Abstract
Objective:
Failure to initiate antiretroviral therapy (ART) and achieve virologic suppression are significant barriers to the United Nations 90–90–90 goals. Identifying resilience resources, or modifiable strength-based factors, among people living with HIV is critical for successful HIV treatment and prevention.
Design:
Prospective cohort study.
Methods:
From July 2014 to July 2015, 500 adults presenting for voluntary counseling and HIV testing who were diagnosed with HIV and were ART-eligible in South Africa (Soweto and Gugulethu) were enrolled and surveyed. Logistic regression models assessed resilience-related predictors of ART initiation within 6 months of voluntary counseling and HIV testing for HIV, and HIV-1 plasma RNA suppression within 9 months, adjusting for sociodemographic factors.
Results:
Within 6 months, 62% initiated ART, and within 9 months, 25% had evidence of an undetectable HIV-1 plasma RNA (<50 copies/ml). Participants who initiated ART relied less on social support from friends [adjusted odds ratio (aOR) 0.94, 95% confidence interval (CI): 0.89–0.99], coped using self-distraction (aOR 1.05, 95% CI: 1.00–1.10) and avoided coping through substance use (aOR 0.79, 95% CI: 0.65–0.97), as compared with participants who did not initiate ART. Those who achieved plasma RNA suppression relied more on social support from a significant other/partner (aOR 1.04, 95% CI: 1.02–1.07), used positive religious coping (aOR 1.03, 95% CI: 1.00–1.07), and were less likely to engage in denial coping (aOR 0.84, 95% CI: 0.77–0.92), compared with those who initiated ART but did not achieve plasma RNA suppression.
Conclusion:
Interventions optimizing resilience resources and decreasing maladaptive coping strategies (e.g., substance use, denial) may present a feasible approach to maximizing ART-based HIV treatment strategies among South African people living with HIV.
Keywords: coping, HIV, resilience resources, social support, South Africa
Introduction
The provision and widespread availability of antiretroviral therapy (ART) has transformed the South African HIV epidemic over the past decade, resulting in an increase in life expectancy by over 10 years, since an all-time low of 52.8 years of age in 2003/2004 [1,2]. Through a unified and unprecedented response from the South African Treasury and multinational donors, South Africa has provided ART for over 3 million [3] people living with HIV (PLWH) as of 2015 [4,5]. Although this reflects a significant uptake in treatment over the past decade, it represents less than half of the population of nearly 7 million PLWH in the country. Given research showing the benefit of ART for prevention of transmission through viral load suppression [6], and additional evidence to support ART initiation to prevent early mortality [7], the WHO has recommended a ‘test and treat’ model of care [8-10]. The efficacy of’test and treat’ will ultimately depend on universal ART initiation at the point of testing and long-term virologic suppression for all PLWH.
Pre-ART loss has been documented consistently throughout sub-Saharan Africa, despite sweeping changes made to increase treatment access and availability [11-18]. Although many studies have focused on programmatic and structural ways to decrease barriers to accessing treatment [19,20], prior research suggests this may not be enough to improve ART uptake [21]. Our group previously identified high rates of ART noninitiation (of 20%) among treatment-eligible adults, despite knowing their HIV status, and having access to free treatment [22]. These data were collected in 2009, during an era when ART was only offered to individuals with advanced disease (CD4+ cell count ≤200 cells/μl). Given that South Africa has expanded treatment to all PLWH as of September 2016 [23], programs need to consider how to minimize attrition post-HIV diagnosis. Eliminating CD4+ cell count thresholds without improved ART initiation procedures will likely not achieve the goal of the 90–90–90 targets set forth by the United Nations [24,25].
Beyond the stressor of a new HIV-diagnosis [26,27], PLWH in South Africa face additional challenges related to unemployment [28], poverty [29], and violence [30]. HIV-associated stigma has consistently been shown to potentiate feelings of isolation, depression, and anxiety within this context, and lead to critical delays in testing and care [31-33]. Behavioral research is needed to inform interventions that aim to increase rates of ART initiation after experiencing this stressor. In particular, research focused on increasing resilience, or individuals’ capacity to overcome situations of adversity and stress to achieve health and well being [34,35], may be especially helpful for identifying modifiable strength-based factors that can promote healthy outcomes [36]. For example, evidence suggests that enhanced social support, including comfort and/or assistance from others, is associated with better HIV-related health symptoms among PLWH, possibly because it buffers people from the negative effects of stressors on physical health [37,38]. Prior research further indicates that one form of resilience, adaptive coping, is associated with acceptance of a new HIV diagnosis by fostering problem solving and emotional expression, while mitigating the perceived risk associated with starting treatment [39,40]. Moreover, interventions for PLWH that increase confidence in the ability to engage in adaptive coping strategies can lead to better mental health [41]. Conversely, maladaptive, negative coping strategies, such as substance use and denial, are associated with worse health outcomes among PLWH [40,42].
The prospective cohort study assessed losses in care across the cascade among ART-eligible PLWH presenting for testing in South Africa in a modern era of widely available treatment, and resilience resources associated with ART initiation and HIV-1 plasma RNA suppression among those who stayed engaged in care. All participants in this cohort faced the stressor of a new HIV diagnosis. Our goal was to understand within this context what factors would be associated with better outcomes for individuals facing this adversity. We differentiated between several sources of social support (i.e., friends, family, partner) and types of coping (i.e., maladaptive coping, through denial and substance use, and adaptive coping, through religious beliefs) to contribute to a nuanced understanding of the association of resilience resources with these outcomes, and to identify key resilience resources that map onto common intervention tools used to enhance linkage to care among PLWH. We hypothesized that these resilience resources would be significantly associated with ART initiation and plasma RNA suppression. This hypothesis was based on our prior research in which PLWH reported social support and adaptive coping were critical to gaining acceptance of a new HIV diagnosis while mitigating the perceived risk associated with starting ART, and previous work that highlighted the deleterious effects of maladaptive coping on the wellbeing of PLWH.
Methods
Study setting
This study was conducted across two townships in South Africa: in Soweto (outside of Johannesburg) we recruited from Zazi Testing Center (affiliated with The Perinatal HIV Research Unit) and a Department of Health Clinic; and in Gugulethu (outside of Cape Town), we recruited from Ministry of Health’s testing center (affiliated with the Desmond Tutu HIV Foundation). Both townships are densely populated urban areas with an overall HIV prevalence of nearly 20% [43]. During the period of recruitment, these sites provided rapid HIV tests to over 500 adults (≥18 years) monthly, along with family planning, and screening for tuberculosis (TB) and sexually transmitted infections (STIs). Clients were supported by clinic-based lay counselors who had undergone training to provide HIV testing and counseling. Individuals presenting for testing were given their HIV results, and, if positive, had blood drawn for a CD4+ cell count. They were advised to return a week later to receive their CD4+ cell count and were referred for treatment as appropriate. A small number of people in this study underwent immediate CD4+ testing using Alere Pima. Upon referral, clients were scheduled to meet with counselors or social workers to discuss ART initiation, which generally occurred within 1–2 weeks. Demographics, HIV test results, including CD4+ cell count, STI, and TB results were recorded in a clinical registry.
Study participants, eligibility, and enrollment
We sequentially recruited and enrolled 500 ART-eligible adults (≥18 years) between July 2014 and July 2015, as they received their CD4+ cell count results after testing HIV-positive. ART eligibility was based on South African guidelines, which changed over the course of the study (CD4+ cell count ≤350 cells/μl before 1 January 2015, and CD4+ cell count ≤500 cells/μl after 1 January 2015). We excluded pregnant women and children, because they qualified for intensive adherence support under South African HIV treatment guidelines. The study protocol and data collection instruments were approved by Human Subjects Committees at Partners Healthcare, the University of Witwatersrand Ethics Committee, the Gauteng Department of Health, and the University of Cape Town Ethics Committee. All participants provided written informed consent. Study data were collected and managed using a secure, web-based, Research Electronic Data Capture tool hosted at Partners Healthcare [44].
Study design
Trained multilingual interviewers administered an inperson survey to ascertain participants’ intent to initiate treatment and measured clinical, structural, and psychosocial factors. Guided by our prior qualitative work [39], resilience resources, including social support from several sources and multiple dimensions of coping, along with clinical, and demographic/structural covariates, were assessed for association with ART initiation within 6 months of testing, and viral load suppression within 9 months of testing.
Data elements
Clinical factors and demographics
Clinical factors included CD4+ cell count at testing (obtained through blood draw or finger prick), prior testing status, and reason for testing (based on clinic records or self-report). Sociodemographic information, including site of testing, sex, age, marital status, children, and employment status were collected during HIV testing and the study interview; study interview date was used to classify participants as engaging in care before or after the change in national guidelines.
Resilience resources
Resilience resources were measured as in two domains: social support and coping strategies. Social support was assessed from significant others, from family, and from friends (four items per subscale from the Multidimensional Scale of Perceived Social Support, from 1 for ‘very strongly disagree’ to 7 for ‘very strongly agree’) [45]. Both effective (reflecting greater resilience) and ineffective (reflecting lower resilience) coping strategies were measured. Individual coping strategies included self-distraction, denial, substance use, disengagement, venting, and self-blame (two items for each of these six subscales from the Brief Cope scale, each item rated from 1 ‘not at all’ to 4 ‘a lot’) [46] as well as religious coping, including positive (e.g., ‘seeking God’s love and care’) and negative religious coping (e.g., ‘wondering whether God has abandoned me’) using the 14-item Brief RCOPE (seven items each for the positive and negative subscale), with response options 1 ‘not at all’ to 4 ‘a great deal’ [47]. Individual survey questions assessing resilience were summed to create each subscale. One participant did not answer questions regarding social support; this individual was excluded from the social support analysis.
Antiretroviral therapy initiation and HIV-1 plasma RNA outcomes
The main outcomes were treatment initiation within 6 months of testing HIV positive; and viral load test indicating HIV-1 plasma RNA suppression (<50 copies/ml) within 9 months of testing among those initiating treatment. These outcomes were ascertained through accessing routine laboratory data collected in South Africa’s National Health Laboratory Service (NHLS), which provides laboratory services to all public-sector facilities in South Africa, including all laboratory testing required for ART monitoring with over 26 million CD4+ cell counts and 13 million HIV-1 plasma RNA tests performed between 2004 and 2015, as well as current ART use (as designated through monitoring of HIV-1 RNA). Data regarding plasma RNA were directly entered into NHLS by clinical providers, and ART start was imputed based on a measure of creatinine, performed prior to initiation of tenofovir, part of a standard first-line ART regimen in South Africa. ARTworkup blood tests as recorded in NHLS have been previously validated as an accurate measure to impute dates of treatment initiation among South African PLWH who are receiving public-sector HIV care [48]. We chose these two outcomes based on clinical relevance and timing of plasma RNA measurements performed in South Africa during this timeframe. Numerous individual, social, and structural factors contribute to timing of engagement with the health system in sub-Saharan Africa, and recent literature reflects the need allow for a significant lag (up to 6 months) between diagnosis to ART initiation, with multiple studies using ART initiation within 6 months of an HIV diagnosis [49,50]. The literature reflects and often amplifies the variability between programs, however, and other time points have been used [12].
Statistical analysis
We compared the study population before and after the change in national guidelines using Chi-squared tests for categorical variables and t tests for continuous variables. We calculated Cronbach’s alpha for each resilience resource measure to assess reliability and calculated mean and SD for each measure. We used the nonparametric Kruskall–Wallis test to compare each resilience resource measure by treatment initiation status and evidence of viral load suppression (among those initiating treatment). We modeled each outcome using logistic models with robust standard errors to account for clustered sampling within clinics (two in Soweto, one in Gugulethu), controlling for baseline characteristics considered to be potential confounders: sex, age below 35, being single, having at least a secondary education, CD4+ cell count below 200 cells/μl at baseline, and illness as the primary reason for testing. We additionally controlled for location (Soweto vs. Gugulethu). As an exploratory analysis, we repeated the analysis stratified by sex to assess the associations of forms of social support with the outcomes. Data analyses were conducted based on a two-sided alpha-level of 0.05, using SAS software (version 9.4; SAS Institute, Cary, North Carolina, USA) and Stata 14.1 (StataCorp, College Station, Texas, USA).
Results
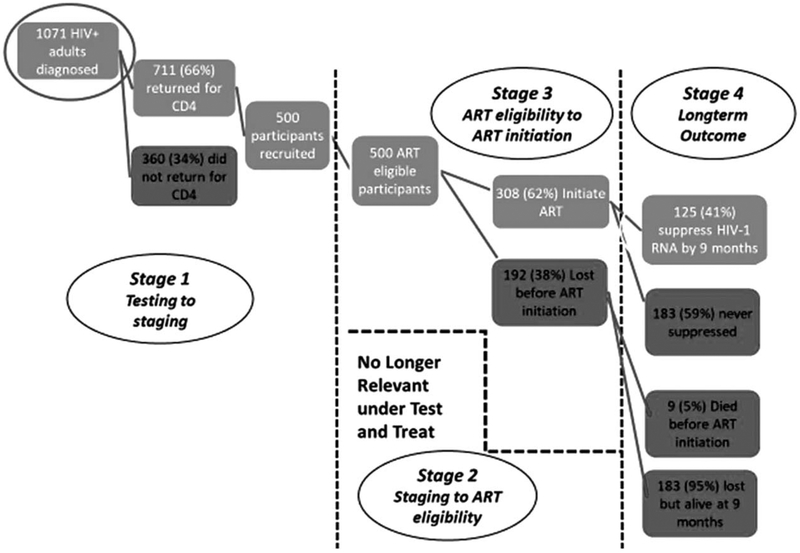
Across sites, 1071 adults presented for testing and were found to be HIV-positive and ART-eligible over the 1-year period of our study. Sixty-six percentage [n=711, median CD4+ cell count: 262 cells/μl, interquartile range (IQR): 141–372 cells/μl] returned for their CD4+ cell count results within 6 weeks of testing. Six-hundred and sixty-seven potential participants were approached, and 500 participants (200 in Soweto and 300 in Gugulethu) were recruited for our study. Baseline characteristics are shown in Table 1. Nearly two-thirds of our population were women (62.6%), with a median age of 35 years old (IQR: 29–42). Sixty percentage presented with CD4+ cell count above 200 cells/μl; median CD4+ cell count was 242 cells/μl (IQR: 135–348). Half were unemployed. Nearly two-thirds (61.9%) were repeat testers, and of those, 55.4% reported a prior positive test. The most common reason given for testing was ‘illness’ (58.8%). Most participants (323 of 500) presented following the change in national guidelines, 249 (77.1%) of them in Gugulethu. There were no differences by sex or age between groups before and after the guideline change; those presenting postguideline change were more likely to cite illness as a reason for testing (68.1 vs. 41.8% before the guidelines changed) but had higher CD4+ cell counts (272 vs. 187).
Table 1.
Characteristics of study participants at baseline.
| Characteristic, N=500 | N | Percentage |
|---|---|---|
| Site | ||
| Soweto | 200 | 40.0 |
| Gugulethu | 300 | 60.0 |
| Women | 313 | 62.6 |
| Age | ||
| 20–25 | 53 | 10.6 |
| 26–30 | 87 | 17.4 |
| 31–35 | 104 | 20.8 |
| 36–40 | 102 | 20.4 |
| >40 | 154 | 30.8 |
| Marital status | ||
| Married | 76 | 15.2 |
| Living together | 36 | 7.2 |
| Single | 365 | 73.0 |
| Widowed, divorced, other | 23 | 4.6 |
| Children | ||
| None | 99 | 19.8 |
| 1 | 138 | 27.6 |
| 2 | 145 | 29.0 |
| 3 or more | 118 | 23.6 |
| Employment status | ||
| Employed | 214 | 42.8 |
| Self-employed | 25 | 5.0 |
| Unemployed | 250 | 50.0 |
| Other | 11 | 2.2 |
| Previously tested for HIV | 309 | 61.8 |
| Previous test positive (N=305) | 169 | 55.4 |
| Reason for testing | ||
| Illness | 294 | 58.8 |
| General check-up | 125 | 25.0 |
| CD4+ check | 73 | 14.6 |
| Other | 8 | 1.6 |
| CD4+ cell count >200 cells/μl | 303 | 60.6 |
Across the two townships, we found significant attrition over the year our study was conducted. Specifically, only 61.6% (308 participants) initiated ART within 6 months of testing (52.5% of those in Soweto vs. 67.7% in Gugulethu), with nine participants dying before ever accessing treatment. Among those who started, 204 had at least one plasma RNA test and only 25% of the entire cohort (125 participants) had a plasma RNA test showing undetectable plasma RNA within 9 months of testing (Fig. 1 – model based on work by Fox and Rosen [51]). ART initiation was more common among those interviewed after the change in national guidelines (64.9 vs. 45.2%); probability of undetectable plasma RNA did not differ among those initiated.
Fig. 1.
Attrition within the care cascade.
Reliability of measures for resilience resources in this sample ranged from very good (Cronbach’s alpha ≥0.80) for each type of social support, for coping through substance use, and both the positive and negative religious coping subscales (Table 2). Interitem reliability for other coping strategies was lower and ranged from 0.56 for coping by venting to 0.68 for coping using disengagement. We compared resilience resources between those initiating treatment and not initiating. Individuals who did not initiate treatment by 6 months expressed stronger social support from friends, greater use of coping through substance use, and higher negative religious coping than those who initiated ART (Table 2); we did not find significant differences based on demonstrated plasma RNA suppression. In adjusted logistic regression models that included all resilience resources (Table 3), reporting greater social support from friends [adjusted odds ratio (aOR) 0.94, 95% confidence interval (CI): 0.89–0.99] and using coping through substance use (aOR 0.79, 95% CI: 0.65–0.96) were associated with lower odds of ART initiation. Self-distraction was associated with higher odds of ART initiation (aOR 1.05, 95% CI 1.00, 1.10). In turn, reporting greater social support from a significant other/partner (aOR 1.04, 95% CI: 1.02–1.07) and using positive religious coping (aOR 1.03, 95% CI: 1.00–1.07) were associated with better odds of plasma RNA suppression among those initiating ART, while engaging in denial as a coping strategy was associated with lower odds of suppression (aOR 0.84, 95% CI: 0.77–0.92). Models stratified by sex showed similar associations between forms of social support and ART initiation. The association between social support from a significant other/partner and plasma RNA suppression was stronger for women than men (aOR 1.07 vs. 1.05); social support from friends was negatively associated with plasma RNA suppression in women (aOR 0.95, 95% CI: 0.91, 0.99) but not among men (models not shown).
Table 2.
Resilience resources for individuals testing positive for HIV.
| ART |
Plasma RNA suppression among those initiating ART, N=308 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, N=500 |
Did not initiate, N=192 |
Initiated, N=308 |
Not suppressed, N=183 |
Suppressed, N=125 |
|||||||
| Social support | Cronbach alpha | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Significant other social support | 0.90 | 17.41 | 3.96 | 20.96 | 3.34 | 20.92 | 3.07 | 20.7 | 3.18 | 21.23 | 2.87 |
| (4–28)a | |||||||||||
| Family social support (4–28)a | 0.90 | 3.94 | 2.09 | 19.92 | 3.84 | 20.32 | 2.98 | 20.22 | 3.09 | 20.47 | 2.81 |
| Friend social support (4–28)a | 0.89 | 3.76 | 1.74 | 17.98 | 4.08 | 17.06 | 3.85 | 17.17 | 3.88 | 16.9 | 3.82 |
| Coping | |||||||||||
| Self-distraction (2–8) | 0.67 | 2.59 | 1.47 | 4.17 | 2.22 | 3.80 | 2.00 | 3.74 | 2.01 | 3.88 | 1.99 |
| Denial (2–8) | 0.57 | 2.67 | 1.39 | 3.97 | 1.90 | 3.63 | 1.63 | 3.74 | 1.63 | 3.47 | 1.62 |
| Substance use (2–8) | 0.90 | 3.08 | 1.66 | 2.93 | 1.85 | 2.38 | 1.12 | 2.34 | 0.96 | 2.43 | 1.32 |
| Disengagement (2–8) | 0.68 | 3.79 | 1.85 | 2.80 | 1.60 | 2.59 | 1.24 | 2.57 | 1.25 | 2.62 | 1.22 |
| Venting (2–8) | 0.56 | 17.92 | 7.89 | 3.29 | 1.79 | 2.95 | 1.56 | 2.95 | 1.55 | 2.94 | 1.58 |
| Self-blame (2–8) | 0.65 | 12.59 | 5.45 | 3.95 | 1.97 | 3.69 | 1.78 | 3.57 | 1.73 | 3.88 | 1.83 |
| Religious coping | |||||||||||
| Positive (4–32) | 0.93 | 10.92 | 7.89 | 18.58 | 7.85 | 17.51 | 7.90 | 17.16 | 8.12 | 18.02 | 7.57 |
| Negative (4–32) | 0.80 | 5.59 | 5.45 | 13.36 | 5.91 | 12.10 | 5.08 | 12.26 | 5.46 | 11.87 | 4.48 |
Bold values indicate P less than 0.05 Kruskal-Wallis rank test. ART, antiretroviral therapy.
N=499/307, one missing observation.
Table 3.
Association of resilience resources with antiretroviral therapy initiation and plasma RNA suppression among individuals testing positive for HiV (N=499).
| ART initiation, N=499 |
Plasma RNA suppression, N=307 |
|||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | |
| Social support | ||||
| Significant other social support | 1.00 | [0.97,1.02] | 1.04 | [1.02,1.07] |
| Family social support | 1.05 | [0.98,1.12] | 1.01 | [0.92,1.11] |
| Friend social support | 0.94 | [0.89,0.99] | 0.98 | [0.93,1.03] |
| Coping scales | ||||
| Self-distraction | 1.05 | [1.00,1.10] | 1.10 | [0.90,1.35] |
| Denial | 0.92 | [0.77,1.10] | 0.84 | [0.77,0.92] |
| Substance use | 0.79 | [0.65,0.96] | 1.06 | [0.98,1.15] |
| Disengagement | 1.08 | [0.94,1.25] | 1.06 | [0.74,1.53] |
| Venting | 1.01 | [0.99,1.04] | 0.94 | [0.67,1.34] |
| Self-blame | 1.02 | [0.89,1.15] | 1.20 | [0.97,1.47] |
| Religious coping | ||||
| Positive | 1.00 | [0.99,1.00] | 1.03 | [1.00,1.07] |
| Negative | 0.99 | [0.96,1.03] | 0.95 | [0.90,1.01] |
Controlling for age, sex, CD4+ more than 200, single, education more than primary, site, illness as reason for testing. Standard errors adjusted for clustering for individual clinic. aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval. Bold value signifies the 95% CI.
Discussion
Among the 500 ART-eligible adults who enrolled in our cohort, 62% initiated treatment within 6 months of voluntary counseling and HIV testing, but ultimately only 25% showed evidence of HIV-1 plasma RNA suppression within 9 months of testing. Our data provide insight into the resilience resources of PLWH who initiated and adhered to ART, and ultimately achieved plasma RNA suppression. Specifically, individuals who initiated ART reported less social support from friends, reported coping using self-distraction, and avoided coping through substance use, as compared with participants who did not initiate ART. In turn, those who achieved viral load suppression amongst those initiating ART relied more on social support from a significant other or partner, used positive religious coping, and were less likely to engage in denial coping, as compared with those who initiated ART but did not demonstrate viral load suppression.
Recent theorists in the field of HIV social and behavioral sciences have conceptualized resilience as a suite of resources that promote ART initiation and viral suppression, and have called for researchers to identify and ultimately strengthen these resilience resources [36,52,53]. Although past work suggests that social support from others, particularly those who are in care and doing well, promotes ART initiation [54], we found that the source of social support mattered. Specifically, patients who received more social support from friends were less likely to initiate ART treatment. This may be because friends may lack knowledge about HIV, have negative views about ART or PLWH, and/or engage in unhealthy behaviors (e.g., substance use) that are a negative influence on ART initiation. In contrast, for PLWH who started treatment, a greater support from a supportive partner or spouse may be critical to maintaining adherence and achieving viral load suppression. Our results are consistent with research examining medication adherence among people with diabetes, which demonstrated increased adherence if daily adherence reminders from spouses were supportive (e.g., showed understanding) [55,56]. Future research should explore the mechanisms by which friends of PLWH negatively impact treatment adoption, and spouses positively impact plasma RNA suppression. Interventions targeting this mechanism may provide a pathway toward training peers or partners to support PLWH in nonjudgmental and nonconfrontational ways (e.g. using motivational interviewing techniques), and may ultimately be effective in helping PLWH to initiate and stay on ART, as has been previously suggested [57,58].
Our study also demonstrated that specific types of coping strategies were significantly associated with ART initiation (self-distraction, avoidance of maladaptive coping) and viral load suppression (using positive religious coping) and may therefore be potential targets for future interventions. These results extend prior research [40], which suggest that individuals who avoid maladaptive coping strategies, including substance use and denial coping, achieve better health-related outcomes. Significantly, other types of cognitive escape (i.e., avoidance of thoughts related to HIV), such as self-distraction, may offer an effective strategy for coping with one’s serostatus. Future research should focus on differentiating maladaptive coping strategies from adaptive self-distraction.
Several limitations to our study should be considered when interpreting its results. Treatment guidelines in South Africa shifted over the course of our study (ART eligibility changed from CD4+ cell count ≤350 to ≤500 cells/μl) and have again changed to allow all PLWH to access treatment as of September 2016. Our data remain limited to the time-period in which this study was performed. In addition, our primary outcomes were obtained through a national database that relies on clinician input into a large registry. Therefore, there may be potential missing data in this database that could not be cross-verified. In particular, 104 individuals who initiated ART did not have a plasma RNA test in the NHLS database; we assumed these individuals were not actively on treatment and hence had not achieved plasma RNA suppression. Although it is possible that this could have biased our results if we misclassified individuals who had achieved HIV-1 RNA suppression as unsuppressed, it is more likely that any misclassification was nondifferential, which would tend to bias results toward the null. In addition, our ability to generalize to all PLWH is limited given our study focused on individuals who returned for their CD4+ cell count. We did not assess the sexual orientation or sex identity of participants, and therefore, we cannot fully determine the impact of religious coping on sexual minority and sex diverse persons who may not be accepted within their religion, and by the relatively low reliability of subscales for venting and denial in this study population. Future studies should seek to continue to examine these constructs in addition to coping and social support. An additional limitation is that this study did not specifically measure resilience, but rather focused on resilience resources as described above. Further research using validated measures of resilience, including the Brief Resilience Scale [59] will be critical to fully defining the impact of resilience on decision-making. Finally, the associations we identified were of relatively modest size and therefore they may be of limited prognostic value for discerning those who initiate ART and/or maintain HIV-1 RNA suppression. Despite these limitations, our research has many strengths including its longitudinal design, the large sample size across multiple sites in South Africa, and the complete dataset as obtained through NHLS. Future research may explore further adaptive coping strategies in addition to the avoidance of maladaptive coping strategies to promote ART initiation and HIV-1 plasma RNA suppression.
In conclusion, our findings suggest there is a persistently high-degree of attrition across the care cascade in South Africa, and PLWH who manage to achieve HIV-1 RNA suppression do so through a combination of resilience resources. Future research should examine resilience resources, as well as resilience, among PLWH in the context of structural barriers to initiation of and adherence to health behaviors (e.g., distance, transport, clinic hours), and whether resilience resources and/or resilience protect PLWH from specific adversities that undermine ART adherence and health, such as violence, poverty, and stigma. Interventions that optimize adaptive rather than maladaptive coping (e.g., substance use, denial), and promote social integration to the experience of PLWH may provide promising strategies for promoting positive health behaviors and outcomes in this vulnerable population.
Acknowledgements
The study would not have been possible with the dedication of the study participants in Soweto and Gugulethu.
I.T.K. designed and led the study with guidance and oversight from C.O., G.G., L.M.B., and D.R.B. G.T. and I.C. led the acquisition of the data. J.J.D., L.M.B., V.A.E., and J.F.M. contributed to measure selection and/or analysis. J.J.D. also assisted with data acquisition and provided oversight for recruitment. D.L., H.I., and H.H.L. performed the analysis with guidance and oversight from G.M.F. I.T.K. wrote the first draft of the article. All authors assisted with the writing and revising of the article and provided final approval of the version to be published. All agreed to be accountable for all aspects of the work.
The publication was made possible with funding from US National Institute for Mental Health K23 MH097667 (I.T.K.). J.F.M. and V.A.E.’s time spent on this article was supported by the US National Institute on Drug Abuse (K23DA041901, K01DA042881). L.M.B.’s time was also supported by P30MH058107 from the US National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). In: Secondary Joint United Nations Programme on HIV/AIDS (UNAIDS) editor. South Africa HIV and AIDS estimates (2015). Secondary South Africa HIV and AIDS estimates (2015) Geneva, Switzerland: UNAIDS; 2015. http://www.unaids.org/en/regionscountries/countries/southafrica. [Accessed 1 December 2018]. [Google Scholar]
- 2.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013;339:961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). Number of people receiving ART In: Secondary Joint United Nations Programme on HIV/AIDS (UNAIDS). editor. Secondary number of people receiving ART Geneva, Switzerland: UNAIDS;2015. http://www.unaids.org/en/regionscountries/countries/southafrica. [Accessed 1 December 2018]. [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS). The gap report In: Secondary Joint United Nations Programme on HIV/AIDS (UNAIDS). editor. Secondary the gap report Geneva, Switzerland: UNAIDS; 2014. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. [Accessed 1 December 2018]. [Google Scholar]
- 5.Katz IT, Bassett IV, Wright AA. PEPFAR in transition – implications for HIV care in South Africa. N Engl J Med 2013; 369:1385–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. , Insight Start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO;Regional Office for the Eastern Mediterranean). HIV test–treat–retain cascade analysis (guide and tools, 2014). World Health Organization;2014. [Google Scholar]
- 9.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373:48–57. [DOI] [PubMed] [Google Scholar]
- 10.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA 2009; 301:2380–2382. [DOI] [PubMed] [Google Scholar]
- 11.Cloete C, Regan S, Giddy J, Govender T, Erlwanger A, Gaynes MR, et al. The linkage outcomes of a large-scale, rapid transfer of HIV-infected patients from hospital-based to community-based clinics in South Africa. Open Forum Infect Dis 2014; 1 :ofu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr 2009; 51:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One 2010;5:e13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindasamy D, van Schaik N, Kranzer K, Wood R, Mathews C, Bekker LG. Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. J Acquir Immune Defic Syndr 2011;58:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox MP, Shearer K, Maskew M, Meyer-Rath G, Clouse K, Sanne I. Attrition through multiple stages of pretreatment and ART HIV care in South Africa. PLoS One 2014;9:e110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plazy M, Dray-Spira R, Orne-Gliemann J, Dabis F, Newell ML. Continuum in HIV care from entry to ART initiation in rural KwaZulu-Natal, South Africa. Trop Med Int Health 2014; 19:680–689. [DOI] [PubMed] [Google Scholar]
- 17.Plazy M, Orne-Gliemann J, Dabis F, Dray-Spira R. Retention in care prior to antiretroviral treatment eligibility in sub-Saharan Africa: a systematic review of the literature. BMJ Open 2015; 5:e006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honge BL, Jespersen S, Nordentoft PB, Medina C, da Silva D, da Silva ZJ, et al. Loss to follow-up occurs at all stages in the diagnostic and follow-up period among HIV-infected patients in Guinea-Bissau: a 7-year retrospective cohort study. BMJ Open 2013;3:e003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc 2014;17:18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson BA, Schnippel K, Ndibongo B, Xulu T, Brennan A, Long L, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr 2012; 61 :e13–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson BA, Schnippel K, Brennan A, Long L, Xulu T, Maotoe T, et al. Same-day CD4 testing to improve uptake of HIV care and treatment in South Africa: point-of-care is not enough. AIDS Res Treat 2013;2013:941493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS 2011;25:2177–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joint United Nations Programme on HiV/AIDS (UNAIDS). South Africa takes bold step to provide HIV treatment for all IN: Secondary Joint United Nations Programme on HIV/AIDS (UNAIDS). editor. Secondary South Africa takes bold step to provide HIV treatment for all Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 24.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90–90–90 An ambitious treatment target to help end the AIDS epidemic In: Secondary Joint United Nations Programme on HIV/AIDS (UNAIDS). editor. Secondary 90–90–90 an ambitious treatment target to help end the AIDS epidemic Geneva, Switzerland: UNAIDS;2014. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Accessed 1 December 2018]. [Google Scholar]
- 25.Jacob Bor SA, Fox MP, Rosen S, Meyer-Rath G, Katz IT, Tanser F, et al. Effect of eliminating CD4-count thresholds on the number of new antiretroviral therapy initiators in South Africa. International AIDS Conference; 2016. [Google Scholar]
- 26.Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burnett P, et al. Posttraumatic stress disorder in response to HIV infection. Gen Hosp Psychiatry 1998;20:345–352. [DOI] [PubMed] [Google Scholar]
- 27.Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress 2010; 23:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levinsohn JA, McLaren Z, Shisana O, Zuma K. HIV status and labor market participation in South Africa. National Bureau of Economic Research Working Paper Series 2011;No. 16901. https://cpb-us-w2.wpmucdn.com/campuspress.yale.edu/dist/c/866/files/2010/10/HIV_labor_33.pdf [Google Scholar]
- 29.Kalichman SC, Simbayi LC, Jooste S, Cherry C, Cain D. Poverty-related stressors and HIV/AIDS transmission risks in two South African communities. J Urban Health 2005;82:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein M, Phillips T, Zerbe A, McIntyre JA, Brittain K, Petro G, et al. Intimate partner violence experienced by HIV-infected pregnant women in South Africa: a cross-sectional study. BMJ Open 2016;6:e011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simbayi LC, Kalichman S, Strebel A, Cloete A, Henda N, Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc Sci Med 2007;64:1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rane MS, Hong T, Govere S, Thulare H, Moosa M-Y, Celum C, et al. Depression and anxiety as risk factors for delayed care-seeking behavior in human immunodeficiency virus–infected individuals in South Africa. Clin Infect Dis 2018;67:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rueda S, Mitra S, Chen S, Gogolishvili D, Globerman J, Chambers L, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open 2016;6:e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungar M Resilience across cultures. Br J Soc Work 2008; 38:218–238. [Google Scholar]
- 35.Rutter M Resilience as a dynamic concept. Dev Psychopathol 2012;24:335–344. [DOI] [PubMed] [Google Scholar]
- 36.Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: moving toward resilience. Am Psychol 2013;68:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull 1985;98:310–357. [PubMed] [Google Scholar]
- 38.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull 1996;119:488–531. [DOI] [PubMed] [Google Scholar]
- 39.Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA, et al. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav 2015;19:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskowitz JT, Hult JR, Bussolari C, Acree M. What works in coping with HIV? A meta-analysis with implications for coping with serious illness. Psycholl Bull 2009;135:121–141. [DOI] [PubMed] [Google Scholar]
- 41.Chesney MA, Chambers DB, Taylor JM, Johnson LM, Folkman S. Coping effectiveness training for men living with HIV: results from a randomized clinical trial testing a group-based intervention. Psychosom Med 2003;65:1038–1046. [DOI] [PubMed] [Google Scholar]
- 42.Vosvick M, Gore-Felton C, Koopman C, Thoresen C, Krumboltz J, Spiegel D. Maladaptive coping strategies in relation to quality of life among HIV+ adults. AIDS Behav 2002;6:97–106. [Google Scholar]
- 43.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African National HIV prevalence, incidence and behaviour survey. HSRC Press;2014. [DOI] [PubMed] [Google Scholar]
- 44.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess 1990; 55:610–617. [DOI] [PubMed] [Google Scholar]
- 46.Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med 1997;4:92–100. [DOI] [PubMed] [Google Scholar]
- 47.Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol 2005;56:519–543. [DOI] [PubMed] [Google Scholar]
- 48.Maskew M, Bor J, Hendrickson C, MacLeod W, Barnighausen T, Pillay D, et al. Imputing HIV treatment start dates from routine laboratory data in South Africa: a validation study. BMC Health Serv Res 2017;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bor J, Ahmed S, Fox MP, Rosen S, Meyer-Rath G, Katz IT, et al. Effect of eliminating CD4-count thresholds on HIV treatment initiation in South Africa: an empirical modeling study. PLoS One 2017;12:e0178249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox MP, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Trop Med Int Health 2012;17:1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox MP, Rosen S. A new cascade of HIV care for the era of ’treat all’. PLoS Med 2017;14:e1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dulin A, Dale S, Earnshaw V, Fava J, Mugavero M, Napravik S, et al. Resilience and HIV: a review of the definition and study of resilience. AIDS Care 1–12[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herrick AL, Lim SH, Wei C, Smith H, Guadamuz T, Friedman MS, et al. Resilience as an untapped resource in behavioral intervention design for gay men. AIDS Behav 2011; 15 (Suppl 1):S25–S29. [DOI] [PubMed] [Google Scholar]
- 54.Parkes-Ratanshi R, Bufumbo L, Nyanzi-Wakholi B, Levin J, Grosskurth H, Lalloo DG, et al. Barriers to starting ART and how they can be overcome: individual and operational factors associated with early and late start of treatment. Trop Med Int Health 2010;15:1347–1356. [DOI] [PubMed] [Google Scholar]
- 55.Stephens MA, Franks MM, Rook KS, Iida M, Hemphill RC, Salem JK. Spouses’ attempts to regulate day-to-day dietary adherence among patients with type 2 diabetes. Health Psychol 2013;32:1029–1037. [DOI] [PubMed] [Google Scholar]
- 56.Martire LM, Helgeson VS. Close relationships and the management of chronic illness: associations and interventions. Am Psychol 2017;72:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steward WT, Sumitani J, Moran ME, Ratlhagana MJ, Morris JL, Isidoro L, et al. Engaging HIV-positive clients in care: acceptability and mechanisms of action of a peer navigation program in South Africa. AIDS Care 2017;30:330–337. [DOI] [PubMed] [Google Scholar]
- 58.Genberg BL, Shangani S, Sabatino K, Rachlis B, Wachira J, Braitstein P, et al. Improving engagement in the HIV care cascade: a systematic review of interventions involving people living with HIV/AIDS as peers. AIDS Behav 2016;20:2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med 2008;15:194–200. [DOI] [PubMed] [Google Scholar]