Abstract
Background.
Infants who are iron-deficient anemic seek and receive less stimulation from their caregivers, predisposing such children to be functionally isolated.
Objective.
To test the above sequence whereby iron deficiency in infancy contributes to children’s disengagement from the environment, which reduces parent stimulation which, in turn, contributes to children’s poor verbal skills.
Methods.
Chilean children (N = 875, 54% male) were studied, 45% of whom were iron deficient or iron-deficient anemic in infancy. We used structural equation modeling to test the sequence outlined above and to examine the effect of infant iron status on children’s verbal performance at ages 5 and 10 years including the roles of child and parent intermediate variables.
Results.
Severity of iron deficiency in infancy was associated with higher levels of children’s dull affect and social reticence at 5 years (β = .10, B = .26, SE = .12, p < .05), and these behaviors were associated with parent unresponsiveness (β = .29, B = .13, SE = .03, p < .001), which related to children’s lower verbal abilities at age 5 (β = −.29, B = −2.33, SE = .47, p < .001) and age 10 (β = −.22, B = −3.04, SE = .75, p < .001). An alternate model where poor iron status related directly to children’s verbal ability was tested but not supported.
Conclusion.
Findings support functional isolation processes resulting from a nutritional deficiency, with iron-deficient anemic infants showing affective and behavioral tendencies that limit developmentally stimulating caregiving which, in turn, hinder children’s verbal abilities.
Keywords: child affect, iron-deficiency anemia, parent stimulation, social reticence, verbal abilities
Introduction
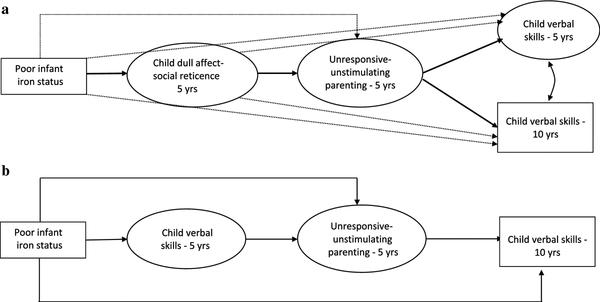
The estimated global prevalence of anemia in children younger than 5 years is 43%, or approximately 273 million children worldwide, mostly due to iron deficiency (Stevens et al., 2013). In the U.S, 1.5 million children are iron deficient, including 15% of 1–2-year-olds (Gupta, Perrine, Mei, & Scanlon, 2016). Like severely malnourished children, iron-deficient anemic infants have been observed to be listless, lethargic, emotionally dull, and show less exploration of their environments (Corapci, Radan, & Lozoff, 2006; Lozoff et al., 2010). At age 4 years, children who were iron-deficient anemic in infancy display less smiling and are less physically active than children who were non-anemic throughout infancy (Chang et al., 2011; Lozoff et al., 2007). Such social-emotional behaviors predispose such children to be “functionally isolated,” or less able to seek and receive environmental inputs crucial for social and cognitive skill development (Brown & Pollitt, 1996; Wachs, 2009). Functional isolation has been described as a two-part process, whereby nutritional deficiencies limit affective and behavioral engagement with the environment, which in turn leads to diminished environmental input (Levitsky & Strupp, 1995). One key environmental input is parents’ responsiveness and stimulation, with several studies showing that mothers of iron-deficient anemic infants are less sensitive to infant cues and less cognitively and socially stimulating than mothers of non-anemic infants (Armony-Sivan et al., 2010; Corapci et al., 2006). Research has not, however, addressed the long-term consequences of these reduced parent-child interactive patterns associated with infant iron deficiency. One study, though, found that parents’ unresponsiveness to children who were anemic in infancy related to children’s social and behavioral problems in adolescence (Authors et al., 2017). Parental responsiveness is particularly instrumental in children’s acquisition of verbal and language skills (Tamis-LeMonda et al., 2001). Drawing on the functional isolation hypothesis, we hypothesized that iron-deficiency anemia in infancy would contribute to children’s dull affect and social reticence at age 5, which would relate to parents’ unresponsiveness and under-stimulation. We further hypothesized that parent unresponsiveness would lead to children’s poor verbal skills at ages 5 and 10 years. A conceptual model illustrating this process is shown in Figure 1a.
Figure 1.
a (top) Model of the tested associations among infant iron status, children’s dull affect-social reticence, parental unresponsiveness, and children’s verbal skills. Bolded pathway represents the process of functional isolation. Children’s verbal skills at age 5 and 10 were correlated a priori.
b (bottom) Model of an alternate ordering of study variables wherein poor infant iron status contributes directly to children's verbal skills.
Several studies also show that children who were iron deficient in infancy have lower cognitive abilities (Carter et al., 2010; McCann & Ames, 2007). Thus, we also considered whether iron-deficiency anemia has a direct effect on children’s verbal skills, which then lead to parent unresponsiveness (Figure 1b). This alternate model was tested to determine whether the behavioral deficits stemming from prior anemia play a more pivotal role in children’s outcomes than the anemia itself.
Methods
Study Design and Participants
Participants were 875 mother and child dyads (53.5% male) who have been studied since the child’s infancy as part of an iron-deficiency anemia preventive trial or neuromaturation study (Lozoff et al., 2003). The preventive trial involved 1,657 6-month-old non-anemic infants who were randomly assigned into one of three groups: 718 infants were fed a high-iron (12 mg/l) formula (from 6 to 12 months); 405 infants were fed a low-iron (2.3 mg/l) formula (6–12 mos), and; 534 infants continued nutrition as usual (6–12 mos), which typically involved formula with no-added iron. (All commercially available infant formula available in Chile at that time had no-added iron.) The neuromaturation study involved 135 6-month-old infants (73 iron-deficient anemic and 62 non-anemic controls) who completed assessments of the main study and additional laboratory assessments. Infants were recruited from community clinics in Santiago, Chile, and all were born healthy at term (birth weight ≥ 3.0 kg) and had no perinatal complications or acute or chronic illnesses. Participants were generally from low-to-middle income families.
At age 5½ years, 888 children who received either high- or no-added iron supplementation as part of the preventive trial or were in the neuromaturation study were assessed. The entire sample was not invited for study at age 5 due to a budget cut (infants in the low-iron supplementation group were not studied). At 5½ years, children’s behaviors and caregiver interactions were assessed. At the 10-year follow-up, funding allowed recruitment from the original infancy sample, of which 1,127 study children participated. (See Supplemental Figure 1 for a flow chart of sample selection).
In order to study participants with the most complete longitudinal data, the current analyses focused on those studied at 5½ years (n = 888). Thirteen of these families had missing data on most study variables and were eliminated from analyses, leaving 875 for the current analytic sample. All 875 child participants had iron status data at infancy, 875 had parent unresponsiveness data at age 5, 871 children had verbal ability data at age 5, and 710 children had verbal ability data at age 10. No differences were found between the 875 children in the current analytic sample and the original sample of 1,792 (i.e., 1657 in the preventive trial, 135 in the neuromaturation study) regarding mothers’ education, IQ, or depression, or any of the study variables (child’s dull affect, verbal skills, parents’ unresponsiveness, etc.). However, compared to children who were not part of the current analytic sample, those who are studied here were less likely to have received iron supplementation in infancy, were breastfed longer, and were from higher socioeconomic status (SES) families (see Supplemental Table 1). We adjust for these variables in our analyses.
Procedures
All study components at children’s infancy and ages 5½ and 10 years were approved by the relevant institutional review boards in the U.S. and Chile. Signed informed consent was obtained from parents at each time point; assent was obtained from children at age 10. All study procedures were in accord with the ethical standards outlined in the Declaration of Helsinki and its later amendments (World Medical Association, 2013).
Measures
At all study time points, Spanish versions of the study measures were used. They were pilot-tested with the study population prior to conducting the study and had good reliability and high equivalence to the English versions. The Cronbach’s coefficient alphas (α) of the multi-item measures are reported below using the current sample.
Infant iron status.
At 6 months, children underwent a finger stick to determine hemoglobin levels. Infants with hemoglobin values ≤ 103 g/L had a venipuncture performed to determine iron status. Anemia at 6 months was defined as a venous hemoglobin concentration ≤ 100 g/L and two of three iron measures in the iron deficient range (mean corpuscular volume < 70 fL, erythrocyte protoporphyrin > 100 μg/L red blood cells, and serum ferritin < 12 mg/L). Infants who were anemic at 6 months were not entered in the preventive trial but were treated and studied in the neuromaturation study. At 12 months, venipuncture blood specimens (7–10 ml) were drawn on all infants; at 18 months, infants in the low- and no-added iron groups received another venipuncture. Anemia at 12 and 18 months was defined as venous hemoglobin < 110 g/L, and iron deficiency was defined as two of the three iron measures in the iron-deficient range (described above). These criteria were the standard in the field (Dallman, Reeves, Driggers, & Lo, 1981). Infants’ iron status was coded as the most severe diagnosis at any of the three assessments (6m, 12m, or 18m), with iron sufficient (i.e., iron sufficient at all 3 assessments) coded as 0, iron deficient-without anemia coded as 1, and iron deficient-with anemia coded as 2. The proportions of the iron status groups are shown in Table 1. Venous blood samples were also obtained at 5 and 10 years. Iron status was generally good after infancy: no child had IDA at age 5 or 10 years and only 5% had ID at 10 years.
Table 1.
Descriptive Statistics of Sample and Study Measures by Participant Age at Assessment (N = 875)
| N | Min | Max | Mean or % | Standard deviation | |
|---|---|---|---|---|---|
| Infant assessment | |||||
| Iron status | |||||
| Iron sufficient | 481 | 0 | 1 | 55.0% | |
| Iron deficient without anemia | 254 | 0 | 1 | 29.0% | |
| Iron deficient with anemia | 140 | 0 | 1 | 16.0% | |
| †Child sex (1 = male) | 875 | 0 | 1 | 53.5% | |
| †Family socioeconomic statusa | 872 | 9 | 43 | 27.06 | 6.19 |
| †Family stressors | 814 | 0 | 30 | 4.66 | 2.66 |
| †Mothers’ educational level | 814 | 0 | 19 | 9.51 | 2.59 |
| †Mothers’ IQ | 873 | 51 | 110 | 83.56 | 9.41 |
| †Infant dull temperament | 678 | 3 | 16 | 7.58 | 2.81 |
| †Duration of exclusive breastfeedingb | 875 | 0 | 18.4 | 3.87 | 3.18 |
| †Iron supplementationc | 835 | 0 | 1 | 55% | |
| †No. children family ≤ 15 years | 872 | 1 | 8 | 2.33 | 1.09 |
| 5 Year Assessment | |||||
| Child age | 875 | 5.42 | 5.83 | 5.51 | 0.03 |
| Child’s dull affect-social reticence | |||||
| Child dull affect | 875 | 6 | 24 | 9.65 | 2.70 |
| Child social reticence | 875 | 8 | 32 | 14.56 | 4.58 |
| Parents’ unresponsivenessd | |||||
| Parents’ low responsiveness | 875 | 0 | 3 | 0.35 | 0.61 |
| Parents’ low language stimulation | 875 | 0 | 4 | 0.72 | 0.83 |
| Parents’ low academic stimulation | 875 | 0 | 5 | 1.95 | 1.32 |
| †Mothers’ depressive symptoms | 872 | 0 | 60 | 19.69 | 13.40 |
| Child verbal skills | |||||
| WPPSI-R verbal std scoree | 872 | 9.96 | 76.36 | 38.59 | 8.62 |
| Preschool Language Scale language std score | 871 | 50 | 123 | 90.80 | 12.13 |
| Woodcock-Munoz Language Survey vocabulary score | 871 | 15 | 30 | 21.34 | 2.29 |
| 10 Year Assessment | |||||
| Child age | 714 | 9.92 | 11.0 | 10.03 | 0.09 |
| WISC-R verbal std scoref | 710 | 13 | 75 | 41.62 | 11.35 |
Note. Bolded variables are latent variables.
Indicates a control variable.
Higher scores indicate greater poverty.
Coded in months.
Supplemented with high- or low-iron formula as part of the preventive trial.
Higher scores indicate lower responsiveness and lower stimulation.
Wechsler Preschool and Primary Scale of Intelligence – revised version.
Wechsler Intelligence Scale for Children- revised version.
Child dull affect and social reticence at 5 years.
Mothers completed the Children’s Adaptive Behavior Inventory (Cowan & Cowan, 1990), which includes scales of dull affect (6 items: e.g., my child shows no smiles, fixed expression, sits idly, not interested in things; α = .75) and social reticence (8 items: e.g., plays alone, is isolated, ignored by others, separates self from others, is inhibited; α = .75). Response options were: never (coded as 1), rarely (2), repeatedly (3), and most of the time (4). Scores were summed across items.
Parents’ responsiveness and stimulation to child at 5 years.
Ratings on the Home Observation for Measurement of the Environment Inventory-Early Childhood version (HOME-EC) were obtained by in-person interview, with mothers indicating their responsiveness and language and academic stimulation to their child (Bradley & Corwyn, 2005). The HOME is a well-established measure that shows good equivalence between in-home observation and in-person interview responses (Han, Leventhal, & Linver, 2004), and includes several features designed to elicit valid information about children’s experiences and not the respondent’s feelings or mental representations (Totsika & Sylva, 2004). Three items were used to index verbal responsiveness (“parent answers child’s questions or requests verbally”), four items indexed parents’ language stimulation (“parent encourages child to talk”), and five items indexed academic stimulation (“child is encouraged to learn to read, … to learn patterned speech such as songs”). Ratings were scored as binary-choice options of yes (1) or no (0) and summed across items. Scores were reversed so that high scores indicate low responsiveness, low language stimulation, and low academic stimulation.
Children’s verbal abilities at age 5.
At 5½ years, children completed three cognitive tests: the Wechsler Preschool and Primary Scale of Intelligence – Revised version (WPPSI-R; Wechsler, 1989), which measures verbal intelligence represented by verbal performance, reasoning and comprehension (possible score range: 5 – 95); the Preschool Language Scales Test - 3rd edition (PLS; Zimmerman, Steiner, & Pond, 1992), which assesses language content, form, and use (possible score range: 50 – 150), and; the Woodcock-Muñoz Language Survey (WMLS; Woodcock & Muñoz-Sandoval, 1993), which is the parallel Spanish version of the Woodcock-Johnson Test of Cognitive Ability. We used the picture vocabulary score from the WMLS, which measures language development and lexical knowledge (possible score range: 0 – 58).
Children’s verbal abilities at age 10.
Children completed the verbal subtests of the Wechsler Intelligence Scale for Children-3rd edition (WISC-III; Wechsler, 1991), which measures verbal performance, expression, reasoning, and comprehension. The total standard verbal score was analyzed (possible score range: 5 – 95).
Covariates
Analyses adjusted for child, parent, and family characteristics that potentially confound the exposure-mediator relationship, the mediator-outcome relationship, or the exposure-outcome relationship (Supplemental Figure 2). These include: child sex, family socioeconomic status (SES), duration of breastfeeding, iron supplementation, family stress, maternal depression, maternal IQ, and number of children in the household, all assessed at the child’s infancy. Family SES was measured using the 13-item Graffar instrument, which assesses living and housing conditions, material possessions, etc. (Graffar, 1956). Duration of breastfeeding (in months) was reported by the participants’ mothers, and iron supplementation was recorded as part of the preventive trial. Family stress was measured using the Social Readjustment Rating Scale (Holmes & Rahe, 1967, 30 items). Maternal depression and maternal IQ were measured with the Center for Epidemiologic Studies-Depression Scale (Radloff, 1977; 20 items, α = .90) and the Wechsler Adult Intelligence Scale-III (Wechsler, 1955), respectively. Dull infant temperament was also included as a covariate when modeling children’s dull affect at 5 years to strengthen interpretation that dull affect results from iron deficiency independent of temperamental dullness (Infant Characteristics Questionnaire dull temperament scale; Bates, Freeland, & Lounsbury, 1979; α = .54). Child age was initially included as a covariate, but because there was little variability in children’s ages and because it was not related to any of the model variables, child age was not considered further. All covariates listed above remained in the model regardless of statistical significance.
Analytic Strategy
We developed a theory-driven diagram that reflects directed pathways between variables according to the functional isolation hypothesis (Figure 1a). We fit a structural equation model (SEM) using Mplus 6.0 (Muthén & Muthén, 2010) to evaluate the strength of each pathway between the exposure (infant iron status) and outcomes (child verbal skills at 5 and 10 years). Verbal skills at 5 and 10 were evaluated as separate outcomes in the primary analyses. We then fit the SEM adjusting for potential confounding variables (described above in Covariates and shown in Supplemental Figure 2). We were also interested in evaluating the role of the two intermediate variables in the path between infant iron status and children’s verbal skills: child dull affect-social reticence and unresponsive–unstimulating parenting. These two intermediate variables and children’s verbal skills at age 5 were analyzed as latent variables. Each latent variable was derived from two or more observed variables selected a priori.
We used the INDIRECT command in MPLUS to decompose the total effect of infant iron status on children’s verbal skills. Because we consider two potential intermediate variables, we estimated three indirect pathways: (1) exposure → intermediate 1→ outcome, (2) exposure → intermediate 2 → outcome, and (3) exposure → intermediate 1 → intermediate 2 → outcome. The sum of these pathways is equivalent to the total indirect effect.
Secondarily, we evaluated an alternative ordering of the model variables. This alternate path (Figure 2b) posits that infant iron status directly affects children’s verbal skills at age 5, which affects parent unresponsiveness, which then affects children’s verbal skills at age 10.
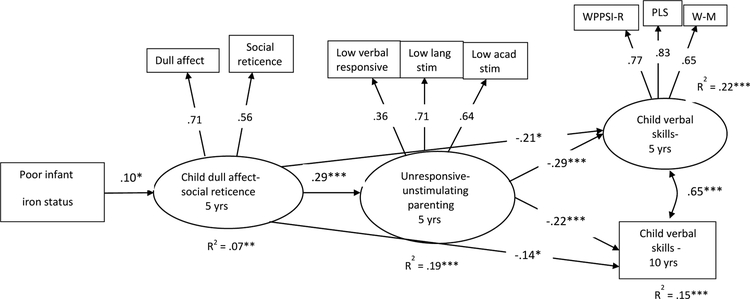
Figure 2.
Poor iron status was coded to reflect deficiency severity: 0 = iron sufficient, 1 = iron deficient, 2 = iron-deficient anemic (N = 875). Standardized coefficients are shown. All paths shown in Figure 1a were tested, only significant paths are shown.
Model fit: χ2 (102) = 205.57, CFI = .950, RMSEA = .034, SRMR = .029. WPPSI-R: Wechsler Preschool and Primary Scale of Intelligence – revised version. PLS: Preschool Language Scales. W-M: Woodcock-Muñoz Language Survey.
*p < .05. **p < .01. ***p < .001.
Model fit was indicated by the chi-square, comparative fit index (CFI > .90), root mean square error of approximation (RMSEA < .06), and standardized root mean square residual (SRMR < .08; Kline, 2011). Analyses were conducted using maximum likelihood estimators, which are robust to non-normality. Standard errors are estimated using the delta method to estimate standard errors (Muthén & Muthén, 2010). All cases were retained using the full information maximum likelihood method (FIML), which fits the model being tested directly onto the non-missing data for each participant (Muthén & Muthén, 2010). FIML has been shown to be superior to other missing data strategies (Enders & Bandalos, 2001). Missing data were < 20% for any single variable, which is an acceptable level when implementing FIML (Enders & Bandalos, 2001).
Modeling Assumptions
Several underlying assumptions of the SEM approach guide interpretation of the results. The SEM approach assumes that the directionality of the presumed effects, as noted by one-directional arrows between variables in our conceptual framework, are correctly specified. We, a priori, identified a plausible alternate ordering of study variables and report findings from both models (Figures 1a, 1b). This approach also assumes no unmeasured confounding of the exposure-outcome, exposure-mediator and mediator-outcome relationships, and that variables are measured without error. Further, we assume that observed variables adequately measure each latent variable and that the latent variables yield better estimates of complex constructs compared to individual observed variables. For all latent variables, we present factor loadings for observed variables, which can be interpreted as standardized regression coefficients (Figure 2).
Results
Latent Variables
The measurement model of the latent variables shown in Figure 2 had good fit: χ2 (17) = 47.66, CFI = .976, RMSEA = .045, SRMR = .030. The individual factor loadings for the three latent variables were ≥ .36 (all p < .001). When tested separately by child sex in a model where all paths varied freely, the pattern of loadings was nearly identical for males and females. Table 2 displays the bivariate correlations among the study variables.
Table 2.
Unadjusted correlations among model variables
| Model variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Iron deficiency severitya - infancy | - | ||||||||
| 2. Child dull affect - 5y | .08* | - | |||||||
| 3. Child social reticence - 5y | .06 | .40*** | - | ||||||
| 4. Low parent responsivenessb -5y | −.03 | 15*** | .02 | - | |||||
| 5. Low parent academic stimb -5y | −.02 | .15*** | .06 | .19*** | |||||
| 6. Low parent language stimb - 5y | −.02 | .20*** | .09* | .29*** | .44*** | - | |||
| 7. WPPSI-R verbal abilities - 5y | −.06 | −.12** | −.19*** | −.08* | −.21*** | −.21*** | - | ||
| 8. Preschool Language Scales - 5y | −.04 | −.16*** | −.20*** | −.09** | −.29*** | −.21*** | .64*** | - | |
| 9. Woodcock-Munoz vocabulary- 5y | −.08* | −.13** | −.12** | −.05 | −.20*** | −.13*** | .48*** | .56*** | - |
| 10. WISC-R verbal abilities - 10y | −.04 | −.14*** | −.16*** | −.04 | −.20*** | −.24*** | .55*** | .56*** | .47*** |
Note.
Coded as most severe diagnosis at 6m, 12m, or 18m as: 0 = iron sufficient, 1 = iron deficient, 2 = iron-deficient anemic.
Higher scores indicate lower responsiveness and stimulation.
p < .05.
p < .01.
p < .001.
Modeling Results
The model testing functional isolation processes had good model fit (Figure 2). Standardized beta estimates are shown in Figure 2, and we report the standardized and unstandardized regression coefficients (β and B, respectively) below. Results indicate that increasing iron deficiency severity was associated with higher levels of children’s dull affect-social reticence (β = .10, B = .26, SE = .12, p < .05), and children’s dull affect-social reticence was strongly associated with parent unresponsiveness (β = .29, B = .13, SE = .03, p < .001). Parents’ unresponsiveness at 5 years was related to children’s lower verbal scores at age 5 (β = −.29, B = −2.33, SE = .47, p < .001) and age 10 (β = −.22, B = −3.04, SE = .75, p < .001). Children’s dull affect-social reticence also had direct negative effects on their verbal scores at ages 5 and 10 (β = −.21, B = −.73, SE = .38, p < .05 and β = −.14, B = −.81, SE = .46, p < .05, respectively). Children’s poor iron status at infancy was not directly related to unresponsive parenting (β = .03, B = .04, SE = .05), children’s verbal skills at age 5 (β = −.04, B = −.33, SE = .34), or verbal skills at age 10 (β = −.01, B = −.15 SE = .58).
Results of the indirect effect tests (Table 3) indicate that the pathway depicting functional isolation (i.e., poor iron status → children’s dull affect-social reticence → parents’ unresponsiveness) was statistically significant (Est = .032, SE = .016). The indirect pathways from child dull affect-social reticence to parent unresponsiveness to children’s verbal skills (at age 5, and separately, age 10) were also significant. The two full-model pathways linking infant iron status to children’s verbal skills (at age 5 and, separately, age 10) approached significance. When decomposing the total effect of the model’s pathways, poor iron status was associated with children’s lower verbal skills at age 5 (Est = −.508, SE = .356). This effect was partially mediated by child dull affect-social reticence and parent unresponsiveness (total indirect effect Est = −.178, SE = .172), with approximately one-third of the total effect explained by these mediators. Poor iron status was also associated with children’s lower verbal skills at age 10 (Est = −.349, SE = .591), with the two mediators partially mediating this effect (total indirect effect Est = −.195, SE = .211). Approximately 56% of the total effect was explained by the child and parent mediators.
Table 3.
Parameter estimates and standard errors for the total effects and indirect effects under investigation (shown in Figure 2)
| Pathways | Estimate (SE) |
|---|---|
| Iron statusa → child dull affect-social reticence → parent unresponsiveness | |
| Total Effect | .004 (.047) |
| Total Indirect Effect: | |
| Iron status → child dull affect-social reticence → parent unresponsiveness | .032* (.016) |
| Iron statusa → child dull affect-social reticence → parent unresponsiveness → child verbal skills 5y | |
| Total Effect | −.508 (.356) |
| Total Indirect Effect | −.178 (.172) |
| Iron status → child dull affect-social reticence → child verbal skills 5y | −.196† (.144) |
| Iron status → parent unresponsiveness → child verbal skills 5y | .084 (.114) |
| Iron status → child dull affect-social reticence → parent unresponsiveness → child verbal skills 5y | −.075† (.040) |
| Iron statusa → child dull affect-social reticence → parent unresponsiveness → child verbal skills 10y | |
| Total Effect | −.349 (.591) |
| Total Indirect Effect | −.195 (.211) |
| Iron status → child dull affect-social reticence → child verbal skills 10y | −.208 (.140) |
| Iron status → parent unresponsiveness → child verbal skills 10y | .110 (.150) |
| Iron status → child dull affect-social reticence → parent unresponsiveness → child verbal skills 10y | −.098† (.054) |
| Child dull affect-social reticence → parent unresponsiveness → child verbal skills 5y | |
| Total Effect | −1.025** (.382) |
| Total Indirect Effect: | |
| Child dull affect-social reticence → parent unresponsiveness → child verbal skills 5y | −.293*** (.082) |
| Child dull affect-social reticence → parent unresponsiveness → child verbal skills 10y | −1.195** (.461) |
| Total Effect | |
| Total Indirect Effect | |
| Child dull affect-social reticence → parent unresponsiveness → child verbal skills 10y | −.382*** (.117) |
Note.Above are listed all indirect effects in the main model presented in Figure 2. Analyses adjusted for: child sex, iron supplementation group in the prevention trial, duration breastfed, family SES, maternal education, family stress, maternal depression, number of children in the household, and mothers’ IQ. Child dull affect-social reticence was also adjusted for infant dull temperament.
Iron status was coded to reflect increasing deficiency severity: 0 = iron sufficient, 1 = iron deficient, 2 = iron-deficient anemic.
p < .09.
p < .05.
p < .01.
p < .001.
Test of Alternate Model
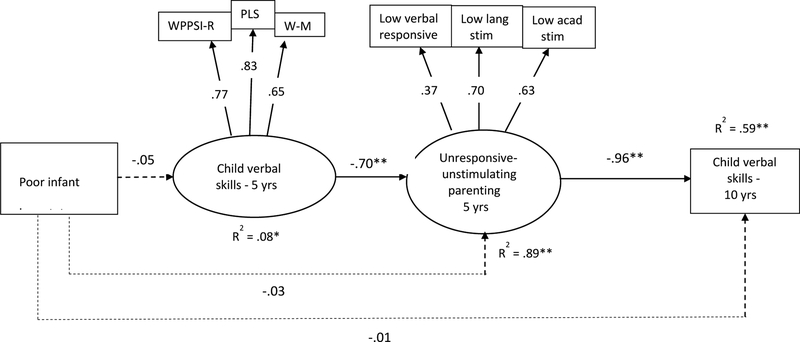
Fit indices of the model testing alternate ordering (and including identical covariates as the previous model) indicated acceptable fit (Figure 3). Fit would have improved had we regressed 5-year verbal skills onto 10-year verbal skills. However, we were interested in the direct effects of the model variables on 10-year verbal scores, not in the change in verbal scores from age 5 to age 10. The path coefficients in the model showed that iron deficiency severity was not related to children’s verbal skills at age 5 (ϐ = −.05, B = −.48 SE = .36, p >.18), nor to parents’ unresponsiveness, or to children’s verbal skills at age 10 (Figure 3). Children’s lower verbal skills at age 5, though, were strongly related to parent unresponsiveness (ϐ = −.70, B = −.06, SE = .01, p < .001), and parent unresponsiveness was highly related to children’s lower verbal scores at age 10 (ϐ = −.96, B = −20.70, SE = 3.28, p < .001). Results of the indirect effect tests are shown in Supplemental Table 2. Only the indirect path involving 5-year verbal skills → parent unresponsiveness → 10-year verbal skills was statistically significant (Est = .668, SE = .033, p < .001). When decomposing the total effect of the association between iron status and 10-year verbal skills (Est = −.354, SE = .593), the total indirect effect involving 5-year verbal skills and parent unresponsiveness explained one-quarter of the total effect (Est = .093, SE = 1.116).
Figure 3.
Model of alternate ordering of variables. Poor iron status coded to reflect increasing deficiency severity: 0 = iron sufficient, 1 = iron deficient, 2 = iron-deficient anemic (N = 875). Standardized coefficients are shown. All direct and indirect paths were tested. Dotted lines represent nonsignificant paths. Model fit: χ2 (67) = 343.89, CFI = .848, RMSEA = .069, SRMR = .037. WPPSI-R: Wechsler Preschool and Primary Scale of Intelligence – revised version. PLS: Preschool Language Scales. W-M: Woodcock-Muñoz Language Survey. *p < .01. **p < .001.
Discussion
Findings support functional isolation mechanisms resulting from poor infant iron status, with severity of iron deficiency relating to children’s solemn and reticent behaviors, which set into motion a series of effects that diminished children’s verbal and language skills. These results highlight a psychosocial pathway linking an early nutritional deficit to adverse outcomes, with inadequately nourished children showing behaviors that produce less social stimulation, which then hampers their growth and development (Wachs, 2009). Tests of an alternate model showed that infant iron status did not contribute directly to children’s verbal scores or to parent responsiveness. Thus, children’s behavioral deficits stemming from prior iron deficiency appear to play a more pivotal role in their verbal performance skills than the iron deficiency itself. We maintain, however, that there could be long-lasting direct effects of iron-deficiency anemia on other cognitive outcomes depending on the brain region or neurocircuitry affected by the prior anemia (Georgieff, 2011).
Findings extend earlier research in several ways. First, very few studies have directly tested functional isolation, with studies focused on the effects of nutritional deficiency on children’s behavior (Lozoff et al., 2010) or parents’ behavior (e.g., Armony-Sivan et al., 2010), but not both. Studies have also examined functional isolation as child inactivity resulting from undernutrition (e.g., Gardner et al., 1995), but this does not incorporate a key component of the functional isolation process, which emphasizes deficits affecting children’s ability to elicit social responses from others (Brown & Pollitt, 1996). The current study attempted to address the functional isolation hypothesis in full, incorporating an initial nutritional deficiency, children’s social and emotional disengagement, parent unresponsiveness-low stimulation, and a pivotal developmental outcome likely to be affected by low environmental input.
Second, results show that the limited parent stimulation to children who had poor iron status as infants continues into early childhood and is associated with children’s lower verbal abilities at age 10. Thus, functional isolation processes last into early childhood and interfere with children’s verbal development well into middle childhood. However, children in the current study tested broadly in the normal intelligence range, such that effects on children’s verbal scores should not be interpreted as significant impairment but, rather, as lower scores within the normal range.
Limitations and Strengths
Certain study limitations are important for interpreting the findings. Child behavior ratings and parent responsiveness ratings were both completed by mothers, leaving open the possibility of shared-reporter bias. In addition, our sample was low-to-middle income; results may not generalize to families of other socioeconomic levels. Although we adjusted for many child, family, and home characteristics, unmeasured features in the environment could have contributed to the relations found. Participants in the current sample differed from non-responders in that they were breastfed longer, had higher SES, and were less likely to receive iron supplementation as part of the preventive trial. We adjusted for these variables in our model. Nonetheless, there remains the possibility that we were unable to fully account for all variables that contributed to the probability of selection into the sample. Additionally, iron status was not determined for most mothers. Poor maternal iron status may have contributed to their unresponsiveness. The current results also may not generalize to samples outside of Chile. Different cultures have different norms toward child independence and parent-child interdependence, which can affect parents’ stimulation and responsiveness to their children (Quintana et al., 2006). Finally, due to the observational nature of the data and the cited limitations, we are cautious to infer causality among the variables analyzed, and we encourage other researchers to replicate this work.
Strengths are the large sample studied for 10 years. Participants were studied when there was no national program of infant iron supplementation, resulting in relatively high rates of iron deficiency and iron-deficiency anemia, allowing for a robust test of effects associated with these conditions. All children were born healthy of uncomplicated pregnancies. Thus, there were no known health problems confounding children’s health status. Almost all children had good iron status in childhood, making it unlikely that chronic iron deficiency and/or anemia contributed to outcomes. Mothers’ depression was incorporated as a key covariate, which had not been ruled out in earlier studies that found that the mothers of iron-deficient anemic infants were less responsive (Lozoff et al., 2007). Additionally, we used several assessments measuring children’s verbal intelligence at both age 5 and 10, including their vocabulary, comprehension, reasoning, verbal expression and language development.
Conclusions
Findings point to the behavioral consequences of an early nutritional deficiency and its effects on environmental input as contributors to poor outcomes and not to the nutrient deficiency itself, a finding that has intervention implications. For example, given a history of iron-deficiency anemia, healthcare providers should check for aberrations in child affect, energy and social engagement. If present, a discussion with parents encouraging responsive and appropriately stimulating parenting would be beneficial (Landry, Smith, Swank, & Guttentag, 2008). A home-visiting program that supported responsive parenting in mothers of anemic infants showed improvements in children’s cognitive and socioemotional behavior and could be helpful (Lozoff et al., 2010). The persistence of negative outcomes related to infant iron-deficiency anemia found here highlights the need for vigilant prevention, screening, and treatment to lessen the long-term effects of this pervasive nutrient disorder.
Supplementary Material
Significance:
Infants who are iron-deficient anemic have affective and behavioral tendencies indicative of social disengagement, and parents of such children are less stimulating and responsive. Results of the current study support the progression of these mechanisms across time and confirm their adverse effects for children’s verbal abilities in middle childhood. Findings point to the behavioral consequences of an early nutritional deficiency and its effects on environmental input as contributors to poor outcomes and not to the nutrient deficiency itself.
References
- Armony-Sivan R, Kaplan-Estrin M, Jacobson SW, & Lozoff B (2010). Iron-deficiency anemia (IDA) in infancy and mother-infant interaction during feeding. Journal of Developmental and Behavioral Pediatrics, 31, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JE, Freeland CB, & Lounsbury ML (1979). Measurement of infant difficultness. Child Development, 50, 794–803. [PubMed] [Google Scholar]
- Bradley RH, & Corwyn RF (2005). Caring for children around the world: A view from HOME. International Journal of Behavioral Development, 29, 468–478. [Google Scholar]
- Brown JL, & Pollitt E (1996). Malnutrition, poverty and intellectual development. Scientific American, 274, 38–43. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, … & Jacobson SW (2010). Iron deficiency anemia and cognitive function in infancy. Pediatrics, 126(2), e427–e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Wang L, Wang Y, Brouwer ID, Kok FJ, Lozoff B, & Chen C (2011). Iron deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics, 127, e927–e933. [DOI] [PubMed] [Google Scholar]
- Corapci F, Radan AE, & Lozoff B (2006). Iron deficiency in infancy and mother-child interaction at 5 years. Journal of Developmental and Behavioral Pediatrics, 27, 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan PA, & Cowan CP (1990). Becoming a family: Research and intervention In Sigel I & Brody A (Eds.), Family research (pp. 1–51). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Dallman PR, Reeves JD, Driggers DA, & Lo YTE (1981). Diagnosis of iron deficiency: The limitations of laboratory tests in predicting response to iron treatment in 1-year-old infants. Journal of Pediatrics, 99, 376–381. [DOI] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural equation modeling, 8(3), 430–457. [PubMed] [Google Scholar]
- Gardner JMM, Grantham-McGregor SM, Himes J, & Chang S (1999). Behaviour and development of stunted and nonstunted Jamaican children. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 40(5), 819–827. [DOI] [PubMed] [Google Scholar]
- Georgieff MK (2011). Long-term brain and behavioral consequences of early iron deficiency. Nutrition Reviews, 69(1), S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffar M (1956). A method for social classification of samples of population.Courier, 6, 455–459. [Google Scholar]
- Gupta PM, Perrine CG, Mei Z, & Scanlon KS (2016). Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients, 8(6), 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WJ, Leventhal T, & Linver MR (2004). The home observation for measurement of the environment in middle childhood: A study of three large-scale data sets. Parenting, 4(2–3), 189–210. [Google Scholar]
- Holmes TH, & Rahe RH (1967). The Social Readjustment Rating Scale. Journal of Psychosomatic Research, 11, 213–218. [DOI] [PubMed] [Google Scholar]
- Kline RB (2011). Principles and practice of structural equation modeling (3rd ed.). New York: Guilford Press. [Google Scholar]
- Landry SH, Smith KE, Swank PR, & Guttentag C (2008). A responsive parenting intervention: The optimal timing across early childhood for impacting maternal behaviors and child outcomes. Developmental Psychology, 44(5), 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky DA, & Strupp BJ (1995). Malnutrition and the brain: Changing concepts, changing concerns. Journal of Nutrition, 125, 2212S–20S. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawa S, & Black M (2007). Preschool-aged children with iron deficiency anemia show altered affect and behavior. Journal of Nutrition, 137, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, & Pino P (2003). Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics, 112, 846–854. [PubMed] [Google Scholar]
- Lozoff B, Smith JB, Clark KM, Perales CG, Rivera F, & Castillo M (2010). Home intervention improves cognitive and social-emotional scores in iron-deficient anemic infants. Pediatrics, 126, e884–e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JC, & Ames BN (2007). An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. American Journal of Clinical Nutrition, 85, 931–45. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2010). Mplus user’s guide (6th ed.). Los Angeles: Authors. [Google Scholar]
- Quintana SM, Aboud FE, Chao RK, Contreras-Grau J, Cross WE, Hudley C, … & Vietze DL (2006). Race, ethnicity, and culture in child development: Contemporary research and future directions. Child Development, 77, 1129–1141. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F … & Ezzati M (2013). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet: Global Health, 1, e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, & Baumwell L (2001). Maternal responsiveness and children’s achievement of language milestones. Child Development, 72(3), 748–767. [DOI] [PubMed] [Google Scholar]
- Totsika V, & Sylva K (2004). The home observation for measurement of the environment revisited. Child and Adolescent Mental Health, 9(1), 25–35. [DOI] [PubMed] [Google Scholar]
- Wachs TD (2009). Models linking nutritional deficiencies to maternal and child mental health. American Journal of Clinical Nutrition, 89, 935S–39S. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1955). The Manual for the Wechsler Adult Intelligence Scale. Oxford, England: Psychological Corp. [Google Scholar]
- Wechsler D (1989). Wechsler preschool and primary scale of intelligence-revised. San Antonio. TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1991). Manual for the Wechsler intelligence scale for children-(WISC-III). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Woodcock RW, & Muñoz-Sandoval AF (1993). Woodcock-Muñoz language survey, Spanish form. Itasca, IL: Riverside Publishing. [Google Scholar]
- World Medical Association (2013). Declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association, 310, 2191–94. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, & Pond RE (1992). Preschool language Scale, 3rd Edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.