Abstract
Erythropoietin (EPO) is a glycoprotein mainly produced by the adult kidney in response to hypoxia and is the crucial regulator of red blood cell production. EPO receptors (EPORs), however, are not confined to erythroid cells, but are expressed by many organs including the heart, brain, retina, pancreas, and kidney, where they mediate EPO-induced, erythropoiesis-independent, tissue-protective effects. Some of these tissues do also produce and locally release small amounts of EPO in response to organ injury as a mechanism of self-repair.
Growing evidence shows that EPO possesses also important immune modulating effects. Monocytes can produce EPO and autocrine EPO/EPOR signaling in these cells is crucial in maintaining immunological self-tolerance. New data in mice and humans also indicate that EPO has a direct inhibitory effect on effector/memory T cells, while it promotes formation of regulatory T cells.
This review examines the non-erythropoietic effects of EPO, with a special emphasis on its modulating activity on innate immune cells and T cells and on how it affects transplant outcomes.
1. Introduction
Over a century ago, two French scientists reported that plasma from rabbits with anemia increased red blood cell production when injected into non anemic animals (1). As investigators hypothesized that this red blood cell stimulating activity was caused by a single plasma protein, they gave it a variety of names, including erythropoietic-stimulating factor, and, ultimately, “erythropoietin”.
Only in the 1950s and ‘60s, several American investigators conclusively showed that a hormone stimulated red cell production, that the kidneys were the primary source of erythropoietin (EPO), and that low oxygen was the main driver of EPO production. Shortly thereafter, researchers found that patients with anemia often display high EPO levels to stimulate red blood cell production (2).
EPO is a 34 kDa glycoprotein of 165 amino acids (2). While the original name(s) mainly refer to its function as erythropoietic hormone, this molecule also exerts important, unanticipated effects as a cytokine and growth factor that affect multiple organs, including the immune system.
2. EPO and EPO receptors
EPO is made by the fetal liver (2), while in adults it is predominantly produced by kidney perivascular interstitial fibroblasts (3). The primary physiological stimulus of increased EPO gene transcription is tissue hypoxia, which can induce up to a 1,000-fold increase in circulating serum EPO protein levels (4). Hypoxia, generally associated with anemia, stabilizes hypoxia inducible factor (HIF)-1α transcription factor that translocates into the nucleus of renal peritubular interstitial cells, where it dimerizes with the constitutively expressed HIF-1β subunit and mediates transcription of target genes, including EPO (2).
EPO induces erythropoiesis through the stimulation of the EPO receptor (EPOR) on erythroid precursor cells in the bone marrow, thereby increasing their survival, proliferation, and differentiation, ultimately enhancing the oxygen-carrying capacity of blood (5). Upon EPO binding, EPOR forms a homodimer capable of signaling through activation of the JAK2, MAPK, and PI3K kinases, and STAT5 phosphorylation (5). Inactivation of JAK2 results in the development of anemia whereas constitutively activating JAK2 mutations lead to increased red blood cell mass and polycythemia. Clinically, dysregulated EPO expression results in the development of anemia when serum EPO levels are inadequately low or polycythemia as a result of EPO overproduction (2) (Figure 1A).
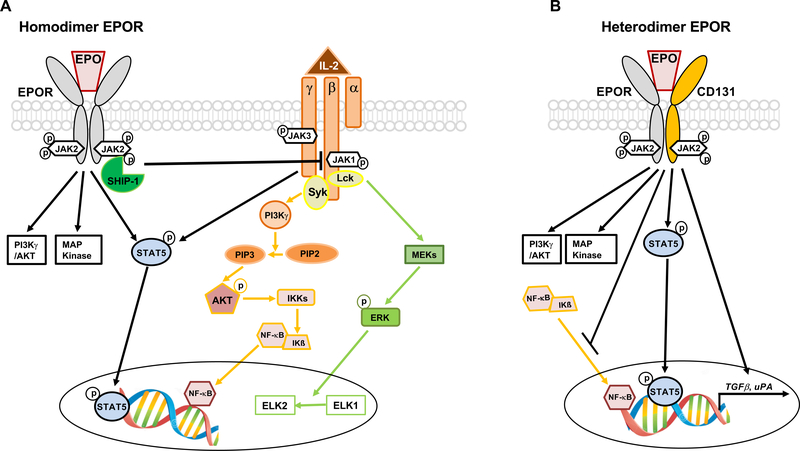
Figure 1.
Homodimer and heterodimer EPOR signaling in T cells and APCs. A) EPO signaling through homodimer EPOR induces SHIP-1 activation that, through a cross-talk mechanism, silences signaling downstream IL-2Rβ. Since conventional T cells (Tconv) rely on IL-2Rβ signaling for activation, EPO/EPOR interaction inhibits their proliferation. In regulatory T cells (Tregs), signaling downstream IL-2Rβ is silenced by constitutive phosphatases, while signaling downstream IL-2Rγ, important for their function, is unaffected by EPO. Modified from (34). B) Both T cells and APC express heterodimer EPOR. While its signaling has no known effect in T cells, its activation in APCs increases transcription and translation of TGFβ and uPA genes. Similar to homodimer receptor, it also activates PI3K, MAPK and STAT5 phosphorylation (7). Other signaling pathways initiated by heterodimer EPOR include inhibition of the binding activity of NF-κB family members.
Affinity of homodimer EPOR for EPO is 1,000 folds higher than that of heterodimer (7).
EPO also binds to a heterodimer complex composed of one chain of the EPOR and the ubiquitous β-common receptor (βcR, CD131, colony-stimulating factor 2 receptor-β) (6). The EPOR/CD131 heterodimer, the activation of which requires much higher concentrations of EPO compared with that of the homodimeric EPOR (7), does not mediate erythropoiesis, but transduces similar signals to those of the homodimer, including PI3K, MAPK activation and STAT5 phosphorylation (7). Other signaling pathways initiated by EPOR/CD131 include regulation of the binding activity of NF-κB family members (7) (Figure 1B).
3. Non erythropoietic functions of EPO
EPOR is expressed in non-hematopoietic tissues, including, amongst others, heart, brain, small bowel, uterus, kidney and pancreatic islets (8). Therefore, it is not surprising that, besides promoting erythropoiesis, EPO exerts cytoprotective effects on non-erythroid cells.
3.1. Heart
Data from various groups converge to indicate a protective effect of EPO in ischemia-reperfusion injury (IRI), especially during myocardial infarction. In vitro experiments indicated that EPO has direct, protective effects on cardiomyocytes and in vivo studies in rats show that administration of EPO halves cardiomyocyte loss upon myocardial infarction, an extent sufficient to normalize hemodynamic function within one week after reperfusion (9).
Building upon these experimental data, short-term EPO therapy has been tested as a strategy to ameliorate outcomes in humans with myocardial ischemia. While clinical studies failed to demonstrate a beneficial effect of EPO on infarct size or left ventricular function at 6 weeks after myocardial infarction (primary endpoints), EPO treatment has been overall safe and associated with decreased incidence of microvascular obstruction and short-term adverse cardiovascular events in secondary analyses (10).
3.2. Brain
Brain cells produce EPO as part of the intrinsic response to hypoxic insults. Locally-produced or systemically administered EPO protects rats against hypoxic injury through revascularization of the ischemic zone (11) that improves oxygen delivery to the brain. Different cell types express the EPOR in the brain, including neural progenitor cells (NPC), astrocytes, neurons and oligodendrocytes. In rats, exposure of NPC to EPO promotes their proliferation and differentiation into astrocytes or oligodendrocytes, an effect associated with ERK and NF-κB activation (12).
In experimental models of stroke, EPO administration reduces the size of lesions and brain damage (13). A randomized controlled study in 142 patients with ischemic stroke showed that two consecutive doses of EPO did not reduce the 90-day combined endpoint of recurrent stroke or death (primary endpoint), but significantly improved long-term neurological outcomes compared to placebo (14). Despite potential benefits of acute EPO administration, chronic treatment with EPO associates with hypertension (15) and stroke, especially in patients with chronic kidney disease (16).
An area where EPO has been extensively studied is the prevention of brain injury and subsequent neurodevelopmental disabilities in preterm infants (17). The mechanisms responsible for the neuroprotective effect of EPO in this setting include 1) prevention of cell apoptosis, inflammation, and neurotoxicity, 2) antioxidant activity, and 3) promotion of neural regeneration, injury repair, and normal development. With the increasing number of premature infants, recombinant EPO is increasingly being used in neonatal medicine as a substitute for blood transfusions due to its tissue-protective effects (17).
3.3. Other organs
Numerous studies have shown the beneficial effects of EPO on many other non-hematopoietic tissues [for a comprehensive review of these effects please refer to (8)].
Several papers demonstrated a protective effect of EPO on pancreas islet cells. He et al. showed that EPO promotes proliferation and prevents apoptosis of neonatal porcine islet cells through the upregulation of Bcl-2 and downregulation of BAX and CASP3 (18). In diabetic mice, EPO also improves glucose metabolism and reduces pancreatic β-cell damage (19).
The retina, a highly metabolically-active tissue that is extremely sensitive to reductions in oxygen tension or traumas, expresses EPOR and produces EPO (20). Numerous studies have shown the protective effects of EPO in non-inherited or inherited retinal degenerations (21). This phenomenon is independent of erythropoiesis and is most likely mediated by the heterodimer EPOR (21). Intriguingly, polymorphisms in the EPO gene are associated with the development of diabetic microvascular complications (22–24).
EPO can also affect bone homeostasis. One of the first experimental connections between EPO and bone formation was observed in rats where bleeding significantly increased mineral apposition rate, osteoblast number, and serum levels of osteogenic growth peptide (25). Subsequent studies demonstrated that EPO treatment enhanced both bone volume and biomechanical properties in multiple murine femoral fracture repair models. Importantly, osteoblasts produce EPO, as a local mechanism to regulate erythropoiesis and bone remodeling (26).
Functional EPOR is expressed by both proximal and distal renal tubular cells and it is thought that EPO/EPOR signaling prevents tubular cell apoptosis and facilitates recovery after injury. However, IRI reduces the expression levels of EPO in the kidney (27). In the model of cisplatinum-induced acute kidney injury in rats, treatment with recombinant EPO accelerates functional renal recovery (28). Similarly, in rats with unilateral ureteral obstruction, both recombinant EPO and a non-erythropoietic EPO derivative which activates the EPOR heterodimer decrease tubular apoptosis and interstitial fibrosis (29).
Despite these encouraging experimental data, a meta-analysis of 7 clinical trials failed to detect a significant benefit of EPO treatment in the incidence of acute kidney injury in humans (30).
A prospective, double-blinded cross-over study in 16 healthy subjects showed that treatment with EPO increases renal vascular resistance and decreased renal plasma flow (31), which may at least in part account for the lack of nephroprotective effects of EPO in humans despite regenerative properties on tubular cells.
Overall, these data document the wide range of EPOR expressing cells and the many, erythropoietic-independent effects of EPO in the body.
4. EPO in transplantation
Anemia occurs frequently during the first weeks after renal transplantation and associates with increased cardiovascular morbidity and mortality. Due to its erythropoietic, tissue-protective, and immune modulating effects, EPO represents an attractive therapeutic option to improve patient and graft outcomes after organ transplantation.
Consistent with its protective effects in experimental models of IRI, EPO has been shown to protect the kidney graft from ischemic injury in rat transplant models (32). However, clinical studies failed to consistently show a significant protective effect of EPO on delayed graft function, nor on 1-year graft survival after renal transplantation (30).
In 2012, Cassis et al. tested the effects of EPO in a rat model of chronic kidney transplant rejection (33). EPO treatment entirely corrected post-transplant anemia and prevented progressive graft dysfunction and fibrosis. Conversely, normalization of post-transplant hemoglobin levels by blood transfusions had no impact on chronic allograft injury, indicating that EPO-mediated graft protection was independent from anemia correction. The effects of EPO were associated with upregulation of the anti-apoptotic factor Bcl-2 in the graft, suggesting that EPO protects the graft from chronic injury through its nephroprotective activity. However, the fact that EPO treatment was associated also with reduced inflammatory cell infiltrates suggests that EPO-associated immune modulating effects are also involved.
To specifically test the hypothesis that immune modulating effects of EPO prolong graft survival, we treated heart transplant recipient mice with a short-term course of EPO or vehicle control (34). EPO treatment significantly prolonged graft survival, a finding that was associated with reduced alloreactive conventional T cells (Tconv) and increased regulatory T cell (Treg) frequencies. Intriguingly, while heart transplants are rapidly rejected, kidney grafts in mice are spontaneously tolerated across certain strain combinations. Since the kidney is the main source of EPO, we reasoned that EPO is responsible for the protolerogenic properties of the kidney. Consistent with our hypothesis, we showed that acriflavine-induced EPO downregulation in mice prevents Treg-mediated spontaneous acceptance of allogeneic kidney transplants. Adding back recombinant EPO rescued these acriflavine-induced deleterious effects, supporting the conclusion that kidney-derived EPO is a crucial regulator of T cell alloimmunity after kidney transplantation (34).
These data provide a potential explanation for the lower rates of acute rejection in kidney compared with heart or lung transplant recipients (35) - kidneys are the major source of EPO, while the heart and the lungs produce only minimal amounts (36) - and for the better outcomes in recipients of combined kidney/heart transplants from the same donor versus recipients of hearts alone (37). EPO-dependent immunoregulation could additionally account for the observation that spontaneous human kidney transplant acceptance/tolerance occurs, albeit infrequently (38), whereas spontaneous human heart transplant acceptance/tolerance has not been described. Importantly, the other major source of EPO besides the kidney is the liver (39), an organ with well-known protolerogenic effects (40). Altogether, these data provide associative evidence supporting an inverse relationship between levels of EPO production and organ immunogenicity.
The nephroprotective effects of EPO, together with its immune modulating activity, formed the basis for a controlled trial where 104 kidney transplant recipients at high risk for delayed graft function were randomized to 4-dose EPO therapy during the first two weeks after transplant or no treatment. EPO treatment was well tolerated, but the two groups showed no significant differences in the incidence of delayed graft function, acute rejection, or graft function at three months after transplant (41). These data contrast with those from other randomized trials where EPO treatment to fully normalize hematocrit significantly reduced graft function decline (42) and increased 2-year graft survival (43) in kidney transplant recipients over lower EPO doses to partially correct hematocrit. Discrepancies across studies testing the impact of EPO on graft outcomes (41–43) may depend on dosing, treatment duration, and patient characteristics and warrant further studies to define optimal therapeutic strategies.
Altogether, these data document that EPO exerts immune modulating effects that positively impact graft outcomes and may, at least partially, explain the unique tolerogenic properties of kidney grafts.
5. Immune modulating mechanisms of EPO
Functional EPOR is expressed on immune cells, including monocytes, T and B cells (34, 44). Reports from the early 2000s indicate that mice treated with pharmacological doses of EPO exhibit amelioration of experimental arthritis, colitis, and autoimmune encephalomyelitis (EAE) (45–47). Mechanisms involve effects on both innate and adaptive immune cells, including macrophages (46, 48) and T cells (45, 49), respectively.
5.1. EPO and innate immune cells
EPOR is highly expressed on the monocyte/macrophage membranes. In vitro data show that EPO inhibits production of murine macrophages-derived inflammatory mediators such as TNF-α, IL-6 and inducible nitric oxide synthase (iNOS) by counteracting NF-κB-inducible pathways (46). Salmonella typhimurium-infected mice treated with EPO demonstrated a higher bacterial load and reduced expression of IL-6, TNF and Nos2 as compared to vehicle-treated controls (46), overall documenting a direct, anti-inflammatory effect of EPO on murine monocytes. In vitro experiments show that exposure of human monocytes to EPO decreases their IL-6 and IL-8 production upon toll like receptor (TLR) ligation.
Macrophages can also produce and release EPO in response to “find-me” signal sphingosine 1-phosphate (S1P) initiated by dying cells. Through an autocrine signaling loop, EPO signals on macrophage-expressed EPOR to increase peroxisome proliferator activated receptor γ (PPARγ) and enhance clearance of apoptotic cells. Consistently, EPOR-deficient macrophages exhibit impaired apoptotic cell phagocytosis and macrophage-specific Epor−/− mice develop lupus-like symptoms, while EPO treatment ameliorates disease progression in lupus-prone mice (48).
In contrast to the above anti-inflammatory effects, evidence also exists that, in certain contexts, EPO may reinvigorate immune responses. In vitro, EPO upregulates expression of the costimulatory molecules CD80 and CD86 as well as that of HLA-DR in peripheral blood dendritic cells (DCs) and monocyte-derived DCs (MoDCs). Functionally, EPO-exposed MoDCs increase antigen uptake and IL-12 secretion. When applied to immature MoDCs, EPO by itself induced their maturation (50).
Data from EPO transgenic mice and administration of recombinant EPO showed increased neutrophil counts associated with increased EPO levels, but no evidence supports a direct effect of EPO on neutrophil function (51). However, in an experimental colitis model, EPO was associated with reduced disease severity and fewer neutrophil infiltration in the colon mucosa, possibly mediated by a decreased integrin expression (52).
5.2. EPO and T cells
At resting state, murine and human T cells express low levels of EPOR on their surface, but they quickly up-regulate it upon activation through the T cell receptor (TCR). In the T helper 17 (TH17) mouse model of EAE, EPO treatment is associated with significant reduction of disease severity, fewer TH17 cells, and increased Tregs (45).
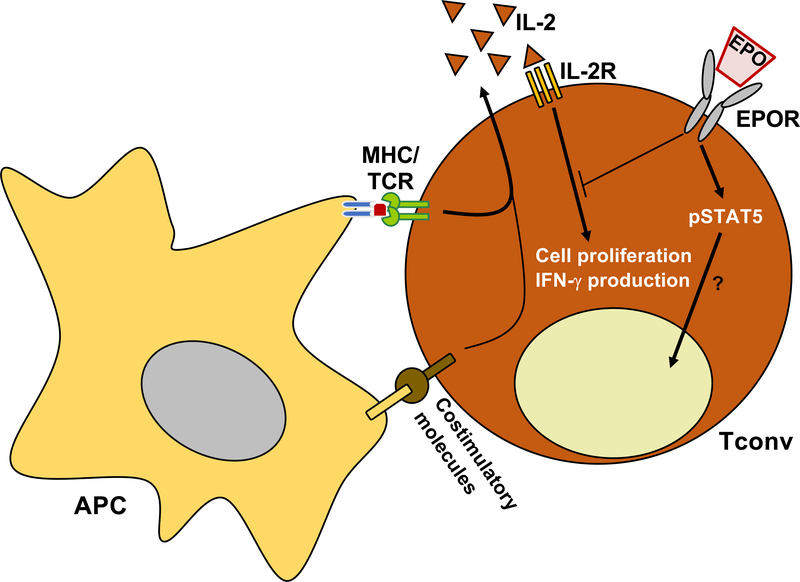
In 2014, we showed that EPO directly inhibits naïve and memory conventional T cell (Tconv) proliferation by signaling through the homodimer EPOR (49). Transfer of human T cells into NOD scid γcnull mice (lacking T and B cells and functional NK cells) results in a strong human anti-mouse T cell xeno-response, that is inhibited by human EPO administration, indicating that EPO inhibits human T cells also in this in vivo system. From a mechanistic standpoint, EPO inhibits Tconv activation through the homodimer EPOR. Similar to what previously reported in other cells, EPO/EPOR signaling in T cells induces STAT5 phosphorylation, but only in the presence of T cell receptor engagement (53). Upon activation by the TCR and costimulation molecules, T cell start producing IL-2 that through an autocrine loop fuels their expansion. EPO/EPOR signaling inhibits IL-2-dependent signals transmitted through the β chain of the IL-2 receptor (IL-2R). This occurs through the activation of SH-2 containing inositol-5-phosphatase 1 (SHIP-1) by EPOR that cross-talks with IL-2Rβ, inhibiting AKT and ERK phosphorylation, thereby preventing T cell activation and proliferation (Figure 1A and 2). Although not formally tested, SHIP-1 phosphatase may also inhibit signaling downstream the TCR (54), providing an additional mechanism responsible for EPOR-induced T cell inhibition.
Figure 2. EPO directly inhibits Tconv.
EPO directly inhibits conventional T cell (Tconv) expansion and IFN-γ production by activating homodimer EPOR that cross-talks with IL-2 receptor to silence its downstream signaling. EPO/EPOR signaling also induces STAT5 phosphorylation, but its impact on Tconv function is unclear.
Tregs, a key T cell subset implicated in maintaining tolerance towards self and alloantigens, are also dependent on IL-2 signaling for their survival and proliferation. Similar to Tconv cells, also Treg express the EPOR on their surface. However, despite EPO-induced inhibition of signaling downstream IL-2R, EPO does not affect their function. In vitro studies showed that EPO-induced, SHIP-1–dependent inhibition of IL-2Rβ signaling spares Treg because EPO does not impair IL-2Rγ/STAT5 signaling required for IL-2-induced Treg proliferation, and because AKT signaling (among other pathways) is constitutively prevented by endogenous phosphatases preferentially expressed in Treg (Figure 1A). While SHIP-1 has been shown to impair Treg function and expansion (55, 56), EPO does not affect Treg proliferation or suppressive capacity. This could be, at least in part, explained by the fact that EPO/EPOR signaling in Treg, while it activates SHIP-1, it also increases STAT5 phosphorylation.
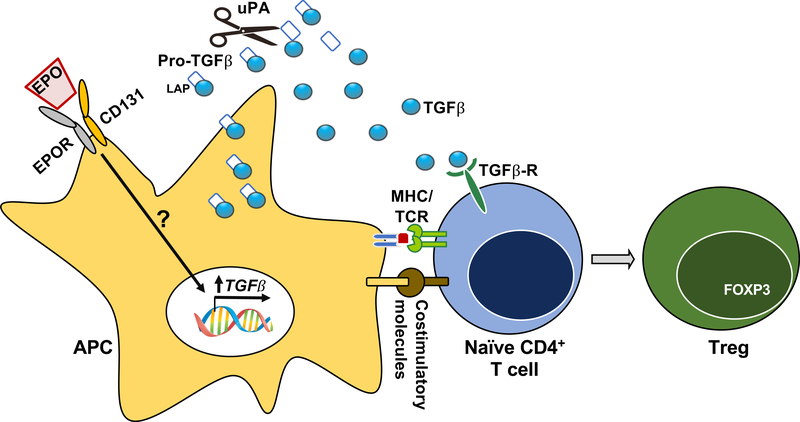
Treg can originate from the thymus or be generated in the periphery from naïve CD4+ T cells (induced or iTreg) through Transforming Growth Factor β (TGFβ)-dependent induction and stabilization of FOXP3. Starting from the evidence that EPO inhibits Tconv, while leaving Treg unaffected, our group tested the effect of EPO on Treg induction building upon data in rodents showing that EPO induces TGFβ synthesis by monocytes (57) and tubular cells (58). In vitro and in vivo data demonstrate that EPO converts naïve CD4+ T cells into functional iTreg by stimulating local production of functionally active TGFβ by antigen presenting cells (APCs). EPO-induced TGFβ production requires CD131 on APCs, supporting the conclusion that this effect is mediated through EPO ligating the EPOR heterodimer (34) (Figure 3).
Figure 3. EPO increases Treg induction by increasing active TGFβ production in APCs.
In antigen presenting cells (APCs), EPO signaling through the heterodimer EPOR increases TGFβ and uPA transcripts. Urokinase-type plasminogen activator (uPA) cleaves the latency associated peptide (LAP) from pro-TGFβ to activate it and promote conversion of naïve CD4+ T cells into functional regulatory T cells (iTregs). See Figure 1 for further details.
In a prospective-cohort study, EPO administered at doses used to correct anemia augmented frequencies of circulating Tregs in patients with chronic kidney disease, suggesting that the effects detected in vitro and in vivo in mice, do also apply to humans in vivo (34).
6. EPO: an evolutionary perspective
Epo genes have been identified in vertebrate species ranging from human to fish, suggesting that EPO signaling evolved earlier than the vertebrate lineage. Intriguingly, functional studies revealed EPO-mediated neuroprotection and neuroregeneration also in insects, indicating that EPO-like signaling was already established in both vertebrates’ and insects’ last common ancestor. Functioning originally as a tissue-protective response to unfavorable physiological situations, cell injury, and pathogen invasion, EPO was possibly later adapted as a humoral regulator of erythropoiesis in the vertebrate lineage (59).
The reason why the kidney represents the major source of EPO is also intriguing. Phylogenetically and ontogenetically, the kidney is not an erythropoietic organ. Investigators have hypothesized that kidney capacity to sense both oxygen tension and extracellular volume makes it the ideal organ to regulate red cell mass through EPO production and plasma volume control (60). However, available data also support the fascinating idea that EPO synthesis within the kidney has been evolved as a mechanism of peripheral immune tolerance to protect this organ from inflammatory responses against the urinary microbiome, high concentration of environmental antigens, and sodium.
7. Summary
Initially discovered for its erythropoietic effects, EPO has improved the quality of life and reduced morbidity and mortality of individuals with anemia due to end-stage renal disease or hematologic malignancies. Over the years, this glycoprotein has gained further attention due to its erythropoietic-independent effects that protect multiple tissues from IRI and apoptosis.
The non-erythropoietic effects of EPO also include immune modulating activities on innate immune cells (mainly monocytes/macrophages) and T cells. These effects have been shown to prolong graft survival in mice and in humans. Evidence that the immune system is involved also in IRI, tissue repair, and regeneration (61) suggest that the immune modulating effects of EPO may be implicated in its tissue protective effects as well.
Development of new molecules targeting the heterocomplex EPOR devoid of erythropoietic activity may allow long-term treatments, opening the avenue to novel strategies to ameliorate transplant outcomes.
Acknowledgments
PC is supported by the National Institutes of Health (NIAID) R01 0255A141.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- APCs
Antigen presenting cells
- EAE
Autoimmune encephalomyelitis
- βcR
β-common receptor
- Tconv
Conventional T cells
- DCs
Dendritic cells
- EPO
Erythropoietin
- EPOR
Erythropoietin receptor
- HIF
Hypoxia inducible factor
- IL-2R
IL-2 receptor
- iTreg
Induced regulatory T cell
- iNOS
Inducible nitric oxide synthase
- IRI
Ischemia-reperfusion injury
- LAP
Latency associated peptide
- MoDCs
Monocyte-derived DCs
- NPC
Neural progenitor cells
- PPARγ
Peroxisome proliferator activated receptor γ
- Treg
Regulatory T cells
- SHIP-1
SH-2 containing inositol-5-phosphatase 1
- S1P
Sphingosine 1-phosphate
- TCR
T cell receptor
- TH17
T helper 17
- TLR
Toll like receptor
- TGFβ
Transforming Growth Factor β
- uPA
Urokinase-type Plasminogen activator
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Carnot P, Deflandre C. Sur l’activite hemopoietique des differents organeau au cours de la regeneration du sang. CR Searces Acad Sci 1906;143:432–435. [Google Scholar]
- 2.Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med 2013;3(3):a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souma T, Yamazaki S, Moriguchi T, Suzuki N, Hirano I, Pan X et al. Plasticity of renal erythropoietin-producing cells governs fibrosis. J Am Soc Nephrol 2013;24(10):1599–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood 1999;94(6):1864–1877. [PubMed] [Google Scholar]
- 5.Elliott S, Sinclair AM. The effect of erythropoietin on normal and neoplastic cells. Biologics 2012;6:163–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A 2008;105(31):10925–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med 2013;210(2):205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M. Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol 2011;82(10):1291–1303. [DOI] [PubMed] [Google Scholar]
- 9.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A 2003;100(8):4802–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fokkema ML, Kleijn L, van der Meer P, Belonje AM, Achterhof SK, Hillege HL et al. Long term effects of epoetin alfa in patients with ST- elevation myocardial infarction. Cardiovasc Drugs Ther 2013;27(5):433–439. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Zhu C, Wang X, Gerwien JG, Schrattenholz A, Sandberg M et al. The nonerythropoietic asialoerythropoietin protects against neonatal hypoxia-ischemia as potently as erythropoietin. J Neurochem 2004;91(4):900–910. [DOI] [PubMed] [Google Scholar]
- 12.Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 2010;41(5):1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatagner A, Huppi PS, Ha-Vinh Leuchter R, Sizonenko S. [Erythropoietin and neuroprotection]. Arch Pediatr 2010;17 Suppl 3:S78–84. [DOI] [PubMed] [Google Scholar]
- 14.Tsai TH, Lu CH, Wallace CG, Chang WN, Chen SF, Huang CR et al. Erratum to: Erythropoietin improves long-term neurological outcome in acute ischemic stroke patients: a randomized, prospective, placebo-controlled clinical trial. Crit Care 2016;20:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal R Mechanisms and mediators of hypertension induced by erythropoietin and related molecules. Nephrol Dial Transplant 2018;33(10):1690–1698. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361(21):2019–2032. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Sun H, Xu F, Kang W, Gao L, Guo J et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol 2016;80(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H, Wu T, Xiong J, Chen K, Mo Z. Effect of erythropoietin on the proliferation and apoptosis of neonatal porcine islet cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2010;35(11):1115–1122. [DOI] [PubMed] [Google Scholar]
- 19.Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med 2010;207(13):2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo W, Hu L, Wang F. The protective effect of erythropoietin on the retina. Ophthalmic Res 2015;53(2):74–81. [DOI] [PubMed] [Google Scholar]
- 21.Colella P, Iodice C, Di Vicino U, Annunziata I, Surace EM, Auricchio A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum Mol Genet 2011;20(11):2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Xu H, Li Y, Zhao D, Ma B. Associations between erythropoietin polymorphisms and risk of diabetic microvascular complications. Oncotarget 2017;8(68):112675–112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooyaart AL, Valk EJ, van Es LA, Bruijn JA, de Heer E, Freedman BI et al. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 2011;54(3):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Z, Yang Z, Patel S, Chen H, Gibbs D, Yang X et al. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci U S A 2008;105(19):6998–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas TS, Bab IA, Lian JB, Stein GS, Jazrawi L, Majeska RJ et al. Stimulation of systemic bone formation induced by experimental blood loss. Clin Orthop Relat Res 1997(340):267–275. [DOI] [PubMed] [Google Scholar]
- 26.Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 2012;149(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Zou YR, Zhong X, Deng HD, Pu L, Peng K et al. Erythropoietin pretreatment ameliorates renal ischaemia-reperfusion injury by activating PI3K/Akt signalling. Nephrology (Carlton) 2015;20(4):266–272. [DOI] [PubMed] [Google Scholar]
- 28.Vaziri ND, Zhou XJ, Liao SY. Erythropoietin enhances recovery from cisplatin-induced acute renal failure. Am J Physiol 1994;266(3 Pt 2):F360–366. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura H, Isaka Y, Takabatake Y, Imamura R, Suzuki C, Takahara S et al. Nonerythropoietic derivative of erythropoietin protects against tubulointerstitial injury in a unilateral ureteral obstruction model. Nephrol Dial Transplant 2008;23(5):1521–1528. [DOI] [PubMed] [Google Scholar]
- 30.Elliott S, Tomita D, Endre Z. Erythropoiesis stimulating agents and reno-protection: a meta-analysis. BMC Nephrol 2017;18(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aachmann-Andersen NJ, Christensen SJ, Lisbjerg K, Oturai P, Johansson PI, Holstein-Rathlou NH et al. Recombinant erythropoietin acutely decreases renal perfusion and decouples the renin-angiotensin-aldosterone system. Physiol Rep 2018;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Zhao D, Na N, Li H, Miao B, Hong L et al. Renoprotective effect of erythropoietin via modulation of the STAT6/MAPK/NF-kappaB pathway in ischemia/reperfusion injury after renal transplantation. Int J Mol Med 2018;41(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassis P, Gallon L, Benigni A, Mister M, Pezzotta A, Solini S et al. Erythropoietin, but not the correction of anemia alone, protects from chronic kidney allograft injury. Kidney Int 2012;81(9):903–918. [DOI] [PubMed] [Google Scholar]
- 34.Purroy C, Fairchild RL, Tanaka T, Baldwin WM 3rd, Manrique J, Madsen JC et al. Erythropoietin Receptor-Mediated Molecular Crosstalk Promotes T Cell Immunoregulation and Transplant Survival. J Am Soc Nephrol 2017;28(8):2377–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matas AJ, Smith JM, Skeans MA, Lamb KE, Gustafson SK, Samana CJ et al. OPTN/SRTR 2011 Annual Data Report: kidney. Am J Transplant 2013;13 Suppl 1:11–46. [DOI] [PubMed] [Google Scholar]
- 36.Jelkmann W Regulation of erythropoietin production. J Physiol 2011;589(Pt 6):1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill J, Shah T, Hristea I, Chavalitdhamrong D, Anastasi B, Takemoto SK et al. Outcomes of simultaneous heart-kidney transplant in the US: a retrospective analysis using OPTN/UNOS data. Am J Transplant 2009;9(4):844–852. [DOI] [PubMed] [Google Scholar]
- 38.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 2010;120(6):1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacombe C, Da Silva JL, Bruneval P, Casadevall N, Camilleri JP, Bariety J et al. Erythropoietin: sites of synthesis and regulation of secretion. Am J Kidney Dis 1991;18(4 Suppl 1):14–19. [PubMed] [Google Scholar]
- 40.Hull TD, Benichou G, Madsen JC. Why some organ allografts are tolerated better than others: new insights for an old question. Curr Opin Organ Transplant 2019;24(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez F, Kamar N, Pallet N, Lang P, Durrbach A, Lebranchu Y et al. High dose epoetin beta in the first weeks following renal transplantation and delayed graft function: Results of the Neo-PDGF Study. Am J Transplant 2010;10(7):1695–1700. [DOI] [PubMed] [Google Scholar]
- 42.Tsujita M, Kosugi T, Goto N, Futamura K, Nishihira M, Okada M et al. The effect of maintaining high hemoglobin levels on long-term kidney function in kidney transplant recipients: a randomized controlled trial. Nephrol Dial Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto-Viguier E, Toupance O et al. Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 2012;23(2):360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisowska KA, Debska-Slizien A, Bryl E, Rutkowski B, Witkowski JM. Erythropoietin receptor is expressed on human peripheral blood T and B lymphocytes and monocytes and is modulated by recombinant human erythropoietin treatment. Artif Organs 2010;34(8):654–662. [DOI] [PubMed] [Google Scholar]
- 45.Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS One 2008;3(4):e1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I et al. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-kappaB-inducible immune pathways. Immunity 2011;34(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuzzocrea S, Mazzon E, di Paola R, Genovese T, Patel NS, Britti D et al. Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum 2005;52(3):940–950. [DOI] [PubMed] [Google Scholar]
- 48.Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R et al. Erythropoeitin Signaling in Macrophages Promotes Dying Cell Clearance and Immune Tolerance. Immunity 2016;44(2):287–302. [DOI] [PubMed] [Google Scholar]
- 49.Cravedi P, Manrique J, Hanlon KE, Reid-Adam J, Brody J, Prathuangsuk P et al. Immunosuppressive effects of erythropoietin on human alloreactive T cells. J Am Soc Nephrol 2014;25(9):2003–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prutchi Sagiv S, Lifshitz L, Orkin R, Mittelman M, Neumann D. Erythropoietin effects on dendritic cells: potential mediators in its function as an immunomodulator? Exp Hematol 2008;36(12):1682–1690. [DOI] [PubMed] [Google Scholar]
- 51.Avneon M, Lifshitz L, Katz O, Prutchi-Sagiv S, Gassmann M, Mittelman M et al. Non-erythroid effects of erythropoietin: are neutrophils a target? Leuk Res 2009;33(10):1430–1432. [DOI] [PubMed] [Google Scholar]
- 52.Cuzzocrea S, Mazzon E, Di Paola R, Patel NS, Genovese T, Muia C et al. Erythropoietin reduces the development of experimental inflammatory bowel disease. J Pharmacol Exp Ther 2004;311(3):1272–1280. [DOI] [PubMed] [Google Scholar]
- 53.Lisowska KA, Debska-Slizien A, Jasiulewicz A, Jozwik A, Rutkowski B, Bryl E et al. Flow cytometric analysis of STAT5 phosphorylation and CD95 expression in CD4+ T lymphocytes treated with recombinant human erythropoietin. J Recept Signal Transduct Res 2011;31(3):241–246. [DOI] [PubMed] [Google Scholar]
- 54.Hebeisen M, Baitsch L, Presotto D, Baumgaertner P, Romero P, Michielin O et al. SHP-1 phosphatase activity counteracts increased T cell receptor affinity. J Clin Invest 2013;123(3):1044–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collazo MM, Wood D, Paraiso KH, Lund E, Engelman RW, Le CT et al. SHIP limits immunoregulatory capacity in the T-cell compartment. Blood 2009;113(13):2934–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collazo MM, Paraiso KH, Park MY, Hazen AL, Kerr WG. Lineage extrinsic and intrinsic control of immunoregulatory cell numbers by SHIP. Eur J Immunol 2012;42(7):1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mausberg AK, Meyer Zu Horste G, Dehmel T, Stettner M, Lehmann HC, Sheikh KA et al. Erythropoietin ameliorates rat experimental autoimmune neuritis by inducing transforming growth factor-beta in macrophages. PLoS One 2011;6(10):e26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gobe GC, Bennett NC, West M, Colditz P, Brown L, Vesey DA et al. Increased progression to kidney fibrosis after erythropoietin is used as a treatment for acute kidney injury. Am J Physiol Renal Physiol 2014;306(6):F681–692. [DOI] [PubMed] [Google Scholar]
- 59.Heinrich R, Gunther V, Miljus N. Erythropoietin-Mediated Neuroprotection in Insects Suggests a Prevertebrate Evolution of Erythropoietin-Like Signaling. Vitam Horm 2017;105:181–196. [DOI] [PubMed] [Google Scholar]
- 60.Donnelly S Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis 2001;38(2):415–425. [DOI] [PubMed] [Google Scholar]
- 61.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009;15(2):192–199. [DOI] [PubMed] [Google Scholar]