Abstract
Microchimerism is the presence of cells from one individual in another genetically distinct individual. Pregnancy is the main cause of natural microchimerism through transplacental bi-directional cell trafficking between mother and fetus. In addition to a variety of cell-free substances, it is now well-recognized that some cells are also exchanged in pregnancy. Furthermore, it is now known that microchimerism persists decades later both in mother and in her progeny. The consequences of pregnancy-related microchimerism are under active investigation. However, many authors have suggested a close relationship linking fetal microchimerism and the development of autoimmune diseases. Fetal microchimerism is emerging as a potential contributing factor in certain diseases, including cancer. Parallel studies in animal and human pregnancy suggest that microchimeric fetal cells play a role in wound healing. Role of these microchimeric cells in human health and disease is discussed here.
Keywords: Autoimmune disease, fetal-maternal trafficking, microchimerism, pregnancy
INTRODUCTION
In mythology, the term “chimera” is related to a monster which is composed of snippets of various animals. According to Greek mythology, chimera, a monstrous fire breathing composite creature of Lycia in Asia Minor, composed of the parts of three animals – a lion, a snake and a goat. Usually depicted as a lion, with the head of a goat arising from its back, and a tail that ended in snake's head [Figure 1].
Figure 1.
Chimera: An individual organ
The term chimerism was first used by Liegeois et al. in the 1970s. Chimera is a single organism that is made up of two or more different populations of genetically distinct cells that originated from different zygotes involved in sexual reproduction. If the different cells have arisen from the same zygote, the organism is called a mosaic. Chimeras are formed from at least four parent cells (two fertilized eggs or early embryos fused together). Each population of cells retains its own character and the resulting organism has a mixture of tissues.[1,2]
Microchimerism was defined by many authors in different ways in the past. Galofre defined microchimerism as the presence of alien cells within an individual tissue with genetically different background.[3]
According to Waszak et al., it is defined as the coexistence of cells of different genetic origin within one individual.
Microchimerism may be defined as the presence of two genetically distinct and separately derived populations of cells, one population being at a low concentration, in the same individual or an organ such as the bone marrow according to Dawe GS (cell migration from the baby to mother). Hence, microchimerism refers to the harboring of small numbers of cells (or DNA) that originated in a genetically different individual.[3,4,5,6]
Microchimerism is a topic of current interest due to several reasons as it plays an important role in autoimmune diseases, cancer and wound healing, etc. During pregnancy, some cells transfer from the mother to the fetus and vice versa. Interestingly, a small number of cells from mother persist in her offspring and persists till adult life, whereas a small number of cells from previous pregnancies persist in mother for many years. We are just beginning to understand the implications of these “cells” which may be beneficial or detrimental for the health of the host.
TYPES OF MICROCHIMERISM
Microchimeric cells have two possible lineages.
Natural – Examples of natural microchimerism are pregnancy, miscarriage and twinning or sexual intercourse
Artificial – Examples of artificial microchimerism are organ/tissue transplant and blood transfusion. Pregnancy is a major source of natural microchimerism.[3,7]
Both types are further divided into following types [Figure 2] [Table 1]:
Figure 2.
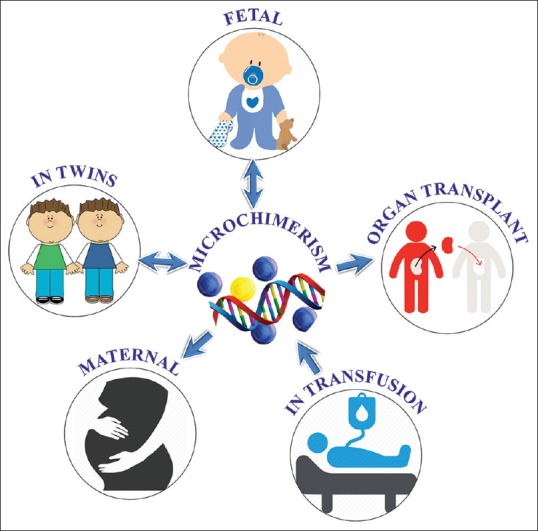
Types of microchimerism
Table 1.
Types of Microchimerism
| Natural microchimerism | Artificial microchimerism |
|---|---|
| Fetal microchimerism | Transfusion-associated microchimerism |
| Maternal microchimerism | Microchimerism in organ transplantation |
| Microchimerism in twins | Microchimerism in relation to bone marrow transplantation |
Natural microchimerism
During normal pregnancy, there is reversible transfer of maternal, fetal and placental cells[7,8] [Figure 3].
Figure 3.
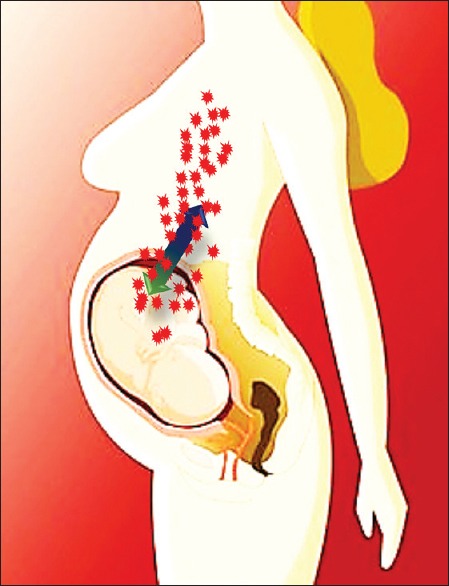
The bidirectional fetomaternal trafficking of cells across the placenta
Natural microchimerism is broadly divided into three categories as follows:
Fetal microchimerism: fetal to maternal bleed during pregnancy or parturition
Maternal microchimerism: maternal to fetal bleed during pregnancy or parturition
Microchimerism in twins: exchange of cells between the fetuses in the uterus.
Fetal microchimerism
This is the most common form of natural microchimerism in which there is transfer of intact living fetal cells from the fetal circulation into the maternal circulation and occurs in all pregnancies and increases as the gestational age advances.[3,6,9,10,11,12,13,14,15]
This transfer of fetal hematopoietic pluripotent progenitor cells starts in the 4th or 5th week after fertilization and continues throughout the pregnancy. The presence of these fetal microchimeric cells can be detected for up to 30 days in the maternal postpartum bloodstream.[9,16]
Postabortion and the incidence of fetal microchimerism
As the placenta is being destroyed during abortion, there is an increased incidence of fetal to maternal transfer of fetal undifferentiated progenitor cells. The amount of fetal DNA found in maternal circulation following a first-trimester abortion was found to be higher in women who underwent a surgical abortion than in women who had a chemical abortion[6] [Figure 4].
Figure 4.
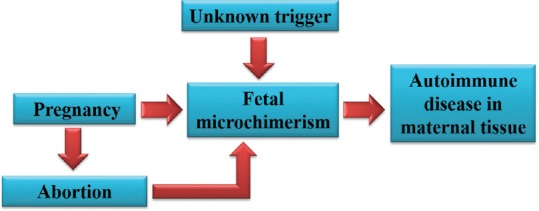
Elective abortion increases the degree of fetal microchimerism which favors the development of autoimmune disease in the postabortion women
Maternal microchimerism
The process of microchimerism is bidirectional, and the transfer of maternal cells into the fetal circulation is known as maternal microchimerism. The concept of maternal microchimerism was reported by Desai et al. in the 1960s who found maternal leukocytes in the umbilical cord blood from newborn infants. More appropriately, during fetal life and in early neonatal life, maternal microchimeric cells were found in various organs such as the skin, thymus, spleen, liver and thyroid.[3] Maternal microchimerism was less common than fetal microchimerism in all cellular subsets tested. Maternal microchimerism was found in more than one-third of healthy women within lymphoid and myeloid lineages.[17]
Role of maternal microchimeric cells on fetal immunity
Fetuses developed Tregs-specific for non-inherited maternal antigens in the presence of transforming growth factor-beta (TGFβ) leading to tolerance to alloantigens later in life. Maternal MC and more particularly T cells may produce cytokines changing the TGFβ dominant environment which might affect fetal Treg numbers and/or function leading later in life to a potential autoimmune disease [Figure 5].
Figure 5.
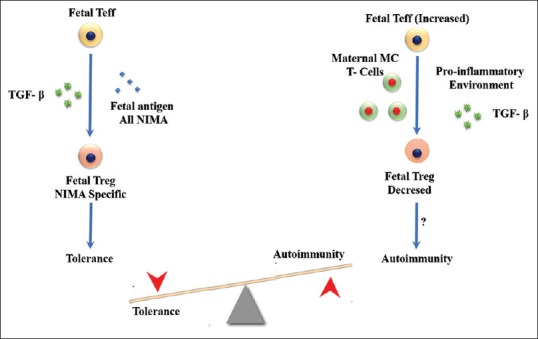
Role of maternal microchimeric cells on fetal immunity
Microchimerism in twins
Microchimerism may also occur other than the transfer of cells between the mother and the fetus. An exchange of cells can also take place between bigeminal fetuses in the uterus. Microchimerism may also originate from a “spectral twin.”[18]
Artificial microchimerism
Microchimerism and blood transfusion
Transfusion-associated microchimerism (TA-MC) appears to be a common and recently identified complication of blood transfusion.[19] Microchimerism from nonleukoreduced cellular blood products has been found to persist from months to years after transfusion.[20]
It is now widely accepted that the transfusion-related reaction is likely the result of antigen antibody reactivity between the donor's plasma and the recipient's blood cells or vice versa. The antibodies recognize targets on the recipient's white blood cells, including human leukocyte antigen (HLA) Class I and II antigens.[20]
Thus far, TA-MC has been detected when a severely injured patient received blood transfusion. Injury induces an immunosuppressive and inflammatory milieu in which fresh blood products with replication-competent leukocytes can sometimes cause TA-MC.[19]
Microchimerism in organ transplantation
As recently proposed by Starlz et al., that exchange of migratory leukocytes between the organ transplant and the recipient lead to the development of long-term tolerance. This phenomenon is known as microchimerism. The hypothesis is that the presence of donor cells in the recipients of organ transplant correlates with the tolerance and allows for withdrawal or reduction of immunosuppression.
This unique form of cell transfer from host to donor and vice versa is thought to have important consequences in transplantation. It was proposed that the phenomenon of cell migration can cause speed up rejection, graft versus host disease (GVHD) or may be the basis of tolerance. On the other hand, it is entirely possible that these cells may simply be an innocent spectator.[21]
Microchimerism and bone marrow transplantation
GVHD causes serious damage and even death in the recipients of bone marrow transplantation. It has been postulated that organ transplant implies a migratory flux of donor “passenger” leukocytes out of the graft into the recipient tissue or organs to establish a persistent condition of “microchimerism.” An equally important phenomenon is the reciprocal migration of circulating recipient leukocytes, which repopulate the interstitium of whole-organ allografts. This bidirectional exchange and interaction of bone marrow-derived cells after organ transplantation are considered a seminal event in the acceptance of allografts and in the induction of donor-specific tolerance.[22]
ROLE OF MICROCHIMERISM IN AUTOIMMUNE DISEASE
Various types of fetal cells can cross the placenta to the maternal circulation. Transferred fetal hematopoietic stem cells can be detected in the circulation of women up to 27 years after delivery. In addition, persisting fetal stem cells are able to differentiate into mature immune-competent cells such as lymphocytes, monocytes and natural killer cells. These cells may recognize mother-specific antigens and be activated under certain conditions. It seems reasonable to suppose that fetal cells persisting in the maternal circulation or tissues might mediate a GVHD leading to the development of autoimmune disease.[14]
About 80% of all people with autoimmune diseases are women. Several hypotheses have been proposed to explain the reasons for the gender difference such as hormones or stronger immune responses in women. Progressive systemic sclerosis (PSS), also known as scleroderma, is an autoimmune disease that primarily affects women in their postpartum years and bears a striking resemblance to graft-versus-host disease. Researchers have begun looking at the association of microchimerism in certain autoimmune diseases that primarily affect women.[19]
Examples of autoimmune diseases where fetal Y-chromosomes were detected decades after pregnancy
Figure 6.
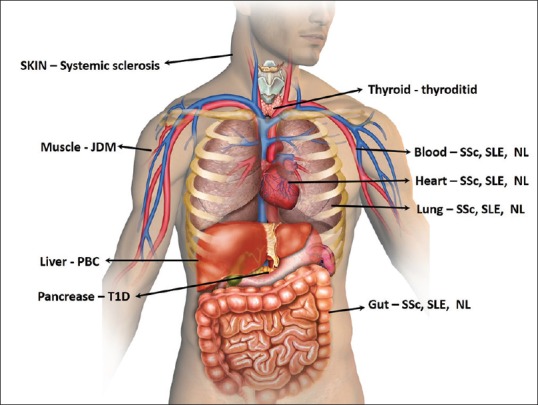
Organs affected by autoimmunity diseases caused by microchimeric cells
ROLE OF MICROCHIMERISM IN ORAL DISEASES
Microchimerism and oral lichen planus
Lichen planus (LP) is a chronic papulosquamous disease of unknown etiology, most commonly affecting middle-aged patients. Females have a three times higher prevalence than men. Histologically, LP is characterized by infiltration of the lower levels of epithelium by T lymphocytes causing basal cell destruction, apoptosis and secondary changes in epithelial thickness and maturation. These features together with experimental evidence are thought to represent a T-cell-mediated autoimmune response as the underlying mechanism for LP.
Cutaneous and oral lesions resembling LP clinically and histologically are also found in chronic GVHD. Hence, it has been suggested that, in some cases, the pathomechanism of LP may resemble that of GVHD.
The fetal microchimerism may trigger a fetus versus host reaction and therefore may play a role in the pathogenesis of autoimmune diseases including LP.
There are many studies done to check whether microchimerism plays any role in the pathogenesis of oral lichen planus (OLP).
In the studies, mucosal biopsies of 12 women with OLP were tested for the presence of both male cells and male DNA originating from prior pregnancies or prior blood transfusions. Male patients with OLP served as a control group. Biopsies were analyzed for the presence of Y-chromosome-positive cells by fluorescence in situ hybridization (FISH) with X- and Y-specific DNA probes. To confirm the FISH findings, fluorescent polymerase chain reaction (PCR) was used to identify Y-chromosome sequences in DNA extracted from mucosal lesions. All the biopsy samples showed good hybridization results. In females, Y-chromosome-specific signals were not detected by FISH at any site of the lesions. Male DNA microchimerism was not present in any of the investigated lesions, suggesting that microchimerism because of persisting male fetal cells is unlikely to play a major role in the pathogenesis of OLP.[23]
Microchimerism in the pathogenesis of Sjögren's syndrome
SS, an autoimmune rheumatic disease of the exocrine glands, also has many clinical and pathological similarities to chronic GVHD. Microchimerism has recently been investigated in SS, a disease in which some histological patterns mirror the lesions seen in chronic GVHD.[14,24] The strong female predilection and increased incidence of SS after childbirth suggest a relationship between SS and pregnancy. These observations have led to the hypothesis that fetal microchimerism may be involved in the pathogenesis of SS as well as systemic sclerosis. To explore this hypothesis, various studies were carried out, where DNA extracted from the affected minor salivary glands was analyzed for the Y-chromosome-specific gene using a nested PCR test. In the minor salivary gland specimens, the Y-chromosome-positive fetal cells were identified by in situ hybridization with a Y-chromosome-specific DNA probe.[25]
In the study carried out by Endo et al., in salivary glands, fetal DNA was detected in 11 of 20 women with SS but in only one of eight normal controls using PCR test. In addition, fetal cells were clearly detected in three out of eight women with SS by the use of in situ hybridization.[25]
Hence, the identification of fetal cells in salivary glands suggests that anti-maternal GVHD may be involved in the development of SS.
ROLE OF MICROCHIMERISM IN CANCER
Although fetal microchimerism (FMc) has been implicated as a mechanism of autoimmune disease, it may confer a beneficial effect with immune surveillance of malignant cells.[26]
Fetal microchimerism also plays a protective role in suppressing tumor development in case of breast cancer in women that have been pregnant. Allogeneic fetal microchimeric cells might provide immunosurveillance for breast cancer in parous women, and further that parous women that do develop breast cancer may have a reduced source of acquired allogeneic immunity.
Pregnancy at older ages has been associated with a reduced risk for ovarian cancer. Given the facts that the number of microchimeric cells in parous women declines as a function of time elapsed after pregnancy, and that ovarian cancer develops at the highest frequency in postmenopausal women, it is possible that fetal microchimerism may play a protective role in ovarian cancer as well.
Microchimeric fetal cells have also been shown to cluster in lung tumors in women decades after pregnancy. Their frequency in tumors was severalfold higher in lung tumors than in surrounding healthy lung tissue. The fetal cells may be recruited from the bone marrow to the tumor sites where they assume their role in immunosurveillance and tissue repair.
As is the case in their involvement in noncancerous diseases where they sometimes fight the disease and other times contribute to the disease, these cells sometimes help to suppress tumor development by taking on an immunosurveillance role, and other times they may behave like cancer stem cells and contribute to the growth of tumors.[27]
ROLE OF MICROCHIMERISM IN THE WOUND HEALING
While persistent fetal cells were first implicated in autoimmune disease, parallel studies in animal and human pregnancy now suggest that microchimeric fetal cells also play an important role in the response to tissue injury.
Microchimeric fetal cells also expressed Collagen I, III and TGF-β3 in healed maternal scars. Identification of male-presumed fetal cells in healed maternal cesarean section scars after pregnancy suggests that, possibly in response to signals produced by maternal skin injury at cesarean section, fetal cells migrate to the site of damage to become involved in maternal tissue repair, or proliferate locally.[28]
METHODS OF DETECTION OF MICROCHIMERISM
A few studies have used the identification of male DNA or male cells in a woman as the demonstration of microchimerism. The results represent presumptive evidence of fetal microchimerism in women with prior male pregnancies or, in transplantation, of male donor microchimerism in female recipients. Male DNA is detected by PCR amplification of a Y-chromosome-specific sequence. Male cells are detected by FISH with the labeling of the X and Y chromosomes.
CONCLUSION
Microchimerism is defined by the presence of circulating cells, bidirectionally transferred from one genetically distinct individual to another. It can occur either physiologically during pregnancy or iatrogenically after blood transfusion and organ transplants. The migrated MC cells may persist for long periods and even decades. Much controversy exists around the role of microchimeric cells in the pathogenesis of various diseases and around their role in tissue repair. Microchimerism has been investigated in different autoimmune disorders such as systemic sclerosis, systemic lupus erythematosus, autoimmune thyroid diseases, primary biliary cirrhosis and juvenile inflammatory myopathies.
Recent research data have demonstrated the promising role of microchimeric cells in the maternal response to tissue injuries by differentiating into many lineages. Therefore, further understanding of fetal-maternal microchimerism may help in anticipating its implications in disease as well as in more general women's health issues.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Polejaeva I, Mitalipov S. Stem cell potency and the ability to contribute to chimeric organisms. Reproduction. 2013;145:R81–8. doi: 10.1530/REP-12-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlitt HJ, Ko S, Deiwick A, Hundrieser J. Microchimerism in organ transplantation. In: Timmerman W, Gassel HJ, Ulrichs K, Zhong R, Thiede A, editors. Organ Transplantation in Rats and Mice. 1st ed. Berlin: Springer Berlin Heidelberg; 1998. pp. 285–98. [Google Scholar]
- 3.Galofré JC. Microchimerism in Graves' disease. J Thyroid Res. 2012;2012:724382. doi: 10.1155/2012/724382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson JL. Microchimerism in human health and disease. Autoimmunity. 2003;36:5–9. doi: 10.1080/0891693031000067304. [DOI] [PubMed] [Google Scholar]
- 5.Gammill HS, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol. 2010;54:531–43. doi: 10.1387/ijdb.082767hg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawe GS, Tan XW, Xiao ZC. Cell migration from baby to mother. Cell Adh Migr. 2007;1:19–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Boyon C, Collinet P, Boulanger L, Rubod C, Lucot JP, Vinatier D, et al. Fetal microchimerism: Benevolence or malevolence for the mother? Eur J Obstet Gynecol Reprod Biol. 2011;158:148–52. doi: 10.1016/j.ejogrb.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93:705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miech RP. The role of fetal microchimerism in autoimmune disease. Int J Clin Exp Med. 2010;3:164–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson AM, Papadogiannakis N, Granath A, Haggstrom J, Schaffer M, Uzunel M, et al. Maternal microchimerism in juvenile tonsils and adenoids. Pediatr Res. 2010;68:199–204. doi: 10.1203/PDR.0b013e3181eb2eb4. [DOI] [PubMed] [Google Scholar]
- 11.Kara RJ, Bolli P, Karakikes I, Matsunaga I, Tripodi J, Tanweer O, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klonisch T, Drouin R. Fetal-maternal exchange of multipotent stem/progenitor cells: Microchimerism in diagnosis and disease. Trends Mol Med. 2009;15:510–8. doi: 10.1016/j.molmed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Lambert NC, Lo YM, Erickson TD, Tylee TS, Guthrie KA, Furst DE, et al. Male microchimerism in healthy women and women with scleroderma: Cells or circulating DNA? A quantitative answer. Blood. 2002;100:2845–51. doi: 10.1182/blood-2002-01-0295. [DOI] [PubMed] [Google Scholar]
- 14.Giacomelli R, Matucci-Cerinic M, Bombardieri S. Microchimerism in Sjögren's syndrome. Ann Rheum Dis. 2002;61:1039–40. doi: 10.1136/ard.61.12.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurel MC, Kanellopoulos-Langevin C. Heredity – Venturing beyond genetics. Biol Reprod. 2008;79:2–8. doi: 10.1095/biolreprod.107.065607. [DOI] [PubMed] [Google Scholar]
- 16.Vogelgesang A, Scapin C, Barone C, Tam E, Blumental Perry A, Dammann CE. Cigarette smoke exposure during pregnancy alters fetomaternal cell trafficking leading to retention of microchimeric cells in the maternal lung. PLoS One. 2014;9:e88285. doi: 10.1371/journal.pone.0088285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loubière LS, Lambert NC, Flinn LJ, Erickson TD, Yan Z, Guthrie KA. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest. 2006;86:1185–92. doi: 10.1038/labinvest.3700471. [DOI] [PubMed] [Google Scholar]
- 18.Waszak M, Cieślik K, Wielgus K, Słomski R, Szalata M, Skrzypczak-Zielińska M. Microchimerism in twins. Arch Med Sci. 2013;9:1102–6. doi: 10.5114/aoms.2013.39212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knippen MA. Microchimerism: Sharing genes in illness and in health. ISRN Nurs. 2011;2011:893819. doi: 10.5402/2011/893819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TH, Paglieroni T, Utter GH, Chafets D, Gosselin RC, Reed W. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 21.Jindal RM, Sahota A. The role of cell migration and microchimerism in the induction of tolerance after solid organ transplantation. Postgrad Med J. 1997;73:146–50. doi: 10.1136/pgmj.73.857.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perico N, Remuzzi G. Acquired transplant tolerance. Int J Clin Lab Res. 1997;27:165–77. doi: 10.1007/BF02912453. [DOI] [PubMed] [Google Scholar]
- 23.Weger W, Bauer M, Odell E, Pertl B, Cerroni L, Kerl H. Role of microchimerism in the pathogenesis of oral lichen planus. Exp Dermatol. 2006;15:125–9. doi: 10.1111/j.1600-0625.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 24.Carlucci F, Priori R, Alessandri C, Valesini G, Stoppacciaro A. Y chromosome microchimerism in Sjögren's syndrome. Ann Rheum Dis. 2001;60:1078–9. doi: 10.1136/ard.60.11.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo Y, Negishi I, Ishikawa O. Possible contribution of microchimerism to the pathogenesis of Sjögren's syndrome. Rheumatology (Oxford) 2002;41:490–5. doi: 10.1093/rheumatology/41.5.490. [DOI] [PubMed] [Google Scholar]
- 26.Gadi VK, Nelson JL. Fetal microchimerism in women with breast cancer. Cancer Res. 2007;67:9035–8. doi: 10.1158/0008-5472.CAN-06-4209. [DOI] [PubMed] [Google Scholar]
- 27.Sawicki JA. Fetal microchimerism and cancer. Cancer Res. 2008;68:9567–9. doi: 10.1158/0008-5472.CAN-08-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood U, O'Donoghue K. Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism. 2014;5:40–52. doi: 10.4161/chim.28746. [DOI] [PMC free article] [PubMed] [Google Scholar]


