Abstract
Objective
To examine the association between cortisol response to stress and suicidal ideation (SI) cross-sectionally and longitudinally in our sample of bereaved and non-bereaved youth.
Methods
The sample included 114 youth bereaved by sudden parental death and 109 non-bereaved controls, mean age of 12.3 (SD=3.6), evaluated at four time-points over an average follow-up period of 7 years. The Trier Social Stress Test (TSST) was conducted on average 6 years after bereavement. We used latent class analyses to examine the trajectories of SI over follow-up and up to the time of the TSST and compare them on cortisol measures. We examined whether cortisol measures predicted future SI at 18.5 months on average after the TSST.
Results
Bereavement was associated with higher cortisol reactivity after controlling for covariates [β=0.96, 95% CI (0.28, 1.65), p<0.01, d=0.41]. Cortisol reactivity to stress was higher in those belonging to the high SI trajectory [β=1.23, 95% CI (0.41, 2.06), p=0.004, d=0.23] compared to the low SI trajectory. Higher baseline cortisol showed small to medium effect size in predicting future SI [β=2.34, 95% CI (0.17, 4.51), p=0.03, d=0.38].
Conclusion
The persistence of SI is associated with higher cortisol reactivity to stress, and higher baseline cortisol may predict future SI. These results emphasize the importance of HPA-axis activity in youth exposed to major stressors, and those with SI. More research is needed to further clarify biological mechanisms linking SI and behavior, bereavement, and HPA axis response to stress, to better identify at-risk subjects for targeted prevention and intervention efforts.
INTRODUCTION
Parental death occurs in 4% of youth under the age of 18 and is an extremely stressful form of childhood adversity (1). It has a major impact on the psychological development of children and increases the risk for a wide range of psychopathology, including depression, post-traumatic stress disorder (PTSD), anxiety, conduct problems, substance abuse, and functional impairment, all of which are risk factors for suicidal behavior (2, 3). Parental death from any cause during childhood was found to be a significant risk factor for suicide among offspring, especially in those who were bereaved by suicide (4–6).
While the association between parental death and risk for suicidal behavior has been well-established, the mechanisms by which parental death leads to increased risk for suicidal behavior are not known. One possible mechanism is via alterations in the hypothalamic-pituitary-adrenal axis (HPA) axis. In fact, parental death has also been associated with altered HPA axis response to stress (7). Altered basal cortisol or cortisol response to stress have also been linked to risk for suicidal behavior (8). However, there are no studies that concomitantly link parental death, alterations in HPA axis function, and risk for suicidal behavior.
Studies suggest that HPA axis dysregulation occurs more commonly in individuals who lost their parents as children (9). Adults who experienced parental death during childhood had been reported to have higher diurnal cortisol, especially if they experienced childhood abuse and family conflict (10). Children who lost a caregiver in the 9/11 terrorist attack exhibited persistently higher basal cortisol levels compared to non-bereaved children (11). We previously showed that sudden parental death is associated with higher total cortisol output in response to social stress in bereaved youth (7).
The role of the HPA-axis in suicidal behavior was robustly demonstrated in a meta-analysis where non-suppression in response to the Dexamethasone Suppression Test (DST) was related to a 4.5-fold increased risk for suicide in adult inpatients (12). Melhem and colleagues found lower baseline and total cortisol output during the Trier Social Stress Test (TSST) in early-onset suicide attempters compared to high-risk subjects who never attempted suicide and healthy controls (13). This blunted HPA axis profile was observed mostly in suicide attempters who also had a family history of an attempt. O’Connor et al. reported similar findings in demonstrating that suicide attempters, especially those with a family history of suicidal behavior, and those who made the attempt within the last year, had the lowest total cortisol output in response to the TSST compared to controls (14). O’Connor et al. also found that blunted cortisol response to the TSST in suicide attempters was associated with a higher level of suicidal ideation one month after the TSST. Similarly, Keilp et al. found that adult suicide attempters had lower baseline cortisol levels compared to non-attempters (15) and McGirr et al. found blunted cortisol response in first-degree relatives of those who died by suicide compared to controls (16).
Melhem et al. extended these findings to show that blunted HPA axis activity most likely precedes suicide attempt (17). They found that psychiatric inpatients admitted for a suicide attempt had lower hair cortisol concentrations (HCC), which provides a measure of overall cortisol production in previous months, compared to those admitted for suicidal ideation but had never attempted suicide and to healthy controls. Specifically, in the above-noted study, HCC obtained from 2–3 cm hair segments closest to the scalp reflected cortisol levels for the prior 2–3 months, which would have been prior to the attempt in Melhem et al.’s study design. However, other studies found that hyper-responsiveness of the HPA axis to social stress was associated with current and future suicidal ideation (18). These discrepant findings could be due to differences in outcome measures (ideation vs. attempt), age differences, and the population studied with respect to their burden of psychopathology, history of childhood adversity, and whether they were responding to acute or chronic stress.
Thus, the relationships of the HPA axis to both suicidality and bereavement have been investigated independently. Here, we examine the relationship of HPA-axis response to stress and suicidal ideation in our sample of children bereaved by sudden parental death. We hypothesize that bereavement and suicidal ideation will each, independently be related to baseline cortisol, total cortisol output, and cortisol reactivity in response to the TSST, and that cortisol measures will predict future suicidal ideation.
METHODS
Study sample
The sample in this paper is drawn from our longitudinal study on the impact of sudden parental death on children and families. A total of 427 bereaved and non-bereaved youth were enrolled in the study, of whom 223 (52%) completed the acute laboratory social stress task and were available for follow-up. The 223 youths included 114 offspring from 79 parentally-bereaved families and 109 offspring from 65 non-bereaved families with a mean age of 12.3 (standard deviation [SD] =3.6, range: 6–25 years). The demographics and clinical variables’ characteristics of the study groups are presented in supplementary table 1. The deceased parents (probands) died within 24 hours of either suicide (n = 29), accidental death (n = 17), or sudden natural death (n = 33). Families in which there were multiple deaths or injuries were excluded. The accidental deaths consisted of 8 drug-overdoses, 4 motor vehicle accidents, 1 accidental fall, and 4 others (e.g., drowning, exposure to cold). The sudden natural deaths were due to myocardial infarction (n = 26), infection (n = 1), and 6 less frequent causes (e.g., diabetes mellitus, stroke, aneurysm, gastric bypass surgery). Non-bereaved offspring had two living biological parents, lived in the home of at least one of them, and had no first-degree relatives who had died within the two years prior to recruitment. Bereaved and non-bereaved families were frequency matched on sex, age, and neighborhood of the deceased proband. This study was approved by the University of Pittsburgh Institutional Review Board, and all participants gave written consent or assent.
Most of the demographic and clinical characteristics of our sample who participated in the TSST did not differ from the rest of our sample that did not. Those who participated in the TSST were less likely to be bereaved (51.1% vs. 62.8%, χ21=5.86, p=0.02) and more likely to be Caucasian (85.7% vs. 77.0%, χ21=5.34, p=0.02). Probands of offspring participating in the TSST were less likely to have a history of mood disorder (40.5% vs. 56.2%, χ21=10.43, p=0.01) and anxiety disorder (18.7% vs. 30.3%, χ21 = 7.60, p = 0.01) prior to death.
Assessments
Offspring and surviving caregivers completed assessments at their homes, which consisted of a structured diagnostic interview and questionnaires regarding psychiatric symptoms, health risk behaviors and other measures of well-being. For the bereaved group, the first assessment was around 9 months after the death. Follow-up assessments were conducted at 21, 33, 62, and 84 months after the death by experienced clinical interviewers, with offspring and parents interviewed separately to keep them blind to one another.
Retrospective assessment was used to determine if disorders had their onset before or after parental death. For non-bereaved offspring, we recorded “new-onset disorder” as having occurred in the 9 months prior to the baseline assessment. The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version and the Structured Clinical Interview for DSM-IV (SCID) Axis I Disorders (19) were used for the diagnostic assessment of children and adults, respectively. A psychological autopsy was conducted to assess the deceased parents’ lifetime history of psychiatric disorders (20). Personality disorders in adult participants were assessed with the Structured Interview for DSM-IV Personality Disorders (21). The Longitudinal Interview for Follow-Up Evaluations was used to document the course of disorders in offspring (22). High inter-rater reliability was maintained for psychiatric diagnoses (κ’s=0.74–0.85, N=107) and for functioning (intraclass correlation=0.92, 95% CI 0.90–0.93, N=734). To assess general functioning, we used the Children’s Global Assessment Scale (CGAS) as a basic quantification of functioning at home and school for participants younger than 18 years old and the Global Assessment of Functioning (GAF) for those who are older than 18 years old (23, 24). Socioeconomic status (SES) was assessed using the Hollingshead’s scale at intake (25).
Suicidal ideation was assessed using the Suicidal Ideation Questionnaire-Junior (SIQ-JR) (26). This questionnaire is designed to measure and quantify suicidal wishes and intent. Items content range from general death wishes to specific thoughts of self-injurious behavior including method and time. For each of the 15 items of the SIQ-JR, the respondent is asked to assess the frequency in which the thought has occurred in the past month. Responses are recorded on a 7- point Likert format scale ranging from “I never had this thought” to “Almost every day”. The items are scored from 0 to 6 in terms of increasing frequency of suicidal cognitions. The total scale score ranges from 0 (none of the thoughts has ever occurred) to 00 (each cognition has happened almost every day). Reynolds et al. investigated the reliability of the SIQ-JR and found it to have Cronbach’s alpha of 0.91 (26).
Cortisol response to stress
During follow-up, bereaved and nonbereaved offspring participated in a modified version of the Trier Social Stress Test (TSST) (27), a procedure designed to induce a moderate stress response. The TSST was conducted on average 6 years (SD=1.3, range: 2.8–9.2) after bereavement in bereaved youth. Participants were asked to refrain from alcohol or smoking in the 24 hours prior to the test, and from eating, and ingesting caffeine or dairy products in the 2 hours prior to the test. The TSST was administered in the afternoon (median time 3:00 PM). Offspring watched a 10-minute relaxing travel video after which baseline cortisol sample was obtained (time 0 minutes). For the next 15 minutes, participants were asked to prepare and deliver a brief speech, and then perform a mental arithmetic task while being observed by research staff. Salivary cortisol samples were obtained immediately after the social stress task (15 minutes after baseline), and 5, 10, 20 minutes after the social stress task (20, 25, 35 minutes after baseline). Containers were kept at −20°C until they were assayed. Saliva samples were sent to Salimetrics, Inc, State College, Pennsylvania, where they were extracted and assayed in duplicate using a high-sensitive enzyme immunoassay with a range of sensitivity from 0.007 to 1.8 ug/dl. The average intra- and inter-assay coefficients of variation were 4.13% and 8.89%, respectively. When using mixed effects linear regression, we found a significant increase in cortisol levels over the time throughout the TSST (β=0.11, 95% CI: 0.05, 0.17, p=0.001), with the peak observed 20–25 minutes after the stressor consistent with expectations. For our analyses, we used: (1) baseline cortisol (pre-stress); (2) total cortisol output; and (3) cortisol reactivity. Total cortisol output was computed using the area under the curve with respect to ground (AUCg), and cortisol reactivity using area under the curve with respect to increase (AUCi) following the trapezoid method via raw values (28). These cortisol measures are complementary to each other: (1) baseline cortisol level represents the cortisol level prior to the introduction to the acute social stress, whereas both AUCg and AUCi capture the responsiveness of the HPA axis to the acute stressor. AUCi represents cortisol reactivity to stress, which is the area under the curve between baseline and peak cortisol levels. AUCg is the total cortisol output throughout the stressor taking into account baseline cortisol levels. The correlation was high between baseline cortisol and AUCg (Pearson r=0.9, p<0.01), low between baseline cortisol and AUCi (Pearson r=−0.11, p=0.09) and low but significant between AUCg and AUCi (Pearson r=0.26, p<0.01).
Statistical analysis
We examined the distribution of the three cortisol measures and used a natural log transformation of baseline and total cortisol output (micrograms/deciliter units) since they were right-skewed. We examined the relationships of each cortisol measure with suicidal ideation at the time of the TSST using linear regressions with each cortisol measure as the dependent variable and suicidal ideation at the time of the TSST as the independent variable and controlling for bereavement. We examined the relationship of cortisol measures with demographic and clinical variables to identify significant covariates to include in our regression models (Table 1) and controlled for covariates known to potentially affect cortisol levels (29, 30). The covariates included in the final model were age, sex, race, SES, time of the day when the TSST occurred, body-mass index (BMI), smoking status, family history of suicide attempt, family history of psychopathology, and the participant’s lifetime history of psychopathology up-to-the time of the TSST. We used Bonferroni correction to account for multiple comparisons with α =0.017 (0.05/3) as our threshold for significance.
Table 1:
Demographic and clinical characteristics by baseline cortisol, total cortisol output, and cortisol reactivity
| Baseline (ln) | AUCg (ln) (total cortisol) | AUCi (cortisol reactivity) | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Bereavement | 0.14 (−0.04, 0.32) | 0.12 | 0.22 (0.31, 0.40) | 0.02 | 0.47 (−0.06, 1.02) | 0.08 |
| Suicidal ideation at the time of TSST | 0.0004 (−0.01, 0.01) | 0.90 | 0.0002 (−0.01, 0.01) | 0.96 | 0.25 (−0.52, 1.03) | 0.52 |
| Demographics | ||||||
| Age at parent’s death | 0.46 (0.02, 0.07) | 0.001 | 0.04 (0.02, 0.07) | 0.002 | −0.02 (−0.08, 0.05) | 0.59 |
| Female Sex | −0.18 (−0.35, −0.01) | 0.03 | −0.22 (−0.38, −0.03) | 0.02 | −0.09 (−0.66, 0.48) | 0.76 |
| Race | 0.31 (0.06, 0.55) | 0.01 | 0.37 (0.16, 0.58) | 0.001 | 0.38 (−0.01, 0.78) | 0.06 |
| Socio-economic status | −0.01 (−0.01, −0.001) | 0.03 | −0.004 (−0.01, 0.002) | 0.22 | 0.02 (−0.004, 0.04) | 0.11 |
| BMI | −0.001 (−0.01, 0.01) | 0.90 | −0.002 (−0.01, 0.01) | 0.66 | −0.01 (−0.04, 0.03) | 0.68 |
| Participant ate§ | −0.19 (−0.41, 0.04) | 0.10 | −0.21 (−0.42, −0.01) | 0.04 | 0.28 (−0.59, 1.16) | 0.52 |
| Participant had caffeine§ | 0.16 (−0.01, 0.33) | 0.06 | 0.18 (−0.003, 0.37) | 0.05 | −0.04 (−0.59, 0.51) | 0.89 |
| Participant had milk products§ | −0.04 (−0.21, 0.13) | 0.61 | −0.03 (−0.21, 0.15) | 0.76 | 0.07 (−0.46, 0.60) | 0.80 |
| Participant smoked§ | 0.24 (−0.01, .048) | 0.06 | 0.14 (−0.09, 0.36) | 0.24 | −1.00 (−1.78, −0.23) | 0.01 |
| Participant rinsed mouth† | −0.017 (−0.33, 0.29) | 0.91 | −0.20 (−0.50, 0.11) | 0.20 | −1.20 (−2.51, 0.12) | 0.07 |
| Participant on medication(s) | −0.01 (−0.18, 0.16) | 0.93 | −0.04 (−0.22, 0.14) | 0.65 | −0.31(−0.85, 0.24) | 0.27 |
| Offspring Lifetime Psychiatric Disorder up to TSST | ||||||
| Physical/sexual abuse | −0.10 (−0.31, 0.1) | 0.33 | −0.19 (−0.40, 0.05) | 0.12 | −0.33 (−0.85, 0.12) | 0.22 |
| Attention deficit hyperactivity disorder | 0.03 (−0.20, 0.27) | 0.80 | −0.02 (−0.28, 0.24) | 0.88 | −0.54 (−1.14, 0.05) | 0.07 |
| Alcohol and substance abuse | 0.26 (0.04, 0.49) | 0.02 | 0.24 (0.01, 0.47) | 0.04 | −0.18 (−0.98, 0.62) | 0.66 |
| Anxiety | −0.05 (−0.21, 0.12) | 0.55 | −0.12 (−0.29, 0.06) | 0.19 | −0.33 (−0.85, 0.19) | 0.21 |
| Behavioral disorder | −0.11 (−0.41, 0.12) | 0.48 | −0.18 (−0.47, 0.10) | 0.20 | −0.63 (−1.37, 0.10) | 0.09 |
| Depression | 0.08 (−0.09, 0.26) | 0.35 | 0.07 (−0.11, 0.25) | 0.44 | −0.12 (−0.68, 0.44) | 0.67 |
| Bipolar disorder | 0.18 (−0.20, 0.57) | 0.35 | 0.10 (−0.30, 0.51) | 0.61 | −0.31 (−1.45, 0.84) | 0.60 |
| Any mood disorder | 0.09 (−0.06, 0.23) | 0.24 | 0.06 (−0.09, 0.21) | 0.41 | −0.13 (−0.57, −0.31) | 0.55 |
| Posttraumatic stress disorder | 0.18 (−0.10, 0.47) | 0.20 | 0.22 (0.004, 0.44) | 0.05 | −0.38 (−1.44, 0.67) | 0.47 |
| Family History up to TSST | ||||||
| Family history of psychopathology | 0.68 (0.25, 0.38) | 0.67 | 0.02 (−0.35, 0.39) | 0.91 | −0.47 (−1.68, 0.75) | 0.45 |
| Family history of suicidality | 0.06 (−0.15, 0.27) | 0.57 | 0.08 (−0.15, 0.31) | 0.50 | 0.16 (−0.48, 0.81) | 0.62 |
On day of TSST
Before TSST
To examine whether cortisol levels predict future suicidal ideation at the next follow-up assessment, which took place on average 18.5 months (SD=6.9, range 0–40) after the TSST, we first examined the distribution the SIQ score, which was highly skewed to the right, even after applying various transformations. Hence, we conducted the analysis using generalized estimate equations (GEE) in which the continuous suicidal ideation score was used as the dependent variable and each cortisol measure as the predictor variable.
We also examined the relationship between cortisol measures and the trajectories of suicidal ideation up to the time of the TSST using linear regression and controlling for the above-mentioned covariates. The trajectories were identified using Latent Class Analysis, beginning with a 1-class model and increasing the number of classes until the model that best fit the data was obtained. The 2-class model was used because the Lo-Mendell-Rubin Adjusted Likelihood Ratio Test identified the 2-class model to have a better fit compared to the 3-class model (χ2=98.01, df=3, P= 0.29).
Statistical analyses were conducted using Stata version 15 (2015; Stata Corp LP, Stata Statistical Software, College Station, TX) and Mplus 8 (Muthén & Muthén, 1998–2018) for Latent Class Analysis.
RESULTS
Relationships of bereavement and suicidal ideation at the time of the TSST on individual cortisol measures
Table 1 shows that bereavement and suicidal ideation at the time of TSST were not significantly associated with any of the cortisol measures at p<0.017. Younger and Caucasian participants had a significantly higher baseline and total cortisol output. Smoking on the day of the TSST was negatively associated with cortisol reactivity. When controlling for covariates, there were no main effects of suicidal ideation on any of the cortisol measures. Bereavement was associated with higher cortisol reactivity after controlling for covariates [β= 0.96, 95% CI (0.28, 1.65), p<0.01, d=0.41] (Supplementary Table 2). When examining the bereavement by suicidal ideation interactions as related with cortisol measures, the results were non-significant (p’s>0.06).
Trajectories of suicidal ideation, bereavement, and cortisol measures
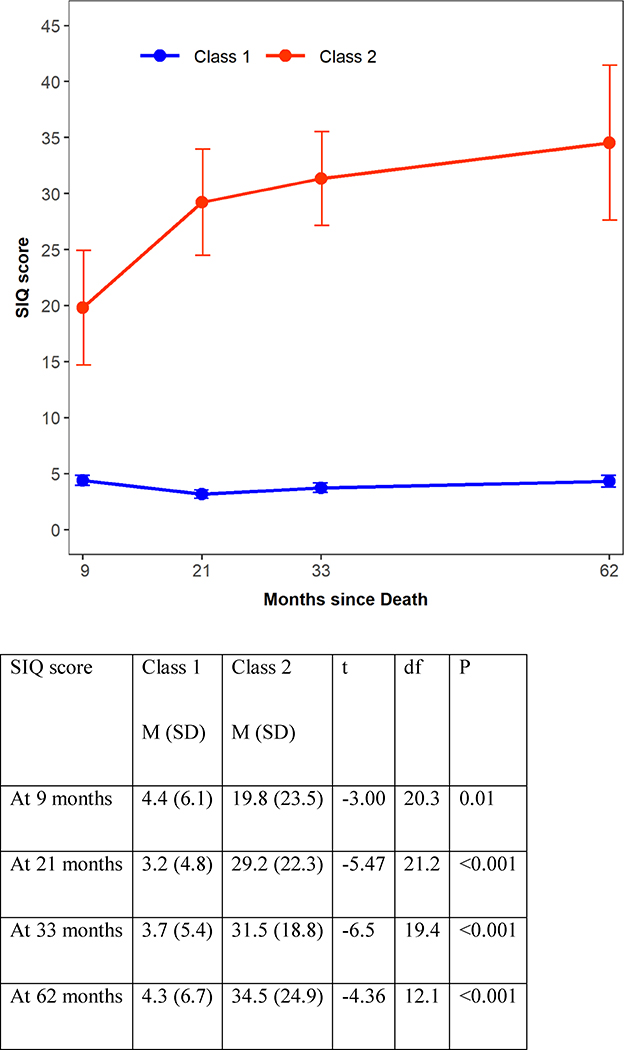
Latent class analysis for suicidal ideation trajectory up-to-the time of the TSST identified two distinct classes: Class 1 with 199 participants who consistently showed low SIQ scores and Class 2 with 22 participants who consistently had high scores on the SIQ. The SIQ scores of these latent classes are shown in Figure 1. We compared these two classes on baseline demographic and clinical characteristics. Class 2, the trajectory with consistently higher suicidal ideation, was more likely than those in Class I to be bereaved (47.2% in class I vs. 81.8 % in class 2, χ2=9.48, p=0.002, Cohen’s d=0.71), have any history of psychopathology (20.3% in class I vs. 40.9% in class 2, Fisher Exact Test (FET), p=0.05, Cohen’s d=0.50), mood disorders (2.5% in class I vs. 13.6% in class 2 for Bipolar Disorder, 33.7% in class I vs. 77.3% in class 2 for Major Depression Disorder, FET, p<0.001, Cohen’s d=1.24) , PTSD (7.5% in class I vs. 22.7% in class 2, FET, p=0.03, Cohen’s d=0.54), and to show increased functional impairment (CGAS score of 79.7 in class I vs. 74.0 in class 2, t=2.26, p=0.02, Cohen’s d=1.18) (Table 2).
Figure 1:
Two-class Latent Class Analysis of suicidal ideation over time.
Table 2.
Demographic and Clinical Characteristics of the study classes.
| Class 1 (low SIQ) | Class 2 (high SIQ) | Test | P | d | |
|---|---|---|---|---|---|
| N | 199 | 22 | |||
| Bereaved, N (%) | 94 (47.2) | 18 (81.8) | χ2=9.48 | 0.002 | 0.71 |
| Sex, N (% male) | 108 (54.3) | 7 (31.8) | χ2=4.00 | 0.04 | 0.45 |
| Race, N (% white) | 172 (86.4) | 17 (77.3) | FET | 0.33 | 0.26 |
| Age at parent’s death, M (SD) | 12.4 (3.6) | 11.6 (3.4) | t=0.95 | 0.34 | 0.21 |
| Socio-economic status, M (SD) | 46.7 (12.5) | 44 (13.6) | t=0.87 | 0.38 | 0.22 |
| Diagnosis up to TSST, N (%) | |||||
| Physical/sexual abuse | 20 (10.1) | 4 (18.2) | FET | 0.27 | 0.26 |
| ADHD | 36 (18.1) | 7 (31.8) | FET | 0.15 | 0.35 |
| Alcohol and substance abuse | 30 (15.1) | 5 (22.7) | FET | 0.36 | 0.21 |
| Anxiety | 44 (22.1) | 7 (31.8) | χ2=1.05 | 0.30 | 0.23 |
| Behavioral disorder | 22 (11.1) | 2 (9.1) | FET | >0.99 | 0.06 |
| Mood disorder - bipolar§ | 5 (2.5) | 3 (13.6) | FET | <0.001 | 1.24 |
| Mood disorder - depression§ | 67 (33.7) | 17 (77.3) | |||
| Posttraumatic stress disorder | 15 (7.5) | 5 (22.7) | FET | 0.03 | 0.54 |
| Any diagnosis | 119 (59.8) | 21 (95.5) | χ2=10.85 | 0.001 | 0.76 |
| Past diagnosis before death | 40 (20.3) | 9 (40.9) | FET | 0.05 | 0.50 |
| Family history of suicidality up to TSST | 49 (24.6) | 8 (36.4) | χ2=1.43 | 0.23 | 0.27 |
| Family lifetime psychopathology up to TSST | 183 (92) | 22 (100) | FET | 0.38 | 0.31 |
| BMI, M (SD) | 25.5 (6.5) | 25.5 (7.3) | t=−0.04 | 0.96 | 0.01 |
| GAS at baseline, M (SD) | 79.7 (11.2) | 74.0 (4.6) | t=2.26 | 0.02 | 1.18 |
| Cortisol | |||||
| Baseline (ln) | −2.1 (0.6) | −2.1 (0.5) | t=0.54 | 0.59 | 0.12 |
| Total (ln) | 1.5 (0.6) | 1.5 (0.6) | t=−0.21 | 0.83 | −0.05 |
| Reactivity | −0.02 (2.0) | 0.7 (1.7) | t=−1.52 | 0.13 | −0.34 |
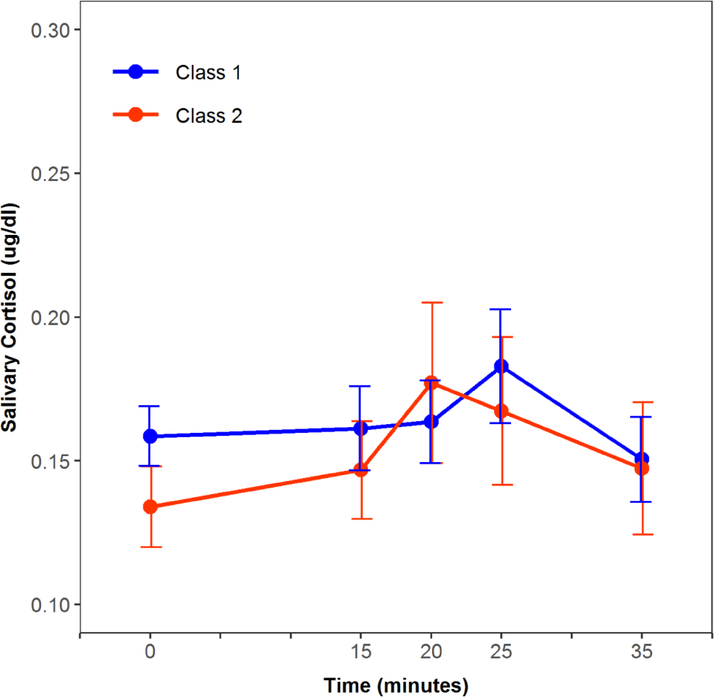
When controlling for covariates, participants in Class 2 showed higher cortisol reactivity compared to those in Class 1 [β =1.23, 95% CI (0.41, 2.06), p=0.004, d=0.23] (Table 3, Figure 2). In this model, bereavement was also associated with higher cortisol reactivity (β =0.87, 95% CI (0.3, 1.43), p=0.003, d=0.23). There were no statistically significant differences between the classes on baseline and total cortisol output, and the bereavement by class interaction was statistically non-significant too (p’s>0.11).
Table 3.
Relatioship between suicidal ideation (SI) Classes and cortisol reactivity (AUCi).
| β (95% CI) | t | P | d | |
|---|---|---|---|---|
| High SI class§ | 1.23 (0.41, 2.06) | 2.98 | 0.004 | 0.23 |
| Bereaved | 0.87 (0.30, 1.43) | 3.05 | 0.003 | 0.23 |
| Family history of suicidality up to TSST | −0.16 (−0.96, 0.64) | −0.40 | 0.69 | 0.03 |
| Family history of psychopathology up to TSST | −0.17 (−0.95, 0.61) | −0.42 | 0.67 | 0.03 |
| Offspring’s history of psychopathology up to TSST | −0.57 (−1.24, 0.09) | −1.70 | 0.09 | 0.13 |
| Age at parent’s Death | 0.02 (−0.05, 0.09) | 0.51 | 0.61 | 0.04 |
| Female sex | 0.04(−0.57, 0.65) | 0.12 | 0.9 | 0.01 |
| White race | 0.38 (−0.17, 0.94) | 1.36 | 0.18 | 0.10 |
| Socio-economic status | 0.01 (−0.02, 0.04) | 0.87 | 0.38 | 0.07 |
| Time of TSST | −0.24 (−0.45, −0.03) | −2.24 | 0.03 | 0.17 |
| BMI | −0.002 (−0.05, 0.05) | −0.06 | 0.95 | 0.01 |
| Smoking | −1.50 (−2.54, −0.45) | −2.85 | 0.005 | 0.22 |
d=Cohen’s d.
As compared to Low SI class, the reference category
Figure 2.
Cortisol response to the social stress test by suicidal ideation (SI)-class
Class 1: low SI, Class 2: high SI.
Cortisol response to stress as predictors of suicidal ideation
Higher baseline cortisol was a predictor of suicidal ideation 18.5 months (SD=6.9, range 0–40) after the TSST [β =2.34, 95% CI (0.17, 4.51), p=0.03, d=0.38]; however, the p value did not reach the significance level of 0.017. Total cortisol output and cortisol reactivity did not predict suicidal ideation 18.5 months later.
DISCUSSION
There were no significant relationships between baseline cortisol, total cortisol output, and cortisol reactivity with suicidal ideation at the time of the TSST. However, participants belonging to the high suicidal ideation trajectory showed greater cortisol reactivity. There were no significant interactions between bereavement and suicidal ideation on each of the cortisol measures. As hypothesized, we found that parental bereavement was associated with greater cortisol reactivity.
These results should be interpreted in light of the following strengths and limitations. These results come from a large prospective study of bereavement due to sudden parental death in which the sample is well characterized in terms of psychopathology for 84 months or a 7 -year period after parental death. The TSST was conducted around 6 years after parental death and as such, it is difficult to assess whether bereavement or the psychiatric consequences of bereavement resulted in alterations in cortisol response to stress. However, we controlled for lifetime history of psychopathology and suicidal ideation and behavior in the offspring and their parents up to the time of the TSST. While the TSST has been validated and widely used in prior studies, cortisol responses to an experimental stressor may not necessarily reflect responses to real-life stressful situations. Finally, these results are limited to offspring bereaved by sudden parental death and may not generalize to those bereaved by other types of death (e.g., due to illness, homicide) and the loss of other loved ones (e.g., siblings).
In this sample, we did not find significant associations between each of baseline and total cortisol output levels with suicidal ideation at the time of TSST. Both HPA axis hypo- and hyper- activity are described in attempters and subjects at risk for suicidal behavior (16, 31–39). However, these results are consistent with our prior studies showing that only suicide attempters have a distinct HPA axis profile. We previously reported that suicide attempters, offspring of parents with mood disorders, showed blunted HPA-axis activity as reflected by lower baseline and total cortisol output compared to other high-risk offspring with suicide-related behavior (including ideation) but never attempted, non-suicidal offspring, and healthy controls and controlling for psychopathology (13). Other independent studies similarly showed suicide attempters to have a lower total cortisol output in response to social stress compared to age and sex-matched healthy controls (13–15). The absolute levels of baseline cortisol and total cortisol output in our bereaved youth with suicidal ideation are comparable to those with suicide-related behavior in our high-risk sample of offspring of parents with mood disorders, which were also similar to non-suicidal and healthy controls.
However, we found that the trajectory with high suicidal ideation had significantly higher cortisol reactivity to stress vs. those belonging to the trajectory of low suicidal ideation throughout the study and up to the time of the TSST (figure 2). This is consistent with prior findings showing that hyper-responsiveness of the HPA axis to social stress is associated with suicidal ideation (18). In contrast to our findings of no relationship between suicidal ideation at the time of the TSST and cortisol reactivity, participants belonging to the trajectory of high suicidal ideation represent a more severe group with chronic suicidal ideation. Nevertheless, there are a few discrepant findings in the literature. For example, Melhem et al showed that cortisol reactivity is not different in suicide attempters compared to other high-risk groups and healthy controls, and O’Connor et al did not find differences in any cortisol measure between those with suicidal ideation and healthy controls (13, 14). We also found that parental bereavement was associated with greater cortisol reactivity. We previously reported that youth bereaved by sudden parental death have higher total cortisol output in response to social stress compared to controls (7). We also showed that bereaved youth had a higher incidence of depression, PTSD, and long-lasting functional impairment compared to non-bereaved (3, 40–42). Dysregulation of the HPA-axis has been linked to a variety of mental disorders. Both hyper- as well as hypo-activity of the HPA-axis, have been associated with heightened risk for developing psychopathology (18). There is also evidence of blunted or normal cortisol responses to stress in depression (39, 40) and normal basal cortisol levels in PTSD (43); that the type and chronicity of depression are associated with distinct HPA axis dysregulations (44–46); and that low cortisol in PTSD is only observed under certain conditions (e.g., abuse, females)(47). Childhood adversity is also associated with blunted HPA axis activity (48). When trying to reconcile these mixed results it should be considered that the age groups and the burden of psychopathology examined in these studies were different. Previous studies have shown cortisol response to stress to differ by age. It was suggested accordingly that there is a non-linear inverted U-shaped relationship between cortisol levels and suicidal ideation where both high and low levels of cortisol are harmful and associated with suicidal ideation (14, 18). In this study, we focused on suicidal ideation in youth (mean age of 12), whereas in Melhem et al.’s study they focused on a higher-risk sample of young adults (mean age of 27) and included suicide attempts and in O’Connor et al.’s study they focused on adult females (mean age of 26). Future prospective studies are needed to further clarify and examine HPA axis responses to stress across the spectrum of psychopathology and suicidal risk profile and to deepen our understanding of the biological mechanisms linking suicidality, bereavement or adversity, and HPA axis dysregulation.
We found that higher baseline cortisol levels may predict more suicidal ideation 18.5 months following the TSST [β =2.34, 95% CI (0.17, 4.51), p=0.03, d=0.38]. Although the result did not reach statistical significance when correcting for multiple comparisons, there was a small to medium effect size. In contrast to these findings, O’Connor et al. found that lower levels of total cortisol secreted in response to the stress test (AUCg) were significantly associated with higher levels of future suicidal ideation, at 1-month following the stress test in suicide attempters (14). The differences in sample characteristics (e.g. different age, sex, bereavement status, burden of psychopathology), type of stress test (The Maastricht Acute Stress Test vs. TSST) and time interval between the TSST and suicidal ideation (18.5 months on average in our study vs. 1 and 6 months in O’Connor’s study) could explain these discrepant results. In addition, chronic activation of the HPA axis in response to chronic stress has been hypothesized to result in downregulation and as such, these results may not be completely discrepant. Our study and others are limited in capturing the dynamic nature of the HPA axis because of the lack of repeated assessment of cortisol responses to stress as well as the lack of a comprehensive assessment of HPA axis activity. Future prospective research is needed to better understand the interplay between biological stress responses, bereavement and adversity, and suicidal ideation in order to help better identify at-risk subjects for targeted prevention and intervention. An example for a psychological oriented intervention which effects the cortisol system was previously introduced by Phillips and colleagues who demonstrated that cognitive-behavioral stress management reduces serum cortisol and increases relaxation in women who suffer from breast cancer (49). The ability to influence serum cortisol through a psychological intervention might also be relevant in the high-risk groups of bereaved youth with high suicidal ideation.
In conclusion, we have demonstrated in our unique sample of parentally bereaved and non-bereaved control youths, that elevated cortisol reactivity to stress is associated with a higher trajectory of suicidal ideation. In addition, higher baseline cortisol may predict higher suicidal ideation one year and a half later. These results further support the importance of the HPA axis as related to bereavement and suicidal ideation. Future prospective research is needed to better understand the interplay between biological stress responses, bereavement and adversity, and suicidal ideation in order to help better identify at-risk subjects for targeted prevention and intervention efforts.
Supplementary Material
Highlights.
Suicidal Ideation is associated with higher cortisol reactivity to stress
Higher baseline cortisol may predict future Suicidal Ideation
HPA axis is related to bereavement and suicidal ideation
Funding and Acknowledgements
This study was supported by grants R01-MH65368 and K01-MH077930 from the National Institute of Mental Health (NIMH); and by a Young Investigator Award from the American Foundation for Suicide Prevention. The authors would like to thank the studies’ participants and their families and to the Herman Dana Foundation which provided stipend for the research activities of the corresponding author.
Footnotes
Conflict of interest: Dr. David Brent receives research support from the National Institutes of Mental Health, American Foundation for Suicide Prevention, the Once Upon a Time Foundation, and the Beckwith Foundation, receives royalties from Guilford Press, from the electronic self-rated version of the C-SSRS from eRT, Inc., and from performing duties as an UptoDate Psychiatry Section Editor, and receives consulting fees from Healthwise. Dr. Nadine Melhem is supported by the National Institutes of Mental Health, American Foundation for Suicide Prevention, and the Brain and Behavior Research Foundation. Dr. Amit Shalev, Ms. Giovanna Porta, Dr. Candice Biernesser, Dr. Jamie Zelazny and Ms. Monica Walker-Payne report no competing interests.
Disclosures: Dr. David Brent receives research support from the National Institutes of Mental Health, American Foundation for Suicide Prevention, the Once Upon a Time Foundation, and the Beckwith Foundation, receives royalties from Guilford Press, from the electronic self-rated version of the C-SSRS from eRT, Inc., and from performing duties as an UptoDate Psychiatry Section Editor, and receives consulting fees from Healthwise. Dr. Nadine Melhem is supported by the National Institutes of Mental Health, American Foundation for Suicide Prevention, and the Brain and Behavior Research Foundation. Dr. Amit Shalev, Ms. Giovanna Porta, Dr. Candice Biernesser, Dr. Jamie Zelazny and Ms. Monica Walker-Payne report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Administration SS. Intermediate assumptions of the 2000 trustees report. Washington, DC: Office of the Chief Actuary of the Social Security Administration; 2000. [Google Scholar]
- 2.Kaplow JB, Saunders J, Angold A, Costello EJ. Psychiatric symptoms in bereaved versus nonbereaved youth and young adults: a longitudinal epidemiological study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(11):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brent D, Melhem N, Donohoe MB, Walker M. The incidence and course of depression in bereaved youth 21 months after the loss of a parent to suicide, accident, or sudden natural death. The American journal of psychiatry. 2009;166(7):786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guldin MB, Li J, Pedersen HS, Obel C, Agerbo E, Gissler M, et al. Incidence of Suicide Among Persons Who Had a Parent Who Died During Their Childhood: A Population-Based Cohort Study. JAMA psychiatry. 2015;72(12):1227–34. [DOI] [PubMed] [Google Scholar]
- 5.Pitman AL, Osborn DP, Rantell K, King MB. Bereavement by suicide as a risk factor for suicide attempt: a cross-sectional national UK-wide study of 3432 young bereaved adults. BMJ open. 2016;6(1):e009948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitman A, Osborn D, King M, Erlangsen A. Effects of suicide bereavement on mental health and suicide risk. The Lancet Psychiatry. 2014;1(1):86–94. [DOI] [PubMed] [Google Scholar]
- 7.Dietz LJ, Stoyak S, Melhem N, Porta G, Matthews KA, Walker Payne M, et al. Cortisol response to social stress in parentally bereaved youth. Biological psychiatry. 2013;73(4):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Heeringen K, Mann JJ. The neurobiology of suicide. The lancet Psychiatry. 2014;1(1):63–72. [DOI] [PubMed] [Google Scholar]
- 9.Luecken LJ, Hagan MJ, Sandler IN, Tein JY, Ayers TS, Wolchik SA. Longitudinal mediators of a randomized prevention program effect on cortisol for youth from parentally bereaved families. Prevention science : the official journal of the Society for Prevention Research. 2014;15(2):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29(8):1012–8. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary Cortisol and Psychopathology in Children Bereaved by the September 11, 2001 Terror Attacks. Biological psychiatry. 2007;61(8):957–65. [DOI] [PubMed] [Google Scholar]
- 12.Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP. Can biological tests assist prediction of suicide in mood disorders? The international journal of neuropsychopharmacology. 2006;9(4):465–74. [DOI] [PubMed] [Google Scholar]
- 13.Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, Stanley B, et al. Blunted HPA Axis Activity in Suicide Attempters Compared to those at High Risk for Suicidal Behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(6):1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC. Cortisol reactivity and suicidal behavior: Investigating the role of hypothalamic-pituitary-adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology. 2017;75:183–91. [DOI] [PubMed] [Google Scholar]
- 15.Keilp JG, Stanley BH, Beers SR, Melhem NM, Burke AK, Cooper TB, et al. Further evidence of low baseline cortisol levels in suicide attempters. Journal of affective disorders. 2016;190:187–92. [DOI] [PubMed] [Google Scholar]
- 16.McGirr A, Diaconu G, Berlim MT, Pruessner JC, Sable R, Cabot S, et al. Dysregulation of the sympathetic nervous system, hypothalamic-pituitary-adrenal axis and executive function in individuals at risk for suicide. Journal of psychiatry & neuroscience : JPN. 2010;35(6):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melhem NM, Munroe S, Marsland A, Gray K, Brent D, Porta G, et al. Blunted HPA axis activity prior to suicide attempt and increased inflammation in attempters. Psychoneuroendocrinology. 2017;77:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giletta M, Calhoun CD, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ. Multi-Level Risk Factors for Suicidal Ideation Among at-Risk Adolescent Females: The Role of Hypothalamic-Pituitary-Adrenal Axis Responses to Stress. Journal of abnormal child psychology. 2015;43(5):807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First M, Spitzer R, Gibbon M, Williams J, Benjamin L. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID) New York: Biometrics Research Department: New York State Psychiatric Institute; 2002. [Google Scholar]
- 20.Hawton K, Appleby L, Platt S, Foster T, Cooper J, Malmberg A, et al. The psychological autopsy approach to studying suicide: A review of methodological issues. Journal of affective disorders. 1998;50(2–3):269–76. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Benjamin LS, Gibbon M, Spitzer RL, Williams JB. Structured clinical interview for DSM-IV Axis II personality disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 22.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of general psychiatry. 1987;44(6):540–8. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS). Archives of general psychiatry. 1983;40(11):1228–31. [DOI] [PubMed] [Google Scholar]
- 24.Association AP, Association AP. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association; 2000;75:78–85. [Google Scholar]
- 25.de Belmont Hollingshead A Four Factor Index of Social Status: Yale University, Department of Sociology; 1975. [Google Scholar]
- 26.Reynolds WM, Mazza JJ. Assessment of suicidal ideation in inner-city children and young adolescents: reliability and validity of the Suicidal Ideation Questionnaire-JR. School Psychology Review. 1999;28(1):17. [Google Scholar]
- 27.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. [DOI] [PubMed] [Google Scholar]
- 28.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. [DOI] [PubMed] [Google Scholar]
- 29.Golden SH, Wand GS, Malhotra S, Kamel I, Horton K. Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. European journal of epidemiology. 2011;26(7):511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and cellular endocrinology. 2012;349(1):20–9. [DOI] [PubMed] [Google Scholar]
- 31.Jokinen J, Nordstrom AL, Nordstrom P. ROC analysis of dexamethasone suppression test threshold in suicide prediction after attempted suicide. Journal of affective disorders. 2008;106(1–2):145–52. [DOI] [PubMed] [Google Scholar]
- 32.Jokinen J, Nordstrom AL, Nordstrom P. CSF 5-HIAA and DST non-suppression--orthogonal biologic risk factors for suicide in male mood disorder inpatients. Psychiatry Res. 2009;165(1–2):96–102. [DOI] [PubMed] [Google Scholar]
- 33.Jokinen J, Ouda J, Nordstrom P. Noradrenergic function and HPA axis dysregulation in suicidal behaviour. Psychoneuroendocrinology. 2010;35(10):1536–42. [DOI] [PubMed] [Google Scholar]
- 34.Kamali M, Saunders EF, Prossin AR, Brucksch CB, Harrington GJ, Langenecker SA, et al. Associations between suicide attempts and elevated bedtime salivary cortisol levels in bipolar disorder. Journal of affective disorders. 2012;136(3):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGirr A, Diaconu G, Berlim MT, Turecki G. Personal and family history of suicidal behaviour is associated with lower peripheral cortisol in depressed outpatients. Journal of affective disorders. 2011;131(1–3):368–73. [DOI] [PubMed] [Google Scholar]
- 36.Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, Stanley B, et al. Blunted HPA Axis Activity in Suicide Attempters Compared to those at High Risk for Suicidal Behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connor DB, Ferguson E, Green JA, O’Carroll RE, O’Connor RC. Cortisol levels and suicidal behavior: A meta-analysis. Psychoneuroendocrinology. 2015;63:370–9. [DOI] [PubMed] [Google Scholar]
- 38.Pfennig A, Kunzel HE, Kern N, Ising M, Majer M, Fuchs B, et al. Hypothalamus-pituitary-adrenal system regulation and suicidal behavior in depression. Biological psychiatry. 2005;57(4):336–42. [DOI] [PubMed] [Google Scholar]
- 39.Yerevanian BI, Feusner JD, Koek RJ, Mintz J. The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. Journal of affective disorders. 2004;83(2–3):103–8. [DOI] [PubMed] [Google Scholar]
- 40.Melhem NM, Porta G, Shamseddeen W, Walker Payne M, Brent DA. Grief in children and adolescents bereaved by sudden parental death. Archives of general psychiatry. 2011;68(9):911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melhem NM, Walker M, Moritz G, Brent DA. Antecedents and sequelae of sudden parental death in offspring and surviving caregivers. Archives of pediatrics & adolescent medicine. 2008;162(5):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham S, Porta G, Biernesser C, Walker Payne M, Iyengar S, Melhem N, et al. The Burden of Bereavement: Early-Onset Depression and Impairment in Youths Bereaved by Sudden Parental Death in a 7-Year Prospective Study. The American journal of psychiatry. 2018;175(9):887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37(3):317–31. [DOI] [PubMed] [Google Scholar]
- 44.Booij SH, Bouma EM, de Jonge P, Ormel J, Oldehinkel AJ. Chronicity of depressive problems and the cortisol response to psychosocial stress in adolescents: the TRAILS study. Psychoneuroendocrinology. 2013;38(5):659–66. [DOI] [PubMed] [Google Scholar]
- 45.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular psychiatry. 2013;18(6):692–9. [DOI] [PubMed] [Google Scholar]
- 46.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosomatic medicine. 2011;73(2):114–26. [DOI] [PubMed] [Google Scholar]
- 47.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. The British journal of psychiatry : the journal of mental science. 2007;191:387–92. [DOI] [PubMed] [Google Scholar]
- 48.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. The American journal of psychiatry. 2003;160(9):1554–65. [DOI] [PubMed] [Google Scholar]
- 49.Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre MM, Avisar E, et al. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosomatic medicine. 2008;70(9):1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.