Abstract
Objective:
To investigate the adoption of abiraterone and enzalutamide by urologists. Abiraterone and enzalutamide are oral therapies approved for the treatment of metastatic castration-resistant prostate cancer, a disease most commonly treated by medical oncologists.
Methods:
Using the Medicare Part D Public Use Files from 2013 to 2016, we identified total abiraterone and enzalutamide prescriptions 2013–2016 and urologists who prescribed moderate to high volumes of these drugs. We then characterized the urologist practices of those urologists according to practice context (e.g., single-specialty group) using data from the Centers for Medicare and Medicaid Services, and the geographic distribution of those providers.
Results:
We found abiraterone prescriptions increased from 71,423 in 2013 to a peak of 100,371 in 2015 and enzalutamide prescriptions continued to increase from 29,572 in 2013 to 100,980 in 2016. Prescriptions by urologists increased between 2013–2016 while prescriptions by other specialties plateaued. The number of moderate-high prescribing urologists increased from 98 (abiraterone) and 22 (enzalutamide) in 2013, to 301 (abiraterone) and 671 (enzalutamide) by 2016 with 1,063 unique urologists prescribing moderate-high volumes of either drug between 2013–2016. Among urologists who prescribe androgen deprivation therapy, 5% were moderate-high prescribers of abiraterone and 12% of enzalutamide in 2016. The majority of moderate-high prescribing urologists were in single-specialty groups (70%).
Conclusion:
Urologists are increasingly prescribing oral therapies for metastatic castration-resistant prostate cancer. Understanding the distribution of urologists specializing in castration-resistant prostate cancer therapeutics will help guide future interventions to optimize the care for this important patient population.
Keywords: prostate cancer, castration-resistant, claims data, urologists
Introduction
Urologists are the first point of contact for patients in the diagnosis and management of prostate cancer, including the detection of recurrent disease. If a patient recurs after treatment, urologists also commonly initiate systemic androgen deprivation therapy (ADT) in the form of injectable gonadotropin-releasing hormone analogs. Progression to castration-resistant disease generally had required chemotherapy, including docetaxel and mitoxantrone. Because they lack expertise in delivering chemotherapy and managing its associated toxicities, urologists traditionally referred patients with castration-resistant prostate cancer to medical oncologists.
The introduction of well-tolerated targeted oral agents for castration-resistant prostate cancer has the potential to affect these practice patterns. Abiraterone and enzalutamide are two drugs approved for metastatic castration-resistant prostate cancer based on clinical trials demonstrating a survival benefit.1–4 Both drugs are well-tolerated compared with chemotherapeutic agents, making them more attractive to deliver by urologists. Indeed, some experts have advocated for urologists to continue treating their patients through end of life.5–7 By providing care across the lifespan of the disease, urologists may capitalize on their longstanding relationship with the patient in a delivery model that is convenient and reduces care fragmentation. However, implicit in this relationship is the responsibility for managing toxicities and late complications (e.g., early recognition of cord compression or pathologic fractures), as well as attention to end-of-life care for a disease that is invariably lethal. The American Urological Association, the leading professional society, has facilitated movement of urologists into this space through educational workshops and dissemination of clinical care guidelines.8
The extent to which urologists have broadened their scope of practice to include men with castration-resistant prostate cancer is unknown. Understanding this potential sea change in practice is important to ensure that educational efforts (e.g., dissemination of newly-identified adverse reactions) and new trial opportunities are appropriately focused. For this reason, we used national Medicare data to assess national trends in prescribing patterns of abiraterone or enzalutamide by urologists. We further explored associations between practice context and adoption to better understand the continued expansion of urologists into this space.
Methods
Data sources
The Medicare Part D Prescriber Public Use File is a publicly available database for prescriptions filled through Medicare Part D. The file includes details about the providers prescribing oral therapies, such as address and specialty. All providers are included in the aggregated totals in the file, but to protect patient confidentiality, the file describes the number of patients and prescriptions for only those providers who prescribe more than 10 prescriptions for a medication.
We enumerated the number of prescriptions for abiraterone and enzalutamide by provider and the number of patients associated with these prescriptions annually between 2013 and 2016. We sorted prescribing providers into one of three groups (low, moderate, and high). “Low prescribers” prescribed either drug at least once but wrote for ≤10 prescriptions. Both “high prescribers” and “moderate prescribers” wrote for >10 individual prescriptions of abiraterone or enzalutamide in a year: “high prescribers” to >10 patients per year and “moderate prescribers” to ≤10 patients per year. Specialty information and practice location are available for “high” and “moderate” prescribers.
The Public Use File also includes total drug cost, which is reported as the sum of the amounts paid by the Part D drug plan, the patient, the government through subsidies, and third-party payers. These payments are inclusive of the cost of the medication itself, dispensing fees, sales tax, and administration fees.
Scope of practice
Recognizing not all urologists care for men with prostate cancer, we used a 20% sample of fee-for-service Medicare beneficiaries to identify urologists who treat men with advanced prostate cancer and provide perspective for trends in abiraterone and enzalutamide prescribing patterns observed. We identified those urologists billing an evaluation and management code for at least one patient with a primary diagnosis of prostate cancer (ICD-9 185 or ICD-10 C61) and prescribing androgen deprivation to their patients, defined as at least two of the CPT codes for any of the depot injections of leuprolide, goserelin, degarelix or triptorelin to the same patient within a 365-day period.
Urologist practice organization
Urologists practice in widely variable practice types, ranging from solo practices to large multi-specialty groups of over 100 physicians. To demonstrate the trends in scope of practice for different types of urology groups, we characterized the clinical practice context among urologists in national Medicare claims and those whose identity was available in the Medicare Part D Prescriber Public Use File (i.e., “moderate” and “high” users of enzalutamide or abiraterone). For this purpose, we used data from the Centers for Medicare and Medicaid Services provided in the Medicare Data on Provider Practice and Specialty file. Urologists were assigned to their group practice for each year based on tax-identification number, using previously established methods.9 We further characterized practices based on their constitution and size and sorted them into groups: a group with 1–2 urologists total was considered a solo practice, a single-specialty group consisted of more than 2 urologists with more than 50% of the physicians in the group being urologists, and for those practices with less than 50% of the physicians in the group identified as urologists, either a specialty group if there were no primary care providers, or multi-specialty if there was at least one primary care provider such as internal medicine, family practice, geriatrics. Those practices that did not include a urologist were categorized as Other and not included in the final analysis.
Statistical Analysis
Aggregated totals of the use of abiraterone and enzalutamide for each year and cost for each treatment were extracted. Using the aggregated totals, we were able to determine the average time patients were on abiraterone or enzalutamide by dividing the total number of prescriptions by the total number of patients treated in a given year since each prescription is for one month of therapy under Medicare Part D.10 Moderate and high prescribers of the two oral therapies were differentiated from the low prescribers and then categorized by specialty.
We calculated the proportion of urologists prescribing moderate- to high-volumes of abiraterone and enzalutamide among those urologists who treat patients with ADT. We also calculated the proportion of moderate-high prescribing urologists in different practice contexts.
To visualize the geographic distribution of urologists prescribing moderate-high volumes of abiraterone and enzalutamide, we plotted the moderate-high prescribers on a map of the United States.
All analyses were performed using SAS® 9.4 software. The maps were generated using Tableau 2018.1.3. This study was determined “Not Regulated” by the University of Michigan Internal Review Board since the research did not interact with nor obtain identifiable private information about human subjects.
Results
Figure 1 illustrates the number of patients treated with abiraterone or enzalutamide and the number of total providers prescribing these therapies through Medicare Part D by year. The number of patients treated with abiraterone rose from 14,187 in 2013 to 17,044 in 2014 before declining to 16,247 by 2016. In contrast, the number of patients treated with enzalutamide increased throughout the study time period, increasing from 7,326 in 2013 (half the number of patients prescribed abiraterone in the same year), to 17,789 patients by 2016. (Table 1) These patients received a total of 71,423 prescriptions for abiraterone in 2013 and 96,756 prescriptions in 2016. In contrast, prescriptions for enzalutamide increased throughout the years studied from 29,572 in 2013 to 100,980 in 2016. Overall, this translated into patients receiving 5.0–6.0 months of abiraterone and 4.0–5.7 months of enzalutamide with an average cost of $50,657 per patient for a course of abiraterone and $51,018 per patient for a course of enzalutamide in 2016. (Table 1)
Figure 1. Frequency of Beneficiaries Treated and Providers Prescribing Abiraterone or Enzalutamide in Medicare Part D.
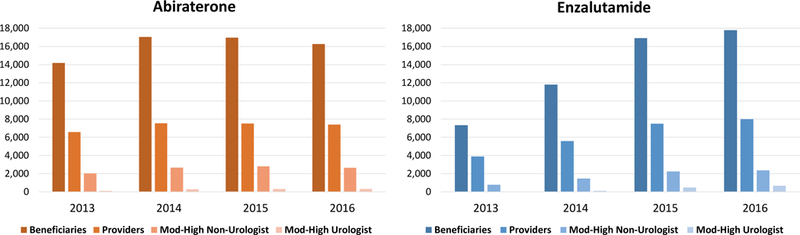
The bars indicate the frequency of patients prescribed abiraterone (orange) and enzalutamide (blue) each year and the frequency of providers writing prescriptions for the treatments, including those urologists and non-urologists who were moderate-high prescribers.
Table 1.
Medicare Part D Public Use File – Abiraterone and Enzalutamide
| 2013 | 2014 | 2015 | 2016 | Total | |
|---|---|---|---|---|---|
| Abiraterone | |||||
| Beneficiaries | 14,187 | 17,044 | 16,963 | 16,247 | - |
| Claims | 71,423 | 98,469 | 100,371 | 96,756 | 367,019 |
| Prescribers | 6,563 | 7,528 | 7,512 | 7,397 | - |
| Low users | 4,523 | 5,398 | 4,398 | 4,464 | - |
| Urologist – moderate | 95 | 278 | 301 | 289 | - |
| Urologist - high | 3 | 11 | 11 | 12 | - |
| Non-urologist – moderate | 1,925 | 2,536 | 2,669 | 2,490 | - |
| Non-urologist – high | 107 | 138 | 133 | 142 | - |
| Cost | $469,537,430 | $707,097,865 | $790,008,643 | $823,026,651 | $2,789,670,589 |
| Cost/beneficiary | $33,096 | $41,487 | $46,572 | $50,657 | - |
| Cost/claim | $6,574 | $7,181 | $7,871 | $8,506 | - |
| Claims/beneficiary | 5 | 5.8 | 5.9 | 6 | - |
| Enzalutamide | |||||
| Beneficiaries | 7,326 | 11,800 | 16,911 | 17,789 | - |
| Claims | 29,572 | 53,980 | 90,112 | 100,980 | 274,644 |
| Prescribers | 3,879 | 5,582 | 7,495 | 7,977 | - |
| Low users | 3,071 | 4,774 | 4,780 | 4,940 | - |
| Urologist – moderate | 21 | 82 | 464 | 651 | - |
| Urologist - high | 1 | 5 | 16 | 20 | - |
| Non-urologist – moderate | 742 | 1,371 | 2,102 | 2,228 | - |
| Non-urologist – high | 44 | 94 | 133 | 138 | - |
| Cost | $231,402,020 | $447,311,084 | $790,628,577 | $907,560,035 | $2,376,901,716 |
| Cost/beneficiary | $31,586 | $37,908 | $46,752 | $51,018 | - |
| Cost/claim | $7,825 | $8,287 | $8,774 | $8,988 | - |
| Claims/beneficiary | 4 | 4.6 | 5.3 | 5.7 | - |
Moderate prescribers prescribed > 10 prescriptions in a year for abiraterone or enzalutamide but to 10 or fewer beneficiaries total. High prescribers prescribed abiraterone or enzalutamide to greater than 10 beneficiaries total in the year. The Total column only includes totals for the number of claims and cost since number of prescribers and beneficiaries can overlap year to year.
Figure 1 and Table 1 also show the frequency of urologists and non-urologists prescribing moderate- to high-volumes of abiraterone and enzalutamide each year, illustrating the majority of providers writing prescriptions for these therapies prescribed a low-volume of therapies (10 or fewer prescriptions each year). Throughout the study timeframe, non-urologists were the majority moderate and high prescribers of abiraterone and enzalutamide. However, the increase in number of urologists who became moderate-high prescribers of abiraterone was substantially higher than the increase among non-urologists. The number of urologists who were moderate-high prescribers of abiraterone increased by 307% from 98 to 301 between 2013 and 2016, as opposed to increasing by 30% for non-urologists (2,032 to 2,632). The number of urologists that were moderate-high prescribers of enzalutamide increased by >3000% from 22 to 671 between 2013 and 2016, as opposed to non-urologist prescriptions which increased by 301% during the same timeframe (786 to 2,366). (Table 1) In total, 1,063 unique urologists were moderate-high prescribers of either abiraterone or enzalutamide between 2013–2016.
To better understand the numbers of urologists prescribing abiraterone and enzalutamide in moderate-high volumes, presumably for patients with castration-resistant disease already receiving ADT, we described the proportion of moderate-high prescribers in the setting of total urologists treating patients for prostate cancer with ADT. The number of urologists treating patients with prostate cancer with ADT through gonadotropin-releasing hormone analog injectables remained relatively stable throughout the study period, ranging from 5,337 to 5,848, with a mean of 5,653 urologists. Therefore, we estimated that in 2016, 5% of urologists who administered androgen deprivation therapy to their patients were moderate to high prescribers of abiraterone and 12% were moderate to high prescribers of enzalutamide.
We then characterized the context in which these urologists practiced to determine which practice contexts (e.g. solo practice, single-specialty, multi-specialty) were most responsible for the increase in use of these therapies. Most moderate–high prescribing urologists were in single-specialty groups (67–68%), 17–18% in solo practices, and 14–15% in multispecialty groups. (Table 2) There were few moderate-high-prescribing urologists in specialty groups (1–2%), which are groups with fewer than 50% urologists and no primary care physicians.
Table 2.
Medicare Part D Public Use File – Urologist Use of Abiraterone and Enzalutamide by Practice Type
| Abiraterone | 2013 | 2014 | 2015 | ||||||
| Total (n=98) | Moderate (n=95) | High (n=3) | Total (n=289) | Moderate (n=278) | High (n=11) | Total (n=312) | Moderate (n=301) | High (n=11) | |
| Solo | 15 | 15 | 0 | 42 | 42 | 0 | 50 | 50 | 0 |
| Single- specialty |
72 | 70 | 2 | 198 | 188 | 10 | 208 | 198 | 10 |
| Specialty | 0 | 0 | 0 | 5 | 5 | 0 | 6 | 6 | 0 |
| Multi-specialty | 11 | 10 | 1 | 44 | 43 | 1 | 48 | 47 | 1 |
| Enzalutamide | 2013 | 2014 | 2015 | ||||||
| Total (n=22) | Moderate (n=21) | High (n=1) | Total (n=87) | Moderate (n=82) | High (n=5) | Total (n=479) | Moderate (n=463) | High (n=16) | |
| Solo | 4 | 4 | 0 | 13 | 13 | 0 | 83 | 83 | 0 |
| Single- specialty |
14 | 13 | 1 | 63 | 60 | 3 | 326 | 311 | 15 |
| Specialty | 0 | 0 | 0 | 1 | 1 | 0 | 4 | 4 | 0 |
| Multi-specialty | 4 | 4 | 0 | 10 | 8 | 2 | 66 | 65 | 1 |
Moderate prescribers prescribed > 10 prescriptions in a year for abiraterone or enzalutamide but to 10 or fewer beneficiaries total. High prescribers prescribed abiraterone or enzalutamide to greater than 10 beneficiaries total in the year. Solo (1–2 physicians total); Single-specialty (>2 physicians and >50% urologists); Specialty (< 50% urologists and no primary care provider); Multi-specialty (< 50% urologists and >1 primary care provider such as internal medicine, family practice, geriatrics). Practice type for urologists who were prescribers in 2016 was not available, so only practice types for those urologists prescribing these drugs in 2013–2015 is shown here.
Figure 2 illustrates where moderate-high prescribing urologists practice in the United States and the increase in number of practices adopting these therapies between 2013 and 2016. In general, the moderate-high prescribing urologists tend to be situated in the eastern and coastal regions of the United States.
Figure 2. Geographic Distribution of Urologists Who Are Moderate-High Prescribers of Abiraterone and Enzalutamide.
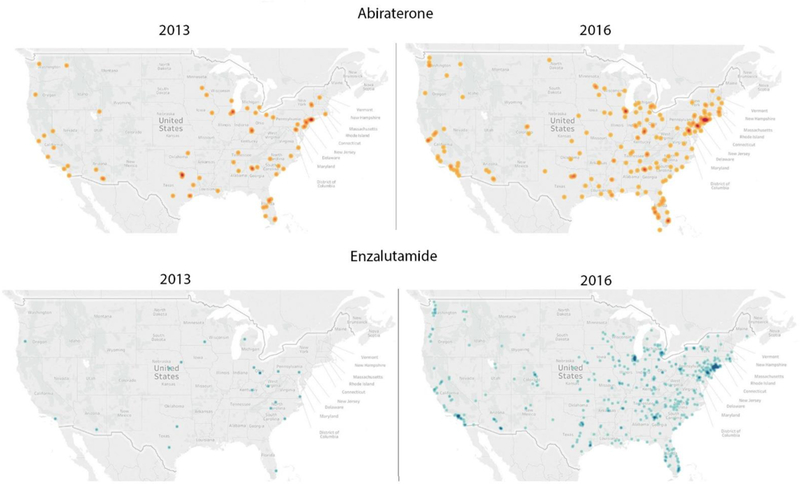
Geographic distribution of urologists who are moderate-high prescribers of abiraterone (top panels) in 2013 and 2016 and moderate-high prescribers of enzalutamide (bottom panels) in 2013 and 2016, demonstrating the increase in urologists prescribing these therapies. Moderate prescribers prescribed > 10 prescriptions in a year for abiraterone or enzalutamide but to 10 or fewer beneficiaries total. High prescribers prescribed abiraterone or enzalutamide to greater than 10 beneficiaries total in the year.
Discussion
Between 2013 and 2016, use of abiraterone and enzalutamide increased, with prescriptions for enzalutamide increasing at a faster rate than those for abiraterone. By 2016, enzalutamide had surpassed abiraterone as the most commonly prescribed secondary oral androgen inhibitor. Even though the majority of prescriptions for abiraterone and enzalutamide were written by low prescribing urologists (≤10 prescriptions each year) and non-urologists, urologists who were moderate–high volume prescribers of abiraterone and enzalutamide increased substantially between 2013 and 2016. By 2016, almost one in eight urologists who treated patients with ADT prescribed moderate- to high-volumes of abiraterone or enzalutamide. Those urologists in single-specialty urology groups appeared to be driving much of the increased prescriptions of both abiraterone and enzalutamide between 2013 and 2016.
Previous work has demonstrated that urologists have been quick to adopt and expand the use of systemic therapies for patients with prostate cancer, mostly through the use of ADT by way of injectable gonadotropin releasing hormone analogs.9 Urologists have also historically expressed interest in delivering and being reimbursed for chemotherapy,5 and are more likely to administer sipuleucel-T than medical oncologists.11 Given urologists’ previous rapid uptake in administering systemic ADT, their expressed desire to broaden the treatment they can offer their patients, and the encouragement and support being offered by their professional organization to do so,8 it is not surprising that urologists have been increasingly adopting use of abiraterone and enzalutamide for patients with advanced prostate cancer.
Since this study was conducted the indications for abiraterone and enzalutamide have continued to expand, further supporting the importance of understanding the extent of urologists’ desired involvement in advanced prostate cancer care. Specifically, abiraterone is now approved for use in the metastatic castration-sensitive setting,12,13 and enzalutamide is approved for use in the non-metastatic castration-resistant setting.14 In addition, apalutamide and darolutamide are oral therapies similar to enzalutamide that have each demonstrated improvement of metastasis-free survival in the non-metastatic castration-resistant setting, similar to enzalutamide.15 These new indications hinge on additional factors such as PSA doubling times, and the extent of metastatic disease (i.e. high and low volume). Providers offering these therapies will be tasked with having an informed discussion about the risks and benefits of different oral therapies in additional disease settings and risks and benefits of other treatment options not traditionally offered by urologists, such as docetaxel in patients with a new diagnosis of metastatic high-volume castration-sensitive disease.
Some patients and urologists may be encouraged and relieved by the prospect of urologists maintaining their active role in treating the cancer as it advances as it may present an opportunity to improve continuity of care provided to these patients and also to expand access to patients in parts of the country where oncology providers are scarce. As urologists increasingly expand their scope of practice into this area there are several important aspects of care they will need to focus on to be successful. Monitoring and addressing toxicities of these oral therapies, both medical and financial, can be addressed at frequent visits and potentially with close coordination with the patient’s primary care provider. Both abiraterone and enzalutamide are oral specialty medications that are only filled through specific specialty pharmacies and commonly associated with high out of pocket expenses. Practices that prescribe these medications frequently are involved with initiating prior authorizations and facilitating financial assistance for patients (e.g. copay coupons, assistance with free drug program forms, and foundation funding), which could potentially be the case for those urology practices that move into this space and increasingly move from being non or low prescribers to moderate-high prescribers. Furthermore, all patients with castration-resistant disease will eventually succumb to their cancer and require discussions surrounding goals of care and aggressive end-of-life management. Many patients may choose to forego treatment in certain situations and opt for palliative care depending on their willingness to accept the risks of therapy, including the financial risk to their family for these oral therapies. Urologist involvement in more of these discussions may present an important opportunity for them to become an active part of a patient’s disease course for which they had not previously been involved.16–20
There were several limitations to this analysis that were mostly related to the database restrictions. First, providers who prescribe 10 or fewer prescriptions for a certain medication are not included in the detailed Medicare Part D Public Use File to protect patient confidentiality. We found the majority of prescribers of abiraterone and enzalutamide were low-volume, so there are likely to be more urologists prescribing abiraterone and enzalutamide during the years studied that we did not capture in our moderate-high volume totals. Furthermore, beneficiary count information is not included for those providers who prescribed more than 10 prescriptions but to fewer than 11 beneficiaries, but we were still able to use aggregated totals to calculate the average number of months patients were on a medication and the average cost per patient. Second, since the public use file reports on data aggregated at the patient level, we conducted our analysis at the provider level and were unable to look at patient factors that may influence prescribing of these therapies, including quality metrics of patients prescribed these medications by different specialists and in the different practice types. Finally, it is important to note that the Medicare Part D plans only encompass two thirds of the patients receiving care through Medicare and mostly accounts for patients over 65. Nevertheless, it is likely that the patterns of treatment by providers we observed would likely apply to prostate cancer patients who are younger, or have other forms of health insurance.
Conclusion
Urologists are prescribing oral therapies for advanced prostate cancer at increasing rates each year and mostly in larger single-specialty practices. Understanding the distribution of urologists specializing in these advanced prostate cancer therapeutics will help guide future interventions aimed at optimizing the value of care provided to patients, something that will increase in importance as more oral therapies are approved (e.g. apalutamide and darolutamide) and become approved earlier in the disease course of patients. The increase in urologists providing care to patients in the later stages of their disease course may provide an important opportunity for urology practices to partner with patients’ primary care providers, social workers, and palliative care to become more involved in patients’ care through monitoring of toxicities, financial counseling, and end of life care. However, it is important that all providers treating patients with these drugs, regardless of specialty or oncology fellowship training, understand the indications for their use and are able to have an informed discussion with patients about all available therapies and their toxicities so that the treatment chosen for patients best aligns with their values and preferences.
Acknowledgement of Research Support:
This work was supported by funding from AHRQ (R01 HS 025707). Dr. Skolarus is funded by the National Cancer Institute (R01 CA222885-01).
Footnotes
Conflicts of interest: The authors have no competing interests and nothing to disclose.
References
- 1.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187–97, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford ED: The role of the urologist in treating patients with hormone-refractory prostate cancer. Rev Urol 5 Suppl 2:S48–52, 2003 [PMC free article] [PubMed] [Google Scholar]
- 6.Sartor AO, Fitzpatrick JM: Urologists and oncologists: adapting to a new treatment paradigm in castration-resistant prostate cancer (CRPC). BJU Int 110:328–35, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Shore ND: Chemotherapy for prostate cancer: when should a urologist refer a patient to a medical oncologist? Prostate Cancer Prostatic Dis 16:1–6, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Lowrance WT, Roth BJ, Kirkby E, et al. : Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2015. J Urol 195:1444–52, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Shahinian VB, Kuo YF, Freeman JL, et al. : Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst 98:839–45, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caram MEV, Borza T, Min HS, et al. : Early National Dissemination of Abiraterone and Enzalutamide for Advanced Prostate Cancer in Medicare Part D. J Oncol Pract 13:e694–e702, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caram MEV, Ross R, Lin P, et al. : Factors Associated With Use of Sipuleucel-T to Treat Patients With Advanced Prostate Cancer. JAMA Netw Open 2:e192589, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 377:352–360, 2017 [DOI] [PubMed] [Google Scholar]
- 13.James ND, de Bono JS, Spears MR, et al. : Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 377:338–351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain M, Fizazi K, Saad F, et al. : Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 378:2465–2474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MR, Saad F, Chowdhury S, et al. : Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 378:1408–1418, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Suwanabol PA, Kanters AE, Reichstein AC, et al. : Characterizing the Role of U.S. Surgeons in the Provision of Palliative Care: A Systematic Review and Mixed-Methods Meta-Synthesis. J Pain Symptom Manage 55:1196–1215 e5, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Suwanabol PA, Reichstein AC, Suzer-Gurtekin ZT, et al. : Surgeons’ Perceived Barriers to Palliative and End-of-Life Care: A Mixed Methods Study of a Surgical Society. J Palliat Med 21:780–788, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergman J, Lorenz KA, Acquah-Asare S, et al. : Urologist attitudes toward end-of-life care. Urology 82:48–52, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Griebling TL: Re: Community-partnered collaboration to build an integrated palliative care clinic: the view from urology. J Urol 193:1617, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Galante JM, Bowles TL, Khatri VP, et al. : Experience and attitudes of surgeons toward palliation in cancer. Arch Surg 140:873–8; discussion 878–80, 2005 [DOI] [PubMed] [Google Scholar]


