Abstract
Background
We previously reported the novel activity of alloprimed CD8+ T cells that suppress post transplant alloantibody production. The purpose of the current study is to investigate the expression and role of CXCR5 on antibody-suppressor CD8+ T cell function.
Methods
C57BL/6 mice were transplanted with FVB/N hepatocytes. Alloprimed CD8+ T cells were retrieved on day 7 from hepatocyte transplant recipients. Unsorted or flow-sorted (CXCR5+CXCR3− and CXCR3+CXCR5−) alloprimed CD8+ T cell subsets were analyzed for in vitro cytotoxicity and capacity to inhibit in vivo alloantibody production following adoptive transfer into C57BL/6 or high alloantibody-producing CD8 KO hepatocyte transplant recipients. Alloantibody titer was assessed in CD8 KO mice reconstituted with naïve CD8+ T cells retrieved from C57BL/6, CXCR5 KO or CXCR3 KO mice. Antibody suppression by OVA-primed monoclonal OT-I CXCR5+ or CXCR3+ CD8+ T cell subsets was also investigated.
Results
Alloprimed CXCR5+CXCR3−CD8+ T cells mediated in vitro cytotoxicity of alloprimed “self” B cells while CXCR3+CXCR5−CD8+ T cells did not. Only flow-sorted alloprimed CXCR5+CXCR3−CD8+ T cells (not flow-sorted alloprimed CXCR3+CXCR5−CD8+ T cells) suppressed alloantibody production and enhanced graft survival when transferred into transplant recipients. Unlike CD8+ T cells from wild-type or CXCR3 KO mice, CD8+ T cells from CXCR5 KO mice do not develop alloantibody-suppressor function. Similarly, only flow-sorted CXCR5+CXCR3− (and not CXCR3+CXCR5−) OVA-primed OT-I CD8+ T cells mediated in vivo suppression of anti-OVA antibody production.
Conclusion
These data support the conclusion that expression of CXCR5 by antigen-primed CD8+ T cells is critical for the function of antibody-suppressor CD8+ T cells.
Introduction
A key challenge in the field of transplantation is the lack of definitive approaches to suppress the development of alloantibody production or to treat antibody-mediated rejection (AMR). Clinical and experimental data indicate that de novo production of MHC-directed alloantibodies after transplant has pathologic and clinical consequences contributing to acute and chronic rejection of solid-organ (reviewed in1) and cellular transplants.2,3 A successful therapeutic approach to suppress the production of post transplant alloantibody would not only prevent AMR but also enhance long-term graft survival. New immunotherapies to suppress post transplant humoral alloimmunity require enhanced understanding of the immune mechanisms that regulate alloantibody production.
Conventional approach to modulating post transplant humoral alloimmunity has focused on the suppression of CD4+ T cells,4 which “help” B cells produce antibody.5,6 However, despite the use of T cell depletion induction immunotherapies and conventional maintenance immunosuppressive agents which target CD4+ T cells, the development of de novo donor-specific antibody (DSA) occurs in ~20%−40% of solid organ(reviewed in7) and also after hepatocyte2 or islet cell3 transplant. Promising results with co-stimulatory blockade therapies, which suppressed alloantibody production and rejection in experimental transplant models,8–13 paved the way for clinical trials testing the efficacy of costimulatory blockade in humans. Unfortunately, clinical trials testing the efficacy of recombinant humanized monoclonal antibody targeting CD154 in humans were associated with thromboembolic complications which resulted in the early suspension of these trials.14,15 More recently clinical trials testing the efficacy of humanized fusion protein targeting CTLA-4 (Belatacept) reported an acceptable safety profile with improved allograft function, allograft survival, and significant reduction in the incidence of alloantibody production compared to cyclosporine-based immunosuppression. However, an unexpectedly higher rate and severity of early acute rejection occurred in Belatacept-treated recipients.16 Thus, new immunotherapeutic approaches which suppress the development of humoral alloimmunity and prevent AMR are needed. Our group has focused on a novel CD8-dependent immunoregulatory mechanism which downregulates post transplant alloantibody production.17 We reported that these antibody-suppressor CD8+ T cells (CD8+ TAb-supp cells) mediate alloantigen-specific suppression of post transplant alloantibody by an IFN-γ-dependent mechanism, which involves cytotoxic killing of alloprimed B cells18 and inhibition of IL-4+CD4+ T cells.17
Since we previously noted that the suppression of alloantibodies occurs, in part, due to CD8-dependent killing of host MHC I+ alloprimed IgG+ B cells18 and that host alloprimed CD8+ T cells and alloprimed IgG+ B cells co-localize in lymphoid depots, we reasoned that antibody-suppressor CD8+ T cells might migrate to lymphoid tissue via expression of the lymphoid-homing chemokine receptor, CXCR5, to mediate their effector functions. The current studies were designed to investigate the expression and role of CXCR5 for antibody-suppressor CD8+ T cell function.
Materials and Methods
Experimental animals
FVB/N (H-2q MHC haplotype, Taconic), C57BL/6 (wild-type; WT), CD8 KO, mOVA Tg, OT-I Tg, CXCR5 KO, and CXCR3 KO mice (all H-2b) and B10.BR (H-2k) mouse strains (all 6–10 weeks of age, Jackson Labs) were used in this study. Transgenic FVB/N mice expressing human α−1 antitrypsin (hA1AT) were the source of “donor” hepatocytes, as previously described.19 Male and female mice of 6–10 weeks of age were used in these studies. All experiments were performed in compliance with the guidelines of the IACUC of The Ohio State University (Protocol 2008A0068-R2).
Hepatocyte isolation, purification, and transplantation
Hepatocyte isolation and purification was completed, as previously described.19 Hepatocyte viability and purity was >95%. Donor FVB/N hepatocytes (2×106) were transplanted by intrasplenic injection with circulation of donor hepatocytes to the host liver.19 Graft survival was determined by detection of secreted hA1AT in serial recipient serum samples by ELISA.19,20
CD8+ T cell isolation
Isolation of CD8+ T cells from naïve or primed hosts was performed using negative selection columns as per the manufacturer’s recommendations (R&D Systems, Minneapolis, MN; purity routinely >90%). In some cases, primed CD8+ T cells were sorted into CXCR5+CXCR3− and CXCR3+CXCR5− CD8+ T cell populations by FACS Aria flow cytometer (Becton Dickinson, Franklin Lakes, NJ) using anti-CXCR5 (clone 2G8) and anti-CXCR3 mAbs (clone CXCR3–173; both Becton Dickinson).
Preparation of Primed CD8+ T cells
Alloprimed CD8+ T cells were isolated from spleens of FVB/N hepatocyte recipients (day 7). To retrieve OVA-primed OT-I CD8+ T cells, naïve OT-I CD8+ T cells were adoptively transferred into OVA-primed CD8 KO hosts [i.p. injection of hepatocyte lysate from mOVA Tg mice as a source of OVA peptide (1 μg OVA)]. mOVA Tg hepatocytes (2 × 106) underwent 5 freeze/thaw cycles to create cell-free lysate.
B cell isolation
B cells (B220) and primed IgG+ B cells were purified from splenocytes using anti-mouse B220 or anti-IgG magnetic beads following the manufacturer’s instructions (Miltenyi Biotech, Auburn, CA; purity routinely >95%).
Donor-reactive alloantibody titer
To quantitate alloantibody titer, we analyzed the recipient serum using published methods.21 Briefly, serum was serially diluted and incubated with allogeneic target splenocytes. Splenocytes were then stained with FITC-conjugated goat anti-mouse IgG Fc (Organon Teknika, Durham, NC). Background staining using anti-mouse IgG Fc with allogeneic splenocytes (alone or with naïve serum) is negligible. The mean fluorescence intensity (MFI) was measured for each sample and the dilution that returned the MFI observed when splenocytes were incubated with a 1:4 dilution of naïve serum was divided by two and recorded as the titer.
Detection of serum anti-OVA IgG
Anti-OVA IgG levels in mOVA hepatocyte-lysate treated mice were detected using the mouse anti-OVA IgG antibody assay kit following the manufacturer’s instructions (Chondrex, Redmond, WA).
In vitro cytotoxicity assay
Cytotoxicity was measured using a LIVE/DEAD cell-mediated cytotoxicity kit (Invitrogen, Eugene, OR) and performed according to the manufacturer’s instructions. In brief, target B cells were stained with CFSE. CD8+ T cells and B cells were co-cultured at a 10:1 ratio for 4 hours. Propidium iodide (PI) was added to the co-cultures to assess cell death and PI uptake in CFSE+ B cells was immediately analyzed by flow cytometry, as previously described.22
Flow cytometric lymphocyte subset analysis and intracellular cytokine staining
Splenocytes were isolated from FVB/N transplant or FVB/N hepatocyte lysate recipients (day 7) and incubated for 4 hours with Leukocyte Activation Cocktail (PMA, ionomycin, and Brefeldin A; Becton Dickinson). Next, splenocytes were stained with Live/Dead Fixable Aqua Dead Cell Stain Kit following manufacture’s recommendations (Thermo Fisher, Grand Island, NY). Following Live/Dead cell staining, splenocytes were treated with anti-FcγR mAb [supernatant from 2.4G2 hybridoma (ATCC; Manassas, VA)]. Splenocyte T (CD4: clone GK1.5, CD8: clone 53–6.7) and B (B220: clone RA3-GB2) cell subsets were subsequently stained and analyzed for expression of markers of activation (CD44: clone IM7, CD62L: clone MEL-14), co-inhibition (PD-1: clone RMPI-30), chemokine receptors (CXCR5: clone 2G8, CXCR3: clone CXCR3–173), and germinal center markers (GL-7). Intracellular staining was performed using FIX&PERM cell permeabilization kit (Thermo Fisher). Analysis for intracellular cytokines IFN-γ (clone XMG1.2), IL-21 (clone RM0268–6G53) and IL-4 (clone 11B11) was performed following Becton Dickinson’s recommendations. Flow cytometric analysis was performed by gating on lymphocyte populations (viable, single cells) of CD8+ T cells, or CXCR5+PD-1+CD4+ T cells. Fluorescence-minus-one (FMO) was utilized as negative controls to set the positive/negative boundary for protein expression.23
Statistical analysis
General linear models were fit for each continuous outcome and contrasts used to compare relevant groups to test the primary hypothesis/hypotheses in each experiment. Model assumptions were assessed and violations to the normality assumption were addressed by transforming the data to the natural log scale. Log rank tests were used to compare time to graft rejection between groups. Multiple comparisons between groups were adjusted using Tukey-Kramer method (analysis of in vitro cytotoxicity), Bonferroni’s method (analysis of transplant survival), or Dunnett’s method (all other figures) to maintain the overall type 1 error rate at 5% for each outcome respectively. All analyses were conducted using SAS statistical Software Version 9.4 (SAS Institute, Inc., Cary, NC). To demonstrate the distribution of the data, results are listed as the mean ± standard error.
Results
Alloprimed CD8+ T cells that express CXCR5 mediate in vitro killing of self IgG1+ B cells
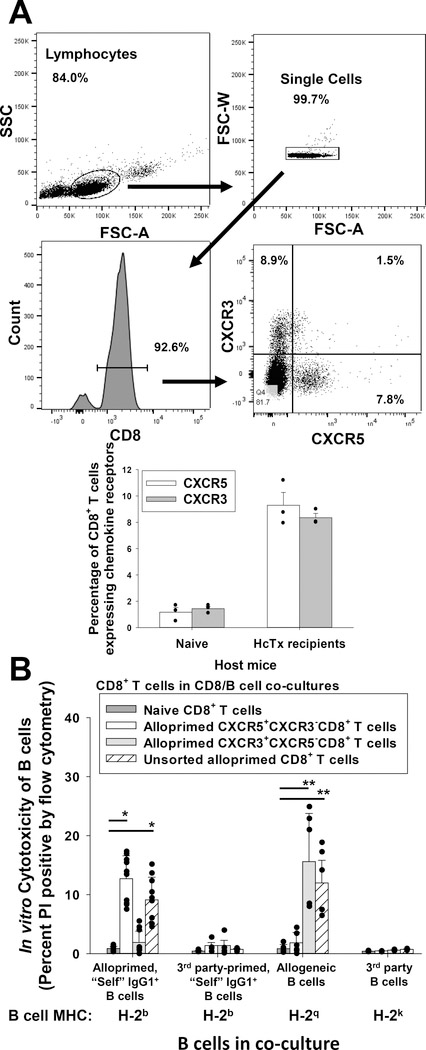
CXCR5 is a chemokine receptor important for homing to the germinal center in lymphoid tissue where B cell maturation occurs.24–26 CXCR3 is a chemokine receptor important for homing to sites of tissue inflammation (including allografts)27–32 and for cell-mediated rejection.33,34 In order to investigate the expression of chemokine receptors CXCR5 and CXCR3 by alloprimed CD8+ T cells, C57BL/6 mice underwent FVB/N hepatocyte transplantation. On day 7 post transplant, alloprimed CD8+ T cells were retrieved, analyzed for CXCR5 and CXCR3 expression by flow cytometry and flow-sorted populations were tested for in vitro killing of self IgG1+ B cells, an assay which correlates with antibody-suppressor CD8+ T cell function.18
We found that bulk, unsorted CD8+ T cells from alloprimed recipients expressed CXCR5 or CXCR3 (very few double positive cells) (Figure 1A). Alloprimed CXCR5+CXCR3− (and CXCR3+CXCR5−) CD8+ T cells were predominantly CD44+ (>80%) (Figure S1). Alloprimed CD8+ T cells (from day 7 C57BL/6 hepatocyte transplant recipients) were purified and flow-sorted into CXCR5+CXCR3−CD8+ and CXCR3+CXCR5−CD8+ T cell subsets. Flow-sorted CD8+ T cells were co-cultured with alloprimed “self” IgG1+ B cells (H-2q primed C57BL/6 B cells) or allogeneic B cells (FVB/N, H-2q) in an in vitro cytotoxicity assay. Third-party primed “self” IgG1+ B cells (H-2k primed C57BL/6 B cells) and B cells expressing third-party alloantigens (H-2k, B10BR) were used as control target cells. Unsorted alloprimed CD8+ T cells and naïve CD8+ T cells served as positive and negative control effector cells, respectively. Positive control unsorted alloprimed CD8+ T cells induced significant cytotoxicity to both alloprimed self IgG1+ B cells (9.1±3.8%; p<0.0001) and allogeneic B cells (12.0±5.5%; p=0.002) compared to naïve control CD8+ T cells (0.9±.04% and 0.8±0.7%, respectively; Figure 1B). Alloprimed CD8+ T cells sorted for the CXCR5+CXCR3−CD8+ T cell subset also mediated significant cytotoxicity to alloprimed self IgG1+ B cells (12.7±1.8%; p<0.0001) compared to naïve control CD8+ T cells but not to allogeneic B cells (1.8±1.8%; p=ns). In contrast, sorted CXCR3+CXCR5−CD8+ T cells mediated cytotoxicity to allogeneic B cells (15.6±8.8%; p<0.0001) but did not mediate cytotoxicity to alloprimed self IgG1+ B cells (1.9±1.9%; p=ns) compared to co-cultures with naïve control CD8+ T cells. No significant cytotoxicity was observed for any CD8+ T cell group against control third-party primed “self” IgG1+ B cells or third-party party B cells. These results suggested that antibody-suppressor CD8+ T cells express CXCR5 but not CXCR3. Next, we investigated the in vivo function of CXCR5+ and CXCR3+ alloprimed CD8+ T cell subsets.
Figure 1. CXCR5+CD8+ T cells mediate in vitro cytotoxicity of alloprimed self IgG+ B cells.
C57BL/6 (wild-type, WT; H-2b) mice were transplanted with FVB/N (H-2q) hepatocytes. On day 7 post transplant, splenic CD8+ T cells were retrieved and purified. A) Flow cytometric analysis of purified CD8+ T cells shows that CXCR5+CXCR3− (9.3±1.0%) and CXCR3+CXCR5− (8.4±0.3%) CD8+ T cell subsets are detected (CD8+ T cells pooled from 10–15 mice) for cell sorting; data for n= 3 sorts are shown in the bar graph. B) In an in vitro cytotoxicity assay, flow-sorted alloprimed CXCR5+CXCR3− or CXCR3+CXCR5− CD8+ T cell populations and B cell targets were co-cultured at a 10:1 ratio for 4 hours and analyzed for cytotoxicity (propidium iodide (PI) uptake). Naïve CD8+ T cells and unsorted alloprimed CD8+ T cells were utilized as negative and positive controls (effector cells), respectively. Significant cytotoxicity of alloprimed, self IgG1+ B cells was observed in co-cultures with CXCR5+CXCR3−CD8+ T cells (12.7±1.8%, n=10) or with unsorted alloprimed CD8+ T cells (9.1±3.8, p<0.0001 for both signified by “*”; n=10) in comparison to co-cultures with naïve CD8+ T cells (0.9±0.4%, n=9) but no significant cytotoxicity was detected in co-cultures with CXCR3+CXCR5−CD8+ T cells (1.9±1.9%, p=ns, n=10). Significant cytotoxicity of allogeneic B cells was observed in co-cultures with CXCR3+CXCR5−CD8+ T cells (15.6±8.8%; n=6) or with unsorted alloprimed CD8+ T cells (12.0±5.5%; n=6, p<0.002 for both signified by “**”), but not with CXCR5+CXCR3−CD8+ T cells (1.8±1.8%; n=6, p=ns) in comparison to control co-cultures with naïve CD8+ T cells (0.8±0.7%; n=5). No significant cytotoxicity was detected against third-party (3rd) party primed “self” IgG1+ B cells (H-2b targets) or 3rd party B cells (H-2k targets) in any co-cultures. Error bars indicate standard error from triplicate experiments.
CXCR5 is critically important for in vivo CD8+ T cell alloantibody-suppressor function
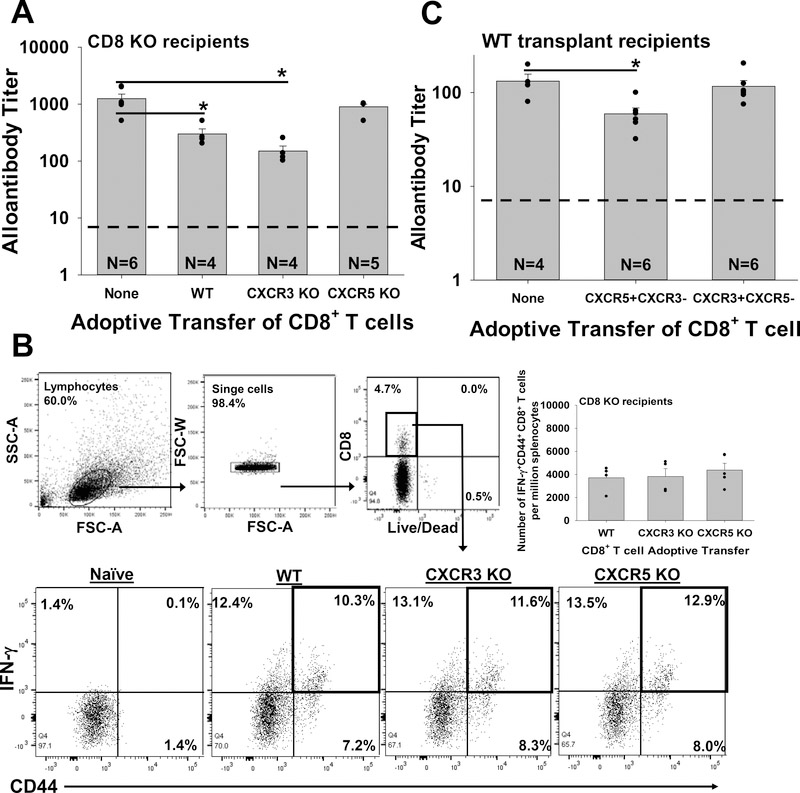
To determine the functional relevance of chemokine receptor expression by activated CD8+ T cells, we adoptively transferred CD8 KO hepatocyte recipients on day 0 (relative to transplant) with naïve CD8+ T cells isolated from WT C57BL/6, CXCR5 KO, or CXCR3 KO mice. Recipients were assayed for alloantibody production on day 14 post transplant. As in previous studies, we found that adoptive transfer of WT CD8+ T cells significantly inhibits alloantibody production by more than three-fold (p=0.004 compared to no CD8+ T cell transfer; Figure 2A).17,18 Adoptive transfer of CXCR3 KO CD8+ T cells also significantly suppressed day 14 alloantibody production (p=0.001 compared to no CD8+ T cell transfer). In contrast, adoptive transfer of CXCR5 KO CD8+ T cells had no effect on the amount of alloantibody produced by day 14 post transplant (p=ns compared to no CD8+ T cell transfer). The failure to suppress alloantibody production by CXCR5 KO CD8+ T cells was not due to a failure to become activated as demonstrated by the upregulation of IFN-γ expression by CD8+ T cells from all groups (WT, CXCR3 KO and CXCR5 KO) (Figure 2B). Furthermore, the quantity of IFNγ+CD44+CD8+ T cells retrieved on day 7 from recipient spleen was equivalent between WT (3,700±500 cells per million splenocytes), CXCR3 KO (3,800±700 cells per million splenocytes) and CXCR5 KO (4,400±600 cells per million splenocytes) groups suggesting equivalent CD8+ T cell reconstitution. Thus, the absence of CXCR5 (but not CXCR3) expression abrogates the capacity for alloprimed CD8+ T cells to suppress in vivo alloantibody production.
Figure 2. CXCR5 is critical to CD8+ T cell-mediated suppression of antibody production.
A) CD8 KO mice were transplanted with FVB/N hepatocytes. On day 0, recipients were adoptively transferred (AT) with 10×106 naïve CD8+ T cells (WT, CXCR5 KO or CXCR3 KO). Recipient serum was analyzed for alloantibody titer on day 14 post transplant. Significantly reduced alloantibody titer was observed in transplant mice that received AT of WT (300±70; n=4) or CXCR3 KO (150±30; n=4) CD8+ T cells (p<0.004 for both signified by “*”) compared to recipients that received AT with CXCR5 KO CD8+ T cells (900±100; n=5) or no AT (1,250±250; n=6). B) All groups of recipients that underwent transplant and adoptive transfer of WT, CXCR3 KO or CXCR5 KO CD8+ T cells developed alloprimed CD8+ T cells that were activated. Flow cytometric analysis gating on total leukocytes and CD8+ T revealed that by day 7 post transplant, approximately 10–13% of CD8+ T cells from all three groups (WT, CXCR3 KO, CXCR5 KO) of AT mice were IFN-γ+CD44+CD8+ T cells; similarly the quantity of IFN-γ+CD44+CD8+ T cells per million splenocytes was similar in all three groups. C) WT mice were transplanted with FVB/N hepatocytes and analyzed for production of alloantibody on day 14 post transplant. Transplant recipients that received AT (on day 5 post transplant) of flow-sorted (day 7) alloprimed CXCR5+CXCR3− WT CD8+ T cells exhibited significant reduction of alloantibody titer (60±10; n=5) compared to recipients which received AT of flow-sorted (day 7) alloprimed CXCR3+CXCR5−CD8+ T cells (120±20; n=6) or no AT (130±20; n=4, p<0.02 for both signified by “*”). The dashed line on Figures 2A and 2C represents negative control sera from naïve mice. Error bars indicate standard error from duplicate experiments.
To avoid any potential experimental biases associated with CD8 KO hosts, we repeated these studies using WT hepatocyte transplant recipients. Alloantibody production in WT hepatocyte recipients is also CD8-regulated and a substantial increase in alloantibody titer is observed in CD8+ T cell-depleted recipients.17 Low titer alloantibody (25±5) is detected by day 5 in WT recipients and peaks on day 14 after transplant.17 For these studies, we retrieved alloprimed CD8+ T cells from C57BL/6 hepatocyte transplant hosts on day 7 post transplant and flow-sorted alloprimed CD8+ T cells into CXCR5+CXCR3− and CXCR3+CXCR5− subsets. These CD8+ T cell populations were adoptively transferred (1×106 cells) into WT hepatocyte transplant recipients (day 5 post transplant). Recipient mice were assayed for alloantibody titer on day 14 post transplant (9 days after adoptive transfer of alloprimed CD8+ T cells). Only the CXCR5+CXCR3−CD8+ T cell population downregulated alloantibody production (p=0.02 compared to WT recipients with no CD8+ T cell transfer; Figure 2C).
CXCR5+CD8+ T cell-mediated suppression of alloantibody enhances hepatocyte allograft survival
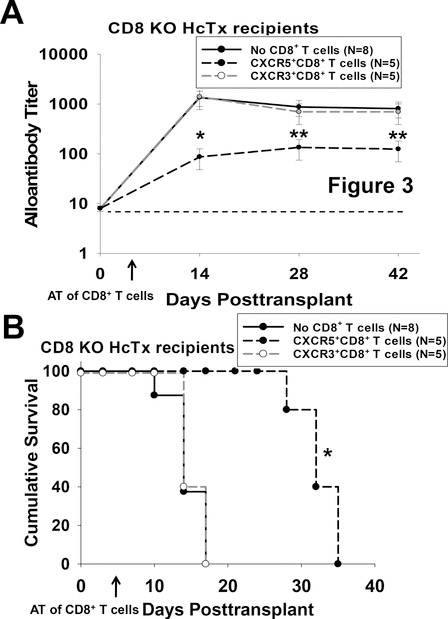
In order to investigate the consequence of CD8-mediated alloantibody suppression upon hepatocyte allograft survival in a model where rejection is antibody-mediated, CXCR5+CXCR3−CD8+ T cells were adoptively transferred into high alloantibody producing CD8 KO hepatocyte recipients.17 Rejection in CD8 KO hepatocyte recipients is alloantibody-dependent and macrophage-mediated.35,36 On day 5 post transplant, cohorts of CD8 KO hepatocyte recipients were adoptively transferred with 2×106 flow-sorted alloprimed CXCR5+CXCR3− or CXCR3+CXCR5−CD8+ T cells. CD8 KO recipients that received CXCR5+CXCR3−CD8+ T cells had significantly decreased quantity of alloantibody by day 14 post transplant compared to the control group that did not receive adoptive transfer of CD8+ T cells (titer=90±40 versus titer=1,300±500 respectively; p<0.0001, Figure 3A). This suppression of alloantibody was accompanied by significantly enhanced graft survival post transplant (MST=32 days versus MST=14 days; p=0.002, Figure 3B). CD8 KO recipients that received CXCR3+CXCR5−CD8+ T cells produced quantities of alloantibody (1,400±600, p=ns) similar to the control group and had similar allograft survival (MST=14 days) compared to CD8 KO recipients that did not receive CD8+ T cells (alloantibody titer=1,300±500; MST=14 days, p=ns). Additionally, we find that unlike bulk alloprimed CD8+ T cells, alloprimed flow-sorted CXCR5+CD8+ T cells do not initiate acute rejection when transferred into immunoincompetent RAG1 KO hepatocyte recipients (not shown). These data demonstrate that alloprimed CXCR5+CXCR3−CD8+ T cells (but not CXCR3+CXCR5−CD8+ T cells) significantly downregulate alloantibody production which is accompanied by prolonged allograft survival. We next evaluated the potential impact of antibody-suppressor CXCR5+CXCR3−CD8+ T cells on quantity of alloprimed B cells and CD4+ TFH cells in the germinal center.
Figure 3. CXCR5+CD8+ T cell-mediated suppression of alloantibody enhances hepatocyte allograft survival.
CD8 KO mice were transplanted with FVB/N hepatocytes. On day 5, cohorts of transplant recipients received adoptive transfer of flow-sorted (day 7) alloprimed CXCR5+CXCR3−CD8+ T cells, CXCR3+CXCR5−CD8+ T cells, or no CD8+ T cells. Mice were observed for alloantibody production and hepatocyte survival. A) Adoptive transfer of CXCR5+CXCR3−CD8+ T cells into CD8 KO recipients significantly inhibited alloantibody titer on day 14 (90±40; n=5, p<0.0001 signified by “*”) compared to no AT (1,300±500; n=8) while adoptive transfer of CXCR3+CXCR5−CD8+ T cells did not (1,400±600; n=5, p=ns). Alloantibody titer remained suppressed after day 14 in CD8 KO mice that received AT of CXCR5+CXCR3−CD8+ T cells (p<0.005 for days 28 and 42 post transplant, “**”). The dashed line represents negative control sera from naïve mice. Error bars indicate standard error. B) CD8 KO hepatocyte recipients that received adoptive transfer of CXCR5+CXCR3−CD8+ T cells had prolonged allograft survival (MST=day 32; p=0.002, signified by “*”) compared to those that received AT of CXCR3+CXCR5−CD8+ T cells (MST= day 14) or those with no AT (MST= day 14).
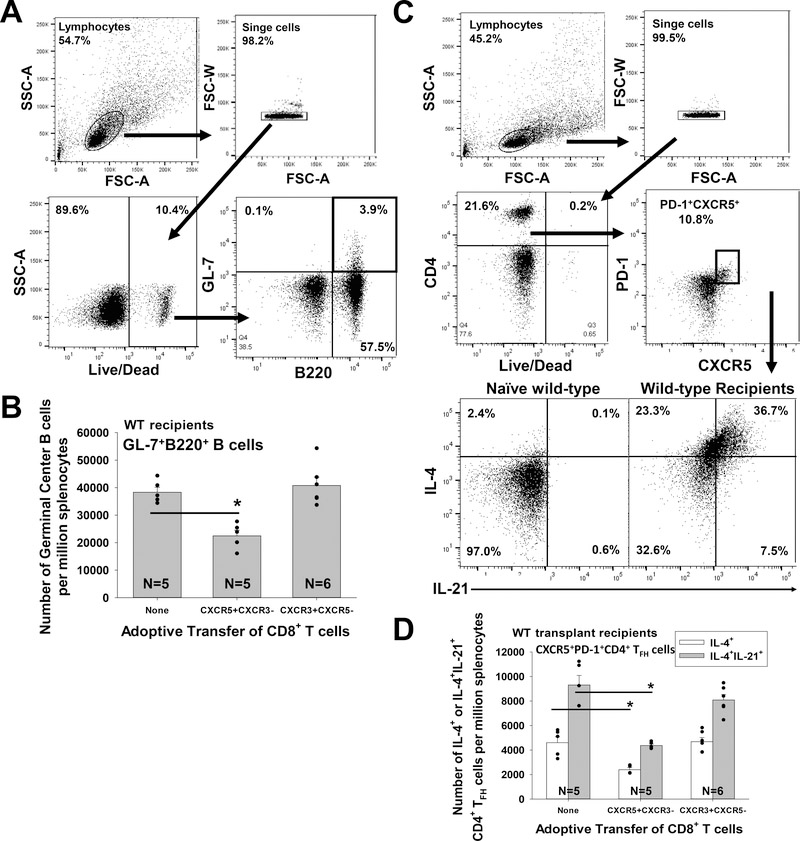
Suppression of alloantibody production by alloprimed CXCR5+CD8+ T cells is accompanied by a reduction in the quantity of Germinal Center B cells and CD4+ TFH cells
Germinal centers are the primary site for T follicular helper (CD4+ TFH) cell and primed B cell interactions, which give rise to long-lived plasma cells and memory B cells (reviewed in37). Since germinal center trafficking relies on CXCR525,26 and we previously reported that CD8+ T cells kill alloprimed B cells18, we investigated the consequence of alloprimed CXCR5+CD8+ T cells adoptive transfer upon the quantity of germinal center (GC) B cells and CD4+ TFH cells in WT hepatocyte transplant recipients. We analyzed the number of GC B cells (GL-7+B220+) as well as the number of IL-4 or IL-21 expressing CD4+ TFH cells (CXCR5+PD-1+CD4+) on day 14 post transplant after adoptive transfer of flow-sorted CXCR5+CXCR3− or CXCR3+CXCR5−CD8+ T cells into WT hepatocyte transplant recipients, as described above (as in Figure 2C). We find that while adoptive transfer of CXCR5+CXCR3− CD8+ T cells significantly inhibits the quantity of GC B cells (p=0.001), IL-4+CD4+ TFH cells (p=0.04), and dual IL-4+IL-21+ CD4+ TFH cells (p=0.003) that are detected post transplant, adoptive transfer of CXCR3+CXCR5−CD8+ T cells does not (Figure 4A–D).
Figure 4. Suppression of alloantibody production by alloprimed CXCR5+CD8+ T cells is accompanied by a reduction in the quantity of Germinal Center B cells and CD4+ TFH cells.
WT mice were transplanted with FVB/N hepatocytes (day 0) and on day 5 received adoptive transfer (AT) of sorted alloprimed CXCR5+CXCR3−CD8+ or CXCR3+CXCR5−CD8+ T cells. On day 14 post transplant, recipient splenocytes were analyzed for the number of germinal center (GC) B cells and CD4+ TFH by flow cytometry. A) GC B cells (GL-7+B220+) were analyzed by gating on lymphocytes. B) Recipients which received AT of CXCR5+CXCR3−CD8+ T cells exhibited significantly reduced number of GC B cells (22,000±2,000 per million splenocytes; n=5) compared to WT recipients without AT (38,000±2,000 per million splenocytes; n=5, p=0.001 signified by “*”). In contrast, the number of GC B cells was not significantly altered in recipients which received AT of CXCR3+CXCR5−CD8+ T cells (41,000±3,000 per million splenocytes; n=6, p=ns) compared to control recipients without CD8+ T cell transfer. C) CD4+ TFH cells were analyzed by gating on lymphocytes, CD4+ T cells, and PD-1+CXCR5+ cells. D) Recipients which received AT of CXCR5+CXCR3−CD8+ T cells exhibited significantly reduced number of IL-4+IL-21+ CD4+ TFH cells (4,400±100 per million splenocytes; n=5, p=0.003 signified by “*”) compared to WT recipients without AT (9,300±800 per million splenocytes; n=5). AT of CXCR5+CXCR3−CD8+ T cells was associated with a reduced number of IL-4+ CD4+ TFH cells (2,400±100 per million splenocytes; n=5, p=0.04) compared to control recipients without CD8+ T cell transfer (4,600±500 per million splenocytes). In contrast, the number of IL-4+ (4,600±300 per million splenocytes; n=6) and IL-4+IL-21+ CD4+ TFH cells (8,100±500 per million splenocytes; n=6) was not significantly altered after AT of CXCR3+CXCR5−CD8+ T cells compared to the control group with no CD8+ T cell transfer (p=ns for both IL-4+ and IL-4+IL-21+ CD4+ TFH cells). Error bars indicate standard error from duplicate experiments.
We have previously reported that in vivo CD8+ T cell-mediated inhibition of alloantibody production and in vitro cytotoxicity to alloprimed self IgG1+ B cell is allospecific.18 Likewise, in these studies alloprimed CD8+ T cell mediated inhibition of CD4+ TFH cells is allospecific as third-party primed (B10BR; H-2k) CD8+ T cells do not reduce the quantity of IL-4+ or IL-4+IL-21+ CD4+ TFH cells detected after allogeneic hepatocyte transplant (FVB/N; H-2q) into CD8 KO hosts (H-2b; Figure S2). Further, in prior co-culture work we found that bulk alloprimed CD8+ T cells do not mediate cytotoxic killing of CD4+ TFH cells (Figure S3A) but rather mediate IFN-γ-dependent suppression of IL-4 and IL-21 expression by alloprimed CD4+ TFH cells (Figure S3B). Together, these data suggest that CXCR5+CXCR3−CD8+ T cells downregulate humoral alloimmunity by trafficking to germinal centers where they kill and/or impair GC B cells and CD4+ TFH cells. In order to study monoclonal antigen-specific antibody-suppressor CD8+ T cells, we performed studies using OVA-peptide-specific OT-I CD8+ T cells.
Dose-dependent downregulation of antibody to OVA-peptide by OT-I antibody-suppressor CD8+ T cells
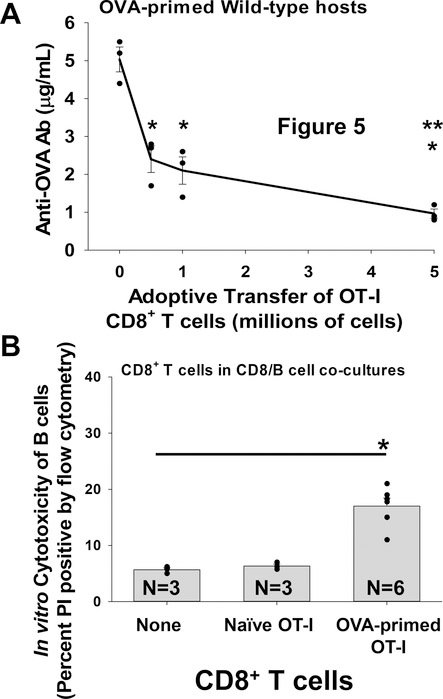
Monoclonal TCR transgenic (Tg) CD8+ T cells from OT-I Tg mice have specificity for OVA peptide SIINFEKL257–264. To stimulate the development of primed OT-I CD8+ T cells, CD8 KO mice were adoptively transferred with OT-I CD8+ T cells and primed with mOVA lysate (membrane-bound OVA) derived from mOVA Tg mice (mOVA.B6, H-2b). To investigate the suppressive function and dose response of unsorted OVA-primed OT-I CD8+ T cells on anti-OVA antibody production, we adoptively transferred increasing numbers of OVA-primed OT-1 cells (0.5, 1, or 5 ×106 cells) on day 0 into cohorts of WT (C57BL/6) mice immunized with mOVA.B6 lysate (day 0) and measured serum anti-OVA antibody levels on day 14. Adoptive transfer of OVA-primed OT-I CD8+ T cells significantly suppressed the production of OVA-specific antibody in WT hosts (p<0.0004 for all quantities of CD8+ T cells transferred; Figure 5A). In addition, the adoptive transfer of 5×106 OVA-primed OT-I CD8+ T cells inhibited anti-OVA antibody production to a greater extent than 0.5 or 1.0×106 cells (p<0.04 for both). These data indicate that antigen-specific, antibody-suppressor CD8+ T cells mediate downregulation of anti-OVA antibody production in a dose-dependent fashion. For reference, 5×106 OVA-primed WT (C57BL/6) CD8+ T cells also inhibited anti-OVA antibody production following adoptive transfer into mOVA-primed WT hosts (p=0.04, data not shown).
Figure 5. OVA-primed OT-I CD8+ T cells suppress anti-OVA antibody production in a dose-dependent manner and mediate in vitro cytotoxicity of OVA-primed B cells.
A) WT mice were primed with mOVA lysate (i.p. injection) on day 0. Cohorts of WT mice received adoptive transfer (AT) on day 0 of increasing quantities of OVA-primed OT-I TCR transgenic CD8+ T cells (0, 0.5, 1, 5×106 cells i.v.). Mouse serum was assayed for anti-OVA antibodies (Ab) by ELISA on day 14 following antigen stimulation. WT mice primed with mOVA lysate exhibit maximal levels of serum anti-OVA antibodies on day 14 following antigen stimulation (5.0±0.3 μg/mL; n=3). AT of 0.5, 1, or 5×106 OVA-primed OT-I CD8+ T cells on day 0 inhibited anti-OVA antibody production in these mice (2.4±0.4, 2.1±0.4, and 1.0±0.1 μg/mL respectively; n=3 and p<0.0004 for all groups signified by “*”). The AT of 5×106 OVA-primed OT-I CD8+ T cells inhibited anti-OVA antibody production significantly more than 0.5 or 1×106 OVA-primed OT-I CD8+ T cells (p<0.04 for both signified by “**”). B) OVA-primed OT-I CD8+ T cells or naïve OT-I CD8+ T cells were co-cultured with OVA-primed IgG1+ B cell targets. CD8+ T cells and B cells were co-cultured at a 10:1 ratio for 4 hours and analyzed for cytotoxicity. Significant cytotoxicity of OVA-primed IgG1+ B cells was observed in co-cultures with OVA-primed OT-I CD8+ T cells (17±1.4%; n=6, p<0.001 signified by “*”) compared to negative control cultures with no CD8+ T cells (5.7±0.3%) or with naïve OT-I CD8+ T cells (6.3±0.3%). Error bars indicate standard error.
Primed OT-I CD8+ T cells (isolated on day 7 following mOVA lysate stimulation) were co-cultured with OVA-primed IgG1+ B cells and in vitro cytotoxicity was assayed. OVA-primed OT-I CD8+ T cells mediated significant cytotoxicity to OVA-primed IgG1+ B cells (17±1.4%; n=6) compared to negative control cultures with no CD8+ T cells (5.7±0.3%) or cultures with naïve OT-I CD8+ T cells (6.3±0.3%) (p<0.001 for both; Figure 5B). OVA-primed OT-I CD8+ T cells did not mediate cytotoxicity to FVB/N alloprimed IgG1+ B cells (5.1±0.6%, p=ns compared to controls). These results suggest that primed OT-I CD8+ T cells suppress OVA antibody production, in part, by mediating cytotoxic killing of OVA-primed IgG1+ B cells. Next, we investigated the expression of CXCR5 and CXCR3 on OVA-primed OT-I cells and the capacity of flow-sorted subsets to mediate suppression of OVA antibody production.
OVA-primed OT-I CXCR5+CD8+ T cells inhibit anti-OVA antibody production and reduce the quantity of GC B cells, and IL-21+CD4+ TFH cells
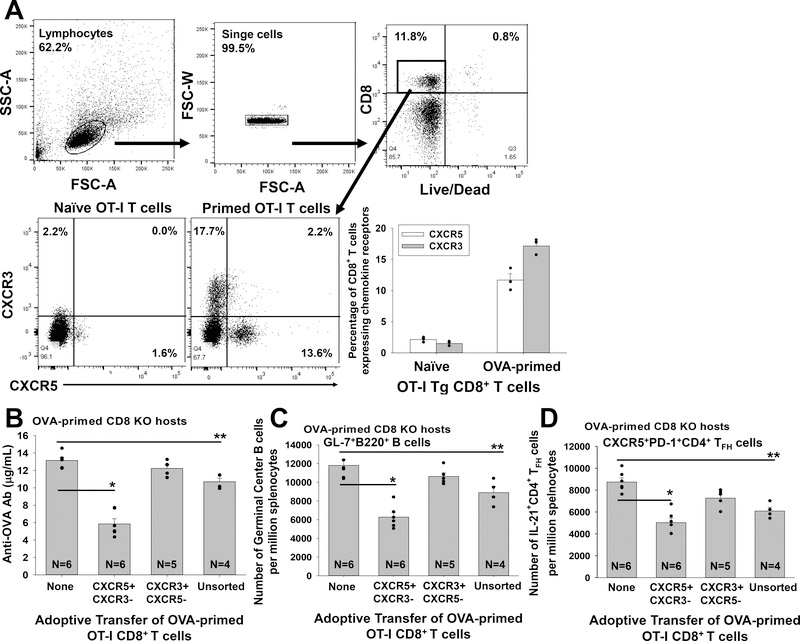
OT-I CD8+ T cells were adoptively transferred into OVA-primed CD8 KO hosts (mOVA lysate). OVA-primed OT-I cells were retrieved on day 7 post transfer and analyzed by flow cytometry for CXCR5 and CXCR3 chemokine receptor expression. OVA-primed OT-I CD8+ T cells upregulated expression of CXCR5 (11.7±1.0% of OT-I cells expressed CXCR5+CXCR3− phenotype) and CXCR3 (17.2±0.7% of OT-I cells expressed CXCR3+CXCR5− phenotype; Figure 6A). In order to further analyze the role of CXCR5 expression on antigen-specific antibody-suppressor CD8+ T cells, OVA-primed OT-I cells were flow-sorted for CXCR5+CXCR3−CD8+ or CXCR3+CXCR5−CD8+ subsets (day 7 post stimulation). These primed OT-I cell subsets (1×106) were adoptively transferred into OVA-primed CD8 KO hosts (day 5 post stimulation). Adoptive transfer of control unsorted, primed OT-I cells into OVA-primed CD8 KO hosts significantly inhibited anti-OVA antibody production (p=0.007). Similar to what was observed using polyclonal alloprimed T cells (Figure 2B), monoclonal OT-I CXCR5+CXCR3−CD8+ T cells downregulated anti-OVA antibody production (p<0.0001; tested on day 14) while OT-I CXCR3+CXCR5−CD8+ T cells did not (p=ns, Figure 6B). As a control, primed OT-I CD8+ T cells do not inhibit alloantibody production in hepatocyte transplant recipients (data not shown).
Figure 6. OVA-primed OT-I CXCR5+CD8+ T cells mediate suppression of anti-OVA antibody production accompanied by reduced quantity of Germinal Center B cells and IL-21+CD4+ TFH cells.
A) Naïve OT-I CD8+ T cells were adoptively transferred (AT) into OVA-primed CD8 KO recipients. 7 days later, OT-I CD8+ T cells were isolated and analyzed by flow cytometry for expression of CXCR5 and CXCR3. A representative flow plot shows OVA-primed OT-I CD8+ T cells include CXCR5+CXCR3− (11.7±1.0%) and CXCR3+CXCR5− (17.2±0.7%) OT-I CD8+ T cell subsets. B) Unsorted or flow-sorted CXCR5+CXCR3− or CXCR3+CXCR5− OVA-primed OT-I CD8+ T cells (1×106) were adoptively transferred into OVA-primed CD8 KO mice (day 5 post mOVA lysate stimulation). The amount of anti-OVA Ab produced in mice that received AT of CXCR5+CXCR3− OT-I CD8+ T cells (5.8±0.6 μg/mL; n=6, p<0.0001 signified by “*”) or unsorted OT-I CD8+ T cells (10.7±0.4 μg/mL, n=4, p=0.008 signified by “**”) was significantly less than the amount produced in CD8 KO recipients without AT of CD8+ T cells (13.4±0.3 μg/mL, n=6). AT of CXCR3+CXCR5− OT-I CD8+ T cells did not suppress anti-OVA antibody production (12.2±0.4 μg/mL; n=5, p=ns). C) The quantity of recipient spleen germinal center (GC) B cells (GL-7+B220+) and IL-21+CD4+ TFH cells (IL-21+CXCR5+PD-1+CD4+) was analyzed in OVA-primed CD8 KO mice (day 14 following mOVA lysate stimulation). The quantity of GC B cells in mice that received AT of CXCR5+CXCR3− OT-I CD8+ T cells (6,200±500 per million splenocytes; n=6, p<0.0001 signified by “*”) or with unsorted OT-I CD8+ T cells (8,900±700; n=4, p=0.008 signified by “**”) was significantly less than the quantity in CD8 KO recipients without AT (11,800±700 per million splenocytes; n=6). In contrast, AT of CXCR3+CXCR5− OT-I CD8+ T cells was not associated with reduction in the quantity of GC B cells (10,600±400 per million splenocytes; n=6, p=ns). D) The quantity of IL-21+ CD4+ TFH cells in mice that received AT of CXCR5+CXCR3− OT-I CD8+ T cells (5,000±400 per million splenocytes; n=6, p<0.0001 signified by “*”) or unsorted OT-I CD8+ T cells (6,100±300, n=4, p=0.001 signified by “**”) was significantly reduced compared to the quantity in CD8 KO recipients without AT (8,800±400 per million splenocytes; n=6). In contrast, AT with CXCR3+CXCR5− OT-I CD8+ T cells was not associated with a reduction in the quantity of IL-21+ CD4+ TFH cells (7,200±400 per million splenocytes; n=6, p=ns). Error bars indicate standard error from duplicate experiments.
When we analyzed the quantity of GC B cells and IL-21+CD4+ TFH cells on day 14 following mOVA lysate stimulation we found that adoptive transfer of primed OT-I CXCR5+CXCR3−CD8+ T cells significantly reduced the quantity of GC B cells (p<0.0001) and IL-21+CD4+ TFH cells (p<0.0001) detected post transplant while adoptive transfer of primed OT-I CXCR3+CXCR5−CD8+ T cells did not (p=ns for both; Figure 6C, D). Adoptive transfer of unsorted primed OT-I cells also inhibited the quantity of GC B cells and IL-21+CD4+ TFH cells (p<0.008 for both). Taken together, these results indicate that OVA-primed monoclonal OT-I CXCR5+CD8+ T cells and alloprimed polyclonal WT CXCR5+CD8+ T cells manifest similar phenotype and in vivo antibody-suppressor function.
Discussion
Our group is the first to report that humoral alloimmunity is CD8-regulated and that suppression of alloantibody production occurs, in part, by killing of alloprimed self IgG1+ B cells (MHC I-restricted alloantigen presentation) which is detected both in vitro and in vivo.18 The current study demonstrates that only antigen-primed CXCR5+CXCR3−CD8+ T cells manifest antibody-suppressor function. Furthermore, expression of CXCR5 is critical to the function of antibody-suppressor CD8+ T cells as alloprimed CXCR5 KO or flow-sorted WT CXCR5−CD8+ T cells do not downregulate alloantibody production. In previous studies we found that IFN-γ is also critical to antibody-suppressor CD8+ T cell function since adoptive transfer of CD8+ T cells from IFN-γ KO mice (unlike IL-4 KO and WT mice) did not suppress alloantibody production.17 Flow cytometric studies demonstrate that the majority (>70%) of alloprimed CD44+CXCR5+CD8+ T cells express IFN-γ (Figure S1). Based on these collective findings, antibody-suppressor CD8+ T cell phenotype can be refined to CXCR5+IFNγ+CD8+ T cells. Our data also show that alloantibody suppression by CXCR5+IFNγ+CD8+ TAb-supp cells is accompanied by a reduction in the number of GC B cells and cytokine-expressing CD4+ TFH cells. This supports the hypothesis that CXCR5+IFNγ+CD8+ TAb-supp cells have a functional niche in the germinal center. This hypothesis is strengthened by the detection of human CXCR5+CD8+ T cells in lymphoid tissue as in Chu et al.’s publication which reports that human CXCR5+CD8+ T cells are present in the tonsil germinal center (and correlates with decreased TFH and plasma cell differentiation in an in vitro co-culture).38 CD8+ TAb-supp cells only mediate suppression of humoral immunity and are distinguished from CD4+ T follicular regulatory (Tfr) cells which appear to both limit GC responses,39–41 and in other circumstances augment antibody responses.42,43 However, it is not understood what controls Tfr suppressor versus helper functions (reviewed in44). Furthermore, unlike CD4+ TFR cells, CD8+ TAb-supp cells do not express FoxP3 or PD-1 (Figure S1).
We observed a two- to three-fold reduction in antibody production by monoclonal and polyclonal CD8+ TAb-supp cells. It has been reported in human kidney transplant recipients that a two-fold reduction in donor specific antibody (by mean fluorescence intensity) correlates with a lower risk for developing AMR.45 In our murine studies, we show that CD8+ TAb-supp cell mediated suppression of alloantibody production is biologically significant since it is accompanied by prolongation of allograft survival. Furthermore, the capacity for CD8+ TAb-supp cells to downregulate a primed humoral response is indicated by the reduction of peak alloantibody production even after adoptive transfer on day 5 after transplant.
Comparison of the alloprimed CXCR5+IFNγ+CD8+ TAb-supp cells reported in the current study with regulatory CD8+ T cell (CD8+ Treg) subsets reported in the literature (reviewed in45) supports the conclusion that these cells are a unique subset of CD8+ T cells. CD8+ TAb-supp cells are IFN-γ-dependent; they do not express FoxP3, IL-10, CD103, ICOSL, or PD-1 (Figure S1) and are IL-15 and IL-10 independent (data not shown). Thus, CD8+ TAb-supp cells are distinguished from all reported CD8+ regulatory T cells including ICOSL+ IL-15-producing Qa-1-restricted CD8+ T cells which regulate autoreactive Qa-1+CD4+ T cells,46,47 IFN-γ-independent CD8+ Treg cells which suppress IgE in allergy models,48,49 and FoxP3+CD8+ Treg cells which inhibit proliferation50,51 or cytokine production52,53 of CD4+ T cells. They do not resemble CD8+ Treg cells that are reported to be IL-10-dependent54–57 or CD103+CD8+ Treg cells associated with spontaneous liver tolerance58; Treg cells express FoxP3 and TGF-β but not IFN-γ.58–61 Importantly, none of these other CD8+ T cell subsets have been reported to kill B cells or directly suppress B cell function.
Anti-viral CD8+ T cells that develop during acute infection do not express CXCR562,63 and instead require CXCR3 to recruit to the infection site.27–30 In chronic viral infections, “exhausted” CXCR5+CD8+ T cells have been detected in the T cell (not B cell) zones of lymph nodes, express high levels of the inhibitory molecule PD-1 and exhibit stem cell like properties proliferating and differentiating from CXCR5+CD8+ T cells into CXCR5−CD8+ T cells.64–67 However, in the current studies CD8+ TAb-supp cells develop in response to acute antigen stimulation and these cells do not express PD-1. Recently an IL-21+CXCR5+ICOS1+PD-1+ CD8+ T cell subset was reported to acquire CD4+ TFH functions and weakly enhance production of autoantibody; however these cells are not similar to CD8+ TAb-supp cells since they enhance rather than suppress antibody production and are only detected in mice with CD4+ Treg deficits (such as IL-2 KO and scurfy mice) and are not detected in WT mice.68 Thus the CXCR5+IFNγ+CD8+ TAb-supp cells reported in the current studies differ from other CXCR5+CD8+ T cell subsets by phenotype (PD-1−) and function (B cell killing and antibody suppression).69–71 Overall, our work to date indicates that CD8+ TAb-supp cells are Ag-specific,18 IFN-γ-dependent,17 self-MHC Class I-restricted,18 CXCR5+CXCR3−IFN-γ+ and are phenotypically and functionally distinguished from all other CD8+ T cell subsets reported in the literature.
While our published studies report the activity of this novel CD8+ TAb-supp cell subset after hepatocyte transplant,17,18,22 we have subsequently determined that their activity is also observed following islet and skin transplantation (not shown). These CD8+ TAb-supp cells are likely also important for regulation of humoral alloimmunity after kidney, heart, and aorta transplant since others have reported increased levels of alloantibodies in these recipients when they are CD8-deficient.72–74 Similarly, CD8-deficient mice exhibit increased antigen-specific antibody production in mouse models of allergy, bacterial infection, viral infection, and platelet transfusion.18,73–81 In the current studies we extended our investigations with polyclonal alloreactive CD8+ T cells to monoclonal OT-I TCR transgenic CD8+ T cells. Our results demonstrate that antigen-specific OT-I CXCR5+CD8+ T cells suppress humoral immunity and similar to studies with polyclonal alloprimed CD8+ TAb-supp cells this is accompanied by a reduction in GC B cells and CD4+ TFH cells. Thus, activity of CD8+ TAb-supp cells is not limited to alloimmune responses and likely these cells play a critical role in regulation of humoral immunity more broadly.
In conclusion, these studies for the first time clarify the critical role of CXCR5 and the distinct CXCR5+IFNγ+ (FoxP3−IL-10−CD103−ICOSL−PD-1−) phenotype of this novel CD8+ TAb-supp cell subset that mediates antigen-specific suppression of humoral immunity. We extend prior studies reporting that these cells mediate cytotoxic killing of self IgG1+ B cells18 by correlating the in vivo suppression of antibody production with a concomitant reduction in germinal center B cells and CD4+ TFH cells. Interestingly, we have reported that CD8+ T cell antibody-suppressor function is impaired by calcineurin inhibition but not mTOR inhibition22 both commonly used for maintenance immunosuppressive therapy in clinical transplantation. The implication is that suppression of de novo post transplant alloantibody production may be optimized with mTOR inhibition, in part, by preservation of antibody-suppressor CD8+ T cell function.
Supplementary Material
Acknowledgments
Support- This work was supported by a grant from the National Institutes of Health grants AI083456 (GLB), CA016058, the OSU Division of Transplant Surgery, and the OSU College of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations Page
- AT
adoptive transfer
- AMR
antibody-mediated rejection
- DSA
donor specific antibody
- hA1AT
human alpha-1 antitrypsin
- IFN
interferon
- IL
interleukin
- I.P.
intraperitoneal
- KO
knockout
- MHC
major histocompatibility complex
- MFI
mean fluorescence intensity
- MST
median survival time
- PI
propidium iodide
- TFH
T follicular helper
- Tg
transgenic
- WT
wild-type
Footnotes
Disclosures- The authors declare no conflicts of interest.
Reference
- 1.Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies. J Clin Invest. 2017;127(7):2492–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorns C, Nowak G, Nemeth A, et al. De Novo Donor-Specific HLA Antibody Formation in Two Patients With Crigler-Najjar Syndrome Type I Following Human Hepatocyte Transplantation With Partial Hepatectomy Preconditioning. Am J Transplant. 2016;16(3):1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks AM, Carter V, Liew A, et al. De Novo Donor-Specific HLA Antibodies Are Associated With Rapid Loss of Graft Function Following Islet Transplantation in Type 1 Diabetes. Am J Transplant. 2015;15(12):3239–3246. [DOI] [PubMed] [Google Scholar]
- 4.Heidt S, Roelen DL, Eijsink C, et al. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol. 2010;159(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard D, Gaillard C, Hermann P, et al. Role of CD40 antigen and interleukin-2 in T cell-dependent human B lymphocyte growth. Eur J Immunol. 1994;24(2):330–335. [DOI] [PubMed] [Google Scholar]
- 6.Steele DJ, Laufer TM, Smiley ST, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183(2):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela NM, Hickey MJ, Reed EF. Antibody Subclass Repertoire and Graft Outcome Following Solid Organ Transplantation. Front Immunol. 2016;7:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levisetti MG, Padrid PA, Szot GL, et al. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159(11):5187–5191. [PubMed] [Google Scholar]
- 9.Lin H, Bolling SF, Linsley PS, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178(5):1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science 1992;257(5071):789–792. [DOI] [PubMed] [Google Scholar]
- 11.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. [DOI] [PubMed] [Google Scholar]
- 12.Adams AB, Shirasugi N, Durham MM, et al. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes. 2002;51(2):265–270. [DOI] [PubMed] [Google Scholar]
- 13.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–453. [DOI] [PubMed] [Google Scholar]
- 14.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Andrews D, Colvin RB, et al. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. [DOI] [PubMed] [Google Scholar]
- 16.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535–546. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerer JM, Pham TA, Sanders VM, et al. CD8+ T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J Immunol. 2010;185(12):7285–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerer JM, Pham TA, Wright CL, et al. Alloprimed CD8(+) T cells regulate alloantibody and eliminate alloprimed B cells through perforin- and FasL-dependent mechanisms. Am J Transplant. 2014;14(2):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bumgardner GL, Heininger M, Li J, et al. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65(1):53–61. [DOI] [PubMed] [Google Scholar]
- 20.Bumgardner GL, Gao D, Li J, et al. Rejection responses to allogeneic hepatocytes by reconstituted SCID mice, CD4 KO, and CD8 KO mice. Transplantation. 2000;70(12):1771–1780. [DOI] [PubMed] [Google Scholar]
- 21.Bickerstaff A, Nozaki T, Wang JJ, et al. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008;8(3):557–566. [DOI] [PubMed] [Google Scholar]
- 22.Avila CL, Zimmerer JM, Elzein SM, et al. mTOR Inhibition Suppresses Posttransplant Alloantibody Production through Direct Inhibition of Alloprimed B Cells and Sparing of CD8+ antibody-suppressing T cells. Transplantation. 2016;100(9):1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tung JW, Heydari K, Tirouvanziam R, et al. Modern flow cytometry: a practical approach. Clin Lab Med. 2007;27(3):453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes NM, Allen CD, Lesley R, et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179(8):5099–5108. [DOI] [PubMed] [Google Scholar]
- 25.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192(11):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192(11):1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hokeness KL, Deweerd ES, Munks MW, et al. CXCR3-Dependent Recruitment of Antigen-Specific T Lymphocytes to the Liver during Murine Cytomegalovirus Infection. J Virol. 2007;81(3):1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadel SA, Bromley SK, Medoff BD,et al. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur J Immunol. 2008;38(12):3376–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Chan YK, Lu B, et al. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol. 2008;180(4):2641–2649. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi Y, Lu B, Gerard C, et al. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seung E, Cho JL, Sparwasser T, et al. Inhibiting CXCR3-dependent CD8+ T cell trafficking enhances tolerance induction in a mouse model of lung rejection. J Immunol. 2011;186(12):6830–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalasani G, Li Q, Konieczny BT, et al. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J Immunol. 2004;172(12):7813–7820. [DOI] [PubMed] [Google Scholar]
- 33.Schnickel GT, Hsieh GR, Garcia C, et al. Role of CXCR3 and CCR5 in allograft rejection. Transplant Proc. 2006;38(10):3221–3224. [DOI] [PubMed] [Google Scholar]
- 34.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192(10):1515–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horne PH, Zimmerer JM, Fisher MG, et al. Critical Role of Effector Macrophages in Mediating CD4-dependent Alloimmune Injury of Transplanted Liver Parenchymal Cells. J Immunol. 2008;181(2):1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerer JM, Liu XL, Blaszczak A, et al. Critical Role of Macrophage FcgammaR Signaling and Reactive Oxygen Species in Alloantibody-Mediated Hepatocyte Rejection. J Immunol. 2018;201(12):3731–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papa I, Vinuesa CG. Synaptic Interactions in Germinal Centers. Front Immunol. 2018;9:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu F, Neelapu SS. CXCR5+CD8+ T cells are localized in B cell follicles and germinal centers and exhibit regulatory and anti-tumor function. J Immunother Cancer. 2015;3(Suppl 2):P321. [Google Scholar]
- 39.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wollenberg I, Agua-Doce A, Hernández A, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187(9):4553–4560. [DOI] [PubMed] [Google Scholar]
- 42.Wu H, Chen Y, Liu H, et al. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol. 2016;46(5):1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laidlaw BJ, Lu Y, Amezquita RA, et al. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol. 2017;2(16):eaan4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie MM, Dent AL. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front Immunol. 2018;9:1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J. Am. Soc. Nephrol. 2010;21(8):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bézie S, Anegon I, Guillonneau C. Advances on CD8+ Treg Cells and Their Potential in Transplantation. Transplantation. 2018;102(9):1467–1478. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Verbinnen B, Tang X, et al. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998;160(2):566–571. [PubMed] [Google Scholar]
- 49.Thomas MJ, MacAry PA, Kemeny DM. CD8 T-lymphocyte-mediated regulation of ovalbumin-specific murine IgE responses. Int Arch Allergy Immunol. 1999;118(2–4):289–291. [DOI] [PubMed] [Google Scholar]
- 50.Thomas MJ, MacAry PA, Noble A, et al. T cytotoxic 1 and T cytotoxic 2 CD8 T cells both inhibit IgE responses. Int Arch Allergy Immunol. 2001;124(1–3):187–189. [DOI] [PubMed] [Google Scholar]
- 51.Jarvis LB, Matyszak MK, Duggleby RC, et al. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35(10):2896–2908. [DOI] [PubMed] [Google Scholar]
- 52.Xystrakis E, Dejean AS, Bernard I, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104(10):3294–3301. [DOI] [PubMed] [Google Scholar]
- 53.Rifa’i M, Shi Z, Zhang SY, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20(7):937–947. [DOI] [PubMed] [Google Scholar]
- 54.Lee YH, Ishida Y, Rifa’i M, et al. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180(2):825–832. [DOI] [PubMed] [Google Scholar]
- 55.Adams B, Dubois A, Delbauve S, et al. Expansion of regulatory CD8+ CD25+ T cells after neonatal alloimmunization. Clin Exp Immunol. 2011;163(3):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou Q, Wu B, Xue J, et al. CD8+ Treg cells suppress CD8+ T cell-responses by IL-10-dependent mechanism during H5N1 influenza virus infection. Eur J Immunol. 2014;44(1):103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endharti AT, Rifa’l M, Shi Z, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175(11):7093–7097. [DOI] [PubMed] [Google Scholar]
- 58.Liu Q, Zheng H, Chen X, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(−) regulatory T cells. Cell Mol Immunol. 2014;12(6):708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu L, Yu Y, Li G, et al. CD8(+)CD103(+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9(5):546–548. [DOI] [PubMed] [Google Scholar]
- 60.Ho J, Kurtz CC, Naganuma M, et al. A CD8+/CD103high T Cell Subset Regulates TNF-Mediated Chronic Murine Ileitis. J Immunol. 2008;180(4):2573–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beres AJ, Haribhai D, Chadwick AC, et al. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012;189(1):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lerret NM, Houlihan JL, Kheradmand T, et al. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant. 2012;12(9):2335–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Lemos C, Christensen JE, Nansen A, et al. Opposing effects of CXCR3 and CCR5 deficiency on CD8+ T cell-mediated inflammation in the central nervous system of virus-infected mice. J Immunol. 2005;175(3):1767–1775. [DOI] [PubMed] [Google Scholar]
- 64.Hoji A, Rinaldo CR Jr. Human CD8+ T cells specific for influenza A virus M1 display broad expression of maturation-associated phenotypic markers and chemokine receptors. Immunology. 2005;115(2):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mylvaganam GH, Rios D, Abdelaal HM, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci USA. 2017;114(8):1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He R, Hou S, Liu C, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537(7620):412–428. [DOI] [PubMed] [Google Scholar]
- 68.Leong YA, Chen Y, Ong HS, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17(10):1187–1196. [DOI] [PubMed] [Google Scholar]
- 69.Valentine KM, Davini D, Lawrence TJ, et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J Immunol. 2018;201(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quigley MF, Gonzalez VD, Granath A, et al. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. 2007;37(12):3352–3362. [DOI] [PubMed] [Google Scholar]
- 71.Jiang H, Li L, Han J, et al. CXCR5+ CD8+ T Cells Indirectly Offer B Cell Help and Are Inversely Correlated with Viral Load in Chronic Hepatitis B Infection. DNA Cell Biol. 2017;36(4):321–327. [DOI] [PubMed] [Google Scholar]
- 72.Kohei N, Tanaka T, Tanabe K, et al. Natural killer cells play a critical role in mediating inflammation and graft failure during antibody-mediated rejection of kidney allografts. Kidney Int. 2016;89(6):1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan SY, DeBruyne LA, Goodman RE, et al. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59(8):1155–1161. [PubMed] [Google Scholar]
- 74.Ensminger SM, Spriewald BM, Witzke O, et al. Intragraft interleukin-4 mRNA expression after short-term CD154 blockade may trigger delayed development of transplant arteriosclerosis in the absence of CD8+ T cells. Transplantation. 2000;70(6):955–963. [DOI] [PubMed] [Google Scholar]
- 75.Ensminger SM, Spriewald BM, Sorensen HV, et al. Critical role for IL-4 in the development of transplant arteriosclerosis in the absence of CD40-CD154 costimulation. J. Immunol. 2001;167(1):532–541. [DOI] [PubMed] [Google Scholar]
- 76.Horne PH, Lunsford KE, Eiring AM, et al. CD4+ T-cell-dependent immune damage of liver parenchymal cells is mediated by alloantibody. Transplantation. 2005;80(4):514–521. [DOI] [PubMed] [Google Scholar]
- 77.Coutelier JP. Enhancement of IgG production elicited in mice by treatment with anti-CD8 antibody. Eur J Immunol. 1991;21(10):2617–2620. [DOI] [PubMed] [Google Scholar]
- 78.Byrom B, Barbet AF, Obwolo M, et al. CD8(+) T cell knockout mice are less susceptible to Cowdria ruminantium infection than athymic, CD4(+) T cell knockout, and normal C57BL/6 mice. Vet Parasitol. 2000;93(2):159–172. [DOI] [PubMed] [Google Scholar]
- 79.Sayeh E, Sterling K, Speck E, et al. IgG antiplatelet immunity is dependent on an early innate natural killer cell-derived interferon-gamma response that is regulated by CD8+ T cells. Blood. 2004;103(7):2705–2709. [DOI] [PubMed] [Google Scholar]
- 80.Thomas MJ, Noble A, Sawicka E, et al. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J Immunol. 2002;168(1):216–223. [DOI] [PubMed] [Google Scholar]
- 81.Salagianni M, Wong KL, Thomas MJ, et al. An essential role for IL-18 in CD8 T cell-mediated suppression of IgE responses. J Immunol. 2007;178(8):4771–4778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.