Abstract
Background
With the introduction of glucagon-like peptide-2 (GLP-2) in the treatment of short bowel syndrome (SBS), there is emerging evidence that GLP-2 may play a role in the restoration of the disturbed homeostatic feedback in the gut-liver axis and may ameliorate SBS-associated liver damage.
We have previously presented that daily subcutaneous injections with 1 and 10 mg of glepaglutide improved intestinal function in patients with SBS. As exploratory endpoints, we here assessed the effect of glepaglutide on liver function.
Methods
Liver tests, transient elastography (TE) with controlled attenuation parameter (CAP), indocyanine green (ICG) kinetics, soluble CD163 (sCD163), soluble mannose receptor (sMR), and lipopolysaccharide binding protein (LBP) were assessed in 18 patients with SBS in a randomised, cross-over, dose-finding phase 2 trial before and after three weeks of treatment with glepaglutide. This trial is completed and registered at ClinicalTrials.gov: NCT02690025.
Findings
Between Feb 2016 and Jan 2017, 22 patients with SBS were screened. Of these, 18 patients were randomised and treated with glepaglutide; 16 patients completed the trial. Treatment with glepaglutide was associated with increase in TE and ICG-elimination. In the 10 mg dose group, glepaglutide increased sCD163 by 0·44 mg/mL (P = 0·0498), and alkaline phosphatase (ALP) decreased in the 1 mg dose group by 33 U/L (P = 0·032). CAP, sMR, LBP, liver transaminases, and INR were not affected.
Interpretation
Glepaglutide may improve hepatic excretory function, but at the same time activate resident liver macrophages and increase liver stiffness. The excretory and the stiffness findings may to some extent relate to increased splanchnic blood flow which would not influence the marker of macrophage activation. Thus, glepaglutide exerted diverse effects on liver status that call for attention in future studies.
Funding
Zealand Pharma.
Keywords: Short bowel syndrome, Transient elastography, Indocyanine green, Soluble CD163, Soluble mannose receptor
Abbreviations: ALAT, Alanine Transaminase; ALP, Alkaline Phosphatase; ANCOVA, Analysis of Covariance; ASAT, Aspartate Transaminase; CAP, Controlled Attenuation Parameter; CI, Confidence Interval; C4, 7α-Hydroxy-4-Cholesten-3-One; ELISA, Enzyme-Linked Immunosorbent Assay; FGF, Fibroblast Growth Factor; FXR, Farnesoid X Receptor; GLP, Glucagon-Like Peptide; HBsAg, Hepatitis B Surface Antigen; ICG, Indocyanine Green; IF, Intestinal Failure; IFALD, Intestinal Failure Associated Liver Disease; II, Intestinal Insufficiency; LBP, Lipopolysaccharide Binding Protein; LLN, Lower Limits of Normal; PS, Parenteral Support; PDR, Plasma Disappearance Rate; R15, Retention Rate after 15 min; SBS, Short Bowel Syndrome; sCD163, Soluble CD163; sMR, Soluble Mannose Receptor; TE, Transient Elastography; ULN, Upper Limits of Normal
Research in context.
Evidence before this study
In patients with SBS, extensive intestinal resections, the provision of PS and its composition as well as an altered homeostatic feedback in the so-called gut-liver axis may induce liver damage which includes a spectrum of chronic hepatic diseases, with IFALD being the most aggressive phenotype, which can lead to liver failure.
To identify clinical trials with the aim to investigate the effect of exogenous GLP-2 administration on the compromised hepatic function in patients with SBS, we searched PubMed and MEDLINE for articles published between Jan 1, 1990 and March 31, 2019 with the search terms “short bowel syndrome”, “glucagon-like peptide-2”, “glucagon-like peptide-2 analogues”, “hepatic function”, “transient elastography”, “indocyanine green elimination”, “soluble CD163”, “soluble mannose receptor”, “lipopolysaccharide binding protein”, “conventional liver tests”, and “adults”. The search retrieved no clinical trials investigating the impact of a GLP-2 analogue treatment on markers of liver status in patients with SBS. Therefore, the current study represents a first-in-class trial in this patient population.
Glepaglutide is a novel long-acting GLP-2 analogue with an effective plasma half-life of approx. 50 h giving this analogue the potential for less than once daily dosing. In a recently published article, we reported findings from a randomised, double-blind, dose-finding, single-centre, proof-of-concept, phase 2 trial, where glepaglutide in the active doses of 1 mg and 10 mg, given subcutaneously once daily, significantly reduced faecal output in SBS patients with intestinal insufficiency or failure [19]. In addition, glepaglutide was associated with increased intestinal absorption, improved hydration level and renal function, and was observed to be intestinotrophic and prolong gastrointestinal transit time.
Added value of this study
Our findings in the present article are based on secondary and exploratory endpoints from the phase 2 trial and provide the first clinical evidence for potential therapeutic benefit of glepaglutide on the compromised liver function in patients with SBS. Moreover, our findings provide a deeper insight into the complex pathophysiology of SBS. We have demonstrated that three weeks of treatment with glepaglutide, primarily at the highest dose level of 10 mg, might improve the compromised liver excretory function, but at the same time increase liver stiffness and activate resident hepatic macrophages in patients with SBS. Increased splanchnic blood flow, which is a known effect of exogenous GLP-2 administration, may to some extend explain the findings on the excretory liver function and liver stiffness, but not the activation of resident hepatic macrophages.
Implications of all the available evidence
Our results in the present article suggest that glepaglutide may play a role in the restoration of the disturbed homeostatic feedback in the gut-liver axis and thereby SBS associated liver damage. Thus, glepaglutide may have therapeutic potential in the treatment of IFALD for which no currently approved medical treatment options exists. Ongoing large multicentre, multinational, controlled pivotal phase 3 trial (EudraCT No: 2017–004394-14) is currently recruiting to form the basis for regulatory approval of glepaglutide for treatment of patients with SBS in the U.S. and EU.
Alt-text: Unlabelled Box
1. Introduction
It is believed that long-term parenteral support (PS) and the associated provision of parenteral lipids are major causes of intestinal failure (IF) associated liver disease (IFALD) [1,2]. However, recent studies indicate that the progressive liver injury may also be inflicted independent of PS due to the altered homeostatic feedback in the so-called gut-liver axis as a consequence of the disrupted enterohepatic circulation following intestinal resection, leading to biliary hypersecretion, bile acid dysmetabolism, and microbial dysbiosis [3,4].
Mechanisms underlying liver damage and the development of IFALD are poorly understood, and current diagnostic tools and non-invasive surveillance approaches, as well as therapeutic options, are sparse and suboptimal. Although liver biopsy remains the gold standard for assessing liver inflammation and for staging liver fibrosis, its invasive character and potential post-procedure complications limit its use as a preferred surveillance tool. Unfortunately, clinical features and current biochemical markers and score systems also tend to fail to properly detect the degree and progression of liver damage [5], making it difficult to initiate preventive measures and early therapeutic approaches.
In line with these unmet needs, non-invasive, easy-to-perform, reproducible, and ultimately reliable diagnostic tools are needed. Recently, transient elastography (TE) has been shown to correlate with histological cholestasis and fibrosis demonstrated in one adult and one paediatric IF population, respectively [6,7]. TE is an ultrasonographic imaging modality that measures shear wave velocity which reflects liver stiffness. However, increase in the portal blood flow as seen in relation to meal ingestion has been shown to increase the liver stiffness [8,9]. At the same time, this technique can detect and quantify steatosis by measuring the ultrasound attenuation.
Indocyanine green (ICG) elimination [10] is another technique known for decades as a measure of liver function. ICG is a water-soluble, tricarbocyanine dye which binds completely to albumin and beta-lipoprotein after intravenous injection. ICG is taken up from the plasma almost exclusively by the liver parenchymal cells and is excreted unchanged by the canicular membrane into the bile [10]. Thus, decreased levels of elimination rate reflect impaired parenchymal liver function, and bile excretion. The ICG elimination kinetics implies that the substance clearance is influenced by splanchnic perfusion [10,11].
Lipid content in PS and recurrent sepsis may lead to activation of resident liver macrophages (Kupffer cells) [1]. Since the activation of the Kupffer cells plays an important role in the progressive fibrotic process, circulating macrophage-specific markers are proposed as non-invasive biomarkers for the diagnosis and monitoring of liver fibrosis [1,12]. In this regard, soluble CD163 (sCD163) and soluble mannose receptor (sMR) have received special attention. Both sCD163 and sMR are endocytic macrophage surface receptors which are shed by activated resident liver macrophages in response to inflammation and can be measured in serum [12]. sCD163 in serum is predominantly present as an ectodomain cleaved form that is generated by metalloprotease action (tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) upon inflammatory macrophage activation. The protein may originate from any population of activated macrophages; however, the biomarker is especially sensitive to liver inflammation, due to the Kupffer Cells comprising the majority of the total macrophage pool of the body [12,34].
At present, there is no evidence-based treatment option for IFALD. However, the introduction of glucagon-like peptide-2 (GLP-2) in the treatment of short bowel syndrome (SBS) seems to impart a new possibility for the restoration of the impaired liver function in this patient population [3,13]. For instance, in preclinical IF settings, GLP-2 has been shown to increase splanchnic blood flow [14,15] and improve cholestasis and liver injury [3]. Native GLP-2 was also associated with increased intestinal blood flow in a single clinical SBS trial [16]. On the other hand, cholecystitis, new-onset cholelithiasis, and one episode of cholestasis have been reported in patients with SBS in relation to treatment with the marketed GLP-2 analogue teduglutide [17,18].
We have previously presented that three weeks of treatment with daily subcutaneous injections of 1 mg and 10 mg glepaglutide resulted in significant reductions in faecal output and improvement in intestinal wet weight and macronutrient absorption in patients with SBS [19]. As exploratory outcomes in this phase 2 trial, we aimed to assess changes in marker of liver status following treatment with glepaglutide. Based on our previous experience, we expected that treatment with glepaglutide would decrease markers of bacterial translocation leading to decreased levels of sCD163 and sMR and improve excretory liver function as measured by ICG elimination. Moreover, we hypothesized that glepaglutide would decrease liver stiffness and reduce biochemical markers of liver injury and cholestasis.
2. Methods
2.1. Study design and participants
The trial protocol was approved by Danish Medicines Agency and the Regional Committee on Health Research Ethics and trial procedures were conducted in accordance with the ethical standards of the Helsinki Declaration and the principles of Good Clinical Practice. Written informed consent was signed by the patients before entrance to the trial and before any trial-related activity was carried out. Patients could withdraw consent at any time during the trial.
Patients with SBS associated intestinal insufficiency (II) and IF were included from the Department of Gastroenterology and Hepatology at Rigshospitalet, Copenhagen, Denmark, after approval from the Danish Medicines Agency and the Regional Committee on Health Research Ethics (Project-ID: H − 15,015,411) and based on a malabsorption with faecal wet weight output of greater than or equal to 1500 g/day. Carriers of Hepatitis B surface antigen (HBsAg) or Hepatitis C antibodies and patients with a history of significant alcohol or drug abuse within one year prior to screening were not included. Patients were also excluded, if they tested positive for HIV antibodies or had a history of cancer, unless it could be documented that the patient had been in a disease-free state for at least five years (except colon cancer: patients with a history of colon cancer were generally excluded). Details on other main inclusion/exclusion criteria have been published previously [19].
Patients were treated with glepaglutide in a randomised, double-blind, dose-finding, single-centre, proof-of-concept, phase 2 trial (ClinicalTrials.gov: NCT02690025). In a two-period cross-over design, patients received two of three different doses (0·1 mg, 1 mg, and 10 mg) of glepaglutide as daily subcutaneous injections for a total of three weeks. The two treatment periods were separated by a wash-out period of four to eight weeks. The primary endpoint of the trial was defined as the absolute change from baseline in faecal wet weight output assessed by metabolic balance studies performed before and after each treatment period [19]. As an exploratory endpoint, the trial also aimed to evaluate changes from baseline in liver tests related to treatment with glepaglutide. To minimize the effect of time as a confounding factor, all assessments were performed in the morning after an oral overnight fast during hospitalisations for the metabolic balance studies prior to and at the end of each treatment period.
2.2. Randomisation and masking
Full randomization and masking procedures have been covered previously [19]. In summary, the effect of glepaglutide was assessed in a two-period, three-treatment (0.1 mg, 1.0 mg, 10 mg) cross-over design. Six sequences (A, B, C, D, E, F) were used, corresponding to all ordered pairs of the three treatments: A ~10 mg/1 mg, B~10 mg/0.1 mg, C~1 mg/10 mg, D~1 mg/0.1 mg, E~0.1 mg/10 mg and F~0.1 mg/1 mg. A sequence label thus uniquely determined the treatments allocated for periods 1 and 2. Five blocks of length 6 were prepared; each block representing a random ordering of A:B:C:D:E:F.
The patients were enrolled by the trial investigator, who assigned them their patient numbers. The randomization process linked the assigned patient numbers to the first available randomization number, each corresponding to a sequence label. Investigators, patients and other care providers remained masked throughout the trial.
2.3. Procedures
2.3.1. Transient elastography
Bedside transient elastography (TE) with controlled attenuation parameter (CAP) was measured after an oral overnight fast by FibroScan® 502 Touch (Echosens, Paris, France) using the FibroScan M probe. The patient was placed in a semi-recumbent position with the right arm in maximal abduction [20]. The tip of the probe transducer was placed between the ribs perpendicularly against the skin overlying the liver. At least ten measurements were performed after normal expiration, targeting a success rate of ≥60% and IQR/median of ≤30% in case the median exceeded 7·1 kPa [21]. For TE the results were expressed in kPa and for CAP the results were expressed in decibels per meter (dB/m).
2.3.2. Indocyanine green clearance examination
ICG kinetics, as measured by plasma disappearance rate (PDR, %/min) and retention after 15 min (R15, %), were assessed after a bolus of 0·25 mg/kg ICG (ICG-Pulsion®, Pulsion Medical Systems SE, Feldkirchen, Germany) was injected intravenously and flushed with saline. The validated bedside pulse spectroscopy device LiMON (Pulsion, Maquet Holding B.V. & Co., Rastatt, Germany) was applied with a near-infrared finger clip sensor placed on the index finger (LiMON Module and Sensor by LiMON PULSION) [[22], [23], [24]]. During the treatment metabolic balance studies, ICG-PDR and ICG-R15 were measured after injection with glepaglutide. The principles behind the method have been described previously [10].
2.3.3. Blood samples
Blood samples were collected for sCD163, a validated marker of macrophage activation, sMR, and lipopolysaccharide binding protein (LBP), a marker of gut integrity. For sCD163, sMR, and LBP, plasma was stored at −80 °C for later analysis. Serum sCD163 and sMR were analysed by in-house sandwich enzyme-linked immunosorbent assays (ELISAs) [25,26]. LBP was measured using a commercially available ELISA kit (Duoset, R&D Systems, Minneapolis, MN, USA).
Blood samples were also collected for conventional liver tests, consisting of alkaline phosphatase (ALP), total bilirubin, international normalised ratio (INR), albumin, and liver transaminases (ASAT and ALAT).
2.3.4. Outcomes
As reported previously, the primary outcome of the trial was the absolute change from baseline to the end of the three-week treatment periods of wet weight of faecal output measured separately over each of the two treatment periods [19]. Main secondary and exploratory endpoints that are in the scope of the present paper included changes in: 1) stiffness of liver (TE) and CAP assessed by FibroScan, 2) ICG elimination, 3) sCD163 and sMR, 4) LBP, and 5) conventional liver tests. A more detailed list of additional secondary and exploratory endpoints has been provided previously [19].
All the endpoints were assessed as changes from baselines of the individual dose groups and were evaluated during hospitalisations for metabolic balance studies as previously described [19]. The assessments were done prior to and at the end of each treatment period. All measurements started after an oral overnight fast.
2.3.5. Statistical analysis
Since the effect of glepaglutide had not been assessed in a pilot study prior to this phase 2 trial, no formal sample size calculation was performed.
Baseline characteristics are reported as either median (min; max) or mean ± standard deviation (SD). An analysis of covariance (ANCOVA) was used to assess changes from baseline within each dose group using Statistical Analysis Software (version 9·4; SAS Institute, Cary, NC) as detailed elsewhere [19]. Patient ID was included in the model as a random effect to deal with within-subject correlation. The individual, period-specific baseline values of the endpoint as well as the baseline food intake, and the period (1 or 2) as a factor were also added to the model. Two-sided tests were performed using a 5% significance level, and results were presented as adjusted means, 95% confidence intervals (CI) and P-values. Since this was a dose-finding phase 2 trial, comparison between the different dose groups were included in the ANCOVA model. Type 3 analysis in the model was used to assess dose dependency.
The Spearman rank correlations were performed to assess any association between variables. The trial was monitored by a contract research organisation (Larix A/S, Denmark) and is registered at ClinicalTrials.gov with registration number NCT02690025.
3. Results
Of the 18 patients randomised and treated with glepaglutide (13 patients with SBS-IF and five with SBS-II) between February 2016 and May 2017, 16 patients completed the trial, thereby comprising the full analysis set group. The last patient's follow-up visit was May 4, 2017. Demographics and baseline characteristics are shown in Table 1.
Table 1.
Individual baseline demographics.
| ID | Sex/Age |
Diagnosis | Anatomy |
SBS | PS |
TE |
CAP |
PDR |
R15 |
sCD163 |
sMR |
LBP |
ALP |
ASAT |
ALAT |
INR |
Albumin |
Platelets |
Bilirubin |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | SB (cm) |
CiC (%) |
(mL) | 2–7 (kPa) |
<248 (dB/m) |
18–25 (%/min) |
0–10 (%) |
0·89–3·95 (mg/L) |
0·10–0·43 (mg/L) |
5–20 (μg/mL) |
35–105 (U/L) |
15–35 (U/L) |
10–45 (U/L) |
<1·2 | 36–45 (g/L) |
145–390 (X10 [9]/L) |
Total 5–25 (μmol/L) |
Conjugated 0–4 (μmol/L) |
Unconjugated F: <17 μmol/L M: <22 μmol/L |
|||
| 01 | M/68 | UC | 200 | 0 | IF | 2000 | 8·4 ↑ | 349↑ | 17·7↓ | 7·0 | 0·78↓ | 0·32 | 1·7↓ | 95 | 28 | 19 | 1·0 | 30↓ | 197 | 7 | 3 | 5 |
| 02 | M/57 | UC | 250 | 0 | II | 0 | 7·9↑ | 241 | 22·7 | 3·3 | 2·12 | 0·26 | 2·5↓ | 75 | 22 | 17 | 1·1 | 33↓ | 215 | 9 | 3 | 6 |
| 03 | F/52 | CD | 300 | 0 | II | 0 | 13·6↑ | 294↑ | 17·7↓ | 7·0 | 3·08 | 0·36 | 1·9↓ | 83 | 21 | 19 | 0·9 | 33↓ | 249 | 5 | 2 | NV |
| 04 | F/65 | CD | 70 | 0 | IF | 2153 | 5·5 | 180 | 26·6↑ | 1·8 | 2·15 | 0·27 | 1·4↓ | 61 | 27 | 9 | 1·0 | 36 | 187 | 4↓ | 2 | NV |
| 05 | F/61 | CD | 150 | 0 | II | 0 | 5·1 | 154 | 19·1 | 5·7 | 2·57 | 0·20 | 2·0↓ | 119↑ | 48↑ | 21 | 1·3 | 29↓ | 187 | 12 | 4 | 8 |
| 06 | M/76 | MVD | 30 | 0 | IF | 5750 | 12·1↑ | 323↑ | ND | ND | 2·70 | 0·60↑ | 4·6↓ | 164↑ | 37↑ | 31 | 2·1↑ | 30↓ | 167 | 8 | 4 | 4 |
| 07 | M/56 | CD | UK | 0 | II | 0 | 5·0 | 278↑ | 20·8 | 4·4 | 3·08 | 0·12 | 12·8 | 95 | 24 | 24 | 1·0 | 33↓ | 223 | 3↓ | 2 | NV |
| 08 | F/57 | MVD | 80 | 0 | IF | 3500 | 5·5 | 328↑ | 17·0↓ | 7·8 | 2·27 | 0·28 | 2·1↓ | 167↑ | 42↑ | 69↑ | 1·2 | 36 | 374 | 18 | 8↑ | 10 |
| 09 | F/63 | MVD | 50 | 0 | IF | 2250 | 5·3 | 167 | 10·7↓ | 20·1↑ | 6·23↑ | 0·28 | 1·7↓ | 255↑ | 32 | 21 | 1·1 | 34↓ | 95↓ | 12 | 6↑ | 7 |
| 10 | F/73 | CD | 30 | 0 | IF | 3130 | NV | NV | 8·7↓ | 27·1↑ | 8·14↑ | 0·64↑ | 2·9↓ | 203↑ | 43↑ | 29 | 2·4↑ | 30↓ | 194 | 13 | 6↑ | 7 |
| 11 | F/58 | MVD | 85 | 50 | IF | 1750 | 2·6 | 298↑ | 18·5 | 6·2 | 2·32 | 0·26 | 4·1↓ | 81 | 26 | 33 | 1·2 | 32↓ | 297 | 11 | 5↑ | 6 |
| 12 | F/64 | CD | 150 | 0 | IF | 1000 | 4·6 | 178 | 15·9↓ | 9·2 | 1·65 | 0·16 | 1·7↓ | 70 | 28 | 19 | 1·1 | 34↓ | 169 | 10 | 3 | 7 |
| 13 | M/59 | CD | 140 | 0 | II | 0 | 6·0 | 273↑ | 27·8↑ | 1·5 | 2·76 | 0·32 | 5·1 | 65 | 17 | 15 | 1·2 | 31↓ | 342 | 5 | 2 | 3 |
| 14 | M/56 | CD | 70 | 0 | IF | 2560 | NV | NV | ND | ND | 3·07 | 0·41 | 9·9 | 113↑ | 27 | 12 | 1·0 | 30↓ | 181 | 13 | 6↑ | 7 |
| 15 | M/72 | CD | UK | 70 | IF | 2000 | 4·7 | 313↑ | 28·4↑ | 1·4 | 1·92 | 0·33 | 12·0 | 86 | 55↑ | 82↑ | 1·0 | 29↓ | 326 | 9 | 4 | 5 |
| 16 | M/71 | MVD | 40 | 0 | IF | 2750 | 6·1 | 304↑ | 17·6↓ | 7·1 | 3·28 | 0·32 | 4·6↓ | 96 | 23 | 24 | 1·1 | 29↓ | 140↓ | 8 | 3 | 5 |
| 17 | M/43 | SC | 70 | 0 | IF | 2865 | 8·8↑ | 322↑ | 15·8↓ | 9·3 | 3·26 | 0·26 | 12·2 | 61 | 33 | 30 | 1·0 | 35↓ | 249 | 11 | 5↑ | 6 |
| 18 | F/55 | MVD | 50 | 0 | IF | 3000 | 4·8 | 202 | 25·2↑ | 2·3 | 4·14↑ | 0·33 | 2·4↓ | 813↑ | 23 | 37 | 1·7↑ | 34↓ | 238 | 7 | 3 | 4 |
ALAT = Alanine Transaminase; ALP = Alkaline Phosphatase; ASAT = Aspartate Transaminase; CAP = Controlled Attenuation Parameter; CD = Crohn's Disease; CiC = Colon in continuity; F = Female; ICG = Indocyanine Green; IF = Intestinal Failure; II = Intestinal Insufficiency; INR = International Normalised Ratio; LBP = Lipopolysaccharide Binding Protein; M = Male; MVD = Mesenteric Vascular Disease; ND = Not done; NV = Not valid; PDR = Plasma Disappearance Rate; PS = Parenteral Support; R15 = Retention Rate after 15 min; SB = Small Bowel; SBS = Short Bowel Syndrome; SC = Surgical Complications; sCD163 = Soluble CD163; sMR = Soluble Mannose Receptor; TE = Transient Elastography; UC = Ulcerative Colitis; UK = Unknown.
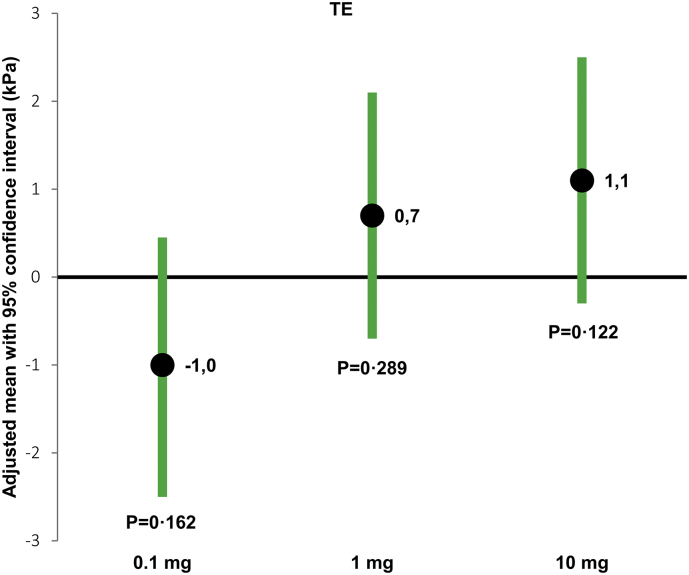
No patients had ascites and successful baseline TE was performed in 16 out of 18 (89%) patients. Mean TE (±SD) was 6·6 ± 2·9 kPa (median 5·5 kPa (min: 2·6; max: 13·6)) and mean CAP was 262 ± 66 dB/m (median 286 dB/m (154; 349)) at baseline. Of the 16 patients, five patients had TE >7 kPa (cut-offs for liver fibrosis stage ≥ F1 [20]); two out of five patients (40%) in SBS-II group and three out of 11 patients (27%) in the SBS-IF group. CAP value was above 248 dB/m (cut-offs for steatosis > S0 [27]) in ten out of 16 patients; three out of five (60%) patients with SBS-II and seven out of 11 (64%) patients with SBS-IF. Following treatment with glepaglutide, TE tended to increase in the 1 mg and 10 mg dose groups, but not in the 0·1 mg dose group. However, no dose-dependency was observed (P = .103), and the changes did not reach statistical significance (Table 2 and Fig. 1). CAP did not change in any of the dose groups.
Table 2.
Transient elastography, controlled attenuation parameter, indocyanine green, sCD163, sMR and LBP.
| Changes from baseline (Adjusted means [95% CI]) |
0·1 mg (N = 10) |
1 mg (N = 11) |
10 mg (N = 11) |
|---|---|---|---|
| TE (kPa) | -1·0 [−2·5, 0·45] P = 0·162* |
0·7 [−0·7, 2·1] P = 0·289** |
1.1 [−0·3, 2·5] P = 0·122* |
| CAP (dB/m) | 12 [−19, 42] P = 0·432* |
17 [−10, 45] P = 0·198** |
−11 [−39, 17] P = 0·434* |
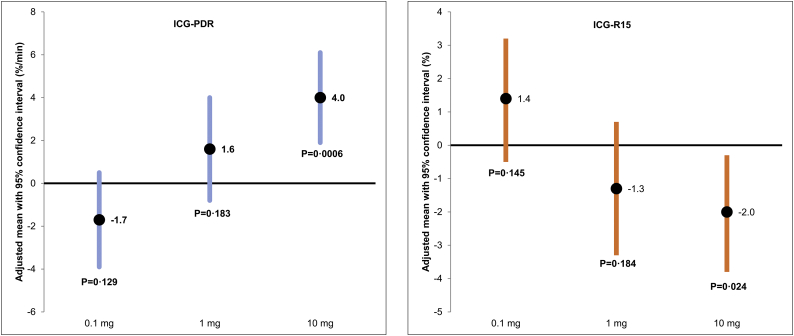
| ICG-PDR (%/min) | −1·7 [−3·9, 0·5] P = 0·129 |
1·6 [−0·8, 4·0] P = 0·183* |
4·0 [1·9, 6·1] P = 0·0006 |
| ICG-R15 (%) | 1·4 [−0·5, 3·2] P = 0·145 |
−1·3 [−3·3, 0·7] P = 0·184* |
−2·0 [−3·8, −0·3] P = 0·024 |
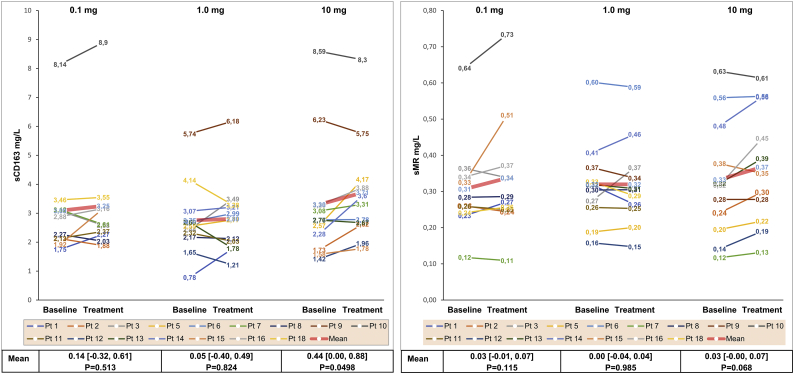
| sCD163 (mg/L) | 0·14 [−0·32, 0·61] P = 0·513 | 0·05 [−0·40, 0·49] P = 0·824 |
0·44 [0·00, 0·88] P = 0·0498 |
| sMR (mg/L) | 0·03 [−0·01, 0·07] P = 0·115 |
0·00 [−0·04, 0·04] P = 0·985 |
0·03 [−0·00, 0·07] P = 0·068 |
| LBP (μg/mL) | −0·21 [−3·24, 2·82] P = 0·881 |
1·33 [−1·57, 4·23] P = 0·330 |
0·77 [−2·07, 3·61] P = 0·561 |
*N = 9; **N = 10; CAP = Controlled Attenuation Parameter; ICG = Indocyanine Green; LBP = Lipopolysaccharide Binding Protein; PDR = Plasma Disappearance Rate; R15 = Retention Rate after 15 min; sCD163 = Soluble CD163; sMR = Soluble Mannose Receptor; TE = Transient Elastography; N = number of patients in full analysis set;
Fig. 1.
Mean changes from baseline in transient elastography.
Mean ICG-PDR at baseline was 19·4 ± 5·7%/min and mean ICG-R15 was 7·6 ± 6·9%. At baseline, ICG-PDR was below the lower limits of normal (LLN) in eight patients; among them seven patients had SBS-IF. ICG-R15 was elevated in two patients with SBS-IF at baseline. A dose response relationship was observed between 1 mg and 10 mg doses of glepaglutide and changes in ICG-PDR and ICG-R15 (Fig. 2). However, only changes in the 10 mg dose group reached statistical significance; ICG-PDR increased by 4%/min (P = 0·0006) and ICG-R15 decreased by 2% (P = 0·024) (Table 2) corresponding to a relative change of 19% and − 37% respectively. No changes were observed in the 0·1 mg dose group.
Fig. 2.
Mean changes from baseline in ICG-PDR and ICG-R15.
Mean sCD163 at baseline was 3·1 ± 1·7 mg/mL, mean sMR was 0·3 ± 0·1 mg/mL and mean LBP was 4·8 ± 4·0 μg/mL. Elevated levels (higher than the reference range 97·5 percentile for healthy individuals [26,28]) of sCD163 (0·89–3·95 mg/L) and sMR (0·10–0·43 mg/L) were observed in three and two SBS-IF patients, respectively. LBP was below LLN in 13 out of 18 (72%) patients. In the 10 mg dose group, sCD163 increased by 0·44 mg/mL (P = 0·0498). In general, sCD163 increased in eight of 11 (73%) patients within the range of 0·01–1·6 mg/mL. However, the post-treatment values for these patients were still within the normal range [28] (Fig. 3). A decrease in serum levels of sCD163 was observed in the 10 mg dose group in two patients with baseline values above upper limits of normal (ULN). A non-significant increase of 0·03 mg/mL (P = 0·068) was observed for sMR in the 10 mg dose group. However, the magnitude of the increase was comparable with changes following 0·1 mg (0·03 mg/mL; P = 0·115). sMR did not change following treatment with 1 mg glepaglutide (0·00; P = 0·985). No changes were observed in LBP following treatment with glepaglutide.
Fig. 3.
Individual serum levels of sCD163 and sMR before and after treatment for each patient.
Biochemical cholestasis as defined by elevated plasma levels of ALP >1·5 fold the ULN [29] was observed at baseline in five of the 18 patients (28%), all having SBS-IF. Conjugated bilirubin was elevated in six of the 18 patients (33%), all having SBS-IF. Total and unconjugated bilirubin values were within the normal range in all patients. INR was above 1·2 in three (17%) patients with SBS-IF; two patients with the shortest small bowel length of 30 cm without colon and one with the need for warfarin due to protein S deficiency. ALAT levels were elevated, but still <2 x UNL in two patients with SBS-IF. Plasma albumin was generally low in all patients (Table 1). In the 1 mg dose group, treatment with glepaglutide resulted in a decrease of 33 U/L (P = 0·032) in plasma ALP from baseline value of 189 ± 216 U/L, and a reduction of 2·1 g/L (P = 0·023) in plasma albumin from baseline value of 32·0 ± 1·8 g/L (Table 3). Minor decreases were observed in plasma total bilirubin (−2 μmol/L, P = 0·016) and unconjugated bilirubin (−1·3 μmol/L, P = 0·035) in the 10 mg dose group. Liver transaminases (ASAT and ALAT), conjugated bilirubin and INR did not change in any dose groups.
Table 3.
Liver tests.
| Changes from baseline (Adjusted means [95% CI]) |
0·1 mg (N = 10) |
1 mg (N = 11) |
10 mg (N = 11) |
|---|---|---|---|
| ALAT (U/L) | 0·0 [−14·0, 14·0] P = 0·999 |
−4·1 [−16·8, 8·7] P = 0·497 |
3·4 [−9·5,16·3] P = 0·568 |
| ALP (U/L) | 9 [−23, 40] P = 0·556 |
−33 [−63, −4] P = 0·032 |
−16 [−46,14] P = 0·255 |
| Total Bilirubin (μmol/L) | −0·2 [−1·8,1·5] P = 0·838 |
−0·3 [−1·8,1·3] P = 0·718 |
−1·9 [−3·4, −0·5] P = 0·016 |
| Conjugated Bilirubin (μmol/L) | 0·4 [−0·4, 1·2] P = 0·287 |
−0·2 [−1·0, 0·5] P = 0·515 |
−0·4 [−1·1, 0·4] P = 0·358 |
| Unconjugated Bilirubin (μmol/L) | 0·2 [−1·0, 1·4] P = 0·710 |
0·2 [−1·0, 1·3] P = 0·732 |
−1·3 [−2·4, 0·1] P = 0·035 |
| INR | −0·02 [−0·09, 0·06] P = 0·682 |
0·07[−0·01, 0·14] P = 0·080 |
−0·00 [−0·08,0·07] P = 0·925 |
| Plasma Albumin (g/L) | −1·2 [−3·1, 0·6] P = 0·173 |
−2·1 [−3·9, −0·4] P = 0·023 |
−1·2 [−3·0, 0·6] P = 0·165 |
ALAT = Alanine Transaminase; ALP = Alkaline Phosphatase; INR = International Normalised Ratio; N = number of patients in full analysis set.
4. Discussion
We have previously published that three weeks of treatment with glepaglutide in a randomised, dose-finding phase 2 trial was associated with improvement of the intestinal function in patients with SBS [19]. In this paper, we report that short term treatment with glepaglutide may improve liver excretory function, increase liver stiffness, and activate resident liver macrophages in patients with SBS as exploratory outcomes from the phase 2 trial. These findings were not in full support of our a priori expectations and are ambiguous in the sense that some of them may be interpreted as beneficial, others as reflecting potentially harmful effects.
Our findings suggest that alterations in markers of liver function are evident in a stable SBS population with or without need for PS at baseline. However, except for TE and CAP, a clear overrepresentation of patients with SBS-IF had biochemical cholestasis, impaired hepatic clearance of ICG as well as elevated levels of sCD163 and sMR. Moreover, small bowel length and the PS volume requirements correlated with baseline values of ALP, ALAT, conjugated and total bilirubin, sMR, and sCD163 (the latter only with small bowel length), but not ICG-PDR/R15 or LBP (appendix page 2–3).
TE in an adult IF population have only been assessed in one previous trial [6], where patients with different pathophysiological aetiology of IF were included with liver function ranging from no significant hepatic alterations to overt clinical signs of severe liver disease [30]. This might explain why the median TE (10·0 (4; 24) kPa) in that study was higher than what we have found in the present trial, where only stable patients with SBS-II and SBS-IF without significant abnormal liver biochemistry were included. Our data do not confirm previous findings concerning the negative correlation between TE and the severity of SBS as indicated by small bowel length and PS volume [6,7]. However, our findings suggest that the severity of SBS at baseline may be a predictor of an increase in TE following treatment with glepaglutide (appendix page 2–3). Since PS volume was kept stable during the trial, we could not assess whether a change in PS volume following the treatment would also induce a change in TE.
Following treatment with glepaglutide, TE seemed to increase which may indicate increased liver stiffness. This is often ascribed to fibrosis, which however is unlikely to develop with our short intervention. Liver stiffness may also result from a number of transient changes such as cellular infiltration as part of inflammation. The increase in sCD163 could support this background and if so would be a cause of attention. However, liver stiffness also increases with splanchnic blood flow [8,31], not measured here but known in other studies to increase by exogenous GLP-2 administration even in a fasting condition [16]. Thus the increase in TE may partly be ascribed to hemodynamic changes which might be taken to be beneficial.
The increase in ICG elimination along with the reduction in ALP and total bilirubin may imply that glepaglutide benefits excretory liver function in patients with SBS. These positive effects might be mediated indirectly through the improved intestinal absorption, allowing a greater portal circulation of fluids and nutrients. This was supported by the positive correlations between changes in ICG-PDR/R15 and changes in wet weight absorption, as well as negative correlations between baseline values of ALP/total bilirubin and wet weight absorption (appendix page 2–3). However, changes in splanchnic perfusion may influence the results since ICG clearance is dependent on hepatic blood flow [10]. Other possible explanatory mechanisms may include activation of the FXR-FGF19-C4 (FXR = Farnesoid X Receptor, FGF19 = Fibroblast Growth Factor 19, C4 = 7α-Hydroxy-4-Cholesten-3-One) gut-liver axis, alterations in bile acid composition and output as well as improvements in the enterohepatic circulation.
Recent experimental and clinical evidence available may suggest that the main trigger in IFALD is resident liver macrophage activation caused by synergistic effects of parenteral plant sterols present in intravenous lipid preparations and bacterial translocation [32,33]. The resulting portal inflammation then leads to suppression of transporters for bile acids, sterols, phospholipids and bilirubin. Somewhat contrary to this hypothesis, alterations in markers of liver function were observed at baseline independent of LBP, which was not elevated in any patients included in this trial and did not seem to correlate with either PS volume or small bowel length (appendix page 2–3). Moreover, glepaglutide improved ICG and markers of cholestasis, but also increased sCD163 without significant effects on LBP that in any case is a relatively insensitive marker of LPS load.
The increase in sCD163 in the 10 mg dose group indicate some degree of inflammatory activation of resident liver macrophages. sCD163 did not seem to correlate with either changes in ICG-PDR or the increase in wet weight absorption (appendix page 2–3) suggesting that the effects of glepaglutide on ICG clearance and sCD163 were independent of each other. The increase in sCD163, contrary to ICG clearance and liver stiffness, cannot be ascribed to hemodynamic or other confounding effects.
Given that our findings are based on results from the exploratory endpoints of the phase 2 trial with a limited number of patients and with no sample size calculation, the results and the level of significance presented in this paper should be interpreted with care. Type 2 errors are possible due to the small sample size. However, type 1 errors should also be considered when interpreting these results given the number of tests we have performed in this small sample, including the correlation analysis. Moreover, as far as we know, the methods used in this study, apart from conventional liver tests, have not been used previously to assess liver function in a stable chronic SBS population with II or IF. The assessments were performed with at least three weeks gap; i.e. we neither have data on the acute changes after glepaglutide administration, nor the consequences of long-term, potentially chronic treatment, which would be of interest. The clinical relevance of our findings should be addressed in larger studies with more robust designs that include patients with overt IFALD to validate the methods and assess their suitability as diagnostic or surveillance tools that would eventually help in proper detection of the degree and progression of hepatic damage in patients with SBS.
In conclusion, short-term treatment with glepaglutide induced increases in TE, ICG elimination, sCD163 as well as a reduction in plasma ALP, and total bilirubin. CAP and gut integrity as assessed by LBP were not measurably affected. Although there was no significant difference between the 1 mg and 10 mg dose groups regarding any of the abovementioned assessments, changes were primarily seen in the 10 mg dose group. No treatment effect was observed for the 0·1 mg dose, which was congruent with our previous findings since glepaglutide at this dose level did not impact intestinal function [19]. The increase in elimination of ICG may imply an improved liver excretory function supported by the reductions in ALP and total bilirubin. The increase may also be suggestive of an increase in splanchnic and thereby hepatic blood flow as supported by the positive correlations between changes in ICG elimination and changes in wet weight absorption. The increases in sCD163 and TE may imply liver macrophage activation and an increased hepatic stiffness, respectively, both reflecting cellular inflammation in the liver, although the stiffness could also increase by higher splanchnic blood flow.
Contributors
RM Naimi, M Hvistendahl, and PB Jeppesen generated the trial concept, contributed to the trial design, conducted the trial, analysed and interpreted the data, and drafted the manuscript with input from all authors. PB Jeppesen supervised the project.
N Nerup, R Ambrus, M Achiam, and LB Svendsen were involved in assessing liver function by indocyanine green (ICG) elimination. They performed the measurements, processed the experimental data, performed the analysis, and aided in interpreting the results.
H Grønbæk, HJ Møller, and H Vilstrup worked out the technical details and contributed to the processing, analysis, and interpretation of sCD163, sMR, and LBP.
A Steensberg contributed to the trial design, interpretation of data, and drafting of the manuscript.
All authors provided critical feedback and helped shape the research, analyses, and manuscript. They have critically revised the manuscript and agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors had access to the trial data and reviewed and approved the final manuscript.
Declaration of interests
RM Naimi was an employee at Zealand Pharma as an industrial PhD fellow during the conduct of this trial. A Steensberg is an employee at Zealand Pharma. PB Jeppesen serves as consultant and trial investigator for Zealand Pharma. The remaining authors have nothing to disclose.
Role of the funding source
The funding source represented by the author AS was involved in the trial design and conduct. AS has reviewed the manuscript and suggested changes, but had no role in patient recruitment, data collection and analysis, and the final decision to publish. The corresponding author had full access to all the data in the trial and had final responsibility for the decision to submit. All authors had full access to the trial data and reviewed and approved the final manuscript.
Data sharing
Any shared data collected for the trial, including datasets containing individual participant data will be in a deidentified or anonymous format. The results of the trial will be made available on one or more public portals according to standard disclosure requirements for clinical trials.
Acknowledgment
We would like to gratefully acknowledge and thank Jette Christiansen, Birgitte Schou, and Dorte Christensen from the Department of Medical Gastroenterology and Hepatology, Rigshospitalet, for their technical assistance.
Our thanks are also extended to the trial statisticians, Henrik Wachmann from Larix A/S and Kim Mark Knudsen from Zealand Pharma A/S, for their assistance and support in the review of statistics, statistical analysis and interpretation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.016.
Appendix A. Supplementary data
Supplementary material
References
- 1.Cavicchi M., Beau P., Crenn P., Degott C., Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann. Intern. Med. 2000;132(7):525–532. doi: 10.7326/0003-4819-132-7-200004040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kelly D.A. Intestinal failure-associated liver disease : what do we know today? Gastroenterology. 2006;130(2 Suppl 1):70–77. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Lim D.W., Wales P.W., Josephson J.K. Glucagon-like peptide 2 improves cholestasis in parenteral nutrition-associated liver disease. JPEN J. Parenter. Enteral Nutr. 2016;40(1):14–21. doi: 10.1177/0148607114551968. [DOI] [PubMed] [Google Scholar]
- 4.Pereira-Fantini P.M., Lapthorne S., Joyce S.A. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J. Hepatol. 2014;61(5):1115–1125. doi: 10.1016/j.jhep.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Sasdelli A.S., Agostini F., Pazzeschi C., Guidetti M., Lal S., Pironi L. Assessment of intestinal failure associated liver disease according to different diagnostic criteria. Clin. Nutr. 2018;S0261–5614(18):30170–30175. doi: 10.1016/j.clnu.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Van Gossum A., Pironi L., Messing B. Transient Elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN J. Parenter. Enteral Nutr. 2015;39(6):719–724. doi: 10.1177/0148607114538057. [DOI] [PubMed] [Google Scholar]
- 7.Hukkinen M., Kivisaari R., Lohi J. Transient elastography and aspartate aminotransferase to platelet ratio predict liver injury in paediatric intestinal failure. Liver Int. 2016;36(3):361–369. doi: 10.1111/liv.12887. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez D., Orozco F., Mella J.M., Anders M., Antinucci F., Mastai R. Meal ingestion markedly increases liver stiffness suggesting the need for liver stiffness determination in fasting conditions. Gastroenterol. Hepatol. 2015;38(7):431–435. doi: 10.1016/j.gastrohep.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Barone M., Iannone A., Brunetti N.D. Liver stiffness and portal blood flow modifications induced by a liquid meal consumption: Pathogenetic mechanisms and clinical relevance. Scand. J. Gastroenterol. 2015;50(5):560–566. doi: 10.3109/00365521.2014.1003396. [DOI] [PubMed] [Google Scholar]
- 10.Levesque E., Martin E., Dudau D., Lim C., Dhonneur G., Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016;35(1):49–57. doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Møller S., la Cour Sibbesen E., Madsen J.L., The Bendtsen F. Indocyanine green retention test in cirrhosis and portal hypertension. Accuracy and relation to severity of disease. J. Gastroenterol. Hepatol. 2018:1–7. doi: 10.1111/jgh.14470. [DOI] [PubMed] [Google Scholar]
- 12.Andersen E.S., Rødgaard-Hansen S., Moessner B., Christensen P.B., Møller H.J., Weis N. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: a pilot study. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33(1):117–122. doi: 10.1007/s10096-013-1936-3. [DOI] [PubMed] [Google Scholar]
- 13.Lim D.W., Wales P.W., Mi S. Glucagon-like Peptide-2 alters bile acid metabolism in parenteral nutrition–associated liver disease. JPEN J. Parenter. Enteral Nutr. 2016 jan;40(1):22–35. doi: 10.1177/0148607115595596. [DOI] [PubMed] [Google Scholar]
- 14.Guan X., Karpen H.E., Stephens J. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130(1):150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Guan X., Stoll B., Lu X. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets. Gastroenterology. 2003;125(1):136–147. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 16.Høyerup P., Hellström P.M., Schmidt P.T. Glucagon-like peptide-2 stimulates mucosal microcirculation measured by laser Doppler flowmetry in end-jejunostomy short bowel syndrome patients. Regul. Pept. 2013;180:12–16. doi: 10.1016/j.regpep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen P.B., Pertkiewicz M., Messing B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473–1481.e3. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz L.K., O'Keefe S.J., Fujioka K. Long-term Teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin. Transl. Gastroenterol. 2016;7 doi: 10.1038/ctg.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naimi R.M., Hvistendahl M., Enevoldsen L.H. Glepaglutide, a novel long-acting glucagon-like peptide-2 analogueue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;1253:1–10. doi: 10.1016/S2468-1253(19)30077-9. [DOI] [PubMed] [Google Scholar]
- 20.Castera L., Forns X., Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008;48(5):835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Boursier J., Zarski J.P., de Ledinghen V. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57(3):1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 22.Cheung T.T., Chan S.C., Chok K.S. Rapid measurement of indocyanine green retention by pulse spectrophotometry: a validation study in 70 patients with child-Pugh a cirrhosis before hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11(3):267–271. doi: 10.1016/s1499-3872(12)60159-3. [DOI] [PubMed] [Google Scholar]
- 23.Purcell R., Kruger P., Jones M. Indocyanine green elimination: a comparison of the LiMON and serial blood sampling methods. ANZ J. Surg. 2006;76(1–2):75–77. doi: 10.1111/j.1445-2197.2006.03643.x. [DOI] [PubMed] [Google Scholar]
- 24.Sakka S.G., Reinhart K., Meier-Hellmann A. Comparison of invasive and noninvasive measurements of indocyanine green plasma disappearance rate in critically ill patients with mechanical ventilation and stable hemodynamics. Intensive Care Med. 2000;26(10):1553–1556. doi: 10.1007/s001340000639. [DOI] [PubMed] [Google Scholar]
- 25.Møller H.J., Hald K., Moestrup S.K. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand. J. Clin. Lab. Invest. 2002;62(4):293–299. doi: 10.1080/003655102760145852. [DOI] [PubMed] [Google Scholar]
- 26.Rødgaard-Hansen S., Rafique A., Christensen P.A. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin. Chem. Lab. Med. 2014;52(3):453–461. doi: 10.1515/cclm-2013-0451. [DOI] [PubMed] [Google Scholar]
- 27.Karlas T., Petroff D., Sasso M. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Møller H.J. Soluble CD163. Scand. J. Clin. Lab. Invest. 2012;72(1):1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 29.Beath S., Pironi L., Gabe S. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation. 2008;85(10):1378–1384. doi: 10.1097/TP.0b013e31816dd513. [DOI] [PubMed] [Google Scholar]
- 30.Pironi L., Arends J., Baxter J. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015;34(1):171–180. doi: 10.1016/j.clnu.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Kjærgaard M., Thiele M., Jansen C. High risk of misinterpreting liver and spleen stiffness using 2D shear-wave and transient elastography after a moderate or high calorie meal. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0173992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Kasmi K.C., Anderson A.L., Devereaux M.W. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology. 2012;55(5):1518–1528. doi: 10.1002/hep.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutanen A., Lohi J., Heikkilä P., Jalanko H., Pakarinen M.P. Liver inflammation relates to decreased Canalicular bile transporter expression in pediatric onset intestinal failure. Ann. Surg. 2018;268(2):332–339. doi: 10.1097/SLA.0000000000002187. [DOI] [PubMed] [Google Scholar]
- 34.Etzerodt A., Maniecki M.B., Møller K., Møller H.J., Moestrup S.K. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J. Leukoc. Biol. 2010;88(6):1201–1205. doi: 10.1189/jlb.0410235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material