Key Points
Question
Would extending statin therapy to patients with borderline 10-year absolute risk of atherosclerotic cardiovascular disease (5.0%-7.4%) and high levels of low-density lipoprotein cholesterol be cost-effective?
Findings
This microsimulation cohort study of 100 cohorts, each including 1 million hypothetical patients, found that adding treatment of patients with borderline risk and low-density lipoprotein cholesterol levels of 160 to 189 mg/dL to standard care would be cost-saving; treating patients at borderline risk with low-density lipoprotein cholesterol levels of 130 to 159 mg/dL would be cost-saving; and treating all patients at borderline risk, regardless of low-density lipoprotein cholesterol level, would be highly cost-effective.
Meaning
Results suggest that statin therapy for patients at borderline risk of ASCVD and with a low-density lipoprotein cholesterol level of 160 mg/dL or more could yield lifetime health benefits similar to those achieved by treating higher-risk patients with lower low-density lipoprotein cholesterol levels and could save health care costs.
This simulation cohort study estimates the cost-effectiveness of adding preventive statin treatment to standard care of patients at borderline risk of atherosclerotic cardiovascular disease with high levels of low-density lipoprotein cholesterol and the cost-effectiveness of statin treatment across population strata.
Abstract
Importance
American College of Cardiology/American Heart Association cholesterol guidelines prioritize primary prevention statin therapy based on 10-year absolute risk (AR10) of atherosclerotic cardiovascular disease (ASCVD). However, given the same AR10, patients with higher levels of low-density lipoprotein cholesterol (LDL-C) experience greater absolute risk reduction from statin therapy.
Objectives
To estimate the cost-effectiveness of expanding preventive statin treatment eligibility from standard care to patients at borderline risk (AR10, 5.0%-7.4%) for ASCVD and with high levels of LDL-C and to estimate cost-effectiveness of statin treatment across ranges of age, sex, AR10, and LDL-C levels.
Design, Setting, and Participants
This study evaluated 100 simulated cohorts, each including 1 million ASCVD-free survey respondents (50% men and 50% women) aged 40 years at baseline. Cohorts were created by probabilistic sampling of the 1999-2014 US National Health and Nutrition Examination Surveys from the perspective of the US health care sector. The CVD Policy Model microsimulation version projected lifetime health and cost outcomes. Probability of first-ever coronary heart disease or stroke event was estimated by analysis of 6 pooled US cohort studies and recalibrated to match contemporary event rates. Other model variables were derived from national surveys, meta-analyses, and published literature. Data were analyzed from May 15, 2018, through June 10, 2019.
Exposures
Four statin treatment strategies were compared: (1) treat all patients with AR10 of at least 7.5%, diabetes, or LDL-C of at least 190 mg/dL (standard care); (2) add treatment for borderline risk and LDL-C levels of 160 to 189 mg/dL; (3) add treatment for borderline risk and LDL-C levels of 130 to 159 mg/dL; and (4) add treatment for remainder of patients with AR10 of at least 5.0%. Statin treatment was also compared with no statin treatment in age, sex, AR10, and LDL-C strata.
Main Outcomes and Measures
Lifetime quality-adjusted life-years (QALYs) and costs (2019 US dollars) were projected and discounted 3.0% annually. The primary outcome was the incremental cost-effectiveness ratio.
Results
In these 100 simulated cohorts, each with 1 million patients aged 40 years at baseline (50% women and 50% men), adding preventive statins to individuals with borderline AR10 and LDL-C levels of 160 to 189 mg/dL would be cost-saving; further treating borderline AR10 and LDL-C levels of 130 to 159 mg/dL would also be cost-saving; and treating all individuals with AR10 of at least 5.0% would be highly cost-effective ($33 558/QALY) and would prevent the most ASCVD events. Within age, AR10, and sex categories, individuals with higher baseline LDL-C levels gained more QALYs from statin therapy. Cost-effectiveness increased with LDL-C level and AR10.
Conclusions and Relevance
In this study, lifetime statin treatment of patients in a hypothetical cohort with borderline ASCVD risk and LDL-C levels of 160 to 189 mg/dL was found to be cost-saving. Results suggest that treating all patients at borderline risk regardless of LDL-C level would likely be highly cost-effective.
Introduction
Hydroxymethylglutaryl–coenzyme A reductase inhibitors (statins) reduce low-density lipoprotein cholesterol (LDL-C) levels and are a cornerstone of primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD).1,2,3,4,5 Recent cholesterol treatment guidelines recommended selecting patients for statin treatment based on predicted 10-year absolute risk (AR10) of ASCVD.6,7 In addition to recommending primary prevention statin treatment for adults with diabetes and LDL-C levels of at least 190 mg/dL (to convert to millimoles per liter, multiply by 0.0259), the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommended statins in adults with AR10 of at least 7.5%.6 A 2018 ACC/AHA update suggested statins be considered for primary prevention in patients without diabetes and with borderline AR10 (5.0%-7.4%) if the patients have risk-enhancing factors.2 Among those risk-enhancing factors is an LDL-C level of at least 160 mg/dL.
A purely AR10-based approach counts on a heterogeneous group of patients with high AR10 gaining equal benefits from statin therapy. However, statin treatment trials consistently found (1) an approximately linear association between the degree of lowering of LDL-C level and ASCVD risk reduction and (2) absolute reduction of LDL-C levels directly proportional to pretreatment LDL-C levels.8,9 Therefore, statin benefit is a function of AR10 and baseline LDL-C level.10 Absolute statin benefit (absolute ASCVD risk reduction) may be equivalent between individuals with low AR10 and high baseline LDL-C level and those with high AR10 and low baseline LDL-C level.11,12,13
The primary objective of this study was to assess the lifetime costs, health benefits, and cost-effectiveness of integrating AR10 and LDL-C level into decision-making for statin treatment. A population-level analysis estimated statin cost-effectiveness in patients with borderline AR10 and high LDL-C levels (160-189 mg/dL or 130-159 mg/dL). Stratified analyses compared statin therapy with no treatment across age, sex, AR10, and LDL-C level strata.
Methods
Computer Simulation Model
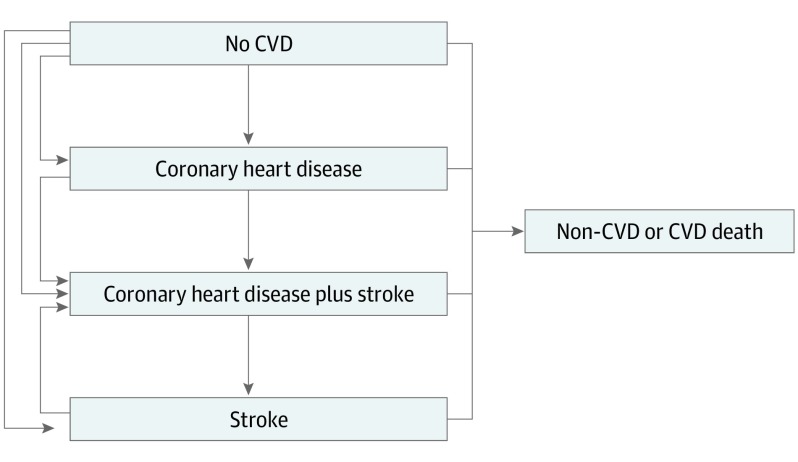
This study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline. All data analyzed were deidentified data from studies that completed data collection in the past. This analysis was reviewed and approved by the Columbia University Medical Center institutional review board. The Cardiovascular Disease (CVD) Policy Model evaluated statin treatment cost-effectiveness in prior analyses.14,15,16,17 For this analysis, a microsimulation version of the CVD Policy Model was developed in TreeAge Pro, version 2018 (TreeAge, Inc). The model estimates lifetime individual-level survival, health-related quality of life, and costs of different statin treatment strategies (eMethods, eTables 1-6 in the Supplement). All individuals started simulations without ASCVD and each year were at risk of coronary heart disease, stroke, combined coronary heart disease and stroke, or death (Figure 1). Annual ASCVD event risk was estimated using time-varying risk factor exposures (ie, age, sex, race, body mass index, systolic blood pressure, LDL-C level, high-density lipoprotein cholesterol level, smoking, and diabetes) and accounted for competing non-ASCVD mortality risk (eFigure 1 in the Supplement).
Figure 1. Cardiovascular Disease (CVD) Policy Model Microsimulation Version Structure With Annual Health-State Transitions.
Annual ASCVD event and non-ASCVD mortality probabilities were estimated using Cox proportional hazards regression functions in the Columbia University National Heart, Lung, and Blood Institute Pooled Cohorts data set (eTable 1 in the Supplement).18 The CVD Policy Model microsimulation version was recalibrated to match contemporary ASCVD and all-cause mortality incidence rates from the previously validated, Fortran-based, population simulation version of the CVD Policy Model that shares the same input data (eFigure 2 in the Supplement).19 Estimated ASCVD event rates were validated by comparing simulation output to cumulative survival curves derived from US life tables and CVD incidence rates from the Columbia University National Heart, Lung, and Blood Institute Pooled Cohorts project (eFigure 2 in the Supplement). The ASCVD event case-fatality rates were derived from Fortran model inputs and adjusted to reflect contemporaneous US data.20
Simulation Cohort and Risk Factor Exposures
We simulated 100 cohorts of 1 million US adults aged 40 years at baseline by sampling, with replacement, individuals from a pooled data set of 1999-2014 National Health and Nutrition Examination Surveys.21 Each cohort consisted of 500 000 men and 500 000 women, which were simulated independently. For each of the 100 simulated cohorts, model input distributions (Table 1) were probabilistically sampled. Mean health and cost outcomes were calculated across these 100 simulations. Lifetime exposure trajectories for LDL-C levels and other risk factors were assigned from ages 40 to 89 years to National Health and Nutrition Examination Surveys participants by randomly matching, with replacement, 1:1 to Columbia University National Heart, Lung, and Blood Institute Pooled Cohorts project participants (eMethods, eTable 2, and eFigure 3 in the Supplement).22
Table 1. Statin Treatment Simulation Parameters.
| Variable | Base Case | Distribution for PSA | Lower Bound | Upper Bound | Source |
|---|---|---|---|---|---|
| Reduction in baseline LDL-C level with statin, % | |||||
| Moderate intensity | 29.0 | Beta | 14.0 | 38.0 | Baigent et al,23 2010 |
| High intensity | 43.0 | Beta | 39.0 | 46.0 | Baigent et al,23 2010 |
| Relative risk per 1 mmol/L (38.67 mg/dL) in LDL-C level reduction | |||||
| CHD | 0.76 | Beta | 0.73 | 0.79 | Mihaylova et al,8 2012 |
| Stroke | 0.85 | Beta | 0.80 | 0.89 | Mihaylova et al,8 2012 |
| Statin-induced type 2 diabetes, absolute incidence increase, % | 0.50 | Log normal | 0.00 | 1.00 | Sattar et al,24 2010 |
| Pill-taking disutility (reduction in QALY)a | 0.002 | Beta | 0.000 | 0.004 | Pandya et al,25 2015 |
| Treatment adherence, % | |||||
| Year 1 | 67.0 | Beta | 50.0 | 84.0 | Greving et al,32 2011 |
| Year 2 | 53.0 | Beta | 40.0 | 66.0 | Greving et al,32 2011 |
| Subsequent years | 50.0 | Beta | 38.0 | 63.0 | Greving et al,32 2011 |
| Statin costs, 2019 US $ | |||||
| Moderate intensity | 127.6 | Gamma | 77.4 | 436.2 | Medical Expenditure Panel Survey 201533 |
| High intensity | 163.5 | Gamma | 100.5 | 2408.8 | Medical Expenditure Panel Survey 201533 |
| Screening and treatment visit costs, 2019 US $ | |||||
| Checkup visit during treatment | 77.6 | Gamma | 58.2 | 97.0 | Centers for Medicare & Medicaid Services,40 2018 |
| Screening visit during treatment | 77.6 | Gamma | 58.2 | 97.0 | Centers for Medicare & Medicaid Services,40 2018 |
| Other costs, 2019 US $ | |||||
| Lipid panel test | 23.9 | Gamma | 17.9 | 29.9 | Heller et al,14 2017 |
| Liver panel test | 1.5 | Gamma | 1.1 | 1.8 | Heller et al,14 2017 |
| Weighted statin-induced diabetes cost | 9.8 | Gamma | 7.3 | 12.2 | Heller et al,14 2017 |
| Office visit frequency | |||||
| Time between screening visits, y | 5.0 | NAb | NAb | NAb | Grundy et al,2 2018 |
| Time between primary care checkups during treatment, y | 1.25 | Gamma | 0.0 | 3.0 | Grundy et al,2 2018 |
| Discount rates, % | |||||
| Health benefits | 3.0 | NAb | 0.0 | 6.0 | Sanders et al,34 2016 |
| Costs | 3.0 | NAb | 0.0 | 6.0 | Sanders et al,34 2016 |
Abbreviations: CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; NA, not applicable; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year.
Pill-taking disutility subtracted from health state utility value in every cycle that an individual receives treatment.
Implies that the variable was not varied in sensitivity analysis.
Statin Treatment Strategies
Following preventive guideline recommendations, AR10 was calculated at 40 years of age using the 2013 ACC/AHA 10-year CVD risk score and updated every 5 years in untreated patients to inform treatment decisions.6 Following ACC/AHA guidelines, statin treatment was initiated for patients with baseline or incident diabetes or LDL-C levels of at least 190 mg/dL in all treatment scenarios. Patients with LDL-C levels of at least 190 mg/dL or diabetes and AR10 of at least 7.5% received high-intensity statins.6 Patients with diabetes and AR10 of less than 7.5% received moderate-intensity statins. Treatment was continued but not initiated in patients 75 years or older.
In the overall population analysis, the following 4 statin initiation strategies were compared that incrementally expanded treatment eligibility: (1) treat patients with AR10 of at least 7.5%, diabetes, or LDL-C level of at least 190 mg/dL (current standard treatment); (2) add treatment with moderate-intensity statins for AR10 of 5.0% to 7.4% and LDL-C level of 160 to 189 mg/dL; (3) add treatment with moderate-intensity statins for AR10 of 5.0% to 7.4% and LDL-C level of 130 to 159 mg/dL; and (4) add treatment with moderate-intensity statins for all individuals with AR10 of at least 5.0% regardless of LDL-C level. In stratified analyses, preventive statin therapy was compared with no treatment in US adults aged 40, 50, and 60 years, stratified by baseline AR10 and LDL-C level. For these analyses, individuals with diabetes, LDL-C level of at least 190 mg/dL, or baseline or incident ASCVD were censored.
Statin Treatment Inputs
Statin treatment–related model input variables reflected current knowledge of statin-related costs, benefits, and risks (Table 1 and eTables 3-5 in the Supplement). Moderate-intensity statin therapy reduced baseline LDL-C levels by 29%, and high-intensity statin therapy reduced baseline LDL-C levels by 43%.23 Each 1.0-mmol/L (38.67-mg/dL) reduction in LDL-C level corresponded to a 0.76 relative risk for primary coronary heart disease and 0.85 relative risk for stroke; we assumed no statin benefit for secondary CVD events.8 Patients initiating statin therapy received a lipid and liver panel test only in their first year of treatment. A proportion of individuals developed statin-induced type 2 diabetes each year (0.5%).24,25 Diabetes increased annual weighted costs to persistent statin users and proportionally increased risk of CVD events and non-CVD mortality.14
Although a proportion of patients who initiated statin therapy may have myalgias and other minor adverse effects of medication, meta-analyses show that persistent statin use does not induce these effects.26,27,28,29 Adverse statin effects were accounted for in the rate of medication discontinuation and rate of follow-up office visits, but no adverse event disutility was applied. We assumed 4.7% of persistent statin users experienced mild adverse events and 0.006% experienced severe adverse events.30 Costs associated with these events were $178 and $7033, respectively.25 As was the case for statin-induced type 2 diabetes, a weighted annual cost was applied to persistent statin users. A recent modeling study suggested that statins may lead to net harm in individuals at intermediate risk during a 10-year horizon.31 In light of this, a threshold analysis was performed to determine the annual treatment-related disutility from statin therapy necessary to cause net loss in health-related quality of life for patients at borderline risk with LDL-C levels of 160 to 189 mg/dL.
Adherence to treatment (ie, proportion persisting in taking statin beyond persistence observed in clinical trials) was 67% in the first, 53% in the second, and 50% in the subsequent years of treatment.32 Statin adherence decay attenuated LDL-C level reduction, adverse effect risks, and treatment-related costs. Annual statin cost inputs represent survey-weighted median costs to all payers, including patient out-of-pocket costs, of moderate- and high-intensity statins in the Medical Expenditure Panel Survey 201533 (eMethods and eTable 7 in the Supplement).
Statistical Analysis
Data were analyzed from May 15, 2018, through June 10, 2019. Primary outcomes were lifetime quality-adjusted life-years (QALYs) gained, costs in 2019 US dollars, and incremental cost-effectiveness ratios (ICERs). All analyses were performed from a US health care sector perspective. All formal health care costs (ie, treatment-related, ASCVD, and non-CVD) were included in the analysis regardless of payer.34 Future QALYs and costs were discounted at a rate of 3.0% annually.34 Strategies were classified as cost-saving (ie, incremental increase in QALYs with lower costs), highly cost-effective (ICER ≥ $0 per QALY but < $50 000 per QALY gained), intermediately cost-effective (ICER ≥ $50 000 per QALY but < $150 000 per QALY gained), or not cost-effective (ICER ≥ $150 000 per QALY gained).35
One-way sensitivity analyses explored the results for the primary outcomes at upper and lower uncertainty bounds of the Table 1 statin treatment inputs. Using the cost and QALY results from the 100 probabilistic analyses described above, we constructed a cost-effectiveness acceptability curve to show the probability of each treatment strategy being the most cost-effective option across a range of willingness-to-pay thresholds.
Scenario analyses explored the effect of model variables on cost-effectiveness outcomes. We performed a scenario analysis in which persistent statin users incurred weighted annual utility decrements of 0.005 and 0.038 QALYs associated with mild and severe statin-related adverse events, respectively.36 Other analyses included assuming no checkup visits in years subsequent to treatment initiation, very high annual statin costs ($1520 and $3040, reflecting brand-name statin medication costs), assuming 100% medication adherence, and estimating cost-effectiveness at time horizons of 10, 20, 30, and 40 years (to reflect perspectives of different payer types, eg, private vs government insurers). A final analysis considered the cost-effectiveness of providing intermediate-intensity statins to individuals at borderline risk with chronic kidney disease (defined as estimated glomerular filtration rate of <60 mL/min/1.73 m2). Chronic kidney disease was examined because it is one of the risk-enhancing factors of the 2018 ACC/AHA guidelines and a characteristic routinely evaluated in clinical practice.
Results
Study Cohort Characteristics and Outcomes Without Statin Treatment
Characteristics of the simulation cohort of 1 000 000 patients at 40, 50, and 60 years of age without statin treatment are shown in eTable 8 in the Supplement (50% men and 50% women). Mean (SD) AR10 increased from 1.8% (1.4%) at 40 years of age to 7.4% (4.0%) at 60 years of age. Mean LDL-C levels increased from 125.8 (32.1) mg/dL at 40 years of age to 126.9 (34.7) mg/dL at 50 years of age, then decreased to 122.3 (33.5) mg/dL at 60 years of age. Without statin treatment, approximately 5.3% of the baseline cohort was projected to have an incident ASCVD event by 50 years of age and 12.6% by 60 years of age.
Population Analysis
We projected that expanding statin treatment from current standard care (about 35 million statin-eligible US adults in 2019) to include individuals with borderline risk and LDL-C levels of 160 to 189 mg/dL would add about 2 million US adults, to include those with borderline risk and LDL-C levels of 130 to 159 mg/dL would add another 4 million, and to include the remainder of individuals with borderline risk would add another 5 million. We estimated that 1200 to 5400 ASCVD events could be prevented and 1200 to 3200 QALYs gained per 1 million individuals treated in the borderline risk group (Table 2). Similar relative ASCVD event reduction was estimated to occur for 10 years (eTable 9 in the Supplement).
Table 2. Lifetime Costs, Health Benefits, and Cost-effectiveness of Risk- and Cholesterol Level–Based Statin Treatment Strategies in a Cohort of 500 000 US Men and 500 000 US Women Aged 40 Years at Baseline.
| Policy | Patient-Years of Treatment Eligibility | No. of ASCVD Events | Discounted QALYs | Discounted Costs, 2019 US $ | ICER ($/QALY)a |
|---|---|---|---|---|---|
| Women | |||||
| Standard care | 8 485 471 | 354 209 | 11 582 459 | 125 046 215 620 | Reference |
| Add AR10 of 5.0%-7.4% and LDL-C level of 160-189 mg/dL | 8 698 683 | 353 782 | 11 582 673 | 125 044 868 352 | Cost-saving |
| Add AR10 of 5.0%-7.4% and LDL-C level of 130-159 mg/dL | 9 099 545 | 353 166 | 11 582 850 | 125 048 146 932 | 18 487 |
| Add remainder AR10 ≥5.0% | 9 850 750 | 352 211 | 11 583 006 | 125 060 057 499 | 76 576 |
| Men | |||||
| Standard care | 11 153 224 | 480 658 | 10 914 126 | 90 574 138 606 | Reference |
| Add AR10 of 5.0%-7.4% and LDL-C level of 160-189 mg/dL | 11 454 862 | 479 925 | 10 915 020 | 90 562 851 415 | Cost-saving |
| Add AR10 of 5.0%-7.4% and LDL-C level of 130-159 mg/dL | 12 105 382 | 478 761 | 10 916 180 | 90 558 695 685 | Cost-saving |
| Add remainder AR10 of ≥5.0% | 13 316 940 | 477 281 | 10 917 063 | 90 581 623 102 | 25 977 |
| Combined women and men | |||||
| Standard care | 19 638 695 | 834 867 | 22 496 585 | 215 620 354 226 | Reference |
| Add AR10 of 5.0%-7.4% and LDL-C level of 160-189 mg/dL | 20 153 545 | 833 707 | 22 497 693 | 215 607 719 767 | Cost-saving |
| Add AR10 of 5.0%-7.4% and LDL-C level of 130-159 mg/dL | 21 204 927 | 831 927 | 22 499 030 | 215 606 842 617 | Cost-saving |
| Add remainder AR10 of ≥5.0% | 23 167 690 | 829 492 | 22 500 068 | 215 641 680 601 | 33 558 |
Abbreviations: AR10, 10-year absolute risk for atherosclerotic cardiovascular disease (ASCVD); ICER, incremental cost-effectiveness ratio; LDL-C, low-density lipoprotein cholesterol; QALY, quality-adjusted life-year.
Incremental to prior most effective, nondominated strategy.
In women and men, adding moderate-intensity statin treatment of borderline AR10 and LDL-C levels of 160 to 189 mg/dL would be cost-saving compared with the current standard (Table 2 and eFigure 4 in the Supplement). Taking the further step of treating borderline AR10 with LDL-C levels of 130 to 159 mg/dL would be highly cost-effective in women (ICER, $18 487 per QALY gained) and cost-saving in men. Adding treatment of all men and women with borderline AR10, regardless of LDL-C level, would be highly cost-effective, with an ICER of $33 558 per QALY gained.
Stratified Analysis
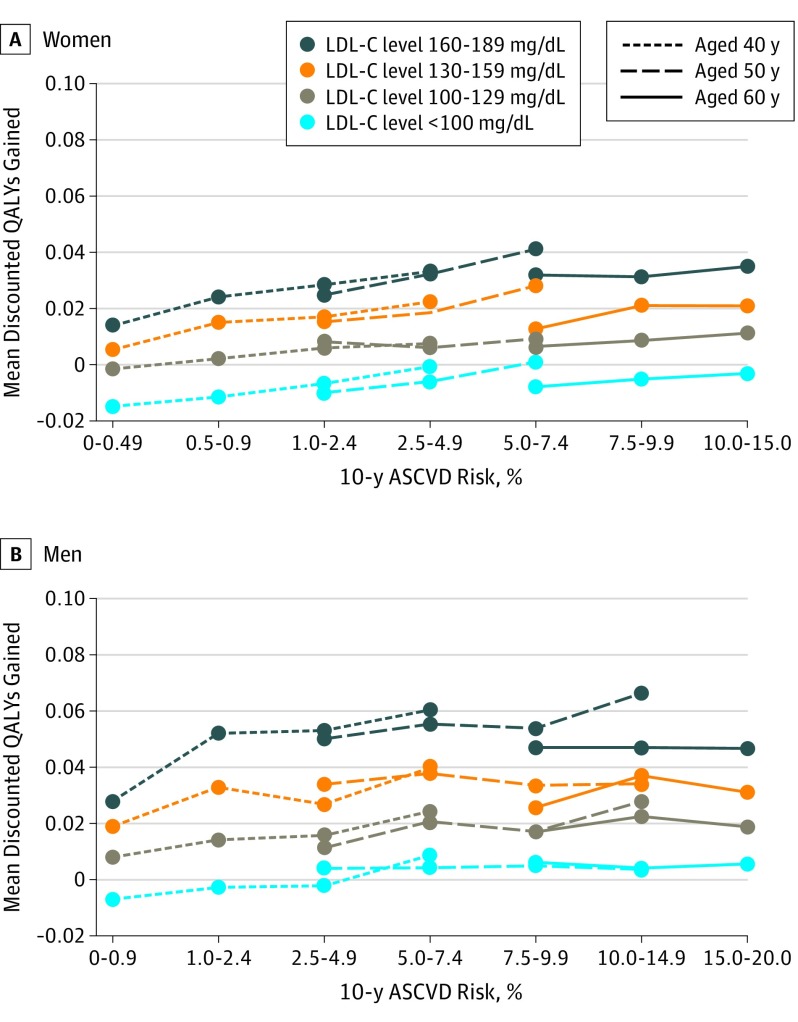
At the same age of treatment initiation, lifetime statin benefits increased with increasing AR10. For each age, AR10, and sex category, lifetime QALYs gained with statin treatment were also greater for individuals with higher baseline LDL-C levels compared with those with lower LDL-C levels (Figure 2). Indeed, patients with high LDL-C levels and low AR10 achieved similar lifetime statin benefits compared with individuals with high AR10 and low LDL-C levels. For example, statin treatment in 40-year-old men with LDL-C levels of 160 to 189 mg/dL and AR10 of 0.0% to 0.9% resulted in similar health gains (0.030 QALYs) to treating 50-year-old men with AR10 of 10.0% to 14.9% and LDL-C levels of 130 to 159 mg/dL. Similarly, 40-year-old women with AR10 of 0.0% to 0.9% and LDL-C levels of 160 to 189 mg/dL achieved similar health gains (0.015 QALYs) to 60-year-old women with AR10 of 10.0% to 15.0% and LDL-C levels of 100 to 139 mg/dL. Notably, men and women with low LDL-C levels experienced health loss (negative QALYs) or negligible health benefit from treatment regardless of baseline AR10 (Figure 2) because small absolute ASCVD risk reductions achieved in this population are dominated by adverse contributions of diabetes risk and pill-taking disutility.
Figure 2. Mean Discounted Quality-Adjusted Life-Year (QALY) Gains From Statin Therapy .
Baseline 10-year absolute risk (AR10) of atherosclerotic cardiovascular disease (ASCVD) is stratified by age and baseline low-density lipoprotein cholesterol (LDL-C) level. To convert LDL-C to millimoles per liter, multiply by 0.0259.
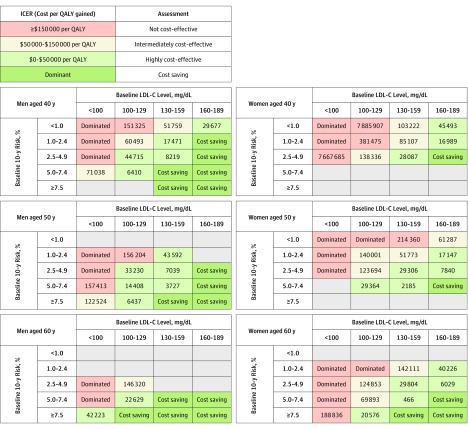
Compared with no treatment, treating patients with borderline AR10 and LDL-C levels of 160 to 189 mg/dL with moderate-intensity statins was cost-saving or highly cost-effective in all age and sex groups (Figure 3). Extending statin treatment to patients with LDL-C levels of 130 to 159 mg/dL and AR10 as low as 2.5% to 4.9% would also be cost-saving or highly cost-effective. Conversely, treating LDL-C levels of less than 100 mg/dL was not cost-effective or only intermediately cost-effective in almost all age and sex groups.
Figure 3. Cost-effectiveness of Moderate-Intensity Statin Therapy.
Data are presented as incremental cost-effectiveness ratios (ICERs) comparing the statin treatment and no-treatment scenarios starting at 40, 50, and 60 years of age for a range of 10-year absolute risk (AR10) of atherosclerotic cardiovascular disease and low-density lipoprotein cholesterol (LDL-C) level subgroups. Results are stratified by sex. Empty gray cells indicate fewer than 25 individuals in National Health and Nutrition Examination Surveys data set. QALY indicates quality-adjusted life-year.
Sensitivity Analysis
Variability in ICER estimates owing to uncertainty surrounding individual model variables is presented in tornado diagrams representing 1-way sensitivity analysis results (eFigure 5 in the Supplement). Generally, uncertainty was greater for women than men, owing to lower ASCVD event rates in women. For both men and women, efficacy of statin therapy, statin cost, and frequency of monitoring checkups during treatment were strong determinants of cost-effectiveness.
In a scenario analysis in which follow-up monitoring costs were eliminated, expanding treatment to all individuals at borderline risk with AR10 of at least 5.0% would be cost-saving (eTables 10 and 11 in the Supplement). Assuming statin users persist in taking statins despite enduring chronic treatment-related myalgias made all strategies marginally less cost-effective. Treatment generally was more cost-effective as the time horizon of the study increased. Expanding statin treatment from standard care to patients with borderline risk and LDL-C levels of 160 to 189 mg/dL would lead to a net reduction in QALYs (ie, less effective than standard care) if annual statin-related adverse event disutility reached 0.007 and 0.010 for women and men, respectively (eFigure 6 in the Supplement). The ICER associated with statins for individuals at borderline risk with chronic kidney disease was $8798 per QALY gained (eTable 12 in the Supplement).
Probabilistic analyses led to similar conclusions for men and women (eFigures 7 and 8 in the Supplement). At very low willingness-to-pay (<$10 000 per QALY gained), standard care plus treatment of patients at borderline risk with LDL-C levels of 160 to 189 mg/dL had the highest probability of being cost-effective. As willingness-to-pay increased, the probability increased that adding statin treatment for patients with borderline risk and LDL-C levels of 130 to 159 mg/dL would be optimal. If decision makers were willing to pay at least $40 000 per QALY gained, treating all individuals at borderline risk with AR10 of at least 5.0% would be the preferred strategy.
Discussion
The 2013 and 2018 ACC/AHA cholesterol treatment guidelines strongly recommended statin treatment for most patients with LDL-C levels of at least 190 mg/dL, diabetes, or 10-year ASCVD risk of at least 7.5%. The 2018 ACC/AHA guideline update further recommended considering statin treatment in patients with borderline AR10 (5.0%-7.4%) if risk-enhancing factors are present. One of those factors is LDL-C level of at least 160 mg/dL. Our cost-effectiveness analysis adhered to best-practice guidelines for conducting and reporting economic evaluations of health care interventions (eTable 13 in the Supplement). We consistently found that treating patients with borderline AR10 and with LDL-C levels of 160 to 189 mg/dL is cost-saving. This finding provides a health economic justification for the ACC/AHA 2018 recommendation. Adding treatment of the remainder of patients with borderline AR10 and LDL-C levels of at least 130 mg/dL—a group not recommended for treatment by current guidelines—may be cost-saving in men and highly cost-effective in women and could be considered in future guidelines. We also found that further extending treatment to all patients with AR10 of at least 5.0% regardless of LDL-C level would prevent the greatest number of ASCVD events and be highly cost-effective. We demonstrated that patients with high LDL-C levels gain the greatest statin treatment benefits within each age and AR10 band and that lifetime benefits from statin therapy are comparable for individuals with low AR10 and high baseline LDL-C levels and those with high AR10 and low LDL-C levels.
Our results further suggested that treatment of all patients with borderline ASCVD risk (AR10, 5.0%-7.4%), regardless of LDL-C level, would be highly cost-effective at currently accepted willingness-to-pay thresholds. However, traditional willingness-to-pay thresholds may be set too high.37,38,39 Society may be unwilling to support the increase in overall health care budget, statin prescription burden, and patient out-of-pocket costs associated with expanding treatment eligibility to all patients at borderline risk, representing about 11 million US adults. Younger patients may find taking statins daily for decades unacceptable, and non-CVD adverse events may be underestimated in long-term users. Such potential unintended consequences are reflected in our scenarios of high pill-taking disutility and high adverse event rates. Currently, individuals without diabetes and with AR10 of at least 7.5% and LDL-C levels of 70 to 100 mg/dL are recommended statin therapy for the primary prevention of CVD. Our analysis suggests that individuals with LDL-C levels of less than 100 mg/dL (3.7 million US adults) are not cost-effective to treat and in some cases may receive net negative health benefits from statins.
Our results are generally in line with those of prior modeling studies. Pandya et al25 estimated the cost-effectiveness of statin therapy at a range of AR10 thresholds. We entered their chronic and acute health state costs and utilities, monitoring costs, treatment-related costs, and treatment-related disutility values into the CVD Policy Model microsimulation version, applying CVD case-fatality rates that aligned with the time of their study. The ICER associated with adding statin treatment in the group of patients with AR10 of 5.0% to 7.4% was approximately $22 000 per QALY gained according to our model and $27 000 per QALY gained in the analysis by Pandya et al25 (eTable 14 in the Supplement). A second analysis by Heller et al14 considered the cost-effectiveness of several approaches to statin prioritization. When using their model variables, we replicated their finding that the ACC/AHA 2013 standard of treating patients with AR10 of at least 7.5%, LDL-C levels of at least 190 mg/dL, or diabetes is cost-saving compared with achieving Adult Treatment Panel III recommendations in men and women.
Limitations
The 2018 ACC/AHA guideline recommended considering statin treatment in patients with borderline AR10 (5.0%-7.4%) if any of 10 risk-enhancing factors are present. Our analysis focused on the risk-enhancing factors of LDL-C of at least 160 mg/dL and chronic kidney disease only; we did not study decision-making about statin therapy associated with other factors, including metabolic syndrome, history of preeclampsia or premature menopause, chronic inflammatory disorders, high-risk ethnicity, persistent hypertriglyceridemia, or elevated levels of apolipoprotein B, C-reactive protein, or lipoprotein(a).2 At the same time, of all these risk-enhancing factors, only LDL-C level is currently immediately available to patients and health care professionals during initial risk assessment and decision-making. This study assumed a direct association between baseline LDL-C levels and statin benefit (relative risk reduction directly associated with unit reduction in LDL-C level) and did not account for statin effects independent of change in LDL-C level. Specifically, statin benefits were modeled based on change in LDL-C level and not on alternative measures of statin efficacy, such as change in non–high-density lipoprotein cholesterol or apolipoprotein B level.
An additional limitation is that the risk reduction associated with each unit of decrease in LDL-C in our analysis was derived from a meta-analysis that combined primary and secondary prevention statin trials, and our analysis focused on statins for primary prevention.8 However, relative risk reductions per unit of LDL-C in the meta-analysis were similar when stratified by ASCVD risk, including a high-risk stratum that encompassed CVD history; slightly more favorable relative risk reduction was actually found in the lowest-risk stratum. Computer simulation models have the relative advantages of synthesizing evidence from multiple sources and simulating a lifetime of treatment and follow-up, but combining data from heterogeneous sources may lead to nongeneralizable results.
Another consideration is that relatively low statin adherence rates were applied in our analysis. These rates aligned with those used in previous analyses of statin cost-effectiveness.14,25 However, adherence may differ between risk- and LDL-C–based subgroups of the US population. Further analysis should consider the costs and benefits associated with interventions that aim to increase patient and physician adherence to guidelines for reducing cholesterol levels.
Conclusions
This computer-simulated health economic study found that adding treatment of borderline AR10 of ASCVD (5.0%-7.4%) and LDL-C levels of 160 to 189 mg/dL to the current standard of statin treatment eligibility is cost-saving and that further adding treatment of borderline AR10 and LDL-C levels of 130 to 159 mg/dL may be cost-saving in men and highly cost-effective in women. Our results further suggest that treating all patients with borderline AR10 would be highly cost-effective if society is willing to devote significant health care resources toward ASCVD prevention.
eMethods. Background, Model Structure, Model Inputs, and CVD Policy Model Microsimulation Version Discrimination and Recalibration
eTable 1. Logistic Risk Functions Determining Incident Event Probability in the CVD Policy Model Microsimulation Version
eTable 2. Difference Between NHANES Observed and NHLBI Pooled Cohort Imputed CVD Risk Factors
eTable 3. Probabilities for Nonincident CHD and Stroke Events in the CVD Policy Model Microsimulation Version
eTable 4. Chronic and Acute Utilities Used in CVD Policy Model Microsimulation Version
eTable 5. Health State and Acute Event Costs Used in CVD Policy Model Microsimulation Version
eTable 6. Comparison of CVD Policy Model Microsimulation Version and O’Sullivan et al’s Health Care Costs
eTable 7. Statin-Intensity Classifications
eTable 8. Characteristics of the Simulation Cohort at Ages 40, 50, and 60 Years Without Statin Treatment
eTable 9. Number of ASCVD Events Prevented Over 10 Years for Risk- and Cholesterol-Based Statin Treatment Strategies
eTable 10. Scenario Analyses Showing the Costs, QALYs, and ICERs When Changing Model Assumptions
eTable 11. Scenario Analyses Showing the ICERs When Changing Model Assumptions
eTable 12. Cost-Effectiveness of Statins for Borderline Risk Individuals With Chronic Kidney Disease
eTable 13. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement
eTable 14. Cross-Validation of the CVD Policy Model Microsimulation Version vs Pandya et al’s ASCVD Risk Threshold Statin Cost-Effectiveness Analysis
eFigure 1. CVD Policy Model Microsimulation Version Structure, Within-Cycle Events
eFigure 2. Validation of the CVD Policy Model Microsimulation Version
eFigure 3. Untreated LDL Cholesterol Lifetime Trajectory
eFigure 4. Cost-Effectiveness Plane for Base Case Analysis
eFigure 5. One-Way Sensitivity Analysis Tornado Diagram
eFigure 6. One-Way Sensitivity Analysis of Statin-Related Adverse Event Disutility on Discounted QALY Gains for Standard Care vs Standard Care Plus Treat LDL 160-189 mg/dL
eFigure 7. Cost-Effectiveness Acceptability Curve
eFigure 8. Incremental Cost-Effectiveness Scatter Plot
eReferences.
References
- 1.Taylor F, Huffman MD, Macedo AF, et al. . Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, et al. . 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;139(25):e1082-e1143. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Network. Risk Estimation and the Prevention of Cardiovascular Disease. Edinburgh, United Kingdom: Scottish Intercollegiate Guidelines Network; July 2017:79. [Google Scholar]
- 4.Anderson TJ, Grégoire J, Pearson GJ, et al. . 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263-1282. doi: 10.1016/j.cjca.2016.07.510 [DOI] [PubMed] [Google Scholar]
- 5.National Clinical Guideline Centre (UK) Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London, United Kingdom: National Institute for Health and Care Excellence; 2014. http://www.ncbi.nlm.nih.gov/books/NBK248067/. Accessed October 20, 2018. [PubMed]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. https://www.ahajournals.org/doi/full/10.1161/01.cir.0000437738.63853.7a. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316(19):1997-2007. doi: 10.1001/jama.2016.15450 [DOI] [PubMed] [Google Scholar]
- 8.Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarese EP, Robinson JG, Kowalewski M, et al. . Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319(15):1566-1579. doi: 10.1001/jama.2018.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanassoulis G, Williams K, Altobelli KK, Pencina MJ, Cannon CP, Sniderman AD. Individualized statin benefit for determining statin eligibility in the primary prevention of cardiovascular disease. Circulation. 2016;133(16):1574-1581. doi: 10.1161/CIRCULATIONAHA.115.018383 [DOI] [PubMed] [Google Scholar]
- 11.Thanassoulis G, Sniderman AD, Pencina MJ. A long-term benefit approach vs standard risk-based approaches for statin eligibility in primary prevention. JAMA Cardiol. 2018;3(11):1090-1095. doi: 10.1001/jamacardio.2018.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pletcher MJ, Pignone M, Jarmul JA, Moran AE, Vittinghoff E, Newman T. Population impact & efficiency of benefit-targeted versus risk-targeted statin prescribing for primary prevention of cardiovascular disease. J Am Heart Assoc. 2017;6(2):e004316. doi: 10.1161/JAHA.116.004316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soran H, Schofield JD, Durrington PN. Cholesterol, not just cardiovascular risk, is important in deciding who should receive statin treatment. Eur Heart J. 2015;36(43):2975-2983. doi: 10.1093/eurheartj/ehv340 [DOI] [PubMed] [Google Scholar]
- 14.Heller DJ, Coxson PG, Penko J, et al. . Evaluating the impact and cost-effectiveness of statin use guidelines for primary prevention of coronary heart disease and stroke. Circulation. 2017;136(12):1087-1098. doi: 10.1161/CIRCULATIONAHA.117.027067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman L, Weinstein MC, Goldman PA, Williams LW. Cost-effectiveness of HMG-CoA reductase inhibition for primary and secondary prevention of coronary heart disease. JAMA. 1991;265(9):1145-1151. doi: 10.1001/jama.1991.03460090093039 [DOI] [PubMed] [Google Scholar]
- 16.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. . Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150(4):243-254. doi: 10.7326/0003-4819-150-4-200902170-00005 [DOI] [PubMed] [Google Scholar]
- 17.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124(2):146-153. doi: 10.1161/CIRCULATIONAHA.110.986349 [DOI] [PubMed] [Google Scholar]
- 18.Oelsner EC, Balte PP, Cassano PA, et al. . Harmonization of respiratory data from 9 US population-based cohorts: the NHLBI Pooled Cohorts Study. Am J Epidemiol. 2018;187(11):2265-2278. doi: 10.1093/aje/kwy139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazi DS, Moran AE, Coxson PG, et al. . Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743-753. doi: 10.1001/jama.2016.11004 [DOI] [PubMed] [Google Scholar]
- 20.Krumholz HM, Normand S-LT, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999-2011. Circulation. 2014;130(12):966-975. doi: 10.1161/CIRCULATIONAHA.113.007787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey data Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 1999. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/DSPI.htm. Revised December 2016. Accessed June 15, 2018.
- 22.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. . Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course [published online December 7, 2018]. Int J Epidemiol. doi: 10.1093/ije/dyy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sattar N, Preiss D, Murray HM, et al. . Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735-742. doi: 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 25.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150. doi: 10.1001/jama.2015.6822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finegold JA, Manisty CH, Goldacre B, Barron AJ, Francis DP. What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur J Prev Cardiol. 2014;21(4):464-474. doi: 10.1177/2047487314525531 [DOI] [PubMed] [Google Scholar]
- 27.He Y, Li X, Gasevic D, et al. . Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Ann Intern Med. 2018;169(8):543-553. doi: 10.7326/M18-0808 [DOI] [PubMed] [Google Scholar]
- 28.Mills EJ, Wu P, Chong G, et al. . Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104(2):109-124. doi: 10.1093/qjmed/hcq165 [DOI] [PubMed] [Google Scholar]
- 29.Newman CB, Preiss D, Tobert JA, et al. ; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council . Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38-e81. doi: 10.1161/ATV.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Plutzky J, Skentzos S, et al. . Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526-534. doi: 10.7326/0003-4819-158-7-201304020-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yebyo HG, Aschmann HE, Puhan MA. Finding the balance between benefits and harms when using statins for primary prevention of cardiovascular disease: a modeling study [published online December 4, 2018]. Ann Intern Med. doi: 10.7326/M18-1279 [DOI] [PubMed] [Google Scholar]
- 32.Greving JP, Visseren FLJ, de Wit GA, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? cost-effectiveness analysis. BMJ. 2011;342:d1672. doi: 10.1136/bmj.d1672 [DOI] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey 2015. http://meps.ahrq.gov/mepsweb/. Published 2015. Accessed November 30, 2018.
- 34.Sanders GD, Neumann PJ, Basu A, et al. . Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093-1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 35.Anderson JL, Heidenreich PA, Barnett PG, et al. ; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines . ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345. doi: 10.1161/CIR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 36.Lee KK, Cipriano LE, Owens DK, Go AS, Hlatky MA. Cost-effectiveness of using high-sensitivity C-reactive protein to identify intermediate- and low-cardiovascular-risk individuals for statin therapy. Circulation. 2010;122(15):1478-1487. doi: 10.1161/CIRCULATIONAHA.110.947960 [DOI] [PubMed] [Google Scholar]
- 37.Claxton K, Martin S, Soares M, et al. . Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomas J, Claxton K, Martin S, Soares M. Resolving the “cost-effective but unaffordable” paradox: estimating the health opportunity costs of nonmarginal budget impacts. Value Health. 2018;21(3):266-275. doi: 10.1016/j.jval.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 39.Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929-935. doi: 10.1016/j.jval.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Medicare & Medicaid Services. License for Use of Current Procedural Terminology, Fourth Edition (“CPT”). http://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=0&T=0&HT=0&CT=3&H1=99212&M=5 Published April 2018. Accessed June 22, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Background, Model Structure, Model Inputs, and CVD Policy Model Microsimulation Version Discrimination and Recalibration
eTable 1. Logistic Risk Functions Determining Incident Event Probability in the CVD Policy Model Microsimulation Version
eTable 2. Difference Between NHANES Observed and NHLBI Pooled Cohort Imputed CVD Risk Factors
eTable 3. Probabilities for Nonincident CHD and Stroke Events in the CVD Policy Model Microsimulation Version
eTable 4. Chronic and Acute Utilities Used in CVD Policy Model Microsimulation Version
eTable 5. Health State and Acute Event Costs Used in CVD Policy Model Microsimulation Version
eTable 6. Comparison of CVD Policy Model Microsimulation Version and O’Sullivan et al’s Health Care Costs
eTable 7. Statin-Intensity Classifications
eTable 8. Characteristics of the Simulation Cohort at Ages 40, 50, and 60 Years Without Statin Treatment
eTable 9. Number of ASCVD Events Prevented Over 10 Years for Risk- and Cholesterol-Based Statin Treatment Strategies
eTable 10. Scenario Analyses Showing the Costs, QALYs, and ICERs When Changing Model Assumptions
eTable 11. Scenario Analyses Showing the ICERs When Changing Model Assumptions
eTable 12. Cost-Effectiveness of Statins for Borderline Risk Individuals With Chronic Kidney Disease
eTable 13. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement
eTable 14. Cross-Validation of the CVD Policy Model Microsimulation Version vs Pandya et al’s ASCVD Risk Threshold Statin Cost-Effectiveness Analysis
eFigure 1. CVD Policy Model Microsimulation Version Structure, Within-Cycle Events
eFigure 2. Validation of the CVD Policy Model Microsimulation Version
eFigure 3. Untreated LDL Cholesterol Lifetime Trajectory
eFigure 4. Cost-Effectiveness Plane for Base Case Analysis
eFigure 5. One-Way Sensitivity Analysis Tornado Diagram
eFigure 6. One-Way Sensitivity Analysis of Statin-Related Adverse Event Disutility on Discounted QALY Gains for Standard Care vs Standard Care Plus Treat LDL 160-189 mg/dL
eFigure 7. Cost-Effectiveness Acceptability Curve
eFigure 8. Incremental Cost-Effectiveness Scatter Plot
eReferences.