Abstract
Background
Anorexia nervosa and bulimia nervosa are complex mental disorders, and their etiology is still not fully understood. This paper reviews the literature on diffusion tensor imaging studies in patients with anorexia nervosa and bulimia nervosa to explore the usefulness of white matter microstructural analysis in understanding the pathophysiology of eating disorders.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to identify diffusion tensor imaging studies that compared patients with an eating disorder to control groups. We searched relevant databases for studies published from database inception to August 2018, using combinations of select keywords. We categorized white matter tracts according to their 3 main classes: projection (i.e., thalamo–cortical), association (i.e., occipital–parietal–temporal–frontal) and commissural (e.g., corpus callosum).
Results
We included 19 papers that investigated a total of 427 participants with current or previous eating disorders and 444 controls. Overall, the studies used different diffusion tensor imaging approaches and showed widespread white matter abnormalities in patients with eating disorders. Despite differences among the studies, patients with anorexia nervosa showed mainly white matter microstructural abnormalities of thalamo–cortical tracts (i.e., corona radiata, thalamic radiations) and occipital–parietal–temporal–frontal tracts (i.e., left superior longitudinal and inferior fronto-occipital fasciculi). It was less clear whether white matter alterations persist after recovery from anorexia nervosa. Available data on bulimia nervosa were partially similar to those for anorexia nervosa.
Limitations
Study sample composition and diffusion tensor imaging analysis techniques were heterogeneous. The number of studies on bulimia nervosa was too limited to be conclusive.
Conclusion
White matter microstructure appears to be affected in anorexia nervosa, and these alterations may play a role in the pathophysiology of this eating disorder. Although we found white matter alterations in bulimia nervosa that were similar to those in anorexia nervosa, white matter changes in bulimia nervosa remain poorly investigated, and these findings were less conclusive. Further studies with longitudinal designs and multi-approach analyses are needed to better understand the role of white matter changes in eating disorders.
Introduction
Anorexia nervosa and bulimia nervosa are complex and serious mental disorders.1 They are characterized by altered eating behaviours; intense preoccupations with weight, eating and body shape; and specific physical signs.1 Although it is widely accepted that the etiology of eating disorders can be multifactorial and may comprise biological, psychological and social factors (e.g., Kaye and colleagues,2 Zipfel and colleagues3), this etiology is not fully understood and there are no widely accepted and targeted treatment strategies for the different eating disorders (e.g., Zipfel and colleagues,3 Frank and Kaye4).
In recent decades, the development of several neuroimaging tools has helped us to better understand the neurobiological substrates of eating disorders. Structural neuroimaging studies on anorexia nervosa using voxel-based morphometry have revealed that patients with acute anorexia nervosa have global and regional grey matter decreases, global white matter decreases and cerebrospinal fluid increases (for meta-analyses, see Seitz and colleagues5 and Titova and colleagues6). These structural alterations seem to be largely (e.g., Castro-Fornieles and colleagues7 and Mainz and colleagues8) or completely (e.g., Lazaro and colleagues,9 Nickel and colleagues10 and Bang and colleagues11) restored after recovery from anorexia nervosa. Furthermore, some structural studies have investigated cortical thickness changes in patients with anorexia nervosa, showing that cortical thinning occurred in patients with acute anorexia nervosa and that such alterations were fully reversible after recovery (e.g., Nickel and colleagues,10 King and colleagues12 and Bernardoni and colleagues13).
Only a few studies have investigated structural brain abnormalities in eating disorders other than anorexia nervosa (for a review, see van den Eynde and colleagues14). In particular, voxel-based morphometry studies in patients with bulimia nervosa have indicated grey matter increases in frontal and ventral striatal areas.14 Increases in grey matter volume have also been found bilaterally in the somatosensory regions, the precuneus and the paracentral lobule in patients with bulimia nervosa with a long duration of disease.15 On the other hand, functional neuroimaging studies have shown alterations in response to specific tasks4,16 and at rest.17,18 Task-related functional MRI (fMRI) studies have mainly shown functional abnormalities in neural circuits relating to reward, taste and executive control (for reviews, see Kaye and colleagues2 and Frank and Kaye4) and also in those related to body image perception and processing (for a review, see Gaudio and Quattrocchi16). In resting-state fMRI studies, patients with anorexia nervosa have shown functional abnormalities in areas and/or networks mostly implicated in cognitive control and visual and homeostatic integration (for a review, see Gaudio and colleagues17). Few resting-state fMRI studies have investigated patients with bulimia nervosa, but altered functional connectivity in somatosensory, visual and limbic systems has been shown.18,19
In recent years, microstructural white matter changes in eating disorders have been investigated using diffusion tensor imaging (DTI).20 This technique is sensitive to the random movement of water in the cells of a target tissue, yielding measures of the magnitude and orientation of movement.21,22 Diffusion tensor imaging allows for the investigation of several white matter parameters, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). Fractional anisotropy is usually considered to reflect better white matter integrity because of greater intravoxel coherence of fibre orientation, axon density and diameter and/or myelination.23,24 Mean diffusivity is considered to be particularly sensitive to extracellular volume25 and inflammation.26 Axial diffusivity is usually related to axon morphological changes27 and RD to the myelination process.28,29 In particular, AD and RD parameters seem to provide complementary information that can help in understanding FA or MD changes, reflecting the diffusion parallel with and perpendicular to the axon, respectively.30 Overall, DTI parameter alterations can have several causes, and the biological mechanisms underpinning white matter diffusivity measures have not been entirely explained.31
Different approaches can be used to analyze DTI data, such as region-of-interest and whole-brain analyses. Whole-brain analyses seem to provide better comparability across studies than region-of-interest analyses, because they appear to overcome the bias of region-placement preference and the absence of meaningful voxels outside the selected regions.32
The most used whole-brain approaches are voxel-based analysis (VBA; which evaluates local voxel-wise differences across the whole brain33) and tract-based spatial statistics (TBSS; which evaluates changes in a skeleton, comprising the centre of the white matter tracts).34 Another approach is fibre tractography analysis, which is based on directional data from the tensor and can provide information about the 3-dimensional white matter connectivity of the human brain.35,36
To date, different methodological approaches have been used in studies of eating disorders (e.g., Kaufmann and colleagues,37 Kazlouski and colleagues38 and Pfuhl and colleagues39), and the literature is growing rapidly.
The aim of this paper, in addition to expanding a previous review of 6 DTI studies in anorexia nervosa,40 is to systematically review DTI studies in patients with eating disorders (i.e., anorexia nervosa and bulimia nervosa) and explore whether this technique provides useful insights into the pathophysiology of eating disorders and contributes to the development of more targeted therapeutic strategies. To this aim, we will consider the results and interpretations of different DTI studies and approaches to evaluate whether there is consistency in the white matter tracts/brain regions affected. Finally, we will discuss methodological implications, relevant themes and future considerations for the ultimate usefulness of DTI in eating disorder research.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.41 The guidelines consist of a checklist of recommended items to be reported and a 4-step flow diagram (Appendix 1, available at jpn.ca).
Search strategy and inclusion criteria
We used the following databases for the search: PubMed (from database inception to August 2018) and Scopus (from database inception to August 2018). We searched using the following terms: “anorexia nervosa,” “bulimia nervosa” OR “eating disorders” AND “diffusion tensor imaging,” “diffusion tensor,” “DTI” or “white matter.” We scrutinized the reference lists of examined full-text papers for additional relevant publications. We also contacted expert colleagues in the field for suggestions of further studies that were not considered in our search. To be included in the review, studies had to: (1) be written in English; (2) investigate a sample of participants who currently had or were recovered from anorexia nervosa or bulimia nervosa; (3) be of cross-sectional, case–control or longitudinal design; (4) adopt measures of diffusion imaging of brain white matter and use a whole-brain approach or investigate the major white matter tracts (i.e., projection, association and commissural fibres). Because of the limited number of peer-reviewed studies on eating disorders, we did not consider confounding factors such as sample inhomogeneity (e.g., anorexia nervosa subtypes) or the presence of psychiatric comorbidity or pharmacological history, which may have limited some of the studies included. Because of the lack of sufficient papers using similar acquisition sequences and/or methodological approaches, we were unable to perform a meta-analysis.
Quality assessment and data abstraction
To reduce the risk of bias, we followed PRISMA recommendations for systematic literature analysis. Two authors (S.G. and C.P.) independently selected paper abstracts and titles, analyzed the full papers that met the inclusion criteria, and resolved disagreements through consensus.
The data extracted from each study were as follows: sample type, study design, sample size, scanning methods and selected findings. In particular, considering that hydration state affects brain structure (e.g., see Streitburger and colleagues42), we extracted evaluation procedures for hydration state from DTI studies on patients with current anorexia nervosa.43
Results
Nineteen DTI studies on participants with eating disorders were included in the review (PRISMA flow diagram, Appendix 1). Overall, the studies included a total of 427 participants with a current or past eating disorder and 444 control participants. Table 1 reports the sample characteristics of each eating disorder study and the evaluation procedures for hydration state in studies of participants with current anorexia nervosa. Of the 19 included studies, 16 were cross-sectional studies (1 also included a longitudinal study) in participants with current or past anorexia nervosa, for a total of 367 patients and 371 controls; 2 were cross-sectional studies in participants with current bulimia nervosa, for a total of 48 patients and 49 controls; and 1 longitudinal study included participants with a restrictive eating disorder (i.e., anorexia nervosa restrictive type and other specified feeding or eating disorder [OSFED] restrictive type). Considering the symptomatological similarities between anorexia nervosa and OSFED restrictive type,1 we have summarized the longitudinal study on participants with restrictive eating disorder in the anorexia nervosa section.
Table 1.
Sample characteristics of DTI studies and evaluation procedures for hydration state
| Study | Participants, n | Age, yr mean ± SD | BMI, kg/m2 mean ± SD | Eating disorder duration, yr mean ± SD | Evaluation of hydration status |
|---|---|---|---|---|---|
| Cross-sectional studies in participants with anorexia nervosa | |||||
| Frank et al.44 | AN = 19 | 15.4 ± 1.4 | 16.2 ± 1.1 | NR | Supervised food and fluid intake |
| HC = 22 | 14.8 ± 1.8 | 21.3 ± 1.9 | — | ||
| Gaudio et al.45 | AN = 14 | 15.7 ± 1.6 | 16.2 ± 1.2 | 0.4 ± 0.1 | Voxel-based morphometry analysis of grey matter, white matter and cerebrospinal fluid volumes |
| HC = 15 | 16.3 ± 1.5 | 21.1 ± 1.9 | — | ||
| Hayes et al.46 | AN = 8 | 35 ± 11 | NR | 16.25 ± 6.4 | NR |
| HC = 8 | 36 ± 9 | — | — | ||
| Hu et al.47 | AN = 8 | 17.6 ± 2.2 | 14.3 ± 1.3 | 0.9 ± 6.2 | At least 1 week of supervised meals and hydration |
| HC = 14 | 19.1 ± 3.1 | 20.1 ± 1.7 | — | ||
| Kaufmann et al.37 | AN = 25 | 22.84 ± 4.75 | 13.83 ± 1.33 | 16.04 ± 2.63 | Supervised food and fluid intake; volumes of the third and lateral ventricles as covariates; correction for free water at the voxel level |
| HC = 25 | 23.36 ± 3.35 | 21.07 ± 1.93 | — | ||
| Kazlouski et al.38 | AN = 16 | 23.9 ± 7 | 16.5 ± 1 | 7.5 ± 8 | Supervised food and fluid intake; exclusion criteria: gross electrolyte or complete blood count abnormalities |
| HC = 17 | 25.1 ± 4 | 21.5 ± 1 | — | ||
| Nagahara et al.48 | AN = 17 | 23.8 ± 6.68 | 13.6 ± 1.3 | 4.93 ± 4.9 | Electrolytes and complete blood count |
| HC = 18 | 26.2 ± 5.6 | 19.9 ± 2.0 | — | ||
| Travis et al.49 | AN = 15 | 16.6 ± 1.4 | 16.0 ± 1.2 | 1.4 ± 1.0 | NR |
| HC = 15 | 17.1 ± 1.3 | 21.4 ± 2.1 | — | ||
| Via et al.50 | AN = 19 | 28.37 ± 9.55 | 17.03 ± 1.09 | 6.5 ± 6.0 | Supervised food and fluid intake |
| HC = 19 | 28.63 ± 8.58 | 21.09 ± 1.80 | — | ||
| Vogel et al.51 | AN = 22 | 15.03 ± 1.60 | 15.36 ± 1.08 | 1.20 ± 1.30 | Urine specific gravity |
| ANd = 9* | 14.76 ± 2.30 | 17.45 ± 1.43 | NR | ||
| HC = 21 | 15.17 ± 1.28 | 20.34 ± 2.59 | — | ||
| Cross-sectional and longitudinal studies in participants with anorexia nervosa | |||||
| von Schwanenflug et al.52 | AN = 56 | 15.9 ± 2.9 | 14.7 ± 1.3 | 1.2 ± 1.8 | Urine specific gravity |
| ANf = 44 | 15.7 ± 2.3 | 18.7 ± 1.1 | NR | ||
| HC = 60 | 16.2 ± 2.9 | 20.6 ± 2.4 | — | ||
| Cross-sectional studies in participants with current and past anorexia nervosa | |||||
| Frieling et al.53 | AN = 12 | 26.84 ± 6.94 | 15.18 ± 1.39 | NR | NR |
| ANrec = 9 | 27.44 ± 5.32 | 19.31 ± 1.39 | NR | ||
| HC = 20 | 24.80 ± 2.60 | 19.60 ± 0.94 | — | ||
| Pfuhl el al.39 | AN = 35 | 16.1 ± 2.8 | 14.70 ± 1.31 | NR | Urine specific gravity |
| ANrec = 35 | 22.5 ± 3.0 | 21.09 ± 1.91 | NR | ||
| HC v. AN = 31 | 16.4 ± 2.6 | 20.75 ± 2.98 | — | ||
| HC v. ANrec = 31 | 22.5 ± 2.9 | 21.34 ± 2.18 | — | ||
| Cross-sectional studies in participants recovered from anorexia nervosa | |||||
| Bang et al.54 | ANrec = 21 | 27.6 ± 5.1 | 20.4 ± 1.7 | 2.8 ± 2.3 | — |
| HC = 21 | 26.1 ± 4.7 | 21.8 ± 1.8 | — | ||
| Shott et al.55 | ANrec = 24 | 30.25 ± 8.13 | 20.83 ± 2.37 | 5.90 ± 5.21 | — |
| HC = 24 | 27.42 ± 6.28 | 21.64 ± 1.26 | — | ||
| Yau et al.56 | ANrec = 12 | 28.7 ± 7.9 | 21.2 ± 1.5 | 5.7 ± 5.2 | — |
| HC = 10 | 26.7 ± 5.4 | 22.0 ± 1.1 | — | ||
| Longitudinal studies in participants with restrictive eating disorders | |||||
| Olivo et al.57 | RED = 12 | 15.3 ± 1.5 | 18.7 ± 2.9 | 0.7 ± 0.46 | NR |
| REDf = 12 | 16.4 ± 1.5 | 21.1 ± 2.7 | NR | ||
| HC = 24 | 14.1 ± 1.0 | 20.6 ± 2.6 | — | ||
| Cross-sectional studies in participants with bulimia nervosa | |||||
| He et al.58 | BN = 28 | 21.32 ± 6.11 | 21.95 ± 2.13 | 5.9 ± 6.4 | — |
| HC = 28 | 20.61 ± 6.12 | 22.18 ± 2.14 | — | ||
| Mettler et al.59 | BN = 20 | 25.2 ± 5.3 | 22.59 ± 5.69 | 6.2 ± 5.3 | — |
| HC = 21 | 27.5 ± 6.6 | 21.55 ± 1.19 | — | ||
AN = anorexia nervosa; ANd = anorexia nervosa at discharge; ANf = anorexia nervosa at follow-up; ANrec = anorexia nervosa, recovered; BN = bulimia nervosa; BMI = body mass index; DTI = diffusion tensor imaging; HC = healthy control; NR = not reported; RED = restrictive eating disorder; rEDf = restrictive eating disorder at follow-up; SD = standard deviation.
An exploratory longitudinal study was also conducted.
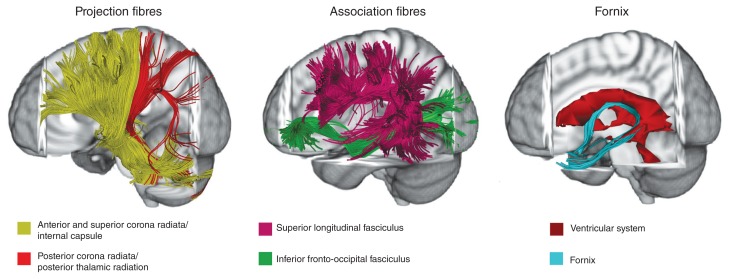
Table 2 reports scanning methods, main results and main clinical interpretations from the 19 included studies. Investigated tracts were typically selected from a white matter anatomic atlas and mapped on either individual or group-average FA maps. Figure 1 reports the main white matter tracts affected in eating disorders. In the following sections, DTI studies will be summarized based on eating disorder diagnosis, considering white matter tracts as projection, association and commissural fibres (e.g., see Catani and colleagues,62 Mori and colleagues63 and Wakana and colleagues64).
Table 2.
Scanning methods, main results and main clinical interpretation from DTI studies in patients with eating disorders (part 1 of 5)
| Study | Methods | Main results | Main clinical interpretation | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Field strength/ sequence | No. of directions | Tool | Type of analysis | DTI measures | Atlas | |||
| Cross-sectional studies in participants with anorexia nervosa | ||||||||
| Frank et al.44 | 3 T/NR | 25 | NordicICE | VBA | FA, ADC | Hutchins | Lower FA values in left fornix, bilateral CG, right forceps major, right superior and left posterior CR. Higher FA values in left SLF, bilateral anterior CR and bilateral IFOF. Higher ADC values in left fornix, right CC, right corticospinal tract, right posterior CR, bilateral corticopontine tract and bilateral SLF. | Altered white matter integrity may be related to impaired taste, reward and emotional processing. |
| Gaudio et al.45 | 1.5 T/ SE, EP | 12 | FDT | TBSS | FA, MD, AD, RD | JHU white matter labels | Lower FA values in left anterior and superior CR, left SLF, fornix and body of the CC. Lower AD values in the left and right SLF, left superior and anterior CR, and the external capsule, posterior limb of internal capsule and posterior thalamic radiation (including optic radiation) of the right hemisphere. | White matter alterations may be related to altered cognitive flexibility and body-image disturbances. |
| Hayes et al.46 | 3 T/DSE, EP | 60 | FDT, 3D slicer | eXtended streamline tractography, ROI (subcallosal cingulate), ROI (fornix crus, PTR/SLF, CC splenium, ALIC, anterior CG, IFOF, posterior CG, CC genu) | Fibre connections, FA, AD, RD | DTI-81 atlas | Higher connectivity from the subcallosal cingulate white matter in the prefrontal and left parieto-occipital cortices, and lower connectivity in the thalamus identified by deterministic multitensor tractography. Lower FA values, associated with lower AD and higher RD, were found within the anterior limb of the internal capsule, right anterior CG, and left fornix crus and IFOF. Higher RD values were found in the anterior limb of the internal capsule and IFOF. | White matter tract alterations may be related to altered processing of affective stimuli, self-perception and interoception in anorexia nervosa. |
| Hu et al.47 | 3 T/SS, EP | 25 | DTI Studio, SPM 8, in-house made | VBA, ROI (based on group comparison results) | FA | — | VBA: significant decrease in FA maps in the left superior frontal gyrus, medial frontal gyrus, anterior cingulate cortex, middle frontal gyrus, inferior frontal gyrus, thalamus and bilateral insula. ROI: significantly positive correlations between the mean FA value of the left inferior frontal gyrus, insula and thalamus and BMI in patients with anorexia nervosa. | White matter alterations may be involved in pathophysiology of anorexia nervosa. |
| Kaufmann et al.37 | 3 T/SENSE, EP | 64 | FDT† | TBSS | FA (ROI) | Juelich Histological Atlas | ROI-based approach: fornix. No FA values differences between anorexia nervosa patients and controls after correction for free water. | FA values alterations of forniceal fibres seem significantly biased by partial volume effects in anorexia nervosa. |
| Kazlouski et al.38 | 3 T/NR | 25 | DTI Studio | VBA, ROI (based on group comparison results) | FA, ADC | Mori (2005) | Lower FA values in bilateral fimbria-fornix region, fronto-occipital fasciculus and posterior CG. Higher ADC values in frontoparietal and parietal–occipital white matter (with uncorrected threshold). | Altered white matter integrity in brain areas that integrate emotion, reward and cognitive behaviours may be related to pathophysiology of anorexia nervosa. |
| Nagahara et al.48 | 3 T/SS, SE, EP | 32 | FDT | TBSS | FA, MD | Johns Hopkins University | Lower FA values in cerebellum. Higher MD values in fornix. | Fornix and cebebellum white matter alterations may be related to the pathophysiology of anorexia nervosa. |
| Travis et al.49 | 3 T/DSE, EP | 96 | mrDiffusion | AFQ | FA* R1* | — | Lower and higher FA values in 6 tracts (18 examined) and 1 subdivision of the CC (8 examined). Lower R1 in 6 cerebral tracts (18 examined) and 5 subdivisions of the CC (8 examined). | White matter changes seem to be related to changes in myelin content. |
| Via et al.50 | 1.5 T/SS, SE, EP | 25 | FDT | TBSS | FA, MD, RD,* AD* | Mori (2007) | Lower FA values in the parietal part of the left SLF, associated with higher MD and RD values. Higher MD values in the fornix associated with lower FA and higher RD and AD values. | Altered white matter integrity may be related to body image distortion, altered weight regulation and reward-processing alterations. |
| Vogel et al.51† | 3 T/ DSE, EPI | 30 | FDT | TBSS, ROI (based on group comparison results) | FA, MD, RD, AD | — | Higher FA values in the bilateral superior CR, anterior CC, anterior and posterior thalamic radiation, anterior and posterior limb of internal capsule, and left inferior longitudinal fasciculus. Altered FA values were mainly related to lower RD and MD values but not to altered AD values. Exploratory longitudinal study showed similar results with a greatly reduced level of significance. | Altered FA values could be related to an acute state of anorexia nervosa. |
| Cross-sectional and longitudinal studies in participants with anorexia nervosa | ||||||||
| von Schwanenflug et al.52 | 3 T/NR | 32 | FDT | TBSS | FA, MD, AD, RD | Juelich Histological Atlas | Baseline: Lower FA and higher MD, AD and RD values in the body of CC. Longitudinal: No differences between follow-up patients and controls. Higher FA and lower AD, MD and RD v. baseline in the fornix, bilateral optic radiation and CC. Reduced FA and increased MD and RD v. baseline in right corticospinal tract. | Altered microstructural properties in people with anorexia nervosa normalized rapidly during nutritional therapy. These findings underline that structural brain alterations associated with the disorder are highly dynamic and more likely to represent consequences of starvation state than pre-existing or white matter degeneration. |
| Cross-sectional studies in participants with current and past anorexia nervosa | ||||||||
| Frieling et al.53 | 3 T/ SS, SE, EP | 15 | Native | VBA | FA | Talairach Daemon | Lower FA values in the posterior thalamic radiation bilaterally and the left mediodorsal thalamus in patients with anorexia nervosa compared with controls. Additional regional lower FA values in parts of the posterior CR bilaterally, the left middle cerebellar peduncle, and in parts of the left SLF. A volume-of-interest-based post hoc analysis of these regions (posterior thalamic radiation bilaterally and the left mediodorsal thalamus) showed no significant differences between patients with acute anorexia nervosa and patients recovered from anorexia nervosa. | FA value alterations may contribute to altered processing of the body image and impairments in cognitive domains. |
| Pfuhl el al.39 | 3 T/SE | 30 | TRACULA | Tractography, cluster analysis | FA, MD, RD, AD | — | Tractography: No differences in FA, MD, RD and AD between the acute anorexia nervosa subjects and controls. Supplementary cluster analysis: no significant differences. No significant differences in both the main and supplementary cluster analyses between people recovered from anorexia nervosa and controls. | White matter microstructure is preserved in patients with acute anorexia nervosa and patients recovered from anorexia nervosa. |
| Cross-sectional studies in participants recovered from anorexia nervosa | ||||||||
| Bang et al.54 | 3 T/SS, EP | 32 | FDT | TBSS, voxel-wise correlation | FA, MD, AD, RD | — | No significant differences between patients with anorexia nervosa and controls in white matter microstructure (FA, MD, RD and AD values). | White matter alterations observed during the acute phase of anorexia nervosa are reversible in long-term recovered patients. |
| Shott et al.55 | 3 T/NR | 25 | FDT | Probabilistic tractography, ROI (taste-reward circuit), TBSS | Fibre connections, FA | Mori (2005) | Seed-based approach: taste-reward-related white matter tracts. Higher white matter connectivity between bilateral insula regions and ventral striatum, left insula and middle orbitofrontal cortex, and right insula projecting to gyrus rectus and medial orbitofrontal cortex. Seed-based FA analysis: Lower FA values in some tracts between insula subregions and ventral striatum and orbitofrontal cortex regions. Whole-brain FA analysis: lower FA values in the anterior CR, external capsule and cerebellum including the corticopontine tract, CC, anterior thalamic radiation, inferior and middle cerebellar peduncle, as well as inferior fronto-occipital and uncinate fasciculi. | White matter alterations suggest altered connectivity within taste-reward pathways and may impair reward circuit functions that drives food intake. |
| Yau et al.56 | 3 T/SS, EP | 55 | FDT | TBSS | FA, MD, AD,* RD* | — | Lower MD values in 6 clusters encompassing parietal, CG and frontal white matter tracts. Lower LD and/or RD in these regions. No FA values alterations between patients recovered from anorexia nervosa and controls. | Lower MD values may be related to premorbid behavioural traits (harm avoidance and heightened concern for mistakes) through an exaggerated cognitive control. |
| Longitudinal studies in participants with restrictive eating disorders | ||||||||
| Olivo et al.57 | 3 T/EP | 48 | FDT | TBSS | FA, MD, AD, RD | ICBM-DTI-81 white matter labels | Analysis at baseline: Lower FA values in the CC (genu, body and splenium), anterior and superior CR bilaterally, right posterior CR, posterior thalamic radiation and right tapetum. Higher RD values in the aforementioned tracts. Longitudinal analysis: no differences between follow-up patients and baseline controls, or between baseline and follow-up patients. | White matter alterations may have a role in the alteration in food-related cognitive processing present in adolescents with restrictive eating disorders. |
| Cross-sectional studies in participants with bulimia nervosa | ||||||||
| He et al.58 | 3 T/ SS, SE, EP | 25 | FDT | TBSS | FA, MD, AD, RD | Johns Hopkins University | A priori hypothesis analysis: lower FA values in the forceps minor and major, SLF, IFOF, anterior thalamic radiation, corticospinal tract, uncinate fasciculus and CG in both hemispheres. Exploratory analysis (RD): Higher RD values in many of the same tracts: forceps minor and major, left SLF, IFOF, anterior thalamic radiation, corticospinal tract and CG. | These results suggest that white matter is affected in bulimia nervosa. Altered white matter tracts may have a role in the persistence of impaired self-regulation in bulimia nervosa. |
| Mettler et al.59 | 3 T/NR | 25 | NordicICE | VBA | FA, ADC | Hutchins and Atlas of Brain Function Orrison | Lower FA values in the bilateral CR, CC, right subinsula, and right fornix. Higher ADC values in the bilateral CR, CC, inferior fronto-occipital and uncinate fasciculi. | Bulimia nervosa is associated with white matter alterations that may contribute to altered trait anxiety, mood disturbance and altered reward processing. |
AD = axial diffusivity; ADC = apparent diffusion coefficient; AFQ = automated fibre quantification; ALIC = anterior limb of internal capsule; BMI = body mass index; CC = corpus callosum; CG = cingulum; CR = corona radiata; DSE = double psin echo; DTI = diffusion tensor imaging; EP = echo planar; EPI = echo-planar imaging; FA = fractional anisotropy; IFOF = inferior fronto-occipital fasciculus; LD = longitudinal diffusivity; MD = mean diffusivity; NR = not reported; PTR/SLF = junction of the posterior thalamic radiation/superior longitudinal fasciculus; R1 = relaxation rate; RD = radial diffusivity; ROI = region of interest; SE = spin echo; SENSE = sensitivity encoding; SLF = superior longitudinal fasciculus; SS = single-shot; TBSS = tract-based spatial statistics; VBA = voxel-based analysis.
Investigated in regions that showed group differences in fractional anisotropy or mean diffusivity.
An exploratory longitudinal study was also conducted.
Fig. 1.
Main white matter tracts affected in eating disoders. This figure represents white matter tracts mainly altered in anorexia nervosa and bulimia nervosa studies on a representative subject (tractographic reconstruction). See Table 2 for specific details of tract alterations. Three-dimensional fibre tractography was performed using the diffusion tensor imaging track module provided in MedINRIA software (ASCLEPIOS Research Team, Sophia Antipolis Cedex, v 1.9.0 France, www-sop.inria.fr/asclepios). For more details on white matter fibre reconstruction, see Wakana and colleagues,36 Mori and colleagues60 and Nagae and colleagues.61 Projection fibres Association fibres
DTI studies in patients with anorexia nervosa
A total of 14 studies investigated patients with current anorexia nervosa (Table 1 and Table 2). Among these, 1 study included a longitudinal design,52 2 studies also included a sample of participants who were recovered from anorexia nervosa,39,53 and 1 study recruited both restrictive OSFED and anorexia nervosa restrictive type and adopted a longitudinal design.57 Of the 14 studies, 10 evaluated the hydration state of participants. Seven studies adopted a TBSS analysis, 4 adopted a voxel-based analysis and 3 adopted a tractographic approach. Only 4 studies compiled samples of more than 20 patients with anorexia nervosa,37,39,51,52 while 2 studies enrolled less than 10 patients with anorexia nervosa.46,47
The DTI studies on anorexia nervosa showed widespread alterations in the projection, association and commissural white matter fibres, with differences in the direction of DTI measures and laterality.
Five studies showed white matter abnormalities in the corona radiata in patients with anorexia nervosa.44,45,51,53,57 Specifically, several studies showed lower FA values in the anterior, superior and posterior corona radiata44,45,53,57 (Table 2). Five studies found white matter alterations of the thalamic radiations in patients with anorexia nervosa.45,46,51,53,57 In particular, lower FA values53,57 and lower connectivity46 were found in both the posterior and anterior thalamic radiation. On the other hand, Vogel and colleagues51 pointed out higher FA values in the above-mentioned white matter tracts in a sample of 22 patients with anorexia nervosa. Lower AD values were also found in the posterior thalamic radiation.45 Moreover, lower FA values (with lower AD and higher RD values) were found in the anterior limb of the internal capsule,46 and higher FA51 and lower AD45 values were found in the posterior limb of internal capsule.
On the whole, patients with anorexia nervosa showed microstructural white matter alterations of the main white matter tracts that connect the thalamus to the cerebral cortex and the frontoparietal cortex to the subcortical nuclei.
Three studies pointed out white matter alterations of the inferior fronto-occipital fasciculus (IFOF) in patients with anorexia nervosa.38,44,46 In particular, both Kazlouski and colleagues38 and Frank and colleagues44 found lower FA values. Four DTI studies found superior longitudinal fasciculus (SLF) abnormalities.44,45,50,53 Lower FA values were found in the left SLF in patients with anorexia nervosa,45,50,53 mainly involving the first and the second component of the tract (i.e., SLF I and SFL II; for details, see also Makris and colleagues65). The FA alterations were associated with higher MD and RD50 and lower AD values,45 respectively. Higher FA and apparent diffusion coefficient values were also found in the left and bilateral SLF, respectively.44
Six studies showed white matter alterations of the fornix in patients with anorexia nervosa.38,44–46,48,50 In particular, most of the studies highlighted lower FA values. On the other hand, Kaufmann and colleagues37 found no fornix differences between a large sample of patients with anorexia nervosa and controls adopting a free water elimination approach. They used this approach, which removes partial volume effects, considering that fornix alterations may be determined by ventricular enlargement. Furthermore, a significant increase in FA values in the fornix was found comparing a large sample of patients with acute anorexia nervosa at baseline and after partial weight restoration.52
Three DTI studies showed microstructural white matter alterations of the cingulum.38,46,44 In particular, lower FA values were found in the posterior,38 right anterior46 and bilateral44 cingulum. In summary, the main association white matter tracts may be specifically affected in patients with anorexia nervosa and be related to altered occipital–parietal–temporal–frontal connections. Of note, although the fornix seems to be affected in anorexia nervosa, no abnormalities of the fornix were found using a new statistical approach.37
Regarding commissural fibres, 5 studies pointed out corpus callosum alterations, with differences in localizations and white matter change directions in patients with anorexia nervosa.44,45,49,51,52 These studies pointed out both lower45,49,52 and higher FA values.51 In particular, a recent study showed FA decreases in the body of the corpus callosum comparing a large anorexia nervosa sample with controls.52 At the same time, these authors, adopting a longitudinal design, found no FA differences between the anorexia nervosa sample and controls after partial weight restoration.52 Higher AD values and lower T1 relaxometry (R1) values were also found.44,49
Regarding the cerebellum, altered FA values of different cerebellar white matter tracts were also found.48,53
Finally, Hu and colleagues47 demonstrated a significant FA decrease in patients with anorexia nervosa in several cortical regions. However, because the authors mainly localized their results at the level of the grey matter, their approach limited comparison of their findings with the other DTI studies in patients with anorexia nervosa.
DTI studies in patients recovered from anorexia nervosa
Three DTI studies recruited participants who were recovered from anorexia nervosa and used a TBSS analysis (1 also adopted a tractographic approach; Table 1 and Table 2).54–56 Two additional studies39,53 recruited a sample of patients with current anorexia nervosa (reported above) and participants who were recovered from anorexia nervosa; this study used tractography. One additional longitudinal study57 assessed a sample of patients with restrictive eating disorder using a TBSS approach (in this section the results of the follow-up stage are reported). Three of the studies investigated samples larger than 20 patients recovered from anorexia nervosa.39,54,55
Two studies showed no white matter differences between patients who were recovered from anorexia nervosa and controls using TBSS54 and tractography.39 Olivo and colleagues57 reported no white matter differences at follow-up assessment between patients with restrictive eating disorder (restrictive OSFED and anorexia nervosa restrictive type) and controls. On the other hand, 2 studies found white matter alterations in participants who were recovered from anorexia nervosa using a TBSS approach56 and an integrated approach comprising tractography and TBSS analysis.55 In particular, Yau and colleagues56 showed mainly lower MD values in some thalamo–cortical (i.e., posterior and superior corona radiata and posterior limb of internal capsule) and parietal–frontal (i.e., SLF and cingulum) tracts. Shott and colleagues55 showed mainly higher white matter fibre connectivity between the insula and some brain regions using a tractographic approach, and lower FA values in some projection fibres (i.e., anterior corona radiata and thalamic radiations) and association fibres (i.e., inferior fronto-occipital and uncinate fasciculi) using TBSS analysis.
Interestingly, the DTI studies on patients who were recovered from anorexia nervosa included people who were recovered for at least 1 year.39,54–56 In particular, the participants recruited by Yau and colleagues56 and Shott and colleagues55 had a longer anorexia nervosa duration compared with those in the other 2 studies.39,54
In summary, it is still unclear whether white matter alterations are fully reversible after recovery from anorexia nervosa, particularly in people who are recovered from long-lasting anorexia nervosa.
DTI studies in bulimia nervosa
Two studies investigated patients with bulimia nervosa and enrolled 20 or more patients58,59 (Table 1 and Table 2). One study adopted a VBA approach,59 and 1 study used TBSS analysis.58 Details of the DTI measures assessed in the studies are reported in Table 2.
These studies showed several white matter alterations in projection, association and commisural tracts.58,59 In particular, both studies highlighted that patients with bulimia nervosa had lower FA values in the inferior fronto-occipital and uncinate fasciculi. White matter alterations of the corona radiata, corpus callosum, forceps minor and major, SLF and cingulum were also found.
In summary, white matter microstructure seems to be specifically affected in bulimia nervosa. However, it remains poorly explored.
Discussion
To the best of our knowledge, this is the first systematic review on DTI studies in eating disorders (anorexia nervosa and bulimia nervosa). Our aim was to systematically review the DTI studies, emphasizing the rapidly growing literature in the field since the first systematic review of 6 DTI studies in people with anorexia nervosa.40 This systematic review included 19 papers that used DTI in patients with current or past eating disorders. The majority of the studies were conducted in participants with current or past anorexia nervosa (n = 16). One study was carried out in a sample of patients with anorexia nervosa restrictive type and restrictive OSFED;57 we have discussed this study in the context of the anorexia nervosa studies. Few studies have been conducted in patients with bulimia nervosa (n = 2).58,59 Overall, the reviewed studies used different types of DTI analysis (i.e., VBA, TBSS, tractography) and different white matter atlases (Table 2). We will discuss the DTI results dividing the white matter tracts into projection, association and commissural fibres, plus the cerebellum white matter tracts (for details, see Catani and colleagues,62 Mori and colleagues,63 Wakana and colleagues64 and Makris and colleagues65).
Main DTI findings
The DTI studies on anorexia nervosa showed multiple white matter alterations with relatively consistent overlap in the altered white matter tracts in patients with current anorexia nervosa (Table 2 and Fig. 1). Furthermore, although DTI findings were characterized mainly by lower FA values, white matter abnormalities were only partially consistent when considering the direction of DTI measures (i.e., lower values or higher values) and laterality.
Overall, the included studies on anorexia nervosa showed altered thalamo–cortical white matter connections (i.e., corona radiata, thalamic radiations and internal capsule)44,45,51,53,57 and occipital–parietal–temporal–frontal white matter connections (i.e., IFOF, SLF and cingulum).44–46,50,53 They also found alterations of interhemispheric connections (i.e., corpus callosum)44,45,49,51,52 and cerebellar connections,48,53 with differences in the localization of white matter abnormalities and the direction of DTI measures. In particular, a recent study with a large anorexia nervosa sample and a longitudinal design showed a rapid normalization of the FA alterations in the corpus callosum after partial weight restoration.52 Although several studies have showed fornix alterations in patients with acute anorexia nervosa,44–46,48,50 a more recent analytical approach has suggested that such alterations might be mainly related to ventricular enlargement.37
The majority of the included studies showed lower FA values in patients with anorexia nervosa than in controls (Table 2). However, some studies also showed higher FA values.44,51 The other DTI measures (MD, AD and RD) have been less explored and showed partially consistent results (for details on DTI measures, see the introduction). In particular, as expected, MD, AD and RD changes seem to be mainly coupled with FA alterations.
Considering the typical symptomatology of anorexia nervosa (i.e., weight loss, malnourishment, starvation etc.), a number of processes (e.g., neuronal–glial remodelling, altered hydration state) may contribute to white matter alterations in patients.12 In addition, considering that DTI findings are particularly sensitive to macroscopic head motion,66 this possible confounding factor could have led to spurious group differences.66 However, DTI findings in the current review showed altered thalamo–cortical and occipital–parietal–temporal–frontal white matter alterations in particular, suggesting a specific vulnerability of these white matter tracts in anorexia nervosa and that they could be involved in the pathophysiology of anorexia nervosa.
Discrepancies among the studies in patients with anorexia nervosa may have been primarily due to differences in sample composition (e.g., age, age range, duration of eating disorder, eating disorder severity, etc.).43,67 White matter abnormalities and directions of DTI measures may change during the course of anorexia nervosa in relation to the duration of disease/long-lasting underweight and to developmental factors and resilience processes.44,45,49,51 In this context, it is useful to remember that FA values can be altered for a variety of reasons, and the biological meaning of white matter diffusivity measures is not entirely clear.31 Furthermore, the reviewed studies used different approaches to DTI analyses (i.e., VBA, TBSS, tractography) and different acquisition sequences and processing pipelines. Therefore, the differences among the anorexia nervosa studies may also be related to different analysis techniques and methodological approaches.43 Interestingly, Pfuhl and colleagues39 found no white matter differences between patients with anorexia nervosa and controls when adopting a global probabilistic tractographic approach, suggesting that different DTI analyses may or may not detect subtle or more localized white matter alterations.52 In addition, considering that some studies did not take into account the hydration state (dehydration/hyperhydration) of patients with anorexia nervosa, this possible confounding factor42 may have contributed to the differences among study results. These last limitations and methodological points will be discussed in depth in Methodological Implications and Limitations of the Current Literature, below.
Few studies have been conducted in participants who have recovered from anorexia nervosa, but those that have showed white matter abnormalities that were partially consistent with those found in patients with current anorexia nervosa, mainly involving thalamo–cortical connections (i.e., corona radiata, thalamic radiations and internal capsule) and occipital–parietal–temporal–frontal connections (i.e., superior longitudinal, inferior fronto-occipital and uncinate fasciculi and cingulum).55,56 Interestingly, the 2 studies reported lower MD56 and lower FA values,55 respectively. These differences in DTI measure directions are still to be explored. On the other hand, other studies have shown no differences in white matter diffusivity between participants who have recovered from anorexia nervosa and controls.39,54,57 It is still unclear whether white matter alterations persist after recovery from anorexia nervosa. Interestingly, the participants recruited by Yau and colleagues56 and Shott and colleagues55 had a longer duration of anorexia nervosa than participants in other studies.39,54 The difference in disease duration and the use of different DTI approaches could explain the discrepancies among these studies. Further research is needed to better clarify whether white matter alterations are fully reversible after recovery from anorexia nervosa and, in particular, in people who have recovered from long-lasting anorexia nervosa.
To date, only 2 studies have investigated patients with bulimia nervosa, and they showed widespread microstructural white matter alterations, but findings were only partially consistent between them.58,59 In particular, both studies found lower FA values in the inferior fronto-occipital and uncinate fasciculi. Altered white matter microstructure was also found in other projection (e.g., corona radiata, anterior thalamic radiation), association (e.g., SLF, cingulum) and commissural (e.g., forceps major and minor and corpus callosum) fibres.
White matter microstructural changes remain poorly explored in bulimia nervosa; further studies are warranted to better clarify white matter alterations and their possible role in this disease.
Altered white matter connections in eating disorders: clinical implications
Altered white matter connections in anorexia nervosa
The current DTI literature shows that white matter microstructure can be specifically affected in the acute stage of anorexia nervosa and may play a role in the pathophysiology of anorexia nervosa. Nonetheless, we cannot exclude the possibility that peculiar anorexia nervosa symptoms (e.g., starvation, weight loss, brain atrophy and altered hydration state) may be involved in white matter changes (e.g., fornix alterations37). Moreover, it is still unclear whether white matter alterations persist after recovery from anorexia nervosa.39,55
Despite discrepancies in the specific DTI measures involved and their directionality, several DTI studies found altered thalamo–cortical connections in patients with anorexia nervosa, characterized mainly by microstructural white matter alterations of the corona radiata, thalamic radiations and internal capsule.44–46,51,53,57 Moreover, abnormalities of the corona radiata and the thalamic radiations were also found in participants recovered from anorexia nervosa.55 These white matter tracts mainly connect the thalamus to the cerebral cortex and vice versa, and their tissue boundaries are not completely defined (see Catani and colleagues62 and Mori and colleagues63). They are involved in several functions and have been also considered as a neuroanatomical backbone of motor and perceptual functions and cognitive processes (e.g., Catani and colleagues62). In particular, corona radiata lesions appear to have a role in central taste disorders.68 Furthermore, the anterior corona radiata seems mainly to connect the anterior cingulate cortex to other structures69 and seems to be involved in executive control functions, the resolution of conflicting stimuli that affect decision making, and self-regulation.69–71 Considering this evidence, it has been suggested that white matter alterations in the anterior corona radiata could play a role in altered state processing and cognitive impairment in anorexia nervosa.44,45,55,57 On the other hand, the posterior thalamic radiation, which is included in the posterior corona radiata,63 connects the posterior part of the thalamus to the occipital, parietal and temporal cortices and also includes the optic radiation.63 It has been suggested that posterior thalamic radiation injuries are related to sensorimotor function deficits,72 including touch and proprioception alterations. As well, parietal and occipital areas are involved in body-image perception (e.g., Peelen and Downing73) and identification of one’s own body.74 As a result, it has been hypothesized that white matter alterations in the posterior thalamic radiation may play a role in body-image disturbances in anorexia nervosa.53 Frieling and colleagues,53 considering that functional alterations in visual and prefrontal cortices occurred in response to food images,75 also hypothesized that alterations in the posterior thalamic radiation may be related to cognitive biases toward food.17
In summary, thalamo–cortical fibres may be specifically affected in anorexia nervosa, and these white matter alterations may be involved in the pathophysiology of anorexia nervosa.
Several DTI studies also found white matter alterations in the association fibres (which connect the occipital, parietal, temporal and frontal areas) in people with anorexia nervosa. The main association fibres involved were the superior longitudinal and inferior fronto-occipital fasciculi, the cingulum and the fornix. Some DTI studies found alterations in the left SLF, mainly affecting the first (SLF I) and second (SLF II) subcomponents.44,45,50,53 The SLF connects the parietal, occipital and temporal lobes with the frontal cortex and vice versa.63,65,76 In particular, the SLF I primarily connects the superior parietal areas (e.g., the precuneus and superior parietal lobe) to the superior frontal cortex and the dorsal prefrontal areas.65 The SLF II mainly connects the posterior-inferior parietal cortex (i.e., the angular gyrus) and the superior temporal lobe to the lateral prefrontal cortex.65 The parietal cortex is involved in several functions, such as proprioception, spatial orientation and integration of visual information, and it seems to play a role in own-body perception (e.g., Peelen and Downing73 and Hodzic and colleagues74) and in body-schema representations (e.g., Schwoebel and Coslett77). In particular, a network including the superior parietal regions (e.g., the precuneus) and prefrontal cortex seems to be involved in the manipulation of mental images and the mental representation of the self.73 It has also been shown that the left hemisphere plays a key role in self-recognition.78 Considering these findings, it has been suggested that the white matter alterations of the left SLF could be involved in body-image disturbances in anorexia nervosa.45,50 In line with this interpretation, event-related fMRI studies in patients with anorexia nervosa have suggested that the superior parietal areas and the prefrontal cortex are related to the perceptive and affective component of body image distortion, respectively.16
Some DTI studies showed alterations of the IFOF in patients with anorexia nervosa.38,44,46 The IFOF primarily connects the frontal lobe to the occipital, posterior parietal and temporal lobe (e.g., Catani and colleagues,62 Mori and colleagues63 and Martino and colleagues79). In the frontal lobe, this association tract merges with the frontal projection of the uncinate fasciculus (e.g., Catani and colleagues62 and Mori and colleagues63). Although the role of the IFOF is still poorly understood (e.g., Martino and colleagues79), it seems to be involved in several functions such as visual and semantic processing.62,79 In particular, considering that the IFOF connects brain areas related to body perception (e.g., the fusiform gyrus and posterior parietal regions; e.g., Peelen and Downing73), IFOF abnormalities might play a role in altered body perception in anorexia nervosa.38,44,46 Interestingly, the IFOF appears to connect visual and emotion-related areas and could be involved in impairments of emotional recognition.80
On the whole, the main occipital–parietal–temporal–frontal tracts (i.e., the left SLF and IFOF) seem to be specifically affected in anorexia nervosa and could play a role in its pathophysiology.
Regarding the other association fibres, cingulum abnormalities were found in its posterior38 and anterior parts.46,44 The cingulum contains fibres of different lengths and mainly connects frontal regions with the temporal lobe and hippocampus.62,63 It belongs to the limbic system and seems to be mainly involved in emotional and cognitive processing (e.g., Catani and colleagues62). Although white matter alteration of the cingulum is not consistent in regard to its specific part (i.e., posterior or anterior),44 white matter alterations may be related to altered emotional and cognitive processes in anorexia nervosa.38,46 In addition, cingulum alterations were found in both structural (e.g., voxel-based morphometry)7,81,82 and functional (i.e., resting-state)83,84 neuroimaging studies in patients with anorexia nervosa, leading to consideration of a possible role for the cingulum in the pathophysiology of anorexia nervosa. Further studies are needed to elucidate the role of this association tract in anorexia nervosa.
Several DTI studies showed alterations of the fornix in patients with anorexia nervosa.44–46,48,50 It mainly connects the hypothalamus and mammillary bodies to the medial temporal lobe (e.g., Catani and colleagues62). The fornix is part of the limbic system and seems to be involved in emotion processing by frontal brain areas85 and memory function.62 Interestingly, lesions of the fornix in rodents seem to lead to altered reward processing,86 altered feeding and drinking patterns,87 and resistance to behaviour extinction.88 Considering these findings, a possible role for the fornix in long-lasting food refusal/restriction and altered food reward processing in patients with anorexia nervosa has been suggested.38,44 Nevertheless, a more recent DTI study showed that fornix alterations may be due primarily to ventricular enlargement.37 Further studies are needed to better understand the role of the fornix in anorexia nervosa, taking into account partial volume effects.37
Regarding the commissural fibres, some DTI studies in patients with anorexia nervosa found alterations of different portions of the corpus callosum.44,45,49,51,57 The corpus callosum connects left and right cerebral hemispheres and is divided into an anterior portion (genu), a central portion (body) and a posterior portion (splenium and tapetum) based on its anatomic connections (e.g., Catani and colleagues62). The anterior portion connects the orbitofrontal and prefrontal regions; the central portion connects precentral frontal regions and parietal lobes; and the posterior portion connects the occipital lobes (splenium) and temporal lobes (tapetum). The corpus callosum, which connects the 2 hemispheres, is involved in motor, perceptual and cognitive functions (e.g., Catani and colleagues62). Although there are differences among the studies concerning the alterations in the different portions of the corpus callosum, the results seem to suggest that altered interhemispheric connections may be present in anorexia nervosa. Interestingly, altered interhemispheric functional connectivity at rest was found in patients with anorexia nervosa.89 Further studies are needed to better understand the possible role of the corpus callosum and interhemispheric connections in the pathophysiology of anorexia nervosa.
Finally, 2 studies showed white matter abnormalities of the left cerebellum in patients with anorexia nervosa.48,53 Reduced FA values were also found in the middle cerebellar peduncle in adult women who were recovered from anorexia nervosa.55 In particular, Nagahara and colleagues48 showed white matter abnormalities of the lateral zone of the cerebellum48 and, considering the connections between the dentate nucleus and specific hypothalamic nuclei,90 suggested that the cerebellar white matter alterations might be mainly involved in altered food intake behaviour in anorexia nervosa. Interestingly, cerebellar volume decrease has been found in participants with current and past anorexia nervosa.81,91 Furthermore, altered resting-state functional connectivity was found in the cerebellum of patients with anorexia nervosa.92 Although these findings seem to suggest a possible involvement of the cerebellar white matter tracts in anorexia nervosa, further studies are needed to better understand their role in the pathophysiology of anorexia nervosa.
Altered white matter connections in bulimia nervosa
To date, only 2 DTI studies have explored white matter changes in patients with bulimia nervosa.58,59 These 2 studies showed that multiple white matter tracts are affected in bulimia nervosa. The thalamo–cortical (e.g., corona radiata) and occipital–parietal–temporal–frontal (e.g., IFOF and cingulum) regions seem to be particularly involved, although with some discrepancies between studies (Table 2). In particular, the 2 studies found abnormalities in the IFOF and uncinate fasciculi.58,59 Considering that the IFOF connects the occipital–parietal–temporal areas to frontal areas, it has been suggested that these alterations may sustain altered processing of body image in bulimia nervosa.58 On the other hand, it has been suggested that alterations in the corona radiata and cingulum may be related to altered reward and taste processing in bulimia nervosa.58,59 Although the studies in patients with bulimia nervosa are few, their findings seem to be partially consistent with the studies in patients with anorexia nervosa and lead to preliminary suggestions that both thalamo–cortical and occipital–parietal–temporal–frontal connections may play a role in both anorexia nervosa and bulimia nervosa. However, further studies are needed to better understand the role of white matter alterations in the pathophysiology of bulimia nervosa.
Methodological implications and limitations of the current literature
Several factors should be taken into consideration when attempting a DTI study in eating disorders. First, several studies have reported evidence for continuing maturation of the white matter from childhood into adulthood,39,55,93 and the values for the DTI parameters may vary with age.94 Therefore, discrepancies among DTI studies in eating disorders may be related to the age and age range of the sample enrolled. The duration of the eating disorder should also be carefully considered, because longer disease duration might be associated with more pronounced changes in white matter microstructure.51 Furthermore, several studies included patients with psychiatric comorbidities and under different treatment conditions (e.g., pharmacological treatment). These conditions can influence DTI results (e.g., King and colleagues43) and should be taken into account to improve understanding of neuroimaging results in eating disorders and comparability among studies (see Frank and colleagues67).
Second, several methodological approaches have been used to investigate white matter changes in eating disorders, limiting our ability to compare results from different studies. In fact, VBA and TBSS analyses can detect abnormalities of large-scale brain systems, while tractography allows the examination of anatomic connectivity.39,95 Because the guidelines for optimizing DTI acquisition for fibre tracking are similar to those for DTI optimization in general, future studies should perform multi-approach analyses, in which tractography is used in conjunction with TBSS/VBA analyses. In this way, it would be possible to better compare results from different studies and conduct a deeper investigation of white matter microstructure in eating disorders.
Third, hydration state may affect DTI results (e.g., Elvsahagen and colleagues96). Typically, anorexia nervosa symptomatology (e.g., food refusal, starvation) may influence hydration levels.43 To date, only a small number of studies has assessed the hydration state of patients with anorexia nervosa, and they have used different procedures (Table 1). Although no gold standard for the assessment of hydration state has been defined97 and the effects of this factor on neuroimaging findings in eating disorders are still a matter for debate,67 future studies should consider this index in DTI analysis design. For instance, Armstrong97 recommended the use of 2 or more indices to evaluate body hydration. This approach could be adopted in DTI studies in patients with anorexia nervosa and bulimia nervosa (e.g., urine specific gravity, urine osmolality and plasma osmolality).67 Furthermore, patients could have a period of controlled nutritional conditions before scanning to minimize the effects of dehydration/hyperhydration on brain measurements.67
Another aspect that needs to be taken into consideration is the influence of ventricular enlargement on diffusion properties. In particular, anorexia nervosa is typically characterized by reduced brain mass and corresponding increased cerebrospinal fluid in the sulci and ventricles (e.g., Seitz and colleagues5 and Titova and colleagues6). Kaufmann and colleagues37 recently introduced a new method to correct DTI images in regions prone to cerebrospinal fluid contamination, such as the fornix. Such an approach should be adopted in future research, including other white matter tracts that are likely to be involved in the pathophysiology of anorexia nervosa.98 Finally, considering that head motion can influence DTI findings,66 this additional confounding factor could be taken into consideration, and future studies on eating disorder patients may consider head motion as a nuisance regressor in white matter analyses.66
Conclusion
The literature pertaining to the use of DTI in eating disorders has grown rapidly in recent years. The current literature has primarily investigated patients with current anorexia nervosa or patients who were recovered from anorexia nervosa; few studies investigated patients with bulimia nervosa. On the whole, DTI studies found widespread microstructural white matter abnormalities that affect projection, association and commissural white matter tracts in both anorexia nervosa and bulimia nervosa, but showing only partially consistent results. Differences among the studies may have been related mainly to heterogeneity in sample composition (e.g., age, age range, eating disorder duration) and methodology (e.g., TBSS, VBA, tractography). Considering eating disorder symptomatology (e.g., altered eating and/or weight loss/malnutrition), we cannot rule out the possibility that white matter alterations may be to some extent related to disease activity. However, in spite of the differences among the studies, the studies in patients with anorexia nervosa pointed out partially consistent results with respect to white matter abnormalities of the thalamo–cortical tracts (i.e., corona radiata and thalamic radiations) and occipital–parietal–temporal–frontal tracts (i.e., superior longitudinal and inferior fronto-occipital fasciculi), mainly characterized by decreases in FA values. On the other hand, it is still unclear if white matter abnormalities persist after anorexia nervosa recovery. Overall, our review leads us to preliminarily suggest that thalamo–cortical and occipital–parietal–frontal connections could be affected in anorexia nervosa. This preliminary evidence suggests that altered thalamo–cortical connections (involved in taste, cognitive and emotional processes, and body-image processing) and occipital–parietal–temporal–frontal connections (involved in complex body image and emotional processing) may play a role in the main symptoms of anorexia nervosa, such as rumination on food and weight, body image disturbances, and food refusal. Although multiple white matter alterations have been found in patients with bulimia nervosa that are partially similar to those of patients with anorexia nervosa, white matter microstructure in bulimia nervosa remains poorly studied, and these findings are less conclusive.
It is critical to improve our knowledge about white matter changes in eating disorders. Further studies with larger and more homogeneous samples, considering confounding factors (e.g., altered hydration state, nutritional state, partial volume effects), and with longitudinal designs are needed to better elucidate the role and consequences of white matter abnormalities in the pathophysiology of eating disorders. Furthermore, considering the differences between the different DTI processing algorithms (e.g., TBSS, VBA, tractography), studies adopting a multi-approach analysis should be encouraged to better explore white matter microstructure in eating disorders. Diffusion tensor imaging is a means of improving our knowledge of the pathophysiology of eating disorders, and it can help us define more targeted treatment strategies.
Footnotes
Competing interests: None declared.
Contributors: S. Gaudio designed the study. S. Gaudio, F. Carducci, C. Piervincenzi and G. Olico acquired the data, which S. Gaudio, F. Carducci, C. Piervincenzi and H. Schiöth analyzed. S. Gaudio and F. Carducci wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington (DC): APA; 2013. [Google Scholar]
- 2.Kaye WH, Wierenga CE, Bailer UF, et al. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110–20. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zipfel S, Giel KE, Bulik CM, et al. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2:1099–111. doi: 10.1016/S2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]
- 4.Frank GK, Kaye WH. Current status of functional imaging in eating disorders. Int J Eat Disord. 2012;45:723–36. doi: 10.1002/eat.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seitz J, Herpertz-Dahlmann B, Konrad K. Brain morphological changes in adolescent and adult patients with anorexia nervosa. J Neural Transm. 2016;123:949–59. doi: 10.1007/s00702-016-1567-9. [DOI] [PubMed] [Google Scholar]
- 6.Titova OE, Hjorth OC, Schioth HB, et al. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. BMC Psychiatry. 2013;13:110. doi: 10.1186/1471-244X-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro-Fornieles J, Bargallo N, Lazaro L, et al. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res. 2009;43:331–40. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Mainz V, Schulte-Ruther M, Fink GR, et al. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med. 2012;74:574–82. doi: 10.1097/PSY.0b013e31824ef10e. [DOI] [PubMed] [Google Scholar]
- 9.Lazaro L, Andres S, Calvo A, et al. Normal gray and white matter volume after weight restoration in adolescents with anorexia nervosa. Int J Eat Disord. 2013;46:841–8. doi: 10.1002/eat.22161. [DOI] [PubMed] [Google Scholar]
- 10.Nickel K, Joos A, Tebartz van Elst L, et al. Recovery of cortical volume and thickness after remission from acute anorexia nervosa. Int J Eat Disord. 2018;51:1056–69. doi: 10.1002/eat.22918. [DOI] [PubMed] [Google Scholar]
- 11.Bang L, Ro O, Endestad T. Normal gray matter volumes in women recovered from anorexia nervosa: a voxel-based morphometry study. BMC Psychiatry. 2016;16:44. doi: 10.1186/s12888-016-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King JA, Geisler D, Ritschel F, et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol Psychiatry. 2015;77:624–32. doi: 10.1016/j.biopsych.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Bernardoni F, King JA, Geisler D, et al. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. Neuroimage. 2016;130:214–222. doi: 10.1016/j.neuroimage.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Van den Eynde F, Suda M, Broadbent H, et al. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur Eat Disord Rev. 2012;20:94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- 15.Amianto F, Caroppo P, D’Agata F, et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Res. 2013;213:210–6. doi: 10.1016/j.pscychresns.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Gaudio S, Quattrocchi CC. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci Biobehav Rev. 2012;36:1839–47. doi: 10.1016/j.neubiorev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Gaudio S, Wiemerslage L, Brooks SJ, et al. A systematic review of resting-state functional-MRI studies in anorexia nervosa: evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci Biobehav Rev. 2016;71:578–89. doi: 10.1016/j.neubiorev.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Lavagnino L, Amianto F, D’Agata F, et al. Reduced resting-state functional connectivity of the somatosensory cortex predicts psychopathological symptoms in women with bulimia nervosa. Front Behav Neurosci. 2014;8:270. doi: 10.3389/fnbeh.2014.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Kong QM, Li K, et al. Altered intrinsic functional brain architecture in female patients with bulimia nervosa. J Psychiatry Neurosci. 2017;42:414–23. doi: 10.1503/jpn.160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 21.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 22.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 23.Beaulieu C, Does MD, Snyder RE, et al. Changes in water diffusion due to Wallerian degeneration in peripheral nerve. Magn Reson Med. 1996;36:627–31. doi: 10.1002/mrm.1910360419. [DOI] [PubMed] [Google Scholar]
- 24.Caminiti R, Carducci F, Piervincenzi C, et al. Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: comparing data from histology and magnetic resonance imaging diffusion tractography. J Neurosci. 2013;33:14501–11. doi: 10.1523/JNEUROSCI.0761-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury — a review. NMR Biomed. 2002;15:561–9. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- 26.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R, Nguyen HD, Macey PM, et al. Regional brain axial and radial diffusivity changes during development. J Neurosci Res. 2012;90:346–55. doi: 10.1002/jnr.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett IJ, Madden DJ, Vaidya CJ, et al. Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–31. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Donnell LJ, Westin CF. An introduction to diffusion tensor image analysis [viii.] Neurosurg Clin N Am. 2011;22:185–96. doi: 10.1016/j.nec.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 32.Astrakas LG, Argyropoulou MI. Shifting from region of interest (ROI) to voxel-based analysis in human brain mapping. Pediatr Radiol. 2010;40:1857–67. doi: 10.1007/s00247-010-1677-8. [DOI] [PubMed] [Google Scholar]
- 33.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Mori S, van Zijl PC. Fiber tracking: principles and strategies — a technical review. NMR Biomed. 2002;15:468–80. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 36.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann LK, Baur V, Hanggi J, et al. Fornix under water? Ventricular enlargement biases forniceal diffusion magnetic resonance imaging indices in anorexia nervosa. Biol Psychiatry. 2017;2:430–7. doi: 10.1016/j.bpsc.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Kazlouski D, Rollin MD, Tregellas J, et al. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011;192:109–16. doi: 10.1016/j.pscychresns.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfuhl G, King JA, Geisler D, et al. Preserved white matter microstructure in young patients with anorexia nervosa? Hum Brain Mapp. 2016;37:4069–83. doi: 10.1002/hbm.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin Monzon B, Hay P, Foroughi N, et al. White matter alterations in anorexia nervosa: a systematic review of diffusion tensor imaging studies. World J Psychiatry. 2016;6:177–86. doi: 10.5498/wjp.v6.i1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Streitburger DP, Moller HE, Tittgemeyer M, et al. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7:e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King JA, Frank GKW, Thompson PM, et al. Structural neuroimaging of anorexia nervosa: future directions in the quest for mechanisms underlying dynamic alterations. Biol Psychiatry. 2018;83:224–34. doi: 10.1016/j.biopsych.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank GK, Shott ME, Hagman JO, et al. Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2013;52:1066–75. doi: 10.1016/j.jaac.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaudio S, Quattrocchi CC, Piervincenzi C, et al. White matter abnormalities in treatment-naive adolescents at the earliest stages of anorexia nervosa: a diffusion tensor imaging study. Psychiatry Res Neuroimaging. 2017;266:138–45. doi: 10.1016/j.pscychresns.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Hayes DJ, Lipsman N, Chen DQ, et al. Subcallosal cingulate connectivity in anorexia nervosa patients differs from healthy controls: a multi-tensor tractography study. Brain Stimulat. 2015;8:758–68. doi: 10.1016/j.brs.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Hu SH, Feng H, Xu TT, et al. Altered microstructure of brain white matter in females with anorexia nervosa: a diffusion tensor imaging study. Neuropsychiatr Dis Treat. 2017;13:2829–36. doi: 10.2147/NDT.S144972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagahara Y, Nakamae T, Nishizawa S, et al. A tract-based spatial statistics study in anorexia nervosa: abnormality in the fornix and the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:72–7. doi: 10.1016/j.pnpbp.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Travis KE, Golden NH, Feldman HM, et al. Abnormal white matter properties in adolescent girls with anorexia nervosa. Neuroimage Clin. 2015;9:648–59. doi: 10.1016/j.nicl.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Via E, Zalesky A, Sanchez I, et al. Disruption of brain white matter microstructure in women with anorexia nervosa. J Psychiatry Neurosci. 2014;39:367–75. doi: 10.1503/jpn.130135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogel K, Timmers I, Kumar V, et al. White matter microstructural changes in adolescent anorexia nervosa including an exploratory longitudinal study. Neuroimage Clin. 2016;11:614–21. doi: 10.1016/j.nicl.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Schwanenflug N, Muller DK, King JA, et al. Dynamic changes in white matter microstructure in anorexia nervosa: findings from a longitudinal study. Psychol Med. 2018 doi: 10.1017/S003329171800212X. [DOI] [PubMed] [Google Scholar]
- 53.Frieling H, Fischer J, Wilhelm J, et al. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa — a voxel based diffusion tensor imaging (DTI) study. J Psychiatr Res. 2012;46:1237–42. doi: 10.1016/j.jpsychires.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Bang L, Ro O, Endestad T. Normal white matter microstructure in women long-term recovered from anorexia nervosa: a diffusion tensor imaging study. Int J Eat Disord. 2018;51:46–52. doi: 10.1002/eat.22802. [DOI] [PubMed] [Google Scholar]
- 55.Shott ME, Pryor TL, Yang TT, et al. Greater insula white matter fiber connectivity in women recovered from anorexia nervosa. Neuropsychopharmacology. 2016;41:498–507. doi: 10.1038/npp.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yau WY, Bischoff-Grethe A, Theilmann RJ, et al. Alterations in white matter microstructure in women recovered from anorexia nervosa. Int J Eat Disord. 2013;46:701–8. doi: 10.1002/eat.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivo G, Wiemerslage L, Swenne I, et al. Limbic-thalamo-cortical projections and reward-related circuitry integrity affects eating behavior: a longitudinal DTI study in adolescents with restrictive eating disorders. PLoS One. 2017;12:e0172129. doi: 10.1371/journal.pone.0172129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He X, Stefan M, Terranova K, et al. Altered white matter microstructure in adolescents and adults with bulimia nervosa. Neuropsychopharmacology. 2016;41:1841–8. doi: 10.1038/npp.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mettler LN, Shott ME, Pryor T, et al. White matter integrity is reduced in bulimia nervosa. Int J Eat Disord. 2013;46:264–73. doi: 10.1002/eat.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori S, Wakana S, Nagae-Poetscher LM, et al. MRI atlas of human white matter. 1st ed. Amsterdam: Elsevier; 2005. [Google Scholar]
- 61.Nagae LM, Hoon AH, Jr, Stashinko E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007;28:1213–22. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–32. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–82. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 65.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 66.Yendiki A, Koldewyn K, Kakunoori S, et al. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank GKW, Favaro A, Marsh R, et al. Toward valid and reliable brain imaging results in eating disorders. Int J Eat Disord. 2018;51:250–61. doi: 10.1002/eat.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Onoda K, Ikeda M, Sekine H, et al. Clinical study of central taste disorders and discussion of the central gustatory pathway. J Neurol. 2012;259:261–6. doi: 10.1007/s00415-011-6165-z. [DOI] [PubMed] [Google Scholar]
- 69.Niogi S, Mukherjee P, Ghajar J, et al. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Front Neuroanat. 2010;4:2. doi: 10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang YY, Lu Q, Geng X, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci U S A. 2010;107:15649–52. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin X, Han Y, Ge H, et al. Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp. 2013;34:796–813. doi: 10.1002/hbm.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peelen MV, Downing PE. The neural basis of visual body perception. Nat Rev Neurosci. 2007;8:636–48. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- 74.Hodzic A, Kaas A, Muckli L, et al. Distinct cortical networks for the detection and identification of human body. Neuroimage. 2009;45:1264–71. doi: 10.1016/j.neuroimage.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 75.Brooks SJ, O’Daly O, Uher R, et al. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS One. 2012;7:e34000. doi: 10.1371/journal.pone.0034000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamali A, Flanders AE, Brody J, et al. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219:269–81. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwoebel J, Coslett HB. Evidence for multiple, distinct representations of the human body. J Cogn Neurosci. 2005;17:543–53. doi: 10.1162/0898929053467587. [DOI] [PubMed] [Google Scholar]
- 78.Devue C, Bredart S. The neural correlates of visual self-recognition. Conscious Cogn. 2011;20:40–51. doi: 10.1016/j.concog.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Martino J, Brogna C, Robles SG, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–9. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 80.Philippi CL, Mehta S, Grabowski T, et al. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29:15089–99. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaudio S, Nocchi F, Franchin T, et al. Gray matter decrease distribution in the early stages of anorexia nervosa restrictive type in adolescents. Psychiatry Res. 2011;191:24–30. doi: 10.1016/j.pscychresns.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Muhlau M, Gaser C, Ilg R, et al. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry. 2007;164:1850–7. doi: 10.1176/appi.ajp.2007.06111861. [DOI] [PubMed] [Google Scholar]
- 83.Gaudio S, Piervincenzi C, Beomonte Zobel B, et al. Altered resting state functional connectivity of anterior cingulate cortex in drug naive adolescents at the earliest stages of anorexia nervosa. Sci Rep. 2015;5:10818. doi: 10.1038/srep10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee S, Ran Kim K, Ku J, et al. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res. 2014;221:43–8. doi: 10.1016/j.pscychresns.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:583–9. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 86.Salinas JA, White NM. Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behav Neurosci. 1998;112:812–26. doi: 10.1037//0735-7044.112.4.812. [DOI] [PubMed] [Google Scholar]
- 87.Osborne B, Dodek AB. Disrupted patterns of consummatory behavior in rats with fornix transections. Behav Neural Biol. 1986;45:212–22. doi: 10.1016/s0163-1047(86)90783-1. [DOI] [PubMed] [Google Scholar]
- 88.Osborne B, Silverhart T, Markgraf C, et al. Effects of fornix transection and pituitary-adrenal modulation on extinction behavior. Behav Neurosci. 1987;101:504–12. doi: 10.1037//0735-7044.101.4.504. [DOI] [PubMed] [Google Scholar]
- 89.Canna A, Prinster A, Monteleone AM, et al. Interhemispheric functional connectivity in anorexia and bulimia nervosa. Eur J Neurosci. 2017;45:1129–40. doi: 10.1111/ejn.13507. [DOI] [PubMed] [Google Scholar]
- 90.Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28:469–78. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boghi A, Sterpone S, Sales S, et al. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res. 2011;192:154–9. doi: 10.1016/j.pscychresns.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 92.Amianto F, D’Agata F, Lavagnino L, et al. Intrinsic connectivity networks within cerebellum and beyond in eating disorders. Cerebellum. 2013;12:623–31. doi: 10.1007/s12311-013-0471-1. [DOI] [PubMed] [Google Scholar]
- 93.Asato MR, Terwilliger R, Woo J, et al. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20:2122–31. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Gan J, Zhong M, Fan J, et al. Abnormal white matter structural connectivity in adults with obsessive-compulsive disorder. Transl Psychiatry. 2017;7:e1062. doi: 10.1038/tp.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elvsashagen T, Norbom LB, Pedersen PO, et al. Widespread changes in white matter microstructure after a day of waking and sleep deprivation. PLoS One. 2015;10:e0127351. doi: 10.1371/journal.pone.0127351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr. 2007;26(Suppl):575S–84S. doi: 10.1080/07315724.2007.10719661. [DOI] [PubMed] [Google Scholar]
- 98.Seitz J. Readdressing fornix pathology in anorexia nervosa. Biol Psychiatry. 2017;2:386–7. doi: 10.1016/j.bpsc.2017.05.002. [DOI] [PubMed] [Google Scholar]