Abstract
Alzheimer's disease (AD) is a highly prevalent neurodegenerative condition that presents with cognitive decline. The current understanding of underlying disease mechanisms remains incomplete. Genetically modified mouse models have been instrumental in deciphering pathomechanisms in AD. While these models were typically generated by classical transgenesis and genome editing, the use of adeno‐associated viruses (AAVs) to model and investigate AD in mice, as well as to develop novel gene‐therapy approaches, is emerging. Here, we reviewed literature that used AAVs to study and model AD and discuss potential gene therapy strategies.
Linked Articles
This article is part of a themed section on Therapeutics for Dementia and Alzheimer's Disease: New Directions for Precision Medicine. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.18/issuetoc
Abbreviations
- AAV
adeno‐associated virus
- AAVR
AAV receptor
- AD
Alzheimer's disease
- APOE4
apolipoprotein E4
- APP
Aβ precursor protein
- Aβ
amyloid‐β
- BBB
blood–brain barrier
- BDNF
brain‐derived neurotrophic factor
- CMV
cytomegalovirus
- CNO
clozapine N‐oxide
- CRISPR
clustered regularly interspaced short palindromic repeats
- DG
dentate gyrus
- DREADD
designer receptors exclusively activated by a designer drug
- EC
entorhinal cortex
- exo‐AAV
exosome‐associated AAV
- FDA
Food and Drug Administration
- FTD
frontotemporal dementia
- GFP
green fluorescence protein
- gRNA
guide RNA
- MAPT
microtubule associate protein τ
- NFT
neurofibrillary tangle
- NHP
non‐human primate
- ORF
open reading frame
- p75ECD
ectodomain of the p75NTR
- p75NTR
p75 neurotrophic receptor
- rh
rhesus
- scFvs
single‐chain antibodies
1. ALZHEIMER'S DISEASE AND MOUSE MODELS
Alzheimer's disease (AD) is the most common neurodegenerative disease and the major form of dementia. Clinically, AD presents with cognitive decline that manifests initially as mild cognitive dementia and progresses eventually to severe intellectual deficits and the inability to cope with daily life. Neuropathologically, AD brain shows deposition of amyloid‐β (Aβ) as extracellular plaques and intraneuronal neurofibrillary tangles (NFTs) made up from hyperphosphorylated microtubule‐associated Tau protein (see Goedert & Spillantini, 2006). Frontotemporal dementia (FTD) is the second most common form of dementia (Cairns et al., 2007). The clinical lead symptoms of FTD are behavioural and/or language deficits often associated with dementia and motor problems (see Burrell et al., 2016). Notably, approximately half of FTD are due to an underlying τ pathology, that is, NFTs in the absence of overt Aβ plaques (Sieben et al., 2012). Hence, Tau pathology is sufficient to drive neurodegeneration, and investigating FTD has contributed to understanding AD. In AD, however, Aβ and Tau protein are connected in a range of molecular processes that contribute to neuronal dysfunction and loss (Ittner et al., 2010; Ittner et al., 2016; Roberson et al., 2007). The prevailing theory for AD pathogenesis remains the amyloid cascade hypothesis that states Aβ accumulation, due to its increased formation or its decreased clearance (Mawuenyega et al., 2010), as the disease‐initiating event (see Hardy & Selkoe, 2002). However, there are a number of alternative or complementing theories that have been put forward over time (Area‐Gomez et al., 2018; Atwood & Bowen, 2015; Bloom, 2014; Friedland‐Leuner, Stockburger, Denzer, Eckert, & Muller, 2014; Leuner et al., 2007).
The vast majority of AD cases are sporadic. In addition, pathogenic mutations have been identified in familial AD. These include mutations in the Aβ precursor protein encoding APP gene, and PSEN1 and PSEN2 that encode the presenilins that are part of the γ‐secretase complex that cleaves APP to release Aβ (Bertram & Tanzi, 2012; Guerreiro &Hardy, 2014). All these pathogenic mutations have been linked to early disease onset with changes in Aβ formation and ratios of Aβ1–40/Aβ1–42, thereby increasing the percentage of aggregation‐prone types of Aβ peptides. An exception to this is the A673T point mutation identified mainly in Icelandic and Scandinavian populations that reduces the formation of Aβ as well as the prevalence of AD (Jonsson et al., 2012). In addition to pathogenic mutations, a growing number of genetic risk factors have been identified in addition to age that increase the likeliness to develop AD. These include apolipoprotein E4 (APOE4) as the major genetic risk factor, as well as BDNF, TREM2, and many others (see Ulrich, Ulland, Colonna, & Holtzman, 2017). Although no familial AD mutations have been identified in the Tau‐encoding microtubule associated protein Tau (MAPT) gene, pathogenic mutations in this locus have been found to underlie familial FTD with Tau pathology (Forrest et al., 2018). The identification of genetic mutations in familial AD and FTD paved the way for developing the first transgenic mouse models that reproduced the human neuropathology and resulted in progressive functional deficits (see Gotz & Ittner, 2008). Specifically, expression of human mutant APP in transgenic mice produced in vivo models with human‐like Aβ plaque pathology and memory deficits. Similarly, transgenic mice expressing human mutant Tau protein resulted in AD/FTD‐like neuropathology with deposition of hyperphosphorylated Tau protein and formation of NFTs. Individual AD/FTD mouse models will be introduced together with the discussion of studies they have been used in below. While transgenic mouse models remain the predominant disease model to study AD as well as develop and test new treatments, more recently available gene delivery mechanisms have added new tools to investigate and potentially treat neurodegenerative diseases. In the present review, we review publications using recombinant adeno‐associated virus vectors (rAAVs) to model, understand, or treat AD in mice (Figure 1).
Figure 1.
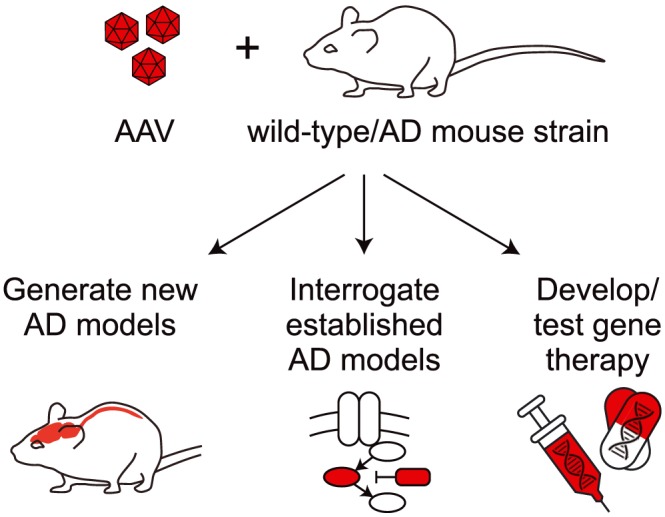
Potential of AAV in advancing AD studies and therapies. Combining AAV with either wild‐type (non‐transgenic) or established genetically modified lines (including AD models) offers the opportunity to generate new AD models and interrogate existing AD models to understand underlying disease mechanisms. Furthermore, AAV has been used to interfere with pathological processes in AD mouse models, establishing proof‐of‐concept for potential gene therapies
2. ADENO‐ASSOCIATED VIRUSES—RESEARCH TOOLS AND GENE THERAPY
AAVs have emerged as the vectors of choice for CNS‐targeted gene delivery to study disease mechanisms in animal models but also for gene therapy of neurodegenerative diseases. The popularity of AAV as a gene delivery system, particularly for CNS applications, can be explained by their non‐pathogenic nature, their ability to transduce neurons and convey long‐term transgene expression as well as mild immune responses. Moreover, the simplistic capsid surface can be easily manipulated to engineer the vectors with targeted cellular tropism and enhanced transduction efficiency (von Jonquieres et al., 2018). Wild‐type AAV is a member of the parvovirus family of non‐enveloped single‐stranded DNA viruses. AAV is small (25 nm) and naturally replication deficient, thus their replication requires a helper virus such as the name‐giving adenovirus. The simplistic 4.7‐kb genome contains three open reading frames (ORFs) that are flanked by 145‐nt inverted terminal repeats, which are T‐shaped hairpin packaging signals required for second strand synthesis. The rep ORF encodes four proteins essential for DNA replication and other regulatory functions including site‐specific integration into human chromosome 19 (Kotterman & Schaffer, 2014). The cap ORF gene encodes three structural proteins (VP1, VP2, and VP3) that form the 60‐mer viral capsid at a 1:1:10 ratio. The third ORF encodes the assembly‐activating protein that is critical for capsid formation of most AAV serotypes (Grimm & Buning, 2017; Sonntag, Schmidt, & Kleinschmidt, 2010). The wild‐type AAV genome can be turned into a recombinant AAV vector genome by gutting rep and cap genes and replacing them with an expression cassette of choice consisting of a promoter that is active in the target tissue, the gene of interest, and a polyadenylation signal. Most commonly AAV2‐derived inverted terminal repeat containing cassettes are used for efficient cross‐packaging into serotype‐specific virions (Rabinowitz et al., 2002), resulting in pseudotyped vectors according to a numeric nomenclature that informs about the encapsidated recombinant vector genome followed by the capsid, for example, AAV2/9.
2.1. AAV‐mediated long‐term gene expression in the brain
Scalable production and purification of AAV vectors can be adopted by standard research laboratories given user‐friendly protocols (Crosson, Dib, Smith, & Zolotukhin, 2018; During, Young, Baer, Lawlor, & Klugmann, 2003; Grieger, Soltys, & Samulski, 2016; Lock et al., 2010). Packaging is mostly done by standard transfection of the AAV plasmid into HEK293 cells because they express as subset of adenoviral genes required for AAV replication. The complementing adenoviral helper functions, as well as the rep and cap genes of the desired serotypes, are supplied by transient transfection. AAVs lacking Rep proteins do not integrate into the host genome. However, long‐term transgene expression occurs even in post‐mitotic target tissues because of AAV genome concatemerization and stable episomal persistence within the nucleus (Duan et al., 1998). As such, AAVs are advantageous over other virus systems such as the lentivirus, as AAV lacks the position effects as a consequence of genomic integration of lentivirus (Piguet, Alves, & Cartier, 2017). Furthermore, AAVs are more stable for long‐term gene expression over other viral vehicles. AAV with glial fibrillary acidic protein promoter‐driven ApoE expression was still detectable in the mouse brain at 12 months after injection, while the same construct delivered via an adenovirus ceased expression after 6 weeks (Feng, Eide, Jiang, & Reder, 2004). Impressively, a recent report showed persistent vector genomes and expression of the therapeutic dopamine‐synthesizing enzymes 15 years after AAV delivery to treat the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐lesioned non‐human primate (NHP) model of Parkinson's disease (Sehara et al., 2017). These data suggest that AAV‐mediated transgene expression in the CNS following a single administration is long‐lasting, and possibly lifelong. This property of AAV vectors is not only important for disease modelling in rodents but is essential for clinical gene therapy.
2.2. AAV serotypes and brain cell transduction
Transduction of host cells with AAV vectors is a multistep process which includes binding, uptake, trafficking, and lastly, nuclear uncoating (Grimm & Buning, 2017). While all AAVs infect cells via a universal cellular AAV receptor (AAVR), they differ in their distinctive molecular interaction with AAVR in a serotype‐dependent manner as well as specificity for other receptors and co‐receptors (Pillay et al., 2016; Pillay et al., 2017). Currently, 13 human and NHP AAV serotypes and over 150 genotypes have been identified that have partially overlapping yet distinct tropism (Gao et al., 2004).
Serotypes 1, 2, 5, 8, 9, and rhesus (rh) 10 have been the most widely used for CNS applications in mice (Aschauer, Kreuz, & Rumpel, 2013; Broekman, Comer, Hyman, & Sena‐Esteves, 2006; Cearley & Wolfe, 2006; Davidson et al., 2000; Taymans et al., 2007) and other species (Burger et al., 2004; Klein, Dayton, Tatom, Henderson, & Henning, 2008; Lawlor, Bland, Mouravlev, Young, & During, 2009; Table 1).
Table 1.
Selected studies comparing AAV serotypes for CNS expression in mice/rats
| Serotypes compared | Mode of delivery (age) | Reporter (promoter) | Mouse/rat strain (background) | Analysis after injection | CNS transduction | Reference |
|---|---|---|---|---|---|---|
| rAAV1,2,5,6,8,9 | Stereotaxic (4–8 weeks) | GFP (CMV) | Wild‐type (C57Bl/6J mice) | 21 days |
Striatum: all CNS cell types; oligodendrocytes rAAV8, neurons rAAV1 > rAAV9 Hippocampus: all CNS cell types; astrocytes rAAV8 > rAAV5 Cortex: all CNS cell types; neurons rAAV9 > rAAV1 (retrograde transduction only by rAAV5) |
Aschauer et al. (2013) |
| rAAV9,rh.10 | i.v. (neonatal) | GFP (CMV) | Tg‐SMN1/2 (FVB‐Cg mice) | 1 month |
Cortex, hippocampus, thalamus rAAV9 = rAAVrh.10 Medulla, cerebellum, spinal cord rAAVr.h10 > rAAV9 |
Tanguy et al. (2015) |
|
rAAV1,2,5,6, 6.2,7,8,9,rh.8, rh.10,rh.39, rh.43 |
i.v. (10 weeks) | GFP (miR‐1 and miR‐122) | Wild‐type (C57Bl/6 mice) | 21 days | CNS and spinal cord: rAAVrh.8, rAAVrh.10, rAAV9 > rAAV7, rAAV8, rAARrh.39, rAAVrh.43 > rAAV6, rAAV6.2 > rAAV1 (rAAV2, rAAV5 no relevant transduction; rAAVrh.8 less peripheral expression than rAAVrh.10 and rAAV9) | Yang et al. (2014) |
| rAAV9,PHP.B | i.v. (6 weeks) | GFP (CAG) | Wild‐type (C57Bl/6 mice) | 21 days | CNS: rAAV‐PHP.B > > rAAV9 | Deverman et al. (2016) |
| rAAV8,9, Exo‐AAV8,9 | i.v. (adult) | GFP (CBA) | Wild‐type (BALB/c mice) | 21–28 days | CNS: exo‐AAV9 > rAAV9; exo‐AAV8 > > rAAV8 | Hudry et al. (2016) |
| rAAV‐PHP.B,‐PHP.EB | i.v. (6 weeks) or stereotaxic | GFP | Wild‐type (Sprague–Dawley rats) | 4 weeks | CNS global or localized: rAAV‐PHP.EB > rAAV‐PHP.B | Dayton et al. (2018) |
Different AAV serotypes have the general ability to transduce all major brain cell types; however, expression levels depend on both promoters and serotypes used (Aschauer et al., 2013). Interestingly, the cytomegalovirus (CMV) promoter or the CMV/chicken β‐actin hybrid promoter have been reported to exert activity in neurons. However, swapping CMV‐based promoters for glia‐specific promoters achieved cell lineage‐selective green fluorescence protein (GFP) expression in astrocytes and oligodendrocytes in mice (Georgiou et al., 2017; von Jonquieres et al., 2013; von Jonquieres et al., 2016; von Jonquieres et al., 2018; Watakabe et al., 2015). Similar promoter selectivity has been confirmed in mouse models of neurodegenerative diseases (Georgiou et al., 2017; von Jonquieres et al., 2018) and also in rat and NHP (Bassil et al., 2017; Lawlor et al., 2009; Mudannayake, Mouravlev, Fong, & Young, 2016). Targeting expression to microglia has been less successful. Here, a variant of rAAV6 together with microglia specific promoters F4/80 and CD68 has overcome some of these limits (Rosario et al., 2016). Taken together, these data suggest that the differential activity of the viral promoter variants has expanded the AAV toolkit for AD researchers interested in genetically modifying glia function. Another possible future avenue for cell type‐specific transduction is the presentation of high affinity ligands on the surface of AAV variants to target them to CNS cells specific cell surface receptors, similar to an approach used for immune cells (Muik et al., 2017). In addition to serotype and promoter, transduction efficiency is influenced by a number of factors including vector purity (Klein, Dayton, Tatom, Henderson, & Henning, 2008), maturity of the host CNS (Foust et al., 2009; von Jonquieres et al., 2013), species (Gray et al., 2011; Watakabe et al., 2015), and route of administration (Hocquemiller, Giersch, Audrain, Parker, & Cartier, 2016).
2.3. Systemic delivery of AAV for CNS expression
Over the years, different strategies have been developed to deliver genes to the CNS (Figure 2; see Hocquemiller et al., 2016; Simonato et al., 2013). While direct AAV delivery into the brain parenchyma using stereotaxic surgery is safe and efficient in many preclinical models of neurological disease, it is invasive and vector spread is limited despite axonal transport facilitating anatomical AAV distribution (Aschauer et al., 2013; Cearley & Wolfe, 2006; Franich et al., 2008). For comparison, intraventricular and intracisternal delivery of AAV are associated with widespread expression, including in NHP (Rosenberg et al., 2014), but require considerable surgical skills and infrastructure; i.v. administration of AAV as a method of delivery is technically less challenging and has gained traction with Rh.10 and AAV9 emerging as vectors of choice. For example, AAV9 has been shown to transduce CNS cells following i.v. administration in rodents, cats, pigs, and NHPs (Bevan et al., 2011; Gray et al., 2011; Samaranch et al., 2012). Similarly, Rh.10 has been used for CNS gene expression upon systemic delivery in several species, including NHP (Yang et al., 2014). Nevertheless, species differences in receptors required for AAV transduction need to be considered during translation to larger models or humans. Both Rh.10 and AAV9 readily cross the blood–brain barrier (BBB) and upon i.v. administration, results in robust transduction of CNS neurons in neonatal mice (Tanguy et al., 2015; Zhang et al., 2011). However, this AAV9 tropism via i.v. delivery shifted towards glial cells in adult mice (Foust et al., 2009). This transduction shifted to also include spinal cord in addition to brain neurons in AAV9 i.v.‐treated NHP (Gray et al., 2011). Furthermore, the kinetics of expression varied greatly between different serotypes, with AAV7 and 9 having the fastest onset of expression (Zincarelli, Soltys, Rengo, & Rabinowitz, 2008).
Figure 2.
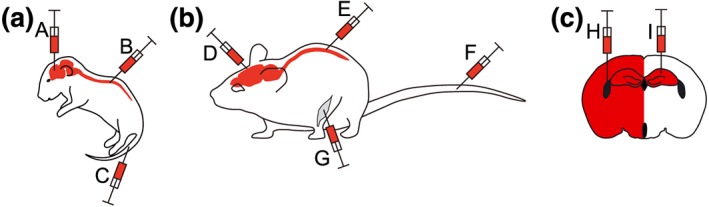
Different modes of delivering AAV for CNS gene expression in mice. (a) Common methods reported for delivering AAV‐mediated gene expression in neonatal mice include (A) direct stereotaxic brain infusion, (B) spinal cord injections, and (C) intravascular delivery. (b) Techniques most frequently reported for delivering AAV‐mediated gene expression in mature mice include (D) direct stereotaxic brain infusion, (E) spinal cord injections, (F) intravascular, and (G) i.m. delivery. (c) Two commonly used methods for intracerebral delivery of AAV in adult brains are (H) intracerebrovascular injection for widespread gene expression or (I) into distinct brain areas for localized expression. Both techniques require the use of stereotaxic devices for guided injections
The mechanism by which i.v. AAV9 enters the CNS parenchyma is not fully understood. Co‐administered mannitol did not affect central AAV9 passage suggesting an active uptake (Gray et al., 2011). In a cellular BBB‐like model, AAV9 showed lower transduction of endothelial cells while it was readily able to pass through these cells (Merkel et al., 2017). For comparison, AAV2 showed higher transduction of endothelial cells but fails to pass across the BBB, which is in line with the different abilities to pass the BBB between AAV2 and AAV9 (Merkel et al., 2017). Despite its ability to cross the BBB, the main transduction remains in peripheral organs, in particular the liver. In an attempt to reduce peripheral transgene expression (=detargeting), the intracardial injection of rAAV9 achieved neuronal gene expression with limited expression in peripheral organs (Iwata et al., 2013). Moreover, peripheral detargeting despite i.v. delivery was accomplished by engineering tissue‐specific complementary miRNA‐binding sites into the rAAV9 genome (Ahmed et al., 2013; Xie et al., 2011).
Using a complex in vivo screening approach in C57BL/6 mice, Deverman and colleagues have generated an improved BBB‐crossing AAV9 variant, named AAV.PHP.B, by inserting a 7 amino acid peptide “TLAVPFK” between amino acids 588 and 589 of VP1 gene of AAV2/9 (Deverman et al., 2016). AAV.PHP.B transduced CNS neurons and glia 40–70× more efficient than the parental AAV9 following single i.v. injections to adult C57Bl/6 mice (Deverman et al., 2016) and might be even more pronounced when used in conjunction with self‐complementary genomes (Rincon et al., 2018). The improved neuronal targeting by AAV.PHP.B was observed in the peripheral nervous system and was leveraged for gene therapy in a mouse model of synucleinopathy (Morabito et al., 2017). AAV.PHP.B delivery was moderately more or similarly efficient as compared to its parent AAV9 in adult rats (Jackson, Dayton, Deverman, & Klein, 2016) or NHP (Hordeaux et al., 2018; Matsuzaki et al., 2018). A dose response study in adult rats by i.v. delivery of an enhanced variant, AAV.PHP.EB, showed robust CNS expression in the presence of marked transduction of liver and heart (Dayton, Grames, & Klein, 2018). Enhanced CNS tropism of AAV.PHP.B over AAV9 may be restricted to the model in which it was originally selected from, that is, the C57Bl/6J mouse strain, as neither another mouse strain (BALB/cJ) nor NHP showed the improved brain tropism of AAV‐PHP.B (Hordeaux et al., 2018). Like the development of the enhanced variant of AAV.PHP.B, further exploration of vector variants is required to bridge strain and species limitations. In the meantime, both AAV.PHP.EB and its predecessor remain valuable tools to tackle both modelling and mechanistic issues in AD mouse models.
Other methods of AAV vector evolution were adopted and one such method led to the rational engineering of a chimeric capsid with combined receptor binding properties from the parental AAV2 and AAV9 serotypes. This AAV2g9 was subsequently used to encode guide RNAs targeting the schizophrenia risk gene MIR137 in CRISPR/Cas9 knockin mice and showed exceptional and specific transduction of the mouse CNS with limited to no off‐target expression in the liver (Murlidharan et al., 2016). Furthermore, screening of an AAV2‐based library identified a variant, rAAV2‐retro, that confers expression in neurons after taking up the viruses via their axonal projections and subsequent retrograde transport to the soma (Tervo et al., 2016). However, these engineered capsids have yet to be shown to cross the species barrier into NHP for example and its translational potential into clinics is yet to be shown. Despite this, this rational engineering approach to vector development opens new and hopefully promising possibilities in CNS targeting.
Another promising avenue for advancing CNS delivery of AAV was the discovery that AAV associates with exosomes. Exosome‐associated AAV9s (exo‐AAV9), purified from medium rather than cell lysates during production, were shown to efficiently cross the BBB and infect brain cells after systemic or intravitreal injections (Hudry et al., 2016; Volak et al., 2018; Wassmer, Carvalho, Gyorgy, Vandenberghe, & Maguire, 2017). Interestingly, exo‐AAV8 conferred similar CNS expression to exo‐AAV9 or AAV9, although AAV8 shows limited BBB passage and brain cell infection after systemic delivery (Hudry et al., 2016). Using tetraspanin CD9, a known exosome marker, further boosted the production of exo‐AAV (Schiller, Lemus‐Diaz, Rinaldi Ferreira, Boker, & Gruber, 2018). Another advantage of exo‐AAV is that they can evade very common human neutralizing anti‐AAV antibodies that can significantly reduce transduction rate in the CNS of standard AAV preparations after systemic administration (György, Fitzpatrick, Crommentuijn, Mu, & Maguire, 2014). However, it remains to be shown if this translates to other species.
2.4. AAV‐based CNS gene therapy developments
More recently, gene editing tools like zinc finger nucleases and CRISPR/Cas have been employed to generate targeted genetic manipulations in vivo. AAV represents an ideal gene platform, albeit with its size limitation of around 5 kb. A recent report coupled intravitreal AAV.PHP.B delivery with Cas9/guide RNA (gRNA) selective targeting of the mutant allele as treatment in a mouse model of retinitis pigmentosa (Giannelli et al., 2018). The different components were packaged as separate vectors and co‐delivered to the eye. In a recent first report, Zahur and colleagues generated a single AAV1/2 vector encoding a zinc finger nuclease pair for robust, yet incomplete, reduction of neuronal cathepsin D expression in the mouse brain (Zahur, Tolo, Bahr, & Kugler, 2017). Alternatively, cell‐type specific knockout of genes in adult mice can be achieved by AAV‐mediated delivery of gRNA into mice with Cre‐dependent Cas9 expression, combined with cell‐type specific Cre mouse lines (Platt et al., 2014). While optimization is required for these systems, they may be instrumental in future studies aiming at ablating dominantly acting disease alleles in AD.
Gene therapy has traditionally been employed to treat monogenic diseases by introduction of the functional gene into a loss‐of‐function background. The remarkable commercial translation of gene therapy products in the rare disease space has seen the approval of Glybera, the first gene therapy product to treat lipoprotein lipase deficiency. In addition, Strimvelis, an ex vivo gene therapy product to treat adenosine deaminase deficiency patients was also approved in Europe (Piguet et al., 2017). More recently, Luxturna, which uses AAV2 to deliver a functional copy of the RPE65 gene into the retinal pigment epithelial cells of patients with biallelic RPE‐65 mutation‐associated retinal dystrophy, was approved by the FDA as the first directly administered gene therapy in the United States. Of highest relevance for the neuroscience community, an AAV9‐based gene therapy encoding SMN protein for the treatment of the devastating childhood disorder spinal muscular atrophy has shown promising safety data and outstanding benefit in clinical trials (Mendell et al., 2017). Biologics License Application for the corresponding drug AVXS‐101 has recently been accepted by the FDA with anticipated regulatory action in May 2019. Interestingly, the AAV9 vector was delivered i.v. to paediatric patients at doses of 2 × 1014 vg·kg−1, the highest AAV exposure to humans ever tested. Remarkably, this treatment triggered elevated serum aminotransferase levels in only few patients and these were attenuated by prednisolone. In contrast, a separate study employing pigs and NHPs exposed to dose‐matched AAV9 expressing the same transgene and administered similarly showed massive vector and transgene‐related toxicity, motor neuron degeneration, and even fatalities (Hinderer et al., 2018). The discrepancy between large animal models and clinical observations is difficult to reconcile but may highlight the inherent limitations of animal models as predictors of outcome for human studies. Finally, recent reports of a placebo‐controlled phase 1 trial employing intraparenchymal AAV2 delivery encoding nerve growth factor showed safety and long‐term transgene expression in post‐mortem brains but no clinical benefits (Rafii et al., 2018; Tuszynski et al., 2015).
Neurodegenerative disease is a broad umbrella term for a group of disorders that has a wide and heterogeneous clinical and pathological presentation. This further highlights the very complex nature of any proposed gene therapy. However, the successes of gene therapy of rare disorders like retinal dystrophy provide the basis and foundation for gene therapy in complex conditions like AD.
AD gene therapy is in its infancy but is gaining traction in recent years. Therapeutic success will depend on the route of administration enabling efficient and widespread CNS transduction. A recent report described the preclinical development of AAVrh.10 encoding the human APOE2 cDNA and optimization of the route of administration in NHP (Rosenberg et al., 2018). The clinical grade vector was administered via one of the three routes: multisite injection to the hippocampus; intracisternally to the cisterna magna; and i.c.v. to the lateral ventricle. The delivery, intracisternally to the cisterna magna, was safe and resulted in ApoE2‐HA immunoreactivity throughout the CNS, suggesting successful targeting of AD‐associated regions. In fact, an optimized delivery protocol into the CSF of NHP was reported with translational implications for ApoE2 and other candidate targets in AD (Hinderer et al., 2018; Katz, Goode, Hinderer, Hordeaux, & Wilson, 2018). There are many other examples for potential gene therapy approaches in AD and three recent examples targeting very different pathways are outlined below: (a) The α‐secretase cleavage product of APP, APPsα, has also neurotrophic properties and has therefore been suggested for gene therapy of AD (Mockett, Richter, Abraham, & Muller, 2017). Accordingly, AAV‐mediated expression of APPsα in AD mice with established deficits improved memory deficits and synaptic changes (Fol et al., 2016). (b) Neuronal network aberrations that have been reported in human AD and mouse models (Ittner et al., 2016; Ittner, Gladbach, Bertz, Suh, & Ittner, 2014; Palop & Mucke, 2009; Verret et al., 2012; Vossel et al., 2013). Here, an elegant therapeutic approach that is currently undergoing clinical translation; optogenetic proteins (=surface channels that can be activated/inactivated by light) were delivered into the brain of the 5xFAD AD mouse model to enhance the compromised neuronal network activity and improve functional deficits by means of light exposure (Iaccarino et al., 2016). (c) A recent study identified destabilization of the polyglutamine binding protein 1 underlying synaptic and memory deficits in AD mouse models and possibly humans. Here, AAV‐mediated expression of polyglutamine binding protein 1 rescued synaptic and behavioural deficits in the two AD mouse models 5xFAD and APP‐KI (Tanaka et al., 2018). The large number of potential gene therapy targets in AD warrant detailed discussion in future reviews.
3. AAV‐BASED AD MOUSE MODELS
AAVs offer the potential to directly generate transgenic mice to investigate pathways in vivo, using naïve mice (Table 2). This eliminates the need to hold and maintain large colonies, as well as crossbreed different genetically modified lines. Using AAV allows for the combinatorial testing of several genes/variants in parallel. For example, rAAV1/2 was injected into the hippocampus of mice to express concurrently either K670M/M671L (Swedish)/T714I (Austrian)/V717I (London) mutant APP (APP.SLA) or non‐mutant Tau or P301L mutant Tau to model AD (Jaworski et al., 2009). While AAV‐APP.SLA‐injected mice developed plaques only 6 months after the injection together with a moderate loss of CA neurons in the hippocampus, AAV‐P301L Tau‐injected mice showed pronounced degeneration of CA neurons within weeks (<3) after administration. Similar rapid degeneration of CA neurons was observed when a non‐mutant version of Tau protein was expressed via AAVs in the hippocampus of mice (Jaworski et al., 2009). The degeneration occurred in the absence of NFT formation but was associated with significant neuroinflammation and up‐regulating of cell cycle marker proteins. A follow‐up study revealed that the initial degeneration of hippocampal neurons affects the apical dendrites (Jaworski et al., 2011). While previous studies used stereotaxic delivery of AAVs into specific brain regions of interest, Cook and colleagues delivered rAAV1‐P301L Tau protein into the brains of neonatal mice, resulting in a widespread expression of transgenic Tau, though, for comparison, at slightly lower levels than in P301L Tau transgenic mice (rTg4510 line; Santacruz et al., 2005; Cook et al., 2015). At 6 months of age, mice bearing AAV1‐P301L Tau protein mice showed significant Tau hyperphosphorylation and NFT formation throughout their brains. This was associated with significant neuroinflammation and behavioural deficits.
Table 2.
Summary of AAV‐based AD rodent models discussed in the review article
| AD/FTD gene | AAV serotype | Promoter | Rodent strain | Injection site | Phenotype (time after injection) | Reference |
|---|---|---|---|---|---|---|
| K670M/M671L (Swedish)/T714I (Austrian)/V717I (London) triple mutant APP (APP.SLA) (isoform: APP695) | rAAV1/2 | Human synapsin1 | FVB/N mice | Hippocampus | Aβ plaques, CA neuron loss (6 months) | Jaworski et al. (2009) |
| Non‐mutant Tau (isoform: 2N/4R) | rAAV1/2 | Human synapsin1 | FVB/N mice | Hippocampus | CA neuron loss (<3 weeks) | Jaworski et al. (2009) |
| P301L mutant Tau (isoform: 2N/4R) | rAAV1/2 | Human synapsin1 | FVB/N mice | Hippocampus | CA neuron loss (<3 weeks) | Jaworski et al. (2009) |
| P301L mutant Tau | rAAV1 | Cytomegalovirus enhancer/chicken β‐actin | C57Bl/6 mice | Neonatal brain | Tau hyperphosphorylation, NFTs, neuroinflammation, behavioural deficits (6 months) | Cook et al. (2015) |
| P301L mutant Tau | rAAV1 | Chicken β‐actin | db/db mice (Jax# 000697) | Neonatal | Increase glucose resistance and Tau hyperphosphorylation | Platt et al. (2016) |
| Human M146L mutant PS1 and/or KM670/671NL (Swedish)/V717I (London) mutant APP (isoform: APP751) | rAAVrh10 | Cytomegalovirus enhancer/chicken β‐actin | C57Bl/6J mice | Hippocampus (bilateral) | Synaptic deficits, higher Aβ levels in double than in single transgenic mice | Audrain et al. (2016) |
| KM670/671NL (Swedish) mutant APP | rAAV1 | Chicken β‐actin | Wistar rats | Hippocampus (bilateral) | Cognitive deficits, increased Aβ levels (3 months) | Lawlor et al. (2007) |
| BRI‐Aβ40 | rAAV1 | Chicken β‐actin | Wistar rats | Hippocampus (bilateral) | Cognitive deficits, increased Aβ levels (3 months) | Lawlor et al. (2007) |
| BRI‐Aβ42 | rAAV1 | Chicken β‐actin | Wistar rats | Hippocampus (bilateral) | Cognitive deficits, increased Aβ levels, Aβ plaques (3 months) | Lawlor et al. (2007) |
| P301L Tau | rAAV8 | Cytomegalovirus enhancer/chicken β‐actin | Sprague–Dawley rat | Substantia nigra | Loss of dopaminergic neurons (4 weeks) | Klein et al. (2006) |
| P301L Tau | rAAV9 or rAAVrh10 | Cytomegalovirus enhancer/chicken β‐actin | Sprague–Dawley rat | Substantia nigra | Loss of dopaminergic neurons (2 weeks) | Klein et al. (2006) |
| P301L Tau or TDP‐43 | rAAV9 | Cre recombination‐dependent promoter | Long Evans‐Tg (TH‐Cre) rat | Substantia nigra | Loss of dopaminergic neurons (4 weeks) | Grames et al. (2018) |
AAVs can furthermore be used to drive Tau pathology in models of other common diseases to study the effects of co‐morbidities or shared disease mechanisms. For example, AD and diabetes mellitus have been linked epidemiologically and mechanistically with increased Tau phosphorylation and NFT pathology in P301L Tau transgenic mice subjected to experimental diabetes (Ke, Delerue, Gladbach, Gotz, & Ittner, 2009). rAAV1 has been used to express P301L Tau protein in neonatal db/db mice, a model of Type 2 diabetes mellitus (Platt, Beckett, Kohler, Niedowicz, & Murphy, 2016). Glucose resistance and body weight of AAV‐P301L Tau‐injected mice increased, as compared to control mice injected with the same virus. This was associated with significantly increased Tau phosphorylation in the brain, suggesting pathomechanistic feedback between insulin resistance and Tau pathology. More recently discovered AAV serotypes have been used to model AD in mice. Accordingly, rAAVrh.10‐mediated expression of human M146L mutant PS1 and KM670/671NL (Swedish)/V717I (London) mutant APP (APPSL) in naïve C57Bl/6J mice induced synaptic deficits associated with Aβ formation (Audrain et al., 2016). As expected, Aβ levels were higher in AAV‐APP/PS1 than in AAV‐APP‐ and APP‐PS1‐injected mice respectively. Taken together, a range of AAV serotypes has been used to model Aβ and Tau pathology in mice, providing readily available models to study disease mechanisms.
Apart from mice, AAVs have been used early on to model AD in rats. For example, rAAV1 was used to express KM670/671NL (Swedish) mutant APP or the Aβ40 or Aβ42 sequence linked to the BRI protein individually or in combination in the hippocampus of adult Wistar rats (Lawlor et al., 2007). All constructs resulted in cognitive deficits and increased Aβ levels in the rats 3 months after the surgery. Interestingly, only AAV‐BRI‐Aβ42 rats expressing Aβ42 together with the secretory linker BRI to facilitate release from cells developed plaque pathology, but plaques were structurally different from BRI‐Aβ42 transgenic mice (McGowan et al., 2005), possibly due to different incubation times. In another study, rAAV8 conferred robust GFP expression in the hippocampus or substantia nigra of 3‐month‐old Sprague–Dawley rats, when compared to rAAV1 (less expression), rAAV2, and rAAV5 (both hardly expressing; Klein et al., 2006); Tau protein has been linked to Parkinson's disease (Kurosinski, Guggisberg, & Gotz, 2002). When rAAV8 was used to express P301L mutant Tau protein in the substantia nigra of the rats, a pronounced loss of dopaminergic neurons was found 4 weeks after the injection (Klein et al., 2006), consistent with the rapid degeneration found in mice (Jaworski et al., 2009). Loss of dopaminergic neurons was observed even earlier (within 2 weeks) when rAAV9 or rAAV10 was used to express P301L Tau protein in the nigrostriatal system of rats (Klein, Dayton, Tatom, Diaczynsky, & Salvatore, 2008). Furthermore, loss of dopaminergic neurons and neuroinflammation were more severe when AAV‐P301L Tau protein was injected in aged (20 months old) compared to adult (3 months old) rats (Klein, Dayton, Diaczynsky, & Wang, 2010). A recent study expanded previous work by using rAAV9 to express Cre‐recombinase‐dependent vectors in rats that express Cre under the control of the TH promoter in dopaminergic neurons (Grames et al., 2018). Delivering vectors with loxP‐flanked inverted (inactive) coding sequences allows the testing of several pathogenic proteins, including P301L Tau, under similar experimental conditions in Cre‐expressing cells that flip the CDS and therefore allow transcription. Comparable loss of dopaminergic neurons was achieved by expression of P301L Tau protein and the FTD‐associated TAR‐DNA binding protein 43 in this model. Taken together, while the availability of transgenic AD rat models limits their use for studying pathomechanisms or treatments, using AAV provides an opportunity to overcome these limitations in a cost and time‐sensitive manner.
4. AAVs TO INVESTIGATE AD MECHANISMS
The body of literature using AAVs to investigate the role of specific genes in established AD mouse models continues to grow. Besides providing insight into disease mechanisms, some of these studies may become the foundation of future gene therapy development. Here, we selected three current areas of research to illustrate the potential of AAVs as a tool to investigate disease mechanisms in AD, rather than attempting to comprehensively reflect the use of AAVs in AD research. The discussed areas include brain‐derived neurotrophic factor (BDNF) signalling in the context of Aβ, post‐synaptic Tau mechanisms, and spreading of Tau pathology.
4.1. BDNF signalling in AD
BDNF promotes survival and functions of neurons. In AD, BDNF mRNA, and protein levels were found to be decreased, possibly contributing to memory deficits and neuronal loss (reviewed in Toh, Ng, Tan, Tan, & Chan, 2018; Zhao et al., 2017). Similarly, BDNF expression is lowered in neurodegenerative conditions with Tau pathology (Belrose, Masoudi, Michalski, & Fahnestock, 2014). Furthermore, polymorphisms in BDNF have been linked to an increased risk for late‐onset AD (Guerreiro & Hardy, 2014). Aβ decreases BDNF levels in cells (Garzon & Fahnestock, 2007) and Tau protein can similarly decrease BDNF levels in neuroblastoma cells (Rosa et al., 2016) and when expressed in transgenic mice (pR5 line; Gotz, Chen, Barmettler, & Nitsch, 2001; Jiao et al., 2016). Interestingly, the decreased BDNF in Aβ forming APP transgenic mice (APP23 line; Sturchler‐Pierrat et al., 1997) was restored when Tau protein was deleted in these mice (Rosa et al., 2016). BDNF expression via rAAV8 injected into the lateral ventricle of 3‐month‐old pR5 mice prevented memory deficits and synaptic/neuron loss at 12 months of age but did not change Tau pathology (Jiao et al., 2016). While BDNF is neuroprotective, its precursor proBDNF has the opposing effects. AAV‐mediated expression of proBDNF in Aβ forming AD mice (APP/PS1 line; Jankowsky et al., 2004) at 5 months of age augmented memory deficits, Aβ levels, and pathology 8 weeks after intraventricular virus injections (Chen et al., 2017). The neurodegenerative effects of AAV‐proBDNF in primary neurons were neutralized by blocking or knocking out the p75 neurotrophic receptor (p75NTR). Similarly, the disease‐worsening effects of AAV‐proBDNF in APPswePS1dE9 mice were prevented by co‐administration of p75NTR blocking antibodies (Chen et al., 2017). This suggested that the effects of proBDNF were mediated via the p75NTR, which confers both protective and detrimental signals depending on interacting receptors/ligands (see Meeker & Williams, 2015). Interestingly, p75NTR has also been described as an Aβ receptor in the pathogenesis of AD (Coulson, 2006). Conversely, the ectodomain of the p75NTR (p75ECD) which is extracellularly released upon α‐secretase cleavage protects from Aβ toxicity (Zhou & Wang, 2011). Accordingly, intracerebral delivery of an AAV to express the p75ECD in the brains of Aβ forming APP/PS1 mice improved their memory deficits (Yao et al., 2015). An elegant follow‐up study showed that peripheral expression of the p75ECD by i.m. rAAV8 injection at 3 months of age in APP/PS1 mice improved memory deficits when tested at 12 months of age (Wang et al., 2016). AAV‐p75ECD treatment reduced τ hyperphosphorylation and mitigated neuroinflammation in APP/PS1 mice. Furthermore, treated mice showed reduced Aβ burden, possibly by providing a “peripheral sink” for brain Aβ. The lack of overt peripheral effects of p75ECD expression together with the beneficial central effects in APP/PS1 supports the use of p75ECD as a gene therapy in AD (Wang et al., 2016).
4.2. Post‐synaptic Tau protein in AD
According to the amyloid cascade theory, accumulation of Aβ pathology is disease initiating, and events such as hyperphosphorylation, aggregation, and deposition of τ are downstream events in AD (Hardy & Selkoe, 2002). In both cell culture and animal models Aβ requires Tau protein to mediate toxicity (Ittner et al., 2010; Rapoport, Dawson, Binder, Vitek, & Ferreira, 2002; Roberson et al., 2007). In this context, we have previously shown that Aβ toxicity is mediated by post‐synaptic Tau protein, a process that involves protein complexes of the post‐synaptic density protein 95 (PSD95), the Src kinase Fyn, and Tau protein downstream of N‐methyl‐D‐aspartate receptors (Ittner et al., 2010; Ittner & Gotz, 2011). More recently, we showed that the PSD95/Fyn/Tau complex is regulated by the p38 kinase isoform γ (p38γ) by phosphorylating Tau protein at tyrosine (T)205 (amino acid [aa] numbering according to the longest Tau isoform 2N4R; 441aa; Ittner et al., 2016; Ittner & Ittner, 2018). Accordingly, p38γ activity generates T205 phosphorylation of post‐synaptic Tau protein resulting in dissociation of the PSD95/Fyn/τ complex and consequently prevents Aβ toxicity. AAV‐mediated expression of an active variant of p38γ in primary neurons mitigated Aβ toxicity and, more importantly, prevented memory deficits and neuronal network aberrations in mutant APP transgenic mice (APP23 line; Sturchler‐Pierrat et al., 1997; Ittner et al., 2016). Illustrating the relevance of T205 phosphorylation in this process, AAVs were used to reconstitute Mapt‐deficient mice with point mutant variants; while expression of non‐mutant and T205A variants of τ rendered Mapt‐deficient neurons sensitive to excitotoxicity again, neurons expressing the phosphorylation‐mimicking variant T205E, remained protective (Ittner et al., 2016). This work highlights the potential of AAVs to test several variants of a protein in a time sensitive manner in vivo. Conceptually, this work challenged the long‐standing idea of Tau phosphorylation being a purely disease‐driven event (Ittner & Ittner, 2018) and suggested that further work is required to examine the physiological and pathological relevance of phosphorylation of Tau protein at individual sites. AAVs offer the opportunity to perform testing of larger number of Tau variants in relevant neuronal culture and in vivo (i.e., disease) models. Therapeutically, reducing Tau levels has been proposed as an avenue to prevent progression of AD in humans (Ittner & Ittner, 2018). Using antisense oligonucleotides to reduce human Tau levels in P301S mutant Tau transgenic mice (PS19 line; Yoshiyama et al., 2007) prevented neuronal loss, improved functional deficits, and extended survival (DeVos et al., 2017). Antisense oligonucleotides for Tau protein also reduced levels of this protein in a NHP model (DeVos et al., 2017). In contrast, AAV mediated expression of shRNA to target Tau mRNA and reduce its protein levels in adult (7 months old) naïve C57Bl/6 mice resulted in motor problems and memory deficits (Velazquez et al., 2018). These deficits were reversible when transient Tau knockdown ended, suggesting that this protein is required for memory formation in the adult mammalian brain. Although the exact mechanisms underlying this physiological function of Tau protein remain to be shown, acute Tau reduction was associated with lower levels of BDNF and synaptic proteins as well as decreased spine density. Together, these findings may be of concern for Tau‐reducing therapeutic strategies for AD and related neurodegenerative conditions with Tau pathology. Hence, targeting Tau‐dependent disease mechanisms more specifically may need to be explored. Accordingly, neuron‐specific expression of p38γ variants to specifically phosphorylate protective site of post‐synaptic Tau protein may be a new avenue for gene therapy in AD (Ittner & Ittner, 2018).
4.3. Spreading of Tau pathology in AD
The spatial distribution of Tau pathology in the brain correlates with the progression of clinical symptoms in AD. NFT pathology is initiated in the entorhinal cortex (EC) and spread from there via neuronal connections from one brain area to the next, following a disease‐specific pattern (Braak & Braak, 1995). Extending the ages of studied brain to include teens suggested that pre‐tangle Tau pathology is found in brainstem neurons as early as 10 years of age, although it remains unclear if these would have progressed to disease (Braak, Thal, Ghebremedhin, & Del Tredici, 2011). The spreading of Tau pathology can be modelled in mice using localized transgenic Tau protein expression but only within the limitation of current expression technologies (Harris et al., 2012). Furthermore, both injection of AD brain extracts and recombinant Tau fibrils induced NFT pathology when injected into the brains of human Tau transgenic mice (ALZ17 line; Probst et al., 2000, and PS19 line; Yoshiyama et al., 2007, respectively; Clavaguera et al., 2013; Iba et al., 2013). Furthermore, AAV has been used for localized expression of Tau protein in specific brain regions to investigate spreading of Tau pathology in mouse models. Expression of human P301L mutant Tau protein in the EC of naïve CD‐1 mice using rAAV2/9 resulted in degeneration of the perforant pathway of the hippocampus and, 10 weeks after injection, spread of Tau protein to dentate gyrus (DG) neurons (Siman, Lin, Malthankar‐Phatak, & Dong, 2013). This occurs with no transfer of GFP within 14 weeks when mice were injected with just AAV expressing GFP into the EC (Siman et al., 2013). A follow‐up study further showed transfer of rAAV2/9‐GFP from EC to DG neurons when co‐injected with rAAV2/9 expressing P301L Tau protein (Siman, Cocca, & Dong, 2015), highlighting a possible problem of AAV transfer rather than protein transfer between neurons may underlie spreading in these models. Notably, rapamycin prevented the trans‐synaptic transfer of AAVs and neurodegeneration of the EC (Siman et al., 2015). In another study, rAAV2/6 was used to locally express P301L mutant full‐length Tau protein in the medial EC of C57Bl/6 mice (Asai et al., 2015). After 28 days, human Tau protein was detectable in interconnected neurons of the DG, sowing rapid spreading in vivo. Interestingly, depletion of microglia and inhibition of exosome formation in these mice markedly reduced the spreading of Tau protein, suggesting that microglial uptake and processing of Tau protein released from neurons is an integral part of neuron‐to‐neuron spreading of Tau pathology in disease (Asai et al., 2015). Co‐injected AAVs expressing GFP were used to show that this model is based on spread of Tau protein rather than spread or diffusion of AAVs into the DG. Different findings of AAV spread between studies may also be due to the different AAV serotypes used. A different approach to study intraneuronal spreading of Tau protein was introduced by using an AAV construct that encodes human P301L mutant Tau together with eGFP linked by a 2A self‐cleavage sequence (AAV‐eGFP‐2A‐huTauP301L; Wegmann et al., 2015). The 2A sequence undergoes post‐translational auto‐cleavage resulting in Tau and GFP expression in “donor” neurons transduced by rAAV8, while non‐infected “recipient” neurons that have taken up Tau protein released from expressing interconnected cells only harbour Tau, but not GFP. Local injection of AAV‐eGFP‐2A‐huTauP301L into the EC of naïve C57Bl/6 mice resulted in spreading of Tau protein to DG recipient neurons (Wegmann et al., 2015); Tau protein is released from neurons in an activity dependent manner, as shown by microdialysis paired with electrical stimulation in brains for freely moving mice (Yamada et al., 2014). In a recent study, Schultz and colleagues followed up on this work and used AAV‐mediated gene expression to study the effects of neuronal activity on spreading of non‐mutant human Tau protein in primary neurons and mice (Schultz et al., 2018). In this study, full‐length Tau (2N4R) was expressed together with an excitatory designer receptors exclusively activated by a designer drug (DREADD) using rAAV2 and rAAV8 respectively. Treatment with clozapine N‐oxide of cells expressing Tau protein/DREADD resulted in dose‐dependent increase of both neuronal activity (as determined by Ca2+ imaging) and Tau protein release. When injected into the ventral hippocampus of naïve, 11‐week‐old mice, twice daily administration of CNO over 8 weeks resulted in significantly more pronounced Tau pathology in the injected brain areas and spreading of Tau protein to interconnected brain areas when compared to vehicle‐treated animals (Schultz et al., 2018). The spreading was associated with signs of neurodegeneration in recipient brain regions. Similar results were obtained when AAV‐Tau/DREADD were injected into Mapt −/− mice and treated with CNO (Schultz et al., 2018), confirming the findings from transgenic mice with localized P301L Tau expression on a Mapt −/− background that endogenous Tau protein is not required for spreading of transgenic Tau (Wegmann et al., 2015).
5. GENE THERAPY STRATEGIES IN AD MOUSE MODELS
Immunotherapy targeting Aβ has advanced through numerous clinical trials with some recent promising outcomes. In AD mouse models, both active and passive vaccination against Aβ has cleared plaque pathology and improved memory deficits (Schenk et al., 1999). Here, AAVs have been used to express antibodies or antigen in preclinical studies in AD mice. For example, AAVs encoding the Aβ42 sequence fused to cholera toxin B (AAV‐CB‐Aβ42) were administered via different routes (oral, i.m., and intranasal) into V717I (London) or V717F (Indiana) mutant APP transgenic mice (APP22; Sturchler‐Pierrat et al., 1997, or PDAPP line; Games et al., 1995; note: unclear which line has been used) to obtain robust circulating anti‐Aβ antibody levels (Zhang et al., 2003). Both, preventive treatment at 1 month of age and therapeutic intervention in aged (12 months) PDAPP mice improved memory deficits and reduced Aβ burden, irrespective of the route of AAV delivery (Zhang et al., 2003). In another study, oral application of an AAV encoding different variants of Aβ (AAV/Aβ), namely, Aβaa1–43 and Aβaa1–21 resulted with expression in epithelial cells of the intestine together with circulating antibody titers against Aβ for over 6 months after the treatment (Hara et al., 2004). AAV/Aβ‐treated mutant APP transgenic mice (Tg2576 line; Hsiao et al., 1996) showed reduced Aβ load in the absence of a detectable T cell response. In a follow‐up study, 10‐month‐old Tg2576 mice were treated orally with AAV/Aβ, resulting in improved memory performance at 12–13 months of age and reduced Aβ deposition (Mouri et al., 2007). These improvements occurred in the absence of lymphatic infiltration of the brain. Recent work suggested that the antibody‐induced clearance of Aβ after AAV/Aβ‐treatment of APP transgenic mice was mediated via autophagy (Wang et al., 2015). These finding triggered the idea to use AAV to express antibodies in AD mouse models as a passive immunization‐like approach (Robert, Gilbert, & Gaillet, 2017). Accordingly, an anti‐Aβ antibody was delivered i.m. via a rAAV1 in Tg2576 mice, using a CMV promoter‐driven construct that expresses anti‐Aβ IgG heavy and light chains connected by an auto‐cleavable furin 2A linker (Shimada et al., 2013). Treated mice showed sustained circulating anti‐Aβ antibodies and markedly reduced Aβ load in two cohorts of Tg2576 mice injected at 5 (preventive) and 10 (therapeutic) months of age respectively. Similarly, rAAVrh.10 was injected bilaterally into the hippocampus of 2‐ to 3‐month‐old P301S Tau transgenic mice (hTau.P301S line; Allen et al., 2002) to express the sequence of the anti‐phosphorylated Tau antibody PHF1 (Liu et al., 2016). At 6 months of age, abundance of phosphorylated and insoluble Tau protein was markedly reduced in the treated mice, and hippocampal volumes increased.
An alternative approach is the expression of single‐chain antibodies (scFvs) against Aβ from AAV in APP transgenic mice. scFv are truncation variants of IgGs comprising the variable, antigen recognizing regions of the IgG heavy, and light chains coupled by a linker sequence (Manoutcharian, Perez‐Garmendia, & Gevorkian, 2017). scFvs retain the ability to bind the antigen but lack antigen cross‐linking and T cell receptor activation properties. Recombinant scFvs against Aβ and Tau protein have been used to reduce pathology and improve deficits in mutant APP and Tau transgenic mouse lines respectively. Intrahippocampal injection of rAAV1 to express a M13 phage library‐derived scFv against Aβ in triple transgenic mice expressing mutant APP, PS1, and Tau protein (3xTgAD line; Oddo et al., 2003) reduced Aβ load, Tau phosphorylation, and memory deficits (Ryan et al., 2010). Using the CMV promoter will have driven anti‐Aβ scFv expression in different CNS cell types in this approach. Therefore, effects may have been mediated by secreted and/or intracellular scFvs. Similarly, intraventricular delivery of rAAV5 to express scFv against Aβ in double transgenic mutant APP/PS1 mice at 10 months of age reduced brain and CSF Aβ load determined 5 months later (Kou et al., 2011). Different from the previous study, micro‐haemorrhages together with increased cerebral amyloid angiopathy were found in treated mice. A CMV enhancer/β‐actin promoter was used to drive anti‐Aβ scFv expression. Interestingly, the same construct delivery with a rAAV1 cause neuronal apoptosis and microglial response, while using a rAAV2 resulted only limited expression (Kou et al., 2011). Variants of scFvs called intrabodies have been used to target intracellular Aβ when delivered via rAAV2 into the brains of 2‐month‐old 3xTgAD mice, significantly reducing both Aβ and Tau pathology burden (Sudol et al., 2009). scFvs targeting Tau protein have also shown efficacy in preventing neuropathology and deficits in pR5 mice (Nisbet et al., 2017). Similarly, AAV‐expressed scFvs against human Tau protein after intraventricular delivery at birth decreased Tau phosphorylation and deposition in ageing PS19 mice in the absence of a proinflammatory response (Ising et al., 2017). Furthermore, rAAV5 injected into the hippocampus of 3‐month‐old P301L Tau transgenic mice (JNPL3 line; Lewis et al., 2000) to express an scFv derived from the conformational anti‐Tau antibody MC1 (Vitale et al., 2018). Four months after the injection, AAV‐scFv‐MC1‐treated JNPL3 mice showed significantly reduced Tau hyperphosphorylation, aggregation, and insolubility. Taken together, AAV‐mediated scFv expression showed therapeutic efficacy in both APP and τ‐based AD mouse models, with a favourable inflammatory profile. Notably, only a small proportion of circulating antibodies enters the brain. This may limit the use of AAV for peripheral antibody expression similar to that of conventional active and passive vaccination. Direct expression of antibodies or their variants in the brain using AAV circumvents these imitations, but translation to humans remains to be shown.
The introduction of CRISPR/Cas9 genome editing has not only revolutionized the generation of genetically modified mice (and other species; Delerue & Ittner, 2015) but raised the idea of corrective gene therapy in different diseases (Giannelli et al., 2018). Pairing CRISPR/Cas9 genome editing with AAV gene delivery may prove to be a viable avenue here. Accordingly, György and colleagues used exo‐AAV9 injected into the hippocampus of adult Tg2576 mice to deliver mutant KM670/671NL APP (APPswe) allele‐specific gRNA and Cas9 (György et al., 2018). They found specific disruption (i.e., indel formation) in 1.3% of APPswe alleles in all (n = 5) injected mice. For comparison, the rate of indel formation was much higher in fibroblasts from human APPswe carriers, which carry only two copies in their genome (György et al., 2018). This proof‐of‐concept study highlighted the possibility of gene targeting as a potential avenue for treating familial AD and the potential role of AAVs herein. Intensive research in this area is expected to deliver significant advances in the near future, focusing on gene correction.
6. SUMMARY
While transgenic mouse models remain the major in vivo “tools” used for AD research, the use of AAV in the field is steadily increasing. Most frequently used to study the effects of candidate genes on AD neuropathology or functional phenotypes, AAV provides the advantage of testing gene functions and variants directly in established mouse models of AD, without the need to first intercross different genetic lines. This makes transgenic mice assessible for a larger community, for which standard housing and breeding of standard genetically modified mouse models is prohibitively expensive or limited by capacities of facilities. However, intrinsic limitations of AAVs remain, including the relatively small size packaging capacity of 5 kb, which limits the use for studying large DNAs. Furthermore, inter‐animal variability of expression levels and/or patterns upon AAV delivery due to heterogeneity and proportions of transduced cells requires larger number of animals for experiments and assessment of correct anatomical targeting and transgene expression in all test subjects. Finally, producing larger quantities of AAV requires specialized facilities and staff associated with significant costs. Nevertheless, AAV has and will continue to expand the toolbox for studying AD in mouse models, complementing but not replacing transgenic mouse strains. Lastly, AAV offers the opportunity to study AD in other species, including NHP.
In addition to their value for studying mechanisms in disease, AAV is likely to play an eminent role in potential gene‐therapies for neurodegenerative diseases, including AD. With first AAV‐based gene therapies approved by the FDA for use in rarer human conditions, past barriers to consider AAV as therapeutic options for more common disease will continue to fall. Besides preclinical testing of novel gene‐therapies in relevant AD animal models, much development of new AAV vectors is required to overcome species barriers for translation and avoid invasive delivery methods, such as intracranial injections.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017a,b; Alexander, Peters et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors received funding from the National Health and Medical Research Council (Grants 1081916, 1123564, 1132524, 1136241, 1143848, and 1143978) and the Australian Research Council (Grants DP150104321, DP170100781, and DP170100843).
Ittner LM, Klugmann M, Ke YD. Adeno‐associated virus‐based Alzheimer's disease mouse models and potential new therapeutic avenues. Br J Pharmacol. 2019;176:3649–3665. 10.1111/bph.14637
REFERENCES
- Ahmed, S. S. , Li, H. , Cao, C. , Sikoglu, E. M. , Denninger, A. R. , Su, Q. , … Gao, G. (2013). A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS Gene therapy in Canavan mice. Molecular Therapy, 21(12), 2136–2147. 10.1038/mt.2013.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174, S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, B. , Ingram, E. , Takao, M. , Smith, M. J. , Jakes, R. , Virdee, K. , … Goedert, M. (2002). Abundant τ filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S τ protein. The Journal of Neuroscience, 22(21), 9340–9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area‐Gomez, E. , de Groof, A. , Bonilla, E. , Montesinos, J. , Tanji, K. , Boldogh, I. , … Schon, E. A. (2018). A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death & Disease, 9, 335 10.1038/s41419-017-0215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, H. , Ikezu, S. , Tsunoda, S. , Medalla, M. , Luebke, J. , Haydar, T. , … Ikezu, T. (2015). Depletion of microglia and inhibition of exosome synthesis halt τ propagation. Nature Neuroscience, 18(11), 1584–1593. 10.1038/nn.4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer, D. F. , Kreuz, S. , & Rumpel, S. (2013). Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS ONE, 8, e76310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood, C. S. , & Bowen, R. L. (2015). A unified hypothesis of early‐ and late‐onset Alzheimer's disease pathogenesis. Journal of Alzheimer's Disease, 47, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain, M. , Fol, R. , Dutar, P. , Potier, B. , Billard, J. M. , Flament, J. , … Braudeau, J. (2016). Alzheimer's disease‐like APP processing in wild‐type mice identifies synaptic defects as initial steps of disease progression. Molecular Neurodegeneration, 11, 5 10.1186/s13024-016-0070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil, F. , Guerin, P. A. , Dutheil, N. , Li, Q. , Klugmann, M. , Meissner, W. G. , … Fernagut, P. O. (2017). Viral‐mediated oligodendroglial α‐synuclein expression models multiple system atrophy. Movement Disorders, 32(8), 1230–1239. 10.1002/mds.27041 [DOI] [PubMed] [Google Scholar]
- Belrose, J. C. , Masoudi, R. , Michalski, B. , & Fahnestock, M. (2014). Increased pro‐nerve growth factor and decreased brain‐derived neurotrophic factor in non‐Alzheimer's disease tauopathies. Neurobiology of Aging, 35, 926–933. [DOI] [PubMed] [Google Scholar]
- Bertram, L. , & Tanzi, R. E. (2012). The genetics of Alzheimer's disease. Progress in Molecular Biology and Translational Science, 107, 79–100. [DOI] [PubMed] [Google Scholar]
- Bevan, A. K. , Duque, S. , Foust, K. D. , Morales, P. R. , Braun, L. , Schmelzer, L. , … Kaspar, B. K. (2011). Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Molecular Therapy, 19(11), 1971–1980. 10.1038/mt.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, G. S. (2014). Amyloid‐β and τ: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurology, 71, 505–508. 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- Braak, H. , & Braak, E. (1995). Staging of Alzheimer's disease‐related neurofibrillary changes. Neurobiology of Aging, 16, 271–278. discussion 278‐284 [DOI] [PubMed] [Google Scholar]
- Braak, H. , Thal, D. R. , Ghebremedhin, E. , & Del Tredici, K. (2011). Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. Journal of Neuropathology and Experimental Neurology, 70, 960–969. [DOI] [PubMed] [Google Scholar]
- Broekman, M. L. , Comer, L. A. , Hyman, B. T. , & Sena‐Esteves, M. (2006). Adeno‐associated virus vectors serotyped with AAV8 capsid are more efficient than AAV‐1 or ‐2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience, 138, 501–510. [DOI] [PubMed] [Google Scholar]
- Burger, C. , Gorbatyuk, O. S. , Velardo, M. J. , Peden, C. S. , Williams, P. , Zolotukhin, S. , … Muzyczka, N. (2004). Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Molecular Therapy, 10(2), 302–317. [DOI] [PubMed] [Google Scholar]
- Burrell, J. R. , Halliday, G. M. , Kril, J. J. , Ittner, L. M. , Gotz, J. , Kiernan, M. C. , & Hodges, J. R. (2016). The frontotemporal dementia‐motor neuron disease continuum. Lancet, 388(10047), 919–931. 10.1016/S0140-6736(16)00737-6 [DOI] [PubMed] [Google Scholar]
- Cairns, N. J. , Bigio, E. H. , Mackenzie, I. R. , Neumann, M. , Lee, V. M. , Hatanpaa, K. J. , … Consortium for Frontotemporal Lobar Degeneration . (2007). Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathologica, 114(1), 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley, C. N. , & Wolfe, J. H. (2006). Transduction characteristics of adeno‐associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Molecular Therapy, 13, 528–537. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Zhang, T. , Jiao, S. , Zhou, X. , Zhong, J. , Wang, Y. , … Xu, Z. (2017). proBDNF accelerates brain amyloid‐β deposition and learning and memory impairment in APPswePS1dE9 transgenic mice. Journal of Alzheimer's Disease, 59(3), 941–949. 10.3233/JAD-161191 [DOI] [PubMed] [Google Scholar]
- Clavaguera, F. , Akatsu, H. , Fraser, G. , Crowther, R. A. , Frank, S. , Hench, J. , … Tolnay, M. (2013). Brain homogenates from human tauopathies induce τ inclusions in mouse brain. Proceedings of the National Academy of Sciences of the United States of America, 110(23), 9535–9540. 10.1073/pnas.1301175110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, C. , Kang, S. S. , Carlomagno, Y. , Lin, W. L. , Yue, M. , Kurti, A. , … Fryer, J. D. (2015). τ deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model. Human Molecular Genetics, 24(21), 6198–6212. 10.1093/hmg/ddv336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson, E. J. (2006). Does the p75 neurotrophin receptor mediate Aβ‐induced toxicity in Alzheimer's disease? Journal of Neurochemistry, 98, 654–660. [DOI] [PubMed] [Google Scholar]
- Crosson, S. M. , Dib, P. , Smith, J. K. , & Zolotukhin, S. (2018). Helper‐free production of laboratory grade AAV and purification by iodixanol density gradient centrifugation. Molecular Therapy ‐ Methods & Clinical Development, 10, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, B. L. , Stein, C. S. , Heth, J. A. , Martins, I. , Kotin, R. M. , Derksen, T. A. , … Chiorini, J. A. (2000). Recombinant adeno‐associated virus type 2, 4, and 5 vectors: Transduction of variant cell types and regions in the mammalian central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 97(7), 3428–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton, R. D. , Grames, M. S. , & Klein, R. L. (2018). More expansive gene transfer to the rat CNS: AAV PHP.EB vector dose‐response and comparison to AAV PHP.B. Gene Therapy, 25, 392–400. [DOI] [PubMed] [Google Scholar]
- Delerue, F. , & Ittner, L. M. (2015). Genome editing in mice using CRISPR/Cas9: Achievements and prospects. Cloning and Transgenesis, 4, 2. [Google Scholar]
- Deverman, B. E. , Pravdo, P. L. , Simpson, B. P. , Kumar, S. R. , Chan, K. Y. , Banerjee, A. , … Gradinaru, V. (2016). Cre‐dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature Biotechnology, 34(2), 204–209. 10.1038/nbt.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVos, S. L. , Miller, R. L. , Schoch, K. M. , Holmes, B. B. , Kebodeaux, C. S. , Wegener, A. J. , … Miller, T. M. (2017). τ reduction prevents neuronal loss and reverses pathological τ deposition and seeding in mice with tauopathy. Science Translational Medicine, 9(374). 10.1126/scitranslmed.aag0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, D. , Sharma, P. , Yang, J. , Yue, Y. , Dudus, L. , Zhang, Y. , … Engelhardt, J. F. (1998). Circular intermediates of recombinant adeno‐associated virus have defined structural characteristics responsible for long‐term episomal persistence in muscle tissue. Journal of Virology, 72(11), 8568–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During, M. J. , Young, D. , Baer, K. , Lawlor, P. , & Klugmann, M. (2003). Development and optimization of adeno‐associated virus vector transfer into the central nervous system. Methods in Molecular Medicine, 76, 221–236. [DOI] [PubMed] [Google Scholar]
- Feng, X. , Eide, F. F. , Jiang, H. , & Reder, A. T. (2004). Adeno‐associated viral vector‐mediated ApoE expression in Alzheimer's disease mice: Low CNS immune response, long‐term expression, and astrocyte specificity. Frontiers in Bioscience, 9, 1540–1546. [DOI] [PubMed] [Google Scholar]
- Fol, R. , Braudeau, J. , Ludewig, S. , Abel, T. , Weyer, S. W. , Roederer, J. P. , … Müller, U. C. (2016). Viral gene transfer of APPsα rescues synaptic failure in an Alzheimer's disease mouse model. Acta Neuropathologica, 131(2), 247–266. 10.1007/s00401-015-1498-9 [DOI] [PubMed] [Google Scholar]
- Forrest, S. L. , Kril, J. J. , Stevens, C. H. , Kwok, J. B. , Hallupp, M. , Kim, W. S. , … Halliday, G. M. (2018). Retiring the term FTDP‐17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain, 141(2), 521–534. 10.1093/brain/awx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust, K. D. , Nurre, E. , Montgomery, C. L. , Hernandez, A. , Chan, C. M. , & Kaspar, B. K. (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature Biotechnology, 27, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franich, N. R. , Fitzsimons, H. L. , Fong, D. M. , Klugmann, M. , During, M. J. , & Young, D. (2008). AAV vector‐mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington's disease. Molecular Therapy, 16, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland‐Leuner, K. , Stockburger, C. , Denzer, I. , Eckert, G. P. , & Muller, W. E. (2014). Mitochondrial dysfunction: Cause and consequence of Alzheimer's disease. Progress in Molecular Biology and Translational Science, 127, 183–210. [DOI] [PubMed] [Google Scholar]
- Games, D. , Adams, D. , Alessandrini, R. , Barbour, R. , Berthelette, P. , Blackwell, C. , et al. (1995). Alzheimer‐type neuropathology in transgenic mice overexpressing V717F β‐amyloid precursor protein. Nature, 373(6514), 523–527. [DOI] [PubMed] [Google Scholar]
- Gao, G. , Vandenberghe, L. H. , Alvira, M. R. , Lu, Y. , Calcedo, R. , Zhou, X. , & Wilson, J. M. (2004). Clades of adeno‐associated viruses are widely disseminated in human tissues. Journal of Virology, 78(12), 6381–6388. 10.1128/JVI.78.12.6381-6388.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon, D. J. , & Fahnestock, M. (2007). Oligomeric amyloid decreases basal levels of brain‐derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. The Journal of Neuroscience, 27, 2628–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou, E. , Sidiropoulou, K. , Richter, J. , Papaneophytou, C. , Sargiannidou, I. , Kagiava, A. , … Kleopa, K. A. (2017). Gene therapy targeting oligodendrocytes provides therapeutic benefit in a leukodystrophy model. Brain, 140(3), 599–616. 10.1093/brain/aww351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli, S. G. , Luoni, M. , Castoldi, V. , Massimino, L. , Cabassi, T. , Angeloni, D. , … Broccoli, V. (2018). Cas9/sgRNA selective targeting of the P23H Rhodopsin mutant allele for treating retinitis pigmentosa by intravitreal AAV9.PHP.B‐based delivery. Human Molecular Genetics, 27, 761–779. 10.1093/hmg/ddx438 [DOI] [PubMed] [Google Scholar]
- Goedert, M. , & Spillantini, M. G. (2006). A century of Alzheimer's disease. Science, 314, 777–781. [DOI] [PubMed] [Google Scholar]
- Gotz, J. , Chen, F. , Barmettler, R. , & Nitsch, R. M. (2001). τ filament formation in transgenic mice expressing P301L τ. The Journal of Biological Chemistry, 276, 529–534. 10.1074/jbc.M006531200 [DOI] [PubMed] [Google Scholar]
- Gotz, J. , & Ittner, L. M. (2008). Animal models of Alzheimer's disease and frontotemporal dementia. Nature Reviews. Neuroscience, 9, 532–544. [DOI] [PubMed] [Google Scholar]
- Grames, M. S. , Dayton, R. D. , Jackson, K. L. , Richard, A. D. , Lu, X. , & Klein, R. L. (2018). Cre‐dependent AAV vectors for highly targeted expression of disease‐related proteins and neurodegeneration in the substantia nigra. The FASEB Journal, 32, 4420–4427. 10.1096/fj.201701529RR [DOI] [PubMed] [Google Scholar]
- Gray, S. J. , Matagne, V. , Bachaboina, L. , Yadav, S. , Ojeda, S. R. , & Samulski, R. J. (2011). Preclinical differences of intravascular AAV9 delivery to neurons and glia: A comparative study of adult mice and nonhuman primates. Molecular Therapy, 19, 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger, J. C. , Soltys, S. M. , & Samulski, R. J. (2016). Production of recombinant adeno‐associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Molecular Therapy, 24, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, D. , & Buning, H. (2017). Small but increasingly mighty: Latest advances in AAV vector research, design, and evolution. Human Gene Therapy, 28, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Guerreiro, R. , & Hardy, J. (2014). Genetics of Alzheimer's disease. Neurotherapeutics, 11, 732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- György, B. , Fitzpatrick, Z. , Crommentuijn, M. H. , Mu, D. , & Maguire, C. A. (2014). Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials, 35, 7598–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- György, B. , Lööv, C. , Zaborowski, M. P. , Takeda, S. , Kleinstiver, B. P. , Commins, C. , … Ingelsson, M. (2018). CRISPR/Cas9 mediated disruption of the Swedish APP allele as a therapeutic approach for early‐onset Alzheimer's disease. Molecular therapy Nucleic acids, 11, 429–440. 10.1016/j.omtn.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, H. , Monsonego, A. , Yuasa, K. , Adachi, K. , Xiao, X. , Takeda, S. , … Tabira, T. (2004). Development of a safe oral Aβ vaccine using recombinant adeno‐associated virus vector for Alzheimer's disease. Journal of Alzheimer's Disease, 6(5), 483–488. [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, J. , & Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science, 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Harris, J. A. , Koyama, A. , Maeda, S. , Ho, K. , Devidze, N. , Dubal, D. B. , … Mucke, L. (2012). Human P301L‐mutant τ expression in mouse entorhinal‐hippocampal network causes τ aggregation and presynaptic pathology but no cognitive deficits. PLoS ONE, 7(9), e45881 10.1371/journal.pone.0045881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer, C. , Bell, P. , Katz, N. , Vite, C. H. , Louboutin, J. P. , Bote, E. , … Wilson, J. M. (2018). Evaluation of intrathecal routes of administration for adeno‐associated viral vectors in large animals. Human Gene Therapy, 29(1), 15–24. https://doig.org/10.1089/hum.2017.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer, C. , Katz, N. , Buza, E. L. , Dyer, C. , Goode, T. , Bell, P. , … Wilson, J. M. (2018). Severe toxicity in nonhuman primates and piglets following high‐dose intravenous administration of an adeno‐associated virus vector expressing human SMN. Human Gene Therapy, 29(3), 285–298. 10.1089/hum.2018.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocquemiller, M. , Giersch, L. , Audrain, M. , Parker, S. , & Cartier, N. (2016). Adeno‐associated virus‐based gene therapy for CNS diseases. Human Gene Therapy, 27, 478–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux, J. , Wang, Q. , Katz, N. , Buza, E. L. , Bell, P. , & Wilson, J. M. (2018). The neurotropic properties of AAV‐PHP.B are limited to C57BL/6J mice. Molecular Therapy, 26, 664–668. 10.1016/j.ymthe.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao, K. , Chapman, P. , Nilsen, S. , Eckman, C. , Harigaya, Y. , Younkin, S. , … Cole, G. (1996). Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science, 274(5284), 99–102. [DOI] [PubMed] [Google Scholar]
- Hudry, E. , Martin, C. , Gandhi, S. , Gyorgy, B. , Scheffer, D. I. , Mu, D. , … Maguire, C. A. (2016). Exosome‐associated AAV vector as a robust and convenient neuroscience tool. Gene Therapy, 23(4), 380–392. 10.1038/gt.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino, H. F. , Singer, A. C. , Martorell, A. J. , Rudenko, A. , Gao, F. , Gillingham, T. Z. , … Tsai, L.‐H. (2016). γ frequency entrainment attenuates amyloid load and modifies microglia. Nature, 540, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba, M. , Guo, J. L. , McBride, J. D. , Zhang, B. , Trojanowski, J. Q. , & Lee, V. M. (2013). Synthetic τ fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's‐like tauopathy. The Journal of Neuroscience, 33, 1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising, C. , Gallardo, G. , Leyns, C. E. G. , Wong, C. H. , Stewart, F. , Koscal, L. J. , … Holtzman, D. M. (2017). AAV‐mediated expression of anti‐τ scFvs decreases τ accumulation in a mouse model of tauopathy. The Journal of Experimental Medicine, 214(5), 1227–1238. 10.1084/jem.20162125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner, A. , Chua, S. W. , Bertz, J. , Volkerling, A. , van der Hoven, J. , Gladbach, A. , … Ittner, L. M. (2016). Site‐specific phosphorylation of τ inhibits amyloid‐β toxicity in Alzheimer's mice. Science, 354(6314), 904–908. [DOI] [PubMed] [Google Scholar]
- Ittner, A. , & Ittner, L. M. (2018). Dendritic τ in Alzheimer's disease. Neuron, 99, 13–27. [DOI] [PubMed] [Google Scholar]
- Ittner, A. A. , Gladbach, A. , Bertz, J. , Suh, L. S. , & Ittner, L. M. (2014). p38 MAP kinase‐mediated NMDA receptor‐dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer inverted question marks disease. Acta Neuropathologica Communications, 2, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner, L. M. , & Gotz, J. (2011). Amyloid‐β and τ—A toxic pas de deux in Alzheimer's disease. Nature Reviews. Neuroscience, 12, 67–72. [DOI] [PubMed] [Google Scholar]
- Ittner, L. M. , Ke, Y. D. , Delerue, F. , Bi, M. , Gladbach, A. , van Eersel, J. , … Götz, J. (2010). Dendritic function of τ mediates amyloid‐β toxicity in Alzheimer's disease mouse models. Cell, 142(3), 387–397. 10.1016/j.cell.2010.06.036 [DOI] [PubMed] [Google Scholar]
- Iwata, N. , Sekiguchi, M. , Hattori, Y. , Takahashi, A. , Asai, M. , Ji, B. , … Saido, T. C. (2013). Global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice. Scientific Reports, 3, 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, K. L. , Dayton, R. D. , Deverman, B. E. , & Klein, R. L. (2016). Better targeting, better efficiency for wide‐scale neuronal transduction with the synapsin promoter and AAV‐PHP.B. Front Mol Neurosci, 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky, J. L. , Fadale, D. J. , Anderson, J. , Xu, G. M. , Gonzales, V. , Jenkins, N. A. , … Borchelt, D. R. (2004). Mutant presenilins specifically elevate the levels of the 42 residue β‐amyloid peptide in vivo: Evidence for augmentation of a 42‐specific γ secretase. Human Molecular Genetics, 13(2), 159–170. [DOI] [PubMed] [Google Scholar]
- Jaworski, T. , Dewachter, I. , Lechat, B. , Croes, S. , Termont, A. , Demedts, D. , … Van Leuven, F. (2009). AAV‐τ mediates pyramidal neurodegeneration by cell‐cycle re‐entry without neurofibrillary tangle formation in wild‐type mice. PLoS ONE, 4(10), e7280 10.1371/journal.pone.0007280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski, T. , Lechat, B. , Demedts, D. , Gielis, L. , Devijver, H. , Borghgraef, P. , … Van Leuven, F. (2011). Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for τ‐mediated neurodegeneration. The American Journal of Pathology, 179(4), 2001–2015. 10.1016/j.ajpath.2011.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, S. S. , Shen, L. L. , Zhu, C. , Bu, X. L. , Liu, Y. H. , Liu, C. H. , … Wang, Y. J. (2016). Brain‐derived neurotrophic factor protects against τ‐related neurodegeneration of Alzheimer's disease. Translational Psychiatry, 6(10), e907 10.1038/tp.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson, T. , Atwal, J. K. , Steinberg, S. , Snaedal, J. , Jonsson, P. V. , Bjornsson, S. , … Stefansson, K. (2012). A mutation in APP protects against Alzheimer's disease and age‐related cognitive decline. Nature, 488(7409), 96–99. 10.1038/nature11283 [DOI] [PubMed] [Google Scholar]
- Katz N, Goode T, Hinderer C, Hordeaux J, & Wilson JM (2018). Standardized method for intra‐cisterna magna delivery under fluoroscopic guidance in nonhuman primates. Hum Gene Ther Methods. [DOI] [PubMed] [Google Scholar]
- Ke, Y. D. , Delerue, F. , Gladbach, A. , Gotz, J. , & Ittner, L. M. (2009). Experimental diabetes mellitus exacerbates τ pathology in a transgenic mouse model of Alzheimer's disease. PLoS ONE, 4, e7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, R. L. , Dayton, R. D. , Diaczynsky, C. G. , & Wang, D. B. (2010). Pronounced microgliosis and neurodegeneration in aged rats after τ gene transfer. Neurobiology of Aging, 31, 2091–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, R. L. , Dayton, R. D. , Leidenheimer, N. J. , Jansen, K. , Golde, T. E. , & Zweig, R. M. (2006). Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of τ or green fluorescent proteins. Molecular Therapy, 13, 517–527. 10.1016/j.ymthe.2005.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, R. L. , Dayton, R. D. , Tatom, J. B. , Diaczynsky, C. G. , & Salvatore, M. F. (2008). τ expression levels from various adeno‐associated virus vector serotypes produce graded neurodegenerative disease states. The European Journal of Neuroscience, 27, 1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, R. L. , Dayton, R. D. , Tatom, J. B. , Henderson, K. M. , & Henning, P. P. (2008). AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: Effects of serotype, promoter and purification method. Molecular Therapy, 16, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman, M. A. , & Schaffer, D. V. (2014). Engineering adeno‐associated viruses for clinical gene therapy. Nature Reviews Genetics, 15, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou, J. , Kim, H. , Pattanayak, A. , Song, M. , Lim, J. E. , Taguchi, H. , … Fukuchi, K. (2011). Anti‐amyloid‐β single‐chain antibody brain delivery via AAV reduces amyloid load but may increase cerebral hemorrhages in an Alzheimer's disease mouse model. Journal of Alzheimer's Disease, 27(1), 23–38. 10.3233/JAD-2011-110230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosinski, P. , Guggisberg, M. , & Gotz, J. (2002). Alzheimer's and Parkinson's disease—Overlapping or synergistic pathologies? Trends in Molecular Medicine, 8, 3–5. [DOI] [PubMed] [Google Scholar]
- Lawlor, P. A. , Bland, R. J. , Das, P. , Price, R. W. , Holloway, V. , Smithson, L. , … Golde, T. E. (2007). Novel rat Alzheimer's disease models based on AAV‐mediated gene transfer to selectively increase hippocampal Aβ levels. Molecular Neurodegeneration, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, P. A. , Bland, R. J. , Mouravlev, A. , Young, D. , & During, M. J. (2009). Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Molecular Therapy, 17, 1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner, K. , Hauptmann, S. , Abdel‐Kader, R. , Scherping, I. , Keil, U. , Strosznajder, J. B. , … Müller, W. E. (2007). Mitochondrial dysfunction: The first domino in brain aging and Alzheimer's disease? Antioxidants & Redox Signaling, 9(10), 1659–1675. [DOI] [PubMed] [Google Scholar]
- Lewis, J. , McGowan, E. , Rockwood, J. , Melrose, H. , Nacharaju, P. , Van Slegtenhorst, M. , … Hutton, M. (2000). Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) τ protein. Nature Genetics, 25(4), 402–405. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Zhao, L. , Blackman, B. , Parmar, M. , Wong, M. Y. , Woo, T. , … Paul, S. M. (2016). Vectored intracerebral immunization with the anti‐τ monoclonal antibody PHF1 markedly reduces τ pathology in mutant τ transgenic mice. The Journal of Neuroscience, 36(49), 12425–12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock, M. , Alvira, M. , Vandenberghe, L. H. , Samanta, A. , Toelen, J. , Debyser, Z. , & Wilson, J. M. (2010). Rapid, simple, and versatile manufacturing of recombinant adeno‐associated viral vectors at scale. Human Gene Therapy, 21, 1259–1271. 10.1089/hum.2010.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoutcharian, K. , Perez‐Garmendia, R. , & Gevorkian, G. (2017). Recombinant antibody fragments for neurodegenerative diseases. Current Neuropharmacology, 15, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki, Y. , Konno, A. , Mochizuki, R. , Shinohara, Y. , Nitta, K. , Okada, Y. , & Hirai, H. (2018). Intravenous administration of the adeno‐associated virus‐PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neuroscience Letters, 665, 182–188. 10.1016/j.neulet.2017.11.049 [DOI] [PubMed] [Google Scholar]
- Mawuenyega, K. G. , Sigurdson, W. , Ovod, V. , Munsell, L. , Kasten, T. , Morris, J. C. , … Bateman, R. J. (2010). Decreased clearance of CNS β‐amyloid in Alzheimer's disease. Science, 330, 1774 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, E. , Pickford, F. , Kim, J. , Onstead, L. , Eriksen, J. , Yu, C. , … Golde, T. (2005). Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron, 47(2), 191–199. 10.1016/j.neuron.2005.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker, R. B. , & Williams, K. S. (2015). The p75 neurotrophin receptor: At the crossroad of neural repair and death. Neural Regeneration Research, 10, 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell, J. R. , Al‐Zaidy, S. , Shell, R. , Arnold, W. D. , Rodino‐Klapac, L. R. , Prior, T. W. , … Kaspar, B. K. (2017). Single‐dose gene‐replacement therapy for spinal muscular atrophy. The New England Journal of Medicine, 377(18), 1713–1722. 10.1056/NEJMoa1706198 [DOI] [PubMed] [Google Scholar]
- Merkel, S. F. , Andrews, A. M. , Lutton, E. M. , Mu, D. , Hudry, E. , Hyman, B. T. , … Ramirez, S. H. (2017). Trafficking of adeno‐associated virus vectors across a model of the blood‐brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. Journal of Neurochemistry, 140(2), 216–230. 10.1111/jnc.13861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett, B. G. , Richter, M. , Abraham, W. C. , & Muller, U. C. (2017). Therapeutic potential of secreted amyloid precursor protein APPsα. Frontiers in Molecular Neuroscience, 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito, G. , Giannelli, S. G. , Ordazzo, G. , Bido, S. , Castoldi, V. , Indrigo, M. , … Broccoli, V. (2017). AAV‐PHP.B‐mediated global‐scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Molecular Therapy, 25(12), 2727–2742. 10.1016/j.ymthe.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri, A. , Noda, Y. , Hara, H. , Mizoguchi, H. , Tabira, T. , & Nabeshima, T. (2007). Oral vaccination with a viral vector containing Aβ cDNA attenuates age‐related Aβ accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. The FASEB Journal, 21, 2135–2148. [DOI] [PubMed] [Google Scholar]
- Mudannayake, J. M. , Mouravlev, A. , Fong, D. M. , & Young, D. (2016). Transcriptional activity of novel ALDH1L1 promoters in the rat brain following AAV vector‐mediated gene transfer. Molecular Therapy ‐ Methods & Clinical Development, 3, 16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik, A. , Reul, J. , Friedel, T. , Muth, A. , Hartmann, K. P. , Schneider, I. C. , … Buchholz, C. J. (2017). Covalent coupling of high‐affinity ligands to the surface of viral vector particles by protein trans‐splicing mediates cell type‐specific gene transfer. Biomaterials, 144, 84–94. 10.1016/j.biomaterials.2017.07.032 [DOI] [PubMed] [Google Scholar]
- Murlidharan, G. , Sakamoto, K. , Rao, L. , Corriher, T. , Wang, D. , Gao, G. , … Asokan, A. (2016). CNS‐restricted transduction and CRISPR/Cas9‐mediated gene deletion with an engineered AAV vector. Molecular Therapy‐‐Nucleic Acids, 5(7), e338 10.1038/mtna.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet, R. M. , Van der Jeugd, A. , Leinenga, G. , Evans, H. T. , Janowicz, P. W. , & Gotz, J. (2017). Combined effects of scanning ultrasound and a τ‐specific single chain antibody in a τ transgenic mouse model. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo, S. , Caccamo, A. , Shepherd, J. D. , Murphy, M. P. , Golde, T. E. , Kayed, R. , … LaFerla, F. M. (2003). Triple‐transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron, 39(3), 409–421. [DOI] [PubMed] [Google Scholar]
- Palop, J. J. , & Mucke, L. (2009). Epilepsy and cognitive impairments in Alzheimer disease. Archives of Neurology, 66, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet, F. , Alves, S. , & Cartier, N. (2017). Clinical gene therapy for neurodegenerative diseases: Past, present, and future. Human Gene Therapy, 28, 988–1003. 10.1089/hum.2017.160 [DOI] [PubMed] [Google Scholar]
- Pillay, S. , Meyer, N. L. , Puschnik, A. S. , Davulcu, O. , Diep, J. , Ishikawa, Y. , … Carette, J. E. (2016). An essential receptor for adeno‐associated virus infection. Nature, 530(7588), 108–112. 10.1038/nature16465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay, S. , Zou, W. , Cheng, F. , Puschnik, A. S. , Meyer, N. L. , Ganaie, S. S. , … Carette, J. E. (2017). AAV serotypes have distinctive interactions with domains of the cellular receptor AAVR. Journal of Virology. 10.1128/JVI.00391-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, R. J. , Chen, S. , Zhou, Y. , Yim, M. J. , Swiech, L. , Kempton, H. R. , … Zhang, F. (2014). CRISPR‐Cas9 knockin mice for genome editing and cancer modeling. Cell, 159(2), 440–455. 10.1016/j.cell.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, T. L. , Beckett, T. L. , Kohler, K. , Niedowicz, D. M. , & Murphy, M. P. (2016). Obesity, diabetes, and leptin resistance promote τ pathology in a mouse model of disease. Neuroscience, 315, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst, A. , Gotz, J. , Wiederhold, K. H. , Tolnay, M. , Mistl, C. , Jaton, A. L. , … Goedert, M. (2000). Axonopathy and amyotrophy in mice transgenic for human four‐repeat τ protein. Acta Neuropathologica (Berlin), 99(5), 469–481. [DOI] [PubMed] [Google Scholar]
- Rabinowitz, J. E. , Rolling, F. , Li, C. , Conrath, H. , Xiao, W. , Xiao, X. , & Samulski, R. J. (2002). Cross‐packaging of a single adeno‐associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. Journal of Virology, 76(2), 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii, M. S. , Tuszynski, M. H. , Thomas, R. G. , Barba, D. , Brewer, J. B. , Rissman, R. A. , … AAV2‐NGF Study Team . (2018). Adeno‐associated viral vector (serotype 2)‐nerve growth factor for patients with Alzheimer disease: A randomized clinical trial. JAMA Neurology, 75(7), 834–841. 10.1001/jamaneurol.2018.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport, M. , Dawson, H. N. , Binder, L. I. , Vitek, M. P. , & Ferreira, A. (2002). τ is essential to β‐amyloid‐induced neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 99, 6364–6369. 10.1073/pnas.092136199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon, M. Y. , de Vin, F. , Duque, S. I. , Fripont, S. , Castaldo, S. A. , Bouhuijzen‐Wenger, J. , … Holt, M. W. (2018). Widespread transduction of astrocytes and neurons in the mouse central nervous system after systemic delivery of a self‐complementary AAV‐PHP.B vector. Gene Therapy, 25, 83–92. [DOI] [PubMed] [Google Scholar]
- Roberson, E. D. , Scearce‐Levie, K. , Palop, J. J. , Yan, F. , Cheng, I. H. , Wu, T. , … Mucke, L . (2007). Reducing endogenous τ ameliorates amyloid β‐induced deficits in an Alzheimer's disease mouse model. Science, 316(5825), 750–754. [DOI] [PubMed] [Google Scholar]
- Robert, M. A. , Gilbert, R. , & Gaillet, B. (2017). Antibody delivery mediated by recombinant adeno‐associated virus for the treatment of various chronic and infectious diseases. Current Gene Therapy, 16, 363–374. 10.2174/1566523217666170102111251 [DOI] [PubMed] [Google Scholar]
- Rosa, E. , Mahendram, S. , Ke, Y. D. , Ittner, L. M. , Ginsberg, S. D. , & Fahnestock, M. (2016). τ downregulates BDNF expression in animal and cellular models of Alzheimer's disease. Neurobiology of Aging, 48, 135–142. 10.1016/j.neurobiolaging.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario, A. M. , Cruz, P. E. , Ceballos‐Diaz, C. , Strickland, M. R. , Siemienski, Z. , Pardo, M. , … Chakrabarty, P. (2016). Microglia‐specific targeting by novel capsid‐modified AAV6 vectors. Molecular Therapy ‐ Methods & Clinical Development, 3, 16026 10.1038/mtm.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, J. B. , Kaplitt, M. G. , De, B. P. , Chen, A. , Flagiello, T. , Salami, C. , … Crystal, R. G. (2018). AAVrh.10‐mediated APOE2 central nervous system gene therapy for APOE4‐associated Alzheimer's disease. Human Gene Therapy. Clinical Development, 29(1), 24–47. 10.1089/humc.2017.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, J. B. , Sondhi, D. , Rubin, D. G. , Monette, S. , Chen, A. , Cram, S. , … Crystal, R. G. (2014). Comparative efficacy and safety of multiple routes of direct CNS administration of adeno‐associated virus gene transfer vector serotype rh.10 expressing the human arylsulfatase A cDNA to nonhuman primates. Human Gene Therapy. Clinical Development, 25(3), 164–177. 10.1089/humc.2013.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, D. A. , Mastrangelo, M. A. , Narrow, W. C. , Sullivan, M. A. , Federoff, H. J. , & Bowers, W. J. (2010). Aβ‐directed single‐chain antibody delivery via a serotype‐1 AAV vector improves learning behavior and pathology in Alzheimer's disease mice. Molecular Therapy, 18, 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch, L. , Salegio, E. A. , San Sebastian, W. , Kells, A. P. , Foust, K. D. , Bringas, J. R. , … Bankiewicz, K. S. (2012). Adeno‐associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Human Gene Therapy, 23(4), 382–389. 10.1089/hum.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz, K. , Lewis, J. , Spires, T. , Paulson, J. , Kotilinek, L. , Ingelsson, M. , … Ashe, K. H. (2005). τ suppression in a neurodegenerative mouse model improves memory function. Science, 309(5733), 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk, D. , Barbour, R. , Dunn, W. , Gordon, G. , Grajeda, H. , Guido, T. , … Seubert, P. (1999). Immunization with amyloid‐β attenuates Alzheimer‐disease‐like pathology in the PDAPP mouse. Nature, 400(6740), 173–177. [DOI] [PubMed] [Google Scholar]
- Schiller, L. T. , Lemus‐Diaz, N. , Rinaldi Ferreira, R. , Boker, K. O. , & Gruber, J. (2018). Enhanced production of exosome‐associated AAV by overexpression of the tetraspanin CD9. Molecular Therapy ‐ Methods & Clinical Development, 9, 278–287. 10.1016/j.omtm.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, M. K. Jr. , Gentzel, R. , Usenovic, M. , Gretzula, C. , Ware, C. , Parmentier‐Batteur, S. , … Zariwala, H. A. (2018). Pharmacogenetic neuronal stimulation increases human τ pathology and trans‐synaptic spread of τ to distal brain regions in mice. Neurobiology of Disease, 118, 161–176. 10.1016/j.nbd.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Sehara, Y. , Fujimoto, K. I. , Ikeguchi, K. , Katakai, Y. , Ono, F. , Takino, N. , … Muramatsu, S. I. (2017). Persistent expression of dopamine‐synthesizing enzymes 15 years after gene transfer in a primate model of Parkinson's disease. Human Gene Therapy. Clinical Development, 28(2), 74–79. 10.1089/humc.2017.010 [DOI] [PubMed] [Google Scholar]
- Shimada, M. , Abe, S. , Takahashi, T. , Shiozaki, K. , Okuda, M. , Mizukami, H. , … Okuda, K. (2013). Prophylaxis and treatment of Alzheimer's disease by delivery of an adeno‐associated virus encoding a monoclonal antibody targeting the amyloid β protein. PLoS ONE, 8(3), e57606 10.1371/journal.pone.0057606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben, A. , Van Langenhove, T. , Engelborghs, S. , Martin, J. J. , Boon, P. , Cras, P. , … Cruts, M. (2012). The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathologica, 124(3), 353–372. 10.1007/s00401-012-1029-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman, R. , Cocca, R. , & Dong, Y. (2015). The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early‐stage Alzheimer‐type tauopathy. PLoS ONE, 10, e0142340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman, R. , Lin, Y. G. , Malthankar‐Phatak, G. , & Dong, Y. (2013). A rapid gene delivery‐based mouse model for early‐stage Alzheimer disease‐type tauopathy. Journal of Neuropathology and Experimental Neurology, 72, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato, M. , Bennett, J. , Boulis, N. M. , Castro, M. G. , Fink, D. J. , Goins, W. F. , … Glorioso, J. C. (2013). Progress in gene therapy for neurological disorders. Nature Reviews Neurology, 9(5), 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag, F. , Schmidt, K. , & Kleinschmidt, J. A. (2010). A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proceedings of the National Academy of Sciences of the United States of America, 107, 10220–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler‐Pierrat, C. , Abramowski, D. , Duke, M. , Wiederhold, K. H. , Mistl, C. , Rothacher, S. , … Sommer, B. (1997). Two amyloid precursor protein transgenic mouse models with Alzheimer disease‐like pathology. Proceedings of the National Academy of Sciences of the United States of America, 94, 13287–13292. 10.1073/pnas.94.24.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol, K. L. , Mastrangelo, M. A. , Narrow, W. C. , Frazer, M. E. , Levites, Y. R. , Golde, T. E. , … Bowers, W. J. (2009). Generating differentially targeted amyloid‐β specific intrabodies as a passive vaccination strategy for Alzheimer's disease. Molecular Therapy, 17(12), 2031–2040. 10.1038/mt.2009.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, H. , Kondo, K. , Chen, X. , Homma, H. , Tagawa, K. , Kerever, A. , … Okazawa, H. (2018). The intellectual disability gene PQBP1 rescues Alzheimer's disease pathology. Molecular Psychiatry, 23(10), 2090–2110. 10.1038/s41380-018-0253-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy, Y. , Biferi, M. G. , Besse, A. , Astord, S. , Cohen‐Tannoudji, M. , Marais, T. , & Barkats, M. (2015). Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Frontiers in Molecular Neuroscience, 8, 36 10.3389/fnmol.2015.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans, J. M. , Vandenberghe, L. H. , Haute, C. V. , Thiry, I. , Deroose, C. M. , Mortelmans, L. , … Baekelandt, V. (2007). Comparative analysis of adeno‐associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Human Gene Therapy, 18(3), 195–206. [DOI] [PubMed] [Google Scholar]
- Tervo, D. G. , Hwang, B. Y. , Viswanathan, S. , Gaj, T. , Lavzin, M. , Ritola, K. D. , … Karpova, A. Y. (2016). A designer AAV variant permits efficient retrograde access to projection neurons. Neuron, 92(2), 372–382. 10.1016/j.neuron.2016.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh, Y. L. , Ng, T. , Tan, M. , Tan, A. , & Chan, A. (2018). Impact of brain‐derived neurotrophic factor genetic polymorphism on cognition: A systematic review. Brain and Behavior: A Cognitive Neuroscience Perspective, 8, e01009 10.1002/brb3.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski, M. H. , Yang, J. H. , Barba, D. , Hoi‐Sang, U. , Bakay, R. A. , Pay, M. M. , … Nagahara, A. H. (2015). Nerve growth factor gene therapy: Activation of neuronal responses in Alzheimer disease. JAMA Neurology, 72, 1139–1147. 10.1001/jamaneurol.2015.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, J. D. , Ulland, T. K. , Colonna, M. , & Holtzman, D. M. (2017). Elucidating the role of TREM2 in Alzheimer's disease. Neuron, 94, 237–248. [DOI] [PubMed] [Google Scholar]
- Velazquez, R. , Ferreira, E. , Tran, A. , Turner, E. C. , Belfiore, R. , Branca, C. , & Oddo, S. (2018). Acute τ knockdown in the hippocampus of adult mice causes learning and memory deficits. Aging Cell, 17(4), e12775 10.1111/acel.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret, L. , Mann, E. O. , Hang, G. B. , Barth, A. M. , Cobos, I. , Ho, K. , … Palop, J. J. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell, 149(3), 708–721. 10.1016/j.cell.2012.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, F. , Giliberto, L. , Ruiz, S. , Steslow, K. , Marambaud, P. , & d'Abramo, C. (2018). Anti‐τ conformational scFv MC1 antibody efficiently reduces pathological τ species in adult JNPL3 mice. Acta Neuropathologica Communications, 6, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volak, A. , LeRoy, S. G. , Natasan, J. S. , Park, D. J. , Cheah, P. S. , Maus, A. , … Maguire, C. A. (2018). Virus vector‐mediated genetic modification of brain tumor stromal cells after intravenous delivery. Journal of Neuro‐Oncology, 139(2), 293–305. 10.1007/s11060-018-2889-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jonquieres, G. , Fröhlich, D. , Klugmann, C. B. , Wen, X. , Harasta, A. E. , Ramkumar, R. , … Klugmann, M. (2016). Recombinant human myelin‐associated glycoprotein promoter drives selective AAV‐mediated transgene expression in oligodendrocytes. Frontiers in Molecular Neuroscience, 9, 13 10.3389/fnmol.2016.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jonquieres, G. , Mersmann, N. , Klugmann, C. B. , Harasta, A. E. , Lutz, B. , Teahan, O. , … Klugmann, M. (2013). Glial promoter selectivity following AAV‐delivery to the immature brain. PLoS ONE, 8(6), e65646 10.1371/journal.pone.0065646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jonquieres, G. , Spencer, Z. H. T. , Rowlands, B. D. , Klugmann, C. B. , Bongers, A. , Harasta, A. E. , … Klugmann, M. (2018). Uncoupling N‐acetylaspartate from brain pathology: Implications for Canavan disease gene therapy. Acta Neuropathologica, 135(1), 95–113. 10.1007/s00401-017-1784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel, K. A. , Beagle, A. J. , Rabinovici, G. D. , Shu, H. , Lee, S. E. , Naasan, G. , … Mucke, L. (2013). Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurology, 70(9), 1158–1166. 10.1001/jamaneurol.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. C. , Zhang, T. , Kuerban, B. , Jin, Y. L. , Le, W. , Hara, H. , … Chui, D.‐H. (2015). Autophagy is involved in oral rAAV/Aβ vaccine‐induced Aβ clearance in APP/PS1 transgenic mice. Neuroscience Bulletin, 31(4), 491–504. 10.1007/s12264-015-1546-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. H. , Wang, Y. R. , Zhang, T. , Jiao, S. S. , Liu, Y. H. , Zeng, F. , … Wang, Y. J. (2016). Intramuscular delivery of p75NTR ectodomain by an AAV vector attenuates cognitive deficits and Alzheimer's disease‐like pathologies in APP/PS1 transgenic mice. Journal of Neurochemistry, 138(1), 163–173. 10.1111/jnc.13616 [DOI] [PubMed] [Google Scholar]
- Wassmer, S. J. , Carvalho, L. S. , Gyorgy, B. , Vandenberghe, L. H. , & Maguire, C. A. (2017). Exosome‐associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Scientific Reports, 7, 45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe, A. , Ohtsuka, M. , Kinoshita, M. , Takaji, M. , Isa, K. , Mizukami, H. , … Yamamori, T. (2015). Comparative analyses of adeno‐associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neuroscience Research, 93, 144–157. 10.1016/j.neures.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Wegmann, S. , Maury, E. A. , Kirk, M. J. , Saqran, L. , Roe, A. , DeVos, S. L. , … Hyman, B. T. (2015). Removing endogenous τ does not prevent τ propagation yet reduces its neurotoxicity. The EMBO Journal, 34(24), 3028–3041. 10.15252/embj.201592748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , Xie, Q. , Zhang, H. , Ameres, S. L. , Hung, J. H. , Su, Q. , … Gao, G. (2011). MicroRNA‐regulated, systemically delivered rAAV9: A step closer to CNS‐restricted transgene expression. Molecular Therapy, 19(3), 526–535. 10.1038/mt.2010.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K. , Holth, J. K. , Liao, F. , Stewart, F. R. , Mahan, T. E. , Jiang, H. , … Holtzman, D. M. (2014). Neuronal activity regulates extracellular τ in vivo. The Journal of Experimental Medicine, 211(3), 387–393. 10.1084/jem.20131685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Li, S. , Wang, H. , Guo, Y. , Gessler, D. J. , Cao, C. , … Gao, G. (2014). Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Molecular Therapy, 22(7), 1299–1309. 10.1038/mt.2014.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X. Q. , Jiao, S. S. , Saadipour, K. , Zeng, F. , Wang, Q. H. , Zhu, C. , … Wang, Y. J. (2015). p75NTR ectodomain is a physiological neuroprotective molecule against amyloid‐β toxicity in the brain of Alzheimer's disease. Molecular Psychiatry, 20(11), 1301–1310. 10.1038/mp.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama, Y. , Higuchi, M. , Zhang, B. , Huang, S. M. , Iwata, N. , Saido, T. C. , … Lee, V. M. (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron, 53(3), 337–351. [DOI] [PubMed] [Google Scholar]
- Zahur, M. , Tolo, J. , Bahr, M. , & Kugler, S. (2017). Long‐term assessment of AAV‐mediated zinc finger nuclease expression in the mouse brain. Frontiers in Molecular Neuroscience, 10, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Yang, B. , Mu, X. , Ahmed, S. S. , Su, Q. , He, R. , … Gao, G. (2011). Several rAAV vectors efficiently cross the blood‐brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Molecular Therapy, 19, 1440–1448. 10.1038/mt.2011.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Wu, X. , Qin, C. , Qi, J. , Ma, S. , Zhang, H. , … He, W. (2003). A novel recombinant adeno‐associated virus vaccine reduces behavioral impairment and β‐amyloid plaques in a mouse model of Alzheimer's disease. Neurobiology of Disease, 14(3), 365–379. [DOI] [PubMed] [Google Scholar]
- Zhao, H. , Alam, A. , San, C. Y. , Eguchi, S. , Chen, Q. , Lian, Q. , & Ma, D. (2017). Molecular mechanisms of brain‐derived neurotrophic factor in neuro‐protection: Recent developments. Brain Research, 1665, 1–21. 10.1016/j.brainres.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Zhou, X. F. , & Wang, Y. J. (2011). The p75NTR extracellular domain: A potential molecule regulating the solubility and removal of amyloid‐β. Prion, 5, 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli, C. , Soltys, S. , Rengo, G. , & Rabinowitz, J. E. (2008). Analysis of AAV serotypes 1‐9 mediated gene expression and tropism in mice after systemic injection. Molecular Therapy, 16, 1073–1080. [DOI] [PubMed] [Google Scholar]


