Abstract
Acidification of the gastric lumen poses a barrier to transit of potentially pathogenic bacteria and enables activation of pepsin to complement nutrient proteolysis initiated by salivary proteases. Histamine-induced activation of the PKA signaling pathway in gastric corpus parietal cells causes insertion of proton pumps into their apical plasma membranes. Parietal cell secretion and homeostasis are regulated by signaling pathways that control cytoskeletal changes required for apical membrane remodeling and organelle and proton pump activities. Helicobacter pylori colonization of human gastric mucosa affects gastric epithelial cell plasticity and homeostasis, promoting epithelial progression to neoplasia. By intervening in proton pump expression, H pylori regulates the abundance and diversity of microbiota that populate the intestinal lumen. We review stimulation–secretion coupling and renewal mechanisms in parietal cells and the mechanisms by which H pylori toxins and effectors alter cell secretory pathways (constitutive and regulated) and organelles to establish and maintain their inter- and intracellular niches. Studies of bacterial toxins and their effector proteins have provided insights into parietal cell physiology and the mechanisms by which pathogens gain control of cell activities, increasing our understanding of gastrointestinal physiology, microbial infectious disease, and immunology.
Keywords: Gastric Organoids, Acid Secretion, Bacteria, Syntelin, H pylori
Infection with Helicobacter pylori is the most clearly identified risk factor for gastric cancer—the third leading cause of cancer mortality worldwide in men, and the fifth in women.1 In 2017, there were an estimated 950,000 cases worldwide, and 723,000 deaths.2 The risk of gastric cancer involves interactions among H pylori strain–specific virulence factors, patient genotype, and environmental factors. Perturbation of gastric acid secretion is an acute and chronic outcome of H pylori infection that promotes gastric carcinogenesis.3–5 The acute inhibitory effects of H pylori on acid secretion are transitory and normal acid secretion can be restored after H pylori is eradicated.6 In contrast to acute H pylori infection, which induces hypochlorhydria, chronic infection can induce an antrum-predominant phenotype associated with gastrin-mediated acid hypersecretion or a corpus-predominant phenotype associated with acid hyposecretion—this results from H pylori–induced proton pump suppression and activities of cytokines produced by infiltrating immune cells.7 H pylori’s ability to reduce proton pump expression allows it to regulate gastric luminal acidity and alter the persistence or transit of other gastrointestinal microbes.7
Acid secretion is regulated by differentiated parietal cells located in secretory glands formed by invagination of the epithelial monolayer that lines the fundus and body of the mammalian stomach. The secretory glands also contain other highly differentiated cell types that protect the stomach and support its function. These include mucus neck cells, which secrete mucus; chief cells, which secrete pepsinogen; and oxyntic enterochromaffin-like (ECL) cells, which regulate acid secretion by secreting histamine in response to paracrine gastrin stimulation originating in antral G cells. Acid secretion by parietal cells is mediated by the membrane-bound enzyme H+,K+-ATPase, found in high density on intracellular tubulovesicular and secretory canalicular membranes.8,9 The enzyme is a heterotetramer of 2 ATP4A subunits, which have catalytic ATPase and cation transport activity, and 2 ATP4B subunits, which are smaller and glycosylated.
In parietal cells, ATP4A and ATP4B are translated on endoplasmic reticulum–bound ribosomes into integral membrane polypeptides that localize to cytoplasmic tubulovesicles with limited K+ permeability.10,11 ATP4B subunits facilitate assembly of functional proton pumps and migration of the nascent enzyme complex to tubulovesicular and canalicular membranes and protect the complex from degradation.12–14 The relative K+ impermeability of tubulovesicular membranes ensures that ATP4A activity, responsible for the H+-K+ counter-transport functions of the enzyme, remains latent.10 A distinct set of intracytoplasmic RAB11-positive vesicles express the K+ voltage–gated channels KCNQ1 and KCNE2.15 Nutrient-induced neural, hormonal, and paracrine stimulation of parietal cells, in the form of acetylcholine activation of calcium-dependent signaling pathways, gastrin activation of histamine release from ECL cells, and histamine-mediated increases in intracellular adenosine 3′,5′-cyclic monophosphate (cAMP), activates protein kinases. These kinases cause fusion of H,K-ATPase–containing tubulovesicles and K+ channel–containing vesicles with collapsed invaginations of the parietal cell apical membrane. The resulting expanded membrane-delimited pan-cellular microvillous secretory canalicular compartment is characteristic of actively secreting parietal cells. Delivery of K+ channels (KCNQ1, KCNE2, KCNJ10, and KCNJ15)16–19 to canalicular membranes provides access of cytoplasmic K+ to luminally oriented K+-binding sites on ATP4A, initiating ATP binding and hydrolysis on its cytoplasmic side, which promotes electroneutral exchange of luminal K+ for cytoplasmic protons with unitary H+:K+:ATP stoichiometry.20 Under maximal secretory conditions, H,K-ATPase activity establishes a million-fold proton gradient across the canalicular membrane.21–23 Recent high-resolution (2.8 Å) crystal structures of H,K-ATPase complexed with the K+-competitive acid secretory inhibitors vonoprazan or SCH28080 indicate that the conformational change of the cation-binding site, induced by ATP hydrolysis, lowers the pKa value of Glu820 in ATP4A, enabling release of protons into the pH 1.0 environment of the stomach.24
Regulation of Gastric Acid Secretion
Stimulants of parietal cell acid secretion include gastrin, secreted by antral G cells (hormonal pathway)25,26; histamine, secreted from oxyntic ECL cells (paracrine pathway)27; and acetylcholine, secreted from oxyntic (gastric body and fundus) and antral intramural post-ganglionic neurons (neural pathway).28 Parietal cells express specific receptors for each of these secretagogues (gastrin or CCK2, H2, and M3, respectively). Gastrin is the major hormonal stimulant secreted during meal ingestion, inducing release of histamine from ECL cells. Gastrin also stimulates the parietal cell directly, acting via CCK2 receptors coupled to an increase in [Ca2+]i. Parietal cell histamine H2 receptors are coupled to adenylate cyclase, which catalyzes formation of cAMP. In rat hepatoma-derived cells transfected with canine histamine H2 receptor, histamine elicited transient increases in intracellular calcium ([Ca2+]i) with generation of inositol trisphosphate.29 Acetylcholine stimulates parietal cells directly through M3 muscarinic receptors coupled to an increase in [Ca2+]i, and indirectly by inhibiting M4 muscarinic receptor-mediated somatostatin secretion from oxyntic D cells.30 Somatostatin, secreted from oxyntic and antral D cells, is the primary physiological inhibitor of acid secretion. Although there are few D cells in oxyntic mucosa, compared to parietal cells, D cells are functionally coupled to parietal and ECL cells via cytoplasmic processes or via local circulation.31,32 Under resting conditions, somatostatin places tonic restraint on parietal cell acid secretion, ECL cell histamine secretion, and G cell gastrin secretion. Removal of this restraint (disinhibition) by activation of cholinergic neurons stimulates acid secretion. Direct evidence for Ca2+ signaling in histamine-dependent acid secretion in mice was provided by organelle-targeted Ca2+ imaging and direct patch-clamping of apical vacuolar membranes of gastric parietal cells.33 This study revealed that a lysosome calcium channel, mucolipin 1 (MCOLN1 or TRPML1), co-localized with tubulovesicles that express ATP4A, was required for histamine-induced PKA-dependent release of Ca2+ from tubulovesicular stores and for acid secretion.
Proton pump inhibitors, such as omeprazole, lansoprazole, rabeprazole, and esomeprazole, have revolutionized the treatment of gastroesophageal reflux disease, peptic ulcer disease, and Zollinger-Ellison syndrome (gastrinoma). Proton pump inhibitors are substituted benzimidazoles that inhibit gastric acid secretion by binding covalently to luminally exposed cysteine residues on ATP4A, altering its conformation. Vonoprazan is a lipophilic, weak base pyrrole derivative that blocks acid secretion by accumulating in acidic parietal cell canaliculi and prevents proton pump activation by competing with K+ on the luminal (intracanalicular) surface of ATP4A.34 Advantages of using vonoprazan vs conventional proton pump inhibitors include rapid onset of action, longer duration of acid suppression, and less variation among individuals, at the possible cost of increased hypergastrinemia.35
Parietal Cell Activation
Parietal cells have characteristic morphology closely related to their function.36 The cells are generally pyramidal in shape and are packed with mitochondria and other membrane-bound structures. The apex of the pyramid, which faces the glandular lumen, is surrounded by tight junctions to adjacent cells. Three-dimensional nano-tomography analysis of mouse parietal cells revealed intricate networks of mitochondria, which are evenly distributed throughout the cytoplasm, and abundant roughly spherical lysosomes, which are mostly adjacent to mitochondria.37 Tubular and vesicular membrane organelles (tubulovesicles) that express ATP4A and ATP4B in high density are distributed throughout the parietal cell cytoplasm. Resting or non-secreting cells have a limited area of apical membrane in direct apposition to the gland lumen, but more extensive luminal exposure is provided by a distinctive invagination of the apical membrane deep into the cytoplasm, forming a residual collapsed secretory canaliculus. Further studies are needed to determine whether parietal cell organelle interactions involved in histamine-induced acid secretion are affected by H pylori infection.
Changes in parietal cell morphology that accompany stimulation of acid secretion result from fusion of intracellular tubulovesicles with the residual secretory canalicular membranes, leading to elongation of intra-canalicular microvilli and the concomitant disappearance of cytoplasmic tubulovesicles.11 These changes in vesicle trafficking, membrane interactions, and actin cytoskeleton arrangement are mediated by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), which are found in different membranes and intracellular locations. Initial searches for parietal cell SNARE proteins identified 6 SNAREs: VAMP; syntaxins 1, 2, 3, and 4; and SNAP25.38,39 Live-cell imaging with fluorescently labeled VAMP2 demonstrated the translocation of VAMP2 from tubulovesicular membranes to the apical canalicular membrane of parietal cells upon stimulation of acid secretion.40 The functional importance of VAMP2 in stimulating acid secretion was demonstrated by concomitant inhibition of acid secretion by parietal cells exposed to tetanus toxin, a Zn-dependent proteinase that specifically cleaves VAMP2.40,41
Although identification of VAMP2 as a v-SNARE in parietal cells was anticipated, the identification of syntaxin 3 on tubulovesicles was unexpected. This prototypical t-SNARE localizes to vesicular membranes of parietal cells and may mediate homotypic fusion of tubulovesicles, accounting for the rapid apical morphologic changes associated with active acid secretion. Parietal cell stimulation was accompanied by translocation of co-localized syntaxin 3 and ATP4A from tubulovesicles to the apical membrane.42 The importance of syntaxin 3 in acid secretion was demonstrated in studies with streptolysin O–permeabilized gastric glands. In these studies, recombinant syntaxin 3 competed for endogenous protein.43 Ezrin, a membrane-cytoskeletal linker with sequence homology to talin and erythrocyte band 4.1, has been associated with the remodeling of parietal cell apical membrane that occurs with cAMP-dependent protein kinase stimulation. Atomic force microscopy studies revealed that ezrin phosphorylation and conformational change allowed binding of syntaxin 3 to the N-terminus of ezrin.44 SNARE proteins therefore mediate recognition and docking events, but additional mechanisms, such as partition of a hydrophobic domain of a membrane protein into an adjacent closely apposed membrane, could promote thermodynamic fusion of membranes.11,45
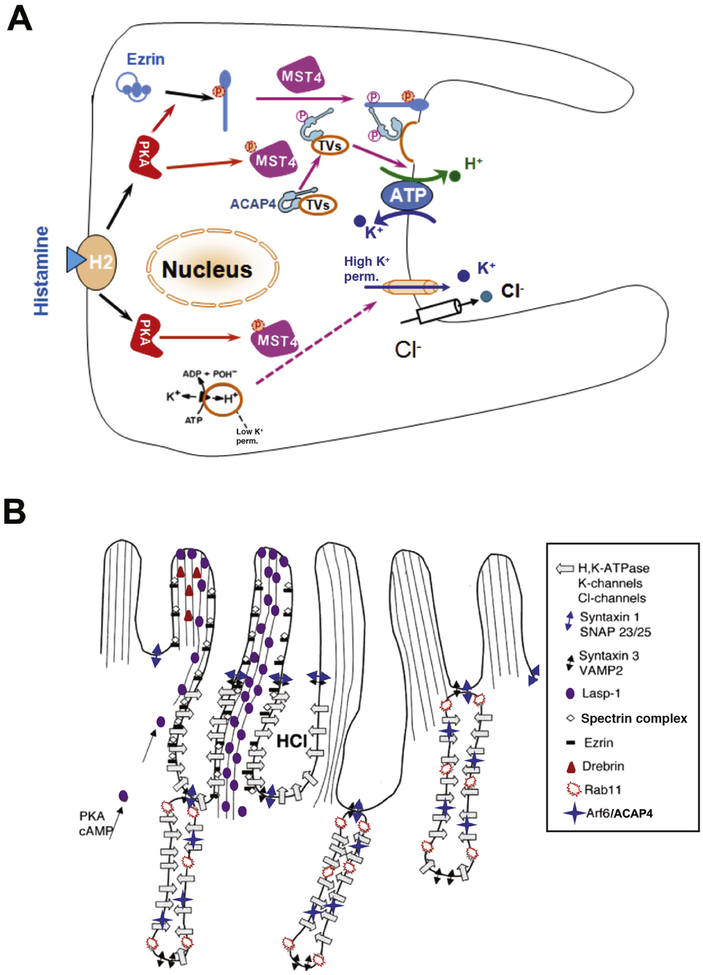
Other molecular effectors of parietal cell morphologic transformation are Rab GTPases, which are members of the Ras GTPase superfamily that regulate many steps of membrane trafficking. Rabs are often tethered to membranes through 2 C-terminal prenyl groups,46 and switch between GDP-bound and GTP-bound forms depending on activation, dissociation, displacement, and exchange factors.47 RAB11 is involved in regulating recycling endosomes in transferrin recycling models and is also required for trafficking from trans-Golgi network to the plasma membrane.48 Initial screening of parietal cells found a high level of RAB11 mRNA49 and RAB11 protein localized to tubulovesicles that contain ATP4A.50 Expression of a dominant-negative form of RAB11 (RAB11N124I) in parietal cells inhibited acid secretion.51 Inhibition correlated with impaired membrane translocation from tubulovesicles to the apical plasma membrane. Interestingly, RAB11 interacts with another small GTPase, ARF6.52 Like ATP4A, native ARF6 redistributes from predominantly cytoplasmic membranes to apical canalicular membranes when cells are stimulated.53 In parietal cells, ARF6 is activated by an Arf-GAP containing a coiled-coil ACAP4.54 ACAP4 interacts with ezrin, which is regulated by protein phosphorylation.55 Histamine stimulation of parietal cell acid secretion activates PKA signaling via STK26 (or MST4),56 leading to phosphorylation of ACAP4 at Thr545. This phosphorylation enables ACAP4 to interact with ezrin.57 Given the location of Thr545 between the GTPase-activating protein domain and the first ankyrin repeat of ACAP4, it is likely that MST4 phosphorylation induces a conformational change that allows ACAP4 to interact with ezrin (Figure 1A and B).
Figure 1.
Mechanisms of parietal cell acid secretion. (A) In the resting state, H,K-ATPase and ACAP4 are sequestered in tubulovesicles with low K+ permeability. Histamine stimulation activates PKA signaling via MST4, resulting in conformational changes in ezrin and ACAP4 that allow them to interact and induce tubulovesicle translocation. MST4 then phosphorylates ACAP4 at Thr545, promoting its association with ezrin. Phosphorylation of ezrin by PKA at Ser66 causes a conformational change, which promotes docking and anchoring of H,K-ATPase- and ACAP4-containing tubulovesicles at the apical plasma membrane, where K+ permeability is high. MST4 might mediate K+ channel activation, but the mechanisms of this process have not been determined. (B) Model of apical membrane cytoskeletal remodeling. Histamine stimulation activates PKA, resulting in phosphorylation and activation of MST4 kinase. Activated MST4 mediates formation of the apical signaling machinery, comprising ARF6, ACAP4, and ezrin, which docks tubulovesicles to the apical membrane by interactions with Lasp-1, drebrin, spectrin, and other proteins, such as syntaxin 1 and SNAP23/25. This leads to reorganization of apical microvillous structures with extended apical surface and onset of proton pumping. The exact mechanisms underlying tubulovesicle fusion with the apical membrane and insertion of proton pump into the apical membrane remain less illustrated.
Although somatostatin, EGF, and TGFα signals inhibit acid secretion, withdrawal of the secretory stimulus alone appears to be sufficient to turn off acid secretion.36,58 Initial studies of the mechanisms of ATP4A recovery from secretory membrane and its recycling led to discovery of a tyrosine-based internalization motif at the cytoplasmic, N-terminus of ATP4B.59 In studies of transgenic mice, substitution of this tyrosine with an alanine resulted in continued localization of ATP4B to the parietal cell surface and sustained secretion of acid. These mice develop gastric ulcers and hypertrophic gastropathy. Post-secretory endocytosis of the proton pump involves the tetraspanin CD63, a membrane protein whose expression and co-localization with ATP4B in apical membrane induces redistribution of ATP4B to intracellular compartments.60 CD63 interacts, through a tyrosine-based motif, with adaptor protein complexes 2 and 3, linking its interaction partners to the endocytic machinery of the cell.60 Dynamin and clathrin also associate with the apical canalicular membrane in resting and stimulated cells.42,61
The complex apical membrane-cytoskeletal structure of parietal cells gives rise to innumerable intracellular canalicular membrane microvilli. Electron microscopy has shown that, in the resting state, abundant short (approximately 0.5 μm) parietal cell microvilli on the apical canalicular membrane have distinct F-actin bundles radially organized along the length of the microvilli, in addition to filamentous extensions that project from the base of each microvillus 1–2 μm into the cytosol.36,62 Upon parietal cell stimulation, apical membrane surface area is increased significantly due to mass tubulovesicle fusion, which leads to expansion of the canalicular membrane surface into the cytoplasm, displacing and crowding together the numerous mitochondria that energize acid secretion. The radial F-actin bundles, now in significantly elongated microvilli, have the same appearance as F-actin bundles in resting cells, providing structural support for the increased microvillous surface extensions. After withdrawal of the stimulus, the canalicular spaces remodel over the next 15–30 minutes as HCl secretion ceases. Highly ordered longitudinal F-actin filaments within the collapsed microvilli are disassembled into short, disordered bundles that extend into the cytoplasm.63
The mechanisms underlying actin filament plasticity during parietal cell activation and membrane recycling have been studied using small molecule inhibitors, such as latrunculin B (Lat B), which binds to actin monomers. These analyses identified a pool of actin that binds Lat B and mediates motility of cultured cells, and a Lat B-resistant pool of actin that may constitute microvillous filaments essential for secretion. Furthermore, cell stimulation appears to increase the effects of Lat B—most likely through Lat B binding to monomeric actin generated by turnover of the actin filament pool.64 Dynamic pools of actomyosin and F-actin are required to maintain apical membrane structures in resting cells and for trafficking of tubulovesicles to the apical plasma membranes during histamine stimulation.65
Ezrin also has important functions in parietal cell apical membrane remodeling. In mice with conditional knockdown of ezrin, there is no fusion of parietal cell tubulovesicles with the apical canalicular membrane, preventing acid secretion.66 In addition, proteolysis of parietal cell ezrin by activation of calpain I prevented histamine-stimulated acid secretion, measured by decreased uptake of [14C]-amino-pyrine, a membrane-permeable weak base (pKa = 5.0) that diffuses into and is protonated in intracellular acid compartments.67 Importantly, persistent expression of the Thr567 phospho-mimicking ezrin mutant redirected ATP4A to basolateral membrane,68 inhibiting acid secretion and inducing an unexpected repolarization of the cells. Ezrin, F-actin, and ATP4A all accumulated at the basolateral membrane in the form of long and extensive microvillous or filopodial extensions.68,69 Several groups, using various cell systems, found that ezrin Thr567Asp mutagenesis increased surface projections,70,71 but specific membrane localization was not described in detail. A membrane fixation effect of ezrin Thr567Asp was demonstrated by fluorescence recovery after photo-bleaching, indicating that mutagenized ezrin, in a permanently unfolded conformation, binds tightly to the basolateral membrane, resulting in repolarization of epithelial cells.72 These data led to a model in which turnover of ezrin Thr567 phosphorylation is required for optimal plasticity in membrane remodeling.45,73,74 Studies are needed to delineate how ezrin links parietal cell stimulation29 to apical membrane remodeling.
Effects of H pylori on Gastric Acid Secretion
Colonization of gastric mucosa by H pylori perturbs the primary function of gastric parietal cells, secretion of hydrochloric acid at concentrations sufficient to sterilize nutrient intake. The discovery of H pylori as an etiologic agent of gastritis and peptic ulceration was accompanied by studies associating acute H pylori infection with transient hypochlorhydria, including reports of iatrogenic H pylori inoculation through contaminated gastric endoscopes and nasogastric tubes. Gastric pH, 1–4 weeks after initial infection, was reported to range from 6.4 to 7.6,75–78 with acid secretion returning to baseline levels within a few weeks or months.75–82 Infection of dogs, ferrets, and Mongolian gerbils with Helicobacter species inhibited acid secretion.83–85 Co-incubation of various H pylori species or H pylori sonicates with rabbit and ferret gastric epithelial cells, gastric glands, or isolated guinea pig or human parietal cell populations inhibited acid secretion.86–92 In most studies, decreased cellular uptake of [14C]-aminopyrine was a marker of acid secretory inhibition.
These findings associated H pylori infection with gastric hypochlorhydria, consistent with acid inhibition being mediated by H pylori–induced interruptions of interactions between gastrin-secreting G cells, histamine-secreting ECL cells, somatostatin-secreting D cells, and parietal cells. However, there could also be a direct interaction of H pylori with parietal cells. H pylori have been observed, by transmission electron microscopy of human gastric biopsies, to penetrate the cytoplasm of epithelial cells, the intraepithelial intercellular spaces, and the underlying lamina propria.93,94 More specifically, H pylori penetration into gastric glands and even the cytoplasm of parietal cells was observed in histologic, immunohistochemical, and ultra-structural analyses of H pylori–infected cats and humans. H pylori were found clustered within gastric glands on the apical surfaces of parietal cells and even sequestered within parietal cell secretory canaliculi.95–98 Contrary to common belief,97–99 H pylori survive at pH <3.0, which is characteristic of the gastric environment. H pylori’s adaptation to this acidic microenvironment has been extensively documented,100–103 and involves a proton-gated inner membrane channel that allows diffusion of urea from the gastric lumen into the bacterium, where it is hydrolyzed by cytoplasmic urease into ammonia and CO2. Subsequent diffusion of NH3 into the periplasm creates a protective higher pH microenvironment essential for survival of the bacteria. This mechanism, one of several strategies deployed by bacteria to facilitate survival and colonization of acidic environments,104 is particularly relevant in the context of potential direct H pylori inhibition of parietal cell acid secretion, previously considered unlikely, given the bactericidal properties of gastric acid.
It is important to understand the mechanisms of acute H pylori–induced hypochlorhydria because they recapitulate the effects of anti-secretory drugs. H pylori–induced hypochlorhydria does not involve loss of parietal cells or atrophy because normal numbers of parietal cells were found in the stomachs of acutely infected Mongolian gerbils and in gastric biopsies from patients.75,80,85 An alternative hypothesis is that H pylori interferes with transcription of ATP4A in parietal cells. Gastric adenocarcinoma (AGS) cells were transfected with a plasmid carrying the 5′ promoter sequence of human or rat ATP4A fused to a luciferase reporter gene.105 Addition of histamine to these cells produced a dose-dependent increase in cAMP, [Ca2+]I, and activity of the ATP4A promoter. Addition of H pylori to the transfected cells reduced basal and histamine-stimulated activity of the ATP4A promoter, in a dose-dependent manner. H pylori also inhibited ATP4A promoter activity induced by phorbol myristate acetate or the diacylglycerol analog 1-oleoyl-2-acetyl-sn-glycerol, both of which were sensitive to staurosporine and calphostin C. The data indicated that H pylori inhibits expression of ATP4A via PKA and PKC signaling pathways.105 Interestingly, human gastric epithelial cells transfected with a human ATP4A–Luc reporter construct were more sensitive to H pylori–induced repression than cells transfected with a rat ATP4A–Luc reporter construct. These findings are consistent with the observation that human and non-human primate stomachs are the principal foci of H pylori infection.106
H pylori–induced repression of ATP4A gene expression was later observed in human gastric biopsies. Co-culture of biopsies with H pylori for 15 or 24 hours reduced levels of ATP4A mRNA, caused virtual disappearance of ATP4A protein, and prevented acid secretion.107 The findings could not be attributed to cell death because control (mock-infected) gastric biopsies had normal levels of ATP4A protein and acid secretory capacity. These findings are consistent with those from a microarray analysis of parietal cell mRNAs from germ-free and H pylori–infected mice, which found a 5.3-fold down-regulation of ATP4A gene expression in mice infected with H pylori.108 However, the comprehensive acute H pylori–induced elimination of ATP4A expression and acid secretory capacity observed in human biopsies107 indicates that, in addition to regulating transcription of ATP4A, H pylori also affects post-transcriptional phases of ATP4A synthesis and activation. These findings support the direct intervention of H pylori virulence factors with parietal cell mechanisms of acid secretion.
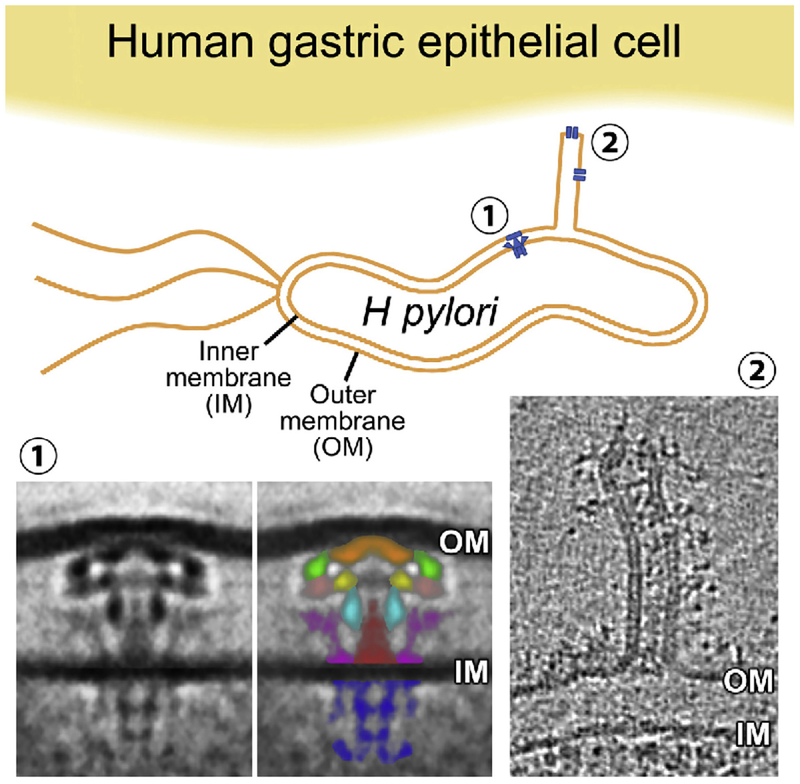
Some of the mechanisms underlying H pylori–induced perturbation of ATP4A expression and translation have been determined.109,110 H pylori strains expressing a type IV secretory system (T4SS) can transfer virulence factors through the T4SS-associated pilus into gastric epithelial cells. Structural T4SS proteins are encoded by a cytotoxin-associated gene (cag) pathogenicity island (PAI). The architecture of the T4SS has been elucidated by electron cryotomography111 (Figure 2). The H pylori T4SS protein, CagL, interacts with α5β1 integrins112 to facilitate injection of the oncogenic bacterial protein CagA into cells. This activates multiple signaling pathways and up-regulates expression of the pro-inflammatory transcription factor NF-κB.113 The α5β1 integrins are expressed on basolateral membranes of epithelial cells, where they mediate morphologic and signaling interactions with cells in the lamina propria. H pylori disrupts the tight junction barriers that separate the luminal and serosal sides of the gastric epithelium114–117 and protect the submucosa against gastric acid. This disruption allows H pylori to interact with parietal cell basolateral membrane receptors, activating intracellular signaling pathways, which are inaccessible from the epithelial apical surface. H pylori infection of cultured gastric epithelial cells inhibits expression of ATP4A by CagA oncoprotein- and peptidoglycan (soluble lytic transglycosylase)-dependent, ERK1- and ERK2-mediated binding of the NF-κB p50 homodimer to the ATP4A promoter.118 Acute H pylori infection causes CagL to dissociate ADAM17 from α5β1 integrins. This activates ADAM17-dependent, NF-κB–mediated repression of ATP4A.119 The requirement for T4SS structural integrity for repression of ATP4A was confirmed in studies of H pylori cagPAI isogenic mutants, which are deficient in cagE, cagM, or cagL. Levels of ATP4A mRNA in human gastric biopsies were unaffected by co-culture with these mutants.107 However, co-culture of biopsies with wild-type H pylori for 15 hours inhibited basal and histamine-stimulated acid secretion, measured by microphysiometry, confirming dependency on a functional bacterial T4SS, and consistent with direct H pylori–mediated activation of NF-κB in parietal cells. However, the observation does not exclude paracrine influences from other cells in the biopsies.107
Figure 2.
Electron micrograph of the Helicobacter pylori Cag T4SS.111 Using electron cryotomography, Chang et al111 determined the structure of the H pylori Cag T4SS, a transmembrane multimeric translocation apparatus assembled when H pylori encounters gastric epithelial cells. The cag T4SS is a 5-barrel structure (1) in close proximity to a tube-like structure that projects from the bacterium (2) and may serve to translocate virulence factors into parietal cells. OM, H pylori outer membrane; IM, H pylori inner membrane; green, α-subunit; light brown, β-subunit; cyan, γ-subunit; purple, wing subunit; brown, stalk subunit; and blue, cytoplasmic Cag5/VirD4 coupling protein. Perturbation of the structural integrity of T4SS complexes might obviate pathogenesis. Integration of cryo-electron tomography and correlative fluorescence microscopy163 can be used to visualize the 3-dimensional ultrastructural features during H pylori infection of gastric parietal cells in organoids. Comparisons of ultrastructural changes in gastric organoids after exposure to different strains of H pylori could be used to study mechanisms of H pylori infection and develop therapeutic interventions.
H pylori also perturbs post-transcriptional regulation of ATP4A mRNA. In silico analysis of the ATP4A mRNA 3′ untranslated region identified microRNA 1289 as a highly conserved potential regulator of ATP4A mRNA translation.120 H pylori infection of AGS cells transfected with ATP4A 3′ untranslated region-Luc reporter significantly reduced luciferase activity, whereas infection with ΔcagA or Δslt H pylori had no effect on luciferase activity. Gastric biopsies from patients infected with cagA-positive H pylori had a significant increase in microRNA 1289 compared with biopsies from uninfected patients or those infected with cagA-negative H pylori.120 Only parietal cells express ATP4A mRNA, so the finding that H pylori up-regulates microRNA 1289 is consistent with direct interaction of specific H pylori virulence factors with parietal cells.
Virulence factors other than those that mediate CagA translocation or interleukin (IL)-8 secretion can repress expression of ATP4A by activating NF-κB.121 AGS cells transfected with ATP4A promoter–Luc reporter constructs containing an intact or mutant NF-κB binding site were infected with wild-type H pylori strain 7.13, isogenic mutants that lack the cag PAI genes responsible for CagA translocation and/or IL8 induction (cagA, cagζ, cagε, cagZ, and cagβ), or strains of H pylori that lack genes encoding 2 peptidoglycan hydrolases (slt and cagγ). Measurement of H pylori–induced activity of the ATP4A promoter in AGS cells, translocation of CagA, IL-8 secretion, and acid secretion in human oxyntic biopsies showed that ATP4A repression was independent of IL-8 expression. Furthermore, the data showed that CagA translocation and H pylori transglycosylases, encoded by slt and cagγ, participate in NF-κB–dependent repression of ATP4A and acid inhibition.121
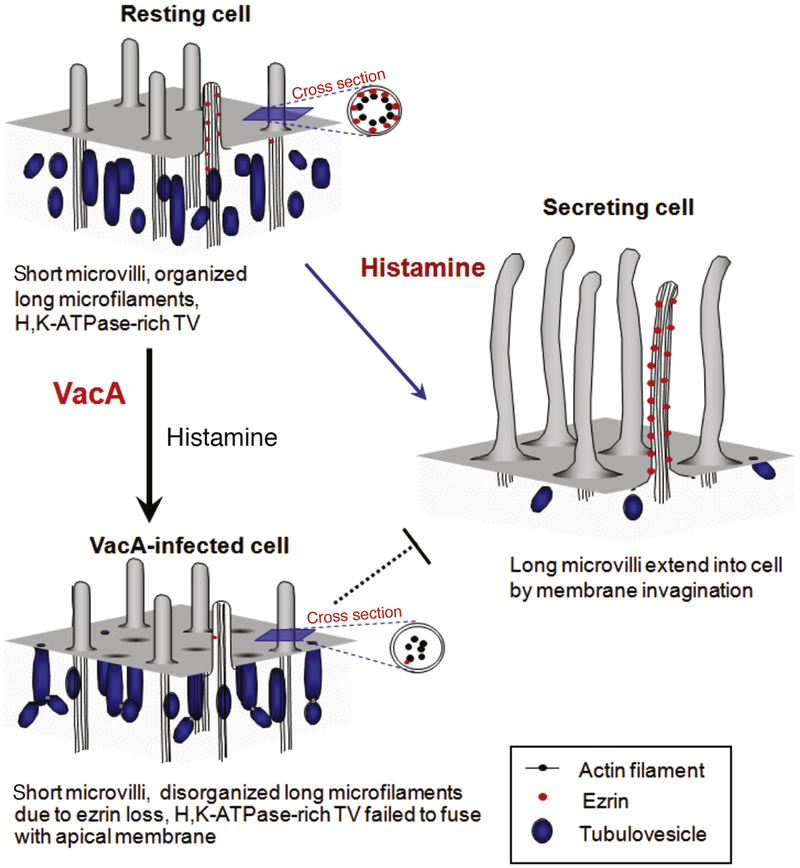
Another virulence factor secreted by H pylori, vacuolating toxin (VacA), contributes to H pylori–induced hypochlorhydria.122 Incubation of epithelial cells with VacA causes formation of anion-selective pores in the plasma membrane and large intracellular vacuoles,123–125 apoptosis,126 epithelial monolayer permeabilization,122 and stimulates the autophagy pathway.127 Exposure of isolated rabbit gastric glands to VacA induced an influx of extracellular calcium, which activated calpain, leading to proteolysis of ezrin. The loss of ezrin from the parietal cell apical membrane disrupted recruitment of tubulovesicles that contain ATP4A and thereby inhibited acid secretion (Figure 3).128 In addition to direct suppression of acid secretion by transfer of virulence factors into parietal cells, H pylori also mobilizes cell proteins that inhibit acid secretion. Gastric H pylori infection increases T helper type 1 cell secretion of IL-1β, a pleiotropic pro-inflammatory cytokine that is a potent inhibitor of acid secretion.129 Also, studies of rat oxyntic mucosa mounted in Ussing chambers showed that H pylori perfusion activated intramural calcitonin gene– related peptide sensory neurons in oxyntic mucosa that are coupled to somatostatin secretion; this subsequently suppresses acid secretion by inhibiting ECL cell histamine secretion and G cell gastrin secretion.130
Figure 3.
Modeling changes in the cytoskeleton at the apical membranes of gastric parietal cells. Model of VacA-induced hypochlorhydria. In resting cells, apical microvilli are supported by microfilaments that extend deep into the cytoplasm. Ezrin (red dots) links actin filaments with the apical plasma membrane. Histamine stimulation leads to docking and fusion of ATP4A-rich tubulovesicles to the apical plasma membrane, expanding the apical surface. Interactions between the apical membrane and microfilaments, via ezrin, reorganize the expanded surface into long microvilli. VacA induces proteolysis of ezrin, disrupting the interactions between the apical membrane and microfilaments, and preventing recruitment of ATP4A to the apical membrane. The ensuing parietal cell inability to secrete acid results in hypochlorhydria.
H pylori’s activation of neural pathways may explain how initially patchy colonization of superficial gastric mucosa acutely and profoundly inhibits acid secretion. In any event, direct virulence factor–dependent or indirect neural-dependent acid secretory inhibition probably occurs when parietal cells are in a resting state, when bacterial penetration into oxyntic glands and adherence to parietal cells is not impeded by the intra-glandular high-pressure counter-flow that is generated during active secretion.131
Progression to Neoplasia
Once regarded as a sterile environment, the gastric mucosa is now understood to host many bacterial genera collectively known as the gastric microbiota.132 Molecular genotyping identifies the most abundant bacterial phyla in the healthy human gastric microbiota as Proteobacteria, Firmicutes, Bacteroidetes, Fusobacteria, and Actinobacteria. The most prominent genera are Provotella, Streptococcus, Rothia, Pasteurellaceae, Fusobacterium, Actinomyces, Neisseria, Hemophilus, and Porphyromonas.132–134 In individuals infected with H pylori, this organism becomes the single most abundant gastric phylotype,135 and the relative abundance of non–H pylori Proteobacteria, Spirochaetes, and Acidobacteria increases, whereas that of Actinobacteria, Bacteroidetes, and Firmicutes decreases.136 Depending on an individual’s environmental, genetic, and bacterial risk factors, H pylori infection may initiate a sequence of pathologies, starting with chronic inflammation, that leads to atrophic gastritis (including autoimmune gastritis with pernicious anemia), intestinal metaplasia, dysplasia, and eventually gastric adenocarcinoma.137
Chronic infection with H pylori also reduces acid secretion, which may alter the abundance, diversity, and relative prevalence of bacterial species in the gastric microbiota. The role of acid in shaping the composition of the gastric microbiota is evident from studies of patients who use proton pump inhibitors or H2-antagonists. These patients develop gastric bacterial overgrowth and have increases in non–H pylori bacteria.138,139 H pylori–positive patients with and without gastric cancer and H pylori–negative patients all have significant differences in their gastric microflora.140–142 These changes can alter the plasticity of the gastric mucosa and contribute to malignancy. Specific communities of the gastric microbiota other than H pylori might therefore contribute to H pylori–induced pathogenesis, in addition to H pylori virulence factors, host genetic polymorphisms, and diet.140,143 Next-generation gene profile analyses of gastric microbial compositions revealed that patients with gastric carcinoma have a microbial dysbiosis, characterized by lower diversity, decreased abundance of H pylori, and higher genotoxic potential, compared to the gastric microbiota of patients with chronic gastritis.144 Eradication of H pylori increased bacterial diversity and restored the relative abundance of other bacteria to levels similar to H pylori–negative individuals.145
During homeostasis, turnover and regeneration of gastric epithelial cells are mediated by differentiation of stem cells located in the isthmus region.146,147 Upon infection with H pylori, mature parietal and chief cells are replaced by proliferating cells that secrete mucins and wound repair proteins in a lesion called spasmolytic polypeptide-expressing metaplasia (SPEM).148,149 In studies of mice, SPEM was found to arise from a subpopulation of chief cells that express the G protein-coupled receptor Lgr5, a stem cell marker regulated by Wnt signaling.150 In gastric pyloric epithelium, Lgr5+ cells are recruited back into the cell division cycle to serve as stem cells that repair gland damage in the short term, and in the longer term can also promote SPEM that persists as a precancerous lesion.151 Chief cells that have not attained full functional maturation are impaired in the ability to trans-differentiate into SPEM, and lose that ability as they age.152 However, the mechanisms by which differentiated cells gain re-entry into the cell division cycle are poorly understood. It is important to learn more about the mechanisms that regulate H pylori–perturbed parietal cell homeostasis and progression into neoplasia. Several lines of evidence indicate that H pylori and the secreted oncoprotein CagA have the potential to reprogram epithelial cells and induce stem cell–like properties in gastric epithelial cells, exemplifying the complex interactions between H pylori and progenitor cells in the gastric mucosa.153–155 Interestingly, CagA-induced gastric carcinogenesis occurs via iterative mechanisms, whereas the oncogenic activities of CagA are successively replaced by a series of genetic and/or epigenetic alterations in pre-cancer cells during long-term infection with CagA-positive H pylori.155
Given the disparity of gastric cancer incidence between Asian/Pacific Islanders and African Americans,156 studies of cell phenotypes in gastric biopsy samples from these populations might provide insights into the mechanisms that mediate the interactions among gastric microbiota, H pylori, and parietal cells (Figure 4).157 These studies would require precise analyses of cell proliferation, chromosome stability during cell division, and acid secretory activity in parietal cells. An example of this approach is the comprehensive evaluation of 295 primary gastric adenocarcinomas included in the Cancer Genome Atlas Research Network that classified gastric cancer into 4 subtypes, based on 6 types of molecular features, including DNA methylation profile, Epstein-Barr virus status, whole-exome sequence, and changes in copy number of somatic genes.158 Samples were also examined for microsatellite instability, a common feature of gastric tumors, which can be used to determine mechanisms of pathogenesis and the contributions of the gastric microbiome and genetic factors.
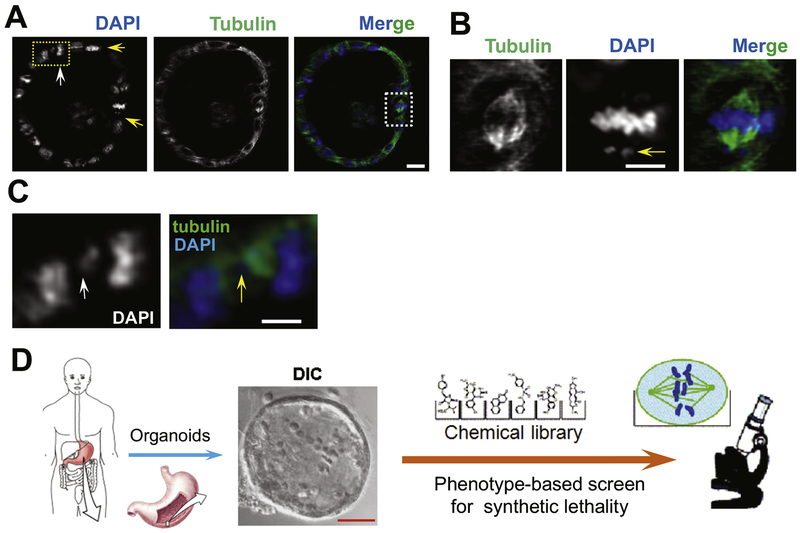
Figure 4.
Gastric stem cell division and plasticity and parietal cell functions in gastric organoids. (A) Non-secreting mouse gastric organoids were maintained in cimetidine and fixed for immunocytochemical staining of α-tubulin (green), and 4′,6-diamidino-2-phenylindole (DAPI) (blue). The light-sheet micrograph shows 4 mitotic cells with apparent different spindle orientations. Two metaphase-like cells are indicated by 2 yellow rows. Insets show 1 prometaphase (yellow arrow) and 1 aberrant anaphase-like cell with apparent perturbations of chromosome segregation (indicated by yellow arrow). Visualization of chromosome segregation in gastric organoids can be used to study chromosome stability and morphology of cancer cells. Light-sheet micrography of gastric epithelial and stem cells can be used to study their responses to different extracellular cues or therapeutic agents. For example, organoids derived from gastric cancer cells of patients might be used to identify the most effective treatments. Real-time spectral imaging could be used to study the effects of different combinations of agents. Scale bar = 20 μm. (B) Magnified image from (A). This mitotic cell has a lagging chromosome and spindle orientation error (arrow). Scale bar = 10 μm. (C) Magnified image from (A). This anaphase cell has a chromosome bridge readily apparent in DAPI staining (white arrow) and merged image with tubulin. Scale bar = 10 μm. (D) Effects of different combinations of therapeutic agents on gastric cancer cells. The schematic illustration shows how patient-derived samples can be used to screen for compounds and/or regimens that specifically tailored to specific cancers with different genetics and epigenetics.
New Systems and Technologies to Study Parietal Cells
The gastric mucosal epithelium constantly renews itself and the stem cells that mediate this process reside in the gastric glands.146,147 As observed during renewal of mouse enterocytes,159 symmetric divisions of stem cells and neutral drift lead to monoclonal antral gastric units.151 It is unclear whether symmetric divisions of stem cells are responsible for epithelial renewal in other gastric regions, such as the corpus. Identification of markers of gastric stem cells in mice, including LGR5 and Troy (a member of the tumor necrosis factor receptor superfamily), has facilitated understanding of the gastric epithelial cell lineage.150,160 Lineage tracing revealed Lgr5+ cells to be self-renewing, multipotent stem cells responsible for the long-term renewal of the gastric epithelium. Using an in vitro culture system, long-lived organoids resembling mature pyloric epithelium have been generated in culture from single Lgr5+ cells.147 In addition, temporal manipulation of the FGF, WNT, BMP, retinoic acid, and EGF signaling pathways allowed for de novo generation of 3-dimensional human gastric organoids from human pluripotent stem cells.161
To date, studies of host–pathogen or host–commensal interactions have relied on experimental models, such as primary or transformed cell lines and infected animals.162 These models have elucidated unprecedented details of H pylori pathophysiology, but the organism’s classification as an A1 carcinogen has necessarily limited experimental infection of humans. The mechanisms of H pylori’s persistence and ability to colonize the stomachs of about half of the world’s population163 are poorly understood. Although chronic H pylori infection can have serious pathological sequelae, including gastric ulceration and gastric adenocarcinoma, the temporal profile of H pylori–mediated pathogenesis has not been established. The development of human gastric organoids therefore provides a powerful tool to study human gastric epithelial development, gastric stem cell plasticity, and the response of gastric epithelial cells to H pylori infection and other ecological perturbations (Figure 4).
Light sheet fluorescence microscopy provides an advanced approach for rapid acquisition of 3-dimensional images over large fields of view and over long durations.164 In essence, 3-dimensional images are compiled from successive light sheet illuminations of thin, 2-dimensional optical sections. The high spatial and temporal resolution of the imaging enables tracking of every cell in relatively thick samples, such as embryos over long periods of time.164 A light sheet microscopy experiment provided high spatial resolution of mitotic stem cells in murine gastric organoids labeled with α-tubulin antibody and the nuclear dye 4′,6-diamidino-2-phenylindole (Figure 4A–C). This study provided a proof of principle for applying light sheet microscopy in real-time imaging of mitotic cellular dynamics in organoids combined with gene editing. Future studies using patient-derived gastric organoids will afford insights into the molecular mechanisms underlying H pylori–elicited pathogenesis, and provide valuable personalized drug discovery opportunities. In this case, the strategy of synthetic lethality can be used to screen for better regimens of precision medication.
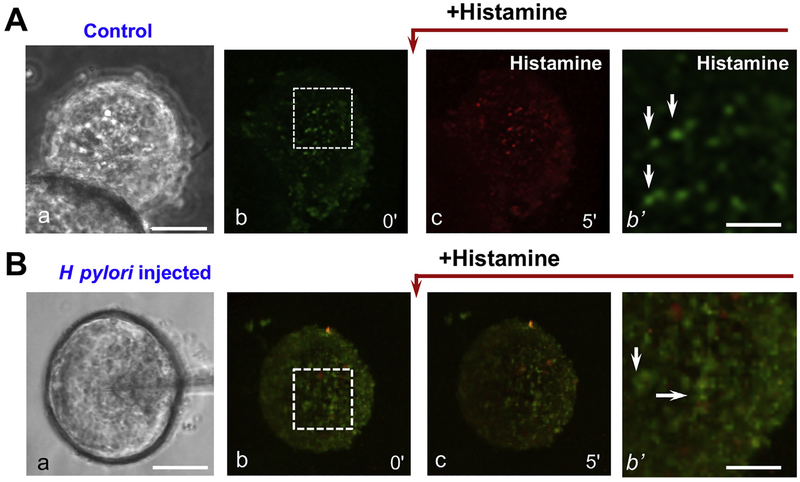
Live fluorescence imaging of mouse gastric organoids exposed to the lipophilic weak base acridine orange, which accumulates in acid spaces in parietal cells (eg, Figure 5A; b’, arrows), has been used to study acid secretion. Using light sheet fluorescence microscopy of gastric organoids, researchers can visualize acid secretion by individual parietal cells as a shift in acridine orange fluorescence from green to red in response to the secretagogues. When organoids preincubated with acridine orange were exposed to 100 μM histamine, red fluorescence intensity in parietal cells increased within 5 minutes, indicating the onset of histamine-induced parietal cell acid secretion (Figure 5A; b). Researchers assessed acid secretion in response to H pylori infection by injecting H pylori into the lumen of gastric organoids (Figure 5B; b). Two hours after the injection of H pylori into the lumen, gastric organoids were loaded with acridine orange for live fluorescence imaging, followed by histamine stimulation. Acute H pylori infection of gastric organoids suppressed acid secretion from parietal cells, visualized as little or no change in residual green fluorescence after addition of histamine (Figure 5B). These observations indicate that human gastric organoids in culture might be used to study H pylori–induced pertubations of parietal cell biology leading to disease. Such organoids might also provide opportunities for parietal cell and other gastric cell phenotype–based screening of small molecule libraries for potential therapeutic applications.
Figure 5.
Effects of acute Helicobacter pylori infection on acid secretion in gastric organoids. Control mouse gastric organoids or organoids exposed to microinjected H pylori for 4 hours were loaded with acridine orange (AO) and exposed to histamine. (A) Differential interference contrast (DIC) light micrograph of control organoid (a), and fluorescence micrographs of organoids before and after histamine stimulation (b) and (c) were collected and presented. Parietal cells are readily identified in the organoids by the AO labeling in magnified area (b’, arrows). (B) DIC micrograph of a gastric organoid undergoing microinjection with H pylori, and fluorescence micrographs of injected organoids before and after histamine stimulation (b) and (c). Parietal cells are identified by AO labeling (b’, arrows). H pylori infection suppressed the parietal cell response to histamine, as indicated by minimized AO fluorescence shift from green to red. Light micrograph scale bars = 100 μm. Fluorescence micrograph scale bars = 25 μm.
Future Directions
A recently developed lattice light-sheet microscopy with adaptive optics has enabled non-invasive aberration-free imaging of subcellular processes, including endocytosis, organelle contacts, and remodeling during mitosis in live human intestinal organoids and even live zebrafish.165 This technology reveals the phenotypic diversity within cells from different organisms and developmental stages and might be used to determine how cells adapt to different physiologic settings.166 Combinations of advanced optical imaging protocols, such as lattice light-sheet microscopy with adaptive optics and photoactivatable complementary fluorescence,167 spectral imaging analyses,165 and correlative light and cryo-electron microscopic tomography,168 could increase our understanding of parietal cell organelle interactions and the cellular adaptations to H pylori infection. In this context, inclusion of immune cell lineages in gastric epithelial organoids would allow study of host–pathogen interactions with great precision in vitro. The expanding collection of gene-edited mice, (epi)genetically defined tissues from patients,169 and gastric organoids derived from gene-edited mice, will allow modeling of cellular and molecular events underlying pathogenesis of gastric cancer, and development of optimal therapeutic interventions.
Acknowledgments
We thank Drs Richard Peek, Vincent Yang, and Lydia Wroblewski for insightful discussion and Dr Xing Liu and Xu Liu for providing organoids images. Author contributions: Xuebiao Yao and Adam Smolka were responsible for the analyses and interpretation of published data, critical presentation of intellectual content, and drafting of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China Grants 31320103904, 31430054, 81630080, 31621002, 91854203 and W.M. Keck Foundation; National Institutes of Health Grants CA-164133, DK-115812, DK-56292 and DK-064371.
Abbreviations used in this paper:
- cAMP
adenosine 3′,5′-cyclic mono-phosphate
- ECL
enterochromaffin-like
- IL
interleukin
- Lat B
latrunculin B
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SPEM
spasmolytic polypeptide-expressing metaplasia
- T4SS
type IV secretory system
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Lederman L Gastric cancer: local and global burden. Am J Manag Care 2017;4. [Google Scholar]
- 3.Hagiwara T, Mukaisho KI, Nakayama T, et al. Long-term proton pump inhibitor administration worsens atrophic corpus gastritis and promotes adenocarcinoma development in Mongolian gerbils infected with Helicobacter pylori. Gut 2011;60:624–630. [DOI] [PubMed] [Google Scholar]
- 4.Judd LM, Andringa A, Rubio CA, et al. Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(−/−)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol 2005;20:1266–1278. [DOI] [PubMed] [Google Scholar]
- 5.Ahn JS, Eom CS, Jeon CY, et al. Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol 2013;19:2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuta T, Baba S, Takashima M, et al. H+/K+-adenosine triphosphatase mRNA in gastric fundic gland mucosa in patients infected with Helicobacter pylori. Scand J Gastroenterol 1999;34:384–390. [DOI] [PubMed] [Google Scholar]
- 7.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20:1161–1181. [DOI] [PubMed] [Google Scholar]
- 8.Forte JG, Hanzel DK, Urushidani T, et al. Pumps and pathways for gastric HCl secretion. Ann N Y Acad Sci 1989;574:145–158. [DOI] [PubMed] [Google Scholar]
- 9.Sachs G The gastric proton pump: the H+, K+-ATPase In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. 2nd ed. New York: Raven, 1987:865–881. [Google Scholar]
- 10.Lee HC, Breitbart H, Berman M, et al. Potassium-stimulated ATPase activity and hydrogen transport in gastric microsomal vesicles. Biochim Biophys Acta 1979;553:107–131. [DOI] [PubMed] [Google Scholar]
- 11.Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol 2003;65:103–131. [DOI] [PubMed] [Google Scholar]
- 12.Spicer Z, Miller ML, Andringa A, et al. Stomachs of mice lacking the gastric H,K-ATPase alpha-subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem 2000;275:21555–21565. [DOI] [PubMed] [Google Scholar]
- 13.Asano S, Kawada K, Kimura T, et al. The roles of carbohydrate chains of the beta-subunit on the functional expression of gastric H(+),K(+)-ATPase. J Biol Chem 2000;275:8324–8330. [DOI] [PubMed] [Google Scholar]
- 14.Bakkelund KE, Waldum HL, Nordrum IS, et al. Long-term gastric changes in achlorhydric H(+)/K(+)-ATPase beta subunit deficient mice. Scand J Gastroenterol 2010;45:1042–1047. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen N, Kozer-Gorevich N, Gliddon BL, et al. Independent trafficking of the KCNQ1 K+ channel and H+/K+ ATPase in gastric parietal cells from mice. Am J Physiol 2013;304:G157–G166. [DOI] [PubMed] [Google Scholar]
- 16.Roepke TK, Anantharam A, Kirchhoff P, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem 2006;281:23740–23747. [DOI] [PubMed] [Google Scholar]
- 17.Song P, Groos S, Riederer B, et al. KCNQ1 is the luminal K+ recycling channel during stimulation of gastric acid secretion. J Physiol 2009;587:3955–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song P, Groos S, Riederer B, et al. Kir4.1 channel expression is essential for parietal cell control of acid secretion. J Biol Chem 2011;286:14120–14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He W, Liu W, Chew CS, et al. Acid secretion-associated translocation of KCNJ15 in gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 2011;301:G591–G600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabon EC, McFall TL, Sachs G. The gastric [H,K] ATPase:H+/ATP stoichiometry. J Biol Chem 1982;257:6296–6299. [PubMed] [Google Scholar]
- 21.Black JA, Forte TM, Forte JG. Structure of oxyntic cell membranes during conditions of rest and secretion of HCl as revealed by freeze-fracture. Anat Rec 1980;196:163–172. [DOI] [PubMed] [Google Scholar]
- 22.Smolka A, Helander HF, Sachs G. Monoclonal antibodies against gastric H+ + K+ ATPase. Am J Physiol 1983;245:589–596. [DOI] [PubMed] [Google Scholar]
- 23.Soroka CJ, Chew CS, Hanzel DK, et al. Characterization of membrane and cytoskeletal compartments in cultured parietal cells: immunofluorescence and confocal microscopic examination. Eur J Cell Biol 1993;60:76–87. [PubMed] [Google Scholar]
- 24.Abe K, Irie K, Nakanishi H, et al. Crystal structures of the gastric proton pump. Nature 2018;556:214–218. [DOI] [PubMed] [Google Scholar]
- 25.Beales I, Blaser MJ, Srinivasan S, et al. Effect of Helicobacter pylori products and recombinant cytokines on gastrin release from cultured canine G cells. Gastroenterology 1997;113:465–471. [DOI] [PubMed] [Google Scholar]
- 26.DelValle J, Sugano K, Yamada T. Progastrin and its glycine-extended posttranslational processing intermediates in human gastrointestinal tissues. Gastroenterology 1987;92:1908–1912. [DOI] [PubMed] [Google Scholar]
- 27.Bitziou E, Patel BA. Simultaneous detection of gastric acid and histamine release to unravel the regulation of acid secretion from the guinea pig stomach. Am J Physiol 2012;303:G396–G403. [DOI] [PubMed] [Google Scholar]
- 28.Wilkes JM, Kajimura M, Scott DR, et al. Muscarinic responses of gastric parietal cells. J Membr Biol 1991;122:97–101. [DOI] [PubMed] [Google Scholar]
- 29.DelValle J, Wang L, Gantz I, et al. Characterization of H2 histamine receptor: linkage to both adenylate cyclase and [Ca2+](i) signaling systems. Am J Physiol 1992;263:G967–G972. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi K, Endoh T, Hayashi S, et al. Activation of muscarinic acetylcholine receptor subtype 4 is essential for cholinergic stimulation of gastric acid secretion: relation to D cell/somatostatin. Front Pharmacol 2016;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson LI, Goltermann N, de Magistris L, et al. Somatostatin cell processes as pathways for paracrine secretion. Science 1979;205:1393–1395. [DOI] [PubMed] [Google Scholar]
- 32.Martinez V, Curi AP, Torkian B, et al. High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology 1998;114:1125–1132. [DOI] [PubMed] [Google Scholar]
- 33.Sahoo N, Gu M, Zhang X, et al. Gastric acid secretion from parietal cells is mediated by a Ca(2+) efflux channel in the tubulovesicle. Dev Cell 2017;41:262–273.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otake K, Sakurai Y, Nishida H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther 2016;33:1140–1157. [DOI] [PubMed] [Google Scholar]
- 35.Sugano K Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Therap Adv Gastroenterol 2018;11 1756283x17745776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forte TM, Machen TE, Forte JG. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology 1977;73:941–955. [PubMed] [Google Scholar]
- 37.Lo HG, Jin RU, Sibbel G, et al. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev 2017;31:154–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calhoun BC, Goldenring JR. Two Rab proteins, vesicle-associated membrane protein 2 (VAMP-2) and secretory carrier membrane proteins (SCAMPs), are present on immunoisolated parietal cell tubulovesicles. Biochem J 1997;325(Pt 2):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng XR, Yao X, Chow DC, et al. Association of syntaxin 3 and vesicle-associated membrane protein (VAMP) with H+/K(+)-ATPase-containing tubulovesicles in gastric parietal cells. Mol Biol Cell 1997;8:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karvar S, Yao X, Duman JG, et al. Intracellular distribution and functional importance of vesicle-associated membrane protein 2 in gastric parietal cells. Gastroenterology 2002;123:281–290. [DOI] [PubMed] [Google Scholar]
- 41.Lehnardt S, Ahnert-Hilger G, Bigalke H, et al. Acid secretion of parietal cells is paralleled by a redistribution of NSF and alpha, beta-SNAPs and inhibited by tetanus toxin. Histochem Cell Biol 2000;114:387–391. [DOI] [PubMed] [Google Scholar]
- 42.Calhoun BC, Lapierre LA, Chew CS, et al. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am J Physiol 1998;275:C163–C170. [DOI] [PubMed] [Google Scholar]
- 43.Ammar DA, Zhou R, Forte JG, et al. Syntaxin 3 is required for cAMP-induced acid secretion: streptolysin O-permeabilized gastric gland model. Am J Physiol Gastrointest Liver Physiol 2002;282:G23–G33. [DOI] [PubMed] [Google Scholar]
- 44.Yu HJ, Zhou JJ, Takahashi H, et al. Spatial control of proton pump H,K-ATPase docking at the apical membrane by phosphorylation-coupled ezrin-syntaxin 3 interaction. J Biol Chem 2014;289:33333–33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forte JG, Zhu L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol 2010;72:273–296. [DOI] [PubMed] [Google Scholar]
- 46.Sanford JC, Foster L, Kapadia Z, et al. Analysis of the stoichiometry of Rab protein prenylation. Anal Biochem 1995;224:547–556. [DOI] [PubMed] [Google Scholar]
- 47.Deneka M, Neeft M, van der Sluijs P. Regulation of membrane transport by rab GTPases. Crit Rev Biochem Mol Biol 2003;38:121–142. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Feng Y, Chen D, et al. Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 1998;9:3241–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldenring JR, Shen KR, Vaughan HD, et al. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem 1993;268:18419–18422. [PubMed] [Google Scholar]
- 50.Goldenring JR, Soroka CJ, Shen KR, et al. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol 1994;267:G187–G194. [DOI] [PubMed] [Google Scholar]
- 51.Duman JG, Tyagarajan K, Kolsi MS, et al. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am J Physiol 1999;277:C361–C372. [DOI] [PubMed] [Google Scholar]
- 52.Schonteich E, Pilli M, Simon GC, et al. Molecular characterization of Rab11-FIP3 binding to ARF GTPases. Eur J Cell Biol 2007;86:417–431. [DOI] [PubMed] [Google Scholar]
- 53.Matsukawa J, Nakayama K, Nagao T, et al. Role of ADP-ribosylation factor 6 (ARF6) in gastric acid secretion. J Biol Chem 2003;278:36470–36475. [DOI] [PubMed] [Google Scholar]
- 54.Fang ZY, Miao Y, Ding X, et al. Proteomic identification and functional characterization of a novel ARF6 GTPase-activating protein, ACAP4. Mol Cell Proteomics 2006; 5:1437–1449. [DOI] [PubMed] [Google Scholar]
- 55.Ding X, Deng H, Wang DM, et al. Phospho-regulated ACAP4-ezrin interaction is essential for histamine-stimulated parietal cell secretion. J Biol Chem 2010; 285:18769–18780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang H, Wang WW, Zhang Y, et al. Cell polarity kinase MST4 cooperates with cAMP-dependent kinase to orchestrate histamine-stimulated acid secretion in gastric parietal cells. J Biol Chem 2015;290:28272–28285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan X, Yao PY, Jiang JY, et al. MST4 kinase phosphorylates ACAP4 protein to orchestrate apical membrane remodeling during gastric acid secretion. J Biol Chem 2017;292:16174–16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawaguchi A, Aoyama F, Ohashi M, et al. Ultrastructural transformation of gastric parietal cells reverting from the active to the resting state of acid secretion revealed in isolated rat gastric mucosa model processed by high-pressure freezing. J Electron Microsc (Tokyo) 2006;55:97–105. [DOI] [PubMed] [Google Scholar]
- 59.Courtois-Coutry N, Roush D, Rajendran V, et al. A tyrosine-based signal targets H/K-ATPase to a regulated compartment and is required for the cessation of gastric acid secretion. Cell 1997;90:501–510. [DOI] [PubMed] [Google Scholar]
- 60.Duffield A, Kamsteeg EJ, Brown AN, et al. The tetraspanin CD63 enhances the internalization of the H,K-ATPase beta-subunit. Proc Natl Acad Sci U S A 2003;100:15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto CT, Duman JG, Tyagarajan K, et al. Clathrin in gastric acid secretory (parietal) cells: biochemical characterization and subcellular localization. Am J Physiol Cell Physiol 2000;279:C833–C851. [DOI] [PubMed] [Google Scholar]
- 62.Osawa W, Ogata T. A scanning electron microscopic study on the fractured rat parietal cells in resting state and after stimulation with tetragastrin. Arch Histol Jpn 1978;41:141–155. [DOI] [PubMed] [Google Scholar]
- 63.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 1993;236:333–340. [DOI] [PubMed] [Google Scholar]
- 64.Ammar DA, Nguyen PN, Forte JG. Functionally distinct pools of actin in secretory cells. Am J Physiol Cell Physiol 2001;281:C407–C417. [DOI] [PubMed] [Google Scholar]
- 65.Natarajan P, Crothers JM Jr, Rosen JE, et al. Myosin IIB and F-actin control apical vacuolar morphology and histamine-induced trafficking of H-K-ATPase-containing tubulovesicles in gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 2014;306:G699–G710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamura A, Kikuchi S, Hata M, et al. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol 2005;169:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao X, Thibodeau A, Forte JG. Ezrin-calpain I interactions in gastric parietal cells. Am J Physiol 1993;265:C36–C46. [DOI] [PubMed] [Google Scholar]
- 68.Zhou R, Zhu L, Kodani A, et al. Phosphorylation of ezrin on threonine 567 produces a change in secretory phenotype and repolarizes the gastric parietal cell. J Cell Sci 2005;118:4381–4391. [DOI] [PubMed] [Google Scholar]
- 69.Zhu L, Hatakeyama J, Chen C, et al. Comparative study of ezrin phosphorylation among different tissues: more is good; too much is bad. Am J Physiol Cell Physiol 2008;295:C192–C202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dard N, Louvet-Vallee S, Santa-Maria A, et al. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev Biol 2004;271:87–97. [DOI] [PubMed] [Google Scholar]
- 71.Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol 2000;150:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu L, Liu Y, Forte JG. Ezrin oligomers are the membrane-bound dormant form in gastric parietal cells. Am J Physiol Cell Physiol 2005;288:C1242–C1254. [DOI] [PubMed] [Google Scholar]
- 73.Zhu L, Crothers J Jr, Zhou R, et al. A possible mechanism for ezrin to establish epithelial cell polarity. Am J Physiol Cell Physiol 2010;299:C431–C443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu L, Zhou R, Mettler S, et al. High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol 2007;293:C874–C884. [DOI] [PubMed] [Google Scholar]
- 75.Ramsey EJ, Carey KV, Peterson WL, et al. Epidemic gastritis with hypochlorhydria. Gastroenterology 1979;76:1449–1457. [PubMed] [Google Scholar]
- 76.Gledhill T, Leicester RJ, Addis B, et al. Epidemic hypochlorhydria. Br Med J (Clin Res Ed) 1985;290:1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol 1987;82:192–199. [PubMed] [Google Scholar]
- 78.Sobala GM, Crabtree JE, Dixon MF, et al. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 1991;32:1415–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall BJ. Helicobacter pylori in peptic ulcer: have Koch’s postulates been fulfilled? Ann Med 1995;27:565–568. [DOI] [PubMed] [Google Scholar]
- 80.Graham DY, Alpert LC, Smith JL, et al. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol 1988;83:974–980. [PubMed] [Google Scholar]
- 81.El-Omar EM, Oien K, El-Nujumi A, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 1997;113:15–24. [DOI] [PubMed] [Google Scholar]
- 82.Harford WV, Barnett C, Lee E, et al. Acute gastritis with hypochlorhydria: report of 35 cases with long term follow up. Gut 2000;47:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee A, Krakowka S, Fox JG, et al. Role of Helicobacter felis in chronic canine gastritis. Vet Pathol 1992;29:487–494. [DOI] [PubMed] [Google Scholar]
- 84.Fox JG, Blanco MC, Yan L, et al. Role of gastric pH in isolation of Helicobacter mustelae from the feces of ferrets. Gastroenterology 1993;104:86–92. [DOI] [PubMed] [Google Scholar]
- 85.Takashima M, Furuta T, Hanai H, et al. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut 2001;48:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffman JS, King WW, Fox JG, et al. Rabbit and ferret parietal cell inhibition by Helicobacter species. Dig Dis Sci 1995;40:147–152. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi H, Kamiya S, Suzuki T, et al. The effect of Helicobacter pylori on gastric acid secretion by isolated parietal cells from a guinea pig. Association with production of vacuolating toxin by H. pylori. Scand J Gastroenterol 1996;31:428–433. [DOI] [PubMed] [Google Scholar]
- 88.Vargas M, Lee A, Fox JG, et al. Inhibition of acid secretion from parietal cells by non-human-infecting Helicobacter species: a factor in colonization of gastric mucosa? Infect Immun 1991;59:3694–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jablonowski H, Hengels KJ, Kraemer N, et al. Effect of Helicobacter pylori on dibutyryl c-AMP-stimulated acid secretion by human parietal cells. Hepatogastroenterology 1994;41:546–548. [PubMed] [Google Scholar]
- 90.Cave DR, Vargas M. Effect of a Campylobacter pylori protein on acid secretion by parietal cells. Lancet 1989;2:187–189. [DOI] [PubMed] [Google Scholar]
- 91.Beil W, Birkholz C, Wagner S, et al. Interaction of Helicobacter pylori and its fatty acids with parietal cells and gastric H+/K(+)-ATPase. Gut 1994;35:11761180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jablonowski H, Hengels KJ, Kraemer N, et al. Effects of Helicobacter pylori on histamine and carbachol stimulated acid secretion by human parietal cells. Gut 1994;35:755–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Necchi V, Candusso ME, Tava F, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 2007;132:1009–1023. [DOI] [PubMed] [Google Scholar]
- 94.Bjorkholm B, Zhukhovitsky V, Lofman C, et al. Helicobacter pylori entry into human gastric epithelial cells: a potential determinant of virulence, persistence, and treatment failures. Helicobacter 2000;5:148–154. [DOI] [PubMed] [Google Scholar]
- 95.Chen XG, Correa P, Offerhaus J, et al. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am J Clin Pathol 1986;86:575–582. [DOI] [PubMed] [Google Scholar]
- 96.Scanziani E, Simpson KW, Monestiroli S, et al. Histological and immunohistochemical detection of different Helicobacter species in the gastric mucosa of cats. J Vet Diagn Invest 2001;13:3–12. [DOI] [PubMed] [Google Scholar]
- 97.Taniguchi Y, Kimura K, Satoh K, et al. Helicobacter pylori detected deep in gastric glands: an ultrastructural quantitative study. J Clin Gastroenterol 1995;21(Suppl 1):S169–S173. [PubMed] [Google Scholar]
- 98.Rollason TP, Stone J, Rhodes JM. Spiral organisms in endoscopic biopsies of the human stomach. J Clin Pathol 1984;37:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tagkalidis PP, Royce SG, Macrae FA, et al. Selective colonization by Helicobacter pylori of the deep gastric glands and intracellular canaliculi of parietal cells in the setting of chronic proton pump inhibitor use. Eur J Gastroenterol Hepatol 2002;14:453–456. [DOI] [PubMed] [Google Scholar]
- 100.Meyer-Rosberg K, Scott DR, Rex D, et al. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 1996;111:886–900. [DOI] [PubMed] [Google Scholar]
- 101.Athmann C, Zeng N, Kang T, et al. Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells. J Clin Invest 2000; 106:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weeks DL, Eskandari S, Scott DR, et al. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 2000;287:482–485. [DOI] [PubMed] [Google Scholar]
- 103.Sachs G, Kraut JA, Wen Y, et al. Urea transport in bacteria: acid acclimation by gastric Helicobacter spp. J Membr Biol 2006;212:71–82. [DOI] [PubMed] [Google Scholar]
- 104.Merchant JL. Inflammation, atrophy, gastric cancer: connecting the molecular dots. Gastroenterology 2005;129:1079–1082. [DOI] [PubMed] [Google Scholar]
- 105.Gooz M, Hammond CE, Larsen K, et al. Inhibition of human gastric H(+)-K(+)-ATPase alpha-subunit gene expression by Helicobacter pylori. Am J Physiol 2000;278:G981–G991. [DOI] [PubMed] [Google Scholar]
- 106.Dubois A, Fiala N, Heman-Ackah LM, et al. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology 1994;106:1405–1417. [DOI] [PubMed] [Google Scholar]
- 107.Saha A, Hammond CE, Beeson C, et al. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut 2010;59:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mills JC, Syder AJ, Hong CV, et al. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci U S A 2001;98:13687–13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol 2012;47:609–618. [DOI] [PubMed] [Google Scholar]
- 110.Smolka AJ, Schubert ML. Helicobacter pylori-induced changes in gastric acid secretion and upper gastrointestinal disease. Curr Top Microbiol Immunol 2017;400:227–252. [DOI] [PubMed] [Google Scholar]
- 111.Chang YW, Shaffer CL, Rettberg LA, et al. In vivo structures of the Helicobacter pylori cag type IV secretion system. Cell Rep 2018;23:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 2007;449:862–866. [DOI] [PubMed] [Google Scholar]
- 113.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol 2008;10:1573–1581. [DOI] [PubMed] [Google Scholar]
- 114.Suzuki K, Kokai Y, Sawada N, et al. SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch 2002;440:318–324. [DOI] [PubMed] [Google Scholar]
- 115.Wroblewski LE, Shen L, Ogden S, et al. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 2009;136:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wroblewski LE, Noble PJ, Pagliocca A, et al. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 2003;116:3017–3026. [DOI] [PubMed] [Google Scholar]
- 117.Weydig C, Starzinski-Powitz A, Carra G, et al. CagA-independent disruption of adherence junction complexes involves E-cadherin shedding and implies multiple steps in Helicobacter pylori pathogenicity. Exp Cell Res 2007;313:3459–3471. [DOI] [PubMed] [Google Scholar]
- 118.Saha A, Hammond CE, Trojanowska M, et al. Helicobacter pylori-induced H,K-ATPase {alpha}-subunit gene repression is mediated by NF-{kappa}B p50 homodimer promoter binding. Am J Physiol 2008;294:G795–G807. [DOI] [PubMed] [Google Scholar]
- 119.Saha A, Backert S, Hammond CE, et al. Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase alpha subunit. Gastroenterology 2010;139:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang YM, Noto JM, Hammond CE, et al. Helicobacter pylori-induced post-transcriptional regulation of H,K-ATPase alpha subunit gene by miRNA. Am J Physiol 2014;306:G606–G613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hammond CE, Beeson C, Suarez G, et al. Helicobacter pylori virulence factors affecting gastric proton pump expression and acid secretion. Am J Physiol 2015;309:G193–G201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol 2005;3:320–332. [DOI] [PubMed] [Google Scholar]
- 123.Szabo I, Brutsche S, Tombola F, et al. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. Embo j 1999;18:5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Bernard M, Arico B, Papini E, et al. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol 1997;26:665–674. [DOI] [PubMed] [Google Scholar]
- 125.Ye D, Willhite DC, Blanke SR. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J Biol Chem 1999;274:9277–9282. [DOI] [PubMed] [Google Scholar]
- 126.Cover TL, Krishna US, Israel DA, et al. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res 2003;63:951–957. [PubMed] [Google Scholar]
- 127.Terebiznik MR, Raju D, Vazquez CL, et al. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy 2009;5:370–379. [DOI] [PubMed] [Google Scholar]
- 128.Wang F, Xia P, Wu F, et al. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J Biol Chem 2008;283:26714–26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 1998;42:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zaki M, Coudron PE, McCuen RW, et al. H. pylori acutely inhibits gastric secretion by activating CGRP sensory neurons coupled to stimulation of somatostatin and inhibition of histamine secretion. Am J Physiol 2013;304:G715–G722. [DOI] [PubMed] [Google Scholar]
- 131.Holm L, Agren J, Persson AE. Stimulation of acid secretion increases the gastric gland luminal pressure in the rat. Gastroenterology 1992;103:1797–1803. [DOI] [PubMed] [Google Scholar]
- 132.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 2006;103:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Delgado S, Cabrera-Rubio R, Mira A, et al. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol 2013;65:763–772. [DOI] [PubMed] [Google Scholar]
- 134.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012;13:260270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andersson AF, Lindberg M, Jakobsson H, et al. Comparative analysis of human gut microbiota by bar-coded pyrosequencing. PLoS One 2008;3:e2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J 2011;5:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–789. [DOI] [PubMed] [Google Scholar]
- 138.Vesper BJ, Jawdi A, Altman KW, et al. The effect of proton pump inhibitors on the human microbiota. Curr Drug Metab 2009;10:84–89. [DOI] [PubMed] [Google Scholar]
- 139.Sanduleanu S, Jonkers D, De Bruine A, et al. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther 2001;15:379–388. [DOI] [PubMed] [Google Scholar]
- 140.Sheh A, Fox JG. The role of the gastrointestinal micro-biome in Helicobacter pylori pathogenesis. Gut Microbes 2013;4:505–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao L, Yu J. Effect of Helicobacter pylori infection on the composition of gastric microbiota in the development of gastric cancer. Gastrointest Tumors 2015;2:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang I, Woltemate S, Piazuelo MB, et al. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep 2016;6:18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wroblewski LE, Peek RM Jr. Helicobacter pylori, cancer, and the gastric microbiota. Adv Exp Med Biol 2016;908:393–408. [DOI] [PubMed] [Google Scholar]
- 144.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018;67:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li TH, Qin Y, Sham PC, et al. Alterations in gastric microbiota after H. pylori eradication and in different histological stages of gastric carcinogenesis. Sci Rep 2017;7:44935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 1993;236:259–279. [DOI] [PubMed] [Google Scholar]
- 147.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25–36. [DOI] [PubMed] [Google Scholar]
- 148.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 2008;134:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol 2018;15:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- 151.Leushacke M, Ng A, Galle J, et al. Lgr5(+) gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep 2013;5:349–356. [DOI] [PubMed] [Google Scholar]
- 152.Weis VG, Petersen CP, Weis JA, et al. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 2017;312:G67–G76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 2016;150:64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015;148:126–136 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hatakeyama M Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014;15:306–316. [DOI] [PubMed] [Google Scholar]
- 156.Ashktorab H, Kupfer SS, Brim H, et al. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017;153:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu X, Yao Y, Wang W, et al. Mitotic motor CENP-E cooperates with PRC1 in temporal control of central spindle assembly. J Mol Cell Biol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.The Cancer Genome Atlas Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010;143:134–144. [DOI] [PubMed] [Google Scholar]
- 160.Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013;155:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCracken KW, Cata EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014;516:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.O’Rourke JL, Lee A. Animal models of Helicobacter pylori infection and disease. Microbes Infect 2003;5:741–748. [DOI] [PubMed] [Google Scholar]
- 163.Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol 2013;11:385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Keller PJ, Schmidt AD, Wittbrodt J, et al. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 2008;322:1065–1069. [DOI] [PubMed] [Google Scholar]
- 165.Liu TL, Upadhyayula S, Milkie DE, et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 2018;360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yao X, Fang G. Visualization and orchestration of the dynamic molecular society in cells. Cell Res 2009;19:152–155. [DOI] [PubMed] [Google Scholar]
- 167.Xia P, Liu X, Wu B, et al. Superresolution imaging reveals structural features of EB1 in microtubule plus-end tracking. Mol Biol Cell 2014;25:4166–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tao CL, Liu YT, Sun R, et al. Differentiation and characterization of excitatory and inhibitory synapses by cryo-electron tomography and correlative microscopy. J Neurosci 2018;38:1493–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ren J, Liu Z, Gao X, et al. MiCroKit 3.0: an integrated database of midbody, centrosome and kinetochore. Nucleic Acids Res 2010;38:D155–D160. [DOI] [PMC free article] [PubMed] [Google Scholar]