Abstract
Insights into animal behaviour play an increasingly central role in species-focused conservation practice. However, progress towards incorporating behaviour into regional or global conservation strategies has been more limited, not least because standardized datasets of behavioural traits are generally lacking at wider taxonomic or spatial scales. Here we make use of the recent expansion of global datasets for birds to assess the prospects for including behavioural traits in systematic conservation priority-setting and monitoring programmes. Using International Union for Conservation of Nature Red List classifications for more than 9500 bird species, we show that the incidence of threat can vary substantially across different behavioural categories, and that some types of behaviour—including particular foraging, mating and migration strategies—are significantly more threatened than others. The link between behavioural traits and extinction risk is partly driven by correlations with well-established geographical and ecological factors (e.g. range size, body mass, human population pressure), but our models also reveal that behaviour modifies the effect of these factors, helping to explain broad-scale patterns of extinction risk. Overall, these results suggest that a multi-species approach at the scale of communities, continents and ecosystems can be used to identify and monitor threatened behaviours, and to flag up cases of latent extinction risk, where threatened status may currently be underestimated. Our findings also highlight the importance of comprehensive standardized descriptive data for ecological and behavioural traits, and point the way towards deeper integration of behaviour into quantitative conservation assessments.
This article is part of the theme issue ‘Linking behaviour to dynamics of populations and communities: application of novel approaches in behavioural ecology to conservation’.
Keywords: behavioural ecology, birds, indicators, latent risk, macroecology, priority-setting
1. Introduction
Conservation biologists and behavioural ecologists have repeatedly called for closer links between their respective fields on the grounds that behavioural insights can contribute significantly to the success of conservation action [1–5]. However, this cross-disciplinary integration has progressed slowly, in part because the methods and central questions of behavioural ecology do not align closely with the needs of conservation practitioners [4]. For example, much of behavioural ecology focuses at the level of the individual, and identifies selective mechanisms acting on genes or organisms, whereas conservation typically operates at the level of populations [6]. This misalignment is perhaps most pronounced at macroecological scales where global analyses are playing a vital role in conservation science and policy (e.g. [7]) but generally include only the most basic behavioural information.
One reason for the low profile of behaviour in comprehensive broad-scale analyses is because it is difficult and costly to measure standardized behavioural traits across species, space and time [8]. The major contributions of behavioural research to conservation have dealt with factors such as individual movements, sensory ecology, animal personality or cultures, and the extent to which they mediate various kinds of human pressures, including disturbance, habitat loss and hunting [4,9]. The key behavioural metrics under this framework are context-dependent, highly plastic both within and between individuals, and typically estimated through detailed observation and experimentation. They are often inappropriate for quantitative assessments at the wider level of communities or ecosystems because they are (i) only available for a small fraction of species, and (ii) not readily incorporated into species-level analyses. For instance, the case-dependent intricacies of how behaviour influences effective population size (Ne) are useful to conservation [8], but we are decades away from having these data available for comprehensive global studies.
Global or regional conservation assessments are largely restricted to comprehensive species-level datasets accessible at the relevant scale (figure 1). Most macroecological analyses have therefore tested whether species conservation status is predicted by human impacts, biogeographic factors such as latitude or range size, and environmental factors such as climate or habitat [10–15], or reversed the process to predict the conservation status of poorly known species [16,17]. Using freely available geographical information system layers, these socio-economic, biogeographic and environmental variables can be extracted for specimen localities or geographical range polygons, which in some vertebrate groups are reasonably accurate. The other main components of macro-scale assessments have been demographic factors, including population size and density, and rates of population decline, all of which are theoretically related to extinction risk [15,17]. In general, only crude population estimates are included in global-scale analyses because very few attempts have been made to quantify population sizes and trends across entire global ranges [18,19]. Previous studies have shown that both extrinsic biogeographic and demographic factors are correlated with extinction risk, leading to their widespread inclusion in regional and international conservation status assessments.
Figure 1.
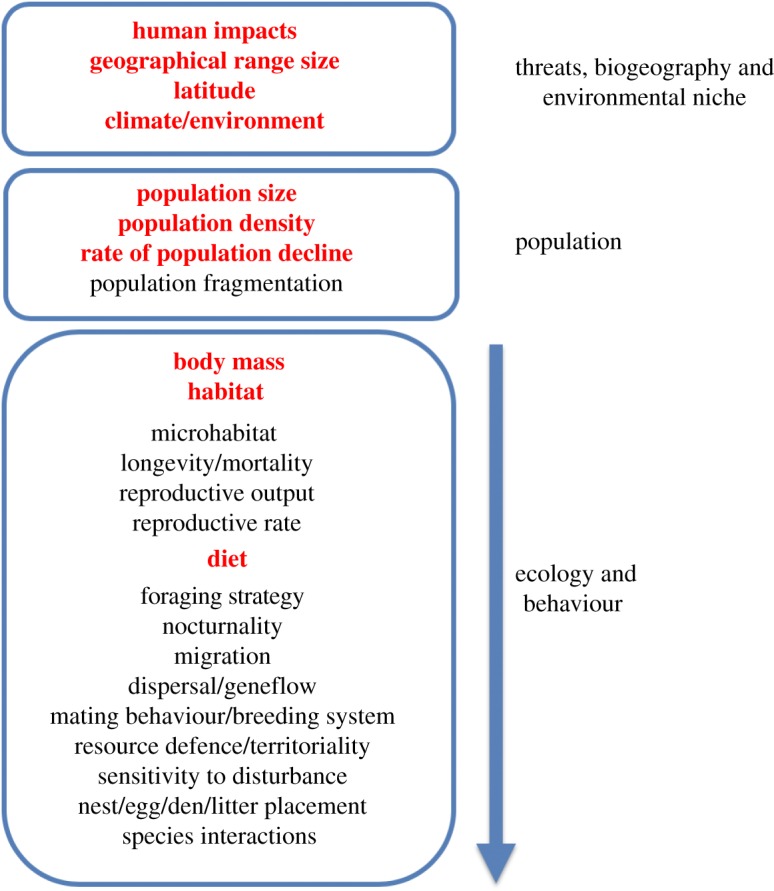
Extrinsic and intrinsic factors associated with extinction risk or conservation status at global scales. Extrinsic factors include anthropogenic threats to species and the biogeographic and environmental context; intrinsic factors include population and ecological niche dimensions. This diagram summarizes the types of traits that are either available or desirable when constructing models of conservation risk at macroecological (continental or global) scales; numerous additional factors may impinge on conservation assessments in particular clades or species. Red/bold text indicates datasets currently available for all species in well studied groups like birds. Availability of data is currently biased towards environmental, biogeographic and population attributes, whereas data tend to be unavailable, uncertain or sparse for most ecological variables, and absent for behavioural variables. (Online version in colour.)
Perhaps the most influential global assessment is the International Union for Conservation of Nature (IUCN) Red List [20], an indicator of biodiversity status and change linked to international convention targets [21]. The conservation status categories systematically generated by the Red Listing process are enshrined in legislation and widely used in macroecological research [22]. Previous assessments of predictors of Red List status have generally focused on standard biogeographic or climatic variables, without delving far into behavioural or ecological factors. Indeed, the only ecological and behavioural traits incorporated into most global models of conservation risk are body mass, diet and habitat preferences [7,14,15]. To convert these variables into species-level traits, body mass is typically averaged from small numbers of published estimates, while diet and habitat are classified into broad categories on the basis of published descriptions in secondary literature [23]. By contrast, many other behavioural or ecological variables have not been comprehensively estimated at global scales and are often difficult to convert into species-level traits (figure 1).
The most relevant behavioural traits to conservation assessment include those that mediate sensitivity to habitat loss, fragmentation, and climate change [4]. Factors relating to dispersal behaviour are particularly pertinent because they impinge on the ability of species to cross unsuitable habitat and thus maintain interconnected metapopulations after habitat fragmentation [24]. Dispersal-related traits may also regulate the ability of species to track shifting geographical ranges in response to climate change [25,26], and predict susceptibility to threats like wind farms [27]. In addition, behavioural dimensions of species interactions may be important determinants of responses to a variety of threats. For example, studies focused at the level of species pairs or communities find evidence that interspecific competition leads to population declines or local extinction following habitat loss and fragmentation [28,29], while reproductive interference may threaten populations of closely related species interacting or hybridizing when climate-driven range shifts lead to secondary contact [4,30]. However, while standardized estimates of dispersal ability and interspecific competition are available for restricted samples of species, they are not readily available at macroecological scales, except in the form of extremely coarse categories (e.g. whether an organism can fly or not; [15]).
Other variables potentially relevant to conservation status can be placed on a continuum from primarily ecological to primarily behavioural (figure 1). At the ecological end are aspects such as microhabitat preferences, while other factors such as foraging mode, migration, sexual selection, territoriality, reproductive strategy and nesting behaviour have an increasingly behavioural dimension. Previous research suggests that species sensitivity to land-use or climate change can be related to microhabitat (e.g. in the form of vertical stratum of vegetation), foraging behaviour (e.g. gregarious foraging), and reproductive strategy (e.g. breeding system) [31,32]. Similarly, territorial strategy is linked to species sensitivity to habitat fragmentation [33], suggesting that elevated interspecific competition via behavioural mechanisms can increase threats associated with land-use and climate change [29,34]. Until recently, such inferences were based on relatively restricted species sampling, but this constraint is changing as the compilation and dissemination of global trait datasets gathers pace.
To assess whether recent progress in data availability can pave the way for behavioural perspectives to be explicitly included in global conservation strategies, we compiled information on a variety of ecological and behavioural traits for all bird species, including estimates of sexual selection [35,36], breeding system [37], foraging strategy [38,39], territorial behaviour [40], and nest placement [41]. We then ran multivariate models to evaluate the extent to which behaviour predicts IUCN Red List status at macroecological scales and in relation to a range of standard biogeographic and environmental variables. Unlike many studies focused on explaining variation in tolerance to human-induced environmental changes [42,43], the aim is not to examine how behaviour influences sensitivity to particular threats, particularly as this would require a different analytical approach. Instead, our goal is to assess the current landscape of behavioural data availability and the prospects for more nuanced conservation assessments and priority-setting.
2. Methods
(a). Data
We assembled data on species threat status from the 2016 Red List [44] along with a range of potential drivers of variation in status, including biogeographic, ecological and behavioural traits, as well as the exposure of each species to human impacts. Geographical range size is consistently identified as the strongest predictor of threat status [14,16]. Although this is not surprising given that two of the main Red List criteria (A and B) are partly based on either Extent of Occurrence (EOO) or Area of Occupancy (AOO), it is nonetheless important to include range size when modelling threat predictors and their correlates. We estimated range size (EOO) for each species based on maps of species breeding distributions [45]. Human population pressure is also known to influence extinction risk [12,46,47]. To quantify the exposure of species to human impacts, we first extracted polygon range maps onto an equal area grid (resolution of 110 km ≈ 1° at the equator) and used this grid to sample human population density, human appropriation of net primary productivity (HANPP) and night-time light intensity, an indicator of urbanization and development. We calculated the mean value of each metric, averaged across all grid cells overlapping with each species range.
We collated data on a selection of ecological traits, including mean species body mass (g), habitat type, diet and island dwelling, all of which have been linked to extinction risk [10,11,13,14,16]. We extracted body mass from Wilman et al. [23]. Using literature to score habitat use, we assigned species to broad habitat categories (coastal, terrestrial, freshwater, sea) according to the predominant habitat used across their geographical distribution. We assigned species to one of 10 dietary categories: aquatic animals, aquatic plants, terrestrial invertebrates, terrestrial vertebrates, terrestrial carrion, nectar, seeds, fruit, other terrestrial plant matter (e.g. leaves), and omnivore, based on the dominant resource present in their diet (see the electronic supplementary material). Data on proportional resource use were first obtained from Wilman et al. [23], and then modified and updated based on comprehensive literature searches. Our dietary classification differs from Wilman et al. [23] in that we subdivided each animal or plant-based resource type into separate aquatic and terrestrial categories (see [39]). This helps us to avoid highly heterogenous categories such as invertivores, which spans a wide variety of species from insectivorous warblers to squid-eating albatrosses and crustacean-eating flamingos [23]. Our approach separates warblers (diet: ‘terrestrial invertebrates’) into a different category from albatrosses and flamingos (diet: ‘aquatic animals’). Using the geographical range polygons described above, we classified species as island dwelling if more than 25% of their geographical range occurred on small islands (landmass <2000 km2). Further details of data compilation methods are given in the electronic supplementary material.
To assess the association between IUCN threat status and key behavioural traits, we assembled data on foraging strategy, nest placement, breeding system, mating behaviour, the mean clutch size of broods, territoriality and migratory behaviour (figure 2). Following the method described by Felice et al. [39], we used literature searches to assign species to one of eight foraging strategies (‘aerial screen’, ‘bark glean’, ‘aerial sally’, ‘arboreal glean’, ‘ground forage’, ‘aquatic plunge’, ‘aquatic surface’ and ‘aquatic dive’). We classified each species according to the predominant behavioural strategy used to acquire resources, and assigned species using multiple foraging strategies as generalists (i.e. nine categories in total, see the electronic supplementary material). Nest placement was scored into a simple three-way system: ground, elevated or cavity (see [41] for details). We used a binary score of breeding system based on a published classification of cooperative and noncooperative breeders [37]. Mating behaviour was scored as strict monogamy, monogamy with infrequent (less than 5% males) polygyny, monogamy with frequent (5–20% males) polygyny, and polygamy (greater than 20% males and females). These categories are based on the index of sexual selection developed by Dale et al. [35]. Clutch size data was based on Jetz et al. [48]. Using data from Tobias et al. [40], we assigned all species to three categories according to the degree of territoriality: ‘strong’ (territories maintained throughout year), ‘weak’ (weak or seasonal territoriality, including species with broadly overlapping home ranges or habitually joining mixed species flocks), and ‘none’ (never territorial or at most defending very small areas around nest sites). Finally, we assigned the migratory behaviour of species as either sedentary, partially migratory (minority of population migrates long distance or most individuals migrate short distances) and migratory (majority of population undertakes long-distance migration) [40].
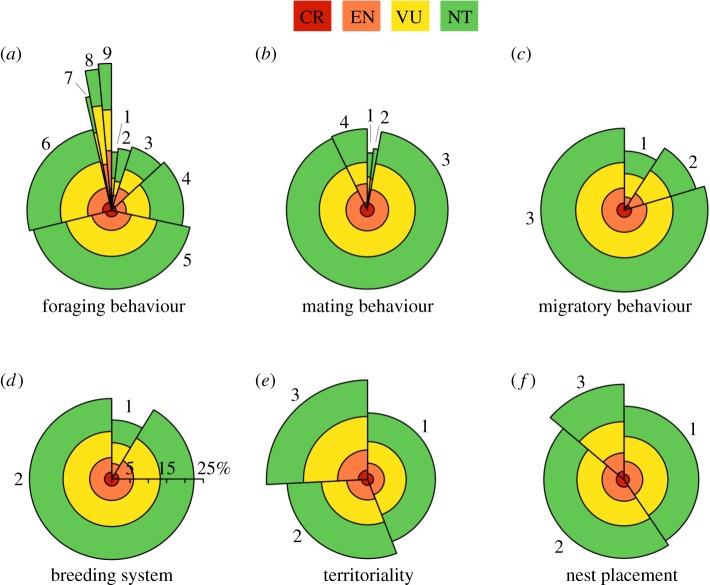
Figure 2.
The percentage of threatened species in different behavioural categories: (a) foraging behaviour (1 foraging generalist, 2 bark gleaning, 3 aerial screening, 4 aerial sallying, 5 arboreal gleaning, 6 ground foraging, 7 aquatic plunge, 8 aquatic dive, 9 aquatic surface); (b) mating behaviour (1 monogamy with infrequent polygyny, 2 monogamy with frequent polygyny, 3 monogamy, 4 polygyny), (c) migratory behaviour (1 migrant, 2 partial or short-distance migrant, 3 sedentary); (d) breeding system (1 cooperative, 2 non-cooperative); (e) territoriality (1 weak, 2 strong, 3 none); (f) nest placement (1 cavity, 2 exposed elevated, 3 exposed ground). The width of each segment indicates the proportion of all species (n = 9576) in each behavioural category. Segment heights indicate the percentage of species threatened in each category. Colours indicate threat level (Critically endangered (CR), Endangered (EN), Vulnerable (VU) and Near Threatened (NT)).
Most variables were available for the vast majority (i.e. greater than 99%) of species but the identity of species with missing values differed across variables. For categorical predictors, we imputed missing values using the modal class for each genus, if the genus contained at least two species and the modal class was present across at least 75% of species. If these conditions were not met, we used the same criteria to impute missing values at the family level. After removing all species with any missing values, our final dataset included n = 9658 species.
(b). Statistical analysis
To model the effects of each predictor variable on extinction risk, we treated threat as a binary variable (0, 1) according to the IUCN Red List categories. All species listed as Vulnerable, Endangered, Critically Endangered, Extinct (including Extinct in the Wild) were classified as Threatened; the remainder (Near Threatened, Least Concern and Data Deficient) were classified as non-Threatened. We modelled threat using a generalized linear mixed effects model in the R package ‘lme4’ [49]. We implemented a binomial error structure and included taxonomic family as a random effect to control for the phylogenetic non-independence of species when identifying predictors of threat. To ensure our results were robust to way random effects were modelled, we repeated our main analysis using a phylogenetic generalized mixed model using the R package ‘phylolm’ [50].
We assessed collinearity between predictor variables by first estimating Pearson correlation coefficients between each pair of continuous variables. We used a threshold of 0.7 as an indicator of potential collinearity. On this basis we excluded HANPP from our analysis because it was strongly correlated with human population density (0.74), which is a standard predictor of extinction risk used in many previous studies. In order to deal with possible associations among categorical predictors we used generalized variance inflation factors (GVIF) accounting for the number of degrees of freedom associated with each predictor. A GVIF value of 5 or 10 is commonly used as a threshold to remove collinear predictors [51]. GVIF values for each predictor were always less than two and so all other predictors were retained in our analysis (electronic supplementary material, table S1). Predictor variables exhibiting right skew were log transformed prior to analysis.
In contrast to previous assessments of the predictors of extinction risk in birds (e.g. [14]), we are particularly interested in how behaviour and its covariation with other putative drivers of extinction risk alter the incidence of threat. First, to assess the overall association between each predictor and threat, we ran a series of single predictor (i.e. univariate) models. Second, we fitted a full multivariate model including all predictor variables. We assessed the contribution of each predictor by removing, and then reinserting, each term from the model and calculating the change in the Akaike information criterion (ΔAIC). Third, to assess the overall effect of behaviour, we ran a model including all ecological predictors along with metrics of human exposure and range size, but excluding all behavioural traits. Finally, to examine how behaviour may mediate the effects of other extinction drivers, we tested for significant interactions between each behavioural trait and each of the core predictors of threat identified in our full model (range size, body size and human population density). We first added and then removed each individual interaction term from our full model to identify those contributing to a significant improvement in model fit (ΔAIC > 2). We then included all of the significant interaction terms in the full model and performed stepwise model simplification, removing those interaction terms resulting in the smallest change in model support. We stopped when the removal of any interaction term resulted in a ΔAIC > 2.
To examine how the definition of threat may influence the predictors of extinction risk, we repeated our analysis considering only threatened species (n = 1251), predicting lower (0 (Vulnerable)) or higher (1 (Endangered, Critically Endangered, Extinct)) levels of threat. Given that range size was included as a predictor in our model, we also repeated our analysis removing the 321 species that were listed as threatened owing to small or declining geographical range sizes (i.e. criteria B). To assess how the predictors of threat may change across broad habitat types, we repeated analyses on different subsets of our data including all species (n = 9658), terrestrial species (n = 8495) and aquatic (n = 767) species. We excluded habitat type as a predictor when fitting models to terrestrial and aquatic species. In addition, we excluded diet and mating behaviour when fitting models to threatened and aquatic species, respectively, because models including these terms failed to converge.
3. Results
(a). Overall predictors of threat in birds
Our results identified a number of core predictors of threat status that align closely with previous assessments indicting that variation in threat across all birds arises as a combination of geography, ecology and human impacts (figure 3). Specifically, the strongest predictor of threat status is geographical range size, with additional strong effects of body mass, island dwelling and the mean human population density across the species geographical range, a metric of exposure to human impact. In both univariate and multivariate models, the incidence of threat decreases with geographical range size (figure 4a) and increases with body size (figure 4b; electronic supplementary material, table S2). When tested in isolation, the incidence of threat is higher on islands. However, in the full multivariate model accounting for other factors including range size, this effect is reversed, with a lower incidence of threat on islands (figure 4d; electronic supplementary material, table S2). We note that this counterintuitive pattern of a lower risk of threat among island dwelling species when accounting for their smaller geographical range size has previously been reported [52]. Similarly, in a univariate model, we found that threat decreases with human population density, but this switches to a positive effect after accounting for variation in geographical range size in the full multivariate model (figure 4c; electronic supplementary material, table S2). In contrast to the positive effect of human population density on threat, threat was only weakly and inconsistently related to night light density (figure 3; electronic supplementary material, table S2). Finally, while there was no consistent relationship between habitat type and threat, we found significant variation in the likelihood of threat across dietary categories, with the highest threat among scavengers, aquatic predators and vertivores compared to invertivores and primary consumers (i.e. frugivores, granivores, nectarivores and herbivores) (figure 4e).
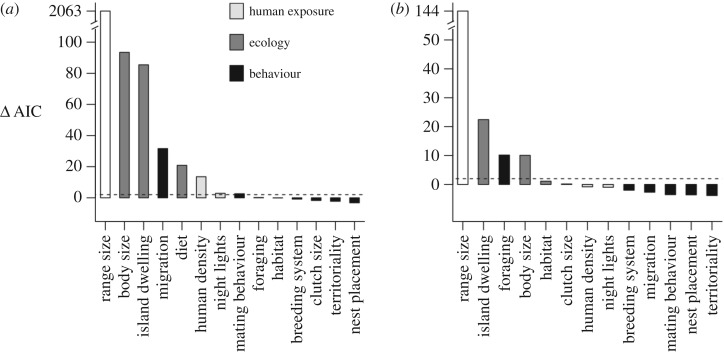
Figure 3.
The relative contribution of anthropogenic, ecological and behavioural predictors to explaining (a) threat across all birds (n = 9658) and (b) level of threat (i.e. Vulnerable versus Endangered, Critically Endangered or Extinct) among threatened species (n = 1251). The contribution of each predictor is quantified as the difference in AIC between the full model and a model excluding each variable. Predictors are shaded according to variable type. The dashed line indicates a difference of two AIC units indicating strong support for predictor inclusion.
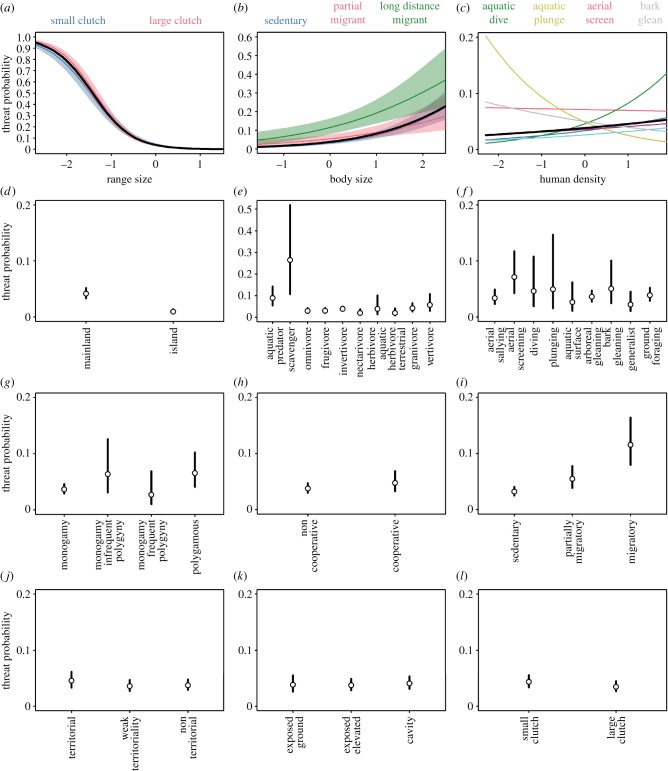
Figure 4.
The influence of behaviour on levels of threat across the world's birds (n = 9658). (a) Effects of range size mediated by clutch size, (b) effects of body size mediated by migratory behaviour, (c) effects of human population density mediated by foraging behaviour, (d) island dwelling, (e) diet, (f) foraging behaviour, (g) mating behaviour, (h) breeding system, (i) migratory behaviour, (j) territoriality, (k) nest placement, and (l) clutch size. Results are from a generalized linear mixed effects model including all predictor variables and family as a random effect. Clutch size is a continuous variable but is here shown as a binary trait (small or large clutch size) to illustrate the interaction with range size (a). Bars indicate the 95% prediction interval. (Online version in colour.)
In addition to these established predictors, we also identified a significant effect of behaviour on extinction risk (figure 3). Although the improvement in explanatory power is modest (marginal R2 [53] excluding versus including behaviour = 0.48 versus 0.51 respectively), a full multivariate model including all predictors is significantly better supported than a model excluding behavioural traits (ΔAIC = 60). All of these key results relating to the core predictors of threat and the role behaviour were robust to the exclusion of species listed as threatened on the basis of small or declining range size and when modelling the non-independence of species on the basis phylogenetic relatedness rather than taxonomy (electronic supplementary material, table S2 and figures S1 and S2).
(b). The effects of behavioural traits on threat
The strongest behavioural predictor of threat in birds was migratory behaviour (figure 3), whereby long-distance migration confers a higher risk of threat (figure 4i). We note that, in a univariate model, long distance migrants are significantly less threatened than partial migrants or sedentary species (figure 3c; electronic supplementary material, table S2). This contrasting finding arises because on average migrants have larger breeding ranges than sedentary species (electronic supplementary material, figure S3a). Thus, while our multivariate model shows that migratory behaviour promotes threat, migrants are nonetheless less likely to be threatened overall because of their large geographical ranges. In addition, we found that the effect of migratory behaviour is also mediated by body size. Specifically, threat increases more rapidly with body size among sedentary compared to partially migratory species (figure 4b). Another key predictor was clutch size, which was inversely related to the incidence of threat. Although not supported as a main effect in the full multivariate model (figure 3), we detected a significant interaction between clutch size and range size, indicating that large clutch size increases threat among species with restricted geographical distributions but reduces threat among large-ranged species (figure 4a).
In contrast to migratory behaviour, some behavioural traits were unrelated to threat, regardless of whether they were considered in isolation or in the full multivariate model. In particular, we found no support for an effect of nest placement (figure 4k) or breeding system (figure 4h) in our models (figure 2). In other cases, threat exhibited significant associations with behaviour, but with effects that varied depending on whether we accounted for other putative drivers of extinction risk (electronic supplementary material, table S2). When tested in isolation, weakly territorial species are less likely to be threatened than non-territorial species but this effect of territoriality is not supported in the full multivariate model accounting for other predictors of threat (figures 2 and 4j). Conversely, when tested in isolation, we found no effect of mating behaviour on threat (figure 3b; electronic supplementary material, table S2), while in the full multivariate model, the likelihood of threat is significantly higher among polygamous than monogamous species (figures 2 and 4g). This suggests that polygamy may enhance the risk of extinction but that its effects are masked because of covariation with other factors that decrease threat. Indeed, polygamous species have smaller body size on average than monogamous species, potentially explaining why the effect of mating behaviour is only evident in a multivariate model including body size (electronic supplementary material, figure S3b).
Models including or excluding foraging behaviour received almost equal support (figure 2), but an effect of foraging behaviour was nevertheless statistically significant (electronic supplementary material, table S2). The incidence of threat is relatively high in species using aquatic plunging and diving behaviours. In addition, while threatened status is currently low among bark gleaning and aerial screening birds, our models show that these foraging strategies may nevertheless promote threat (figure 4f). By contrast, our models show that threat is lower among foraging generalists suggesting that behavioural niche breadth may buffer species from extinction (figure 4f). In addition to these main effects, we found that threat generally increases with human population density but that within some foraging strategies this relationship was weak or even reversed (figure 4c), suggesting that foraging behaviour may mediate the effects of exposure to higher human population density.
(c). Behavioural predictors across different threat levels and environments
Our results suggest that the role of behaviour in predicting threat varies across different thresholds of extinction risk in birds (figure 3). In particular, we found that while migratory behaviour is a core predictor of whether species are threatened or not, it does not predict the level of threat (i.e. whether a species is Vulnerable versus Endangered, Critically endangered or Extinct). As a result, a model excluding all behavioural traits is more strongly supported than a full model incorporating all predictors (electronic supplementary material, table S2). The only behavioural trait that is strongly supported as a predictor of threat level is foraging behaviour (figure 2). Finally, we found that the core predictors of threat and the effects of behaviour varied depending on the environment (electronic supplementary material, figure S1). As with our overall analysis, our models highlighted the primary role of migratory behaviour and weaker effects of foraging and mating behaviour among terrestrial species (electronic supplementary material, figure S4). This is expected given that the majority of all birds are terrestrial. By contrast, foraging strategy was the only behaviour significantly associated with threatened status of aquatic species (electronic supplementary material, figure S5), which was instead primarily driven by range size, human exposure and island dwelling (electronic supplementary material, figure S1).
4. Discussion
We have shown that global-scale ecological and behavioural datasets predict variation in IUCN Red List status of birds. Some behavioural traits were only significant predictors when behaviour was analysed independently (e.g. territoriality), becoming non-significant when other core predictors of threat were included in the model. Conversely, other behavioural traits (e.g. mating behaviour) were not significant predictors when tested in isolation, and their effect was only evident when accounting for correlations with factors such as body size, geographical range size and human impacts. These findings are consistent with previous reports that most ecological and behavioural traits have relatively weak associations with conservation status when incorporated into regional or global models as a species-level trait [7,14,15]. However, although we find little evidence that the recent expansion of behavioural datasets can revolutionize conservation strategies at these wider scales, our results nonetheless show that behavioural traits act as modifiers that can improve explanatory power in conservation assessments, and thus presumably in other predictive exercises (e.g. range shift modelling).
The traits with strongest influence on conservation status were foraging strategy and migration. Although migratory species are less threatened overall than sedentary species, this trend is driven by the larger breeding range size of migratory species and, having accounted for this, we found the migratory behaviour promotes extinction risk. This is expected because migrants are sensitive to human pressures not only in their breeding distribution but also along their migratory routes and in their wintering range [54]. We also show that this effect of migration interacts with body size to determine threat. Specifically, threat increases with body size more rapidly among sedentary compared to partially migratory species, perhaps indicating that poorly dispersing large bodied species are particularly at risk. In the case of foraging, we found that significant relationships between behaviour and conservation status were mainly driven by a subset of strategies. For example, bird species foraging by diving or plunging from air to water are highly threatened and these strategies appear to promote extinction risk. Other foraging strategies that appear to promote threat include aerial screening and bark gleaning but the level of threat is currently lower in these categories. One possibility is that species using these foraging strategies have been less exposed to human pressure but this seems unlikely given that we found little or no effect of human population density on threat in these groups (figure 4c). A more likely explanation, therefore, is that there are other as yet unknown traits associated with these foraging strategies that reduce sensitivity. A number of other species-level behaviours, including variation in breeding system, territoriality, and nest placement, had little predictive power in explaining variation in IUCN Red List status regardless of how they were entered into models. This does not necessarily indicate that such factors are unimportant to conservation, as it is well known that they play a role in some contexts (e.g. nest design and placement has important implications for predation risk in modified landscapes; [55]). However, our models show that these effects are relatively minor and often overwhelmed by other non-behavioural factors at global scales.
Behaviour has proved difficult to integrate into global conservation assessment frameworks, including the IUCN Red List criteria. Our results do not point to any straightforward method of achieving this integration, at least in birds. However, the accuracy of Red List assessments might be improved by using life history and behaviour to scale terms in the criteria which are difficult to assess or define, such as ‘number of mature individuals’, ‘future rate of decline’ and ‘severe fragmentation’ [20]. These factors are typically judged with a considerable degree of inference (see [18,19]). The IUCN Red List Guidelines [56] on how to assess parameters such as these could usefully be augmented with further guidance in relation to ecological and behavioural factors such as mating systems, sex ratios, reproductive rate and predation pressure, dispersal ability, gap-crossing ability and ecological specialism. Moreover, for Red List assessors considering what constitutes ‘severe fragmentation’, future versions of the criteria may be improved with guidelines on how best to account for dispersal ability, gap-crossing ability and ecological specialism.
(a). Challenges
Previous case studies have highlighted the many vital contributions behavioural insights can offer conservation, including more broadly when identifying behavioural factors that predict tolerance to environmental change [42,43]. However, our findings highlight the key challenge of applying behavioural data over larger spatial and taxonomic scales, namely that behavioural traits can have a major influence in particular species or contexts, yet only reduced effect in global analyses. This occurs for two main reasons. First, behavioural traits are often highly flexible, varying within and between individuals and over time, according to factors such as age, season and context. This makes them relatively difficult to estimate by averaging across entire species or populations. Second, behaviour is often not consistently or independently associated with extinction risk in the same way as, for example, low population size, small geographical range and slow reproductive output [13,14].
This point can be illustrated by year-round territoriality, a system of resource defence most widespread in tropical birds [40]. Intense year-round territorial behaviour can increase the risk of extinction in some contexts, such as mountaintop species driven to extinction through costly agonistic interactions with lower elevation replacements moving upslope in response to climatic warming [34,57]. The costs of territoriality are asymmetric, producing both lower-elevation winners and upper-elevation losers. Moreover, the pattern of non-overlapping elevational ranges for highly territorial species holds largely true for some species pairs and localities [58], but not others [59], particularly in lowland systems where species do not tend to occupy rare climatic niches or to share parapatric range boundaries with close ecological competitors. Given that the relationship between territoriality and extinction risk is bidirectional and context-dependent, it makes sense that we find no overall link between territoriality and IUCN Red List status.
An important viewpoint to bear in mind is that the models presented here treat behaviour as an independent species-level trait whereas the influence of behaviour is often dependent on inter-relationships among species. Staying with the example of territoriality, the key factor is not so much whether a particular species aggressively defends territories year-round, but whether it directly competes with a closely related taxon that does the same. Thus, future versions of global models or associated conservation assessments should consider scoring behavioural interactions rather than behaviour per se. Advancing towards this goal is particularly urgent given that species interactions are sensitive to environmental effects. Both climate and land-use change can potentially influence the behaviour of multiple interacting species, as well as their phenology, physiology and relative abundance, and we ideally need to quantify a range of behavioural interactions and responses to understand how environmental changes affect interaction-based ecosystems [60,61]. Again, the key challenge is that the role of behaviour in heterotrophic systems can be complex and highly flexible [62], creating difficulties for multi-species models. Nonetheless, we may improve predictions by incorporating behaviour in more sophisticated ways using interaction-based models, starting at local scales and expanding to larger scale ecological networks when data become available.
A related point is that, although we have largely focused on how particular behaviours may influence extinction risk, such factors may yet prove to be less important than behavioural flexibility itself [63]. Individual organisms with the ability to modify their behaviour through adaptability (i.e. plasticity) may be better able to survive when confronted with novel environmental conditions and selection pressures imposed by anthropogenic change. Defining and developing general indices of behavioural flexibility and innovation remains a challenge [64], but may nevertheless be broadly predictable by morphometric traits that are increasingly available at large scales [65]. For instance, differences in relative brain size across species is positively associated with rates of behavioural innovation in birds, an effect that may explain the apparently greater success of large brained species in colonizing and persisting in more unpredictable environments [66,67], including cities, the most highly altered of human environments (i.e. the ‘cognitive buffer’ hypothesis) [43].
(b). Opportunities
Although they extend the number of behavioural traits compiled across a major global radiation, our analyses are limited by the patchy availability of trait datasets and thus remain highly incomplete (figure 1). A major omission is dispersal behaviour, which we only include as a simple score of migration. Dispersal has long been considered relevant to the conservation of fragmented populations and the optimum design of reserve networks [2]. However, despite the likely importance of dispersal to understanding biodiversity responses to habitat loss and fragmentation, most broad-scale models (e.g. [68,69]) lack estimates of dispersal behaviour simply because they are generally not available as a standardized organismal trait at macroecological scales. This problem may be addressed by the fast-moving field of movement ecology, with global positioning system trackers and loggers deployed over increasing numbers of species [70], and data compilation accelerated by new satellite tracking systems, such as ICARUS (https://icarusinitiative.org). Given that it could take decades for these technological innovations to generate comprehensive dispersal estimates across major taxonomic groups, one potential stopgap solution is to use morphometric indices of dispersal or flight ability. Dispersal indices, such as hand-wing index in birds, can be estimated by measuring museum specimens to provide a fuller picture of spatial ecology and movement behaviour across multiple species in macroecological analyses (e.g. [71]) and comparative studies of anthropogenic threats (e.g. [27]). Such indices, along with further missing data on factors such as reproductive rate and sensitivity to disturbance (figure 1) should be compiled and applied to conservation assessments at global scales.
Another area where behavioural indices may prove useful is ecological forecasting. At present, dispersal is usually ignored in global range shift models, or only included on the basis of crude metrics, such as geographical range size (e.g. [72]). Similarly, species interactions are difficult to quantify and, while most range shift forecasting models acknowledge the limitation, they are generally not included in analyses. Future models should explore the possibility of estimating the strength of species interactions using either pairwise morphometric trait divergence or scores of territorial behaviour, both of which have been shown to limit geographical range overlap in pairs of avian sister species [58,73]. Theoretically, suites of behavioural traits and associated morphometric indices can be incorporated into species distribution modelling in much the same way proposed for detailed physiological traits [74].
The associations we detect between behaviour and conservation status (figure 3) suggest that future research could use similar techniques to identify ‘threatened behaviours’ or suites of behaviours. Using global analyses to look beyond species conservation and instead to identify behaviours that are rare or declining might be a useful step towards targeting conservation action towards maintaining behavioural trait diversity. Similarly, the completion of rich behavioural trait datasets for entire taxonomic groups would pave the way towards multi-dimensional community-based analyses of behavioural diversity (BD) metrics, adopting methods from the functional diversity literature [75,76]. Setting strategic conservation priorities based on rare behaviours or BD may have important implications for ecosystem function, particularly when focusing on behavioural traits linked to key ecological processes, such as trophic interactions (pollination, seed dispersal, etc.). In addition, there are opportunities for including behaviours in models designed to pinpoint likely future shifts in conservation status by estimating latent extinction risk [77]. The way these models work is to predict threat status for any taxon based on a wide range of attributes and then compare predictions with their observed threat status, thus flagging up any species currently ‘flying under the radar’ (i.e. probably more threated, and thus a higher conservation priority, than indicated by their current conservation status).
5. Conclusion
Over recent years, there have been repeated calls for behavioural ecologists to increase their focus on conservation, not least because their study organisms are being driven to extinction by anthropogenic change [3]. Previous authors have suggested that bridging the gulf between these fields might be achieved by applying the experimental or mechanistic approaches predominant in behavioural ecology to conservation research [78], or else returning to more descriptive forms of behavioural ecology potentially relevant to conservation [6]. However, neither of these approaches are exactly suited to the needs of global conservation assessments which call for simple standardized classifications of basic behavioural traits at ambitious scales, including natural history observations and morphometric measurements. Our analyses show how global behavioural classifications are now within reach for some major taxa, highlighting the need for continued sampling of basic descriptive information for massive samples of species and pointing the way forward to a deeper integration of the resultant datasets into conservation assessments at the scale of clades, communities and ecosystems.
Supplementary Material
Acknowledgements
We are grateful to Jakob Bro-Jorgenson for inviting us to contribute to this special issue. We thank Stu Butchart for sharing insights into Red List processes, and numerous collaborators and assistants for help with compiling and organizing data, including Monte Neate-Clegg and Ruth Brandt.
Data accessibility
Most datasets used in the analyses are openly available in published sources cited in the methods. Where we have used primary data these are provided in the electronic supplementary material.
Authors' contributions
J.A.T. and A.L.P. developed the concepts and compiled data; A.L.P. conducted analyses and produced figures; J.A.T. wrote the manuscript with substantial input from A.L.P.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by a Natural Environment Research grant (NE/I028068/1 to J.A.T.) and a Royal Society University Research Fellowship (to A.L.P.).
References
- 1.Clemmons JR, Buchholtz R. 1997. Behavioral approaches to conservation in the wild. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Caro T. 1999. The behavior-conservation interface. Trends Ecol. Evol. 14, 366–369. ( 10.1016/S0169-5347(99)01663-8) [DOI] [PubMed] [Google Scholar]
- 3.Caro T, Sherman PW. 2011. Endangered species and a threatened discipline: behavioural ecology. Trends Ecol. Evol. 26, 111–118. ( 10.1016/j.tree.2010.12.008) [DOI] [PubMed] [Google Scholar]
- 4.Greggor AL, et al. 2016. Research priorities from animal behaviour for maximising conservation progress. Trends Ecol. Evol. 31, 953–964. ( 10.1016/j.tree.2016.09.001) [DOI] [PubMed] [Google Scholar]
- 5.Berger-Tal O, Polak T, Oron A, Lubin Y, Kotler BP, Saltz D. 2011. Integrating animal behavior and conservation biology: a conceptual framework. Behav. Ecol. 22, 236–239. ( 10.1093/beheco/arq224) [DOI] [Google Scholar]
- 6.Caro T. 2007. Behavior and conservation: a bridge too far? Trends Ecol. Evol. 22, 394–400. ( 10.1016/j.tree.2007.06.003) [DOI] [PubMed] [Google Scholar]
- 7.Newbold T, et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. ( 10.1038/nature14324) [DOI] [PubMed] [Google Scholar]
- 8.Anthony LL, Blumstein DT. 2000. Integrating behaviour into wildlife conservation: the multiple ways that behaviour can reduce Ne. Biol. Conserv. 95, 303–315. ( 10.1016/S0006-3207(00)00037-9) [DOI] [Google Scholar]
- 9.Brakes P, et al. 2019. Animal cultures matter for conservation. Science 363, 1032–1034. ( 10.1126/science.aaw3557) [DOI] [PubMed] [Google Scholar]
- 10.Bennett PM, Owens IPF. 1997. Variation in extinction risk among birds: chance or evolutionary predisposition? Proc. R. Soc. Lond. B 264, 401–408. ( 10.1098/rspb.1997.0057) [DOI] [Google Scholar]
- 11.Owens IPF, Bennett PM. 2000. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl Acad. Sci. USA 97, 12 144–12 148. ( 10.1073/pnas.200223397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, 909–914. ( 10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds OR, Sechrest W, Orme CD, Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241. ( 10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 14.Lee T, Jetz W. 2011. Unravelling the structure of species extinction risk for predictive conservation science. Proc. R. Soc. B 278, 1329–1338. ( 10.1098/rspb.2010.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keinath DA, Doak DF, Hodges KE, Prugh LR, Fagan W, Sekercioglu CH, Buchart SH, Kauffman M. 2017. A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob. Ecol. Biogeogr. 26, 115–127. ( 10.1111/geb.12509) [DOI] [Google Scholar]
- 16.Jetz W, Freckleton RP. 2015. Towards a general framework for predicting threat status of data-deficient species from phylogenetic, spatial and environmental information. Phil. Trans. R. Soc. B 370, 20140016 ( 10.1098/rstb.2014.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santini L, Butchart SHM, Rondinini C, Benítez-López A, Hilbers JP, Schipper AM, Cengic M, Tobias JA, Huijbregts MAJ. In press Applying habitat and population density models to land cover time series to inform IUCN Red List assessments. Conserv. Biol. ( 10.1111/cobi.13279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobias JA, Seddon N. 2002. Estimating population size in the subdesert mesite (Monias benschi): new methods and implications for conservation. Biol. Conserv. 108, 199–212. ( 10.1016/S0006-3207(02)00106-4) [DOI] [Google Scholar]
- 19.Tobias JA, Brightsmith D. 2007. Distribution, ecology and conservation status of the blue-headed macaw Primolius couloni. Biol. Conserv. 139, 126–138. ( 10.1016/j.biocon.2007.06.009) [DOI] [Google Scholar]
- 20.IUCN. 2001. IUCN Red List Categories and Criteria: Version 3.1. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK See http://app.iucn.org/webfiles/doc/SSC/RedList/redlistcatsenglish.pdf.
- 21.Butchart SHM, Stattersfield AJ, Bennun LA, Akcakaya HR, Baillie JEM, Stuart SN, Hilton-Taylor C, Mace GM. 2005. Using Red List indices to measure progress towards the 2010 target and beyond. Phil. Trans. R. Soc. B 1454, 255–268. ( 10.1098/rstb.2004.1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues ASL, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM. 2006. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 21, 72–76. ( 10.1016/j.tree.2005.10.010) [DOI] [PubMed] [Google Scholar]
- 23.Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W. 2014. EltonTraits 1.0: species-level foraging attributes of the world's birds and mammals. Ecology 95, 2027 ( 10.1890/13-1917.1) [DOI] [Google Scholar]
- 24.Lees AC, Peres CA. 2009. Gap-crossing movements predict species occupancy in Amazonian forest fragments. Oikos 118, 280–290. ( 10.1111/j.1600-0706.2008.16842.x) [DOI] [Google Scholar]
- 25.Early R, Sax DF. 2011. Analysis of climate paths reveals potential limitations on species range shifts. Ecol. Lett. 14, 1125–1133. ( 10.1111/j.1461-0248.2011.01681.x) [DOI] [PubMed] [Google Scholar]
- 26.Howard C, Stephens PA, Tobias JA, Sheard C, Butchart SHM, Willis SG. 2018. Flight range, fuel load, and the impact of climate change on the journeys of migrant birds. Proc. R. Soc. B 285, 20172329 ( 10.1098/rspb.2017.2329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaxter CB, et al. 2017. Bird and bat species’ global vulnerability to collision mortality at wind farms revealed through a trait-based assessment. Proc. R. Soc. B 284, 20170829 ( 10.1098/rspb.2017.0829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bregman TP, Lees AC, Seddon N, MacGregor HEA, Darski B, Aleixo A, Bonsall MB, Tobias JA. 2015. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 96, 2692–2704. ( 10.1890/14-1731.1) [DOI] [PubMed] [Google Scholar]
- 29.Grether GF, Peiman KS, Tobias JA, Robinson BW. 2017. Causes and consequences of behavioral interference between species. Trends Ecol. Evol. 32, 760–772. ( 10.1016/j.tree.2017.07.004) [DOI] [PubMed] [Google Scholar]
- 30.Hochkirch A, Groning J, Bucker A. 2007. Sympatry with the devil: reproductive interference could hamper species coexistence. J. Anim. Ecol. 76, 633–642. ( 10.1111/j.1365-2656.2007.01241.x) [DOI] [PubMed] [Google Scholar]
- 31.Kokko H, Brooks R. 2003. Sexy to die for? Sexual selection and the risk of extinction. Ann. Zool. Fennici 40, 207–219. [Google Scholar]
- 32.Bueno AS, Dantas SM, Henriques LMP, Peres CA. 2018. Ecological traits modulate bird species responses to forest fragmentation in an Amazonian anthropogenic archipelago. Divers. Distrib. 24, 387–402. ( 10.1111/ddi.12689) [DOI] [Google Scholar]
- 33.Ulrich W, Banks-Leite C, De Coster G, Habel JC, Matheve H, Newmark WD, Tobias JA, Lens L. 2017. Environmentally and behaviourally mediated co-occurrence of functional traits in bird communities of tropical forest fragments. Oikos 127, 274–284. ( 10.1111/oik.04561) [DOI] [Google Scholar]
- 34.Jankowski JE, Robinson SK, Levey DJ. 2010. Squeezed at the top: interspecific aggression may constrain elevational ranges in tropical birds. Ecology 91, 1877–1884. ( 10.1890/09-2063.1) [DOI] [PubMed] [Google Scholar]
- 35.Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 36.Cooney CR, Tobias JA, Weir JT, Botero CA, Seddon N. 2017. Sexual selection, speciation, and constraints on geographical range overlap in birds. Ecol. Lett. 20, 863–871. ( 10.1111/ele.12780) [DOI] [PubMed] [Google Scholar]
- 37.Jetz W, Rubenstein DR. 2011. Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 1–7. ( 10.1016/j.cub.2011.02.025) [DOI] [PubMed] [Google Scholar]
- 38.Pigot A, Trisos CH, Tobias JA. 2016. Functional traits reveal the expansion and packing of ecological niche space underlying an elevational diversity gradient in passerine birds. Proc. R. Soc. B 283, 20152013 ( 10.1098/rspb.2015.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felice RN, Tobias JA, Pigot AL, Goswami A. 2019. Dietary niche and the evolution of cranial morphology in birds. Proc. R. Soc. B 286, 20182677 ( 10.1098/rspb.2018.2677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias JA, Sheard C, Seddon N, Meade A, Cotton AJ, Nakagawa S. 2016. Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4, 74 ( 10.3389/fevo.2016.00074) [DOI] [Google Scholar]
- 41.Stoddard MC, Yong EH, Akkaynak D, Sheard C, Tobias JA, Mahadevan L. 2017. Avian egg shape: form, function and evolution. Science 356, 1249–1254. ( 10.1126/science.aaj1945) [DOI] [PubMed] [Google Scholar]
- 42.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. ( 10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 43.Sol D, Lapiedra O, Gonzalez-Lagos C. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. ( 10.1016/j.anbehav.2013.01.023) [DOI] [Google Scholar]
- 44.IUCN. 2016. The IUCN Red List of Threatened Species. Version 2016-2 See http://www.iucnredlist.org Downloaded on 15 July 2016.
- 45.Birdlife International. 2012. Bird species distribution maps of the world. Version 2.0. Cambridge, UK, Arlington, TX: BirdLife International/NatureServe. [Google Scholar]
- 46.Scharlemann JPW, Balmford A, Green RE. 2005. The level of threat to restricted-range bird species can be predicted from mapped data on land use and human population. Biol. Conserv. 123, 317–326. ( 10.1016/j.biocon.2004.11.019) [DOI] [Google Scholar]
- 47.Davies RG, et al. 2006. Human impacts and the global distribution of extinction risk. Proc. R. Soc. B 273, 2127–2133. ( 10.1098/rspb.2006.3551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jetz W, Sekercioglu CH, Bohning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, 2650–2657. ( 10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 50.Ho LST, Ane C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 51.Dormann CF, et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. ( 10.1111/j.1600-0587.2012.07348.x) [DOI] [Google Scholar]
- 52.Manne L, Brooks TM, Pimm SL. 1999. Relative risk of extinction of passerine birds on continents and islands. Nature 399, 258–261. ( 10.1038/20436) [DOI] [Google Scholar]
- 53.Nakagawa S, Schielzeth HA. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 54.Hardesty-Moore M, et al. 2018. Migration in the Anthropocene: how collective navigation, environmental system and taxonomy shape the vulnerability of migratory species. Phil. Trans. R. Soc. B 373, 1746 ( 10.1098/rstb.2017.0017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilcove DS. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66, 1211–1214. ( 10.2307/1939174) [DOI] [Google Scholar]
- 56.IUCN Red List Standards and Petitions Committee. 2018. Red List Guidelines See https://www.iucnredlist.org/resources/redlistguidelines.
- 57.Freeman BG, Scholer MN, Ruiz-Gutierrez V, Fitzpatrick JW. 2018. Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl Acad. Sci. USA 115, 11 982–11 987. ( 10.1073/pnas.1804224115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freeman BG, Tobias JA, Schluter D. 2019. Behavior influences range limits and patterns of coexistence across an elevational gradient in tropical bird diversity. bioRxiv 2019, 528950 ( 10.1101/528950) [DOI] [Google Scholar]
- 59.Boyce AJ, Martin TE, 2019. Interspecific aggression among parapatric and sympatric songbirds on a tropical elevational gradient. Behav. Ecol. 30, 541–547. ( 10.1093/beheco/ary194) [DOI] [Google Scholar]
- 60.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 61.Meise K, Franks DW, Bro-Jørgensen J. 2019. Using social network analysis of mixed-species groups in African savannah herbivores to assess how community structure responds to environmental change. Phil. Trans. R. Soc. B 374, 20190009 ( 10.1098/rstb.2019.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ness JH, Bressmer K. 2005. Abiotic influences on the behaviour of rodents, ants, and plants affect an ant-seed mutualism. Ecoscience 12, 76–81. ( 10.2980/i1195-6860-12-1-76.1) [DOI] [Google Scholar]
- 63.Sol D, Sayol F, Ducatez S, Lefebvre L. 2016. The life-history basis of behavioural innovations. Phil. Trans. R. Soc. B 371, 20150187 ( 10.1098/rstb.2015.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Audet JN, Lefebvre L. 2017. What's flexible in behavioral flexibility? Behav. Ecol. 28, 943–947. ( 10.1093/beheco/arx007) [DOI] [Google Scholar]
- 65.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D. 2016. Environmental variation and the evolution of large brains in birds. Nat. Commun. 7, 13971 ( 10.1038/ncomms13971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sol D, Bacher S, Reader SM, Lefebvre L. 2008. Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 172, S63–S71. ( 10.1086/588304) [DOI] [PubMed] [Google Scholar]
- 68.Newbold T, Scharlemann JPW, Butchart SHM, Sekercioglu CH, Alkemade R, Booth H, Purves DW. 2013. Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc. R. Soc. B 280, 20122131 ( 10.1098/rspb.2012.2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bregman TP, Sekercioglu CH, Tobias JA. 2014. Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation. Biol. Conserv. 169, 372–383. ( 10.1016/j.biocon.2013.11.024) [DOI] [Google Scholar]
- 70.Kays R, Crofoot MC, Jetz W, Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478 ( 10.1126/science.aaa2478) [DOI] [PubMed] [Google Scholar]
- 71.Pigot A, Tobias JA. 2015. Dispersal and the transition to sympatry in vertebrates. Proc. R. Soc. B 282, 20141929 ( 10.1098/rspb.2014.1929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hof C, Voskamp A, Biber MF, Böhning-Gaese C, Engelhardt EK, Niamira A, Willis SG, Hickler T. 2018. Bioenergy cropland expansion may offset positive effects of climate change mitigation for global vertebrate diversity. Proc. Natl Acad. Sci. USA 115, 13 294–13 299. ( 10.1073/pnas.1807745115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pigot A, Tobias JA. 2013. Species interactions constrain geographic range expansion over evolutionary time. Ecol. Lett. 63, 330–338. ( 10.1111/ele.12043) [DOI] [PubMed] [Google Scholar]
- 74.Chown SL. 2012. Trait-based approaches to conservation physiology: forecasting environmental change risks from the bottom up. Phil. Trans. R. Soc. B 367, 1615–1627. ( 10.1098/rstb.2011.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petchey OL, Gaston KJ. 2002. Functional diversity (FD), species richness and community composition. Ecol. Lett. 5, 402–411. ( 10.1046/j.1461-0248.2002.00339.x) [DOI] [Google Scholar]
- 76.Villéger S, Mason NWH, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. ( 10.1890/07-1206.1) [DOI] [PubMed] [Google Scholar]
- 77.Cardillo M, Mace GM, Gittleman JL, Purvis A. 2006. Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161. ( 10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linklater WL. 2004. Wanted for conservation research: behavioral ecologists with a broader perspective. Bioscience 54, 352–360. ( 10.1641/0006-3568(2004)054[0352:WFCRBE]2.0.CO;2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most datasets used in the analyses are openly available in published sources cited in the methods. Where we have used primary data these are provided in the electronic supplementary material.