimaging craving: urge intensity matters. Addiction 110:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA (2005) Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res 7:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA (2004) Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci 7:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001) Ch 14, in Functional MRI: An Introduction to Methods In: Statistical analysis of activation images Jezzard P, Matthews PM, Smith SM (eds). OUP. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992) A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12:900–918. [DOI] [PubMed] [Google Scholar]
- Zachary R (1986) Shipley Institute of Living Scale: Revised Manual Los Angeles: Western Pyschological Services. [Google Scholar]


