Abstract
Aims
Cannabidiol (CBD) is a cannabis‐derived medicinal product with potential application in a wide‐variety of contexts; however, its effective dose in different disease states remains unclear. This review aimed to investigate what doses have been applied in clinical populations, in order to understand the active range of CBD in a variety of medical contexts.
Methods
Publications involving administration of CBD alone were collected by searching PubMed, EMBASE and ClinicalTrials.gov.
Results
A total of 1038 articles were retrieved, of which 35 studies met inclusion criteria covering 13 medical contexts. Twenty‐three studies reported a significant improvement in primary outcomes (e.g. psychotic symptoms, anxiety, seizures), with doses ranging between <1 and 50 mg/kg/d. Plasma concentrations were not provided in any publication. CBD was reported as well tolerated and epilepsy was the most frequently studied medical condition, with all 11 studies demonstrating positive effects of CBD on reducing seizure frequency or severity (average 15 mg/kg/d within randomised controlled trials). There was no signal of positive activity of CBD in small randomised controlled trials (range n = 6–62) assessing diabetes, Crohn's disease, ocular hypertension, fatty liver disease or chronic pain. However, low doses (average 2.4 mg/kg/d) were used in these studies.
Conclusion
This review highlights that CBD has a potential wide range of activity in several pathologies. Pharmacokinetic studies as well as conclusive phase III trials to elucidate effective plasma concentrations within medical contexts are severely lacking and highly encouraged.
Keywords: cannabidiol, cannabinoid, dose, dosing, therapeutics
What is already known about this subject
Due to its favourable toxicity and side effect profile, cannabidiol is under increasing investigation in the commercial and medical industry to treat many clinical indications.
What this study adds
This study identifies the wide active dosing range of cannabidiol (<1 to 50 mg/kg/d) within a variety of medical conditions including epilepsy, anxiety and graft‐vs‐host disease.
This review indicates that studies that used higher doses tended to have better therapeutic outcomes compared to lower doses overall.
This study identifies a strong existing need for dose‐ranging clinical studies to be conducted in which plasma concentrations can provide a better indication of the therapeutic range of cannabidiol.
1. INTRODUCTION
Cannabidiol (CBD) is a non‐intoxicating major constituent of the Cannabis sativa plant that has been increasing in interest due to its potentially diverse range of therapeutic properties and its favourable safety and tolerability profile.1 Side effects are generally mild and infrequent, such as sleepiness, diarrhoea or increased temperature. It is also reported that clinically significant drug‐interactions pose a low risk.2 There is no evidence for dependency or abuse potential with CBD use, as concluded by the World Health Organisation Expert Committee on Drug Dependence.1 The purported effects of CBD include analgesic, anti‐inflammatory, antioxidant, anxiolytic, anticonvulsant and cytotoxic effects, which are mediated through signalling mechanisms including the cannabinoid receptor 1 (weak agonist), the cannabinoid receptor 2 (inverse agonist), the serotonin 1a receptor (5‐HT1A), G protein‐coupled receptor 55 (GPR55), G protein‐coupled receptor 18 (GPR18) and the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptors, amongst others.3
Clinically, CBD is being investigated in multiple disease states including neurodegeneration, anxiety disorder, orphan childhood diseases with a prevalence of <5 in 10 000 individuals (e.g. tuberous sclerosis complex) and addiction (ongoing trials in cannabis and cocaine craving).4, 5, 6 Epidiolex has recently become the first Food and Drug Administration‐approved CBD medicine, indicated for use in Lennox–Gastaut or Dravet syndrome (childhood epilepsy) by oral administration. Sativex is an oromucosal spray containing both CBD and δ‐9‐tetrahydrocannibinol, which is licenced in the EU and Canada for the treatment of multiple sclerosis associated spasticity. At the time of writing, there are 49 clinical trials registered on clinicaltrials.gov investigating CBD alone (either not yet recruiting, recruiting or active) and there have been at least a further 100 clinical trials previously registered containing CBD, indicating a significant clinical interest with an ongoing need to ensure that human volunteers engaged in these trials are given doses that are optimised for efficacy and safety. Surprisingly, none of the 49 currently registered trials have explicitly included a study design to investigate the dose‐ranging efficacy of CBD.
Hemp‐derived CBD is commercially available and is currently used as a health and food supplement commonly for anxiety and pain relief. This market represents a flourishing industry expected to rise financially and globally.7 However, the blurred lines between CBD as a licensed medicine and CBD as an over‐the‐counter remedy contribute to the overall lack of understanding of what dose of CBD may be considered therapeutic. This is further hampered by the lack of standardisation in over‐the‐counter CBD products and their unregulated labelled doses.
Despite the prevalence of CBD use and current hype, guidance on dose recommendations has not advanced and is not clear, additionally hampered by the striking lack of accessible pharmacokinetic and bioavailability data of CBD in humans.8 No published study to date has reported the absolute oral bioavailability of CBD in humans.8 Limited dose‐determination studies have left a paucity in data surrounding desired plasma concentrations to achieve minimum effective doses. Additionally, the lack of information on the role of different formulations and routes of administration on absorption are also apparent. The aim of this review was to comprehensively collate all published data relating to CBD administration in clinical populations to describe the range of CBD doses assessed across different pathological states.
2. METHODS
2.1. Search strategy
The systematic review was carried out in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines. A systematic search of PubMed, EMBASE (including MEDLINE) and clinicaltrials.gov was conducted to retrieve all articles reporting CBD administration in clinical populations using ‘CBD or Cannabidiol’ as search terms. Searches were restricted to ‘humans’ and ‘clinical trials and case reports’ in PubMed and EMBASE, with no restrictions on clinicaltrials.gov. The searches were carried out by 8 August 2018 by 2 independent researchers.
2.2. Eligibility criteria
The titles and abstracts of retrieved studies were examined by 2 independent researchers, and inappropriate articles were rejected. Inclusion criteria were as follows: an original, peer‐reviewed published paper that involved administration of CBD to a clinical population, or reported on clinicaltrials.gov, and included an outcome measurement to assess the efficacy of CBD i.e. improvement in disease. Exclusion criteria were: administration in healthy participants only; CBD administered in combination with other cannabinoids such as with δ‐9‐tetrahydrocannibinol or as whole cannabis extracts; article not in English; no stated concentration of CBD used; or no statistical results reported. The reference lists of included studies were hand‐searched for additional relevant studies.
2.3. Data acquisition and analysis
The included articles were analysed, and the following data extracted: sample size, clinical population/medical context; study design and length; administration route of CBD; source of CBD; dose of CBD; side effects; and primary outcome results. All data entry was checked by an additional independent researcher. Risk of bias of the 15 randomised controlled trials was assessed using the 2011 Cochrane Collaboration's tool for assessing risk of bias.
As this review included studies of participants of all ages (from infants to adults), dosing is reported in mg/kg of body weight to allow for comparison. Where not available as mg/kg (24 studies), dose was converted for adults using an average adult body weight of 62 kg.9 In only 1 publication, a case report on a child, an average child weight of 40 kg had to be used to convert reported mg/d dose into mg/kg/d.10
A positive effect of CBD was determined by the presence of a significant improvement in primary end points(s) or outcomes reported compared to placebo or baseline. A lack of positive effect was determined if no significant improvements were reported. Mixed findings were reported for example in case reports wherein some patients improved, others did not, or where a primary outcome was not specified (exploratory study) and in which some endpoints improved while others worsened (1 study) or remained unchanged.
3. RESULTS
The initial search yielded 1038 records, from which 896 abstracts were reviewed, and 35 articles were included in the final analysis, comprising a total number of 1223 participants. A flow chart of article retrieval and selection is presented in Figure 1. Fifteen studies were randomised controlled trials (RCTs), 8 were clinical trials but not both randomised and controlled in design (for example open‐label trials), and 12 articles were case reports/series. A description of each study is presented in tables 1, 2, 3 according to study design. Results of the risk of bias assessment of the RCTs are presented in Figure 2. A component of blinding was included in 74% of the RCTs . No study was reported with a high risk of selection bias, detection bias, or reporting bias. Overall, most information was from studies at low risk of bias. No study reported plasma concentrations of CBD. All studies reported oral administration of CBD, either as an oral solution (n = 11), capsules (n = 13), spray/sublingual (n = 4), or orally but unspecified (n = 6).
Figure 1.
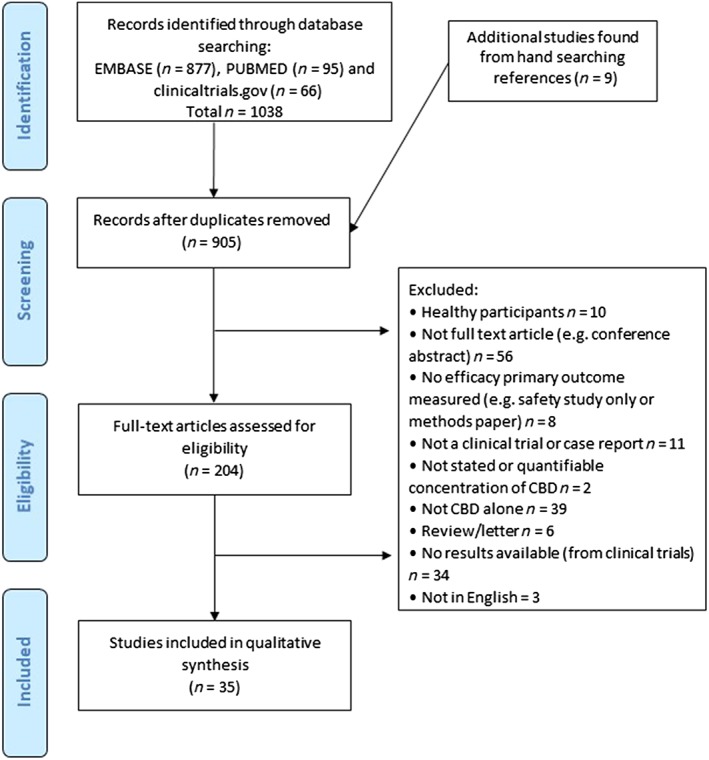
Flow chart of study retrieval and selection
Table 1.
Summary of included studies: randomised controlled trials
| Study | Clinical population | Total n | Design | Trial length | CBD dose (mg) and approx. mg/kg/da | Route of admin. | CBD source | Resultsb: Primary endpoint(s) | + effect | Side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| McGuire, 2018 (NCT02006628) 11 | Schizophrenia, adults | 88 | Phase II exploratory double‐blind, parallel‐group, RCT. Add‐on therapy to anti‐psychotic drugs. | 8 wk | 1000 mg/d (16.7 mg/kg/d) | Oral solution | GW | Positive psychotic symptoms reduced. Negative, overall and general psychotic symptoms unchanged. Higher proportion of CBD treated patients rated as improved. No differences in functionality. No significant improvement in cognitive function except for motor speed. Overall reported as clinically significant improvements with CBD. | Yes | Rates of adverse events similar between CBD and placebo groups |
| Thiele, 2018 (NCT02224690) 12 | Seizures (Lennox–Gastaut syndrome), ages 2–55 y | 171 | Double‐blind, phase III, RCT. Add‐on therapy to AEDs. | 14 wk | 20 mg/kg/d | Oral solution | GW | Monthly frequency of drop seizures decreased by a median of 43.9% in the CBD group, significantly more than in the placebo group | Yes | Diarrhoea, somnolence, pyrexia, decreased appetite, vomiting |
| Devinsky, 2018 (NCT02224560) 13 | Lennox–Gastaut syndrome (epilepsy), ages 2–55 y | 225 | Phase III, double‐blind, RCT. Add‐on therapy to AEDs. | 14 wk | 10 or 20 mg/kg/d | Oral solution | GW | Significantly greater reduction in CBD groups in drop seizure frequency than in placebo | Yes | 9% taking CBD had elevated liver aminotransferases. Somnolence, decreased appetite, diarrhoea, upper respiratory tract infection, pyrexia, vomiting. |
| Boggs, 2018 (NCT00588731) 14 | Schizophrenia, adults | 36 | Double‐blind, parallel group, RCT. Add‐on therapy to anti‐psychotic drugs. | 6 wk | 600 mg/d (10 mg/kg/d) | Oral capsules | STI | No effect on cognition or symptoms | No | Similar rates between placebo and CBD, with exception of sedation which was higher in CBD group. |
| Naftali, 2017 (NCT01037322) 15 | Crohn's disease, adults | 19 | RCT | 8 wk | 20 mg/d (0.3 mg/kg/d) | Orally, sublingual | On‐site | No difference in disease index | No | None observed |
| Devinsky, 2017 (NCT02091375) 16 | Treatment resistant Dravet syndrome (epilepsy), aged 2–18 y | 120 | Double‐blind, RCT. Add‐on therapy to AEDs. | 14 wk | 20 mg/kg/d | Oral solution | GW | Reduction in frequency of convulsive seizures compared to baseline, significantly greater reduction than with placebo | Yes | Diarrhoea, vomiting, fatigue, pyrexia, somnolence, abnormal results on liver‐function: tests were higher in the CBD group than placebo |
| Jadoon, 2016 (NCT01217112) 17 | Type 2 diabetes patients, adults | 62 | Double‐blind, RCT | 13 wk | 200 mg/d (3.3 mg/kg/d) | Oral | GW | No change in HDL‐cholesterol concentrations or glycaemic control. | No | Well tolerated |
| Chagas, 2014 18 | Parkinson's disease, adults | 21 | Double‐blind exploratory RCT. Add‐on therapy to anti‐Parkinson's drugs. | 6 wk | 75 or 300 mg/d (1.25 or 5 mg/kg/d) | Oral capsules | THC | No effect on motor and general symptoms; 300‐mg dose improved well‐being and quality of life scores. | Mixed | None reported |
| Leweke, 2012 19 | Schizophrenia, adults | 42 | Phase II, double‐blind, parallel‐group, RCT | 4 wk | 800 mg/d (max: 13.3 mg/kg/d) | NA | NA | Significant improvement of psychotic symptoms compared to baseline | Yes | Well tolerated |
| Bergamaschi, 2011 20 | Generalised SAD, adults | 24 | Double‐blind, RCT | Acute | 600 mg (10 mg/kg) | Oral capsule | STI and THC | Reduction in anxiety, cognitive impairment, discomfort in speech performance. Alert factors in anticipatory speech were also reduced. | Yes | None reported |
| Tomida, 2006 21 | Ocular hypertension, adults | 6 | Double‐blind, 4‐way cross‐over, RCT | Acute | 20 or 40 mg (0.3 or 0.7 mg/kg) | Oromucosal spray | GW | 20 mg of CBD was ineffective, while 40 mg slightly increased intraocular pressure. | No | Mild—e.g. oral discomfort. |
| Notcutt, 2004 22 | Chronic pain, adults | 24 | Double‐blind, 4‐way cross‐over, RCT. Add‐on therapy to pain medication. | 8 wk | Approx. 9 sprays/d, equivalent of 22.5 mg/d (0.4 mg/kg/d) | Sublingual spray | GW | Symptom control or sleep duration was not improved with CBD; however, sleep quality was. | No | Mid—drowsiness, dry mouth |
| Consroe, 1991 23 | Huntington's disease, adults | 15 | Double‐blind, cross‐over, RCT | 6 wk | 10 mg/kg/d | Oral capsules | US NIDA | CBD was ineffective | No | Similar between CBD and placebo |
| Cunha, 1980 24 | Epilepsy, adults | 15 | Double‐blind, RCT study. Add‐on therapy to AEDs. | Up to 4.5 months | 200–300 mg/d (5 mg/kg/d) | Oral capsules | NA | All but 1 patient improved condition | Yes | Well tolerated |
| * NCT01284634 25 | Fatty liver disease, adults | 25 | Partially‐blinded, phase II, RCT | 8 wk | 200, 400 or 800 mg/d (3.3, 6.7, or 13.3 mg/kg/d) | Oral capsules | GW | No differences in liver triglyceride levels | No | Similar between CBD and placebo |
If not supplied, mg/kg/d was calculated based on average adult weight of 62 kg to enable comparisons.
Significant compared to placebo/control (P < .05) unless stated otherwise.
Registered clinical trial identifier: not published in any peer‐reviewed journal but results available from clinicaltrials.gov.
AEDs, anti‐epileptic drugs; CBD, cannabidiol; GW, GW Pharmaceuticals; HDL, high density lipoprotein; NA, not available; NIDA, National Institute on Drug Abuse; RCT, randomised controlled trial; SAD, social anxiety disorder; STI, STI Pharmaceuticals; THC, THC Pharm.
Table 2.
Summary of included studies: clinical studies
| Study | Clinical population | Total n | Design | Trial length | CBD dose (mg) and approx. mg/kg/da | Route of admin. | CBD source | Resultsb: Primary endpoint(s) | + effect | Side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Rosenberg, 2017 26 | Epilepsy, 1–30 y | 48 | Open label clinical study | 12 wk | 2–5 mg/kg/d titrated up to 50 mg/kg/d or intolerance | Oral solution or by gastric tube | GW | Improvement in quality of life as well as some cognitive functions (memory and control) | Yes | Somnolence, drowsiness, fatigue |
| Devinsky, 2016 27 | Drug‐resistant epilepsy, ages 1–30 y | 137 | Prospective, open‐label trial | 12 wk | 2–5 mg/kg/d, up‐titrated to 25 or 50 mg/kg/d | Oral solution or gastric tube | GW | Monthly motor seizures reduced by a median of 35.5% from baseline | Yes | Somnolence, fatigue, diarrhoea, decreased appetite, weight loss, status epilepticus (6%). |
| Hess, 2016 28 | Drug‐resistant epilepsy in tuberous sclerosis complex, 2–31 y | 18 | Prospective study | 6–12 months | 5 mg/kg/d titrated up to 50 mg/kg/d if tolerated | Oral solution | GW | Decreased seizure frequency | Yes | Drowsiness, ataxia, diarrhoea |
| Yeshurun, 2015 (NCT01385124) 29 | Cell transplant, (GVHD), adults | 48 | Prospective, phase II clinical trial | 37‐day | 300 mg/d (5 mg/kg/d) | Oral solution | STI | No patients developed acute GVHD. Significantly reduced risk ratio compared to historical case controls. | Yes | None reported |
| Crippa, 2011 5 | Generalised SAD, adults | 10 | Double‐blind, placebo‐controlled study | Acute | 400 mg (6.7 mg/kg) | Oral capsule | THC | Reduced subjective anxiety | Yes | None reported |
| Hallak, 2010 30 | Schizophrenia, adults | 28 | Placebo‐controlled study | Acute | 300 or 600 mg (5 or 10 mg/kg) | Oral capsules | Gift | No beneficial effects on selective attention | No | None reported |
| Zuardi, 2009 31 | Psychosis in Parkinson's disease, adults | 6 | Open‐label pilot study | 4 wk | 150 mg/d, increased by 150 mg each week to a total of 400 mg/d (6.7 mg/kg/d) | Oral capsule | THC | Decrease in psychotic symptoms and Parkinson's disease rating compared to baseline | Yes | None reported |
| Consroe, 1986 32 | Dystonic movement disorder, adults | 5 | Preliminary open pilot study | 6 wk | 100–600 mg/d, increased weekly (1.7–10 mg/kg/d) | Oral capsules | NA | Dose‐related improvement in dystonia disability | Yes | Mild—drop in standing blood pressure |
If not supplied, mg/kg/d was calculated based on average adult weight of 62 kg to enable comparisons.
Significant compared to placebo/control (P < .05) unless stated otherwise.
CBD, cannabidiol; GW, GW Pharmaceuticals; GVHD, graft‐vs‐host disease; STI, STI Pharmaceuticals; SAD, social anxiety disorder; THC, THC Pharm.
Table 3.
Summary of included studies: case studies
| Study | Clinical population | Total n | Design | Trial length | CBD dose (mg) and approx. mg/kg/da | Route of admin. | CBD source | Resultsb: Primary endpoint(s) | + effect | Side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaplan, 2017 33 | Refractory seizures in Sturge–Weber syndrome, children | 5 | Case‐series | 14 wk | 5–25 mg/kg/d | Oral solution | GW | Decreases in seizure frequency | Yes | Mild |
| Warren, 2017 34 | Brain tumour related epilepsy, aged 17–40 y | 3 | Case series | 2–10 mo | 10–50 mg/kg/d | Oral | GW | Improvement in seizure frequency (n = 2) and severity (n = 3) | Yes | Diarrhoea |
| Gofshteyn, 2017 35 | Febrile infection‐related epilepsy syndrome, children | 7 | Open‐label case series | Acute and up to 48 weeks | 15–25 mg/kg/d | Oral solution | GW | Improvements in frequency and duration of seizures | Yes | Dizziness, decreased appetite, weight loss |
| Shannon, 2016 10 | Anxiety and insomnia in PTSD, child | 1 | Case report | 5 mo | 25 mg/d (0.6 mg/kg/d) | Oral capsule and spray | CannaVest Corp | Increased sleep quality and duration, and decreased anxiety secondary to PTSD | Yes | None observed |
| Saade, 2015 36 | Seizures, 10‐month old infant | 1 | Case report | 6 mo | 25 mg/kg/d | Oral solution | GW | Substantial reductions in seizures | Yes | None reported |
| Chagas, 2014 37 | RBD in Parkinson's disease, adults | 4 | Case series | 6 wk | 75 mg/d (1.25 mg/kg/d) | NA | NA | Substantial reduction in RBD‐associated events compared to baseline | Yes | None reported |
| Crippa, 2013 38 | Cannabis dependency, adult | 1 | Case report | 10 d | 300 mg/d increased to 600 mg/d (5–10 mg/kg/d) | Oral capsule | THC | Absence of withdrawal symptoms | Yes | None reported |
| Zuardi, 2010 39 | Bipolar disorder, adults | 2 | Case series | 30 d | 600 mg/d increased to 1200 mg/d (20 mg/kg/d) | Oral | STI and THC | CBD was ineffective for manic episode | No | None observed |
| Zuardi, 1995 40 | Schizophrenia, adult | 1 | Case report | 4 wk | 1500 mg/d (25 mg/kg/d) | Oral capsules | NA | Improvements in psychiatric ratings | Yes | Well tolerated; none reported |
| Zuardi, 2006 41 | Treatment‐resistant schizophrenia, adults | 3 | Case series | 30 d | 40 mg/d, increased to 1280 mg/d (21.3 mg/kg/d) | Oral | GW | 1 patient showed mild improvement to baseline and discontinuing treatment worsened symptoms | No | Well tolerated; none observed |
| Snider, 1985 42 | Parkinson's disease, adult | 1 | Case report | 4 wk | 100–400 mg/d (3.3 mg/kg/d) | Oral | NA | Improvement of dyskinesia up to 200 mg/d, worsening of Parkinson disease symptoms with 300–400 mg/d | Mixed | Dizziness, drowsiness, increased Parkinson symptoms |
| Snider, 1984 43 | Meige syndrome, adult | 1 | Case report | Long‐term | Initially 100 mg/d increased to 400 mg/d (6.6 mg/kg/d) | Oral | NA | 50% improvement in spasm frequency and severity | Yes | Dry mouth, headache, sedation |
If not supplied, mg/kg/d was calculated based on average adult weight of 62 kg to enable comparisons.
Significant compared to placebo/control (P < .05) unless stated otherwise.
CBD, cannabidiol; GW, GW Pharmaceuticals; PTSD, post‐traumatic stress disorder; RBD, rapid eye movement sleep behaviour disorder; STI, STI Pharmaceuticals; THC, THC Pharm.
Figure 2.
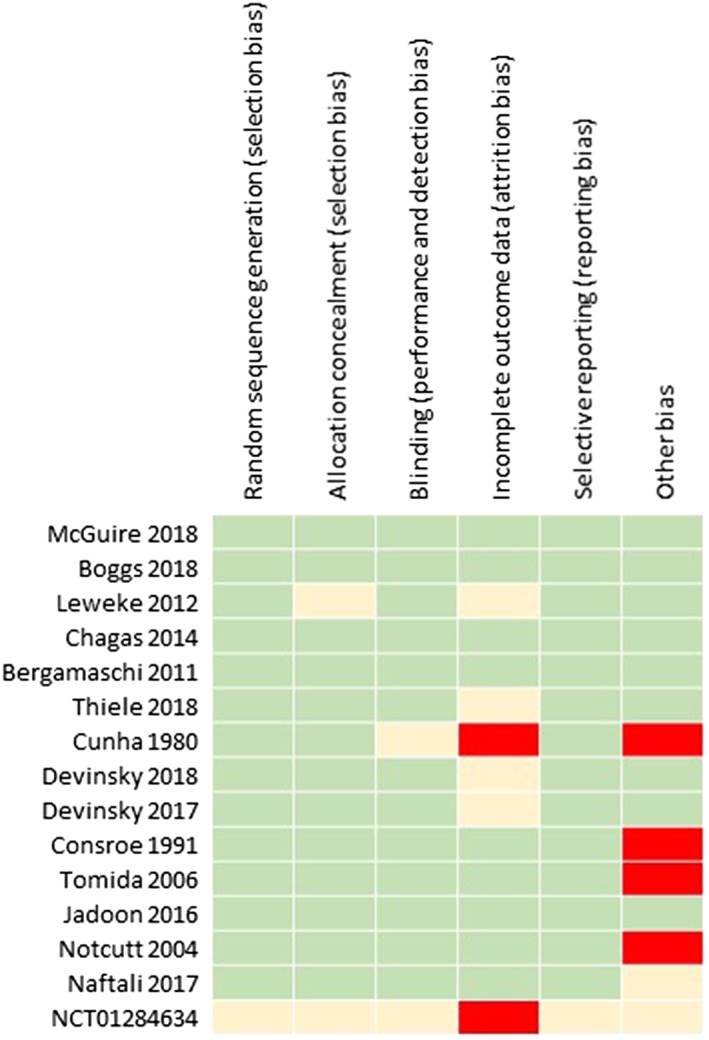
Risk of bias summary of the randomised controlled trials included in the systematic review. Green indicates low‐risk bias, red indicates high‐risk bias, and yellow indicates intermediate or unclear risk
Of the 15 RCTs, the range of doses investigated varied from <1 mg/kg up to 20 mg/kg per day (average 9 mg/kg/d).11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 22, 23, 24, 25 Seven RCTs reported CBD efficacy (average dose 14 mg/kg/d),11, 12, 13, 16, 19, 20, 24 7 studies describe neutral effects of CBD (average dose 5 mg/kg/d)14, 15, 17, 21, 22, 23, 25 and 1 study showed both positive and negative outcomes.18 In the remaining 8 clinical trials of various study design, 7 studies reported CBD positively (average dosing 23 mg/kg/d)5, 26, 27, 28, 29, 31, 32 and 1 study was neutral (8 mg/kg/d).30 Within the 12 case studies and case series, 9 described positive effects of CBD (average dosing 16 mg/kg/d),10, 33, 34, 35, 36, 37, 38, 40, 43 2 were neutral (average dosing 21 mg/kg/d)39, 41 and 1 study described mixed results (3 mg/kg/d).42
Epilepsy was the most frequently studied medical condition, with all 11 studies describing beneficial effects of CBD in reducing the severity or frequency of seizures.12, 13, 16, 24, 26, 27, 28, 33, 34, 35, 36 Within the 4 conducted RCTs (n = 531), an average dosing of 15 mg/kg/d was used where CBD was administered successfully as an add‐on therapy to usual anti‐epileptic drugs.12, 13, 16, 24 Significant improvements were observed compared to placebo as an add‐on therapy. Within the other 3 clinical trials of prospective open‐label design (n = 203), CBD was administered at an average dosing of 42 mg/kg/d and significant improvements in quality of life and seizure frequency compared to baseline were observed.26, 27, 28 3 case series and 1 case report (total n = 16) reported beneficial effects of CBD on seizure frequency, duration and severity with an average administered dose of 21 mg/kg/d.33, 34, 35, 36
Seven studies were conducted in the context of schizophrenia and bipolar disorder. Within the RCTs, 2 conducted with an average dosing of 15 mg/kg/d over 4 or 8 weeks reported positive reductions in psychotic or psychiatric symptoms and a better side effect profile (n = 130).11, 19 One of these compared CBD against an active control (amisulpride), and the other as an add‐on therapy to usual medication compared to placebo as an add‐on therapy. However, a third RCT employing CBD as an add‐on therapy did not report any improvements in cognition or symptoms of schizophrenia after a lower average dose of 10 mg/kg/d over 6 weeks (n = 36).14 An acute dose of 5 or 10 mg/kg/d did not improve selective attention in a placebo‐controlled trial of 28 schizophrenia patients.30 A number of case studies have also been conducted by Zuardi and colleagues in this medical context. In 2 patients with bipolar disease, 20 mg/kg/d was ineffective in treating manic episodes.39 CBD was similarly unable to improve symptoms in 3 schizophrenia patients, although 1 patient described mild improvement.41 Another case report described improvement in psychiatric ratings following an average dose of 25 mg/kg/d over 4 weeks.40
Results are mixed within Parkinson's disease studies. Within an RCT in 21 patients, 1.25 or 5 mg/kg/d CBD had no effect on motor and general symptoms. However, the 5 mg/kg/d dose improved well‐being and quality of life scores.18 The remaining studies are case studies in which CBD decreased psychotic symptoms and Parkinson's disease ratings (n = 6; 7 mg/kg/d),31 improved rapid eye movement sleep behaviour disorder (n = 4; 1 mg/kg/d),37 decreased dyskinesia with 2 to 3 mg/kg/d doses (n = 1), but exaggerated Parkinson's disease symptoms with 5 and 7 mg/kg/d doses.42
CBD did not change therapeutic outcome variables in a double‐blind RCT in Huntington disease patients compared to placebo (n = 15; 10 mg/kg/d for 6 weeks),23 but improved dystonia disability in an open pilot study (n = 5; 10 mg/kg/d for 6 weeks),32 and improved spasm frequency and severity in a case report in 1 patient with Meige syndrome (7 mg/kg/d).43
Within the RCTs, CBD did not significantly change the primary outcomes in diabetes (n = 62), Crohn's disease (n = 19), ocular hypertension (n = 6), chronic pain (mostly neuropathic; n = 24), or fatty liver disease (n = 25).15, 17, 21, 22, 25 However, an average dose of 2.4 mg/kg/d (range 0.3–13.3 mg/kg/d) was used in these studies, which is very low in the clinical and clinical trial setting compared to other studies. Low doses (10 mg/kg) did, however, produce positive responses in generalised social anxiety disorder (SAD) in a double‐blind RCT in 24 patients.20 Likewise, in another double‐blind placebo‐controlled study, a dose of 6.7 mg/kg reduced subjective anxiety in 10 adults with generalised SAD.5 Additionally, in a case report in a child, 0.6 mg/kg/d increased sleep quality and duration, and decreased anxiety secondary to PTSD.10
Lastly, it was found that doses of 5 mg/kg/d prevented occurrence of graft‐vs‐host disease in a phase II clinical trial (n = 48) and 5–10 mg/kg/d doses have been shown in a case report to remove withdrawal symptoms from a patient with cannabis dependency.29, 38
Within studies that compared CBD against a placebo or control (n = 17 publications), only 1 compared CBD against an active control (and a greater clinical improvement and side effect profile was observed with CBD against amisulpride), 8 compared CBD against a placebo (monotherapy), and 8 studies compared CBD as an add‐on therapy (adjunctive to antipsychotic medication, antiepileptic medication, anti‐Parkinson medication or pain medication) against placebo. Analysis of these data revealed that a greater proportion of studies reported a beneficial effect of CBD in the add‐on therapy group compared to the monotherapy group (n = 6 and n = 2 respectively). However, higher doses were used overall within the add‐on therapy group compared to the monotherapy group (average 11 and 6 mg/kg/d, respectively) and, due to such a small data set and heterogeneity of studies, we did not perform any further analysis.
4. DISCUSSION
To our knowledge, this is the first study to compile and compare all publications in which CBD was administered to clinical populations. The aim of this systematic review was to better understand the range of doses of CBD used in clinical studies. In total, 13 medical contexts were included in this review amongst 35 studies including clinical trials and case reports. A positive effect of CBD was reported in 66% of studies, covering disorders including schizophrenia, SAD, epilepsy, cannabis dependency and graft‐vs‐host disease, with doses ranging between <1 and 50 mg/kg/d (i.e. <62–3100 mg/d for an adult). Although we acknowledge that these results mix widely heterogeneous studies, it appears well founded to highlight the differences in average dosing for positive effect studies against those without positive effects, which is confirmed when analysing studies per medical context within each study design format. This suggests that CBD potentially displays a wide therapeutic range, and variable minimum doses are required for effect depending on primary outcomes assessed and the population group. However, it is vital to note that no conclusions can be drawn on the efficacy of CBD as larger phase III and conclusive efficacy trials have not been conducted, with exception of epilepsy. A number of phase III clinical trials are registered on clinicaltrials.gov, which should provide more evidence in the coming years in the contexts of pain, anxiety, Crohn's disease, bipolar disorder, Fragile X syndrome, epilepsy and more.
CBD is increasingly popular, both as a food and health supplement and as a licensed medicine. Within this review, 51% of studies have been published in the last 5 years (since 2013); however, the included articles span over decades, with prominent publications first appearing in the 1980s and early 1990s.24, 40 Despite its long history of sole administration to patients, there is surprisingly little published about the pharmacokinetic properties of CBD, particularly its bioavailability, making it difficult to estimate true effective doses.8 Historically, there is a striking lack of dose‐ranging studies and, looking forward, there are no registered trials on clinicaltrials.gov including specific dose‐ranging investigations in their study design. Ideally, this review would have compared plasma concentrations of CBD in order to more accurately estimate therapeutic concentrations, but, due to the lack of reporting, this was not possible.
Different effective plasma concentrations of CBD may be required for achieving different endpoints across clinical populations, which is a recognised trait in a number of other drugs and diseases. For example, aspirin (acetylsalicylic acid) is used at low doses for antiplatelet therapy, and at higher doses as an analgesic agent.44, 45 With CBD, lower doses may be effective in anxiety relief, while higher doses may be required for effective reduction in epileptic seizures. In studies where there are good rationales for CBD use (e.g. Crohn's disease and chronic pain46, 47), neutral results may be secondary to subtherapeutic dosing, and dose‐escalation trials with embedded pharmacokinetic studies are the next logical step.15, 22 Studies in this review using higher doses concluded that CBD was generally well‐tolerated with the most frequent side effects including drowsiness, nausea, somnolence, fatigue and vomiting.
Among the clinical trial records retrieved from clinicaltrials.gov, only 60% of completed trials had results uploaded and available. This may represent a significant publication bias and is suggestive of disregard for the priority of publication of negative results, which is a well‐recognised problem.48 Unfortunately, this may potentially skew the findings presented in this review and so should be interpreted with caution and is acknowledged as a limitation. We also acknowledge that despite all routes of administration being oral, there may be further bias introduced between studies as one dose cannot be directly compared to another due to lack of standardisation of formulations and pharmacokinetic activity, including differences in bioavailability between an oral spray and an oral capsule.
Future studies should also consider the safety of drug interactions with CBD. CBD is a known inhibitor of the cytochrome P450 (CYP) system49 and can therefore increase plasma concentrations of medicines already in use, in particular antiepileptic drugs. Indeed, this has been reported in a number of publications investigating concomitant use of CBD and antiepileptic drugs.50 Similarly, CYP inhibitors are predicted to increase CBD plasma concentrations which should be equally monitored. Where possible, further well designed trials with CBD may disentangle whether CBD offers unique therapeutic potential in addition to benefits seen when used as an add‐on treatment.
5. CONCLUSION
Although larger confirmatory and efficacy clinical trials examining dosing in more detail for each medical context is required, this review summarises that CBD appears to offer a wide‐range of activity between 1 and 50 mg/kg/d, and there was a tendency of studies with positive outcomes to have used higher doses of CBD. We recommend pharmacokinetic dosing schedules in subsequent trials to consider this range along with safety data and individual patient requirements. Finally, we implore all completed trial results to be made readily available so the research community can progress and learn from equally important positive and negative outcomes for the ultimate benefit of patients.
COMPETING INTERESTS
A.S.Y. and S.E.O. are paid consultants for Artelo Biosciences and the UK Centre for Medicinal Cannabis. All other authors declare no competing interests.
CONTRIBUTORS
S.E.O. and S.A.M.: substantial contributions to the conception or design of the work. S.M.: writing of the manuscript. S.A.M., Z.D.B. and N.L.S.: database searching and data extraction. All authors: analysis and interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
This work was supported by the Biotechnology and Biological Sciences Research Council (S.M. and N.S.) [Grant number BB/M008770/1] and Artelo Biosciences (Z.B.).
Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O'Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85:1888–1900. 10.1111/bcp.14038
REFERENCES
- 1. WHO . Cannabidiol (CBD): World Health Organisation Expert Committee on Drug Dependence Thirty‐ninth Meeting. 2017. Available from: https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf. Accessed July 12, 2019.
- 2. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86‐95. 10.3109/03602532.2013.849268 [DOI] [PubMed] [Google Scholar]
- 3. McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9) ‐tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737‐753. 10.1111/bph.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campos AC, Fogaca MV, Sonego AB, Guimaraes FS. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119‐127. 10.1016/j.phrs.2016.01.033 [DOI] [PubMed] [Google Scholar]
- 5. Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol (Oxford, England). 2011;25(1):121‐130. 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- 6. Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433‐2436. 10.1016/j.addbeh.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 7. Hazekamp A. The trouble with CBD oil. Med Cannabis Cannabinoids. 2018;1(1):65‐72. 10.1159/000489287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Millar SA, Stone NL, Yates AS, O'Sullivan SE. A systematic review on the pharmacokinetics of Cannabidiol in humans. Front Pharmacol. 2018;9:1365 10.3389/fphar.2018.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walpole SC, Prieto‐Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12(1):439 10.1186/1471-2458-12-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shannon S, Opila‐Lehman J. Effectiveness of Cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J. 2016;20(4):108‐111. 10.7812/tpp/16-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225‐231. 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- 12. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018. 10.1016/s0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 13. Devinsky O, Patel AD, Cross JH, et al. Effect of Cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. N Engl J Med. 2018;378(20):1888‐1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 14. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923‐1932. 10.1007/s00213-018-4885-9 [DOI] [PubMed] [Google Scholar]
- 15. Naftali T, Mechulam R, Marii A, et al. Low‐dose Cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Dig Dis Sci. 2017;62(6):1615‐1620. 10.1007/s10620-017-4540-z [DOI] [PubMed] [Google Scholar]
- 16. Devinsky O, Cross JH, Wright S. Trial of Cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;377(7):699‐700. 10.1056/NEJMc1708349 [DOI] [PubMed] [Google Scholar]
- 17. Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of Cannabidiol and Tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, parallel group pilot study. Diabetes Care. 2016;39(10):1777‐1786. 10.2337/dc16-0650 [DOI] [PubMed] [Google Scholar]
- 18. Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double‐blind trial. J Psychopharmacol (Oxford, England). 2014;28(11):1088‐1098. 10.1177/0269881114550355 [DOI] [PubMed] [Google Scholar]
- 19. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment‐naive social phobia patients. Neuropsychopharmacology. 2011;36(6):1219‐1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomida I, Azuara‐Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma. 2006;15(5):349‐353. 10.1097/01.ijg.0000212260.04488.60 [DOI] [PubMed] [Google Scholar]
- 22. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1′ studies. Anaesthesia. 2004;59(5):440‐452. 10.1111/j.1365-2044.2004.03674.x [DOI] [PubMed] [Google Scholar]
- 23. Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40(3):701‐708. [DOI] [PubMed] [Google Scholar]
- 24. Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175‐185. [DOI] [PubMed] [Google Scholar]
- 25. clinicaltrials.gov . Study to Assess the Effect of Cannabidiol on Liver Fat Levels in Subjects With Fatty Liver Disease. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01284634. Accessed July 12, 2019.
- 26. Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D. Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open‐label clinical study with cannabidiol. Epilepsia. 2017;58(8):e96‐e100. 10.1111/epi.13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. The Lancet Neurology. 2016;15(3):270‐278. 10.1016/s1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- 28. Hess EJ, Moody KA, Geffrey AL, et al. Cannabidiol as a new treatment for drug‐resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(10):1617‐1624. 10.1111/epi.13499 [DOI] [PubMed] [Google Scholar]
- 29. Yeshurun M, Shpilberg O, Herscovici C, et al. Cannabidiol for the prevention of graft‐versus‐host‐disease after allogeneic hematopoietic cell transplantation: results of a phase II study. Biol Blood Marrow Transplant. 2015;21(10):1770‐1775. 10.1016/j.bbmt.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 30. Hallak JE, Machado‐de‐Sousa JP, Crippa JA, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Revista Brasileira de Psiquiatria (Sao Paulo, Brazil: 1999). 2010;32(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 31. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol (Oxford, England). 2009;23(8):979‐983. 10.1177/0269881108096519 [DOI] [PubMed] [Google Scholar]
- 32. Consroe P, Sandyk R, Snider SR. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci. 1986;30(4):277‐282. [DOI] [PubMed] [Google Scholar]
- 33. Kaplan EH, Offermann EA, Sievers JW, Comi AM. Cannabidiol treatment for refractory seizures in Sturge‐weber syndrome. Pediatr Neurol. 2017;71:18‐23.e2. 10.1016/j.pediatrneurol.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 34. Warren PP, Bebin EM, Nabors LB, Szaflarski JP. The use of cannabidiol for seizure management in patients with brain tumor‐related epilepsy. Neurocase. 2017;23(5–6):287‐291. 10.1080/13554794.2017.1391294 [DOI] [PubMed] [Google Scholar]
- 35. Gofshteyn JS, Wilfong A, Devinsky O, et al. Cannabidiol as a potential treatment for febrile infection‐related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol. 2017;32(1):35‐40. 10.1177/0883073816669450 [DOI] [PubMed] [Google Scholar]
- 36. Saade D, Joshi C. Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol. 2015;52(5):544‐547. 10.1016/j.pediatrneurol.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 37. Chagas MH, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep‐related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014;39(5):564‐566. 10.1111/jcpt.12179 [DOI] [PubMed] [Google Scholar]
- 38. Crippa JA, Hallak JE, Machado‐de‐Sousa JP, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013;38(2):162‐164. 10.1111/jcpt.12018 [DOI] [PubMed] [Google Scholar]
- 39. Zuardi A, Crippa J, Dursun S, et al. Cannabidiol was ineffective for manic episode of bipolar affective disorder. J Psychopharmacol (Oxford, England). 2010;24(1):135‐137. 10.1177/0269881108096521 [DOI] [PubMed] [Google Scholar]
- 40. Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485‐486. [PubMed] [Google Scholar]
- 41. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment‐resistant schizophrenia. J Psychopharmacol (Oxford, England). 2006;20(5):683‐686. 10.1177/0269881106060967 [DOI] [PubMed] [Google Scholar]
- 42. Snider SR, Consroe P. Beneficial and adverse effects of cannabidiol in a Parkinson patient with sinemet‐induced dystonic dyskinesia. Neurology. 1985;35:201. [Google Scholar]
- 43. Snider SR, Consroe P. Treatment of Meige's syndrome with cannabidiol. Neurology. 1984;34:147. [Google Scholar]
- 44. Paez Espinosa EV, Murad JP, Khasawneh FT. Aspirin: pharmacology and clinical applications. Thrombosis. 2012;2012:173124 10.1155/2012/173124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Derry S, Moore RA. Single dose oral aspirin for acute postoperative pain in adults. Cochrane Database Syst Rev. 2012;(4):Cd002067 10.1002/14651858.CD002067.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Couch DG, Tasker C, Theophilidou E, Lund JN, O'Sullivan SE. Cannabidiol and palmitoylethanolamide are anti‐inflammatory in the acutely inflamed human colon. Clin Sci (London, England: 1979). 2017;131(21):2611‐2626. 10.1042/cs20171288 [DOI] [PubMed] [Google Scholar]
- 47. Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4(1):245‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867‐872. [DOI] [PubMed] [Google Scholar]
- 49. Zendulka O, Dovrtelova G, Noskova K, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17(3):206‐226. [DOI] [PubMed] [Google Scholar]
- 50. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586‐1592. 10.1111/epi.13852 [DOI] [PubMed] [Google Scholar]


