Abstract
The objective of this study was to examine whether patient income has an impact on likelihood of being prescribed an antimicrobial agent at the county level. A better understanding of factors that influence antimicrobial prescription is needed to efficiently mitigate rates of antimicrobial agents prescribed.
This cross-sectional study used data from two publicly available datasets. The 2015 Medicare Part D PUF data quantifies the antimicrobial prescription rate at the county level and data from US Census Bureau provides information on socioeconomic status at the county level.
At the county level, we explained 48% of the variation in antimicrobial prescriptions by socioeconomic status, age, gender, and race. More specifically, socioeconomic status accounted for 26% of the variation in antimicrobial rate and as income increased, correlation with antimicrobial prescription rate trended down.
We determined patient income and other sociodemographics to influence the prescription of antimicrobial agents. Interventions should consider these factors to effectively evaluate antimicrobial prescription methods. Findings from this study can help guide intervention efforts which aim to minimize the number of inappropriate antimicrobials prescribed, such as antimicrobial stewardship programs. Effective interventions have the capability of decreasing levels of inappropriate antimicrobials prescribed and prevent future cases of resistance.
Keywords: anti-bacterial agents, drug resistance, medicare part D, prescriptions, socioeconomic factors, US
1. Introduction
The World Health Organization (WHO) has recognized antimicrobial resistance as a leading threat to public health[1] and links adverse health outcomes related to antimicrobial resistant bacteria and antimicrobial agent consumption.[2] The Centers for Disease Control and Prevention (CDC) reported that over 50% of antimicrobial agents prescribed in the United States are not correctly prescribed.[3] Also, several studies have confirmed higher rates of resistance in countries that have higher antimicrobial use.[4–7] The findings from these studies support the conclusion that the misuse and overuse of antimicrobial agents is a leading cause in the emergence of antimicrobial resistant bacteria.[8,9]
We need a better understanding of factors that influence antimicrobial prescription to inform approaches targeting antimicrobial stewardship. Sociodemographic factors influence the care demand, access, delivery, and overall health outcomes,[10] placing low-income populations at considerably greater risk of healthcare inequities when compared to higher income individuals.[11] While research has shown an association between income inequality and antimicrobial resistance,[12–14] little research exists which examines the role income has on antimicrobial agent prescription rate.
The prescription of antimicrobials is influenced by several factors, including: background of country, clinical autonomy, patient's cultural beliefs and demands, the background of the prescriber, and general sociocultural factors.[15] However, the role patient income has on being prescribed an antimicrobial has been rarely studied. This study applied a syndemic framework[16] to hypothesize that the prescription rates of antimicrobials is correlated with socioeconomic factors.[12–14] An examination of this relationship is vital to suggest effective public health intervention. The purpose of this study, therefore, was to examine the relationship between antimicrobial agent prescription rates and income.
2. Methods
This cross-sectional study used data from two publicly available datasets. Datasets included the Medicare Provider Utilization and Payment Data: Part D[17] and the American Community Survey (ACS) from US Census Bureau.[18] The study utilized aggregate prescription data and did not require IRB approval.
2.1. Rates of antimicrobial agent claims at the county level
The primary outcome variable of interest in this study was the percentage of outpatient antimicrobial agent claims prescribed per 10,000 people in a county. This estimate was done using the 2015 Medicare Part D Prescriber Public User File (PUF). Medicare Part D provides outpatient prescription drug coverage for disabled and elderly people.[19] Medicare Part D Prescriber PUF contains information on prescription drug counts from 36 million Medicare beneficiaries with a part D prescription drug plan including people who are dual eligible.[17] We isolated information related to antimicrobial agents and defined the outcome variable as the total claims of antimicrobials, including refills, from providers listed in the PUF. These data allowed us to obtain the number of antimicrobial claims given by prescribers. We then summarized claims by the provider's zip code. Finally, we matched each zip code with its corresponding county to summarize claims per county. To estimate the antimicrobial prescription rate, we summarized antimicrobial prescription drug claims per county, divided by county population, and multiplied by 10,000.
2.2. Demographic variables
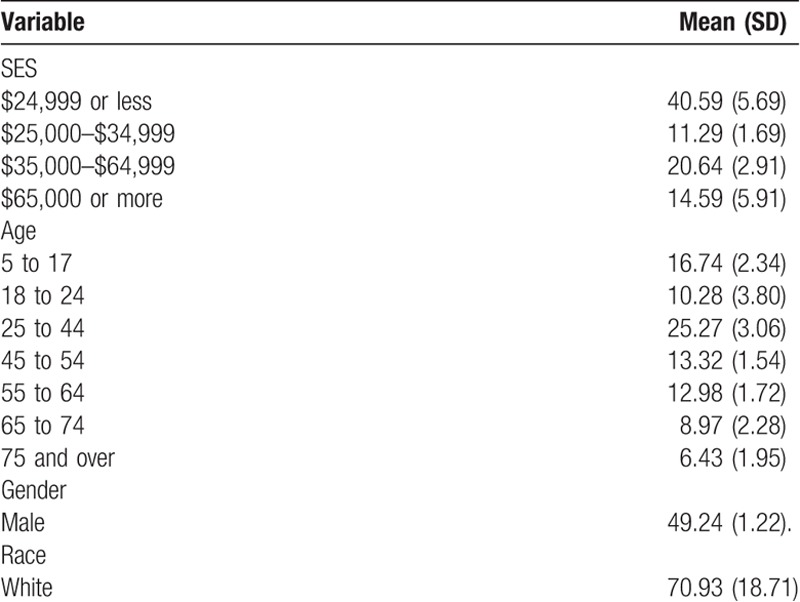
The primary exposure variable of interest was patient income. We assessed this using estimated income levels reported for counties within the 2012–2016 American Community Survey (ACS) 5-year estimate dataset.[18] Income was obtained for each county from the 2012–2016 ACS 5-year estimates, a component of the publicly available data provided by US Census Bureau.[18] Income was examined at the county level and assigned specific median income ranges.[20] Annual household income was grouped into the following brackets: $24,999 or less, $25,000–$34,999, $35,000–$64,999, and $65,000 or more.
We further examined the association other sociodemographic characteristics have with antimicrobial prescription rate. The ACS was used to obtain additional sociodemographic information.[18] The following explanatory variables were included in our analysis: first, age, second, percentage of males, and third, percentage of white population.
The number of antimicrobial agents prescribed has been shown to vary according to age group.[21] Age was grouped into seven brackets (age: 5–17, 18–24, 25–44, 45–54, 55–64, 65–74, 75, and so on) to examine antimicrobial rates for several age ranges. Previous reports suggest that women are more likely to be prescribed antimicrobials than men.[21] Therefore, men were examined with women used as the reference group. Little literature exists that examines the role race has on prescription rates. However, recent studies have shown that white patients are more likely to be prescribed antimicrobials.[22,23] Thus, for the purposes of this study, white individuals were used as the reference group.
The analysis of this study is limited to counties in the United States who had available data containing information on levels of income and the number of antimicrobial agents prescribed. Of the counties (n = 3,141) in the 50 states and District of Columbia, 807 counties included data on number of antimicrobial prescriptions and sociodemographics of interest and were therefore included in our eligible sample.
2.3. Analysis
The county population was used as a weight for study analysis to account for oversampling. Descriptive statistics and bivariate correlations were utilized for all study variables. This study utilized linear regression analyses to strongly assess the association between our variables and to identify potential confounders. We standardized beta to determine appropriate coefficient values. Linear regression analyses examined relationships between patient income and rate of antimicrobial agents prescribed. A univariable regression was used to determine the unadjusted relationship between income and antimicrobial prescription rates. Multivariable linear regressions were completed to examine a cross-tabulation of the outcome, age, gender, and race by the exposure (Table 1). For analyses, statistical normality (Shapiro–Wilk) tests were performed to verify the statistical test assumptions. A P value of < .05 was used to indicate statistical significance and statistical tests were two-sided. All statistical analyses were performed using Stata 14.0[24] and accounted for complex survey design. We also conducted a hot spot analysis to obtain a visual relationship of counties and antimicrobial prescription rate on a national level. Values in the analysis represent z-scores, with positive z-scores indicating hot spots and negative z-scores indicating cold spots.
Table 1.
Summary of variables used in study analysis.
3. Results
We analyzed 54,382,436 prescriptions reported by health providers in the 2015 Medicare Part D dataset, that were prescribed in 807 counties to nearly 36 million beneficiaries. After stratifying for income and relevant variables, we determined the overall average annual antimicrobial prescription rate to be 1.30 claims per beneficiary that received a prescription for an antimicrobial agent.
A univariable linear regression was first performed to examine the relationship between the main exposure and outcome of interest. Income was broken down into four ascending brackets of monetary amounts and served as our proxy for socioeconomic status. All income brackets showed statistical significance with a prediction, P < .05 (Table 2). In a county level, 26% of the variation in antimicrobial agent rate was associated with income (Pmodel < .001, R2 = 0.26).
Table 2.
Univariate linear regression analysis of total antibiotic prescription rate with income at the county level.
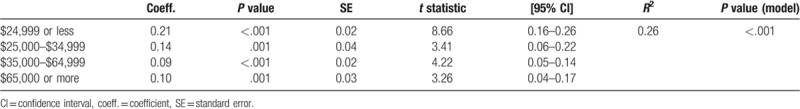
Interestingly, the coefficient for each income bracket tended to lower given increase in income. An overall trend was noted in the brackets revealing that as income increased, the correlation with rate of antimicrobial prescription decreased (Fig. 1). Our lowest income bracket ($24,999 or less) yielded a 0.21 increase in the total antimicrobial prescription rate per 1 SD increase in percentage of individuals included in this income in a given county. At the county level, the coefficient nearly halved to 0.11 for an increase in percentage of individuals in our highest income bracket ($65,000 or more). Meaning that a 1 SD increase in percentage of individuals in a given county whom reported an income of $65,000 or more yielded only a 0.11 increase in total antimicrobial prescription rate.
Figure 1.
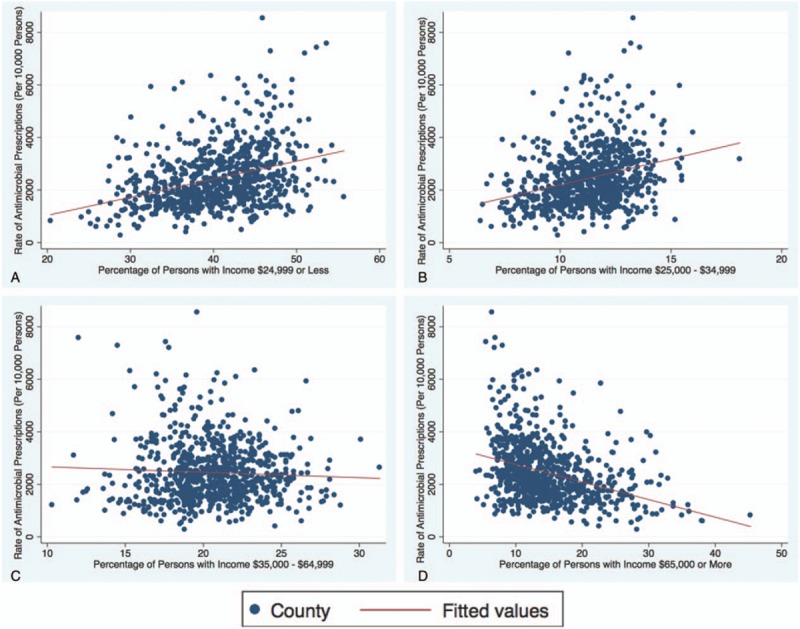
Scatter plot(s) of bivariate data visualizing relationship between antimicrobial agent prescription rate and socioeconomic status.
Additional univariable linear regressions were performed to identify other sociodemographics that were believed to be significantly associated with antimicrobial prescription rates. The number of antimicrobials prescribed were seen to be positively associated with age and race (% of white population). For our analysis, age was ordered into seven ascending range brackets. Conversely, a negative association was found related to the number of antimicrobial prescribed and gender (% of male population). Multivariable linear regressions at the county level were then performed with the significant predictors to evaluate for potential associations between antimicrobial prescription rate and income,
At the county level, 37% of the variation in antimicrobial prescription rate was explained income and age (Pmodel < .001, R2 = 0.37). Multivariable analysis revealed the seven age brackets to add statistical significance to the prediction, P < .05. However, only two of the income brackets ($24,999 or less and $35,000–$64,999) continued to add significance (Table 3). Similar to the univariable regression done examining income, the coefficients of the income brackets decreased as the income range increased. Given age constant, a 1 SD increase in percentage of persons in a given county whom reported an income of $24,999 or less yielded a 0.12 increase in total antimicrobial prescription rate.
Table 3.
Multivariate linear regression analysis of total antibiotic prescription rate with sociodemographics at the county level.
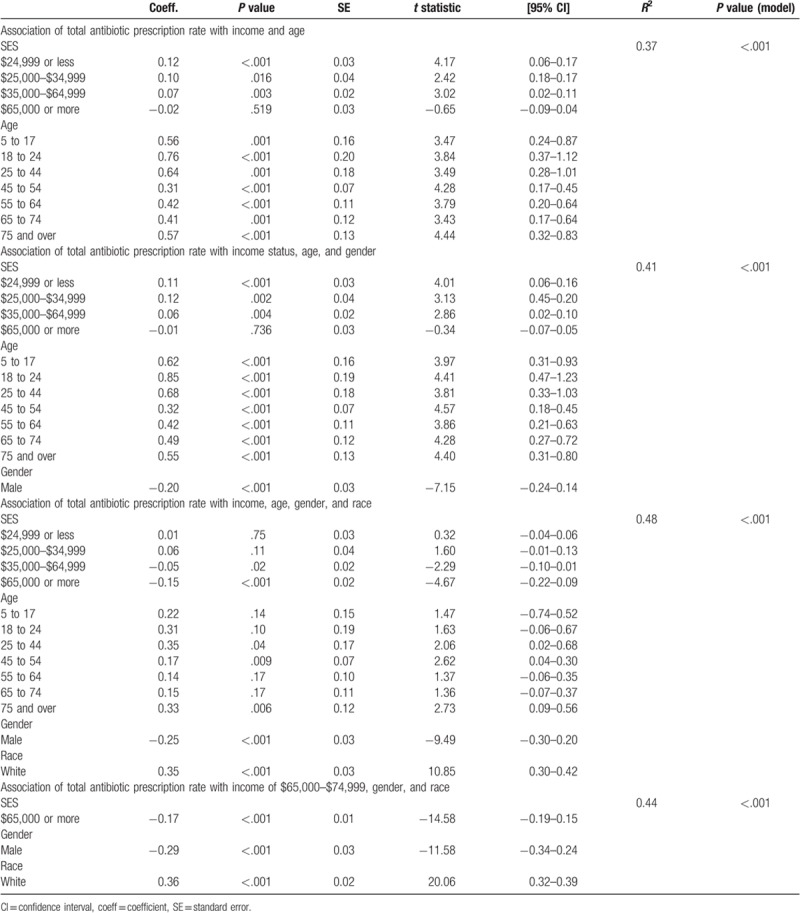
As in the univariate analysis, age positively associated with antimicrobial prescription rate. When examining age and income in multivariable analysis, results indicated the strongest positive association to be for those aged 18 to 24. We found that a 1 SD increase of individuals in this age range in a county yielded a 0.76 increase in total antimicrobial prescription rate. The coefficient of this result directly precedes the regressions finding for those aged 25 to 44. Where, as percentage of individuals aged 25 to 44 increased by 1 SD in a given county, an increase of 0.64 in total antimicrobial prescription rate was observed.
We then examined antimicrobial prescription rates given income, age, and gender (% of male population). The gender variable added statistical significance to the prediction, P < .05 while all of the age variables and three of the income variables ($24,999 or less, $25,000–$34,999, and $35,000–$64,999) continued to provide statistical significance (Table 3). At the county level, 43% of the variation in antimicrobial rate is explained by income, age, and gender (Pmodel < .001, R2 = 0.43). The most significant association was again seen to be for those in our lowest income bracket. More specifically, with gender and age constant, a 1 SD increase in percentage of individuals in a county with an income of $24,999 or less yielded a 0.11 increase in total antimicrobial prescription rate. As seen in the univariate analysis, gender was negatively associated with rate of antimicrobial prescription. When included in multivariable analysis, results found an increase of 1 SD in % of males in a county to yield a 0.2 decrease in antimicrobial prescription rate.
Moreover, we performed a multivariable linear regression that examined antimicrobial prescription rates given income, age, gender, and race (% of white population). At the county level, 48% of the variation in antimicrobial rate can be explained by these sociodemographics (Pmodel < .001, R2 = 0.48). Race was seen to reduce the heterogeneity of prediction, P < .05. However, the only additional variables that further helped discriminate findings were gender and a reported income of $65,000 or more (Table 3). Notably, white individuals were found to have a 0.35 increase in antimicrobial prescription rate per 1 SD increase in percentage of white populace in a county.
By our third regression, the remaining statistically significant income brackets lost their positive relationship with our main exposure of interest. However, with the addition of race in multivariable analysis, results determined a negative association concerning antimicrobial prescription rate and our highest income bracket. At the county level, this analysis determined that with gender, race, and age held constant, a 1 SD increase in percentage of individuals with an income of $65,000 or more in a county yielded a decrease of −0.15 in total antimicrobial prescription rate.
While the lowest income bracket lost its significance in this regression, the reemergence of significance for the highest income bracket warrants further examination. An income of $65,000 or more was the bracket which lost significance in our previous multivariable analyses. Unlike other income brackets ($25,000–$34,999 & $35,000–$64,999), the highest income bracket did not regain significance in the multivariable regressions concerning age and gender. However, with the addition of race in analysis, the ≥$65,000 bracket was the only income range to have a significant association. Further, the association was found to have a negative relationship with antimicrobial prescription rate. This differed from the positive relationship that was yielded in the initial univariate linear regression on income.
Finally, we examined the remaining significant variables (Table 3). At the county level, 44% of the variation in antimicrobial prescription rate was explained by gender, race, and an income of $65,000 or more (Pmodel < .001, R2 = 0.44). Race was still seen to be positively associated with antimicrobial prescription rate whereas gender and an income of $65,000 or more continued to be negatively associated. This analysis indicates that, given race and gender constant, a 1 SD increase in percentage of individuals with an income of $65,000 or more in a given county yielded a −0.17 decrease in rate of antimicrobials prescribed.
The findings from the hot spot analysis were visually apparent (Fig. 2). From a national perspective, we see that the Southwest and Rocky Mountain region of the United States were mostly determined to have a nonsignificant relationship between rates of antimicrobial prescriptions and county cluster. However, we found a cluster of counties with low (cold spots) antimicrobial prescription rates in the Northeast and the Pacific Coast that were highly significant. Conversely, we determined a highly significant cluster of counties with high (hot spots) antimicrobial prescription rates primarily in the Southeast. The hot spot analysis suggests that the Southeast region of the United States is most likely to have a large cluster of counties with high rates of antimicrobial prescriptions.
Figure 2.
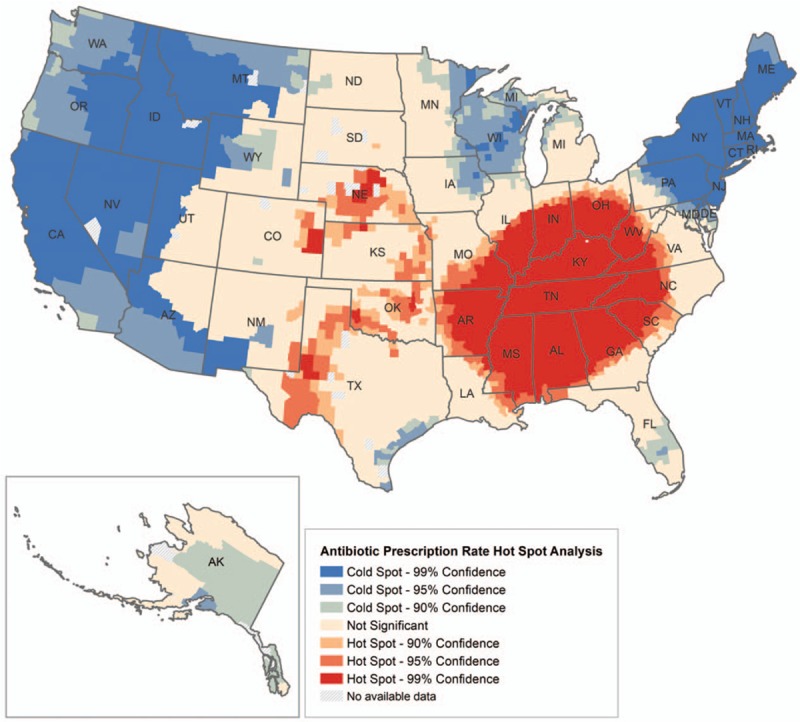
Antimicrobial prescription rates across the United States, 2015.
4. Discussion
Our study combined data from the Medicare Part D PUF and US Census Bureau to further examine a potential relationship. Results found patient income to account for a considerable amount of the variation in antimicrobial prescription rate at the county level. For this study, we examined income in four ascending ranges of monetary values. The coefficients in income brackets generally decreased given higher income bracket. Given previous research showing an association between income inequality and antimicrobial resistance globally,[12–14] we predicted a higher antimicrobial prescription rate for individuals of lower income. Our findings also revealed rates of antimicrobial prescriptions in a given county correlate with age and race (% white) of patients but negatively correlate with gender (% male) of patients. Additionally, analysis showed with these age, race, and gender held constant, that as the percentage of individuals in a county with an income of at least $65,000 increases, the rates of antimicrobial prescriptions decreases.
This analysis demonstrates the importance patient income has on rates of antimicrobial prescriptions. Prior to this study, the association between income and antimicrobial prescription rate in the United States had not been examined in such a large level. The main finding from this study provides new insight as to sociodemographic relationship related to antimicrobial agent prescribing. A better understanding as to how antimicrobials are prescribed can help focus policies that moderate antimicrobial prescription rates effectively.
We may partially explain this finding by using a syndemic model of health. Typically, a person's likelihood of disease decreases as their income increases.[25] Health is consistently worse for those of lower income with research showing that people with a higher SES report fewer adverse health outcomes than those of lower SES.[26] The syndemic framework hypothesizes that health consists of interactions involving the environment, social factors, and negative effects of disease interaction.[16] Using a syndemic approach, individuals of lower income may be at higher need of antimicrobials compared to individuals of higher income because they are more likely to seek medical treatment in which antimicrobial agents are typically prescribed.
The multivariable linear regressions performed using age differed from consistent findings in previous literature. Typically, the elderly have a higher likelihood of being prescribed antimicrobials.[21] However, this study found that counties with a higher percentage of individuals aged 18 to 24 had the highest increase in total antimicrobial prescription rate compared to those of all other age groups in a county. Further, the second highest age groups were those aged 25 to 44. This study finding may relate to research showing that the highest percentage of unnecessary antimicrobial prescriptions are for individuals in these age ranges.[27]
This study provided interesting findings related to race. While previous research has found individuals from disadvantaged areas, identify as a minority, or are older, are groups that are more often prescribed antimicrobial agents,[28] more recent research has found that white individuals are more likely to be prescribed such agents.[22,23] Our study determined that an increase in percentage of white individuals in a county was associated with an increased risk of being prescribed antimicrobial agents when compared to individuals of other racial backgrounds. One possible explanation for the observation of greater antibiotic use among whites could be because of greater access to healthcare due to insurance status. In US, whites are more likely than any other racial or ethnic group to have health insurance.[29] These disparities extend to the Medicare dataset used as white individuals comprise 76% of Medicare beneficiaries.[30] Further, a recent study found that racial minorities who have Medicare are less likely to utilize health services.[31]
Gender has been associated with differences in outpatient drug prescriptions.[32] Our findings determined that an increase in percentage of men in a county resulted in a lower likelihood of being prescribed an antimicrobial agent. This finding matches well with a recent meta-analysis that determined women received a significantly higher number of prescriptions of outpatient antimicrobial agents compared to men.[32] This disparity in prescribing is predicted to be primarily due to behavioral and social differences. Research has shown that men have a higher reluctance to attend health appointments and engage in health behaviors.[33] A gap in seeking treatment makes men less likely to receive an antimicrobial prescription. Additionally, women are at increased risk of certain infectious conditions such as a urinary tract infection.[32]
Our hot spot analysis provides a national visual of the substantial geographic variation in antimicrobial prescription rates. The Southeast region had a large cluster of counties with a high rate of antimicrobial prescriptions. This is relevant to our main study finding as the highest incidence of poverty in the United States is concentrated in this region.[34] Moreover, the Northeast and the Pacific Coast were likely to have high cluster of counties with lower antimicrobial prescription rates. The Northeast result is of particular importance as this is the region that has the greatest number of high-SES counties in the United States.[35]
Limitations exist with the datasets used for the purposes of this study. Our cross-sectional study design is limited due to the inability to establish a temporal relationship between the exposure and outcome and difficulty in controlling additional confounding. However, our study inferred that the sociodemographics examined in this study will persist overall beyond the study's conclusion. A specific limitation for this study involved the being unable to account for individuals who receive health treatment in counties other than the county they reside in. Further, the data used in this study relied on self-reported measures, including information on income. A common problem in survey research is the overestimation of income.[36] Therefore, the number of income brackets may be overestimated with the data on people in the lowest income bracket being most distorted. While the final models included three variables for analysis, there is the possibility of other variables contributing to rates of antimicrobial agents prescribed. Also, the findings from this study lack the ability to be generalizable given data from individual cases was not available for analysis. Instead, the study relied on data collected at the county level. Additionally, the data are limited to the years 2012–2016 for the sociodemographic data and 2014–2015 for the Medicare data and may not accurately represent the general populace. However, the Medicare data used on antimicrobial agent claims includes information on Part D beneficiaries, who comprise nearly two-thirds of those who received Medicare and comprise 15% of the total US population.[37] Additionally, this study was one of the first to utilize national data to examine antimicrobial rates at the county-level. Moreover, the data examined in this study were collected by trained interviewers and is likely to be reliable.
5. Conclusion
Examining the way that antimicrobial agents are prescribed is a prudent step in addressing the crisis of antibiotic resistance. Antimicrobial prescriptions are increasing annually in the United States[3] and the findings from this study are thus likely to intensify. Future research should pay particular attention to the long-term outcomes of individuals from the sociodemographics at increased risk found in this study. This research can help determine whether these individuals are in fact at greater risk for the negative outcomes associated with antimicrobial resistant bacteria. Our findings suggest that specific sociodemographics are more likely to receive antimicrobial prescriptions. Stakeholders should consider our study results when attempting to implement antimicrobial stewardship programs to effectively minimize inappropriate prescription rates. Moreover, our findings suggest that specific geographic areas may benefit more from implementation of such stewardship programs. This study determined an existing inequity that may ultimately lead to poorer health outcomes for individuals of particular sociodemographics. Therefore, it is prudent that the findings from this study are further examined to suggest appropriate health policy.
This study was one of the first to show an association between patient income and antimicrobial prescription rate and found a county-level association between lower income and rate of antimicrobial prescriptions. We also determined the Southeast to be the region at highest risk of having a high rate of antimicrobial prescriptions. Findings from this study can help guide intervention efforts which aim to minimize the number of inappropriate antimicrobials prescribed, such as antimicrobial stewardship programs. Effective interventions have the capability of decreasing levels of inappropriate antimicrobials prescribed and prevent future cases of resistance.
Author contributions
Conceptualization: Connor Volpi, Eleftherios Mylonakis.
Data curation: Connor Volpi, Fadi Shehadeh.
Formal analysis: Connor Volpi, Fadi Shehadeh.
Investigation: Connor Volpi.
Visualization: Fadi Shehadeh.
Writing – original draft: Connor Volpi.
Writing – review & editing: Connor Volpi, Fadi Shehadeh, Eleftherios Mylonakis.
Footnotes
Abbreviations: ACS = American Community Survey, CDC = The Centers for Disease Control and Prevention, PUF = Medicare Part D Prescriber Public User File, WHO = World Health Organization.
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization. 2018 Antibiotic Resistance. Fact Sheet. Available at: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/ Accessed November 19, 2018. [Google Scholar]
- [2].Kåhrström C. Entering a post-antibiotic era? Nat Rev Microbiol 2013;11:146. [Google Scholar]
- [3].Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance. Division of Healthcare Quality Promotion. Available at: https://www.cdc.gov/drugresistance/about.html Accessed November 19, 2018. [Google Scholar]
- [4].Austin D, Kristinsson K, Anderson R. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A 1999;96:1152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goossens H, Ferech M, Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579–87. [DOI] [PubMed] [Google Scholar]
- [6].Hicks L, Chien Y, Taylor T, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996−2003. Clin Infect Dis 2011;53:631–9. [DOI] [PubMed] [Google Scholar]
- [7].Riedel S, Beekmann S, Heilmann K, et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur J Clin Microbiol Infect Dis 2007;26:485. [DOI] [PubMed] [Google Scholar]
- [8].Lee G, Reveles K, Attridge R, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 2014;12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ventola C. The antibiotic resistance crisis: part 1: causes and threats. P T 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- [10].National Academies of Sciences Engineering and Medicine. Accounting for Social Risk Factors in Medicare Payment. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- [11].Berenson J, Doty M, Abrams M, et al. Achieving better quality of care for low-income populations: the roles of health insurance and the medical home in reducing health inequities. Issue Brief (Commonw Fund) 2012;11:1–8. [PubMed] [Google Scholar]
- [12].Alvarez-Uria G, Gandra S, Laxminarayan R. Poverty and prevalence of antimicrobial resistance in invasive isolates. Int J Infect Dis 2016;52:59–61. [DOI] [PubMed] [Google Scholar]
- [13].Kirby A, Herbert A. Correlations between income inequality and antimicrobial resistance. PLoS One 2013;8:e73115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nomamiukor B, Horner C, Kirby A, et al. Living conditions are associated with increased antibiotic resistance in community isolates of Escherichia coli. J Antimicrob Chemother 2015;70:3154–8. [DOI] [PubMed] [Google Scholar]
- [15].Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014;5:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singer M, Bulled N, Ostrach B, et al. Syndemics and the biosocial conception of health. Lancet 2017;389:941–50. [DOI] [PubMed] [Google Scholar]
- [17].Centers for Medicare & Medicaid Services. Part D Prescriber Data CY 2015. Medicare Provider Utilization and Payment Data. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/PartD2015.html Accessed November 19, 2018. [Google Scholar]
- [18].United States Census Bureau. General Economic Characteristics. 2012–2016 American Community Survey 5-Year Estimates. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml Accessed November 19, 2018. [Google Scholar]
- [19].Jung J, Feldman R, Cheong C, et al. Coverage for hepatitis C drugs in Medicare Part D. Am J Manag Care 2016;22: (6 Spec No.):SP220-6. [PMC free article] [PubMed] [Google Scholar]
- [20].Berkowitz S, Traore C, Singer D, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res 2015;50:398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions-United States, 2011. Apporpriate Antibiotic Use: Community. Available at: https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2011.html Accessed November 19, 2018. [Google Scholar]
- [22].Gerber J, Prasad P, Localio A, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics 2013;131:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goyal M, Johnson T, Chamberlain J, et al. Racial and ethnic differences in antibiotic use for viral illness in emergency departments. Pediatrics 2017. e20170203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- [25].Schiller J, Lucas J, Ward B, et al. Summary health statistics for US adults: national health interview survey, 2010. Vital Health Stat 2012;10:1–207. [PubMed] [Google Scholar]
- [26].Adler N, Rehkopf D. US disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health 2008;29:235–52. [DOI] [PubMed] [Google Scholar]
- [27].Centers for Disease Control and Prevention. Antibiotic Use in the United States, 2017: Progress and Opportunities. Antibiotic Use. Available at: https://www.cdc.gov/antibiotic-use/stewardship-report/index.html Accessed November 19, 2018. [Google Scholar]
- [28].Ackerman S, Gonzales R. The context of antibiotic overuse. Ann Intern Med 2012;157:211–2. [DOI] [PubMed] [Google Scholar]
- [29].Artiga S, Stephens J, Damico A. The impact of the coverage gap in states not expanding Medicaid by race and ethnicity. San Francisco, CA: Henry J Kaiser Family Foundation; 2015. [Google Scholar]
- [30].Kaiser Family Foundation. Profile of Medicare Beneficiaries by Race and Ethnicity: A Chartpack. Medicare. Available at: https://www.kff.org/report-section/profile-of-medicare-beneficiaries-by-race-and-ethnicity-tables/ Accessed November 19, 2018. [Google Scholar]
- [31].Gandhi K, Lim E, Davis J, et al. Racial disparities in health service utilization among medicare fee-for-service beneficiaries adjusting for multiple chronic conditions. J Aging Health 2018;30:1224–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schröder W, Sommer H, Gladstone B, et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J Antimicrob Chemother 2016;71:1800–6. [DOI] [PubMed] [Google Scholar]
- [33].Rosu M, Oliffe J, Kelly M. Nurse practitioners and men's primary health care. Am J Mens Health 2017;11:1501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Farrigan T. Rural Poverty & Well-being. Rural Economy & Population. Available at: https://www.ers.usda.gov/topics/rural-economy-population/rural-poverty-well-being/ Accessed November 19, 2018. [Google Scholar]
- [35].US Census Bureau. Median Household Income by County for 2010–2014. Census Infographics & Visualizations. Available at: https://www.census.gov/library/visualizations/2015/acs/2010-2014-acs-hh-income.html Accessed November 19, 2018. [Google Scholar]
- [36].Shengelia B, Murray C, Adams O. Health Systems Performance Assessment: Debates, Methods and Empiricism. Geneva: WHO; 2003. [Google Scholar]
- [37].Hoadley J, Cubanski J, Neuman T. Medicare Part D in 2016 and Trends Over Time. Medicare. Available at: http://www.kff.org/medicare/report/medicare-part-d-in-2016-and-trends-over-time Accessed November 19, 2018. [Google Scholar]


