Abstract
Introduction.
Among patients with hepatitis C virus (HCV) infection, alcohol synergistically increases the risk of cirrhosis, hepatocellular carcinoma, and death. Randomized controlled trials of integrated models of HCV-alcohol treatment have been recommended but only performed in patients with severe alcohol use disorders.
Objectives.
This pragmatic randomized controlled trial seeks to compare clinical effectiveness and cost-effectiveness of integrated alcohol treatment compared to enhanced treatment as usual (TAU) on alcohol consumption and economic outcomes among patients ever infected with HCV.
Methods.
Patients recruited from three liver centers who had current or prior chronic HCV and qualifying alcohol screener scores were randomly assigned to enhanced TAU or the Hepatitis C-Alcohol Reduction Treatment (Hep ART) intervention. All patients received enhanced TAU, consisting of a patient-administered alcohol screener and care from medical providers who were trained in Screening, Brief Intervention and Referral to Treatment (SBIRT), including brief motivational interviewing counseling. The Hep ART intervention combined enhanced TAU with up to six months of integrated co-located individual and/or group therapy that provided motivational, cognitive, and behavioral strategies to reduce alcohol consumption. The Timeline Followback (TLFB) Method was used to evaluate alcohol use at baseline, 3, 6, and 12 months. Primary outcomes are alcohol abstinence and fewer heavy drinking days, and for the cost-effectiveness analysis, measures included grams of alcohol consumed.
Discussion.
This study will determine whether Hep ART, a six-month integrated alcohol treatment, compared to enhanced TAU, is both clinically effective and cost-effective in patients with a history of comorbid HCV and alcohol use.
Keywords: Behavioral, integrated care, therapy, addiction, hepatitis C, alcohol
1. Introduction
Chronic hepatitis C virus (HCV) infection affects an estimated 1.0% of people (approximately 2.7 million) aged six and older in the United States (US).1 Among Veterans, the prevalence of HCV infection is even higher (5.4%).2 With chronic infection over 20 to 30 years, fibrosis can progress to cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma.3,4 With an HCV prevalence of 2.6%, Americans aged 50–70 are now experiencing serious HCV-related complications, resulting in increasing hospitalization rates and morbidity.5 Chronic HCV is the most common indication for liver transplants in the US.6 Although likely attenuated with the advent of effective antiviral treatment, numerous US population projections of the HCV epidemic have suggested that liver mortality, morbidity, and disease related costs will rise this decade.7,8.
Compared to US residents without HCV infection, people with HCV infection are almost eight times more likely to consume over three alcoholic drinks per day (2% vs. 19%).9 Alcohol consumption works synergistically with HCV to increase the risk of liver cirrhosis,10,11 hepatocellular carcinoma,12–14 and mortality.15,16 Even if cured of HCV, patients with advanced fibrosis or cirrhosis are recommended to abstain from alcohol to prevent progression to liver-related complications, such as portal hypertension, ascites, gastrointestinal bleeding, hepatic encephalopathy, and liver cancer.17 Yet alcohol use is vastly under-treated in the US.18 There is a documented shortage of needed alcohol treatment among patients with HIV-HCV co-infection,19 but such studies among patients with HCV only are lacking.
Few integrated alcohol treatment models for HCV-infected patients have been tested, yet there exists a window of opportunity to engage patients at point of care with liver providers.20 Previously studied HCV-alcohol integrated treatments have mostly focused on people who inject drugs recruited from needle exchange settings,21 or involved patients with severe alcohol use disorders.22 Although limited by nonrandomized study designs, two studies examining integrated care for patients with less severe alcohol use showed increased initiation of HCV antiviral therapy and reduced or abstinent alcohol consumption.23,24 These studies suggested that integrated HCV-alcohol treatment for less severe alcohol use may be beneficial and encouraged studies using randomized designs. An additional study that did use a randomized design but tested a less intensive form of integrated care (four sessions of motivational enhancement therapy delivered by psychologists) for patients with both HCV and alcohol use disorder found more days of alcohol abstinence in the treatment group.25
Extending our previously conducted pilot feasibility study of an integrated HCV-alcohol intervention,26 we describe herein a pragmatic randomized controlled trial of this intervention on alcohol consumption for patients with varying alcohol use severity. All patients received enhanced treatment as usual (TAU) in the form of Screening, Brief Intervention and Referral to Treatment (SBIRT) delivered by trained hepatology providers; and half additionally were offered six months of intensive integrated Hepatitis C-Alcohol Reduction Treatment (Hep ART). This study will determine the degree of benefit, if any, of intensive integrated alcohol treatment over enhanced TAU alone for patients with current or prior HCV infection.
2. Design and methods
This study employs a randomized controlled trial (RCT) design. It compares enhanced treatment as usual (TAU), described below, with a six-month integrated Hep ART treatment. Primary outcomes are alcohol abstinence and fewer heavy drinking days, and for the cost-effectiveness analysis, grams of alcohol consumed. Secondary outcomes include fewer drinks per week, fewer days of illicit drug use, and fewer positive urine toxicology screens.
Designed to be a pragmatic trial, a key feature of our study is recruitment of participants from three diverse real-world liver clinic settings: a private university liver clinic, a public university liver clinic, and a Veterans Affairs (VA) hospital liver clinic. Consistent with what implementation science researchers have designated a hybrid design, our study design examines clinical outcomes while implementing it under diverse circumstances and collecting process data. The loosening of tight control over some study characteristics in this study, such as clinic settings, paired with the collection of process data allows for earlier identification of barriers to widespread implementation and possible modifications to maximize future implementation.27
2.1. Overview of study design and research hypotheses
Patients are recruited from three different health system clinics who had current or prior chronic HCV infection and qualifying alcohol screener scores were randomly assigned to enhanced TAU or the Hep ART intervention. Enhanced TAU utilizes the Screening, Brief Intervention and Referral to Treatment (SBIRT) model that includes a patient-administered alcohol screener, medical provider-delivered brief motivational interviewing counseling, and referral to alcohol treatment. The Hep ART intervention combines enhanced TAU with up to six months of integrated co-located individual and/or group therapy that provides supportive, motivational, cognitive, and behavioral strategies to reduce or abstain from alcohol consumption and emphasizes the relationship between alcohol use and HCV. A key feature of the Hep ART intervention is structuring integrated care between HCV medical providers and addiction therapists to address alcohol use and liver health, with an integrated medical-behavioral plan for liver health. In the enhanced TAU condition, patients receive enhanced TAU, which includes referral out for alcohol treatment in their local community, which is unlikely to focus on liver health. The aims and hypotheses are:
Aim 1: To implement a randomized controlled trial to evaluate alcohol abstinence in an integrated six-month treatment model (Hep ART) compared to enhanced TAU, among patients with current or prior chronic HCV infection who consume alcohol.
Hypothesis 1: The Hep ART intervention, compared to enhanced TAU, will significantly improve alcohol abstinence rates in a six-month period and significantly decrease alcohol relapse rates six months after the treatment intervention ends.
Aim 2: To determine differences in secondary outcomes of the Hep ART intervention versus enhanced TAU.
Hypothesis 2: Hep ART intervention participants will report fewer heavy drinking days, fewer drinks per week, fewer days of illicit drug use, and fewer positive urine toxicology screens.
Aim 3: To conduct a cost effectiveness analysis (CEA) of the Hep ART intervention versus enhanced TAU.
Hypothesis 3: The Hep ART intervention will be cost-effective, falling below $50,000 per quality-adjusted life year gained, making it comparable to well-accepted medical interventions in the health economics literature.
2.2. Conceptual model for study
Our conceptual model hinges on patients with current or prior chronic HCV recognizing the need for alcohol treatment, combined with an intervention to decrease barriers to alcohol treatment. Specifically, the Health Beliefs Model proposes that treatment participation and adherence are at least partly a function of a person’s beliefs about susceptibility to illness, perceived severity of illness, perceived benefits and barriers to treatment, and cues to action.28,29 These factors readily apply to patients with HCV who consume alcohol. For example, patients with more advanced liver disease may be more motivated to participate in alcohol treatment.23 Also, patient understanding of the negative effects of drinking and the benefits of abstinence to their liver health as a person with current or prior chronic HCV infection likely affects their alcohol treatment participation and adherence.
In this study, we enroll patients with current or prior chronic HCV infection who consume alcohol, and therefore may perceive themselves as susceptible to liver disease. In addition, the Hep ART intervention focuses on barriers to alcohol treatment and removing those barriers. Accordingly, addiction treatment is located in the liver clinic, which may be convenient as a familiar location where multiple services are received, and which may be a safe location that destigmatizes alcohol care. In all involved liver clinics, providers across disciplines collaborate on patient care, including alcohol treatment, without assuming that patients can or should coordinate this care themselves. We see substance use and psychiatric illness as potential barriers to engaging in alcohol treatment and important co-morbidities that we address through psychiatric care, when needed, and through individually tailored sessions with the addiction therapist. We also attempt to remove transportation barriers through offering therapy over the phone, as well as bus and parking passes.
2.3. Study settings
This study is being conducted at three health systems providing liver care. One system is Duke University Health System, which includes several academically affiliated gastroenterology (GI), hepatology, and infectious diseases (ID) clinics based on the campus and in the community of Durham, North Carolina, a county with a population of approximately 295,000.30 In the current study, participants reported traveling an average of 72 minutes one-way to reach the clinic. Another system is the University of North Carolina (UNC) Medical Center – Chapel Hill, which includes academically affiliated public liver clinics based on the campus and an off-site clinic located in Chapel Hill, a city nested in Orange County with a population of roughly 140,000.31 UNC study participants seen at the UNC Medical Center, a state public hospital that receives patients with liver issues from across North Carolina, reported taking on average 92 minutes one-way to access the hospital. The third system is the Durham VA Medical Center where VA study participants reported commuting on average 121 minutes one-way. Several subclinics within the Durham VA Medical Center are participating in the study, including the GI, liver, ID-GI, and HCV treatment management clinics. Despite the diversity among these clinics, we refer to them collectively as “liver clinics” for simplicity.
This study was approved by each institution’s local IRB: the Duke Medical Center Institutional Review Board, the UNC Medical Center Institutional Review Board, and the Durham VA Institutional Review Board. It was registered in ClinicalTrials.gov on June 26, 2014 ().
2.4. Enhanced treatment as usual (TAU) delivered in both conditions
Figure 1 depicts the study’s flow and treatment arms. The integrated approach begins with universal alcohol screening using the self-administered Alcohol Use Disorders Identification Test (AUDIT)32 in the liver clinic setting, followed by the medical provider and patient reviewing the patient’s AUDIT answers. For men with scores 8 and higher and for women with scores 4 and higher, as recommended in the National Institute of Alcohol Abuse and Alcoholism clinician’s guide,33 the medical providers have been trained in and are encouraged to conduct Screening, Brief Intervention and Referral to Treatment (SBIRT).34,33 SBIRT is a 5–10 minute brief intervention with patients about their thoughts on their alcohol use following the principles of Motivational Interviewing, which is “a person-centered, goal-oriented method of communication for eliciting and strengthening intrinsic motivation for positive change” and has a strong evidence base.35,36 This conversation uses the FRAMES steps: providing Feedback on how the patient’s alcohol use may affect their current and future health; noting that it is the patient’s Responsibility to change behavior; giving Advice to stop drinking based on medical concern; giving a Menu of options for cutting down on drinking; expressing Empathy; and reinforcing the patient’s Self-efficacy to change.37,38 Medical providers are encouraged to conduct SBIRT during every HCV clinic visit for patients indicating current alcohol use.
Figure 1. Study flow and treatment arms.
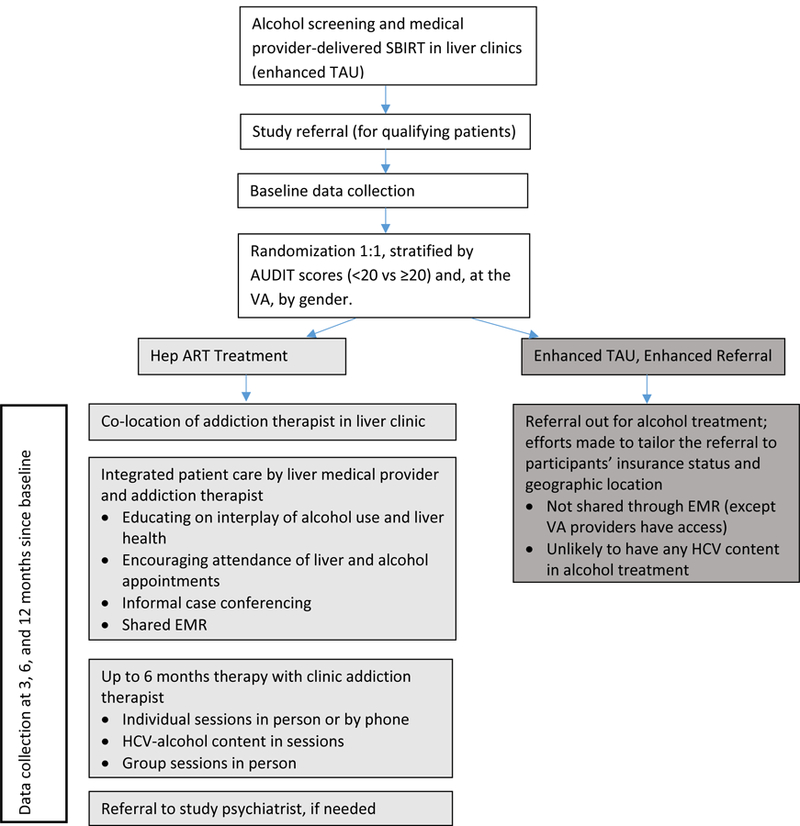
Notes:
SBIRT = screening, brief intervention and referral to treatment
AUDIT = Alcohol Use Disorders Identification Test (Alcohol screener)
EMR = electronic medical record
Despite SBIRT effectiveness,39,40 studies have found its diffusion problematic among physicians.41,42 In particular, implementation of SBIRT delivery by primary care physicians, including brief advice, has been difficult to achieve even with financial incentives.43 These difficulties underline the need for formalized SBIRT implementation programs that incorporate the use of evidence-based strategies.
We train all participating liver providers on SBIRT methods during in-person trainings led by SBIRT experts and involving videos and role plays. Prior to these trainings, this study’s medical providers reported being generally unfamiliar with SBIRT. To assist medical providers in remembering the steps, we provide a checklist and ask them to indicate for each patient which FRAMES steps they do and do not complete, which also provides us with process data about the variability in delivery of SBIRT techniques. Medical providers conclude alcohol counseling sessions by highlighting the importance of alcohol abstinence in the presence of current HCV infection and/or following HCV cure if the patient has liver fibrosis or cirrhosis.
All study liver clinics enhanced their treatment as usual for HCV patients to include both alcohol screening using the AUDIT and medical-provider conducted brief alcohol counseling using the structured SBIRT process. This enhancement was new for the clinics, and we debated whether or not to include SBIRT for the referral out arm of the study and ultimately decided to include it because the 2011–2013 US Department of Health and Human Services Viral Hepatitis Action Plan indicated in strategy 2.3.1 that several federal agencies would disseminate and train on brief alcohol interventions for hepatitis providers,44 and even if these brief alcohol interventions were not standard of care yet, they soon could be. It should therefore be noted that study patients in both treatment arms receive an enhanced standard of care.
Participants randomly assigned to the enhanced TAU only group are referred for alcohol treatment. They are asked their insurance status, geographic location, and transportation resources. They are then given alcohol treatment referral options, tailored as well as possible to their individual needs. Example referral sites include university-affiliated outpatient programs in which licensed addiction specialists offer individual or group therapy, with length and frequency of treatment tailored to the patient. Occasionally short-term residential detox or longer residential addiction treatment is recommended. Participants have also been referred to Alcoholics Anonymous or Narcotics Anonymous alone or in conjunction with other referral options. This degree of care during referral-making is beyond the scope of what liver clinic medical providers normally give, because they do not have clinic social workers to help them, so this careful referral process can be considered an additional enhancement to TAU. Further, participation in data collection with detailed assessment of recent alcohol use and questions about drinking motivations may be considered a minor intervention.
2.5. Hep ART treatment group intervention
The Hep ART treatment group receives integrated HCV-alcohol treatment. A key aspect of this integrated care is co-locating addiction therapists in liver clinics. In addition to the convenience and trust that the liver clinic location affords patients, the co-location allows the addiction therapist and medical provider to communicate regularly through on-site contact. They jointly develop treatment plans and share notes via the electronic medical record (EMR).
During the early phases of this study, we determined the clinical information medical and addiction providers would need to be able to integrate their patient care. We then ensured that this information was communicated through the EMR and often verbally, as well. In general, medical providers wanted to know if treatment participants are engaged in alcohol therapy and if they are experiencing something that could affect their medical care (e.g., homelessness, desiring medical detoxification). They were also interested in knowing whether patients are stable enough to adhere to HCV antiviral treatment. Conversely, the addiction therapists wanted to know the patient’s disease status, including changes in laboratory tests and virus levels, and upcoming medical appointments. Prior to an upcoming medical appointment, the addiction therapists often alert the patient’s medical provider as to whether or not the patient is engaging in alcohol therapy, so the medical provider can praise the patient or encourage attendance, accordingly.
To further integrate care, the Hep ART treatment arm used a 24-session manual-based treatment that includes content on both HCV and alcohol use. The addiction therapist offers up to 12 individual sessions and 24 weekly group therapy sessions, spaced as recommended by the addiction therapist and agreeable to the patient. The therapy is conducted using principles of Cognitive Behavioral Therapy,45 Motivational Enhancement Therapy,46 and substance abuse treatment, evidence-based approaches that can be applied to both alcohol and substance use. The session content integrates HCV and alcohol issues in alcohol treatment, liver health, and personal realms. The backbone of the manual came from our prior pilot feasibility study26 and was expanded by two clinical psychologists and two social workers. The manual includes didactic content and questions to guide participant discussion, and homework for skill-building, activity replacement, alternate coping strategies, journaling, and craving management. All participants begin with 3 core sessions, which provide a road map of addiction depicting: knowledge about HCV, alcohol, and liver health; motivation to decrease alcohol use; multiple, detailed skills for decreasing alcohol use; alcohol reduction behaviors and meaningful activities; and improved health and well-being outcomes. The addiction therapists strive to help participants visualize the many contributors to their individual alcohol use and cognitive and behavioral strategies to temper alcohol use. After these core modules, sessions are jointly chosen by the participant and addiction therapist to be most relevant to the participant; there are more session modules available to draw on than any single participant will receive. A full list of sessions is included in the Appendix. The information and skills are imparted in the context of having HCV, which may increase and maintain motivation for alcohol reduction.
Participants are encouraged to engage in in-person group therapy. We offer individual therapy by phone but generally recommend that the first session be conducted in person, so the addiction therapist can build rapport and better assess the participant’s demeanor and affect. Group therapy and in-person individual therapy are held for the Duke site in the liver clinic, for the UNC site within a short walk of the liver clinic, and for the VA site outside of the clinic but in the same building. Therapy emphasizes alcohol reduction, but substance use, when it is a comorbid condition, is also addressed.
Psychiatric treatment is included for participants who request a consult or based on the opinion of the addiction therapist. Participants with mental health issues beyond what their liver medical provider and addiction therapist can treat are referred to a study psychiatrist who provides services in the liver clinic setting and charts in the shared EMR. We include psychiatric treatment to minimize the barriers that mental health issues pose to participation in alcohol treatment; decreasing these obstacles allows us to provide a patient safety net as should occur in practice. For participants who need it, psychiatrists conduct a comprehensive psychiatric evaluation. Treatment recommendations are tailored to the individual participant and may consist of prescribing anxiety, depression, or alcohol relapse-prevention medications as appropriate. The participant may or may not be followed by the study psychiatrist for the remaining time in the 6-month treatment, depending on treatment recommendations and participant preference.
Thus, the Hep ART intervention condition includes the components of individual therapy, group therapy, and possibly psychiatric treatment. The maximum number of sessions for individual plus group therapy combined could not exceed 36. Psychiatric treatment had to occur within the six-month intervention period. Despite this maximum number of sessions, we did not set a minimum number, favoring instead to allow the addiction therapist to individualize the number of sessions necessary to motivate the participant to engage to the extent judged clinically beneficial. This pragmatic approach will allow the findings to reflect an intervention dosage consistent with real-world clinical practice in which liver clinics do not incentivize alcohol treatment attendance.
2.6. Treatment fidelity/therapist adherence to the treatment manual
The 24 module-based treatment manual provides detailed subsections that the addiction therapist is expected to deliver during each session, including didactic content, skill-building exercises, and discussion questions to facilitate helpful dialog. Having a therapist manual that outlines the content of each module promotes therapist fidelity and adherence to the treatment manual to ensure patients are getting similar treatment. To ensure therapist fidelity and adherence, all group and individual therapy sessions are audio-recorded. A subset of sessions from each study therapist is listened to by a study investigator (a clinical psychologist with expertise in developing and delivering protocol-driven psychosocial treatments). Audio-recorded sessions are reviewed and a protocol fidelity checklist is completed to indicate the degree of concordance with subsections of each module. The study investigator meets with each therapist to provide feedback on fidelity to the written module.
2.7. Participant eligibility and recruitment procedures
Current patients attending appointments with liver care providers at the three study sites are eligible. For HCV status, our initial eligibility criteria included currently having confirmed chronic HCV. However, after we initiated the study, HCV treatment changed dramatically, achieving extremely high rates of sustained virologic response (SVR) with less toxicity. The majority of HCV patients were being referred to direct-acting antiviral agent (DAA) therapy, with many of them starting DAA therapy. We sought to capture patients before, during, and after DAA therapy; patients who are post-SVR may have liver damage that can be exacerbated by alcohol use. We therefore expanded our eligibility criteria to include patients who previously had confirmed chronic HCV, irrespective of HCV treatment status (before, during or after DAA therapy). In addition, eligible patients must have qualifying AUDIT scores of ≥4 for women and ≥8 for men. Eligible patients must also have had at least one alcoholic drink in the past 60 days. Additional eligibility criteria included being age 18 years or older; understanding and speaking English; not currently being engaged in effective substance abuse treatment; willing to be involved in the study for 12 months; and having adequate transportation to attend at least one individual therapy session. Exclusion criteria are intentionally few; only patients with active psychosis are ineligible.
Medical providers in the liver clinics initiate recruitment. They know which patients have current or prior HCV and they receive the patient’s self-administered AUDIT screener and conduct the brief SBIRT counseling. They examine the AUDIT for qualifying scores. For qualifying patients, the medical provider introduces the study. If the patient expresses interest, the medical provider introduces the patient to a study staff member, or, if unavailable, notifies the study team of an interested and eligible patient. At two sites, study staff engages the patient in the consent process. If the patient has questions regarding the alcohol therapy component, the on-site addiction therapist is brought in to answer those questions. Following consent, study staff conduct a baseline interview and urine. At times, these activities require scheduling an additional research appointment to complete the baseline research assessment. The third site uses a two-staged consent approach in which patients complete the first phase of the consent process to consent to be screened for eligibility. Once that patient consents to be screened for eligibility, the process is the same as described for the other sites. At all sites, study staff are required to administer a breathalyzer test before proceeding with consenting and all research assessment interviews; blood alcohol levels must fall below 0.08 to proceed.
2.8. Randomization and blinding
After enrollment and baseline data collection, each participant is randomly assigned to the Hep ART treatment or to enhanced TAU on a 1:1 basis. The randomization by site stratifies participants based on their AUDIT score to ensure equal allocation of participants scoring a 20 or higher, which indicates likely alcohol dependence.47 In addition, the randomization stratifies patients based on their gender at the VA site. Random assignment codes were generated through statistical software then uploaded into an electronic data capture system called REDCap for the research interviewer.
It is not possible to blind participants, medical providers, or research interviewers to participants’ study assignment for obvious reasons.
2.9. Baseline and follow-up assessment
Data from both the Hep ART and enhanced TAU participants are collected at baseline and 3, 6, and 12 months. Because the Hep ART intervention lasts up to 6 months, the 12-month assessment allows us to assess alcohol use and relapse after the intervention has ended. Assessment at each time point includes a urine sample for toxicology testing and a participant interview. The assessments are conducted by study interview staff who have received extensive training on specific study measures, such as the Timeline Followback, described below.
2.10. Outcome measures
Table 1 depicts all outcome measures, by study aim and time point.
Table 1.
Measures by study aim and time point
| Measure | Aim | Baseline | 3-mo | 6-mo | 12-mo |
|---|---|---|---|---|---|
| Alcohol Use Disorders Identification Test (AUDIT)32 | X | ||||
| Urine toxicology screen (alcohol, 10-drug panel) | 2 | X | X | X | X |
| Timeline Followback (for alcohol)48 | 1 & 2 | X | X | X | X |
| Addiction Severity Index-Lite (based on ASI-Lite alcohol and drug subscale)49 | 2 | X | X | X | X |
| Positive and Negative Affect Schedule (I-PANAS Short Form)*50 | X | X | X | X | |
| Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES 8A)*51 | MofC | X | X | X | X |
| Alcohol Abstinence Self-Efficacy (AASE)*52 | MofC | X | X | X | X |
| Mental Health Continuum Short Form (MHC – SF)53 | X | X | X | X | |
| Patient Health Questionnaire-8 (PHQ-8) depression scale*54 | X | X | X | X | |
| Generalized Anxiety Disorder-7 (GAD-7)*55 | X | X | X | X | |
| EQ-5D (generic health status, provides info to calculate quality-adjusted life years)*56 | 3 | X | X | X | X |
| Alcohol Use Disorders - AUD criteria† | X | ||||
| Medical visits, hospitalizations, medical costs and health insurance coverage† | 3 | X | X | X | X |
| Utilization of mental and physical health and addiction services† | 3 | X | X | X | X |
| Work Productivity and Activity Impairment Measure57 | 3 | X | X | X | X |
| Legal Consequences (based on ASI-Lite)49 | 3 | X | X | X | X |
| Drinking Motives Questionnaire (DMQ)*58 | MofC | X | X | X | X |
| Attitudes and Beliefs about Alcohol Use† | MofC | X | X | X | X |
| Knowledge of HCV and Alcohol59 | MofC | X | X | X | X |
| Alcohol skills*† | MofC | X | X | X | X |
| Access to HCV testing and liver care† | X | ||||
| Brief Pain Inventory*60 | X | X | X | X | |
| Demographic items: | |||||
| Gender, marital status, sexual orientation, education, HIV status, HCV year of diagnosis | X | ||||
| Employment status, income, financial situation, health insurance, transportation, housing | X | X | X | X | |
Notes:
Study Aim 1 is alcohol primary outcomes; Aim 2 is secondary outcomes; Aim 3 is CEA.
MofC = Mechanisms of change, which will be tested in addition to the aims.
Baseline column includes questions that were asked in eligibility screening and at baseline.
The Drinking Motives Questionnaire was only asked post-baseline if alcohol use was reported on the Timeline Followback at that time point.
Self-admistered option
Items developed internally
2.10.1. Primary outcomes
Our primary outcomes are assessed using the Timeline Followback, which is a reliable and validated interview measure for alcohol use outcomes. Participants are asked to recall the type and amount of alcohol consumed per day for the past 90 or 180 days, assisted by identifying special dates on calendars and creating patterns of drinking.48 Self-reported alcohol use data based on Timeline Followback methods have been shown to be more complete across time and to indicate higher amounts of alcohol use than data from blood tests and from reports from friends and family members.61,62
Our primary outcomes are: a) alcohol abstinence, defined as no alcohol consumed in the past 30 days at 6 months and b) decreased alcohol relapse, defined as the number of heavy drinking days (i.e., 5 or more drinks in one day for men and 4 or more for women) occurring in the 6- to 12-month period after enrollment.63 The primary analyses will be conducted on self-reported data.
2.10.2. Secondary outcomes
We have two sets of secondary outcomes. The first set is of alcohol outcomes and the second set is of substance use outcomes. Using the Timeline Followback, three of the secondary outcomes are measured and will be used to compare differences between the treatment groups on: 1) the change in the average grams of alcohol consumed per week at 6 months; 2) the change in the average grams of alcohol consumed per week at 12 months; 3) the number of heavy drinking days in the 0 to 6-month period after enrollment; and 4) the number of alcohol-abstinent days at 12 months.
The substance use outcomes, which are exploratory only, are: 1–2) toxicology screens positive for illicit drug use at 6 months and separately at 12 months; 3–4) the change in the percentage of days with any illicit substance use (self-reported using items adapted from the Addiction Severity Index-5 (ASI)49) at 6 months and separately at 12 months; and 5–6) the change in the percentage of days with any illicit substance use excluding marijuana (self-reported on the ASI) at 6 months and separately at 12 months.
As partial validation of patient reported data, we will compare self-reported alcohol use (i.e., using the Timeline Followback measure) with the urinalysis, which provides an objective assessment of alcohol consumption over the prior 80 hours.
2.10.3. Cost effectiveness analysis
The primary effectiveness outcome for the cost effectiveness analysis is change in past 30- and 90-day grams of alcohol consumed. Quality of life, as measured using the EQ-5D,56 will also be evaluated in a secondary analysis. Our economic outcomes include potential expenditures and savings to society: 1) primary and specialty care medical visits, hospitalizations, and emergency department usage64; 2) medical expenditures via direct measurement of healthcare utilization and applying Centers for Medicare & Medicaid Services reimbursement rates; 3) legal consequences of alcohol and substance use as measured by items adapted from the ASI-Lite; 4) transportation costs; and 5) work absenteeism for both the participant and anyone driving the participant to alcohol treatment appointments, using the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0.57
2.10.4. Mechanisms of change
In addition to collecting data to evaluate the three specific aims, we also collect data to understand the mechanisms of change in alcohol use behaviors. Specifically, we base our conceptualization on the Integrated Model, a theoretical model which combines the Theory of Reasoned Action, the Theory of Planned Behavior, and other major behavioral theories.65,66 This integrated theoretical model of an individual’s behavior change (not to be confused with integrating clinical care across providers) postulates that behavior change is caused most proximally by the intention to perform the behavior. Influencing intention is a key aspect of many health behavior change theories. Specifically for this study, we measure behavior change as change in alcohol use, measured by the Timeline Followback, and we measure intention to change using the SOCRATES stages of change measure,51 which measures Recognition, Ambivalence, and Taking Steps.
Figure 2 depicts the components of the Integrated Model that the Hep ART treatment attempts to impact. The Integrated Model postulates that intention to change is influenced by attitudes and personal agency (as well as perceived norms, which we do not attempt to change in Hep ART).65 Attitudes are how favorable or unfavorable a behavior is to a person. One’s attitude toward a behavior is influenced by their feelings about the behavior (e.g., drinking is enjoyable or necessary for me to cope), as well as one’s thoughts about whether the behavior will lead to good outcomes (e.g., decreasing alcohol use will be very beneficial for me since I have HCV). We hope the Hep ART treatment, through individual and group therapy and messages from medical providers, will make participants less favorable to alcohol use. To measure this, in the participant interview, we include the HCV-Alcohol Knowledge Scale,59 as well as participant attitudes and beliefs about alcohol use.
Figure 2. Hypothesized mechanisms of change for the Hep ART treatment, based on the Integrated Model.
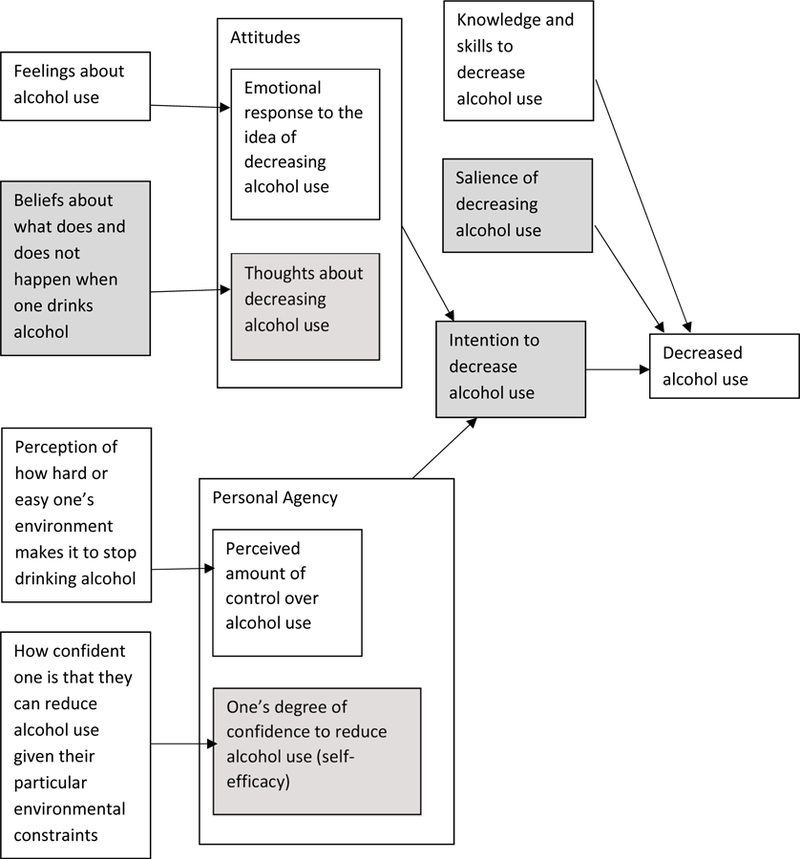
Notes: The Integrated Theoretical Model of Behavior Change65 contains other components (e.g., social norms) that are not included below. This figure depicts the pathways hypothesized for Hep ART.
The addiction therapists facilitate change in all areas below. Shaded boxes indicate where medical providers also facilitate change.
The Integrated Model also proposes that intention to change is influenced by personal agency, which is using one’s influence within one’s environment.65 Personal agency is affected by 1) perceived control, which depends on one’s perception of how hard or easy one’s environment makes it to stop drinking alcohol; and 2) self-efficacy, which is influenced by how confident one is that they can reduce alcohol use given their particular environmental constraints. In this context, the Hep ART intervention seeks to have participants feel increased perceived control in the following areas: during times of negative affect; during times when social situations make alcohol use tempting; and during times of experiencing urges and withdrawal symptoms. It also seeks to have participants gain confidence that they can reduce their alcohol use, even given their environmental realities. To measure perceived control, we include in the participant interview the Drinking Motives measure that assesses drinking to be social, to forget about problems, to feel better, and to fit in,58 and to measure self-efficacy we include the Alcohol Abstinence Self-Efficacy scale.52
Because the Integrated Model additionally indicates that skills and knowledge can lead to behavior change, the Hep ART treatment attempts to increase knowledge about how to reduce alcohol use through individual and group therapy (e.g., learning what triggers for alcohol use are), and also works to teach skills (e.g., what to do to avoid a trigger or when a trigger is inevitable). The participant interview includes items on the particular skills taught in Hep ART. To reflect the importance of the salience of behavior change in the Integrated Model, the Hep ART treatment has medical providers and addiction therapists repeatedly remind patients with current or prior HCV infection that decreasing and abstaining from alcohol use can make an important difference in liver health.
2.11. Statistical analysis
Tests of primary outcomes will be conducted based on intent-to-treat (ITT) principles. Statistical tests will be conducted at the two-sided significance level, α= 0.05. We will examine demographic and patient characteristics for baseline comparability between treatment groups; proportions (categorical variables) and means (continuous variables) with associated 95% confidence intervals will be provided for each variable within each of the Hep ART treatment and enhanced TAU groups. If significant differences are observed, we will explore the impact of relevant factors by including them in tests of hypothesis as covariates. Appropriate statistical methods (e.g., assessing type of missingness and imputation methods) will be applied to address missing data as needed. We will descriptively examine whether outcomes vary across the three participating sites.
In addition to the ITT approach used to test the primary outcomes, we will conduct a per-protocol analysis, in which we will examine outcomes for Hep ART treatment condition participants who attend at least four sessions. We chose four sessions based on our primary outcome of abstinence and Project MATCH’s Motivational Enhancement Therapy condition, which involved four sessions and found that 23% of participants were alcohol-abstinent for three months, when assessed nine months post-intervention67. We will additionally examine outcomes by number of time periods with a minimum clinical dose (e.g., each two-week period with at least one session).
2.11.1. Planned statistical analyses
Baseline demographic and patient characteristics, including HIV status, race, gender, income level, length of time since HCV diagnosis, liver health status, and alcohol dependence status will be compared as described above.
Aim 1, primary outcomes: To compare the Hep ART treatment group to the enhanced TAU group, two primary outcomes will be considered: 1) the alcohol abstinence rate for the 30 days comprising their sixth month since enrollment and 2) relapse rate defined as the number of heavy drinking days during the time period of 6–12 months since enrollment. We will obtain strata-adjusted point estimates and the corresponding 95% confidence intervals for the difference between treatment groups in the proportions of abstinent patients and the mean difference in the number of heavy drinking days. The stratified t-test or appropriate non-parametric equivalent will be used to determine if differences are statistically significant. If significant differences are found in baseline demographic and patient characteristics, we will also include relevant measures in the model as potential confounding risk factors.
Aim 2, secondary outcomes: The Hep ART treatment group will be compared to the enhanced TAU group on the following 30-day alcohol outcomes, at their sixth month since enrollment: 1) grams of alcohol consumed per week, 2) number of heavy drinking days, and 3) number of abstinent days and substance abuse outcomes: 1) percentage of days with illicit drug use and 2) positive drug screens. Weekly alcohol consumption will be analyzed using repeated measures analysis of variance with multiple comparisons to determine where differences are observed.68 For the number of heavy drinking days and abstinent days, we will report point estimates for the mean differences and the corresponding 95% confidence intervals. The t-test or appropriate non-parametric equivalent will be used to determine if differences are statistically significant.
Aim 3: To conduct a cost effectiveness analysis (CEA) of the Hep ART intervention compared to enhanced TAU, our primary economic endpoint will be the incremental cost-effectiveness ratio (ICER) (CIntervention – CControl) / (EIntervention – EControl), where C represents costs and E represents effectiveness. The ICER determines the value of an intervention by dividing its additional cost by its additional benefit relative to the control intervention. The standard effectiveness measure is quality-adjusted life years saved (QALYs) to reflect length of life and disease morbidity. In general, interventions with ICERs falling below $50,000 to $100,000 per QALY gained are considered to be “cost-effective.”69
One of the strengths of this study is that we are being comprehensive in the costs we are capturing and the concurrent plan. Capturing all those costs requires multiple data sources and a detailed analysis plan. The CEA will occur in two steps: (1) building the economic model and (2) evaluating the cost-effectiveness. Our economic model will be a base-case economic analysis conducted from the societal perspective. We measure: intervention cost (induced costs and savings), health care utilization (changes due to the intervention), and patient costs (travel, productivity loss, and work absenteeism). We will use the Consumer Price Index to standardize costs observed across study years. We also measure quality of life data in the repeated patient interviews using the Euro-QoL (EQ-5D) as our primary effectiveness measure.56,70
Intervention Cost.
The cost of the intervention and comparison treatments involves mostly labor inputs, so we will use micro-costing to estimate intervention cost per patient.71 We will time and keep logs for: 1) training of medical providers and addiction specialists to conduct the treatments; 2) weekly group and bi-weekly intervention therapy sessions; and 3) retention specialist activities (e.g., mailing reminders, searching for participants). These times will be aggregated and multiplied by their respective wage and fringe benefit rates to derive total professional costs for each study arm. Because timing and keeping logs is too burdensome for health providers, we will ask providers to estimate the percentage of a typical visit devoted to alcohol counseling and apply that percentage to Medicare’s reimbursement rate for a clinic visit as the cost for alcohol counseling. This unit cost for alcohol counseling will be applied to all initial and follow-up physician visits for each study arm. Lastly, psychiatric visits will be multiplied by the appropriate Medicare reimbursement rate.
Health Care Utilization.
Participants who become alcohol or drug abstinent may have fewer primary care or emergency department (ED) visits or hospitalizations than those who do not. During the follow-up research interviews, we ask participants to report on their primary care, ED, and hospitalization visits and provide a brief reason for each visit.72 When possible, we will confirm these data with medical records from our three sites. Because costs differ across the three study sites, particularly at the VA, we will standardize unit costs by using Medicare reimbursement rates and appropriate procedural (CPT) codes for the primary care and ED visits and diagnosis (DRG) codes for hospitalizations.
Patient Costs.
During the follow-up interviews, we ask participants to report time and distance required for travel to attend the alcohol counseling, group and individual therapy sessions, and any psychiatric visits. We inquire if they or a family member had to miss work in order to attend these counseling and therapy sessions. For example, the IRS reimbursement rate for medical travel will be used to monetize distance traveled. The US average hourly labor wage rate will be used for productivity loss.
Cost-Effectiveness Analysis.
To estimate the long-term cost-effectiveness, extending clinical trial alcohol outcomes and their impact on the risk of liver fibrosis and advanced liver disease11,14,15,73–75 beyond the time horizon of the study typically involves decision analysis, a prescriptive, normative approach to decision-making in the face of uncertainty. Markov or simulation models track the proportion of the cohort that is alive each year until all cohort members have died. By crediting the cohort for the proportion alive each year and for their annual medical care expenditure or other costs, the simulation estimates the average life expectancy and lifetime expenditures for each strategy. Economic model development will build on an existing natural history HCV model.7
Because costs are discounted, life expectancy will also be discounted in accordance with cost-effectiveness recommendations using the 3% annual discount rate.76 The analysis will provide deterministic estimates of the cost-effectiveness of interventions, and because uncertainty surrounds all data estimates, extensive sensitivity analyses will be performed, varying each variable or groups of variables over their plausible ranges. From these models, we will calculate our primary endpoint, the ICER using EQ5D, as described above. We will also calculate the cost-effectiveness ratio using alternative measures (e.g., alcohol abstinence).
Exploratory analyses: Participants will vary in terms of HCV treatment status (before, during and after DAA therapy). During the first six months of the study, some will be naïve to treatment and others will be at various stages of initiating or ending HCV antiviral treatment, or being assessed for SVR. We will explore potential differences between HCV treatment status groups in baseline characteristics and also in study outcomes.
2.11.2. Sample size
We initially sought to enroll 230 participants in this trial. We report here the statistical power available with the final recruited sample of 182 randomized patients based on effect size assumptions from our R21 pilot study for the primary outcomes: 1) the proportion of participants at 6 months follow-up with no alcohol consumed in the past 30 days; and 2) the number of heavy drinking days during the time period of 6–12 months since enrollment.
In the pilot study, the six-month abstinence rate was 39% for baseline dependent drinkers and 57% for risky/harmful drinkers. For dependent drinkers, we assumed this same 39% abstinence rate for the Hep ART treatment group and 10% for the enhanced TAU group. For risky/harmful drinkers, we assumed a 57% abstinence rate for the Hep ART treatment group and 25% for the enhanced TAU group. Power estimation is based on the Cochran-Mantel-Haenszel test stratified by AUDIT score. Drawing on our baseline data (n=182), under the observed numbers of patients in each AUDIT strata (83% risky/harmful) and the efficacy assumptions described above, greater than 90% power is achieved to detect the hypothesized difference (2-sided α=0.05). The strata-adjusted difference in proportions between treatment groups and corresponding 95% confidence intervals will be computed; differences in proportions abstinent and 95% confidence intervals will also be estimated within each AUDIT stratum.
For the power analysis of our primary outcome of number of heavy drinking days, we considered other sources to hypothesize the baseline number of heavy drinking days (“binge drinking”) among participants in the Hep ART treatment group and the enhanced TAU group and the expected differences between 6 and 12 months. One in six adults in the United States drinks heavily four times per month77. Since at baseline the number of heavy drinking days will be measured in the prior three months, we will average the numbers of drinking days in the 6–12 months post intervention in two three-month segments. Based on an assumption that all participants will be among the 17% that do binge, we hypothesize the number of heavy drinking days at baseline to be 12 over 3 months for participants assigned to either group. We also identified the Project TrEAT study that tested an intervention of two, 15-minute physician interventions followed by two nurse phone calls.78 In this trial, 25% and 46% decreases in the number of heavy drinking days between baseline and 6 months were observed in the control and intervention arms, respectively. Because we expect our enhanced TAU intervention to have a greater impact than the intervention in the TrEAT study, we assume a slightly higher decrease in the enhanced TAU group (29%). We also sought to detect a slightly lower yet clinically meaningful decrease in the Hep ART treatment group (42%). Based on these assumptions we expect to see differences of 3.5 and 5 days, respectively, in the mean numbers of heavy drinking days between baseline (measured over prior three months) and 6 months (analyzed as the average of the 6–9 and 9–12 month periods). These differences correspond to reductions from 12 to 8.5 heavy drinking days in the enhanced TAU group and from 12 to 7 heavy drinking days in the Hep ART treatment group. No preliminary data are available on the standard deviations. We therefore assume a coefficient of variation of 0.7 for both differences, resulting in standard deviations of 2.5 (TAU) and 3.5 (Hep ART), respectively. Based on these assumptions, with 182 patients studied, approximately 91% power is achieved (2-sided α=0.05).
3. Results
3.1. Recruitment
Recruitment in the Duke liver clinics occurred from October 2014 through September 2017, and in the UNC liver clinics from October 2015 through August 2017. Recruitment in the Durham VA liver clinics occurred from August 2016 through September 2017. We launched recruitment in the Duke liver clinics first, because they had been involved in the pilot study and any recruitment lessons learned from them in 2014 could be applied to the other two sites. It took longer than expected to establish all systems and approvals at each site. Specifically, IRB approvals and data sharing agreements and procedures required concerted effort for many months, partly because data capabilities and security requirements vary among sites and have undergone multiple shifts during these years. Informed consent was obtained from all participants from each local IRB.
3.2. Baseline characteristics
Table 2 depicts the demographic characteristics of study participants by site. Of the 182 participants enrolled across sites, 29.1% are female, 60.4% identify as single-race African-American or Black and 31.9% as single-race White, and 30.9% are married or living with a significant other. Monthly income is low, with a mean income of $1,572 (SD=$2,137) and 22.0% earning less than $500/month. 12.6% are uninsured and 5.5% are homeless. Based on interview questions that approximate Alcohol Use Disorder diagnostic criteria; 22.0% met moderate and 68.7% met severe Alcohol Use Disorder criteria.
Table 2.
Description of Hep ART participants
| Total (N=182) | Duke (N=110) | UNC (N=53) | VA (N=19) | |
|---|---|---|---|---|
| %(n) or M(SD) | %(n) or M(SD) | %(n) or M(SD) | %(n) or M(SD) | |
| Gender | ||||
| Female | 29.1% (53) | 29.1% (32) | 35.8% (19) | 10.5% (2) |
| Male | 70.9% (129) | 70.9% (78) | 64.2% (34) | 89.5% (17) |
| Race | ||||
| Black | 60.4% (110) | 65.5% (72) | 43.4% (23) | 78.9% (15) |
| White | 31.9% (58) | 25.5% (28) | 50.9% (27) | 15.8% (3) |
| Bi/multi-racial | 4.9% (9) | 5.5% (6) | 3.8% (2) | 5.3% (1) |
| Other single race | 2.2% (4) | 2.7% (3) | 1.9% (1) | 0.0% (0) |
| Native American | 0.5% (1) | 0.9% (1) | 0.0% (0) | 0.0% (0) |
| Hispanic | 3.8% (7) | 4.5% (5) | 3.8% (2) | 0.0% (0) |
| Married/cohabitating | 30.9% (54/175) | 32.0% (33/103) | 28.3% (15) | 31.6% (6) |
| Education | ||||
| High school or below | 58.8% (107) | 58.2% (64) | 64.2% (34) | 47.4% (9) |
| Some college or Associate degree | 31.9% (58) | 33.6% (37) | 26.4% (14) | 36.8% (7) |
| Bachelor degree or above | 9.3% (17) | 8.2% (9) | 9.4% (5) | 15.8% (3) |
| Employment | ||||
| Full time | 28.0% (51) | 29.1% (32) | 32.1% (17) | 10.5% (2) |
| Part time | 11.5% (21) | 12.7% (14) | 11.3% (6) | 5.3% (1) |
| Unemployed | 18.7% (34) | 14.5% (16) | 28.3% (15) | 15.8% (3) |
| Disabled | 34.6% (63) | 35.5% (39) | 24.5% (13) | 57.9% (11) |
| Other | 7.1% (13) | 8.2% (9) | 3.8% (2) | 10.5% (2) |
| Individual monthly income in dollars | 1,572 (2,137) | 1,663 (2,517) | 1,394 (1,474) | 1,543 (1,119) |
| Health insurance | ||||
| Private | 31.3% (57) | 37.3% (41) | 30.2% (16) | 0.0% (0) |
| Government: Medicare, Medicaid etc. | 44.0% (80) | 50.9% (56) | 32.1% (17) | 36.8% (7) |
| Other public: VA etc. | 9.9% (18) | 0.9% (1) | 9.4% (5) | 63.2% (12) |
| Uninsured | 13.2% (24) | 8.2% (9) | 28.3% (15) | 0.0% (0) |
| Project Access | 1.6% (3) | 2.7% (3) | 0.0% (0) | 0.0% (0) |
| AUDIT ≥ 20 | 17.0% (31) | 16.4% (18) | 13.2% (7) | 31.6% (6) |
| Alcohol Use Disordera | ||||
| None | 0.5% (1) | 0.9% (1) | 0.0% (0) | 0.0% (0) |
| Mild | 8.8% (16) | 10.9% (12) | 5.7% (3) | 5.3% (1) |
| Moderate | 22.0% (40) | 17.3% (19) | 26.4% (14) | 36.8% (7) |
| Severe | 68.7% (125) | 70.9% (78) | 67.9% (36) | 57.9% (11) |
| Housing | ||||
| Own or rent apartment, room or house | 71.4% (130) | 73.6% (81) | 69.8% (37) | 63.2% (12) |
| Someone else’s apartment, room or house | 19.2% (35) | 18.2% (20) | 24.5% (13) | 10.5% (2) |
| Shelter | 3.3% (6) | 3.6% (4) | 3.8% (2) | 0.0% (0) |
| Homeless | 2.2% (4) | 1.8% (2) | 0.0% (0) | 10.5% (2) |
| Other | 3.8% (7) | 2.7% (3) | 1.9% (1) | 15.8% (3) |
| Transport time to and from clinic (minutes) | 83 (70) | 72 (57) | 92 (89) | 121 (63) |
| Years since HCV diagnosis | 9.0 (9.9) | 8.6 (9.2) | 9.5 (11.0) | 10.3 (10.3) |
| HIV status | 6.0% (11) | 7.3% (8) | 5.7% (3) | 0.0% (0) |
| Illegal drug useb; past 30 days | ||||
| Marijuana | 47.5% (86/181) | 45.0% (49/109) | 54.7% (29) | 42.1% (8) |
| Other drugsc | 29.7% (54) | 28.2% (31) | 34.0% (18) | 26.3% (5) |
Note.
Alcohol Use Disorder was not assessed clinically but was based on responses to structured research interview questions.
Illegal drug use was based on self-report.
Other drugs include cocaine, heroin, non-prescription methadone, and opioids and sedatives in amounts greater than prescribed.
4. Discussion
For people with chronic HCV infection, alcohol use increases the likelihood and rate of liver fibrosis, which leads to development of cirrhosis, hepatocellular carcinoma, and ultimately liver-related mortality. Compared with HCV patients who do not drink alcohol, HCV patients with excessive alcohol use have a 2- to 3-fold greater risk of developing cirrhosis.10,12,73,79–81 In one study, 58% in the excessive alcohol group (60 grams/day for men) became cirrhotic by the end of the second decade compared to only 10% in the non-drinking group.73 Patients with HCV infection and chronic alcohol use also have increased risk of hepatocellular carcinoma12–14 and mortality.15,16 Patients who have been cured of HCV but who have a compromised liver and drink alcohol remain at risk for poor liver health, due to HCV-related liver fibrosis and ongoing damage from alcohol use.82 If effective, six months of co-located integrated alcohol and liver health treatment may significantly improve patient health, especially if patients can maintain alcohol abstinence.
The calls for integrated models of care that incorporate treatment for alcohol use, substance use, and mental health comorbidities have asked for patient-centered care, especially for behavioral health integration like that in Hep ART, for patients with chronic liver disease.83 These calls have also emphasized the importance of conducting randomized controlled trials while acknowledging the difficulties of patient recruitment; in the current study, we collaborate with multiple liver clinics to achieve a large enough sample for sufficient statistical power. Few empirically tested models exist. Willenbring has called for integrated care in the HCV clinic setting for HCV patients with psychiatric and substance use disorders, and noted the absence of RCTs in this area.84
This study will add to the scientific knowledge base on the benefit, or lack thereof, of integrated care. At first blush, the concept of integrated care sounds beneficial and like a logical step. However, integrated care is difficult to implement and adds care costs; studies assessing whether it is beneficial, to whom, and to what extent are lacking. The six-month Hep ART treatment condition is innovative in its use of several levels of integrated care, including co-locating addiction therapists in the clinics, having medical and addiction providers integrate care for patients, and incorporating substantial content about HCV in the alcohol treatment manual. The intervention is also pragmatic in that it recognizes the difficulties that many participants have attending weekly group therapy or even the ideal number of individual therapy sessions by phone. We chose not to compensate participants financially for therapy attendance because clinics are unlikely to have these funds in the absence of research funding, thus improving this study’s generalizability to clinical practice. We do offer psychiatric care resources that may be out of reach for some clinics or health care systems. We chose to do this because many patients with HCV and alcohol use have other co-morbidities, including depression and serious mental illness such as schizophrenia. Including psychiatric resources ensures a safety net for participants and may inform us as to whether alcohol use can be reduced when more ideal levels of care (i.e., psychiatric care) are available. We realize that participants in need of psychiatric care may not choose to access it even when it is free, because of barriers such as transportation, time, and stigma.
Decreasing alcohol use requires behavioral intervention; this study will add to the literature by comparing an enhanced standard of care to an integrated behavioral intervention. Comparing less intensive to more intensive alcohol interventions is particularly important given that an evidence base exists for brief alcohol counseling in the form of SBIRT decreasing alcohol use in non-dependent drinkers.85,86 However, once patients have HCV and have been told to abstain from alcohol use, their lack of abstinence may suggest that medical provider-delivered SBIRT is not enough without more intensive addiction counseling.
A particularly important aspect of this study is the cost effectiveness analysis (CEA). Beyond clinical efficacy and safety, the rising costs of health care worldwide has led to greater interest in economic outcomes. Clinical trials are increasingly including an economic analysis as many countries consider the clinical effectiveness of new technologies and their economic value for both regulatory and reimbursement health policy decision-making.87 Thus, the results of the CEA will inform third-party health insurers, governments, delivery systems, healthcare professionals, and patients about the potential economic impact of offering integrated alcohol-HCV treatment in liver clinics. Decreasing alcohol use among persons with HCV should reduce risk of hepatic fibrosis progression, hepatocellular carcinoma, cirrhosis, and liver-related mortality, among other potential health and societal benefits. Through these liver benefits, we hypothesize that the six-month alcohol treatment model will be cost effective. Demonstrating clinical and cost-effectiveness would have important implications for services that should be packaged and reimbursement for the management of HCV and alcohol use disorders.
Prevention of liver cirrhosis will also lead to less burden on the US healthcare system. The proportion of HCV-infected patients with cirrhosis is projected to increase from 25% in 2010 to 45% in 2030, and peak at 1 million persons in 2020, resulting in health complications including hepatic decompensation and liver-related death.8 HCV-related morbidity is also projected to result in direct medical care costs exceeding $1 billion per year.7 Widespread adoption of a proven efficacious alcohol treatment specific to persons with HCV would reduce morbidity and decrease the high medical cost burden.
This study has limitations worth noting. Since recruitment began in 2014, remarkable advances in HCV antiviral treatment have occurred, including the introduction of highly effective treatments with few adverse effects in the form of directly acting antivirals (DAAs). Thus, patients we enrolled early in the study may differ from those enrolled later in terms of their motivation to stop drinking. At one time, alcohol abstinence was considered an eligibility criterion for interferon-based HCV treatment and a way to prolong liver health and attenuate treatment side effects. Although abstinence is now less important for those reasons, it remains important for patients needing DAA authorization by their insurance carriers. An additional concern is that liver clinics are likely not using the SBIRT paradigm or integrated care as TAU, which would hamper broad-based adoption of these strategies in real-world clinical settings outside of research. Regardless, this study’s findings will contribute to our knowledge of whether medical provider-delivered SBIRT versus intensive integrated alcohol treatment leads to decreased alcohol use in HCV-infected patients.
Among this study’s strengths is the use of a pragmatic trial. It is well-documented that alcohol therapy, if attended, reduces alcohol use.88–90 What is difficult, however, is getting people who consume alcohol to attend therapy and maintain motivation for as long as needed to achieve alcohol reduction goals. Therefore, including pragmatic elements in trials in this area is essential. The Hep ART study uses a pragmatic effectiveness-implementation design.27 This design, which includes randomization, will generate findings as to whether there is a signal that the integrated care and alcohol therapy offered Hep ART participants leads to reductions in alcohol use or not. The study design is pragmatic in that it includes broad inclusion criteria; for example, participants can use substances other than alcohol and can have co-morbidities such as HIV. The study is also pragmatic in its use of highly interpretable and clinical outcomes, such as grams of alcohol consumed per week. In addition, the study is implemented in three diverse clinics, which should offer lessons on how to implement integrated care for optimal uptake in diverse settings.
In conclusion, decreasing alcohol use remains challenging, including among patients with a history of HCV. For those patients who do not stop after several warnings from medical providers, evidence-based intensive behavioral treatments are needed. Our approach, if supported by positive trial results, will decrease barriers to alcohol treatment for patients with current or prior HCV infection and make possible one of the best actions HCV-infected patients can take – alcohol reduction – to live a long and healthy life.
Acknowledgments
We are grateful to the many study interventionists who implement and provide feedback on the study. These include addiction therapists Cathryn Mainville and Hayden Dawes; liver clinic medical providers Janet Jezsick, Elizabeth Goacher, Krista Edelman, Gabe Mansouraty, Dawn Piercy, Dawn Harrison, Danielle Cardona, Jama Darling, Steven Choi, Kimberly Pollis, Cynthia Moylan, Nancy Shen, Colleen Boatright, Joyce Davis; addiction psychiatrists J.C. Garbutt and Roy Stein; and Durham VA Social Work Supervisor for Mental Health Larry Rhodes. We also thank our study interviewers, including Courtenay Pierce, Kelly Keefe, Carla Mena, Becca Heine, and Lavanya Vasudevan, our data managers including Donna Safley and Ceci Chamorro, and Malik Muhammed Sohail for literature review. This research was supported by the Duke University Center for AIDS Research (CFAR), an NIH-funded program (5P30 AI064518).
Funding: This work was supported by the National Institutes of Health [grant number R01AA021133-01A1]. The funding source had no involvement in the manuscript.
Abbreviations
- HCV
Hepatitis C
- AUDIT
Alcohol Use Disorders Identification Test
- SBIRT
Screening, Brief Intervention, and Referral to Treatment
- HCV
hepatitis C virus
- TAU
treatment as usual
- SBIRT
Screening, Brief Intervention and Referral to Treatment
- CEA
cost effectiveness analysis
- TLFB
Timeline Followback
- US
United States
- Hep ART
Hepatitis C-Alcohol Reduction Treatment
- AUDIT
Alcohol Use Disorders Identification Test
- VA
Veterans Affairs
- EMR
electronic medical record
- SVR
sustained virologic response
- DAA
direct-acting antiviral agent
Appendix
Group Therapy Sessions
Module 1: Health/ Attitudes /Activities
Module 2: Hep C/ Triggers
Module 3: Effects of Alcohol/ Triggers
Module 4: Effects of Alcohol/ Mindfulness
Module 5: Neurobiology of Addiction
Module 6: Replacing Unhelpful Thoughts
Module 7: Reducing Anxiety
Module 8: Managing Feelings
Module 9: Examining Drinking Attitudes
Module 10: Managing Stigma
Module 11: Thought Stopping/ Mindfulness
Module 12: Pros and Cons/ Refusal Skills
Module 13: Motivation and Values
Module 14: Spirituality/Attending AA
Module 15: Liver Health/ Making Friends
Module 16: Families and Alcohol
Module 17: Communicating with Others
Module 18: Resisting Triggers
Module 19: Overcoming Challenges/ Rewarding Successes
Module 20: Sober When Ill/ 12-Step sayings
Module 21: Communicating with Doctors
Module 22: DBT Dialectical Abstinence
Module 23: DBT Distress Tolerance
Module 24: DBT Clear Mind
Individual Therapy Sessions
Module 1: Assessment
Module 2: HCV and Alcohol/Triggers/Pleasant Activities/Stage of Change
Module 3: HCV Info/ Motivation
Module 4: Pleasant Activities
Module 5: Values and Goals
Module 6: Managing Depression
Module 7: Managing Anxiety
Module 8: Managing Anger
Module 9: Refusal Skills
Module 10: Communicating with Others
Module 11: Alcohol and Families
Module 12: Exploring Spirituality
Module 13: Replacing Unhelpful Thoughts
Module 14: Accepting Emotions/ Mindfulness
Module 15: Making Friends/ Support System
Module 16: Pros and Cons of Drinking
Module 17: Termination/ Celebrating Successes
Module 18: Crisis Intervention/Other
Module 19: Relapse Prevention
Module 20: Managing Stigma
Module 21: Sleep
Module 22: DBT Dialectical Abstinence
Module 23: DBT Distress Tolerance
Module 24: DBT Clear Mind
Footnotes
Conflicts of interest
Dr. Evon receives research grants from Gilead, Inc.
Dr. Wong is an invited Panel Member of the American Association for the Study of Liver Disease and Infectious Diseases Society of America, Recommendations for Testing, Managing, and Treating Hepatitis C.
Dr. Mannelli has received consultation fees from Guidepoint Global and research funding from Orexo and Alkermes Inc., and served on Scientific Advisory Boards for Alkermes.
Dr. Naggie has received research grants from AbbVie, Bristol Meyers Squibb, Gilead, Janssen, Merck and Tacere.
Dr. Muir has received research grants and served on advisory boards for AbbVie, Bristol Myers Squibb, Gilead, Janssen, and Merck.
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology 2005;41(1):88–96. [DOI] [PubMed] [Google Scholar]
- 3.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48(2):418–431. [DOI] [PubMed] [Google Scholar]
- 4.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology 1999;29(4):1311–1316. [DOI] [PubMed] [Google Scholar]
- 5.Sie L, Gatto NM, Bancroft E. Hospitalizations due to hepatitis C in Los Angeles County, 2007–2009: case characteristics and factors associated with mortality. J Viral Hepat 2013;20(9):628–637. [DOI] [PubMed] [Google Scholar]
- 6.Bhamidimarri KR, Satapathy SK, Martin P. Hepatitis C virus and liver transplantation. Gastroenterol Hepatol (NY) 2017;13(4):214–220. [PMC free article] [PubMed] [Google Scholar]
- 7.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health 2000;90(10):1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010;138(2):513–521, 521 e511–516. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144(10):705–714. [DOI] [PubMed] [Google Scholar]
- 10.Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology 1998;27(6):1730–1735. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349(9055):825–832. [DOI] [PubMed] [Google Scholar]
- 12.Noda K, Yoshihara H, Suzuki K, et al. Progression of type C chronic hepatitis to liver cirrhosis and hepatocellular carcinoma--its relationship to alcohol drinking and the age of transfusion. Alcohol Clin Exp Res 1996;20(1 Suppl):95A–100A. [DOI] [PubMed] [Google Scholar]
- 13.Donato F, Tagger A, Chiesa R, et al. Hepatitis B and C virus infection, alcohol drinking, and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC Study. Hepatology 1997;26(3):579–584. [DOI] [PubMed] [Google Scholar]
- 14.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36(5):1206–1213. [DOI] [PubMed] [Google Scholar]
- 15.Pessione F, Ramond MJ, Peters L, et al. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver international : official journal of the International Association for the Study of the Liver 2003;23(1):45–53. [DOI] [PubMed] [Google Scholar]
- 16.Shiomi S, Kuroki T, Minamitani S, et al. Effect of drinking on the outcome of cirrhosis in patients with hepatitis B or C. J Gastroenterol Hepatol 1992;7(3):274–276. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson IM, Lim JK, Fried MW. American Gastroenterological Association Institute Clinical Practice Update-Expert Review: Care of Patients Who Have Achieved a Sustained Virologic Response After Antiviral Therapy for Chronic Hepatitis C Infection. Gastroenterology 2017;152(6):1578–1587. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings 2014; https://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm#7.3.3. Accessed 9 August, 2017.
- 19.Palepu A, Cheng DM, Kim T, et al. Substance abuse treatment and receipt of liver specialty care among persons coinfected with HIV/HCV who have alcohol problems. J Subst Abuse Treat 2006;31(4):411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonner JE, Barritt ASt, Fried MW, Evon DM. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci 2012;57(6):1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein MD, Charuvastra A, Maksad J, Anderson BJ. A randomized trial of a brief alcohol intervention for needle exchangers (BRAINE). Addiction 2002;97(6):691–700. [DOI] [PubMed] [Google Scholar]
- 22.Willenbring ML, Olson DH. A randomized trial of integrated outpatient treatment for medically ill alcoholic men. Arch Intern Med 1999;159(16):1946–1952. [DOI] [PubMed] [Google Scholar]
- 23.Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol 2006;101(10):2254–2262. [DOI] [PubMed] [Google Scholar]
- 24.Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics 2010;51(2):149–156. [DOI] [PubMed] [Google Scholar]
- 25.Dieperink E, Fuller B, Isenhart C, et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: a randomized controlled trial. Addiction 2014;109(11):1869–1877. [DOI] [PubMed] [Google Scholar]
- 26.Proeschold-Bell RJ, Patkar AA, Naggie S, et al. An integrated alcohol abuse and medical treatment model for patients with hepatitis C. Dig Dis Sci 2012;57(4):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly GR, Mamon JA, Scott JE. Utility of the health belief model in examining medication compliance among psychiatric outpatients. Soc Sci Med 1987;25(11):1205–1211. [DOI] [PubMed] [Google Scholar]
- 29.Becker MH. The Health belief model and personal health behavior Thorofare, N.J.: Slack; 1974. [Google Scholar]
- 30.The City of Durham, NC. Demographics 2017; https://durhamnc.gov/386/Demographics. Accessed August 4, 2017.
- 31.Orange County, NC. Population, Demographics, and Population Projections. 2017; Census Bureau 2010 Census. Note: 2014 estimate calculated by North Carolina’s Office of State Budget & Management Available at: http://www.orangecountync.gov/departments/planning_and_inspections/census_demographics.php. Accessed August 4, 2017.
- 32.Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 33.National Institute of Alcohol Abuse and Alcoholism (NIAAA). Helping patients who drink too much: A clinician’s guide 2005; http://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/clinicians_guide.htm. Accessed May 15, 2011.
- 34.Moyer VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med 2013;159(3):210–218. [DOI] [PubMed] [Google Scholar]
- 35.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71(5):843–861. [DOI] [PubMed] [Google Scholar]
- 36.Miller WR, Rollnick S. Motivational Interviewing: Preparing people to change Second edition ed. New York: Guilford Press; 2002. [Google Scholar]
- 37.Governor’s Institute. Screening, Brief Intervention and Referral to Treatment (SBIRT) 2017; http://addictionmedicineupdates.org/screening-brief-intervention-and-referral-to-treatment/. Accessed 21 June, 2017.
- 38.Hester RK, Miller WR. Handbook of alcoholism treatment approaches Second ed. Boston, MA: 1995. [Google Scholar]
- 39.Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med 2005;165(9):986–995. [DOI] [PubMed] [Google Scholar]
- 40.Kaner E, Bland M, Cassidy P, et al. Screening and brief interventions for hazardous and harmful alcohol use in primary care: a cluster randomised controlled trial protocol. BMC Public Health 2009;9:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Beurden I, Anderson P, Akkermans RP, Grol RP, Wensing M, Laurant MG. Involvement of general practitioners in managing alcohol problems: a randomized controlled trial of a tailored improvement programme. Addiction 2012;107(9):1601–1611. [DOI] [PubMed] [Google Scholar]
- 42.Mertens JR, Chi FW, Weisner CM, et al. Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: the ADVISe cluster randomized controlled implementation trial. Addict Sci Clin Pract 2015;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Medicaid and Medicare Services MLS. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services 2014, June.
- 44.US Department of Health and Human Services. Combatting the silent epidemic of viral hepatitis: Action plan for the prevention, care & treatment of viral hepatitis,2011–2013 2011; https://www.hhs.gov/ash/initiatives/hepatitis/actionplan_viralhepatitis2011.pdf. Accessed 21 June, 2017.
- 45.Beck AT, Wright FD, Newman CF. Cocaine abuse. In: Freeman A, Dettilio F, eds. Comprehensive Casebook of Cognitive Therapy New York, NY: Plenum; 1992:1185–1192. [Google Scholar]
- 46.Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior New York: Guilford Press; 1991. [Google Scholar]
- 47.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT, the Alcohol Use Disorders Identification Test: guidelines for use in primary care Geneva, Switzerland: World Health Organization, Dept. of Mental Health and Substance Dependence;2001. [Google Scholar]
- 48.Sobell LC, Sobell MB. Alcohol Timeline Followback (TFLB). In: Association AP, ed. Handbook of Psychiatric Measures Washington, DC: American Psychiatric Association; 2000:477–479. [Google Scholar]
- 49.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 1992;9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 50.Thompson ER. Development and Validation of an Internationally Reliable Short-Form of the Positive and Negative Affect Schedule (PANAS). J Cross Cult Psychol 2007;38(2):227–242. [Google Scholar]
- 51.Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Psychol Addict Behav 1996;10(2):81–89. [Google Scholar]
- 52.DiClemente CC, Carbonari JP, Montgomery RP, Hughes SO. The Alcohol Abstinence Self-Efficacy scale. J Stud Alcohol 1994;55(2):141–148. [DOI] [PubMed] [Google Scholar]
- 53.Keyes CLM. The mental health continuum: from languishing to flourishing in life. J Health Soc Behav 2002;43(2):207–222. [PubMed] [Google Scholar]
- 54.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 55.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 56.Oppe M, Devlin NJ, van Hout B, Krabbe PF, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health 2014;17(4):445–453. [DOI] [PubMed] [Google Scholar]
- 57.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4(5):353–365. [DOI] [PubMed] [Google Scholar]
- 58.Cooper ML. Motivations for alcohol use among adolescents: development and validation of a four-factor model. Psychol Assess 1994;6(2):117–128. [Google Scholar]
- 59.Proeschold-Bell RJ, Yao J, Gorthala S, Muir A. Development of a Measure of Hepatitis C-alcohol Knowledge. J Alcohol Drug Educ 2014;58(3):7–18. [PMC free article] [PubMed] [Google Scholar]
- 60.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 2004;20(5):309–318. [DOI] [PubMed] [Google Scholar]
- 61.Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: Measuring drinking outcomes in clinical trials. J Stud Alcohol 2000;61(1):55–63. [DOI] [PubMed] [Google Scholar]
- 62.Sobell LC, Sobell MB. Self-report issues in alcohol abuse: state of the art and future directions. Behavioral Assessment 1990;12(1):77–90. [Google Scholar]
- 63.Wang SJ, Winchell CJ, McCormick CG, Nevius SE, O’Neill RT. Short of complete abstinence: an analysis exploration of multiple drinking episodes in alcoholism treatment trials. Alcohol Clin Exp Res 2002;26(12):1803–1809. [DOI] [PubMed] [Google Scholar]
- 64.Watson JM, Crosby H, Dale VM, et al. AESOPS: a randomised controlled trial of the clinical effectiveness and cost-effectiveness of opportunistic screening and stepped care interventions for older hazardous alcohol users in primary care. Health Technol Assess 2013;17(25):1–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montaño DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. Health behavior and health education: theory, research, and practice 4th ed. San Francisco: Jossey-Bass; 2008:67–96. [Google Scholar]
- 66.Fishbein M An integrative model for behavioral prediction and its application to health promotion. In: DiClemente RJ, Crosby RA, Kegler MC, eds. Emerging theories in health promotion practice and research San Francisco, CA: Jossey-Bass; 2009:215–234. [Google Scholar]
- 67.Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol 1997;58(1):7–29. [PubMed] [Google Scholar]
- 68.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis Hoboken, NJ: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 69.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 70.Group EuroQol. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 71.Smith MW, Barnett PG. Direct measurement of health care costs. Med Care Res Rev 2003;60(3 Suppl):74S–91S. [DOI] [PubMed] [Google Scholar]
- 72.Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev 2006;63(2):217–235. [DOI] [PubMed] [Google Scholar]
- 73.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology 1998;28(3):805–809. [DOI] [PubMed] [Google Scholar]
- 74.Loguercio C, Di Pierro M, Di Marino MP, et al. Drinking habits of subjects with hepatitis C virus-related chronic liver disease: prevalence and effect on clinical, virological and pathological aspects. Alcohol Alcohol 2000;35(3):296–301. [DOI] [PubMed] [Google Scholar]
- 75.Peters MG, Terrault NA. Alcohol use and hepatitis C. Hepatology 2002;36:S220–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gold MR. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-effectiveness in health and medicine New York: [et al. ]: Oxford University Press; 1996. [Google Scholar]
- 77.Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual Total Binge Drinks Consumed by U.S. Adults, 2015. Am J Prev Med 2018;54(4):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleming MF, Mundt MP, French MT, Manwell LB, Stauffacher EA, Barry KL. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res 2002;26(1):36–43. [PubMed] [Google Scholar]
- 79.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998;27(4):914–919. [DOI] [PubMed] [Google Scholar]
- 80.Danta M, Dore GJ, Hennessy L, et al. Factors associated with severity of hepatic fibrosis in people with chronic hepatitis C infection. Med J Aust 2002;177(5):240–245. [DOI] [PubMed] [Google Scholar]
- 81.Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 1985;142(11):1259–1264. [DOI] [PubMed] [Google Scholar]
- 82.Serfaty L Follow-up of patients with chronic hepatitis C and a sustained viral response. Liver international : official journal of the International Association for the Study of the Liver 2016;36 Suppl 1:67–71. [DOI] [PubMed] [Google Scholar]
- 83.Verma M, Navarro V. Patient-centered care: a new paradigm for chronic liver disease. Hepatology 2015;62(4):988–990. [DOI] [PubMed] [Google Scholar]
- 84.Willenbring ML. Integrating care for patients with infectious, psychiatric, and substance use disorders: concepts and approaches. AIDS 2005;19(S3):S227–S237. [DOI] [PubMed] [Google Scholar]
- 85.O’Donnell A, Anderson P, Newbury-Birch D, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol 2014;49(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saitz R Alcohol screening and brief intervention in primary care: absence of evidence for efficacy in people with dependence or very heavy drinking. Drug and Alcohol Review 2010;29(6):631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18(2):161–172. [DOI] [PubMed] [Google Scholar]
- 88.Witbrodt J, Ye Y, Bond J, Chi F, Weisner C, Mertens J. Alcohol and drug treatment involvement, 12-step attendance and abstinence: 9-year cross-lagged analysis of adults in an integrated health plan. J Subst Abuse Treat 2014;46(4):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collins SE, Malone DK, Larimer ME. Motivation to change and treatment attendance as predictors of alcohol-use outcomes among project-based Housing First residents. Addict Behav 2012;37(8):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dale V, Coulton S, Godfrey C, et al. Exploring treatment attendance and its relationship to outcome in a randomized controlled trial of treatment for alcohol problems: secondary analysis of the UK Alcohol Treatment Trial (UKATT). Alcohol Alcohol 2011;46(5):592–599. [DOI] [PubMed] [Google Scholar]


