Abstract
The Caprini risk assessment model (RAM) has been validated in over 250 000 patients in more than 100 clinical trials worldwide. Ultimately, appropriate treatment options are dependent on precise completion of the Caprini RAM. As the numerical score increases, the clinical venous thromboembolism rate rises exponentially in every patient group where it has been properly tested. The 2013 Caprini RAM was completed by specially trained medical students via review of the presurgical assessment history, medical clearances, and medical consults. The Caprini RAM was completed for every participant both preoperatively and predischarge to ensure that any changes in the patient’s postoperative course were captured by the tool. This process led to the development of completion guidelines to ensure consistency and accuracy of scoring. The 2013 Caprini scoring system provides a consistent, thorough, and efficacious method for risk stratification and selection of prophylaxis for the prevention of venous thrombosis.
Keywords: thromboprophylaxis, risk stratification, deep vein thrombosis, pulmonary embolism, Caprini risk assessment
Background
Pulmonary embolism (PE) and deep vein thrombosis (DVT), collectively known as venous thromboembolism (VTE), represent a major public health dilemma that affects 350 000 to 600 000 Americans annually.1 Venous thromboembolism remains the most preventable cause of death in hospitalized patients and is known to cause significant morbidity with associated health-care expenditure.2 After a primary thromboembolic episode, VTE recurs in approximately 25% of patients over the subsequent 10 years. Complications associated with VTE include postthrombotic syndrome after DVT (20%-50% incidence) and chronic thromboembolic pulmonary hypertension after PE (0.1%-3.8% incidence).3 These conditions negatively impact quality of life and individual productivity while adding burden to the patient, their support system, and the health-care system at large.
There has been a marked increase in federal and national efforts over the last decade to increase both awareness and treatment of this avoidable outcome. In 2008, the Surgeon General and the Director of the National Heart, Lung and Blood Institute announced a “Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism.” The Call to Action emphasized public awareness about risk factors, triggering events, and symptoms of venous thrombosis and PE and encouraged the development of evidence-based practices for screening, prevention, diagnosis, and treatment of venous thrombosis and PE.4 From 2006 through 2008 in recognition of the high attributable risk of DVT and PE due to hospitalization, the National Quality Forum, the Joint Commission, and the Centers for Medicare and Medicaid Services all instituted policies and measures to reduce VTE and promote appropriate prophylaxis to at-risk patients in the hospital setting.5 In 2008, the eighth edition of the American College of Chest Physicians (AT8) guidelines for the prevention of VTE endorsed the need for an “active, formal strategy” to prevent hospital-induced VTE. The authors felt that available risk assessment models (RAMs) were minimally validated and impractical to use.6 They proposed that thrombosis prophylaxis should be provided for groups of patients where clinical trial data were available. It has been subsequently shown that since many patients in clinical practice did not fit the criteria for clinical trials, a specific analysis of each individual’s risk factors was a better approach. The availability of the electronic medical record has facilitated collection of these data and assisted in the implementation of RAMs. As a result, over the past 5 years, individual thrombosis risk assessment has become accepted practice in most surgical specialties.
There are multiple quantitative VTE RAMs available for use in clinical practice. The Padua Prediction Score and IMPROVE RAMs were designed to address VTE risk in medical patients.7,8 The ninth edition of the American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis guideline (AT9) has recognized only 2 risk assessment tools in the nonorthopaedic surgical population, the Rogers Score, and the Caprini Score.9 The Rogers Score has been validated in general, vascular, and thoracic surgery in a single study. The score is based on variables that were found to be independent predictors of VTE risk such as surgical procedure, female sex, and a variety of other individual patient characteristics.10 Unlike the Caprini RAM, the Rogers score does not take into account certain VTE risk factors including any personal or family history of VTE and thrombophilia. Several other issues have been identified with using this model. Firstly, the categorizations by which variables are assigned point values are not easy to follow and have been noted as “cumbersome” to use.9,10 Secondly, the study that was used to validate this RAM did not clearly state which patients received prophylactic measures for VTE, and what type they received (ie, mechanical, pharmacological).9
Caprini Risk Assessment Tool for VTE
A group of physicians, nurses, and scientists led by Dr Caprini developed a risk assessment scoring system first published in 1991.11 Individual risk factors were assigned one or more points according to their relative risk of resulting in a thrombotic event. Factors such as surgery or cancer received 2 points, whereas varicose veins or oral contraception were assigned one point. The initial assignment of points was based on the relative risk of thrombosis for each risk factor using current literature at that time. For example, cancer or past history of thrombosis were stronger risk factors than swollen legs. The total score for each patient was compared to the incidence of clinically evident VTE events at 30 days to validate the model. An exponential increase in thrombotic events with increasing score has been observed for every group properly tested. This allowed for categorizing patients into low-, moderate-, and high-risk groups based on the thrombosis event rate for each group. Using this stratification data, the type, duration, and strength of prophylaxis could be tailored to the level of risk. The clinical VTE event rate for each group could be compared to the patients’ risk of bleeding, resulting in prophylaxis tailored to provide optimal thrombosis protection.
Since its introduction in 1991, the Caprini RAM has been validated in over 250 000 patients in more than 100 clinical trials worldwide.12–22 One should note that the cutoff score between risk groups varies depending on the surgical population that is tested. AT9 defines the high-risk group as a score of 5 or greater for general surgery patients.9 This cutoff point, however, is not appropriate for all surgical specialties. Since then, a score of 12 has been shown to be the very high-risk cutoff for those with hip fractures.12 A companion manuscript submitted for publication in this journal involving total joint arthroplasty included 1078 patients to establish the very high-risk cutoff in this population. Krauss et al found that total joint arthroplasty patients with a score of 10 or greater were at high risk of VTE, and the authors presented data that this very high-risk group would benefit from traditional anticoagulants.23 Patients with a lower score could safely receive aspirin according to their findings. A large, prospective, multicenter trial to test these concepts is currently in development.
Drugs administered to prevent VTE carry their own risks, specifically minor to major bleeding events. Thus, individualization of prophylaxis treatment based on calculated risk factors will prevent unnecessary prophylaxis for patients deemed low risk while providing prophylaxis for patients who are high risk. Ultimately, the appropriate treatment options are dependent on precise completion of the Caprini RAM. An inaccurate medical history or incorrect interpretation of criteria contained in the RAM could result in an erroneous Caprini score, thereby leading to an incorrect treatment assignment for the patient.
Recently, Pannucci and colleagues performed a meta-analysis of selected publications using Caprini scores to investigate benefits and harms of chemoprophylaxis among surgical patients individually risk stratified for VTE. They evaluated 13 studies; 11 (n = 14 776) contained data for VTE events, and 8 (n = 7590) contained data for clinically relevant bleeding with and without chemoprophylaxis. The most important finding was a 14-fold increase in VTE risk (from 0.7% to 10.7%) among surgical patients who did not receive chemoprophylaxis, and patients with higher Caprini scores were significantly more likely to have a thrombotic event. The benefit of perioperative VTE chemoprophylaxis was only found among surgical patients with Caprini scores equal to or greater than 7.24 One must remember that this study selected the purest 13 of the nearly 100 clinical trials, and these results may not apply to all patient groups. This meta-analysis, however, demonstrates the value of the Caprini score in classifying patient risk to selectively use anticoagulants without exposing entire populations to anticoagulants, as was popular in the past.
The 2013 version is the most recent iteration of the Caprini RAM.25 This version differs from preceding versions in that it includes additional risk factors not tested in validation studies but shown in the literature to be associated with thrombosis. These identified risk factors include BMI above 40,26,27 smoking,28,29 diabetes requiring insulin,30,31 chemotherapy,32,33 blood transfusions,34,35 and length of surgery over 2 hours.36,37
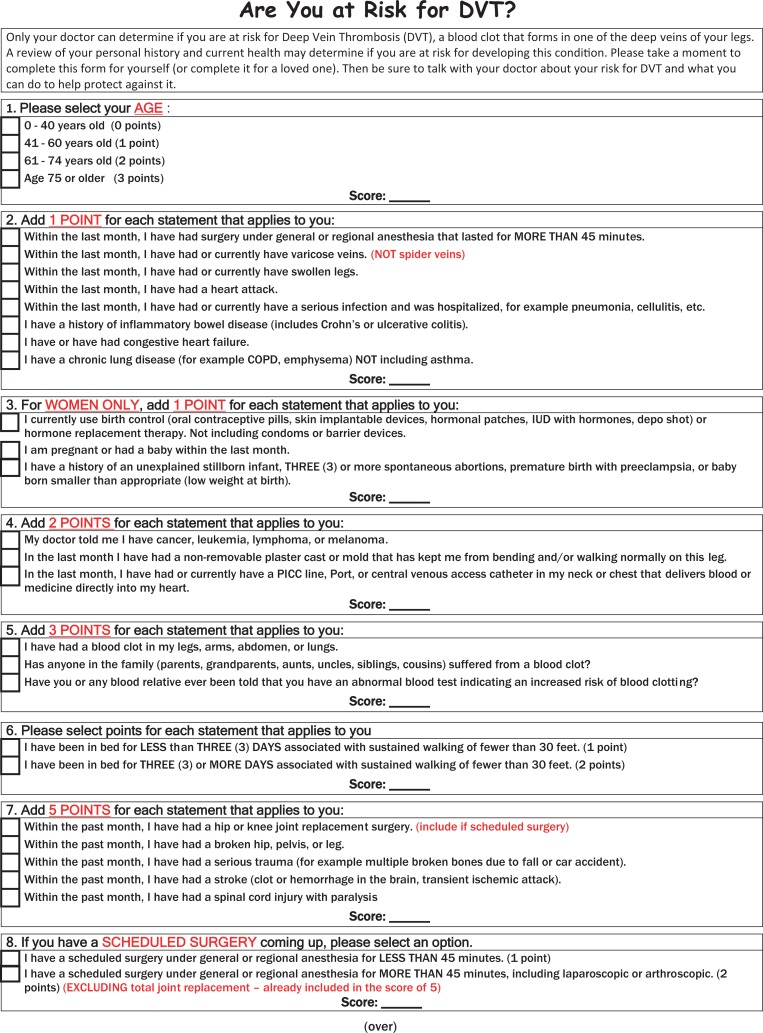
During our validation study of the 2013 Caprini RAM in arthroplasty patients, scoring was completed by specially trained medical students via review of the presurgical assessment history, medical clearances, and medical consults. Any questions or concerns were escalated to Dr Caprini. The Caprini RAM was completed for every participant both preoperatively and predischarge to ensure that any changes in the patient’s postoperative course were captured by the tool (Figure 1).
Figure 1.
Caprini Risk Assessment Model (version 2013).
Completion Guidelines
The patient or the patient’s health-care advocate should complete the risk assessment tool.38 In 2017, Fuentes et al tested multiple versions of a patient-friendly Caprini risk score worksheet until they created a tool that, when completed first by the patient, and then by a physician trained to interpret the Caprini RAM, yielded a high correlation of agreement. The average time to complete the tool was 5 minutes for the patient and then 6 minutes for the physician to finalize the score.38 Having the patient complete the validated patient-friendly risk assessment in advance of the day of surgery in the presence of family members is vital, especially to provide details concerning family history of thrombosis and obstetrical complications (where applicable). Personal experience has taught us that on many occasions, questions regarding family history of thrombosis in first-, second-, and third-degree relatives are not asked or are addressed in a superfluous manner.39 Patients who have this history have an increased level of risk, equivalent to those who have a personal thrombophilic defect.40
Pannucci and Fleming have stressed the importance of face-to-face patient interviews rather than relying on record review.41 One of the most common errors is failure to ask all of the pertinent questions. Retrospective database reviews are flawed since it is not known if all of the questions were presented to the patient or if their responses reflected an accurate interpretation of the question. This is especially true of obstetrical complications which may reflect possible antiphospholipid syndrome being carried by a preoperative patient later in life. These antibodies are a powerful stimulus for VTE. Family history is the most frequent issue that is overlooked. To reemphasize, a history of past VTE or family VTE history is one of the most prevailing risk factors responsible for postoperative thrombosis, including untimely death.38
The immediate preoperative period on the day of surgery is strongly discouraged for completion of the Caprini RAM. It is an anxious time for the patient and family and a comprehensive, accurate assessment may not be captured. We have found that the presurgical testing visit is an optimal opportunity for the patient and family to fill out the patient-friendly form. The entire document is then reviewed by the health-care provider, preferably the person responsible for the preoperative history and physical. Knowledge regarding a detailed history and physical is necessary to precisely complete the tool for optimal patient outcomes with respect to thrombosis.
The Caprini RAM is a dynamic tool, requiring ongoing evaluation of the patient during their hospital course and the postoperative recovery period. Changes in clinical status could result in a change in the score, thereby resulting in a new score and potentially a revised treatment option. For example, a postoperative infection necessitating a central line for antibiotics would increase a patient’s score, and subsequently, the incidence of VTE. A patient sent home after a short hospitalization may be unexpectedly immobile due to postoperative pain or weakness and might develop leg swelling due to inactivity. This may increase their chance of suffering a thrombotic event in the outpatient setting.
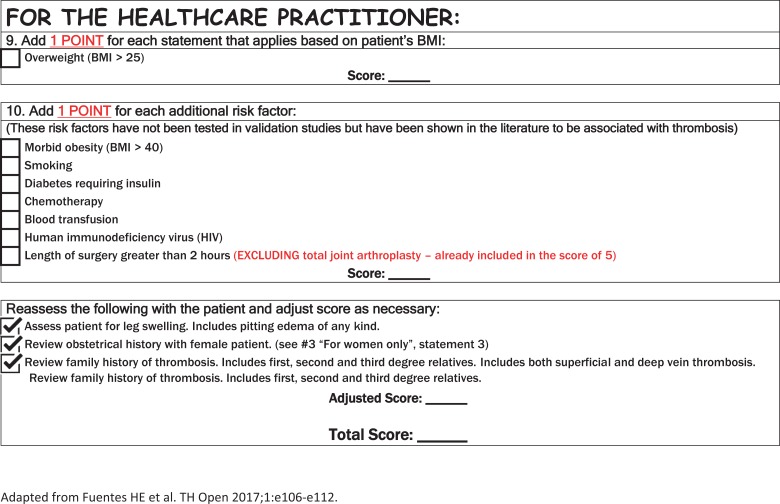
Accurate interpretation of the 2013 version of the Caprini RAM is discussed below. The Caprini RAM requires detailed patient information for successful risk assessment. Thus, a tool with patient-friendly text has been created for the 2013 version of the Caprini RAM and is now completed by all of our patients in presurgical testing, which occurs up to 21 days prior to surgery (Figure 2).
Figure 2.
Patient-friendly tool (Caprini RAM version 2013) - side 1. Completed by the patient -side 2. Completed by the Healthcare Provider.
Conclusion
The Caprini RAM is a dynamic tool, requiring ongoing evaluation of the patient during their hospital course and the postoperative recovery period. Changes in clinical status could result in a change in the score, thereby resulting in a new score and potentially a revised treatment option. The 2013 Caprini scoring system provides a consistent, accurate, and efficacious method for risk stratification and selection of prophylaxis.
| Add 1 POINT for each of the following criteria that apply NOW OR WITHIN THE PAST MONTH: | Criteria interpretation for the health-care provider |
|---|---|
| □ Age 41-60 years | |
| □ Minor surgery (less than 45 minutes) is planned | The length of surgery must also include the anesthesia time |
| □ Past major surgery (more than 45 minutes) within the last month | Major surgery within the past month only |
| □ Visible varicose veins | Patients with visible bulging veins would receive a score of 1. This risk factor does not refer to a patient with spider veins or a patient with a history of surgically removed varicose veins |
| □ History of inflammatory bowel disease (IBD; eg, Crohns disease or ulcerative colitis) | This risk factor include both active and inactive inflammatory bowel diseases such as ulcerative colitis or regional ileitis. This would not include irritable bowel syndrome or diverticulosis |
| □ Swollen legs (current) | Swollen legs include pitting edema of any level, loss of definition of the bony prominences, obscured surface foot veins, or indentation of the leg when a stocking is removed. This factor refers to either 1 or 2 legs affected |
| □ Overweight or obese (body mass index [BMI] above 25 kg/m2) | This weight level was associated with patients developing symptomatic thrombosis following total hip replacement. The combination of BMI greater than 25 kg/m2 and oral contraceptive use in women increased the thrombotic risk 10-fold42–44 |
| □ Heart attack | This refers to acute myocardial infarction (MI) that occurred within the past month |
| □ Congestive heart failure | This risk factor include patients who have had an episode within the last month. Additionally, patients who are currently being treated with medication for CHF are included, even if they had not had an acute episode within the past month. An ejection fraction alone should not be used when determining whether a patient meets this criteria |
| □ Serious infection (eg, pneumonia) | A “serious infection” is defined as a patient who requires hospitalization and intravenous antibiotics for treatment. For example, if a patient has a cellulitis requiring hospitalization with IV antibiotics they would receive one point for this risk factor. Treatments that are less severe and are managed on an outpatient basis with oral antibiotics are not included. Serious infections would include diverticulitis, bacterial infection of the bladder and lungs, and septicemia |
| □ Lung disease (eg, emphysema or COPD) | In addition to emphysema or COPD this risk factor also includes any interstitial lung disease or patients with abnormal pulmonary function tests. This would include, but not limited to, any patient with sarcoidosis, pulmonary fibrosis, pulmonary hypertension, and bronchiectasis. Patients who present with more than one diagnosis meeting the criteria for lung disease will receive a point for each diagnosis. For example, if the patient has a diagnosis of sarcoidosis and COPD they would receive a total of 2 points for this risk assessment. Asthma is not considered a “lung disease” for the purpose of the risk assessment score. Additionally, patients with restrictive pulmonary disease related to obesity would not be included in this criteria |
| □ On bed rest or restricted mobility, including a removable leg brace for less than 72 hours | Restricted mobility (bedrest) is defined as any individual who is unable to ambulate continuously more than 30 feet. “Restricted mobility” would also apply to any patient who is unable to ambulate using both leg muscles. For example, a patient who requires crutches and is nonweight bearing would be considered as restricted mobility even though they can ambulate 30 feet. Patients who are using a cane or walker for stability are not included in this group if they are using their calf muscles for ambulation45 |
| Other risk factors (1 POINT each) that apply NOW OR WITHIN THE PAST MONTH: | These risk factors have not been tested in validation studies but have been shown in the literature to be associated with thrombosis |
| □ BMI above 40 | 26,27 |
| □ Smoking within the last month | Smoking is defined as the inhalation of anything that burns, including tobacco, marijuana, or vaping28,29 |
| □ Diabetes requiring insulin | Only insulin products are included in the risk assessment score. This does not include any other oral or parenteral medications used for the treatment of diabetes30,31 |
| □ Chemotherapy | Chemotherapy treatments used for any medical condition are included in the scoring. For example, a patient receiving methotrexate for Rheumatoid Arthritis, regardless of the dose given, would receive a point for this risk factor. Patients diagnosed with essential thrombocytosis taking hydroxyurea would also receive a point here, in addition to 3 points for “personal history of positive blood test indicating increased risk for blood clotting”32,33 |
| □ Blood transfusion(s) | One point is added for one or more blood transfusions34,35 |
| □ Length of surgery over 2 hours | Actual current surgery time exceeding 2 hours, including anesthesia time. Do not add to the “5” for total hip or knee replacement surgery36,37 |
| FOR WOMEN ONLY: Add 1 POINT for each of the following criteria: | Criteria interpretation for the health-care provider |
| □ Current use of birth control or hormone replacement therapy (HRT) | This includes estrogen contraceptives of any type. This also includes estrogen-like drugs, including raloxifene, tamoxifen, anastrozole, and letrozole. Exemestane has not been shown to increase the risk of DVT. Recent publications have shown that there is no increased risk of DVT in men who are on long-term testosterone therapy; therefore, testosterone is excluded |
| □ Pregnant or had a baby within the last month | |
| □ History of unexplained stillborn infant, recurrent spontaneous abortion (3 or more), premature birth with toxemia or growth restricted infant | Recurrent fetal loss is associated with antiphospholipid antibody syndrome, procoagulant platelet microparticles and some inherited thrombophilias such as Factor V Leiden. There have been reports of both heritable and acquired thrombophilias being associated with preeclampsia, intrauterine growth restriction (IUGR), and abruption. However, these associations are not consistently reported with hereditary thrombophilias46 |
| Add 2 POINTS for each of the following criteria: | Criteria interpretation for the health-care provider |
| □ Age 61-74 years | |
| □ Current or past malignancies (excluding skin cancer but including melanoma) | Whether the cancer diagnosis is remote or recent the patient will receive a score of 2. This is because patients with a remote history of cancer are always at risk of occult metastasis, which would increase their risk of thrombosis. Every incidence of cancer is considered separately and scored accordingly. For example, a patient who has a remote history of breast cancer and is recently diagnosed with uterine cancer would receive a score of 4 (2 points for each episode of cancer). For the purpose of this document, Ductal Carcinoma in situ (DCIS) would also receive a score of 2 as there is always the potential of an invasive cancer. Myelodysplastic syndrome (MDS) would be scored as 2 points only if the disease requires chemotherapy treatment. The patient would also receive an additional point for the chemotherapy treatment |
| □ Planned major surgery lasting longer than 45 minutes (including laparoscopic and arthroscopic) | Do not add to the “5” for total hip or knee replacement surgery. One additional point should be added if the surgery lasts longer than 2 hours, including anesthesia time. See “Other risk factors (1 point each)” |
| □ Nonremovable plaster cast or mold that prevents leg movement within last month | The intent of this risk factor is to capture any limitation in leg mobility which would interfere with calf muscle pumping action such as a leg brace or cast. Patients using crutches who are nonweight bearing on one leg would also be included. The use of an assistive device for stability, such as a walker, would not meet the criteria if the patient is using their calf muscles |
| □ Tube in blood vessel in neck or chest that delivers blood or medicine directly to the heart within the last month (eg, central venous access, PICC line, port) | |
| □ Confined to bed for 72 hours or more (unable to ambulate continuously 30 feet). | “Confined to bed” is confusing terminology and should be referred to as impaired mobility. The patient is unable to ambulate continuously 30 feet. This would also apply to any patient who is unable to ambulate using both leg muscles. For example, a patient who requires crutches and is nonweight bearing would be considered as restricted mobility even though they can ambulate 30 feet. Patients who are using a cane or walker for stability are not included in this group if they are using their calf muscles for ambulation45 |
| Add 3 POINTS for each of the following criteria: | Criteria interpretation for the health-care provider |
| □ Age 75 or over | |
| □ History of blood clots, either deep vein thrombosis (DVT) or pulmonary embolism (PE). This also includes history of superficial venous thrombosis (SVT). | Arterial blood clots are not included in the scoring. A CVA due to a paradoxical embolus (eg, PFO) would be given 3 points; however, a DVT must be documented by an objective measure in this case. Each episode of a DVT or PE is captured as a separate event for scoring. For example, a patient with a medical history of DVT in 2014 and PE in 2015 would be given a cumulative score of 6. However, PE and DVT events that occur simultaneously would be scored as 3 points. SVT must be captured and scored a 3 here as well47 |
| □ Family history of blood clots | Family history should include first-degree relatives (sibling, son/daughter, parent, grandparent), second-degree relatives (maternal half-sibling, paternal half-sibling, niece/nephew), and third-degree relatives (cousin). Younger age of first venous thromboembolism (VTE) and male relative increase the risk39 |
| □ Personal or family history of positive blood test indicating an increased risk of blood clotting (eg, genetic or acquired thrombophilia) | A patient will receive a score of 3 points for each genetic thrombophilia marker. If a family member has a proven genetic marker the patient will receive a score of 3 unless it has been confirmed that the patient does not have this genetic marker. Genetic (inherited) factors: Factor V Leiden/activated protein C resistance, antithrombin III deficiency, protein C & S deficiency, dysfibrinogenemia, 20210A prothrombin mutation. Acquired factors: lupus anticoagulant, antiphospholipid antibodies, myeloproliferative disorders (including thrombocytosis), disorders of plasminogen and plasmin activation, heparin-induced thrombocytopenia, hyperviscosity syndromes, and homocysteinemia.48 HIV infection is an acquired thrombophilia49 |
| Add 5 POINTS for each of the following criteria that apply NOW OR WITHIN THE PAST MONTH: | Criteria interpretation for the health-care provider |
| □ Elective hip or knee joint replacement surgery | 5 points are given for each surgical procedure; therefore, patients having staged or bilateral arthroplasty surgery are to be scored as 10 points |
| □ Broken hip, pelvis, or leg | Fractures requiring surgical repair would receive 5 points for the fracture and will also be assessed additional points depending on the type of surgery. Patients undergoing an ORIF would be given 2 points for “surgery over 45 minutes.” Patients requiring a hemiarthroplasty would receive 5 points “for elective hip replacement surgery.” For example, a patient with a fractured ankle undergoing an ORIF would receive a score of 7, 5 points for the fracture and 2 points for the surgical repair. An additional 2 points would be added if a cast or brace is applied |
| □ Serious trauma (eg, multiple broken bones due to a fall or car accident) | Now or within the past month |
| □ Spinal cord injury resulting in paralysis | Now or within the past month |
| □ Experienced a stroke | Now or within the past month |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebral vascular accident; IV, intravenous; ORIF, open reduction internal fixation; PFO, patent foramen ovale; VTE, venous thromboembolism.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: MaryAnne Cronin, MS, PharmD, BCPS  https://orcid.org/0000-0002-7498-1564
https://orcid.org/0000-0002-7498-1564
References
- 1. Maynard G, Stein J. Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29(2):159–166. doi:10.1007%2Fs11239-009-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahan CE, Spyropoulos AC. Venous thromboembolism prevention: a systematic review of methods to improve prophylaxis and decrease events in the hospitalized patient. Hosp Pract. 2010;38(1):97–108. doi:10.3810/hp.2010.02.284. [DOI] [PubMed] [Google Scholar]
- 3. Becattini C, Agnelli G. Treatment of venous thromboembolism with new anticoagulant agents. J Am Coll Cardiol. 2016;67(16):1941–1955. doi:10.1016/j.jacc.2016.01.072. [DOI] [PubMed] [Google Scholar]
- 4. Wakefield TW, McLafferty RB, Lohr JM, et al. Call to action to prevent venous thromboembolism. J Vasc Surg. 2009;49(6):1620–1623. doi:10.1016/j.jvs.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 5. Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism a public health concern. Am J Prev Med. 2010;38(4S):S495–S501. doi:10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 6. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism. American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):381S–453S. doi:10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 7. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457. doi:10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 8. Spyropoulos AC, Anderson FA, FitzGerald G, et al. Predictive and associative models to identify hospitalized medication patients at risk for VTE. Chest. 2011;140(3);706–714. doi:10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients. antithrombotic therapy and prevention of thrombosis, 9th edition: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S. doi:10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laryea J, Champagne B. Venous thromboembolism prophylaxis. Clin Colon Rectal Surg. 2013;26(3):153–159. doi:10.1055/s-0033-1351130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical Assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304–312. doi:10.1136/bmj.g5334. [PubMed] [Google Scholar]
- 12. Luksameearunothai K, Sa-Ngasoongsong P, Kulachote N, et al. Usefulness of clinical predictors for preoperative screening of deep vein thrombosis in hip fractures. BMC Musculoskelet Disord. 2017;18(1):208 doi:10.1186/s12891-017-1582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatef D, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excision body contouring surgery. Plast Reconstr Surg. 2008;122(1):269–279. doi:10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 14. Seruya M, Venturi M. Efficacy and safety of venous thromboembolism prophylaxis in highest risk plastic surgery patients. Plast Reconstr Surg. 2008;122(6):1701–1708. doi:10.1097/PRS.0b013e31818dbffd. [DOI] [PubMed] [Google Scholar]
- 15. Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350. doi:10.1097/SLA.0b013e3181b7fca6. [DOI] [PubMed] [Google Scholar]
- 16. Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212(1):105–112. doi:10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shuman AG, Hu HM, Pannucci CJ, Jackson CR, Bradford CR, Bahl V. Stratifying the risk of venous thromboembolism in otolaryngology. Otolaryngol Head Neck Surg. 2012;146(5):719–724. doi:10.1177/0194599811434383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroud W, Whitworth JM, Miklic M, et al. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol. 2014;134(1):160–163. doi:10.1016/j.ygyno.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 19. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically Ill surgical patients. JAMA Surg. 2015;150(10):941–948. doi:10.1001/jamasurg.2015.1841. [DOI] [PubMed] [Google Scholar]
- 20. Lobastov K, Barinov V, Schastlivtsev I, Laberko L, Rodoman G, Boyarintsev V. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4(2):153–160. doi:10.1016/j.jvsv.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 21. Krasnow R, Gelpi-Hammerschmidt F, Preston M, et al. Validation of the Caprini risk assessment model in radical cystectomy patients. J Urology. 2016;95(4 suppl):e823–e824. doi:10.1016/j.juro.2016.02.937. [Google Scholar]
- 22. Saragas NP, Ferrao PN, Jacobson BF, Saragas E, Strydom A. The benefit of pharmacological venous thromboprophylaxis in foot and ankle surgery. S Afr Med J. 2017;107(4):327–330. doi:10.1016/j.fas.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 23. Krauss ES, Segal A, Cronin M, Dengler N, Lesser ML, Ahn S, Caprini JA. Implementation and Validation of the 2013 Caprini Score for Risk Stratification of Arthroplasty Patients in the Prevention of Venous Thrombosis. Clinical and Applied Thrombosis/Hemostasis; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pannucci CJ, Swistun L, MacDonald JK, Kenke PK, Brooke BS. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients. Ann Surg. 2017;265(6):1094–1103. doi:10.1097/SLA.0000000000002126. [DOI] [PubMed] [Google Scholar]
- 25.Illinois Medical Society. https://www.venousdisease.com/caprini-dvt-risk-assessment.pdf.
- 26. Rocha AT, de Vasconcellos AG, da Luz Neto ER, Araújo DM, Alves ES, Lopes AA. Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg. 2006;16(12):1645–1655. doi:10.1381/096089206779319383. [DOI] [PubMed] [Google Scholar]
- 27. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308–1311. doi:10.1111/jth.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sweetland S, Parkin L, Balkwill A, et al. Smoking, surgery and venous thromboembolism risk in women. United Kingdom cohort study. Circulation. 2013;127(12):1276–1282. doi:10.1161/CIRCULATIONAHA.113.001428. [DOI] [PubMed] [Google Scholar]
- 29. Enga KF, Brækkan SK, Hansen-Krone IJ, le Cessie S, Rosendaal FR, Hansen JB. Cigarette smoking and the risk of venous thromboembolism: the Tromsø Study. J Thromb Haemost. 2012;10(10):2068–2074. doi:10.1111/j.1538-7836.2012.04880.x. [DOI] [PubMed] [Google Scholar]
- 30. Chung W, Lin C, Kao C. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb Haemost. 2015;114(4):812–818. doi:10.1160/TH14-10-0868. [DOI] [PubMed] [Google Scholar]
- 31. van Schouwenburg IM, Mahmoodi BK, Veeger NJGM, et al. Insulin resistance and risk of venous thromboembolism: results of a population-based cohort study. J Thromb Haemost. 2012;10(6):1012–1018. doi:10.1111/j.1538-7836.2012.04707.x. [DOI] [PubMed] [Google Scholar]
- 32. Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118(5):555–568. doi:10.1016/j.thromres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 33. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119(3):648–655. doi:10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 34. Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151 doi:10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vasan SK, Rostgaard K, Majeed A, et al. ABO blood group and risk of thromboembolic and arterial disease. A study of 1.5 million blood donors. Circulation. 2016;133(15):1449–1457. doi:10.1161/CIRCULATIONAHA.115.017563. [DOI] [PubMed] [Google Scholar]
- 36. Borow M, Goldson H. Postoperative venous thrombosis. Evaluation of five methods of treatment. Am J Surg. 1981;141(2):245–251. [DOI] [PubMed] [Google Scholar]
- 37. Pannucci CJ, Shanks A, Moote MJ, Bahl V, Cederna PS. Identifying patients at high risk for venous thromboembolism requiring treatment after outpatient surgery. Ann Surg. 2012;255(6):1093–1099. doi:10.1097/SLA.0b013e3182519ccf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuentes HE, Paz LH, Al-Ogaili A, et al. Validation of a patient-completed Caprini risk score for venous thromboembolism risk assessment. TH Open. 2017;1(2):e106–e112. doi:10.1055/s-0037-1607339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zöller B, Ohlsson H, Sundquist J, Sundquist K. Familial risk of venous thromboembolia in first-, second- and third-degree relatives: a nationwide family study in Sweden. Thromb Haemost. 2013;109(3):458–463. doi:10.1160/TH12-10-0743. [DOI] [PubMed] [Google Scholar]
- 40. Bezemer ID, van der Meer FJ, Eikenbook JC, Rosendall FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med. 2009;169(6):610–615. doi:10.1001/archinternmed.2008.589. [DOI] [PubMed] [Google Scholar]
- 41. Pannucci CJ, Fleming KI. Comparison of face-to-face interaction and the electronic medical record for venous thromboembolism risk stratification using the 2005 Caprini score. J Vasc Surg Venous Lymphat Disord. 2018;6(3):304–311. doi:10.1016/j.jvsv.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 42. White RH, Henderson MC. Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med. 2002;8(5):375–371. [DOI] [PubMed] [Google Scholar]
- 43. White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343(24):1758–1764. doi:10.1056/NEJM200012143432403. [DOI] [PubMed] [Google Scholar]
- 44. Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89(3):493–498. [PubMed] [Google Scholar]
- 45. Amin AN, Girard F, Samama MM. Does ambulation modify venous thromboembolism risk in acutely ill medical patients? Thromb Haemost. 2010;104(5):955–961. doi:10.1160/TH10-04-0236. [DOI] [PubMed] [Google Scholar]
- 46. Greer IA. Thrombophilia: implications for pregnancy outcome. Thromb Res. 2003;109(2-3):73–81. doi:10.1016/S0049-3848(03)00095-1. [DOI] [PubMed] [Google Scholar]
- 47. Roach RE, Lijfering WM, van Hylckama Vlieg A, et al. The risk of venous thrombosis in individuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood. 2013;122(26):4264–4269. doi:10.1182/blood-2013-07-518159. [DOI] [PubMed] [Google Scholar]
- 48. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 suppl):S3–10. doi:10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 49. Shen YM, Frenkel EP. Thrombosis and a hypercoagulable state in HIV-infected patients. Clin Appl Thromb Hemost. 2004;10(3):277–280. doi:10.1177/107602960401000311. [DOI] [PubMed] [Google Scholar]