Abstract
Background
Patients with diabetes mellitus (DM) commonly receive statins to suppress vulnerability to adverse cardiovascular events. It has been clinically proven that hepatotoxicity is one of the most severe adverse effects of statins.
Material/Methods
We constructed diabetic rat models by feeding rats with high-fat food and by injection of low-dose STZ. Rats were randomized into 2 groups: a DM group (n=10) and a control (CON) group (n=5). CON rats received a normal diet, whereas DM rats ate high-fat food. Rats in the DM group underwent intraperitoneal STZ (35 mg/kg) injection following 6-week diet restriction. On the seventh day following STZ or blank injection, rats with FBG concentration over 11.1 mM were regarded as successfully established models and were used for further research.
Results
We showed that severe liver injury occurred in diabetic rats treated with 20 mg/kg atorvastatin, as evidenced by attenuation of liver enzyme activities, elevation of bilirubin levels, and alterations in the hepatic architecture, including hepatocyte death by necrosis, lymphocyte infiltration, and fibrosis. We also found that atorvastatin increased the secretion of pro-inflammatory factors such as L-1, TNF, IL-6, and IL-18 by enhancing activation of the NF-B signal pathway in the livers of diabetic rats. Atorvastatin elevated the levels of ROS and reduced the antioxidant enzyme (SOD and CAT) activities. Atorvastatin also increased the expression of anti-apoptotic protein BCL2 and decreased the expression of pro-apoptotic protein BAX in the livers of diabetic rats.
Conclusion
Atorvastatin exerts potentially hepatotoxic effects on diabetic rats by modulating oxidative/antioxidative status, pro-inflammatory cytokine production, and apoptosis inhibition.
MeSH Keywords: Metabolic Detoxication, Phase I; Mitochondrial Diseases; Respiratory Burst
Background
Diabetes mellitus (DM) commonly entails hypercholesterolemia, which is thought to accelerate atherosclerotic complication generation [1]. Patients with DM commonly receive statins aimed at suppression of vulnerability to cardiovascular events [2]. Nevertheless, it has been clinically proven that hepatotoxicity is one of the most severe adverse effects of statins [3–6]. Decreased regulation of coenzyme Q10 and selenoprotein activity can contribute to hepatic malfunction related to statins [7]. Previous studies have shown that Akt phosphorylation is an important indicator of vulnerability to statin-triggered toxicity [8].
Statins suppress HMG-CoA reductase (hydroxymethylglutaryl-CoA synthase), the rate-limiting enzyme in the mevalonate pathway of cholesterol generation, and thereby reduce cholesterol concentration [9]. Atorvastatin is the most frequently and widely applied statin [10]. Metabolic transformation from atorvastatin to ortho- as well as para-hydroxy atorvastatin is conducted via CYP3A/CYP3A4 in humans and rats [11]. Atorvastatin is also a substrate of SLCO1B1, which has important roles in hepatic elimination of atorvastatin [12]. The influence of oxidative stress (OS) on hepatotoxicity generation has been proven in animal experiments and clinical trials [13–15]. OS affects cellular constituents, including DNA, proteins, and lipids. Injury of DNA and membrane lipids triggered by OS can bring about apoptosis and tissue injury. On the other hand, hepatic OS increases liver inflammation. Upregulation of the expression of inflammatory mediators, including TNF-α, is another indicator of hepatotoxicity. In fact, enhanced generation of lipid metabolites, reactive oxygen intermediates, and inflammatory mediators, including TNF-α, has been found in some hepatotoxic models [16,17].
Liver injury involves a complex interaction of events that include Kupffer cell activation, neutrophil infiltration, generation of reactive oxygen species, and release of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), ultimately followed by endothelial cell and hepatocyte death. The Toll-like receptor (TLR) family, particularly Toll-like receptor 4 (TLR4), is one of the molecular mechanisms mediating the deleterious effects that occur during liver injury. Triggering the TLR pathway leads to activation of nuclear factor kappaB (NF-κB) and subsequently upregulates the expression of inflammatory genes and inflammatory factors (e.g., TNF-α, IL-1β, and IL-6). Drugs inhibiting the activity of the TLR inflammatory system can have beneficial effects for the liver, as do transgenic methods of blocking TLR4/NF-κB-related genes.
The present study focused on determining atorvastatin-triggered hepatic damage in diabetic rats and elucidating the relationship between liver toxicity and change in OS and inflammation. Our research proves that atorvastatin triggers liver toxicity in diabetic rats by stimulation of OS, the apoptosis-counteracting pathway, and inflammation.
Material and Methods
Animals
Male Sprague-Dawley rats weighing 100–120 g were from Sino-British Sipper & BK Lab Animals (Shanghai, China) and maintained at 50±5% relative humidity and 12-h light/dark cycle at 22±2°C. Rats had free access to feed and water. Animals were treated in conformity with the Guide for the Care and Use of Laboratory Animals and the Animal Ethics Committee of the Third Xiangya Hospital, Central South University.
Diabetic rat models and drug administration
We constructed diabetic rat models by feeding rats a high-fat diet and by injection of low-dose STZ, as described previously, but with slight alterations [18]. Rats were randomized into 2 groups: a DM group (n=10) and a control (CON) group (n=5). CON rats received a normal diet, whereas DM rats ate high-fat food (provided by TROPHIC Animal Feed High-tech Co., Nantong, People’s Republic of China). Rats in the DM group underwent intraperitoneal STZ (35 mg/kg) injection after 6-week diet restriction. On the seventh day after STZ or blank injection, rats with FBG concentration over 11.1 mM were regarded as successfully established models and were used for further research.
Fifteen DM rats were randomized into 3 groups: the DM group (n=5) did not receive atorvastatin supplementation; the atorvastatin-supplemented (DM-AM) group (n=5) consisted of 5 rats orally administered atorvastatin (20 mg/kg) every day for 10 days; and the DM-AM+NAC group (n=5) were orally administered atorvastatin (20 mg/kg) and NAC (2 mg/kg) every day for 10 days.
Hepatic histological evaluation
Liver tissues were sectioned into 0.3–0.5 cm blocks and underwent immersion in 10% formalin. Paraffin embedding was applied following dehydration. Slices were cut into sections 4 μm thick and stained with hematoxylin-eosin (HE). An inverted microscope (ECLIPSE Ti-S, Nikon, Japan) was used for semi-quantification of every section by an expert who was blind to group assignments. Liver damage severity was evaluated based on the following criteria: (1) vacuolization, (2) infiltration of inflammatory cells, (3) nuclei of pyknotic hepatocytes, and (4) sinusoidal dilatation. They were respectively classified as: 3, severe response; 2, moderate response; 1, mild response; and 0, no response. The highest score was 12, indicating the most severe liver damage.
Identification of biochemical and physiological markers
Reagent kits were bought from Jiancheng Bioengineering Institute, Nanjing, China to assess liver toxicity markers – alanine aminotransferase (ALT), total bilirubin (TBIL), alkaline phosphatase (AKP), and aspartate aminotransferase (AST) – as well as circulatory markers linked with OS – catalase (CAT), ROS, malondialdehyde (MDA), and superoxide dismutase (SOD).
ELISA
ELISA Kits (R&D Systems) were used to evaluate the pro-inflammatory factors IL-1, IL-6, IL-1β, and TNF-α.
Western blotting (WB) assay
Hepatic lysates underwent homogenization with lyses buffer (Beyotime, China) and protein was quantified via Bradford assay (Bio-Rad, Hercules, CA). Standard SDS-PAGE was used for protein assessment. Enhanced chemiluminescence (ECL) plus detection reagent (Pierce, Rockford, IL) were used to measure immunoreactive bands, which underwent analysis with the Omega 16ic Chemiluminescence Imaging System (UltraLum, CA). Primary antibodies used in our research were as follows: rabbit anti-Phospho-IKKα/β (2697, Cell Signaling Technology, USA), β-actin (1: 1000, Sigma, USA), rabbit anti-Phospho-NF-κB p65 (3033, Cell Signaling Technology, USA), Bcl-2 (1: 1000, CST, 2876, USA), and BAX (1: 1000, CST, 2772S, USA).
Statistical analysis
Results are presented as mean ±S.D. One-way ANOVA prior to Tukey’s post hoc test was used for outcome evaluation. A nonparametric test was applied to assess differences among groups, if needed. The level of significance was set at P<0.05.
Results
Atorvastatin triggered hepatic toxicity in DM rats
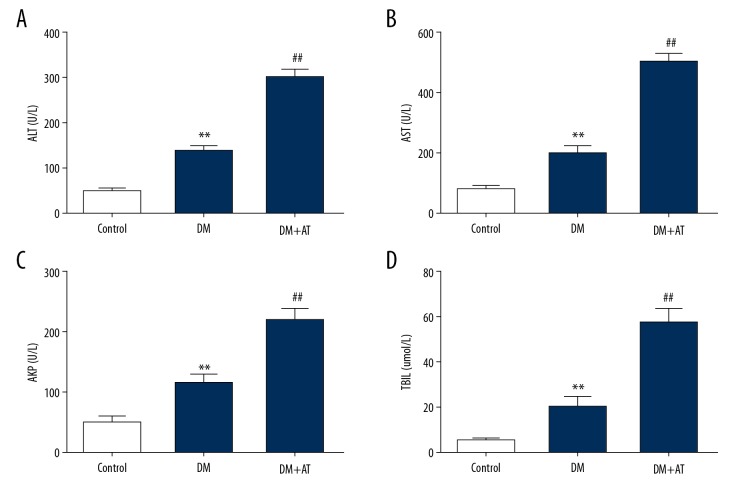
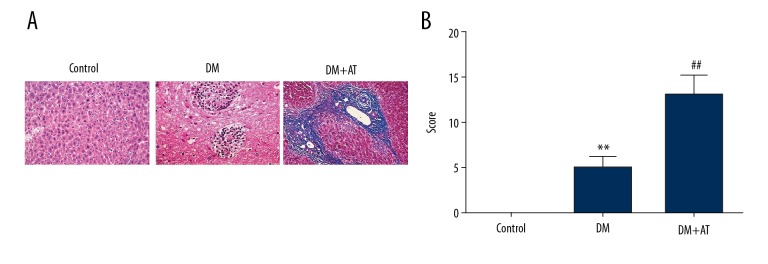
DM rats had markedly elevated concentrations of liver toxicity markers, and atorvastatin supplementation excessively promoted the elevation (Figure 1). Concentrations of ALT, AKP, AST, and TBIL were elevated in DM-AM rats in comparison to DM rats. Histopathological evaluation revealed that atorvastatin supplement provoked lipid degradation and vacuolization, apoptosis, infiltration of inflammatory cells, and diffused hemorrhaging (Figure 2). DM-AM rats had elevated injury scores, in conformity with the change in liver toxicity markers. These findings suggest that atorvastatin triggered serious liver damage in diabetic cells.
Figure 1.
Concentrations of ALT (A), AST (B), AKP (C), and DBIL (D) in control rats (CON), diabetic rats (DM), and DM rats that underwent 10-day atorvastatin supplement (20 mg/kg; DM+AT). Mean ± standard deviation (n=5) was used to describe the results, ** p<0.01 vs. CON, ## p<0.01 vs. DM.
Figure 2.
Histological assessment of the liver of CON, DM-AM, and DM rats. (A) Every section was subjected to HE staining. (B) Histopathological injury scores for every rat. Mean ± standard deviation (n=5) was used to describe the results, ** p<0.01 vs. CON, ## p<0.01 vs. DM.
Atorvastatin promoted OS-associated markers in diabetic rats
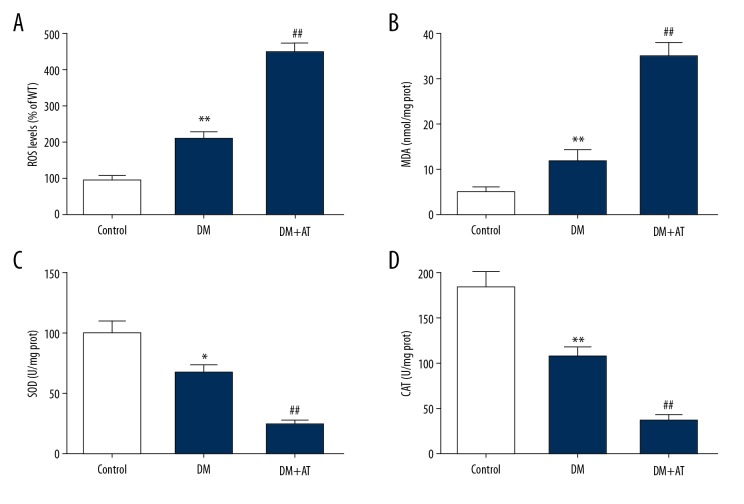
Markers associated with OS were examined, showing that atorvastatin suppressed CAT and SOD function and promoted hepatic concentrations of MDA and ROS (Figure 3). These findings suggest that OS contributes to atorvastatin-triggered liver toxicity of diabetic rats.
Figure 3.
Concentrations of ROS (A), MDA (B), SOD (C), and CAT (D) of CON, DM, and DM-AM rats. Mean ± standard deviation (n=5) was used to describe the results, * p<0.05, ** p<0.01 vs. CON, ## p<0.01 vs. DM.
Atorvastatin elevated the production of pro-inflammatory factors in diabetic rats
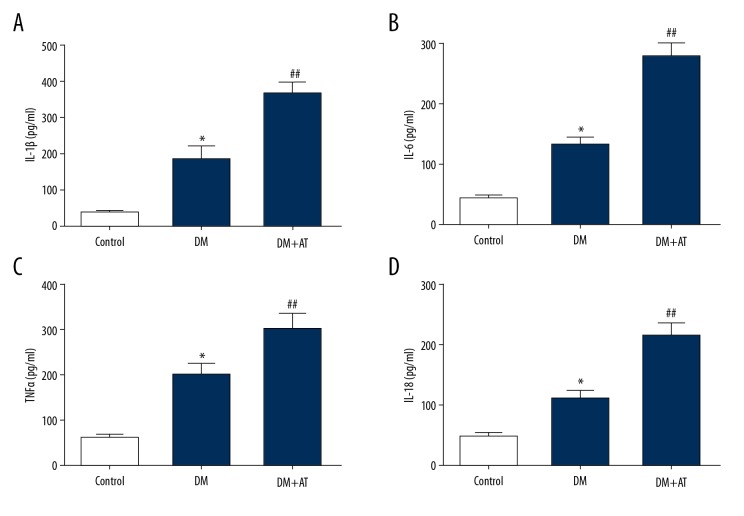
Because inflammation is an essential contributor to hepatic injury, we examined the concentrations of inflammation-promoting factors using ELISA. We discovered that DM-AM rats displayed increased concentrations of IL-18, IL-6, IL-1β, and TNF-α compared to DM rats (Figure 4). These findings indicate that inflammation plays a role in atorvastatin-triggered liver toxicity in diabetic rats.
Figure 4.
Concentrations of IL-1β (A), IL-6 (B), TNF-α (C) and IL-18 (D) of CON, DM, and DM-AM rats. Mean ± standard deviation (n=5) was used to describe the results, * p<0.05 vs. CON, ## p<0.01 vs. DM.
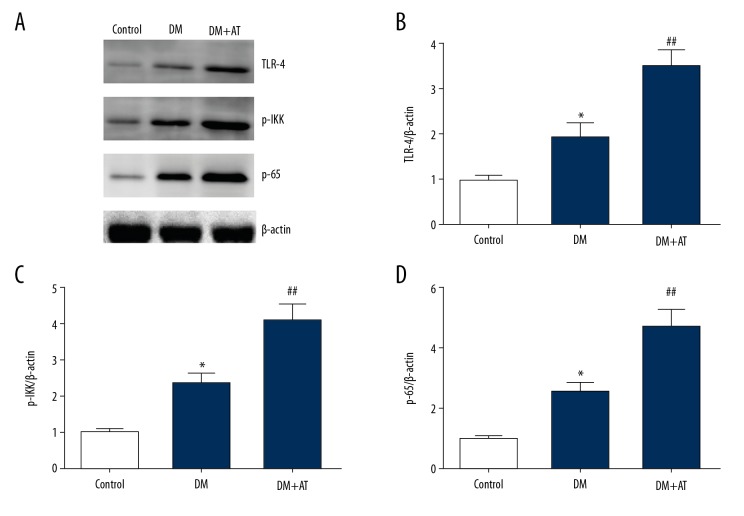
Atorvastatin promoted stimulation of the NF-κB pathway in diabetic rats
The NF-κB pathway is an essential contributor to generation of inflammation-promoting factors. Our research revealed that atorvastatin remarkably upregulated TLR-4 and promoted phosphorylation of IKK and p65 in rat livers (Figure 5). Our research findings suggest that atorvastatin promoted production of pro-inflammatory factors by enhancing activation of the NF-κB signal pathway in diabetic rats.
Figure 5.
Representative immunoblots (A) and quantitative assessment of TLR-4 (B), p-IKK (C), and p-p65 (D) of CON, DM, and DM-AM rats. Mean ± standard deviation (n=5) was used to describe the results, * p<0.05 vs. CON, ## p<0.01 vs. DM.
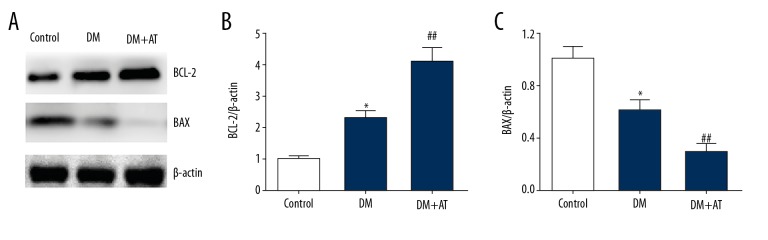
Atorvastatin inhibited the expression of apoptosis-related proteins in diabetic rats
WB analysis was used to evaluate expression of proteins associated with cell death. Apoptosis counteracting BCL-2 was noticeably upregulated, while apoptosis promoting BAX was remarkably downregulated in DM-AM rats compared to diabetic rats (Figure 6). Taken together, these results suggest that cell death suppression plays a role in atorvastatin-triggered liver toxicity in diabetic rats.
Figure 6.
Representative immunoblots (A) and quantitative assessment of BCL-2 (B) and BAX (C) of CON, DM, and DM-AM rats. Mean ± standard deviation (n=5) was used to describe the results, * p<0.05 vs. CON, ## p<0.01 vs. DM.
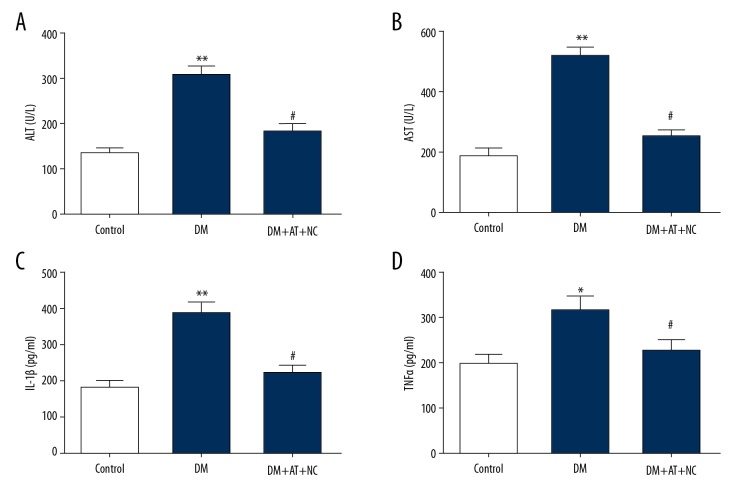
Inhibition of ROS reversed the effects of atorvastatin on hepatic toxicity and inflammation in diabetic rats
Oxidative stress (OS) plays an important role in hepatotoxicity and inflammation; therefore, we explored whether atorvastatin induced liver toxicity via oxidative stress. Diabetic rats were pretreated with ROS inhibitor NAC and then treated with atorvastatin. As shown in Figure 7, atorvastatin excessively elevated the levels of ALT and AST in DM rats and this effect was reversed by NAC treatment. In addition, atorvastatin significantly increased IL-1β and TNF-α production in DM rats, but this alternation was prevented by NAC treatment. Taken together, these results demonstrate that atorvastatin triggers liver toxicity in diabetic rats by promoting oxidative stress.
Figure 7.
Inhibition of ROS reversed the effects of atorvastatin on hepatic toxicity and inflammation in diabetic rats. (A, B) Concentrations of ALT (A) and AST (B) in diabetic rats (DM), in DM rats treated with ten-day atorvastatin (20 mg/kg; DM+AT), and in DM rats treated with 10-day 20 mg/kg atorvastatin and 2 mg/kg NAC (DM+AT+NAC). (C, D) Levels of IL-1β (C) and TNF-α (D) in different groups. Mean ± standard deviation (n=5) was used to describe the results, * p<0.05, ** p<0.01 vs. DM, # p<0.05 vs. DM+AT.
Discussion
Our research mainly revealed that atorvastatin caused serious liver toxicity in diabetic rats, demonstrated by noticeable changes in liver toxicity markers in the circulation. Histological assessment additionally verified the toxicity.
The liver is susceptible to various kinds of chronic damage because of its special anatomic location and function [19]. The liver is able to eradicate free radicals produced from metabolism of multiple drugs and xenobiotics, relying on its cellular antioxidant system. The increased production of free radicals and suppressed antioxidant defense in liver cells accelerates OS development and brings about liver malfunction [20,21]. OS is an essential contributor to the etiology of multiple chronic liver disorders (CLDs). Growing evidence verifies that OS participates in the etiology of CLD triggered by chemicals as well as by drugs [22,23]. Chronic hepatic damage is characterized by cholestasis, cirrhosis, necrosis, and fibrosis. It has previously been proven that long-term treatment with immunosuppressive, anti-tubercular, and anti-retroviral drugs can trigger free-radical production during liver biotransformation [24,25]. Drug-triggered CLD has a wide spectrum of pathological features, such as chronic hepatitis, cholestasis, vascular injury, acute hepatic necrosis, hepatic fibrosis, and generation of malignancies. For example, long-term administration of anti-tubercular drugs in patients with HIV and tuberculosis (TB) co-infection induces OS-triggered liver toxicity [26]. Treatment with anti-tubercular drugs, including isoniazid(INH), pyrazinamide, and rifampicin, leads to OS at therapeutic doses in humans, and at toxic doses in animals [27]. Reactive metabolites are secreted during liver biotransformation of those drugs, thereby disturbing liver cell membranes and inducing OS-triggered necrosis. Atorvastatin increased concentrations of ALT, AKP, TBIL, and AST. Moreover, histopathological assessment revealed that atorvastatin provoked lipid degradation and vacuolization, infiltration of inflammatory cells, necrosis, and diffused hemorrhaging. Additionally, atorvastatin suppressed CAT and SOD function while elevating liver concentrations of MDA and ROS. Inhibition of ROS reversed the effects of atorvastatin on hepatic toxicity and inflammation in diabetic rats. These findings suggest that atorvastatin triggers serious liver damage in diabetic rats by OS stimulation.
Various toxic, inflammatory, and metabolic reactions lead to hepatic damage and disorders. Cell death accounts for physiological elimination of undesired cells, including injured or senescent cells, in mature or developing tissues. In balance with mitosis, cell death is a dominant mechanism for protecting hepatic homeostasis in normal cell alteration, and for regulating hepatic proliferation and reproduction [28,29]. Nevertheless, the crucial influence of cell death in modulating liver size has been proven. First, mice lacking the death receptor Fas/CD95, a dominant modulator of liver cell death, present noticeable liver hyperplasia [30]. Second, remission of liver hyperplasia results from cell death, as proven in various models [31–33]. Furthermore, in 2 biliary epithelial hyperplasia rat models, remission of aberrant proliferation of bile ducts is attributable to biliary epithelial apoptosis [34]. Moreover, cell death can enhance liver renovation by releasing growth signals that trigger proliferation of progenitor cells. Mice lacking caspase 3 or caspase 7, the 2 crucial mediators of cell death, display suppressed liver renovation [35]. Our research demonstrated that BCL-2 was noticeably upregulated and BAX was remarkably downregulated in DM-AM rats compared to DM rats. These findings indicate that cell death suppression could be a factor in atorvastatin-triggered liver toxicity in diabetic rats.
Kupffer cells (KCs) are the resident hepatic macrophages in lymph nodes, the portal tract, and sinusoids [36]. KC stimulation is an essential contributor to hepatic injury etiology and development, as proven in research that reveals KC exhaustion impairs insulin resistance, fibrosis, and inflammatory development [37]. Like other macrophages, KCs can experience multiple types of stimulation, depending on the local environment. PAMPs are dominant stimulators that propel the classic “M1” phenotype of KCs, such as LPS. Additionally, other gut bacteria metabolites bind to TLRs of KCs, leading to generation of inflammation-promoting cytokines and chemokines. This is a crucial stage in stimulating local inflammatory reactions and exacerbating liver cell damage, bringing about DAMP release. DAMPs stimulate KCs through TLR pathways, consequently inducing a malignant inflammation cycle [38,39]. Our research revealed that atorvastatin promoted NF-κB stimulation, TLR-4 expression, p65, and IKK phosphorylation, which contribute to generation of inflammation-promoting factors. These findings indicate that inflammation plays a role in atorvastatin-triggered liver toxicity in diabetic rats.
Conclusions
In summary, atorvastatin triggers liver toxicity in diabetic rats through stimulation of inflammation, OS, and cell death suppression.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Koo BK, Lee CH, Yang BR, et al. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: A National Health Insurance Database Study. PLoS One. 2014;9:e110650. doi: 10.1371/journal.pone.0110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagheri B, Alikhani A, Mokhtari H, Rasouli M. Esterification of HDL-cholesterol is decreased in diabetes mellitus and CAD and enhanced following treatment with statins. Med Arch. 2018;72:197–201. doi: 10.5455/medarh.2018.72.197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karahalil B, Hare E, Koc G, et al. Hepatotoxicity associated with statins. Arh Hig Rada Toksikol. 2017;68:254–60. doi: 10.1515/aiht-2017-68-2994. [DOI] [PubMed] [Google Scholar]
- 4.Licata A, Giammanco A, Minissale MG, et al. Liver and atatins: A critical appraisal of the evidence. Curr Med Chem. 2018;25:5835–46. doi: 10.2174/0929867325666180327095441. [DOI] [PubMed] [Google Scholar]
- 5.Bril F, Portillo Sanchez P, Lomonaco R, et al. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: Post hoc analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102:2950–61. doi: 10.1210/jc.2017-00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moctezuma-Velázquez C, Abraldes JG, Montano-Loza AJ. The use of statins in patients with chronic liver disease and cirrhosis. Curr Treat Options Gastroenterol. 2018;16(2):226–40. doi: 10.1007/s11938-018-0180-4. [DOI] [PubMed] [Google Scholar]
- 7.Busanello ENB, Marques AC, Lander N, et al. Pravastatin chronic treatment sensitizes hypercholesterolemic mice muscle to mitochondrial permeability transition: Protection by creatine or coenzyme Q10. Front Pharmacol. 2017;8:185. doi: 10.3389/fphar.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Docrat TF, Nagiah S, Krishnan A, et al. Atorvastatin induces MicroRNA-145 expression in HEPG2 cells via regulation of the PI3K/AKT signalling pathway. Chem Biol Interact. 2018;287:32–40. doi: 10.1016/j.cbi.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Lewicki M, Ng I, Schneider AG. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database Syst Rev. 2015:CD010480. doi: 10.1002/14651858.CD010480.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurukovska-Nospal M, Arsova V, Levchanska J, Sidovska-Ivanovska B. Effects of statins (atorvastatin) on serum lipoprotein levels in patients with primary hyperlipidemia and coronary heart disease. Prilozi. 2007;28(2):137–48. [PubMed] [Google Scholar]
- 11.He BX, Shi L, Qiu J, et al. The effect of CYP3A4*1G allele on the pharmacokinetics of atorvastatin in Chinese Han patients with coronary heart disease. J Clin Pharmacol. 2014;54(4):462–67. doi: 10.1002/jcph.229. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Tian Y, Lv P, et al. The effect of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and 2-hydroxyatorvastatin in healthy Chinese people. Pharmazie. 2017;72:365–68. doi: 10.1691/ph.2017.6944. [DOI] [PubMed] [Google Scholar]
- 13.Shan S, Shen Z, Song F. Autophagy and acetaminophen-induced hepatotoxicity. Arch Toxicol. 2018;92:2153–61. doi: 10.1007/s00204-018-2237-5. [DOI] [PubMed] [Google Scholar]
- 14.Khodayar MJ, Kalantari H, Khorsandi L, et al. Betaine protects mice against acetaminophen hepatotoxicity possibly via mitochondrial complex II and glutathione availability. Biomed Pharmacother. 2018;103:1436–45. doi: 10.1016/j.biopha.2018.04.154. [DOI] [PubMed] [Google Scholar]
- 15.Bajt LM, Ramachandran A, Yan HM, et al. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifi-Rigi A, Heidarian E, Amini SA. Protective and anti-inflammatory effects of hydroalcoholic leaf extract of Origanum vulgare on oxidative stress, TNF-α gene expression and liver histological changes in paraquat-induced hepatotoxicity in rats. Arch Physiol Biochem. 2019;125(1):56–63. doi: 10.1080/13813455.2018.1437186. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Tan X, Lv Z, et al. Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci Rep. 2016;6:37157. doi: 10.1038/srep37157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Zhang M, Hu MY, et al. Increased glucagon-like peptide-1 secretion may be involved in antidiabetic effects of ginsenosides. J Endocrinol. 2013;217:185–96. doi: 10.1530/JOE-12-0502. [DOI] [PubMed] [Google Scholar]
- 19.Fruci B, Giuliano S, Mazza A, et al. Nonalcoholic fatty liver: A possible new target for type 2 diabetes prevention and treatment. Int J Mol Sci. 2013;14:22933–66. doi: 10.3390/ijms141122933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Echeverría F, Valenzuela R, Bustamante A, et al. Attenuation of high-fat diet-induced rat liver oxidative stress and steatosis by combined hydroxytyrosol-(HT-) eicosapentaenoic acid supplementation mainly relies on HT. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/5109503. 5109503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiao MM, Lin YJ, Yu HR, et al. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018;17:178. doi: 10.1186/s12944-018-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briggs D, Lok E, Nera EA, et al. Short-term effects of butylated hydroxytoluene on the Wistar rat liver, urinary bladder and thyroid gland. Cancer Lett. 1989;46:31–36. doi: 10.1016/0304-3835(89)90211-5. [DOI] [PubMed] [Google Scholar]
- 23.Homma T, Kurahashi T, Lee J, et al. Double knockout of peroxiredoxin 4 (Prdx4) and superoxide dismutase 1 (Sod1) in mice results in severe liver failure. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/2812904. 2812904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arab JP, Arrese M. Old remedies to heal the liver: Novel effects of digoxin in hepatic sterile inflammation. Hepatology. 2019;69:904–6. doi: 10.1002/hep.30188. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Kikuchi H, Ohta M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–62. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- 26.Pich J. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy. Int J Nurs Pract. 2018;24:e12627. doi: 10.1111/ijn.12627. [DOI] [PubMed] [Google Scholar]
- 27.Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J Gastroenterol. 2018;19:56–64. doi: 10.1016/j.ajg.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Vitaglione P, Morisco F, Mazzone G, et al. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52:1652–61. doi: 10.1002/hep.23902. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Liu Y, Chen H, et al. TIM-1 attenuates the protection of ischemic preconditioning for ischemia reperfusion injury in liver transplantation. Am J Transl Res. 2017;9:3665–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 31.Tucker MJ, Kalinowski AE, Orton TC. Carcinogenicity of cyproterone acetate in the mouse. Carcinogenesis. 1996;17:1473–76. doi: 10.1093/carcin/17.7.1473. [DOI] [PubMed] [Google Scholar]
- 32.Columbano A, Ledda-Columbano GM, Coni PP, et al. Occurrence of cell death (apoptosis) during the involution of liver hyperplasia. Lab Invest. 1985;52:670–75. [PubMed] [Google Scholar]
- 33.Grasl-Kraupp B, Rossmanith W, Ruttkay-Nedecky B, et al. Levels of transforming growth factor beta and transforming growth factor beta receptors in rat liver during growth, regression by apoptosis and neoplasia. Hepatology. 1998;28:717–26. doi: 10.1002/hep.510280318. [DOI] [PubMed] [Google Scholar]
- 34.Bhathal PS, Gall JA. Deletion of hyperplastic biliary epithelial cells by apoptosis following removal of the proliferative stimulus. Liver. 2010;5:311–25. doi: 10.1111/j.1600-0676.1985.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 35.Bertrand MJ, Milutinovic S, Dickson KM, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Sheng M, Weng Y, et al. Berberine protects against ischemia/reperfusion injury after orthotopic liver transplantation via activating Sirt1/FoxO3α induced autophagy. Biochem Biophys Res Commun. 2017;483(2):885–91. doi: 10.1016/j.bbrc.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Scott CL, Guilliams M. The role of Kupffer cells in hepatic iron and lipid metabolism. J Hepatol. 2018;69:1197–99. doi: 10.1016/j.jhep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv Q, Gao R, Peng C, et al. Bisphenol A promotes hepatic lipid deposition involving Kupffer cell M1 polarization in male mice. J Endocrinol. 2017;234(2):143–54. doi: 10.1530/JOE-17-0028. [DOI] [PubMed] [Google Scholar]
- 39.Luo W, Xu Q, Wang Q, et al. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. doi: 10.1038/srep44612. [DOI] [PMC free article] [PubMed] [Google Scholar]