Summary
Objective:
Over a third of all patients with epilepsy are refractory to treatment and there is an urgent need to develop new drugs that can prevent the development and progression of epilepsy. Epileptogenesis is characterized by distinct histopathological and biochemical changes, which include astrogliosis and increased expression of the adenosine metabolizing enzyme adenosine kinase (ADK; EC 2.7.1.20). Increased expression of ADK contributes to epileptogenesis and is therefore a target for therapeutic intervention. Here we tested the prediction that the transient use of an ADK inhibitor administered during the latent phase of epileptogenesis can mitigate the development of epilepsy.
Methods:
We used the intrahippocampal kainic acid (KA) mouse model of temporal lobe epilepsy, which is characterized by ipsilateral hippocampal sclerosis with granule cell dispersion and the development of recurrent hippocampal paroxysmal discharges (HPDs). KA injected mice were treated with the ADK inhibitor 5-iodotubercidin (5-ITU, 1.6 mg/kg bid i.p.) during the latent phase of epileptogenesis from day 3–8 after injury; the time period when gradual increases in hippocampal ADK expression begin to manifest. HPDs were assessed at 6 and 9 wks post KA followed by epilepsy histopathology including assessment of granule cell dispersion, astrogliosis and ADK expression.
Results:
5-ITU significantly reduced the percent time in seizures by at least 80% in 56% of mice at 6 wks post KA. This reduction in seizure activity was maintained in 40% of 5-ITU treated mice at 9 wks. 5-ITU also suppressed granule cell dispersion, and prevented maladaptive ADK increases in these protected mice.
Significance:
Our results show that the transient use of a small molecule ADK inhibitor, given during the early stages of epileptogenesis, has antiepileptogenic disease modifying properties, which provides the rationale for further investigation into the development of a novel class of antiepileptogenic ADK inhibitors with increased efficacy for epilepsy prevention.
Keywords: Epileptogenesis, disease modification, adenosine kinase, adenosine kinase inhibitor, hippocampus
Introduction
Epilepsy is a prevalent neurological condition affecting about 1% of the population. Despite the availability of over 40 different anti-seizure drugs (ASDs), a third of all patients remains refractory to treatment and there is an urgent need for the development of rationally designed therapeutic strategies capable to prevent epilepsy and its progression1. A significant proportion of epilepsies that emerge in the adult population are acquired, i.e. caused by a precipitating injury, which can be as diverse as traumatic brain injury, febrile seizure, status epilepticus, or brain infection. Strikingly, diverse insults trigger common inflammatory responses entailing microglial and astroglial activation, which contribute to the development of epilepsy2. Astroglial activation is associated with overexpression of the adenosine regulating enzyme adenosine kinase (ADK), which is linked to epileptogenesis3–5 and is a target for novel antiepileptogenic therapies.
Adenosine is a well-characterized endogenous anticonvulsant and seizure terminator of the brain that suppresses neuronal activity through increased activation of Gi/o-coupled adenosine A1Rs6–8. Consequently, overexpression of ADK in the epileptic brain directly contributes to seizure generation by depleting the extracellular concentration of adenosine5. Conversely, the local therapeutic replenishment of adenosine via stem cell-derived brain implants engineered to release therapeutic amounts of adenosine effectively suppresses seizures in rodent models of temporal lobe epilepsy9. In addition to adenosine receptor mediated anti-seizure effects, adenosine has previously unrecognized adenosine receptor independent effects in the cell nucleus, which regulate DNA methylation as a molecular basis for the disease modifying effects of adenosine10. Specifically, a nuclear splice variant of ADK (ADK-L) regulates DNA methylation by determining the flux of methyl groups through the S-adenosyl-homocysteine dependent transmethylation pathway11. Consequently, the increased expression of ADK, which has been associated with epileptogenesis promotes increased DNA methylation10, which has independently been shown to be a contributing factor for epilepsy development and its progression12. Therefore, the therapeutic blockade of ADK, and specifically of ADK-L, would be a rational therapeutic approach for the prevention of epilepsy.
ADK inhibitors have already been in preclinical drug development during the early 2000s for the indications seizures, chronic pain, and inflammation13, 14 based on the rationale that the systemic blockade of ADK would lead to enhanced beneficial A1R activation, leading to a bias for the identification of agents that act on the cytoplasmic form of ADK (ADK-S), which regulates the extracellular tissue tone of adenosine (Boison, 2013). In addition, the symptomatic treatment of those conditions would require the long-term use of such agents, which is problematic due to concerns of liver toxicity and cardiovascular side effects11, 13. Those hurdles for the development of novel antiepileptogenic strategies could be alleviated by (i) the development of novel ADK inhibitors with increased specificity for the nuclear isoform ADK-L15 or (ii) the short term use of non-selective ADK inhibitors during a critical time period of epileptogenesis.
The goal of the present study was to assess whether a transient dose of a non-selective ADK inhibitor can affect epileptogenesis long-term. We chose the mouse intrahippocampal kainic acid (KA) model of temporal lobe epilepsy, which is a model with a distinct ‘latent period’ of epileptogenesis followed by the onset of spontaneous hippocampal paroxysmal discharges (HPD) 2 weeks after the KA injection16–18. The non-selective ADK inhibitor 5-iodotubercidin (5-ITU) given transiently for 5 days during the latent period of epileptogenesis attenuated epilepsy development and progression in >50% of all subjects when assessed at 6 and 9 weeks after status epilepticus (SE). Epilepsy prevention was associated with the suppression of the epilepsy-associated pathologies of granule cell dispersion and overexpression of ADK. Our findings support the further development of ADK inhibitors for antiepileptogenic therapies.
Methods
Intrahippocampal Kainic Acid.
All animals were social housed under standardized conditions of light, temperature and humidity, environmental enrichment, and had access to food and water ad libitum. C57BL/6 (000664, Jackson Laboratory) adult male mice weighing at least 30g were used for all studies. Unilateral intrahippocampal kainic acid (KA) was injected into the CA1 to induce epileptogenesis. Under general anesthesia (1.5% isoflurane in 100% O2) 400 ng of KA KA in sterile saline, pH 7.4 (K0250, Sigma-Aldrich) in a volume of 200 nl was injected into the hippocampus via a 31-gauge internal cannula inserted into the lumen of a 26-gauge steel guide cannula. (Plastics One Inc.) Control mice received 200 nl vehicle (sterile saline solution). KA or vehicle were infused over a 1 min timeframe. Following injection, the cannula was kept in place for 5 min prior to retraction. The stereotaxic coordinates for injection were AP: −2.1 mm, ML: −1.8 mm and DV: −1.7 from bregma. For mice that received the ADK inhibitor, twice daily intraperitoneal injections of 1.6 mg/kg 5-ITU in 20% DMSO (I-100, 5-ITU, Sigma-Aldrich) were administered from 3–8 days post KA injection. Control mice received intraperitoneal injections of 20% DMSO.
EEG Monitoring.
Five weeks after KA or control injection, 5-ITU-treated and control mice were affixed with electroencephalogram (EEG) recording electrodes and headset under general anesthesia (1.5% isoflurane, 100% 02). Stereotaxic surgery was performed to implant a twisted bipolar coated stainless-steel electrode (80µm in diameter; Plastics One Inc.) into the ipsilateral CA1 (AP: −2.1 mm, ML: −1.8 mm and DV: −1.7 from bregma). Two additional monopolar electrodes were placed onto the frontal cortex (to record cortical EEG) and cerebellum (as reference). Recording electrodes were inserted into a headset and fixed in place with dental acrylate. EEG recordings were performed at 6wk and 9wks post KA injection. Mice were single housed while tethered for acquisition of the EEG recordings. Electrical brain activity was monitored using a Nervus EEG recording system connected with a Nervus Magnus 32/8 Amplifier, and filtered (high-pass filter 50 Hz cut off, low-pass 1 Hz). The digital EEG signal was recorded and stored using a NicoletOne-System (Viasys Healthcare Inc).
Immunohistochemistry.
Mice were transcardially perfused with 4% paraformaldehyde in phosphate buffer (0.15 M, pH 7.4). The brains were postfixed in the same fixative at 4°C overnight and cryoprotected in 30% sucrose in PBS before being cut into 40-μm coronal sections. For the intrahippocampal KA time-course experiment, adjacent sections were either Nissl stained with cresyl violet or stained for ADK protein using a rabbit anti-ADK polyclonal antibody (A304–280A, Bethyl Laboratories) developed with 3,3’-diaminobenzidine (DAB) following standard protocols19. To assess epilepsy histopathology following 5-ITU treatment, adjacent coronal sections were stained for Nissl, ADK or GFAP. For the immunofluorescence detection of GFAP and ADK, we followed our previously published procedures19. ADK and GFAP sections were counter stained with DAPI (Invitrogen). For each hippocampus 3 independent sections were stained for each method. Sections damaged by surgery artefacts (intracranial injection, electrode implantation) were discarded. Representative sections as close as possible to the KA injection were analyzed. High resolution digital images were acquired using equivalent settings with either a Leica DM1000 bright field microscope equipped with the DCF295 camera and Leica LAS AF Image Acquisition Software (Leica Microsystems Inc.) or a Leica DMLB microscope using a high-resolution Zeiss Axiocam camera.
Results
Increased ADK expression accompanies epilepsy development in the mouse intrahippocampal KA model.
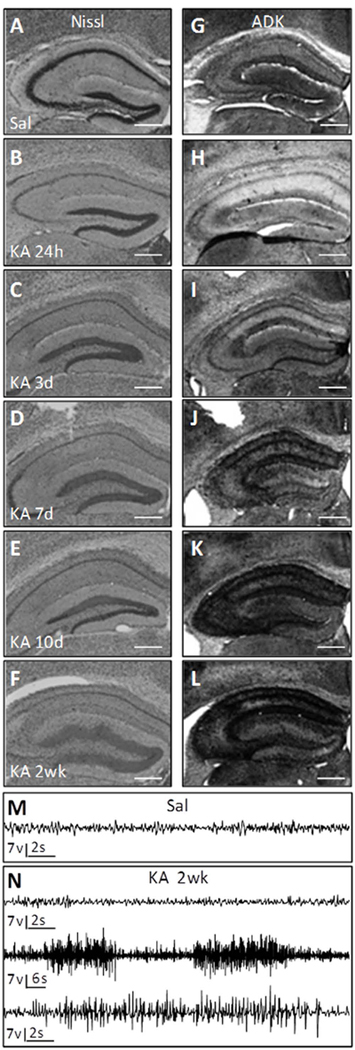
Because antiepileptogenesis studies ideally require the development of a robust and reproducible epilepsy phenotype in 100% of all therapy-naïve control animals, we chose to use a stronger version of the intrahippocampal KA model by injecting a total of 400 ng KA into the dorsal hippocampus. Because maladaptive changes in ADK expression are intrinsically involved in the development of epilepsy4, 5, 20 and in order to select appropriate time points for therapeutic intervention, we first analyzed the ADK expression profile during epileptogenesis in the mouse intrahippocampal KA model of TLE. Adult male C57Bl/6 mice received intrahippocampal injections of KA or saline and brains were prepared for histopathological analysis at 24h, 3d, 7d, 10d, and 2 weeks (N = 4–7 per time point). Nissl staining revealed loss of CA1 pyramidal and dentate hilar neurons as early as 24 hours after the KA-induced status epilepticus (SE) (Fig. 1B), compared to intrahippocampal saline injected controls (Fig. 1A). This acute injury is a direct consequence of the KA-induced SE. Subsequently, granule cell dispersion developed with a robust and reproducible phenotype of dispersion at 2 weeks after KA (Fig. 1F), which coincides with the occurrence of spontaneous HPDs (Fig. 1N). ADK immunohistochemistry of the ipsilateral hippocampal formation showed a biphasic response: acute reduction of ADK at 24h (Fig. 1H) followed by gradual increases in ADK expression (Fig. 1I–L). Again, and in line with our prior work20, 21, robust overexpression of ADK coincided with spontaneous HPD onset at 2 weeks after KA injection (Fig. 1L, N).
Figure 1. Histopathology during epileptogenesis.
Adult male C57BL/6 mice were injected with unilateral intrahippocampal kainic acid (KA) or saline (Sal). (A–F) Representative images of the hippocampus of Nissl stained coronal sections harvested at 24h, 3d, 7d, 10d and 2wk post KA injection and 4wk post Sal injection. (G–L) Representative images of ADK immunoreactivity from adjacent sections. Note that following KA injection at 24 hours there is CA1 cell loss and decreased ADK protein. By 3d post injury ADK is increased and continues to increase throughout epileptogenesis. N = 4 – 7 mice per time point. Scale bars = 500µm. (M–N) Representative hippocampus electroencephalogram (EEG) traces following intrahippocampal injection of (M) saline at 4wk and (N) KA at 2wk post surgery. (M) EEG recording from the saline injected control has normal baseline activity void of epileptiform activity. (N) Intrahippocampal KA injection show periods of normal baseline activity (upper trace) and periods of spontaneous hippocampal paroxysmal discharge (HPD) activity (middle and lower traces). Lower trace is high resolution of a single HPD event from the middle trace.
The ADK inhibitor 5-ITU exerts antiepileptogenic effects in the mouse intrahippocampal KA model.
In line with the results from our longitudinal study of epileptogenesis in the intrahippocampal KA model (Fig. 1), we predicted that timing ADK inhibition to coincide with the onset of increased ADK expression might sufficiently interfere with a key component of epileptogenesis and result in sustained suppression of epilepsy development. Three days after SE, 5-ITU or vehicle was systemically injected for a restricted time span of 5d. Six weeks and 9 weeks after the SE, HPDs were quantified by intrahippocampal EEGs and brains were processed for histopathology (Fig. 2A). A representative 30 min of EEG trace shows multiple HPD events in the KA/vehicle mice (Fig. 2B1). These events are characterized by high-amplitude rhythmic discharges. See Fig. 2B2 for a high-resolution example of a seizure demarcated by the dashed box in Fig. 2B1. Conversely, in KA/5-ITU mice that had a low seizure burden there is evidence of high-amplitude spikes (Fig. 2C1), but the spikes are typically more isolated and do not form organized HPDs (Fig. 2C2). EEG analysis shows that the KA/vehicle-injected control group developed a robust epileptiform phenotype by 6 weeks after the SE (Time spent in seizure: 28.9±9.4%; HPD Rate: 20.0±5.5 HPD/h; HPD duration: 53.9±20.1 sec, n=16). In contrast, KA/5-ITU mice had significant reductions in seizure activity (Time spent in HPD: 13.0±15.0%; HPD rate: 11.0±12.4 HPD/h; HPD duration: 32.5±21.3 sec, n=16) (Fig. 2D–F). Importantly, 56% of all KA/5-ITU mice (9 out of 16) were characterized with >80% reduction in the time spent in seizures. Three of which were completely seizure free (Fig. 2D). HPD activity was re-assessed at 9 weeks after KA to determine if 5-ITU sustains suppression or delays the onset of epileptiform activity. For the KA/vehicle mice HPD activity at 9 weeks was similar to 6 weeks indicating that the epilepsy phenotype was stable (Time spent in seizure: 26.2±8.4%; HPD rate: 21.7±5.9 HPD/h; HPD duration: 44.6±14.3 sec, n=12). For the KA/5-ITU mice, seizure activity was still significantly reduced at 9 weeks (Time spent in seizures: 12.7±12.11; HPD rate: 13.4±13.2 HPD/h; HPD duration: 25.5±17.1 sec, n=15) (Fig. 2G–I) and 40% of the mice had maintained a >80% reduction of time spent in seizure (Fig. 2G). Together, these data demonstrate a robust and lasting antiepileptogenic effect of the ADK-inhibitor 5-ITU given transiently during the latent period of epileptogenesis.
Figure 2. Transient systemic treatment with 5-ITU during the latent phase is antiepileptogenic.
(A) Schematic of experimental design: Adult male C57BL/6 mice injected with unilateral intrahippocampal KA received 1.6mg/kg 5-ITU or 20% DMSO vehicle (Veh) injections (i.p., b.i.d.) from 3–8d post injury. EEG recordings were acquired at 6 and 9wks post KA injection. Tissue was harvested at 9wk for histopathology analysis. (B–C) Representative EEG traces from (B) KA-Veh and (C) protected KA-5-ITU treated mice. Panels B1 and C1 depict 30 min of continuous EEG recordings. Panel B2 is a high-resolution trace of a single hippocampal paroxysmal discharge (HPD) event demarcated by the box B1. Panel C2 is a high-resolution trace of an EEG that shows epileptiform activity (spikes), but no organized HPD events. (D–F) At 6wk post KA injection there was a significant decrease in the percent time in HPD (D, t(30)=3.58), average HPDs/hour (E, Welch-corrected t(20.8)=2.67), and average HPD duration (F, t(30)=6.52) for KA-5-ITU (n=16) vs. KA-Veh (n=16) mice. (G–I) At 9wks KA-ITU (n=15) mice had a significant decrease in the percent time in HPD (G, t(25)=3.28), average HPDs/hour (H, Welch-corrected t(20.2)=2.20), and average HPD duration (I, t(25)=3.09) vs. KA-Veh (n=12) mice. Note EEG recordings at the 9wk timepoint were excluded for a subset of mice with lost/damaged recording headsets. Data are shown as the mean±SD and analyzed by Student’s unpaired t-test (F, G, I) or Welch’s unpaired t-test (D, E, H) for comparison of groups with unequal variances.
5-ITU prevents granule cell dispersion in the mouse intrahippocampal KA model.
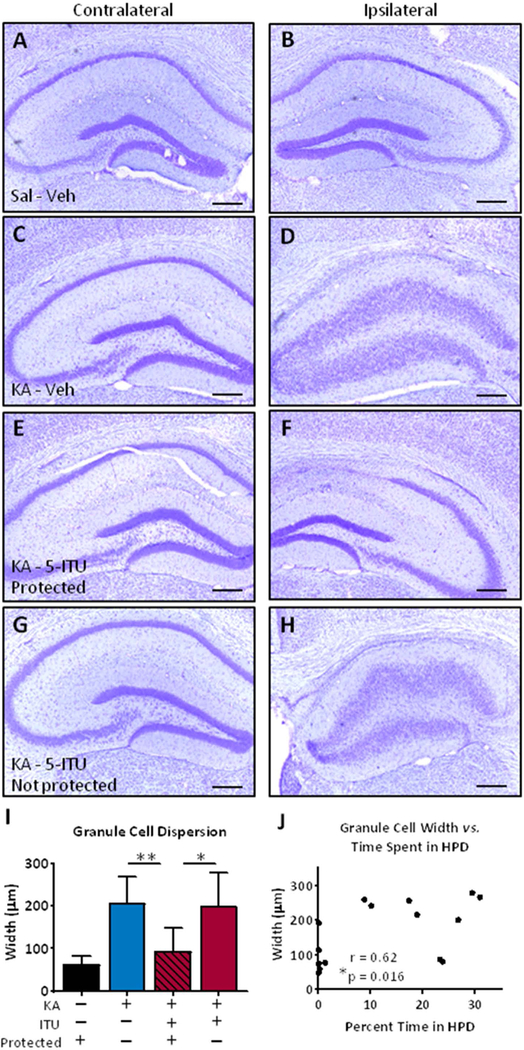
After completion of the EEG recordings animals were euthanized and subjected to histopathological analysis. We first assessed granule cell dispersion in Nissl stained hippocampal sections. Regardless of group, the contralateral hippocampus shows minimal to no morphological changes in the hippocampus (Fig 3A,C,E,G). In the ipsilateral hippocampus of all KA injected mice there is CA1 thinning (Fig. 3D,F,H), which is indicative of SE sufficient to cause injury as a precipitating factor for subsequent epileptogenesis. In the KA/Veh animals we observed a consistent expansion of the dentate granule cell layer (Fig. 3D), compared to saline injected controls (Fig. 3B). However, in KA/5-ITU mice that were protected from developing seizures (mice with >80% HPD reduction at 9 weeks) there is an absence of granule cell dispersion (Fig. 3F). In contrast, KA/5-ITU mice that were not protected had changes in granule cell dispersion that match the KA/Veh mice (Fig. 3D,H). We quantified granule cell dispersion by measuring the width of the dentate granular cell layer. The protected KA/5-ITU mice have a significant decrease in granule cell layer width, compared to either KA/Veh or KA/5-ITU non-protected mice (Fig. 3I). To assess if HPD activity is related to granule cell dispersion, we assessed the correlation of time in seizure against granule cell width within each KA/5-ITU mouse (Fig. 3J). In doing so, we show that reduced HPD activity is associated with a reduction in granule cell thickness (Fig. 3J). Together these data show that administration of 5-ITU after an injury-inducing SE is sufficient to prevent granule cell dispersion as a morphological correlate to seizure prevention.
Figure 3. Transient systemic treatment with 5-ITU reduces granule cell dispersion in epilepsy.
Adult male C57BL/6 mice injected with unilateral intrahippocampal kainic acid (KA) received 1.6mg/kg 5-ITU or 20% DMSO vehicle (Veh) from 3–8d post injury (i.p., b.i.d.). Control mice had intrahippocampal saline (Sal) injection followed by Veh injections. Tissue was harvested at 9wk post intrahippocampal injection. (A–H) Representative coronal hippocampal sections Nissl stained to visualize granule cell dispersion in Sal-Veh (A,B), KA-Veh (C,D), KA-5-ITU – Protected (E,F) and KA-5-ITU – Not protected. The contralateral (panels A,C,E,G) and ipsilateral (panels B,D,F,H) hemispheres are presented for each mouse. Scale bars = 250µm. (I) Dentate granule cell layer width is significantly (F(3,32)=9.43, p=0.0001) reduced in KA-5-ITU-Protected mice (n=6), compared to KA-Veh (n=16) and KA-5-ITU-Not Protected mice (n=10), but not Sal-Veh mice (n=4). Data are shown as the mean±SD and analyzed by One-way ANOVA and Tukey’s multiple comparisons post hoc test. (J) Nonparametric Spearman correlation of all KA-5-ITU treated mice shows a significant relationship between dentate granule cell layer width and percent time in HPD at 9wks post KA (n=15 pairs, Spearman r=0.62, 95% CI=0.14 to 0.86).
5-ITU attenuates increased ADK expression in the mouse intrahippocampal kainic acid model.
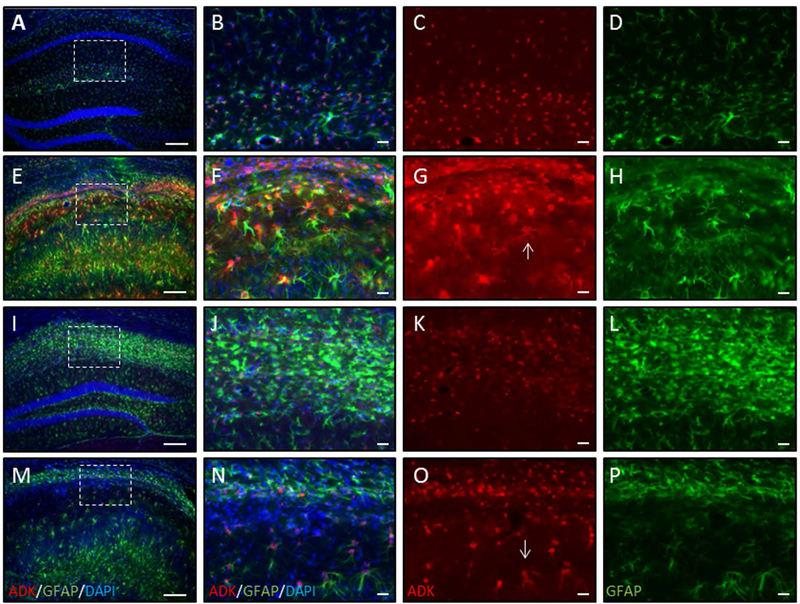
We also assessed the effects of transient 5-ITU treatment on the maladaptive development of increased astrocytic ADK protein levels and astrogliosis within the epileptic hippocampus. Representative images of the ipsilateral hippocampus of Sal/Veh, KA/Veh, protected KA/5-ITU, and non-protected KA/5-ITU mice stained for ADK, GFAP and DAPI are shown in Figure 4. A high-resolution image of the CA1 subfield field (box in panel A) shows normal astrocytes (GFAP) with co-expression of ADK in Sal/Veh control mice (Fig. 4A–D). However, in KA/Veh mice there is profound increase in both ADK and GFAP expression (Fig. 4E–H). The increase in GFAP expression is indicative of astrogliosis. Further, there is an increase in ADK in the reactive astrocytes (Fig. 4G, arrow). Similar to the effects on granule cell dispersion, in mice with >80% reduction in seizure activity, transient 5-ITU treatment prevented the increase in ADK expression (Fig. 4I–K) despite the increase in the number of GFAP positive astrocytes (Fig. 4I,J,L). Finally, in KA/5-ITU mice that were not protected we see increased GFAP in conjunction with increased ADK expression (Fig. 4M–N). Together, these data show that a transient blockade of ADK selectively prevents maladaptive increases in ADK, despite astrogliosis; further, those findings indicate that the increase in ADK, rather than astrogliosis per se plays a major role in epileptogenesis in line with our prior findings22.
Figure 4. Transient systemic treatment with 5-ITU reduces ADK overexpression in mice protected from epilepsy.
Adult male C57BL/6 mice were injected with unilateral intrahippocampal kainic acid (KA) received 1.6mg/kg 5-ITU or 20% DMSO vehicle (Veh) injections (i.p., b.i.d.) from 3–8d post injury. Control mice had intrahippocampal saline (Sal) injection followed by Veh injections. Tissue was harvested at 9wk post intrahippocampal injection. ADK (red), GFAP (green) and DAPI (blue) immunofluorescence in coronal ipsilateral hemisphere of Sal-Veh (A–D), KA-Veh (E–H) and KA-5-ITU-Protected (I–L) and KA-5-ITU-Not protected (M–P). (A,E,I,M) Low magnification images. Note that DAPI staining shows that in the KA-Veh (E), KA-5-ITU-Protected (I) and KA-5-ITU-Not protected (M) mice there is CA1 thinning, but in only the KA-Veh and KA-5-ITU-Not protected mice there is compression of the CA1 subfield and granule cell dispersion. Higher resolution images of the CA1 subfield demarcated by the white box in Sal (B–D), KA-Veh (F–H), KA-5-ITU-Protected (J–L), and KA-5-ITU-Not protected (N–O) mice. (B–D) Sal hippocampus has ADK confined largely to the nucleus and perinuclear region of GFAP stained astrocytes. (F–H) KA-Veh hippocampus has increased ADK expression that is present in the cytoplasm (arrow in G) of reactive astrocytes that have increased GFAP immunoreactivity. (J–L) KA-5-ITU-Protected hippocampus has an increase in GFAP immunoreactive astrocytes that are ADK positive. (N–P) KA-5-ITUnon-protected hippocampus has an increase in GFAP immunoreactive astrocytes with increased ADK expression (arrow in O). Scale bars = 150µm (A,E,I,M); 25µm (B–D,F–H,J–L,N–P).
Discussion
In this first-in-kind therapeutic proof-of-concept study we demonstrate that epileptogenesis is preventable through a transient pharmacological approach, using a small molecule ADK inhibitor. Importantly, we show that a delayed – beginning 3 days after the epilepsy triggering insult – treatment for only 5 days provides robust and lasting antiepileptogenic disease-modifying outcome as evidenced by reduction of seizure burden, prevention of granule cell dispersion, and maladaptive ADK expression. To the best of our knowledge, this is the first successful study to demonstrate a robust antiepileptogenic effect in the mouse intrahippocampal KA model. Prior attempts using AMPA/NMDA blockers, or rapamycin were not effective in antiepileptogenic outcome23, 24. Importantly, the timing of our antiepileptogenic intervention was closely matched to the time course of maladaptive changes in ADK expression (Fig. 1). Our findings are in line with a newly identified epigenetic mechanism of adenosine previously shown to be antiepileptogenic10. The underlying epigenetic mechanisms contributing to the development of epilepsy and its progression12, 25, 26 offer novel therapeutic opportunities for the prevention of epilepsy – an unmet clinical need. Several aspects of our study warrant further discussion.
The adenosine kinase hypothesis of epileptogenesis.
The ribokinase ADK is a high affinity enzyme for the metabolism of adenosine and therefore a key regulator of adenosine concentrations in the brain27. In the adult brain, ADK is primarily expressed in astrocytes19 and increases in ADK have consistently been linked to the epileptogenic process5, 20, 28–30. Because increases in ADK are both a marker for epileptogenesis and a target for its prevention5 it is important to precisely tailor the timing of therapeutic intervention to maladaptive changes in ADK expression. ADK expression changes were followed after the epilepsy-triggering KA injection (Fig. 1). In line with our prior findings20, although at an accelerated time scale due to the use of a higher dose of KA (400 ng as opposed to 200 ng) we found an acute downregulation of ADK in the KA-injected hippocampus at 24 hours followed by gradual increases in ADK expression from 3 days onwards. Overexpression of ADK in the epileptic brain is expected to have two main effects: (i) We have previously shown that the transgenic overexpression of cytoplasmic ADK-S is sufficient to generate spontaneous seizures, in line with reduced extracellular adenosine and reduced adenosine A1R activation5, 31. This is in line with clinical data showing lower levels of adenosine just prior to a seizure6. (ii) As a key metabolic enzyme ADK regulates the S-adenosylhomocysteine-dependent transmethylation pathway, which also controls DNA and histone methylation. ADK deficient patients32, 33, mice11, and plants34 suffer from the effects of reduced methylation causing developmental abnormalities. High levels of nuclear ADK-L expression in turn drive increased DNA methylation10 thought to be a major mechanistic contributor for the development of epilepsy and its progression12, 25, 26. If increased ADK is causally linked to the development of epilepsy, then its inhibition during a critical time span should prevent epilepsy development. Our EEG data (Fig. 2) directly demonstrate that blocking ADK pharmacologically via a small molecule drug prevents or reduces the development of epilepsy in a subset of our test subjects. Those findings support the adenosine kinase hypothesis of epileptogenesis and suggest maladaptive expression changes of ADK after an epileptogenesis triggering injury play a causal role in epileptogenesis.
Putative mechanism.
We have previously demonstrated that adenosine can prevent the progression of epilepsy through an epigenetic mechanism, by which a transient dose of adenosine delivered locally through a brain implant of adenosine releasing silk blocked maladaptive DNA hypermethylation associated with overexpression of ADK10. In our current proof-of-concept study for a more translatable therapeutic strategy, we employed the ADK inhibitor 5-ITU, as one of the most effective pharmacological strategies to raise adenosine in the brain13, 14. We acknowledge that 5-ITU may also affect hepatic enzymes, albeit at an IC50, which is about 1000-fold higher than the IC50 for ADK (26 nM). More interesting is the fact that 5-ITU also blocks adenosine transport at a similar IC5035. This is of therapeutic interest as it would allow 5-ITU to trap adenosine inside the cell and thereby enable adenosine receptor-independent epigenetic activity previously demonstrated to play a key role in epilepsy prevention. Unfortunately, it is currently not practical to experimentally determine the concentrations of the extracellular versus intracellular adenosine pools.
Metabolism, diet, and epigenetic mechanisms.
Our data show a key contribution of a major metabolic enzyme with a lasting disease modifying effect. Adenosine, regulated by ADK, is mechanistically linked to DNA methylation. Importantly, adenosine can also be regulated by dietary intervention. It has been shown that a high fat low carbohydrate ketogenic diet – clinically used for the treatment of epilepsy for almost a hundred years36–38 – reduces the expression levels of adenosine kinase and thereby suppresses seizures through an adenosine A1 receptor dependent mechanism31. Newer findings suggest that a ketogenic diet has additional antiepileptogenic effects, likely mediated through an adenosine-dependent epigenetic mechanism39. Therefore, the exciting possibility exists, that diet and metabolism can influence the process of epileptogenesis through an epigenetic mechanism.
Rationale for the clinical development of antiepileptogenic ADK inhibitors.
Our study is a proof-of-concept study to support the future clinical development of novel small-molecule ADK inhibitors as antiepileptogenic drugs. Our strategy is translatable for the following reasons: (i) The transient use of an ADK inhibitor would avoid toxicities (e.g. hepatic) associated with the chronic use of ADK inhibitors. In addition, side effects such as sedation might be acceptable if short-term dosing would yield long-term benefits. (ii) ADK inhibitors, such as 5-ITU, which also affect adenosine transport would yield increased intracellular adenosine thus mobilizing antiepileptogenic epigenetic effects of adenosine. (iii) The use of methanocarba-based ADK inhibitors may allow the development of more selective inhibitors of the enzyme15. In conclusion, we propose that the development of novel ADK inhibitors is a tractable strategy for the prevention of epilepsy.
Statistical Methods.
Experimental Design:
Adult male C57BL/6 mice injected with unilateral intrahippocampal KA received 1.6mg/kg 5-ITU or 20% DMSO vehicle injections (i.p., b.i.d.) from 3–8d post injury. EEG recordings were acquired at 6wks post KA injection in 16 KA/vehicle and 16 KA/5-ITU mice. HPD activity was re-assessed at 9 weeks after KA in 12 KA/vehicle and 15 KA/5-ITU mice (n=4 KA/vehicle and n=1 KA/5-ITU mice had damaged EEG headset by 9 wks). Tissue was harvested at 9wk for histopathology analysis. All mice included in the study had CA1 thinning, indicative of a surgery that resulted in SE sufficient to induce injury and subsequent epileptogenesis.
EEG Analysis:
EEGs were analyzed manually by two investigators blinded to treatment group. The first hour of EEG recording was excluded from analysis to allow animals to habituate to the recording arena. The following 5 hours of continuous EEG recording were analyzed in entirety for HPDs, defined as high-amplitude rhythmic discharges that clearly represented a new pattern of tracing and which lasted at least 15 s. HPDs occurring with an interval less than 5 s without the EEG returning to baseline were defined as belonging to the same event. EEG data are reported as the group average ± Standard Deviation (SD) for the percent time spent in HPD activity seizure, HPD rate (s events/hour) and HPD duration.
Dentate Granule Cell Dispersion Analysis:
The width of the ipsilateral dentate granule cell layer was measured using Image J software. Five measurements per blade were obtained per section with 3 sections per animal. The averages were calculated for each blade (upper: lengths 1–5 and lower: lengths 6–10) as well as an overall average (lengths 1–10). Granule cell dispersion is reported as the group average ± SD with the 5-ITU treated mice divided into two subgroups, protected (≥80% reduction in HPDs) and non-protected mice. We also show correlation of dentate granule cell against percent time in HPDs for all 5-ITU treated mice.
Statistical Analyses:
Statistics were conducted and data were analyzed with GraphPad Prism7 (GraphPad Prism). For EEG data, Student’s unpaired t-test and Welch’s unpaired t-test for comparison of groups with unequal variances were used. For dentate granule cell dispersion data, one-way ANOVA followed by Tukey’s multiple comparison was used for group comparisons and Spearman correlation was used for within mouse comparisons of HPD to granule cell width. Statistical significance was assumed at p<0.05 (*p<0.05, **p<0.01, ***p<0.001) and the details of individual outcomes are reported in the figure legends.
Key Point Box:
Administration of a systemic ADK inhibitor attenuates epileptogenesis.
Systemic administration of 5-ITU during the latent phase of epilepsy reduces epilepsy development in >50% of mice.
5-ITU-induced disease modification is characterized by reduced seizure burden, granule cell dispersion, and ADK expression.
Acknowledgements:
This work was supported by a grant from Citizens United for Research in Epilepsy (CURE) and the NIH (NS088024, NS065957).
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure: Author DB is a co-Founder of PrevEp LLC and has served as paid consultant for Hoffman LaRoche AG. Authors USS, MY, RB, JLF, and BS have no conflicts of interest.
References:
- 1.Stefan H, da Silva FHL, Loscher W, et al. Epileptogenesis and rational therapeutic strategies. Acta Neurologica Scandinavica 2006;113:139–55. [DOI] [PubMed] [Google Scholar]
- 2.Klein P, Dingledine R, Aronica E, et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia 2018;59:37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond ML, Ritter AC, Jackson EK, et al. Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia 2015;56:1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedele DE, Gouder N, Güttinger M, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain 2005;128:2383–95. [DOI] [PubMed] [Google Scholar]
- 5.Li T, Ren G, Lusardi T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv 2008;118:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 1992;32:618–24. [DOI] [PubMed] [Google Scholar]
- 7.Lado FA, Moshe SL. How do seizures stop? Epilepsia 2008;49:1651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther 1989;249:31–7. [PubMed] [Google Scholar]
- 9.Fedele DE, Koch P, Brüstle O, et al. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci Lett 2004;370:160–5. [DOI] [PubMed] [Google Scholar]
- 10.Williams-Karnesky RL, Sandau US, Lusardi TA, et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Inv 2013;123:3552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boison D, Scheurer L, Zumsteg V, et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA 2002;99:6985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobow K, Kaspi A, Harikrishnan KN, et al. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta neuropathologica 2013;126:741–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGaraughty S, Cowart M, Jarvis MF, et al. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem 2005;5:43–58. [DOI] [PubMed] [Google Scholar]
- 14.Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs 2000;9:551–64. [DOI] [PubMed] [Google Scholar]
- 15.Toti KS, Osborne D, Ciancetta A, et al. South (S)- and North (N)-Methanocarba-7-Deazaadenosine Analogues as Inhibitors of Human Adenosine Kinase. Journal of Medicinal Chemistry 2016;59:6860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki F, Junier MP, Guilhem D, et al. Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience 1995. February;64:665–74. [DOI] [PubMed] [Google Scholar]
- 17.Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia 2003;44:877–85. [DOI] [PubMed] [Google Scholar]
- 18.Haussler U, Bielefeld L, Froriep UP, et al. Septotemporal position in the hippocampal formation determines epileptic and neurogenic activity in temporal lobe epilepsy. Cereb Cortex 2012;22:26–36. [DOI] [PubMed] [Google Scholar]
- 19.Studer FE, Fedele DE, Marowsky A, et al. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience 2006;142:125–37. [DOI] [PubMed] [Google Scholar]
- 20.Gouder N, Scheurer L, Fritschy J-M, et al. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci 2004;24:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Lan JQ, Fredholm BB, et al. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology 2007;3:353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biology 2008;4:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schidlitzki A, Twele F, Klee R, et al. A combination of NMDA and AMPA receptor antagonists retards granule cell dispersion and epileptogenesis in a model of acquired epilepsy. Sci Rep 2017;7:12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo D, Zeng L, Brody DL, et al. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 2013;8:e64078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller-Delaney SF, Bryan K, Das S, et al. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 2015;138:616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobow K, El-Osta A, Blumcke I. The methylation hypothesis of pharmacoresistance in epilepsy. Epilepsia 2013;54 Suppl 2:41–7. [DOI] [PubMed] [Google Scholar]
- 27.Boison D Adenosine kinase: exploitation for therapeutic gain. Pharmacological Reviews 2013;65:906–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aronica E, Sandau US, Iyer A, et al. Glial adenosine kinase - A neuropathological marker of the epileptic brain. Neurochem Int 2013;63:688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronica E, Zurolo E, Iyer A, et al. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia 2011;52:1645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boison D, Steinhauser C. Epilepsy and astrocyte energy metabolism 2018;66:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masino SA, Li T, Theofilas P, et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Inv 2011;121:2679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staufner C, Lindner M, Dionisi-Vici C, et al. Adenosine kinase deficiency: expanding the clinical spectrum and evaluating therapeutic options. Journal of inherited metabolic disease 2016;39:273–83. [DOI] [PubMed] [Google Scholar]
- 33.Bjursell MK, Blom HJ, Cayuela JA, et al. Adenosine kinase deficiency disrupts the methionine cycle and causes hypermethioninemia, encephalopathy, and abnormal liver function. Am J Hum Genet 2011;89:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffatt BA, Stevens YY, Allen MS, et al. Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol 2002;128:812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foga IO, Geiger JD, Parkinson FE. Nucleoside transporter-mediated uptake and release of [3H]L-adenosine in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol 1996;318:455–60. [DOI] [PubMed] [Google Scholar]
- 36.Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Frontiers in pharmacology 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kossoff EH. The ketogenic diet: an appropriate first-line therapy? Expert review of neurotherapeutics 2010;10:843–5. [DOI] [PubMed] [Google Scholar]
- 38.Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Advances in pediatrics 2010;57:315–29. [DOI] [PubMed] [Google Scholar]
- 39.Lusardi TA, Akula KK, Coffman SQ, et al. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 2015;99:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]