Abstract
Red blood cells (RBC) are actually exploited as innovative drug delivery systems with unconventional and convenient properties. Because of a long in vivo survival and a non-random removal from circulation, RBC can be loaded with drugs and/or contrasting agents without affecting these properties and maintaining the original immune competence. However, native or drug-loaded RBC, can be modified decorating the membrane with peptides, antibodies or small chemical entities so favoring the targeting of the processed RBC to specific cells or organs. Convenient modifications have been exploited to induce immune tolerance or immunogenicity, to deliver antibodies capable of targeting other cells, and to deliver a number of constructs that can recognize circulating pathogens or toxins. The methods used to induce membrane processing useful for biomedical applications include the use of crosslinking agents and bifunctional antibodies, biotinylation and membrane insertion. Another approach includes the expression of engineered membrane proteins upon ex vivo transfection of immature erythroid precursors with lentiviral vectors, followed by in vitro expansion and differentiation into mature erythrocytes before administration to a patient in need. Several applications have now reached the clinic and a couple of companies that take advantage from these properties of RBC are already in Phase 3 with selected applications. The peculiar properties of the RBC and the active research in this field by a number of qualified investigators, have opened new exciting perspectives on the use of RBC as carriers of drugs or as cellular therapeutics.
Keywords: RBC targeting, RBC carriers, RBC membrane modifications, RBC circulation, drug targeting
Introduction
For many years, drug-loaded red blood cells (RBC) have been exploited as delivery systems for the release in circulation of active agents, for the increase in the life-span in circulation of therapeutic agents, for the protection by immune inactivation of therapeutic enzymes, and for a prolonged circulation of contrasting agents useful in diagnostic applications (reviewed in Rossi et al., 2016; Villa et al., 2016; Pierigè et al., 2017). In addition, native or drug-loaded RBC could be conveniently modified by membrane decoration with peptides, antibodies, receptors, nanoparticles and other constructs expanding the use and scope in processing RBC for therapeutic applications. This review will summarize these approaches providing information on the potential use of these engineered RBC and highlighting the limits of each of the methods developed.
Red Blood Cell Membrane and Osmotic Loading Procedure for Therapeutic Applications
The possibility of using RBC as carriers takes advantage from the discovery performed many years ago (Ihler et al., 1973) that these non-nucleate cells could swell under hypotonic conditions and that pores could open on the RBC membrane. Once the pores are open, these can be easily crossed by extracellular agents (drugs, enzymes, nanoparticles, etc.), which are later entrapped into the RBC simply restoring the physiological osmolarity followed by pore closing. Several variants of this process have been described during the years (Pierigè et al., 2008; Rossi et al., 2016) but only with the development of specific medical devices capable of processing blood under sterile and non-pyrogenic conditions, the use of RBC as carriers entered into the clinic (Ropars et al., 1987; Hunault-Berger et al., 2015; Mambrini et al., 2017). Nowadays several trials are ongoing and the most advanced are in Phase 3 (ClinicalTrials.gov Identifier: NCT02770807; NCT03563053; NCT01518517). Several biomedical applications of RBC as carriers of therapeutic or contrasting agents require the resealing of the membrane pores produced during the loading procedure and the annealing of the RBC membrane to the cytoskeleton. These requirements are mandatory to prevent the release of the encapsulated drugs, to preserve the RBC immunogenicity avoiding the induction of anti-carrier immune responses and to maintain native phospholipid asymmetry preventing RBC clearance by macrophages. In fact, the lipid composition of the RBC membrane is mainly represented by equal weight of cholesterol and phospholipids. The latter are asymmetrically distributed with the prevalence of phosphatidylcholine and sphingomyelin in the outer monolayer and phosphatidylethanolamine and phosphatidylserine in the inner monolayer (Mohandas and Gallagher, 2008). This phospholipid asymmetry is not spontaneous but maintained by energy dependent and independent enzymes. This asymmetry prevents the adhesion of RBC to vascular endothelial cells and the recognition as well as the removal of phosphatidylserine-exposing RBC by liver and spleen macrophages (McEvoy et al., 1986). Thus, it is responsible for RBC survival in circulation. The phospholipid bilayer is also associated directly and indirectly to the RBC cytoskeleton. The 2-dimensional spectrin based cytoskeleton together with ankyrin, actin and protein 4.1R are connected with selected membrane proteins including but not limited to band 3, glycophorin C and others. The cohesion of the membrane proteins with the cytoskeleton enables the RBC to maintain their favorable membrane surface area preventing vesiculation and preserving the cell integrity (Diez-Silva et al., 2010). Thus, all osmotic-based procedures used to encapsulate agents into RBC should carefully consider not only restoring the physiological osmolarity of the cell but also the annealing of the lipid bilayer with the cell cytoskeleton to preserve the cell integrity, the normal biconcave shape of RBC, and the membrane surface area in excess to permit RBC deformability. In addition, cell-volume regulation by several membrane proteins with transport functions regulates cytoplasmic viscosity and ultimately RBC deformability (Milanick and Hoffman, 1986). All these processes are energy demanding. Thus, annealing of the lipid bilayer with the cytoskeleton, normalization of cytosol viscosity and cell deformability, require the addition, during the resealing of RBC submitted to the osmotic encapsulation of therapeutic agents, of compounds useful to produce ATP, and other relevant metabolic intermediates in the RBC, in adequate amounts and for an adequate time at 37°C (Magnani and DeLoach, 1992). An alternative approach to produce RBC as carriers of therapeutic agents is based on the in vitro generation of engineered erythrocytes to express therapeutic molecules inside the cells starting from hematopoietic precursor cells. For example, this strategy has been used to generate erythrocytes containing an enzyme able to metabolize phenylalanine that are entering a phase I clinical trial for the treatment of patients with phenylketonuria1.
The Red Blood Cell Membrane Can Be Conveniently Modified to Improve the Delivery of Therapeutic Agents
Early methods for coupling therapeutic agents on the RBC membrane were based on the use of crosslinking agents including tannic acid and chromium chloride (Muzykantov et al., 1987, 1993; Chiarantini et al., 1992) with limited specificity and orientation. More than 35 years ago Samokhin et al. (1983) showed that the RBC membrane can be modified by biotinylation in order to couple selected antibodies by way of an avidin bridge. The system was very efficient and up to 80,000–100,000 molecules per cell could be easily coupled on the RBC membrane. Unfortunately, Muzykantov et al. (1991) showed that avidin causes complement activation via alternative pathway and leads to RBC lysis. This problem was subsequently solved by reducing the number of biotin molecules per cell or by reducing the amount of bound streptavidin molecules per cell (Muzykantov et al., 1996). The same system was also used to deliver therapeutic enzymes (Magnani et al., 1992a). Optimization of RBC biotinylation depends on a series of factors, i.e., the number of biotin molecules coupled, the selected biotinylation chemistry and the biotin spacer length (Magnani et al., 1994). Other approaches have explored the possibility of targeting complement receptor 1 (CR1) which is present almost exclusively on RBC membrane.
Taylor et al. (1991) have prepared bispecific cross-linked antibodies to target antigens or ligands to the human RBC membrane via CR1. Spitzer et al. (2004) produced instead a fusion protein by linking a murine red blood cell restricted surface antigen (a scFv specific for TER-119) to the amino-terminus of the human complement regulatory protein (CRP) decay-accelerating factor (DAF). This construct was safe without affecting the circulation and stability of the RBC in mice.
However, a significant improvement was obtained by coupling the drug of interest, i.e., tissue type plasminogen activator (tPA) to an antibody able to recognize human CR1 (Zaitsev et al., 2006). RBC modified by the monoclonal antibody coupled to tPA had a normal viability in a proper preclinical animal model and were effective in preventing occlusive clots (Danielyan et al., 2008). The major expert in the field (Muzykantov, 2010) demonstrated that RBC carrying up to 105 tPA molecules do not induce complement activation, hemolysis, phagocytosis and accelerated clearance in preclinical animal models. Furthermore, normal hemostasis is not affected and tPA is protected from plasma inhibitors by the RBC glycocalyx. The estimated therapeutic window of RBC/PA in humans may vary from hours to days or even weeks depending on the dose. The approach was found to be effective especially in thromboprophylaxis of brain ischemia and stroke (Danielyan et al., 2008). More recently the use of cross-linked antibody-tPA conjugate has been substituted by an antigen binding single chain variable fragment (scFv) fused with a mutated recombinant tPA. The RBC target was also changed using glycophorin A (GPA) instead of CR1 (Zaitsev et al., 2010). Subsequently Zaitsev et al. (2012) studied the function and efficacy of an antibody fragment against Ter-119 fused to the extracellular domain of mouse trombomodulin (TM). They demonstrated that murine RBC receiving this construct were stable and capable of preventing platelet activation and vascular occlusion by clots. Of interest, it became later evident that the target selection on the RBC membrane was also relevant. In fact, binding fusions to RBC on band 3/GPA and RhCE (Rh17) similarly endowed RBC with hTM activity, but differed in their effects on RBC physiology. hTM-scFv targeted to band 3/GPA increased membrane rigidity and sensitized RBC to hemolysis induced by mechanical stress; in contrast, binding of hTM-scFv to RhCE did not alter deformability or sensitivity to mechanical and osmotic stress (Villa et al., 2018). Thus, RBC can be conveniently modified, covalently or non-covalently, to deliver membrane bound therapeutic agents but target specificity, number of target sites on the RBC membrane, and the resulting effect of coupling the therapeutic agent must be carefully investigated to prevent RBC damage and ultimately hemolysis and/or fast removal from circulation. The historical experience about crosslinking agents and the recent data from Muzykantov laboratory (Villa et al., 2016) have clearly documented the limits and the potential solutions to the problem.
Other approaches have been developed during the years. In the nineties, proteins carrying a transmembrane domain were successfully electro-inserted into the RBC membrane providing stable constructs with near normal in vivo survival (Mouneimne et al., 1990; Mouneimne et al., 1991; Zeira et al., 1991). Others (Müller et al., 2000) have used coupling to non-specific NH2 groups on the RBC membrane apparently without damaging the same (only in vitro data are available). Proteins, in particular Decay accelerating factor (DAF) or CD59 insertion, were coupled via a lipid anchor, glycosylphosphatidylinositol (GPI), on the RBC membrane (Civenni et al., 1998). This approach resulted in the formation of functional constructs but released more easily the inserted proteins than native RBC.
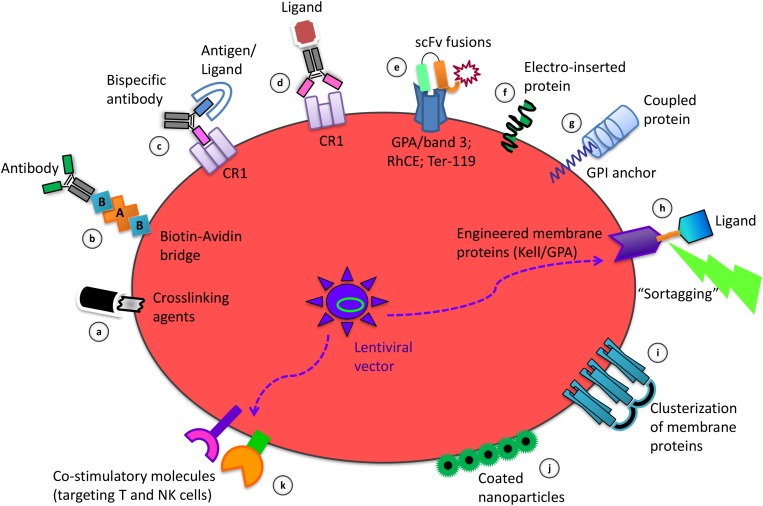
The methods for coupling therapeutic agents on the RBC membrane herein described are schematized in Figures 1a–g.
FIGURE 1.
Strategies of RBC membrane processing to obtain the delivery and targeting of therapeutic agents and to induce immune tolerance or immunogenicity. Among the coupling modalities for binding of molecules to the RBC surface there are: the use of crosslinking agents for chemical coupling of ligands (a); the biotinylation to couple selected antibodies by an avidin bridge (b); the use of bispecific antibodies targeting antigens/ligands to the RBC specific protein target CR1 (c); the crosslinking of antibodies carrying a specific ligand to Complement Receptor 1 (CR1) (d); the use of scFv-based fusion constructs targeting GPA/band 3, RhCE, Ter-119, on the RBC surface (e); the electro-insertion of transmembrane proteins into the RBC membrane (f); the coupling of proteins using the GPI lipid anchor (g); the Sortase A-mediated tagging (“sortagging”) of molecules to genetically engineered membrane proteins, expressed upon ex vivo transfection of erythroid precursors with lentiviral vectors, followed by their differentiation into enucleated RBC (h); the clusterization of RBC membrane proteins for selective targeting (i); the non-covalent attachment of coated nanoparticles to the RBC surface (j); the lentivirus-driven co-expression of combinations of co-stimulatory molecules on engineered RBC (k).
Immunogenicity or Immune Tolerance?
One key question arisen during the years on the experimental use of agents coupled to the RBC membrane is related to the potential immunogenicity of the modified cells. Smith et al. (2012) showed that mice receiving transfusions of RBC expressing human GPA did not produce anti hGPA antibodies and became tolerant to further immunizations with the same antigen. Ryder et al. (2014) observed that RBC antigens trigger immune responses in mice depending on antigen properties as well as on donor and recipient features. This conclusion apparently describes also the situation in humans although the mechanisms for alloimmunization are not completely clear (Hendrickson and Tormey, 2016). Others and we have reported that RBC can perform as antigen delivery systems inducing an immunological response that overcomes also the use of adjuvants (Magnani et al., 1992b; Chiarantini et al., 1997). This response was also partially protective in a feline model of retroviral infection (Chiarantini et al., 1998). Furthermore, these constructs were able to induce CTL responses and neutralizing antibodies (Corinti et al., 2002; Dominici et al., 2003). Richards et al. (2016) showed that inflammation should be considered a key factor in the induction of erythrophagocytosis or tolerance involving different antigen presenting cells. In particular, macrophages in the spleen and liver appear to induce a tollerogenic phenotype while plasmacytoid dendritic cells and monocytes may be associated with humoral alloimmunization. Cremel et al. (2013) provided evidence that macrophages in the liver and spleen represent “tollerogenic” antigen-presenting cells and that the ability to target RBC-loaded with an antigen to these districts can be used conveniently to induce immune tolerance. Murray et al. (2006) instead demonstrated that humoral immune responses can be evoked by using antigen-loaded carrier erythrocytes without adjuvants. The main difference among these experimental approaches was based on the stability of the antigen-loaded RBC. Cremel et al. (2013) intentionally modified the carrier RBC to be quickly removed in the liver and spleen while Murray et al. (2006) produced antigen-loaded RBC with near-normal survival in circulation. Apparently, antigen-carrier RBC induce an immune response if the constructs have a near-normal survival while they induce immune tolerance if they are rapidly removed from bloodstream by the antigen-presenting cells of liver and spleen. Taking into consideration also previous data cited above, it is possible to conclude that antigens taken up by antigen presenting cells in the absence of an inflammatory context induce anergy or immune tolerance (Baekkeskov et al., 2017). Hubbell and colleagues (Kontos et al., 2013) developed a strategy of antigen coupling to mouse RBC by decorating the immunogenic selected protein with the peptide sequence WMVLPWLPGTLD (ERY1) with high specificity for GPA. An antigen specific deletion of reactive CD4 and CD8 T cells was induced when these constructs were administered to mice. Antigens targeted by RBC but not in soluble form were able to activate Tregs. Moreover, Grimm et al. (2015) observed that one antigenic epitope could modulate responses to other epitopes in the same protein antigen. Of interest Lorentz et al. (2015) demonstrated that binding of E. coli L-Asparaginase (a therapeutic enzyme) to erythrocytes abrogated development of antibody titer by >1,000-fold and extended the pharmacodynamic (PD) effect of the drug 10-fold in mice.
Lodish and coworkers (Shi et al., 2014) developed a further innovative approach based on the possibility to attach payloads to selected RBC surface proteins by exploiting sortase-mediated site-specific cut. However, the transpeptidase Sortase A recognizes the LPXTG motif near the C-terminus of the substrate. Thus, the RBC membrane proteins must be first modified to express this target motif that is not naturally present on RBC membrane proteins. Starting from tissue culture of erythrocyte progenitors, normal murine and human red cells can be obtained; Lodish et al. introduced genes into the progenitor cells encoding membrane surface proteins that can be modified by sortases. Two sortase-modifiable membrane proteins, the blood group antigen Kell and GPA, were expressed in erythroid progenitors (Figure 1h). The Kell is a type II membrane protein with the C-terminus exposed to the extracellular milieu while GPA is a type I membrane protein with the N-terminus extracellularly disposed. Of interest, to target the RBC to a specific cell type, the red cells were modified by linking on their surface a single domain antibody with full retention of binding specificity. These constructs show near normal circulation in mice and mature in vitro to enucleated reticulocytes up to 50–60% of retroviral transduced precursor cells. Later on, Pishesha et al. (2017) demonstrated that modified RBC expressing specific antigens blunt the immune response of the main immune effector cells (B, CD4 T cells, and CD8 T cells). The encouraging results obtained in different mouse models of autoimmune diseases led to conclude that this strategy may be applied in therapeutic approaches and prophylactic measures.
Overall, the apparent discrepancy arisen from the results of the above mentioned studies about the different immune responses evoked by the engineered RBC, maybe be due to several reasons: a) membrane proteins used to bind the antigens; b) half-life in bloodstream of the engineered RBC; c) experimental protocol characteristics, e.g., number of RBC administrations, experiment duration, time-interval between RBC administrations. Therefore, these observations suggest that a number of variants could be responsible for the final immunological outcome and that a unique protocol to mount either immunogenicity or immune tolerance versus an antigen delivered by RBC is not yet available.
The Red Blood Cell Membrane Can Be Conveniently Modified to Facilitate Drug Targeting
The possibility of coupling proteins and different types of ligands on the RBC external membrane without compromising RBC survival in circulation, prompted investigators to target RBC to selected target cells or circulating compounds. Several examples have already been illustrated above but other relevant considerations and approaches are summarized here. Chiarantini et al. (1992) demonstrated the possibility of selective targeting of T cells by coupling a specific antibody on the RBC membrane. In, Chiarantini et al. (1995) showed that controlled modification of RBC membrane proteins can conveniently target the RBC to liver and spleen macrophages. In case the RBC have been previously loaded with molecules of interest, the same will also be selectively transferred to these phagocytic cells (Magnani et al., 1992c, 1996; Rossi et al., 1998). The mechanism identified was based on the use of agents (Zn, BS3 or both) able to induce clusterization of RBC membrane associated proteins (Figure 1i). The extension of the clusterization was responsible for the observed removal of processed RBC from circulation. Many years later Cremel et al. (2013) used only BS3 for a fast and efficient method to target RBC to liver and spleen phagocytic cells confirming the previous observations. Thus, RBC targeting to spleen and liver phagocytic cells is feasible, can be modulated in the rate of processed cells removed from circulation, and can be adopted also for the targeting of RBC previously loaded with drugs or agents of interest. Huang et al. (2017) demonstrated that, by using RBC expressing chimeric proteins that consisted of single-domain camelid antibodies (VHHs) against botulinum neurotoxin A fused with GPA or Kell, a prolonged protection against bacterial toxins was attainable. The system was very efficient in mice conferring resistance to a lethal dose in the order of 10,000-times the effect observed using free antibodies. Anselmo et al. (2013) showed that it is possible to increase the amount of nanoparticles (NPs) in circulation and their persistence in the lungs over 24 h by a non-covalent attachment of NPs to RBC, while reducing their removal by liver and spleen phagocytic cells. A further increase in lung targeting and retention of NPs is achievable by linking anti-ICAM-1 antibody to the exposed surface of NPs attached to RBC. Thus, a new, indirect, drug targeting system was developed by using NPs coated with specific antibodies and non-covalently bound on the RBC membrane (Figure 1j). More recently, Brenner et al. (2018) have reported clear evidences for a general targeting platform based on nanocarrier hitching onto RBC for a selective targeting to different organs according to the site of injection. This platform is of general interest since it works with a large variety of nanocarriers. Actually, https://www.rubiustx.com/ is developing engineered RBC obtained from precursor erythroid cells transfected with lentiviral vectors to co-express combination of co-stimulatory molecules on the RBC membrane to target T cells and NK cells aiming at killing tumor cells (Figure 1k).
Conclusion
For several years, RBC have not only been exploited as oxygen carriers but also as drug delivery and targeting agents. The RBC have unique properties that outperform conventional and new drug delivery systems. These interesting properties permit: to load the RBC with agents of interest without affecting the in vivo RBC circulation and the RBC immunological properties; to couple or decorate the RBC membrane by agents useful to target the cells to selected cells and/or organs; to express antibodies able to inactivate toxins; to carry antigens for the induction of immune tolerance or induce immunogenicity. This review focuses on these last properties and considers different modalities for coupling or inserting peptides, proteins or antibodies on the RBC membrane. Having shown that different modalities illustrated by many groups listed in this review produce RBC with different properties, the researchers can be guided in selecting the most appropriate approach for the intended application (Table 1). For example, we know now that chemical linking of proteins or peptides on the RBC membrane is rarely the modality to be preferred if survival in circulation is the necessary requirement. In addition, the engagement of selected cell determinants during the coupling procedure could affect some important physiological functions of the RBC. Finally, when ligands or proteins are inserted into the membrane, their membrane distribution and the stability of the constructs should be considered. Of interest, recent observations that describe the possibility of RBC-hitching of nanocarriers open additional perspectives. Finally, the isolation of RBC precursors, their transfection with lentiviral vectors, the expansion and in vitro maturation of enucleated reticulocytes and mature RBC, will also open new unexplored possibilities for the expression on the human RBC membrane of new therapeutic agents. These possibilities should be exploited case-by-case since they could also be associated with unwanted secondary effects. Similarly, some limitations can also arise when therapeutic molecules are administered confined inside RBC such as: (a) premature uptake of drug-loaded erythrocytes from bloodstream when a prolonged permanence in circulation is required; (b) limited kinetics of erythrocyte transmembrane transport of substrates or products when therapeutic enzymes are loaded inside; (c) drug leaking across cell membranes; (d) possible alterations operated by some drugs on RBC structure (Leuzzi et al., 2016). Considering the peculiarity of human RBC versus other animal species, the experimental evidences should not be limited to animal models but derived also from clinical trials. In conclusion, the use of RBC is continuing to open new possibilities for realizing carriers endowed with enormous potential for the benefit of patients in need and for the improvement of therapeutic agents with limited or poor pharmacokinetic (PK)/PD properties. These approaches have reached the clinic and at least two companies are already in Phase 3 for selected applications2,3. The activities in place, and the excellent amount of publications in this field, make the scientific community optimistic about the possibility of a fast clinical development and approval of the use of RBC as carriers and delivery systems in many conditions with unmet medical needs.
TABLE 1.
Coupling modalities for the binding of molecules of interest to the RBC membrane.
| Coupling modality | Advantages | Limits | References |
| Chromium Chloride | High efficiency | Limited specificity/orientation | Muzykantov et al., 1987, 1993; Chiarantini et al., 1992 |
| Biotin-Avidin | High efficiency | Complement activation and cell lysis at high copy number | Muzykantov et al., 1991; Magnani et al., 1994 |
| Crosslinking of antibodies to Complement Receptor 1 (CR1) | No complement activation, no hemolysis, no phagocytosis, no accelerated clearance | Significant variation in CR1 expression levels among individuals and limited dosing CR1 conjugates | Taylor et al., 1991; Birmingham and Herbert, 2001; Zaitsev et al., 2006; Danielyan et al., 2008; Muzykantov, 2010 |
| Crosslinking of antibody fragments (scFv) to glycophorin A (GPA)/band 3 | Stability, high efficiency, no RBC aggregation, no hemolysis, no uptake by RES | Membrane rigidity and hemolysis induced by mechanical stress | Zaitsev et al., 2010; Villa et al., 2018 |
| Crosslinking of antibody fragments (scFv) to Ter-119 | High efficiency, no RBC damage, no survival RBC alteration | Unknown | Zaitsev et al., 2012 |
| Crosslinking of antibody fragments (scFv) to RhCE | No deformability or sensitivity to mechanical and osmotic stress, no impact on RBC physiology, presence on the RBC of 100% of the human population | Unknown | Villa et al., 2018 |
| Electro-insertion of transmembrane proteins into the RBC membrane | Almost normal in vivo survival of RBC | Unknown | Mouneimne et al., 1990, 1991; Zeira et al., 1991 |
| Coupling of proteins via a glycosylphosphatidylinositol (GPI)-anchor on the RBC membrane | Formation of functional constructs | Early release of the inserted proteins respect to the endogenous GPI-anchored proteins | Civenni et al., 1998 |
| Coupling of antigens to RBC upon conjugation to the linear peptide sequence (ERY1) with high specificity for GPA | Antigen specific deletion of reactive CD4 and CD8 T cells | Unknown | Kontos et al., 2013 |
| Sortase-mediated site-specific covalent attachment of “cargo” to engineered surface proteins (Kell, GPA) in erythroid precursors | Acceptable in vitro maturation of retroviral transduced precursor cells to enucleated reticulocytes Near normal circulation | Expensive and time-consuming | Shi et al., 2014; Pishesha et al., 2017 |
| Clusterization of RBC surface membrane proteins by crosslinking agents (Zn, BS3) | Targeting of RBC (loaded or not with agents) to liver and spleen macrophages | Unknown | Magnani et al., 1992c; Chiarantini et al., 1995; Magnani et al., 1996; Rossi et al., 1998; Cremel et al., 2013 |
| Non-covalent attachment of nanoparticles (NPs), coated with the agents of interest, to RBC | Increased blood levels of NPs. Lung targeting and retention. Reduced uptake by liver and spleen | Unknown | Anselmo et al., 2013,Brenner et al., 2018 |
| RBC precursors engineered to co-express combinations of co-stimulatory molecules | Targeting of T and NK cells | Unknown | https://www.rubiustx.com/ |
Author Contributions
All authors have provided substantial contributions to the concept of the work, analysis and interpretation of the data for the work, drafting the work and revising it critically, approval for publication, and agreed to be accountable for all aspects of the work.
Conflict of Interest Statement
MM and LR hold shares in EryDel SpA a company with interests in the technology of RBC-based drug delivery. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We greatly acknowledge the colleagues and the students who during the years have contributed to these developments. Furthermore, we thank the agencies that supported our work.
Funding. This work was partially supported by the University of Urbino and FanoAteneo, Italy.
References
- Anselmo A. C., Gupta V., Zern B. J., Pan D., Zakrewsky M., Muzykantov V., et al. (2013). Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano 7 11129–11137. 10.1021/nn404853z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Hubbell J. A., Phelps E. A. (2017). Bioengineering strategies for inducing tolerance in autoimmune diabetes. Adv. Drug Deliv. Rev. 114 256–265. 10.1016/j.addr.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Birmingham D. J., Herbert L. A. (2001). CR1 and CR1-like: the primate immune adherence receptors. Immunol. Rev. 180 100–111. [DOI] [PubMed] [Google Scholar]
- Brenner J. S., Pan D. C., Myerson J. W., Marcos-Contreras O. A., Villa C. H., Patel P., et al. (2018). Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 9:2684. 10.1038/s41467-018-05079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarantini L., Argnani R., Zucchini S., Stevanato L., Zabardi P., Grossi M. P., et al. (1997). Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: immunogenicity and protection in mice. Vaccine 15 276–280. [DOI] [PubMed] [Google Scholar]
- Chiarantini L., Droleskey R., Magnani M., DeLoach J. R. (1992). In vitro targeting of erythrocytes to cytotoxic T-cells by coupling of Thy-1.2 monoclonal antibody. Biotechnol. Appl. Biochem. 15 171–184. [PubMed] [Google Scholar]
- Chiarantini L., Matteucci D., Pistello M., Mancini U., Mazzetti P., Massi C., et al. (1998). AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: homologous erythrocytes as a delivery system for preferential immunization with putative protective antigens. Clin. Diagn. Lab. Immunol. 5 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarantini L., Rossi L., Fraternale A., Magnani M. (1995). Modulated red blood cell survival by membrane protein clustering. Mol. Cell. Biochem. 144 53–59. [DOI] [PubMed] [Google Scholar]
- Civenni G., Test S. T., Brodbeck U., Bütikofer P. (1998). In vitro incorporation of GPI-anchored proteins into human erythrocytes and their fate in the membrane. Blood 91 1784–1792. [PubMed] [Google Scholar]
- Corinti S., Chiarantini L., Dominici S., Laguardia M. E., Magnani M., Girolomoni G. (2002). Erythrocytes deliver Tat to interferon-gamma-treated human dendritic cells for efficient initiation of specific type 1 immune responses in vitro. J. Leukoc. Biol. 71 652–658. [PubMed] [Google Scholar]
- Cremel M., Guérin N., Horand F., Banz A., Godfrin Y. (2013). Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int. J. Pharm. 443 39–49. 10.1016/j.ijpharm.2012.12.044 [DOI] [PubMed] [Google Scholar]
- Danielyan K., Ganguly K., Ding B. S., Atochin D., Zaitsev S., Murciano J. C., et al. (2008). Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation 118 1442–1449. 10.1161/CIRCULATIONAHA.107.750257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Silva M., Dao M., Han J., Lim C. T., Suresh S. (2010). Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. 35 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici S., Laguardia M. E., Serafini G., Chiarantini L., Fortini C., Tripiciano A., et al. (2003). Red blood cell-mediated delivery of recombinant HIV-1 Tat protein in mice induces anti-Tat neutralizing antibodies and CTL. Vaccine 21 2073–2081. [DOI] [PubMed] [Google Scholar]
- Grimm A. J., Kontos S., Diaceri G., Quaglia-Thermes X., Hubbell J. A. (2015). Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci. Rep. 5:15907. 10.1038/srep15907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson J. E., Tormey C. A. (2016). Understanding red blood cell alloimmunization triggers. Hematology Am. Soc. Hematol. Educ. Program. 2016 446–451. 10.1182/asheducation-2016.1.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N. J., Pishesha N., Mukherjee J., Zhang S., Deshycka R., Sudaryo V., et al. (2017). Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin. Nat. Commun. 8:423. 10.1038/s41467-017-00448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault-Berger M., Leguay T., Huguet F., Leprêtre S., Deconinck E., Ojeda-Uribe M., et al. (2015). A phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study. Am. J. Hematol. 90 811–818. 10.1002/ajh.24093 [DOI] [PubMed] [Google Scholar]
- Ihler G. M., Glew R. H., Schnure F. W. (1973). Enzyme loading of erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 70 2663–2666. 10.1073/pnas.70.9.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos S., Kourtis I. C., Dane K. Y., Hubbell J. A. (2013). Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl. Acad. Sci. U.S.A. 110 E60–E68. 10.1073/pnas.1216353110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzzi V., Rossi L., Gabucci C., Nardecchia F., Magnani M. (2016). Erythrocyte-mediated delivery of recombinant enzymes. J. Inherit. Metab. Dis. 39 519–530. 10.1007/s10545-016-9926-0 [DOI] [PubMed] [Google Scholar]
- Lorentz K. M., Kontos S., Diaceri G., Henry H., Hubbell J. A. (2015). Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci. Adv. 1:e1500112. 10.1126/sciadv.1500112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani M., Casabianca A., Fraternale A., Brandi G., Gessani S., Williams R., et al. (1996). Synthesis and targeted delivery of an azidothymidine homodinucleotide conferring protection to macrophages against retroviral infection. Proc. Natl. Acad. Sci. U.S.A. 93 4403–4408. 10.1073/pnas.93.9.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani M., Chiarantini L., Mancini U. (1994). Preparation and characterization of biotinylated red blood cells. Biotechnol. Appl. Biochem. 20 335–345. [DOI] [PubMed] [Google Scholar]
- Magnani M., DeLoach J. R. (1992). The Use of Resealed Erythrocytes as Carriers and Bioreactors. New York, NY: Plenum Press. [Google Scholar]
- Magnani M., Chiarantini L., Vittoria E., Mancini U., Rossi L., Fazi A. (1992b). Red blood cells as an antigen-delivery system. Biotechnol. Appl. Biochem. 16 188–194. [PubMed] [Google Scholar]
- Magnani M., Mancini U., Bianchi M., Fazi A. (1992a). Comparison of uricase-bound and uricase-loaded erythrocytes as bioreactors for uric acid degradation. Adv. Exp. Med. Biol. 326 189–194. [DOI] [PubMed] [Google Scholar]
- Magnani M., Rossi L., Brandi G., Schiavano G. F., Montroni M., Piedimonte G. (1992c). Targeting antiretroviral nucleoside analogues in phosphorylated form to macrophages: in vitro and in vivo studies. Proc. Natl. Acad. Sci. U.S.A. 89 6477–6481. 10.1073/pnas.89.14.6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambrini G., Mandolini M., Rossi L., Pierigè F., Capogrossi G., Salvati P., et al. (2017). Ex vivo encapsulation of dexamethasone sodium phosphate into human autologous erythrocytes using fully automated biomedical equipment. Int. J. Pharm. 517 175–184. 10.1016/j.ijpharm.2016.12.011 [DOI] [PubMed] [Google Scholar]
- McEvoy L., Williamson P., Schlegel R. A. (1986). Membrane phospholipid asymmetry as adeterminant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. U.S.A. 83 3311–3315. 10.1073/pnas.83.10.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanick M. A., Hoffman J. F. (1986). Ion transport and volume regulation in red blood cells. Ann. N. Y. Acad. Sci. 488 174–186. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Gallagher P. G. (2008). Red cell membrane: past, present, and future. Blood 112 3939–3948. 10.1182/blood-2008-07-161166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouneimne Y., Tosi P. F., Barhoumi R., Nicolau C. (1990). Electroinsertion of full length recombinant CD4 into red blood cell membrane. Biochim. Biophys. Acta 1027 53–58. [DOI] [PubMed] [Google Scholar]
- Mouneimne Y., Tosi P. F., Barhoumi R., Nicolau C. (1991). Electroinsertion of xeno proteins in red blood cell membranes yields a long lived protein carrier in circulation. Biochim. Biophys. Acta 1066 83–89. 10.1016/0005-2736(91)90254-6 [DOI] [PubMed] [Google Scholar]
- Müller M., Büchi L., Woodtli K., Haeberli A., Beer J. H. (2000). Preparation and characterization of ‘heparinocytes’: erythrocytes with covalently bound low molecular weight heparin. FEBS Lett. 468 115–119. 10.1016/s0014-5793(00)01204-7 [DOI] [PubMed] [Google Scholar]
- Murray A. M., Pearson I. F., Fairbanks L. D., Chalmers R. A., Bain M. D., Bax B. E. (2006). The mouse immune response to carrier erythrocyte entrapped antigens. Vaccine 24 6129–6139. 10.1016/j.vaccine.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R. (2010). Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin. Drug Deliv. 7 403–427. 10.1517/17425241003610633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R., Murciano J. C., Taylor R. P., Atochina E. N., Herraez A. (1996). Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal. Biochem. 241 109–119. 10.1006/abio.1996.0384 [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Sakharov D. V., Domogatsky S. P., Goncharov N. V., Danilov S. M. (1987). Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am. J. Pathol. 128 276–285. [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R., Smirnov M. D., Samokhin G. P. (1991). Avidin attachment to biotinylated erythrocytes induces homologous lysis via the alternative pathway of complement. Blood 78 2611–2618. [PubMed] [Google Scholar]
- Muzykantov V. R., Smirnov M. D., Zaltzman A. B., Samokhin G. P. (1993). Tannin-mediated attachment of avidin provides complement-resistant immunoerythrocytes that can be lysed in the presence of activator of complement. Anal. Biochem. 208 338–342. 10.1006/abio.1993.1057 [DOI] [PubMed] [Google Scholar]
- Pierigè F., Bigini N., Rossi L., Magnani M. (2017). Reengineering red blood cells for cellular therapeutics and diagnostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9 e1454. 10.1002/wnan.1454 [DOI] [PubMed] [Google Scholar]
- Pierigè F., Serafini S., Rossi L., Magnani M. (2008). Cell-based drug delivery. Adv. Drug Deliv. Rev. 60 286–295. 10.1016/j.addr.2007.08.029 [DOI] [PubMed] [Google Scholar]
- Pishesha N., Bilate A. M., Wibowo M. C., Huang N. J., Li Z., Deshycka R., et al. (2017). Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc. Natl. Acad. Sci. U.S.A. 114 3157–3162. 10.1073/pnas.1701746114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. L., Hendrickson J. E., Zimring J. C., Hudson K. E. (2016). Erythrophagocytosis by plasmacytoid dendritic cells and monocytes is enhanced during inflammation. Transfusion 56 905–916. 10.1111/trf.13497 [DOI] [PubMed] [Google Scholar]
- Ropars C., Avenard G., Chassaigne M. (1987). Large-scale entrapment of drugs into resealed red blood cells using a continuous-flow dialysis system. Methods Enzymol. 149 242–248. [DOI] [PubMed] [Google Scholar]
- Rossi L., Brandi G., Schiavano G. F., Balestra E., Millo E., Scarfì S., et al. (1998). Macrophage protection against human immunodeficiency virus or herpes simplex virus by red blood cell-mediated delivery of a heterodinucleotide of azidothymidine and acyclovir. AIDS Res. Hum. Retroviruses 14 435–444. 10.1089/aid.1998.14.435 [DOI] [PubMed] [Google Scholar]
- Rossi L., Pierigè F., Antonelli A., Bigini N., Gabucci C., Peiretti E., et al. (2016). Engineering erythrocytes for the modulation of drugs’ and contrasting agents’ pharmacokinetics and biodistribution. Adv. Drug Deliv. Rev. 106 73–87. 10.1016/j.addr.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Ryder A. B., Zimring J. C., Hendrickson J. E. (2014). Factors influencing RBC alloimmunization: lessons learned from murine models. Transfus. Med. Hemother. 41 406–419. 10.1159/000368995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhin G. P., Smirnov M. D., Muzykantov V. R., Domogatsky S. P., Smirnov V. N. (1983). Red blood cell targeting to collagen-coated surfaces. FEBS Lett. 154 257–261. [DOI] [PubMed] [Google Scholar]
- Shi J., Kundrat L., Pishesha N., Bilate A., Theile C., Maruyama T., et al. (2014). Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl. Acad. Sci. U.S.A. 111 10131–10136. 10.1073/pnas.1409861111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. H., Hod E. A., Spitalnik S. L., Zimring J. C., Hendrickson J. E. (2012). Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs. Blood 119 1566–1569. 10.1182/blood-2011-09-382655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer D., Unsinger J., Bessler M., Atkinson J. P. (2004). ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement. Mol. Immunol. 40 911–919. [DOI] [PubMed] [Google Scholar]
- Taylor R. P., Sutherland W. M., Reist C. J., Webb D. J., Wright E. L., Labuguen R. H. (1991). Use of heteropolymeric monoclonal antibodies to attach antigens to the C3b receptor of human erythrocytes: a potential therapeutic treatment. Proc. Natl. Acad. Sci. U.S.A. 88 3305–3309. 10.1073/pnas.88.8.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa C. H., Anselmo A. C., Mitragotri S., Muzykantov V. (2016). Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 106 88–103. 10.1016/j.addr.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa C. H., Pan D. C., Johnston I. H., Greineder C. F., Walsh L. R., Hood E. D., et al. (2018). Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2 165–176. 10.1182/bloodadvances.2017011734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev S., Danielyan K., Murciano J. C., Ganguly K., Krasik T., Taylor R. P., et al. (2006). Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood 108 1895–1902. 10.1182/blood-2005-11-012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev S., Kowalska M. A., Neyman M., Carnemolla R., Tliba S., Ding B. S., et al. (2012). Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood 119 4779–4785. 10.1182/blood-2011-12-398149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev S., Spitzer D., Murciano J. C., Ding B. S., Tliba S., Kowalska M. A., et al. (2010). Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fibrinolysis. J. Pharmacol. Exp. Ther. 332 1022–1031. 10.1124/jpet.109.159194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeira M., Tosi P. F., Mouneimne Y., Lazarte J., Sneed L., Volsky D. J., et al. (1991). Full-length CD4 electroinserted in the erythrocyte membrane as a long-lived inhibitor of infection by human immunodeficiency virus. Proc. Natl. Acad. Sci. U.S.A. 88 4409–4413. 10.1073/pnas.88.10.4409 [DOI] [PMC free article] [PubMed] [Google Scholar]