Abstract
Lead (Pb) is an environmental neurotoxicant, and has been implicated in several neurological disorders of dopaminergic dysfunction; however, the molecular mechanism of its toxicity has yet to be fully understood. This study investigated the effect of Pb exposure on dopaminergic neurodegeneration and function, as well as expression level of several dopaminergic signaling genes in wild type (N2) and protein kinase C (pkc) mutant Caenorhabditis elegans. Both N2 and pkc mutant worms were exposed to Pb2+ for 1 h. Thereafter, dopaminergic (DAergic) neurodegeneration, behavior and gene expression levels were assessed. The results revealed that Pb2+ treatment affects dopaminergic cell morphology and structure in worms expressing green fluorescent protein (GFP) under a DAergic cell specific promoter. Also, there was a significant impairment in dopaminergic neuronal function as tested by basal slowing response (BSR) in wild-type, N2 worms, but no effect was observed in pkc mutant worms. Furthermore, Pb2+ exposure increased dat-1 gene expression level when compared with N2 worms, but no alteration was observed in the pkc mutant strains. LC–MS analysis revealed a significant decrease in dopamine content in worms treated with Pb2+ when compared with controls. In summary, our results revealed that Pb2+ exposure induced dopaminergic dysfunction in C. elegans by altering dat-1 gene levels, but pkc mutants showed significant resistance to Pb2+ toxicity. We conclude that PKC activation is directly involved in the neurotoxicity of Pb.
Keywords: Pb, Neurotoxicant, PKC, Dopaminergic neuron, dat-1
1. Introduction
In humans, dopaminergic (DAergic) neurotransmission is linked with several behaviour such as locomotion, motivation and recognition activity [[1], [2], [3]]. Studies have shown that an imbalance or dysfunction in dopamine (DA) signaling will result in neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer disease (AD) [2,4]. In humans, the DAergic signaling system is similar to C. elegans [5]. For example, DA is synthesized from L-tyrosine by tyrosine hydroxylase (CAT-2, C. elegans), rate-limiting step in DA synthesis, to L-3,4-dihydroxyphenylalanine (L-DOPA) which is then converted to DA by DOPA decarboxylase. After synthesis, DA is stored in presynaptic storage vesicles by the vesicular monoamine transporter (CAT-1, C. elegans), where it remains until being released into the presynaptic cleft in response to an action potential. Once in the synapse, DA binds to D1-like (DOP-1) and D-2 like receptors (DOP-2 and DOP-3) that are positioned at the post synaptic neuron. Unbound DA is taken back up into the presynaptic cell via re-uptake mechanism by the dopamine transporter (DAT-1) [5,6].
DAT-1, an integral membrane protein, is located on the presynaptic neurons and it regulates DA levels during dopaminergic neurotransmission by the re-uptake of DA back to the presynaptic neuron [2,6]. This action is accompanied by co-transport of Na+ and Cl− down electrochemical gradients.
Previous studies have highlighted the role of kinases such as protein kinase C (PKC), calcium-calmodulin dependent kinase II (CAMKII) and extracellular signal-regulated protein kinase (ERK) in DAT functions [7,8]. Multiple DAT functions are regulated by PKC, with activation of the enzyme leading to reduced transport Vmax, elevated efflux Vmax, and enhanced transporter internalization [[7], [8], [9]]. Each of these actions resulted in increased extracellular DA levels, implicating PKC as a positive regulator of DAT function and DA neurotransmission. Recently, study by Hovde et al. [10] have both shown that DAT possesses several phosphorylation sites for protein kinases and that treatment with PKC activators such as phorbol 12-myristate 13-acetate (PMA) alter DAT expression and activity. Notably, previous studies have shown that several heavy metals affect PKC phosphorylation system [[11], [12], [13]], but their effect on DAT function remains poorly understood.
Lead (Pb) is an environmental neurotoxicant and a non-essential metal in the ecosystem. Pb has been reported to be toxic mostly to children because of their developing nervous system and pika behavior [14]. It has been reported that one of the major route of exposure to Pb is via diet [15,16]. The neurotoxic effect of Pb in children has attracted lots of attention and had continue to be a global concern especially in the developing countries; however, the mechanism of action is yet to be fully clarified. Previous work by Loikkanen et al. [17] showed that Pb altered Ca2+-mediated cellular processes and mimics Ca2+ in binding to regulatory proteins. Also, Pb has been reported to affect the release and reuptake of several neurotransmitters controlled by voltage gated Ca2+ channels [18,19], and Zhang et al. [20] demonstrated that Pb causes tau hyperphosphorylation and alpha-synuclein accumulation in the hippocampus, thus, leading to apoptosis and autophagy. Rogers et al. [21] demonstrated that amyloid precursor protein (APP) plays a significant role in Pb toxicity via iron regulatory pathways using human dopaminergic SH-SY5Y neuroblastoma cells. In addition, studies from both animal and human studies have shown that exposure to Pb induces ultrastructural/molecular alterations in the hippocampus and alters postnatal cholinergic and monoaminergic systems [[22], [23], [24], [25], [26]]. Pb has also been shown to activate PKC leading to ROS production [27,28].
Based on the fact that PKC plays a role in DAT function and Pb readily induces oxidative stress via PKC activation resulting in neurotoxicity, we therefore hypothesized that Pb exposure would alter DAT expression via PKC. The present study sought to investigate the effect of Pb exposure on DAergic signaling and DAT gene expression level in C. elegans model, taking advantage of PKC null mutants worm strains.
2. Materials and methods
2.1. Reagents
Reagents such as lead (II) acetate trihydrate [Pb(CH3COO)2], cholesterol, albumin and polymerase chain reaction (PCR) primers were purchased from Sigma (St. Louis, MO, USA). TaqMan primers used for real-time quantitative reverse transcription PCR (qRT-PCR) analysis were obtained from Life Technologies (Carlsbad, CA, USA). Agar, peptone and agarose were purchased from BD (Franklin Lakes, NJ, USA). Trizol and SuperSignal West Pico chemiluminescent substrate were purchased from Thermo Fisher Scientific (Waltham, MA, USA). All other reagents used were of analytical grade.
2.2. C. elegans strains and maintenance
The following worm strains; Bristol N2 (wild-type; WT), BY200 (dat-1:GFP (vtIs1) V), RB781 (pkc-1(ok563) V), VC127 (pkc-2(ok328) X), MT15620 (cat-2(n4547) and VP596 (dvls19[pAF15(gst-4::GFP::NLS)];vsls33[dop-3::RFP]) were used in this study. The strains were obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota, MN, USA.
Caenorhabditis elegans strains were handled and maintained at 20 °C. Gravid worms were maintained in plates containing agar 8 P (3 g/L NaCl, 25 g/L agar; 20 g/L peptone; 1 mM CaCl2, 5 mg/L cholesterol, 1 mM MgSO4, 25 mM KPO4) seeded with NA22 Escherichia coli. During experiments, worms were maintained on plates containing nematode growth medium (NGM: 3 g/L NaCl, 17 g/L agar, 2.5 g/L peptone, 1 mM CaCl2, 5 mg/L cholesterol, 1 mM MgSO4, 25 mM KPO4 with 1.25 mL nystatin and 50 mg/L streptomycin sulfate) and E. coli OP50 strain was used as food source. Synchronization of nematode cultures was achieved by bleaching treatment of gravid hermaphrodites using hypochlorite solution (1% NaOCl, 0.25 M NaOH). Eggs were isolated from cell debris by a 30% sucrose gradient, washed with sterile water and resuspended in M9 buffer (42 mM Na2HPO4, 22 mM KH2PO4, 8.5 mM NaCl and 1 mM MgSO4) and the obtained eggs were allowed to hatch overnight in M9 buffer at 20 °C. After 13 h, synchronized L1 worms were used for the experiment as described below.
2.3. Pb treatment and survival assay
Nematodes were exposed to Pb(CH3COO)2 prepared in K-medium (0.032 M KCl and 0.015 NaCl) as described by Williams and Dusenbery [29]. Briefly, worms were synchronized by alkaline hypochlorite treatment of gravid adults and collected egg populations were placed on unseeded nematode growth media (NGM) plates to grow to the first larval stage (L1). About 2500 synchronized L1 worms were treated with Pb for 1 h. After Pb treatment, worms were placed on nematode growth media (NGM) plates for survival assay,
For the survival assay, synchronized L1 worms [N2 (wild-type; WT), BY200 (dat-1:GFP (vtIs1) V), RB781 (pkc-1(ok563) V) and VC127 (pkc-2(ok328) X)] were treated with Pb at different high concentrations (0–50 mM) in siliconized tubes for 1 h due to the fact that worm has thick cuticle [30]. Tubes containing treated worms were centrifuged at 7000 rpm for 2 min and washed 4 times with K-medium. Then, 30–50 worms were then plated onto OP50-seeded NGM plates in triplicates. After forty-eight (48) hours, the surviving worms were counted and the results were expressed as a percentage of surviving worms relative to day 0. Dose-response survival curves in the various strains were constructed and the median lethal dose (LD50) was calculated. The assay was repeated in six independent worm preparations for each tested strain. Doses close to the LD50 calculated in this study was used for subsequent analysis.
2.4. Dopaminergic neurodegeneration assay
Dopaminergic neurodegeneration was evaluated using BY200 (dat-1:GFP) worms express green fluorescent protein (GFP) in DAergic neurons. Briefly, 2500 synchronized L1 worms were treated with Pb concentrations and a no-Pb medium control as described earlier. After washing, worms were placed onto OP50-seeded NGM plates to recover. At 2 h post treatment, 20 worms per condition were mounted onto 4% agarose pads (in M9 buffer) and anesthetized with 3 mM levamisole. The presence or absence of DAergic degeneration in the worms was evaluated with an epifluorescence microscope (Nikon Eclipse 801, Nikon Corporation, Tokyo, Japan) equipped with a Lambda LS Xenon lamp (Sutter Instrument Company) and Nikon Plan Fluor 20×dry and Nikon Plan Apo 60 × 1.3 oil objectives. Each BY200 worm was scored for absence (0) or presence (1) of any of the following morphological changes representing degeneration; dendritic puncta formation, shrunken soma and loss of dendrites or soma. Confocal images acquired for illustration were captured through Plan-Apochromat 20× objective on a LSM510 confocal microscope (Carl Zeiss MicroImaging, Inc) scanning every 200 nm for XZ sections. Images were taken and processed as previously described. Each experiment was repeated independently four times (n = 4).
2.5. Behavioral test – basal slowing response (BSR)
This behavioral assay assesses the functionality of the worm’s dopaminergic system as described by Sawin et al. [31]. Briefly, 2500 synchronized L1 worms were treated with high doses of Pb (0, 2.5 or 5 mM) for 1 h because worms have thick cuticle and were later transferred to OP50-seeded NGM plates. Twenty (20) hours before the test, assay plates were prepared as follows; seeded assay plates were prepared by adding 20 μL OP50 bacteria onto 60 mm plates and spread in a circle with the bottom of a small glass culture tube to give a ring-shaped bacterial lawn and incubated overnight at 37 °C. The BSR test was performed 48 h after Pb treatment and consists of washing the worms three times with S-basal buffer (100 mM NaCl, 5 mg/L cholesterol, 50 mM KPO4, pH 6.0) to remove residual bacteria. Then five worms were placed on the center of a 60 mm NGM plate with or without bacteria, respectively. After a habituation period of 5 min, the number of bends that each worm performed in the anterior region of their body was counted over a period of 20 s to determine its mobility rate. This was done manually by an experimenter who was blnded to the worms conditions. The results were expressed as the difference (Δ) between the numbers of body bends in the plate with and without bacteria (food). A low Δ value means greater mobility on the food, indicating deficits in dopaminergic function. The cat-2 mutant strain which lacks CAT-2 (homolog for tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of DA) was used as a positive control in this test. This assay was repeated independently five times in each strain.
2.6. RNA isolation and real-time polymerase chain reaction (PCR)
RNA was isolated from 50,000 synchronized L1 worms after Pb exposure. The worms were washed with M9 and lysed with 1 mL Trizol by freezing the worms in liquid nitrogen and thawing repeatedly at 37 °C for three times. Samples were centrifuged (14,000 rpm, 10 min, 4 °C) and 200 μL chloroform was added for complete extraction of proteins and other undesirable substances. After further centrifugation, the upper aqueous phase was transferred to RNAse free polypropylene tubes. Nucleic acids were precipitated by adding cold isopropanol, the tubes were inverted several times and incubated overnight at - 20 °C. Two microlitres (2 μL) of glycogen was added before isopropanol to allow RNA pellet. After precipitation, the precipitate was washed by adding 300 μL 75% ethanol and then resuspended in 25 μL sterile RNase-free water (Thermo Scientific, San Jose, CA, USA). The RNA concentration was quantified using NanoDrop 2000 Spectrophotometer (Thermo Scientific, San Jose, CA, USA). Only samples with 260/280 nm ratio between 1.8 and 2.0 and concentration above 200 ng/μL were converted to cDNA. The cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription kit (Thermo Scientific, San Jose, CA, USA) with a standard amount of 1 μg of total RNA for each sample.
Real-time PCR was then performed in a thermocycler CFX96 real-time system (Bio-Rad, Hercules, CA, USA). Gene expression was determined from the cDNAs to synthesize above which serve as the templates and a pre-designed TaqMan probes (Life Technologies) used to detect C. elegans dat-1 (Ce02450891_g1) and cat-2 (Ce02426736_g1) expression while tba-1 (Ce02412618_gH) was used as a housekeeping gene. The amplification reaction was performed by initial denaturation at 50 °C for 2 min followed by 95 °C for 10 min and 40 thermal cycles of 95 °C for 15 s and 60 °C for 1 min. All the real-time PCRs were performed in a final volume of 20 μL. Experiment for both target genes as well as endogenous control were carried out in triplicate of four independent worm preparation (n = 4). The threshold cycle (Ct) used for the analysis was the arithmetic average of the triplicates of the target and endogenous control genes. The relative expression of each gene was calculated by 2−ΔΔCt method.
2.7. Dopamine content analysis in worms by LC/MS
L1 synchronized WT N2 worms were exposed to Pb or no-Pb medium for 1 h. Next, they were washed off the plates several times with M9. 200,000 worms per group were homogenized, using a tissue dismembrator, in 100–750 u L of 0.1 M TCA, which contains 0.01 M sodium acetate, 0.0001 M EDTA, and 10.5% methanol (pH 3.8). Ten microliters of the homogenate are used for quantification of protein levels. Then samples were centrifuged at 10,000 g for 20 min at 4 °C. The supernatant obtained is removed and used for dopamine analysis.
DA levels were determined by a highly sensitive and specific liquid chromatography/mass spectrometry (LC/MS) methodology following derivatization of analytes with benzoyl chloride (BZC). 20 u L of tissue extract is added to a 1.5 mL microcentrifuge tube containing 60 u L of a solution composed of 80% acetonitrile in water with 0.5% formic acid. Each tube is vortexed for 20 s and spun in a microcentrifuge at 10,000 g for 2 min at 20 °C. 5 u L of the supernatant is combined in an LC/MS vial with ten microliters each of 500 mM NaCO3 and 2% BZC in acetonitrile. After two minutes, the reaction is stopped by the addition of 20 u L internal standard solution (in 20% acetonitrile containing 3% sulfuric acid) and 40 u L water. The samples are then ready for LC/MS analysis.
LC was performed on a 2.0 x 100 mm, 1.7 μm particle Kinetix biphenyl column (Phenomenex, Torrance, CA, USA) using a Waters Acquity UPLC (Waters Corporation, Milford, MA USA). Mobile phase A was 0.15% aqueous formic acid and mobile phase B was acetonitrile. Samples were separated by a gradient of 98–5% of mobile phase A over six minutes at a flow rate of 450 μL/min prior to delivery to a SCIEX 6500+ QTrapmass spectrometer. Each experiment was performed within four independent worm preparations (n = 4).
2.8. Relative Pgst‑4:GFP fluorescence intensity in worms
For this experiment, we used the transgenic C. elegans VP596 line, which expresses one fluorescent construct to monitor glutathione-S-transferases (GSTs) and another as a standard for worm number normalization (Pgst-4::GFP and Pdop-3::RFP, respectively). Synchronized L1 VP596 worms were exposed to K-medium, vehicle or Pb for I h, thereafter, they were washed several times with M9 and S-buffer, and was later transferred to a 96 microplate (20,000 worms per well) for GFP fluorescence analysis. GFP fluorescence was quantified according to Leung et al. [32] (filters: GFP 485/20ex 528/20em). Since gst-4 is inducible by reactive oxygen species (ROS), its fluorescence intensity was taken as a marker for oxidative stress in the living worms [33]. Each experiment was performed in triplicate and repeated independently four times (n = 4).
2.9. Monoamine oxidase assay
Synchronized L1 N2 worms were exposed to K-medium or Pb for I h, thereafter, they were washed several times with M9 and homogenized in phosphate buffer. The homogenate was centrifuged at 10,000 g for 10 min at 4 °C. The supernatant obtained is removed and used for monoamine oxidase-A (MAO-A) activity. This analysis was carried out using monoamine oxidase assay ELISA kit which provides a convenient fluorimetric means to measure MAO enzyme activity. In the assay, MAO reacts with p-tyramine, a substrate for both MAO-A and MAO-B, resulting in the formation of H2O2, which is determined by a fluorimetric method (λem/ex = 585/530 nm). Each experiment was performed in triplicate and repeated independently four times (n = 4).
2.10. Protein assay
Protein concentration was determined by BCA Protein Assay Kit (Thermo Scientific). Ten microliter tissue homogenate was distributed into 96-well plate and 200 μL of mixed BCA reagent (25 mL of Protein Reagent A was mixed with 500 μL of Protein Reagent B) was added. Plates were incubated at room temperature for two hours allowing for color development. A BSA standard curve was run at the same time. Absorbance was measured by a microplate reader (POLARstar Omega).
2.11. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 for Windows (GraphPad Software Inc., La Jolla, CA, USA). All the results were expressed as the mean ± SEM (standard error of the mean) of at least four independent experiments. Data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. Results were considered statistically significant when p < 0.05.
3. Results
3.1. Lethality test
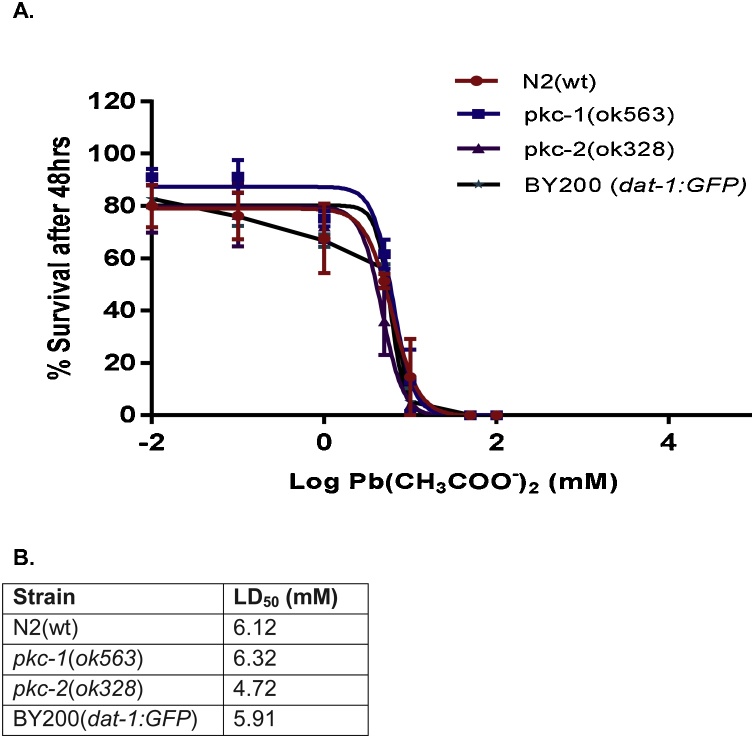
The survival test was performed in L1 worms exposed to Pb (0–50 mM). The result as presented in Fig. 1, revealed that there was no significant difference in LD50 values among the C. elegans strains (N2, BY200, RB781, and VC127) tested (Fig. 1).
Fig. 1.
Dose-response lethality curves of C. elegans after exposure to Pb (CH3COO)2 for 1 h. Synchronized L1 worms were exposed to increasing concentrations of Pb2+ acetate (0–50 mM) for I h. Thereafter, 30–50 worms were plated and score for lethality 48 h later. (A) Data represent the percentage of surviving worms and (B) Median lethal dose (LD50) which was calculated by non-linear regression.
Sigmoidal dose-response model was used to plot the % survival curves and determine the respective LD50. Results were analyzed by two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test.
3.2. Dopaminergic neurodegeneration in C. elegans following Pb acetate treatment
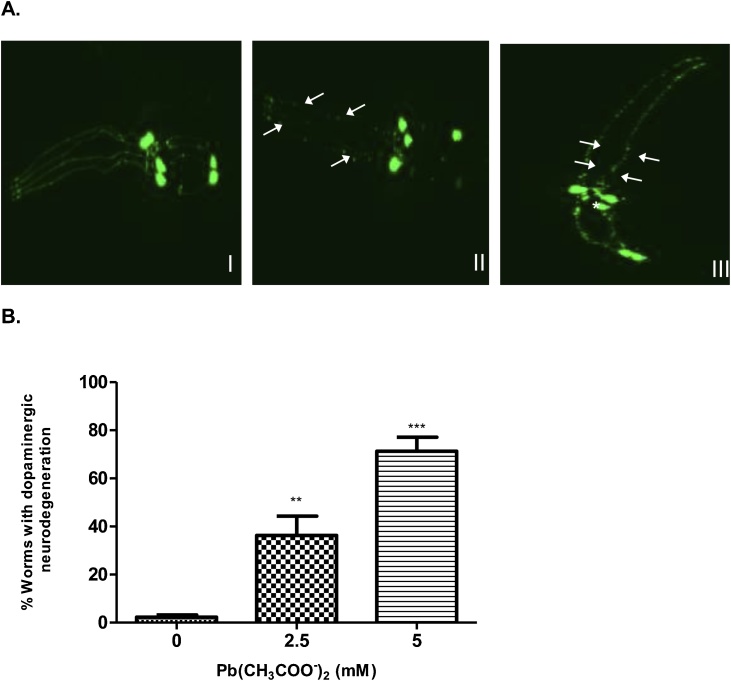
BY200 (dat-1:GFP) worms (which has green fluorescent protein (GFP) expressed in
dat-1 promoter) was used to determine Pb-induced dopaminergic neurodegeneration. As shown in Fig. 2, there was a significant (P < 0.05) decrease in a number of intact DAergic neurons following Pb treatment for 1 h. Treatment with Pb at doses close to LD50 (2.5 and 5 mM) in this study showed degenerating neurons in worms as observed by the presence of puncta and loss of dendrites (Fig. 2).
Fig. 2.
Dopaminergic neurodegeneration in BY200 worms following Pb acetate treatment. (A) Representative confocal images of cephalic (CEP) dopaminergic neurons in head of L1 BY200 (dat-1:GFP) worms treated with Pb acetate (0, 2.5 or 5 mM), I – show intact neurons while II and III – show degenerating neurons in worms.
(B) Data represent the percentage of worms with dopaminergic neurodegeneration. Results were expressed as mean ± S.E.M. (n = 6). Statistical analysis was performed by Two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. **p < 0.01,***p < 0.001 compared to control group treated with K-medium.
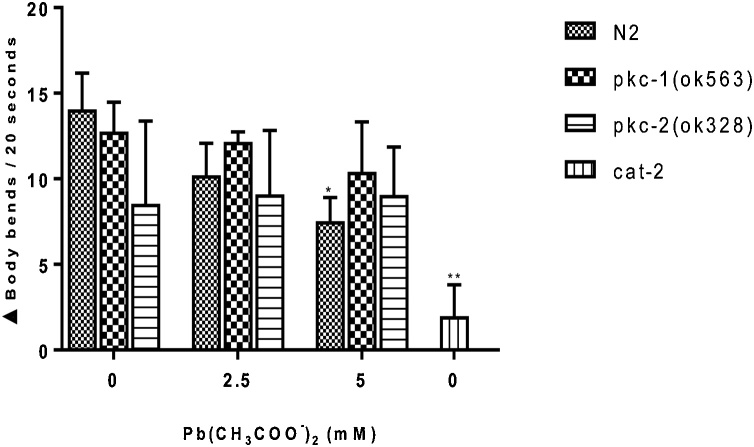
3.3. Basal slowing response (BSR)
Basal slowing response (BSR) is a behavioral assay designed to assess the function of the dopaminergic neuron. We tested this behavior in N2 and pkc mutant worms exposed to Pb by measuring the change (Δ) in body bends per 20 s. According to Fig. 3, there was a significant (p < 0.05) impairment on BSR in wild-type N2 strain exposed to 5 mM Pb acetate when compared to the WT control group. However, the pkc-1 and pkc-2 null mutant strains did not exhibit any significant impairment on BSR (Fig. 3).
Fig. 3.
Basal slowing response (BSR) in N2 and null mutant (pkc-1 and pkc-2) worms following Pb acetate treatment for 1 h (0, 2.5 and 5 mM). BSR was carried out 48 h after Pb2+ exposure. Worms were washed off plates with S-basal and five worms were pipetted in plate’s presence or absence of OP50 E. coli. Worms were allowed to acclimatize for about 5 min. on the new plate and thereafter the body bends were counted in 20 s intervals for each worm to obtain an average. The difference in the average number of body bends in the presence and absence of food was calculated as the BSR. cat-2 mutants, with deficiency in tyrosine hydroxylase, were used as positive control. Data were expressed as mean ± S.E.M. (n = 5). Statistical analysis was performed by Two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. *p < 0.05,**p < 0.01 compared to control group treated with K-medium.
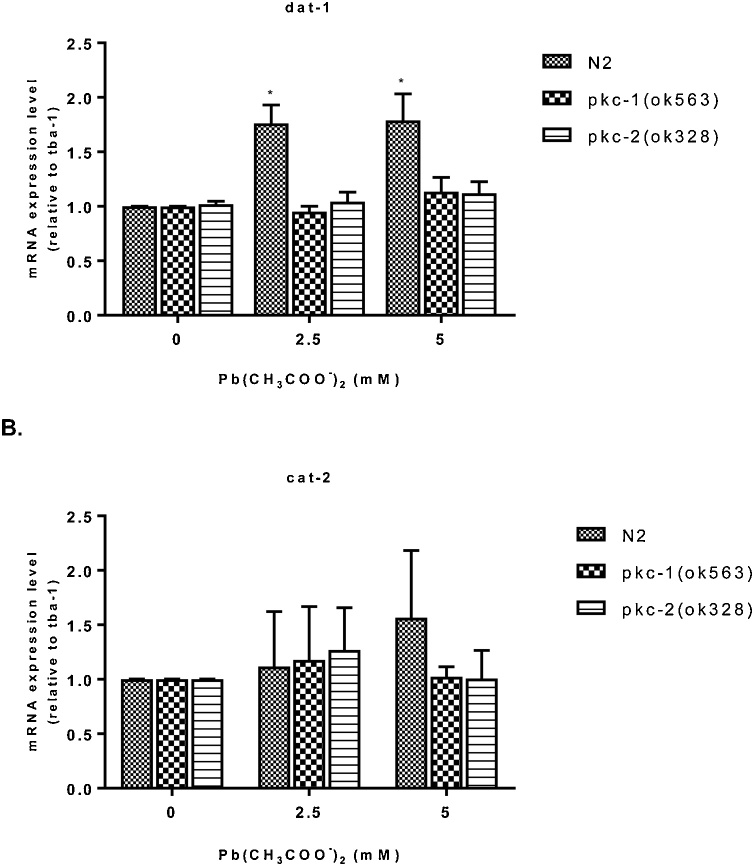
3.4. mRNA expression levels following Pb treatment
The effect of Pb exposure on cat-2 and dat-1 gene expressions level was assessed using RT-PCR analysis. One-way ANOVA revealed that Pb exposure caused a significant (p < 0.05) increase in dat-1 gene expression level at 2.5 and 5 mM when compared with control without Pb treatment for wild-type N2 worms, but no alteration was observed for the mutant strains (Fig. 4A).
Fig. 4.
mRNA expression level of N2 or null mutants pkc-1 and pkc-2 worms exposed to Pb acetate for 1 h (0, 2.5 and 5 mM). (A) dat-1 expression level and (B) cat-2 expression level relative to the constitutive gene tba-1 and normalized to the N2 control group. Data were expressed as mean ± S.E.M. (n = 6). Statistical analysis was performed by Two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. *p < 0.05,**p < 0.01 compared to control group treated with K-medium.
Furthermore, two-way ANOVA revealed that cat-2 gene expression levels were not altered in any of the doses tested for N2, pkc-1 or pkc-2 strains (Fig. 4B).
3.5. Dopamine content analysis
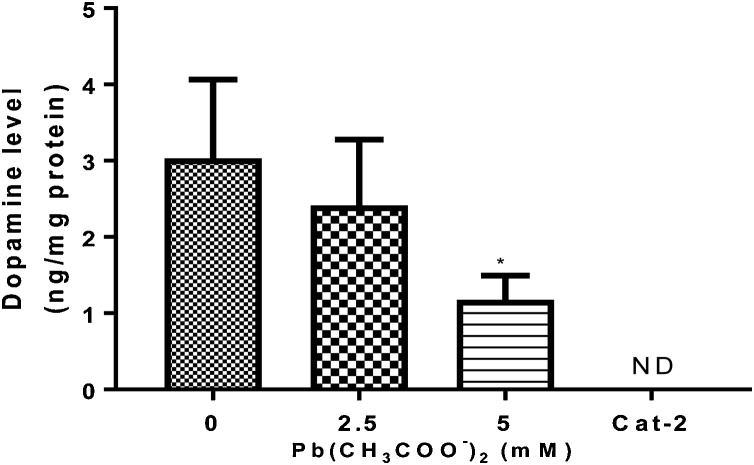
Dopamine content was analyzed by one-way ANOVA and the result revealed a significant (p < 0.05) decrease in dopamine levels for N2 worms exposed to Pb for 1 h when compared with a control group without Pb. Furthermore, the DA level was not detected in cat-2 loss-of-function mutants (Fig. 5).
Fig. 5.
Dopamine (DA) levels in N2 worms following Pb acetate treatment for 1 h (0, 2.5 and 5 mM). After 1 h treatment, DA levels were quantified by LC/MS analysis in extracts from L1 worms. Data were expressed as mean ± S.E.M. (n = 5). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. *p < 0.05 compared to control group treated with K-medium.
ND – Not detected
3.6. GFP fluorescence intensity
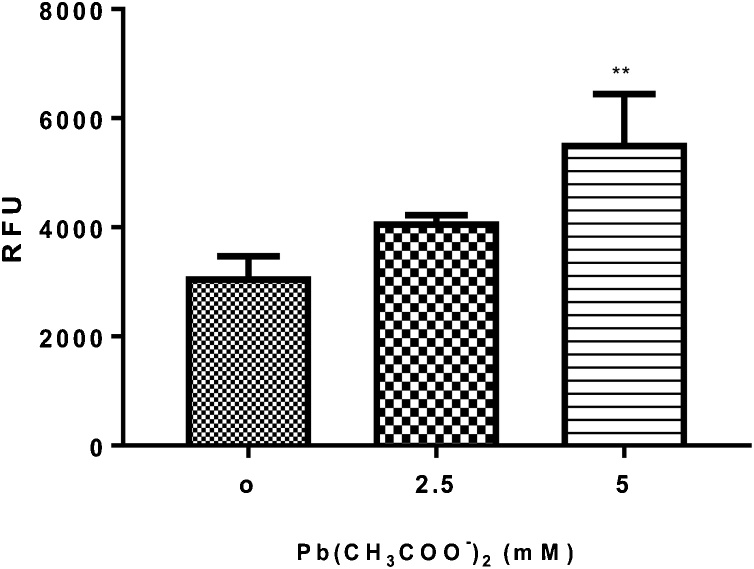
This experiment was conducted as a marker for oxidative stress since gst-4 is induced by ROS. One-way ANOVA showed that Pb exposure at the highest dose tested (5 mM) caused a significant (p < 0.05) increase on relative Pgst-4::GFP fluorescence intensity in VP596 worms (Fig. 6).
Fig. 6.
Quantification of GFP fluorescence in VP596 worms expressing GFP under the gst-4 promoter following Pb acetate treatment for 1 h (0, 2.5 and 5 mM). Transgenic worms expressing GFP under the gst-4 promoter were treated with Pb2+ for 1 h (0, 2.5 and 5 mM) and GFP fluorescence was measured as an indicator of oxidative stress and SKN-1 activity. Data were expressed as mean ± S.E.M. (n = 5). Statistical analysis was performed by Two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. **p < 0.01 compared to control group treated with K-medium.
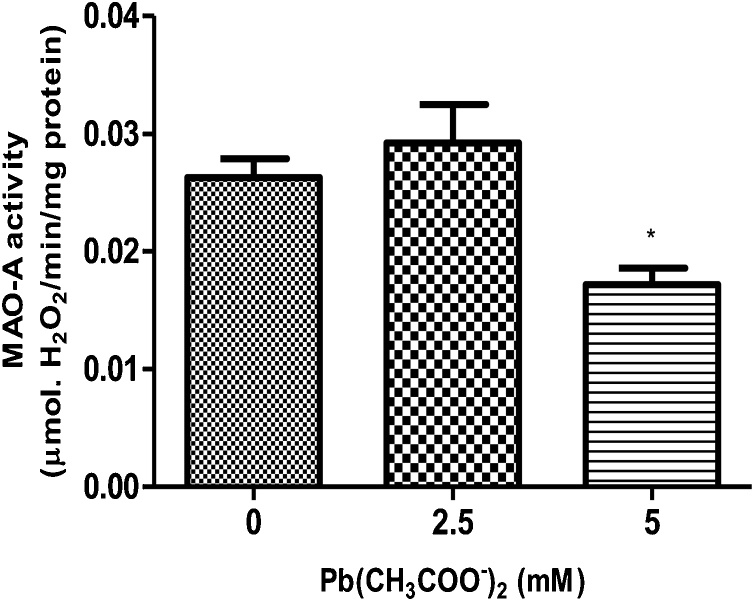
3.7. Monoamine oxidase activity
One-way ANOVA showed that Pb exposure at the highest dose tested (5 mM) caused a significant (p < 0.05) decrease in monoamine oxidase (MAO-A) activity when compared with control group.
4. Discussion
Several studies have highlighted the neurotoxic potential of Pb exposure in children and adults [[34], [35], [36]]. However, the molecular mechanism of action of Pb-induced neurotoxicity remains unclear. In the present study, we demonstrated for the first time, the ability of Pb to interfere with the dopamine transporter (DAT-1) via PKC activation in C. elegans model.
According to McDonald et al. [37], the dopaminergic (DAergic) systems of C. elegans are orthologues to humans given that they harbor the same genes responsible for DA synthesis, storage, release, re-uptake and signaling. These neurons have been reported to be sensitive to neurotoxins and pesticides such as 6-OHDA, MPP+ and rotenone or paraquat [[38], [39], [40]], thereby making them an optimal model for studying neurodegenerative diseases.
In this study, we observed morphological alterations induced by Pb exposure in the worm DA neurons (Fig. 2). Treatment of BY200 worms with Pb caused an increased in the number of shrunken soma and decreased the number of worms with intact CEP neurons. This result is consistent with previous studies where heavy metals alter dopaminergic neuronal structure in C. elegans [41,42]. Also, several studies in both rats and human have highlighted the combined toxic effect of Pb with other heavy metals on dopaminergic neurons [[43], [44], [45], [46]]. This observed neurodegenerative effects of Pb exposure were concomitant with neurobehavioral deficit (Fig. 3). Hence, we could confirm that Pb-induced neuronal damage in C. elegans model.
DA signaling has been implicated in controlling locomotion behavior in C. elegans. According to Sawin et al. [31], well-fed, wild-type animals with normal dopamine contents slow their locomotion rate in the presence of bacteria than in the absence of bacteria whereas this behavior is lost in cat-2 mutants or animals in which the dopaminergic neurons have been ablated. This behavioral assay known as basal slowing response (BSR) evaluates the functionality of the DAergic signaling system in C. elegans [31]. There are three classes of DAergic neurons namely; CEP, ADE, and PDE that have been implicated in this response, mediating locomotor activity. As presented in Fig. 3, Pb-exposed N2 worms increased their locomotor rate on food when compared with control (Fig. 3). This finding is in agreement with previous studies where Pb exposure affect behaviors associated with DA function in rat model [[24], [25], [26], [27],47]. This is an indication that the DAergic system is altered by Pb. However, worms deficient in pkc-1 or pkc-2 did not show alteration in BSR upon treatment with Pb when compared with control. This suggest that protein kinase C (PKC) might play an important role in Pb induced dopaminergic neurotoxicity.
Regulatory enzyme PKC has been discovered to be present in almost any type of tissue and studies have shown they play a critical role in a wide variety of cellular processes [48]. Previous studies have shown that activation or inhibition of PKC affects DA signaling DAT thereby suggesting that PKC phosphorylation plays an important regulatory mechanism in DAT function [[49], [50], [51], [52]]. It has been suggested that PKC regulation of DAT is mediated by direct phosphorylation of DAT protein based on studies that have revealed the presence of phosphorylation site for PKC [[52], [53], [54]].
The present study demonstrated that Pb exposure increased dat-1 gene expression level in wild-type N2 worms, but no alteration was observed for pkc-1 and pkc-2 mutant strains (Fig. 4). This result suggests that Pb exposure affect DAT function and this action might be mediated via PKC. Several studies have highlighted the role of PKC activation in Pb toxicity [[55], [56], [57]].
DAT is an integral membrane protein whose primary function is to terminate DAergic neurotransmission by the re-uptake of released DA back to the presynaptic neuron [54,58,59]. The increased in dat-1 gene expression as observed in this study would result in increased DAT function and ultimately excess DA levels in the presynaptic neuron. Dysfunction of DAT would affect DA signaling and has been linked to several neurological disorders. It is noteworthy that Parkinson’s disease (PD) brains showed a 50–70% loss of DAT function [60], consistent with experimental models of DAergic toxicity, including Mn [61] and antipsychotics [62]. Measurement of dopamine levels showed that Pb-exposed worms had reduced DA levels when compared with control (Fig. 5). These reduced dopamine levels appear to be related to the inhibition of monoamine oxidase-A (MAO) activity upon Pb treatment (Fig. 7). MAOs are a family of flavin enzymes located in the outer mitochondrial membrane of astrocytes and neuronal cells and they catalyze the oxidation of monoamines [63]. Several studies have shown that MAO activity regulates levels of biogenic amines and neuronal activity in the nervous system [27,63,64]. Our results are in agreement with reported studies where MAO activity was decreased in the cortex, striatum, cerebellum, and hippocampus of the offspring following exposure to heavy metals [27,63,64]. This inhibition could be attributed to the ability of Pb to bind with cysteine residues of MAO thereby resulting in disrupted structure and decreased MAO function [65]. Recently, it was suggested that alterations in MAO activity plays a key role in initiating of cell apoptosis through ROS generation [27,65].
Fig. 7.
Monoamine oxidase (MAO-A) activity in WT N2 worms following Pb acetate treatment for 1 h (0, 2.5 and 5 mM). Data were expressed as mean ± S.E.M. (n = 5). Statistical analysis was performed by Two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. *p < 0.05 compared to control group treated with K-medium.
Furthermore, excessive DA can oxidize dopamine to generate unstable quinones, which are highly reactive and cause lipid peroxidation [30,66]. Dopaminergic cell loss in PD patients and experimental PD models invokes excessive reactive oxygen species (ROS) production [[67], [68], [69]]. Skn-1 is the C. elegans homolog of nuclear factor (erythroid-derived-2)-like 2 (Nrf2), and it is constitutively expressed in dopaminergic neurons where it regulates a number of phase II detoxification enzymes including GSTs [30,70]. As shown in Fig. 6, Pb exposure at 5 mM increased the relative Pgst-4::GFP fluorescence intensity in transgenic VP596 worms. This is an indication that Pb induced activation of GST enzymes which reflects a neuroprotective defense mechanism against ROS generated from Pb exposure. Nrf2 is a nuclear transcription factor that controls the expression and coordinated induction of defensive genes encoding detoxifying enzymes and antioxidant proteins such as SOD and GST. This is a mechanism of critical importance for cellular protection and cell survival. Activated Nrf2 splits from its inhibitor protein Keap1, causing its translocation to the cell nucleus. The released Nrf2 binds to antioxidant response element (ARE), triggering the transcription and expression of multiple genes, including the endogenous antioxidant genes, phase II detoxification enzymes, and other cellular defensive genes [30,[71], [72], [73]]. In the present study, Pb increased the activity of GST, which is a downstream target of Nrf2. Thus, Pb plays a critical role in the activation of Nrf2 due to increasing ROS production. This result agrees with previous studies where lead-induced oxidative stress by activating the Nrf2 pathway [[74], [75], [76]].
In conclusion, we demonstrated that Pb caused damages to DAergic neuron morphology, which are probably related to alterations on DAT that might lead to decreased extracellular DA levels and consequent neurotoxicity. These results provide new insights to PD pathophysiology and should facilitate the search for new therapeutic modalities; however, additional studies must be carried out to further evaluate whether the specific effects of Pb on DAT function are secondary to direct or indirect phosphorylation by PKC.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The first author and OMI are a recipient of IBRO/ISN postdoctoral fellowship and wish to thank the organization for their support. Also, dopamine analysis was performed in the Neurochemistry Core at Vanderbilt University. The Core receives support from the EKS NICHD of the NIH under Award #U54HD083211
References
- 1.Iversen S.D., Iversen L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen A.S., Andersen J., Jorgensen T.N., Sorensen L., Eriksen J., Loland C.J., Stromgaard K., Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 3.Felton C.M., Johnson C.M. Dopamine signaling in C. elegans is mediated in part by HLH-17-dependent regulation of extracellular dopamine levels. G3 Gene. 2014;4:1081–1089. doi: 10.1534/g3.114.010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pramod A.B., Foster J., Carvelli L., Henry L.K. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol. Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase D.L., Koelle M.R. Vol. 20. 2007. Biogenic amine neurotransmitters in C. elegans; pp. 1–15. (WormBook). Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nirenberg M.J., Vaughan R.A., Uhl G.R., Kuhar M.J., Pickeli V.M. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci. 1996;76:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.German C.L., Hanson G.R., Fleckenstein A.E. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J. Neurochem. 2012;123:288–297. doi: 10.1111/j.1471-4159.2012.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaughan R.A., Foster J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melikian H.E., Buckley K.M. Membrane trafficking regulates the activity of the human dopamine transporter. J. Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovde M.J., Larson G.H., Vaughan R.A., Foster J.D. Model systems for analysis of dopamine transporter function and regulation. Neurochem. Int. 2019;123:13–21. doi: 10.1016/j.neuint.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami K., Feng G., Chen S.G. Inhibition of brain protein kinase C subtypes by lead. J. Pharmacol. Exp.Ther. 1993;264:757–761. [PubMed] [Google Scholar]
- 12.Rajanna B., Chetty C.S., Rajanna S., Hall E., S Fail, Yallapragada P.R. Modulation of protein kinase C by heavy metals. Toxicol. Lett. 1985;81:197–203. doi: 10.1016/0378-4274(95)03433-1. [DOI] [PubMed] [Google Scholar]
- 13.Jan A.T., Azam M., Siddiqui K., Ali A., Choi I., Haq Q.M.R. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015;16:29592–29630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein Y., Markowitz M.E., Rosen J.F. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res. Rev. 1998;27:168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 15.Antoine J.M.R., HooFung L.A., Grant C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017;4:181–187. doi: 10.1016/j.toxrep.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain M.T., Hassi U., Huq S.M.I. Assessment of concentration and toxicological (Cancer) risk of lead, cadmium and chromium in tobacco products commonly available in Bangladesh. Toxicol. Rep. 2018;5:897–902. doi: 10.1016/j.toxrep.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loikkanen J.J., Naarala J., Savolainen K.M. Modification of glutamateinduced oxidative stress by lead: the role of extracellular calcium. Free Radic. Biol. Med. 1998;24:377–384. doi: 10.1016/s0891-5849(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 18.Bressler J.P., Belloni-Olivi L., Forman S., Goldstein G.W. Distinct mechanisms of neurotransmitter release from PC 12 cells exposed to lead. J. Neurosci. Res. 1998;46:678–685. doi: 10.1002/(SICI)1097-4547(19961215)46:6<678::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Suszkiw J.B. Presynaptic disruption of transmitter release by lead. Neurotoxicology. 2004;25:599–604. doi: 10.1016/j.neuro.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Cai T., Zhao F., Yao T., Chen Y., Liu X., Luo W., Chen J. The role of alpha synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int. J. Biol. Sci. 2012;8:935–944. doi: 10.7150/ijbs.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers J.T., Venkataramani V., Washburn C., Liu Y., Tummala V., Jiang H., Smith A., Cahill C.M. A role of amyloid precursor protein translation to restore iron homeostasis and ameliorate lead (Pb) neurotoxicity. J. Neurochem. 2016;138:479–494. doi: 10.1111/jnc.13671. [DOI] [PubMed] [Google Scholar]
- 22.Fortune T., Lurie D.I. Chronic low-level lead exposure affects the monoaminergic system in the mouse superior olivary complex. J. Comp. Neurol. 2009;513:542–558. doi: 10.1002/cne.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cory-Slechta D.A., Merchant-Borna K., Allen J., Liu S., Weston D., Conrad K. Enhanced stimulus sequence-dependent repeated learning in male offspring after prenatal stress alone or in conjunction with lead exposure. Neurotoxicology. 2012;33:1188–1202. doi: 10.1016/j.neuro.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basha D.C., Rani M.U., Devi C.B., Kumar M.R., Reddy G.R. Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: reversal effect of calcium co-administration. Int. J. Dev. Neurosci. 2012;30:343–350. doi: 10.1016/j.ijdevneu.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Mansouri M.T., Naghizadeh B., López-Larrubia P., Cauli O. Behavioral deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem. Int. 2013;62:232–239. doi: 10.1016/j.neuint.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Saritha S., Davuljigari C.B., Kumar K.P., Reddy G.R. Effects of combined arsenic and lead exposure on the brain monoaminergic system and behavioral functions in rats: reversal effect of MiADMSA. Toxicol. Ind. Health. 2019;35:89–108. doi: 10.1177/0748233718814990. Saritha et al., 2019. [DOI] [PubMed] [Google Scholar]
- 27.Hilliard A., Ramesh A., Zawia N.H. Correlation between lead-induced changes in cerebral ornithine decarboxylase and protein kinase C activities during development and in cultured PC 12 cells. Int. J. Dev. Neurosci. 1999;17:777–785. doi: 10.1016/s0736-5748(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Su P., Zhang J., Wang S., Aschner M., Cao Z., Zhao F., Wang D., Chen J., Luo W. Genistein alleviates lead-induced neurotoxicity in vitro and in vivo: involvement of multiple signalling pathways. Neurotoxicology. 2016;53:153–164. doi: 10.1016/j.neuro.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Williams P.L., Dusenbery D.R. Acute toxicity testing using the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 1990;9:1285–1290. [Google Scholar]
- 30.Reckziegel P., Chen P., Caito S., Gubert P., Soares F.A.A., Fachinetto R., Aschner R. Extracellular dopamine and alterations on dopamine transporter are related to reserpine toxicity in Caenorhabditis elegans. Arch. Toxicol. 2016;90:633–645. doi: 10.1007/s00204-015-1451-7. [DOI] [PubMed] [Google Scholar]
- 31.Sawin E.R., Ranganathan R., Horvitz H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 32.Leung C.K., Deonarine A., Strange K., Choe K.P. High-throughput screening and biosensing with fluorescent C. elegans strains. J. Vis. Exp. 2011;51:1–5. doi: 10.3791/2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kampkötter A., Nkwonkam C.G., Zurawski R.F., Timpel C., Chovolou Y., Wätjen W. R. Kahl Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and foxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007;81:849–858. doi: 10.1007/s00204-007-0215-4. [DOI] [PubMed] [Google Scholar]
- 34.Miranda M.L., Kim D., Galeano M.A., Paul C.J., Hull A.P., Morgan S.P. The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environ. Health Perspect. 2007;115:1242–1247. doi: 10.1289/ehp.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders T., Liu Y., Buchner V., Tchounwou P.B. Neurotoxic effects and biomarkers of lead exposure: a review. Rev. Environ. Health. 2009;24:15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason L.H., Harp J.P., Han D.Y. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res. Int. 2014;840547:8. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald P.W., Hardie S.L., Jessen T.N., Carvelli L., Matthies D.S., Calixto J.B., Rocha J.B., Ferreira J. Valeriana officinalis does not alter the orofacial dyskinesia induced by haloperidol in rats: role of dopamine transporter. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1478–1486. doi: 10.1016/j.pnpbp.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Nass R., Hall D.H., Miller D.M., Blakely R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braungart E., Gerlach M., Riederer P., Baumeister R., Hoener M.C. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener. Dis. 2004;1:175–183. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- 40.Ved R., Saha S., Westlund B., Perier C., Burnam L., Sluder A., Hoener M., Rodrigues C.M., Alfonso A., Steer C., Liu L., Przedborski S., Wolozin B. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J. Biol. Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen P., Martinez-Finley E.J., Bornhorst J., Chakraborty S., Aschner M. Metal-induced neurodegeneration in C. elegans. Front. Aging Neurosci. 2013;5:18. doi: 10.3389/fnagi.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu C., Svoboda K.R., Lenz K.A., Pattison C., Ma H. Toxicity interactions between manganese (Mn) and lead (Pb) or cadmium (Cd) in a model organism the nematode C. elegans. Environ. Sci. Pollut. Res. 2018;25:15378–15389. doi: 10.1007/s11356-018-1752-5. [DOI] [PubMed] [Google Scholar]
- 43.Tsatsakis A.M., Docea A.O., Calina D., Buga A.M., Zlatian O., Gutnikov S., Kostoff R.N., Aschner M. Hormetic Neurobehavioral effects of low dose toxic chemical mixtures in real-life risk simulation (RLRS) in rats. Food Chem. Toxicol. 2019;125:141–149. doi: 10.1016/j.fct.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 44.Hernández A.F., Tsatsakis A.M. Human exposure to chemical mixtures: challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017;103:188–193. doi: 10.1016/j.fct.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Kostoff R.N., Goumenou M., Tsatsakis A. The role of toxic stimuli combinations in determining safe exposure limits. Toxicol. Rep. 2018;5:1169–1172. doi: 10.1016/j.toxrep.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsatsakis A.M., Vassilopoulou L., Kovatsi L., Tsitsimpikou C., Karamanou M., Leon G., Liesivuori J., Hayes A.W., Spandidos D.A. The dose response principle from philosophy to modern toxicology: the impact of ancient philosophy and medicine in modern toxicology science. Toxicol. Rep. 2018;5:1107–1113. doi: 10.1016/j.toxrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang J., Kueon C., Kim J. Influence of lead on repetitive behavior and dopamine metabolism in a mouse model of iron overload. Toxicol. Res. 2014;30:267–276. doi: 10.5487/TR.2014.30.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang K.P., Huang F.L. How is protein kinase C activated in CNS? Biochem. Int. 1993;22:417–433. doi: 10.1016/0197-0186(93)90037-6. [DOI] [PubMed] [Google Scholar]
- 49.Vaughan R.A., Huff R.A., Uhl G.R., Kumar M.J. Protein kinase-C mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J. Biol. Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- 50.Pristupa Z.B., McConkey F., Liu F., Man H.Y., Lee F.J.S., Wang Y.T., Niznik H.B. Protein kinase-mediated bidirectional trafficking and functional regulation of the dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 51.Chang M.Y., Lee S.H., Kim J.H., Lee K.H., Kim Y.S., Son H., Lee Y.S. Protein kinase C-mediated functional regulation of dopamine transporter is not achieved by direct phosphorylation of the dopamine transporter protein. J. Neurochem. 2001;77:754–761. doi: 10.1046/j.1471-4159.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 52.Foster J.D., Cervinski M.A., Gorentla B.K., Vaughan R.A. Regulation of the dopamine transporter by phosphorylation. Handb. Exp. Pharmacol. 2006;175:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- 53.Giros B., el Mestikawy S., Bertrand L., Caron M.G. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- 54.Shimada S., Kitayama S., Lin C.L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- 55.Chen H.H., Ma T., Ho I.K. Protein kinase C in rat brain is altered by developmental lead exposure. Neurochem. Res. 1999;24:415–421. doi: 10.1023/a:1020993802239. [DOI] [PubMed] [Google Scholar]
- 56.Hwang K.Y., Lee B.K., Bressler J.P., Bolla K.I., Stewart W.F., Schwartz B.S. Protein Kinase C Activity and the Relations between Blood Lead and Neurobehavioral Function in Lead Workers Environ. Health Perspect. 2002;110:133–138. doi: 10.1289/ehp.02110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hains A.B., Vua M.A.T., Maciejewski P.K., van Dyck C.H., Gottron M., Arnsten A.F.T. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. PNAS. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cass W.A., Zahniser N.R., Flach K.A., Gerhardt G.A. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J. Neurochem. 1993;61:2269–2278. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 59.Kilty J.E., Lorang D., Amara S.G. Cloning and expression of a cocaine sensitive rat dopamine transporter. Science. 1991;254:578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- 60.Seeman P., Niznik H.B. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 61.Benedetto A., Au C., Avila D.S., Milatovic D., Aschner M. Extracellular dopamine potentiates Mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet. 2010;6:1–18. doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fachinetto R., Villarinho J.G., Wagner C., Pereira R.P., Avila D.S., Burger Felton C.M., Johnson C.M. Dopamine signaling in C. elegans is mediated in part by HLH-17-dependent regulation of extracellular dopamine levels genes. Genomes Genet. 2014;4:1081–1089. doi: 10.1534/g3.114.010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beyrouty P., Stamler C.J., Liu J.N. Effects of prenatal methylmercury exposure on brain monoamine oxidase activity and neurobehaviour of rats. Neurotoxicol. Teratol. 2006;28:251–259. doi: 10.1016/j.ntt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Basha D.C., Reddy S.N., Rani U.M. Age related changes in aminergic system and behavior following lead exposure: protection with essential metal supplements. Neurosci. Res. 2014;78:81–89. doi: 10.1016/j.neures.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Naoi M., Maruyama W., Akao Y. Involvement of type A monoamine oxidase in neurodegeneration: Regulation of mitochondrial signaling leading to cell death or neuroprotection. J. Neural Transm. Suppl. 2006;71:67–77. doi: 10.1007/978-3-211-33328-0_8. [DOI] [PubMed] [Google Scholar]
- 66.Aluf Y., Vaya J., Khatib S., Finberg J.P.M. Alterations in striatal oxidative stress level produced by pharmacological manipulation of dopamine as shown by a novel synthetic marker molecule. Neuropharmacology. 2011;61:87–94. doi: 10.1016/j.neuropharm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Cassarino D.S., Fall C.P., Swerdlow R.H., Smith T.S., Halvorsen E.M., Miller S.W., Parks J.P., Parker W.D., Jr, Bennett J.P., Jr Elevated reactive oxygen species and antioxidant enzyme activities in animaland cellular models of Parkinson’s disease. Biochim. Biophys. Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 68.Wu D.C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoo M.S., Chun H.S., Son J.J., DeGiorgio L.A., Kim D.J., Peng C., Son J.H. Oxidative stress regulated genes in nigral dopaminergic neuronal cells: correlation with the known pathology in Parkinson’s disease. Mol. Brain Res. 2003;110:76–84. doi: 10.1016/s0169-328x(02)00586-7. [DOI] [PubMed] [Google Scholar]
- 70.VanDuyn N., Settivari R., Wong G., Nass R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol. Sci. 2010;118:613–624. doi: 10.1093/toxsci/kfq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H., Davies K.J., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Done A.J., Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krajka-Kuzniak V., Paluszczak J., Baer-Dubowska W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017;69:393–402. doi: 10.1016/j.pharep.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Fang J., Huang S., Chena L., Fanc G., Wang C. The chronic effects of low lead level on the expressions of Nrf2 and Mrp1 of the testes in the rats. Environ. Toxicol. Pharmacol. 2013;35:109–116. doi: 10.1016/j.etap.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Huang H., Tu R., Liu F., Feng D., Liu W., Yuan W., Cheng X., Cui L. Effects of resveratrol on Nrf2 signal pathway of chronic lead-exposed mouse brain tissue. J. Hyg. Res. 2015;44:954–958. [PubMed] [Google Scholar]
- 76.Liu B., Zhang H., Tan X., Yang D., Lv Z., Jiang H., Lu J., Baiyun R., Zhang Z. GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3β in rat kidney. Oncotarget. 2017;8:42226–42237. doi: 10.18632/oncotarget.15033. [DOI] [PMC free article] [PubMed] [Google Scholar]