Relebactam is a novel class A/C β-lactamase inhibitor that restores imipenem in vitro activity against multidrug-resistant and carbapenem-nonsusceptible Pseudomonas aeruginosa. Time-kill analyses were performed to evaluate the potential role of imipenem-relebactam in combination with amikacin or colistin against P. aeruginosa. Ten clinical P. aeruginosa isolates (9 imipenem nonsusceptible) with imipenem-relebactam MICs ranging from 1/4 to 8/4 μg/ml were included.
KEYWORDS: β-lactamase, β-lactamase inhibitor, multidrug, synergy, time-kill
ABSTRACT
Relebactam is a novel class A/C β-lactamase inhibitor that restores imipenem in vitro activity against multidrug-resistant and carbapenem-nonsusceptible Pseudomonas aeruginosa. Time-kill analyses were performed to evaluate the potential role of imipenem-relebactam in combination with amikacin or colistin against P. aeruginosa. Ten clinical P. aeruginosa isolates (9 imipenem nonsusceptible) with imipenem-relebactam MICs ranging from 1/4 to 8/4 μg/ml were included. The isolates had varied susceptibilities to imipenem (1 to 32 μg/ml), amikacin (4 to 128 μg/ml), and colistin (0.5 to 1 μg/ml). Duplicate 24-h time-kill studies were conducted using the average steady-state concentrations (Cssavg) observed after the administration of imipenem-relebactam at 500 mg/250 mg every 6 hours (q6h) alone and in combination with the Cssavg of 25 mg/kg of body weight/day amikacin and 360 mg/day colistin in humans. Imipenem-relebactam alone resulted in 24-h bacterial densities of −2.93 ± 0.38, −1.67 ± 0.29, +0.38 ± 0.96, and +0.15 ± 0.65 log10 CFU/ml at imipenem-relebactam MICs of 1/4, 2/4, 4/4, and 8/4 μg/ml, respectively. No synergy was demonstrated against the single imipenem-susceptible isolate. Against the imipenem-nonsusceptible isolates (n = 9), imipenem-relebactam combined with amikacin resulted in synergy (−2.61 ± 1.50 log10 CFU/ml) against all amikacin-susceptible isolates and in two of three amikacin-intermediate (i.e., MIC, 32 μg/ml) isolates (−2.06 ± 0.19 log10 CFU/ml). Synergy with amikacin was not observed when the amikacin MIC was >32 μg/ml. Imipenem-relebactam combined with colistin demonstrated synergy in eight out of the nine imipenem-resistant isolates (−3.17 ± 1.00 log10 CFU/ml). Against these 10 P. aeruginosa isolates, imipenem-relebactam combined with either amikacin or colistin resulted in synergistic activity against the majority of strains. Further studies evaluating combination therapy with imipenem-relebactam are warranted.
INTRODUCTION
Pseudomonas aeruginosa is an important human bacterial pathogen able to cause serious infections in the lung, bloodstream, wounds, urinary tract, and peritoneal cavity, among other organ systems (1–3). The treatment of infection due to P. aeruginosa is complicated by this organism’s ability to harbor several resistance mechanisms, including β-lactamase expression, efflux pump upregulation, and outer membrane porin loss, which can render several antimicrobials ineffective (4, 5). Antimicrobial resistance has led to limited treatment options for P. aeruginosa infections, resulting in increased patient morbidity and mortality (1, 6).
Historically, carbapenem antibiotics (i.e., meropenem and imipenem) have been reserved for the treatment of multidrug-resistant (MDR) P. aeruginosa infections since they often retained activity in the face of resistance to other antipseudomonal β-lactams. The isolation of carbapenem-resistant P. aeruginosa, however, has now become conventional at many hospitals in North America. Recent surveillance studies report P. aeruginosa carbapenem nonsusceptibility rates of 20% to 25% and up to 35% in the intensive care unit setting (7–9); furthermore, carbapenem nonsusceptibility generally mirrors the prevalence of MDR isolates. As a result, new treatments for carbapenem-resistant and MDR P. aeruginosa are needed (10). Imipenem-relebactam is a carbapenem–β-lactamase inhibitor combination currently in clinical development for the treatment of hospital-acquired bacterial pneumonia (HABP), ventilator-associated bacterial pneumonia (VABP), complicated intra-abdominal infection (cIAI), and complicated urinary tract infection (cUTI) caused by MDR pathogens (ClinicalTrials.gov identifiers NCT02452047 and NCT02493764) (11). The addition of relebactam to imipenem-cilastatin restored its activity in 78% of imipenem-nonsusceptible P. aeruginosa strains (8).
Current Infectious Diseases Society of America (IDSA) guidelines recommend empirical combination therapy with two antipseudomonal agents from different classes to treat HABP/VABP due to P. aeruginosa bacteria in high-risk patients (3). Dual antipseudomonal therapy is also recommended for HABP/VABP patients with risk factors for MDR Gram-negative pathogens, patients in units where more than 10% of Gram-negative isolates are resistant to an agent being considered for monotherapy, and patients in an intensive care unit where local antimicrobial susceptibility rates are not available (3). Combination antimicrobial therapy is an attractive treatment strategy often considered for the most serious P. aeruginosa infections, as it has the potential to broaden empirical coverage, take advantage of potential synergy between the two antibiotics, and prevent the emergence of resistance to one or both of the active agents (12). Despite evidence of synergy exhibited by various antimicrobial combinations in vitro, combination therapy has not been strongly associated with improved clinical outcomes, except in the most severe cases of infection (3, 12–14). As imipenem-relebactam is a potent, yet novel, antibiotic, we sought to assess the potential for synergistic interactions with two other antibiotic classes typically reserved for serious MDR P. aeruginosa cases, amikacin and colistin (3).
Methods for assessing antimicrobial in vitro interactions include disk diffusion tests, checkerboard titrations, time-kill assays, and in vitro pharmacodynamic modeling. The optimal methodology, concentration, and interpretation criteria vary widely within these tests; however, time-kill assays remain a simple and effective testing strategy to assess the rate and extent of bacterial kill with antimicrobial agents (15–17). Utilizing time-kill assays, we assessed the synergistic effect of clinical concentrations of imipenem-relebactam in combination with amikacin and colistin against MDR P. aeruginosa, including carbapenem-nonsusceptible isolates.
RESULTS
P. aeruginosa susceptibility.
The susceptibilities of the 10 selected isolates to imipenem-relebactam, amikacin, colistin, and comparator antibiotics are presented in Table 1. Nine out of the 10 isolates met the definition for MDR and were also nonsusceptible to imipenem and meropenem. Imipenem-relebactam MICs ranged from 1/4 to 8/4 μg/ml. All 10 isolates were susceptible to colistin (<2 μg/ml), and amikacin MICs ranged from 4 to 128 μg/ml.
TABLE 1.
MICs of imipenem-relebactam, amikacin, colistin, and comparator agents against 10 P. aeruginosa isolates
| Isolatea | MIC (μg/ml) forb
: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | ATM | CAZ | CIP | CST | FEP | IPM | I/R | MEM | TZP | TOB | |
| CAIRD M9-44 | 4 | 8 | 2 | 0.25 | 0.5 | 2 | 1 | 1/4 | 0.5 | 4 | 1 |
| CAIRD M8-29 | 8 | 32 | 32 | >16 | 1 | 16 | 4 | 1/4 | 8 | 64 | 1 |
| CDC0508c | 8 | 8 | 16 | >16 | 1 | 16 | 16 | 2/4 | 8 | 64 | 1 |
| CAIRD M3-1 | 128 | 16 | 4 | >16 | 0.5 | 16 | 16 | 2/4 | 8 | 256 | >64 |
| CAIRD M6-6 | 16 | 16 | 32 | >16 | 1 | 16 | 16 | 4/4 | 64 | 16 | 128 |
| CAIRD M23-3 | 32 | 8 | 64 | >16 | 1 | 32 | 32 | 4/4 | 16 | 256 | 128 |
| CAIRD M1-13 | 64 | >64 | >64 | >16 | 1 | >64 | 32 | 4/4 | 32 | 64 | >64 |
| CAIRD M3-34 | 128 | 16 | 8 | >16 | 1 | 16 | 16 | 4/4 | 16 | 256 | >64 |
| CDC0526d | 32 | >64 | >64 | 4 | 0.5 | 64 | 16 | 8/4 | 32 | 32 | >64 |
| CAIRD M1-4 | 32 | 64 | 8 | >16 | 0.5 | 64 | 32 | 8/4 | >64 | 128 | 2 |
Unless noted otherwise, all isolates are clinical respiratory or blood P. aeruginosa isolates collected from the U.S. hospital surveillance study (46).
AMK, amikacin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CST, colistin; FEP, cefepime; IPM, imipenem; I/R, imipenem-relebactam; MEM, meropenem; TZP, piperacillin-tazobactam; TOB, tobramycin.
Obtained from the FDA-CDC Antimicrobial Resistance Isolate Bank (Atlanta, GA). Isolate CDC0508 has chromosomally encoded blaPDC-35 and blaPER-1 β-lactamases.
Obtained from the FDA-CDC Antimicrobial Resistance Isolate Bank. Isolate CDC0526 has a chromosomally encoded blaPDC-19 β-lactamase.
Time-kill analyses.
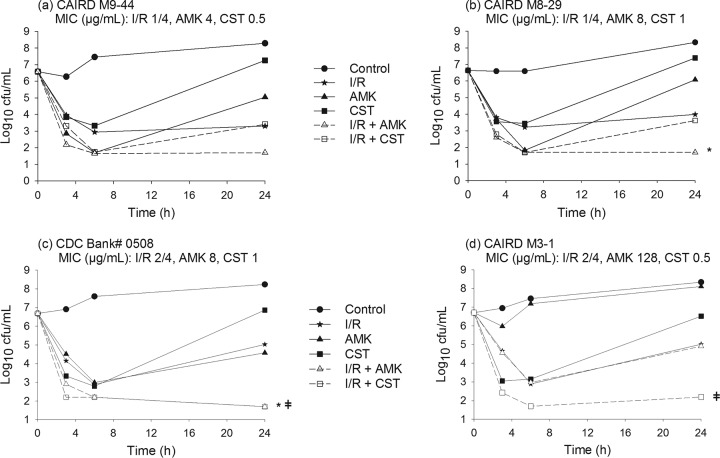
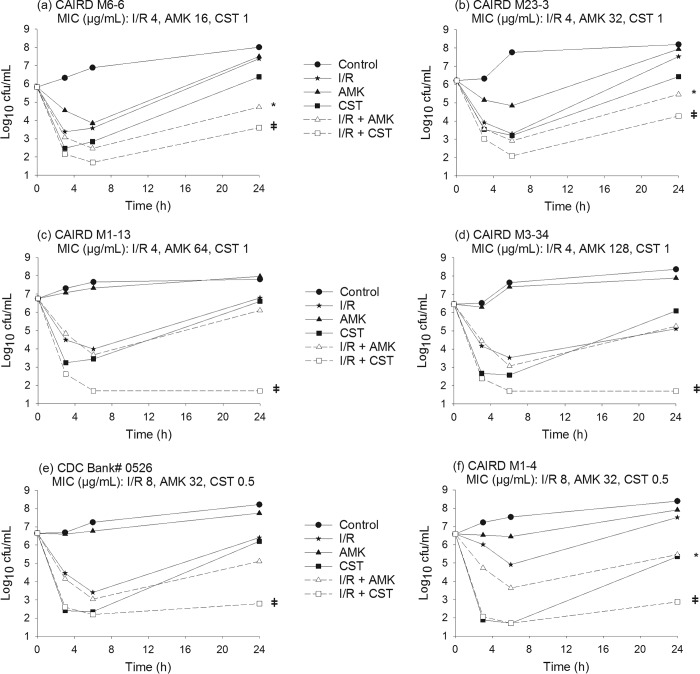
Time-kill curves for imipenem-relebactam, amikacin, and colistin alone and in combination are shown in Fig. 1 and 2. The mean bacterial density of the starting inoculum was 6.55 ± 0.28 log10 CFU/ml across all isolates examined. Drug-free control experiments resulted in CFU increases of 1.69 ± 0.37 log10 CFU/ml after 24 h. Imipenem-relebactam alone resulted in 24-h CFU changes of −2.93 ± 0.38, −1.67 ± 0.29, +0.38 ± 0.96, and +0.15 ± 0.65 log10 CFU/ml for isolates with imipenem-relebactam MICs of 1/4, 2/4, 4/4, and 8/4 μg/ml, respectively. At 24 h, amikacin alone resulted in CFU reduction in isolates with an amikacin MIC of ≤8 μg/ml (−1.52 ± 0.72 log10 CFU/ml, n = 3), while regrowth was observed in remaining isolates with an amikacin MIC of ≥16 μg/ml (+1.39 ± 0.53 log10 CFU/ml), relative to the 0-h controls. Colistin alone produced rapid killing over the first 3 to 6 h in all 10 isolates, followed by growth at 24 h to a mean bacterial density (6.54 ± 0.77 log10 CFU/ml) on par with the 0-h starting inoculum.
FIG 1.
Time-kill experiments displaying the activity of imipenem-relebactam alone and in combination with amikacin or colistin against P. aeruginosa isolates with imipenem-relebactam MICs of 1/4 μg/ml and 2/4 μg/ml. I/R, imipenem-relebactam; AMK, amikacin; CST, colistin; *, synergy with I/R plus AMK; ǂ, synergy with I/R plus CST.
FIG 2.
Time-kill experiments displaying the activity of imipenem-relebactam alone and in combination with amikacin or colistin against P. aeruginosa isolates with imipenem-relebactam MICs of 4/4 μg/ml and 8/4 μg/ml. I/R, imipenem-relebactam; AMK, amikacin; CST, colistin; *, synergy with I/R plus AMK; ǂ, synergy with I/R plus CST.
Table 2 shows the antimicrobial interactions (i.e., synergy, additivity, indifference, and antagonism) observed with imipenem-relebactam in combination with amikacin or colistin against all evaluated isolates. The combination of imipenem-relebactam and amikacin was rapidly bactericidal against all isolates at 6 h. By 24 h, additivity or synergy was observed in 7 isolates. The subgroup of isolates that did not demonstrate synergy were highly resistant to amikacin (MIC, ≥64 μg/ml; n = 3) or susceptible to imipenem (MIC, <2 μg/ml; n = 1). Of note, the combination of imipenem-relebactam and amikacin was additive or synergistic in the 3 isolates with intermediate susceptibility to amikacin (MIC, 32 μg/ml) evaluated in this study.
TABLE 2.
Antimicrobial interactions observed with imipenem-relebactam in combination with amikacin or colistin against 10 P. aeruginosa isolates at 24 h
| Isolate | Interaction for combinationa
: |
|
|---|---|---|
| I/R plus AMK | I/R plus CST | |
| CAIRD M9-44 | Additivity | Indifference |
| CAIRD M8-29 | Synergy | Indifference |
| CDC0508 | Synergy | Synergy |
| CAIRD M3-1 | Indifference | Synergy |
| CAIRD M6-6 | Synergy | Synergy |
| CAIRD M23-3 | Synergy | Synergy |
| CAIRD M1-13 | Indifference | Synergy |
| CAIRD M3-34 | Indifference | Synergy |
| CDC0526 | Additivity | Synergy |
| CAIRD M1-4 | Synergy | Synergy |
AMK, amikacin; CST, colistin; I/R, imipenem-relebactam.
The combination of imipenem-relebactam and colistin was rapidly bactericidal against all isolates at 6 h and met the definition of synergy at 24 h in 8 of the 10 isolates. The 2 isolates that showed bacterial indifference with this combination had the lowest imipenem-relebactam MICs (i.e., 1/4 μg/ml); thus, the addition of colistin did not result in further CFU reduction beyond that with imipenem-relebactam alone.
DISCUSSION
Multidrug resistance in P. aeruginosa is a growing public health problem and is challenging to treat. Combination therapy is routinely used in clinical practice to broaden the antimicrobial spectrum and decrease the risk of initial inappropriate therapy, although its potential for antimicrobial synergy and delaying or preventing antimicrobial resistance remain appealing theoretical indications (12). In this study, imipenem-relebactam-based combination therapy resulted in bactericidal and synergistic effects against the majority of the imipenem-nonsusceptible P. aeruginosa isolates evaluated.
Relebactam is an investigational non-β-lactam–β-lactamase inhibitor in clinical development. Paired with imipenem, relebactam has potent in vitro activity against class A β-lactamases, including KPC-type carbapenemases, and class C β-lactamases (11, 18). In a recent intensive care unit (ICU)-based surveillance study with 538 P. aeruginosa isolates, the respective susceptibility rates of meropenem, imipenem, and imipenem-relebactam were 72.7%, 67.1%, and 91.5% (9). The carbapenem-nonsusceptible and MDR P. aeruginosa isolates utilized in this study are representative of isolates that may be encountered in a critically ill population and where antimicrobial agents such as imipenem-relebactam would be considered for treatment.
Time-kill assays are a reliable in vitro test for the assessment of antimicrobial synergy. Compared with the Etest and checkerboard methodology, time-kill assays examine the magnitude of bacterial killing over time (17). Despite their wide use, debate continues over the optimal antimicrobial concentration to be assessed in time-kill assays; thus, investigators utilize a variety of approaches, including fractions of the isolate’s MIC (16, 19). Consequently, the amikacin and colistin concentrations utilized in this study were based on average steady-state concentrations (Cssavg) achieved in critically ill patients when administered at clinically relevant doses, while imipenem-relebactam, due to its investigational status, was employed at clinically achievable steady-state concentrations based on healthy volunteer data. In contrast to using an MIC fraction, peak, or trough concentration, the free Cssavg methodology reflects the average clinical antimicrobial concentration available to interact with bacteria over 24 h. It is worth noting that critically ill patients tend to have high intra- and interpatient variability in drug pharmacokinetics; thus, antimicrobial steady-state, peak, or trough concentrations obtained from the literature for utilization in in vitro studies may vary. The MIC fraction methodology is also prone to similar challenges given the ±1 2-fold concentration variation associated with MIC determination (20). Importantly, the colistin and amikacin average steady-state concentrations utilized in this study are similar to reported concentrations from other critically ill cohorts (21, 22).
The magnitude of bacterial killing with imipenem-relebactam alone across the range of imipenem-relebactam MICs was consistent with the imipenem-relebactam free Cssavg tested in vitro. Bacterial reductions of >2 log and >1 log were observed at imipenem-relebactam MICs of 1/4 μg/ml and 2/4 μg/ml, respectively, while bacteriostatic effects were observed at MICs of ≥4/4 μg/ml relative to the 0-h controls. Given enhanced imipenem-relebactam activity against P. aeruginosa (8, 10, 18), we included isolates with imipenem-relebactam MICs above the current imipenem breakpoint to assess an MIC distribution that may encompass a potential imipenem-relebactam MIC breakpoint. Notably, P. aeruginosa isolates with imipenem-relebactam MICs of ≥4 μg/ml represent less than 6% of the MIC distribution (8).
Imipenem-relebactam-based combination therapy resulted in synergy against the majority of the isolates evaluated in this study; however, the combination of imipenem-relebactam and amikacin proved to be ineffective against amikacin-resistant P. aeruginosa isolates. The underlying mechanism of β-lactam–aminoglycoside synergy is thought to be mediated by both agents. The inhibition of bacterial cell wall synthesis by β-lactams facilitates the entry of aminoglycosides into the bacterial cell (12, 23), while the ionic binding of positively charged aminoglycoside molecules to negatively charged bacterial cell surface components, including outer membrane proteins, results in increased permeability of aminoglycosides and other antimicrobial agents (24, 25). Irrespective of the imipenem-relebactam MIC, enhanced bacterial killing (i.e., synergy or additivity) with the combination of imipenem-relebactam plus amikacin was diminished as the MIC of the aminoglycoside increased beyond an amikacin MIC of ≥64 μg/ml.
Numerous in vitro studies have examined aminoglycosides in combination with β-lactams against P. aeruginosa, albeit with a variety of drug concentrations (26–31). In a time-kill study by Yadav et al. (31), imipenem combined with either tobramycin or amikacin demonstrated synergy and suppression of resistance against 3 imipenem-resistant P. aeruginosa isolates. Notably, the imipenem concentrations studied included the highest clinically achievable steady-state plasma concentrations possible after the administration of imipenem doses of 4 g/day (31). Though FDA approved, imipenem doses of 4 g/day are not routinely utilized in clinical practice, given a trend toward an increased risk of seizure with doses of >2 g/day (32, 33). The high imipenem concentration utilized in the investigation by Yadav and colleagues (31) may explain the synergistic killing observed among aminoglycoside-resistant P. aeruginosa strains, in contrast to our observations.
Colistin alone, utilized at a steady-state concentration achieved in critically ill patients, was initially bactericidal, but significant regrowth occurred at 24 h in 50% of the isolates, despite in vitro colistin susceptibility. Regrowth of colistin-susceptible P. aeruginosa (i.e., colistin heteroresistance) is well described in the literature (34–37). To mitigate the potential for heteroresistance with colistin monotherapy, combination therapy against P. aeruginosa has been suggested as a possible means by which to increase antimicrobial activity and mitigate resistance development (35, 37). Indeed, a meta-analysis of data (n = 136 P. aeruginosa isolates) from published time-kill studies with either colistin or polymyxin in combination with a carbapenem showed substantial synergy rates (50% [95% confidence interval {CI}, 30% to 69%]) and evidence of resistance suppression in vitro (38). In this study, combining the novel imipenem-relebactam with colistin resulted in synergy in 8 of 9 imipenem-resistant P. aeruginosa isolates evaluated.
In summary, we assessed average steady-state concentrations of imipenem-relebactam in combination with amikacin and colistin against MDR and carbapenem-nonsusceptible P. aeruginosa isolates using in vitro time-kill methodology. These data demonstrate the bactericidal and synergistic antimicrobial effects of an imipenem-relebactam-based combination therapy with no evidence of antagonism. These data support the role of imipenem-relebactam as part of a promising therapeutic strategy for the treatment of P. aeruginosa infections. Further in vitro studies and pharmacokinetic/pharmacodynamic analyses of these combinations are warranted against challenging-to-treat P. aeruginosa strains.
MATERIALS AND METHODS
Bacterial isolates.
Ten clinical P. aeruginosa isolates were selected for this study. Two isolates were obtained from the FDA-CDC Antimicrobial Resistance Isolate Bank (Atlanta, GA), and the remaining 8 isolates were from the Center for Anti-Infective Research and Development (CAIRD) isolate repository of clinical strains collected during a recent U.S.-based surveillance study between July 2017 and June 2018. Isolates were chosen to display a range of imipenem-relebactam MICs of 1/4, 2/4, 4/4, and 8/4 μg/ml. This range of imipenem-relebactam MICs allowed an assessment of isolates at or within 2 dilutions of the current Clinical and Laboratory Standards Institute (CLSI) breakpoint (2 μg/ml) for imipenem against P. aeruginosa (39). Additionally, isolates were selected for a range of amikacin MICs from susceptible to resistant. Ultimately, 9 imipenem-nonsusceptible isolates were included; in addition, 1 imipenem-susceptible isolate was included to determine if synergy is observed regardless of imipenem susceptibility. All isolates were maintained in skim milk (BD Biosciences, Sparks, MD) at −80°C. Each isolate was subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences) and grown for 18 to 20 h at 37°C under 5% CO2 prior to use in the experiments.
Susceptibility testing.
Susceptibilities to amikacin, colistin, imipenem, and imipenem-relebactam, as well as commonly utilized antipseudomonal agents, were determined for all isolates using the broth microdilution methodology, as outlined by the CLSI (39). Antibiotics were obtained as laboratory-grade powders from their respective manufacturers. MIC testing for imipenem-relebactam used a fixed relebactam concentration of 4 μg/ml in combination with doubling dilutions of imipenem. MIC values were obtained in triplicate, and the modal MIC was reported. P. aeruginosa ATCC 27853 was used as a quality control strain, as defined by the CLSI (39). In addition, to assess the appropriate concentration of relebactam in microdilution trays, P. aeruginosa CDC0516, harboring a KPC-2 enzyme, was used as an additional quality control (range, 0.5/4 to 2/4 μg/ml).
Simulated drug exposures.
Time-kill studies were performed using the average steady-state concentration (Cssavg) of imipenem and relebactam alone and in combination with the Cssavg of amikacin and colistin. Imipenem and relebactam plasma clearance (in liters per hour) following the administration of a single dose of imipenem-cilastatin (500 mg) with relebactam (500 mg) to healthy volunteers in a phase 1 study was used to calculate the free area under the concentration-time curve from 0 to 24 h (AUC0–24) of imipenem and relebactam achieved with the administration of 500/250 mg imipenem-relebactam every 6 h (40). After applying a protein binding factor of 20% for imipenem and relebactam (41), the simulated target imipenem and relebactam free Cssavg over a 24-h period were 5.9 μg/ml and 4.2 μg/ml, respectively. Amikacin was simulated to achieve a free Cssavg of 14.5 μg/ml consistent with that achieved with a 25-mg/kg/day dose in a 70-kg critically ill patient with severe sepsis (42). Colistin was simulated as a continuous infusion to achieve the mean free Cssavg (0.73 μg/ml) for critically ill adult patients receiving the currently recommended European Medicines Agency (EMA) dosing regimen of 360 mg daily of colistin base activity (43).
Time-kill studies.
Time-kill analyses were performed on each of the 10 P. aeruginosa isolates. Mueller-Hinton broth (Becton, Dickinson and Company, Sparks, MD) was inoculated with the bacterial suspension to a final suspension of approximately 106 CFU/ml. Duplicate time-kill experiments with imipenem-relebactam alone, amikacin alone, colistin alone, and imipenem-relebactam in combination with amikacin and colistin were conducted. Control experiments without any active agents were conducted simultaneously with the time-kill studies. The final volume for each bacterium-drug concentration was 10 ml, and the suspensions were incubated in a shaking water bath at 37°C. Samples were taken from each experiment at 0, 3, 6, and 24 h, diluted, and incubated for 18 to 24 h on blood agar plates to quantify the mean bacterial densities. The minimal accurately countable number of CFU per milliliter was determined to be 5 × 10−1 CFU/ml. Bactericidal activity of single antibiotics or combinations was defined as a ≥3-log10 reduction in CFU/ml of the initial inoculum after 24 h of incubation. Synergy was defined as a ≥2-log10 decrease in CFU/ml between the antibiotic combination and its most active constituent after 24 h. Additivity was defined as a 1- to <2-log10 decrease in CFU/ml between the antibiotic combination and its most active constituent after 24 h, while indifference was defined by a <1-log10 CFU/ml change between the antibiotic combination and its most active constituent. Antagonism (≥2-log10 increase in CFU/ml at 24 h with the combination compared with that by the most active drug alone) was also noted (44, 45).
ACKNOWLEDGMENTS
We thank Christina Sutherland, Deborah Santini, Jennifer Tabor-Rennie, Sara Giovagnoli, Elizabeth Cyr, Kimelyn Greenwood, Alissa Padgett, Janice Cunningham, Michelle Insignares, Lauren McLellan, Elias Mullane, Safa Abuhussain, Lindsay Avery, and James Kidd from the Center for Anti-Infective Research and Development, Hartford, CT, for their assistance with the conduct of the study.
This study was funded by Merck & Co., Inc. (Kenilworth, NJ) as an investigator-initiated trial. D.P.N. is a member of the advisory board and speaker’s bureau for Merck & Co., Inc. The remaining authors report no conflicts of interest.
REFERENCES
- 1.Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, Pennisi MA, Bello G, Antonelli M. 2013. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 39:682–692. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 2.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quale J, Bratu S, Gupta J, Landman D. 2006. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 50:1633–1641. doi: 10.1128/AAC.50.5.1633-1641.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. 2006. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sader HS, Castanheira M, Arends SJR, Goossens H, Flamm RK. 2019. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997–2016). J Antimicrob Chemother 74:1595–1606. doi: 10.1093/jac/dkz074. [DOI] [PubMed] [Google Scholar]
- 8.Karlowsky JA, Lob SH, Kazmierczak KM, Young K, Motyl MR, Sahm DF. 2018. In vitro activity of imipenem-relebactam against clinical isolates of Gram-negative bacilli isolated in hospital laboratories in the United States as part of the SMART 2016 program. Antimicrob Agents Chemother 62:e00169-18. doi: 10.1128/AAC.00169-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asempa TE, Nicolau DP, Kuti JL. 2019. Carbapenem-nonsusceptible Pseudomonas aeruginosa isolates from intensive care units in the United States: a potential role for new β-lactam combination agents. J Clin Microbiol 57:e00535-19. doi: 10.1128/JCM.00535-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher HW, Talbot GH, Benjamin DK, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D, Infectious Diseases Society of America. 2013. 10 × ‘20 progress–development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagacé-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2018. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem–β-lactamase inhibitor combinations. Drugs 78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 12.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Safdar N, Kethireddy S, Chateau D. 2010. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med 38:1651–1664. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 14.Pletz MW, Hagel S, Forstner C. 2017. Who benefits from antimicrobial combination therapy? Lancet Infect Dis 17:677–678. doi: 10.1016/S1473-3099(17)30233-5. [DOI] [PubMed] [Google Scholar]
- 15.Eliopoulos GM, Eliopoulos CT. 1988. Antibiotic combinations: should they be tested? Clin Microbiol Rev 1:139–156. doi: 10.1128/cmr.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard and E test. Antimicrob Agents Chemother 40:1914–1918. doi: 10.1128/AAC.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doern CD. 2014. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blizzard TA, Chen H, Kim S, Wu J, Bodner R, Gude C, Imbriglio J, Young K, Park Y-W, Ogawa A, Raghoobar S, Hairston N, Painter RE, Wisniewski D, Scapin G, Fitzgerald P, Sharma N, Lu J, Ha S, Hermes J, Hammond ML. 2014. Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin. Bioorg Med Chem Lett 24:780–785. doi: 10.1016/j.bmcl.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 19.Craig WA, Ebert SC. 1990. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl 74:63–70. [PubMed] [Google Scholar]
- 20.Jorgensen JH, Ferraro MJ, Jorgensen JH, Ferraro MJ. 2009. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 21.Duszynska W, Taccone F, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojeńska A, Kübler A. 2013. Therapeutic drug monitoring of amikacin in septic patients. Crit Care 17:R165. doi: 10.1186/cc12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. 2010. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 138:1333–1339. doi: 10.1378/chest.10-0463. [DOI] [PubMed] [Google Scholar]
- 23.Miller MH, Feinstein SA, Chow RT. 1987. Early effects of beta-lactams on aminoglycoside uptake, bactericidal rates, and turbidimetrically measured growth inhibition in Pseudomonas aeruginosa. Antimicrob Agents Chemother 31:108–110. doi: 10.1128/aac.31.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taber HW, Mueller JP, Miller PF, Arrow AS. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev 51:439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serio AW, Keepers T, Andrews L, Krause KM. 2018. Aminoglycoside revival: review of a historically important class of antimicrobials undergoing rejuvenation. EcoSal Plus 2018. doi: 10.1128/ecosalplus.ESP-0002-2018. [DOI] [PubMed] [Google Scholar]
- 26.Weiss K, Lapointe JR. 1995. Routine susceptibility testing of four antibiotic combinations for improvement of laboratory guide to therapy of cystic fibrosis infections caused by Pseudomonas aeruginosa. Antimicrob Agents Chemother 39:2411–2414. doi: 10.1128/aac.39.11.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens RC Jr, Banevicius MA, Nicolau DP, Nightingale CH, Quintiliani R, Quintiliani R. 1997. In vitro synergistic activities of tobramycin and selected beta-lactams against 75 Gram-negative clinical isolates. Antimicrob Agents Chemother 41:2586–2588. doi: 10.1128/AAC.41.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karakoç B, Gerçeker AA. 2001. In-vitro activities of various antibiotics, alone and in combination with amikacin against Pseudomonas aeruginosa. Int J Antimicrob Agents 18:567–570. doi: 10.1016/S0924-8579(01)00458-7. [DOI] [PubMed] [Google Scholar]
- 29.Gerçeker AA, Gürler B. 1995. In-vitro activities of various antibiotics, alone and in combination with amikacin against Pseudomonas aeruginosa. J Antimicrob Chemother 36:707–711. doi: 10.1093/jac/36.4.707. [DOI] [PubMed] [Google Scholar]
- 30.Song W, Woo HJ, Kim JS, Lee KM. 2003. In vitro activity of beta-lactams in combination with other antimicrobial agents against resistant strains of Pseudomonas aeruginosa. Int J Antimicrob Agents 21:8–12. doi: 10.1016/S0924-8579(02)00269-8. [DOI] [PubMed] [Google Scholar]
- 31.Yadav R, Bulitta JB, Nation RL, Landersdorfer CB. 2017. Optimization of synergistic combination regimens against carbapenem- and aminoglycoside-resistant clinical Pseudomonas aeruginosa isolates via mechanism-based pharmacokinetic/pharmacodynamic modeling. Antimicrob Agents Chemother 61:e01011-16. doi: 10.1128/AAC.01011-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koppel BS, Hauser WA, Politis C, van Duin D, Daras M. 2001. Seizures in the critically ill: the role of imipenem. Epilepsia 42:1590–1593. doi: 10.1046/j.1528-1157.2001.34701.x. [DOI] [PubMed] [Google Scholar]
- 33.Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. 2014. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother 69:2043–2055. doi: 10.1093/jac/dku111. [DOI] [PubMed] [Google Scholar]
- 34.Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. 2003. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 47:905–909. doi: 10.1128/AAC.47.3.905-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. 2011. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother 55:5134–5142. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David MD, Gill MJ. 2008. Potential for underdosing and emergence of resistance in Acinetobacter baumannii during treatment with colistin. J Antimicrob Chemother 61:962–964. doi: 10.1093/jac/dkn009. [DOI] [PubMed] [Google Scholar]
- 38.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute (CLSI). 2018. Performance standards for antimicrobial susceptibility testing, 28th ed. CLSI document M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 40.Rhee EG, Rizk ML, Calder N, Nefliu M, Warrington SJ, Schwartz MS, Mangin E, Boundy K, Bhagunde P, Colon-Gonzalez F, Jumes P, Liu Y, Butterton JR. 2018. Pharmacokinetics, safety, and tolerability of single and multiple doses of relebactam, a β-lactamase inhibitor, in combination with imipenem and cilastatin in healthy participants. Antimicrob Agents Chemother 62:e00280-18. doi: 10.1128/AAC.00280-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Racine F, Wismer MK, Young K, Carr DM, Xiao JC, Katwaru R, Si Q, Harradine P, Motyl M, Bhagunde PR, Rizk ML. 2018. Exploring the pharmacokinetic/pharmacodynamic relationship of relebactam (MK-7655) in combination with imipenem in a hollow-fiber infection model. Antimicrob Agents Chemother 62:e02323-17. doi: 10.1128/AAC.02323-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahmoudi L, Mohammadpour AH, Ahmadi A, Niknam R, Mojtahedzadeh M. 2013. Influence of sepsis on higher daily dose of amikacin pharmacokinetics in critically ill patients. Eur Rev Med Pharmacol Sci 17:285–291. [PubMed] [Google Scholar]
- 43.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. 2016. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neu HC, Gootz TD. 1996. Antimicrobial chemotherapy In Baron S. (ed), Medical microbiology, 4th ed University of Texas Medical Branch at Galveston, Galveston, TX. [PubMed] [Google Scholar]
- 45.Pillai SK, Moellering RC, Eliopoulos GM. 2005. Antimicrobial combinations, p 365–440. In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 46.Abuhussain SA, Sutherland CA, Nicolau DP. 2019. Susceptibility of P. aeruginosa to ceftolozane/tazobactam and ceftazidime/avibactam, research snapshot, abstr 548 SCCM, 48th Critical Care Congress, San Diego, CA, USA. [Google Scholar]