Abstract
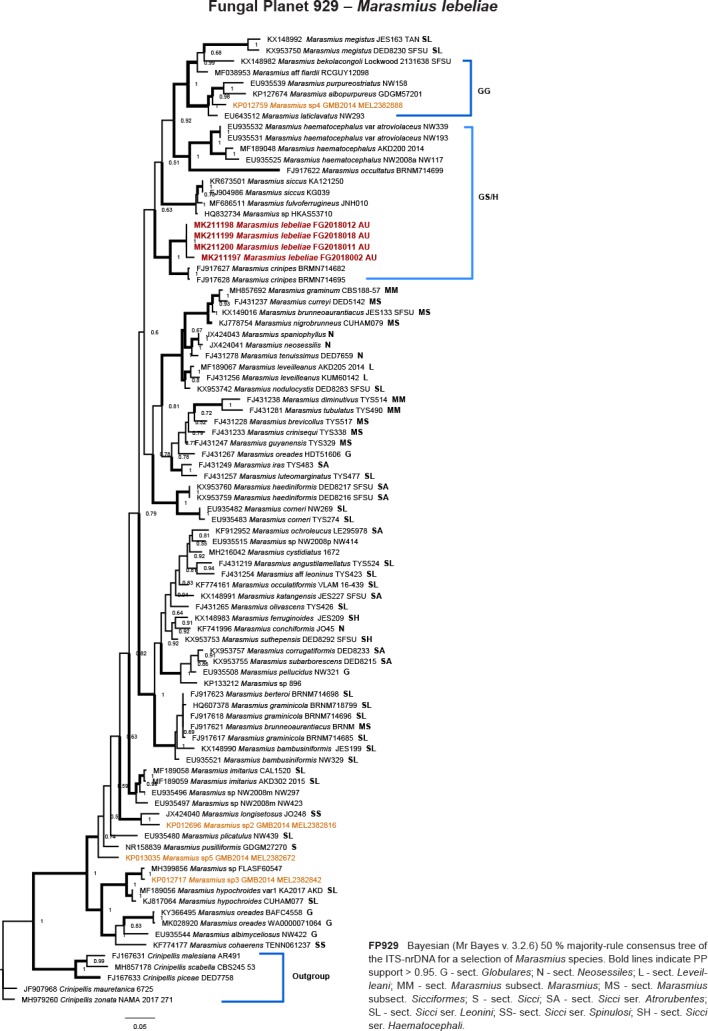
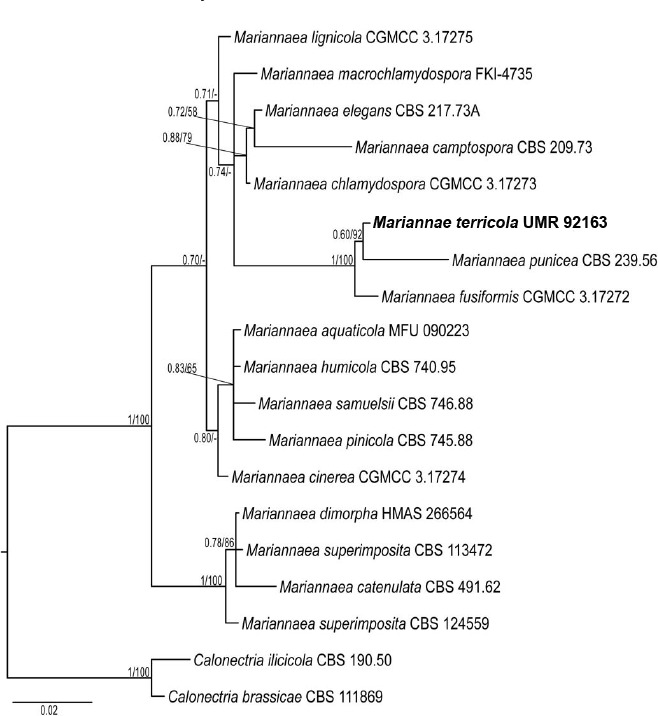
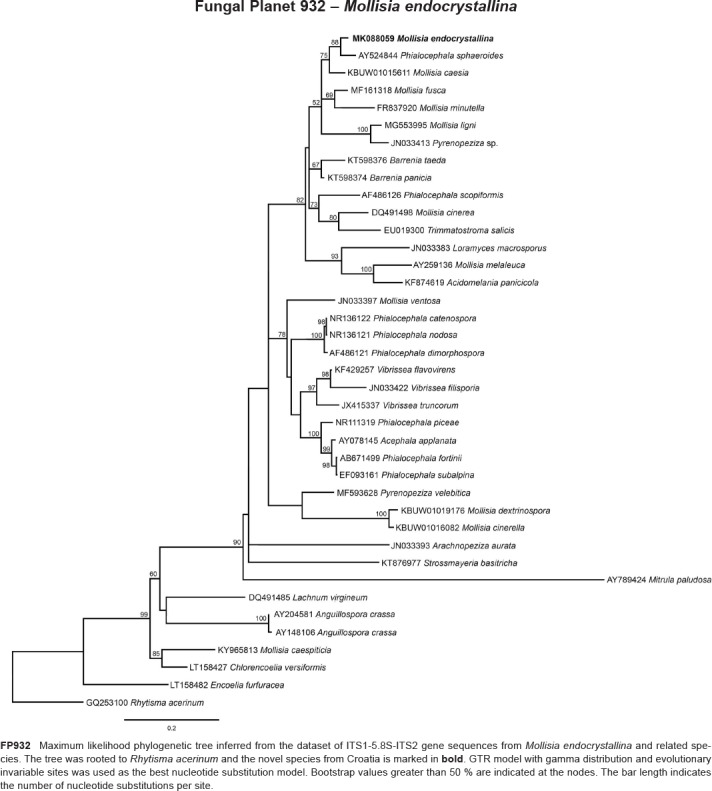
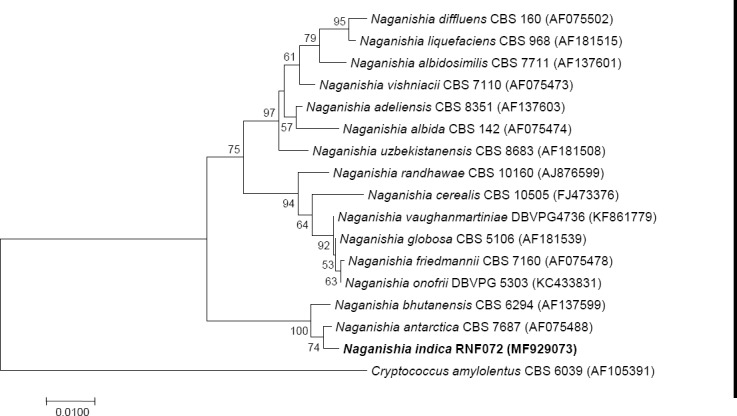
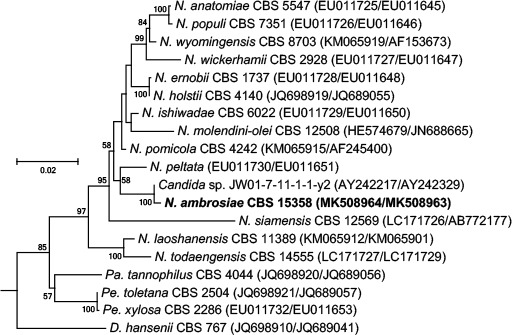
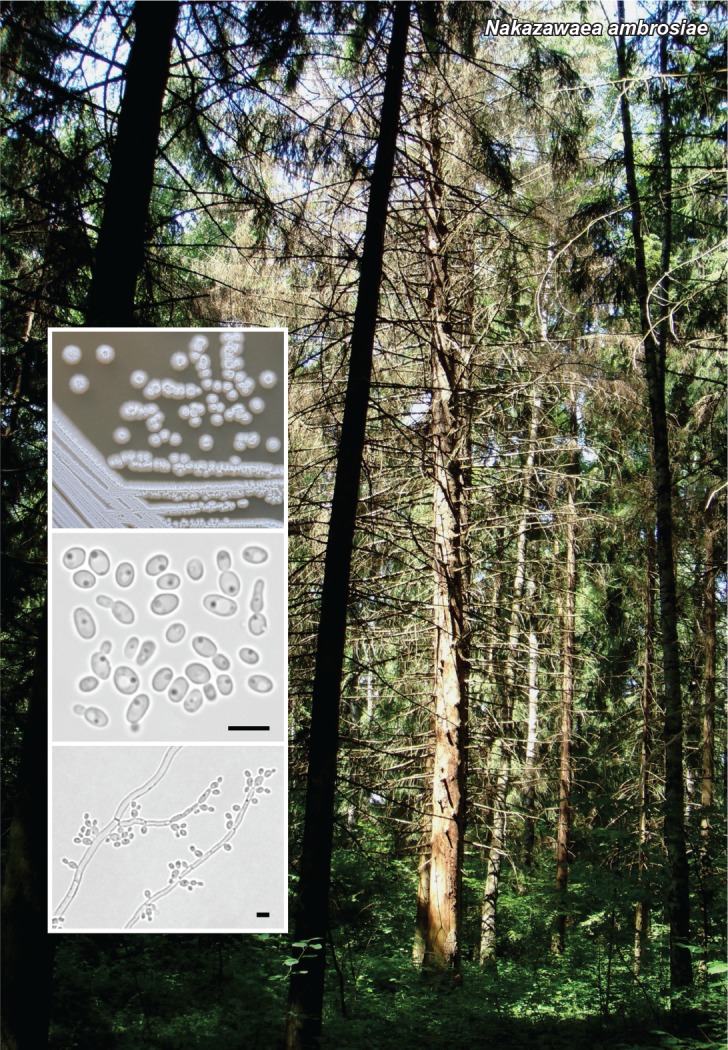
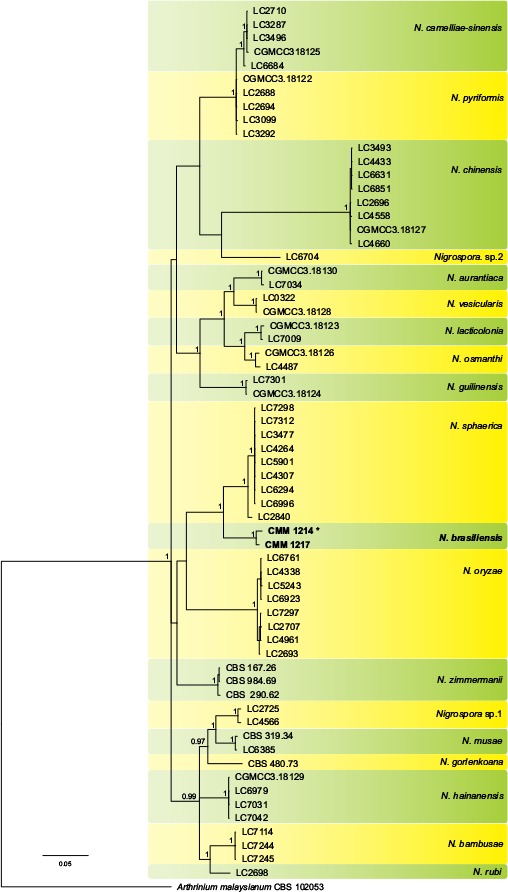
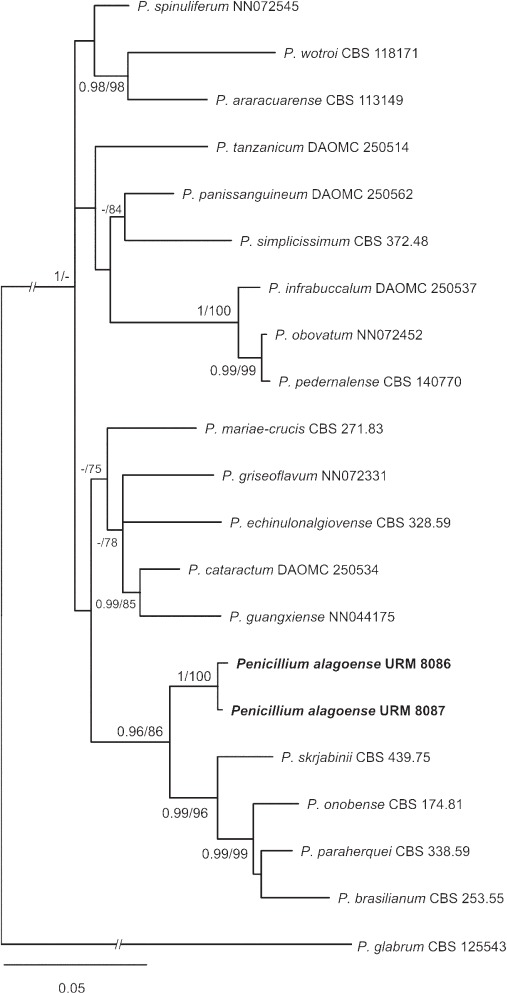
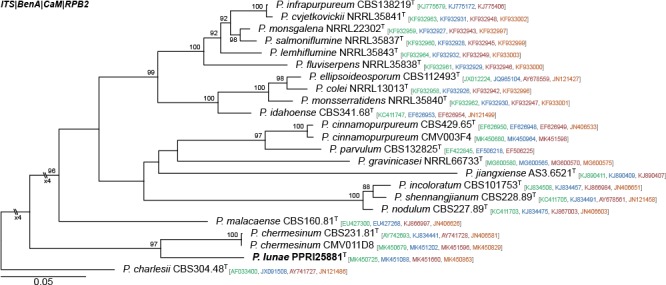
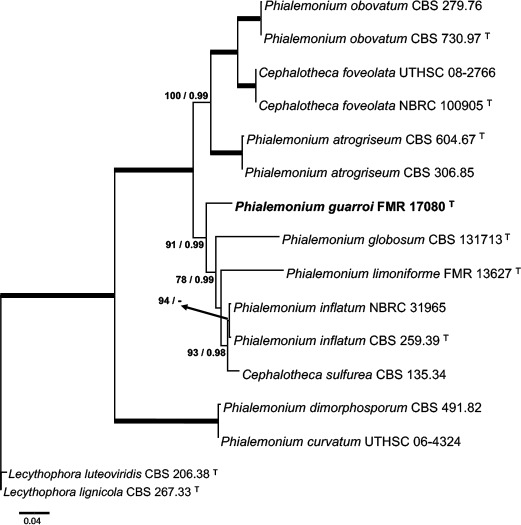
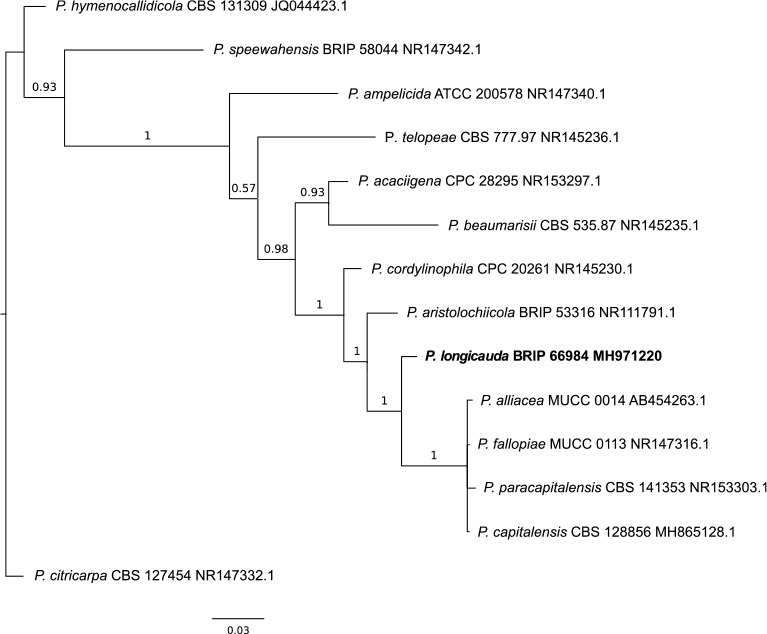
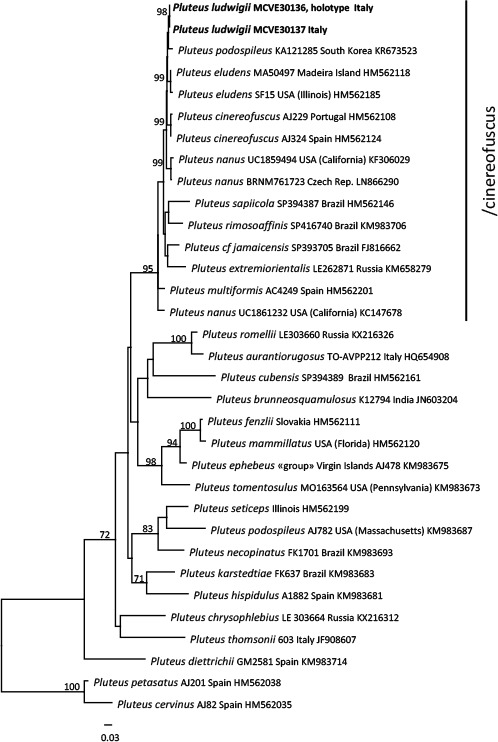
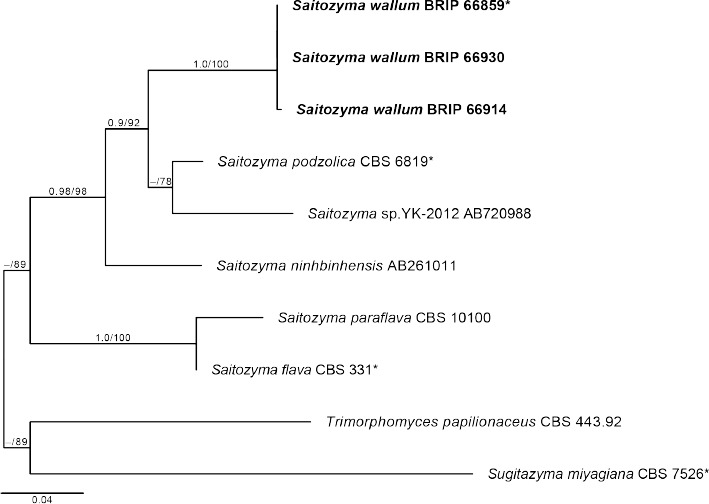
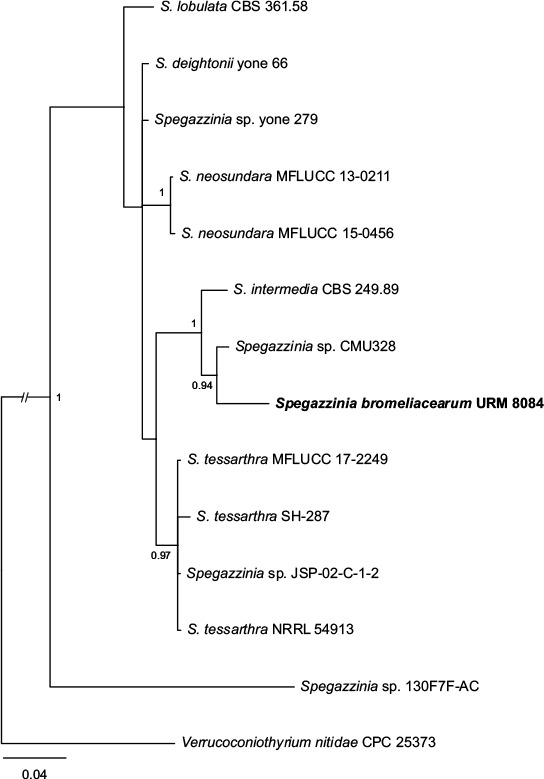
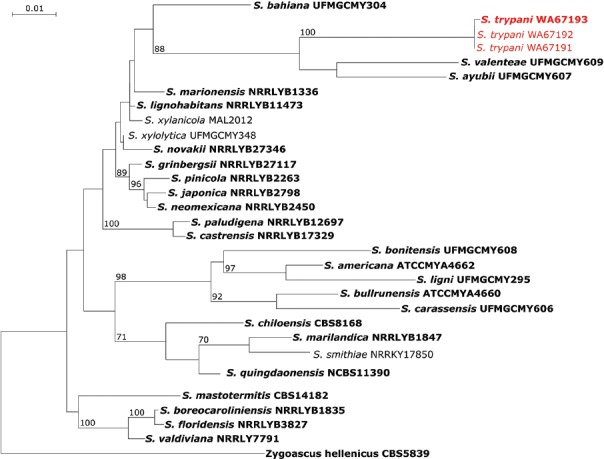
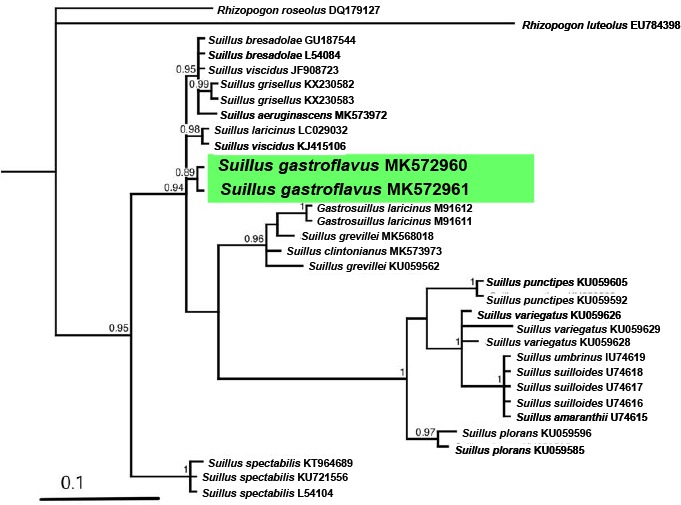
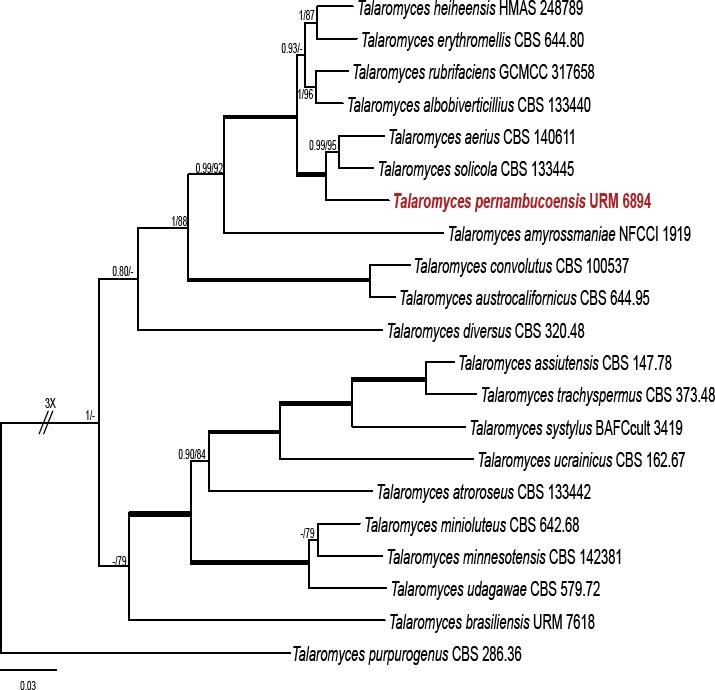
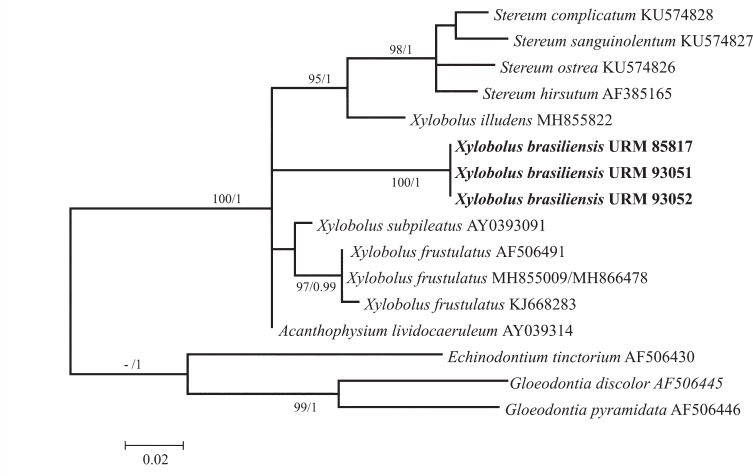
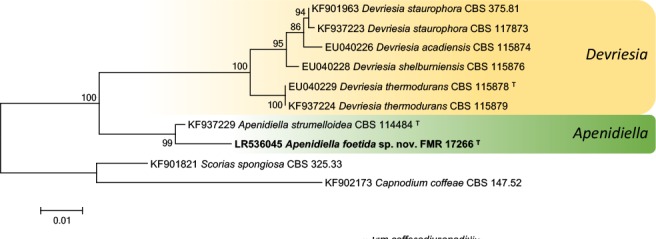
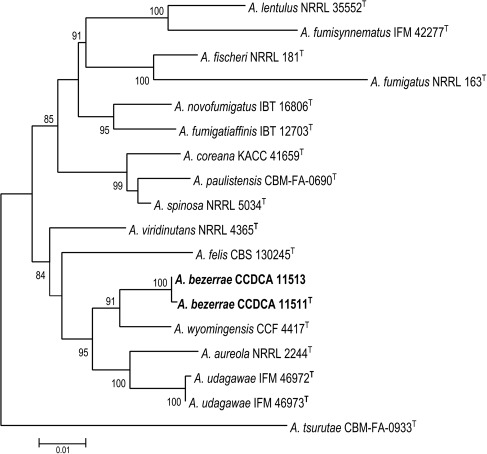
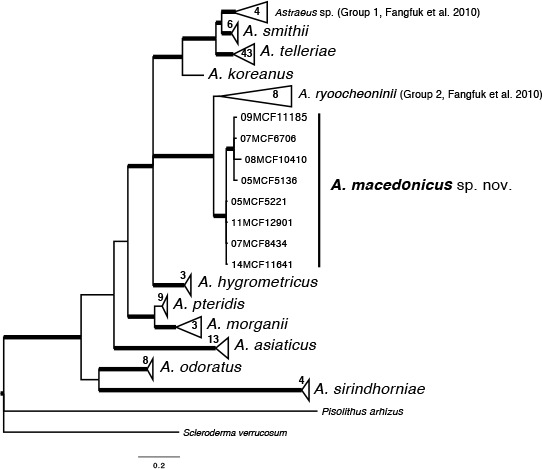
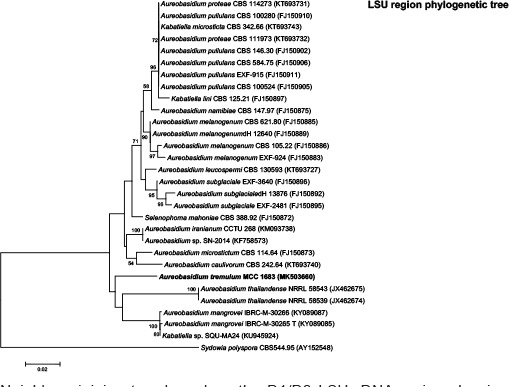
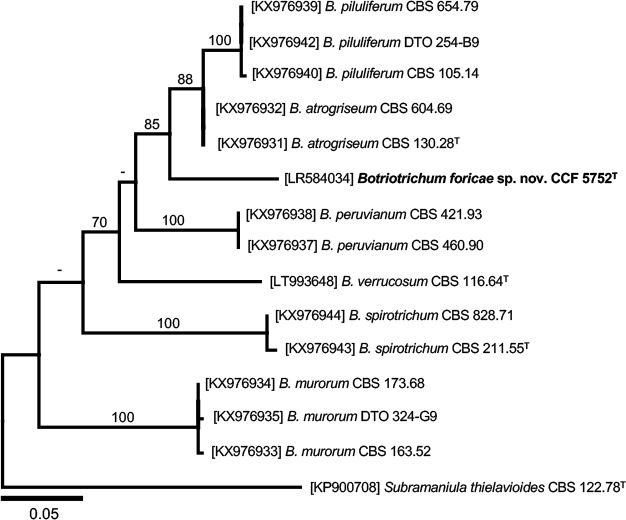
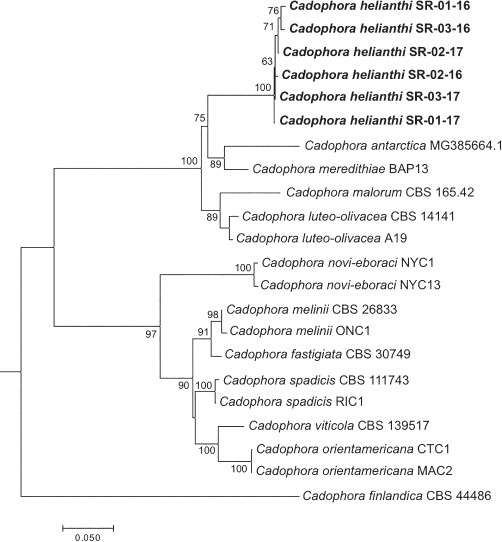
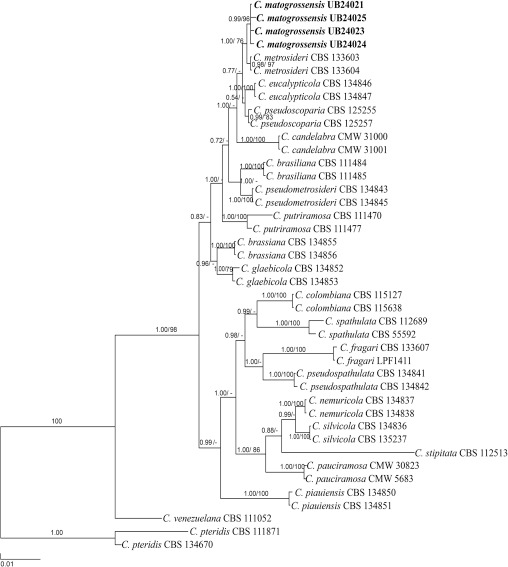
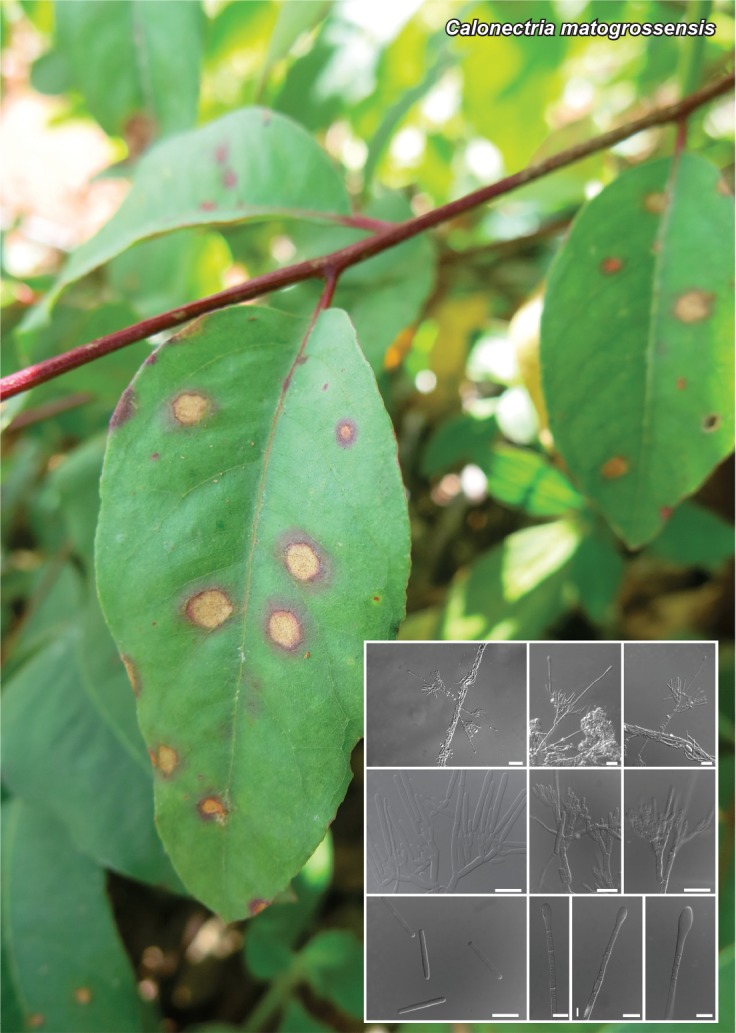
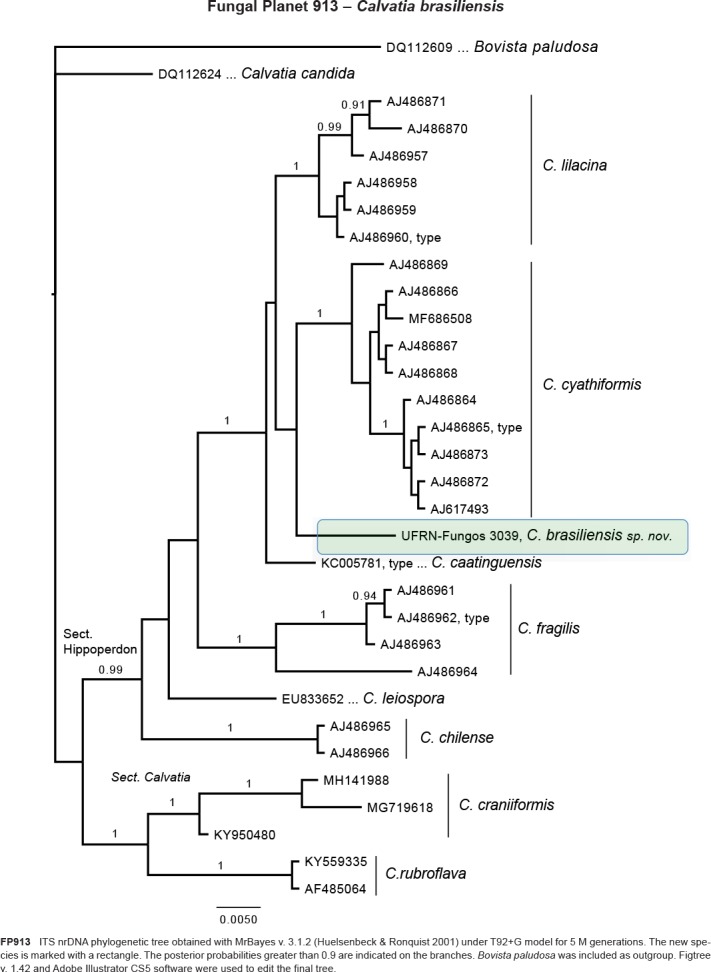
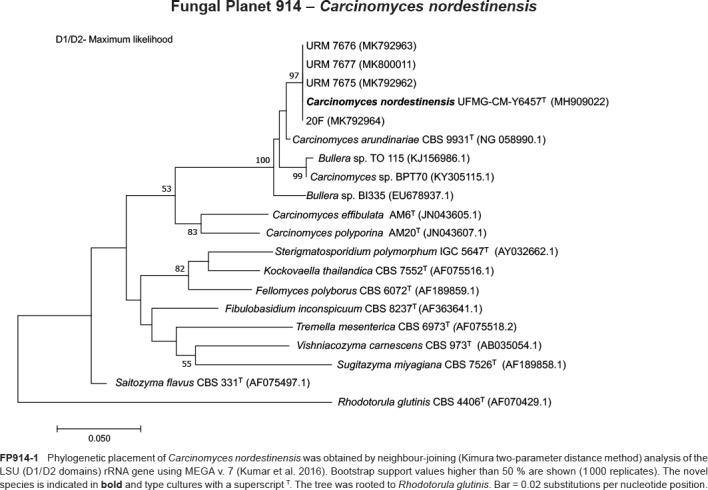
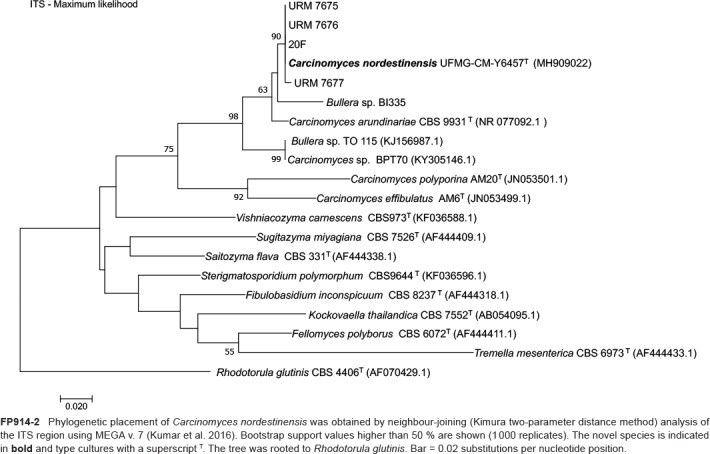
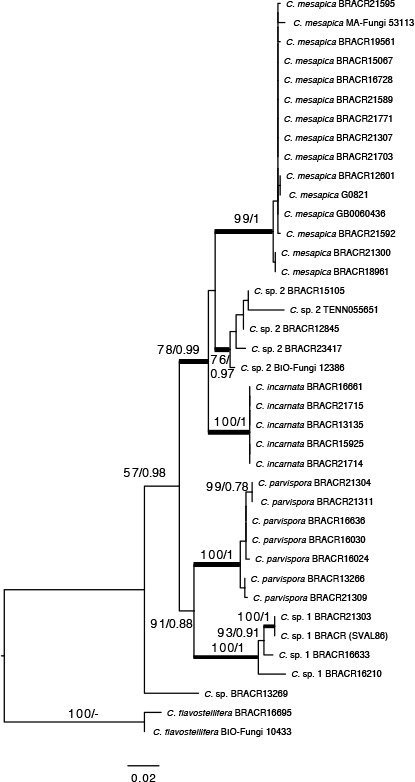
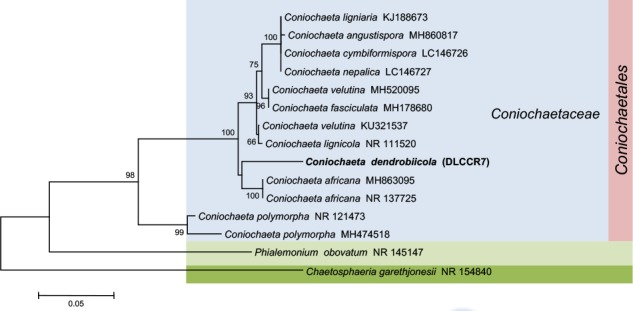
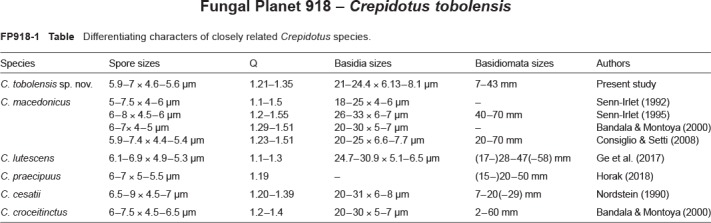
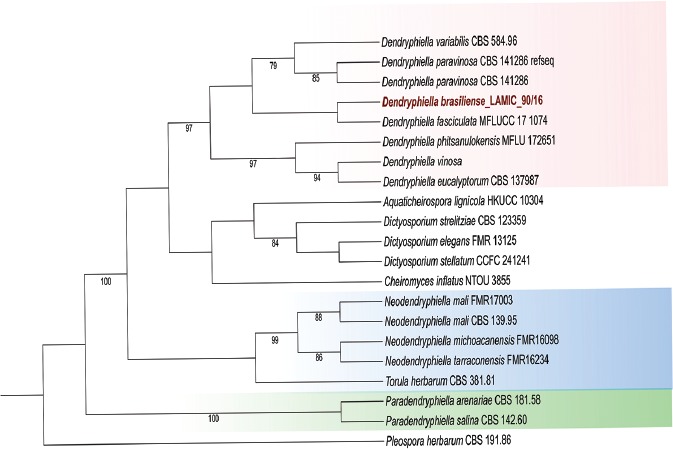
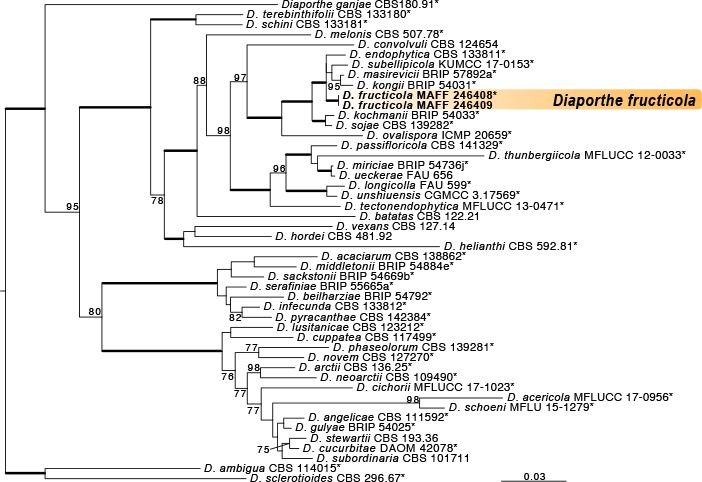
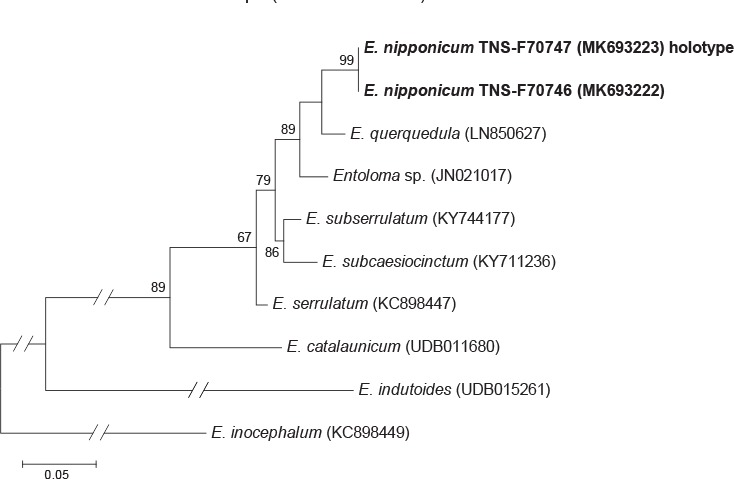
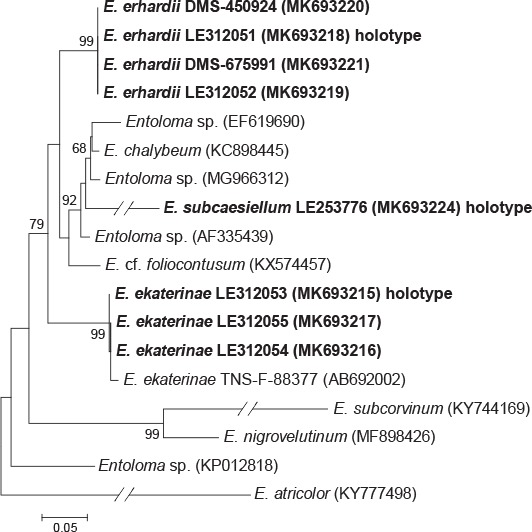
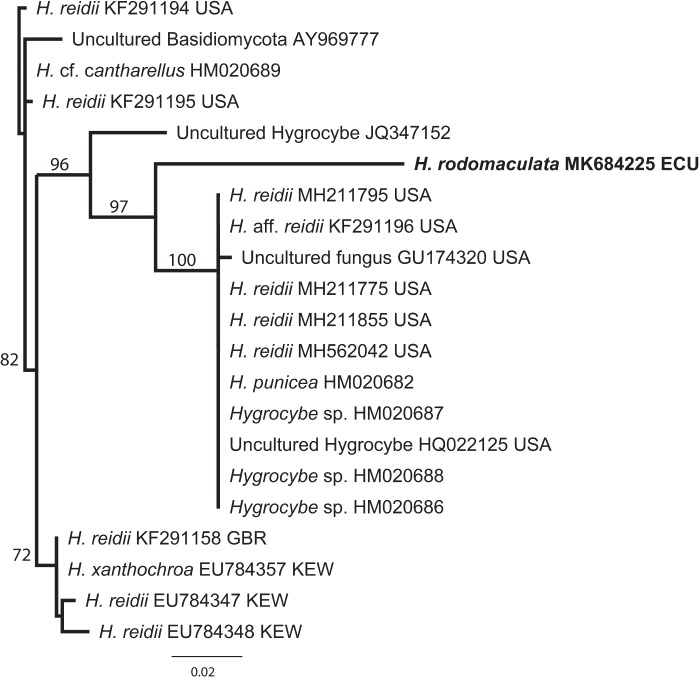
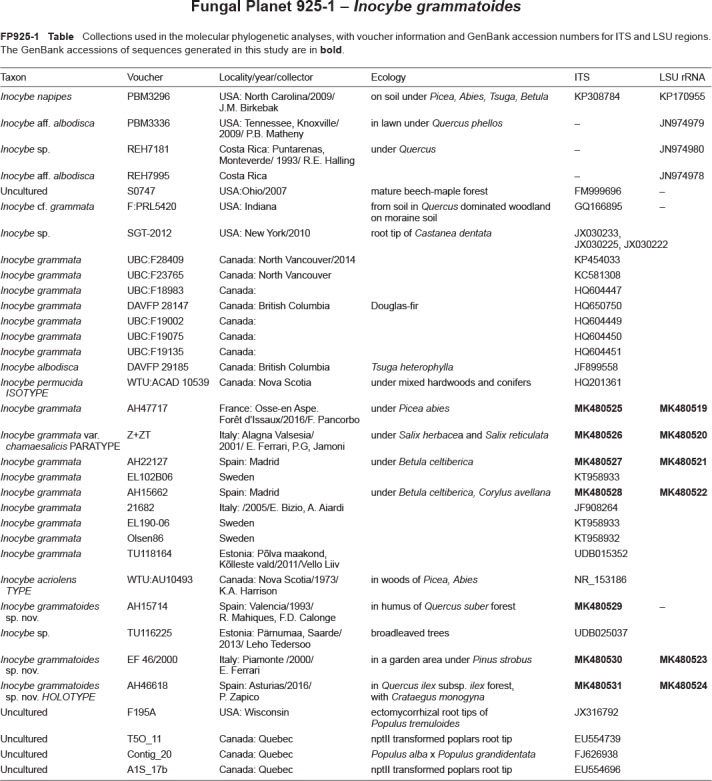
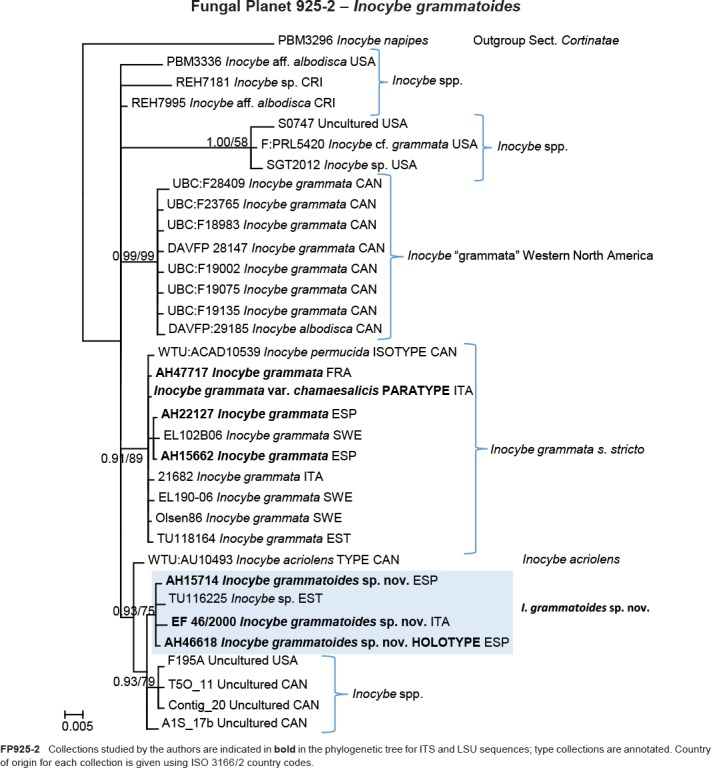
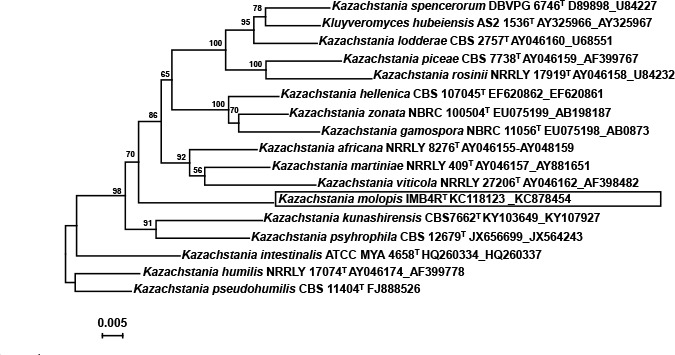
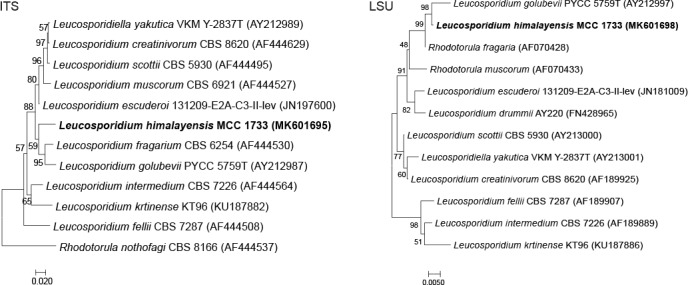
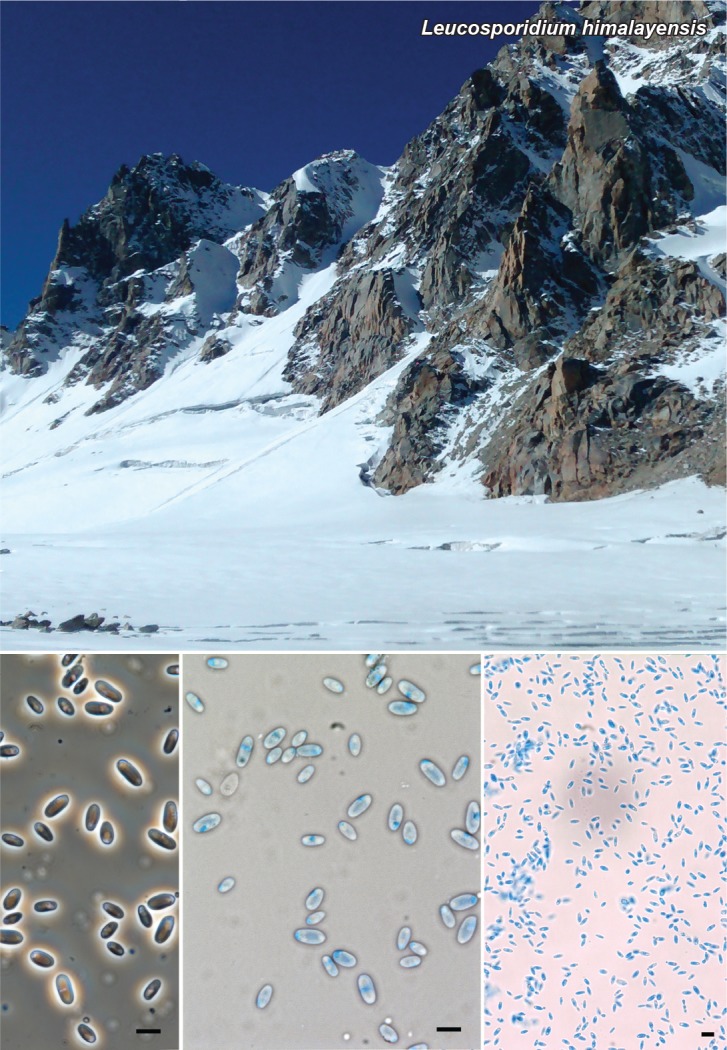
Novel species of fungi described in this study include those from various countries as follows: Australia, Chaetomella pseudocircinoseta and Coniella pseudodiospyri on Eucalyptus microcorys leaves, Cladophialophora eucalypti, Teratosphaeria dunnii and Vermiculariopsiella dunnii on Eucalyptus dunnii leaves, Cylindrium grande and Hypsotheca eucalyptorum on Eucalyptus grandis leaves, Elsinoe salignae on Eucalyptus saligna leaves, Marasmius lebeliae on litter of regenerating subtropical rainforest, Phialoseptomonium eucalypti (incl. Phialoseptomonium gen. nov.) on Eucalyptus grandis × camaldulensis leaves, Phlogicylindrium pawpawense on Eucalyptus tereticornis leaves, Phyllosticta longicauda as an endophyte from healthy Eustrephus latifolius leaves, Pseudosydowia eucalyptorum on Eucalyptus sp. leaves, Saitozyma wallum on Banksia aemula leaves, Teratosphaeria henryi on Corymbia henryi leaves. Brazil, Aspergillus bezerrae, Backusella azygospora, Mariannaea terricola and Talaromyces pernambucoensis from soil, Calonectria matogrossensis on Eucalyptus urophylla leaves, Calvatia brasiliensis on soil, Carcinomyces nordestinensis on Bromelia antiacantha leaves, Dendryphiella stromaticola on small branches of an unidentified plant, Nigrospora brasiliensis on Nopalea cochenillifera leaves, Penicillium alagoense as a leaf endophyte on a Miconia sp., Podosordaria nigrobrunnea on dung, Spegazzinia bromeliacearum as a leaf endophyte on Tilandsia catimbauensis, Xylobolus brasiliensis on decaying wood. Bulgaria, Kazachstania molopis from the gut of the beetle Molops piceus. Croatia, Mollisia endocrystallina from a fallen decorticated Picea abies tree trunk. Ecuador, Hygrocybe rodomaculata on soil. Hungary, Alfoldia vorosii (incl. Alfoldia gen. nov.) from Juniperus communis roots, Kiskunsagia ubrizsyi (incl. Kiskunsagia gen. nov.) from Fumana procumbens roots. India, Aureobasidium tremulum as laboratory contaminant, Leucosporidium himalayensis and Naganishia indica from windblown dust on glaciers. Italy, Neodevriesia cycadicola on Cycas sp. leaves, Pseudocercospora pseudomyrticola on Myrtus communis leaves, Ramularia pistaciae on Pistacia lentiscus leaves, Neognomoniopsis quercina (incl. Neognomoniopsis gen. nov.) on Quercus ilex leaves. Japan, Diaporthe fructicola on Passiflora edulis × P. edulis f. flavicarpa fruit, Entoloma nipponicum on leaf litter in a mixed Cryptomeria japonica and Acer spp. forest. Macedonia, Astraeus macedonicus on soil. Malaysia, Fusicladium eucalyptigenum on Eucalyptus sp. twigs, Neoacrodontiella eucalypti (incl. Neoacrodontiella gen. nov.) on Eucalyptus urophylla leaves. Mozambique, Meliola gorongosensis on dead Philenoptera violacea leaflets. Nepal, Coniochaeta dendrobiicola from Dendriobium lognicornu roots. New Zealand, Neodevriesia sexualis and Thozetella neonivea on Archontophoenix cunninghamiana leaves. Norway, Calophoma sandfjordenica from a piece of board on a rocky shoreline, Clavaria parvispora on soil, Didymella finnmarkica from a piece of Pinus sylvestris driftwood. Poland, Sugiyamaella trypani from soil. Portugal, Colletotrichum feijoicola from Acca sellowiana. Russia, Crepidotus tobolensis on Populus tremula debris, Entoloma ekaterinae, Entoloma erhardii and Suillus gastroflavus on soil, Nakazawaea ambrosiae from the galleries of Ips typographus under the bark of Picea abies. Slovenia, Pluteus ludwigii on twigs of broadleaved trees. South Africa, Anungitiomyces stellenboschiensis (incl. Anungitiomyces gen. nov.) and Niesslia stellenboschiana on Eucalyptus sp. leaves, Beltraniella pseudoportoricensis on Podocarpus falcatus leaf litter, Corynespora encephalarti on Encephalartos sp. leaves, Cytospora pavettae on Pavetta revoluta leaves, Helminthosporium erythrinicola on Erythrina humeana leaves, Helminthosporium syzygii on a Syzygium sp. bark canker, Libertasomyces aloeticus on Aloe sp. leaves, Penicillium lunae from Musa sp. fruit, Phyllosticta lauridiae on Lauridia tetragona leaves, Pseudotruncatella bolusanthi (incl. Pseudotruncatellaceae fam. nov.) and Dactylella bolusanthi on Bolusanthus speciosus leaves. Spain, Apenidiella foetida on submerged plant debris, Inocybe grammatoides on Quercus ilex subsp. ilex forest humus, Ossicaulis salomii on soil, Phialemonium guarroi from soil. Thailand, Pantospora chromolaenae on Chromolaena odorata leaves. Ukraine, Cadophora helianthi from Helianthus annuus stems. USA, Boletus pseudopinophilus on soil under slash pine, Botryotrichum foricae, Penicillium americanum and Penicillium minnesotense from air. Vietnam, Lycoperdon vietnamense on soil. Morphological and culture characteristics are supported by DNA barcodes.
Keywords: ITS nrDNA barcodes, LSU, new taxa, systematics
Overview Mucoromycota, Ascomycota and Basidiomycota phylogeny – part 1.
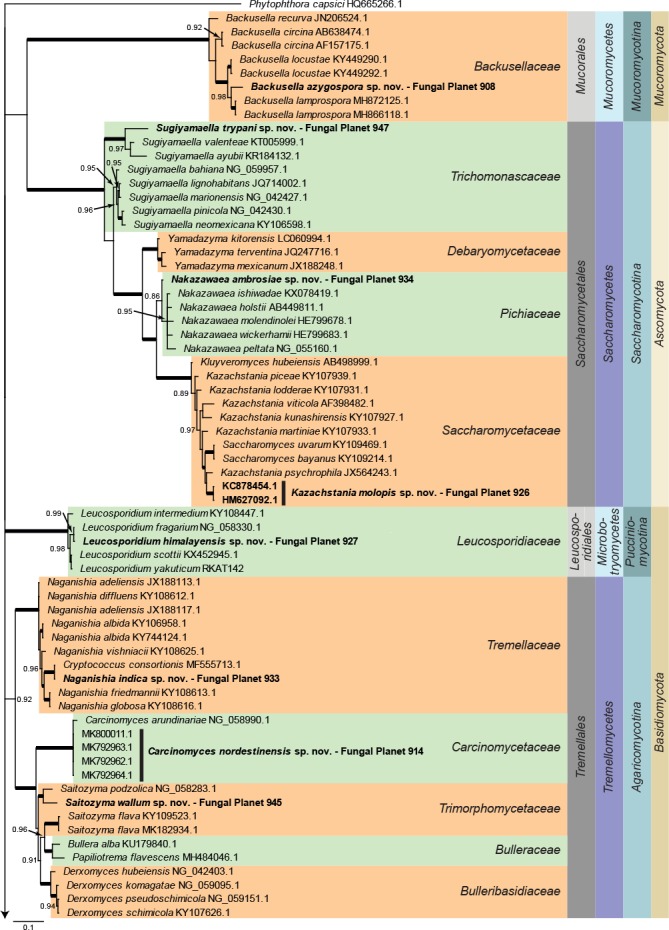
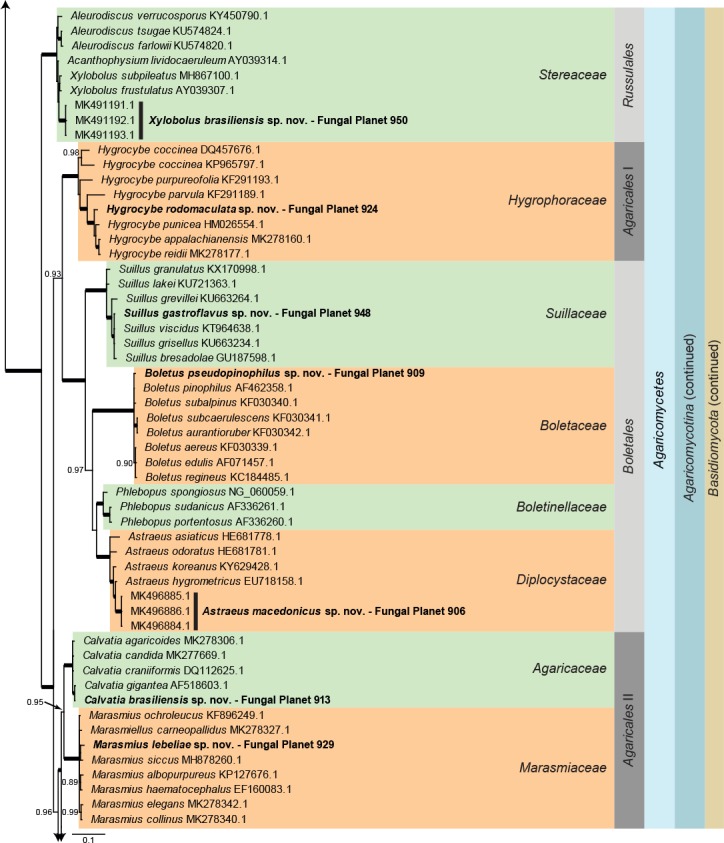
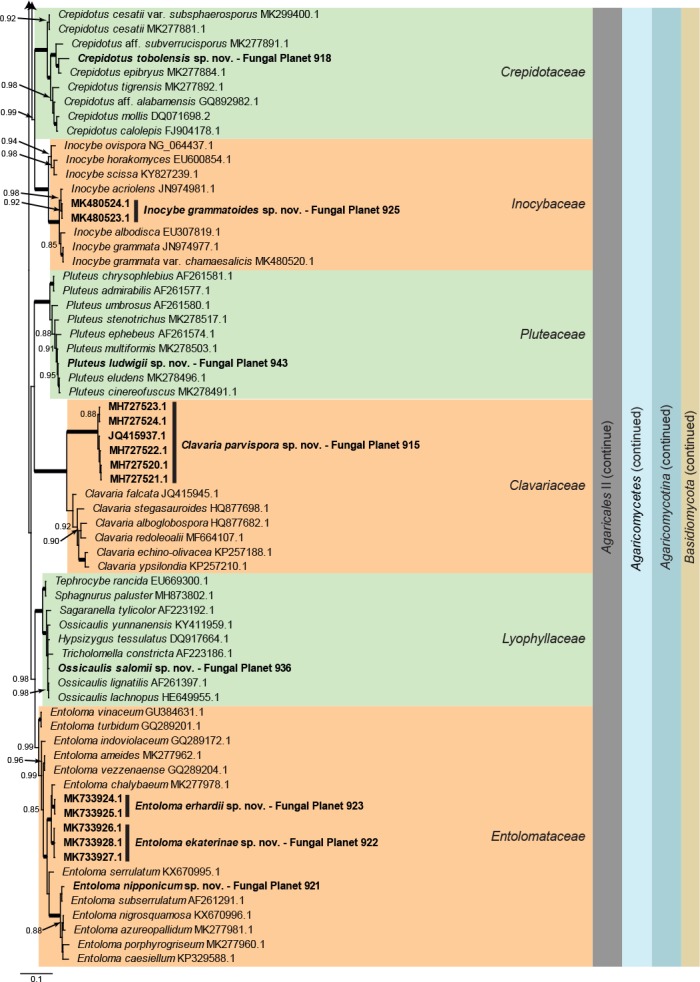
Consensus phylogram (50 % majority rule) of 40 878 trees resulting from a Bayesian analysis of the LSU sequence alignment (188 taxa including outgroup; 947 aligned positions; 656 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders, classes, subdivisions and phyla are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Phytophthora capsici (GenBank HQ665266.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Overview Dothideomycetes phylogeny – part 1.
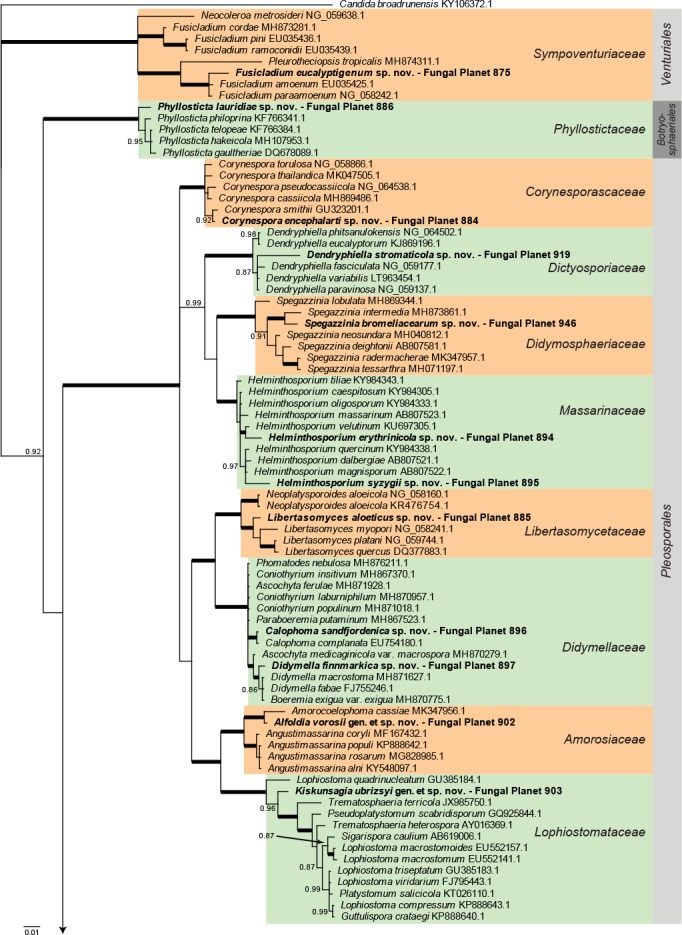
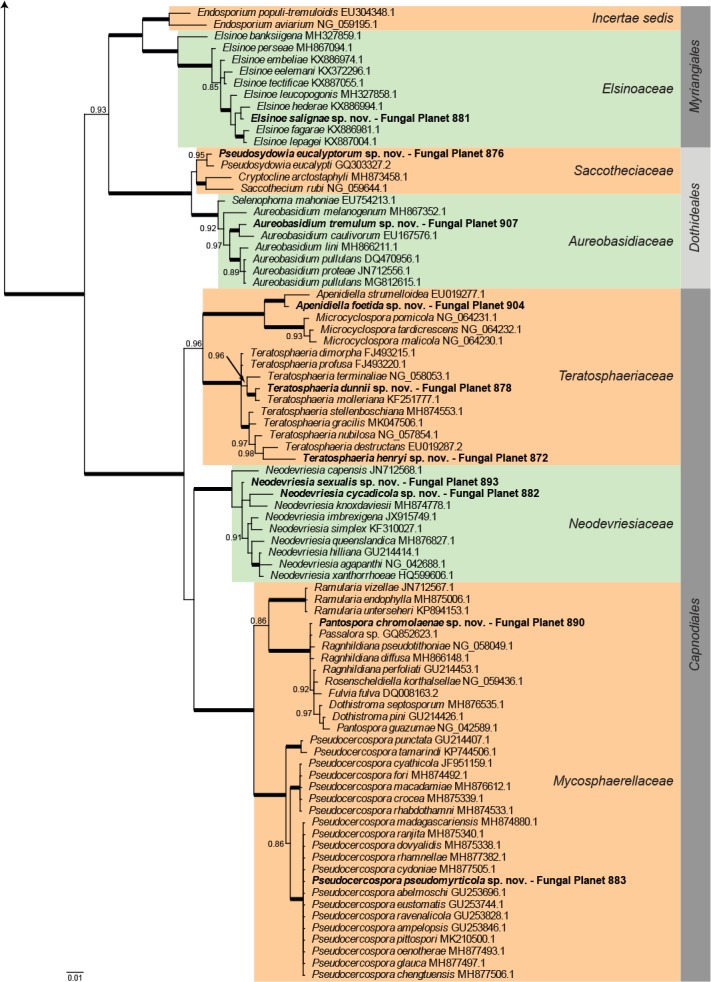
Consensus phylogram (50 % majority rule) of 22 278 trees resulting from a Bayesian analysis of the LSU sequence alignment (164 taxa including outgroup; 809 aligned positions; 394 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Overview Eurotiomycetes phylogeny.
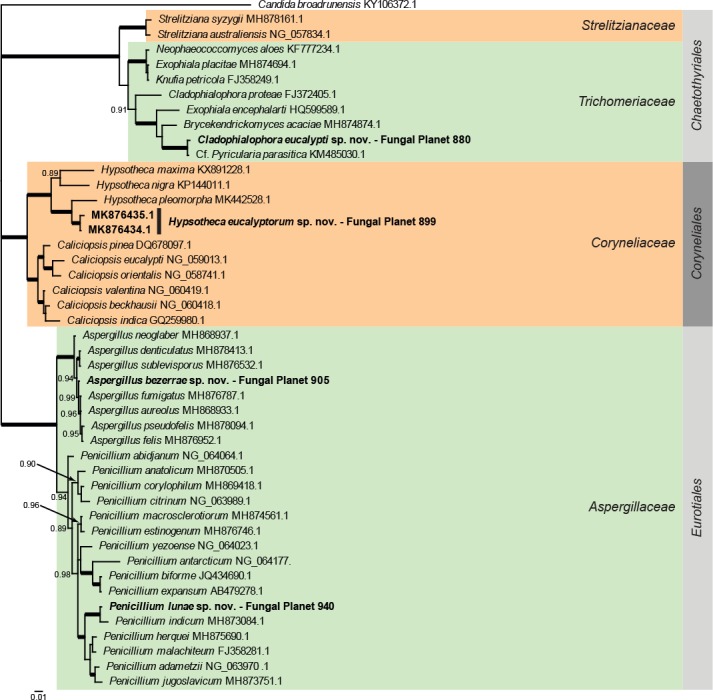
Consensus phylogram (50 % majority rule) of 7 802 trees resulting from a Bayesian analysis of the LSU sequence alignment (46 taxa including outgroup; 816 aligned positions; 282 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Overview Diaporthales and Glomerellales (Sordariomycetes) phylogeny.
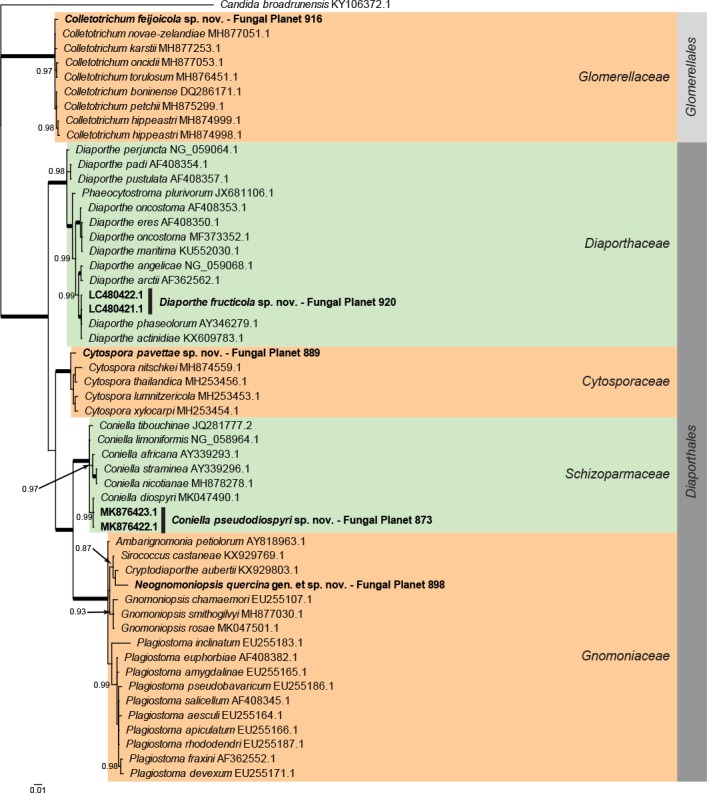
Consensus phylogram (50 % majority rule) of 21 752 trees resulting from a Bayesian analysis of the LSU sequence alignment (54 taxa including outgroup; 781 aligned positions; 185 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Overview Hypocreales (Sordariomycetes) phylogeny.
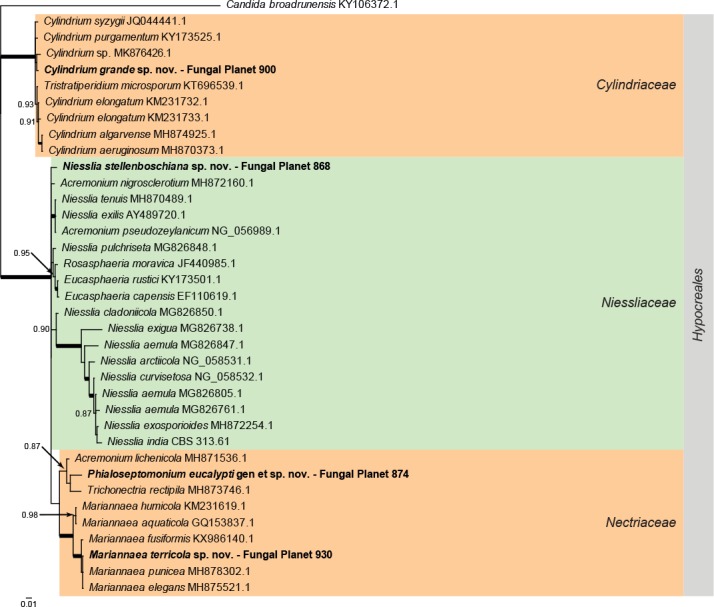
Consensus phylogram (50 % majority rule) of 13 052 trees resulting from a Bayesian analysis of the LSU sequence alignment (37 taxa including outgroup; 761 aligned positions; 181 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Overview other orders (Sordariomycetes) phylogeny.
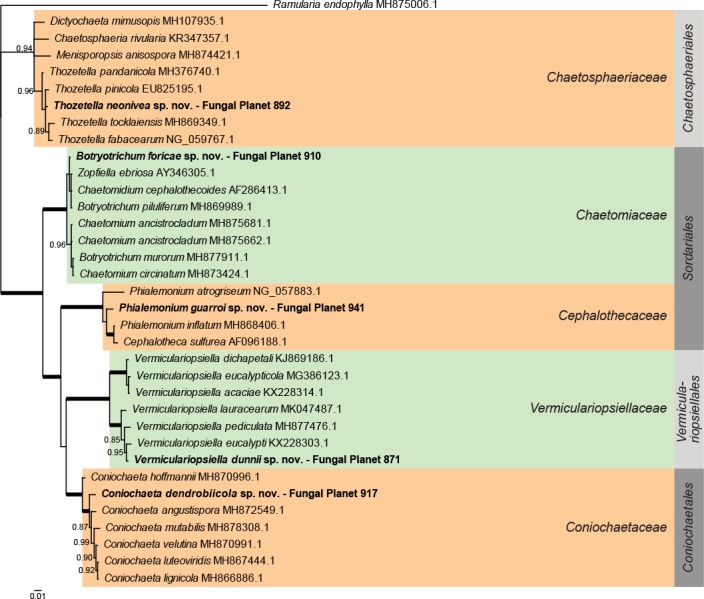
Consensus phylogram (50 % majority rule) of 14 252 trees resulting from a Bayesian analysis of the LSU sequence alignment (35 taxa including outgroup; 724 aligned positions; 192 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Ramularia endophylla (GenBank MH875006.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
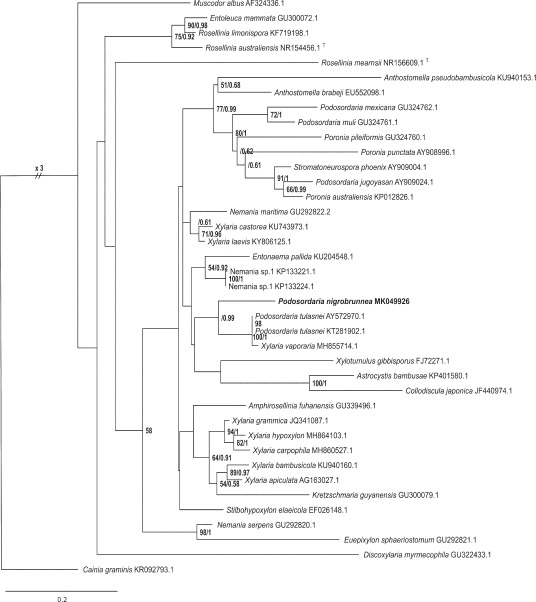
Overview Xylariales (Sordariomycetes) phylogeny.
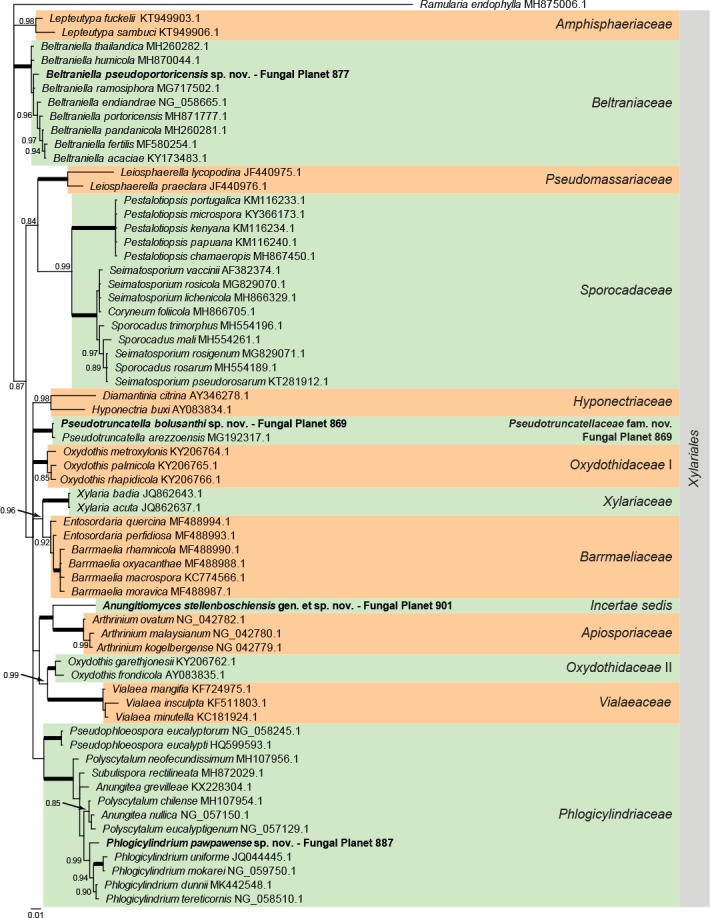
Consensus phylogram (50 % majority rule) of 35 702 trees resulting from a Bayesian analysis of the LSU sequence alignment (65 taxa including outgroup; 736 aligned positions; 194 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Ramularia endophylla (GenBank MH875006.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Overview Orbiliomycetes, Lecanoromycetes and Leotiomycetes phylogeny.
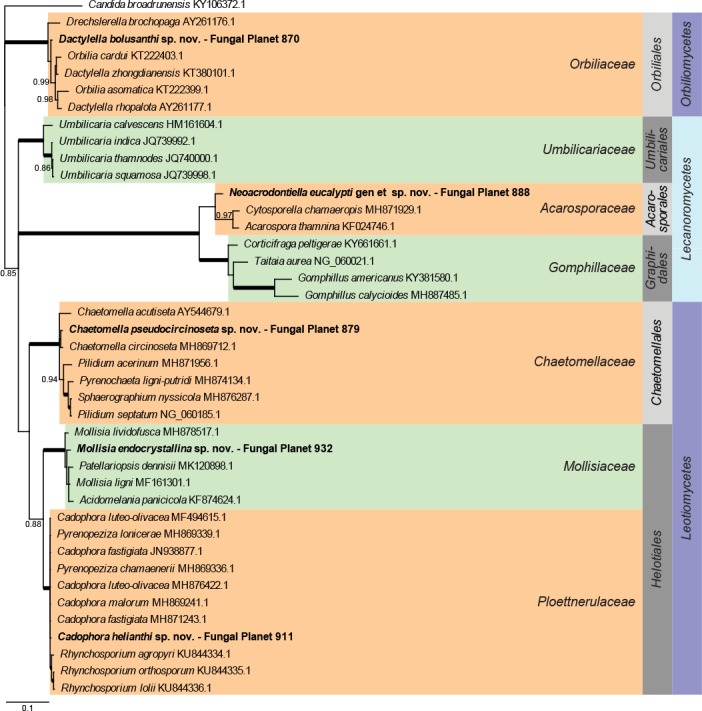
Consensus phylogram (50 % majority rule) of 58 402 trees resulting from a Bayesian analysis of the LSU sequence alignment (41 taxa including outgroup; 812 aligned positions; 350 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) >0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S24386).
Acknowledgments
We wish to thank Fundação de Amparo à Pesquisa do Estado de Mato Grosso (224618/2015) for financial support. The research of Dániel G. Knapp, Ildikó Imrefi and Gábor M. Kovács was supported by the National Research, Development and Innovation Office, Hungary (NKFIH KH-130401) and the ELTE Institutional Excellence Program (1783-3/2018/FEKUTSRAT) of the Hungarian Ministry of Human Capacities. Katerina Rusevska and colleagues received support from the SYNTHESYS Project (http://www.synthesys.info/), which is financed by the European Community Research Infrastructure Action under the FP7 ‘Capacities’ Program. The authors express their gratitude to the Macedonian Ecological Society and Biology Students’ Research Society for arranging collecting trips. Thalline R.L. Cordeiro and co-authors express their gratitude to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for and Fundação de Amparo à Ciência do Estado de Pernambuco (FACEPE) for Master scholarships provided to André L.C.M. de A. Santiago, Diogo X. Lima, Rafael J.V. de Oliveira and Thalline R.L. Cordeiro. This manuscript was financed by the projects ‘Diversity of Mucoromycotina in the different ecosystems of the Atlantic Rainforest of Pernambuco’ (FACEPE – First Projects Program PPP/FACEPE/CNPq–APQ–0842-2.12/14) and Mucoromycotina from Atlantic Rainforest in the semiarid of Pernambuco (CNPq–Chamada Universal–458391/2014-0). Vit Hubka was supported by the project BIOCEV (CZ.1.05/1.1.00/02.0109) provided by the Ministry of Education, Youth and Sports of the Czech Republic and ERDF and by the Charles University Research Centre program No. 204069. Sujit Shah and colleagues thank the University Grants Commission, Nepal; Centre for Co-operation in Science & Technology among Developing Societies, Department of Science and Technology, India; Department of Biotechnology, New Delhi for establishing National Centre for Microbial Resource (NCMR), NCCS, Pune, India wide grant letter no. BT/Coord.II/01/03/2016 dated 6th April 2017. Vladimir I. Kapitonov and colleagues are very grateful to Brigitta Kiss for help in molecular studies, Bálint Dima and László G. Nagy for their critical notes. This study was conducted under the research projects of the Tobolsk Complex Scientific Station of the Ural Branch of the Russian Academy of Sciences (N ÀÀÀÀ-À19-119011190112-5). Taiga Kasuya and co-authors thank Ms Shizuka Ikegawa for her support with morphological observations. The study of Olga V. Morozova, Ekaterina F. Malysheva and Ivan V. Zmitrovich was carried out within the framework of research project of the Komarov Botanical Institute RAS ‘Herbarium funds of the BIN RAS’ (ÀÀÀÀ-À18-118022090078-2). She and her colleagues are also grateful to the staff of the Teberda and Sikhote-Alin Nature Reserves for the permission to collect on their territories and for help in the field work. M.E. Noordeloos and his collaborators thank the Kits van Waveren Foundation (Rijksherbariumfonds Dr E. Kits van Waveren, Leiden, Netherlands) which contributed substantially to the costs of sequencing. Teresa Iturriaga and colleagues thank Angela Bond, Fungarium Manager at IMI, for sending ILLS the loan of the type specimen of Meliola carvalhoi, the E.O. Wilson Biodiversity Laboratory in Gorongosa National Park, to its Associate Director Piotr Naskrecki, to botanist Meg Coates Palgrave from Zimbabwe who identified the host of Meliola gorongosensis, and to the Ella Lyman Cabot Trust for funding to J. Karakehian to collect in the Park. Fernando Esteve-Raventós and co-authors thank D. Bandini, T. Conca, E. Ferrari, M. Laso, P.B. Matheny, J. Rejos and P. Zapico for their valuable collaboration in the elaboration and completion of this work. This study has been partially funded by a project granted by the Spanish Science Council (CGL2017-86540-P) to F. Esteve-Raventós and G. Moreno. Dilnora Gouliamova and colleagues were supported by a grant from the Bulgarian Science Fund (D002-TK-176) and F7 Research and Infrastructure grant - European Consortium of Microbial Resource Centres. The authors express their gratitude for Dr Borislav Guéorguiev from National Museum of Natural History (Sofia, Bulgaria) for the identification of beetles. Alina V. Alexandrova is supported by the RUDN University Program 5-100, Russia. Amanda Lucia Alves and Ana Carla da Silva Santos acknowledge scholarships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Renan N. Barbosa a scholarship from the Conselho Nacional de Pesquisa (CNPq) and Cristina M. Souza-Motta and Patricia Vieira Tiago acknowledge financial support from the Pró-Reitoria de Pesquisa e Pós-Graduação (Propesq). José Leonardo Siquier and Jean-Michel Bellanger acknowledge A. Bidaud and L.A. Parra for help in species identification, P. Alvarado for generating sequences and J. Planas for the composition of the photographic plate. The research of Cobus M. Visagie was supported by a grant from the NRF-FBIP Program (grant nr FBIS170605237212). Elena A. Zvyagina is supported by the KhMAO – Ugra government assignment for Surgut State University; Yury A. Rebriev is supported by a government assignment for South Science Center RAS (AAAA-A19-119011190176-7); Nina A. Sazanova is supported by a government assignment for Institute of Biological Problems of the North FEB RAS (ÀÀÀÀ-À17-117122590002-0). Roberta Cruz and colleagues thank the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco – FACEPE for financial support. Renata S. Chikowski and co-authors would like to thank the Herbarium URM for the loan of exsiccates; PPGBF (UFPE, Brazil), CNPq (SISBIOTA (563342/2010-2), PPBio Semi-Árido (457476/2012-5), PROTAX (562106/2010-3), Universal (472792/2011-3), PQ (307601/2015-3)), CAPES (Capes-SIU 008/13) and FACEPE (APQ 0375-2.03/15) for financial support; CAPES for the master scholarship of R.S. Chikowski and PhD scholarship of Lira, and FACEPE for the PhD scholarship of R.S. Chikowski and post-doctorate scholarship of C.R.S. Lira. Financial support was provided to Renan de L. Oliveira and colleagues by the Coordination of Improvement of Higher Level Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq) for CNPq-Universal 2016 (409960/2016-0) and for CNPq-Pesquisador visitante (407474/2013-7). Areeb Inamdar and Nitin N. Adhapure are thankful to the Institution, Vivekanand Arts, Sardar Dalipsingh Commerce and Science College for providing Institutional support throughout the research work. Rohit Sharma and Mahesh S. Sonawane thank the Department of Biotechnology, New Delhi for financial support for the establishment of National Centre for Microbial Resource (NCMR), Pune wide grant letter no. BT/Coord.II/01/03/2016. Amanda C.Q. Brito, Juliana F. Mello, Cinthia Conforto, Sami J. Michereff & Alexandre R. Machado acknowledge financial support and/or scholarships from CNPq, CAPES and FACEPE. Shiv Mohan Singh, Rohit Sharma and co-authors thank the Department of Biotechnology, New Delhi for financial support for the establishment of National Centre for Microbial Resource (NCMR), Pune wide grant letter no. BT/Coord.II/01/03/2016 dated 6 April 2017. We are also thankful to Indian Council of Agricultural Research (ICAR) for financial support (NBAIM/AMAAS/2014-17/PF/24/21) for research on Himalaya. Shiv Mohan Singh is thankful to Dr Perman and Sharma for help during sampling and Ms Rohita Naik for technical aid. Jadson D.P. Bezerra and colleagues acknowledge financial support and/or scholarships from the CAPES (Finance Code 001), CNPQ/ICMBio (Processes numbers 421241/2017-9 and 310298/2018-0) and FACEPE (APQ-0143-2.12/15). Dayse A. Andrade, Ciro R. Félix and Melissa F. Landell, are thankful for the financial support, permissions and collaboration of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) (process numbers 475378/2013-0 and 408718/2013-7), Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Maria E. Ordoñez and colleagues acknowledge financial support obtained from Secretaria de Educación Superior, Ciencia, Tecnología e Innovación del Ecuador (SENESCYT), Arca de Noé Initiative. Ivona Kautmanová and colleagues were funded by the Operational Program of Research and Development and co-financed with the European Fund for Regional Development (EFRD) ITMS 26230120004: ‘Building of research and development infrastructure for investigation of genetic biodiversity of organisms and joining IBOL initiative’. This study was partially supported by the Spanish Ministerio de Economía, Industria y Competitividad (grant CGL2017-88094-P). Sincere thanks to Dr Teresa Lebel (Royal Botanic Gardens Victoria) for initiating the citizen science ‘fungi-taxonomist’ project in Victoria, and providing molecular and taxonomic expertise. Angus Carnegie acknowledges the support of Forestry Corporation of NSW, Australia. The research of Julia Pawłowska was partially supported by the National Science Centre, Poland, under grant no 2017/25/B/NZ8/00473. Neven Matočec, Ivana Kušan, Margita Jadan, Armin Mešić and Zdenko Tkalčec were supported by the Croatian Science Foundation under the project ForFungiDNA (IP-2018-01-1736) and co-financed by the Public Institution Sjeverni Velebit National Park.
REFERENCES
- Abdalla MY, Al-Rokibah AA. 2003. New record of the pyrenomycete Coniochaeta velutina causing leaf blight of date palm. Journal of King Saud University, Agricultural Sciences 15: 177–184. [Google Scholar]
- Aebi B. 1972. Untersuchungen über Discomyceten aus der Gruppe Tapesia-Trichobelonium. Nova Hedwigia 23: 49–112. [Google Scholar]
- Agustí-Brisach C, Gramaje D, García-Jiménez J, et al. 2013. Detection of black-foot and Petri disease pathogens in natural soils of grapevine nurseries and vineyards using bait plants. Plant and Soil 364: 5–13. [Google Scholar]
- Alfenas RF, Lombard L, Pereira OF, et al. 2015. Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil. Studies in Mycology 80: 89–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfenas RF, Pereira OL, Ferreira MA, et al. 2013. Calonectria metrosideri, a highly aggressive pathogen causing leaf blight, root rot, and wilt of Metrosideros spp. in Brazil. Forest Pathology 43: 257–265. [Google Scholar]
- Alvarez LV, Groenewald JZ, Crous PW. 2016. Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Studies in Mycology 85: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjic V, Whyte G, Hardy GEStJ, et al. 2010. New Teratosphaeria species occurring on eucalypts in Australia. Fungal Diversity 43: 27–38. [Google Scholar]
- Antonín V, Ryoo R, Shin HD. 2012. Marasmioid and gymnopoid fungi of the Republic of Korea. 4. Marasmius sect. Sicci. Mycological Progress 11: 615–638. [Google Scholar]
- Asgari B, Zare R. 2006. Two new Coniochaeta species from Iran. Nova Hedwigia 82: 227–236. [Google Scholar]
- Bandala VM, Montoya L. 2000. A revision of some Crepidotus species related to Mexican taxa. Mycological Research 104: 495–506. [Google Scholar]
- Baral H-O. 1987. Lugol’s solution / IKI versus Melzer’s reagent: hemiamyloidity, a universal feature of the ascus wall. Mycotaxon 29: 399–450. [Google Scholar]
- Barbosa FR, Silva SSD, Fiuza PO, et al. 2011. Conidial fungi from the semi-arid Caatinga biome of Brazil. New species and records for Thozetella. Mycotaxon 115: 327–334. [Google Scholar]
- Barbosa RN, Bezerra JDP, Costa PMO, et al. 2016. Aspergillus and Penicillium (Eurotiales: Trichocomaceae) in soils of the Brazilian tropical dry forest: diversity in an area of environmental preservation. Revista de Biología Tropical 64: 45–53. [DOI] [PubMed] [Google Scholar]
- Barbosa RN, Bezerra JDP, Souza-Motta CM, et al. 2018. New Penicillium and Talaromyces species from honey, pollen and nests of stingless bees. Antonie van Leeuwenhoek 111: 1883–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeli M. 1920. Note sur le genre Meliola Fr. Bulletin du Jardin Botanique de l’État à Bruxelles 7: 89–160. [Google Scholar]
- Benny GL, Benjamin RK. 1975. Observations on Thamnidiaceae (Mucorales). New taxa, new combinations, and notes on selected species. Aliso 8: 301–351. [Google Scholar]
- Bernicchia A, Gorjón SP. 2010. Corticiaceae s.l. Fungi Europaei n° 12. Italia. Candusso. [Google Scholar]
- Bessette AE, Roody W, Bessette AR. 2000. North American boletes – A color guide to the fleshy pored mushrooms. Syracuse University Press. [Google Scholar]
- Bessette AE, Roody W, Bessette AR. 2016. Boletes of Eastern North America. Syracuse University Press. [Google Scholar]
- Bessette AE, Roody W, Bessette AR, et al. 2007. Mushrooms of the Southeastern United States. Syracuse University Press. [Google Scholar]
- Bettucci L, Simeto S, Alonso R, et al. 2004. Endophytic fungi of twigs and leaves of three native species of Myrtaceae in Uruguay. Sydowia 56: 8–23. [Google Scholar]
- Blanchette RA, Held WB, Jurgens JA, et al. 2004. Wood-destroying soft rot fungi in the historic expedition huts of Antarctica. Applied and Environmental Microbiology 70: 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardo D, Traverso M, Vizzini A, et al. 2008. Funghi d’Italia. Zaichelli, Bologna, Italia. [Google Scholar]
- Boekhout T, Fonseca Á, Sampaio JP, et al. 2011. Discussion of teleomorphic and anamorphic basidiomycetous yeasts. In: Kurtzman CP, Fell JW, Boekhout T. (eds), The yeasts. Elsevier, Amsterdam: 1339–1372. [Google Scholar]
- Boertmann D. 2008. Hygrocybe (Fr.) P. Kumm. In: Knudsen H, Vesterholt J. (eds), Funga Nordica. Agaricoid, boletoid and cyphelloid genera: 194–212. Nordsvamp-Copenhagen. [Google Scholar]
- Bottomley AM. 1948. Gasteromycetes of South Africa. Bothalia 4: 473–810. [Google Scholar]
- Cai L, Kurniawati E, Hyde KD. 2010. Morphological and molecular characterization of Mariannaea aquaticola sp. nov. collected from freshwater habitats. Mycological Progress 9: 337–343. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, et al. 2002. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, et al. 2009. Myrtaceae, a cache of fungal biodiversity. Persoonia 23: 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AJ, Sun BD, Houbraken J, et al. 2016. New Talaromyces species from indoor environments in China. Studies in Mycology 84: 119–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Jiang JR, Zhang GZ, et al. 2015. Resolving the Phoma enigma. Studies in Mycology 82: 137–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio G, Setti L. 2008. Il genere Crepidotus in Europa. Trento, Associazione Micologica Bresadola. [Google Scholar]
- Contu M. 2007. Agarics of Sardinia: notes and descriptions. VII. Micologia e Vegetaziones Mediterranea 22: 29–40. [Google Scholar]
- Crous PW. 1999. Species of Mycosphaerella and related anamorphs occurring on Myrtaceae (excluding Eucalyptus). Mycological Research 103: 607–621. [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. 2017. The genera of fungi – G 4: Camarosporium and Dothiora. IMA Fungus 8: 131–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
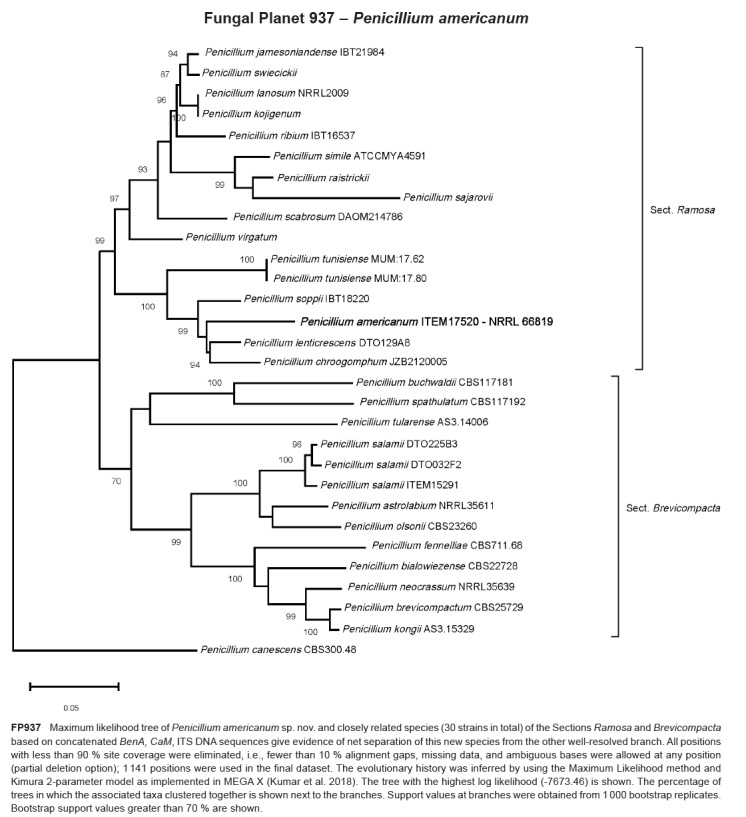
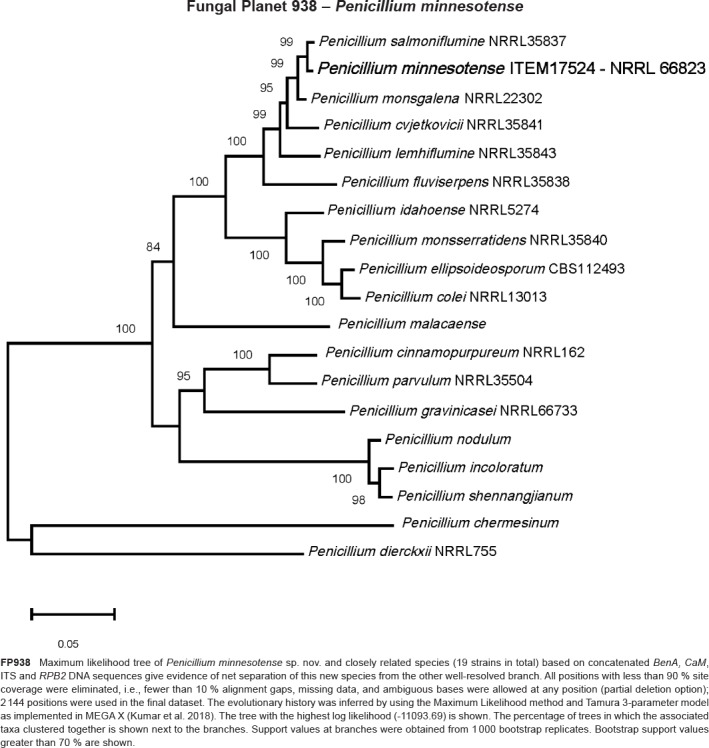
- Crous PW, Luangsaard JJ, Wingfield MJ, et al. 2018a. Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, et al. 2009. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, et al. 2019. New and interesting fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2015a. Fungal Systematics and Evolution: FUSE 1. Sydowia 67: 81–118. [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2018b. New and interesting fungi. 1. Fungal Systematics and Evolution 1: 169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012. Fungal Planet description sheets: 107–127. Persoonia 28: 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ. 1997. Colletogloeopsis, a new coelomycete genus to accommodate anamorphs of two species of Mycosphaerella occurring on Eucalyptus. Canadian Journal of Botany 75: 667–674. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017. Fungal Planet description sheets: 625–715. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2018c. Fungal Planet description sheets: 716–784. Persoonia 40: 240–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015b. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. 2016. Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz R, Santos C, Lima JS, et al. 2013. Diversity of Penicillium in soil of Caatinga and Atlantic Forest areas of Pernambuco, Brazil: an ecological approach. Nova Hedwigia 97: 543–556. [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012. The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Fourie PH, Crous PW. 2010. Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 24: 60–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Sato T, Alizadeh A, et al. 2019. The Colletotrichum draecaenophilum, C. magnum and C. orchidearum species complexes. Studies in Mycology 92: 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TS, Hofstetter RW, Foster JT, et al. 2011. Interactions between the yeast Ogataea pini and filamentous fungi associated with the western pine beetle. Microbial Ecology 61: 626–634. [DOI] [PubMed] [Google Scholar]
- Deighton FC. 1951. New African Meliolaceae. Sydowia Annales Mycologici 5: 1–8. [Google Scholar]
- Di Marco S, Calzarano F, Osti F, et al. 2004. Pathogenicity of fungi associated with a decay of kiwifruit. Australasian Plant Pathology 33: 337–342. [Google Scholar]
- Dimitrov R, Gouliamova D. 2019. Genetic and phenotypic cut-off values for species and genera discrimination of the Kazachstania clade. Comptes rendus de l’Académie Bulgare des Sciences 72: 350–357. [Google Scholar]
- Dissing H, Lange M. 1962. Gasteromycetes of Congo. Bulletin du Jardin Botanique de l’État a Bruxelles 32: 325–416. [Google Scholar]
- Ellis MB. 1976. More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, England. [Google Scholar]
- Ellis MB, Ellis JP. 1997. Microfungi on land plants – an identification handbook. Richmond Publishing Co., England. [Google Scholar]
- Esteve-Raventós F, Calonge FD. 1996. Dos agaricales poco frecuentes e interesantes en la Península Ibérica. Boletín de la Sociedad Micológica de Madrid 2: 293–298. [Google Scholar]
- Fan XL, Barreto RW, Groenewald JZ, et al. 2017. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Studies in Mycology 87: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangfuk W, Petchang R, Toanun C, et al. 2010. Identification of Japanese Astraeus, based on morphological and phylogenetic analyses. Mycoscience 51: 291–299. [Google Scholar]
- Fantinel VS, Muniz MFB, Blume E, et al. 2017. First report of Colletotrichum siamense causing anthracnose on Acca sellowiana fruits in Brazil. Plant Disease 101: 1035. [Google Scholar]
- Farr DF, Rossman AY. 2018. Fungal databases, U.S. National Fungus Collections, ARS, USDA. Retrieved 15 Feb. 2018. Available from https://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Fisher PJ, Webster J. 1983. The teleomorphs of Helicodendron giganteum and H. paradoxum. Transactions of the British Mycological Society 81: 656–659. [Google Scholar]
- Gams W, McGinnis M. 1983. Phialemonium, a new anamorph genus intermediate between Phialophora and Acremonium. Mycologia 75: 977–987. [Google Scholar]
- Gams W, Stielow B, Gräfenhan T, et al. 2019. The ascomycete genus Niesslia and associated monocillium-like anamorphs. Mycological Progress 18: 5–76. [Google Scholar]
- Ge Y, Yang S, Bau T. 2017. Crepidotus lutescens sp. nov. (Inocybaceae, Agaricales), an ochraceous salmon colored species from northeast of China. Phytotaxa 297: 189–196. [Google Scholar]
- Giraldo A, Crous PW. 2019. Inside Plectosphaerellaceae. Studies in Mycology 92: 227–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke C, Pereira O, Stringari D, et al. 2011. Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia 26: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaje D, León M, Santana M, et al. 2014. Multilocus ISSR Markers reveal two major genetic groups in Spanish and South African populations of the grapevine fungal pathogen Cadophora luteo-olivacea. PLOS ONE 9: e110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünig CR, Queloz V, Duò A, et al. 2009. Phylogeny of Phaeomollisia piceae gen. sp. nov.: a dark, septate, conifer-needle endophyte and its relationships to Phialocephala and Acephala. Mycological Research 113: 207–221. [DOI] [PubMed] [Google Scholar]
- Guarnaccia V, Aiello D, Polizzi G, et al. 2014. Emergence of prochloraz-resistant populations of Calonectria pauciramosa and Calonectria polizzii in ornamental nurseries of Southern Italy. Plant Disease 98: 344–350. [DOI] [PubMed] [Google Scholar]
- Guarnaccia V, Vitale A, Cirvilleri G, et al. 2016. Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. European Journal of Plant Pathology 146: 963–976. [Google Scholar]
- Guarro J. 2012. Taxonomía y biología de los hongos causantes de infección en humanos. Enfermedades Infecciosas y Microbiología Clínica 30: 33–39. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, et al. 2010. PHYML Online – a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Research 33 (Web Server issue): W557–W559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleen F, Crous PW, Petrini O. 2003. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australasian Plant Pathology 32: 47–52. [Google Scholar]
- Hamim A, Miché L, Douaik A, et al. 2017. Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems. Comptes Rendus Biologies 340: 226–237. [DOI] [PubMed] [Google Scholar]
- He X-L, Wang D, Peng W-H, et al. 2017. Two new Entoloma s.l. species with serrulatum-type lamellar edge from Changbai Mountains, Northeast China. Mycological Progress 16: 761–768. [Google Scholar]
- Hernández-Restrepo M, Madrid H, Tan YP, et al. 2018. Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia 41: 71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortstam K, Larsson KH, Ryvarden L. 1988. The Corticiaceae of North Europe 8: 1450–1631. [Google Scholar]
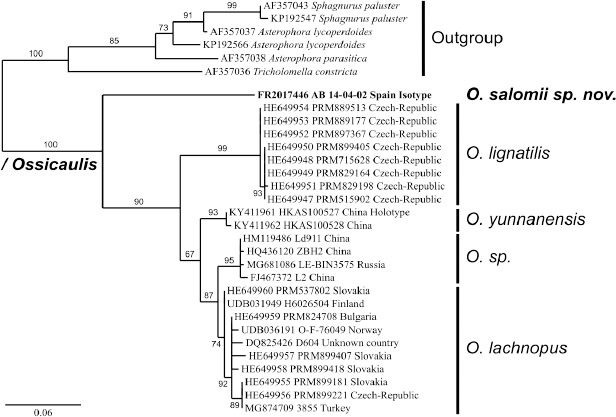
- Holec J, Kolařík M. 2013. Ossicaulis lachnopus (Agaricales, Lyophyllaceae), a species similar to O. lignatilis, is verified by morphological and molecular methods. Mycological Progress 12: 587–597. [Google Scholar]
- Horak E. 2018. Fungi of New Zealand. Volume 6. Agaricales (Basidiomycota) of New Zealand. 2. Brown spored genera p.p. Crepidotus, Flammulaster, Inocybe, Phaeocollybia, Phaeomarasmius, Pleuroflammula, Pyrrhoglossum, Simocybe, Tubaria and Tympanella. Westerdijk Biodiversity Series 16: 1–205. [Google Scholar]
- Hou LW, Liu F, Duan WJ, et al. 2016. Colletotrichum aracearum and C. camelliae-japonicae, two holomorphic new species from China and Japan. Mycosphere 7: 1111–1123. [Google Scholar]
- Hu DM, Wang M, Cai L. 2016. Phylogenetic assessment and taxonomic revision of Mariannaea. Mycological Progress 16: 271–283. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hujslová M, Kubátová A, Chudíčková M, et al. 2010. Diversity of fungal communities in saline and acidic soils in the Soos National Natural Reserve, Czech Republic. Mycological Progress 9: 1–15. [Google Scholar]
- Iturrieta-González I, Gené J, Guarro J, et al. 2018. Neodendryphiella, a novel genus of the Dictyosporiaceae (Pleosporales). MycoKeys 37: 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami F, Marincowitz S, Crous PW, et al. 2018. A new Cytospora species pathogenic on Carpobrotus edulis in its native habitat. Fungal Systematics and Evolution 2: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EBG, Suetrong S, Sakayaroj J, et al. 2015. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Diversity 73: 1–72. [Google Scholar]
- Justo A, Caballero A, Muñoz G, et al. 2011. Taxonomy of Pluteus eugraptus and morphologically similar taxa. Mycologia 103: 646–655. [DOI] [PubMed] [Google Scholar]
- Karadelev M, Rusevska K, Stojkoska K. 2008. Distribution and ecology of the gasteromycete fungi – orders Phallales and Sclerodermatales in the Republic of Macedonia. Proceedings of III Congress of Ecologists of the Republic of Macedonia with International Participation. Struga, 6–9 Oct. 2007, Macedonian Ecological Society, Skopje, 2008: 208–216. [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D, Lindenmayer D, Lowe A, et al. 2014. Heathlands. In: Lindenmayer D, Burns E, Thurgate N, et al. (eds), Biodiversity and environmental change. Monitoring, challenges and direction: 213–281. CSIRO Publishing, Collingwood, Victoria. [Google Scholar]
- Kelly KL. 1964. Inter-society color council - National bureau of standards - color-name charts illustrated with centroid colors. US Government Printing Office, Washington. [Google Scholar]
- Kerry E. 1990. Microorganisms colonizing plants and soil subjected to different degrees of human activity, including petroleum contamination, in the Vestfold Hills and MacRobertson Land, Antarctica. Polar Biology 10: 423–430. [Google Scholar]
- Kinoshita A, Sasaki H, Nara K. 2012. Multiple origins of sequestrate basidiomes within Entoloma inferred from molecular phylogenetic analyses. Fungal Biology 116: 1250–1262. [DOI] [PubMed] [Google Scholar]
- Knapp DG, Kovács GM, Zajta E, et al. 2015. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 35: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DG, Pintye A, Kovács GM. 2012. The dark side is not fastidious – Dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLOS ONE 7: e32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B. 1991. Illustrated key to the filamentous higher marine fungi. Botanica Marina 34: 1–61. [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour, 3rd ed. London, Eyre Methuen. [Google Scholar]
- Kreisel H. 1992. An emendation and preliminary survey of the genus Calvatia (Gasteromycetidae). Persoonia 14: 431–439. [Google Scholar]
- Krieglsteiner L. 2004. Ascomycetenfunde während des Seminars an der Schwarzwälder Pilzlehrschau vom 23. bis 27. Juni 2003. Zeitschrift für Mykologie 70: 49–58. [Google Scholar]
- Kumar S, Stecher G, Li M, et al. 2018. MEGAX: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman CP, Fell JW, Boekhout T. 2011. The yeasts, a taxonomic study. Vol. 3 Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- Kurtzman CP, Robnett CJ, 2007. Multigene phylogenetic analysis of the Trichomonascus, Wickerhamiella and Zygoascus yeast clades, and the proposal of Sugiyamaella gen. nov. and 14 new species combinations. FEMS Yeast Research 7: 141–151. [DOI] [PubMed] [Google Scholar]
- Kuyper TW. 1986. A revision of the genus Inocybe in Europe I. Subgenus Inosperma and the smooth-spored species of subgenus Inocybe. Persoonia supplement 3: 1–247. [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, et al. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Lawrence DP, Holland LA, Nouri MT, et al. 2018. Molecular phylogeny of Cytospora species associated with canker diseases of fruit and nut crops in California, with the descriptions of ten new species and one new combination. IMA Fungus 9: 333–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Bonthond G, Groenewald JZ, et al. 2019. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Studies in Mycology 92: 287–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-Z, Wang Q-M, Göker M, et al. 2015a. Towards an integrated phylogenetic classification of the Tremellomycetes. Studies in Mycology 81: 85–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-Z, Wang Q-M, Theelen B, et al. 2015b. Phylogeny of tremellomycetous yeasts and related dimorphic and filamentous basidiomycetes reconstructed from multiple gene sequence analyses. Studies in Mycology 81: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfeld BD, et al. 2010. Phylogeny and systematic of the genus Calonectria. Studies in Mycology 66: 31–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutchmeah RS. 1992. A new disease of passion fruit in Mauritius: postharvest stem-end rot caused by Phomopsis tersa. Plant Pathology 41: 772–773. [Google Scholar]
- Maerz AJ, Paul MR. 1950. A dictionary of color. 2rd ed. McGraw-Hill Book Company, New York. [Google Scholar]
- Mapperson RR. 2014. Diversity of fungal endophytes in the semi-evergreen vine thickets of the southern Brigalow Belt bioregion and their production of antimicrobial secondary metabolites. PhD thesis, University of Southern Queensland, Australia. [Google Scholar]
- Martín-Sanz A, Rueda S, García-Carneros AB, et al. 2018. Cadophora malorum: a new pathogen of sunflower causing wilting, yellowing, and leaf necrosis in Russia. Plant Disease 102: 823. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA: 1–8. [Google Scholar]
- Milne I, Wright F, Rowe G, et al. 2004. TOPALi: Software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20: 1806–1807. [DOI] [PubMed] [Google Scholar]
- Minnis AM, Kennedy AH, Grenier DB, et al. 2011. Asperisporium and Pantospora (Mycosphaerellaceae): epitypifications and phylogenetic placement. Persoonia 27: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moënne-Loccoz P, Poirier J, Reumaux P. 1990. Fungorum Rariorum Icones Coloratae. Pars XIX. Inocybes critiquables et critiqués. Cramer; Berlin-Stuttgart. [Google Scholar]
- Monod M. 1983. Monographie taxonomique des Gnomoniaceae (Ascomycetes de l’ordre des Diaporthales). I. Beiheft Sydowia 9: 1–315. [Google Scholar]
- Morais CG, Lara CA, Marques S, et al. 2013. Sugiyamaella xylanicola sp. nov., a xylan-degrading yeast species isolated from rottingwood in Brazil. International Journal of Systematic and Evolutionary Microbiology 63: 2356–2360. [DOI] [PubMed] [Google Scholar]
- Morgan AP. 1890. North American fungi. The Gasteromycetes. III. The Journal of the Cincinnati Society of Natural History 12: 163–172. [Google Scholar]
- Munsell Color. 1994. Soil Color Charts (revised edition). Macbeth Division of Kollmorgen Instruments Corporation; New Windsor, New York, USA. [Google Scholar]
- Nguyen L-T, Schmidt HA, Von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T. 1973. Studies on degradation and cellulolytic activity of microfungi. Studia Forestalia Suecica 104 Stockholm, Sweden. [Google Scholar]
- Noordeloos ME. 1987. Entoloma (Agaricales) in Europe. Beihefte zur Nova Hewigia 91 Cramer, Berlin-Stuttgart. [Google Scholar]
- Noordeloos ME. 1992. Entoloma s.l. In: Candusso (ed), Fungi Europaei, vol. 5 Giovanna Biella, Saronno. [Google Scholar]
- Noordeloos ME. 2008. Entoloma in North America. Cryptogamic Studies vol. 2 Fischer Verlag; Stuttgart-New York. [Google Scholar]
- Noordeloos ME, Morozova OV. 2010. New and noteworthy Entoloma species from the Primorsky Territory, Russian Far East. Mycotaxon 112: 231–255. [Google Scholar]
- Nordstein S. 1990. The genus Crepidotus (Basidiomycotina, Agaricales) in Norway. Synopsis Fungorum 2: 1–115. [Google Scholar]
- Norphanphoun C, Doilom M, Daranagama DA, et al. 2017. Revisiting the genus Cytospora and allied species. Mycosphere 8: 51–97. [Google Scholar]
- Nováková A, Hubka V, Dudová Z, et al. 2014. New species in Aspergillus section Fumigati from reclamation sites in Wyoming (U.S.A.) and revision of A. viridinutans complex. Fungal Diversity 64: 253–274. [Google Scholar]
- Osmundson TW, Robert VA, Schoch CL, et al. 2013. Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLOS ONE 8: E62419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantone Colour Finder: https://www.pantone.com/color-finder#/pick?pantoneBook=pantoneSolidCoatedV3M2 [last accessed 30 Nov. 2018].
- Pascoe IG, McGee (Maher) PA, Smith IW, et al. 2018. Caliciopsis pleomorpha sp. nov. (Ascomycota: Coryneliales) causing a severe canker disease of Eucalyptus cladocalyx and other eucalypt species in Australia. Fungal Systematics and Evolution 2: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycook SR, Newhook FJ. 1978. Spore fall as a quantitative method in phylloplane studies. Transactions of the British Mycological Society 71: 453–456. [Google Scholar]
- Perdomo H, Sutton DA, García D, et al. 2011. Molecular and phenotypic characterization of Phialemonium and Lecythophora isolates from clinical samples. Journal of Clinical Microbiology 48: 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RH, Maharachchikumbura SSN, Hyde KD, et al. 2018. An appendage-bearing coelomycete Pseudotruncatella arezzoensis gen. and sp. nov. (Amphisphaeriales genera incertae sedis) from Italy, with notes on Monochaetinula. Phytotaxa 338: 177–188. [Google Scholar]
- Peterson SW, Jurjević Ž, Frisvad JC. 2015. Expanding the species and chemical diversity of Penicillium section Cinnamopurpurea. PLOS ONE 10: e0121987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phosri C, Martín MP, Sihanonth P, et al. 2007. Molecular study of the genus Astraeus. Mycological Research 111: 275–286. [DOI] [PubMed] [Google Scholar]
- Phosri C, Martín MP, Watling R. 2013. Astraeus: hidden dimensions. IMA Fungus 4: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phosri C, Watling R, Suwannasai N, et al. 2014. A new representative of star-shaped fungi: Astraeus sirhindhorniae sp. nov. from Thailand. PLOS ONE 9: e71160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozynski KA, Hodges CS. 1973. New Hyphomyces from South Carolina. Canadian Journal of Botany 51: 157–173. [Google Scholar]
- Pitt JI. 1980. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London. [Google Scholar]
- Polburee P, Lertwattanasakul N, Limtong P, et al. 2017. Nakazawaea todaengensis f.a., sp. nov., a yeast isolated from a peat swamp forest in Thailand. International Journal of Systematic and Evolutionary Microbiology 67: 2377–2382. [DOI] [PubMed] [Google Scholar]
- Posada D. 2008. jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar KC, Crous PW, Groenewald JZ, et al. 2016. Resolving the phylogenetic placement of Porobeltraniella and allied genera in the Beltraniaceae. Mycological Progress 15: 1119–1136. [Google Scholar]
- Ramírez C. 1982. Manual and atlas of the Penicillia. Elsevier Biomedical Press, Amsterdam. [Google Scholar]
- Raper KB, Thom C. 1949. A manual of the Penicillia. The Williams & Wilkins Company, Baltimore. [Google Scholar]
- Rayner RW. 1970. A Mycological Colour Chart. Commonwealth Mycological Institute and British Mycological Society, Kew. [Google Scholar]
- Redhead SA, Ginns JH. 1985. A reappraisal of agaric genera associated with Brown rots of wood. Transactions of the Mycological Society of Japan 26: 349–381. [Google Scholar]
- Rehm H. 1896. Die Pilze Deutschlands, Oesterreichs und der Schweiz. In: Rabenhorst L. (ed), Kryptogamen – flora von Deutschland, Oesterreich und der Schweiz. Verlag von Eduard Kummer, Leipzig. [Google Scholar]
- Ridgway R. 1912. Color standards and color nomenclature. Ridgway, Washington, DC. [Google Scholar]
- Rivera FN, González E, Gómez Z, et al. 2009. Gut-associated yeast in bark beetles of the genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae). Biological Journal of the Linnean Society 98: 325–342. [Google Scholar]
- Rivero M, Hidalgo A, Alastruey-Izquierdo A, et al. 2009. Infections due to Phialemonium species: case report and review. Medical Mycology 47: 766–774. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Aime MC, Farr DF, et al. 2004. The coelomycetous genera Chaetomella and Pilidium represent a newly discovered lineage of inoperculate discomycetes. Mycological Progress 3: 275–290. [Google Scholar]
- Royal Botanic Garden Edinburgh. 1969. Colour identification chart. Flora of British Fungi.
- Ryoo R, Sou H-D, Park H, et al. 2017. Astraeus ryoocheninii sp. nov. from Korea and Japan and phylogenetic relationships within Astraeus. Mycotaxon 132: 63–72. [Google Scholar]
- Saccardo PA. 1880. Fungi Gallici lecti a cl. viris P. Brunaud, Abb. Letendre, A. Malbranche, J. Therry, vel editi in Mycotheca Gallica C. Roumeguèri. Series II. Michelia 2: 39–135. [Google Scholar]
- Saccardo PA. 1895. Supplementum Universale, Pars. III. Sylloge Fungorum 11: 1–753. [Google Scholar]
- Saenz GS, Taylor JW. 1999. Phylogenetic relationships of Leliola and Meliolina inferred from nuclear small subunit rRNA sequences. Mycological Research 103: 1049–1056. [Google Scholar]
- Samson RA. 1974. Paecilomyces and some allied hyphomycetes. Studies in Mycology 6: 1–119. [Google Scholar]
- Samson RA, Visagie CM, Houbraken J, et al. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78: 141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrire BD. 2000. A synopsis of the genus Philenoptera (Leguminosae-Millettiae) from Africa and Madagascar. Kew Bulletin 55: 81–94. [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. 2011. The genera of Hyphomycetes. CBS Biodiversity Series no. 9: 1–997. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. [Google Scholar]
- Sena LMF, Morais CG, Lopes MR, et al. 2017. D -Xylose fermentation, xylitol production and xylanase activities by seven new species of Sugiyamaella. Antonie van Leeuwenhoek 110: 53–67. [DOI] [PubMed] [Google Scholar]
- Senn-Irlet B. 1992. Type studies in Crepidotus-I. Persoonia 14: 615–623. [Google Scholar]
- Senn-Irlet B. 1995. The genus Crepidotus (Fr.) Staude in Europe. Persoonia 16: 1–80.- [Google Scholar]
- Silveira VD. 1943. O gênero Calvatia no Brasil. Rodriguésia 16: 63–80. [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical frontend for RAxML. Organisms Diversity and Evolution 12: 335–337. [Google Scholar]
- Singer R. 1976. Marasmieae (Basidiomycetes, Tricholomataceae). Flora Neotropica 17: 1–347. [Google Scholar]
- Siquier JL, Salom JC, Espinosa J, et al. 2015. Contribució al coneixement micològic de les Illes Balears (Espanya). XXI. Revista Catalana de Micologia 36: 59–88. [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, et al. 2005. The type species of genus Gnomonia, G. gnomon, and the closely related G. setacea. Sydowia 57: 102–119. [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, et al. 2008. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SO, McHugh JV, Pollock DD, et al. 2005. The beetle gut: a hyperdiverse source of novel yeasts. Mycological Research 109: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SO, Zhou JJ. 2011. Kazachstania intestinalis sp. nov., an ascosporogenous yeast from the gut of passalid beetle Odontotaenius disjunctus. Antonie van Leeuwenhoek 100: 109–115. [DOI] [PubMed] [Google Scholar]
- Svrček M. 1987. New or less known Discomycetes. XVI. Česká Mykologie 41: 88–96. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanney JB, Douglas B, Seifert KA. 2016. Sexual and asexual states of some endophytic Phialocephala species of Picea. Mycologia 108: 255–280. [DOI] [PubMed] [Google Scholar]
- Taylor K, Andjic V, Barber PA, et al. 2012. New species of Teratosphaeria associated with leaf diseases on Corymbia calophylla (Marri). Mycological Progress 11: 159–169. [Google Scholar]
- Thirumalachar MJ. 1950. Some new or interesting fungi II. Sydowia 4: 66–81. [Google Scholar]
- Travadon R, Lawrence PD, Rooney-Latham S, et al. 2015. Cadophora species associated with wood-decay of grapevine in North America. Fungal Biology 119: 53–66. [DOI] [PubMed] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2014. Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Diversity 67: 203–229. [Google Scholar]
- Urbina H, Schuster J, Blackwell M. 2013. The gut of guatemalan passalid beetles: a habitat colonized by cellobiose- and xylose-fermenting yeasts. Fungal Ecology 6: 339–355. [Google Scholar]
- Van der Aa HA, Vanev S. 2002. A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Vauras J. 1997. Finnish records on the genus Inocybe (Agaricales). Three new species and I. grammata. Karstenia 37: 35–56. [Google Scholar]
- Vellinga EC. 1988. Glossary. In: Bas C, Kuyper TW, Noordeloos ME, et al. (eds), Flora Agaricina neerlandica vol. 1: 54–64. Balkema, Rotterdam, The Netherlands. [Google Scholar]
- Vellinga EC. 1990. Pluteus. In: Bas C, Kuyper TW, Noordeloos ME, et al. (eds), Flora Agaricina Neerlandica, vol. 2: 31–55. Balkema, Rotterdam, The Netherlands. [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. 2016. All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. 2017. Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Verkley GJM, et al. 2015. The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology 119: 823–843. [DOI] [PubMed] [Google Scholar]
- Visagie CM, Hirooka Y, Tanney T, et al. 2014a. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Studies in Mycology 78: 63–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Frisvad JC, et al. 2014b. Identification and nomenclature of the genus Penicillium. Studies in Mycology 78: 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2017. Corynespora, Exosporium and Helminthosporium revisited – new species and generic reclassification. Studies in Mycology 87: 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom Fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E, Luo J, Naik A, et al. 2015. Barrenia, a new genus associated with roots of switchgrass and pine in the oligotrophic pine barrens. Fungal Biology 119: 1216–1225. [DOI] [PubMed] [Google Scholar]
- Walsh E, Luo J, Zhang N. 2014. Acidomelania panicicola gen. et sp. nov. from switchgrass roots in acidic New Jersey pine barrens. Mycologia 106: 856–864. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu F, Crous PW, et al. 2017. Phylogenetic reassessment of Nigrospora: Ubiquitous endophytes, plant and human pathogens. Persoonia 39: 118–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Houbraken J, Groenewald JZ, et al. 2016. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Studies in Mycology 84: 145–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Yang FY, Meijer M, et al. 2019. Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Studies in Mycology 93: 65–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Binder M, Schoch CL, et al. 2006. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Molecular Phylogenetics and Evolution 41: 295–312. [DOI] [PubMed] [Google Scholar]
- Watling R. 1969. Colour Identification Chart. Edinburgh, Scotland, Her Majesty’s Stationary Office. [Google Scholar]
- Weber E. 2002. The Lecythophora-Coniochaeta complex. I. Morphological studies on Lecythophora species isolated from Picea abies. Nova Hedwigia 74: 159–185. [Google Scholar]
- Weber NS, Smith AH. 1985. A field guide to Southern mushrooms. The University of Michigan Press. [Google Scholar]
- Weir BS, Johnston PR, Damm U. 2012. The Colletotrichum gloeosporioides species complex. Studies in Mycology 73: 115–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikee S, Lombard L, Nakashima C, et al. 2013. A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Studies in Mycology 76: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Addy HD, Tsuneda A, et al. 2004. Phialocephala sphaeroides sp. nov., a new species among the dark septate endophytes from a boreal wetland in Canada. Canadian Journal of Botany 82(5): 607–617. [Google Scholar]
- Woudenberg JHC, Groenewald JZ, Binder M, et al. 2013. Alternaria redefined. Studies in Mycology 75: 171–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SD, Huang HY, Zhao J, et al. 2018. Ossicaulis yunnanensis sp. nov. (Lyophyllaceae, Agaricales) from southwestern China. Mycoscience 59: 33–37. [Google Scholar]
- Yilmaz N, Visagie CM, Houbraken J. 2014. Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology 78: 175–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller SM, Smith AH. 1964. The genus Calvatia in North America. Lloydia 27: 148–186. [Google Scholar]









































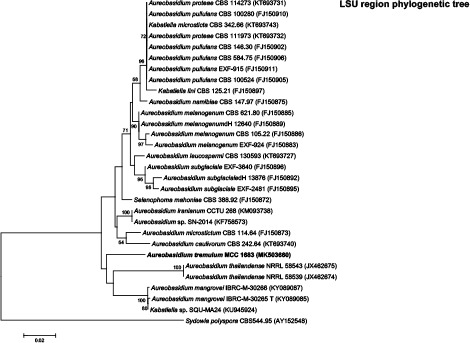
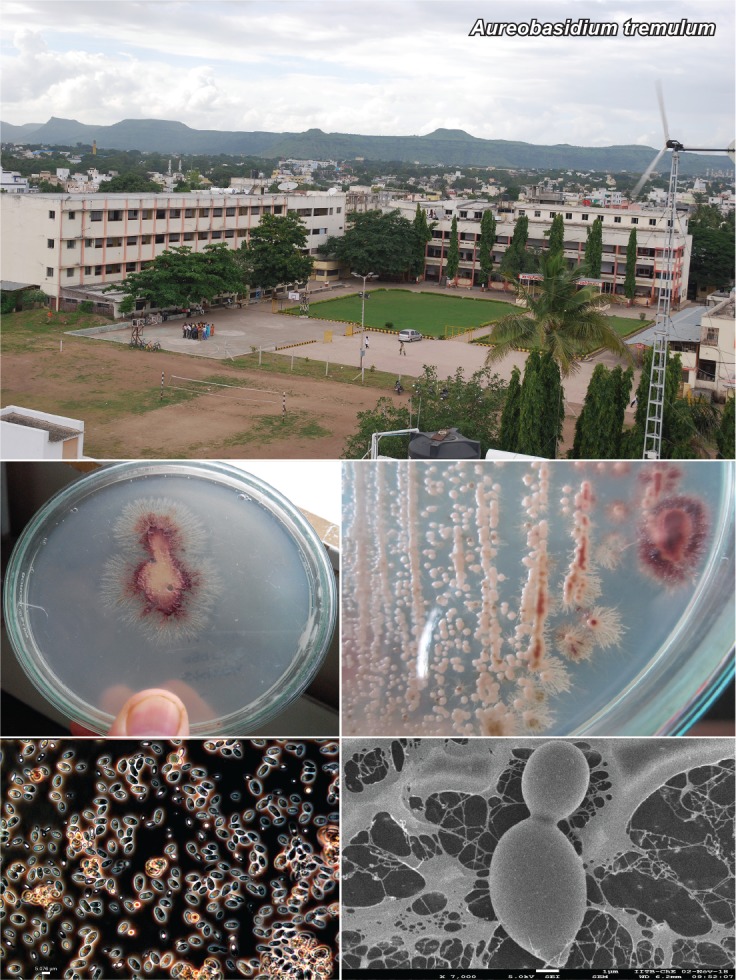
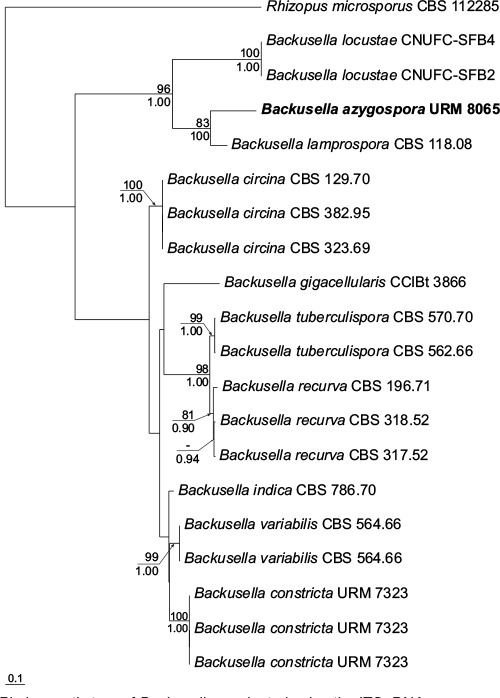

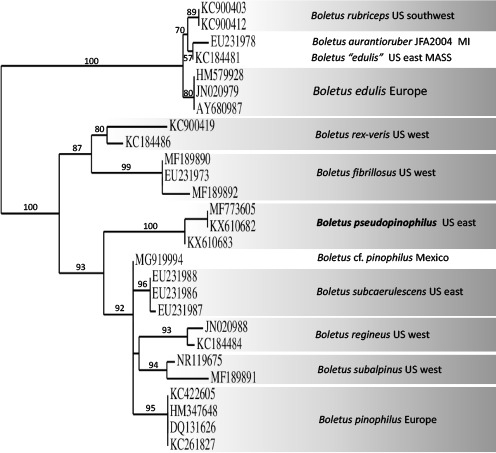


















 k L
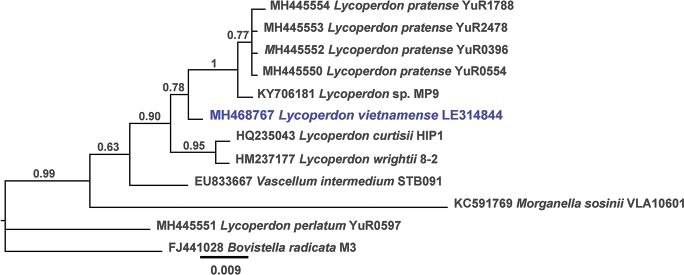
k L k Province, Buôn Đôn District, Krông Na commune, Bąn Đôn, Yok Đôn National Park, alt. 196 m, N12°56’24" E107°43’31", margin of tropical open deciduous forest, on soil, 10 May 2014, A.V. Alexandrova (holotype LE 314844, ITS sequence GenBank
k Province, Buôn Đôn District, Krông Na commune, Bąn Đôn, Yok Đôn National Park, alt. 196 m, N12°56’24" E107°43’31", margin of tropical open deciduous forest, on soil, 10 May 2014, A.V. Alexandrova (holotype LE 314844, ITS sequence GenBank