Abstract
Aim: Patients undergoing percutaneous coronary intervention (PCI) who require both oral anticoagulant (OAC) and antiplatelet therapy (APT) are exposed to a serious risk of bleeding. The aim of this study was to clarify the relationship among nutritional and inflammation status and long-term bleeding in patients requiring both OACs and APT after PCI.
Methods: We performed PCI in 3,718 consecutive patients between April 2011 and March 2017, 302 of whom were treated with both OACs and APT. Patients were followed for up to 3 years for bleeding events, defined as the Bleeding Academic Research Consortium (BARC) class ≥3 bleeding. We retrospectively evaluated the ability of the Geriatric Nutritiosk Index (GNRI) and high-sensitivity C-reactive protein (hs-CRP) to detect bleeding events.
Results: During a median follow-up of 1,080 days, bleeding events were observed in 53 (17.5%) patients. Bleeding events were associated with a low GNRI (≤98) (hazard ratio [HR], 3.16; 95% confidence interval [CI], 1.84–5.45; p<0.0001) and hs-CRP level ≥2.5 mg/L (HR, 2.75; 95% CI, 1.61–4.78; p=0.0003). A low GNRI+high hs-CRP showed a 5.12-fold increase in the incidence of BARC class ≥3 bleeding (95% CI, 2.68–9.91; p<0.0001) compared with a normal GNRI+low hs-CRP. The addition of the GNRI and hs-CRP to the PRECISEDAPT score improved C-statistics from 0.67 to 0.71 and enhanced the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (NRI, 0.36, p<0.0001; IDI, 0.066, p<0.0001).
Conclusions: The GNRI and hs-CRP were novel predictors of the long-term bleeding risk in patients requiring both OACs and APT after PCI.
Keywords: Antiplatelet therapy, Oral anticoagulant, Percutaneous coronary intervention, Bleeding events, Geriatric nutritional risk index
Introduction
Approximately 5%–10% of patients undergoing percutaneous coronary intervention (PCI) require long-term oral anticoagulant (OAC) use because of atrial fibrillation, mechanical heart valves, and other conditions1). In such patients, both antiplatelet therapy (APT) and OACs are indicated; however, great concern regarding bleeding complications (4% – 16% of cases) has been raised2, 3). Various prediction scores for bleeding events have been proposed for patients who are taking antithrombotic agents4, 5), and recently the PRECISEDAPT score has been developed6). However, the performance of these risk scores to detect bleeding events is moderate in patients who require both OACs and APT after PCI7, 8).
Several studies have indicated a relationship between hypoalbuminemia and bleeding events in various conditions9–11). Recently, comprehensive nutritional assessment scores, such as the Geriatric Nutritional Risk Index (GNRI)12) or controlling nutritional status (CONUT) score13), have emerged, and these scores and inflammation markers were reported to be associated with long-term adverse events14–16). However, little is known about the relationship between nutritional status, inflammation, and long-term bleeding events in patients who are taking both OACs and antiplatelet agents after PCI.
Aim
The aim of this study was to investigate the relationship between the GNRI, C-reactive protein (CRP), and long-term bleeding events in patients undergoing PCI who required both OACs and APT.
Methods
Patient Population
The study population was first collected at single Japanese tertiary hospital to evaluate the predictive performance of various bleeding risk scores, and has been described previously8). In brief, among 3,718 patients who underwent PCI from April 2011 to March 2017, patients with indications for long-term OAC treatment were enrolled. Exclusion criteria were as follows: history of intracranial bleeding, cardiogenic shock, patients who did not take antiplatelet agents, peptic ulcer in the previous 6 months, thrombocytopenia (platelet count, <50×109/L), major bleeding (according to the Thrombolysis in Myocardial Infarction criteria17)) in the previous 12 months, and a life expectancy of less than 12 months. The indication for PCI was based on established European18), American19), and Japanese20) guidelines. This study was approved by the research review board of our hospital. Moreover, this study was conducted according to the tenets of the Helsinki Declaration. All patients provided consent for participation in this study.
Antithrombotic Treatment
The details of the antithrombotic treatment also have been described previously8). All patients received at least one antiplatelet agent (thienopyridine [200 mg ticlopidine, 75 mg clopidogrel, or 3.75 mg prasugrel daily] and/or 81 – 100 mg aspirin daily) and an OAC (vitamin K antagonist or a direct OAC [DOAC]). The type and timing of discontinuing antiplatelet agents, and the selection of the OAC were at the discretion of each attending physician. In the periprocedural period, OACs were continued if possible.
GNRI and High-Sensitivity CRP
The patient's serum albumin level, body weight (BW), and height were measured before PCI. The GNRI was calculated by modifying the GNRI as follows21):
GNRI=(14.89×albumin [g/dL]+41.7×BW/ideal BW [iBW]).
The BW/iBW was set to 1 when the patient's BW exceeded the iBW. The iBW in the present study was calculated from a body mass index (BMI) of 22, which is associated with the lowest mortality22). Then, patients were divided into two groups based on previous studies as follows: GNRI ≤ 98 (low GNRI) and >98 (normal GNRI)12).
Serum high-sensitivity CRP (hs-CRP) levels were determined using a latex immune-nephelometry method (NITTOBO MEDICAL, Tokyo, Japan). Receiver operating characteristic curve analyses were carried out to examine the optimal cutoff value of hs-CRP to predict bleeding events. Subsequently, patients were divided into two groups based on the best cutoff value: low hs-CRP and high hs-CRP.
To estimate the combined effects of the GNRI with hs-CRP on predicting bleeding events, patients were divided into four groups: normal GNRI+low hs-CRP; normal GNRI+high hs-CRP; low GNRI+low hs-CRP; and low GNRI+high hs-CRP.
Assessments and Follow-Up
Demographic, angiographic, and procedural data were collected from hospital charts or hospital databases. Follow-up data were obtained from hospital charts or by contacting patients telephonically for up to 3 years. All patient follow-up data were obtained by March 2018.
The primary outcome of this study was out-of-hospital bleeding defined as the Bleeding Academic Research Consortium (BARC)23) class ≥ 3 and occurring 7 days or later after the index PCI; bleeding that occurred earlier was censored. We selected 7 days as a conservative time frame based on the current trend in the length of hospital stay for patients with acute coronary syndrome and to exclude events related to the index PCI procedure.
Anemia was classified as a hemoglobin level <12 g/dL in men and <11 g/dL in women24). The estimated glomerular filtration rate (eGFR) was calculated using the following equation for Japanese patients recommended by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2)=194×serum creatinine−1.094×age−0.287 (×0.739 if female)25).
Statistical Analyses
Continuous variables were summarized as the mean and standard deviation (SD) or the median and inter-quartile range (IQR), and categorical variables are shown as numbers and proportions. Continuous variables were compared using Student's t-test or the Wilcoxon ranksum test based on their distributions. Categorical variables were compared using chi-squared tests.
Bleeding event rates at 3 years were calculated using the Kaplan-Meier method and compared using the log-rank test. Since the PRECISE-DAPT score was best able to stratify bleeding events of patients who required both OACs and APT after PCI among four famous bleeding risk scores (HAS-BLED, ORBIT, PRECISE-DAPT, and PARIS scores)8), we adopted it as the reference bleeding score. Thus, a Cox proportional hazards model was used to estimate the contribution of the PRECISE-DAPT score, GNRI, and continuous values of hs-CRP levels to predict bleeding events.
Model discrimination was assessed using the C-statistic. Improvements in predictive accuracy were determined by calculating the net reclassification improvement and the integrated discrimination improvement26).
All statistical analyses were performed using JMP software version 13.1 (SAS Institute Inc., Cary, NC, USA) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
Results
Patient Characteristics
Of the 3,718 patients who underwent PCI during the study period, we identified 302 eligible patients who took both OACs and antiplatelet agents. Of these 302 patients, 87 had a low GNRI and 215 had a normal GNRI (Fig. 1). The clinical characteristics of the recruited patients are summarized in Table 1. The overall follow-up rate was 92.1%. The median length of follow-up was 787 (IQR=424–1,080) days for the low GNRI group and 1,080 (IQR=671–1,080) days for the normal GNRI group. Advanced age, female sex, and a high prevalence of heart failure were significantly more frequent in the low GNRI group. A low GNRI was also associated with decreased total cholesterol, low-density lipoprotein cholesterol, triglyceride levels, and eGFR, and increased glycated hemoglobin, hs-CRP, and brain natriuretic peptide levels.
Fig. 1.
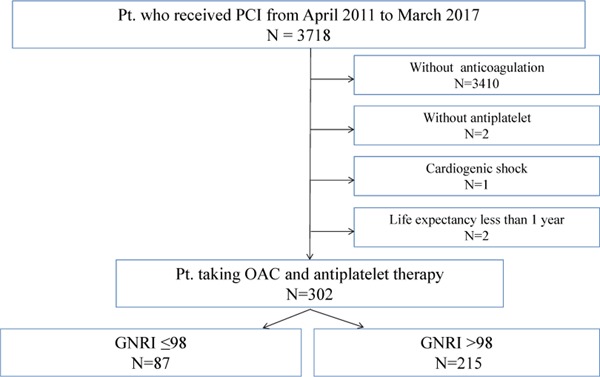
Flowchart detailing the study design.
Pt., patient; PCI, percutaneous coronary intervention; OAC, oral anticoagulant; GNRI, Geriatric Nutritional Risk Index.
Table 1. Patient's clinical characteristics, medication, and procedural characteristics at baseline.
| Patients: n | low GNRI | normal GNRI | p value |
|---|---|---|---|
| (n = 87) | (n = 215) | ||
| Clinical baseline characteristics | |||
| Mean (SD) age (years) | 76.9 (9.0) | 70.2 (8.6) | <0.0001 |
| Male sex | 57 (65.5%) | 178 (82.8%) | 0.001 |
| Mean (SD) BMI (kg/m2) | 21.0 (3.5) | 24.4 (3.5) | <0.0001 |
| Diabetes mellitus | 47 (54.0%) | 90 (41.9%) | 0.05 |
| Hypertension | 62 (71.3%) | 162 (75.4%) | 0.47 |
| Dislipidemia | 42 (48.3%) | 148 (68.8%) | 0.001 |
| Smoking history | 57 (65.5%) | 134 (62.3%) | 0.60 |
| Prior myocardial infarction | 18 (20.7%) | 60 (27.9%) | 0.19 |
| Prior heart failure | 65 (74.7%) | 76 (35.4%) | <0.0001 |
| Prior stroke | 15 (17.2%) | 27 (12.6%) | 0.30 |
| Prior PCI | 31 (35.6%) | 85 (39.5%) | 0.53 |
| Prior CABG | 16 (18.4%) | 42 (19.5%) | 0.82 |
| Serum albumin (g/dl) | 3.6 (0.3) | 4.3 (0.3) | <0.0001 |
| Total cholesterol (mg/dl) | 152.8 (34.4) | 172.4 (41.0) | 0.0001 |
| LDL-cholesterol (mg/dl) | 89.5 (29.2) | 104.1 (35.0) | 0.001 |
| HDL-cholesterol (mg/dl) | 40.3 (12.8) | 42.7 (10.6) | 0.09 |
| Triglyceride (mg/dl) | 90 [60, 128] | 117 [74, 171] | 0.003 |
| Glycated hemoglobin (%) | 6.0 (1.3) | 5.7 (0.9) | 0.03 |
| hs-CRP (mg/dl) | 0.27 [0.13, 0.51] | 0.1 [0.05, 0.24] | <0.0001 |
| BNP (pg/ml) | 291.6 [155.1, 606.2] | 81.6 [39.7, 180.2] | <0.0001 |
| eGFR (ml/min/1.73 m2) | 53.3 (24.7) | 65.2 (19.8) | <0.0001 |
| PRECISE-DAPT score | 35.6 (16.3) | 24.9 (11.3) | <0.0001 |
| Medication at discharge | |||
| Aspirin | 74 (85.1%) | 195 (90.7%) | 0.17 |
| Thienopyridine | 84 (96.6%) | 197 (91.6%) | 0.10 |
| DAPT | 73 (83.9%) | 177 (82.3%) | 0.74 |
| PPI | 83 (95.4%) | 197 (91.6%) | 0.23 |
| Type of OAC | 0.27 | ||
| VKA | 55 (63.2%) | 121 (56.3%) | |
| DOAC | 32 (36.7%) | 94 (43.7%) | |
| Indication for OAC | 0.87 | ||
| Atrial fibrillation | 58 (66.7%) | 150 (69.7%) | |
| Post cardiac surgery | 15 (17.2%) | 34 (15.8%) | |
| Other (apical aneurysm, pulmonary embolism, PAD) | 14 (16.1%) | 31 (14.4%) | |
| DAPT continuation (continue number/survivor, %) | |||
| At 1 month | 77/87 (88.5%) | 180/214 (84.1%) | 0.42 |
| At 3 months | 55/85 (64.7%) | 139/212 (65.6%) | 0.89 |
| At 6 months | 46/84 (54.8%) | 106/211 (50.2%) | 0.52 |
| At 12 months | 24/76 (31.6%) | 75/207 (36.2%) | 0.49 |
| Procedural characteristics | |||
| DES | 66 (75.9%) | 141 (65.6%) | 0.08 |
| Indication of PCI | 0.34 | ||
| STEMI | 27 (31.0%) | 66 (30.7%) | |
| NSTEMI | 3 (3.5%) | 4 (1.9%) | |
| Unstable AP | 13 (14.9%) | 19 (8.8%) | |
| Stable AP | 44 (50.6%) | 126 (58.6%) |
Values are the mean±standard deviation (SD), n (%), or median (interquartile range) as appropriate. GNRI, geriatric nutritional risk index; BMI, body-mass index; PCI, pertcutaneous coronary intervention; CABG, coronary artery bypass graft; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hs-CR, high-sensitivity C-reactive protein; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; DAPT, dual antiplatelet therapy; PPI, proton-pump inhibitor; OAC, oral anticoagulant; VKA, vitamin K antagonist; DOAC, direct oral anticoagulant; PAD, peripheral artery disease; DES, drug eluting stent; STEMI, ST elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; AP, angina pectoris.
The mean PRECISE-DAPT score was significantly higher in the low GNRI group than in the normal GNRI group (PRECISE-DAPT: low GNRI, 35.6 [SD= 16.3]; normal GNRI, 24.9 [SD = 11.3]; p<0.0001).
The DAPT continuation rate was comparable between the two groups at 1, 3, 6, and 12 months (Table 1).
Bleeding Events and Predictive Performance of GNRI and hs-CRP
During the entire follow-up period, the primary outcome (BARC class ≥3 bleeding) occurred in 27 patients (31.0%) in the low GNRI group and 26 patients (12.1%) in the normal GNRI group. The median time to the first occurrence of BARC class ≥3 bleeding was 237 (IQR=76–636) days.
Fig. 2 shows the 3-year risk estimates comparing the groups in terms of BARC class ≥3 bleeding. BARC class ≥3 bleeding for the low and normal GNRI groups occurred in 37.1% and 14.3% of patients, respectively (hazard ratio [HR], 3.16; 95% confidence interval [CI], 1.84–5.45; p<0.0001) (Fig.2A).
Fig. 2.
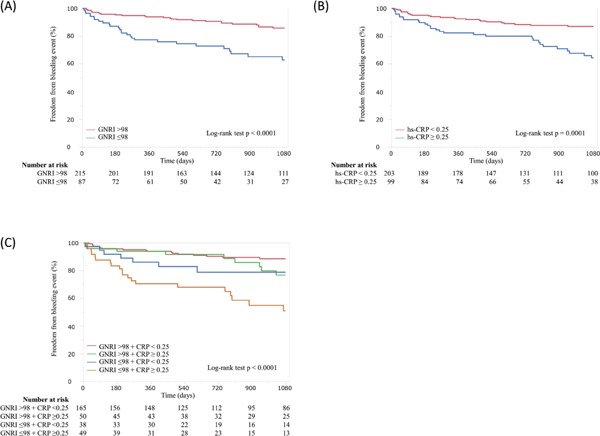
Cumulative incidence of the Bleeding Academic Research Consortium class ≥ 3 bleeding.
Panel A shows the freedom from adverse bleeding events in patients stratified by the GNRI. Panel B shows the freedom from adverse bleeding events in patients stratified by hs-CRP. Panel C shows the freedom from adverse bleeding events in patients stratified by a combination of the GNRI and hs-CRP.
hs-CRP levels were divided using an optimal cut-off value of 2.5 mg/L (area under the curve = 0.64; p = 0.001). Results for the low hs-CRP (<2.5 mg/L) and high hs-CRP (≥2.5 mg/L) groups were BARC class ≥ 3 bleeding in 35.7% and 13.2% of patients, respectively (HR, 2.75; 95% CI, 1.61–4.78; p = 0.0003) (Fig.2B). Regarding the combined effects of GNRI and hs-CRP, low GNRI+high hs-CRP showed a 5.12-fold increase in the incidence of BARC class ≥3 bleeding (95% CI, 2.68 – 9.91; p<0.0001) compared with normal GNRI+low hs-CRP. In contrast, no significant increase was observed in the incidence of bleeding events for normal GNRI+high hs-CRP or low GNRI+low hs-CRP compared with normal GNRI+low hs-CRP (Fig.2C).
Cox proportional hazards modeling is shown in Table 2. After adjustment for the PRECISE-DAPT score, a low GNRI independently predicted adverse bleeding events (HR, 2.13; 95% CI, 1.17–3.87; p = 0.01); after adjustment for both the PRECISE-DAPT score and hs-CRP, a low GNRI tended to be associated with the increased incidence of bleeding events. (HR, 1.75; 95% CI, 0.96–3.20; p=0.07). In contrast, hs-CRP independently predicted bleeding events after adjustment for the PRECISE-DAPT score and GNRI.
Table 2. Predictive value for Adverse Bleeding Events (BARC class ≥ 3 bleeding) by Cox analysis.
| Variables | Univariate | Multivariate (Model 1) | Multivariate (Model 2) | Multivariate (Model 3) | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| PRECISE-DAPT | 1.04 (1.02–1.06) | <0.0001 | 1.03 (1.01–1.05) | 0.001 | 1.04 (1.02–1.06) | <0.0001 | 1.03 (1.02–1.05) | 0.0004 |
| Low GNRI | 3.16 (1.84–5.45) | <0.0001 | 2.13 (1.17–3.87) | 0.01 | 1.75 (0.96–3.20) | 0.07 | ||
| hs-CRP | 1.08 (1.04–1.12) | 0.001 | 1.09 (1.04–1.13) | 0.001 | 1.08 (1.03–1.12) | 0.004 | ||
PRECISE-DAPT, PRECISE-DAPT score; GNRI, geriatric nutritional risk index; hs-CRP, high-sensitivity C-reactive protein; HR, hazard ratio; 95% CI, 95% confidence interval.
The C-statistic for BARC class ≥3 bleeding was 0.67 (95% CI, 0.52–0.64, p=0.001) for the PRECISE-DAPT score alone. Adding the GNRI and hs-CRP to the PRECISE-DAPT score increased the predictive accuracy of bleeding events (Table 3).
Table 3. Discrimination of each predictive model of Adverse Bleeding Events (BARC class ≥ 3 bleeding).
| Variables | C-statistic | p value | NRI | p value | IDI | p value |
|---|---|---|---|---|---|---|
| PRECISE-DAPT | 0.67 | <0.0001 | Reference | Reference | ||
| +BMI | 0.68 | <0.0001 | 0.01 | 0.51 | 0.001 | 0.45 |
| +Alb | 0.67 | <0.0001 | 0.06 | 0.44 | 0.005 | 0.26 |
| +low GNRI | 0.69 | <0.0001 | 0.30 | 0.02 | 0.028 | 0.04 |
| +hs-CRP | 0.70 | <0.0001 | 0.28 | 0.01 | 0.051 | <0.0001 |
| +low GNRI and hs-CRP | 0.71 | <0.0001 | 0.36 | 0.02 | 0.066 | <0.0001 |
PRECISE-DAPT, PRECISE-DAPT score; GNRI, geriatric nutritional risk index; hs-CRP, high-sensitivity C-reactive protein; NRI, net reclassification improvement; IDI, integrated discrimination improvement.
Discussion
In this study, we assessed the comprehensive nutritional assessment score and inflammation marker as risk factors for long-term bleeding events in patients treated with PCI who required both OACs and APT. The main findings of the present study were as follows:
1) The GNRI was able to discriminate bleeding risk in patients who underwent PCI and required both OACs and APT.
2) hs-CRP was an independent predictor of long-term bleeding events by Cox proportional hazards analysis.
3) The combination of the GNRI and hs-CRP could detect the patients with the highest risk of bleeding events.
4) The addition of the GNRI and hs-CRP to the PRECIS-DAPT score, which is an established bleeding risk score, significantly improved the ability to stratify bleeding risk in these patients.
Our study highlighted the clinical impact of nutritional status and inflammation on long-term bleeding events in those who required both OACs and APT after PCI. Bleeding complications are associated with an increased risk of subsequent adverse outcomes, such as death, myocardial infarction, stroke, and stent thrombosis27). Therefore, it is very important to identify high risk patients; however, it is difficult to predict bleeding events4, 6), particularly in patients who require both OACs and APT7). In the present study, the GNRI and hs-CRP could discriminate the bleeding risk in this subset of patients. Moreover, adding the GNRI and hs-CRP increased the accuracy to discriminate bleeding events of the established bleeding risk score (PRECISE-DAPT score). In this regard, the result of the present study offered information of importance.
In previous studies, the GNRI has been recognized as a nutrition-related risk index that makes it possible to stratify the risk of morbidity and mortality in relation to pathologies in elderly patients, which are often associated with malnutrition12). The GNRI appraises the BMI as well as serum albumin levels. In the present study, the addition of BMI alone to the PRECISE-DAPT score did not improve its ability to predict bleeding events compared with the GNRI. There could be various reasons for this. Previous epidemiologic studies have found that underweight patients were associated with a greater incidence of bleeding complications than normal-weight patients4). However, the relationship between BMI and bleeding events was a reverse J-shape or a U-shape, which demonstrated the lowest risk in patients with a normal BMI compared with underweight or overweight patients. Moreover, BMI alone may not distinguish sarcopenic obesity, an expression of the loss of lean body mass and increased fat mass, which has been recognized as a risk for multi-morbidity in some populations28). Therefore, it might be important to assess BMI together with nutritional status, and the GNRI was appropriate for this purpose.
There are several other nutritional screening tools, and each has its advantages and disadvantages. Some are based on subjective data, such as the Subjective Global Assessment29) and Mini-Nutritional Assessment30). Other tools are based on objective biochemical and clinical data, such as the GNRI12) and the CONUT score13). To assess nutritional status correctly, combining both subjective and objective tools is important31). However, for daily clinical practice, an objective and simple tool that uses routinely measured data is desirable. Furthermore, subjective indices require the training and experience of medical practitioners, and it might be affected by the questioner's opinions. The GNRI, which uses only serum albumin level and BMI, is an easy and efficient screening tool to assess the nutritional status of patients undergoing PCI.
It has been suggested that a large proportion of patients at high risk for atherosclerosis also had a high bleeding risk, reflecting an overlap of the risk predictors for thrombosis and bleeding32). A sub-analysis of the DAPT study revealed that polyvascular disease was a strong predictor of bleeding events as well as a predictor of ischemic events33). Inflammation is also known to play an important role in the initiation and progression of atherosclerosis34). In the present study, hs-CRP, which is a popular marker of inflammation, was revealed to be useful for predicting bleeding events after PCI. It is very difficult to show the mechanism underlying the effect of inflammation on the bleeding risks from the results of this observational study. However, several studies have demonstrated that systemic inflammation is correlated with advanced age and sarcopenia35). In the present study, patients with a low GNRI were significantly older and had higher hs-CRP levels than patients with a normal GNRI. When considered comprehensively, bleeding events might be involved in the context of a malnutrition–inflammation–atherosclerosis syndrome, which is a malignant cycle propounded in patients with chronic kidney disease, formed by malnutrition and inflammation36). Therefore, it was suggested that assessing both nutritional and inflammation status as well as established risk factors was required to improve the identification of patients at high risk for bleeding among those who underwent PCI and required both OACs and APT.
The role of bleeding risk scores should be to identify high risk patients for a more careful review and follow-up, as well as to draw attention to reversible factors37). From this perspective, the GNRI is simple, practical, and reversible; until now, data on interventions in nutritional status that improve the risk for long-term bleeding events have been limited. Thus, future studies should investigate if nutritional intervention will improve the risk for bleeding events in patients taking both OACs and antiplatelet agents after PCI.
Study Limitations
This study had several limitations. First, it was performed at a single medical center and followed a non-randomized, retrospective study design. Accordingly, only a limited number of patients were enrolled. Moreover, by the nature of the retrospective observational design, regimens of APT and the duration of the DAPT were at the discretion of the treating physicians. Second, the association among nutritional and inflammation status, and long-term bleeding risks might differ by race. The results of our study might not be generalizable to patients other than Japanese patients. Third, the nutritional status and inflammation marker was measured only at the time of the index PCI. Therefore, these conditions might change during the followup period. Fourth, patients taking a DOAC tended to be included more in recent years. Therefore, DOACs were used more frequently in patients on the SAPT and OAC regimen, although this regimen was employed in less than one-sixth of the patients. Furthermore, we did not examine the comorbidity or frailty scores. Most patients in this study may have been affected to some degree by frailty or comorbidity rather than their nutritional status alone. However, such a bias might be reduced because other factors were taken into account; considering that patients in this study underwent invasive procedures probably reflects patient frailty and comorbidity. Additional studies with large sample sizes are needed to investigate the effect of intervention on nutritional status for patients taking an OAC treated with PCI.
Conclusion
The GNRI and hs-CRP are novel predictors of long-term bleeding risk in patients taking both OACs and antiplatelet agents after PCI. Adding the GNRI and hs-CRP to the PRECISE-DAPT score improved the ability to stratify bleeding risk in these patients.
Conflict of Interest
H.I. received lecture fees from Astellas Pharma Inc., Bayer Pharmaceutical Co., Ltd., and MSD K. K. T.M. received lecture fees from Bayer Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K. K., Pfizer Japan Inc., Sanofi-aventis K. K., and Takeda Pharmaceutical Co., Ltd. T.M. received an unrestricted research grant for the Department of Cardiology, Nagoya University Graduate School of Medicine from Astellas Pharma Inc., Daiichi-Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K. K., Otsuka Pharma Ltd., Pfizer Japan Inc., Sanofi-aventis K. K., Takeda Pharmaceutical Co., Ltd., and Teijin Pharma Ltd. The other authors have nothing to disclose.
Research Funding
This study was supported by a grant from Aichi Kidney Foundation and Grant-in-Aid for Scientific Research (KAKENHI) (No. 1 7 K 0 9 4 9 3) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japanese Society for the Promotion of Science (JSPA).
References
- 1). Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, Kirchhof P, Marin F, European Society of Cardiology Working Group on T : Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/stenting. Thromb Haemost. 2010; 103: 13-28 [DOI] [PubMed] [Google Scholar]
- 2). Lamberts M, Gislason GH, Olesen JB, Kristensen SL, Schjerning Olsen AM, Mikkelsen A, Christensen CB, Lip GY, Kober L, Torp-Pedersen C, Hansen ML: Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013; 62: 981-989 [DOI] [PubMed] [Google Scholar]
- 3). Yoshida R, Morishima I, Takagi K, Morita Y, Tsuboi H, Murohara T: Comparison Between Long-Term Clinical Outcomes of Vitamin K Antagonist and Direct Oral Anticoagulants in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. Circ J. 2018; 82: 2016-2024 [DOI] [PubMed] [Google Scholar]
- 4). Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S: Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J Am Coll Cardiol. 2016; 67: 2224-2234 [DOI] [PubMed] [Google Scholar]
- 5). Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY: A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138: 1093-1100 [DOI] [PubMed] [Google Scholar]
- 6). Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M, Investigators P-DS : Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISEDAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017; 389: 1025-1034 [DOI] [PubMed] [Google Scholar]
- 7). Kiviniemi T, Puurunen M, Schlitt A, Rubboli A, Karjalainen P, Vikman S, Niemela M, Lahtela H, Lip GY, Airaksinen KE: Performance of bleeding risk-prediction scores in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol. 2014; 113: 1995-2001 [DOI] [PubMed] [Google Scholar]
- 8). Yoshida R, Ishii H, Morishima I, Tanaka A, Morita Y, Takagi K, Yoshioka N, Hirayama K, Iwakawa N, Tashiro H, Kojima H, Mitsuda T, Hitora Y, Furusawa K, Tsuboi H, Murohara T: Performance of HAS-BLED, ORBIT, PRECISE-DAPT, and PARIS risk score for predicting long-term bleeding events in patients taking an oral anticoagulant undergoing percutaneous coronary intervention. J Cardiol. 2018. in press [DOI] [PubMed] [Google Scholar]
- 9). Tatami Y, Ishii H, Aoki T, Harada K, Hirayama K, Shibata Y, Sumi T, Negishi Y, Kawashima K, Kunimura A, Kawamiya T, Yamamoto D, Suzuki S, Murohara T: Decreased Serum Albumin Predicts Bleeding Events in Patients on Antiplatelet Therapy After Percutaneous Coronary Intervention. Circ J. 2017; 81: 999-1005 [DOI] [PubMed] [Google Scholar]
- 10). Engelman DT, Adams DH, Byrne JG, Aranki SF, Collins JJ, Jr., Couper GS, Allred EN, Cohn LH, Rizzo RJ: Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999; 118: 866-873 [DOI] [PubMed] [Google Scholar]
- 11). Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K: Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013; 77: 705-711 [DOI] [PubMed] [Google Scholar]
- 12). Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C, Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005; 82: 777-783 [DOI] [PubMed] [Google Scholar]
- 13). Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G: CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005; 20: 38-45 [PubMed] [Google Scholar]
- 14). Kunimura A, Ishii H, Uetani T, Aoki T, Harada K, Hirayama K, Negishi Y, Shibata Y, Sumi T, Kawashima K, Tatami Y, Kawamiya T, Yamamoto D, Suzuki S, Amano T, Murohara T: Impact of nutritional assessment and body mass index on cardiovascular outcomes in patients with stable coronary artery disease. Int J Cardiol. 2017; 230: 653-658. [DOI] [PubMed] [Google Scholar]
- 15). Kunimura A, Ishii H, Uetani T, Aoki T, Harada K, Hirayama K, Negishi Y, Shibata Y, Sumi T, Kawashima K, Tatami Y, Kawamiya T, Yamamoto D, Suzuki S, Amano T, Murohara T: Impact of Geriatric Nutritional Risk Index on cardiovascular outcomes in patients with stable coronary artery disease. J Cardiol. 2017; 69: 383-388 [DOI] [PubMed] [Google Scholar]
- 16). Kawamiya T, Suzuki S, Ishii H, Hirayama K, Harada K, Shibata Y, Tatami Y, Harata S, Kawashima K, Kunimura A, Takayama Y, Shimbo Y, Osugi N, Yamamoto D, Ota T, Kono C, Murohara T: Correlations between geriatric nutritional risk index and peripheral artery disease in elderly coronary artery disease patients. Geriatr Gerontol Intern. 2017; 17: 1057-1062 [DOI] [PubMed] [Google Scholar]
- 17). Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987; 76: 142-154 [DOI] [PubMed] [Google Scholar]
- 18). Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A: 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI).. Eur Heart J. 2014; 35: 2541-2619 [DOI] [PubMed] [Google Scholar]
- 19). Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH: 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011; 58: e44-122 [DOI] [PubMed] [Google Scholar]
- 20). Guidelines for elective percutaneous coronary intervention in patients with stable coronary artery disease (JCS 2011) published in 2012--digest version. Circ J. 2013; 77: 1590-1607 [DOI] [PubMed] [Google Scholar]
- 21). Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H: Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008; 87: 106-113 [DOI] [PubMed] [Google Scholar]
- 22). Shah B, Sucher K, Hollenbeck CB: Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract. 2006; 21: 312-319 [DOI] [PubMed] [Google Scholar]
- 23). Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H: Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011; 123: 2736-2747 [DOI] [PubMed] [Google Scholar]
- 24). Astor BC, Muntner P, Levin A, Eustace JA, Coresh J: Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002; 162: 1401-1408 [DOI] [PubMed] [Google Scholar]
- 25). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 26). Uno H, Tian L, Cai T, Kohane IS, Wei LJ: A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013; 32: 2430-2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Genereux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Yadav M, Francese DP, Palmerini T, Kirtane AJ, Litherland C, Mehran R, Stone GW: Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2015; 66: 1036-1045 [DOI] [PubMed] [Google Scholar]
- 28). Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE: Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016; 7: 126-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). da Silva Fink J, Daniel de Mello P, Daniel de Mello E: Subjective global assessment of nutritional status - A systematic review of the literature. Clin Nutr. 2015; 34: 785-792 [DOI] [PubMed] [Google Scholar]
- 30). Guigoz Y: The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us?. The journal of nutrition, health & aging, 2006; 10: 466-485; discussion 485–467 [PubMed] [Google Scholar]
- 31). Poulia KA, Yannakoulia M, Karageorgou D, Gamaletsou M, Panagiotakos DB, Sipsas NV, Zampelas A: Evaluation of the efficacy of six nutritional screening tools to predict malnutrition in the elderly. Clin Nutr. 2012; 31: 378-385 [DOI] [PubMed] [Google Scholar]
- 32). Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Akasaka T, Hanaoka KI, Kozuma K, Tanabe K, Morino Y, Muramatsu T, Kimura T: Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J Am Heart Assoc. 2018; 7. 10.1161/JAHA.118.008708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Secemsky EA, Yeh RW, Kereiakes DJ, Cutlip DE, Steg PG, Massaro JM, Apruzzese PK, Mauri L, Dual Antiplatelet Therapy Study I : Extended Duration Dual Antiplatelet Therapy After Coronary Stenting Among Patients With Peripheral Arterial Disease: A Subanalysis of the Dual Antiplatelet Therapy Study. JACC Cardiovasc Interv. 2017; 10: 942-954 [DOI] [PubMed] [Google Scholar]
- 34). Shankar A, Li J, Nieto FJ, Klein BE, Klein R: Association between C-reactive protein level and peripheral arterial disease among US adults without cardiovascular disease, diabetes, or hypertension. Am Heart J. 2007; 154: 495-501 [DOI] [PubMed] [Google Scholar]
- 35). Ershler WB, Keller ET: Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000; 51: 245-270 [DOI] [PubMed] [Google Scholar]
- 36). Hwang JH, Ryu J, An JN, Kim CT, Kim H, Yang J, Ha J, Chae DW, Ahn C, Jung IM, Oh YK, Lim CS, Han DJ, Park SK, Kim YS, Kim YH, Lee JP: Pretransplant malnutrition, inflammation, and atherosclerosis affect cardiovascular outcomes after kidney transplantation. BMC Nephrol. 2015; 16: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Roldan V, Marin F: Predicting bleeding risk after coronary surgery: Let's focus on modifiable risk factors and simple, practical decision making. Thromb Haemost. 2017; 117: 647-649 [DOI] [PubMed] [Google Scholar]


