Abstract
The Osteochondral Autograft Transfer System (OATS; Arthrex, Naples, FL) is an excellent option for the treatment of articular cartilage lesions within the knee. Current literature suggests that at early-term to midterm follow-up, patients experience improved function, alleviation of pain, and good satisfaction with acceptable complication rates. Although long-term data are lacking, studies in athletes have shown that the OATS can provide an adequate rate of return to sports. The OATS procedure has traditionally been considered an open procedure. However, with the advancement of arthroscopic techniques, the procedure can now be completed arthroscopically. We discuss this modern operation.
Articular cartilage is vital to the functionality of the knee and is a key area of study to enhance clinical outcomes. It primarily provides a smooth, low-friction surface for the transmission of forces through the joint.1 Hyaline cartilage, however, has a very limited capacity for healing because of its avascularity.1, 2, 3 Chondral defects are an extremely common musculoskeletal pathology, found in up to 60% of knees undergoing arthroscopy.4 Without treatment, these lesions can affect daily activities or sports participation and may lead to degenerative changes and premature osteoarthritis.5, 6, 7, 8 The Osteochondral Autograft Transfer System (OATS; Arthrex, Naples, FL) has been shown to be very effective in treating chondral lesions and achieving positive patient outcomes.2, 3, 5, 9, 10, 11, 12, 13 In addition, studies have suggested that the OATS procedure is superior to a microfracture technique in the treatment of such defects.5, 7, 14, 15
Several studies have reported clinical outcomes using the OATS with an open technique. In a study of 142 patients, Ollat et al.12 reported that this is a reliable technique that yields significantly improved functional scores, good patient satisfaction, and a complication rate of 13% at minimum 5-year follow-up. In a systematic review, Camp et al.3 similarly determined that the procedure alleviated pain, enhanced activity scores, and showed a high rate of survivorship of the transferred tissue with acceptable failure rates. In a study of 152 patients, Emre et al.5 showed excellent results in restoring joint function with no complications at a short-term follow-up of 18 months. Although the goal of the procedure is to delay the progression of degenerative changes, many patients also wish to return to sports. In a study of 13 competitive or well-trained athletes, Muller et al.2 found that 92% returned to sports at an intermediate to high level after 6 months, with excellent functional and clinical scores, no reported instability, no joint space narrowing, and an acceptable complication rate at a mean follow-up of 42 months.
Traditionally, the OATS procedure has been performed using an open technique. However, advances in arthroscopy have allowed this procedure to be performed through an arthroscopic approach, and this modern procedure is the focus of our discussion.
Indications
The indications for the procedure include the following: age younger than 50 years, body mass index lower than 35, previously unsuccessful conservative or surgical interventions, focal grade III to IV osteochondral defects of the femoral condyle diagnosed by magnetic resonance imaging (MRI) (Fig 1) or arthroscopy, normal or correctable alignment, normal or correctable ligamentous stability, normal or correctable meniscal integrity, willingness to comply with the rehabilitation protocol, and realistic postsurgical expectations.3, 5, 6, 7, 9, 13, 15, 16 Although more controversial, the contraindications include obesity, generalized osteoarthritis, osteonecrosis, active infection or bone cancer, and lack of corresponding symptoms.7, 9, 10, 13, 16
Fig 1.
Magnetic resonance imaging of the left knee. (A) T1-weighted image in the coronal plane showing evidence of an osteochondral defect (OCD) along the left medial femoral condyle. T2-weighted image in the coronal plane (B), T1-weighted image in the sagittal plane (C), and T2-weighted image in the sagittal plane (D) showing the same OCD along the left medial femoral condyle.
Surgical Technique
The patient is placed in the supine position on a standard operating bed with a lateral post positioned 1 handbreadth above the superior pole of the patella (Video 1). A tourniquet is then placed on the proximal thigh but left uninflated during the case; it is only used in the rare case of poor visualization related to bleeding. After the induction of general anesthesia, a standard vertical portal incision is made at the intersection of a line transecting the inferior pole of the patella with the lateral facet of the patella (Fig 2). A superolateral outflow portal is then made as part of our standard arthroscopic technique. A 4-mm arthroscope (Synergy; Arthrex) is introduced, standard diagnostic arthroscopy is performed, and any concurrent pathology is treated appropriately. A non-aggressive shaver (Torpedo; Arthrex) and a thermal device (CoolCut; Arthrex) are used to resect a portion of the fat pad to aid in visualization of the cartilage lesion and the planned harvest site. In our demonstration, this is performed in the region of the lateral aspect of the medial femoral condyle at the planned repair site and in the region of the sulcus terminalis, where graft harvest will occur (Fig 3).
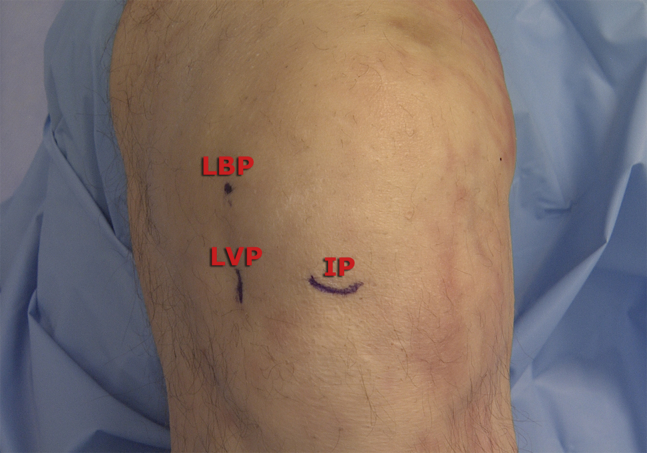
Fig 2.
Photograph of the left knee flexed at 90° showing the lateral viewing portal (LVP), which is made at the intersection of the inferior pole (IP) of the patella with the lateral border of the patella (LBP).
Fig 3.
Arthroscopic image through the lateral viewing portal of the left medial femoral condyle showing the osteochondral defect (OCD).
We prefer to use the Arthrex single-use OATS set for articular cartilage transfer. The cartilage defect is assessed via preoperative MRI, with the size and depth of the defect measured to assist in operative planning. After appropriate visualization of the lesion is obtained, a calibrated probe (3.4-mm hook probe with markings; Arthrex) is useful to confirm the defect size, as well as to assess the cartilage defect for stability of the area surrounding the visible lesion. After an assessment of the defect is performed, a longitudinal incision is made for the accessory anteromedial portal along the border of the patellar tendon to remain as perpendicular to the osteochondral lesion as possible. Options for graft harvest are 6, 8, and 10 mm, so multiple osteochondral plugs may be warranted for cartilage restoration in larger defects. A cannulated guide (Arthrex) is placed on the cartilage surface at the site of the cartilage lesion, and the amount of knee flexion is adjusted to ensure that the guide is placed perpendicularly to the planned recipient site. The guide pin for the OATS is then advanced to a depth of approximately 10 to 12 mm, followed by removal of the cannulated guide. An appropriately sized cannulated reamer is placed over the pin and advanced to the depth of the cystic change on MRI, usually 8 to 10 mm (Fig 4). The central pin is then removed, and an arthroscopic shaver is reintroduced to remove any remaining bony debris and free edge cartilage. Next, a cannulated dilator from the OATS kit (Arthrex) is gently inserted into place with a mallet to obtain depth measurements at the 12-, 3-, 6-, and 9-o’clock positions (Fig 5). If we determine through this process that our positioning is slightly non-perpendicular to the cartilage surface (e.g. one side measures 9 mm whereas another side measures 10 mm), then we will plan to have the donor-site harvest match this same angulation difference.
Fig 4.
Arthroscopic image through the lateral viewing portal showing where a cannulated reamer is used to core out the site of the osteochondral defect on the left medial femoral condyle to prepare the recipient site (RS).
Fig 5.
Arthroscopic image through the lateral viewing portal showing a measuring guide placed perpendicularly to the articular surface and the depth of the recipient site being measured at the 3-, 6-, 9-, and 12-o’clock positions of the left medial femoral condyle (MFC).
At this point, our attention is turned to obtaining the graft. Our usual graft harvest location is slightly anterior to the sulcus terminalis at the junction of the lateral trochlea and lateral femoral condyle (in the non–weight-bearing zone), although an area superior and lateral to the intercondylar notch may be used as an alternative harvest location (Fig 6). On identification of the sulcus terminalis, the anterolateral portal incision is extended longitudinally to a length of approximately 2 cm in preparation for graft harvest. An appropriately sized harvester (Donor Harvester; Arthrex) is then placed on the planned harvest site, positioned perpendicularly to the donor surface or matching the slight angulation of the recipient site as necessary (Fig 7). This is performed with the collared pin slightly prominent from the leading edge of the harvester in an effort to protect the chondral surface. On proper placement, the screw-in core extruder knob is removed, allowing the harvester to seat into the cartilage surface. It is then impacted to a depth of approximately 10 mm. On completion of impaction of the harvester, the T-handle is rotated firmly 90° clockwise and then counterclockwise to fracture the donor graft from the deep bone. An axial load is placed during these turns to assist in graft removal. Ultimately, a click will be heard when the graft bone is fractured; if necessary, another axial load may be performed on the graft if no click is heard during planned extraction. Depending on the size of each plug, one can usually harvest up to 3 grafts from the sulcus terminalis if needed.
Fig 6.
Arthroscopic image through the lateral viewing portal showing an arthroscopic probe identifying the sulcus terminalis (ST) for donor-site harvest from the left knee.
Fig 7.
Arthroscopic image through the lateral viewing portal showing the Osteochondral Autograft Transfer System graft harvester (Arthrex) on the left knee at the anterior border of the sulcus terminalis (ST) and photograph showing the graft harvester being impacted using a mallet to a depth of approximately 10 mm. Care is taken to remain perpendicular to the articular surface when obtaining graft from the donor site.
After the graft is harvested, it is inspected as to the exact length at each of the 4 quadrants and a rongeur is used to shape the bone graft end pertaining to graft length. The bone edges are slightly chamfered to allow for easier placement. The graft is ultimately rongeured to a depth 1 mm shorter than the prepared recipient site to ensure a flush surface, avoiding proud edges (Table 1). In addition, if slight angulation of the graft is observed, then during placement, the graft is oriented to match the angulation of the recipient site (Fig 8).
Table 1.
Pearls and Pitfalls
| Pearls |
| If the recipient site is not prepared perpendicularly to the cartilage surface, match the same angulation difference when harvesting the graft from the donor site. |
| Rongeur the graft to a depth 1 mm shorter than the prepared recipient site to ensure a flush surface. |
| When seating the graft, ensure proper orientation to match any angulation. |
| When obtaining donor graft, perform one-quarter turns to help ensure smooth removal of the graft from the donor site. |
| Pitfalls |
| Flex the knee appropriately to achieve perpendicularity and avoid angulation of the recipient or donor site. |
| When selecting the donor site, identify the sulcus terminalis to avoid graft harvest from a weight-bearing zone. |
Fig 8.
Osteochondral Autograft Transfer System donor plug from left sulcus terminalis. One should note the contoured appearance of the donor plug after it is rongeured to a depth 1 mm shorter than the prepared recipient site to ensure a flush finish.
The cartilage side of the graft is placed inside the plastic delivery tube and reintroduced into the joint, perpendicular to the recipient site, and a bone tamp is used to gently tap the graft into place. This is performed while ensuring that the graft is seated in the appropriate orientation, until there is a flush surface with surrounding host cartilage (Figs 9 and 10). Finally, a backfill plug 1 mm larger than the harvested diameter is cut to the appropriate length and placed into the graft harvest site, tamping to a flush surface; alternatively, it can be left unfilled. Final arthroscopic images are obtained, and portal-site closure is performed. A local anesthetic is injected around the incision sites, and the area is covered with a soft dressing, followed by ice application to the knee postoperatively, as is our standard regimen.
Fig 9.
Arthroscopic image through the lateral viewing portal showing the arthroscopic impactor being used to advance the donor graft (DG) into the recipient site on the left medial femoral condyle (MFC). One should note that overlap occurs between the impactor and the recipient site to prevent countersinking of the donor graft.
Fig 10.
Arthroscopic image through the lateral viewing portal showing the final result after impaction of the donor graft (DG) into the ipsilateral recipient site on the left medial femoral condyle. The well-contoured, flush appearance of the articular surface should be noted.
Rehabilitation
On completion of the procedure, immediate goals of healing include protection of the tissue from load and shear forces to allow for graft incorporation. Phase 1 (weeks 0-6 postoperatively) is aimed at reduction of pain and knee effusion with restoration of full passive extension, regaining quadriceps control, and gradual improvement of knee flexion. The affected extremity remains non–weight bearing and patients sleep in a locked brace for 2 to 4 weeks. Partial weight bearing begins after 2 to 4 weeks with the brace locked in full extension. Continuous passive motion is used during this phase to assist with return of full range of motion.
Phase 2 (weeks 6-12) is geared toward functional activity improvement. Bracing is discontinued at postoperative week 6, and the patient is allowed 25% to 50% weight bearing using crutches. Full weight bearing with discontinuation of crutches is typically expected at weeks 10 to 12, with progression of standing and walking as tolerated.
Phase 3 (weeks 12-26) is the maturation phase and involves progression of muscular strength and endurance. Full range of motion should be regained prior to this phase, and exercises include squats, step ups, lunges, bicycling, and swimming, with a maintenance program typically initiated at weeks 16 to 20.
Phase 4 (weeks 26-52) is the final recovery phase, when patients typically return to full functional and sporting activities. Skating, rollerblading, and cycling are permitted between 6 and 8 months postoperatively. Jogging, running, and aerobics may be performed at 8 to 10 months, and high-impact sports such as tennis, basketball, and baseball are allowed at 12 to 18 months.
Discussion
In our experience, the described procedure offers a dependable technique for treating osteochondral defects of the knee. The steps can be applied to a lesion anywhere on the femoral condyle through careful preparation of the recipient site. This method uses an arthroscopic approach. In addition to being a quicker, less invasive procedure, arthroscopy has been shown in cadaveric models to be identical to, if not better than, the open approach in the precision and accuracy of graft harvesting, site preparation and perpendicularity, plug placement, and overall visualization17, 18 (Table 2). Although more clinical research into this unique approach is needed, our experience with the arthroscopic OATS technique is promising for achieving excellent patient outcomes. This method also uses an autologous graft harvested from a non–weight-bearing compartment of the joint. Several studies have argued that using an allograft eliminates the possibility of donor-site morbidity while generating rates of graft survival and return to sports similar to those with autografts3, 19, 20, 21 (Table 2). However, the limited time frame of allograft viability, large expense of the allograft, and possibility of immunologic reactions suggest that autografting is a more reliable method.3
Table 2.
Advantages and Disadvantages
| Advantages |
| Less invasive than open procedure |
| Less blood loss and shorter operative time than open procedure |
| Ability to treat larger osteochondral defects through harvest of multiple autografts |
| Disadvantages |
| Possible decreased visualization of donor and recipient sites owing to improper portal-site placement |
| Possible donor-site morbidity owing to improper graft harvest location |
A limitation of our technique relates to the size of the defect being treated (Table 3). Most studies using the OATS method have treated defects measuring 3 cm2 or less in diameter and recommended that lesions of 4 to 6 cm2 be the upper limit of eligibility.2, 5, 7, 12, 13 This is said to be because of the limited size of available donor sites. In fact, increasing lesion size, along with increasing patient age and concomitant intra-articular injuries, has been proposed as a negative prognostic indicator.5 However, a study by Baltzer et al.9 concluded that the overall defect surface area was not associated with poor outcomes and that these lesions can be treated with additional transplanted grafts. We argue that, through the use of multiple donor grafts, larger defects can be included for treatment. The risks of the procedure include postoperative hemarthrosis, deep venous thrombosis, donor-site morbidity, graft instability, graft fracture, arthrofibrosis, infection, and chronic pain.
Table 3.
Limitations
| Treatment of larger osteochondral defects |
| Treatment of generalized osteoarthritis and osteonecrosis |
| Patients with abnormal alignment, ligamentous instability, or lack of meniscal integrity |
The prevalence of chondral cartilage defects is becoming increasingly concerning because of their potential to alter daily activities, limit sports participation, and progress to degeneration. The described arthroscopic procedure offers a reliable and reproducible method of filling articular cartilage lesions within the knee.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: D.J.W. is a paid consultant for Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Osteochondral Autograft Transfer System (OATS) procedure through an arthroscopic technique. The osteochondral defect in the left knee is located on the lateral aspect of the medial femoral condyle, and the region of the sulcus terminalis is the harvest site. The patient is positioned supine on a standard operating bed with a lateral post positioned 1 handbreadth above the superior pole of the patella. Arthroscopic portals are made in the following positions: anterolaterally at the intersection of a line transecting the inferior pole of the patella with the lateral facet of the patella, superolaterally to allow for outflow, and anteromedially along the border of the patellar tendon to remain as perpendicular to the osteochondral lesion as possible. Care is taken to place the guide perpendicularly to the planned recipient site before the guide pin for the OATS is advanced to a depth of approximately 10 to 12 mm. A cannulated dilator from the OATS kit is gently inserted into place with a mallet to obtain depth measurements around the circumference of the site to determine the exact depth of the recipient site to prepare for graft acquisition from the donor site. After the graft is harvested and inspected, the cartilage side of the graft is prepared and introduced at the recipient site and a bone tamp is used to place the graft, with care taken to place the graft with the appropriate orientation until there is a flush surface with the surrounding host cartilage; the portal sites are then closed.
References
- 1.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller S., Breederveld R.S., Tuinebreijer W.E. Results of osteochondral autologous transplantation in the knee. Open Orthop J. 2010;4:111–114. doi: 10.2174/1874325001004020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camp C.L., Stuart M.J., Krych A.J. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6:265–273. doi: 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widuchowski W., Widuchowski J., Trzaska T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee. 2007;14:177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Emre T.Y., Ege T., Kose O., Tekdos Demircioglu D., Seyhan B., Uzun M. Factors affecting the outcome of osteochondral autografting (mosaicplasty) in articular cartilage defects of the knee joint: Retrospective analysis of 152 cases. Arch Orthop Trauma Surg. 2013;133:531–536. doi: 10.1007/s00402-013-1680-2. [DOI] [PubMed] [Google Scholar]
- 6.Gracitelli G.C., Meric G., Briggs D.T. Fresh osteochondral allografts in the knee: Comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43:885–891. doi: 10.1177/0363546514565770. [DOI] [PubMed] [Google Scholar]
- 7.Krych A.J., Harnly H.W., Rodeo S.A., Williams R.J., III Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: A retrospective comparative study. J Bone Joint Surg Am. 2012;94:971–978. doi: 10.2106/JBJS.K.00815. [DOI] [PubMed] [Google Scholar]
- 8.Minzlaff P., Feucht M.J., Saier T. Can young and active patients participate in sports after osteochondral autologous transfer combined with valgus high tibial osteotomy? Knee Surg Sports Traumatol Arthrosc. 2016;24:1594–1600. doi: 10.1007/s00167-014-3447-x. [DOI] [PubMed] [Google Scholar]
- 9.Baltzer A.W., Ostapczuk M.S., Terheiden H.P., Merk H.R. Good short- to medium-term results after osteochondral autograft transplantation (OAT) in middle-aged patients with focal, non-traumatic osteochondral lesions of the knee. Orthop Traumatol Surg Res. 2016;102:879–884. doi: 10.1016/j.otsr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Cognault J., Seurat O., Chaussard C., Ionescu S., Saragaglia D. Return to sports after autogenous osteochondral mosaicplasty of the femoral condyles: 25 cases at a mean follow-up of 9 years. Orthop Traumatol Surg Res. 2015;101:313–317. doi: 10.1016/j.otsr.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Filardo G., Kon E., Perdisa F., Balboni F., Marcacci M. Autologous osteochondral transplantation for the treatment of knee lesions: Results and limitations at two years' follow-up. Int Orthop. 2014;38:1905–1912. doi: 10.1007/s00264-014-2322-1. [DOI] [PubMed] [Google Scholar]
- 12.Ollat D., Lebel B., Thaunat M. Mosaic osteochondral transplantations in the knee joint, midterm results of the SFA multicenter study. Orthop Traumatol Surg Res. 2011;97(suppl):S160–S166. doi: 10.1016/j.otsr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Zak L., Krusche-Mandl I., Aldrian S., Trattnig S., Marlovits S. Clinical and MRI evaluation of medium- to long-term results after autologous osteochondral transplantation (OCT) in the knee joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:1288–1297. doi: 10.1007/s00167-014-2834-7. [DOI] [PubMed] [Google Scholar]
- 14.Gudas R., Gudaite A., Pocius A. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40:2499–2508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 15.Gudas R., Kalesinskas R.J., Kimtys V. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066–1075. doi: 10.1016/j.arthro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Wang D., Eliasberg C.D., Wang T. Similar outcomes after osteochondral allograft transplantation in anterior cruciate ligament-intact and -reconstructed knees: A comparative matched-group analysis with minimum 2-year follow-up. Arthroscopy. 2017;33:2198–2207. doi: 10.1016/j.arthro.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Epstein D.M., Choung E., Ashraf I. Comparison of mini-open versus arthroscopic harvesting of osteochondral autografts in the knee: A cadaveric study. Arthroscopy. 2012;28:1867–1872. doi: 10.1016/j.arthro.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Koulalis D., Stavropoulos N.A., Citak M. Open versus arthroscopic mosaicplasty of the knee: A cadaveric assessment of accuracy of graft placement using navigation. Arthroscopy. 2015;31:1772–1776. doi: 10.1016/j.arthro.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Bugbee W., Cavallo M., Giannini S. Osteochondral allograft transplantation in the knee. J Knee Surg. 2012;25:109–116. doi: 10.1055/s-0032-1313743. [DOI] [PubMed] [Google Scholar]
- 20.McCarty E.C., Fader R.R., Mitchell J.J., Glenn R.E., Jr., Potter H.G., Spindler K.P. Fresh osteochondral allograft versus autograft: Twelve-month results in isolated canine knee defects. Am J Sports Med. 2016;44:2354–2365. doi: 10.1177/0363546516648700. [DOI] [PubMed] [Google Scholar]
- 21.Murphy R.T., Pennock A.T., Bugbee W.D. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42:635–640. doi: 10.1177/0363546513516747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Osteochondral Autograft Transfer System (OATS) procedure through an arthroscopic technique. The osteochondral defect in the left knee is located on the lateral aspect of the medial femoral condyle, and the region of the sulcus terminalis is the harvest site. The patient is positioned supine on a standard operating bed with a lateral post positioned 1 handbreadth above the superior pole of the patella. Arthroscopic portals are made in the following positions: anterolaterally at the intersection of a line transecting the inferior pole of the patella with the lateral facet of the patella, superolaterally to allow for outflow, and anteromedially along the border of the patellar tendon to remain as perpendicular to the osteochondral lesion as possible. Care is taken to place the guide perpendicularly to the planned recipient site before the guide pin for the OATS is advanced to a depth of approximately 10 to 12 mm. A cannulated dilator from the OATS kit is gently inserted into place with a mallet to obtain depth measurements around the circumference of the site to determine the exact depth of the recipient site to prepare for graft acquisition from the donor site. After the graft is harvested and inspected, the cartilage side of the graft is prepared and introduced at the recipient site and a bone tamp is used to place the graft, with care taken to place the graft with the appropriate orientation until there is a flush surface with the surrounding host cartilage; the portal sites are then closed.